Abstract
Background
This is the first update of a review published in 2010. While calcium channel blockers (CCBs) are often recommended as a first‐line drug to treat hypertension, the effect of CCBs on the prevention of cardiovascular events, as compared with other antihypertensive drug classes, is still debated.
Objectives
To determine whether CCBs used as first‐line therapy for hypertension are different from other classes of antihypertensive drugs in reducing the incidence of major adverse cardiovascular events.
Search methods
For this updated review, the Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials (RCTs) up to 1 September 2020: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 1), Ovid MEDLINE, Ovid Embase, the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also contacted the authors of relevant papers regarding further published and unpublished work and checked the references of published studies to identify additional trials. The searches had no language restrictions.
Selection criteria
Randomised controlled trials comparing first‐line CCBs with other antihypertensive classes, with at least 100 randomised hypertensive participants and a follow‐up of at least two years.
Data collection and analysis
Three review authors independently selected the included trials, evaluated the risk of bias, and entered the data for analysis. Any disagreements were resolved through discussion. We contacted study authors for additional information.
Main results
This update contains five new trials. We included a total of 23 RCTs (18 dihydropyridines, 4 non‐dihydropyridines, 1 not specified) with 153,849 participants with hypertension. All‐cause mortality was not different between first‐line CCBs and any other antihypertensive classes. As compared to diuretics, CCBs probably increased major cardiovascular events (risk ratio (RR) 1.05, 95% confidence interval (CI) 1.00 to 1.09, P = 0.03) and increased congestive heart failure events (RR 1.37, 95% CI 1.25 to 1.51, moderate‐certainty evidence). As compared to beta‐blockers, CCBs reduced the following outcomes: major cardiovascular events (RR 0.84, 95% CI 0.77 to 0.92), stroke (RR 0.77, 95% CI 0.67 to 0.88, moderate‐certainty evidence), and cardiovascular mortality (RR 0.90, 95% CI 0.81 to 0.99, low‐certainty evidence). As compared to angiotensin‐converting enzyme (ACE) inhibitors, CCBs reduced stroke (RR 0.90, 95% CI 0.81 to 0.99, low‐certainty evidence) and increased congestive heart failure (RR 1.16, 95% CI 1.06 to 1.28, low‐certainty evidence). As compared to angiotensin receptor blockers (ARBs), CCBs reduced myocardial infarction (RR 0.82, 95% CI 0.72 to 0.94, moderate‐certainty evidence) and increased congestive heart failure (RR 1.20, 95% CI 1.06 to 1.36, low‐certainty evidence).
Authors' conclusions
For the treatment of hypertension, there is moderate certainty evidence that diuretics reduce major cardiovascular events and congestive heart failure more than CCBs. There is low to moderate certainty evidence that CCBs probably reduce major cardiovascular events more than beta‐blockers. There is low to moderate certainty evidence that CCBs reduced stroke when compared to angiotensin‐converting enzyme (ACE) inhibitors and reduced myocardial infarction when compared to angiotensin receptor blockers (ARBs), but increased congestive heart failure when compared to ACE inhibitors and ARBs. Many of the differences found in the current review are not robust, and further trials might change the conclusions. More well‐designed RCTs studying the mortality and morbidity of individuals taking CCBs as compared with other antihypertensive drug classes are needed for patients with different stages of hypertension, different ages, and with different comorbidities such as diabetes.
Plain language summary
Calcium channel blockers versus other classes of drugs for hypertension
What is the aim of this review?
In this first update of a review published in 2010, we wanted to find out if calcium channel blockers (CCBs) can prevent harmful cardiovascular events such as stroke, heart attack, and heart failure when compared to other antihypertensive (blood pressure‐lowering) medications used for individuals with raised blood pressure (hypertension).
Background
Appropriate lowering of elevated blood pressure in individuals with hypertension can reduce the amount of major complications of hypertension, such as stroke, heart attack, congestive heart failure, and even death. CCBs are used as a first‐line blood pressure‐lowering medication, but whether this is the best way to reduce harmful cardiovascular events has been a matter of debate.
Search date
We collected and analysed all relevant studies up to 01 September 2020.
Study characteristics
We found 23 relevant studies conducted in Europe, North America, Oceania, Israel, and Japan. The studies compared treatment with CCBs versus treatment with other classes of blood pressure‐lowering medications in people with hypertension and included 153,849 participants. Follow‐up of trial participants ranged from 2 to 5.3 years.
Key results
There was no difference in deaths from all causes between CCBs and other blood pressure‐lowering medications. Diuretics probably reduce total cardiovascular events and congestive heart failure more than CCBs. CCBs probably reduce total cardiovascular events more than beta‐blockers. CCBs reduced stroke when compared to angiotensin‐converting enzyme (ACE) inhibitors and reduced heart attack when compared to angiotensin receptor blockers (ARBs), but increased congestive heart failure when compared to ACE inhibitors and ARBs.
Quality of the evidence
We assessed the quality of the evidence as mostly moderate, although more trials are desirable.
Summary of findings
Background
Description of the condition
Hypertension is a leading cause of death worldwide, and its prevalence has increased dramatically over the past two decades (GBD 2015). In the population‐based ARIC (Atherosclerosis Risk in Communities) study, hypertension was associated with an increased risk of coronary heart disease, stroke, heart failure, and end‐stage renal disease; 25% of all cardiovascular events were attributable to hypertension (Cheng 2014).
Description of the intervention
Antihypertensive therapies have established benefits in reducing the risk for major cardiovascular events. Pharmacotherapy for high blood pressure includes first‐line agents, such as diuretics, angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs), and non‐first‐line agents, such as beta‐blockers and alpha‐blockers (Whelton 2018).
How the intervention might work
Different classes of antihypertensive drugs have different mechanisms of action. Previous meta‐analysis demonstrated that all major antihypertensive drug classes (diuretics, ACE inhibitors, ARBs, beta‐blockers, and CCBs) caused a similar reduction in coronary heart disease events and stroke for a given reduction in blood pressure (Law 2009). The systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults indicated that thiazides were associated with a lower risk of many cardiovascular outcomes compared with other antihypertensive drug classes (Reboussin 2017). CCBs significantly increased the risk of congestive heart failure as compared to diuretics, ACE inhibitors, and ARBs in a review by Thomopoulos(Thomopoulos 2015). One previous review concluded that beta‐blockers reduced total cardiovascular events significantly less than CCBs (Wiysonge 2007).
Why it is important to do this review
The issue of first‐line drug selection is highly relevant for millions of subjects receiving drug therapy for hypertension. The benefits in reducing the risk for major cardiovascular events of any one class of antihypertensive therapies relative to other classes has been a matter of debate. Our first systematic review compared CCBs with other classes of antihypertensive drugs in 2010 (Chen 2010), but since then some head‐to‐head trials of CCBs versus other classes of antihypertensive drugs have been performed. These additional newer trials not included in previous systematic reviews may provide an improved understanding of the relative benefits of each class of antihypertensive therapies. This review update aims to sent the outcome data in a way that best assists clinicians in the choice of a antihypertensive drug.
Objectives
To determine whether CCBs used as first‐line therapy for hypertension are different from other classes of antihypertensive drugs in reducing the incidence of major adverse cardiovascular events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that randomised 100 or more participants and followed participants for at least two years.
Types of participants
We included participants with a baseline blood pressure (BP) of at least 140 mmHg systolic or 90 mmHg diastolic, measured in a standard way on at least two occasions, or participants with diabetes mellitus with a BP of more than 135/85 mmHg. If a trial was not limited to participants with elevated BP, it must have reported outcome data separately for participants with elevated BP as defined above.
Types of interventions
We included trials comparing first‐line CCBs with other first‐line antihypertensive classes. The majority (> 70%) of participants in all study groups must be taking the assigned drug class after one year. Supplemental drugs from drug classes other than CCBs were allowed as stepped therapy.
Types of outcome measures
The main outcomes of the review were as follows.
Primary outcomes
All‐cause mortality
Myocardial infarction (non‐fatal and fatal MI plus sudden or rapid death)
Stroke (non‐fatal and fatal stroke)
Congestive heart failure
Cardiovascular mortality
Major cardiovascular events (MI, congestive heart failure, stroke, and cardiovascular mortality)
Secondary outcomes
Reduction in systolic and diastolic blood pressure
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Hypertension Information Specialist searched the following databases without language or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 01 September 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL, 2020, Issue 1) via CRS‐Web (searched 01 September 2020);
MEDLINE Ovid, MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 01 September 2020);
Embase Ovid (searched 01 September 2020);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 01 September 2020);
World Health Organization International Clinical Trials Registry Platform (www.who.it.trialsearch) (searched 01 September 2020).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, these were combined with subject strategy adaptations of the Highly Sensitive Search Strategy designed by Cochrane for identifying randomised controlled trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.c.)(Higgins 2011). The database search strategies are shown for this update in Appendix 1 and from the previous (2010) review in Appendix 2.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches of CAB Abstracts & Global Health, CINAHL (Cumulative Index to Nursing and Allied Health Literature), ProQuest Dissertations & Theses, and Web of Science for controlled trials.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
Data collection and analysis
Selection of studies
Two review authors (Jiaying Zhu, Ning Chen) independently examined the titles and abstracts of citations identified by the electronic searches for possible inclusion. We retrieved full‐text publications of potentially relevant studies and three review authors (Jiaying Zhu, Jie Zhou and Mengmeng Ma) then independently determined study eligibility. We resolved disagreements about study eligibility by discussion and, if necessary, a fourth review author would arbitrate.
Data extraction and management
Three review authors (Jiaying Zhu, Jie Zhou and Mengmeng Ma) independently extracted data using a standard form, and then cross‐checked them. Muke Zhou and Jian Guo confirmed all numeric calculations and graphic interpolations. We resolved any discrepancies by consensus.
Assessment of risk of bias in included studies
The review authors (Jiaying Zhu and Mengmeng Ma) independently used the Cochrane 'Risk of bias' tool to categorize studies as having low,unclear, or high risk of bias for sequence generation, allocation sequence concealment, loss of blinding, selective reporting,incomplete reporting of outcomes, and other potential sources of bias (Higgins 2011a).
Measures of treatment effect
We based quantitative analysis of outcomes on intention‐to‐treat principles as much as possible. For dichotomous outcomes, we expressed results as the risk ratio (RR) with a 95% confidence interval (CI). For combining continuous variables (systolic blood pressure reduction, diastolic blood pressure reduction), we used the mean difference (with 95% CI).
Unit of analysis issues
The unit of analysis was the individual trial. For trials having more than two arms, we only included arms relevant to this review. For trials included more than one intervention group with a single comparator arm, we included both intervention groups.
Dealing with missing data
We contacted study investigators in the case of missing data. We based the quantitative analyses of outcomes on intention‐to‐treat results.
Assessment of heterogeneity
We used Chi2 and I2statistics to test for heterogeneity of treatment effect among trials.
We assessed values of the I2statistic as follows (Higgins 2011a):
0% to 40%: heterogeneity might not be important;
30% to 60%: moderate heterogeneity;
50% to 90%: substantial heterogeneity;
75% to 100% considerable heterogeneity.
We used the fixed‐effect model when there was homogeneity and used the random‐effects model to test for statistical significance where there was heterogeneity.
Assessment of reporting biases
We planned to assess reporting bias following the recommendations on testing for funnel plot asymmetry as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Data synthesis
We performed data synthesis and analyses using the Cochrane Review Manager software, RevMan 5.4, We describe data results in tables and forest plots. We also give full details of all studies we include and exclude. We have included a standard PRISMA flow diagram.
Subgroup analysis and investigation of heterogeneity
If appropriate, we would perform subgroup analyses.
Heterogeneity among participants could be related to: age,gender, baseline blood pressure, target blood pressure, high‐risk participants, participants with comorbid conditions. Heterogeneity in treatments could be related to: form of drugs,dosage of drugs, or duration of therapy.
Sensitivity analysis
We planned to conduct sensitivity analyses to examine the effects of excluding studies with a moderate or high risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Summary of findings and assessment of the certainty of the evidence
In this updated review, we included 'Summary of findings' tables for comparisons that included more than one trial to present the main findings of the review, which included information about the quality of the evidence, the magnitude of effects, and the sum of the available data on the main outcomes (Schünemann 2011a).
We assessed the quality of a body of evidence according to five GRADE considerations (study limitations, inconsistency of effect, imprecision, indirectness, and publication bias) (Ryan 2016). We downgraded the evidence from 'high certainty by one level where one of these factors was present to a serious degree and two levels if very serious. We used the methods and recommendations described in Chapter 8 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). We justified all decisions to downgrade the quality of the evidence using footnotes and made comments to aid reader's understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The results of the search are shown in the PRISMA diagram (Figure 1), We identified 4,700 records from database searches. 4,649 records remained after removal of duplicates. After screening titles and abstracts, we obtained 65 full‐text articles. Of these articles, we excluded 42 studies based on them not meeting our inclusion criteria.
1.
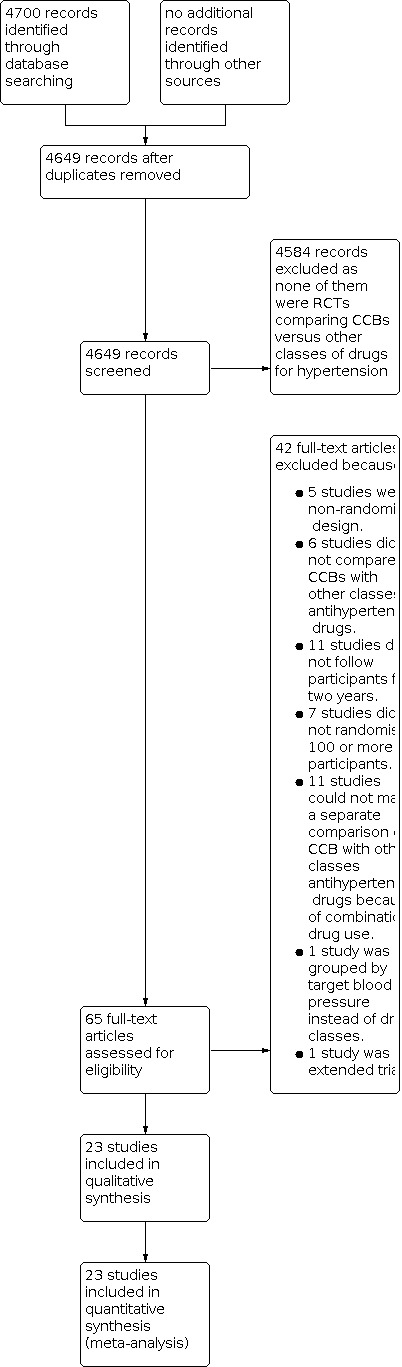
Study flow diagram.
Included studies
See Characteristics of included studies for details.
We included 23 RCTs (AASK; ABCD; ALLHAT; ASCOT‐BPLA; CASE‐J; CONVINCE; ELSA; FACET; HOMED‐BP; IDNT; INSIGHT; INVEST; J‐MIC(B); MIDAS; NAGOYA; NICS‐EH; NORDIL; SHELL; STOP‐Hypertension‐2; TOMHS; VALUE; VART; VHAS) with a total of 153,849 participants. Five of the 23 trials were new in this update (CASE‐J; HOMED‐BP; J‐MIC(B); NAGOYA; VART).
All the included RCTs supplied explicit inclusion and exclusion criteria. Twenty trials included only hypertensive participants, but these were defined differently, as follows: 140/90 mmHg or more (FACET; INVEST; NAGOYA; VART); 150/90 mmHg or more (J‐MIC(B)); more than 160/95 mmHg (VHAS); more than 135/85 mmHg for participants with diabetes mellitus (IDNT); 140 to 179 mmHg systolic and/or 90 to 109 mmHg diastolic (ALLHAT); 150 to 210 mmHg systolic and 95 to 115 mmHg diastolic (ELSA); systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 105 mmHg (STOP‐Hypertension‐2); diastolic BP of 100 mmHg or more, NORDIL, or of 90 to 99 mmHg (TOMHS); treated hypertension with an upper limit of 175/100 mmHg or untreated hypertension of 140 to 190 mmHg systolic or 90 to 110 mmHg diastolic (CONVINCE); BP ≥ 160/100 mmHg for participants with untreated hypertension or BP ≥ 140/90 mmHg for participants on antihypertensive treatment (ASCOT‐BPLA); systolic BP ≥ 150 mmHg and diastolic BP ≥ 95 mmHg, or only systolic BP ≥ 160 mmHg (INSIGHT); only diastolic BP ≥ 95 mmHg, AASK, or 90 to 115 mmHg (MIDAS); 160 to 210/220 mmHg systolic and less than 115 mmHg diastolic (NICS‐EH; VALUE); ≥ 160 mmHg systolic and ≤ 95 mmHg diastolic (SHELL); systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg in participants < 70 years old, or systolic BP ≥ 160 mmHg or diastolic BP ≥ 90 mmHg in participants ≥ 70 years old (CASE‐J); mild‐to‐moderate hypertension (HOMED‐BP). Only one trial did not limit participants to elevated BP (diastolic BP ≥ 80 mmHg) (ABCD), but it reported outcomes on participants with elevated BP (diastolic BP ≥ 90 mmHg) separately, so data for hypertensive participants could be extracted.
Additional inclusion criteria varied for each study, as follows: with other risk factor(s) for coronary heart disease or cardiovascular disease (ALLHAT; ASCOT‐BPLA; CASE‐J; CONVINCE; INSIGHT); with coronary heart disease (INVEST; J‐MIC(B)); with cardiovascular risk factors or cardiovascular disease (VALUE); with type 2 diabetes mellitus (non‐insulin‐dependent diabetes mellitus), ABCD; FACET, or type 2 diabetes mellitus and nephropathy (IDNT); with glucose intolerance (type 2 diabetes or impaired glucose tolerance) (NAGOYA); African‐Americans with hypertensive kidney disease (AASK).
All 23 included RCTs recruited participants of both sexes, but age requirements differed amongst the trials, as follows: ≥ 30 years (VART); > 40 years (HOMED‐BP; MIDAS); > 50 years (INVEST; VALUE); > 55 years (ALLHAT; CONVINCE); > 60 years (NICS‐EH; SHELL); 18 to 70 years (AASK); 30 to 70 years (IDNT); 30 to 75 years (NAGOYA); 40 to 65 years (VHAS); 40 to 74 years (ABCD); 40 to 79 years (ASCOT‐BPLA); 45 to 69 years (TOMHS); 45 to 75 years (ELSA); 50 to 74 years (NORDIL); 55 to 80 years (INSIGHT); under 75 years (J‐MIC(B)); 70 to 84 years (STOP‐Hypertension‐2). In the CASE‐J trial, differing BP levels were required for participants aged < 70 years and ≥ 70 years.
Most trials followed a goal BP in their protocols, mostly less than 140/90 mmHg (ALLHAT; ASCOT‐BPLA; CONVINCE; FACET; INSIGHT; INVEST; VALUE; VART); or less than 150/90 mmHg (J‐MIC(B)); less than 130/80 mmHg for hypertensive participants with glucose intolerance (NAGOYA); less than 130/85 mmHg for participants with diabetes or renal impairment (ASCOT‐BPLA; INVEST); ≤ 135/85 mmHg or a decrease ≥ 10 mmHg systolic for diabetic participants (IDNT); ≤ 160/95 mmHg (STOP‐Hypertension‐2); less than 90 mmHg diastolic, NORDIL, or 95 mmHg (TOMHS); less than 95 mmHg with a fall of at least 5 mmHg (ELSA); less than 90 mmHg with a fall of at least 10 mmHg (MIDAS); reduction more than 20 mmHg or systolic BP ≤ 160 mmHg (SHELL); ≤ 90 mmHg or ≤ 95 mmHg with a reduction of at least 10% from baseline value (VHAS); 75 mmHg or less diastolic in the intensive‐treatment group and 80 to 89 mmHg diastolic in the moderate‐treatment group (ABCD); 102 to 107 mmHg of mean arterial pressure in the usual‐goal group and 92 mmHg or less in the lower‐goal group (AASK); a decrease ≥ 20 mmHg of BP if systolic BP was more than 160 mmHg or diastolic BP was more than 110 mmHg (FACET); 60 years old, systolic BP/diastolic BP 130/85 mmHg; 60 to 69 years old, systolic BP/diastolic BP 140/90 mmHg; 70 to 79 years old, systolic BP/diastolic BP 150/90 mmHg; 80 years old, systolic BP/diastolic BP 160/90 mmHg (CASE‐J); usual control 125 to 134/80 to 84 mmHg and tight control < 125/< 80 mmHg (HOMED‐BP).
Of CCBs for hypertension, dihydropyridines (DHPs) were the most commonly studied, especially amlodipine (AASK; ALLHAT; ASCOT‐BPLA; CASE‐J; FACET; IDNT; NAGOYA; TOMHS; VALUE; VART). Other DHPs studied included nifedipine (INSIGHT; J‐MIC(B)), felodipine (STOP‐Hypertension‐2), nisoldipine (ABCD), nicardipine (NICS‐EH), lacidipine (ELSA; SHELL), and isradipine (MIDAS). Other trials evaluated non‐DHPs such as an aralkylamine derivative verapamil, CONVINCE; INVEST; VHAS, and a benzothiazepine derivative diltiazem (NORDIL). One study did not describe the specific CCBs used (HOMED‐BP). The included RCTs compared one of the above CCBs to other classes of antihypertensive drugs, including: a diuretic (ALLHAT; INSIGHT; MIDAS; NICS‐EH; SHELL; TOMHS; VHAS); a beta‐blocker (AASK; ASCOT‐BPLA; ELSA; INVEST; TOMHS); a diuretic or beta‐blocker, or both, data of which could not be separated for each drug (CONVINCE; NORDIL; STOP‐Hypertension‐2); an alpha‐1‐antagonist (TOMHS); an ACE inhibitor (AASK; ABCD; ALLHAT; FACET; HOMED‐BP; J‐MIC(B); STOP‐Hypertension‐2; TOMHS); or an ARB (CASE‐J; HOMED‐BP; IDNT; NAGOYA; VALUE; VART).
Supplemental antihypertensive agents other than the study drugs were permitted in most of the included trials, often administered sequentially to achieve BP goals (AASK; ABCD; ALLHAT; ASCOT‐BPLA; CASE‐J; CONVINCE; ELSA; HOMED‐BP; IDNT; INSIGHT; INVEST; J‐MIC(B); MIDAS; NAGOYA; NORDIL; SHELL; STOP‐Hypertension‐2; VALUE; VART; VHAS). The FACET trial added the study drug of the other group to participants whose BP was not controlled well. The TOMHS trial studied five classes of first‐line antihypertensive drugs, and added chlortalidone or enalapril, both of which were study drugs, to participants to control BP. NICS‐EH prohibited the use of any other antihypertensive drugs.
Outcomes differed amongst studies, but results for our planned outcomes of cardiovascular events and BP changes were reported in most trials. However, fatal MI, stroke, and heart failure were sometimes contained in death events, and in some trials components of cardiovascular events were not reported separately. As a result, not every trial supplied data to each meta‐analysis for outcomes of this review. Only two trials explicitly presented the mean BP changes with standard deviations (SDs), INVEST, or standard errors, TOMHS, which could be directly inputted into Review Manager 5 for analysis. In some other trials, mean BP change could be calculated by subtracting the baseline value at randomisation from the value reported at the end of the trial, but SDs for changes were not reported. We calculated change‐from‐baseline SDs when baseline and final values were known (Higgins 2011b) (AASK; ALLHAT; FACET; NICS‐EH; NORDIL; VALUE). However, when trials did not supply SDs for the baseline and final BPs, the BP results were not entered into the meta‐analysis (ABCD; ASCOT‐BPLA; CASE‐J; CONVINCE; ELSA; HOMED‐BP; IDNT; INSIGHT; J‐MIC(B); MIDAS; NAGOYA; SHELL; STOP‐Hypertension‐2; VART; VHAS).
Mean duration of follow‐up ranged from 2 to 5.3 years. One trial stated that no participant was lost to follow‐up and no participant refused to continue in the study (STOP‐Hypertension‐2), whilst loss to follow‐up and withdrawal were reported in the other 22 trials. All trials with the exception of NICS‐EH stated that an intention‐to‐treat analysis was performed.
Excluded studies
See Characteristics of excluded studies for details.
The reasons for exclusion included: non‐randomised design (Abascal 1998; Bhad 2011; DHCCP; Pahor 1995; Psaty 1995); the control group used placebo instead of other classes of antihypertensive drugs (Chen 2013; STONE; Syst‐China; Syst‐Eur); the comparison was performed between different kinds of CCBs, without any other classes of antihypertensive drugs (Abe 2013; Kes 2003); the follow‐up was shorter than two years (Espinel 1992; GLANT; Gottdiener 1997; Kereiakes 2012; Leon 1993; Papademetriou 1997; PRESERVE; Schneider 1991; Van Leeuwen 1995; Weir 1990; Zhang 2012); small sample of participants (fewer than 100 were randomised) (Bakris 1996; Bakris 1997; FACTS; Kim 2011; Maharaj 1992; Mesci 2011; Radevski 1999); cannot make a separate comparison of CCB with other classes antihypertensive drugs because of combination drug use (ACCOMPLISH; BEAHIT; Calhoun 2013; Cicero 2012; COLM; DEMAND; FEVER; Kojima 2013; Lauria 2012; OSCAR; Wen 2011); study groups differed in target BPs instead of drug classes (HOT); to avoid repeated inclusion of the research population in extended trial (CASE‐J Ex).
Risk of bias in included studies
Since trials with a small sample were excluded from the current review, most of included trials were large and multicentre with standardised protocols. We evaluated the methodological quality of the included trials in several ways. According to the summary assessment of the risk of bias for each important outcome (Higgins 2011a), we assessed five trials as at low risk of bias (ALLHAT; ASCOT‐BPLA CASE‐J; IDNT; INVEST; J‐MIC(B)), two trials as at high risk of bias (FACET; NICS‐EH), and the remaining 16 trials as at unclear risk of bias. The risk of bias graph (Figure 2) shows judgements of the review authors about each domain presented as percentages across all included studies. The risk of bias summary (Figure 3) shows review authors' judgements about each risk of bias item for each included study.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the included studies were stated as randomised controlled trials. A computer‐generated code for randomisation was often used, but eight trials did not report the methods of allocation (ABCD; HOMED‐BP; INSIGHT; NICS‐EH; NORDIL; STOP‐Hypertension‐2; VART; VHAS). Allocation concealment was seldom described; only four trials stated that their randomisation codes were concealed at the clinical trials centre (ALLHAT; ASCOT‐BPLA; IDNT; INVEST), whilst in the CONVINCE trial, an interactive voice response system for randomising, assigning, and tracking blinded medication was used. Information was insufficient to assess this 'Risk of bias' domain for the remaining trials.
Blinding
All the included trials compared two first‐line antihypertensive drug classes to each other. With exception of the FACET trial, which was open‐label, the included studies were stated as blinded. In some trials active drugs were described as of indistinguishable appearance, but it was still impossible to know the extent of blinding (Higgins 2011a). Nine trials used a Prospective, Randomised, Open‐label, Blinded Endpoint (PROBE) design (ASCOT‐BPLA; CASE‐J; HOMED‐BP; INVEST; J‐MIC(B); NAGOYA; NORDIL; STOP‐Hypertension‐2; VART), which differs from the classical double‐blind method. In a PROBE study, outcomes are evaluated by a blinded endpoint committee to avoid detection bias; in this way treatment allocation might be open to risk of performance bias from participants and doctors (Hansson 1992).
Incomplete outcome data
Missing data caused by loss to follow‐up or withdrawals were on the whole equal amongst the treatment groups, and an intention‐to‐treat analysis, which meant data were analysed according to randomised treatment assignments regardless of the subsequent medications (Fergusson 2002), was performed in most of the included trials, with the exception of the STOP‐Hypertension‐2 trial (with negligible loss) and the NICS‐EH trial. Some sites and their participants were excluded after randomisation because of poor documentation of informed consent, data integrity concerns, or misconduct (ALLHAT; ASCOT‐BPLA; CONVINCE; INSIGHT), which could have led to attrition bias.
Selective reporting
In this review, we judged all included studies to have a low risk of reporting bias.
Other potential sources of bias
In FACET trial, when BP was not controlled well on monotherapy, the other study drug was added. In NORDIL trial, a diuretic or blocker was added in step 3, and any other antihypertensive compound could be added as step 4 in the diltiazem group. This could have affected the evaluation of effect of each study drug.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. CCBs versus diuretic for hypertension.
| CCBs versus diuretic for hypertension | ||||||
| Patient or population: patients with hypertension Settings: outpatients or inpatients Intervention: CCBs versus diuretic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CCBs versus diuretic | |||||
| All‐cause mortality Follow‐up: 2 to 5 years | Study population | RR 0.98 (0.92 to 1.04) | 35,057 (5 studies) | ⊕⊕⊕⊝ moderate1 | NNH 83 (95%CI 53 to 187) | |
| 121 per 1000 | 118 per 1000 (111 to 126) | |||||
| Myocardial infarction Follow‐up: 3 to 5 years | Study population | RR 1.00 (0.92 to 1.08) | 34,072 (5 studies) | ⊕⊕⊕⊝ moderate2 | NNT 146 (95%CI 81 to 729) | |
| 74 per 1000 | 74 per 1000 (68 to 79) | |||||
| Stroke Follow‐up: 3 to 5 years | Study population | RR 0.94 (0.84 to 1.05) | 34,072 (5 studies) | ⊕⊕⊕⊝ moderate2 | NNT 236 (95%CI 120 to 5816) | |
| 40 per 1000 | 37 per 1000 (33 to 42) | |||||
| Congestive heart failure Follow‐up: 3 to 5 years | Study population | RR 1.37 (1.25 to 1.51) | 34,072 (5 studies) | ⊕⊕⊕⊝ moderate2 | NNH 107 (95% CI 71 to 213) | |
| 45 per 1000 | 62 per 1000 (56 to 68) | |||||
| Cardiovascular mortality Follow‐up: 2 to 5 years | Study population | RR 1.02 (0.93 to 1.12) | 32,721 (4 studies) | ⊕⊕⊕⊝ moderate3 | NNT 242 (95% CI 111 to 1377) | |
| 54 per 1000 | 55 per 1000 (50 to 60) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CCB: calcium channel blocker; CI: confidence interval; RR: risk ratio; NNH: number needed to harm; NNT: number needed to treat | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1All studies were blinded, but two of them did not describe the method of blinding. All studies mentioned randomisation, but only three studies provided details; only one study described allocation concealment. 2All studies were blinded, but one of them did not describe the method of blinding. All studies mentioned randomisation, but two of them did not provide details; only one study described allocation concealment. 3All four studies were blinded, but one of them did not describe the method of blinding. All studies mentioned randomisation, but two of them did not provide details; only one study described allocation concealment.
Summary of findings 2. CCBs versus β‐blocker for hypertension.
| CCBs versus β‐blocker for hypertension | ||||||
| Patient or population: patients with hypertension Settings: outpatients or inpatients Intervention: CCBs versus β‐blocker | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CCBs versus β‐blocker | |||||
| All‐cause mortality Follow‐up: 2.7 to 5.5 years | Study population | RR 0.94 (0.88 to 1) | 44,825 (4 studies) | ⊕⊕⊕⊝ moderate1 | NNT 194 (95%CI 99 to 4004) | |
| 79 per 1000 | 74 per 1000 (70 to 79) | |||||
| Myocardial infarction Follow‐up: 3 to 5 years | Study population | RR 0.90 (0.79 to 1.02) | 22,249 (3 studies) | ⊕⊕⊕⊝ moderate2 | NNT 223 (95%CI 102 to 1190) | |
| 45 per 1000 | 41 per 1000 (36 to 46) | |||||
| Stroke Follow‐up: 3 to 5 years | Study population | RR 0.77 (0.67 to 0.88) | 22,249 (3 studies) | ⊕⊕⊕⊝ moderate2 | NNT 104 (95%CI 69 to 210) | |
| 41 per 1000 | 32 per 1000 (27 to 36) | |||||
| Congestive heart failure Follow‐up: 4 to 5 years | Study population | RR 0.83 (0.67 to 1.04) | 19,915 (2 studies) | ⊕⊕⊝⊝ low2,3 | NNT 279 (95%CI 141 to 12238) | |
| 18 per 1000 | 15 per 1000 (12 to 19) | |||||
| Cardiovascular mortality Follow‐up: 2.7 to 5.5 years | Study population | RR 0.90 (0.81 to 0.99) | 44,825 (4 studies) | ⊕⊕⊝⊝ low1,4 | NNT 279 (95%CI 145 to 3783) | |
| 35 per 1000 | 32 per 1000 (29 to 35) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCB: calcium channel blocker; CI: confidence interval; RR: risk ratio; NNT: number needed to treat | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Only two studies described allocation concealment. 2Two studies did not describe allocation concealment. 3Wide 95% CI crossing the line of no effect and low event rate. 4I2 > 60%. Effect size varied considerably.
Summary of findings 3. CCBs versus ACE inhibitor for hypertension.
| CCBs versus ACE inhibitor for hypertension | ||||||
| Patient or population: patients with hypertension Settings: outpatients or inpatients Intervention: CCBs versus ACE inhibitor | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CCBs versus ACE inhibitor | |||||
| All‐cause mortality Follow‐up: 3 to 5 years | Study population | RR 0.97 (0.91 to 1.03) | 27,999 (7 studies) | ⊕⊕⊝⊝ low1,2 | NNT 282 (95%CI 89 to 240) | |
| 126 per 1000 | 122 per 1000 (115 to 130) | |||||
| Myocardial infarction Follow‐up: 3 to 5.3 years | Study population | RR 1.05 (0.97 to 1.14) | 27,999 (7 studies) | ⊕⊕⊝⊝ low1,3 | NNT 235 (95%CI 96 to 541) | |
| 71 per 1000 | 75 per 1000 (69 to 81) | |||||
| Stroke Follow‐up: 3 to 5.3 years | Study population | RR 0.90 (0.81 to 0.99) | 27,999 (7 studies) | ⊕⊕⊝⊝ low1,2 | NNT 185 (95%CI 95 to 2863) | |
| 52 per 1000 | 47 per 1000 (42 to 51) | |||||
| Congestive heart failure Follow‐up: median 3 years | Study population | RR 1.16 (1.06 to 1.28) | 25,276 (5 studies) | ⊕⊕⊝⊝ low4,5 | NNT 94 (95%CI 59 to 222) | |
| 63 per 1000 | 73 per 1000 (66 to 80) | |||||
| Cardiovascular mortality Follow‐up: 3 to 5 years | Study population | RR 0.98 (0.89 to 1.07) | 27,619 (6 studies) | ⊕⊕⊕⊝ moderate6 | NNT 923 (95%CI 148 to 219) | |
| 62 per 1000 | 61 per 1000 (55 to 66) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACE: angiotensin‐converting enzyme; CCB: calcium channel blocker; CI: confidence interval; RR: risk ratio; NNT: number needed to treat | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1In one study, study drugs were administered open‐label. All studies mentioned randomisation, but two of them did not provide details; only three studies described allocation concealment. 2In one study, when BP was not well‐controlled on monotherapy, the other study drug was added. 3I2 > 60%; direction and size of effect inconsistent. 4All studies mentioned randomisation, but two of them did not provide details; only two studies described allocation concealment. 5Wide 95% CI crossing the line of no effect and low event rate. 6All studies mentioned randomisation, but two of them did not provide details; only three studies described allocation concealment.
Summary of findings 4. CCBs versus ARB for hypertension.
| CCBs versus ARB for hypertension | ||||||
| Patient or population: patients with hypertension Settings: outpatients or inpatients Intervention: CCBs versus ARB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CCBs versus ARB | |||||
| All‐cause mortality Follow‐up: 2 to 5.5 years | Study population | RR 1.00 (0.92 to 1.08) | 25,611 (6 studies) | ⊕⊕⊕⊝ moderate1 | NNT 3128 (95%CI 143 to 157) | |
| 81 per 1000 | 81 per 1000 (75 to 88) | |||||
| Myocardial infarction Follow‐up: 2 to 5.5 years | Study population | RR 0.82 (0.72 to 0.94) | 25,611 (6 studies) | ⊕⊕⊕⊝ moderate1 | NNT 157 (95%CI 93 to 492) | |
| 36 per 1000 | 29 per 1000 (26 to 34) | |||||
| Stroke Follow‐up: 2.6 to 5.5 | Study population | RR 0.89 (0.76 to 1.00) | 25,611 (6 studies) | ⊕⊕⊕⊝ moderate2 | NNT 226 (95%CI 115 to 8570) | |
| 34 per 1000 | 30 per 1000 (26 to 34) | |||||
| Congestive heart failure Follow‐up: mean 2.6 years | Study population | RR 1.20 (1.06 to 1.36) | 23,265 (5 studies) | ⊕⊕⊝⊝ low1,3 | NNT 94 (95%CI 59 to 222) | |
| 38 per 1000 | 45 per 1000 (40 to 51) | |||||
| Cardiovascular mortality Follow‐up: mean 2 years | Study population | RR 0.79 (0.54 to 1.15) | 4642 (3 studies) | ⊕⊕⊕⊝ moderate4 | NNT 184 (95% CI 72 to 331) | |
| 25 per 1000 | 20 per 1000 (13 to 29) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: angiotensin receptor blocker; CCB: calcium channel blocker; CI: confidence interval; RR: risk ratio; NNT: number needed to treat | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Only two studies described allocation concealment, and one study had withdrawals. 2Only three studies described allocation concealment, and one study had withdrawals. 3I2 > 60%; direction and size of effect inconsistent. 4One study of three did not describe allocation concealment, and one study had withdrawals.
The diuretic and beta‐blocker subgroup included data from three studies in which a diuretic, a beta‐blocker, or both were used but could not be separately analysed.
All‐cause mortality
The effect of CCBs on death from any cause was not significantly different from that of any other evaluated agents: diuretics (5 trials with 35,057 participants: risk ratio (RR) 0.98, 95% confidence interval (CI) 0.92 to 1.04, I2 = 0%; moderate‐certainty evidence); beta‐blockers (4 trials with 44,825 participants: RR 0.94, 95% CI 0.88 to 1.00, P = 0.54, I2 = 0%; moderate‐certainty evidence); diuretics and beta‐blockers (3 trials with 31,892 participants: RR 1.03, 95% CI 0.94 to 1.12, I2 = 0%; moderate‐certainty evidence); ACE inhibitors (7 trials with 27,999 participants: RR 0.97, 95% CI 0.91 to 1.03, I2 = 0%; low‐certainty evidence); and ARBs (6 trials with 25,611 participants: RR 1.00, 95% CI 0.92 to 1.08, I2 = 0%; moderate‐certainty evidence) (Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1: All‐cause mortality, Outcome 1: CCBs vs other classes of antihypertensive agents
4.

Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 CCBs versus other classes of antihypertensive agents.
MI (non‐fatal and fatal MI plus sudden or rapid death)
The effect of CCBs on MI was not significantly different from that of diuretics (5 trials with 34,072 participants: RR 1.00, 95% CI 0.92 to 1.08, I2 = 0%; moderate‐certainty evidence); beta‐blockers (3 trials with 22,249 participants: RR 0.90, 95% CI 0.79 to 1.02, I2 = 0%; moderate‐certainty evidence); diuretics and beta‐blockers (3 trials with 31,892 participants: RR 1.05, 95% CI 0.93 to 1.19, I2 = 72%; moderate‐certainty evidence); and ACE inhibitors (7 trials with 27,999 participants: RR 1.05, 95% CI 0.97 to 1.14], I2 = 66%; low‐certainty evidence). The incidence of MI was statistically significantly lower (P = 0.004) for CCBs compared to ARBs (6 trials with 25,611 participants: RR 0.82, 95% CI 0.72 to 0.94, I2 = 0%; moderate‐certainty evidence) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Myocardial infarction, Outcome 1: CCBs vs other classes of antihypertensive agents
We found significant statistical heterogeneity between trials comparing CCBs to diuretics and beta‐blockers (I2 = 72%, P = 0.03) and CCBs to ACE inhibitors (I2 = 66%, P = 0.007). A possible explanation for the heterogeneity may be that the type of CCB studied was different in each trial. The three trials involving diuretics and beta‐blockers respectively studied an aralkylamine derivative (verapamil, CONVINCE), a benzothiazepine derivative (diltiazem, NORDIL), and a DHP (felodipine, STOP‐Hypertension‐2). Another possible explanation is difference in participants. In the CONVINCE trial, participants diagnosed as having hypertension and who had one or more additional risk factors for cardiovascular disease were enrolled, but participants enrolled in the other two trials had no additional risk factors for cardiovascular disease. Six of seven trials involving ACE inhibitors studied DHPs, but three of them gave participants amlodipine (AASK; ALLHAT; FACET), and two administered felodipine (STOP‐Hypertension‐2), or nisoldipine (ABCD) and one gave nifedipine (J‐MIC(B)). One study did not describe the the specific ACE inhibitors and CCBs that were used (HOMED‐BP). The pooled RR for the trials comparing amlodipine and ACE inhibitors was 1.00 (95% CI 0.91 to 1.10, I2 = 0%; low‐certainty evidence) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Myocardial infarction, Outcome 2: Amlodipine vs ACE inhibitors
Stroke (non‐fatal and fatal stroke)
The incidence of stroke was not significantly different between CCB and diuretic groups (5 trials with 34,072 participants: RR 0.94, 95% CI 0.84 to 1.05, I2 = 0%; moderate‐certainty evidence) or between CCB and diuretic and beta‐blocker groups (3 trials with 31,892 participants: RR 0.92, 95% CI 0.81 to 1.03, I2 = 55%; moderate‐certainty evidence). Hypertensive participants treated with CCBs had a significantly lower risk of developing a stroke than those treated with a beta‐blocker (3 trials with 22,249 participants: RR 0.77, 95% CI 0.67 to 0.88, I2 = 0%; moderate‐certainty evidence) or an ACE inhibitor (7 trials with 27,999 participants: RR 0.90, 95% CI 0.81 to 0.99, I2 = 28%; low‐certainty evidence). There was no difference in risk of stroke between on CCB and ARB groups (6 trials with 25611 participants: RR 0.87, 95% CI 0.76 to 1.00, p = 0.05, I2 = 15%; moderate‐certainty evidence) (Analysis 3.1), but the incidence of stroke was lower for amlodipine compared to ARBs (5 trials with 23265 participants:RR 0.85, 95% CI 0.74 to 0.98, I2 = 0%)(Analysis 3.2; Figure 5).
3.1. Analysis.

Comparison 3: Stroke, Outcome 1: CCBs vs other classes of antihypertensive agents
3.2. Analysis.

Comparison 3: Stroke, Outcome 2: Amlodipine vs ARBs
5.

Forest plot of comparison: 3 Stroke, outcome: 3.1 CCBs versus other classes of antihypertensive agents.
The reason for significant statistical heterogeneity between trials comparing CCBs to diuretics and beta‐blockers (I2 = 55%, P = 0.11) might be related to the type of CCBs, similar to the description above in the MI results. Explanation for the heterogeneity may be that the type of CCB studied and inclusion criteria of participants were different in each trial. Regarding trials comparing CCBs to ARBs, one trial did not describe the specific CCBs used (HOMED‐BP), whilst five of six trials gave participants amlodipine (CASE‐J; IDNT; NAGOYA; VALUE; VART). The pooled RR for the trials comparing amlodipine to ARBs was 0.85 (95% CI 0.74 to 0.98, I2 = 0%) (Analysis 3.2).
Congestive heart failure
There was no significant difference in development of congestive heart failure between CCB and beta‐blocker groups (2 trials with 19,915 participants: RR 0.83, 95% CI 0.67 to 1.04, I2 = 0%; low‐certainty evidence) and between CCB and diuretic and beta‐blocker groups (3 trials with 31,892 participants: RR 1.15, 95% CI 0.99 to 1.33, I2 = 0%; low‐certainty evidence). However, the risk of developing congestive heart failure was markedly higher in participants given CCBs than those given diuretics (5 trials with 34,072 participants: RR 1.37, 95% CI 1.25 to 1.51, I2 = 17%; moderate‐certainty evidence); ACE inhibitors (5 trials with 25,276 participants: RR 1.16, 95% CI 1.06 to 1.28, I2 = 0%; low‐certainty evidence); and ARBs (5 trials with 23,265 participants: RR 1.20, 95% CI 1.06 to 1.36, I2 = 66%; low‐certainty evidence) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Congestive heart failure, Outcome 1: CCBs vs other classes of antihypertensive agents
The lack of homogeneity between the five trials comparing a CCB to an ARB may be due to the different inclusion criteria of participants: the IDNT trial included hypertensive individuals with type 2 diabetic nephropathy, and the NAGOYA trial included hypertensive individuals with glucose intolerance, whilst the VALUE, CASE‐J, and VART trials only required participants to have hypertension and cardiovascular risk factors. There was a significant increase in congestive heart failure events among the diabetic nephropathic participants in IDNT (RR 1.58, 95% CI 1.17 to 2.14) and glucose intolerance participants in NAGOYA (RR 5.00, 95% CI 1.46 to 17.18]) treated with a CCB compared to those treated with an ARB.
Cardiovascular mortality
We added death caused by cardiovascular disease as a supplemental outcome, which differed from the published protocol, as we judged it to be important and it was reported in most of the included trials.
We found only a marginally lower cardiovascular mortality in the CCBs group compared to the beta‐blocker group (4 trials with 44,825 participants: RR 0.90, 95% CI 0.81 to 0.99, I2 = 62%; low‐certainty evidence). The effect of CCBs on cardiovascular mortality was not significantly different from that of diuretics (4 trials with 32721 participants: RR 1.02, 95% CI 0.93 to 1.12, I2 = 0%; moderate‐certainty evidence); diuretics and beta‐blockers (3 trials with 31892 participants: RR 1.04, 95% CI 0.92 to 1.18, I2 = 0%); ACE inhibitors (6 trials with 27619 participants: RR 0.98, 95% CI 0.89 to 1.07, I2 = 0%; moderate‐certainty evidence) or ARBs (3 trials with 4642 participants: RR 0.79, 95% CI 0.54 to 1.15, I2 = 0%; moderate‐certainty evidence) (Analysis 5.1)
5.1. Analysis.

Comparison 5: Cardiovascular mortality, Outcome 1: CCBs vs other classes of antihypertensive agents
The heterogeneity amongst trials involving beta‐blockers (I2 = 62%, P = 0.05) might be explained by the different types of CCBs that were evaluated: a non‐DHP in the INVEST trial (verapamil) and a DHP in the other three trials (amlodipine in AASK and ASCOT‐BPLA, and lacidipine in ELSA). After deselecting the INVEST trial, the pooled RR was 0.77 (95% CI 0.66 to 0.90, I2 = 0%), still showing a significant decrease in cardiovascular mortality in the CCB group (Analysis 5.2).
5.2. Analysis.

Comparison 5: Cardiovascular mortality, Outcome 2: DHP vs β‐blockers
Major cardiovascular events (MI, congestive heart failure, stroke, and cardiovascular mortality)
Compared to beta‐blockers, CCBs significantly reduced major cardiovascular events (3 trials with 22,249 participants: RR 0.84, 95% CI 0.77 to 0.92, I2 = 0%). In contrast, when compared to diuretics, CCBs probably increased major cardiovascular events (4 trials with 33,643 participants: RR 1.05, 95% CI 1.00 to 1.09, I2 = 0%, P = 0.03). There was no significant difference in total major cardiovascular events when CCBs were compared to diuretics or beta‐blockers (2 trials with 21,011 participants: RR 1.02, 95% CI 0.95 to 1.10, I2 = 0%); to ACE inhibitors (5 trials with 25,186 participants: RR 0.98, 95% CI 0.94 to 1.02, I2 = 45%); or ARBs (3 trials with 6874 participants: RR 0.97, 95% CI 0.78 to 1.22, I2 = 32%) (Analysis 6.1; Figure 6).
6.1. Analysis.

Comparison 6: Major cardiovascular events, Outcome 1: CCBs vs other classes of antihypertensive agents
6.

Forest plot of comparison: 6 Major cardiovascular events, outcome: 6.1 CCBs versus other classes of antihypertensive agents.
The poor methodological quality of the FACET trial might be a source of heterogeneity amongst the five trials comparing CCBs with ACE inhibitors. We undertook a sensitivity analysis on this effect by deselecting the FACET trial; the results were unchanged (4 trials with 24,806 participants: RR 0.98, 95% CI 0.94 to 1.02, I2 = 0%) (Analysis 6.2).
6.2. Analysis.

Comparison 6: Major cardiovascular events, Outcome 2: Sensitivity analysis: CCBs vs ACE inhibitors
Systolic and diastolic BP reduction
Using the weighted mean difference method and the fixed‐effect model, we found that the mean systolic BP reduction of the CCB group was 0.81 mmHg (95% CI 0.56 to 1.06) less than that of the diuretic‐based regimen group, and 3.00 mmHg (95% CI 2.59 to 3.41) less than the diuretic‐and‐beta‐blocker‐based regimen group. Systolic BP reduction was ‐1.11 mmHg (95% CI −1.40 to −0.82) more with CCBs than with ACE inhibitors, and ‐2.10 mmHg (95% CI −2.46 to −1.74]) more than with ARBs. There was no significant difference between the CCB group and beta‐blocker group (P = 0.38), or between the CCB group and alpha‐1‐antagonist group (P = 0.27) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Blood pressure reduction, Outcome 1: Systolic blood pressure reduction
For diastolic BP, the mean reduction of the CCB group was −0.68 mmHg (95% CI −0.84 to −0.52) more than the diuretic group; −0.63 mmHg (95% CI −0.81 to −0.44) more than the ACE inhibitor group; −1.70 mmHg (95% CI −1.91 to −1.49) more than the ARB group; and −1.20 mmHg (95% CI −2.39 to −0.01) more than the alpha‐1‐antagonist group. Mean diastolic changes between the CCB and beta‐blocker groups were not significantly different (Analysis 7.2).
7.2. Analysis.

Comparison 7: Blood pressure reduction, Outcome 2: Diastolic blood pressure reduction
There was heterogeneity (I2 of 85%) for the four trials comparing the effect of CCBs versus ACE inhibitors on systolic BP reduction, however there was no heterogeneity for the same comparison evaluating diastolic BP reduction. The heterogeneity was most likely due to the poor methodological quality of the FACET trial. Sensitivity analyses conducted without the FACET trial resulted in homogeneous significant mean differences for both systolic and diastolic BP: mean difference −1.00 (95% CI −1.29 to −0.70) and −0.62 (95% CI −0.81 to −0.44), respectively (Analysis 7.3).
7.3. Analysis.

Comparison 7: Blood pressure reduction, Outcome 3: Sensitivity analysis: CCBs vs ACE inhibitors
Discussion
Summary of main results
After a systematic search and selection process according to the protocol for this review, we included 23 RCTs with 153,849 participants that assessed cardiovascular outcomes or BP change, or both, among hypertensive participants. The two most important outcomes from the perspective of the patient were total all‐cause mortality and major cardiovascular events. The latter outcome is important as it is a composite of the individual outcomes of stroke, MI, and congestive heart failure. There was no significant difference between first‐line CCBs and any of the other classes of antihypertensive drugs for total mortality. In this update, no new trial comparing CCBs with beta‐blockers or diuretics has been incorporated, therefore the outcomes for these comparisons are consistent with the first version of the review. First‐line CCBs reduced major cardiovascular events as compared to beta‐blockers (moderate‐certainty evidence) and increased major cardiovascular events as compared to diuretics (moderate‐certainty evidence). The reduction in major cardiovascular events with CCBs as compared to beta‐blockers is explained by a significant reduction in stroke (moderate‐certainty evidence) and cardiovascular mortality (low‐certainty evidence). The increase in major cardiovascular events for first‐line CCBs as compared to diuretics is explained by increased congestive heart failure events (moderate‐certainty evidence). The risk difference (RD) for heart failure for the comparison of CCBs versus diuretics was 0.02 and is thus clinically important and consistent with either a protective effect of diuretics or a harmful effect of CCBs for this outcome. The finding that first‐line CCBs increased congestive heart failure as compared to ACE inhibitors (low‐certainty evidence) and ARBs (low‐certainty evidence) is robust after more RCTs were included in this update. The other significant differences found were that first‐line CCBs reduced stroke more than ACE inhibitors (low‐certainty evidence) and reduced MI more than ARBs (moderate‐certainty evidence). With the inclusion of new studies comparing CCBs with ARBs, the advantages of CCBs in reducing stroke over ARBs that were found in the first version of the review no longer exist (Table 1; Table 2; Table 3; Table 4), but in pooled analysis, the incidence of stroke was lower for amlodipine compared to ARBs.(Analysis 3.2)
Blood pressures decreased in all treatment arms of all the included trials, but mean BP reduction differed. First‐line CCB‐based regimens lowered systolic BP less than first‐line diuretic‐based regimens and conventional treatment‐based regimens. In contrast, first‐line CCBs lowered diastolic BP better than diuretic‐based regimens. First‐line CCB‐based regimens also lowered both systolic and diastolic BPs more than ACE inhibitors and ARBs. This could partially explain the differences in stroke outcomes.
Overall completeness and applicability of evidence
Most of the included trials with the exception of TOMHS reported relevant hypertension outcomes, but not all of the desired outcomes were available from each trial. Furthermore, supplemental inclusion criteria were required in several trials, and most trials were event‐driven hypertension studies, which meant that the included participants tended to have more complicated hypertension or advanced disease (Zanchetti 2005). Patients at the two extremes, that is those with uncomplicated hypertension at one extreme and those with severe or acute hypertension and secondary hypertension at the other extreme, were not included in the current analysis.
Although we included 23 studies with a large number of participants comparing several classes of antihypertensive drugs in this update, the number of trials for each of the subgroups was limited. Because of this data were insufficient for some comparisons. This was particularly the case for alpha‐1‐antagonists. Furthermore, most of the included CCBs were dihydropyridines, with evidence for non‐DHPs inadequate.
The prevalence of hypertension amongst adults with diabetes mellitus is approximately 80% (Kannel 1991). Although all major antihypertensive drug classes (i.e. ACE inhibitors, ARBs, CCBs, and diuretics) are useful in the treatment of hypertension in diabetes mellitus (Whelton 2018), guidelines recommended CCBs as a first‐line choice for those with hypertension and diabetes (JNC‐8; Whelton 2018; Williams 2018). Opie and colleagues made the point that the incidence of developing diabetes was less on the amlodipine‐based regimen (Opie 2002). On the other hand, the NAGOYA study found that hypertensive participants with type 2 diabetes mellitus or impaired glucose tolerance in the valsartan group had a significantly lower incidence of heart failure than those in the amlodipine group. A meta‐analysis of RCTs of primary prevention of albuminuria in participants with diabetes mellitus demonstrated a significant reduction in progression of moderately to severely increased albuminuria with the use of ACE inhibitors or ARBs (Palmer 2015), with CCB showing no effect. As we were unable to extract data to separately evaluate the effects on hypertensive participants with diabetes in our review, it is not possible to say whether CCBs have different effects in diabetic hypertensive patients.
Quality of the evidence
We graded the overall quality of the evidence and developed 'Summary of findings' tables, using GRADEpro GDTsoftware.
We found moderate‐certainty evidence that all‐cause mortality was not different between first‐line CCBs and any other antihypertensive classes.
We found moderate‐certainty evidence that first‐line CCBs increased congestive heart failure more than diuretics, and low‐certainty evidence that they increased congestive heart failure more than ACE inhibitors or ARBs.
We found moderate‐certainty evidence that first‐line CCBs reduced stroke, and low‐certainty evidence that they reduced cardiovascular mortality more than beta‐blockers.
We found low‐certainty evidence that first‐line CCBs reduced stroke more than ACE inhibitors, and moderate‐certainty evidence that they reduced myocardial infarction more than ARBs.
Potential biases in the review process
The included trials varied in their designs and methods, baseline and goal BPs, study populations, and drugs, so combining their data to arrive at a conclusion may have some limitations. For example, CCBs are a heterogeneous group of drugs that are subclassified into DHPs and non‐DHPs. The different classes have different in binding sites on the calcium channel pores and thus could have different effects (Opie 2000; Triggle 2007). In the current review, we did not evaluate different types of CCBs in separate comparisons, but it might not be appropriate to combine them in a meta‐analysis. The high I2 values for pooled trials involving both DHPs and non‐DHPs (72% for three trials assessing MI events) are consistent with this possibility (CONVINCE; NORDIL; STOP‐Hypertension‐2). However, in this case dividing the trials into DHPs and non‐DHPs does not explain the heterogeneity. Likewise, heterogenous populations in the included trials might be the cause of the heterogeneity of the effect. In the current review, enrolled participants included those with diabetes, cardiovascular disease, kidney disease, or other conditions. It was not possible to investigate the effect of these subgroup populations on the effect size. In general, there was excellent homogeneity of most effects as shown by an I2 value of 0%, with only a few outcomes associated with I2 > 50%, leading us to believe that the overall conclusions of our review are valid.
Although the benefits of BP lowering for the prevention of cardiovascular disease are well established (BPLTTC 2000; BPLTTC 2003; Ezzati 2002; Thomopoulos 2015; Wright 2018; Xie 2016), which antihypertensive drug class should be prescribed first is still somewhat controversial. In order to achieve the BP goal many patients need to be prescribed more than one antihypertensive agent (Chobanian 2003; Haller 2008; Mancia 2007). This fact leads to another limitation in the review and is perhaps its major weakness. Since additional antihypertensive agents other than first‐line drugs were administered sequentially to reach BP goals in most of the included trials, the results may have been confounded, although they were presumed to reflect the effect of the first drug. Only one small trial included in our review prohibited the use of any other antihypertensive drugs (NICS‐EH), and it concluded that the CCB and diuretic groups had a similar decrease in BPs and cardiovascular events. BP differences between different classes of drugs could have an impact on outcomes (Staessen 2003; Wright 2018), which is a further limitation of this type of review. In addition, three included trials had a 2 X 3 design (AASK; ABCD; HOMED‐BP). Participants were randomised 1) BP tight target versus usual 2) different drug classes. As reported, the effect of different drugs is difficult to differentiate from that of BP targets.
We have tried to reduce the risk of attrition bias by reporting on the intent‐to‐treat population to the greatest degree possible. We do not think publication bias is likely as we have done an extensive search of the pertinent literature, including published and unpublished studies, without any language restrictions.
Agreements and disagreements with other studies or reviews
This review was not designed to assess the effect of CCBs versus placebo or no treatment, but other meta‐analyses have addressed this question and demonstrated that first‐line CCBs reduce stroke and total cardiovascular events. A recent meta‐analysis of 10 RCTs (30, 359 participants) comparing CCBs blood pressure‐lowering treatment with no or less intense treatment showed that significant reductions in stroke, major cardiovascular events, cardiovascular and all‐cause death were obtained with CCBs (Thomopoulos 2015). Another meta‐analysis of 147 RCTs including 464,000 participants with hypertension demonstrated that all major antihypertensive drug classes (diuretics, ACE inhibitors, ARBs, beta‐blockers, and CCBs) caused a similar reduction in coronary heart disease events and stroke for a given reduction in BP (Law 2009). Blood pressure lowering by all classes of antihypertensive drugs is accompanied by significant reductions in stroke and major cardiovascular events, supporting the concept that reduction of these events is due to BP lowering.
In this review, we found that CCBs increased total cardiovascular events as compared to diuretics; within total cardiovascular events, only congestive heart failure events increased with CCB. The results of recent meta‐analyses are consistent with this conclusion: thiazides were associated with a lower risk of heart failure compared with CCBs, whilst there was no difference between groups in other events (Reboussin 2017; Thomopoulos 2015). The increase in total cardiovascular events for first‐line CCBs as compared to diuretics is explained by increased congestive heart failure events with CCBs.
CCBs significantly increased the risk of congestive heart failure as compared to diuretics, ACE inhibitors, and ARBs. This finding is consistent with other reviews (Black 2004; Opie 2000; Thomopoulos 2015). The Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults indicated that thiazides were associated with a lower risk of many cardiovascular outcomes compared with other antihypertensive drug classes (Reboussin 2017). Since CCBs and other drug classes did not have any other advantages as compared to diuretics, this would suggest that diuretics are the preferred first‐line drugs for patients with hypertension.
The results of this review are consistent with the findings of another Cochrane Review evaluating the comparison of beta‐blockers versus first‐line CCBs (Wiysonge 2007). That review concluded that beta‐blockers reduced total cardiovascular events significantly less than CCBs. A similar meta‐analysis including six of the trials included in our review, INSIGHT; MIDAS; NICS‐EH; NORDIL; STOP‐Hypertension‐2; VHAS, concluded that mortality and major cardiovascular events with CCBs were similar to those seen with conventional therapy (diuretics or beta‐blockers) (Opie 2002). A recent meta‐analysis showed that the risk of stroke was significantly higher (25%) with beta‐blockers as compared with CCBs (Thomopoulos 2015). To this point there is no evidence to support the initial use of beta‐blockers for hypertension in the absence of specific cardiovascular comorbidities.
Other authors have claimed that CCBs are more effective than other treatments in decreasing the risk of stroke in hypertensive individuals (Angeli 2004; Verdecchia 2005). However, a previous meta‐analysis found no difference between ARBs and CCBs in risk of stroke in diabetic participants (Turnbull 2005). Our results showed that stroke events are significantly reduced by CCBs as compared to beta‐blockers and ACE inhibitors. In the previous version of this review we found that CCBs reduced the risk of stroke as compared to ARBs (2 trials with 16,391 participants). In this updated version we added 4 new trials for comparison (CASE‐J; HOMED‐BP; NAGOYA; VART),the results indicated no difference between ARBs and CCBs(total 6 trials with 25611 participants). But in a pooled analysis of 5 trials comparing amlodipine of CCBs and ARBs, the incidence of stroke was lower for amlodipine compared to ARBs.This may be due to the greater blood pressure‐lowering effect of CCBs as compared to ACE inhibitors as was found in this review, but it does not explain the difference for beta‐blockers, which did not have a different blood pressure‐lowering effect. It has been hypothesised that CCBs might have anti‐atherosclerotic actions that could be helpful in reducing stroke as well (Angeli 2004).
Authors' conclusions
Implications for practice.
This update changed some conclusions of the previous version of this review. First‐line calcium channel blockers (CCBs) do not affect total mortality as compared to other antihypertensive drug classes. First‐line CCBs reduce major cardiovascular events, stroke, and cardiovascular mortality as compared to beta‐blockers. First‐line CCBs increase major cardiovascular and congestive heart failure events as compared to diuretics. First‐line CCBs reduce stroke as compared to angiotensin‐converting enzyme (ACE) inhibitors and myocardial infarction as compared to angiotensin receptor blockers (ARBs), but they increase congestive heart failure events as compared to both ACE inhibitors and ARBs.
The review shows an advantage of diuretics over CCBs in reducing major cardiovascular mortality and congestive heart failure events. We found evidence supporting CCBs over beta‐blockers in reduce major cardiovascular events, stroke, and cardiovascular mortality. It should be noted that many of the differences found in the current review are not robust, and further trials might change the conclusions. It will therefore be important to follow the research in this field closely and update this review when new data become available.
Implications for research.
More well‐designed randomised controlled trials comparing CCBs with other types of antihypertensive drugs and combinations of CCBs with other antihypertensive drug classes are needed, especially for individuals with comorbidities such as diabetes, coronary heart disease, and nephropathy. These trials must avoid confounding factors to the greatest degree possible, such as by ensuring that the secondary drugs added to each arm of the trial are the same. It is important that all relevant outcomes are well defined and reported.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2022 | New citation required and conclusions have changed | We created 'Summary of findings' tables using GRADEpro software and assessed the overall quality of evidence for each outcome based on GRADE criteria. Some main conclusions were changed. |
| 7 January 2022 | New search has been performed | We updated the literature searches and included five new studies in this updated review. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 8, 2010
| Date | Event | Description |
|---|---|---|
| 1 September 2020 | New search has been performed | The authors finished the first draft of the full review. |
| 1 May 2009 | New citation required and major changes | Protocol re‐published with new authors and amended methods. |
| 23 August 2006 | New citation required and major changes | Protocol withdrawn by authors. |
Notes
This protocol was first published in the Cochrane Library in Issue 2, 2002, by Onder G, Furberg CD, Moore A, Psaty BM, Pahor M. It was subsequently withdrawn by the original authors in June 2006 because they were not able to continue working on it.
This review was updated in 2021.
Acknowledgements
We would like to acknowledge the original authors of this Cochrane Review protocol (Onder G, Furberg CD, Moore A, Psaty BM, Pahor M), who identified the topic and contributed extensively to the background.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to September 01, 2020> Search Date: 2 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp calcium channel blockers/ 2 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw,kf. (63679) 3 (calcium adj2 (antagonist? or block$ or inhibit$)).tw,kf. 4 or/1‐3 5 exp thiazides/ 6 exp sodium chloride symporter inhibitors/ 7 exp sodium potassium chloride symporter inhibitors/ 8 ((loop or ceiling) adj diuretic?).tw,kf. 9 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw,kf. 10 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw,kf. 11 or/5‐10 12 exp angiotensin‐converting enzyme inhibitors/ 13 angiotensin converting enzyme inhibit$.tw,kf. 14 (ace adj2 inhibit$).tw,kf. 15 acei.tw,kf. 16 exp enalapril/ 17 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw,kf. 18 or/12‐17 19 exp Angiotensin Receptor Antagonists/ 20 (angiotensin adj3 receptor antagon$).tw,kf. 21 (angiotensin adj3 receptor block$).tw,kf. 22 (arb or arbs).tw,kf. 23 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw,kf. 24 or/19‐23 25 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 26 (reserpine or serpentina or rauwolfia or serpasil).mp. 27 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 28 exp hydralazine/ 29 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).mp. 30 or/25‐29 31 exp adrenergic beta‐antagonists/ 32 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw,kf. 33 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw,kf. 34 or/31‐33 35 hypertension/ 36 essential hypertension/ 37 (antihypertens$ or hypertens$).tw,kf. 38 exp blood pressure/ 39 (blood pressur$ or bloodpressur$).mp. 40 or/35‐39 41 randomized controlled trial.pt. 42 controlled clinical trial.pt. 43 randomized.ab. 44 placebo.ab. 45 dt.fs. 46 randomly.ab. 47 trial.ab. 48 groups.ab. 49 or/41‐48 50 animals/ not (humans/ and animals/) 51 49 not 50 52 4 and (11 or 18 or 24 or 30 or 34) and 40 and 51
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies Search Date: 2 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM) AND INSEGMENT #2 calcium NEAR2 (antagonist* OR block* OR inhibit*) AND INSEGMENT #3 (#1 OR #2) AND INSEGMENT #4 thiazide* AND INSEGMENT #5 sodium chloride symporter inhibitor* AND INSEGMENT #6 sodium potassium chloride symporter inhibitor* AND INSEGMENT #7 ((loop OR ceiling) NEXT diuretic*) AND INSEGMENT #8 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*) AND INSEGMENT #9 (chlorthalidone OR chlortalidone OR phthalamudine OR chlorphthalidolone OR oxodoline OR thalitone OR hygroton OR indapamide OR metindamide) AND INSEGMENT #10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) AND INSEGMENT #11 angiotensin converting enzyme inhibit* AND INSEGMENT #12 (ace NEAR2 inhibit*) AND INSEGMENT #13 (acei OR aceis) AND INSEGMENT #14 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril) AND INSEGMENT #15 (#11 OR #12 OR #13 OR #14) AND INSEGMENT #16 ((angiotensin NEAR3 receptor antagon*) OR (angiotensin NEAR3 receptor block*)) AND INSEGMENT #17 (arb OR arbs) AND INSEGMENT #18 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten) AND INSEGMENT #19 (#16 OR #17 OR #18) AND INSEGMENT #20 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa) AND INSEGMENT #21 (reserpine OR serpentina OR rauwolfia OR serpasil) AND INSEGMENT #22 (clonidine or adesipress or arkamin or caprysin or catapres* or catasan or chlofazolin or chlophazolin or clinidine or clofelin* or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin* or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or "m‐5041t" or normopresan or paracefan or "st‐155" or "st 155" or tesno timelets) AND INSEGMENT #23 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat) AND INSEGMENT #24 (#20 OR #21 OR #22 OR #23) AND INSEGMENT #25 (beta NEAR2 (adrenergic* OR antagonist* OR block* OR receptor*)) AND INSEGMENT #26 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) AND INSEGMENT #27 (#25 OR #26) AND INSEGMENT #28 (#3 AND (#10 OR #15 OR #19 OR #24 OR #27)) AND INSEGMENT #29 RCT:DE AND INSEGMENT #30 Review:ODE AND INSEGMENT #31 (#29 OR #30) AND INSEGMENT #32 #28 AND #31 AND INSEGMENT
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials (Issue 8, 2020) via Cochrane Register of Studies Search Date: 2 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR calcium channel blockers EXPLODE ALL AND CENTRAL:TARGET #2 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM) AND CENTRAL:TARGET #3 (calcium NEAR2 (antagonist* OR block* OR inhibit*)) AND CENTRAL:TARGET #4 (#1 OR #2 OR #3) AND CENTRAL:TARGET #5 MESH DESCRIPTOR thiazides EXPLODE ALL AND CENTRAL:TARGET #6 MESH DESCRIPTOR sodium chloride symporter inhibitors EXPLODE ALL AND CENTRAL:TARGET #7 MESH DESCRIPTOR sodium potassium chloride symporter inhibitors EXPLODE ALL AND CENTRAL:TARGET #8 ((loop OR ceiling) NEXT diuretic*) AND CENTRAL:TARGET #9 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*) AND CENTRAL:TARGET #10 (chlorthalidone OR chlortalidone OR phthalamudine OR chlorphthalidolone OR oxodoline OR thalitone OR hygroton OR indapamide OR metindamide) AND CENTRAL:TARGET #11 (#5 OR #6 OR #7 OR #8 OR #9 OR #10) AND CENTRAL:TARGET #12 MESH DESCRIPTOR Angiotensin‐Converting Enzyme Inhibitors EXPLODE ALL AND CENTRAL:TARGET #13 angiotensin converting enzyme inhibit* AND CENTRAL:TARGET #14 (ace NEAR2 inhibit*) AND CENTRAL:TARGET #15 acei AND CENTRAL:TARGET #16 MESH DESCRIPTOR enalapril EXPLODE ALL AND CENTRAL:TARGET #17 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril) AND CENTRAL:TARGET #18 (#12 OR #13 OR #14 OR #15 OR #16 OR #17) AND CENTRAL:TARGET #19 MESH DESCRIPTOR Angiotensin Receptor Antagonists EXPLODE ALL AND CENTRAL:TARGET #20 ((angiotensin NEAR3 receptor antagon* OR angiotensin NEAR3 receptor block*)) AND CENTRAL:TARGET #21 (arb OR arbs) AND CENTRAL:TARGET #22 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten) AND CENTRAL:TARGET #23 (#19 OR #20 OR #21 OR #22) AND CENTRAL:TARGET #24 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa) AND CENTRAL:TARGET #25 (reserpine OR serpentina OR rauwolfia OR serpasil) AND CENTRAL:TARGET #26 (clonidine or adesipress or arkamin or caprysin or catapres* or catasan or chlofazolin or chlophazolin or clinidine or clofelin* or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin* or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or "m‐5041t" or normopresan or paracefan or "st‐155" or "st 155" or tesno timelets) AND CENTRAL:TARGET #27 MESH DESCRIPTOR hydralazine EXPLODE ALL AND CENTRAL:TARGET #28 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat) AND CENTRAL:TARGET #29 (#24 OR #25 OR #26 OR #27 OR #28) AND CENTRAL:TARGET #30 MESH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #31 (beta NEAR2 (adrenergic* OR antagonist* OR block* OR receptor*)) AND CENTRAL:TARGET #32 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) AND CENTRAL:TARGET #33 (#30 OR #31 OR #32) AND CENTRAL:TARGET #34 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #35 MESH DESCRIPTOR Essential Hypertension AND CENTRAL:TARGET #36 (antihypertens* OR hypertens*) AND CENTRAL:TARGET #37 MESH DESCRIPTOR Blood Pressure EXPLODE ALL AND CENTRAL:TARGET #38 (blood pressur* OR bloodpressur*) AND CENTRAL:TARGET #39 (#34 OR #35 OR #36 OR #37 OR #38) AND CENTRAL:TARGET #40 (#4 AND (#11 OR #18 OR #23 OR #29 OR #33) AND #39) AND CENTRAL:TARGET
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Embase <1974 to 2020 September 01> Search Date: 2 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp calcium channel blocking agent/ 2 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw,kw. (84571) 3 (calcium adj2 (antagonist? or block$ or inhibit$)).tw,kw. 4 or/1‐3 5 exp thiazide diuretic agent/ 6 exp loop diuretic agent/ 7 ((loop or ceiling) adj diuretic?).tw,kw. 8 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw,kw. 9 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw,kw. 10 or/5‐9 11 exp dipeptidyl carboxypeptidase inhibitor/ 12 angiotensin converting enzyme inhibit$.tw,kw. 13 (ace adj2 inhibit$).tw,kw. 14 acei.tw,kw. 15 enalapril/ 16 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw,kw. 17 or/11‐16 18 exp Angiotensin Receptor Antagonist/ 19 (angiotensin adj3 receptor antagon$).tw,kw. 20 (angiotensin adj3 receptor block$).tw,kw. 21 (arb or arbs).tw,kw. 22 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw,kw. 23 or/18‐22 24 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 25 (reserpine or serpentina or rauwolfia or serpasil).mp. 26 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 27 hydralazine/ 28 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw,kw. 29 or/24‐28 30 exp beta adrenergic receptor blocking agent/ 31 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw,kw. 32 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw,kw. 33 or/30‐32 34 exp hypertension/ 35 (antihypertens$ or hypertens$).tw,kw. 36 exp blood pressure/ 37 (blood pressur$ or bloodpressur$).mp. 38 or/34‐37 39 randomized controlled trial/ 40 crossover procedure/ 41 double‐blind procedure/ 42 (randomi?ed or randomly).tw. 43 (crossover$ or cross‐over$).tw. 44 placebo$.ab. 45 (doubl$ adj blind$).tw. 46 assign$.ab. 47 allocat$.ab. 48 or/39‐47 49 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 50 48 not 49 51 4 and (10 or 17 or 23 or 29 or 33) and 38 and 50
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov Search Date: 2 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition or disease: Hypertension Other terms: randomized Study type: Interventional Studies (Clinical Trials) Intervention/treatment: Calcium Channel Blockers First Posted: 02/18/2019 To 09/02/2020
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform (ICTRP) via Cochrane Register of Studies Search Date: 2 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR calcium channel blockers EXPLODE ALL AND CENTRAL:TARGET #2 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM) AND CENTRAL:TARGET #3 (calcium NEAR2 (antagonist* OR block* OR inhibit*)) AND CENTRAL:TARGET #4 (#2 OR #3) AND CENTRAL:TARGET #5 MESH DESCRIPTOR thiazides EXPLODE ALL AND CENTRAL:TARGET #6 MESH DESCRIPTOR sodium chloride symporter inhibitors EXPLODE ALL AND CENTRAL:TARGET #7 MESH DESCRIPTOR sodium potassium chloride symporter inhibitors EXPLODE ALL AND CENTRAL:TARGET #8 ((loop OR ceiling) NEXT diuretic*) AND CENTRAL:TARGET #9 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*) AND CENTRAL:TARGET #10 (chlorthalidone OR chlortalidone OR phthalamudine OR chlorphthalidolone OR oxodoline OR thalitone OR hygroton OR indapamide OR metindamide) AND CENTRAL:TARGET #11 (#5 OR #6 OR #7 OR #8 OR #9 OR #10) AND CENTRAL:TARGET #12 MESH DESCRIPTOR Angiotensin‐Converting Enzyme Inhibitors EXPLODE ALL AND CENTRAL:TARGET #13 angiotensin converting enzyme inhibit* AND CENTRAL:TARGET #14 (ace NEAR2 inhibit*) AND CENTRAL:TARGET #15 acei AND CENTRAL:TARGET #16 MESH DESCRIPTOR enalapril EXPLODE ALL AND CENTRAL:TARGET #17 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril) AND CENTRAL:TARGET #18 (#12 OR #13 OR #14 OR #15 OR #16 OR #17) AND CENTRAL:TARGET #19 MESH DESCRIPTOR Angiotensin Receptor Antagonists EXPLODE ALL AND CENTRAL:TARGET #20 ((angiotensin NEAR3 receptor antagon* OR angiotensin NEAR3 receptor block*)) AND CENTRAL:TARGET #21 (arb OR arbs) AND CENTRAL:TARGET #22 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten) AND CENTRAL:TARGET #23 (#19 OR #20 OR #21 OR #22) AND CENTRAL:TARGET #24 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa) AND CENTRAL:TARGET #25 (reserpine OR serpentina OR rauwolfia OR serpasil) AND CENTRAL:TARGET #26 (clonidine or adesipress or arkamin or caprysin or catapres* or catasan or chlofazolin or chlophazolin or clinidine or clofelin* or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin* or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or "m‐5041t" or normopresan or paracefan or "st‐155" or "st 155" or tesno timelets) AND CENTRAL:TARGET #27 MESH DESCRIPTOR hydralazine EXPLODE ALL AND CENTRAL:TARGET #28 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat) AND CENTRAL:TARGET #29 (#24 OR #25 OR #26 OR #27 OR #28) AND CENTRAL:TARGET #30 MESH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #31 (beta NEAR2 (adrenergic* OR antagonist* OR block* OR receptor*)) AND CENTRAL:TARGET #32 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) AND CENTRAL:TARGET #33 (#30 OR #31 OR #32) AND CENTRAL:TARGET #34 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #35 MESH DESCRIPTOR Essential Hypertension AND CENTRAL:TARGET #36 (antihypertens* OR hypertens*) AND CENTRAL:TARGET #37 (#34 OR #35 OR #36) AND CENTRAL:TARGET #38 (#4 AND (#11 OR #18 OR #23 OR #29 OR #33) AND #37) AND CENTRAL:TARGET #39 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET #40 http*:SO AND CENTRAL:TARGET #41 (#39 OR #40) AND CENTRAL:TARGET #42 #38 AND #41 AND CENTRAL:TARGET
Appendix 2. Search strategies from the 2010 review
Cochrane Central Register of Controlled Trials (CENTRAL) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. (calcium channel blockers or amlodipine or amrinone or bencyclane or bepridil or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or magnesium sulfate or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil or omega‐agatoxin iva or omega‐conotoxin gvia or omega‐conotoxins).mp. 2. calcium adj2 (inhibit$ or agonist? or exogenous or blockader?).tw. 3. 1 or 2 4. hypertension/ 5. hypertens$.tw. 6. (blood adj pressure).tw. 7. or/4‐6 8. 3 and 7 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Ovid MEDLINE ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. (calcium channel blockers or amlodipine or amrinone or bencyclane or bepridil or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or magnesium sulfate or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil or omega‐agatoxin iva or omega‐conotoxin gvia or omega‐conotoxins).mp. 2. calcium adj2 (inhibit$ or agonist? or exogenous or blockader?).tw. 3. 1 or 2 4. hypertension/ 5. hypertens$.tw. 6. (blood adj pressure).tw. 7. or/4‐6 8. 3 and 7 9. randomized controlled trial.pt. 10. controlled clinical trial.pt. 11. randomized.ab. 12. placebo.ab. 13. drug therapy.fs. 14. randomly.ab. 15. trial.ab 16. groups.ab. 17. or/9‐16 18. animals/ not (humans/ and animals/) 19. 17 not 18 20. 8 and 19 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Ovid Embase ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. Randomized Controlled Trial/ 2. Clinical Trial/ 3. Multicenter Study/ 4. Controlled Study/ 5. Crossover Procedure/ 6. Double Blind Procedure/ 7. Single Blind Procedure/ 8. exp Randomization/ 9. Major Clinical Study/ 10. Placebo/ 11. Meta Analysis/ 12. phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 13. (clin$ adj25 trial$).tw. 14. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. 15. placebo$.tw. 16. random$.tw. 17. control$.tw. 18. (meta?analys$ or systematic review$).tw. 19. (cross?over or factorial or sham? or dummy).tw. 20. ABAB design$.tw. 21. or/1‐20 22. human/ 23. nonhuman/ 24. 22 or 23 25. 21 not 24 26. 21 and 22 27. 25 or 26 28. hypertension/ 29. hypertens$.tw. 30. (blood adj pressure).tw. 31. or/28‐30 32. exp calcium channel blockers/ 33. (calcium channel blockers or amlodipine or amrinone or bencyclane or bepridil or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or magnesium sulfate or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil or omega‐agatoxin iva or omega‐conotoxin gvia or omega‐conotoxins).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 34. calcium adj2 (inhibit$ or agonist? or exogenous or blockader?).tw. 35. or/32‐34 36. 27 and 31 and 35
Data and analyses
Comparison 1. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 CCBs vs other classes of antihypertensive agents | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 CCBs vs diuretics | 5 | 35057 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| 1.1.2 CCBs vs β‐blockers | 4 | 44825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
| 1.1.3 CCBs vs diuretics or β‐blockers | 3 | 31892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.12] |
| 1.1.4 CCBs vs ACE inhibitors | 7 | 27999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
| 1.1.5 CCBs vs ARBs | 6 | 25611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
Comparison 2. Myocardial infarction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 CCBs vs other classes of antihypertensive agents | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 CCBs vs diuretics | 5 | 34072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 2.1.2 CCBs vs β‐blockers | 3 | 22249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 2.1.3 CCBs vs diuretics and β‐blockers | 3 | 31892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.19] |
| 2.1.4 CCBs vs ACE inhibitors | 7 | 27999 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.14] |
| 2.1.5 CCBs vs ARBs | 6 | 25611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.94] |
| 2.2 Amlodipine vs ACE inhibitors | 3 | 19135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
Comparison 3. Stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 CCBs vs other classes of antihypertensive agents | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 CCBs vs diuretics | 5 | 34072 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 3.1.2 CCBs vs β‐blockers | 3 | 22249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.88] |
| 3.1.3 CCBs vs diuretics or β‐blockers | 3 | 31892 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.03] |
| 3.1.4 CCBs vs ACE inhibitors | 7 | 27999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 3.1.5 CCBs vs ARBs | 6 | 25611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 3.2 Amlodipine vs ARBs | 5 | 23265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
Comparison 4. Congestive heart failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 CCBs vs other classes of antihypertensive agents | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 CCBs vs diuretics | 5 | 34072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.25, 1.51] |
| 4.1.2 CCBs vs β‐blockers | 2 | 19915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.04] |
| 4.1.3 CCBs vs diuretics and β‐blockers | 3 | 31892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.99, 1.33] |
| 4.1.4 CCBs vs ACE inhibitors | 5 | 25276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.06, 1.28] |
| 4.1.5 CCBs vs ARBs | 5 | 23265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.06, 1.36] |
Comparison 5. Cardiovascular mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 CCBs vs other classes of antihypertensive agents | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 CCBs vs diuretics | 4 | 32721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 5.1.2 CCBs vs β‐blockers | 4 | 44825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 5.1.3 CCBs vs diuretics or β‐blockers | 3 | 31892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.18] |
| 5.1.4 CCBs vs ACE inhibitors | 6 | 27619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.07] |
| 5.1.5 CCBs vs ARBs | 3 | 4642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.54, 1.15] |
| 5.2 DHP vs β‐blockers | 3 | 22249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.66, 0.90] |
Comparison 6. Major cardiovascular events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 CCBs vs other classes of antihypertensive agents | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 CCBs vs diuretics | 4 | 33643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.00, 1.09] |
| 6.1.2 CCBs vs β‐blockers | 3 | 22249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.77, 0.92] |
| 6.1.3 CCBs vs diuretics and β‐blockers | 2 | 21011 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.10] |
| 6.1.4 CCBs vs ACE inhibitors | 5 | 25186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.02] |
| 6.1.5 CCBs vs ARBs | 3 | 6874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.22] |
| 6.2 Sensitivity analysis: CCBs vs ACE inhibitors | 4 | 24806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.02] |
Comparison 7. Blood pressure reduction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Systolic blood pressure reduction | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 CCBs vs diuretics | 3 | 24963 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.56, 1.06] |
| 7.1.2 CCBs vs β‐blockers | 3 | 23474 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.31, 0.81] |
| 7.1.3 CCBs vs diuretics or β‐blockers | 1 | 10881 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [2.59, 3.41] |
| 7.1.4 CCBs vs ACE inhibitors | 4 | 19368 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐1.40, ‐0.82] |
| 7.1.5 CCBs vs ARBs | 1 | 15245 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐2.46, ‐1.74] |
| 7.1.6 CCBs vs α1‐antagonist | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐3.89, 1.09] |
| 7.2 Diastolic blood pressure reduction | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 CCBs vs diuretics | 3 | 24963 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.84, ‐0.52] |
| 7.2.2 CCBs vs β‐blockers | 3 | 23474 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.16, 0.45] |
| 7.2.3 CCBs vs diuretics or β‐blockers | 1 | 10881 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.07, 0.27] |
| 7.2.4 CCBs vs ACE inhibitors | 4 | 19368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.81, ‐0.44] |
| 7.2.5 CCBs vs ARBs | 1 | 15245 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐1.91, ‐1.49] |
| 7.2.6 CCBs vs α1‐antagonists | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.39, ‐0.01] |
| 7.3 Sensitivity analysis: CCBs vs ACE inhibitors | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 Systolic blood pressure reduction | 3 | 18988 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐1.29, ‐0.70] |
| 7.3.2 Diastolic blood pressure reduction | 3 | 18988 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.81, ‐0.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AASK.
| Study characteristics | ||
| Methods | Long‐term, multicentre, randomised controlled, double‐blind trial, using a 3 × 2 factorial design. Because of acute changes in glomerular filtration rate (GFR), the amlodipine arm was halted about 1 year ahead of the protocol, while most participants in other groups were followed to the planned end, giving a median follow‐up of 4.3 years to the cardiovascular composite outcome. All analyses were intent‐to‐treat. | |
| Participants | Participants (N = 1094) were self‐identified African‐Americans with hypertension (diastolic blood pressure (BP) was 95 mmHg or higher), aged 18 to 70 years, with glomerular filtration rate (GFR) between 20 and 65 mL/min per 1.73 m2. | |
| Interventions | Participants were randomised equally to 1 of 2 blood pressure goals (usual mean arterial pressure (MAP) goal of 102 to 107 mmHg (N = 554) or a lower MAP goal of 92 mmHg (N = 540)), and to treatment with 1 of 3 antihypertensive drugs (metoprolol, 50 to 200 mg/d (N = 441); ramipril, 2.5 to 10 mg/d (N = 436); or a dihydropyridine calcium channel blocker (CCB) amlodipine, 5 to 10 mg/d (N = 217), using a 2:2:1 randomisation ratio). Additional open‐labeled antihypertensives were added if the BP goal could not be achieved by the randomised drug. | |
| Outcomes | Rate of change in GFR and other renal outcomes, and all cardiovascular events including cardiovascular deaths and hospitalisations for miocardial infarction (MI), strokes, heart failure, revascularisation procedures, and other hospitalised cardiovascular events were reviewed. | |
| Notes | Study was carried out at 11 clinical centres in the USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was stratified by city using randomly permuted blocks, and the Data Coordinating Center performed randomisation centrally. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were masked to randomised drug but not blood pressure goal. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal amongst the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
ABCD.
| Study characteristics | ||
| Methods | A prospective, controlled, randomised, double‐blinded trial with a long‐term follow‐up of more than 5 years. Cardiovascular endpoints were analysed using the intention‐to‐treat principle. | |
| Participants | Enrolled participants were diagnosed with non‐insulin‐dependent diabetes mellitus (NIDDM), and had diastolic blood pressure of 80 mmHg or higher and were receiving no antihypertensive medications at the time of randomisation. The current review only focused on the hypertensive cohort (N = 470) (mean baseline diastolic blood pressure ≥ 90 mmHg). | |
| Interventions | Participants randomised to active study medication received either nisoldipine (N = 235) (10 mg per day, with increases to 20, 40, and 60 mg per day, plus placebo for enalapril) or enalapril (N = 235) (5 mg per day, with increases to 10, 20, and 40 mg per day, plus placebo for nisoldipine). Open‐label antihypertensive medications except the study drugs were added when necessary. | |
| Outcomes | Cardiovascular outcomes including death due to cardiovascular events, non‐fatal myocardial infarction (MI), non‐fatal cerebrovascular accident (CVA), heart failure requiring hospital admission, and pulmonary infarction were reviewed. | |
| Notes | Results at the end of the planned 5‐year follow‐up were reported in 1998, and additional follow‐up results were described in 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study drug in each group plus placebo for the other study drug were administered in a double‐blind manner. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
ALLHAT.
| Study characteristics | ||
| Methods | A randomised, double‐blind, multicentre clinical trial with a large sample size and long follow‐up (with a mean length of 4.9 years (standard deviation (SD) 1.4 years)). | |
| Participants | Participants (N = 33,357) were men and women aged 55 years or older who had stage 1 or stage 2 hypertension with at least 1 additional risk factor for coronary heart disease (CHD) events. | |
| Interventions | Treatment with the study drug was initiated the day after randomisation. Participants were randomly assigned to chlortalidone, amlodipine, or lisinopril in a ratio of 1.7:1:1, which meant that 15,255, 9048, and 9054 participants were enrolled in the 3 groups, respectively. Goal blood pressure was less than 140/90 mmHg achieved by titrating the assigned study drug, with additional open‐label agents allowed if necessary. | |
| Outcomes | The primary outcome was fatal CHD or non‐fatal myocardial infarction combined; secondary outcomes included all‐cause mortality, stroke, combined CHD, and combined cardiovascular disease. | |
| Notes | Sponsored by the National Heart, Lung, and Blood Institute and carried out in 623 North American centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme was generated by computer. |
| Allocation concealment (selection bias) | Low risk | It specified that the concealed randomisation scheme was implemented at the clinical trials centre and stratified by centre. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study drugs were encapsulated and identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 625 centres in the United States and Canada participated in the trial; 2 sites were excluded because their 30 participants had poor documentation of informed consent. Participants were recruited in 623 centres, which might have impacted on the results, however an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
ASCOT‐BPLA.
| Study characteristics | ||
| Methods | An independent, investigator‐initiated, investigator‐led, multicentre, prospective, randomised controlled trial, with 5.5 years' median follow‐up. It compared the time to first event on an intention‐to‐treat basis. | |
| Participants | A total of 19,257 participants aged 40 to 79 years were recruited, all of whom had either untreated hypertension (systolic blood pressure ≥ 160 mmHg, diastolic BP ≥ 100 mmHg, or both) or treated hypertension (systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or both). Participants had to have at least 3 other cardiovascular risk factors. | |
| Interventions | Participants were randomised to CCB‐based regimen (amlodipine 5 to 10 mg; N = 9639) or β‐blocker‐based regimen (atenolol 50 to 100 mg; N = 9618). Additional antihypertensive agents were administered to both groups according to a prespecified algorithm: perindopril 4 to 8 mg was added to amlodipine‐based group as required; bendroflumethiazide 1.25 to 2.5 mg was added to atenolol‐based group as required. | |
| Outcomes | Primary endpoints: non‐fatal myocardial infarction (MI) + fatal coronary heart disease Secondary endpoints: all‐cause mortality, total stroke, primary endpoint minus silent MI, all coronary events, total cardiovascular events and procedures, cardiovascular mortality, and non‐fatal and fatal heart failure Tertiary endpoints: silent MI, unstable angina, chronic stable angina, peripheral arterial disease, life‐threatening arrhythmias, development of diabetes, development of renal impairment |
|
| Notes | Participants were recruited between February 1998 and May 2000 in the UK, Ireland, and the Nordic countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was a computer‐generated optimum allocation. |
| Allocation concealment (selection bias) | Low risk | The allocation was blinded for any person involved in the undertaking of the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A PROBE design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 centres with 85 participants were excluded after randomisation, but missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
CASE‐J.
| Study characteristics | ||
| Methods | A prospective, multicentre, randomised, open‐label, active‐controlled, 2‐arm parallel‐group comparison with a response‐dependent dose titration and blinded assessment of the endpoints. Participants were followed for an average of 3.2 years. | |
| Participants | 4728 hypertension patients (systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg in participants < 70 years old or SBP ≥ 160 mmHg or DBP ≥ 90 mmHg in participants ≥ 70 years old) with high risk (high‐risk patients were defined by the presence of any of the following factors: (1) severe hypertension (SBP ≥ 180 mmHg or DBP ≥ 110 mmHg); (2) type 2 diabetes mellitus; (3) a history of stroke or transient ischaemic attack > 6 months before the screening; (4) left ventricular hypertrophy; (5) proteinuria or a serum creatinine concentration ≥ 1.3 mg/dL; or (6) arteriosclerotic peripheral artery obstruction) were enrolled in the study. Mean age was 63.8 years, and 136 participants were lost to follow‐up. | |
| Interventions | Participants were randomly assigned to the treatment groups. Enrolled participants were given candesartan cilexetil or amlodipine besylate. The candesartan was administered orally at a dose of 4 to 8 mg/d and was increased to 12 mg/d when necessary. The amlodipine was administered orally at a dose of 2.5 to 5.0 mg/d and was increased to 10.0 mg/d when necessary. Once a participant was given the assigned medication, use of other angiotensin receptor blockers (ARBS), CCBs, and all of the angiotensin‐converting enzyme inhibitors was prohibited. Participants already being treated with diuretics, α‐blockers, β‐blockers, or α‐ and β‐blockers before enrolment were allowed to continue taking these medications. | |
| Outcomes | Primary endpoint: sudden death, cerebrovascular events, cardiac events and vascular events. Secondary endpoints: all‐cause death and new‐onset diabetes |
|
| Notes | A large‐scale clinical trial in Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by the Automatic Bar Code Data‐Capturing/Allocation, Booking & trial Coding, Data Management (ABCD) system. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded assessment of the endpoint |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were almost equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
CONVINCE.
| Study characteristics | ||
| Methods | Prospective, double‐blind, randomised, active‐controlled, multicentre, international clinical trial with a mean follow‐up of 3 years | |
| Participants | A total of 16,602 participants with hypertension and 1 or more additional risk factors for cardiovascular disease were enrolled, but 126 of them were excluded because of data integrity, so findings from 16,476 participants were reported. | |
| Interventions | Participants were administered standard‐of‐care drug (β‐blocker or diuretic) chosen by the investigator prior to randomisation. They were then randomised to verapamil group (N = 8241) (starting at 180 mg daily, with dose increased or other drugs added when necessary) or standard‐of‐care regimen group (N = 8361) (β‐blocker or diuretic). | |
| Outcomes | Effect in preventing acute myocardial infarction, stroke, or cardiovascular disease related death, and all‐cause mortality. | |
| Notes | Study was conducted at 661 clinical sites in 15 countries; the sponsor closed the study 2 years earlier than the planned 5‐year follow‐up for commercial reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A simple automated system, the interactive voice response system, was used for randomising, assigning, and tracking blinded medication. |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Labeled bottles with active drug or placebo were given to participants; the content of the bottles was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants from 2 sites (n = 126; 62 randomised to controlled‐onset extended‐release verapamil) were excluded because of data integrity concerns; the impact of these exclusions on the results was unclear, but we performed an intention‐to‐treat analysis in the review. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Unclear risk | Withdrawal between groups was imbalanced (115 with verapamil, 207 with atenolol or hydrochlorothiazide). |
ELSA.
| Study characteristics | ||
| Methods | A randomised, double‐blind, multicentre trial with a mean follow‐up of 3.75 years. 49 lacidipine and 43 atenolol participants lost to follow‐up; an intention‐to‐treat analysis was performed. | |
| Participants | All enrolled participants (N = 2334) were aged 45 to 75 years with sitting systolic blood pressure of 150 to 210 mmHg and diastolic blood pressure of 95 to 115 mmHg. | |
| Interventions | Participants were randomised to receive either lacidipine 4 mg once daily (N = 1177) or atenolol 50 mg once daily (N = 1157). If diastolic blood pressure goal was not achieved, the dose of lacidipine could be increased to 6 mg, and atenolol could be increased to 100 mg (month 1), with open‐label hydrochlorothiazide added (12.5 mg daily month 3 and 25 mg daily month 6). | |
| Outcomes | Change in mean maximum intima‐media thickness, proportion of participants with an increase or decrease in plaque number, incidence of cardiovascular events and total mortality | |
| Notes | Study was conducted in 410 clinical units in France, Germany, Greece, Italy, Spain, Sweden, and the UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and study personnel, excluding the Safety Committee, were blinded to treatment assignment for the duration of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
FACET.
| Study characteristics | ||
| Methods | An open‐label, randomised prospective trial. Participants were followed for up to 3.5 years. All analyses were intention‐to‐treat unless otherwise stated. | |
| Participants | Participants (N = 380) with a diagnosis of non‐insulin‐dependent diabetes mellitus and hypertension (systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg) | |
| Interventions | Participants were randomly assigned to open‐label fosinopril (20 mg/day) (N = 189) or amlodipine (10 mg/day) (N = 191). The other study drug was added when necessary. | |
| Outcomes | Serum lipids and diabetes control, cardiovascular events, blood pressure control, and renal function status | |
| Notes | Participants were recruited from an outpatient diabetes clinic in Marino, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study drugs were administered open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | High risk | When BP was not controlled well on monotherapy, the other study drug was added. This could have affected the evaluation of effect of each study drug. |
HOMED‐BP.
| Study characteristics | ||
| Methods | A clinical trial with PROBE design, the last follow‐up (median 5.3 years) | |
| Participants | This trial involved 3518 participants (50% women; mean age 59.6 years) with mild‐to‐moderate hypertension who were 40 years of age or older. | |
| Interventions | Participants were randomised to usual control (125 to 134/80 to 84 mmHg) vs tight control (< 125/< 80 mmHg) of blood pressure self‐measurement at home and to initiation of drug treatment with an angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, or calcium channel blocker. | |
| Outcomes | The primary endpoint was cardiovascular death plus stroke and myocardial infarction. | |
| Notes | Participants were recruited from 457 general practices throughout Japan from 2001 to 2010. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computerised random number function with a minimisation algorithm. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A prospective randomized open‐blinded end point evaluation design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were almost equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported |
| Other bias | Low risk | No other potential bias was found. |
IDNT.
| Study characteristics | ||
| Methods | An international, prospective, randomised, double‐blind, placebo‐controlled, multicentre trial. The mean duration of follow‐up was 2.6 years. 16 enrolled participants never received the study medication, and follow‐up was incomplete in 11 participants; reasons were not specified. All analyses were based on the intention‐to‐treat principle. | |
| Participants | A total of 1715 hypertensive participants (systolic blood pressure > 135 mmHg whilst sitting, diastolic blood pressure > 85 mmHg whilst sitting, or documented treatment with antihypertensive agents) with diabetic nephropathy due to type 2 diabetes mellitus underwent randomisation. | |
| Interventions | Eligible participants were randomised into 1 of 3 groups treated with irbesartan (300 mg daily) (N = 579), amlodipine (10 mg daily) (N = 567), or placebo (N = 569). The target blood pressure was 135/85 mmHg or less in all groups, and other classes of antihypertensive agents were allowed as needed in each group. | |
| Outcomes | The primary endpoint was renal outcomes. The secondary endpoint was the composite of fatal or non‐fatal cardiovascular events, which were not statistically different in the 3 groups. Adverse events were recorded at quarterly visits. |
|
| Notes | Conducted in 209 centres in the Americas, Europe, Israel, and Australasia by the clinical co‐ordinating centre and the various committees of the Collaborative Study Group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate sequence was generated by computer. |
| Allocation concealment (selection bias) | Low risk | To minimise any centre effect, randomisation was blocked by centre. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Probably done because it was stated as a "double‐blind clinical trial", "matched placebo" was given in the control group, and the blinded clinical database was provided to the centre for statistical analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal amongst the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
INSIGHT.
| Study characteristics | ||
| Methods | A prospective, randomised, double‐blind trial. Analysis was done by intention‐to‐treat. 254 participants were excluded after randomisation from centres due to misconduct, and were not included in the analysis. The follow‐up was 3 years. | |
| Participants | A total of 6575 participants aged 55 to 80 years with hypertension (BP ≥ 150/95 mmHg, or ≥ 160 mmHg systolic) were enrolled. Participants also had at least 1 additional cardiovascular risk factor. | |
| Interventions | Participants were randomly assigned to nifedipine 30 mg in a long‐acting gastrointestinal‐transport system formulation (n = 3289) or co‐amilozide (hydrochlorothiazide 25 g plus amiloride 2.5 mg; n = 3286). Dose titration was by dose doubling, and addition of atenolol 25 to 50 mg or enalapril 5 to 10 mg. | |
| Outcomes | Cardiovascular death, MI, heart failure, or stroke | |
| Notes | Study was conducted in 703 centres in 8 countries in Western Europe and Israel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo was administered at the same time of day. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 254 participants (132 and 122 participants in each group) were excluded after randomisation from centres due to misconduct, and were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
INVEST.
| Study characteristics | ||
| Methods | An international, multicentre study with a prospective, randomised, open blinded endpoint evaluation design. Mean follow‐up was 2.7 years. Intention‐to‐treat principle was used in analyses. | |
| Participants | A total of 22,576 hypertensive coronary artery disease (CAD) patients aged 50 years or older were enrolled. | |
| Interventions | Participants were randomly assigned to either verapamil sustained release (240 mg/d) (N = 11,267) or atenolol (50 mg/d) (N = 11,309). Administration of additional antihypertensive agents was allowed to achieve BP goals. | |
| Outcomes | Primary: all‐cause mortality, non‐fatal MI, or non‐fatal stroke Additional outcomes: time to most serious event, cardiovascular death, angina, cardiovascular hospitalisations, BP control, etc. |
|
| Notes | Study recruited participants at 862 sites in 14 countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An internet‐based management system automatically randomised each participant to a treatment strategy. |
| Allocation concealment (selection bias) | High risk | The randomisation result was stored in the central database, but drugs also might be open‐label because of the PROBE design. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It used the blinded endpoint design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
J‐MIC(B).
| Study characteristics | ||
| Methods | A prospective, randomised controlled clinical trial comparing the effect of nifedipine retard versus ACE inhibitors on the incidence of cardiac events and mortality due to cardiovascular disease. The follow‐up was 3 years, and analysis was done on an intention‐to‐treat basis. | |
| Participants | 1650 outpatients aged under 75 years with diagnoses of both hypertension and coronary artery disease | |
| Interventions | Participants in the nifedipine group received nifedipine retard (a long‐acting nifedipine formulation given at a dose of 10 to 20 mg twice daily in Japan) for 3 years, whilst participants in the ACE inhibitor group received an ACE inhibitor (enalapril at 5 to 10 mg, imidapril at 5 to 10 mg, or lisinopril at 10 to 20 mg, once daily as recommended in Japan) for 3 years. | |
| Outcomes | The primary endpoint was the overall incidence of cardiac events (cardiac death or sudden death, myocardial infarction, hospitalisation for angina pectoris or heart failure, serious arrhythmia, and coronary interventions). | |
| Notes | Participants were enrolled at 354 Japanese hospitals specialising in the management of cardiovascular disease between January 1994 and July 1997. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number sequence obtained from an external biostatistician was used for randomisation. |
| Allocation concealment (selection bias) | Low risk | The sealed‐envelope method was used for randomisation of the study drug. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded endpoint design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
MIDAS.
| Study characteristics | ||
| Methods | A multicentre, randomised, double‐blind, controlled clinical trial with a follow‐up of 3 years. All analyses were performed using the intention‐to‐treat approach. | |
| Participants | Enrolled participants (N = 883) all had hypertension (average diastolic BP from 90 to 115 mmHg). | |
| Interventions | Participants were randomised into 2 treatment groups: hydrochlorothiazide, 12.5 to 25 mg twice a day (n = 441) or isradipine, 2.5 to 5.0 mg twice a day (n = 442). If diastolic BP did not reach the planned goal with the highest dose of the study drug, open‐label enalapril was added. | |
| Outcomes | Mean maximum intima‐media thickness, and other findings of carotid artery and vascular events/procedures | |
| Notes | Study was conducted in 9 medical centre clinics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation process was stratified and blocked by clinic. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study was stated to be double‐blind, but method of blinding was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
NAGOYA.
| Study characteristics | ||
| Methods | A prospective, open‐label, randomised controlled trial with a follow‐up of 3.2 years. All analyses were performed using the intention‐to‐treat approach. | |
| Participants | A total of 1150 participants (women: 34%; mean age: 63 years; diabetes mellitus: 82%) were enrolled. Participants were aged between 30 and 75 years with both hypertension and glucose intolerance. | |
| Interventions | Participants were randomised into 2 treatment groups: valsartan 80 mg once daily (n = 575) or amlodipine 5 mg once daily (n = 575). Physicians could increase the respective dose until 160 mg or 10 mg daily after 4 weeks, and other antihypertensive drugs could be added after 8 weeks as needed. | |
| Outcomes | Primary outcome was a composite of acute myocardial infarction, stroke, coronary revascularisation, admission attributed to heart failure, or sudden cardiac death. | |
| Notes | Participants were recruited by 171 cardiologists only from 46 board‐certified medical centres and hospitals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed automatically by a host computer system using the minimisation method. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded endpoint design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Unclear risk | No other potential bias was found. |
NICS‐EH.
| Study characteristics | ||
| Methods | A randomised, double‐blind trial with a follow‐up of 5 years | |
| Participants | Patients ≥ 60 years of age with systolic BP of 160 to 220 mmHg and diastolic BP<115 mmHg were enrolled (N = 429). Participants were without history of cardiovascular complications. | |
| Interventions | Participants were randomly assigned to 20 mg of sustained‐release nicardipine hydrochloride twice daily (N = 215) or 2 mg of trichlormethiazide once daily (N = 214). Doubling of the dose was permitted if BP response was insufficient, but any other antihypertensive drugs were prohibited. | |
| Outcomes | Cardiovascular complications | |
| Notes | Study was conducted in Japan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated as double‐dummy, but details were not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data for withdrawn participants were not included using intention‐to‐treat analysis, but we were able to obtain the missing data to include in our review. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
NORDIL.
| Study characteristics | ||
| Methods | A prospective, randomised, open, blinded‐endpoint, multicentre, parallel‐group study. The mean follow‐up was 4.5 years, and 52 participants (0.5%) were lost to follow‐up. Analysis was done by intention‐to‐treat. | |
| Participants | A total of 10,881 participants, aged 50 to 74 years, with diastolic BP of 100 mmHg or more on 2 occasions, were enrolled. | |
| Interventions | Participants were randomised to a diltiazem‐based regimen (180 to 360 mg daily, N = 5410) or conventional antihypertensive treatment (N = 5471) with diuretics, β‐blockers, or both. Additional antihypertensive treatment could be given to any participant to lower diastolic BP to less than 90 mmHg. | |
| Outcomes | Stroke, MI, and other cardiovascular death | |
| Notes | Recruitment of participants was from 9 October 1992 to 31 October 1999 in 1032 health centres in Norway and Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | Method of concealment was not described, but risk of bias could be limited by strict randomisation and blinded endpoint assessment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded endpoint study, all endpoints were blinded before evaluation by the separate endpoint committee. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Unclear risk | In the diltiazem group, a diuretic or ‐blocker was added in step 3, and any other antihypertensive compound could be added as step 4. |
SHELL.
| Study characteristics | ||
| Methods | A randomised, controlled, multicentre trial conducted in outpatient clinics. Follow‐up visits were made at monthly intervals during the first 3 months after randomisation and thereafter after 6 months and every year for a maximum of 5 years (median 32 months). Data were analysed on an intention‐to‐treat basis by BETA Trial Center. | |
| Participants | Participants (N = 1882) were recruited if sitting systolic BP was 160 mmHg with a diastolic BP ≤ 95 mmHg. | |
| Interventions | Participants were randomly assigned to the administration of chlortalidone 12.5 mg/d (N = 940) or lacidipine 4 mg/d (N = 942). Increased dose of study drug and additional antihypertensive agents could be administered to help control blood pressure. | |
| Outcomes | Primary outcome was composite of cardiovascular and cerebrovascular events, including stroke, sudden death, MI, congestive heart failure, etc. | |
| Notes | Participants were recruited from 134 units located in northern, central, and southern Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was made by BETA Trial Center, Genoa (Italy), using a sequentially based criterion. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
STOP‐Hypertension‐2.
| Study characteristics | ||
| Methods | A prospective, randomised, open blinded endpoint trial with a follow‐up of at least 4 years. No participant was lost to follow‐up, and no participant refused to continue in the study. Analysis was by intention‐to‐treat and of only the first occurrence of each event in question. | |
| Participants | Participants must have hypertension (BP ≥ 180 mmHg systolic, ≥ 105 mmHg diastolic, or both), and were aged 70 to 84 years. | |
| Interventions | There were 3 groups, each of which was given a different class of drugs: conventional antihypertensive drugs (N = 2213) (atenolol 50 mg, metoprolol 100 mg, pindolol 5 mg, or fixed‐ratio hydrochlorothiazide 25 mg plus amiloride 2.5 mg daily), ACE inhibitors (N = 2205) (enalapril 10 mg or lisinopril 10 mg daily), or calcium antagonists (N = 2196) (felodipine 2.5 mg or isradipine 2.5 mg daily). Additional antihypertensive drugs were allowed if necessary. | |
| Outcomes | The major outcomes were cardiovascular death, other cardiovascular event, and blood pressure change. | |
| Notes | Study was conducted in 212 health centres in Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded endpoint design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal amongst the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
TOMHS.
| Study characteristics | ||
| Methods | A randomised, double‐blind, placebo‐controlled clinical trial. Participants were seen at least every 3 months for a median follow‐up of 4.4 years. All analyses were by treatment allocation (intention‐to‐treat). | |
| Participants | A total of 902 participants aged 45 to 69 years, with mild hypertension (diastolic BP was 90 to 99 mmHg at both of the first 2 eligibility visits and averaged 90 to 99 mmHg over the 3 eligibility visits) were included. | |
| Interventions | Sustained nutritional‐hygienic advice to all participants and increase physical activity. Participants were randomly allocated to take (1) placebo (n = 234); (2) chlortalidone (15 mg/d, n = 136); (3) acebutolol (400 mg/d, n = 132); (4) doxazosin mesylate (1 mg/d for 1 month, then 2 mg/d, n = 134); (5) amlodipine maleate (5 mg/d, n = 131); or (6) enalapril maleate (5 mg/d, n = 135). If BP was not well controlled, drug dose was doubled, followed by use of additional drugs when necessary. | |
| Outcomes | Systolic and diastolic BP. Data on morbidity and mortality outcomes were not provided for the different treatment arms. | |
| Notes | Study was conducted in 4 hypertension screening and treatment centres in the USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomisation scheme with stratification was used by clinical centre. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Active drugs and placebo administered to participants were prepared in identical capsule form. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal amongst the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
VALUE.
| Study characteristics | ||
| Methods | A prospective, multinational, double‐blind, randomised, active‐controlled, parallel‐group trial with a mean follow‐up time of 4.2 years. All endpoints and BP values were analysed using the intention‐to‐treat approach. | |
| Participants | Enrolled participants were 50 years of age or older, with treated or untreated (mean sitting systolic BP between 160 and 210 mmHg, and mean sitting diastolic BP of less than 115 mmHg) hypertension at baseline and combinations of cardiovascular risk factors and cardiovascular disease. Additional antihypertensive drugs excluding ARBs could be given to achieve BP goal. | |
| Interventions | Participants were randomised to either valsartan 80 mg (N = 7649) or amlodipine 5 mg (N = 7596). | |
| Outcomes | Time to first cardiac event, incidence of MI, heart failure and stroke, all‐cause mortality, and new‐onset diabetes | |
| Notes | Study was carried out in 31 countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list was computer generated and prepared centrally by the sponsor. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study medication was provided in externally indistinguishable capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 68 participants in 9 centres were excluded after randomisation because of good clinical practice deficiencies, and were not included in intention‐to‐treat analyses, which could result in bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
VART.
| Study characteristics | ||
| Methods | A multicentre, prospective, randomised, open‐label, blinded endpoint trial. The mean follow‐up period was 3.4 years. | |
| Participants | A total of 1021 participants were enrolled. Age ≥ 30 years and recent diagnosis of hypertension (systolic ≥ 140 mmHg or diastolic BP ≥ 90 mmHg, with the patient in a sitting position at a clinic) or previous treatment with antihypertensive agents. | |
| Interventions | Participants were randomised to either valsartan 80 mg (N = 510) or amlodipine 5 mg (N = 511) per day. The doses were increased to 160 or 10 mg per day, respectively, when necessary. | |
| Outcomes | The primary endpoint was a composite of all‐cause death, sudden death, cerebrovascular death, cardiac events, vascular events, and renal events. The secondary endpoints were effects on left ventricular hypertrophy, cardiac sympathetic nerve activity, and renal function. |
|
| Notes | The trial involved 92 medical facilities in Japan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random assignment of participants, data entry, and data collection were performed at the homepage originally produced for the trial, and the participants were assigned randomly to either the valsartan group or the amlodipine group with the minimisation. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded endpoint design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participants withdrew consent and 11 were lost to follow‐up in the valsartan group; 11 withdrew consent and 5 were lost to follow‐up in amlodipine group. An intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
VHAS.
| Study characteristics | ||
| Methods | A multicentre, randomised, double‐blind (for the first 6 months, open subsequently) parallel‐group trial lasting 2 years (prolonged to 4 years for the subgroup of participants evaluated by carotid ultrasonography) | |
| Participants | Inclusion criteria were essential hypertension (systolic BP when seated ≥ 160 mmHg and diastolic BP ≥ 95 mmHg measured at the end of a placebo run‐in period of 3 weeks), age of 40 to 65 years, of either sex. A total of 1414 hypertensive participants were enrolled. | |
| Interventions | The study included a run‐in period (3 weeks), a double‐blind‐treatment period (6 months, either 240 mg sustained release verapamil (n = 707) or 25 mg chlortalidone (n = 707) once a day), and an open‐treatment period (18 months); captopril was added to the treatment of non‐responding participants; free therapy of other drugs was permitted during follow‐up when necessary. | |
| Outcomes | BP reduction, heart rate, clinical safety, cardiovascular events, death, and intima‐media thickness | |
| Notes | Multicentre trial conducted in Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were equal between the treatment groups, and an intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported. |
| Other bias | Low risk | No other potential bias was found. |
PROBE: Prospective, Randomised, Open‐label, Blinded Endpoint
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abascal 1998 | Non‐randomised trial |
| Abe 2013 | Compared 2 kinds of CCBs, cilnidipine and amlodipine; no other classes of antihypertensive drugs were studied |
| ACCOMPLISH | Compared treatment with an angiotensin converting enzyme (ACE) inhibitor combined with amlodipine versus treatment with the same ACE inhibitor combined with a thiazide diuretic |
| Bakris 1996 | Only 52 participants included in trial. |
| Bakris 1997 | Only 34 participants included in trial. |
| BEAHIT | Compared co‐administration of a diuretic or calcium channel blocker (CCB) with an ACE inhibitor |
| Bhad 2011 | Non‐randomised trial |
| Calhoun 2013 | Compared the antihypertensive efficacy and safety of once‐daily triple therapy with amlodipine 10 mg, valsartan 320 mg, and hydrochlorothiazide 25 mg versus dual‐therapy combinations of these components Follow‐up lasted only 4 weeks and 8 weeks. |
| CASE‐J Ex | Another 3 years beyond the experimental period of the CASE‐J trial. No useful data could be extracted from this extended study for the current review. |
| Chen 2013 | A review that evaluated CCB versus placebo |
| Cicero 2012 | 162 participants were allocated to the combination of lercanidipine with ‐blockers, diuretics, ACE inhibitors, and angiotensin receptor blockers (ARBs). |
| COLM | Compared the cardiovascular effects of olmesartan, an ARB, combined with a CCB or a diuretic |
| DEMAND | Compared manidipine/delapril combination with delapril or placebo |
| DHCCP | Design was a combination of 2 case‐control studies and 2 longitudinal studies. |
| Espinel 1992 | Follow‐up lasted only 8 weeks. |
| FACTS | Only 96 participants were randomised to treatment in this study, which was less than the 100 randomised participants specified in the protocol for the current review. |
| FEVER | Compared the incidence of stroke and other cardiovascular events in hypertensive patients receiving a low‐dose diuretic and low‐dose calcium antagonist combination versus those receiving low‐dose diuretic monotherapy |
| GLANT | Follow‐up lasted for 12 months. |
| Gottdiener 1997 | Study primarily evaluated the reduction of left ventricular mass; follow‐up lasted only 1 year. |
| HOT | Participants were randomly assigned to groups with different target diastolic blood pressure instead of groups with different study drug. |
| Kereiakes 2012 | Follow‐up lasted only 12 weeks. |
| Kes 2003 | Compared 2 kinds of CCBs, nifedipine and amlodipine; no other classes of antihypertensive drugs were studied |
| Kim 2011 | Only 32 participants included in trial. |
| Kojima 2013 | Study concerned kidney‐protective effects of azelnidipine versus a diuretic in combination with olmesartan; follow‐up lasted only 6 months. |
| Lauria 2012 | 380 hypertensive participants with albuminuria < 200 µg/min were randomised to at least 3‐year treatment with manidipine (10 mg/day) plus delapril (30 mg/day), delapril (30 mg/day), or placebo. |
| Leon 1993 | Follow‐up lasted only 22 weeks. |
| Maharaj 1992 | Only 30 participants included in trial. |
| Mesci 2011 | Only 80 participants were randomised to treatment in this study, which was less than the 100 randomised participants specified in the protocol for the current review; follow‐up lasted only 6 months. |
| OSCAR | Compared the efficacy of ARB uptitration to an ARB plus CCB combination |
| Pahor 1995 | Prospective cohort study rather than a randomised controlled trial |
| Papademetriou 1997 | Study primarily evaluated the reduction of left ventricular mass; follow‐up lasted only 6 months. |
| PRESERVE | Study primarily evaluated the reduction of left ventricular mass; follow‐up lasted only 12 months. |
| Psaty 1995 | Population‐based case‐control study instead of a randomised design |
| Radevski 1999 | Only 96 participants included in trial. |
| Schneider 1991 | Study aimed to evaluate the effect of therapy on hypertensive urgencies; follow‐up was short. |
| STONE | Non‐randomised, placebo‐controlled trial |
| Syst‐China | Non‐randomised, placebo‐controlled trial |
| Syst‐Eur | Placebo‐controlled trial that did not compare CCBs with any other classes of drugs for hypertension |
| Van Leeuwen 1995 | Study primarily evaluated the comparative effects of diltiazem and lisinopril on left ventricular structure; follow‐up lasted only 6 months. |
| Weir 1990 | Follow‐up lasted only 8 weeks. |
| Wen 2011 | A total of 13,500 participants were randomly assigned to either low‐dose amlodipine + telmisartan group or amlodipine + diuretic group. |
| Zhang 2012 | Follow‐up lasted only 6 months. |
Differences between protocol and review
In the updated review, we added 'Summary of findings' tables and assessed the certainty of evidence for each outcome, which were not mentioned in the protocol.
We amended the 'Types of participants' section in the Methods, adding "participants with diabetes mellitus with a BP of more than 135/85 mmHg".
Contributions of authors
For the current version, Jiaying Zhu and Ning Chen selected and assessed studies. Jiaying Zhu drafted the review. Jiaying Zhu, Jie Zhou, and Mengmeng Ma were responsible for the inclusion or exclusion of trials and data extraction. Jiaying Zhu, Muke Zhou, and Jian Guo performed the analyses.
Cairong Zhu offered expert advice. Li He offered expert advice, reviewed the updated review, and was responsible for developing the review.
Sources of support
Internal sources
-
West China Hospital, Sichuan University, China
This project was supported by National Key R&D program of China (Nos. 2018YFC1311400 and 2018YFC1311401)
External sources
-
no, Other
no
Declarations of interest
Jiaying Zhu, Ning Chen, Muke Zhou, Jian Guo, Cairong Zhu, Jie Zhou, Mengmeng Ma, and Li He declare that they have no competing interests.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
AASK {published data only}
- Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001;285(21):2719-28. [DOI] [PubMed] [Google Scholar]
- Kusek JW, Lee JY, Smith DE, Milligan S, Faulkner M, Cornell CE, et al. Effect of blood pressure control and antihypertensive drug regimen on quality of life: The African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Controlled Clinical Trials 1996;16:40S-6S. [DOI] [PubMed] [Google Scholar]
- Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. American Journal of Kidney Diseases 2006;48(5):739-51. [DOI] [PubMed] [Google Scholar]
- Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288(19):2421-31. [DOI] [PubMed] [Google Scholar]
- Wright JT, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, et al. Design and baseline characteristics of participants in the African American Study of Kidney disease and hypertension (AASK) pilot study. Controlled Clinical Trials 1996;17(4 Suppl):3S-16S. [DOI] [PubMed] [Google Scholar]
ABCD {published data only}
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645-52. [DOI] [PubMed] [Google Scholar]
- Savage S, Johnson Nagel N, Estacio RO, Feig PU, MacCarthy EP, Lukken NJ, et al. The ABCD (Appropriate Blood Pressure Control in Diabetes) trial: rationale and design of a trial of hypertension control (moderate or intensive) in type II diabetes. Online Journal of Current Clinical Trials 1993 Nov 24:Doc No 104. [PubMed]
- Schrier R, Estacio R. Additional follow-up from the ABCD Trial in Patients with Type 2 Diabetes and Hypertension. New England Journal of Medicine 2000;343:1969. [DOI] [PubMed] [Google Scholar]
ALLHAT {published data only}
- Cushman WC, Davis BR, Pressel SL, Cutler JA, Einhorn PT, Ford CE, et al. Mortality and morbidity during and after the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Journal of Clinical Hypertension 2012;14(1):20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT Jr, Cushman WC, et al. Rationale and design for the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). American Journal of Hypertension 1996;9(4 Pt 1):342-60. [DOI] [PubMed] [Google Scholar]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981-97. [DOI] [PubMed] [Google Scholar]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. JAMA 2002;288:2998-3007. [DOI] [PubMed] [Google Scholar]
ASCOT‐BPLA {published data only}
- Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOTBPLA): a multicentre randomised controlled trial. Lancet 2005;366:895-906. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. Journal of Hypertension 2001;19(6):1139-47. [DOI] [PubMed] [Google Scholar]
CASE‐J {published data only}
- Fukui T, Rahman M, Hayashi K, Takeda K, Higaki J, Sato T, et al. Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial of cardiovascular events in high-risk hypertensive patients: rationale, design, and methods. Hypertension Research 2003;26(12):979-90. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Nakao K, Fukui T, Fukiyama K, Ueshima K, Oba K, et al. Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: Candesartan Antihypertensive Survival Evaluation in Japan trial. Hypertension 2008;51(2):393-8. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Ueshima K, Nakao K, Fukiyama K, Oba K, Yasuno S, et al. Long-term effects of candesartan and amlodipine on cardiovascular morbidity and mortality in Japanese high-risk hypertensive patients: the Candesartan Antihypertensive Survival Evaluation in Japan Extension Study (CASE-J Ex). Hypertension Research 2011;34(12):1295-301. [DOI] [PubMed] [Google Scholar]
CONVINCE {published data only}
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 2003;289(16):2073-82. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH Jr, et al. Baseline characteristics and early blood pressure control in the CONVINCE trial. Hypertension 2001;37(1):12-8. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH Jr, et al. Rationale and design for the Controlled Onset Verapamil Investigation of Cardiovascular Endpoints (CONVINCE) Trial. Controlled Clinical Trials 1998;19(4):370-90. [DOI] [PubMed] [Google Scholar]
ELSA {published data only}
- Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palù C, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002;106(19):2422-7. [DOI] [PubMed] [Google Scholar]
FACET {published data only}
- Tatti P, Pahor M, Byington RP, Di Mauro P, Guarisco R, Strollo G, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care 1998;21(4):597-603. [DOI] [PubMed] [Google Scholar]
HOMED‐BP {published data only}
- Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertension Research 2012;35(11):1102-10. [DOI] [PubMed] [Google Scholar]
IDNT {published data only}
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Annals of Internal Medicine 2003;138(7):542-9. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine 2001;245(12):851-60. [DOI] [PubMed] [Google Scholar]
- Rodby RA, Rohde RD, Clarke WR, Hunsicker LG, Anzalone DA, Atkins RC, et al. The Irbesartan Type II Diabetic Nephropathy Trial: study design and baseline patient characteristics. Nephrology Dialysis Transplantation 2000;15(4):487-97. [DOI] [PubMed] [Google Scholar]
INSIGHT {published data only}
- Brown MJ, Palmer CR, Castaigne A, Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000;356(9227):366-72. [DOI] [PubMed] [Google Scholar]
INVEST {published data only}
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290(21):2805-16. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Handberg-Thurmond E, Marks RG, Conlon M, Cooper-DeHoff R, Volkers P, et al. Rationale and design of the International Verapamil SR/Trandolapril Study (INVEST). Journal of the American College of Cardiology 1998;32(5):1228-37. [DOI] [PubMed] [Google Scholar]
J‐MIC(B) {published data only}
- Yui Y, Sumiyoshi T, Kodama K, Hirayama A, Nonogi H, Kanmatsuse K, et al. Comparison of nifedipine retard with angiotensin converting enzyme inhibitors in Japanese hypertensive patients with coronary artery disease: the Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B) randomized trial. Hypertension Research 2004;27(3):181-91. [DOI] [PubMed] [Google Scholar]
MIDAS {published data only}
- Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa-Terris M, Carr AA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS): a randomized controlled trial. JAMA 1996;276(10):785-91. [PubMed] [Google Scholar]
- Borhani NO, Miller ST, Brugger SB, Schnaper HW, Craven TE, Bond MG, et al. MIDAS: hypertension and atherosclerosis, a trial of the effect of antihypertensive drug treatment on atherosclerosis. Journal of Cardiovascular Pharmacology 1992;19(Suppl 3):16-20. [PubMed] [Google Scholar]
- Furberg C, Byington R, Borhani N. Design features: Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). American Journal of Medicine 1989;86(Suppl 4A):37-9. [DOI] [PubMed] [Google Scholar]
NAGOYA {published data only}
- Matsushita K, Muramatsu T, Kondo T, Maeda K, Shintani S, Murohara T, et al. Rationale and design of the NAGOYA HEART Study: comparison between valsartan and amlodipine regarding morbidity and mortality in patients with hypertension and glucose intolerance. Journal of Cardiology 2010;56(1):111-7. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Matsushita K, Yamashita K, Kondo T, Maeda K, Shintani S, et al. Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Hypertension 2012;59(3):580-6. [DOI] [PubMed] [Google Scholar]
NICS‐EH {published data only}
- Kuramoto K, National Intervention Cooperative Study Group. Treatment of elderly hypertensive in Japan: National Intervention Cooperative Study in Elderly Hypertensives. Journal of Hypertension 1994;12(Suppl 6):S35-40. [PubMed] [Google Scholar]
- National Intervention Cooperative Study in Elderly Hypertensives Study Group. Randomized double-blind comparison of a calcium antagonist and a diuretic in elderly hypertensives. Hypertension 1999;34(5):1129-33. [PubMed] [Google Scholar]
NORDIL {published data only}
- Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomised trial of effects of calcium antagonists compared with diuretics and β-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359-65. [DOI] [PubMed] [Google Scholar]
- Hedner T. Progress report on the Nordic diltiazem study (NORDIL): an outcome study in hypertensive patients. Blood Pressure 1999;8(5-6):296-9. [DOI] [PubMed] [Google Scholar]
- NORDIL Study Group. The Nordic Diltiazem Study (NORDIL). A prospective intervention trial of calcium antagonist therapy in hypertension. Blood Pressure 1993;2:312-21. [DOI] [PubMed] [Google Scholar]
SHELL {published data only}
- Malacco E, Mancia G, Rappelli A, Menotti A, Zuccaro MS, Coppini A, et al. Treatment of isolated systolic hypertension: The SHELL Study results. Blood Pressure 2003;12:160-7. [DOI] [PubMed] [Google Scholar]
STOP‐Hypertension‐2 {published data only}
- Dahlöf B, Hansson L, Lindholm LH, Scherstén B, Wester PO, Ekbom T, et al. STOP-Hypertension-2: a prospective intervention trial of "newer" versus "older" treatment alternatives in old patients with hypertension. Blood Pressure 1993;2:136-41. [DOI] [PubMed] [Google Scholar]
- Hansson L, Lindholm LH, Ekbom T, Dahlöf B, Lanke J, Scherstén B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-6. [DOI] [PubMed] [Google Scholar]
- Lindholm LH, Hansson L, Dahlöf B, Ekbom T, Hedner T, De Faire U, et al. The Swedish Trial in Old Patients with Hypertension (STOP-Hypertension-2): a progress report. Blood Pressure 1996;5:300-4. [DOI] [PubMed] [Google Scholar]
TOMHS {published data only}
- Mascioli SR, Grimm RH Jr, Neaton JD, Stamler J, Prineas RJ, Cutler JA, et al. The Treatment of Mild Hypertension Study (TOMHS): characteristics of participants at baseline. American Journal of Cardiology 1990;66(9):32C-5C. [DOI] [PubMed] [Google Scholar]
- Neaton JD, Grimm RH Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, et al. Treatment of Mild Hypertension Study: final results. JAMA 1993;270:713-24. [PubMed] [Google Scholar]
- Stamler J, Prineas RJ, Neaton JD, Grimm RH, McDonald RH, Schnaper HW, et al. Background and design of the new US trial on diet and drug treatment of 'mild' hypertension (TOMHS). American Journal of Cardiology 1987;59(14):51G-60G. [DOI] [PubMed] [Google Scholar]
- The Treatment of Mild Hypertension Research Group. The Treatment of Mild Hypertension Study. A randomized, placebo-controlled trial of a nutritional-hygienic regimen along with various drug monotherapies. Archives of Internal Medicine 1991;151:1413-23. [DOI] [PubMed] [Google Scholar]
VALUE {published data only}
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363(9426):2022-31. [DOI] [PubMed] [Google Scholar]
- Mann J, Julius S. The Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial of cardiovascular events in hypertension. Rationale and design. Blood Pressure 1998;7(3):176-83. [DOI] [PubMed] [Google Scholar]
VART {published data only}
- Narumi H, Takano H, Shindo S, Fujita M, Mizuma H, Kuwabara Y, et al. Effects of valsartan and amlodipine on cardiorenal protection in Japanese hypertensive patients: the Valsartan Amlodipine Randomized Trial. Hypertension Research 2011;34(1):62-9. [DOI] [PubMed] [Google Scholar]
VHAS {published data only}
- Rosei EA, Dal Palù C, Leonetti G, Magnani B, Pessina A, Zanchetti A. Clinical results of the Verapamil in Hypertension and Atherosclerosis Study. Journal of Hypertension 1997;15(11):1337-44. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Rosei EA, Dal Palù C, Leonetti G, Magnani B, Pessina A. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. Journal of Hypertension 1998;16(11):1667-76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abascal 1998 {published data only}
- Abascal VM, Larson MG, Evans JC, Blohm AT, Poli K, Levy D, et al. Calcium antagonists and mortality risk in men and women with hypertension in the Framingham Heart Study. Archives of Internal Medicine 1998;158(17):1882-6. [DOI] [PubMed] [Google Scholar]
Abe 2013 {published data only}
- Abe M, Maruyama N, Suzuki H, Inoshita A, Yoshida Y, Okada K, et al. L/N-type calcium channel blocker cilnidipine reduces plasma aldosterone, albuminuria, and urinary liver-type fatty acid binding protein in patients with chronic kidney disease. Heart Vessels 2013;28:480-9. [DOI] [PubMed] [Google Scholar]
ACCOMPLISH {published data only}
- Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. New England Journal of Medicine 2008;359(23):2417-28. [DOI] [PubMed] [Google Scholar]
Bakris 1996 {published data only}
- Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney International 1996;50(5):1641-50. [DOI] [PubMed] [Google Scholar]
Bakris 1997 {published data only}
- Bakris GL, Mangrum A, Copley JB, Vicknair N, Sadler R. Effect of calcium channel or β-blockade on the progression of diabetic nephropathy in African Americans. Hypertension 1997;29:744-50. [DOI] [PubMed] [Google Scholar]
BEAHIT {published data only}
- Xue C, Zhou C, Yang B, Lv J, Dai B, Yu S, et al. Comparison of efficacy and safety between benidipine and hydrochlorothiazide in fosinopril-treated hypertensive patients with chronic kidney disease: protocol for a randomised controlled trial. BMJ Open 2017;7(2):e013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhad 2011 {published data only}
- Bhad P, Ayalasomayajula S, Karan R, Leon S, Riviere GJ, Sunkara G, et al. Evaluation of pharmacokinetic interactions between amlodipine, valsartan, and hydrochlorothiazide in patients with hypertension. Journal of Clinical Pharmacology 2011;51:933-42. [DOI] [PubMed] [Google Scholar]
Calhoun 2013 {published data only}
- Calhoun DA, Lacourcière Y, Crikelair N, Jia Y, Glazer RD. Effects of demographics on the antihypertensive efficacy of triple therapy with amlodipine, valsartan, and hydrochlorothiazide for moderate to severe hypertension. Current Medical Research and Opinion 2013;29:901-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
CASE‐J Ex {published data only}
- Ogihara T, Ueshima K, Nakao K, Fukiyama K, Oba K, Yasuno S, et al. Long-term effects of candesartan and amlodipine on cardiovascular morbidity and mortality in Japanese high-risk hypertensive patients: the Candesartan Antihypertensive Survival Evaluation in Japan Extension Study (CASE-J Ex). Hypertension Research 2011;34(12):1295-301. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen GJ, Yang MS. The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: a meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PLoS ONE 2013;8(3):e57854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicero 2012 {published data only}
- Cicero AF, Gerocarni B, Rosticci M, Borghi C. Blood pressure and metabolic effect of a combination of lercanidipine with different antihypertensive drugs in clinical practice. Clinical & Experimental Hypertension (New York) 2012;34:113-7. [DOI] [PubMed] [Google Scholar]
COLM {published data only}
- Ogihara T, Saruta T, Rakugi H, Saito I, Shimamoto K, Matsuoka H, et al. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: a randomized, controlled trial. Journal of Hypertension 2014;32(10):2054-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
DEMAND {published data only}
- Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S, et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension 2011;58(5):776-83. [DOI] [PubMed] [Google Scholar]
DHCCP {published data only}
- Bulpitt CJ, Palmer AJ, Beevers DG, Coles EC, Ledingham JG, Petrie JC, et al. Calcium channel blockers and cardiac mortality in the treatment of hypertension: a report from the Department of Health Hypertension Care Computing Project (DHCCP). Journal of Human Hypertension 1997;11:205-11. [DOI] [PubMed] [Google Scholar]
Espinel 1992 {published data only}
- Espinel CH, Bruner DE, Davis JR, Williams JL. Enalapril and verapamil in the treatment of isolated systolic hypertension in the elderly. Clinical Therapeutics 1992;14:835-44. [PubMed] [Google Scholar]
FACTS {published data only}
- Pahor M, Franse LV, Deitcher SR, Cushman WC, Johnson KC, Shorr RI, et al. Fosinopril versus Amlodipine Comparative Treatments Study: a randomized trial to assess effects on plasminogen activator inhibitor-1. Circulation 2002;105:457-61. [DOI] [PubMed] [Google Scholar]
FEVER {published data only}
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A, et al. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. Journal of Hypertension 2005;23(12):2157-72. [DOI] [PubMed] [Google Scholar]
GLANT {published data only}
- The GLANT Study Group. A 12-month comparison of ACE inhibitor and CA antagonist therapy in mild to moderate essential hypertension - The GLANT Study. Study Group on Long-term Antihypertensive Therapy. Hypertension Research 1995;18(3):235-44. [PubMed] [Google Scholar]
Gottdiener 1997 {published data only}
- Gottdiener JS, Reda DJ, Williams DW, Materson BJ, Cushman W, Anderson RJ. Effect of single-drug therapy on reduction of left ventricular mass in mild to moderate hypertension. Circulation 1997;95:2007-14. [DOI] [PubMed] [Google Scholar]
HOT {published data only}
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998;351:1755-62. [DOI] [PubMed] [Google Scholar]
Kereiakes 2012 {published data only}
- Kereiakes DJ, Chrysant SG, Izzo JL Jr, Littlejohn T 3rd, Melino M, Lee J, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double-blind, parallel-group TRINITY study. Cardiovascular Diabetology 2012;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kes 2003 {published data only}
- Kes S, Caglar N, Canberk A, Deger N, Demirtas M, Dortlemez H, et al. Treatment of mild-to-moderate hypertension with calcium channel blockers: a multicentre comparison of once-daily nifedipine GITS with once-daily amlodipine. Current Medical Research and Opinion 2003;19:226-37. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim CJ, Joe BH. Renin-guided versus routine treatment in young untreated hypertension: a randomized comparative pilot study. Journal of Hypertension 2011;29:e58-9. [Google Scholar]
Kojima 2013 {published data only}
- Kojima M, Okubo S, Mizubayashi R, Isaka N, Machida H, Okamoto S, et al. Kidney-protective effects of azelnidipine versus a diuretic in combination with olmesartan in hypertensive patients with diabetes and albuminuria: a randomized study. Nephrology Dialysis Transplantation 2013;28:1802-10. [DOI] [PubMed] [Google Scholar]
Lauria 2012 {published data only}
- Lauria G, Sghirlanzoni A. Effects of manidipine and delapril on type 2 diabetic neuropathy. A randomized, double-blind, placebo-controlled trial (the DEMAND study). Journal of the Peripheral Nervous System 2012;17:S30. [Google Scholar]
Leon 1993 {published data only}
- Leon AS. Efficacy and safety of enalapril versus extended-release nifedipine for the treatment of mild-to-moderate essential hypertension: A multicenter 22-week study. Clinical Therapeutics 1993;15:1094-107. [PubMed] [Google Scholar]
Maharaj 1992 {published data only}
- Maharaj B, Byl K. A comparison of the acute hypotensive effects of two different doses of nifedipine. American Heart Journal 1992;124(3):720-5. [DOI] [PubMed] [Google Scholar]
Mesci 2011 {published data only}
- Mesci B, Tekin M. Are all fixed dose combinations equally effective in blood pressure control? The analysis of four different fixed dose antihypertensive combinations. Obesity Reviews 2011;12(7):568. [Google Scholar]
OSCAR {published data only}
- Kim-Mitsuyama S, Ogawa H, Matsui K, Jinnouchi T, Jinnouchi H, Arakawa K. An angiotensin II receptor blocker-calcium channel blocker combination prevents cardiovascular events in elderly high-risk hypertensive patients with chronic kidney disease better than high-dose angiotensin II receptor blockade alone. Kidney International 2013;83(1):167-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pahor 1995 {published data only}
- Pahor M, Guralnik JM, Corti MC, Foley DJ, Carbonin P, Havlik RJ. Long-term survival and use of antihypertensive medications in older persons. Journal of the American Geriatrics Society 1995;43(11):1191-7. [DOI] [PubMed] [Google Scholar]
Papademetriou 1997 {published data only}
- Papademetriou V, Gottdiener JS, Narayan P, Cushman WG, Zachariah PK, Gottdiener PS, et al. Hydrochlorothiazide is superior to isradipine for reduction of left ventricular mass: results of a multicenter trial. Journal of the American College of Cardiology 1997;30:1802-8. [DOI] [PubMed] [Google Scholar]
PRESERVE {published data only}
- Devereux RB, Palmieri V, Sharpe N, De Quattro V, Bella JN, Simone G, et al. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension. The Prospective Randomized Enalapril Study Evaluating Regression of Ventricular Enlargement (PRESERVE) trial. Circulation 2001;104:1248-54. [DOI] [PubMed] [Google Scholar]
Psaty 1995 {published data only}
- Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA 1995;274:620-5. [PubMed] [Google Scholar]
Radevski 1999 {published data only}
- Radevski I, Skudicky D, Candy G, Sathekge S, Strugo V, Sareli P. Antihypertensive monotherapy with nisoldipine CC is superior to enalapril in black patients with severe hypertension. American Journal of Hypertension 1999;12:194-203. [DOI] [PubMed] [Google Scholar]
Schneider 1991 {published data only}
- Schneider E, Jennings A, Opie L. Captopril, nifedipine and their combination for therapy of hypertensive urgencies. South African Medical Journal 1991;80(6):265-70. [PubMed] [Google Scholar]
STONE {published data only}
- Gong L, Zhang W, Zhu Y, Zhu J, Kong D, Pagé V, et al. Shanghai trial of nifedipine in the elderly (STONE). Journal of Hypertension 1996;14:1237-45. [DOI] [PubMed] [Google Scholar]
Syst‐China {published data only}
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. Journal of Hypertension 1998;16:1823-9. [DOI] [PubMed] [Google Scholar]
Syst‐Eur {published data only}
- Amery A, Birkenhäger W, Bulpitt CJ, Clément D, De Leeuw P, Dollery CT, et al. Syst-Eur: a multicentre trial on the treatment of isolated systolic hypertension in the elderly: objectives, protocol, and organization. Aging Clinical and Experimental Research 1991;3:287–302. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757-64. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Fagard RH, Birkenhäger WH, Arabidze G, Babeanu S, et al. Calcium channel blockade and cardiovascular prognosis in the European trial on isolated systolic hypertension. Hypertension 1998;32:410-6. [DOI] [PubMed] [Google Scholar]
Van Leeuwen 1995 {published data only}
- Leeuwen JT, Smit AJ, May JF, ten Berge BS, Hamer HP, Havinga TK, et al. Comparative effects of diltiazem and lisinopril on left ventricular structure and filling in mild-to-moderate hypertension. Journal of Cardiovascular Pharmacology 1995;26:983-9. [DOI] [PubMed] [Google Scholar]
Weir 1990 {published data only}
- Weir MR, Cassidy CA, Hall PS, Lancaster A, Schubert C, Urick A, et al. Efficacy and tolerability of enalapril and sustained-release verapamil in older patients with mild to moderate essential hypertension. Clinical Therapeutics 1990;12:139-48. [PubMed] [Google Scholar]
Wen 2011 {published data only}
- Wen W, Liyuan M, Dingliang Z, Shuguang L, Shuping M, Xinhua T, al. Chinese hypertension intervention efficacy (CHIEF) study: randomized controlled trial of initial combination CCB-based antihypertensive in patients with hypertension to reduce cardiovascular events. Journal of Hypertension 2011;29:e17-8. [Google Scholar]
Zhang 2012 {published data only}
- Zhang Y, Zhang X, Liu L, Wang Y, Tang X, Zanchetti A, et al. Higher cardiovascular risk and impaired benefit of antihypertensive treatment in hypertensive patients requiring additional drugs on top of randomized therapy: is adding drugs always beneficial? Journal of Hypertension 2012;30:2202-12. [DOI] [PubMed] [Google Scholar]
Additional references
Angeli 2004
- Angeli F, Verdecchia P, Reboldi GP, Gattobigio R, Bentivoglio M, Staessen JA, et al. Calcium channel blockade to prevent stroke in hypertension: a meta-analysis of 13 studies with 103,793 subjects. American Journal of Hypertension 2004;17:817-22. [DOI] [PubMed] [Google Scholar]
Black 2004
- Black HR. Calcium channel blockers in the treatment of hypertension and prevention of cardiovascular disease: results from major clinical trials. Clinical Cornerstone 2004;6(4):53-66. [DOI] [PubMed] [Google Scholar]
BPLTTC 2000
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood pressure-lowering drugs: results of prospectively designed overviews of randomized trials. Lancet 2000;356:1955-64. [DOI] [PubMed] [Google Scholar]
BPLTTC 2003
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure lowering regimens on major cardiovascular events: results of prospectively designed overviews of randomised trials. Lancet 2003;362:1527-35. [DOI] [PubMed] [Google Scholar]
Cheng 2014
- Cheng S, Claggett B, Correia AW, Shah AM, Gupta DK, Skali H, et al. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation 2014;130:820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chobanian 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [DOI] [PubMed] [Google Scholar]
Ezzati 2002
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002 Nov 2;360(9343):1347-60. [DOI] [PubMed] [Google Scholar]
Fergusson 2002
- Fergusson D, Aaron S, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2015
- GBD 2013 Risk Factors Collaborators, Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2287-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haller 2008
- Haller H. Effective management of hypertension with dihydropyridine calcium channel blocker-based combination therapy in patients at high cardiovascular risk. International Journal of Clinical Practice 2008;62:781-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansson 1992
- Hansson L, Hedner T, Dahlöf B. Prospective Randomized Open Blinded End-point (PROBE) study. A novel design for intervention trials. Blood Pressure 1992;1:113-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
JNC‐8
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507-20. [DOI] [PubMed] [Google Scholar]
Kannel 1991
- Kannel WB, Wilson PW, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. American Heart Journal 1991;121:1268-73. [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancia 2007
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2007;28(12):1462-536. [DOI] [PubMed] [Google Scholar]
Opie 2000
- Opie LH, Yusuf S, Kübler W. Current status of safety and efficacy of calcium channel blockers in cardiovascular diseases: a critical analysis based on 100 studies. Progress in Cardiovascular Diseases 2000;43:171-96. [DOI] [PubMed] [Google Scholar]
Opie 2002
- Opie LH, Schall R. Evidence-based evaluation of calcium channel blockers for hypertension: equality of mortality and cardiovascular risk relative to conventional therapy. Journal of the American College of Cardiology 2002;39:315-22. [DOI] [PubMed] [Google Scholar]
Palmer 2015
- Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015;385(9982):2047-56. [DOI] [PubMed] [Google Scholar]
Reboussin 2017
- Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, et al. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017 Nov 13 [Epub ahead of print]. [DOI: 10.1161/HYP.0000000000000067] [DOI]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group, available at cccrg.cochrane.org/author-resources. Version 3.0. 1 December 2016 (accessed prior to 2 January 2020).
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and‘Summary of findings' tables. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011, Available from www.cochrane-handbook.org. [Google Scholar]
Staessen 2003
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview. Journal of Hypertension 2003;21:1055-76. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2015
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs - overview and meta-analyses. Journal of Hypertension 2015;33(2):195-211. [DOI] [PubMed] [Google Scholar]
Triggle 2007
- Triggle DJ. Calcium channel antagonists: clinical uses - past, present and future. Biochemical Pharmacology 2007;74:1-9. [DOI] [PubMed] [Google Scholar]
Turnbull 2005
- Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Archives of Internal Medicine 2005;165(12):1410-9. [DOI] [PubMed] [Google Scholar]
Verdecchia 2005
- Verdecchia P, Reboldi G, Angeli F, Gattobigio R, Bentivoglio M, Thijs L, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension 2005;46:386-92. [DOI] [PubMed] [Google Scholar]
Whelton 2018
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71(6):1269-324. [DOI] [PubMed] [Google Scholar]
Williams 2018
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018;39(33):3021-104. [DOI] [PubMed] [Google Scholar]
Wiysonge 2007
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD002003. [DOI: 10.1002/14651858.CD002003.pub2] [DOI] [PubMed] [Google Scholar]
Wright 2018
- Wright JM, Musini VM. First-line drugs for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD001841. [DOI: 10.1002/14651858.CD001841.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2016
- Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387(10017):435-43. [DOI] [PubMed] [Google Scholar]
Zanchetti 2005
- Zanchetti A. Evidence-based medicine in hypertension: what type of evidence? Journal of Hypertension 2005;23:1113-20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2010
- Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No: CD003654. [DOI: 10.1002/14651858.CD003654.pub4] [DOI] [PubMed] [Google Scholar]
Pahor 2000
- Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Cavazzini C, et al. Health outcomes associated with calcium antagonists compared with other first-line antihypertensive therapies: a meta-analysis of randomised controlled trials. Lancet 2000;356(9246):1949-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


