This secondary analysis of a randomized clinical trial determines whether evoked compound action potentials–controlled, closed-loop spinal cord stimulator is associated with better outcomes compared with fixed-output, open-loop spinal cord stimulator at 24 months following implant.
Key Points
Question
Is a novel closed-loop spinal cord stimulator (SCS) that measures and adjusts to neural response associated with greater pain reduction compared with a fixed-output, open-loop SCS system at 24 months?
Findings
In this secondary analysis of a double-blind randomized clinical trial, closed-loop SCS delivered higher, more consistent neural response within the prescribed therapeutic window and demonstrated superior long-term improvements in pain relief and patient-reported outcomes, with meaningful opioid reduction.
Meaning
Closed-loop SCS sustained the previously published, superior pain relief at 3 and 12 months, suggesting it may be an effective long-term therapy to alleviate chronic pain, improve quality of life, and potentially reduce opioid use.
Abstract
Importance
Chronic pain is debilitating and profoundly affects health-related quality of life. Spinal cord stimulation (SCS) is a well-established therapy for chronic pain; however, SCS has been limited by the inability to directly measure the elicited neural response, precluding confirmation of neural activation and continuous therapy. A novel SCS system measures the evoked compound action potentials (ECAPs) to produce a real-time physiological closed-loop control system.
Objective
To determine whether ECAP-controlled, closed-loop SCS is associated with better outcomes compared with fixed-output, open-loop SCS at 24 months following implant.
Design, Setting, and Participants
The Evoke study was a double-blind, randomized, controlled, parallel arm clinical trial with 36 months of follow-up. Participants were enrolled from February 2017 to 2018, and the study was conducted at 13 US investigation sites. SCS candidates with chronic, intractable back and leg pain refractory to conservative therapy, who consented, were screened. Key eligibility criteria included overall, back, and leg pain visual analog scale score of 60 mm or more; Oswestry Disability Index score of 41 to 80; stable pain medications; and no previous SCS. Analysis took place from October 2020 to April 2021.
Interventions
ECAP-controlled, closed-loop SCS was compared with fixed-output, open-loop SCS.
Main Outcomes and Measures
Reported here are the 24-month outcomes of the trial, which include all randomized patients in the primary and safety analyses. The primary outcome was a reduction of 50% or more in overall back and leg pain assessed at 3 and 12 months (previously published).
Results
Of 134 randomized patients, 65 (48.5%) were female and the mean (SD) age was 55.2 (10.6) years. At 24 months, significantly more closed-loop than open-loop patients were responders (≥50% reduction) in overall pain (53 of 67 [79.1%] in the closed-loop group; 36 of 67 [53.7%] in the open-loop group; difference, 25.4% [95% CI, 10.0%-40.8%]; P = .001). There was no difference in safety profiles between groups (difference in rate of study-related adverse events: 6.0 [95% CI, −7.8 to 19.7]). Improvements were also observed in health-related quality of life, physical and emotional functioning, and sleep, in parallel with opioid reduction or elimination. Objective neurophysiological measurements substantiated the clinical outcomes and provided evidence of activation of inhibitory pain mechanisms.
Conclusions and Relevance
ECAP-controlled, closed-loop SCS, which elicited a more consistent neural response, was associated with sustained superior pain relief at 24 months, consistent with the 3- and 12-month outcomes.
Introduction
Low back pain with or without leg pain has a global prevalence of 7.5% and is the leading cause of years lived with disability.1 The detrimental consequences of chronic pain on functional ability and quality of life are well known.2 Spinal cord stimulation (SCS) is an established treatment strategy for managing chronic pain. However, until recently, the rigor of evidence for SCS efficacy has been lacking (eFigures 1 and 2 and eTable 1 in Supplement 1).3 Furthermore, there have been no objective measures of SCS therapy delivery or adherence owing to the inability to continuously measure spinal cord electrical activity in vivo.
SCS therapy entails implanting electrodes in the dorsal epidural space over the dorsal columns of the spinal cord. Electric stimulation is delivered using preprogrammed parameters (ie, pulse duration, current amplitude, and frequency) to activate the spinal cord.4 SCS targets Aβ sensory fibers in the dorsal columns, the activation of which produces analgesia through subsequent modulation of pain pathways in spinal gray matter.5,6 Extracellular stimulation preferentially activates nerve axons over soma, larger-diameter axons over smaller diameter, and elements closer to the stimulating electrode over those more distant.7 Therefore, SCS preferentially activates large-diameter, myelinated fibers in the dorsal columns over more distant, smaller neurons in the dorsal horn or elsewhere in the spinal cord. Physiological functions such as breathing, heartbeat, and changing posture alters the distance between the spinal cord target fibers and epidural SCS electrodes.8 Therefore, the number of nerve fibers activated by fixed-output, open-loop stimulation continually changes, resulting in inconsistent therapy delivery (ie, understimulation or overstimulation) as the spinal cord moves in and out of the unchanged electric field.9
To overcome these limitations, a novel SCS system has the unique ability to measure and adjust stimulation dose in response to in-vivo human spinal cord electrophysiology in real time for each delivered stimulation pulse. Evoked compound action potentials (ECAPs) are a measure of the neural response elicited by electrical stimulation; they are the summation of action potentials from multiple nerve fibers activated by a given stimulation pulse. ECAPs may be used to confirm activation of targeted fibers (ie, those involved in modulation of pain inhibition pathways) and provide evidence of therapy delivery and adherence. Furthermore, this system can use ECAP recordings to deliver closed-loop stimulation, wherein the strength of each stimulus is adjusted automatically to maintain a consistent neural response, controlling activation of targeted fibers. When operating in closed-loop mode, the system responds in real time to correct for variations in spinal cord activation that occur from cardiorespiratory cycles or body movement. Recording and adjustment occur at the same rate as the stimulation frequency; for example, at a stimulation rate of 50 Hz, the system will make 50 ECAP recordings and subsequent stimulation output adjustments per second, totaling more than 4 million adjustments daily to maintain a consistent neural response (ie, ECAP amplitude) at the level prescribed by the clinician.
This system is being evaluated in a double-blind, randomized clinical trial. The published 3- and 12-month outcomes demonstrated superiority of ECAP-controlled closed-loop SCS over fixed-output, open-loop SCS (fixed dose) in the treatment of chronic back and leg pain, including failed back surgery syndrome and nonsurgical refractory pain (NCT02924129).10 Herein we present the long-term, 2-year, clinical and quality of life results of this study to provide evidence of the longevity and durability of this therapy.
Methods
Study Design
This pivotal, multicenter, double-blind, parallel-arm, randomized clinical trial was conducted at 13 investigation sites throughout the United States under an Investigational Device Exemption to gain US Food and Drug Administration approval. The protocol (published previously11 and available in Supplement 2, along with the statistical analysis plan) was approved by each participating site’s institutional review board. This study was conducted in accordance with US Food and Drug Administration regulatory requirements, Good Clinical Practice, and the ethical principles of the Declaration of Helsinki.12
Participants
SCS candidates with chronic, intractable back and leg pain with a minimum visual analog scale score of 60 mm or higher (where 100 mm indicates the worst imaginable pain) refractory to conservative therapy, who provided written informed consent, were screened for enrollment. An independent medical monitor confirmed consistent interpretation of the inclusion/exclusion criteria prior to patient randomization.10 Patients were screened between January 2017 and February 2018. Data on race and ethnicity were collected by self-report according to US Food and Drug Administration guidelines.
Randomization and Masking
Patients were randomized 1:1 to Evoke ECAP-controlled, closed-loop SCS (investigational group) or fixed-output, open-loop SCS (control group). Treatment allocation was concealed from the patients, investigator, and site staff for the full study duration. Randomization and masking procedures were described previously.10
Procedures
Randomized patients underwent a temporary SCS trial lasting up to 11 days. Patients with 50% or more overall back and leg pain visual analog scale score reduction were eligible for permanent implantation. Follow-up was at 1, 3, 6, 9, and 12 months and biannually thereafter for up to 3 years following permanent implant. Self-selected crossover was offered after 24 months.
During the temporary trial and permanent implant procedures, 2 percutaneous leads were implanted in the dorsal epidural space over the dorsal columns as per standard practice (eAppendix in Supplement 1).
The same neuromodulation system (Evoke System; Saluda Medical) was the investigational and control device, as it offered both ECAP-controlled, closed-loop SCS and fixed-output, open-loop SCS and the ability to measure the neural response in both groups. The closed-loop control system, a proportional-integral-derivative controller, minimizes the difference between the measured mean ECAP amplitude and the ECAP amplitude target by automatically varying the therapeutic current amplitude in real time for every stimulus. This process is frequency dependent and can occur more than 100 times per second. The system thereby maintains a consistent neural response, where the average error between the prescribed ECAP amplitude target and the measured ECAP amplitude is zero.13
Programming was performed as previously described (eAppendix in Supplement 1) to personalize therapy based on individual patient pain threshold.10,14
Outcomes
Pain relief was assessed using the visual analog scale to determine responders (patients with ≥50% reduction) and high responders (≥80% reduction)10 as well as the percent reduction in overall back and leg pain. Additional standardized patient-reported outcome measures per Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations were collected3,15,16,17,18,19,20,21,22,23,24 as well as pain medication usage.25,26
Objective measurements, including program parameters, device performance, patient adherence, and neurophysiological properties associated with spinal cord activation, were collected on the device as described in the eAppendix in Supplement 1. All adverse events were reported by the investigators throughout the study and reviewed and adjudicated by a blinded, independent clinical events committee.
Statistical Analysis
The sample size calculation, primary analysis at 3 months, and additional prespecified analysis at 12 months postpermanent implant have been described previously.10 This report discusses the long-term results at 24 months. The 24-month analysis of the primary outcome of pain included all randomized patients and is described in the eAppendix in Supplement 1, as are the analyses of the secondary outcomes.
For all tests, P values less than .05 (2-tailed tests) were considered significant and are reported together with the 95% CIs where appropriate. All statistical analyses were conducted using SAS statistical software version 9.4 (SAS Institute). Analysis took place between October 2020 and April 2021.
Results
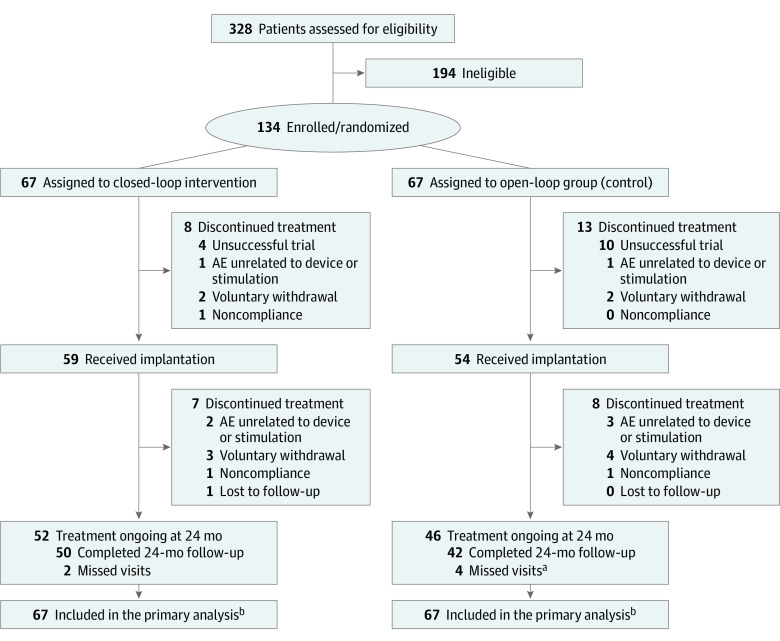
Of 328 screened patients, 134 were enrolled, with 67 patients randomized to each treatment group (Figure 1). Of these, 113 patients underwent implantation (59 in the closed-loop and 54 in the open-loop group). Fifty closed-loop patients and 42 open-loop patients completed 24-month follow-up. The double-blind was maintained for the full study duration.
Figure 1. CONSORT Diagram.
AE indicates adverse event.
aCOVID-19 resulted in 2 missed 24-month visits in the open-loop group.
bAll randomized patients (67 in the closed-loop group and 67 in the open-loop group) were included in the analysis of the primary outcome, visual analog scale pain, using a last value carried forward approach to account for missing data. Patients who completed the 24-month visit (50 in the closed-loop group and 42 in the open-loop group) were included in the analysis of secondary outcomes.
Race (not mutually exclusive) and ethnicity (mutually exclusive) of participants included American Indian or Alaska Native (closed-loop group: 1 [1.5%] vs open-loop group: 2 [3.0%]), Black or African American (closed-loop group: 2 [3.0%] vs open-loop group: 6 [9.0%]), Hispanic/Latino (closed-loop group: 3 [4.5%] vs open-loop group: 6 [9.0%]); non-Hispanic/Latino (closed-loop group: 64 [95.5%] vs open-loop group: 61 [91.0%]); and White (closed-loop group: 63 [94.0%] vs open-loop group: 59 [88.1%]).
There were no significant differences in the baseline diagnoses, prior treatments including opiate use, demographics, or other characteristics between treatment groups. All patients in both groups had a diagnosis of chronic intractable back and leg pain. The most frequent pain etiologies in both groups were radiculopathy (61 of 67 [91.0%] in the closed-loop group and 59 of 67 [88.1%] in the open-loop group), failed back surgery syndrome (38 of 67 [56.7%] in the closed-loop group and 41 of 67 [61.2%] in the open-loop group), and degenerative disc disease (33 of 67 [49.3%%] in the closed-loop group and 42 of 67 [62.7%] in the open-loop group). A total of 54 patients (40%) in both groups did not have prior back surgery. The mean (SD) baseline overall back and leg pain scores were 82.0 (10.7) mm for closed-loop patients and 82.2 (9.2) mm for open-loop patients. Baseline characteristics were similar for the cohort of patients who completed 24-month follow-up (eTable 2 in Supplement 1).
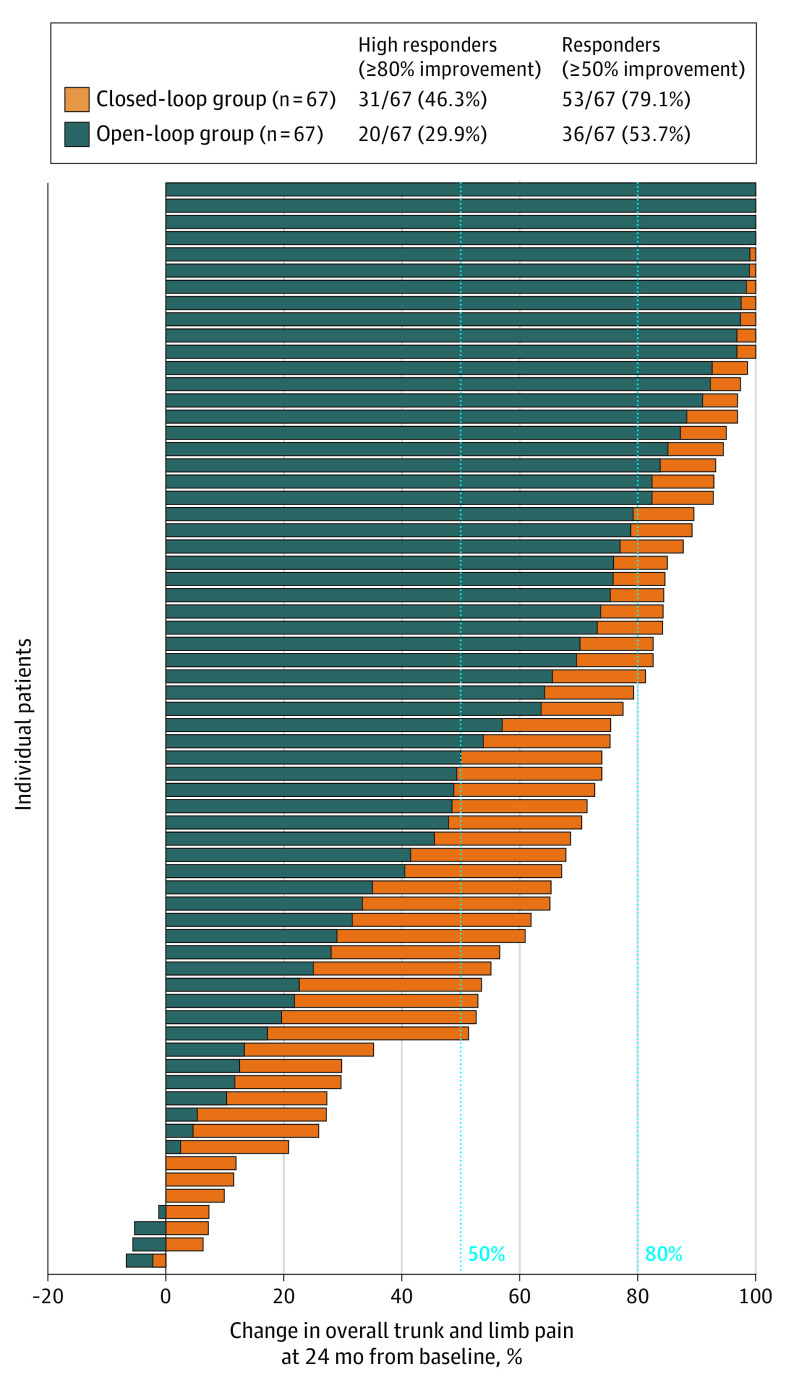
At 24 months, a significantly greater proportion of closed-loop patients were responders (≥50% reduction in overall back and leg pain) than open-loop patients in the randomized population (53 of 67 [79.1%] in the closed-loop group and 36 of 67 [53.7%] in the open-loop group; difference, 25.4% [95% CI, 10.0%-40.8%], P = .001) (Figure 2). Similarly, there was a significantly greater proportion of high responders, (≥80% reduction in overall pain) in the closed-loop group (31 of 67 [46.3%] in the closed-loop group and 20 of 67 [29.9%] in the open-loop group; difference, 16.4% [95% CI, 0.2%-32.6%]; P = .047). The reduction in overall back and leg pain was significantly greater for closed-loop (mean [SD] score, 26.4 [26.0]; point decrease, 55.6; percent decrease, 68.5%) than open-loop patients (mean [SD] score, 38.3 [29.7]; point decrease, 43.9; percent decrease, 53.7%) (between groups: mean score difference, −11.9 [95% CI, −21.4 to 2.3]; P = .02; point decrease difference, 11.7 [95% CI, 2.4-21.0]; P = .01; percent decrease difference, 14.8% [95% CI, 3.5%-26.0%]; P = .01). These results were confirmed by the repeated-measures sensitivity analysis (estimate of the difference, 15.1 [95% CI, 4.3-25.9]; P = .006).
Figure 2. Individual Patient Percent Change From Baseline in Overall Back and Leg Pain at 24 Months.
Positive change indicates improvement. A significantly greater proportion of closed-loop patients were responders and high responders in overall back and leg pain compared with open-loop patients.
For patients with permanent implant, 83.1% (49 of 59) of closed-loop compared with 63.0% (34 of 54) of open-loop were responders (difference, 20.1% [95% CI, 4.0%-36.1%]; P = .01) (eFigures 3, 4, and 5 in Supplement 1).
There was statistically significant and clinically meaningful improvement from baseline at 24 months in both treatment groups in all other patient-reported outcomes including Profile of Mood States, 12-item Short Form Survey, Oswestry Disability Index, Pittsburgh Sleep Quality Index, and European Quality of Life Five-Dimensional Five-Level (Figure 3, Table, and eFigures 6 and 7 in Supplement 1). In general, the improvement was greater in the closed-loop compared with the open-loop group, with significant differences seen in Profile of Mood States Total Mood Disturbance (Figure 3A) and 12-item Short Form Survey mental component summary at 24 months (eFigure 7 in Supplement 1). These results were substantiated by the repeated-measures sensitivity analyses. Furthermore, more than 80% of patients (42 of 50 [84.0%] in the closed-loop group and 34 of 42 [81.0%] in the open-loop group) indicated their health status was very much improved or much improved compared with baseline on the Patient Global Impression of Change.
Figure 3. Additional Patient-Reported Outcomes.
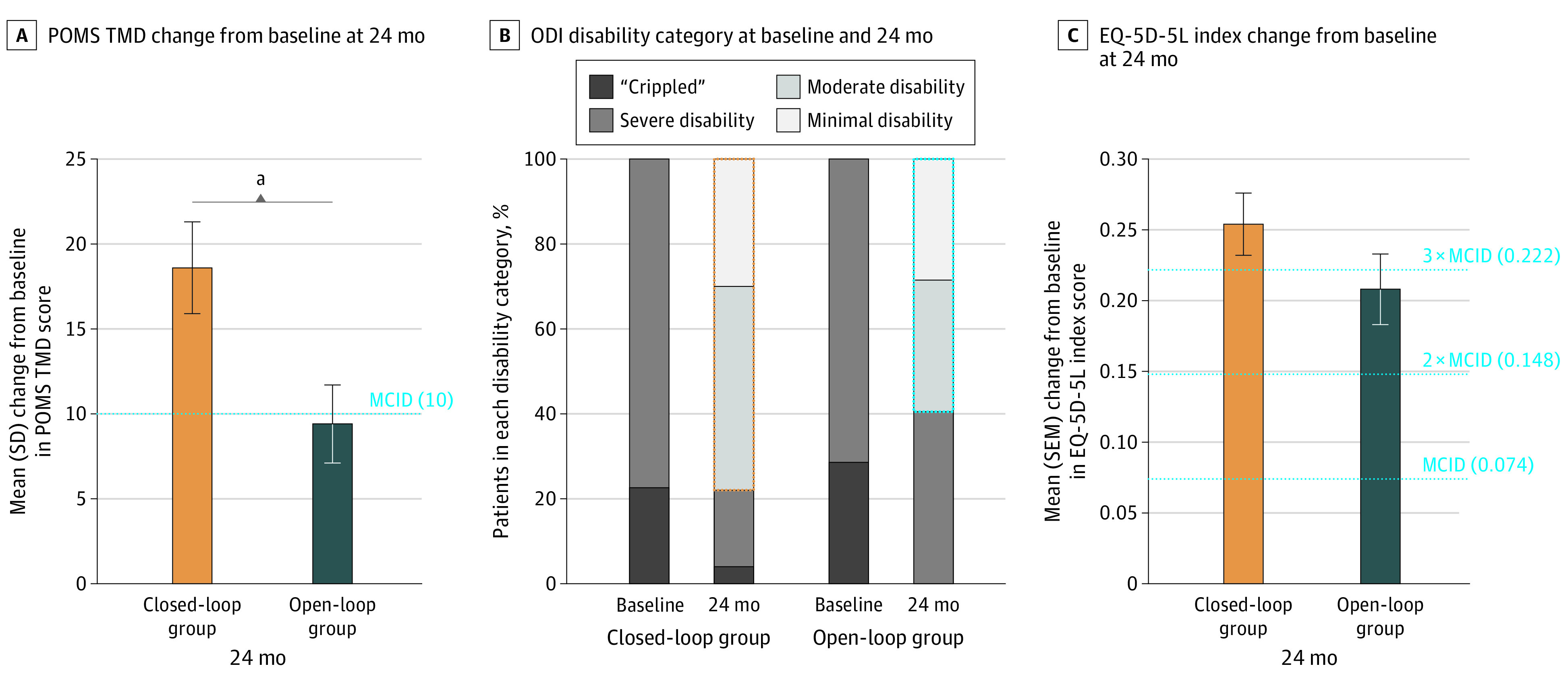
A, Mean change in profile of mood states (POMS) total mood disturbance (TMD) from baseline to 24 months was approximately double in the closed-loop compared with open-loop group, a significant difference (closed-loop group: 18.6; open-loop group: 9.4; difference, 9.2 [95% CI, 1.8-16.5]; P = .02; repeated-measures model: estimate of the difference, 10.7 [95% CI, 3.9-17.6]; P = .002). Additionally, 68.0% of closed-loop compared with 42.9% of open-loop patients had a minimum clinically important difference (MCID) of ≥10 points (difference, 25.1% [95% CI, 5.4%-44.9%]; P = .02). B, For study inclusion, patients were required to be classified as having a severe disability or as “crippled” on the Oswestry Disability Index (ODI) (score, 41-80). At 24 months, 78.0% closed-loop vs 59.5% open-loop patients improved to minimum or moderate disability (score, 0-40). C, The mean improvement in the European Quality of Life Five-Dimensional Five-Level (EQ-5D-5L) index score was greater than 3 times the MCID (MCID = 0.074) in the closed-loop group (mean [SD], 0.254 [0.157]) and greater than 2 times the MCID in the open-loop group (mean [SD], 0.208 [0.163]).
aSignificant difference between treatment groups (P < .05).
Table. Summary of Patient-Reported Outcomes at 24 Months.
| Patient-reported outcomea | No. (%) | |
|---|---|---|
| Closed-loop group (n = 50) | Open-loop group (n = 42) | |
| Oswestry Disability Index | ||
| Change from baseline, mean (SD) | 26.0 (13.6)b | 23.2 (14.5)b |
| % Change from baseline, mean (SD) | 47.8 (24.6)b | 42.2 (27.4)b |
| Minimum clinically important difference (score ≥15) | 41 (82.0) | 30 (71.4) |
| Minimal or moderate disability (score, 0-40)c | 39 (78.0) | 25 (59.5) |
| Profile of mood states total mood disturbance | ||
| Change from baseline, mean (SD) | 18.6 (19.4)b,d | 9.4 (15.1)b |
| Minimum clinically important difference (score ≥10) | 34 (68.0)d | 18 (42.9) |
| Short Form Health Survey Physical Component Summary | ||
| Change from baseline, mean (SD) | 10.1 (11.0)b | 11.0 (10.0)b |
| Minimum clinically important difference (score ≥6) | 33 (66.0) | 26 (63.4) |
| Short Form Health Survey Mental Component Summary | ||
| Change from baseline, mean (SD) | 6.7 (11.6)b,d | −1.4 (10.0) |
| Minimum Clinically Important Difference (score ≥7) | 23 (46.0)d | 9 (22.0) |
| EQ-5D-5L Index score | ||
| Change from baseline, mean (SD) | 0.254 (0.157)b | 0.208 (0.163)b |
| Minimum clinically important difference (score ≥0.074) | 43 (86.0) | 35 (83.3) |
| EuroQol-visual analog scale | ||
| Change from baseline, mean (SD) | 26.9 (19.9)b | 19.5 (21.7)b |
| Pittsburgh Sleep Quality Index | ||
| Change from baseline, mean (SD) | 4.1 (4.3)b | 4.1 (4.7)b |
| Minimum Clinically Important Difference (score ≥3) | 31 (63.3) | 26 (61.9) |
| Patient Global Impression of Change | ||
| Very much improved or much improved | 42 (84.0) | 34 (81.0) |
Positive change indicates improvement. Patient-reported outcomes collected included health-related quality of life measured by the Short Form Health Survey,16 which has a minimum clinically important difference (MCID) of 6 points for the Physical Component Summary and 7 points for the Mental Component Summary,16 and the European Quality of Life Five-Dimensional Five-Level (EQ-5D-5L),17 which has an MCID of 0.07418 for the index score; functional disability measured by the Oswestry Disability Index,19 which has an MCID of 15 points20; emotional functioning measured by the Profile of Mood States,21 which has an MCID of 10 points for Total Mood Disturbance22; sleep quality measured by the Pittsburgh Sleep Quality Index,23 which has an MCID of 3 points24; and Patient Global Impression of Change, which measures the impact of therapy on health status and tends to reflect other aspects such as treatment convenience, cost, and adverse reaction burden.
Significant within-group improvement from baseline (P < .05).
No patients had minimal or moderate severity on the Oswestry Disability Index (score, 0-40) at baseline. For study inclusion, patients were required to be classified as having a severe disability or “crippled” on the Oswestry Disability Index (score, 41-80).
Significant difference between treatment groups (P < .05).
In parallel with pain reduction and improvement in secondary outcomes, voluntary opioid reduction or elimination was observed at 24 months in 66.7% (18 of 27) and 60.9% (14 of 23) of closed-loop and open-loop patients, respectively (P = .77), who were taking opioids at baseline. Further, mean daily morphine milligram equivalents decreased to less than 50 morphine milligram equivalents in both groups (mean [SD]: baseline, 80.1 [105.3] in the closed-loop group and 66.4 [86.2] in the open-loop group; 24 months, 41.9 [47.3] in the closed-loop group and 42.2 [41.5] in the open-loop group), in alignment with current practice guidelines.27,28 The percent reduction was greater for the closed-loop (mean [SD], 42.3% [39.3%]) than for the open-loop group (mean [SD], 24.5% [81.4%]). Eighteen of 27 patients (66.7%) in the closed-loop group and 13 of 23 (56.5%) in the open-loop group had a meaningful decrease in opioid use defined as a morphine milligram equivalents reduction of 20% or more.26 One open-loop patient (4.3%) increased their opiate use at 24 months.
Data collected on the device, including program parameters, device performance, patient adherence, and neurophysiological properties provide objective data on actual therapy received by which to judge the relationship to clinical outcomes.
The stimulation and ECAP parameters (frequency, pulse duration, stimulation amplitude, dose-response relationship, or sensitivity) programmed in clinic in a stable sitting position at the 24-month visit were not significantly different between treatment groups (eFigure 8 in Supplement 1).
However, device performance (ie, the ability of the device to adhere to the prescribed neural response), was significantly different between open- and closed-loop SCS during a series of directed posture changes performed in clinic. Compared with open-loop SCS, there was an 88.2% reduction between the target neural response and the elicited neural response with closed-loop SCS. The elicited neural response with closed-loop SCS deviated 3.2 μV from the target neural response, whereas with open-loop SCS, the deviation was 27.4 μV (P < .001) (example illustrated in Figure 4A).
Figure 4. Device Performance, Dose-Response Relationship, and Therapeutic Window Differences Between Closed- and Open-Loop SCS.
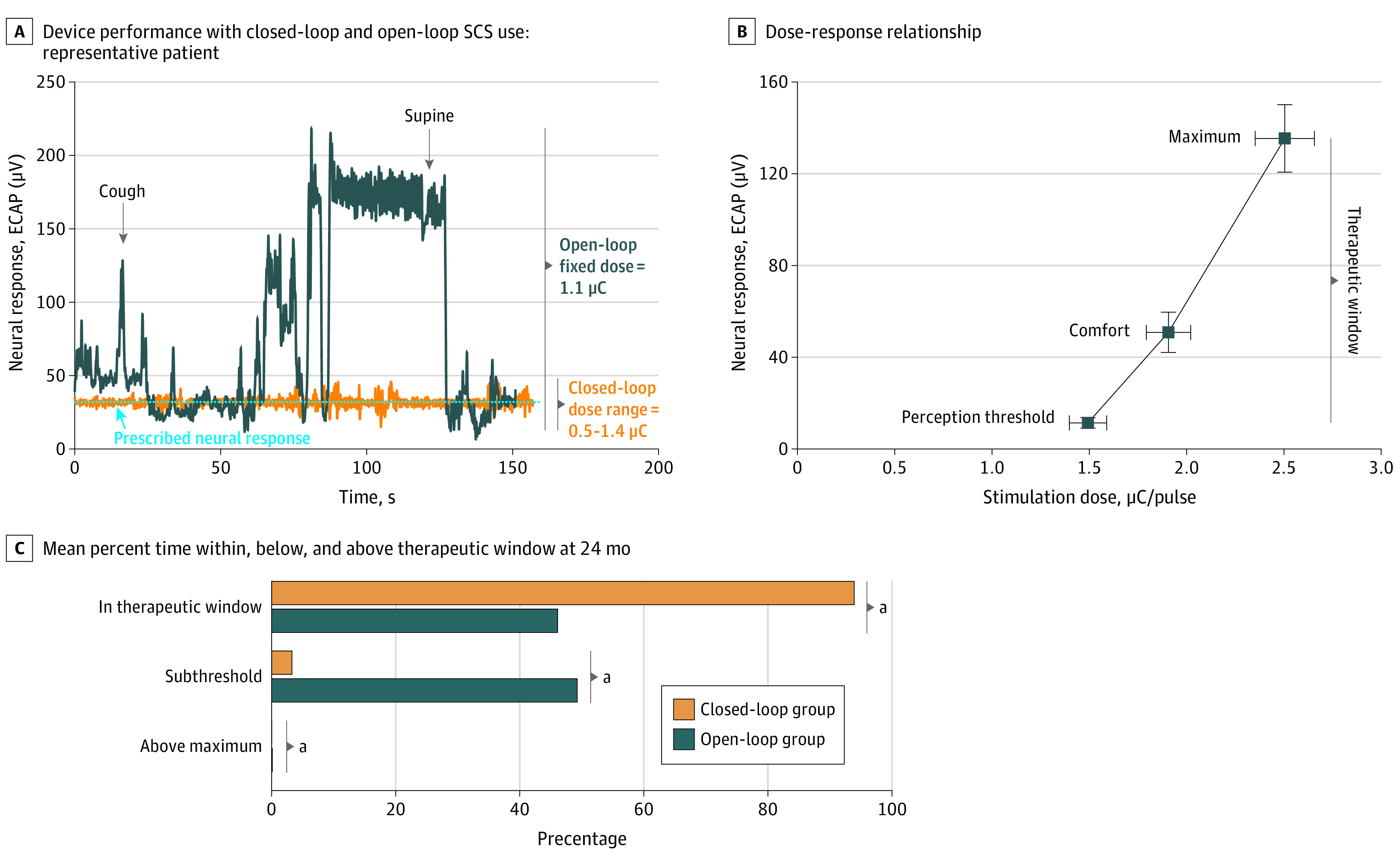
A, Device performance is defined as the ability of the spinal cord stimulation (SCS) device to adhere to the prescribed neural response. Owing to the ever-changing coupling of the electrical field to the target neural tissue, open-loop systems are not capable of continuously adhering to the prescribed neural response. A consistent neural response at the prescribed level may only be achieved with a physiological closed-loop control system that continually adjusts on every stimulation pulse. This figure depicts the neural response of an Evoke study patient performing the same series of postures in clinic in both the open-loop and closed-loop stimulation modes and demonstrates how closed-loop minimizes the error between the prescribed target neural response and the observed elicited neural response pulse by pulse. In this patient, the elicited neural response deviated 22.1 times more from the target neural response with open-loop compared with closed-loop (66.4 μV deviation vs 3.0 μV deviation, respectively). B, In pharmacology, the importance of characterizing the dose-response relationship has long been understood because the concentration of a drug at its site of action controls its effect. In SCS, the charge (stimulation dose [μC/pulse]) produces a neural response (evoked compound action potentials [ECAP]; μV), which results in a clinical effect. ECAP amplitude (ie, a measure of the number of fibers activated) increases with increasing charge.9 In this study, dose-response data (ie, ECAP amplitude and charge) were collected during scheduled programming visits in a sitting position at patients’ perception threshold, comfort, and maximum. Patient perception threshold to maximum defined the patients’ therapeutic window. The mean (standard error) dose-response for the Evoke study patients at 24 months is presented. C, On average, closed-loop patients were nearly always within the therapeutic window (median [IQR], 93.9% [48.0%-98.9%]), while open-loop patients were only with the window half of the time (median [IQR], 46.1% [25.9%-71.9%]), and half of the time below threshold (median [IQR], 49.3% [22.7%-74.1%]); the differences in time spent with, below, and above the therapeutic window were significant between groups (P < .05).
aSignificant difference between treatment groups (P < .05).
Patient adherence (ie, patient compliance to the prescription) is reflected by the patients’ interaction with the device. Device utilization data demonstrated patient adherence was high in both groups with stimulation turned on most of the time (median [IQR], 88.0% [50.6%-96.3%] in the closed-loop group; 95.0% [32.4%-99.3%] in the open-loop group; P = .18). However, full compliance to the prescription was limited by the performance of the stimulation mode.
Outside the clinic, the closed-loop group demonstrated significantly greater consistency in maintaining the prescribed level of neural response as seen by the degree of spinal cord activation and grossly by the amount of time spent within the neural therapeutic window. The mode activation level in closed-loop was 3 times greater than open-loop (median [IQR], 22.5 [4.0-45.0] μV in the closed-loop group; 7.5 [1.0-19.5] μV in the open-loop group; P = .008). On average, the neural response for closed-loop patients was within the therapeutic window (perception threshold to maximum) the majority of the time (median [IQR], 93.9% [48.0%-98.9%] in the closed-loop group; 46.1% [25.9%-71.9%] in the open-loop group; P < .001), whereas it was below the therapeutic window a greater proportion of time in the open-loop group (median [IQR], 3.3% [0.4%-45.7%] in the closed-loop group; 49.3% [22.7%-74.1%] in the open-loop group; P < .001). Although the percentages are small, the open-loop group spent significantly more time above the therapeutic window (median [IQR], 0% [0%-0.3%] in the closed-loop group; 0.1% [0%-3.6%] in the open-loop group; P = .01). In open-loop SCS, to avoid overstimulation events, patients turn stimulation down such that a greater proportion of time was spent below perception threshold, whereas in the closed-loop SCS, maintenance of activation around the prescribed comfort level was provided by the device (Figure 4C and eFigure 9 in Supplement 1).
Neurophysiological properties including antidromic and orthodromic conduction velocity, rheobase, and chronaxie were also collected. While there was individual variability in neurophysiological properties as expected owing to intrinsic patient differences in activation, there was not a significant difference between groups in any parameter (eFigure 10 in Supplement 1).
The type, nature, and severity of adverse events were similar between treatment groups and are described in eTables 3 and 4 in Supplement 1. All patients received the same device and underwent the same procedure; the only difference between groups was the stimulation mode (open-loop or closed-loop SCS), thus, the true indicator of the safety differences between groups. There were no differences between groups in stimulation therapy-related adverse events. There were 2 explants due to loss of efficacy (closed-loop group: 0 [0%]; open-loop group: 2 [3.0%]) and 3 explants due to procedure-related infections (closed-loop group: 2 [3.0%]; open-loop group: 1 [1.5%]).
Discussion
Although the exact mechanisms of action of SCS are still debated, it is commonly accepted that stimulating sensory fibers in the dorsal columns modulates pain pathways to produce analgesia.5,6 What is known is that the neural response to stimulation varies as the distance between the epidural electrodes and the spinal cord target dynamically changes and is also affected by individual patient anatomy and neurophysiology.8 Despite its use for more than 50 years, SCS has been limited by the inability to continuously measure and control the elicited spinal cord response. Without this ability, the therapy invariably fluctuates and may only be improved by trial and error.29 A real-time physiological closed-loop control system, which measures the neural response on every stimulation pulse and continuously adjusts the stimulation dose, works to maintain effective and consistent therapy with minimal adverse effects.10
This study demonstrated that ECAP-controlled, closed-loop SCS provided pain reduction superior to open-loop SCS and that this effect was maintained through 2-year follow-up. The significantly higher degree of spinal cord activation maintained within the therapeutic window in the closed-loop group paralleled the greater improvement in both the primary pain and secondary outcome measures, including physical and emotional functioning and health-related quality of life. The improvements seen in other measures, such as quality-of-life measures reaching population norms, further validate ECAP-controlled, closed-loop SCS as an effective therapy. The quality-of-life improvements observed in this study were better than those seen in many other chronic pain treatments, such as lumbar decompression and fusion,30,31 and rival distinguished interventions for other conditions such as hip and knee arthroplasty.32,33
Prior SCS clinical trials lack objective evidence of device performance and patient adherence and the association with reported clinical outcomes. A most significant limitation has been the inability to differentiate a lack of therapeutic efficacy from a lack of device performance and/or patient adherence. Deviations in patient adherence, in particular, can lead to biased results, reduced statistical power, and improper causal inferences in clinical trials.34 However, the current study measured both device performance and patient adherence via neural response and usage data. While patient adherence was high in both groups (>88% patient utilization), device performance in the open-loop group deviated from the prescribed neural target by 8.6 times more, supporting the observed differences in clinical outcomes between groups.
The utilization and neurophysiological data support an objective approach to SCS through which clinicians may monitor and adjust therapy to improve outcomes. This transparency is essential for patients, physicians, and payers to evaluate cost-effectiveness. Without such objective measurement, outcomes and failures cannot be properly interpreted. With concerns over high explant rates for existing SCS systems,35 these tools permit increased understanding and earlier therapy adjustments by clinicians to prevent loss of efficacy. In the Evoke trial, through 24 months postimplant, there have been no explants due to loss of efficacy in the closed-loop group.
Limitations
The double-blind, parallel-arm randomized clinical trial design of this study, with treatment assignment and blinding maintenance through 24 months and collection of objective data, provides the highest rigor of any study conducted in neuromodulation to date and to our knowledge. One study limitation may be its control arm, which has advantages over other open-loop systems owing to the ability to measure the neural response to inform programming and may improve clinical outcomes. Therefore, the advantages of closed-loop SCS may be even more profound when compared with other open-loop systems. The choice to use the same device in both treatment groups was made to ensure proper double blinding and to facilitate measurement of neural response in both groups. Patients in both groups received identical care, with the same degree of device programming. Blinded investigators documented their oversight to confirm comprehensive programming and optimization for all study participants and to ensure that no bias was introduced.10 The successful randomization and blinding resulted in equivalent baseline characteristics, programming parameters, and underlying neurophysiology, indicating that the observed differences between groups are likely attributed to the differences in spinal cord activation achieved with each of the stimulation modes.
Conclusions
Full adoption of SCS as a therapy for chronic pain has been limited, in part, owing to inconsistent outcomes. The continued superiority of ECAP-controlled, closed-loop SCS compared with open-loop SCS through 24 months is demonstrated by the greater degree of pain relief and is further substantiated by the improvements in secondary outcome measures. This causal inference is supported by the observed equivalent device utilization but greater therapeutic spinal cord activation in closed-loop SCS. Thus, ECAP-controlled SCS has demonstrated to provide a profoundly effective, reliable, and durable treatment option for patients with chronic pain, allowing for the potential of significant voluntary opioid reduction. Continued measurement of neurophysiological properties should lead to greater knowledge of underlying mechanism(s) of action and more efficacious therapy in the long term.36
eAppendix.
eFigure 1. 24-Month RCT Evidence for SCS
eFigure 2. 24-Month RCT Evidence for SCS – Health-Related Quality of Life Outcomes
eFigure 3. Overall Back and Leg VAS Scores through 24 Months
eFigure 4. Percent Reduction in Overall Back and Leg Pain through 24 Months
eFigure 5. ≥50% Reduction (Responder) in Overall Back and Leg Pain through 24 Months
eFigure 6. Mean EQ-5D Index Score at Baseline and 24 Months
eFigure 7. Mean Change from Baseline in SF-12 MCS Score at 24 Months
eFigure 8. Interim Reprogramming Visits per Month per Patient through 24 Months
eFigure 9. Therapeutic Window Width through 24 Months
eFigure 10. Patient Neurophysiological Property Measures at 24 Months
eTable 1. 24-Month RCT Evidence for SCS
eTable 2. Baseline Characteristics for all Randomized Patients and for the Cohort of Patients who Completed 24-month Follow-up
eTable 3. Summary of Study-Related Adverse Events for all Randomized Patients
eTable 4. Rates of Study-Related Adverse Events for All Randomized Patients
eReferences.
Trial protocol
Data Sharing Statement
References
- 1.Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8(6):299-299. doi: 10.21037/atm.2020.02.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wettstein M, Eich W, Bieber C, Tesarz J. Pain intensity, disability, and quality of life in patients with chronic low back pain: does age matter? Pain Med. 2019;20(3):464-475. doi: 10.1093/pm/pny062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz N, Dworkin RH, North R, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: initiative on methods, measurement, and pain assessment in clinical trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain. 2021;162(7):1935-1956. doi: 10.1097/j.pain.0000000000002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caylor J, Reddy R, Yin S, et al. Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectron Med. 2019;5(1):12. doi: 10.1186/s42234-019-0023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato KL, Johanek LM, Sanada LS, Sluka KA. Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth Analg. 2014;118(2):464-472. doi: 10.1213/ANE.0000000000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits H, van Kleef M, Holsheimer J, Joosten EAJ. Experimental spinal cord stimulation and neuropathic pain: mechanism of action, technical aspects, and effectiveness. Pain Pract. 2013;13(2):154-168. doi: 10.1111/j.1533-2500.2012.00579.x [DOI] [PubMed] [Google Scholar]
- 7.Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience. 1999;89(2):335-346. doi: 10.1016/S0306-4522(98)00330-3 [DOI] [PubMed] [Google Scholar]
- 8.Ranger MRB, Irwin GJ, Bunbury KM, Peutrell JM. Changing body position alters the location of the spinal cord within the vertebral canal: a magnetic resonance imaging study. Br J Anaesth. 2008;101(6):804-809. doi: 10.1093/bja/aen295 [DOI] [PubMed] [Google Scholar]
- 9.Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153(3):593-601. doi: 10.1016/j.pain.2011.11.023 [DOI] [PubMed] [Google Scholar]
- 10.Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123-134. doi: 10.1016/S1474-4422(19)30414-4 [DOI] [PubMed] [Google Scholar]
- 11.Levy R, Deer TR, Poree L, et al. Multicenter, randomized, double-blind study protocol using human spinal cord recording comparing safety, efficacy, and neurophysiological responses between patients being treated with evoked compound action potential-controlled closed-loop spinal cord stimulation or open-loop spinal cord stimulation (the Evoke study). Neuromodulation. 2019;22(3):317-326. doi: 10.1111/ner.12932 [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Hellerstein J, Diao Y, Parekh S, Tilbury DM. Feedback Control of Computing Systems. IEEE Press: Wiley; 2004. doi: 10.1002/047166880X [DOI] [Google Scholar]
- 14.US Food and Drug Administration . Investigator responsibilities: protecting the rights, safety, and welfare of study subjects. Published October 2009. Accessed December 17, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigator-responsibilities-protecting-rights-safety-and-welfare-study-subjects
- 15.Dworkin RH, Turk DC, Farrar JT, et al. ; IMMPACT . Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 16.Maruish ME, ed. User’s Manual for the SF-12v2 Health Survey. 3rd ed. QualityMetric Inc; 2012. [Google Scholar]
- 17.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523-1532. doi: 10.1007/s11136-004-7713-0 [DOI] [PubMed] [Google Scholar]
- 19.Savre I, Fairbank J.. Oswestry Disability Index Information Booklet. 1st ed. Mapi Research Trust; 2011. [Google Scholar]
- 20.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine (Phila Pa 1976). 2000;25(24):3115-3124. doi: 10.1097/00007632-200012150-00006 [DOI] [PubMed] [Google Scholar]
- 21.McNair DM, Heuchert JWP. Profile of mood states technical update. Multi-Health Systems Inc; 2005. [Google Scholar]
- 22.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887-895. doi: 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention . Data resources: analyzing opioid prescription data and oral morphine milligram equivalents (MME). Published 2018. Accessed December 17, 2021. https://www.cdc.gov/drugoverdose/resources/data.html
- 26.Dougherty MC, Woodroffe RW, Wilson S, Gillies GT, Howard MA III, Carnahan RM. Predictors of reduced opioid use with spinal cord stimulation in patients with chronic opioid use. Neuromodulation. 2020;23(1):126-132. doi: 10.1111/ner.13054 [DOI] [PubMed] [Google Scholar]
- 27.Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. US Department of Health and Human Services; 2019. https://www.hhs.gov/sites/default/files/pain-mgmt-best-practices-draft-final-report-05062019.pdf
- 28.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 29.Levy RM. The need for mechanism-based medicine in neuromodulation. Neuromodulation. 2012;15(4):273-279. doi: 10.1111/j.1525-1403.2012.00484.x [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Mortaz Hedjri S, Coyte PC, Rampersaud YR. Cost-utility of lumbar decompression with or without fusion for patients with symptomatic degenerative lumbar spondylolisthesis. Spine J. 2012;12(1):44-54. doi: 10.1016/j.spinee.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 31.Pekkanen L, Neva MH, Kautiainen H, Kyrölä K, Marttinen I, Häkkinen A. Changes in health utility, disability, and health-related quality of life in patients after spinal fusion: a 2-year follow-up study. Spine (Phila Pa 1976). 2014;39(25):2108-2114. doi: 10.1097/BRS.0000000000000624 [DOI] [PubMed] [Google Scholar]
- 32.Konopka JF, Lee YY, Su EP, McLawhorn AS. Quality-adjusted life years after hip and knee arthroplasty: health-related quality of life after 12,782 joint replacements. JB JS Open Access. 2018;3(3):e0007. doi: 10.2106/JBJS.OA.18.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmallah RK, Cherian JJ, Jauregui JJ, Bhowmik-Stoker M, Beaver WB, Mont MA. Determining health-related quality-of-life outcomes using the SF-6D preference-based measure in patients following total knee arthroplasty. J Arthroplasty. 2015;30(7):1150-1153. doi: 10.1016/j.arth.2015.01.050 [DOI] [PubMed] [Google Scholar]
- 34.Breckenridge A, Aronson JK, Blaschke TF, Hartman D, Peck CC, Vrijens B. Poor medication adherence in clinical trials: consequences and solutions. Nat Rev Drug Discov. 2017;16(3):149-150. doi: 10.1038/nrd.2017.1 [DOI] [PubMed] [Google Scholar]
- 35.Al-Kaisy A, Royds J, Al-Kaisy O, et al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg Anesth Pain Med. 2020;45(11):883-890. doi: 10.1136/rapm-2020-101681 [DOI] [PubMed] [Google Scholar]
- 36.van der Wilt GJ, Zielhuis GA. Merging evidence-based and mechanism-based medicine. Lancet. 2008;372(9638):519-520. doi: 10.1016/S0140-6736(08)61215-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.
eFigure 1. 24-Month RCT Evidence for SCS
eFigure 2. 24-Month RCT Evidence for SCS – Health-Related Quality of Life Outcomes
eFigure 3. Overall Back and Leg VAS Scores through 24 Months
eFigure 4. Percent Reduction in Overall Back and Leg Pain through 24 Months
eFigure 5. ≥50% Reduction (Responder) in Overall Back and Leg Pain through 24 Months
eFigure 6. Mean EQ-5D Index Score at Baseline and 24 Months
eFigure 7. Mean Change from Baseline in SF-12 MCS Score at 24 Months
eFigure 8. Interim Reprogramming Visits per Month per Patient through 24 Months
eFigure 9. Therapeutic Window Width through 24 Months
eFigure 10. Patient Neurophysiological Property Measures at 24 Months
eTable 1. 24-Month RCT Evidence for SCS
eTable 2. Baseline Characteristics for all Randomized Patients and for the Cohort of Patients who Completed 24-month Follow-up
eTable 3. Summary of Study-Related Adverse Events for all Randomized Patients
eTable 4. Rates of Study-Related Adverse Events for All Randomized Patients
eReferences.
Trial protocol
Data Sharing Statement