Abstract
Objectives
Salivary gland acinic cell carcinoma (AciCC) has recognizable cytomorphologic features that can overlap with benign and malignant entities, creating a diagnostic challenge. AciCC harbors a t(4;9) translocation increasing nuclear receptor subfamily 4 group A member 3 (NR4A3) expression, detectable by immunohistochemistry (IHC) on surgical resection (SR). NR4A3 IHC cytology data are limited. Here, we examine NR4A3 IHC on smears, cell blocks (CBs), and SRs of AciCC and its mimickers.
Methods
Our cohort comprised AciCC (including high-grade transformation), secretory carcinoma, mucoepidermoid carcinoma (MEC), Warthin tumor, pleomorphic adenoma (PA), cellular PA, carcinoma ex-PA, oncocytic carcinoma, oncocytoma, and nodular oncocytosis. NR4A3 IHC (Santa Cruz Biotechnology and Origene antibodies) was positive if more than 5% tumor cells showed nuclear staining.
Results
Among CBs, 90% of AciCC cases and none of the mimickers expressed NR4A3. Among SRs, 100% of AciCC cases showed diffuse NR4A3, whereas one high-grade MEC expressed focal NR4A3. Concordance was 95% with two antibody clones. Sensitivity, specificity, positive predictive value, and negative predictive value were 90%, 100%, 100%, and 94.7% for CBs and 100%, 98.8%, 92.3%, and 100% for SRs, respectively. NR4A3 immunostaining was demonstrable on smears from an AciCC case.
Conclusions
NR4A3 IHC can be a robust diagnostic tool to identify AciCC, especially for cytology specimens.
Keywords: NR4A3, Acinic cell carcinoma, Salivary, Oncocytic
KEY POINTS.
Nuclear receptor subfamily 4 group A member 3 (NR4A3) is highly sensitive and specific in distinguishing acinic cell carcinoma from other primary salivary neoplasms on limited cytologic preparations and in surgical specimens.
NR4A3 expression is retained in cases of acinic cell carcinoma with high-grade transformation.
NR4A3 immunocytochemistry has potential utility on direct smears from acinic cell carcinoma.
INTRODUCTION
Acinic cell carcinoma (AciCC) is a relatively uncommon malignant salivary gland tumor, accounting for approximately 6% of salivary gland neoplasms.1,2 Histologically, low-grade AciCC shows characteristic polygonal serous cells containing basophilic cytoplasmic zymogen granules in various architectural patterns.2 However, a definitive diagnosis of AciCC can be more challenging in fine-needle aspiration (FNA) cytology, since the characteristic features may be less apparent, due to the limited specimen, tumor heterogeneity, lack of architecture, and especially cytomorphologic overlap with other salivary gland entities, including nonneoplastic salivary gland acini and other oncocytic salivary gland neoplasms, including secretory carcinoma (SC), oncocytoma, and mucoepidermoid carcinoma.3-5 Distinguishing AciCC from benign neoplasms, particularly oncocytoma, preoperatively could potentially affect surgical management, including extent of resection, especially if it is closely associated with the facial nerve. Additional diagnostic challenges include zymogen granule-poor AciCC and AciCC with high-grade transformation (HGT), in which the characteristic cytomorphologic features may be absent. Considering these diagnostic challenges, the recently published Milan System for Classification of Salivary Gland Cytology includes most AciCC variants in the salivary gland neoplasm of uncertain malignant potential category under the cellular oncocytic/oncocytoid neoplasm subcategory.4,6
In challenging cases, ancillary tests are essential to render a definitive diagnosis of AciCC on limited FNA material.7 With immunohistochemistry (IHC), AciCCs are positive for discovered on GIST-1 (DOG1) and Sry-related HMG-box gene 10 (SOX10) and negative for p63 and S100, which distinguishes these from most oncocytoid neoplasms.8 However, SOX10 may be expressed in SC, a key entity in the differential diagnosis, although SC typically lacks DOG1 staining.9 Furthermore, neither DOG1 nor SOX10 is entirely specific, as both can stain benign salivary gland and other salivary gland neoplasms.10,11 Finally, using large immunomarker panels may deplete the limited cytologic material and may not be cost-effective for laboratories with limited resources. Thus, there is a need for sensitive and specific markers to classify the tumor preoperatively as malignant as well as to separate AciCC from its mimickers on limited material, particularly benign and nonneoplastic mimics, which may affect surgical management.
Two recent studies from Haller et al12,13 showed that AciCC harbors a unique t(4;9)(q13;q31) rearrangement, placing active chromatin domains of the 4q13 secretory calcium-binding phosphoprotein cluster upstream of the nuclear receptor subfamily 4 group A member 3 (NR4A3) transcription factor gene in 9q31. The net effect of this genetic mechanism, termed enhancer hijacking, is overexpression of the NR4A3 gene and activation of downstream genes involved in cellular metabolism and proliferation (Haller et al12). Importantly, the authors demonstrated that the rearrangement was absent in all other key oncocytic neoplasms. Furthermore, NR4A3 IHC was more than 95% sensitive and specific for AciCC in surgical resections (SRs).13 However, data on NR4A3 IHC in cytology specimens are limited. Two small studies have shown NR4A3 IHC to be effective on FNA CB with superior performance to DOG1 and fluorescence in situ hybridization for the t(4;9) rearrangement.14,15 However, neither study explored the use of NR4A3 immunostaining on direct smear preparations. In the largest cytologic series to date, we investigated the performance of NR4A3 IHC in cytology cell blocks (CBs) and SRs. We further expand the potential applicability of this marker to direct smears of a case of AciCC.
MATERIALS AND METHODS
Study Cohort
This study was approved by the institutional review boards of all the participating institutions. The multi-institutional cohort comprised formalin-fixed FNA CBs (n = 56) and SRs (n = 79). Tumors consisted of conventional AciCC (n = 29 patients, 20 CBs and 9 SRs), AciCC with HGT (n = 3, 3 SRs), SC (n = 10 patients, 7 SRs and 3 CBs), mucoepidermoid carcinoma (MEC; n = 16 patients, 15 SRs and 6 CBs), Warthin tumor (WT; n = 43 patients, 31 SRs and 19 CBs), pleomorphic adenoma (PA; n = 6 patients, 6 SRs and 2 CBs), cellular PA (n = 1 patient, 1 SR and 1 CB), carcinoma ex-PA (n = 2 patients, 2 SRs), oncocytic carcinoma (n = 1 patient, 1 SR), oncocytoma (n = 19 patients, 18 SRs and 5 CBs), and nodular oncocytosis (n = 5 patients, 5 SRs) diagnosed at seven institutions. Matched specimens were available for 19 patients: 4 patients with oncocytoma, 2 patients with PA, 1 patient with cellular PA, 5 patients with MEC, and 7 patients with WT. Cases were selected after review of the H&E-stained CB and SR slides by at least two independent pathologists (T.S. and K.V.) using the current World Health Organization diagnostic criteria.
NR4A3 Immunostaining
IHC was performed using NR4A3 antibodies (1:100, NOR-1 antibody, clone H-7, Santa Cruz Biotechnology; 1:100, NR4A3 mouse monoclonal antibody, clone OTI2B11, Origene Technologies) on paraffin-embedded tissue sections from CBs and SRs on a Leica Bond system using the modified protocol F provided by the manufacturer. The section was pretreated using heat-mediated antigen retrieval with Tris-EDTA buffer (pH 9, epitope retrieval solution 2) for 20 minutes and incubated with the antibodies for 15 minutes at room temperature. NR4A3 was detected using a horseradish peroxidase-conjugated compact polymer system and 3,3’-diaminobenzidine as the chromogen. Each section was counterstained with hematoxylin and mounted with Leica Micromount. NR4A3 IHC was considered positive when nuclear immunostaining was present in more than 5% of tumor cells with any intensity. For immunocytochemistry, smears from one case of AciCC were fixed in 95% ethanol until use. Subsequently, slides were treated in a stepwise manner to first dehydrate and rehydrate the smear as follows: 70% alcohol, 100% alcohol, xylene for 1 minute, 100% alcohol for 1 minute, 95% ethanol for 1 minute, 70% ethanol for 1 minute, and phosphate-buffered saline for 1 minute. The rehydrated smears were then subjected to NR4A3 immunostaining as described above. An SR of an AciCC was used for the initial optimization of NR4A3 staining and subsequently used as a positive control. All AciCCs (including cases of HGT) and SCs were stained with both the Origene and Santa Cruz clones. The remaining non-AciCC and non-SC cases (WT, MEC, PA, cellular PA, carcinoma ex-PA, oncocytoma, oncocytic carcinoma) were stained with the Origene clone only.
RESULTS
Among AciCCs (including with HGT, n = 32 total), 87.5% (n = 28) were primary in the parotid gland, 3.1% (n = 1) were primary in the submandibular gland, and the remainder were either primary in minor salivary glands or metastatic to extra–salivary gland sites (eg, pleura). The median age of the patients was 63 years (range, 12-82 years) with a male-to-female ratio of 0.45:1 for cases without HGT (n = 29) and 82 years (range, 68-82 years) with a male-to-female ratio of 2:1 for cases with HGT (n = 3) TABLE 1.
Table 1.
Clinicopathologic Characteristics for Acinic Cell Carcinoma and Non–Acinic Cell Carcinomas (n = 135)
| Location, No. (%) | |||||
|---|---|---|---|---|---|
| Tumor Type | Females and Males, No. (%) | Age, Median (Range), y | Parotid | Submandibular | Others |
| Acinic cell carcinoma (n = 29) | 20 (62.1) females and 9 (37.9) males | 63 (12-82) | 25 (86.2) | 1 (3.4) | 3 (10.4) |
| Acinic cell carcinoma with high-grade transformation (n = 3) | 1 (33.3) female and 2 (66.7) males | 82 (68-82) | 3 (100) | 0 | 0 |
| Warthin tumor (n = 43) | 17 (39.5) females and 26 (60.5) males | 64 (49-95) | 40 (93) | 0 | 3 (7) |
| Mucoepidermoid carcinoma (n = 16) | 11 (68.7) females and 5 (31.3) males | 48 (13-88) | 12 (75) | 2 (12.5) | 2 (12.5) |
| Secretory carcinoma (n = 10) | 4 (40) females and 6 (60) males | 50 (30-86) | 6 (60) | 1 (10) | 3 (30) |
| Pleomorphic adenoma (n = 6) | 2 (33.3) females and 4 (66.7) males | 69.5 (30-75) | 5 (83.3) | 1 (16.7) | 0 |
| Carcinoma ex-pleomorphic adenoma (n = 2) | 2 (100) females | 76 (69-83) | 2 (100) | 0 | 0 |
| Cellular pleomorphic adenoma (n = 1) | 1 (100) male | 71 (71) | 1 (100) | 0 | 0 |
| Oncocytic carcinoma (n = 1) | 1 (100) male | 64 (64) | 1 (100) | 0 | 0 |
| Oncocytoma (n = 19) | 11 (57.9) females and 8 (42.1) males | 64 (41-89) | 14 (73.6) | 4 (21.1) | 1 (5.3) |
| Nodular oncocytosis (n = 5) | 1 (20) female and 4 (80) males | 82 (45-84) | 3 (60) | 0 | 2 (40) |
Among CBs, 90% (18/20) and 85% (17/20) of AciCCs showed strong NR4A3 staining with the Origene and Santa Cruz antibody clones, respectively. The concordance rate between the two antibody clones for CBs was 95% (19/20 concordant), with one CB that was negative with the Santa Cruz clone but positive with the Origene clone. Overall, with either antibody clone, 90% of AciCCs (18/20) were positive for NR4A3. On the other hand, NR4A3 staining was negative or demonstrated cytoplasmic granular staining in all other neoplasms tested FIGURE 1 and FIGURE 2. In CBs, nuclear NR4A3 staining showed 90% sensitivity and 100% specificity for AciCC. Immunocytochemistry on alcohol-fixed slides from one AciCC case showed strong nuclear NR4A3 staining FIGURE 3.
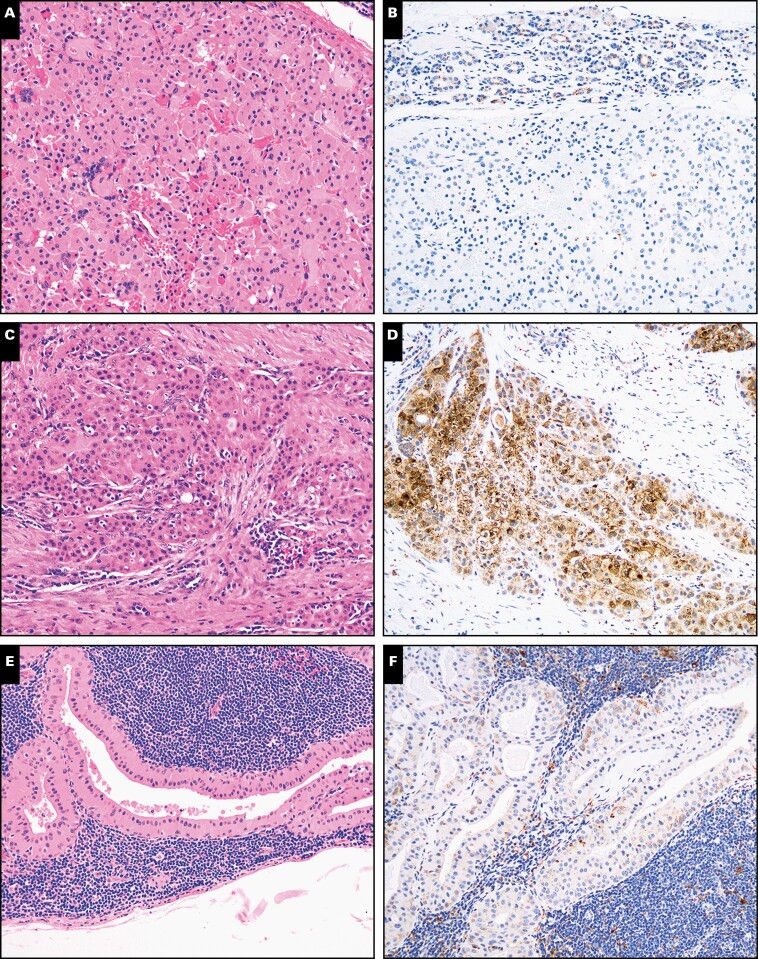
Figure 1.
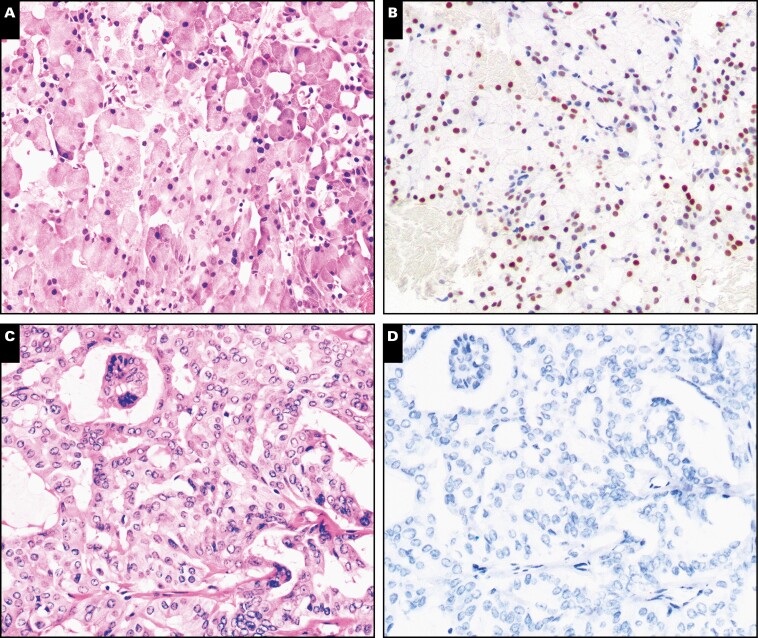
Nuclear receptor subfamily 4 group A member 3 (NR4A3) immunohistochemistry in acinic cell carcinoma and secretory carcinoma in cytology specimens (×100). Cell block preparations from fine-needle aspirates from acinic cell carcinoma (A, B) and secretory carcinoma (C, D), a key entity on the differential diagnosis, were stained with H&E or immunostained with NR4A3. Nuclear NR4A3 was seen only in acinic cell carcinoma cases but not in secretory carcinoma cases.
Figure 2.
Nuclear receptor subfamily 4 group A member 3 (NR4A3) immunohistochemistry in other primary oncocytic salivary gland neoplasms in cytology specimens (×100). Cell block preparations from fine-needle aspirates from oncocytoma (A, B), low-grade mucoepidermoid carcinoma (C, D), and Warthin tumor (E, F) were stained with H&E or immunostained with NR4A3. Nuclear NR4A3 was not identifiable in any of these oncocytic/oncocyotid salivary gland neoplasms. Nonspecific cytoplasmic granular NR4A3 staining was seen in the case of low-grade mucoepidermoid carcinoma.
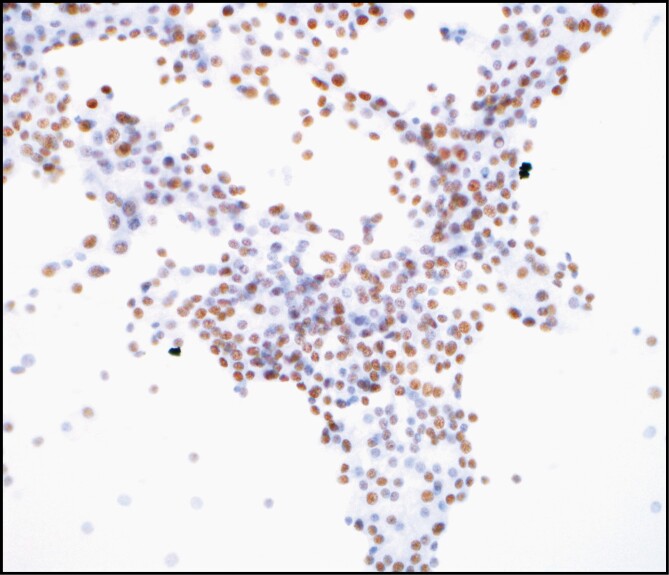
Figure 3.
Nuclear receptor subfamily 4 group A member 3 (NR4A3) immunocytochemistry on ethanol-fixed cytologic smear preparations of acinic cell carcinoma (×400).
In SRs, 89% of AciCCs (8/9) showed strong diffuse NR4A3 staining when using the NR4A3 Origene antibody clone FIGURE 4 and FIGURE 5. All three cases of AciCC with HGT were positive with the NR4A3 Santa Cruz antibody clone FIGURE 6. The single Origene-negative case of AciCC FIGURE 7A and Figure 7B stained positive with the Santa Cruz antibody clone. Overall, with either antibody clone, nuclear NR4A3 was seen in 100% of AciCCs, including the three cases with HGT (12/12; Figures 4 and 6). Among the non-AciCC SRs (n = 86), only one high-grade MEC (1/86, 1.2%) showed focal NR4A3 staining in 5% to 10% of the tumor cells Figure 7C and FIGURE 7D. This was also the only case that demonstrated discordance among the 19 matched non-AciCC cases (18/19) for a concordance rate of 94.7% between CBs and SRs. Finally, NR4A3 expression was not identifiable in normal salivary gland tissue when present (data not shown). Thus, in SRs, NR4A3 showed a 100% sensitivity and 98.8% specificity for AciCC in SRs.
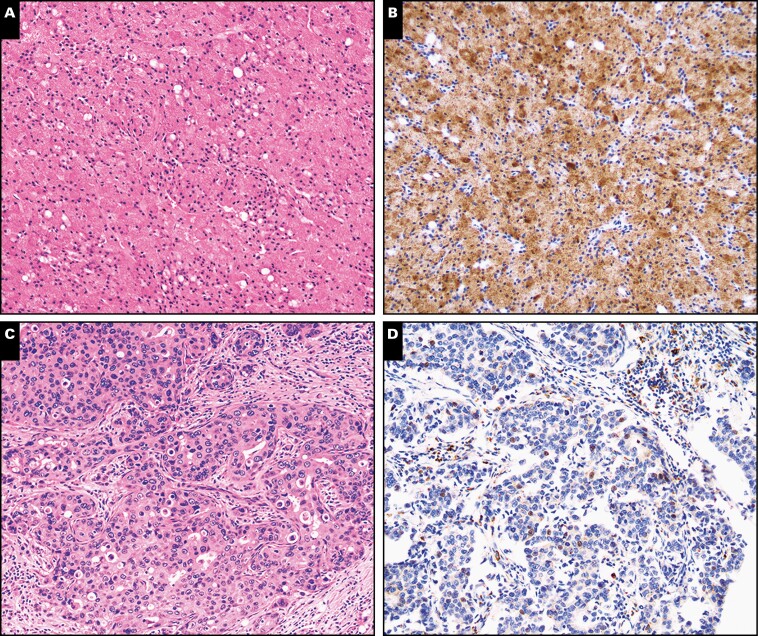
Figure 4.
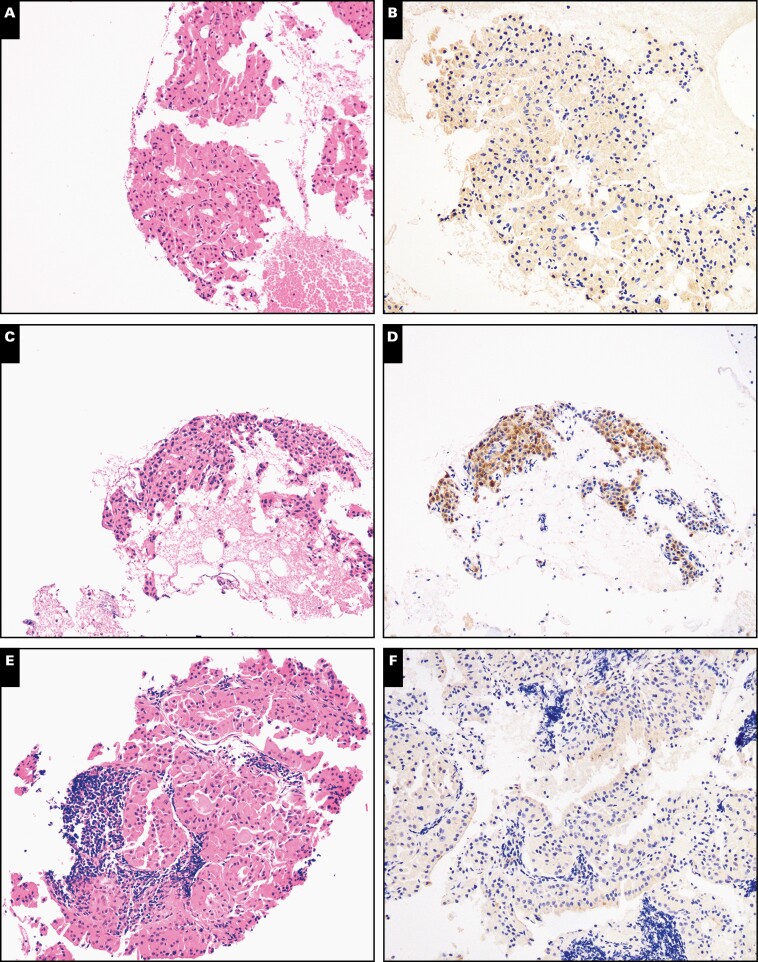
Nuclear receptor subfamily 4 group A member 3 (NR4A3) immunohistochemistry in acinic cell carcinoma and secretory carcinoma in surgical resections (×100). Representative sections from surgical resection specimens of acinic cell carcinoma (A, B) and secretory carcinoma (C, D) were stained with H&E or immunostained with NR4A3. Similar to cytologic preparations, nuclear NR4A3 was seen only in acinic cell carcinoma but not in secretory carcinoma.
Figure 5.
Nuclear receptor subfamily 4 group A member 3 (NR4A3) immunohistochemistry in other primary oncocytic salivary gland neoplasms in surgical resections (×100). Representative sections from surgical resection specimens of oncocytoma (A, B), oncocytic mucoepidermoid carcinoma (C, D), and Warthin tumor (E, F) were stained with H&E or immunostained with NR4A3. Similar to cytologic preparations, nuclear NR4A3 was not seen in other oncocytic salivary gland neoplasms, a few of which showed nonspecific granular cytoplasmic staining.
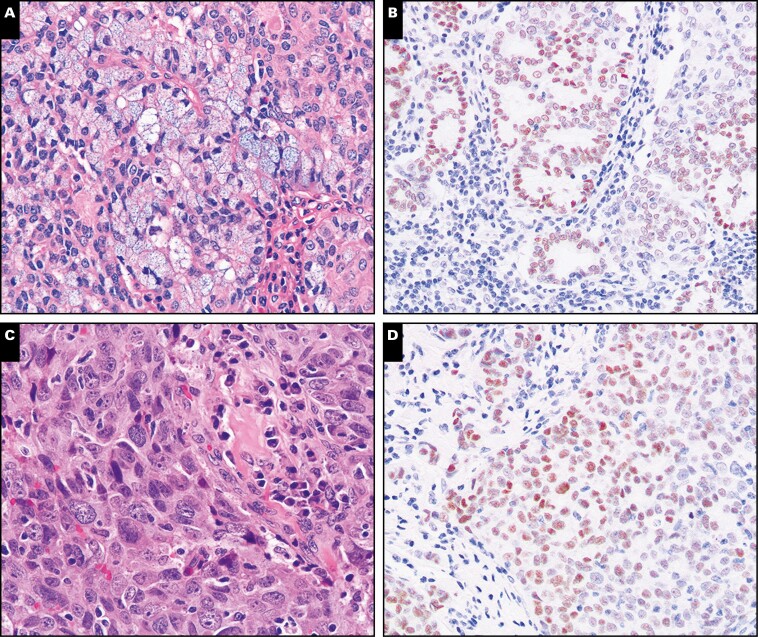
Figure 6.
Nuclear receptor subfamily 4 group A member 3 (NR4A3) expression is retained in acinic cell carcinoma with high-grade transformation. Representative sections from two different resections of acinic cell carcinoma with high-grade transformation (A-D) were stained with H&E or immunostained with NR4A3 (×200).
Figure 7.
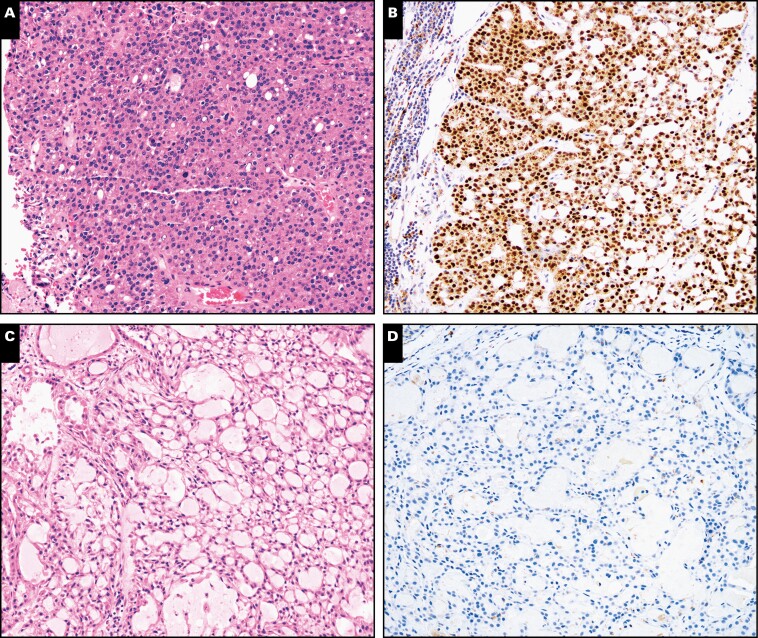
Examples of false-positive and a single negative case of nuclear receptor subfamily 4 group A member 3 (NR4A3) immunohistochemistry. Representative examples of a low-grade acinic cell carcinoma surgical resection negative with the Origene NR4A3 antibody clone (A, B; ×40) and a false-positive high-grade mucoepidermoid carcinoma (C, D; ×100).
Overall, nuclear NR4A3 showed a 90% to 100% sensitivity and 98.8% to 100% specificity for AciCC in CBs and SRs. The positive predictive value (PPV) and negative predictive value (NPV) of NR4A3 IHC for salivary AciCC were calculated to be 100% and 94.7% on CBs and 92.3% PPV and 100% NPV on SRs, respectively TABLE 2.
Table 2.
Performance Characteristics for NR4A3 in Acinic Cell Carcinoma and Other Primary Salivary Gland Neoplasms in Cytologic and Surgical Specimens (n = 135)
| Tumor Type | Specimen Type (No.) | NR4A3 Positive, No. (%) |
|---|---|---|
| Acinic cell carcinoma (n = 29) | SR (9) | 9/9 (100) |
| CB (20) | 18/20 (90) | |
| Acinic cell carcinoma with high-grade transformation (n = 3) | SR (3) | 3/3 (100) |
| Warthin tumor (n = 43) | SR (31) | 0/31 (0) |
| CB (19) | 0/19 (10) | |
| Mucoepidermoid carcinoma (n = 16) | SR (15) | 1/15 (6.7) |
| CB (6) | 0/6 (0) | |
| Secretory carcinoma (n = 10) | SR (7) | 0/7 (0) |
| CB (3) | 0/3 (0) | |
| Pleomorphic adenoma (n = 6) | SR (6) | 0/6 (0) |
| CB (2) | 0/2 (0) | |
| Carcinoma ex-pleomorphic adenoma (n = 2) | SR (2) | 0/2 (0) |
| Cellular pleomorphic adenoma (n = 1) | SR (1) | 0/1 (0) |
| CB (1) | 0/1 (0) | |
| Oncocytic carcinoma (n = 1) | SR (1) | 0/1 (0) |
| Oncocytoma (n = 19) | SR (18) | 0/18 (0) |
| CB (5) | 0/5 (0) | |
| Nodular oncocytosis (n = 5) | SR (5) | 0/5 (0) |
CB, cell block; NR4A3, nuclear receptor subfamily 4 group A member 3; SR, surgical resection.
DISCUSSION
The distinction of AciCC from other salivary gland neoplasms, especially those with oncocytic or oncocytoid features, can be difficult. While the characteristic histologic features may be more definitive on SRs, diagnosing AciCC in cytology aspirates may present a greater challenge.3 Moreover, canonical markers, including DOG1 and SOX10, are not entirely sensitive or specific for AciCC, although they are certainly quite useful adjuvant markers.8-11 These markers may also stain normal salivary tissue. Distinguishing AciCC from nonneoplastic and neoplastic mimickers can affect patient management; hence, it is important to have sensitive and specific IHC markers that can assist in this separation. Since the discovery of the t(4;9) translocation in AciCC in 2019, NR4A3 represents one such potential immunohistochemical marker.12,13
Only two other studies have explored the expression of NR4A3 in AciCC and non-AciCC cytology specimens.14,15 Nguyen et al15 assessed 11 AciCCs and 59 non-AciCCs in CBs and corresponding SRs. In their study, NR4A3 IHC showed a 100% sensitivity for AciCC. Skaugen et al14 showed that NR4A3 IHC had an 82% sensitivity in their cohort of 11 AciCC CBs. Similarly, our CB cohort of AciCCs showed a 90% sensitivity with NR4A3 IHC. All three studies (including ours) showed a 100% specificity of NR4A3 for AciCC. Overall, the sensitivity and specificity of NR4A3 IHC for AciCC were high in cytologic specimens. By comparison, DOG1, which has become the go-to marker for AciCC diagnosis, had a lower sensitivity, ranging from 73% to 82%, and a specificity ranging from 48% to 92% on cytology specimens, depending on the extent of membranous staining.14 Another important point is that in all studies, including ours, NR4A3 IHC expression was not seen in normal salivary gland acini (data not shown). A known potential pitfall for DOG1 and SOX10 is variable staining of normal salivary gland intercalated duct and acinar elements, respectively, that could lead to a false-positive interpretation, especially in cellular acinar cell–rich specimens.9,10,16-18 The combination of NR4A3 with additional IHC markers, including DOG1, in enhancing sensitivity and specificity for CBs and SRs has been proposed. Skaugen et al14 were unable to find any enhancement of NR4A3 performance characteristics with the addition of DOG1 IHC in cytologic specimens. In contrast, Cheng et al 19 reported improvement of NR4A3 performance to a sensitivity of 100% using the same combination in SRs. Thus, additional studies are needed to clarify the utility of NR4A3 IHC with other immunomarkers. Nonetheless, in all of these studies, NR4A3 performed well with high sensitivity and specificity for AciCC on cytology specimens.
In our study, the concordance rate between the two NR4A3 antibody clones was 95% on the CB preparations, with the Origene clone performing slightly better than the Santa Cruz clone. However, we noted that the Origene clone had a higher background signal compared with the Santa Cruz clone that was also previously noted in Haller et al.13 Thus, these factors should be considered when selecting an antibody clone for use in clinical practice.
A unique aspect of our study compared with Nguyen et al15 and Skaugen et al14 is that our cytologic cohort is the largest to date. Furthermore, to our knowledge, we are the first to demonstrate the application of NR4A3 immunocytochemistry on direct smear preparations of a case of AciCC. At times, the CBs can be quite limited, with diagnostic material primarily present on direct smears. In addition, using this approach enables preservation of the CB material should molecular testing be necessary. While we performed testing on smears from only a single case of AciCC, the nuclear NR4A3 staining was robust; thus, our work further expands the potential utility of NR4A3 immunostaining to direct smears, which can be explored on a larger cohort in future studies.
For our surgical cohort, NR4A3 IHC showed a 100% sensitivity when accounting for the positive staining with either antibody clone. However, the imperfect sensitivity was noted in other studies with NR4A3 IHC on SRs, ranging from 92% to 97%.13,20 By comparison to these studies, the sample size for our AciCC SR cohort was smaller and possibly skewed the relative sensitivity of the NR4A3 IHC on SRs. In addition, NR4A3 antibody clone-specific differences were seen: one AciCC SR that was negative for NR4A3 with the Origene clone was positive for the Santa Cruz clone.
However, other potential considerations may explain differences in NR4A3 immunostaining in these studies. First, institution-specific tissue fixation or antigen retrieval protocols might contribute to the differences in NR4A3 detection. Thus, the optimal threshold for considering a tumor NR4A3 positive will likely vary based on institution-dependent staining protocols and the experience level of the reviewing pathologist. Second, alternate NR4A family members (NR4A2 and NR4A1), which share similar downstream targets, may be involved in AciCC tumorigenesis. A recent communication from Haller et al21 demonstrated that NR4A2 was overexpressed in their only NR4A3-negative case of AciCC. Second, in Nguyen et al,15 one case of AciCC with HGT that had recurred and was treated with radiation demonstrated variable NR4A3 expression; the impact of treatment effect on NR4A3 expression in this case is not known and will need further study, including genetic analysis. Despite these challenges, NR4A3 IHC remains a useful marker in limited specimens due to its high PPV and NPV in both CBs and SRs. In addition, NR4A3 IHC has been shown to be far more sensitive for AciCC compared with fluorescence in situ hybridization, due to either the heterogeneous nature of the genomic breakpoints, which all may not be covered by the standard probes, or alternate rearrangements such as the HTN3-MSANTD3.12,13,22,23
Unlike the cytology specimens, NR4A3 IHC was not entirely specific for AciCC on SRs, with one high-grade MEC demonstrating focal weak to moderate staining FIGURE 5. The mechanism underlying the upregulation of NR4A3 in this case is unclear. Nonetheless, by comparison, our AciCC cohort demonstrated extensive to diffuse NR4A3 staining. Wong et al20 identified two cases of polymorphous adenocarcinoma and one case of a pleomorphic adenoma that showed focal, weak to moderate NR4A3 staining. However, both pleomorphic adenoma and polymorphous adenocarcinoma are morphologically distinct from AciCC and should not pose a diagnostic dilemma. Also, none of the other non-AciCC oncocytic neoplasms that share morphologic overlap with AciCC demonstrated any NR4A3 expression. Thus, in that context, NR4A3 IHC appears to be very specific for AciCC in the context of lesions with morphologic overlap and can be used with other ancillary immunohistochemical markers, such as DOG1 and SOX10, toward a definite diagnosis of AciCC.
Diagnosing HGT in AciCC on limited material, especially without a low-grade component, can be challenging given the extensive morphologic overlap with other high-grade salivary gland malignancies, such as salivary duct carcinoma, and the variable expression of IHC markers such as for DOG1 and SOX10.1,24 In our study, all three cases of AciCC with HGT showed strong NR4A3 expression FIGURE 4. Between the cytology and histology studies by Haller et al,13 Wong et al,20 Skaugen et al,14 and Nguyen et al,15 a total of 19 AciCCs with HGT were assessed with NR4A3 IHC. In all HGT cases, NR4A3 expression was present. Thus, NR4A3 can prove helpful in confirming the diagnosis of AciCC with HGT, especially in limited samples of a high-grade, nonspecific carcinoma.
Although our data demonstrate the value of NR4A3 in diagnosing AciCC, a few limitations include the small overall sample size of the AciCC cohort and lack of matched CBs and SRs for our AciCC. The lack of matched CBs and SRs was partly due to insufficient or absent diagnostic material on the concurrent CB or unavailable CB material in some cases. This is similar to other recently published studies and reflects the lower prevalence of these neoplasms.14,15
In summary, in the largest cytologic cohort to date, we demonstrate that NR4A3 is a promising, highly sensitive and specific immunomarker to distinguish AciCC from nonneoplastic and neoplastic salivary gland entities. Moreover, even AciCCs with HGT retain NR4A3 expression. NR4A3 immunostaining can also be potentially applied to cytologic smear preparations as a useful alternative for ancillary testing in the event that there is insufficient diagnostic CB material.
Acknowledgments
Funding and project support for this research were provided by the Center for Translational Pathology at the Department of Pathology and Laboratory Medicine, Weill Cornell Medicine. Funding to Peter M. Sadow and William C. Faquin was also provided by the National Cancer Institute of the National Institutes of Health (1PO1CA240239-01).
REFERENCES
- 1. Thompson LD, Herrera HB, Lau SK. A clinicopathologic series of 685 thyroglossal duct remnant cysts. Head Neck Pathol. 2016;10: 465-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El-Naggar AK, Chan JKC, Grandis JR, et al. WHO Classification of Head and Neck Tumours. Lyon, France: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 3. Hughes JH, Volk EE, Wilbur DC; Cytopathology Resource Committee, College of American Pathologists . Pitfalls in salivary gland fine-needle aspiration cytology: lessons from the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology. Arch Pathol Lab Med. 2005;129:26-31. [DOI] [PubMed] [Google Scholar]
- 4. Griffith CC, Pai RK, Schneider F, et al. Salivary gland tumor fine-needle aspiration cytology: a proposal for a risk stratification classification. Am J Clin Pathol. 2015;143:839-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viswanathan K, Sung S, Scognamiglio T, et al. The role of the Milan system for reporting salivary gland cytopathology: a 5-year institutional experience. Cancer Cytopathol. 2018;126:541-551. [DOI] [PubMed] [Google Scholar]
- 6. Faquin WC, Rossi ED, Baloch Z, et al. The Milan System for Reporting Salivary Gland Cytopathology. Cham, Switzerland: Springer Nature; 2018. [Google Scholar]
- 7. Griffith CC, Siddiqui MT, Schmitt AC. Ancillary testing strategies in salivary gland aspiration cytology: a practical pattern-based approach. Diagn Cytopathol. 2017;45:808-819. [DOI] [PubMed] [Google Scholar]
- 8. Ohtomo R, Mori T, Shibata S, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041-1050. [DOI] [PubMed] [Google Scholar]
- 9. Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol. 2016;56:134-142. [DOI] [PubMed] [Google Scholar]
- 10. Canberk S, Onenerk M, Sayman E, et al. Is DOG1 really useful in the diagnosis of salivary gland acinic cell carcinoma? A DOG1 (clone K9) analysis in fine needle aspiration cell blocks and the review of the literature. Cytojournal. 2015;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JH, Kang HJ, Yoo CW, et al. PLAG1, SOX10, and Myb expression in benign and malignant salivary gland neoplasms. J Pathol Transl Med. 2019;53:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haller F, Bieg M, Will R, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haller F, Skálová A, Ihrler S, et al. Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol. 2019;43: 1264-1272. [DOI] [PubMed] [Google Scholar]
- 14. Skaugen JM, Seethala RR, Chiosea SI, et al. Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma. Cancer Cytopathol. 2021;129:104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen L, Chopra S, Laskar DB, et al. NOR-1 distinguishes acinic cell carcinoma from its mimics on fine-needle aspiration biopsy specimens. Hum Pathol. 2020;102:1-6. [DOI] [PubMed] [Google Scholar]
- 16. Chênevert J, Duvvuri U, Chiosea S, et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919-929. [DOI] [PubMed] [Google Scholar]
- 17. Abd Raboh NM, Hakim SA. Diagnostic role of DOG1 and p63 immunohistochemistry in salivary gland carcinomas. Int J Clin Exp Pathol. 2015;8:9214-9222. [PMC free article] [PubMed] [Google Scholar]
- 18. Khurram SA, Speight PM. Characterisation of DOG-1 expression in salivary gland tumours and comparison with myoepithelial markers. Head Neck Pathol. 2019;13:140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng K, Wang X, Wei X, et al. Expression of NR4A3/NOR-1 in acinic cell carcinoma of the salivary gland [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:1142-1146. [DOI] [PubMed] [Google Scholar]
- 20. Wong KS, Marino-Enriquez A, Hornick JL, et al. NR4A3 immunohistochemistry reliably discriminates acinic cell carcinoma from mimics. Head Neck Pathol. 2021;15:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haller F, Moskalev EA, Kuck S, et al. Nuclear NR4A2 (Nurr1) immunostaining is a novel marker for acinic cell carcinoma of the salivary glands lacking the classic NR4A3 (NOR-1) upregulation. Am J Surg Pathol. 2020;44:1290-1292. [DOI] [PubMed] [Google Scholar]
- 22. Andreasen S, Varma S, Barasch N, et al. The HTN3-MSANTD3 fusion gene defines a subset of acinic cell carcinoma of the salivary gland. Am J Surg Pathol. 2019;43:489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barasch N, Gong X, Kwei KA, et al. Recurrent rearrangements of the Myb/SANT-like DNA-binding domain containing 3 gene (MSANTD3) in salivary gland acinic cell carcinoma. PLoS One. 2017;12:e0171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skálová A, Sima R, Vanecek T, et al. Acinic cell carcinoma with high-grade transformation: a report of 9 cases with immunohistochemical study and analysis of TP53 and HER-2/neu genes. Am J Surg Pathol. 2009;33:1137-1145. [DOI] [PubMed] [Google Scholar]