Abstract
Objectives
Hospital admissions for gout flares have increased dramatically in recent years, despite widely available, effective medications for the treatment and prevention of flares. We conducted a systematic review to evaluate the effectiveness and implementation of interventions in patients hospitalized for gout flares.
Methods
A search was conducted in MEDLINE, Embase and the Cochrane library, from database inception to 8 April 2021, using the terms ‘gout’ and ‘hospital’ and their synonyms. Studies were included if they evaluated the effectiveness and/or implementation of interventions during hospital admissions or emergency department attendances for gout flares. Risk of bias assessments were performed for included studies.
Results
Nineteen articles were included. Most studies were small, retrospective analyses performed in single centres, with concerns for bias. Eleven studies (including five randomized controlled trials) reported improved patient outcomes following pharmacological interventions with known efficacy in gout, including allopurinol, prednisolone, NSAIDs and anakinra. Eight studies reported improved outcomes associated with non-pharmacological interventions: inpatient rheumatology consultation and a hospital gout management protocol. No studies to date have prospectively evaluated strategies designed to prevent re-admissions of patients hospitalized for gout flares.
Conclusion
There is an urgent need for high-quality, prospective studies of strategies for improving uptake of urate-lowering therapies in hospitalized patients, incorporating prophylaxis against flares and treat-to-target optimization of serum urate levels. Such studies are essential if the epidemic of hospital admissions from this treatable condition is to be countered.
Keywords: gout, hospital, admission, emergency, inpatient, prevention, urate-lowering therapy, allopurinol, febuxostat
Rheumatology key messages
There is a paucity of high-quality studies of interventions in patients hospitalized for gout flares.
No studies have prospectively evaluated strategies for optimizing urate-lowering therapies and preventing re-admissions following hospitalizations.
These studies are urgently needed if the epidemic of gout admissions is to be halted.
Introduction
Gout is characterized by recurrent flares of joint pain and swelling, which can necessitate hospital admission when severe. Highly effective, low-cost medications are available for the treatment of gout flares: colchicine, NSAIDs and corticosteroids [1–3]. Flares can be prevented by urate-lowering therapies (ULTs), of which allopurinol is the most widely used [1–3]. British Society for Rheumatology (BSR) and EULAR guidelines recommend offering ULT to all patients with gout, with up-titration to achieve serum urate (SU) levels of 300–360 micromol/l (5–6 mg/dl), to facilitate crystal dissolution [1, 2]. The ACR gout management guideline was recently updated to conditionally recommend initiation of ULT during flares, rather than delayed initiation of ULT after flare resolution [3].
Despite effective treatments, hospitalizations for gout flares have increased dramatically: doubling in the USA between 1993 and 2011, from 4.4 to 8.8 admissions per 100 000 adults, respectively [4]; doubling in Canada between 2000 and 2011, from 3.8 to 7.6 admissions per 100 000 adults [5]; and increasing by 58.4% in England between 2006 and 2017, from 7.9 to 12.5 admissions per 100 000 adults [6]. This contrasts with the decline in hospitalizations from RA [4–6]. There are multiple contributing factors to the epidemic of gout hospitalizations: the prevalence of gout has increased in Western countries in recent years on a background of an ageing population with rising prevalences of obesity and metabolic syndrome [7, 8]; the management of gout is frequently suboptimal in primary care, rheumatology clinics and inpatient settings, and only a minority of patients achieve the SU levels required to prevent flares [8, 9].
Hospital admissions provide a unique opportunity to engage patients in shared decision-making and begin the process of establishing optimal ULT. What is not known is how best to implement evidence-based treatments during hospitalizations for gout. Such strategies are essential if the rising number of gout admissions is to be countered. The objective of this systematic review was to evaluate the evidence for the effectiveness of interventions in patients hospitalized with gout.
Methods
Database search strategy and eligibility criteria
A systematic literature search was conducted using the MEDLINE, Embase and Cochrane library databases. Studies were eligible if they evaluated the effectiveness and/or implementation of interventions in patients aged ≥18 years during hospital admissions or emergency department (ED) attendances for gout flares. Studies involving patients with secondary admission diagnoses of gout were also eligible for inclusion. Search terms utilized included ‘gout’, ‘hospital’ and their synonyms (see Supplementary Data S1 for the full list of search terms used, available at Rheumatology online). Interventions could be pharmacological or non-pharmacological, for example implementation of a management protocol.
Outcomes were selected following consensus discussion around measures felt to be important in the management and follow-up of hospitalized gout patients. Primary outcome measures were the frequency of admission to hospital and/or ED attendances for gout flares; the frequency of gout flares following the intervention; and length of stay in hospital. Additional outcomes of interest were time to resolution of the initial gout flare; time to initiation of treatment; time to first flare re-occurrence; change in pain scores; change in inflammatory markers (CRP, ESR); adverse event rates; and the proportions of patients (i) undergoing joint aspiration and/or steroid injection during admission, (ii) with a SU level measured during admission, (iii) prescribed ULT on or after discharge, (iv) with discharge plans and/or outpatient follow-up for gout, or (iv) attaining target SU levels.
An initial search of databases was performed on 10 February 2021, followed by a re-run of the search on 8 April 2021 to ensure additional relevant studies were included. Eligible study types were randomized controlled trials (RCTs), non-randomized controlled trials, prospective cohort studies, retrospective cohort studies, case–control studies, and case series reporting at least five patients. Case reports were excluded.
The study was performed in accordance with the preferred reporting system for systematic reviews (PRISMA) [10], and was registered with the international prospective register of systematic reviews (PROSPERO registration ID: CRD42021245672).
Data extraction
Two reviewers (M.D.R. and B.D.C.) screened manuscript titles and abstracts. Full texts of relevant studies were reviewed against the eligibility criteria. Data extraction was performed by two reviewers (M.D.R. and B.D.C.). Study characteristics extracted included study type, participant numbers, demographics, disease characteristics, interventions, and outcome measures as detailed above. Discrepancies arising between reviewers during study selection or data extraction were resolved through consensus discussion, with involvement of a third reviewer (J.B.G.) where necessary.
Risk of bias determination
A bias assessment was conducted by two reviewers (M.D.R. and B.D.C.). The Cochrane Risk of Bias 2 (RoB 2) tool was used for RCTs [11]. the Newcastle–Ottawa Scale (NOS) was used for non-randomized studies [12]. Discrepancies were resolved through consensus discussion, with involvement of a third reviewer (J.B.G.) where necessary.
Data synthesis
A narrative synthesis was performed due to the small number of eligible studies and the differing interventions and outcome measures; meta-analysis was not possible for these reasons.
Results
Study characteristics
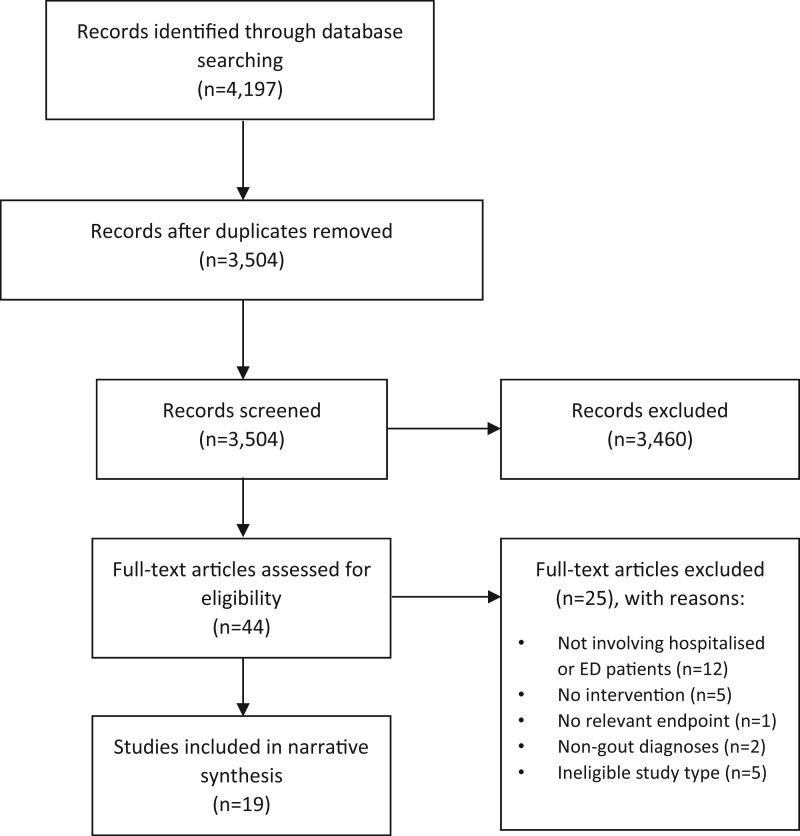
The systematic literature search identified 4197 studies, of which 19 were included (Fig. 1 and Table 1 [13–31]). Of the included studies, five were RCTs, one was a prospective cohort study and 13 were retrospective analyses. Eleven studies assessed outcomes after pharmacological interventions: ULT (six studies), prednisolone vs indomethacin (two studies), indomethacin vs ketorolac (one study), anakinra (one study), and adrenocorticotropic hormone (ACTH) (one study). Eight studies assessed outcomes after non-pharmacological interventions: inpatient rheumatology consultation (seven studies), and an inpatient gout management protocol (one study). Of the five included RCTs, one was deemed to be at high risk of bias [13], three had some concerns for bias [14–16], and one was at low risk of bias [17] (Supplementary Fig. S1, available at Rheumatology online). All non-randomized studies had potential sources of bias (Supplementary Fig. S2, available at Rheumatology online).
Fig. 1.
PRISMA flowchart of studies identified from the systematic literature search. Adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. ED: emergency department
Table 1.
Summary of characteristics of studies included within the systematic review, highlighting key outcome measures and study findings
| Study author/year/country | Study design | Participants | Intervention | Comparator | Outcomes measures | Findings |
|---|---|---|---|---|---|---|
| Rainer et al., 2016, Hong Kong [14] | RCT | 416 patients presenting to EDs (four centres) with gout flares | Prednisolone 30 mg OD orally for 5 days | Indomethacin 50 mg TDS orally for 2 days then 25 mg TDS for 3 days | Improvement in pain (VAS); adverse events; time to resolution of symptoms; length of ED stay; return visits to ED | Equivalent reductions in pain at rest and on activity for prednisolone and indomethacin; no major adverse events; more minor adverse events with indomethacin than prednisolone (P <0.001); no differences in length of ED stay or return visits to ED within 14 days |
| Man et al., 2007, Hong Kong [15] | RCT | 90 patients presenting to ED (single centre) with suspected gout flares | Prednisolone 30 mg OD orally for 5 days | Indomethacin 50 mg TDS orally for 2 days then 25 mg TDS for 3 days | Improvement in pain (VAS); adverse events | Rate of decrease in pain on activity greater for prednisolone than indomethacin (P =0.0026); more adverse events with indomethacin than prednisolone (P < 0.05) |
| Shrestha et al., 1995, USA [13] | RCT | 20 patients presenting to EDs (two centres) with gout flares | Indomethacin 50 mg OD orally single dose | Ketorolac 60 mg i.m. single dose | Improvement in pain (Wong-Baker FACES® Rating Scale); adverse events | Equivalent reductions in pain between the study arms at 2 h; more rebound increases in pain with ketorolac at 6 h (P <0.05); no adverse events |
| Ghosh et al., 2013, USA [18] | Retrospective | 26 patients hospitalized for gout flares; flares resistant to standard treatments and/or contraindications to these treatments | Anakinra, multiple dosing regimens | None | Pain response (VAS <3/10 and able to weight bear); time to resolution of flare; adverse events | Pain response observed in 67% of patients within 24 h and 85% within 48 h; complete resolution of presenting symptoms in 73% by day 5; no attributable adverse events |
| Daoussiset et al., 2012, Greece [19] | Retrospective | 181 patients hospitalized for gout flares (primary or secondary admission diagnoses) | ACTH 1 mg i.m. single dose, followed by repeat dose if indicated | None | Response to treatment (attenuation of inflammation and no requirement for acute gout medications for 2 days); adverse events | 78% of patients responded to the initial ACTH dose; 83% responded to a further dose; few attributable adverse events |
| Pattanaik et al., 2019, USA [20] | Retrospective | 250 patients (US veterans) attending ED with gout flares | ULT | No ULT | Frequency of ED visits | Use of ULT associated with fewer ED visits than no use of ULT (P =0.02) |
| Hutton et al., 2009, New Zealand [21] | Case–control | 48 patients hospitalized for gout at least twice in the preceding year (cases); 48 matched patients with gout but without hospital admissions (controls) | Allopurinol; colchicine prophylaxis | No allopurinol; no colchicine prophylaxis | Hospital admissions | Patients who had been hospitalized were less likely to be on allopurinol than non-hospitalized patients (OR 0.06; P <0.0001) and less likely to have been prescribed colchicine prophylaxis (OR 0.39; P =0.039) |
| Huang et al., 2020, USA [22] | Retrospective | 59 patients with active prescriptions for allopurinol who had been admitted for gout flares | Continuation of ULT/dose increase | Discontinuation of ULT/dose reduction | Frequency of gout flares in the 3 months post-discharge | Dose reduction/discontinuation of allopurinol associated with more repeat gout flares within 3 months of discharge (P =0.03) |
| Hill et al., 2015, USA [16] | RCT | 31 patients with gout meeting ACR criteria for ULT commencement, recruited from EDs and rheumatology clinics within 72 h of initial therapy for gout flares | Allopurinol 100 mg OD orally (days 0–14) then 200 mg daily (days 15–28) | Placebo | Time to resolution of acute flare | No significant difference between allopurinol arm (15.4 days) or placebo arm (13.4 days) (P =0.50) |
| Taylor et al., 2012, USA [17] | RCT | 57 patients with crystal-proven gout flares, recruited from EDs, wards and outpatient clinics within 7 days of flare onset | Allopurinol 300 mg OD orally from day 0 onwards | Placebo (days 0–10) then allopurinol 300 mg OD orally (days 11–30) | Improvement in pain (VAS) by day 10; new or recurrent flares by day 30 | Rapid decrease in pain in both study arms, with no significant differences; flares reported in 7.7% of early initiation group and 12.0% of delayed initiation group (P =0.61); rapid decreases in SU levels by day 10 in the early initiation group |
| Feng et al., 2015, China [23] | Retrospective | 123 patients with gout initiating ULT during acute flares in ward and outpatient settings vs 457 patients initiating ULT after flares | ULT initiation during acute flares | Delayed ULT initiation after flare resolution | Proportion attaining target SU levels; time to attainment of SU target; flare rates | No difference in SU attainment rates (66.7% vs 65.6%); quicker attainment of target SU with immediate ULT (2.5 months vs 3.8 months; P =0.004); numerically more flares with immediate ULT vs delayed ULT in first 12 weeks but not subsequently |
| Kamalaraj et al., 2012, Australia [24] | Retrospective | Patients with gout flares in hospital before (n = 118) and after (n = 89) the introduction of management protocol | Introduction of a gout management protocol | No gout management protocol | Length of stay; treatment delays; proportion continuing ULT on admission | After introduction of protocol, more patients continued baseline allopurinol (P =0.01), treatment delays reduced (P <0.001), length of stay non-significantly shorter (10 vs 11.5 days; P =0.3) |
| Kapadia and Abhishek, 2019, UK [25] | Retrospective | 55 patients with crystal-proven gout flares admitted to a single centre | Inpatient rheumatology consultation | No rheumatology consultation | Proportion with discharge plan to initiate ULT | More patients receiving rheumatology consultation had a discharge plan to initiate ULT (OR 22.25; P =0.007) |
| Teichtahl et al., 2014, Australia [26] | Prospective cohort | 58 patients hospitalized with gout flares (primary or secondary admission diagnoses) in a single centre | Inpatient rheumatology consultation | No rheumatology consultation | Proportion on ULT at discharge; proportion receiving gout discharge plans or outpatient follow-up | Rheumatology consultation associated with non-significantly more ULT on discharge (42% vs 27%; P =0.27); more gout discharge planning (92% vs 24%; P <0.001); more rheumatology outpatient follow-up (42% vs 0%; P <0.001) |
| Sen, 2019, USA [27] | Retrospective | 200 hospitalized patients with diagnoses of gout in a single centre, 27% of whom flared during admission | Inpatient rheumatology consultation | No rheumatology consultation | Length of stay; proportion discharged on ULT or colchicine; proportion with outpatient follow-up | No difference in length of stay (4.7 days vs 5.8 days); more patients with rheumatology input were discharged on ULT or colchicine (100% vs 79%; P <0.04); more patients received outpatient follow-up (62% vs 12%; P <0.002) |
| Gnanenthiran et al., 2011, Australia [28] | Retrospective | 134 patients admitted for gout flares (primary or secondary admission diagnoses) in a single centre | Inpatient rheumatology consultation | No rheumatology consultation | Length of stay; treatment delays; proportion with outpatient follow-up | Length of stay not significantly different (19 vs 17 days; P =0.6); treatment delay not significantly different (2.0 vs 1.7 days; P =0.05); more rheumatology follow-up for those with inpatient rheumatology consultation (53% vs 0%; P <0.001) |
| Kennedy et al., 2015, New Zealand [29] | Retrospective | 90 admissions for gout flares (primary or secondary admission diagnoses) in a single centre | Inpatient rheumatology consultation | No rheumatology consultation | Length of stay; proportion initiating ULT +/– treat-to-target therapy | Length of stay not significantly different (7.1 vs 7.6 days; P =0.81); more patients with rheumatology input commenced allopurinol (53% vs 23%; P =0.04); no difference in treat-to-target therapy (17% vs 7%; P =0.15) |
| Wright et al., 2017, New Zealand [30] | Retrospective | 235 admissions for gout flares (primary or secondary admission diagnoses) in a single centre | Inpatient rheumatology consultation | No rheumatology consultation | Length of stay; proportion undergoing joint aspiration, SU measurement or ULT dose adjustment | Length of stay not significantly different (5.3 vs 6.7 days; P =0.44); more joint aspirations, SU measurement and ULT adjustment with rheumatology input (all P <0.001) |
| Barber et al., 2009, Canada [31] | Retrospective | 138 patients hospitalized with gout flares (primary or secondary admission diagnoses) in a single centre | Inpatient rheumatology consultation | No rheumatology consultation | Proportion initiating ULT +/– treat-to-target therapy; proportion receiving prophylaxis while initiating ULT | Non‐significantly more patients who were consulted by a rheumatologist commenced ULT during/after admission (81% vs 65%; P =0.08); non-significantly more patients received a treat-to-target approach (53% vs 30%; P =0.06); more patients received prophylaxis while initiating ULT (61% vs 29%; P =0.03) |
ACTH: adrenocorticotropic hormone; ED: emergency department; OD: once daily; OR: odds ratio; RCT: randomized controlled trial; SU: serum urate; TDS: three times daily; ULT: urate-lowering therapy; VAS: visual analogue scale for pain.
Pharmacological treatments for gout flares in hospitalized patients
Two RCTs compared NSAIDs and corticosteroids in patients presenting to EDs with gout flares [14, 15]. In both studies, participants were randomized to receive prednisolone 30 mg daily for 5 days or indomethacin 50 mg three times daily for 2 days followed by indomethacin 25 mg daily for 3 days. All participants received concomitant paracetamol (acetaminophen) 1 g, up to 4 times daily as required.
In the larger of the two studies, 416 participants from four EDs were recruited and randomized, of whom 376 participants completed the study [14]. In intention-to-treat and per-protocol analyses, reductions in pain scores were similar between the prednisolone and indomethacin arms, both in ED and by day 14. No serious adverse events occurred with either intervention. Minor adverse events were more frequent with indomethacin than prednisolone during the ED stays (19% vs 6%; P < 0.001) but not subsequently. Length of stay in ED did not differ between the study arms (5 h in both cohorts). There were no significant differences in the proportion of participants returning to ED within 14 days.
In the second RCT (n = 90), the rate of decrease in pain on activity from day 1 to day 14 of follow-up was greater for prednisolone than for indomethacin (−2.9 mm/day vs −1.7 mm/day, respectively; mean difference: 1.2 mm/day; 95% CI: 0.4, 2.0 mm/day; P = 0.003) [15]; however, the absolute differences in pain scores between the interventions were modest, and both cohorts reached the same VAS score by day 14. The indomethacin arm experienced more adverse events than the prednisolone arm (63% vs 27%, respectively; P < 0.05), particularly gastrointestinal bleeding events requiring hospitalization (5 vs 0 events, respectively). Flare relapse rates did not differ significantly between the indomethacin and prednisolone arms (8 vs 5 flares, respectively).
An additional RCT compared the analgesic efficacy of two NSAIDs—oral indomethacin 50 mg, single dose, and i.m. ketorolac 60 mg, single dose—in patients (n = 20) presenting to two EDs with gout flares [13]. Analgesic efficacy was not significantly different between the treatments at 2 h after administration (64% vs 68% reduction in pain scores, respectively). With indomethacin, pain scores remained low at 24 h after treatment. With ketorolac, mean pain scores rebounded after 6 h (from 1.4 to 2.8 on a 0–5 Wong-Baker FACES® Rating Scale; P <0.05), followed by improvements thereafter, such that scores were not significantly different between indomethacin and ketorolac by 24 h after treatment. No adverse effects were reported with either treatment.
A single-centre retrospective study reported outcomes for 26 hospitalized patients who received anakinra for treatment-resistant flares, defined as an inadequate response to colchicine, NSAIDs or corticosteroids and/or contraindications to these medications [18]. Several anakinra dosing regimens were used, depending on each patient’s weight, renal function, extent of joint involvement and response to initial treatment. Multiple courses of anakinra were administered in seven patients, five of whom received the additional courses during different hospital admissions. There was no comparator group. Improvements in pain scores to below 3 on a 10-point scale were observed in 67% of anakinra courses within 24 h of treatment and in 85% by 48 h. Symptom resolution occurred in 73% of patients by day 5; by day 10, all but one patient had fully responded. Anakinra was well tolerated, with no attributable adverse events.
Another single-centre retrospective study reported on the use of i.m. ACTH 1 mg in 181 hospitalized gout patients [19]. There was no comparator group. Response to ACTH, defined as attenuation of signs of inflammation and no requirement for corticosteroids, NSAIDs, colchicine or analgesics for 2 days, was observed in 78% of participants. Most non-responders were re-treated with a further injection of ACTH and, of these, 83% responded. A repeat flare was suffered by 11% of participants, after a median of 4 days. Few attributable adverse events were reported, with local injection site reactions observed in 2% of participants.
ULT for the prevention of gout flares in hospitalized patients
The benefits of ULT on hospitalizations and ED attendances have been evaluated in retrospective analyses. In a single-centre study of US veterans (n = 250) attending ED for gout flares, use of ULT was associated with fewer ED visits for gout flares (determined retrospectively), relative to those with no use of ULT (P =0.02; effect size not provided) [20].
In a case–control study, patients (n = 48) hospitalized for gout at least twice in the preceding year were less likely to have received allopurinol than age-, sex- and ethnicity-matched controls with gout but without hospital admissions (OR 0.06; 95% CI: 0.02, 0.20; P < 0.0001) [21]. The median allopurinol dosages were lower in patients with recurrent admissions than in the comparator group (200 mg vs 300 mg, respectively; P = 0.0019), and hospitalized patients were less likely to have been prescribed colchicine prophylaxis (OR 0.39; 95% CI: 0.17, 0.89; P =0.039). Relative to those without recurrent admissions, patients with recurrent admissions had more comorbidities (6.5 vs 5.1; P =0.011), more comorbid heart disease (71% vs 46%; P =0.013), higher rates of erosive gout (89% vs 46%; P =0.0007) and more tophaceous disease (65% vs 42%; P =0.038). Patients with recurrent hospital admissions for gout were also more likely to have been admitted for other conditions in the preceding year (5.8 vs 0.6 admissions; P <0.0001).
In a retrospective study of patients hospitalized for gout flares while receiving allopurinol (n = 59), dose reductions (or discontinuations) of allopurinol during admissions were associated with higher rates of flares in the 3 months following discharge than admissions during which allopurinol doses were unchanged or increased (53% vs 22%; P =0.03) [22]. The primary reason provided for the allopurinol dose reductions/discontinuations was acute kidney injury, which was present in a higher proportion of this group than that of the comparator group (60% vs 36%). Patients in the dose-reduced/discontinued cohort were less likely to have received flare prophylaxis at discharge than the dose-unchanged/increased cohort (60% vs 27%; P-value not specified), which may have contributed to the observed differences in post-discharge flares.
Whether to initiate ULT during a gout flare has been evaluated in three studies that included participants recruited from EDs and inpatient settings [16, 17, 23]. In an RCT, 31 participants were recruited from EDs and rheumatology clinics within 72 h of initial therapy for a gout flare and randomized to receive allopurinol 100 mg daily (up-titrated to 200 mg daily after 14 days) or placebo [16]. Treatment for the flare was determined by the treating physician, with corticosteroids utilized in over 80% of participants. Both study arms received prophylactic low-dose colchicine. The primary end point of time to resolution of the flares was not significantly different between the allopurinol and the placebo arms (15.4 days vs 13.4 days, respectively; P =0.50). It is of note, however, that post-hoc power calculations suggested 116 subjects per arm were required in order to have demonstrated a significant difference in this end point. Pain and physician global assessment scores declined rapidly in both study arms. As might be expected, SU levels were significantly lower in the allopurinol arm than the placebo arm at study completion (6.4 mg/dl vs 8.3 mg/dl; P =0.012).
In another single-centre RCT, 57 participants recruited from EDs, wards and outpatient clinics within 7 days of onset of gout flares were randomized to receive allopurinol 300 mg daily or placebo for 10 days [17]. After day 10, all participants received open-label allopurinol 300 mg daily. All participants received indomethacin 50 mg three times daily for 10 days and colchicine 0.6 mg twice daily for 90 days. The co-primary end point of participant-reported joint pain normalized rapidly in both study arms, with no significant differences between arms from days 1 to 10. Self-reported new or recurrent gout flares did not differ significantly between study arms by day 30 (7.7% in the early initiation group vs 12.0% in the delayed initiation group; P =0.61), despite rapid decreases in SU levels in the early initiation group. Similarly, in a retrospective study involving patients recruited from hospital or outpatient settings, more rapid attainment of target SU levels was observed with immediate vs delayed initiation of ULT (2.5 vs 3.8 months, respectively; P =0.004) [23]. Repeat flares occurred more frequently in the immediate commencement cohort than in the delayed commencement cohort in the 12 weeks after the initial flare, but were comparable beyond this point.
Non-pharmacological interventions for hospitalized gout patients
Gaps in healthcare providers’ knowledge of gout are an important barrier to optimal care [32]. To address this, one study retrospectively analysed outcomes before and after the introduction of an evidence-based protocol for non-rheumatologists treating hospitalized patients with gout flares [24]. This protocol recommendations to continue baseline ULT, initiate anti-inflammatory medications, perform joint aspiration, and involve rheumatologists in cases of diagnostic uncertainty. Following introduction of the protocol, more patients continued their baseline allopurinol (56% vs 20%; P =0.01), treatment delays were reduced (5% vs 33%; P <0.001), and rheumatology consults increased (52% vs 34%; P =0.01). Admission durations were shorter following introduction of the protocol, albeit non-significantly (10 days vs 11.5 days; P =0.3).
Six retrospective studies and one prospective cohort study have reported outcomes for gout admissions involving inpatient rheumatology consultation, relative to those without inpatient rheumatology consultation [25–31]. The proportion of admissions with rheumatology input varied widely between studies, from 17% to 76%, averaging 40% across all studies. Rheumatology input was consistently associated with more intra-articular joint aspirations and/or steroid injections [26–31]. Those receiving rheumatology input were more likely to have had SU levels measured [28, 30, 31] and more likely to have received outpatient rheumatology follow-up [26–28], relative to patients without rheumatology input. Four studies reported significant associations between rheumatology consultation and increased utilization of ULT [25, 27, 29, 30]. No studies reported significant associations between rheumatology consultation and length of stay in hospital [27–30].
Discussion
In this systematic review, we identified 19 studies reporting associations between interventions and improved outcomes for patients hospitalized with gout. Most were small, retrospective analyses performed in single centres, with concerns for bias. The majority reported on pharmacological interventions known to be effective in the treatment and prevention of gout flares. However, no prospective studies to date have evaluated packages of care designed specifically to prevent further admissions in patients hospitalized for gout flares. There is an urgent need for such studies if the inexorable rise in hospitalizations from this treatable condition is to be stopped.
Hospitalizations provide an opportunity for clinicians to educate patients about gout, engage them in shared decision-making, facilitate self-management and introduce optimal ULT. Sustained reductions in SU levels with optimal use of ULT halts crystal formation and causes dissolution of existing crystals, thereby preventing flares, shrinking tophi and protecting against long-term joint damage [1, 33]. We identified two retrospective analyses that reported associations between the use of ULT and the prevention of hospitalizations and ED attendances [20, 21]. Despite this, most patients do not receive ULT prior to, during or after their admissions [25, 30]. Initiation of ULT is frequently deferred until after discharge due to concerns that initiation of ULT will prolong or worsen the existing flare [17]. Post-discharge recommendations to commence ULT are frequently not acted upon [25], leaving patients at risk of re-admission. The recently updated ACR gout management guideline challenged this practice by conditionally recommending initiation of ULT during flares, supported by their patient panel who advised that the flare may provide additional motivation for patients to commence ULT, although also highlighting the potential for information overload, which could conflate flare management and long-term ULT [3]. ACR’s recommendation is backed by the findings of four studies [16, 17, 23, 34], three of which recruited hospitalized patients or patients attending ED. These studies demonstrated ways of mitigating the risk of flare aggravation while commencing ULT, including gradual up-titration of ULT from a low starting dose and concomitant use of anti-inflammatory medications. Widespread implementation of ACR’s recommendation in patients hospitalized for gout could greatly improve uptake of ULT in this high-risk population and prevent recurrent admissions. Admission affords the time to provide information to patients about both flare management and ULT, addressing the concern of the ACR guideline patient panel.
Only a minority of patients who commence ULT achieve the target SU levels necessary to prevent flares and hospitalizations [8, 9]. Very few studies identified in our search reported on the attainment of target SU levels, and no studies directly evaluated approaches for achieving target SU levels after discharge. Seven studies reported improved outcomes with involvement of rheumatologists during hospitalizations, emphasizing the importance of specialist input in facilitating appropriate diagnosis and management. However, rheumatology consultation does not necessarily equate to the attainment of target SU levels; a recent UK national audit of gout management in outpatient rheumatology clinics reported that target SU levels were achieved in less than half of patients [9]. Furthermore, of the relevant studies in our review, rheumatology input occurred in only 40% of admissions for gout flares, suggesting strategies are needed to increase consultation rates.
Several studies in community settings have evaluated interventions aimed at increasing attainment of target SU levels [33, 35–38], many of which could be applied to hospitalized patients. In an RCT of 517 patients with gout in primary care, research nurses were trained to deliver an individualized package of care, incorporating patient education, shared decision-making, and follow-up visits to guide ULT dose escalation [33]. At 1 year, 95% of patients who received the intervention achieved target SU levels, compared with 30% receiving usual GP care. Gout flares were less frequent following the intervention, and patients’ quality of life improved significantly. In a site-randomized study of 1463 patients receiving new prescriptions for allopurinol, pharmacist-led treat-to-target optimization of allopurinol was compared with usual care [39]. The intervention was delivered through an interactive voice–response system, incorporating reminders and encouragement for patients. At 1 year, patients receiving the intervention were more likely to have been adherent to allopurinol (50% vs 37%; OR 1.68; P <0.001) and more likely to have achieved SU targets (30% vs 15%; OR 2.37; P <0.001) than those receiving usual care. In another study, an electronic visit tool was used to facilitate patient–clinician interaction, treat-to-target ULT, and education for outpatients [38]. Significantly more patients achieved target SU levels following this intervention, relative to a historical cohort (64% vs 34%, respectively; P <0.01). Aspects of all of these interventions could be incorporated into a care package, delivered by nurses or pharmacists, with the aim of establishing patients on dose-optimized ULT following discharge from hospital.
Many interventions that were associated with improved outcomes for hospitalized patients are already included within international gout management guidelines [1–3]. Poor healthcare provider understanding of the long-term health consequences of gout and the importance of treatment are important barriers to optimal care [32]. Strategies to improve implementation of evidence-based interventions in hospitalized patients are needed if outcomes are to be improved and re-admissions prevented. In their study of an inpatient gout management protocol based upon EULAR guidelines, Kamaralaj et al. utilized three implementation approaches: educational sessions for clinicians, electronic health record prompts, and advertising in clinical settings [24]. Multipronged implementation approaches are essential if interventions known to be effective in the management of gout are to be assimilated into clinical practice [40]. Case-notereviews and process mapping will help to identify barriers and facilitators of optimal admitted gout care and the necessary behavioural changes [41]. Only then can interventions be selected to address these barriers, alongside implementation approaches tailored to the inpatient setting [42].
Our systematic review has several limitations, many of which reflect the paucity of available data. Most included studies had small participant numbers, with concerns for bias. The majority of studies reported positive findings, suggesting a degree of publication bias. Many were single-centre analyses, which limits the generalizability of the findings. Outcome measures varied widely between studies, precluding direct comparisons and meta-analysis. This was also reflected in the range of outcome measures selected for our review, which were chosen on the basis of consensus discussion rather than by using specific criteria, such as the OMERACT criteria [43]. Although some outcomes align with those within the OMERACT criteria, adoption of these criteria in future studies would facilitate comparisons of study outcomes. Similarly, diagnostic and inclusion criteria varied substantially between studies, while verification of diagnosis was not possible, which may have resulted in a degree of misclassification bias. Many of the included studies reported pooled results for primary and secondary admission diagnoses of gout, despite differences between these populations, and separate reporting of outcomes in future studies may highlight the need for different management strategies in these populations.
This systematic review highlights an urgent need for prospective studies of strategies to prevent hospitalizations from gout. Gout is a highly treatable yet poorly managed condition, and many admissions from gout are likely to be preventable with better use of existing treatments. Effective implementation of strategies designed to improve uptake of ULT in hospitalized patients, alongside prophylaxis against flares and treat-to-target ULT optimization, is essential if the epidemic of hospital admissions from this treatable condition is to be countered.
Funding: This work is independent research supported by a National Institute for Health Research (NIHR) Doctoral Fellowship for M.D.R. (NIHR300967). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclosure statement: J.B.G. receives speaker fees from Abbvie, Biovitrum, BMS, Celgene, Chugai, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi and UCB. M.D.R. has received honoraria and educational grants from Janssen, Lilly, Menarini, Pfizer and UCB. B.D.C. has received honoraria from Abbvie.
Data availability statement
The data underlying this article are available from research articles in the public domain.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Hui M, Carr A, Cameron S. et al. ; British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology guideline for the management of gout. Rheumatology 2017;56:1246. [DOI] [PubMed] [Google Scholar]
- 2. Richette P, Doherty M, Pascual E. et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 3. FitzGerald JD, Dalbeth N, Mikuls T. et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res 2020;72:744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim SY, Lu N, Oza A. et al. Trends in gout and rheumatoid arthritis hospitalizations in the United States, 1993–2011. JAMA 2016;315:2345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rai SK, Avina-Zubieta JA, McCormick N. et al. Trends in gout and rheumatoid arthritis hospitalizations in Canada from 2000 to 2011. Arthritis Care Res 2017;69:758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell MD, Yates M, Bechman K. et al. Rising incidence of acute hospital admissions due to gout. J Rheumatol 2020;47:619–23. [DOI] [PubMed] [Google Scholar]
- 7. Dehlin M, Jacobsson L, Roddy E.. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol 2020;16:380–90. [DOI] [PubMed] [Google Scholar]
- 8. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M.. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis 2015;74:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roddy E, Packham J, Obrenovic K, Rivett A, Ledingham JM.. Management of gout by UK rheumatologists: a British Society for Rheumatology national audit. Rheumatology (Oxford) 2018;57:826–30. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Shamseer L, Clarke M. et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Altman DG, Gotzsche PC. et al. ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells GA, Shea B, O’Connell D. et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. Ottawa, ON: The Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (15 June 2021, date last accessed).
- 13. Shrestha M, Morgan DL, Moreden JM. et al. Randomized double-blind comparison of the analgesic efficacy of intramuscular ketorolac and oral indomethacin in the treatment of acute gouty arthritis. Ann Emerg Med 1995;26:682–6. [DOI] [PubMed] [Google Scholar]
- 14. Rainer TH, Cheng CH, Janssens HJ. et al. Oral prednisolone in the treatment of acute gout: pragmatic, multicenter, double-blind, randomized trial. Ann Intern Med 2016;164:464–71. [DOI] [PubMed] [Google Scholar]
- 15. Man CY, Cheung IT, Cameron PA, Rainer TH.. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med 2007;49:670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill EM, Sky K, Sit M, Collamer A, Higgs J.. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. J Clin Rheumatol 2015;21:120–5. [DOI] [PubMed] [Google Scholar]
- 17. Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA.. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med 2012;125:1126–34.e7. [DOI] [PubMed] [Google Scholar]
- 18. Ghosh P, Cho M, Rawat G, Simkin PA, Gardner GC.. Treatment of acute gouty arthritis in complex hospitalized patients with anakinra. Arthritis Care Res 2013;65:1381–4. [DOI] [PubMed] [Google Scholar]
- 19. Daoussis D, Antonopoulos I, Yiannopoulos G, Andonopoulos AP.. ACTH as first line treatment for acute gout in 181 hospitalized patients. Joint Bone Spine 2013;80:291–4. [DOI] [PubMed] [Google Scholar]
- 20. Pattanaik D, Ali Z, Freire A.. AB0880 Acute gouty arthritis related emergency department visits among US veterans: characteristics, predictors and areas of improvement. Ann Rheum Dis 2019;78:1907–8. [Google Scholar]
- 21. Hutton I, Gamble G, Gow P, Dalbeth N.. Factors associated with recurrent hospital admissions for gout: a case–control study. J Clin Rheumatol 2009;15:271–4. [DOI] [PubMed] [Google Scholar]
- 22. Huang IJ, Bays AM, Liew JW.. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol 2021;48:467–8. [DOI] [PubMed] [Google Scholar]
- 23. Feng X, Li Y, Gao W.. Significance of the initiation time of urate-lowering therapy in gout patients: a retrospective research. Joint Bone Spine 2015;82:428–31. [DOI] [PubMed] [Google Scholar]
- 24. Kamalaraj N, Gnanenthiran SR, Kathirgamanathan T. et al. Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis 2012;15:512–20. [DOI] [PubMed] [Google Scholar]
- 25. Kapadia A, Abhishek A.. Inpatient rheumatology consultation for gout flares and advice to initiate urate lowering treatment (ULT) in hospital discharge summary increases ULT prescription in primary care. Joint Bone Spine 2019;86:271–2. [DOI] [PubMed] [Google Scholar]
- 26. Teichtahl AJ, Clemens L, Nikpour M, Romas E.. A prospective study of acute inpatient gout diagnoses and management in a tertiary hospital: the determinants and outcome of a rheumatology consultation. Intern Med J 2014;44:1095–9. [DOI] [PubMed] [Google Scholar]
- 27. Sen M. SAT0443 Reconciliation of urate lowering therapies during hospitalization and the impact of rheumatologic consultation on management of inpatient gout flares. Ann Rheum Dis 2019;78:1310–1. [Google Scholar]
- 28. Gnanenthiran SR, Hassett GM, Gibson KA, McNeil HP.. Acute gout management during hospitalization: a need for a protocol. Intern Med J 2011;41:610–7. [DOI] [PubMed] [Google Scholar]
- 29. Kennedy NJ, Healy PJ, Harrison AA.. Inpatient management of gout in a New Zealand hospital: a retrospective audit. Int J Rheum Dis 2016;19:205–10. [DOI] [PubMed] [Google Scholar]
- 30. Wright S, Chapman PT, Frampton C. et al. Management of gout in a hospital setting: a lost opportunity. J Rheumatol 2017;44:1493–8. [DOI] [PubMed] [Google Scholar]
- 31. Barber C, Thompson K, Hanly JG.. Impact of a rheumatology consultation service on the diagnostic accuracy and management of gout in hospitalized patients. J Rheumatol 2009;36:1699–704. [DOI] [PubMed] [Google Scholar]
- 32. Spencer K, Carr A, Doherty M.. Patient and provider barriers to effective management of gout in general practice: a qualitative study. Ann Rheum Dis 2012;71:1490–5. [DOI] [PubMed] [Google Scholar]
- 33. Doherty M, Jenkins W, Richardson H. et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun R, Lu J, Li H. et al. Evaluation of febuxostat initiation during an acute gout attack: a prospective, randomized clinical trial. Joint Bone Spine 2020;87:461–6. [DOI] [PubMed] [Google Scholar]
- 35. Lim AY, Shen L, Tan CH. et al. Achieving treat to target in gout: a clinical practice improvement project. Scand J Rheumatol 2012;41:450–7. [DOI] [PubMed] [Google Scholar]
- 36. Phang KF, Santosa A, Low BPL. et al. A nurse-led, rheumatologist-assisted telemedicine intervention for dose escalation of urate-lowering therapy in gout. Int J Rheum Dis 2020;23:1136–44. [DOI] [PubMed] [Google Scholar]
- 37. Goldfien RD, Ng MS, Yip G. et al. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open 2014;4:e003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yokose C, Jorge A, D’Silva K. et al. Using electronic visits (E-visits) to achieve goal serum urate levels in patients with gout in a rheumatology practice: a pilot study. Semin Arthritis Rheum 2020;50:1382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mikuls TR, Cheetham TC, Levy GD. et al. Adherence and outcomes with urate-lowering therapy: a site-randomized trial. Am J Med 2019;132:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Handley MA, Gorukanti A, Cattamanchi A.. Strategies for implementing implementation science: a methodological overview. Emerg Med J 2016;33:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michie S, van Stralen MM, West R.. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geerligs L, Rankin NM, Shepherd HL, Butow P.. Hospital-based interventions: a systematic review of staff-reported barriers and facilitators to implementation processes. Implement Sci 2018;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schumacher HR, Taylor W, Edwards L. et al. Outcome domains for studies of acute and chronic gout. J Rheumatol 2009;36:2342–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from research articles in the public domain.