Abstract
Axonal spheroids are bubble-like biological features that form on most degenerating axons, yet little is known about their influence on degenerative processes. Their formation and growth has been observed in response to various degenerative triggers such as injury, oxidative stress, inflammatory factors, and neurotoxic molecules. They often contain cytoskeletal elements and organelles, and, depending on the pathological insult, can colocalize with disease-related proteins such as amyloid precursor protein (APP), ubiquitin, and motor proteins. Initial formation of axonal spheroids depends on the disruption of axonal and membrane tension governed by cytoskeleton structure and calcium levels. Shortly after spheroid formation, the engulfment signal, phosphatidylserine (PS), is exposed on the outer leaflet of spheroid plasma membrane, suggesting an important role for axonal spheroids in phagocytosis and debris clearance during degeneration. Spheroids can grow until they rupture, allowing pro-degenerative factors to exit the axon into extracellular space and accelerating neurodegeneration. Though much remains to be discovered in this area, axonal spheroid research promises to lend insight into the etiologies of neurodegenerative disease, and may be an important target for therapeutic intervention. This review summarizes over 100 years of work, describing what is known about axonal spheroid structure, regulation and function.
Keywords: Axonal spheroids, degeneration, neurodegenerative disease, cytoskeleton
Introduction
The elimination of axons is both vital during development and deleterious during pathology (Luo and O’Leary, 2005). As neurons innervate their targets during development, they grow exuberant axon collaterals that are then either retained or eliminated by degeneration (Neukomm and Freeman, 2014). In response to axon injury, a similar process of axonal fragmentation, or Wallerian degeneration (WD), occurs, often resulting in permanent loss of neural function (Waller, 1851; Vargas and Barres, 2007). Despite decades of research on each discrete topic, molecular pathways common to both developmental and pathological degenerative etiologies remain largely obscure.
During neurodegeneration, bubble-like swellings form along the length of the axon, a primary and early effect of dynamic axonal deformation. These “varicosities”, “spheroids”, or “swellings” have been described as beads on a string and branches bearing fruit, and they frequently appear on degenerating axons (Ramón y Cajal, 1928; Sasaki et al., 1989). They have been found in developmental degeneration and the aging brain, as well as in injury, neuropathy, neuroaxonal dystrophy, and many neurodegenerative diseases (Luo and O’Leary, 2005; Kilinc et al., 2008; Lauria et al., 2003; Kikuchi et al., 1990; Stokin et al., 2005). As speculated by Cajal, “these voluminous balls may be the seat of destructive processes, autolytic in nature” (Ramón y Cajal, 1928; Azmitia, 2002). However, little has been done to characterize their regulation and function in the 100 years following Cajal’s description.
Recently, we have found that axonal spheroids are critical for the transition from an initial, quiescent phase in which normal functions continue and gross morphology is unchanged after injury (the “latent phase”) to the “catastrophic degeneration” phase in which the axon rapidly disintegrates (Fig. 1) (Yong et al., 2019; Yong et al., 2020). This headway in identifying their function is complemented by elucidation of their regulation. To-date, light and electron microscopy, together with biochemical analysis and biophysical modeling, have described the mechanisms and functions of spheroid formation in neurodegeneration. In this review, we summarize the features of axonal spheroids across degenerative etiologies, propose a working model for spheroid formation and regulation, and discuss known and potential roles of axonal spheroids in progression of axon degeneration and debris clearance.
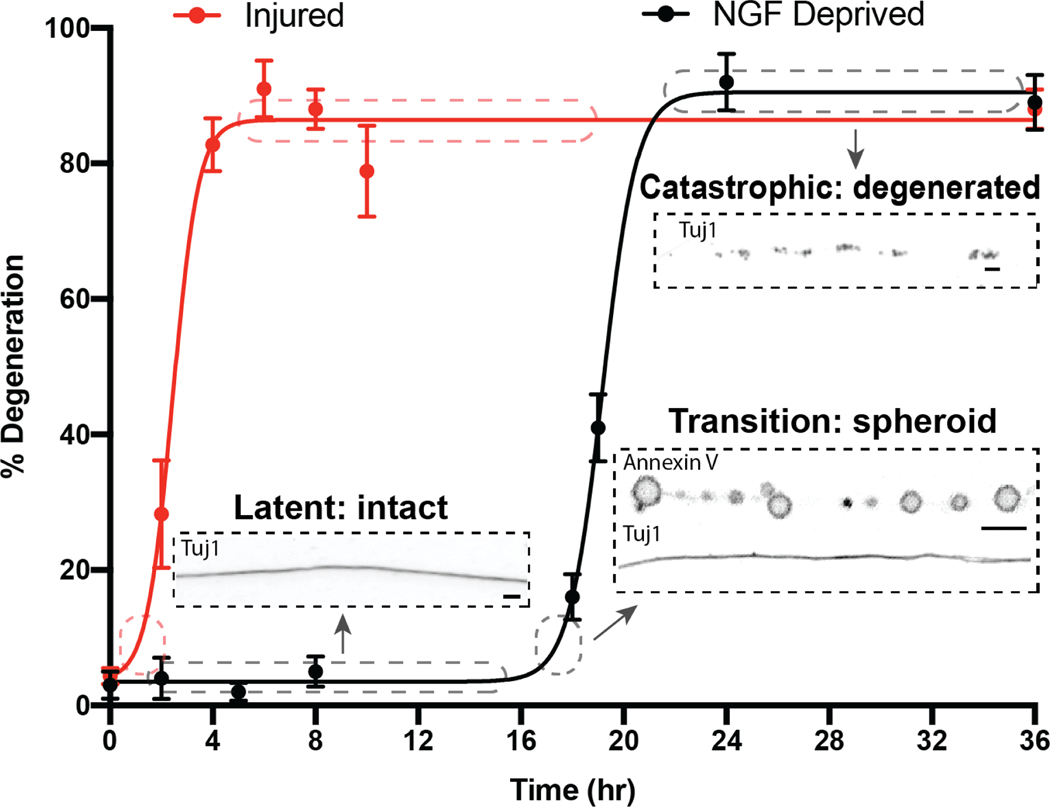
Figure 1:
Stages and morphological hallmarks of degeneration in response to injury and NGF deprivation
Axons are intact and continuous during the latent and transition phases, then become fragmented during the catastrophic phase (Yong et al., 2019). In the transition phase, axons develop spheroids with phosphatidylserine on their outer membranes. Scale bar = 5μm
Aggregates within axonal spheroids
The focal swellings on degenerating axons, axonal spheroids, are often filled with cellular debris such as organelles, pathological proteins, and disorganized cytoskeletal elements. Here, we summarize when and where these contents are found.
Organelles
Neurons require high rates of protein and lipid synthesis, processing, and turnover throughout the cell to support proper function. Because axons grow up to a meter long, neurons rely on a uniquely extended endoplasmic reticulum (ER) network that reaches throughout axonal and dendritic processes, and on anterograde and retrograde transport, in which mitochondria and vesicles are trafficked along the axon via microtubule (MT) tracks (Wu et al., 2017; Hollenbeck and Saxton, 2005). But in response to degenerative triggers, these organelles often accumulate in axonal spheroids, indicating disrupted axonal transport at the site of spheroid formation. Indeed, electron dense bodies, multivesicular bodies (MVBs), mitochondria, and double membrane-bound vesicles have been observed in spheroids in a range of degenerative contexts (Kilinc et al., 2008; Griffiths et al., 1998), though not all (Beirowski et al., 2010). After disrupting MTs via mechanical injury in chick forebrain neurons, mitochondria colocalized with spheroids and microtubule disruptions. However, pharmacological MT stabilizers eliminated spheroid formation, indicating that organelle buildup in spheroids can occur as a result of impaired axonal transport (Kilinc et al., 2008). This deficit in transport could result in axon degeneration and pathology, as proposed in response to observations that membranous dense bodies and mitochondria accumulate at distal paranodes, simultaneous with the appearance of neurofilament (NF) swellings (Griffiths et al., 1998). It is also possible that there is constitutive packaging of malformed organelles within spheroids, regardless of impaired transport. In a mouse model of hereditary spastic paraplegia that disrupted mitochondrial form and function, aberrantly formed mitochondria were observed in spheroids well before detectable functional pathology (Ferreirinha et al., 2004). As such, it seems that spheroidal accumulation of dysfunctional organelles in degeneration could be both functional, sequestering aberrant organelles for degradation, and a correlate of pathology, indicative of impaired cellular transport.
Pathological proteins
Pathological proteins implicated in a variety of neurological disorders have been found in axonal spheroids (Coleman, 2005). Amyloid precursor protein (APP) and ubiquitin accumulate in spheroids across different models of Alzheimer’s disease (AD), hereditary spastic paraplegia (HSP), and traumatic brain injury (TBI) (Griffiths et al., 1998; Ferreirinha et al., 2004; Wirths et al., 2006; Dawson et al., 2010; Ohgami et al., 1992; Johnson et al., 2013). In some cases of neuroaxonal dystrophy and Parkinson’s disease (PD), the degeneration-associated protein α-synuclein has been observed in spheroids (Newell et al., 1999; Martin et al., 2006). Microtubule associated protein (MAP), tau, and phosphorylated tau could also be detected in spheroids in a mouse model of AD (Stokin et al., 2005). However, it should be noted that not all axonal spheroids contain pathological proteins, even when other spheroids from the same neuron do. Instead, some spheroids on injured nerves remain “empty” as lucent cavities under electron microscopy, suggesting that the spheroids do not need to contain pathological proteins in order to form (Beirowski et al., 2010). How these “empty” spheroids differ in fate from their filled counterparts and whether or not they, too, eventually fill remain open questions.
Cytoskeletal elements
Cytoskeletal reorganization underlies morphological changes in degenerating axons, making it unsurprising that most axonal spheroids contain cytoskeletal elements. This is observed across neurodegenerative diseases, mechanical and chemical insults, and developmental contexts. One significant feature of motor neuron disease pathologies such as amyotrophic lateral sclerosis (ALS) is the accumulation of neurofilaments (NF), a subtype of intermediate filaments specific to neurons, in axonal spheroids (Delisle and Carpenter, 1984). Post-mortem analyses of brains and spinal cords from ALS patients reveal strong spheroid positivity for NF, kinesin, and synaptophysin (Takahashi et al., 1997; Toyoshima et al., 1989; Toyoshima et al., 1998). NF has also been observed in spheroids taken from both mouse and human nerves with gracile axonal dystrophy (GAD) (Prineas et al., 1976; Shinzawa et al., 1978). In Alzheimer’s Disease, spheroids containing microtubule-associated protein tau, kinesin-I, and phosphorylated NF have been found preceding established pathological hallmarks such as amyloid deposition in both mouse models and human samples (Stokin et al., 2005). Spheroids containing increased quantities of the cytoskeletal proteins tubulin and actin were observed in both chemical and mechanical insult models such as live cortical neurons treated with hydrogen peroxide and mechanically injured chick forebrain neurons (Kilinc et al., 2008; Barsukova et al., 2012). Corroborating these findings and expanding them to developmental contexts, we have found that tubulin and actin accumulate in injured and neurotrophic growth factor (NGF)-deprived sympathetic axon spheroids (Yong et al., 2019, Yong et al., 2020). As such, it seems that cytoskeletal proteins like NF, tubulin, and actin accumulate in spheroids during cytoskeletal disorganization underlying axon degeneration across a range of degenerative contexts (Ochs et al., 1997; Sabharwal and Koushika, 2019).
Axonal spheroids accumulate cytoskeletal elements in response to a wide range of degenerative triggers including mechanical, chemical, genetic, and trophic insults. Organelles and pathological proteins have also been detected in spheroids across a variety of degenerative processes (Fig. 2). In contrast, some axonal spheroids appear to be empty under light and transmission electron microscopy (Beirowski et al., 2010), which could result from artifacts of fixation, dehydration, and embedding preparations. To determine whether such axonal spheroids are truly “empty” requires the use of imaging techniques that circumvent potential artifacts, such as high-pressure freezing electron microscopy (Mobius et al., 2016). Importantly, how these contents vary as a function of degenerative triggers is unknown, as are the signaling pathways governing their aggregation therein. Moreover, how these contents relate functionally to the formation of spheroids or progression of neurodegeneration requires further investigation.
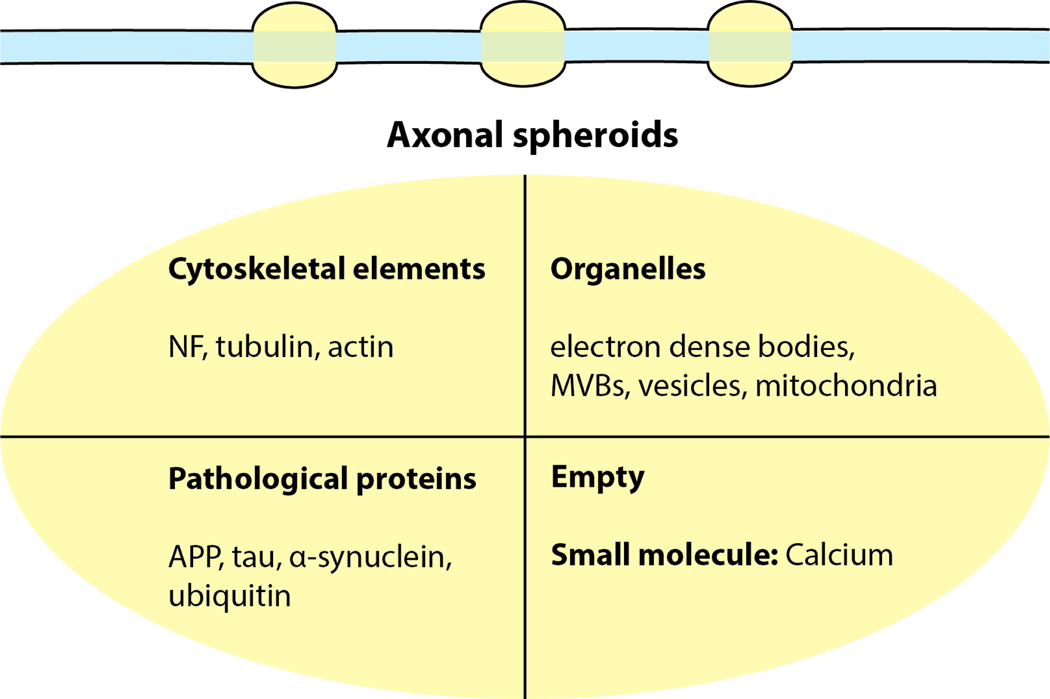
Figure 2:
Aggregates within axonal spheroids
Accumulation of cytoskeletal elements including neurofilaments (NF), tubulin and actin; organelles including electron dense bodies, multivesicular bodies (MVBs), vesicles, and mitochondria; and pathological proteins including amyloid precursor protein (APP), tau, α-synuclein and ubiquitin have been found in axonal spheroids in various neurodegenerative disease models. Some of the axonal spheroids may appear devoid of large organelles, but may still accumulate small molecules like calcium. Although each of these elements has been individually reported to accumulate in spheroids, it is unlikely that their occupancy of spheroids is mutually exclusive.
Axonal spheroid formation and growth
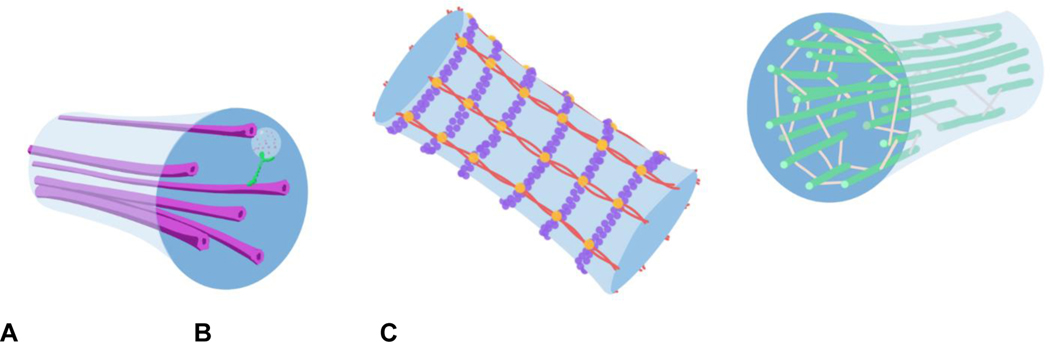
A. Microtubules provide a lengthwise track along which cellular cargo can be transported down the center of the axon, as well as structural stability. B. The membrane periodic skeleton (MPS) is made up of actin polymers linked into rings by actin linking proteins, and spectrin linking actin rings. C. Neurofilaments pack together in the axon, taking up space to keep the axon from collapsing in on itself.
The role of the cytoskeleton in spheroid formation
Axonal spheroids represent a significant disruption in the architecture of the axon, so it follows that changing cytoskeletal structure is involved in their formation. The axonal cytoskeleton is composed primarily of neurofilaments, the membrane periodic skeleton (MPS), and microtubules (MT), each of which play their own roles in shaping the axon’s final structure. Neurofilaments are elastic heteropolymers that organize parallel to the length of the axon, taking up space to provide radial structure that in turn regulates axonal radius and conductivity (Yuan et al., 2012). Actin and spectrin combine to make up the MPS, which forms in a ring-shaped configuration along the length of the axon to provide stability in the radial direction, to anchor the membrane, and to buffer tensile forces (Xu et al., 2013; Dubey et al., 2020). MTs running lengthwise down the center of the axon provide a path along which cellular cargo can be trafficked (Kapitein et al., 2010), but also play a structural role, orchestrating cytoskeleton rearrangement through their associations with MT binding proteins, motor proteins, and actin patches (Coles and Bradke, 2015, Fig. 4)
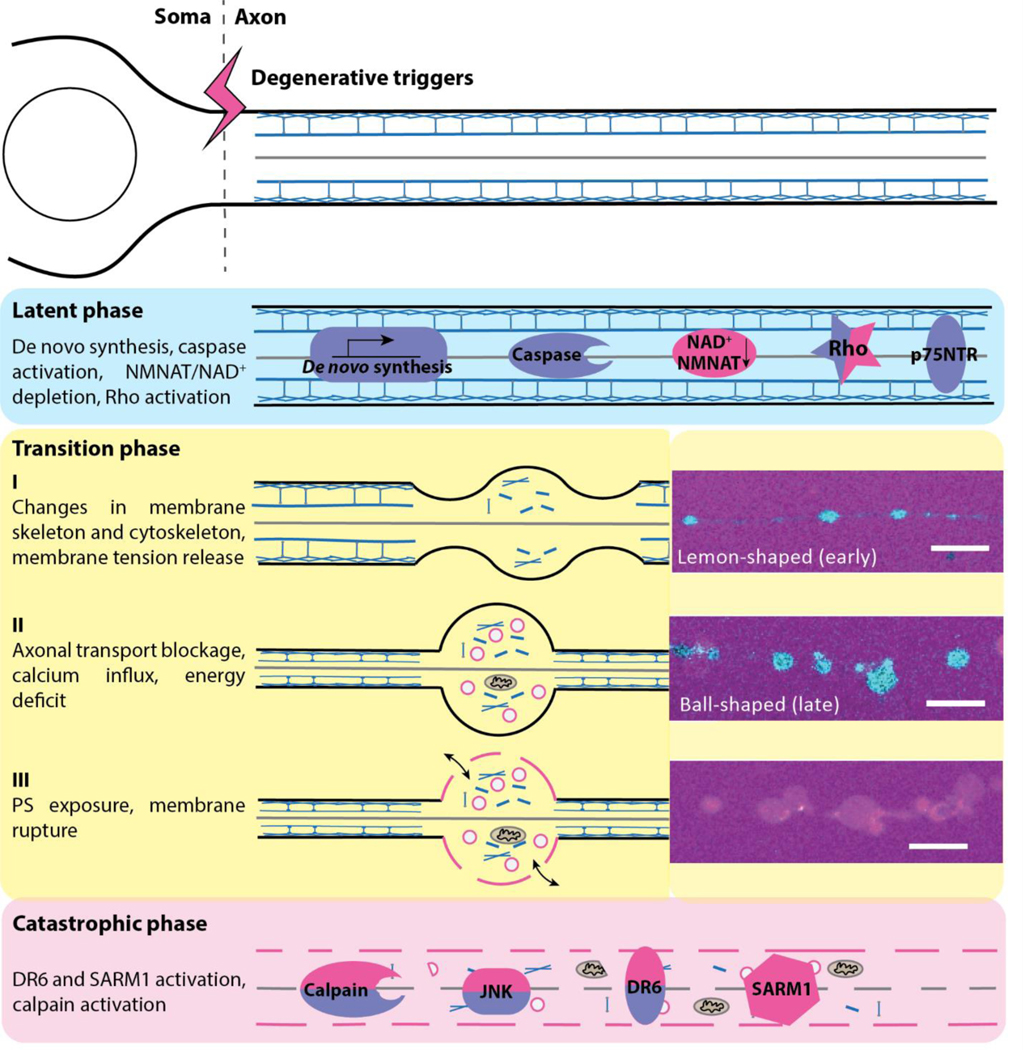
Figure 4:
Proposed model of axonal spheroid formation in axonal degeneration.
Changing the axon from a smooth, cylindrical shape into the “beads on a string” conformation typical of axonal spheroids involves localized constriction and bulging, which is in turn caused by changes in the membrane cytoskeleton (Ochs et al., 1997; Budde and Frank, 2010). This is evidenced by the emergence of spheroids at regions of cytoskeleton disruption after injury or toxic insult, and by the suppression of spheroid formation on axons when pre-incubated with cytoskeleton stabilizers (Kilinc et al., 2008; Barsukova et al., 2012; Tang-Schomer et al., 2012). For instance, we reported that cytochalasin D, an actin-stabilizing drug, delays spheroid formation on both neurotrophin growth factor (NGF)-deprived and injured axons (Yong et al., 2019; Yong et al., 2020). Conversely, destabilizing actin filament (F-actin) with Lactrunculin-A leads to small spheroid formation towards the distal end of the atrophying sensory axons (Datar et al., 2019). Observations such as these are unsurprising, given that the MPS is largely composed of actin and can regulate expansion of axonal diameter and swellings (Costa et al., 2018; Wang et al., 2019), and that neuronal growth cones, made of F-actin, collapse early on in neurodevelopmental degeneration (Unsain et al., 2018).
Similarly to the MPS, microtubules (MTs) are also implicated in spheroid regulation by their structural properties. One form of MTs within the axon can remain intact over long distances to allow efficient trafficking of cargo from soma to terminal, referred to as “stable” MTs (Kapitein and Hoogenraad, 2015). Alternatively, another form of MTs is shorter in length and undergoes rapid de- and repolymerization, a process referred to as “dynamic instability.” This dynamically unstable form of MTs underlies changes in axon structure responding to environmental cues (Coles and Bradke, 2015). Perturbations in MT dynamic instability can cause the formation of axonal spheroids, as inhibiting structure-defining MT depolymerization using detyrosinated MTs and reduced labile tyrosinated tubulin causes the formation of axonal spheroids (Negrete-Hurtado et al., 2020). This observation may represent aberrantly elongated MTs in locations where MTs are typically kept shorter by frequent depolymerization phases, such as en passant boutons (Stettler et al., 2006), pushing out the axonal membrane and giving rise to swellings and spheroids along the axon. In addition to dynamically unstable MTs, stable MTs are also causally linked to spheroid formation, as will be discussed in a later section. Taken together, it is clear that cytoskeletal changes are ubiquitously necessary in forming axonal spheroids. However, how spheroid location is determined relative to cytoskeletal perturbations and what particular cytoskeletal changes accompany that formation have yet to be elucidated.
Regulation of cytoskeletal changes underlying spheroid formation
As discussed previously, cytoskeletal changes such as actin remodeling underlie axon degeneration (Wang et al., 2018). Rho GTPases such as RhoA are key regulators of F-actin and activate downstream signaling via effector proteins like Rho-associated, coiled-coil containing protein kinase 1 (ROCK) (Govek et al., 2005). During developmental degeneration, RhoA/ROCK signaling induces axonal pruning and growth cone collapse by switching F-actin from extending to contractile force generation (Gallo, 2006). Specifically, ROCK activation inhibits myosin phosphatase and activates LIM kinase, which phosphorylates cofilin, resulting in inhibition of actin depolymerization and contributing to actin destabilization (Maekawa et al., 1999).
Given the close overlap between developmental and pathological axon degeneration pathways, RhoA/ROCK signaling is likely important for axonal deformation of all etiologies. Indeed, accumulating evidence suggests that RhoA/ROCK signaling increases in cases of WD, neuroinflammation, and neurodegenerative diseases such as AD, ALS, and spinal cord injury (SCI) (Stankiewicz and Linseman, 2014). In keeping with these observations, we recently found that RhoA activation is involved in axonal spheroid formation (Yong et al., 2019; Yong et al., 2020). Moreover, inhibition of RhoA by CT04 suppresses spheroid formation, while activation of RhoA by CN03 promotes spheroid formation in axons that are normally refractory to degenerative cues, suggesting that Rho activation is both necessary and sufficient to drive the formation of axonal spheroids (Yong et al., 2019; Yong et al., 2020).
However, RhoA can also target another downstream effector of RhoA, mammalian homologue of diaphanous (mDia). mDia recruits profilin, which promotes actin polymerization into elongated stress fibers and, consequently, causes axonal growth (Pantaloni and Carlier, 1993; Watanabe et al., 1999; Arakawa et al., 2003). It seems that RhoA activates ROCK or mDia at the expense of the other (Watanabe et al., 1999), explaining why RhoA activation can result in either axonal degeneration or growth. Taken together, RhoA signaling is a known regulator of spheroid formation in some degenerative contexts such as trophic deprivation and injury, but whether this is true across all degenerative etiologies (e.g. AD, PD, TBI) remains an open question. Additionally, how its downstream effectors such as mDia and ROCK are affected during the formation of axonal spheroids has yet to be elucidated. Finally, inhibition of the RhoA/ROCK pathway provides therapeutic potential for boosting neuroprotection and axonal regeneration after injury (Kubo et al., 2007; Forgione and Fehlings, 2014).
It should be noted that cytoskeleton dysregulation independent of RhoA signaling merits its own attention with respect to axonal spheroid formation. For instance, the axonal actin-spectrin network acts as a tension buffer system, which is required for maintaining axon diameter to release excess mechanical stress (Dubey et al., 2020). Depletion of the actin binding protein adducin results in increased diameter of the actin ring, which may be the structure underlying axonal swellings, or nascent axonal spheroids (Leite et al., 2016; Datar et al., 2019). As such, future research addressing the molecular mechanisms governing membrane tension and regulation of the actin-spectrin network during spheroid formation would provide valuable insight to the field.
Contributions of disrupted axonal transport to spheroid formation
Impaired axonal transport is believed to be sufficient, though not necessary, for spheroid formation. Additionally, it has long been debated whether impaired transport causes spheroid formation and disease, or if spheroid formation precedes disease-induced transport deficits (Mi et al., 2005). In fact, evidence exists for both models, which will also be reviewed below.
The axonal transport system is a unique feature in neurons that allows long distance signal transduction, vesicular transport, and organelle delivery across the neuron via attachment to motor proteins moving along MTs. The slow transport system carries the bulk of axoplasmic constituents in the anterograde direction (i.e. cell body to distal tips of axons); the fast transport system functions bidirectionally and carries small vesicles and other organelles via the dynein and kinesin (Griffin and Watson, 1988). There is a well-established associative link between neurodegenerative disease and transport deficits, with mutations in genes for motor proteins, adaptor proteins, and microtubule network proteins resulting in neuropathologies (Sleigh et al., 2019). These deficits and diseases inevitably coincide with axonal spheroid formation. Light and electron microscopy provide evidence of universally impaired axonal transport across a variety of neuropathologies, revealing focal accumulation of APP, tau, motor proteins, and membrane-bound organelles like mitochondria in axonal spheroids (Ferreirinha et al., 2004; Götz et al., 2006; Kilinc et al., 2008; Tang-Schomer et al., 2012). It remains unclear whether spheroid formation and neurodegeneration drives transport defects or vice versa.
In favor of axonal transport deficits causing spheroid formation and disease, impaired axonal transport is a key step in the pathogenesis of many neurodegenerative diseases (Millecamps and Julien, 2013). In a mouse model of AD, impairing axonal transport by depleting kinesin-I increased amyloid deposition and enhanced the formation of axonal spheroids (Stokin et al., 2005). As such, it is possible that axonal transport defects can trigger spheroid formation and disease pathology, at least in this model of AD. Also consistent with observations that would be expected from buildup of trafficking cargo, axonal transport deficits give rise to organelle-filled spheroids, the formation of which can be rescued with MT-stabilizers (Kilinc et al., 2008). Interestingly, pharmacological disruption of MT dynamics results in the proximal-to-distal formation of axonal spheroids (Datar et al., 2019), which could be the product of a proximal-to-distal loss of anti-degeneration signals constitutively trafficked along MTs (Wang et al., 2012).
The formation of spheroids is unlikely to result from “traffic jams” in many cases, as evidenced by the following observations from models of trophic deprivation, stretch-induced injury, and axotomy: 1. Continuous microtubule tracks were observed in a large fraction of spheroids (Tang-Schomer et al., 2012). 2. No vesicles or mitochondria were seen in axonal spheroids at early stages (Beirowski et al., 2010). 3. The spheroid shapes (lemon-like at early stages and clamshell- or ball-like at late stages, Fig. 3) are typical of a surface maintained under tension, as opposed to a shape defined by a collection of organelles that would look more like a sack of potatoes (Datar et al., 2019). Taken together, these observations suggest that spheroid formation is unlikely to be solely dependent on impaired axonal transport, although it seems that impaired axonal transport can cause spheroid formation in some cases.
Figure 3:
Axonal cytoskeleton structures
Calcium dependent transition in size and shape of spheroids
Disruptions in calcium homeostasis and buffering are common in aging and neurodegenerative disease (Mattson, 2007; Müler et al., 2018), with perturbations like aberrant influx or release from intracellular stores such as those in mitochondria promoting axon degeneration (Stirling and Stys, 2010), and calcium-rich spheroids forming on degenerating axons in both developmental and pathological contexts (Barsukova et al., 2012; Gu et al., 2017; Yong et al., 2019). The precise mechanistic pathways underlying this calcium-driven spheroid formation are still being elucidated; however, extracellular and intracellular calcium seem to coordinate to create fully formed spheroids. Trophically deprived, sympathetic axons still form spheroids when intracellular calcium is depleted, although they are diminished in size and maintain a lemon-like shape instead of clamshell- or ball-like shape (Yong et al., 2019). In contrast, extracellular calcium quenching or removing extracellular calcium can prevent spheroid formation entirely on optic nerves and sensory axons (Beirowski et al., 2010; Johnstone et al., 2019). Consistent with these findings, extracellular calcium is necessary and sufficient for axonal swellings preceding axonal spheroids to form on axons exposed to reactive oxygen species, while intracellular calcium from mitochondria is required for swellings to transition to spheroids (Barsukova et al., 2012). As such, it seems that extracellular calcium coordinates the initial phase of spheroid formation, and intracellular calcium continues the process of spheroid growth. Future research elucidating how calcium mediates spheroid growth will be useful in understanding this phenomenon.
Changing osmolarity also determines spheroid size and shape by providing the driving force for water and ions to leak through channels on the membrane following elastic stresses in the cytoskeleton, inducing swelling (Pullarkat et al., 2006; Fernández and Pullarkat, 2010). For instance, mechanical stress induces spheroid formation by activating the transient receptor potential cation channel subfamily V member 4 (TRPV4) (Gu et al., 2017). The resulting calcium influx creates a local hypertonic environment, relative to extracellular space (Woolums et al., 2020). This increased osmolarity would bring in water, causing volume expansion and activating calmodulin, a calcium-binding messenger protein that inhibits MT-stabilizing protein STOP, promoting further spheroid formation (Gu et al., 2017). Consistent with spheroid growth in response to osmotic shock driven by calcium influx, focal aggregation of the calcium exchanger NCX and subunits of the voltage-gated calcium channel (VGCC) were found at the site of spheroid formation in stressed axons (Barsukova et al., 2012). As such, it is likely that calcium flux and attendant osmolarity changes contribute to growth and maturation of pre-existing axonal spheroids.
Biophysical model for spheroid formation
In the physics community, the morphological changes and formation of spheroids observed on degenerating axons is described as “pearling instability”, the transformation of cylindrical cell extensions into a chain of periodic spheres (Bar-Ziv et al., 1999). Theoretical models and computational simulations of this axonal behavior suggest that spheroid formation is a tension-driven shape instability dependent on interplay between cytoskeletal rigidity and membrane surface tension (Datar et al., 2019; Shao et al., 2020). Based on the biophysical measurements of axonal spheroids, we propose the model of spheroid formation described in the following section (Fig. 3).
Interestingly, initial intracellular calcium rise following axon injury is not driven by influx through the recently sheared axonal membrane, as calcium causes the rapid fusion of vesicles at the injury site, rebuilding the membrane over the injury (Eddleman et al., 1998). Instead, ion channels are opened in the seconds following pathological insult, allowing calcium from extracellular space to enter the axon (Ziv and Spira, 1993). This activates proteases such as calpain, which cleave cytoskeletal elements including spectrin and tubulin (Wang et al., 2012). The resulting structural perturbations lead to localized release of axonal and membrane tension, driving the initial formation of axonal swellings via pearling instability. Stretching the membrane in such a way causes nanoruptures to form and tension-regulated ion channels to open, which allow further ion and water influx (Sterling and Stys, 2010; Witte et al., 2019). This influx expands the swellings into nascent spheroids. Sustained elevated calcium impairs axonal transport, which could result in buildup of organelles within spheroids (Gu et al., 2017), cytoskeletal dysregulation, and energy deficits, escalating the positive feedback loop of spheroid growth and intracellular ion perturbation. The loop ends when, eventually, these spheroid- and axon-derived perturbations accumulate enough calcium to trigger catastrophic axonal degeneration (Barrientos et al., 2011) via release of mitochondrial calcium stores through the mitochondrial permeability transition pore (mPTP) (Rasola and Bernardi, 2007). Interestingly, formation of the mPTP causes mitochondria to take on a swollen morphology (Bernardi et al., 2006), consistent with the aberrantly shaped mitochondria observed within spheroids in a model of hereditary spastic paraplegia (Ferreirinha et al., 2004). In the time leading up to mPTP opening, NAD+, the key product of NMNAT that slows Wallerian degeneration, can localize to the mitochondrial membrane (Yahata et al., 2009), mitigating the ion imbalance to delay catastrophic degeneration as in the case of WLDs mutants (Wang and Barres, 2012).
This model is consistent with observations that extracellular calcium is necessary for the formation of spheroids, while intracellular calcium is required only for expansion of spheroids after their initial formation, as discussed previously. Additionally, this model could account for the observation that degenerating axons thin between spheroids (Datar, 2019). Firstly, if the spectrin-actin network becomes disorganized during spheroid formation, this could cause the disintegration of structures near the axonal membrane and result in axon thinning. Secondly, increasing spheroid volume would demand reallocation of axonal membrane to spheroids, resulting in axonal volume loss between spheroids in an attempt to prevent membrane rupture. While this model is based on the aforementioned empirical observations and biophysical modeling, further experiments are required to verify the spatiotemporal regulation of axoskeleton during spheroid formation in response to different degenerative triggers.
Spheroid formation dynamics
Temporal dynamics of spheroid formation have been studied in vitro for acute insults such as pharmacological cytoskeletal disruption, axotomy, and trophic factor deprivation. In every such case, spheroid formation increases exponentially with time; however, the latent period leading up to spheroid formation becomes shorter with worsening insult severity. Spheroid formation begins within minutes in cases of pharmacological cytoskeleton disruption, within hours in cases of axotomy, and in some 18 hours in cases of trophic factor deprivation (Datar et al., 2019, Yong et al., 2020, Yong et al., 2019). This speaks to the order in which processes underlying spheroid formation occur, with latent degeneration pathways converging on cytoskeleton disruption as a key, final step.
Though in vivo time courses remain scarce, Eyo et al. (2021) found that new beads form out to 48 hours after kainic acid-induced seizures, and, interestingly, that some of these spheroids are resolved in that same window of time. As such, spheroid formation may be prevented or even reversed by environmental factors such as glia and extracellular matrix. In keeping with this idea, spheroids seem to form more readily in the CNS than in the PNS (Coleman, 2005; Beirowski et al., 2005), which have notably different extracellular environments. This is further supported by the fact that spheroids are readily observed on PNS neurons cultured alone in vitro (Yong et al., 2020), but not in ex vivo (Beirowski et al., 2004) and in vivo imaging preparations (Beirowski et al., 2005) in which the extracellular environment remains intact during injury responses. Further examination of how extracellular factors influence spheroid formation would provide insight as to how these processes occur in vivo. Furthermore, while spheroid formation dynamics have been studied in acute insult models, their dynamics in chronic pathologies like Alzheimer’s Disease and ALS have yet to be examined, likely due to the challenges of long term in vivo imaging and the relative nascence of axonal spheroid research.
In the latent phase of degeneration, no morphological change is observed in axons. NGF deprivation (indicated in violet) triggers de novo synthesis of pro-degenerative molecules, caspase activation, p75 neurotrophin receptor (p75NTR) and Rho activation, while injury (indicated in red) leads to NMNAT/NAD+ depletion and Rho activation. These upstream events promote the formation of axonal spheroids in the transition phase. Early changes in membrane skeleton and cytoskeleton promote the release of membrane tension and emergence of the lemon-like shape of spheroids. Subsequent disruption of axonal transport, calcium dysregulation, and energy deficit further promote the growth of axonal spheroids, becoming clamshell- or ball-like shapes. Insets are of injured axons and spheroids visualized using Fluo-4 calcium imaging to show “lemon”- versus ball-shaped spheroids early or late in the transition process, and visualized using 3kDa fluorescent dextran, respectively. These spheroids have phosphatidylserine (PS) exposure on the outer surface of the membrane and develop membrane rupture that allows the transmission of pro-degenerative molecules across the membrane. Ruptured axonal spheroids, together with the activation of calpain, c-jun N-terminal kinase (JNK), death receptor 6 (DR6) and sterile alpha and TIR motif containing 1 (SARM1) promote cytoskeleton breakdown and catastrophic axon degeneration. Other molecules, such as ubiquitin proteasome system (UPS), Bcl-2 associated x-protein (Bax), Axundead (Axed), mitogen-activated protein kinase (MAPK) signaling cascade, have not been assigned to particular phases of degeneration,and their roles in regulating spheroid formation remain unclear.
Axonal spheroid rupture
Both cell death and axon degeneration have early phenotypic representations, like membrane blebbing and spheroid formation, respectively. Plasma membrane rupture is commonly seen in necrotic, but not apoptotic, cell death, and we and others have found that rupture is also a hallmark of spheroid formation and growth during axon degeneration (Zhang et al., 2018; Yong et al., 2019; Yong et al., 2020). Fluorescent neutral dyes less than 10 kDa are able to enter the spheroids via membrane rupture, indicating that the pores on the spheroidal membrane are likely non-selective gaps in cell membrane (Zhang et al., 2018; Yong et al., 2019; Yong et al., 2020).
In addition to permeability caused by membrane rupture, there is also evidence for regulated pore formation in axon degeneration. Recent studies have revealed the involvement of necroptosis activation in axon degeneration. Pharmacological inhibition of the necroptotic kinase RIPK1 (receptor-interacting serine/threonine-protein kinase 1), knockdown of the key necroptotic regulator RIP3 or the downstream effector mixed lineage kinase domain like pseudokinase (MLKL), delayed axonal degeneration of sensory neurons after mechanical and toxic insults (Arrázola et al., 2019). As the final effector of necroptosis, MLKL translocates to the plasma membrane, creating pores capable of allowing molecules less than 10kDa to exchange between the axoplasm and extracellular space (Ros et al., 2017; Heckmann et al., 2019). Whether MLKL pores contribute to spheroidal membrane rupture remains an open question. Interestingly, although MLKL is transiently activated after axonal damage, MLKL does not directly induce membrane rupture and calcium influx in axons as it does in cell bodies. Instead, it has been proposed that MLKL may stimulate sterile alpha and TIR motif containing 1 SARM1-dependent axon degeneration (Arrázola et al., 2019; Ko et al., 2020). Additionally, it has been shown that necroptosis is dispensable in motor neuron degeneration in ALS (Wang et al., 2020). Other studies suggested as data not shown that treatment of necroptosis inhibitor or lentiviral-shRNA knockdown of signaling components of necroptosis pathway like RIP3 and MLKL failed to protect axons (Yang et al., Cell 2015), indicating non-necroptotic mechanism that governs the irreversible spheroidal rupture and axon degeneration. While the contribution of necroptotic mediators in axon degeneration varies in different neuron types, the membrane rupture on axonal spheroids might be regulated by MLKL-independent mechanisms.
Another pathway to induce membrane rupture in dying cells is pyroptosis, in which the pore-forming protein gasdermin D (GSDMD) is activated (McKenzie et al., 2020). Emerging evidence suggests the formation of GSDMD pores in microglia, macrophages, and oligodendrocytes in neurodegeneration (McKenzie et al., 2018). Interestingly, increasing SARM1 in cells induces mitochondrial depolarization to promote GSDMD-dependent pyroptosis (Carty et al., 2019). Neurons with little or low GSDMD expression seem to be susceptible to caspase-1-mediated, but GSDMD-independent cell death (Tsuchiya et al., 2019). However, whether the spheroid rupture is driven by the formation of GSDMD pores or simply the result of disrupted membrane tension requires further investigation.
Relationship between spheroids and myelin
How do spheroids form in myelinated axons? Most of the in vitro studies so far have focused on the spheroids in non-myelinated axons. While most axons are unmyelinated in vivo, especially in the PNS (Carter & Lisney, 1987), some axons are wrapped in a myelin sheath. In response to injury, peripheral axons are fragmented and encapsulated into myelin ovoids (Catenaccio et al., 2017; Beirowski et al., 2005). Given that alterations in myelination are another hallmark of degenerating axons, it is also important to understand how spheroids form in the context of myelinated axons. While the overwhelming majority of in vivo studies have reported spheroids on demyelinated axons as result of neurodegeneration (Rosenfeld and Friedrich, 1983; Lovell and Jones, 1985; Barron et al., 1987; Arsinio-Nunes and Goutieres, 2015), there are few examples of spheroids forming while myelin is still intact (Hirano et al., 1984, Alturkustani et al., 2015). Given these previous observations, it is tempting to speculate that, while spheroid formation does not require demyelination, loss of ensheathment increases the probability of spheroid formation. Moreover, a lack of myelination could allow spheroidal contents that are released after rupture to interact with neighboring axons that are usually protected by myelin to induce their neurodegenerative effect. Alternatively, given the biophysical model of spheroid formation described above, myelination might be physically impeding formation of spheroids from the axons. However, if and how that occurs requires further investigation.
Function of axonal spheroids
What is the evolutionary significance of spheroid formation in the nervous system? Are spheroids causes or symptoms of axon degeneration? Are they protective of distressed axons or causative in the progression of axon degeneration? Perhaps axonal spheroids promote localized degeneration and clearance of debris of an axon without influencing the main neurite and cell body (Coleman, 2005; Luo and O’Leary, 2005). Here, we discuss the potential roles of spheroid rupture in clearance of axon debris and progression of neurodegeneration.
Phosphatidylserine (PS) exposure and clearance
Similarly to the PS exposure on the outer surface of cell membrane during apoptosis, PS exposure was also observed on the membrane of degenerating dorsal root ganglion axons after NGF deprivation or vincristine treatment (Shacham-Silverberg et al., 2018). Interestingly, studies have shown that PS is exposed on non-apoptotic degenerating neurites in a specific spatiotemporal pattern, which causes phagocytes to engulf the neurites (Sapar et al., 2018; Shlomovitz et al., 2019). Consistently, we showed PS outer leaflet exposure specifically on the spheroids of superior cervical ganglion axons after NGF deprivation (Yong et al., 2019), suggesting that PS exposure can be targeted to selective parts of the cell membrane.
As a well defined phagocytotic signal, PS externalization is promoted by phospholipid scramblases mediated by calcium or caspases (Segawa et al., 2014; Nagata et al., 2016). Interestingly, blocking extracellular calcium influx did not prevent PS exposure, while inhibiting caspase activity suppressed PS exposure only in apoptotic axon death (Shacham-Silverberg et al., 2018). Moreover, transected axons of retinal ganglion cells isolated from Wlds rats have suppressed axonal spheroid formation and a delay of PS outer leaflet exposure compared to injured wild-type axons (Almasieh et al., 2017). PS is also exposed on degenerating dendrites after injury and depends on NAD+ depletion but not caspase activity (Sapar et al., 2018). These results suggest that while localized perturbation of PS plasma membrane leaflet asymmetry is common in degenerating axons and dendrites, the trigger for PS exposure on the outer leaflet of axonal spheroids or different neurite domains is context dependent.
As a neuronal “eat-me” signal, localized externalization of PS has been shown to mediate developmental synaptic pruning by microglia in mouse hippocampal neurons, while PS exposure on damaged axons acts as a “save-me” signal to promote axonal fusion after injury in C. elegans (Abay et al., 2017; Scott-Hewitt et al., 2020). Therefore, it is possible that axonal spheroids bearing PS on their outer leaflet function as the trash cans laid out on the curb of damaged axons to promote phagocytosis and clearance. This idea is further supported by localization of lysosome and autophagosome markers to spheroids and swellings (Beirowski et al., 2010), suggesting that neuronal debris may be selectively gathered there for disposal. Indeed, recent work in a rat model of Parkinson’s Disease found that aberrant mitochondria at degenerating dopaminergic synapses are packaged into spheroids, then taken up by astrocytes for destruction by autophagosomes (Morales et al., 2020).
Axonal spheroids are frequently observed in proximity to macrophages (Takahashi et al., 1997), indicating that axonal spheroids may affect macrophage localization in instances such as nerve injury responses. Supporting this notion, PS exposure on axonal spheroids could act as the “eat-me” signal to macrophages in response to pathological insult (Shacham-Silverberg et al., 2018; Yong et al., 2019). Specifically, T-cell immunoglobulin and mucin domain containing 4 (Tim4) binds to PS and is tethered to the macrophage surface. Activated macrophages upregulate the key PS-binding protein MFG-E8 (milk fat globule EGF-like factor-8) and its vitronectin receptor (integrin), and the Mer receptor tyrosine kinase (MerTK), which recognises PS through bridging molecules and leads to Rac1 activation and actin polymerization, both of which are required for phagocytosis (Brown and Neher, 2012; Fricker et al., 2012; Segawa et al., 2014). Interestingly, macrophage recruitment to injured axons is not impaired in Wlds expressing zebrafish, which do not develop axonal spheroids after injury. A novel “nerve scanning” behavior of macrophages was observed in these animals (Rosenberg et al., 2012), indicating that macrophage engulfment behaviors, not recruitment, may be associated with axonal spheroids. From these findings it is tempting to speculate the existence of discrete “find me” and “eat me” signals on injured axons, with PS representing one critical “eat me” signal (Ravichandran, 2011). Both of these signals must be further delineated if we are to fully understand the relationship between axon spheroids and macrophages in promoting axonal protection, clearance of debris and/or axon regeneration. Schwann cells are another major player in PNS degeneration. They are known to collaborate with and recruit macrophages to clear debris at sites of injury (Brosius Lutz et al., 2017; Vaquie et al, 2019). Upon injury, these cells convert into repair Schwann cells (Jessen et al., 2016) to help clean up debris and promote subsequent axon regeneration. As discussed previously, myelination seems to prevent spheroid formation. Identifying this overlap between Schwann cell injury responses and spheroid formation pathways could shed light on how neurodegeneration is regulated in the complex context of a whole organism.
Spheroid rupture and progression of degeneration
Membranes compartmentalize the cell and organize cellular processes, and perturbation of these compartments has been observed in many neurodegenerative diseases (de Groot and Burgas, 2015). In dying neurons, membrane rupture allows the extrusion of intracellular contents, triggering inflammatory responses in and causing damage to neighboring cells (Fricker et al., 2018). It also allows the pathological influx of molecules like calcium from extracellular space to drive axon degeneration (Witte et al., 2019). Interestingly, we found that axonal spheroids often rupture, providing a gateway for electrochemical gradient disruption and toxic axonal content release and thus promoting catastrophic axon degeneration in an autocrine or paracrine manner. Indeed, conditioned media collected after spheroidal rupture is able to hasten entry of intact recipient axons or injured Wlds axons into rapid and irreversible catastrophic phase (Yong et al., 2019; Yong et al., 2020). Thus, ruptured axonal spheroids could be a lynchpin of pro-degenerative factor transmission across the membrane to coordinate degeneration of nearby vulnerable axons or axon fragments.
Specific spheroid derived pro-degenerative factors have yet to be identified. We detected increased calcium levels in conditioned media after spheroidal rupture, which promotes catastrophic degeneration of injured or NGF-deprived axons (Yong et al., 2019; Yong et al., 2020). Importantly, depleting intracellular calcium stores suppressed the increase of extracellular calcium after membrane rupture. Chelating calcium in conditioned media or in the extracellular environment can suppress the pro-degenerative effect (George et al., 1995; Yong et al., 2019). These results suggest that calcium could be one of the pro-degenerative factors transmitted via spheroidal rupture. However, the intracellular calcium level is usually kept relatively low with respect to the extracellular space (Maravall et al., 2000). Spatiotemporal patterning of calcium contributes to neuronal function and signaling transduction (Bagur and Hajnóczky, 2017). How intracellular calcium gets released into the extracellular environment against the overall electrochemical gradient is unknown. It’s possible that the localized spheroidal calcium concentration is higher than the extracellular calcium level prior to membrane rupture. But whether the ruptured spheroids are sufficient to drive a calcium-dependent catastrophic degeneration remains an open question. Likewise, in the context of membrane rupture, intracellular and extracellular calcium concentrations rapidly equilibrate, which may indicate that if degeneration were triggered via transient calcium efflux, it may be acting via a cell surface signaling.
The receptors downstream of spheroid-derived prodegenerative ligands remain to be fully characterized. Previously, we found that death receptor 6 (DR6) likely acts downstream of spheroidal rupture. Indeed, neurons lacking DR6 or Sarm1 are refractory to conditioned media induced entry into catastrophic degeneration (Yong et al., 2019; Yong et al., 2020). DR6 is known to be an orphan receptor so it is unclear how spheroidal contents might be activating it. Whether and how DR6 and Sarm1 work together to coordinate axon degeneration as one of the receptors and adaptor proteins responsive to pro-degenerative factors derived from axonal spheroids require further investigation.
Concluding remarks
Although the field is making headway in elucidating the regulation and function of axonal spheroids in neurodegeneration, a number of questions remain unanswered. First, how do axonal spheroids develop in the context of a myelin sheath? Most evidence related to the regulation of spheroid formation comes from in vitro studies of pure neuronal cultures. However, most axons over a critical diameter are enwrapped by myelin in vivo (Stassart et al., 2018). Histological analysis of brain slices and nerve sections reveal uneven distribution of axonal spheroids across regions (Kikuchi et al., 1990; Beirowski et al., 2010). It’s possible that spheroids are only functionally relevant in degenerating unmyelinated or pre-myelinated axon tracts. Alternatively, they may have a role in coordinating glial response in developmental and pathological degeneration.
Second, is the formation and rupture of axonal spheroids a conduit for disease progression? For example, disease related molecules such as tau, APP, and ɑ-synuclein accumulate in some axonal spheroids, which could potentially be exposed to the extracellular environment and contaminate “by-stander” neurons via spheroid rupture (Ohgami et al., 1992; Newell et al., 1999; Tang-Schomer et al., 2012). Since spheroidal rupture is non-selective, neurotoxic factors in the extracellular space could also enter axon segments to speed up the degeneration process. Further identification of spheroid-derived degenerative molecules is required.
Third, many features of axonal spheroids, such as PS exposure, NF accumulation, RhoA activation, actin remodeling and cation channel aggregation point to cytoskeleton organization and lipid domains on spheroids are distinct from non-spheroidal regions of the axons. What are the membrane and cytoskeleton coupled signaling complexes in the axonal spheroid? Do spheroidal signaling complexes contribute to the progression of degeneration?
As a point of convergence common to all degenerative processes, axonal spheroids represent a worthwhile target for therapeutic consideration. Improving macrophage recruitment based on the recognition of axonal spheroids might protect axons and promote regeneration. Alternatively, suppressing spheroid formation and rupture may prevent the spread of degeneration to bystander neurons in pathological scenarios like TBI and secondary damage in neurodegeneration.
Highlights.
Axonal spheroids accumulate cytoskeletal elements, organelles, and pathological proteins.
Axonal spheroid formation and growth are regulated by ion influx, namely calcium, and consequent structural modifications caused by cytoskeletal changes and membrane rupture.
Spheroids can signal to macrophages via phosphatidylserine, indicating a potential role for them in eliminating cellular debris.
Unchecked, this cellular debris can activate degeneration-inducing receptors such as p75NTR and Death Receptor 6, and activate known degeneration pathways such as necroptosis and pyroptosis.
Acknowledgements
Carol Cho and Tanvika Vegiraju edited the manuscript and formatted the references.
Funding
This work was supported by NIH-NINDS Grant R01NS091617 and the Owens family foundation awarded to C.D.D.
List of abbreviations
- APP
amyloid precursor protein
- PS
phosphatidylserine
- NF
neurofilaments
- WD
Wallerian degeneration
- ALS
amyotrophic lateral sclerosis
- GAD
gracile axonal dystrophy
- ER
endoplasmic reticulum
- MVBs
multivesicular bodies
- AD
Alzheimer’s disease
- HSP
hereditary spastic paraplegia
- TBI
traumatic brain injury
- SCI
spinal cord injury
- PD
Parkinson’s disease
- MAP
microtubule associated protein
- MPS
membrane periodic skeleton
- TRPV4
transient receptor potential cation channel subfamily V member 4
- NCX
sodium calcium exchanger
- VGCC
voltage gated calcium channel
- RIPK1
receptor-interacting serine/threonine-protein kinase 1
- MLKL
mixed lineage kinase domain like pseudokinase
- GSDMD
gasdermin D
- DR6
death receptor 6
- SARM1
sterile alpha and TIR motif containing 1
Footnotes
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript in this journal.
Availability of data and materials
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abay ZC, Wong MY-Y, Teoh J-S, Vijayaraghavan T, Hilliard MA, Neumann B, 2017. Phosphatidylserine save-me signals drive functional recovery of severed axons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 114, E10196–205. doi: 10.1073/pnas.1703807114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, Catrinescu M-M, Binan L, Costantino S, Levin LA, 2017. Axonal Degeneration in Retinal Ganglion Cells Is Associated with a Membrane Polarity-Sensitive Redox Process. J. Neurosci 37, 3824–39. doi: 10.1523/JNEUROSCI.3882-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alturkustani M, Keith J, Hazrati L-N, Rademakers R, and Ang L-C, 2015. Pathologic Staging of White Matter Lesions in Adult-Onset Leukoencephalopathy/Leukodystrophy With Axonal Spheroids. J. Neuropathol. Exp. Neurol 74(3), 233–240. Retrieved from https://academic.oup.com/jnen/article/74/3/233/2614341 [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S, 2003. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 161, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Saribasak H, Buerstedde JM, 2004. Activation-Induced Cytidine Deaminase Initiates Immunoglobulin Gene Conversion and Hypermutation by a Common Intermediate. PLOS Biology 2(7), e179. 10.1371/journal.pbio.0020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrázola MS, Saquel C, Catalán RJ, Barrientos SA, Hernandez DE, Martínez NW., et al. , 2019. Axonal degeneration is mediated by necroptosis activation. J. Neurosci 39, 3832–44. doi: 10.1523/JNEUROSCI.0881-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsinio-Nunes ML, and Goutieres F, 1978. Diagnosis of infantile neuroaxonal dystrophy by conjunctival biopsy. Journal of Neurology, Neurosurgery, and Psychiatry. 41, 511–515. 10.1136/jnnp.41.6.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, 2002. Chapter 8 Cajal’s hypotheses on neurobiones and neurotropic factor match properties of microtubules and S-100β. In: Changing views of cajal’s neuron. Elsevier; pp. 87–100. doi: 10.1016/S0079-6123(02)36010-2. [DOI] [PubMed] [Google Scholar]
- Bagur R, Hajnóczky G, 2017. Intracellular ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell 66, 780–8. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ziv R, Tlusty T, Moses E, Safran SA, Bershadsky A, 1999. Pearling in cells: a clue to understanding cell shape. Proc. Natl. Acad. Sci. USA 96, 10140–5. doi: 10.1073/pnas.96.18.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, Twiss JL, Alvarez J, Court FA, 2011. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci 31(3), 966–78. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron KD, Dentinger MP, Csiza CK, Keegan SM, and Mankes R, 1987. Abnormalities of central axons in a dysmyelinative rat mutant. Experimental and Molecular Pathology. 47(1), 125–142. 10.1016/0014-4800(87)90013-X [DOI] [PubMed] [Google Scholar]
- Barsukova AG, Forte M, Bourdette D, 2012. Focal increases of axoplasmic Ca2+, aggregation of sodium-calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress. J. Neurosci 32, 12028–37. doi: 10.1523/JNEUROSCI.0408-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP, 2004. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J Neurosci. Methods 134, 23–35. Doi: DOI: 10.1016/j.jneumeth.2003.10.016 [DOI] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP, 2005. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 1;6:6. doi: 10.1186/1471-2202-6-6. PMID: 15686598; PMCID: PMC549193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Nógrádi A, Babetto E, Garcia-Alias G, Coleman MP, 2010. Mechanisms of axonal spheroid formation in central nervous system Wallerian degeneration. J. Neuropathol. Exp. Neurol 69, 455–72. doi: 10.1097/NEN.0b013e3181da84db. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronili V, Blachly-Dyson E, Di Lisa F, Forte MA, 2006. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 273, 2077–99. doi: 10.1111/j.1742-4658.2006.05213. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A, Chung W-S, Sloan SA, Carson GA, Zhou L, Lovelett E, Posada S, Zuchero JB, Barres BA, 2017. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. PNAS, 8072–8080. 10.1073/pnas.1710566114 [DOI] [PMC free article] [PubMed]
- Brown G, Neher J, 2014. Microglial phagocytosis of live neurons. Nat Rev Neurosci 15, 209–216. 10.1038/nrn3710 [DOI] [PubMed] [Google Scholar]
- Budde MD, Frank JA, 2010. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc. Natl. Acad. Sci. USA 107, 14472–7. doi: 10.1073/pnas.1004841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DA & Lisney SJW The numbers of unmyelinated and myelinated axons in normal and regenerated rat saphenous nerves. J. Neurol. Sci 80, 163–171 (1987). [DOI] [PubMed] [Google Scholar]
- Carty M, Kearney J, Shanahan KA, Hams E, Sugisawa R, Connolly D, et al. , 2019. Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity. 50, 1412–1424.e6. doi: 10.1016/j.immuni.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Catenaccio A, Llavero Hurtado M, Diaz P, Lamont DJ, Wishart TM, Court FA. Molecular analysis of axonal-intrinsic and glial-associated co-regulation of axon degeneration. Cell Death Dis. 2017. November 9;8(11):e3166. doi: 10.1038/cddis.2017.489. PMID: 29120410; PMCID: PMC5775402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M, 2005. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci 6, 889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coles CH, Bradke F, 2015. August 3. Coordinating neuronal actin-microtubule dynamics. Curr. Biol 25(15), R677–91. doi: 10.1016/j.cub.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Costa AR, Pinto-Costa R, Sousa SC, Sousa MM, 2018. The Regulation of Axon Diameter: From Axonal Circumferential Contractility to Activity-Dependent Axon Swelling. Front Mol. Neurosci 11, 319. doi: 10.3389/fnmol.2018.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datar A, Ameeramja J, Bhat A, Srivastava R, Mishra A, Bernal R, et al. , 2019. The roles of microtubules and membrane tension in axonal beading, retraction, and atrophy. Biophys. J 117, 880–91. doi: 10.1016/j.bpj.2019.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, et al. , 2010. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer’s disease. Neuroscience. 169, 516–31. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot NS, Burgas MT, 2015. Is membrane homeostasis the missing link between inflammation and neurodegenerative diseases? Cell Mol. Life Sci 72, 4795–805. doi: 10.1007/s00018-015-2038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle MB, Carpenter S, 1984. Neurofibrillary axonal swellings and amyotrophic lateral sclerosis. J. Neurol. Sci 63, 241–50. doi: 10.1016/0022-510x(84)90199-0. [DOI] [PubMed] [Google Scholar]
- Dubey S, Bhembre N, Bodas S, Veer S, Ghose A, Callan-Jones A, Pullarkat P, 2020. The axonal actin-spectrin lattice acts as a tension buffering shock absorber. Elife 8;9:e51772. doi: 10.7554/eLife.51772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleman CS, Ballinger ML, Smyers ME, Fishman HM, Bittner GD, 1998. Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axolemma injury. J. Neurosci 18(11), 4029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Haruwaka K, Mo M, Campos-Salazar AB, Wang L, Speros XS, Sabu S, Xu P, Wu LJ, 2021. Microglia provide structural resolution to injured dendrites after severe seizures. Cell Rep. 35(5). doi: 10.1016/j.celrep.2021.109080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández P, Pullarkat PA, 2010. The role of the cytoskeleton in volume regulation and beading transitions in PC12 neurites. Biophys. J 99, 3571–9. doi: 10.1016/j.bpj.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreirinha F, Quattrini A, Pirozzi M, Valsecchi V, Dina G, Broccoli V, et al. , 2004. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J. Clin. Invest 113, 231–42. doi: 10.1172/JCI20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgione N, Fehlings MG, 2014. Sep-Oct. Epub 2013 Jan 5. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 82(3–4), e535–9. doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC, 2018. Neuronal Cell Death. Physiol. Rev 98, 813–80. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, 2006. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J. Cell. Sci 119, Pt 16:3413–23. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW, 1995. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci 15, 6445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Ittner LM, Kins S, 2006. Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer’s disease? J. Neurochem 98, 993–1006. doi: 10.1111/j.1471-4159.2006.03955.x. [DOI] [PubMed] [Google Scholar]
- Govek E-E, Newey SE, Van Aelst L, 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 19, 1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Watson DF, 1988. Axonal transport in neurological disease. Ann. Neurol 23, 3–13. doi: 10.1002/ana.410230103. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. , 1998. Axonal Swellings and Degeneration in Mice Lacking the Major Proteolipid of Myelin. Science. 280(5369), 1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Gu Y, Jukkola P, Wang Q, Esparza T, Zhao Y, Brody D, et al. , 2017. Polarity of varicosity initiation in central neuron mechanosensation. J. Cell Biol 216, 2179–99. doi: 10.1083/jcb.201606065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann BL, Tummers B, Green DR, 2019. Crashing the computer: apoptosis vs. necroptosis in neuroinflammation. Cell Death Differ. 26, 41–52. doi: 10.1038/s41418-018-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A, Hirano H, Sasaki S, and Nakano I, 1984. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol 43(5), 461–470. 10.1097/00005072-198409000-00001 [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM, 2005. The axonal transport of mitochondria. J. Cell Sci 118, Pt. 23, 5411–9. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, and Mirsky R, 2016. The repair Schwann cell and its function in regenerating nerves. The Journal of Physiology, 594, 3521–3531. 10.1113/JP270874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2013. Axonal pathology in traumatic brain injury. Exp. Neurol 246, 35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone AD, de Léon A, Unsain N, Gibon J, Barker PA, 2019. Developmental Axon Degeneration Requires TRPV1-Dependent Ca2+ Influx. Eneuro. 6, doi: 10.1523/ENEURO.0019-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Hoogenraad CC, 2015. August 5. Building the Neuronal Microtubule Cytoskeleton. Neuron. 87(3), 492–506. 10.1016/j.neuron.2015.05.046 [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlagerm MA, Kuijpers M, Wulf PS, van Sporonsen M, Mackintosh FC, et al. , 2010. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol 20, 290–299. doi. 10.1016/j.cub.2009.12.052 [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Mukoyama M, Yamazaki K, Moriya H, 1990. Axonal degeneration of ascending sensory neurons in gracile axonal dystrophy mutant mouse. Acta Neuropathol. 80, 145–151. doi: 10.1007/BF00308917. [DOI] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA, 2008. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol. 212, 422–430. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Ko KW, Milbrandt J, DiAntonio A, 2020. SARM1 acts downstream of neuroinflammatory and necroptotic signaling to induce axon degeneration. J. Cell Biol 219. doi: 10.1083/jcb.201912047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, et al. , 2003. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 61, 631–6. doi: 10.1212/01.wnl.0000070781.92512.a4. [DOI] [PubMed] [Google Scholar]
- Leite SC, Sampaio P, Sousa VF, Nogueira-Rodrigues J, Pinto-Costa R, Peters LL, Brites P, Sousa MM, 2016. April 19. Epub 2016 Apr 7. The Actin-Binding Protein α-Adducin Is Required for Maintaining Axon Diameter. Cell Rep. 15(3), 490–498. doi: 10.1016/j.celrep.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell KL, and Jones MZ, 1985. Axonal and Myelin Lesions in beta-Mannosidosis: Ultrastructural Characteristics*. Acta Neuropathologica. 65, 293–299. [DOI] [PubMed] [Google Scholar]
- Luo L, O’Leary DDM., 2005. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 28, 127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S, 1999. August 6. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 285(5429), 895–8. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Maravall M, Mainen ZF, Sabatini BL, Svoboda K, 2000. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J 78, 2655–67. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, et al. , 2006. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci 26, 41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 2007. Calcium and neurodegeneration. Aging Cell. 6, 337–50. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- McKenzie BA, Dixit VM, 2020. Power C. Fiery cell death: pyroptosis in the central nervous system. Trends Neurosci. 43, 55–73. doi: 10.1016/j.tins.2019.11.005. [DOI] [PubMed] [Google Scholar]
- McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO, et al. , 2018. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc. Natl. Acad. Sci. USA 115, E6065–74. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, Adalbert R, Wagner D, Grumme D, et al. , 2005. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 128, pt. 2:405–16. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Julien JP, 2013. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci 14, 161–176. 10.1038/nrn3380 [DOI] [PubMed] [Google Scholar]
- Möbius W, Nave KA & Werner HB Electron microscopy of myelin: Structure preservation by high-pressure freezing. Brain Res. 1641, 92–100 (2016). [DOI] [PubMed] [Google Scholar]
- Morales I, Sanchez A, Puertas-Avenadno R, Rodriguez-Sabate C, Perez-Barreto A, Rodriguez M, 2020. Neuroglial transmitophagy and Parkinson’s disease. Glia. 68(11), 2277–2299. doi: 10.1002/glia.23839. [DOI] [PubMed] [Google Scholar]
- Nagata S, Suzuki J, Segawa K, Fujii T, 2016. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 23, 952–61. doi: 10.1038/cdd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Hurtado A, Overhoff M, Bera S. et al. , 2020. Autophagy lipidation machinery regulates axonal microtubule dynamics but is dispensable for survival of mammalian neurons. Nat Commun. 11, 1535. 10.1038/s41467-020-15287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukomm LJ, Freeman MR, 2014. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 24, 515–523. doi: 10.1016/j.tcb.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KL., Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, et al. , 1999. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J. Neuropathol Exp. Neurol 58, 1263–8. doi: 10.1097/00005072-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Ochs S, Pourmand R, Jersild RA, Friedman RN, 1997. The origin and nature of beading: A reversible transformation of the shape of nerve fibers. Prog. Neurobiol 52, 391–426. doi: 10.1016/S0301-0082(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Ohgami T, Kitamoto T, Tateishi J, 1992. Alzheimer’s amyloid precursor protein accumulates within axonal swellings in human brain lesions. Neurosci. Lett 136, 75–8. doi: 10.1016/03043940(92)90651-m. [DOI] [PubMed] [Google Scholar]
- Pantaloni D, Carlier MF, 1993. December 3. How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell. 75(5), 1007–14. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Ouvrier RA, Wright RG, Walsh JC, McLeod JG, 1976. Giant axonal neuropathy—a generalized disorder of cytoplasmic microfilament formation. J. Neuropathol. Exp. Neurol 35, 458–70. doi: 10.1097/00005072-197607000-00006. [DOI] [PubMed] [Google Scholar]
- Pullarkat PA, Dommersnes P, Fernández P, Joanny J-F.Ott A, 2006. Osmotically driven shape transformations in axons. Phys. Rev. Lett 96, 048104. doi: 10.1103/PhysRevLett.96.048104. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S., 1928. Degeneration and regeneration of the nervous system.
- Rasola A, Bernardi P, 2007. May. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 12(5), 815–33. doi: 10.1007/s10495-007-0723-y [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, 2011. October 28. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 35(4), 445–55. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros U, Peña-Blanco A, Hänggi K, Kunzendorf U, Krautwald S, Wong WW-L, et al. , 2017. Necroptosis execution is mediated by plasma membrane nanopores independent of calcium. Cell Rep. 19, 175–87. doi: 10.1016/j.celrep.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M, 2012. In vivo nerve-macrophage interactions following peripheral nerve injury. J. Neurosci 32, 3898–909. doi: 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J, and Freidrich VL, 1983. Axonal swellings in jimpy mice: Does lack of myelin cause neuronal abnormalities? Neuroscience. 10(3), 959–966. 10.1016/0306-4522(83)90233-6 [DOI] [PubMed] [Google Scholar]
- Sabharwal V, Koushika SP, 2019. Crowd control: effects of physical crowding on cargo movement in healthy and diseased neurons. Front Cell Neurosci. 13, 470. doi: 10.3389/fncel.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapar ML, Ji H, Wang B, Poe AR, Dubey K, Ren X, et al. , 2018. Phosphatidylserine Externalization Results from and Causes Neurite Degeneration in Drosophila. Cell Rep. 24, 2273–86. doi: 10.1016/j.celrep.2018.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Maruyama S, Yamane K, Sakuma H, Takeishi M, 1989. Swellings of proximal axons in a case of motor neuron disease. Ann Neurol. 25, 520–2. doi: 10.1002/ana.410250520. [DOI] [PubMed] [Google Scholar]
- Scott-Hewitt N, Perrucci F, Morini R, Erreni M, Mahoney M, Witkowska A, et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020;39:e105380. doi: 10.15252/embj.2020105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S, 2014. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 344, 1164–8. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- Shacham-Silverberg V, Sar Shalom H, Goldner R, Golan-Vaishenker Y, Gurwicz N, Gokhman I, et al. , 2018. Phosphatidylserine is a marker for axonal debris engulfment but its exposure can be decoupled from degeneration. Cell Death Dis. 9, 1116. doi: 10.1038/s41419018-1155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Sørensen MH, Xia X, Fang C, Hui TH., Chang RCC, et al. , 2020. Beading of injured axons driven by tension- and adhesion-regulated membrane shape instability. Journal of The Royal Society Interface. 17, 20200331. doi: 10.1098/rsif.2020.0331. [DOI] [Google Scholar]
- Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, et al. , 2008. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J. Neurosci 28, 2212–20. doi: 10.1523/JNEUROSCI.435407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomovitz I, Speir M, Gerlic M, 2019. Flipping the dogma - phosphatidylserine in non-apoptotic cell death. Cell Commun Signal. 17, 139. doi: 10.1186/s12964-019-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh JN, Rossor AM, Fellow AD, Tosolini AP, Schiavo G, 2019. Axonal transport and neurological disease. Nat. Rev. Neurol 15, 691–703. doi: 10.1038/s41582-019-0257-2. [DOI] [PubMed] [Google Scholar]
- Stankiewicz TR, Linseman DA, 2014. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 8, 314. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassart RM, Möbius W, Nave K-A, Edgar JM, 2018. The Axon-Myelin Unit in Development and Degenerative Disease. Front Neurosci. 12, 467. doi: 10.3389/fnins.2018.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD, 2006. March 16. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 49(6), 877–87. 10.1016/j.neuron.2006.02.018 [DOI] [PubMed] [Google Scholar]
- Stirling DP, Stys PK, 2010. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol. Med 16, 160–70. doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. , 2005. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 307, 1282–8. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yagishita S, Amano N, Yamaoka K, Kamei T, 1997. Amyotrophic lateral sclerosis with numerous axonal spheroids in the corticospinal tract and massive degeneration of the cortex. Acta Neuropathol. 94, 294–9. doi: 10.1007/s004010050707. [DOI] [PubMed] [Google Scholar]
- Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH, 2012. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp. Neurol 233, 364–72. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuling E, van Dis V, Wulf PS, Haasdijk ED, Akhmanova A, Hoogenraad CC, Jaarsma D, 2008. A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Hum. Mol. Genet 17(18), 2849–62. doi: 10.1093/hmg/ddn182 [DOI] [PubMed] [Google Scholar]
- Toyoshima I, Sugawara M, Kato K, Wada C, Hirota K, Hasegawa K, et al. , 1998. Kinesin and cytoplasmic dynein in spinal spheroids with motor neuron disease. J Neurol Sci. 159, 38–44. doi: 10.1016/s0022-510x(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Toyoshima I, Yamamoto A, Masamune O, Satake M, 1989. Phosphorylation of neurofilament proteins and localization of axonal swellings in motor neuron disease. J Neurol Sci. 89, 269–77. doi: 10.1016/0022-510x(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, et al. , 2019. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun 10, 2091. doi: 10.1038/s41467-019-09753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsain N, Bordenave MD, Martinez GF, Jalil S, von Bilderling C, Barabas FM, et al. , 2018. Remodeling of the Actin/Spectrin Membrane-associated Periodic Skeleton, Growth Cone Collapse and F-Actin Decrease during Axonal Degeneration. Sci. Rep 8:3007. doi: 10.1038/s41598-018-21232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquié A, Sauvain A, Duman M, Nocera G, Egger B, Meyenhofer F, Falquet L, Bartesaghi L, Chrast R, Maurice CL, Band S, Seung-Ryeol L, Jeon NL, Ruff S, Jacob C, 2019. Injured Axons Instruct Schwann Cells to Build Constricting Actin Spheres to Accelerate Axonal Disintegration. Cell Reports, 27(11), 3152–3166.e7. 10.1016/j.celrep.2019.05.060 [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA, 2007. Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci 30, 153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Waller A, 1851. Experiments on the Section of the Glosso-Pharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Edinb. Med. Surg. J 76, 369–376. [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yu M, Zhang W, Wang Z, and Yuan Y, 2018. AARS2 Compound Heterozygous Variants in a Case of Adult-Onset Leukoencephalopathy With Axonal Spheroids and Pigmented Glia. J. Neuropathol. Exp. Neurol 77(11), 997–1000. 10.1093/jnen/nly087 [DOI] [PubMed] [Google Scholar]
- Wang G, Simon DJ, Wu Z, Belsky DM, Heller E, O’Rourke MK, et al. , 2019. Structural plasticity of actin-spectrin membrane skeleton and functional role of actin and spectrin in axon degeneration. Elife. 8, doi: 10.7554/eLife.38730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA, 2012. Axon degeneration: molecular mechanisms of a self-destruction pathway. J. Cell. Biol 196(1), 7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, and Barres BA, 2012. Axon degeneration: where the Wlds things are. Curr Biol. 22(7), 221–223. doi: 10.1016/j.cub.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Perera ND, Chiam MDF, Cuic B, Wanniarachchillage N, Tomas D, et al. , 2020. Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ. 27, 1728–39. doi: 10.1038/s41418-019-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S, 1999. July. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1(3), 136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Wirths O, Weis J, Szczygielski J, Multhaup G, Bayer TA, 2006. Axonopathy in an APP/PS1 transgenic mouse model of Alzheimer’s disease. Acta. Neuropathol 111, 312–9. doi: 10.1007/s00401-006-0041-4. [DOI] [PubMed] [Google Scholar]
- Witte ME, Schumacher A-M, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, et al. , 2019. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron. 101, 615–624.e5. doi: 10.1016/j.neuron.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolums BM, McCray BA, Sung H. et al. , 2020. TRPV4 disrupts mitochondrial transport and causes axonal degeneration via a CaMKII-dependent elevation of intracellular Ca2+. Nat Commun 11, 2679. 10.1038/s41467-020-16411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, et al. , 2017. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. USA 114, E4859–67. doi: 10.1073/pnas.1701078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X, 2013. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 339(6118), 452–6. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T, 2009. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 29(19), 6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, Greer PA, Tournier C, Davis RJ, Tessier-Lavigne M, 2015. Pathological Axonal Death through a MAPK Cascade that Triggers a Local Energy Deficit. Cell. 160(1–2), 161–176. doi: 10.1016/j.cell.2014.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Y, Gamage K, Cheng I, Barford K, Spano A, Winckler B, et al. , 2019. p75NTR and DR6 Regulate Distinct Phases of Axon Degeneration Demarcated by Spheroid Rupture. J Neurosci. 39, 9503–20. doi: 10.1523/JNEUROSCI.1867-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Y, Gamage K, Cushman C, Spano A, Deppmann CD, 2020. Regulation of degenerative spheroids after injury. Sci. Rep 10,15472. doi: 10.1038/s41598-020-71906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Veeranna, and Nixon RA, 2012. Neurofilaments at a glance. J. Cell Sci 125, 3257–3263. doi: 10.1242/jcs.104729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen X, Gueydan C, Han J, 2018. Plasma membrane changes during programmed cell deaths. Cell Res. 28, 9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Spira ME, 1993. June 1. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. Eur. J. Neurosci 5(6), 657–68. doi: 10.1111/j.1460-9568.1993.tb00531.x [DOI] [PubMed] [Google Scholar]