Abstract
Objective
To investigate all-cause and cause-specific mortality in SLE patients between two time periods, 1997–2005 and 2006–14.
Methods
We used an administrative health database from the province of British Columbia, Canada to match all incident SLE patients to 10 non-SLE individuals on sex, age and index date. Cohorts were divided into two subgroups, according to diagnosis year: early cohort 1997–2005 and late cohort 2006–14. The outcome was death [all-cause, renal disease, cancer, infection, cardiovascular disease (CVD) and other]. Hazard ratios (HR) and 95% CIs were estimated using univariate and multivariable Cox models.
Results
Among 6092 SLE patients and 60 920 non-SLE individuals, there were 451 and 1910 deaths, respectively. The fully adjusted all-cause mortality HR (95% CI) in the overall SLE cohort was 1.85 (1.66, 2.06), with no statistically significant improvement between early and late cohorts [1.95 (1.69, 2.26) vs 1.74 (1.49, 2.04)]. There was excess mortality from renal disease [3.04 (2.29, 4.05)], infections [2.74 (2.19, 3.43)] and CVD [2.05 (1.77, 2.38)], but not cancer [1.18 (0.96, 1.46)], in the overall SLE cohort. There was no statistically significant improvement in cause-specific mortality between early and late cohorts for renal disease [3.57 (2.37, 5.36) vs 2.65 (1.78, 3.93)], infection [2.94 (2.17, 3.98) vs 2.54 (1.84, 3.51)] and CVD [1.95 (1.60, 2.38) vs 2.18 (1.76, 2.71)]. There was no increase in cancer-related mortality in either cohort [1.27 (0.96, 1.69) vs 1.10 (0.82, 1.48)].
Conclusion
This population-based study demonstrates a persisting mortality gap in all-cause and cause-specific deaths in SLE patients, compared with the general population.
Keywords: Key words: systemic lupus erythematosus, epidemiology, mortality, cancer, risk, cohort
Rheumatology key messages
The premature mortality gap in SLE patients compared with the general population has persisted.
SLE patients are at increased risk of mortality from renal disease, infection and CVD.
Cancer-related mortality did not differ between SLE patients and matched comparators.
Introduction
SLE is a complex multi-systemic autoimmune disorder characterized by autoantibody production and a chronic inflammatory state ultimately leading to multiple organ involvement [1, 2]. SLE is associated with significant morbidity and premature mortality, with studies reporting mortality rates at least 2- to 3-fold higher than the general population [3, 4]. There has been a significant improvement in the overall mortality for patients with SLE from the 1970s to the early 2000s [5]. The few contemporary cohorts that have been reported in the past two decades continue to demonstrate increased mortality rates in SLE patients as compared with the general population [6, 7].
Renal disease, infections and cardiovascular disease (CVD) have been reported as the leading causes of mortality in SLE patients [8–13]. While some studies have reported increased risk of mortality from cancer in SLE patients, others have shown this risk to be the same as the general population [3–5, 8]. Previous mortality studies in SLE are limited by the use of prevalent cohorts [8], exclusive selection of hospitalized patients [10, 14], lack of control subjects and inability to adjust for comorbidities and medication use [6, 8]. It is therefore unclear whether recent mortality trends in SLE have improved similar to that seen with other systemic rheumatic disorders including RA or vasculitis [15, 16].
To address this gap in knowledge, we conducted a population-based cohort study evaluating the trends of all-cause and cause-specific mortality in patients with a new diagnosis of SLE and individuals without SLE from the general population from 1997–2005 and 2006–14. Specifically, we compared trends in all-cause mortality and mortality from renal disease, infections, CVD, cancers and other causes.
Methods
Data source
Universal health coverage is available for all residents of British Columbia (BC), Canada (population of ∼4.7 million in 2014). Population Data BC captures data from all provincially funded healthcare services since 1990 including all outpatients [17], hospital admissions and discharges [18], interventions [19], investigations, demographic data [20], cancer registry [21] and vital statistics [20]. Furthermore, Population Data BC encompasses PharmaNet [22], a comprehensive prescription drug database that includes all dispensed medications for BC residents since 1996, regardless of the source of funding. Numerous population-based studies have been successfully conducted using this administrative data set [16, 23–25].
Study design and cohort definition
We used data from Population Data BC to conduct a matched cohort study to determine trends of all-cause and cause-specific mortality among patients with incident SLE compared with individuals without SLE who were randomly selected from the general population (non-SLE cohort).
We created an incident SLE cohort identifying patients diagnosed for the first time between January 1997 and December 2014. Previously published and validated criteria were used to capture incident patients as follows [26]: (i) individuals ≥18 years of age; (ii) one International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for SLE (710.0) by a rheumatologist or from a hospitalization [Tenth Revision (ICD-10) M32.1, M32.8 and 32.9], or two ICD-9-CM codes for SLE (710.0) at least 2 months apart within 2 years by a non-rheumatologist physician; and (iii) at least 7 years of observation time between registration for provincially funded healthcare and an initial ICD for code for SLE (‘run-in’ period). The validity of ICD-9-CM codes for identifying SLE in administrative health databases has been previously studied. In a Swedish registry, a similar definition identified incident SLE cases with a sensitivity of 98.1%, positive predictive value 97.9% and a negative predictive value of 99.9% [27]. Similarly, using US Medicare data, a selection algorithm similar to our case definition was demonstrated to have a sensitivity of 98.2%, specificity of 73% [26] and a positive predictive value of 90% [28].
To improve specificity, individuals were excluded if they had at least two visits, ≥2 months apart, subsequent to the SLE diagnostic visit that resulted in a diagnosis of another systemic autoimmune rheumatic disease (e.g. RA, other CTDs, PsA, AS or other SpAs).
To assemble the non-SLE cohort, we received data from a random sample of >2 000 000 BC residents registered with the provincial medical services plan during the study period, and selected individuals without any history of SLE and matched them to SLE patients (10:1 ratio) on age, sex, and the entry cohort time (i.e. incident SLE diagnosis date). To be comparable to the SLE cohort, we also excluded those non-SLE individuals without at least 7 years’ run-in time before the matched index date.
The SLE sample was divided into two timed cohorts for comparison. SLE patients diagnosed from January 1997 to December 2005 and their respective controls were categorized as the ‘early cohort’, and SLE patients diagnosed from January 2006 to December 2014 were classified as the ‘late cohort’. Participants were followed until their time of death, withdrawal from the health plan through leaving the province or the end of study period, whichever occurred first. The early cohort was right-censored on 31 December 2005 to allow equal observation time for both cohorts. Deaths after this period were not counted.
Assessment of outcome
Our outcomes of interest were all-cause and cause-specific death during the follow-up period. Causes of death were extracted from death certificates using ICD-9 and ICD-10 codes. Cause-specific deaths included those related to renal disease, infections, CVD and cancers (supplementary Table S1, available at Rheumatology online). Deaths from any cause other than these categories was included as a separate category ‘other’. Death certificates in BC allow up to 20 ICD codes, the first one being the primary cause of death and any additional ICD codes identifying additional factors related to the cause of death. The primary cause of death listed on patient charts was used in assessment of all-cause mortality, whereas our analyses for cause-specific mortality utilized all listed ICD codes related to cause of death. Utilization of all available recorded causes is described as an ‘axis-based’ cause-specific mortality analysis.
Covariate assessment
Covariates were assessed in the year prior to study entry date and included comorbidities (angina, chronic obstructive pulmonary diseases, obesity, hypertension, alcoholic liver disease, varicose vein stripping surgery, trauma, fractures, IBD and sepsis), healthcare utilization (number of outpatient medical and hospital visits) and medication use [i.e. anti-diabetes medication (insulin and oral hypoglycemic agents), cardiovascular medications (anti-hypertensives, anti-anginals, anti-arrythmics, nitrates and diuretics), oral glucocorticoids, traditional NSAIDs, cyclooxygenase-2 (COX-2) inhibitors, statins, fibrates, aspirin and HRT]. Additionally, we assessed socioeconomic status using census-derived neighbourhood income quintiles, with quintile 1 representing the lowest socioeconomic status and quintile 5 the highest. Finally, we calculated the Romano adaptation of the Charlson Comorbidity Index for administrative data from the year prior to the index date [29].
Statistical analysis
Patient characteristics were compared between the SLE and non-SLE cohorts. Person-years of follow-up were calculated from the index date to the end of the follow-up period right-censored at 31 December 2005 and 31 December 2014, respectively, for the early and late cohorts allowing equal comparison of both cohorts. Mortality rates for all outcomes were calculated for both SLE and non-SLE cohorts. Cumulative incidence function curves were used to illustrate differences in survival between the SLE and non-SLE cohort.
We used Cox proportional hazard regression models to examine the relation of SLE with all-cause and cause-specific mortality over early and late cohorts, first in a univariate model (matched on age, sex and entry time) and then in a multivariable ‘fully adjusted’ model adjusting for selected confounders. According to the purposeful selection algorithm, we entered confounders one at a time into the Cox models, using forward selection depending on the impact of each confounder on the hazard ratio (HR) of SLE relative to the HR in the model selected in the previous step. Cut-off for the minimum important relative effect at each step was set to 5% [30]. The only confounder meeting this definition from all those assessed was the number of medical visits. Therefore, all reported fully adjusted HRs have been adjusted for the number of medical visits. To assess whether the relation between SLE and all-cause and cause-specific mortality varied over time, we tested an interaction term between cohort period and disease status in the univariable and fully adjusted models. We used SAS Version 9.4 (SAS Institute, Inc., Cary, NC, USA) for all analyses. We calculated 95% CI for all HR. All P-values are two sided and acceptable level of significance was 0.05.
Sensitivity analyses
We performed four sensitivity analysis. First, we estimated HRs for SLE over time-successive nested periods since SLE diagnosis: <1, <2, <3, <4, <5 and ≥5 years, as well as all-time, to determine the impact of SLE duration on mortality. Second, for the cause-specific analyses, we performed sensitivity analysis using proportional hazard models for sub-distribution HRs, thereby assessing the effect of competing risks between specific causes of death on cause-specific mortality [31]. Third, we controlled for stochastically simulated confounders ranging in prevalence from 10% to 20%, and association with each specific mortality outcome and SLE ranging from 1.3–3.0 (odds ratios). Fourth, we fit models ‘maximally adjusted’ for all potential confounders at once. Maximally adjusted HRs for all-cause and cause-specific mortality are included in the Supplementary Data, available at Rheumatology online.
Ethics approval
No personal identifying information was made available in this study. All procedures were in compliance with the British Columbia Freedom of Information and Privacy Protection Act. Ethics approval was received from the University of British Columbia (REB H15-00887).
Results
Patient characteristics
A total of 6092 patients with newly diagnosed SLE and 60 920 individuals without SLE were included in the study, contributing 24 861 and 254 404 person-years of follow-up, respectively. Baseline characteristics of the SLE and non-SLE cohorts are shown in Table 1, divided into early (1997–2005) and late (2006–14) cohorts. The early SLE cohort consisted of 2794 individuals and the late cohort was 3298 individuals.
Table 1.
Baseline characteristics of individuals with and without SLE in early (1997–2005) and late (2006–14) cohorts
| Overall |
Early cohort (1997–2005) |
Late cohort (2006–2014) |
||||
|---|---|---|---|---|---|---|
| Variables | SLE (N = 6092) |
Non-SLE(N = 60920) | SLE (N = 2794) |
Non-SLE(N = 27940) | SLE (N = 3298) |
Non-SLE(N = 32980) |
| Female, n (%) | 5255 (86.3) | 52 550 (86.3) | 2363 (84.6) | 23 630 (84.6) | 2892 (87.7) | 28 920 (87.7) |
| Age, years, mean (s.d.) | 49.5 (15.1) | 49.5 (15.1) | 49.5 (15.1) | 49.5 (15.1) | 49.6 (15.2) | 49.6 (15.1) |
| Hospitalizations, n (%) | 1939 (31.8) | 8276 (13.6) | 915 (32.8) | 3729 (13.4) | 1024 (31.1) | 4547 (13.8) |
| Outpatient visits, mean (s.d.) | 21.6 (17.7) | 8.0 (10.3) | 21.8 (17.9) | 7.8 (9.9) | 21.5 (17.6) | 8.2 (10.6) |
| Medication, n (%) | ||||||
| HRT | 595 (9.8) | 3915 (6.4) | 400 (14.3) | 2718 (9.7) | 195 (5.9) | 1197 (3.6) |
| COX-2 inhibitors | 602 (9.9) | 1576 (2.6) | 366 (13.1) | 920 (3.3) | 236 (7.2) | 656 (2.0) |
| Glucocorticoids | 2418 (39.7) | 2163 (3.6) | 1135 (40.6) | 910 (3.3) | 1283 (38.9) | 1253 (3.8) |
| Cardiovascular drugs | 1886 (31.0) | 10 257 (16.8) | 943 (33.8) | 4832 (17.3) | 943 (28.6) | 5425 (16.5) |
| Anti-diabetic drugs | 272 (4.5) | 2631 (4.3) | 114 (4.1) | 1022 (3.7) | 158 (4.8) | 1609 (4.9) |
| Traditional NSAIDs | 2062 (33.9) | 8230 (13.5) | 1069 (38.3) | 4121 (14.8) | 993 (30.1) | 4109 (12.5) |
| Statins | 448 (7.4) | 3880 (6.4) | 196 (7.0) | 1599 (5.7) | 252 (7.6) | 2281 (6.9) |
| Fibrates | 20 (0.3) | 301 (0.5) | 12 (0.4) | 193 (0.7) | 8 (0.2) | 108 (0.3) |
| Oral contraceptives | 400 (6.6) | 4159 (6.8) | 175 (6.3) | 1847 (6.6) | 225 (6.8) | 2312 (7.0) |
| Aspirin | 130 (2.1) | 507 (0.8) | 58 (2.1) | 203 (0.7) | 72 (2.2) | 304 (0.9) |
| CCI, mean (s.d.) | 0.53 (1.24) | 0.22 (0.79) | 0.48 (1.17) | 0.19 (0.73) | 0.57 (1.28) | 0.24 (0.84) |
| Comorbidity, n (%) | ||||||
| Obesity | 39 (0.6) | 250 (0.4) | 21 (0.8) | 82 (0.3) | 18 (0.6) | 168 (0.5) |
| Angina | 340 (5.6) | 1343 (2.2) | 182 (6.5) | 737 (2.6) | 158 (4.8) | 606 (1.8) |
| Alcoholism with liver disease | 66 (1.1) | 335 (0.6) | 27 (1.0) | 154 (0.6) | 39 (1.2) | 181 (0.6) |
| Hypertension | 1300 (21.3) | 9408 (15.4) | 551 (19.7) | 3832 (13.7) | 749 (22.7) | 5576 (16.9) |
| COPD | 757 (12.4) | 4200 (6.9) | 358 (12.8) | 1914 (6.9) | 399 (12.1) | 2286 (6.9) |
| Sepsis | 132 (2.2) | 204 (0.3) | 51 (1.8) | 79 (0.3) | 81 (2.5) | 125 (0.4) |
| Varicose veins | 87 (1.4) | 665 (1.1) | 33 (1.2) | 304 (1.1) | 54 (1.6) | 361 (1.1) |
| IBD | 56 (0.9) | 221 (0.4) | 17 (0.6) | 83 (0.3) | 39 (1.2) | 138 (0.4) |
| Trauma | 24 (0.4) | 96 (0.2) | 11 (0.4) | 34 (0.1) | 13 (0.4) | 62 (0.2) |
| Fractures | 114 (1.9) | 596 (1.0) | 47 (1.7) | 254 (0.9) | 67 (2.0) | 342 (1.0) |
| Surgery | 69 (1.1) | 512 (0.8) | 38 (1.4) | 286 (1.0) | 31 (0.9) | 226 (0.7) |
| Socioeconomic status, n (%) | ||||||
| Quintile 1 | 1284 (21.1) | 11939 (19.6) | 588 (21.1) | 5507 (19.7) | 696 (21.1) | 6432 (19.5) |
| Quintile 2 | 1187 (19.5) | 11798 (19.4) | 539 (19.3) | 5339 (19.1) | 648 (19.7) | 6459 (19.6) |
| Quintile 3 | 1274 (20.9) | 12381 (20.3) | 586 (21.0) | 5627 (20.1) | 688 (20.9) | 6754 (20.5) |
| Quintile 4 | 1231 (20.2) | 12403 (20.4) | 560 (20.0) | 5745 (20.6) | 671 (20.4) | 6658 (20.2) |
| Quintile 5 | 1116 (18.3) | 12399 (20.4) | 521 (18.7) | 5722 (20.5) | 595 (18.0) | 6677 (20.3) |
COX-2: cyclooxygenase-2; CCI: Charlson Comorbidity Index; COPD: chronic obstructive pulmonary disease. HRT: hormone replacement therapy; IBD: inflammatory bowel disease
The early SLE cohort was comprised of 84.6% females with mean age of 49.5 (s.d. 15.1) years and the late cohort was 87.7% females with mean age 49.6 (s.d. 15.2) years. SLE patients in both cohorts had higher rates of healthcare utilization, with increased hospitalization and more frequent outpatient visits during the 12 months prior to diagnosis as compared with the corresponding non-SLE cohorts. As expected, both early and late SLE cohorts had higher usage of cardiovascular medication, glucocorticoids, NSAIDs and selective COX-2 inhibitors as compared with respective comparison cohorts. The Charlson Comorbidity Index was higher in the early and late SLE cohorts than the non-SLE cohorts. There were no substantial socioeconomic differences between the SLE and non-SLE cohorts.
Association of SLE with all-cause mortality
The risk of mortality for the SLE and non-SLE cohorts using the purposeful selection model is demonstrated in Table 2 and Fig. 1. The mean follow-up time for SLE patients was 4.0 years in the early and 4.1 years in the late cohort, respectively. A total of 451 deaths were observed in the SLE cohort, and 1910 deaths in the non-SLE cohort during the entire study duration. From the total number of deaths in the SLE cohort, SLE was recorded among all listed causes of death in 107 cases, and as the primary cause of death in 51 cases. There were 249 deaths in the early and 202 deaths in the late SLE cohort, with a mortality rate of 22.2 and 14.8 per 1000 person-years, respectively. The mortality rate for the non-SLE cohort showed a similar trend for improvement between the early and late cohort (8.9 vs 6.3 per 1000 person-years).
Table 2.
All-cause mortality in the overall, early and late cohorts
| Variables | Overall |
Early cohort (1997–2005) |
Late cohort (2006–14) |
||||
|---|---|---|---|---|---|---|---|
| SLE (N = 6092) |
Non-SLE (N = 60 920) | SLE (N = 2794) |
Non-SLE (N = 27 940) | SLE (N = 3298) |
Non-SLE (N = 32 980) | P for interaction | |
| Total follow-up (person-years) | 24 861 | 254 404 | 11 213 | 116 169 | 13 648 | 138 235 | N/A |
| Mean follow-up (years) | 4.08 | 4.18 | 4.01 | 4.16 | 4.14 | 4.19 | N/A |
| All-cause mortality | |||||||
| Deaths | 451 | 1910 | 249 | 1034 | 202 | 876 | N/A |
| MR (cases per 1000 person-years) | 18.14 | 7.51 | 22.21 | 8.90 | 14.80 | 6.34 | N/A |
| Age-, sex- and entry time-adjusted HR (95% CI) | 2.58 (2.33, 2.86) | 1 | 2.74 (2.38, 3.15) | 1 | 2.43 (2.08, 2.83) | 1 | 0.26 |
|
Fully adjusted HRa (95% CI) |
1.85 (1.66, 2.06) | 1 | 1.95 (1.69, 2.26) | 1 | 1.74 (1.49, 2.04) | 1 | 0.28 |
In addition to matching variables (age, sex, and entry-time), fully adjusted models were adjusted for the number of medical visits.
MR: mortality rate; HR: hazard ratio; N/A: not applicable.
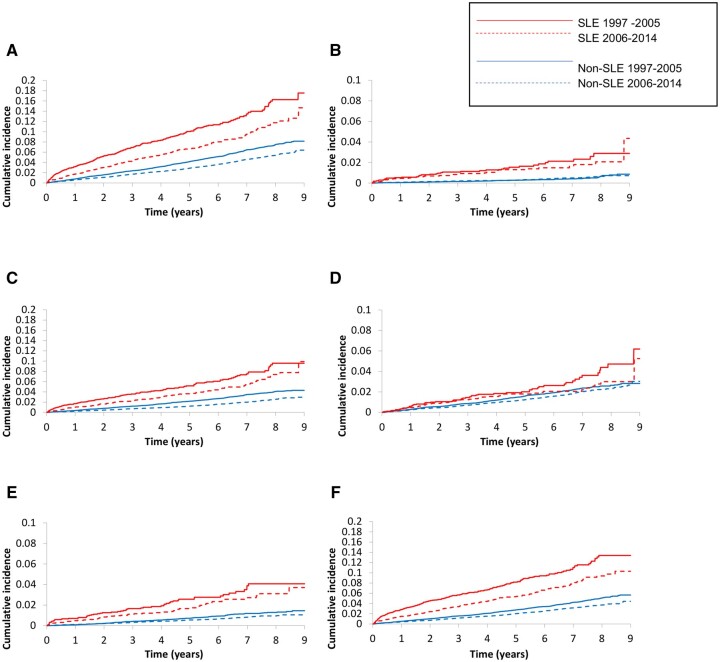
Fig. 1.
Death in SLE and non-SLE cohorts
Cumulative incidence of (A) all-cause, (B) renal disease, (C) cardiovascular disease, (D) cancer, (E) infection and (F) other causes of death in SLE and non-SLE cohorts.
SLE patients had a higher risk of mortality overall as compared with the non-SLE cohort with an age-, sex- and entry time-adjusted HR of 2.58 (95% CI 2.33, 2.86) (Table 2). This risk remained significant even after adjustment [fully adjusted HR (95% CI) 1.85 (1.66, 2.06)] (Table 2, Fig. 1). There was no significant improvement in mortality risk between early and late SLE cohorts in the fully adjusted model [fully adjusted HR (95% CI) 1.95 (1.69, 2.26) for early, and 1.74 (1.49, 2.04) for late SLE cohort] (P-value for interaction = 0.28).
Association of SLE with cause-specific mortality
Cause-specific mortality was evaluated using axis-based analysis with utilization of all recorded causes of death (Table 3, Fig. 1). Renal disease conferred the highest risk of mortality in the overall SLE cohort [fully adjusted HR (95% CI) 3.04 (2.29, 4.05)]. Mortality from renal disease improved over time with fully adjusted HR (95% CI) 3.57 (2.37, 5.36) as compared with 2.65 (1.78, 3.93) for the early and late SLE cohorts, respectively, though this did not achieve statistical significance (P-value for interaction = 0.30). The overall SLE cohort also had a higher risk of mortality from infections and CVD [fully adjusted HR (95% CI) 2.74 (2.19, 3.43) and 2.05 (1.77, 2.38), respectively]. There was no improvement in infection-related mortality over time [fully adjusted HR (95% CI) 2.94 (2.17, 3.98) vs 2.54 (1.84, 3.51)] or CVD-related mortality over time [fully adjusted HR (95% CI) 1.95 (1.60, 2.38) vs 2.18 (1.76, 2.71)] (P-value for interaction >0.4 for both). Cancer-related mortality did not differ between the SLE cohort and the general population [overall fully adjusted HR (95% CI) 1.18 (0.96, 1.46)].
Table 3.
Axis-based analysis of cause-specific mortality in early and late cohorts
| Overall |
Axis-based analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variables |
Early cohort (1997–2005) |
Late cohort (2006–14) |
|||||
| SLE (N = 6092) |
Non-SLE (N = 60 920) | SLE (N = 2794) |
Non-SLE (N = 27 940) | SLE (N = 3298) |
Non-SLE (N = 32 980) | P for interaction | |
| Total follow up (person-years) | 24 860.54 | 254 403.92 | 11 212.67 | 116 168.77 | 13 647.87 | 138 235.15 | N/A |
| Mean follow-up (years) | 4.08 | 4.18 | 4.01 | 4.16 | 4.14 | 4.19 | N/A |
| Renal disease mortality | |||||||
| Deaths | 76 | 172 | 39 | 74 | 37 | 98 | N/A |
| MR (cases per 1000 person-years) | 3.06 | 0.68 | 3.48 | 0.64 | 2.71 | 0.71 | N/A |
| Age-, sex- and entry time-adjusted HR (95% CI) |
4.73 (3.59, 6.22) |
1 |
5.74 (3.86, 8.52) |
1 |
3.99 (2.72, 5.85) |
1 | 0.20 |
| Fully adjusted HRa (95% CI) |
3.04 (2.29, 4.05) |
1 |
3.57 (2.37, 5.36) |
1 |
2.65 (1.78, 3.93) |
1 | 0.30 |
| Cancer mortality | |||||||
| Deaths | 109 | 748 | 57 | 372 | 52 | 376 | N/A |
| MR (deaths per 1000 person-years) | 4.38 | 2.94 | 5.08 | 3.20 | 3.81 | 2.72 | N/A |
| Age-, sex- and entry time-adjusted HR (95% CI) |
1.56 (1.28, 1.91) |
1 |
1.69 (1.28, 2.24) |
1 |
1.44 (1.08, 1.93) |
1 | 0.45 |
| Fully adjusted HRa (95% CI) |
1.18 (0.96, 1.46) |
1 |
1.27 (0.96, 1.69) |
1 |
1.10 (0.82, 1.48) |
1 | 0.48 |
| Infection mortality | |||||||
| Deaths | 113 | 332 | 60 | 175 | 53 | 157 | N/A |
| MR (cases per 1000 person-years) | 4.55 | 1.31 | 5.35 | 1.51 | 3.88 | 1.14 | N/A |
| Age-, sex- and entry time-adjusted HR (95% CI) |
3.85 (3.10, 4.79) |
1 |
4.11 (3.05, 5.55) |
1 |
3.62 (2.64, 4.95) |
1 | 0.56 |
| Fully adjusted HRa (95% CI) |
2.74 (2.19, 3.43) |
1 |
2.94 (2.17, 3.98) |
1 |
2.54 (1.84, 3.51) |
1 | 0.52 |
| CVD mortality | |||||||
| Deaths | 246 | 916 | 132 | 535 | 114 | 381 | N/A |
| MR (cases per 1000 person-years) | 9.90 | 3.60 | 11.77 | 4.61 | 8.35 | 2.76 | N/A |
| Age-, sex- and entry time-adjusted HR (95% CI) |
2.96 (2.57, 3.42) |
1 |
2.82 (2.32, 3.42) |
1 |
3.17 (2.57, 3.92) |
1 | 0.42 |
| Fully adjusted HRa (95% CI) |
2.05 (1.77, 2.38) |
1 |
1.95 (1.60, 2.38) |
1 |
2.18 (1.76, 2.71) |
1 | 0.45 |
| Other causes | |||||||
| Deaths | 366 | 1281 | 203 | 682 | 163 | 599 | N/A |
| MR (cases per 1000 person-years) | 14.72 | 5.04 | 18.10 | 5.87 | 11.94 | 4.33 | N/A |
| Age-, sex- and entry time-adjusted HR (95% CI) |
3.15 (2.80, 3.55) |
1 |
3.40 (2.90, 3.99) |
1 |
2.90 (2.44, 3.45) |
1 | 0.18 |
| Fully adjusted HRa (95% CI) |
2.19 (1.94, 2.47) |
1 |
2.34 (1.99, 2.76) |
1 |
2.03 (1.69, 2.42) |
1 | 0.24 |
In addition to matching variables (age, sex and entry time), fully adjusted models were adjusted for the number of medical visits.
CVD: cardiovascular disease; MR: mortality rate; HR: hazard ratio; N/A: not applicable.
When stratified by year since diagnosis of SLE, all-cause mortality remained persistently elevated for each year of disease duration in the first 5-year period and after (Table 4, supplementary Table S2 and S3, available at Rheumatology online). The highest risk of mortality from renal disease, infection and CVD was within the first year after diagnosis with >5-fold, >4-fold and >3-fold risk of death, respectively. There was no difference in cancer-related mortality between the SLE cohort and the general population for any year of disease duration.
Table 4.
All-cause and cause-specific mortality in SLE from the time of diagnosis
| Years since SLE diagnosis | All-cause | Renal disease | Cancer | Infection | CVD | Other causes |
|---|---|---|---|---|---|---|
| <1 | 2.44 (1.99, 3.00) | 5.10 (3.07, 8.48) | 1.37 (0.91, 2.08) | 4.02 (2.57, 6.28) | 3.17 (2.37, 4.23) | 3.15 (2.51, 3.95) |
| <2 | 2.21 (1.88, 2.59) | 4.14 (2.75, 6.24) | 1.39 (1.02, 1.90) | 3.70 (2.63, 5.20) | 2.47 (1.96, 3.10) | 2.83 (2.36, 3.39) |
| <3 | 2.08 (1.81, 2.39) | 3.64 (2.52, 5.26) | 1.31 (1.00, 1.72) | 3.01 (2.24, 4.04) | 2.23 (1.83, 2.72) | 2.53 (2.16, 2.97) |
| <4 | 2.00 (1.76, 2.27) | 3.56 (2.53, 5.01) | 1.25 (0.97, 1.60) | 2.68 (2.03, 3.53) | 2.17 (1.81, 2.60) | 2.42 (2.09, 2.80) |
| <5 | 1.94 (1.72, 2.19) | 3.58 (2.60, 4.95) | 1.15 (0.91, 1.46) | 2.76 (2.14, 3.55) | 2.11 (1.78, 2.51) | 2.29 (2.00, 2.63) |
| ≥5 | 1.58 (1.24, 2.01) | 1.75 (0.93, 3.30) | 1.37 (088, 2.13) | 2.64 (1.62, 4.33) | 1.87 (1.37, 2.55) | 1.87 (1.42, 2.46) |
| Overall | 1.85 (1.66, 2.06) | 3.04 (2.29, 4.05) | 1.18 (0.96, 1.46) | 2.74 (2.19, 3.43) | 2.05 (1.77, 2.38) | 2.19 (1.94, 2.47) |
Values are hazard ratio (95% CI). CVD: cardiovascular disease.
Sensitivity analysis
To assess the robustness of our results, we performed sensitivity analyses to adjust for unmeasured confounders and determine the effect of competing risks between specific causes of death (Table 5, supplementary Table S4 and S5, available at Rheumatology online). The HRs remained robust even with introduction of the highest level of hypothetical confounders (simulated confounder 20%, odds ratio 3.0) with increased risk of all-cause mortality and death from renal disease, infections and CVD.
Table 5.
Sub-distribution and simulated confounder hazard ratios for all-cause and cause-specific mortality
| All-cause | Renal disease | Cancer | Infection | CVD | Other causes | |
|---|---|---|---|---|---|---|
| Primary analysis | 1.85 (1.66, 2.06) | 3.04 (2.29, 4.05) | 1.18 (0.96, 1.46) | 2.74 (2.19, 3.43) | 2.05 (1.77, 2.38) | 2.19 (1.94, 2.47) |
| Competing risk of death—Cox model | – | 2.78 (2.07, 3.74) | 1.15 (0.93, 1.42) | 2.57 (2.05, 3.24) | 2.00 (1.71, 2.34) | 2.20 (1.93, 2.50) |
| Simulated confounder 10%/OR = 1.3 | 1.84 (1.65, 2.05) | 3.03 (2.28, 4.04) | 1.18 (0.96, 1.45) | 2.72 (2.17, 3.41) | 2.04 (1.76, 2.37) | 2.18 (1.93, 2.46) |
| Simulated confounder 10%/OR = 3.0 | 1.58 (1.42, 1.77) | 2.61 (1.94, 3.51) | 0.96 (0.78, 1.19) | 2.34 (1.86, 2.95) | 1.74 (1.49, 2.03) | 1.88 (1.66, 2.13) |
| Simulated confounder 20%/OR = 1.3 | 1.84 (1.65, 2.05) | 3.03 (2.28, 4.03) | 1.17 (0.95, 1.45) | 2.73 (2.18, 3.42) | 2.05 (1.76, 2.38) | 2.18 (1.93, 2.46) |
| Simulated confounder 20%/OR = 3.0 | 1.52 (1.36, 1.70) | 2.54 (1.89, 3.41) | 0.95 (0.77, 1.17) | 2.24 (1.78, 2.82) | 1.69 (1.45, 1.97) | 1.81 (1.60, 2.05) |
Values are hazard ratio (95% CI).
CVD: cardiovascular disease; OR: odds ratio.
All analyses were repeated using a maximally adjusted model where all potential confounders listed in Table 1 were simultaneously included in the regression models (see supplementary Table S6 and S7, available at Rheumatology online). HRs were similar between maximally adjusted and fully adjusted models with robust HRs.
Discussion
We conducted a large population-based study using administrative health data to evaluate trends of all-cause and cause-specific mortality among patients with newly diagnosed SLE between two time periods. We observed an increased risk of all-cause mortality in SLE patients relative to the general population without significant improvement between the early (1997–2005) and late (2006–14) cohorts with a 95% and 74% increased risk in mortality, respectively. In both time periods, we observed similar excess mortality due to renal disease, infections and CVD. Elevated all-cause and cause-specific mortality persisted for every year of disease duration; however, mortality for renal disease, infections and CVD was particularly heightened within the first year of diagnosis. There was no excess mortality from cancer in either period.
Our findings for increased all-cause mortality are congruent with previous population-based studies with incident SLE cohorts. Using a database representative of the general population in the UK, Jorge et al. [7] demonstrated all-cause mortality HRs (95% CI) of 2.15 (1.63, 2.83) and 2.12 (1.61, 2.80) in two similarly distributed cohorts from 1999–2006 and 2007–14, respectively. Similar to our findings, their results are indicative of excess mortality in SLE patients without improvement in recent years. In another study evaluating time trends in SLE mortality, Tselios et al. [8] demonstrated significant reduction of standardized mortality ratios (SMRs) for all-cause and cause-specific mortality from infections and atherosclerotic disease from 1971 to 2013. However, this reduction is likely driven by the earlier time period included in this study (1971–99), as there is only a modest reduction in SMR (95% CI) of 3.2 (2.4, 4) to 2.2 (1.4, 3.1) between 2000–09 and 2010–13, time frames that are similar to that of our study. Additionally, the SMRs calculated in this study were only adjusted for an age- and sex-matched population, whereas we were able to adjust for additional confounders.
In our cause-specific analysis, we found a >3-fold increased risk of mortality from renal disease in the early cohort, without significant improvement in the late cohort. Additionally, we observed that mortality from renal disease was highest within the first year of SLE diagnosis. Despite the rising incidence of end-stage kidney disease in some SLE populations, few have explored trends in renal-related mortality over time [32]. In a recent study, Jorge et al. [33] reported mortality trends among individuals with incident end-stage kidney disease due to LN on renal replacement therapy, using a national registry showing a decline in mortality rate per 100 patient-years from 11.1 (95% CI 10.4, 11.8) in 1995–99 to 6.7 (95% CI 6.2, 7.2) in 2010–14.
We observed persistently increased mortality from infections and CVD in both our early and late cohorts. This is in contrast to results reported by Jorge et al. [33], who cite a 44% and 63% decline in deaths from CVD and infections in SLE patients over the above-mentioned time periods. However, there are important differences between this study and our patient cohort, namely that Jorge et al. studied patients with LN not all incident SLE cases, and conducted the analysis based on the primary cause recorded in the death files, which may not necessarily be the actual underlying cause of death. For instance, death from causes such as renal disease may be under-represented, as ultimately it may be a cardiac arrest that is recorded as the primary cause of death. Thus, death due to CVD can overwhelm other causes inappropriately and thereby affect subsequent interpretation of mortality trends. This is particularly important in evaluation of causes of mortality based on death records for rare diseases. Falasinnu et al. [34] demonstrated this in a Swedish population-based cohort from 2001–13 wherein only 41% of 1802 deaths among SLE patients reported SLE on their death records, suggesting an underestimation of the true burden of mortality from this disease. Indeed, in our cohort only 107 of 451 records had SLE reported among the primary or non-primary causes of death. However, our approach using axis-based analysis for cause-specific mortality, reviewing all causes of death listed in administrative data as opposed to only the primary cause, addresses this issue.
In the longitudinal study by Tselios et al. [8], while a significant reduction from mortality related to atherosclerosis is observed in the period extending from 1980 to 1999, similar reductions are not reported during the more recent periods extending from 2000 to 2013. These authors also report a modest reduction in death from infections in their more recent cohorts, however the interpretability of their results is limited by the very few numbers of deaths (<10) from infection in these cohorts.
We did not observe an excess mortality from cancer in our study. This is in keeping with two other previous studies reporting cancer-related mortality in patients with SLE [3, 8]. While this is a reassuring observation, it is important to interpret the results of our study with caution. In general, the risk of malignancy in patients with SLE is a complex topic beyond the scope of our study. While some have shown an increased risk of haematologic, lung or hepatobiliary disease [35–39], others have reported decreased incidence of breast, ovarian, endometrial and prostate cancers [35, 40]. Our cohort does not differentiate between subtypes of cancer nor can it be used to comment on incidence of cancers in patients with SLE in general.
Our study has several strengths and limitations. The limitations of our study are those inherent to population-based studies using ICD codes. While we cannot entirely exclude the uncertainty around diagnostic accuracy, we used stringent previously validated criteria with a sensitivity of 98.1%, positive predictive value of 97.9% and negative predictive value of 99.9% for selection of incident cases and additional exclusion criteria to minimize the rate of false positives [26–28]. We required at least 6 years of run-in period before the first SLE diagnosis to ensure incident patients. Despite this, inclusion of false positives would only introduce a conservative bias in which the association would favour the null hypothesis. Our definition for selection of cases only includes adults (age >18 years), therefore the reported mortality trends in this study cannot be extrapolated to the paediatric SLE population. Our data has the strength of being derived from a large population covering an entire province, with incident cases included from both inpatient and outpatient settings, as well as 10 comparisons for each patient with SLE. The administrative database used for this study includes both inpatient and outpatient encounters. Therefore, our results can likely be generalized to the general adult population. Additionally, having access to information on medications and comorbidities allows adjustment of our analysis for a number of possible confounders.
Conclusion
In summary, our population-based study demonstrates no improvement in overall and cause-specific mortality from SLE in the comparison of early and late cohorts. While there have been significant improvements in mortality in population-based studies of RA and vasculitis, patients with SLE continue to have excess mortality compared with the general population [15, 16]. This gap in mortality highlights the need for further interventions in management of SLE patients. This may include development of new therapeutic agents, strategies for earlier detection of disease and more comprehensive measures in the management of SLE-related complications.
Supplementary Material
Acknowledgements
J.A.A.-Z. is the BC Lupus Society Research Scholar and the Walter & Marilyn Booth Research Scholar.
All inferences, opinions, and conclusions drawn in this article are those of the authors, and do not reflect the opinions or policies of the Data Steward(s).
Funding: This work was supported by Canadian Institutes of Health Research [THC 135235].
Disclosure statement: The authors have no conflicts of interest pertaining to the presented information.
Data availability statement
The data underlying this article were accessed from Population Data BC, https://www.popdata.bc.ca/data.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Rahman A, Isenberg DA.. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 2. Lisnevskaia L, Murphy G, Isenberg D.. Systemic lupus erythematosus. Lancet 2014;384:1878–88. [DOI] [PubMed] [Google Scholar]
- 3. Yurkovich M, Vostretsova K, Chen W, Aviña‐Zubieta JA.. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2014;66:608–16. [DOI] [PubMed] [Google Scholar]
- 4. Lee YH, Choi SJ, Ji JD, Song GG.. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 2016;25:727–34. [DOI] [PubMed] [Google Scholar]
- 5. Bernatsky S, Boivin J-F, Joseph L. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 6. Rees F, Doherty M, Grainge MJ. et al. Mortality in systemic lupus erythematosus in the United Kingdom 1999–2012. Rheumatology (Oxford) 2016;55:854–60. [DOI] [PubMed] [Google Scholar]
- 7. Jorge AM, Lu N, Zhang Y, Rai SK, Choi HK.. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology (Oxford) 2018;57:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M.. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971–2013). Ann Rheum Dis 2019;78:802–6. [DOI] [PubMed] [Google Scholar]
- 9. Bultink IEM, de Vries F, van Vollenhoven RF, Lalmohamed A.. Mortality, causes of death and influence of medication use in patients with systemic lupus erythematosus vs matched controls. Rheumatology (Oxford, England) 2021;60:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhital R, Pandey RK, Poudel DR. et al. All-cause hospitalizations and mortality in systemic lupus erythematosus in the US: results from a national inpatient database. Rheumatol Int 2020;40: 393–7. [DOI] [PubMed] [Google Scholar]
- 11. Wu W, Ma J, Zhou Y. et al. Mortality risk prediction in lupus patients complicated with invasive infection in the emergency department: LUPHAS score. Ther Adv Musculoskelet Dis 2019;11:1759720X1988555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aviña-Zubieta JA, To F, Vostretsova K. et al. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res (Hoboken) 2017;69:849–56. [DOI] [PubMed] [Google Scholar]
- 13. Thomas G, Mancini J, Jourde-Chiche N. et al. Mortality associated with systemic lupus erythematosus in France assessed by multiple-cause-of-death analysis. Arthritis Rheumatol 2014;66:2503–11. [DOI] [PubMed] [Google Scholar]
- 14. Anastasiou C, Trupin L, Glidden DV. et al. Mortality among hospitalized individuals with systemic lupus erythematosus in the United States between 2006 and 2016. Arthritis Care Res (Hoboken) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M.. Improvement in 5-year mortality in rheumatoid arthritis compared to the general population – closing the mortality gap. Ann Rheum Dis 2017;76: 1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan JA, Choi HK, Xie H. et al. All-cause and cause-specific mortality in patients with granulomatosis with polyangiitis: a population-based study. Arthritis Care Res (Hoboken) 2019;71:155–63. [DOI] [PubMed] [Google Scholar]
- 17.Medical Services Plan (MSP) payment information file. www.popdata.bc.ca. 2020. https://www.popdata.bc.ca/data/health/msp.
- 18.Discharge Abstracts Database (Hospital Separations file). www.popdata.bc.ca. 2020. https://www.popdata.bc.ca/data/health/dad.
- 19.Ministry of Health B. Health BCMo. Consolidation file (MSP Registration & Premium Billing). 2013. http://popdata.bc.ca/data.
- 20.Vital Statistics Deaths. www.popdata.bc.ca. 2020. https://www.popdata.bc.ca/data/population/vsdeaths
- 21.BC Cancer Registry Data. www.popdata.bc.ca. 2020. https://www.popdata.bc.ca/data/health/bccancer
- 22.PharmaNet. www.popdata.bc.ca. 2020. https://www.popdata.bc.ca/data/health/PharmaNet.
- 23. Amiri N, De Vera M, Choi HK, Sayre EC, Avina-Zubieta JA.. Increased risk of cardiovascular disease in giant cell arteritis: a general population-based study. Rheumatology (Oxford) 2016;55:33–40. [DOI] [PubMed] [Google Scholar]
- 24. Aviña-Zubieta JA, Mai A, Amiri N. et al. Risk of myocardial infarction and stroke in patients with granulomatosis with polyangiitis (Wegener’s): a population-based study. Arthritis Rheumatol 2016;68:2752–9. [DOI] [PubMed] [Google Scholar]
- 25. Li L, Lu N, Avina-Galindo AM. et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population-based study. Rheumatology (Oxford) 2020;60:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernatsky S, Linehan T, Hanly JG.. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol 2011;38:1612–6. [DOI] [PubMed] [Google Scholar]
- 27. Arkema EV, Jönsen A, Rönnblom L. et al. Case definitions in Swedish Register data to identify systemic lupus erythematosus. BMJ Open 2016;6:e007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katz JN, Barrett J, Liang MH. et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum 1997;40:1594–600. [DOI] [PubMed] [Google Scholar]
- 29. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 30. Bursac Z, Gauss CH, Williams DK, Hosmer DW.. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 32. Costenbader KH, Desai A, Alarcón GS. et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 2011;63: 1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jorge A, Wallace ZS, Zhang Y. et al. All-cause and cause-specific mortality trends of end-stage renal disease due to lupus nephritis from 1995 to 2014. Arthritis Rheumatol 2019;71:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falasinnu T, Rossides M, Chaichian Y, Simard JF.. Do death certificates underestimate the burden of rare diseases? The example of systemic lupus erythematosus mortality, Sweden, 2001-2013. Public Health Rep 2018;133:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernatsky S, Ramsey-Goldman R, Labrecque J. et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun 2013;42:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernatsky S, Ramsey-Goldman R, Petri M. et al. Smoking is the most significant modifiable lung cancer risk factor in systemic lupus erythematosus. J Rheumatol 2018;45:393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tallbacka KR, Pettersson T, Pukkala E.. Increased incidence of cancer in systemic lupus erythematosus: a Finnish cohort study with more than 25 years of follow-up. Scand J Rheumatol 2018;47:461–4. [DOI] [PubMed] [Google Scholar]
- 38. Bae EH, Lim SY, Han K-D. et al. Systemic lupus erythematosus is a risk factor for cancer: a nationwide population-based study in Korea. Lupus 2019;28:317–23. [DOI] [PubMed] [Google Scholar]
- 39. Cassaniti I, Cavagna L, Calarota SA. et al. Evaluation of EBV- and HCMV-specific T cell responses in systemic lupus erythematosus (SLE) patients using a normalized Enzyme-Linked Immunospot (ELISPOT) Assay. J Immunol Res 2019;2019:4236503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan K, Clarke AE, Ramsey-Goldman R. et al. Breast cancer in systemic lupus erythematosus (SLE): receptor status and treatment. Lupus 2018;27:120–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were accessed from Population Data BC, https://www.popdata.bc.ca/data.