Abstract
Objective
To characterize the epidemiology of temporal artery biopsy-positive (TAB+) GCA, including trends in incidence, seasonal variation and prevalence in Skåne, the southernmost region of Sweden.
Methods
All histopathology reports of TABs from 1997 through 2019 were reviewed to identify patients diagnosed with TAB+ GCA. Incidence rates based on the 23-year period and the point-prevalence at 31 December 2014 were determined. An alternative prevalence calculation included only TAB+ GCA patients living in the study area and receiving immunosuppressant therapy on the point-prevalence date.
Results
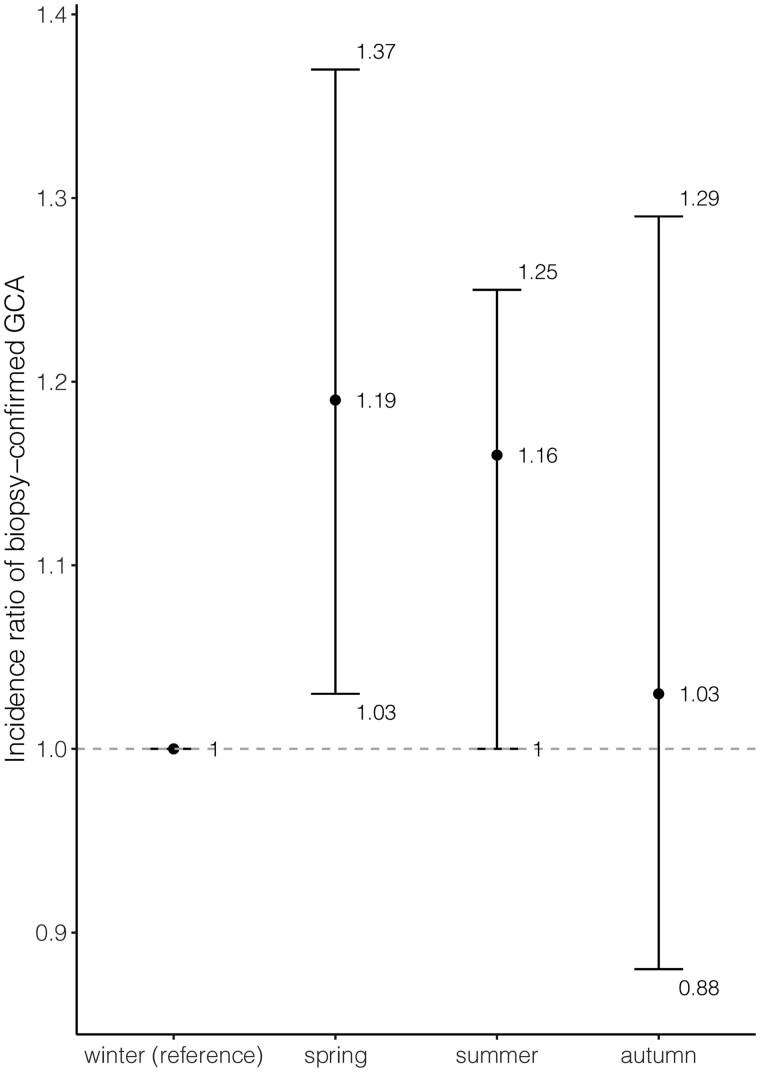
One thousand three hundred and sixty patients were diagnosed with TAB+ GCA (71% female). The average annual incidence 1997–2019 was 13.3 (95% CI: 12.6, 14.0) per 100 000 inhabitants aged ≥50 years and was higher in females (17.8; 95% CI: 16.7, 18.9) than in males (8.2; 95% CI: 7.4, 9.0). The age- and sex-standardized incidence declined from 17.3 in 1997 to 8.7 in 2019, with incidence ratio (IR) of 0.98 per year (95% CI: 0.98, 0.99). A seasonal variation was observed with higher incidence during spring than winter [IR = 1.19 (95% CI: 1.03, 1.39)]. The overall point-prevalence of TAB+ GCA was 127.1/100 000 (95% CI: 117, 137.3) and was 75.5 (95% CI: 67.7, 83.3) when including only patients receiving immunosuppressants.
Conclusion
Over the past 2 decades, the incidence of biopsy-confirmed GCA has decreased by ∼2% per year. Still, a high prevalence of GCA on current treatment was observed. More cases are diagnosed during spring and summer than in the winter.
Keywords: vasculitis, glucocorticoids, registry, demographics, population studies
Introduction
GCA is a systemic vasculitis of unknown aetiology affecting large and medium sized arteries, especially the aorta and its major branches [1]. The disease occurs as three major clinical phenotypes, cranial GCA, supra-aortic large vessel GCA and PMR overlapping with GCA [2]. Phenotypes may be distinct or overlap, and commonly involve constitutional symptoms [2]. Cranial GCA is the most frequently seen, resulting in the typical symptomatology of new onset headache, scalp tenderness and jaw claudication [3, 4]. The diagnosis of GCA is based on clinical characteristics and is usually confirmed by a temporal artery biopsy (TAB) revealing vasculitis. In patients with negative TAB and in those without cranial involvement but with involvement of the large supra-aortic arteries, evidence of vasculitis may be found with computed tomography angiography (CTA), magnetic resonance angiography (MRA), US and PET. US is increasingly used as an alternative to TAB in the diagnostic process [5]. GCA is about three times as common in females as in males and rarely occurs before the age of 50 years [4, 6–9] with incidence peak in the 71–79 year age group [7, 10, 11]. The treatment of GCA mainly relies on glucocorticosteroids (GCs); however, in relapsing or refractory cases, other immunosuppressants may be needed [12, 13]. Patients with GCA are treated with GCs for various periods of time, with a significant number achieving drug-free remission 2–3 years after disease onset [14–17].
GCA is most common in populations of northern European ancestry [18]. The incidence of biopsy-confirmed GCA in Gothenburg, Sweden, has been reported to be 22/100 000 inhabitants aged ≥50 years [8] and 18.8 in Olmsted County, Minnesota, USA [11]. We previously reported the annual incidence rate of TAB+ GCA in southern Sweden to be 14.1/100 000 inhabitants aged ≥50 years with decreasing incidence over time, along with a decrease in the incidence of TAB in the same area [7].
Few studies have examined the prevalence of GCA, and results have been wide ranging [18]. In Germany, the prevalence of GCA was estimated to be 24/100 000 inhabitants aged ≥50 years in 1994, increasing to 44/100 000 in 2006 [19]. The prevalence estimate in Olmsted County, Minnesota, was much higher, 204/100 000 population aged ≥50 years [20]. A similar prevalence was estimated in the UK, 0.25% (95% CI: 0.11, 0.39) of those aged ≥55 years [21]. The prevalence of GCA has been reported lower in southern European and Asian populations [22–24]. There have previously been no reports on the prevalence of GCA in Scandinavia [18].
Differences in environmental exposures and lifestyle factors as well as in diagnostic procedures may influence the reported epidemiological landscape of GCA, and we believe that an update of the epidemiology of GCA is needed. We have established and maintained a population-based registry of all biopsy-confirmed cases of giant cell arteritis in a large stable population in southern Sweden over the course of 23 years. This cohort provides an important opportunity to study trends in incidence over the long-term as well as in seasonal variation. In this report, we present the first prevalence assessment of TAB-positive GCA in Skåne, Sweden and calculate its prevalence including only patients on active immunosuppressant therapy.
Patients and methods
Study area and population
The study took place in Skåne, the southernmost region of Sweden, with a total population of 1 288 908 on 31 December 2014 (13.2% of the population in 2.7% of the area of the country) [25]. Skåne includes both urban and rural areas and has a substantial foreign-born population. Characteristics of the study area are shown in Table 1.
Table 1.
Characteristics of the study area, December 2014.
| Population, total | 1 288 908 |
|---|---|
| Population, ≥50 years (%) | 37.2 |
| Female ≥50 years (%) | 52.3 |
| Population ≥50 years, non-Swedish ancestry (%) | 17.3 |
| Female, % | 52.4 |
| Number of hospitals | 8 |
| Hospitals with pathology department | 4 |
| Number of university hospitalsa | 1 |
| Primary healthcare centres | 152 |
Skåne University Hospital in Lund and Malmö.
The Department of Pathology in Skåne serves the four main hospitals in Skåne (Malmö, Lund, Helsingborg and Kristianstad) and provides histopathology services to all physicians and clinics. A single computerized system is used by the pathology departments, which includes results of all pathology specimens examined and allows search by topographic classification codes, histopathology diagnosis codes, clinical diagnosis, and free-text terms.
The diagnosis of GCA
The case identification and ascertainment of diagnosis has been described [7]. We searched the registry at the Department of Pathology in Skåne for biopsies conducted from 1997 through 2019, using the topographic codes T41 for artery, T42750 for temporal artery and T45 for artery in the head, which yielded a list of all individuals assigned at least one of the codes at some time. All pathology reports were reviewed by two of the authors (A.J.M., P.S.) to establish the diagnosis of TAB+ GCA. In cases of borderline diagnosis, a consensus of three authors was reached (A.J.M., C.T., P.S.).
Patients were diagnosed with GCA if the pathology report stated a diagnosis of giant cell arteritis, temporal arteritis, granulomatous arteritis or unequivocally indicated infiltration of mononuclear cells into the arterial wall with or without giant cells.
The swedish prescribed drug register
The Swedish Prescribed Drug Register (SPDR) contains information regarding drug utilization and expenditure for prescribed drugs for the entire Swedish population [26]. The SPDR is a complete register with >99% coverage in patient identity data [26]. All prescription drugs, irrespective of reimbursement status, are included. Pharmaceutical prescriptions that have been dispensed at least once can be retrieved for every person living in Sweden after July 2005. The unique personal identification number enabled linking our GCA cohort with the SPDR. All information on dispensed prescriptions, including for oral GCs and DMARDs, was extracted through 31 October 2015.
Patients
All persons newly diagnosed with biopsy-confirmed GCA between 1997 and 2019 living in Skåne and aged ≥50 years at the date of diagnosis were eligible to be included in the analysis of incidence and seasonal variation. To be included in the prevalence analysis, patients must have been living in Skåne on the point-prevalence date of 31 December 2014. To be included in the prevalence analysis of TAB+ GCA requiring active immunosuppressive treatment, patients were assumed to be currently treated with GC or DMARD if, according to the SPDR, they had had at least one dispensation of oral GC or a DMARD during the 12 months preceding the point-prevalence date. The use of GCs and DMARDs was identified by searching patient ATC-codes (Supplementary Table SA, available at Rheumatology online).
Statistical analyses
The numerator for the average annual incidence was the total number of patients newly diagnosed with TAB+ GCA from 1 January 1997 through 31 December 2019, and the denominator was the total population aged ≥50 years in Skåne from 31 December 1996 through 31 December 2018. To calculate the incidence of each calendar year, we used as numerator the number of new cases during that year and, as denominator, the population aged ≥50 years 31 December of the prior year. The incidence estimates were standardized for age and sex using the first year (1997) as a reference and direct standardization, with 95% confidence intervals (CIs) estimated assuming Poisson distribution. The age-specific incidence was calculated in four groups representing age at diagnosis: 50–59, 60–69, 70–79 and ≥80 years. To illustrate possible fluctuations in incidence during the 23-year period, we defined four time periods (1997–2002, 2003–08, 2009–14 and 2015–19). To estimate changes in incidence over time, we fitted a Poisson regression model with number of cases as outcome and age, sex, and year as independent variables, taking into account the population at risk. To study possible seasonal variation, seasons were defined as winter (December to February), spring (March to May), summer (June to August) and autumn (September to November). We used Poisson regression on seasonal data to estimate incidence ratios (IRs) among seasons, with winter as reference. The numerator for the point-prevalence estimate included all patients with TAB+ GCA who fulfilled the study definition of prevalent cases, and the denominator was the total population aged ≥50 years living in Skåne on the point-prevalence date (31 December 2014, n = 476 681) [25]. The age-specific point-prevalence was calculated for the four age groups. Statistical analyses were performed using the Statistical Package for the Social Sciences, SPSS v.25.0 for Mac OS (IBM SPSS Statistics) and Stata v.15.
The study was performed in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Regional Ethics Review Board, Lund, Sweden (2010/517).
Results
A total of 6545 TABs were identified during the 23-year study period. The average incidence of TABs per 100 000 in the age group ≥50 years was 64.1 (95% CI: 62.5, 65.6) for the entire study period (Table 2). The average incidence of TABs declined from 74.1/100 000 inhabitants aged ≥50 years (95% CI: 70.7, 77.5) in 1997–2002 to 50.2 (95% CI: 47.4, 53.0) in 2015–19.
Table 2.
Number, outcome and incidence of temporal artery biopsies
| Period | Number | Positive, % | Incidencea (95% CI) |
|---|---|---|---|
| 1997–2019 | 6545 | 20.8 | 64.1 (62.5, 65.6) |
| 1997–2002 | 1801 | 21.2 | 74.1 (70.7, 77.5) |
| 2003–08 | 1822 | 18.6 | 70.5 (67.2, 73.7) |
| 2009–14 | 1687 | 21.0 | 61.5 (58.6, 64.5) |
| 2015–19 | 1235 | 23.2 | 50.2 (47.4, 53.0) |
per 100 000 inhabitants ≥50 years of age.
Incidence of TAB+ GCA
Over the study period, 1360 patients were diagnosed with TAB+ GCA (71% female). The mean age at diagnosis was 75.2 ± 7.9 for all patients, 75.5 ± 7.6 for females vs 74.5 ± 8.4 for males.
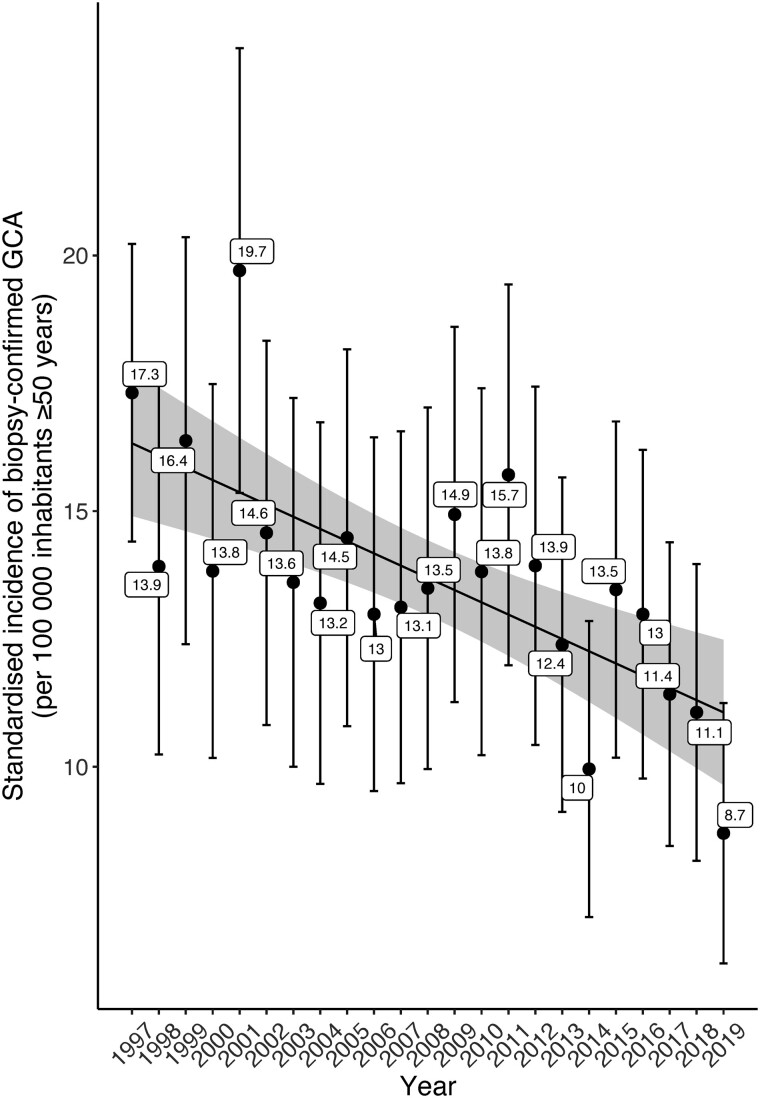
The incidence of TAB+ GCA per 100 000 inhabitants in the age group ≥50 years was estimated to be 13.3 (95% CI: 12.6, 14.0). Sex- and age-specific incidence estimates of TAB+ GCA are summarized in Table 3. The incidence of GCA was higher in females than in males (17.8 vs 8.2/100 000). Incidence increased with age from 1.4 (95% CI: 1.0, 1.8) per 100 000 in individuals age 50–59 to 27.7 (95% CI: 25.0, 30.4) per 100 000 in the age group ≥80 years. The highest incidence was estimated in the 70–79 age group, 28.1/100 000 (95% CI: 25.9, 30.3). Age- and sex-adjusted incidence declined during the study period from 17.3 (95% CI: 14.4, 20.2) in 1997 to 8.7 (95% CI: 6.2, 11.2) in 2019 (Fig. 1 and Table 3). A decreasing trend in annual incidence was observed [IR = 0.98 (95% CI: 0.98, 0.99)].
Table 3.
Sex- and age-specific incidence of biopsy-confirmed GCA per 100 000 inhabitants ≥50 years in Skåne, Sweden, 1997–2019a
| Incidence, sex | |||||
|---|---|---|---|---|---|
| 1997–2019 (95% CI) | 1997–2002 (95% CI) | 2003–2008 (95% CI) | 2009–2014 (95% CI) | 2015–2019 (95% CI) | |
| Allb | 13.3 (12.6–14.0) | 15.7 (14.1–17.2) | 13.1 (11.7–14.5) | 12.9 (11.6–14.3) | 11.6 (10.3–13.0) |
| Female | 17.8 (16.7–18.9) | 22.0 (19.4–24.5) | 18.4 (16.1–20.7) | 16.9 (14.8–19.0) | 14.0 (11.9–16.0) |
| Male | 8.2 (7.4–9.0) | 8.2 (6.6–9.9) | 7.0 (5.5–8.5) | 8.6 (7.0–10.2) | 9.1 (7.4–10.8) |
| Incidence, age | |||||
|---|---|---|---|---|---|
| 1997–2019 (95% CI) | 1997–2002 (95% CI) | 2003–2008 (95% CI) | 2009–2014 (95% CI) | 2015–2019 (95% CI) | |
| 50–59 years | 1.4 (1.0–1.8) | 2.0 (1.1–2.9) | 1.4 (0.6–2.2) | 1.3 (0.6–2.1) | 0.7 (0.1–1.3) |
| 60–69 years | 9.4 (8.3–10.5) | 10.5 (7.9–13.0) | 10.9 (8.5–13.2) | 8.9 (6.9–10.8) | 7.6 (5.6–9.7) |
| 70–79 years | 28.1 (25.9–30.3) | 33.9 (29.0–38.7) | 25.3 (21.0–29.6) | 28.3 (23.9–32.7) | 24.9 (20.8–28.9) |
| ≥80 years | 27.7 (25.0–30.4) | 31.6 (25.7–37.5) | 29.0 (23.6–34.4) | 26.7 (21.6–31.7) | 23.5 (18.3–28.6) |
1360 patients with positive temporal artery biopsy, only 4 patients aged just below the age of 50 years (49.5, 48.7, 48 and 45.1).
With 1997–2002 as reference period, the corresponding age- and sex-standardized incidence rates for 2003–08, 2009–14 and 2015–19 were 13.2 (95% CI: 11.8, 14.7), 13.0 (95% CI: 11.7, 14.4) and 11.3 (95% CI: 10.0, 12.6), respectively.
Fig. 1.
Variations of the annual sex and age-standardized incidenceof biopsy-confirmed GCA in Skåne, Sweden, 1997–2019
Seasonal variation in GCA diagnosis
The seasonal distribution of the number of TAB+ GCA diagnoses was 371 in spring, 360 in summer, 318 in autumn and 311 in winter. IRs using winter as reference indicated higher incidence in spring and summer (Fig. 2).
Fig. 2.
IR with 95% CI of TAB+ GCA diagnoses of seasons with winter as reference.
Point-prevalence of GCA
On the date of point-prevalence estimate, 31 December 2014, 606 patients (439 female, 72%) diagnosed with TAB+ GCA lived in the study area (Table 4). Of these, 360 (269 female) were receiving active immunosuppressive treatment, GCs and/or DMARDs, according to the study definition. Accordingly, the point-prevalence of GCA based on patients on active immunosuppressive therapy was 75.5 (95% CI: 67.7, 83.3)/100 000 inhabitants aged ≥50 years; and was higher in females (Table 4). The prevalence of biopsy-confirmed GCA irrespective of treatment status was 127.1/100 000 (95% CI: 117, 137.3).
Table 4.
Sex- and age-specific point-prevalence of biopsy-confirmed GCA in Skane, Sweden, overall and based only on patients receiving immunosuppressive therapy
| GCA patients receiving immunosuppressive therapy |
All GCA patients |
|||
|---|---|---|---|---|
| n | Point-prevalence (95% CI) | n | Point-prevalence (95% CI) | |
| Patients | 360 | 75.5 (67.7, 83.3) | 606 | 127.1 (117, 137.3) |
| Sex-specific point- prevalence | ||||
| Female | 269 | 107.8 (94.9, 120.7) | 439 | 176 (159.5, 192.4) |
| Male | 91 | 40.1 (31.8, 48.3) | 167 | 73.5 (62.4, 84.7) |
| Age-specific point- prevalence | ||||
| 50–59 | 7 | 4.5 (1.2, 7.9) | 8 | 5.2 (1.6, 8.8) |
| 60–69 | 50 | 33.5 (24.2, 42.8) | 69 | 46.3 (35.3, 57.2) |
| 70–79 | 146 | 136.6 (114.5, 158.8) | 229 | 214.3 (186.6, 242.1) |
| ≥80 | 157 | 237.2 (200.1, 274.3) | 300 | 453.3 (402.0, 504.6) |
Point-prevalence/100 000 age ≥ 50 years.
To assess the proportion of prevalent cases who could had received GCs due to other comorbidities than GCA [asthma, chronic obstructive pulmonary disease (COPD), RA, SLE and IBDs], we found that only 8% (29/360) had been assigned diagnostic codes for other diseases requiring potential treatment with GCs during the last 2 years before the point prevalence date (data not shown).
Discussion
We present an update of the incidence of biopsy-confirmed GCA in a well-defined large geographical area of southern Sweden over a period of 23 years. The age- and sex-standardized incidence of biopsy-confirmed GCA per 100 000 declined from 17.3 in 1997 to 8.7 in 2019, with annual decrease ∼2% (IR = 0.98, 95% CI: 0.98, 0.99). We observed seasonal variation in diagnosis of TAB+ GCA, with more cases diagnosed during spring and summer. We present the first assessment of the prevalence of biopsy-confirmed GCA in Sweden as well as prevalence based on patients receiving active treatment with GCs and/or DMARDs during the year preceding the point-prevalence date.
The overall incidence of TAB+ GCA during the study period was lower than observed in our previous study covering 1997–2010, when the incidence was reported to be 14.1/100 000 inhabitants ≥50 years [7]. Both these estimates are lower than that of a study in Gothenburg, Sweden in the 1990s that reported a rate of 22.2/100 000 [8], suggesting a reduction of cases in Sweden. A similar trend has been reported in Norway, with incidence declining from 29/100 000 inhabitants ≥50 years in 1987–1994 [27] to recently reported estimates of 11.2 and 16.8 [6, 28]. Epidemiological studies from Italy, Spain and Israel have observed a decreasing trend in the incidence of biopsy-confirmed GCA, especially since 2000 [22, 29, 30].
The decreasing incidence of biopsy-confirmed GCA probably cannot be attributed to a single factor, and underlying causes need to be clarified. Dynamics potentially contributing to the downward trend of biopsy-confirmed GCA in our study area may include the increased use of imaging (PET, CT, MRA and CTA) for the diagnosis of large vessel GCA, potentially contributing to a decrease in TABs. This premise is supported by our clinical experience during the study period. In addition, modifiable factors known to influence the occurrence of GCA have altered. In particular, smoking has been associated with an increased risk of GCA [31, 32]. In Sweden, there is evidence of decreasing prevalence of smoking, possibly impacting incidence of GCA [31–33]. A third element may be change in prevalence of comorbidities known to affect the risk of GCA. The increasing prevalence of overweight, obesity and diabetes may influence the incidence of TAB+ GCA, as these factors are inversely correlated with GCA [34–38]. Changes in age distribution in our area during the study period had no impact on incidence, as the standardized incidence was almost identical to the raw data.
As the risk for GCA varies across ethnic groups, one may speculate on the role of changes in the ethnic composition of the population in Sweden in recent years. The percentage of the foreign-born population aged ≥50 years in Skåne increased from 12.1% in 2000 to 19.5% in 2019 [39]. The majority of non-native inhabitants of Sweden during the late 1990s and early 2000s were predominantly citizens of Scandinavia or other European countries [40]. However, the years since 2010 have seen an increase in residents of Sweden from countries known to have lower prevalence of GCA, while the percentage of immigrants from other Scandinavian countries decreased [40].
Throughout the 23-year period of this study, a tendency toward seasonal variation was evident, with more patients diagnosed with TAB+ GCA in spring and summer. The variation may be related to infections as a seasonal exposure triggering the GCA. Epidemiological data, including our previous study, suggest a relationship of certain infections with GCA, especially upper respiratory tract infections [41–44]. Previous studies of seasonal variation have shown divergent results, reporting either no seasonal variation or peaks of new cases in different seasons [8, 45–47]. The conflicting results may be explained by the heterogeneity of patient populations and possibly small sample sizes.
In this study, we presented a novel estimate of the prevalence of biopsy-confirmed GGA, defining as prevalent cases only patients receiving GCs or other immunosuppressive therapy. Previous studies have suggested that a significant proportion of GCA patients achieve sustained drug-free remission [14–17]. The majority of previous studies have presented data on prevalence irrespective of immunosuppressive treatment status. We believe that the number of patients receiving active therapy is more likely to represent a more accurate estimation of the burden of GCA. Our novel prevalence estimate was lower than reported from the USA, UK and Italy, although comparison may be difficult, as some of these studies include TAB-negative GCA [20–22]. Our prevalence estimate was higher than that reported in northern Germany [19] and, as expected, higher than in Asian populations [23, 24].
Strengths of our study include the population-based setting and the relatively large sample size. In addition, the diagnosis of GCA was confirmed by biopsy, with low risk of misclassification or recall bias. Using a consistent method of case identification and ascertainment over a 23-year period allowed a robust estimate of fluctuations in incidence. In addition, we present an alternative, and possibly more informative, estimate of the disease prevalence, utilizing a validated drug register.
A limitation of the study is that we could not estimate incidence or prevalence of TAB-negative GCA or GCA with isolated large vessel disease. Due to the lack of such data, the findings may partly reflect changing practice patterns (i.e. more imaging, less TAB) as opposed to changes in disease. This may also have affected the point prevalence estimate. Future epidemiologic studies that include GCA confirmed by other methods than TAB will be helpful.
Furthermore, the inclusion of only patients with TAB in 1997 or later could lead to a slight underestimation of the prevalence, as patients diagnosed prior to 1997 may have been living and on active treatment on the point-prevalence date but would not have been counted. Another limitation of this study is the lack of data regarding the date of symptoms onset in order to present a more complete picture of seasonality in biopsy-confirmed GCA. Finally, the results may not apply to other populations with a different genetic background.
There has been a declining trend in the incidence of biopsy-confirmed GCA and in TABs conducted in Skåne from 1997 through 2019. Possible explanations may relate to the use of other diagnostic techniques, changes in environmental factors, or may reflect a genuine decrease in incidence of GCA. The observed seasonal variation highlights the need for studies elucidating the effect of seasonal environmental factors, for example infections, on the incidence of GCA. Calculating prevalence taking into account only cases with active immunosuppressive treatment may estimate the magnitude of the disease more accurately. On this basis, the prevalence of GCA in Skåne appears to be higher than reported in many other regions of the world.
Supplementary Material
Acknowledgements
All authors were involved in drafting the manuscript or revising it critically for important intellectual content, and all authors approved the final version to be published. Study conception and design: P.S., C.T., A.J.M.; acquisition of data: P.S., C.T., A.J.M.; analysis and interpretation of data: P.S., A.T., M.E., C.T., A.J.M.
We would like to thank Dr Karl Gisslander for his kind contribution to data collection.
Funding: This study was supported by grants from the Swedish Research Council [2019–01655 to AJM] and King Gustaf V's 80-year foundation [FAI-2016–0277 to AJM].
Disclosure statement: Nothing to disclose.
Data availability statement
Data are protected by the confidentiality laws of Sweden and cannot be shared. All data relevant to the study are included in the manuscript. Please contact corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B.. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017;56:506–15. [DOI] [PubMed] [Google Scholar]
- 3. Pucelj NP, Hočevar A, Ješe R. et al. The incidence of giant cell arteritis in Slovenia. Clin Rheumatol 2019;38:285–90. [DOI] [PubMed] [Google Scholar]
- 4. Tuckwell K, Collinson N, Dimonaco S. et al. ; GiACTA Investigators. Newly diagnosed vs. relapsing giant cell arteritis: baseline data from the GiACTA trial. Semin Arthritis Rheum 2017;46:657–64. [DOI] [PubMed] [Google Scholar]
- 5. Dejaco C, Ramiro S, Duftner C. et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. [DOI] [PubMed] [Google Scholar]
- 6. Brekke LK, Diamantopoulos AP, Fevang BT. et al. Incidence of giant cell arteritis in Western Norway 1972-2012: a retrospective cohort study. Arthritis Res Ther 2017;19:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohammad AJ, Nilsson JA, Jacobsson LT, Merkel PA, Turesson C.. Incidence and mortality rates of biopsy-proven giant cell arteritis in southern Sweden. Ann Rheum Dis 2015;74:993–7. [DOI] [PubMed] [Google Scholar]
- 8. Petursdottir V, Johansson H, Nordborg E, Nordborg C.. The epidemiology of biopsy-positive giant cell arteritis: special reference to cyclic fluctuations. Rheumatology (Oxford) 1999;38:1208–12. [DOI] [PubMed] [Google Scholar]
- 9. Chandran AK, Udayakumar PD, Crowson CS, Warrington KJ, Matteson EL.. The incidence of giant cell arteritis in Olmsted County, Minnesota, over a 60-year period 1950-2009. Scand J Rheumatol 2015;44:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith CA, Fidler WJ, Pinals RS.. The epidemiology of giant cell arteritis. Report of a ten-year study in Shelby County, Tennessee. Arthritis Rheum 1983;26:1214–9. [DOI] [PubMed] [Google Scholar]
- 11. Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE.. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum 2004;51:264–8. [DOI] [PubMed] [Google Scholar]
- 12. Turesson C, Börjesson O, Larsson K, Mohammad AJ, Knight A.. Swedish Society of Rheumatology 2018 guidelines for investigation, treatment, and follow-up of giant cell arteritis. Scand J Rheumatol 2019;48: 259–65. [DOI] [PubMed] [Google Scholar]
- 13. Hellmich B, Agueda A, Monti S. et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 14. Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG.. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003;49:703–8. [DOI] [PubMed] [Google Scholar]
- 15. Chandran A, Udayakumar PD, Kermani TA. et al. Glucocorticoid usage in giant cell arteritis over six decades (1950 to 2009. ). Clin Exp Rheumatol 2015;33:S-102. [PMC free article] [PubMed] [Google Scholar]
- 16. Restuccia G, Boiardi L, Cavazza A. et al. Long-term remission in biopsy proven giant cell arteritis: a retrospective cohort study. J Autoimmun 2017;77:39–44. [DOI] [PubMed] [Google Scholar]
- 17. Albrecht K, Huscher D, Buttgereit F. et al. Long-term glucocorticoid treatment in patients with polymyalgia rheumatica, giant cell arteritis, or both diseases: results from a national rheumatology database. Rheumatol Int 2018;38:569–77. [DOI] [PubMed] [Google Scholar]
- 18. Sharma A, Mohammad AJ, Turesson C.. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: a systematic literature review. Semin Arthritis Rheum 2020;50:1040–8. [DOI] [PubMed] [Google Scholar]
- 19. Herlyn K, Buckert F, Gross WL, Reinhold-Keller E.. Doubled prevalence rates of ANCA-associated vasculitides and giant cell arteritis between 1994 and 2006 in northern Germany. Rheumatology (Oxford) 2014;53:882–9. [DOI] [PubMed] [Google Scholar]
- 20. Crowson CS, Matteson EL.. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum 2017;47:253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yates M, Graham K, Watts RA, MacGregor AJ.. The prevalence of giant cell arteritis and polymyalgia rheumatica in a UK primary care population. BMC Musculoskelet Disord 2016;17:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Catanoso M, Macchioni P, Boiardi L. et al. Incidence, Prevalence, and Survival of Biopsy-Proven Giant Cell Arteritis in Northern Italy During a 26-Year Period. Arthritis Care Res (Hoboken) 2017;69:430–8. [DOI] [PubMed] [Google Scholar]
- 23. Pamuk ON, Donmez S, Karahan B, Pamuk GE, Cakir N.. Giant cell arteritis and polymyalgia rheumatica in northwestern Turkey: clinical features and epidemiological data. Clin Exp Rheumatol 2009;27:830–3. [PubMed] [Google Scholar]
- 24. Kobayashi S, Yano T, Matsumoto Y. et al. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum 2003;49:594–8. [DOI] [PubMed] [Google Scholar]
- 25.Statistics Sweden [A government agency that brings official statistics to the public]. Population Sweden 2014. http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101A/BefolkningNy/ (31 October 2020, date last accessed).
- 26. Wettermark B, Hammar N, Fored CM. et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 27. Gran JT, Myklebust G.. The incidence of polymyalgia rheumatica and temporal arteritis in the county of Aust Agder, South Norway: a prospective study 1987-94. J Rheumatol 1997;24:1739–43. [PubMed] [Google Scholar]
- 28. Andersen JB, Myklebust G, Haugeberg G, Pripp AH, Diamantopoulos AP.. Incidence Trends and Mortality of Giant Cell Arteritis in Southern Norway. Arthritis Care Res (Hoboken) 2020;73:409–14. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez-Gay MA, Miranda-Filloy JA, Lopez-Diaz MJ. et al. Giant cell arteritis in northwestern Spain: a 25-year epidemiologic study. Medicine (Baltimore) 2007;86:61–8. [DOI] [PubMed] [Google Scholar]
- 30. Nesher G, Ben-Chetrit E, Mazal B, Breuer GS.. The Incidence of Primary Systemic Vasculitis in Jerusalem: a 20-year Hospital-based Retrospective Study. J Rheumatol 2016;43:1072–7. [DOI] [PubMed] [Google Scholar]
- 31. Larsson K, Mellstrom D, Nordborg E, Oden A, Nordborg E.. Early menopause, low body mass index, and smoking are independent risk factors for developing giant cell arteritis. Ann Rheum Dis 2006;65:529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brennan DN, Ungprasert P, Warrington KJ, Koster MJ.. Smoking as a risk factor for giant cell arteritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:529–37. [DOI] [PubMed] [Google Scholar]
- 33.Public Health Agency of Sweden. Living conditions and Lifestyle - Tobacco 2019. [updated 28 June 2019]. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/living-conditions-and-lifestyle/alcohol-narcotics-doping-tobacco-and-gambling/tobacco/ (31 October 2020, date last accessed).
- 34.Public Health Agency of Sweden. Living Conditions and Lifestyle - Obesity 2018. [updated 22 May 2018]. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/living-conditions-and-lifestyle/obesity/ (31 October 2020, date last accessed).
- 35. Jakobsson K, Jacobsson L, Warrington K. et al. Body mass index and the risk of giant cell arteritis: results from a prospective study. Rheumatology (Oxford) 2015;54:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ungprasert P, Thongprayoon C, Warrington KJ.. Lower body mass index is associated with a higher risk of giant cell arteritis: a systematic review and meta-analysis. Ann Transl Med 2015;3:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ungprasert P, Upala S, Sanguankeo A, Warrington KJ.. Patients with giant cell arteritis have a lower prevalence of diabetes mellitus: a systematic review and meta-analysis. Mod Rheumatol 2016;26:410–4. [DOI] [PubMed] [Google Scholar]
- 38. Andersson T, Ahlbom A, Carlsson S.. Diabetes prevalence in Sweden at present and projections for year 2050. PloS one 2015;10:e0143084-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Statistics Sweden [A government agency that brings official statistics to the public]. Citizens born outside Sweden 2018. http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101E/InrUtrFoddaRegAlKon/?rxid=bdc00023-f0fb-4b1c-a77c-b355f8c9ed8a (October 2020, date last accessed).
- 40.Statistics Sweden [A government agency that brings official statistics to the public]. Foreign citizens 1997–2019 in Sweden 2019. [updated January 2020]. http://www.statistikdatabasen.scb.se/sq/90454 (October 2020, date last accessed).
- 41. Elling P, Olsson AT, Elling H.. Synchronous variations of the incidence of temporal arteritis and polymyalgia rheumatica in different regions of Denmark; association with epidemics of Mycoplasma pneumoniae infection. J Rheumatol 1996;23:112–9. [PubMed] [Google Scholar]
- 42. Gabriel SE, Espy M, Erdman DD. et al. The role of parvovirus B19 in the pathogenesis of giant cell arteritis: a preliminary evaluation. Arthritis Rheum 1999;42:1255–8. [DOI] [PubMed] [Google Scholar]
- 43. Gilden D, Nagel MA.. Varicella zoster virus triggers the immunopathology of giant cell arteritis. Curr Opin Rheumatol 2016;28:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stamatis P, Turkiewicz A, Englund M. et al. Infections are associated with increased risk of giant cell arteritis - a population-based case-control study from Southern Sweden. J Rheumatol 2021;48:251–7. [DOI] [PubMed] [Google Scholar]
- 45. Bas-Lando M, Breuer GS, Berkun Y. et al. The incidence of giant cell arteritis in Jerusalem over a 25-year period: annual and seasonal fluctuations. Clin Exp Rheumatol 2007;25:S15–7. [PubMed] [Google Scholar]
- 46. Gokoffski KK, Chatterjee A, Khaderi SK.. Seasonal incidence of biopsy-proven giant cell arteritis: a 20-year retrospective study of the University of California Davis Medical System. Clin Exp Rheumatol 2019;37 (Suppl 117):90–7. [PubMed] [Google Scholar]
- 47. De Smit E, Clarke L, Sanfilippo PG. et al. Geo-epidemiology of temporal artery biopsy-positive giant cell arteritis in Australia and New Zealand: is there a seasonal influence? RMD Open 2017;3:e000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are protected by the confidentiality laws of Sweden and cannot be shared. All data relevant to the study are included in the manuscript. Please contact corresponding author.