Abstract
The increasing incidence of food allergy remains a significant public health concern. Food allergy is partially due to a lack, or loss of tolerance to food allergens. Clinical outcomes surrounding early life practices, such as breastfeeding, antibiotic use, and food allergen exposure, indicate the first year of life in children represents a unique time for shaping the immune system to reduce allergic outcomes. Animal models have identified distinctive aspects of when and where dietary antigens are delivered within the intestinal tract to promote oral tolerance prior to weaning. Additionally, animal models have identified contributions from maternal proteins from breastmilk and bacterial products from the gut microbiota in regulating dietary antigen exposure and promoting oral tolerance, thus connecting decades of clinical observations on the benefits of breastfeeding, early food allergen introduction, and antibiotic avoidance in the first year of life in reducing allergic outcomes. Here we discuss how exposure to gut luminal antigens, including food allergens, is regulated in early life to generate protective tolerance and the implications of this process for preventing and treating food allergies.
Keywords: Regulatory T cells, oral tolerance, allergy, early life
Introduction:
Allergic diseases are a heterogeneous set of disorders characterized by T helper type 2 (Th2) immune responses to harmless environmental antigens, also known as allergens. Food allergy affects 8% of young children and 10% of adults in the United States, numbers that represent a significant increase over the past forty years1–3. Polymorphisms in genes related to the generation of Th2 responses are known risk factors for the development of allergy4, but changes in genetics alone is insufficient to explain this rapid increase. Allergic disorders are increased when individuals migrate from countries with a low prevalence of allergies to one with a high prevalence of allergy5, providing strong support for environmental contributors to the risk of allergic outcomes. However, identifying and defining the role of these environmental contributors has been difficult in part due to the time-limited nature of some of the risks or benefits conferred by these environmental exposures.
There is a growing appreciation that allergies may in part be due to loss, or lack, of tolerance to allergens. Food allergies in particular remain a significant public health concern, with exposure to allergens in affected individuals resulting in clinical symptoms ranging from mild urticaria to severe anaphylaxis. Although allergy to any type of food is possible, the most common food allergies in the United States are to peanut, fish, egg, wheat, milk, tree nuts, and soy1,2. In the healthy individual, the immune system develops tolerance to dietary antigens to blunt potential inflammatory responses. This process, termed oral tolerance, is largely mediated via the development of dietary antigen specific Foxp3+ T regulatory cells (Tregs). Treg dysfunction contributes to allergic disorders, as children with allergy have deficiencies in Tregs6,7 and Treg deficiency in mice results in immune skewing reminiscent of an allergic phenotype8. The majority of food allergies are acquired during the first few years of life9, drawing significant interest in how early life events, including antibiotic use and breastfeeding, impact the development of tolerance and allergy later in life. Recent human and animal studies support that there is a critical window of time in early life for the introduction of food allergens to promote tolerance and to decrease the risk of food allergy. Here we will review these observations supporting the existence of this period in early life.
The case for early life food allergen exposure
In sensitized individuals, food allergy manifests as dietary antigen specific IgE response leading to mast cell degranulation, Th2 responses, and clinical symptoms. Due to the fact that exposure to and introduction of food allergens can be controlled, food allergy is potentially the allergic disorder most amenable to dissecting environmental contributors to allergic outcomes. Despite this advantage, it is has taken decades to determine if and when food allergen introduction is beneficial. The current standard of care for food allergy is avoidance of food allergens, and because food allergy more commonly affects children, childhood feeding recommendations included food allergen avoidance in at risk children until recently. In the 1970s more than 70% of American babies were formula-fed, a practice that remained popular until the turn of the century. During this time allergic disorders, including food allergy continued to rise, suggesting that formula-feeding may be contributing to food allergy risk. This, as well as other potential benefits of breastfeeding, and the lack of suitable refrigeration of formulas in third world countries, prompted the resurgence of breastfeeding and the 2001 World Health Organizations recommendation for exclusive breastfeeding for the first six months of life. However, a 2011 retrospective analysis of children whose mothers followed this recommendation found that food allergies actually increased in exclusively breastfed children10. Concurrent with this, observational studies found that children consuming peanuts early in life had reduced peanut allergy11, prompting trials that demonstrated that early introduction of peanut into the diets of susceptible children between the age of 4 and 11 months decreased the rates of allergy development by 5 years of age11–15. Other studies showed similar associations of early introduction of solid food, including allergens other than peanuts, with reduced risk of food allergies16–19. Thus, decades of studies finally arrived at the recommendations of early food allergen consumption as protective for food allergy, yet why these benefits were restricted to this time in life remained unclear.
Early life factors promoting tolerance and avoiding allergy
Insights into why early introduction of food allergens is protective come from observations that the benefits of early exposure to allergens are not restricted to the child’s consumption of allergens. Recently, the role of maternal peanut consumption during breastfeeding and the early introduction of peanut allergen were examined more closely. This study revealed that the lowest incidence of peanut allergy by seven years of age occurred if the mothers both 1) consumed peanuts while breastfeeding and 2) introduced peanuts into the diet of their children before 12 months of age20. Of note, the rates of sensitization to peanut by seven years of age was increased if breastfeeding mothers did not introduce peanut into the diets of their children by this 12 month time point, yet again suggesting a critical role for the early introduction of antigen for the best development of oral tolerance to foods such as peanuts. Other studies found that the benefit of early introduction of a food allergen was most apparent in children who were breastfed21. Thus, complimentary breastfeeding and dietary antigen introduction provided the maximal benefit of early food allergen introduction in decreasing food allergy20,21, even without continual exposure to the food allergen12.
Breastfeeding
Additional insights into the benefits of breastfeeding come from mouse models which have shown complexes of maternal IgG antibodies and dietary antigens in the breastmilk are potent inducers oral tolerance and help prevent future allergic disorders22,23. Free antigens not bound in maternal IgG complexes were also able to induce tolerance in the presence of TGF-β, a cytokine present in the breastmilk with potent tolerogenic properties22,24. Further, breastfeeding is associated with significantly reduced risk of other atopic conditions in children, including asthma, eczema, and allergic rhinitis25. As children with food allergy are significantly more likely to have other atopic conditions26, breastfeeding may confer similar protective effect to the reduced risk of multiple types of allergic disorders regardless of the environmental antigen.
Microbial exposure
Multiple observations indicate that exposure to microbes, and in particular gut microbes, is protective of allergy. Decreased exposure to microbes is associated with increased allergic outcomes in humans27–29, and germ free mice are Th2 skewed30,31. This protective role does not extend to all microbes. Dysbiosis of the gut microbiota is associated with allergy and only specific bacterial taxa from the gut microbiota protect against an allergic phenotype in mice32,33. Interestingly the protective effects of specific gut bacteria on the allergic phenotype may be most pronounced in early life. A recent systematic review pinpointed the association of antibiotic use and development of childhood allergy to the first 6–12 months of life34. Cephalosporin use was associated with an increased risk of cow’s milk allergy (CMA) development35, and cohort studies revealed patients with CMA were found to have significant intestinal microbial dysbiosis compared to healthy controls, with healthy children having increased levels of Clostridial species, which were critical for the protection against an allergic response to food33. Further supporting the importance of this period in life for protective effects of the gut microbiota on allergic outcomes, children with allergic outcomes later in life had a transient dysbiosis of the gut microbiota in the first 100 days of life36. Other environmental factors that correlate with reduced risk of allergic disorders include exposure to a domesticated pet, such as a dog or cat37,38, which could impact the development of the gut microbiota in early life39. During early life, the pioneering microbiota in the neonate develops and matures into the microbiota found after weaning9,40. Breastfeeding, aside from having direct beneficial effects on protection from allergy, also contributes to early microbial colonization by providing a number of nutrients such as maternal IgA, human milk oligosaccharides (HMOs), and lactoferrin which help shape microbial communities in the neonate41–44. The combination of environmental factors such as breastfeeding, domestic pets, and avoidance of antibiotics all converge to facilitate the development of a healthy intestinal microbiota that is protective of allergic outcomes.
Establishing Oral Tolerance:
Over the past decade, multiple lines of work have converged to connect mechanisms regulating exposure to antigens and the necessity of the commensal microbiota in establishing oral tolerance. Tregs expressing the transcription factor Foxp3, have been shown as indispensable players in oral tolerance by suppressing subsequent immune responses45. Tregs that are raised against dietary or microbial antigens are peripherally derived Tregs (pTregs) that differentiate extrathymically in the lymph nodes draining the gut45,46. During their generation in the gut draining lymph nodes, these pTregs become preferentially imprinted with gut homing molecules allowing them to localize in the intestinal lamina propria47. The intestinal lamina propria contains a large population of Tregs, including pTregs induced in the gut draining lymph nodes, due in part to the favorable environment for inducing pTregs48. Notably, TGF-β present in the intestine, as well as retinoic acid (RA) from the diet, and microbial metabolites such as butyrate and other short-chain fatty acids are critical factors in the induction of Foxp3+ Tregs49–52. Tregs present in the intestine are potent suppressors of future inflammatory responses including food allergies and colitis driven responses against commensals53.
M cells and Peyer’s patches
Importantly, the induction of pTregs requires the acquisition, processing, and presentation of the cognate antigen by antigen presenting cells, indicating that the development of dietary antigen, or microbial antigen, specific pTregs is dependent upon these antigens traversing the epithelial barrier. Paracellular transport, or leak, is a well-known and studied pathway by which substances cross the epithelium by passing through the space between intestinal epithelial cells54. However, the size of peptides or amino acids delivered by paracellular leak are generally too small to generate antigen specific T cell responses55. One route to traverse the epithelial barrier to generate antigen specific T cell responses is through microfold cells (M cells), a specialized subset of intestinal epithelial cells found in the follicle-associated epithelium of Peyer’s patches, cecal patches, and isolated lymphoid follicles56. Through a variety of cellular transport mechanisms, including pinocytosis, macrocytosis, receptor-mediated endocytosis, luminal antigens transverse M cells and are delivered to underlying mononuclear phagocytic cells or antigen presenting cells, including macrophages and dendritic cells57. Whether the Peyer’s patch antigen presenting cells migrate to the MLN and are significant contributors to the induction of dietary and microbial antigen specific pTregs in the MLN is unclear, however Peyer’s patches have been observed to be dispensable for oral tolerance58,59. The primary immunological outcome for most luminal antigens delivered via M cells to Peyer’s patch follicles is induction of IgA secreting plasma cells60. Selective IgA deficiency has been associated with an increased risk of food allergy61,62, and presence of high levels of luminal IgA in early life has been associated with reduced risk of IgE-mediated diseases including food allergy63,64. M cells are present on the follicle associated epithelium overlying Peyer’s patches beginning in utero, and have not been shown to change in density or transport capacity during early life. In aged mice, the density and function of M cells decreases65,66, though this suggests M cell mediated transport represents a consistent pathway for antigen delivery from early life through most of adulthood.
Dendritic cell-mediated antigen acquisition
Dendritic cells in the lamina propria have the capacity to extend dendrites between epithelial cells into the gut lumen in response to bacterial metabolites as a potential mechanism to directly sample luminal contents67–69. Mice deficient in the chemokine receptor CX3CR1 lack these trans-epithelial dendrites and are deficient in oral tolerance, yet can still generate antigen specific T cell responses to dietary antigens in the gut draining lymph nodes68,70,71. This indicates that delivery of dietary antigen to the gut draining lymph nodes is not impaired in the absence of trans-epithelial dendrite extension, and suggests that the loss of oral tolerance may be due to other effects of the absence of CX3CR1. Further the extension of trans-epithelial dendrites is lacking in mouse strains which are not impaired in oral tolerance72, suggesting the presence of other pathways of antigen delivery supporting oral tolerance.
Goblet cell-associated antigen passages
Goblet cells are the second most abundant intestinal epithelial cell lineage and play a crucial role in maintaining the epithelial barrier by secreting mucins and anti-microbial peptides73. Similar to M cells, goblet cells have also been shown as capable of translocating luminal antigens to underlying immune cells through the formation of goblet cell-associated antigen passages (GAPs)74,75. Goblet cells are located on non-follicle bearing epithelium overlaying lamina propria, which represents the vast expanse of the intestinal landscape where a majority of the immune cells reside. The lamina propria has been shown to be a major site for antigen acquisition by antigen presenting cells for the induction of tolerance. Thus, GAPs are potentially a major pathway for antigens to cross the epithelium to be acquired by antigen presenting cells in the lamina propria and generate T cell responses. Compared to M cells, GAP formation by goblet cells is a dynamic process with multiple regulatory mechanisms to control when antigen exposure occurs. Just prior to the time of weaning (21 days old in most standard specific pathogen free mouse facilities) GAPs form in the small intestine in the healthy state and presumably persist throughout life74,75,76. Antigen delivered via small intestinal GAPs induce and maintain pTregs within the small intestinal environment77. Small intestinal Tregs developing in adult life require frequent dietary antigen exposure53, delivered via GAPs77, to be maintained, revealing a limitation of oral tolerance generated during adult life. Cessation of antigen exposure either through lack of ingestion or inhibition of delivery via GAPs resulted in a fairly rapid decrease in pTregs53,77. There are times in life when tolerance is not the desired outcome of immune responses to luminal antigens, and indeed during times of infection, GAP formation, and therefore antigen exposure is inhibited by inflammatory cytokines present as part of the immune response to the pathogen78. Ignorance of luminal antigens through temporarily halting luminal delivery would prevent the development of undesired inflammatory responses to innocuous antigens79–81. This apparent plasticity in the adult pTreg response subject to disruption during infection or cessation of antigen delivery suggests functional, though potentially transient, oral tolerance induction occurs during adulthood and could underlie why later introduction of food allergens is less effective at reducing food allergy (Figure 1).
Figure 1:
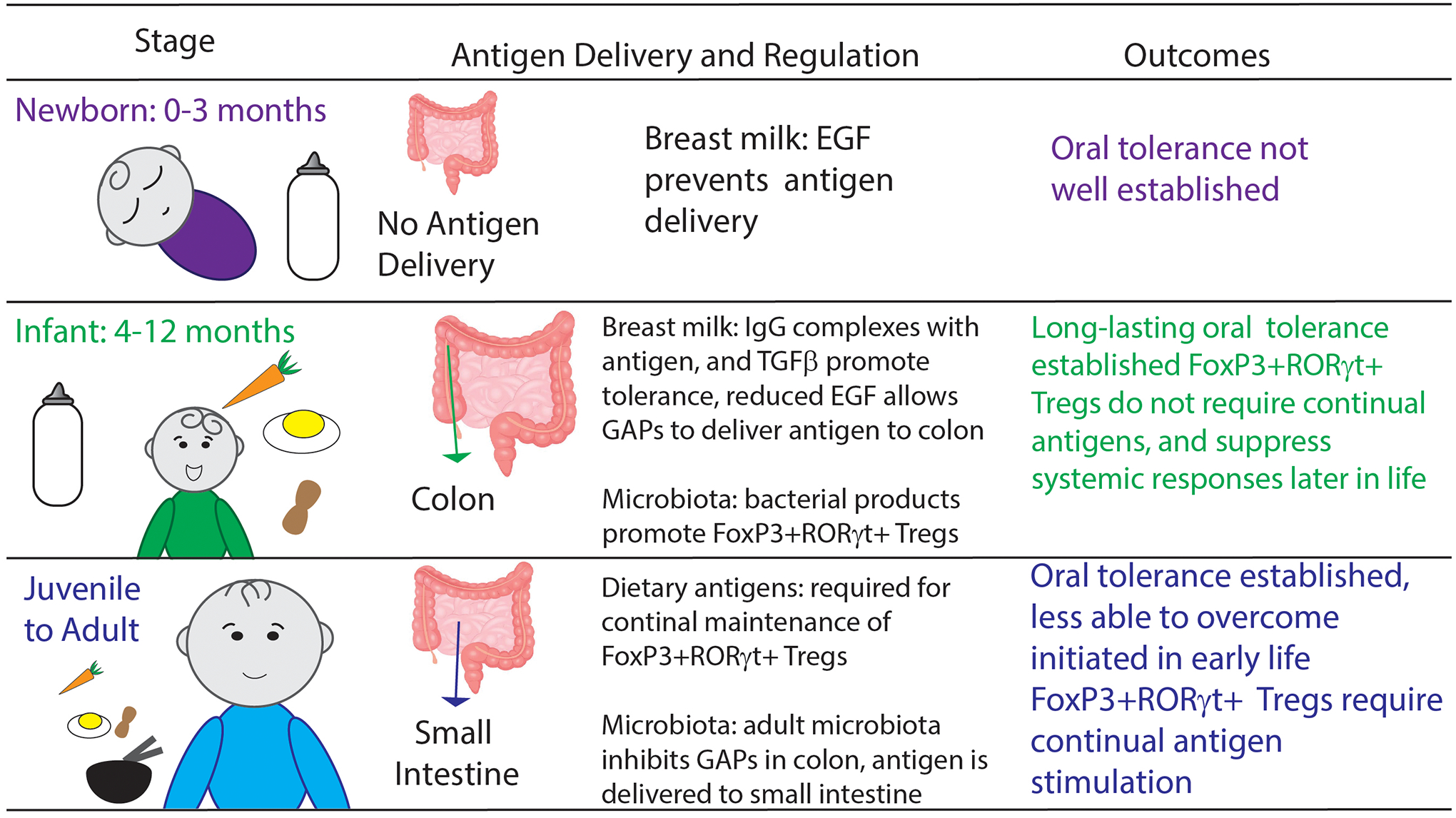
Oral antigens are encountered through distinct routes throughout life. How these delivery pathways are regulated and the outcomes following antigen delivery are unique to each phase of life.
Defining antigen delivery and oral tolerance in early life:
While tolerance to dietary antigens can be induced both in early life and adulthood, observations in humans and animals indicate there are differences in the outcomes of dietary antigen introduction at these times in life. Breastfeeding is unique to early life and contributes to these differences by controlling when and where dietary antigens are encountered by the gut immune system. Breastmilk contains high levels of epidermal growth factor receptor (EGFR) ligands including EGF, amphiregulin, HB-EGF, and TGFα which promote intestinal barrier function by supporting intestinal epithelial cell development82 and IgA development83. EGF levels in breastmilk are highest immediately after parturition and decrease through lactation until weaning84. Activation of EGFR on goblet cells in the intestine inhibits delivery of luminal antigens by inhibiting GAP formation75. In nursing mice EGF levels in the gut lumen have a proximal to distal gradient and a temporal decrease throughout nursing reflective of the origin of EGF, the breastmilk. High levels of EGFR ligands in the gut lumen in the first week of life result in no GAP formation and limited antigen delivery across the intestine76,85, a time when oral tolerance is difficult to induce, which is reversed after the first week of life86,87 (Figure 1). Around day of life 10 in nursing mice, the EGF levels decrease in the colonic lumen to allow colonic GAPs to form, but remain higher in the small intestinal lumen and inhibits small intestinal GAPs, directing antigen delivery to the colonic immune system resulting in tolerance to dietary and commensal antigens76,85. That tolerance to dietary antigens introduced pre-weaning would be induced in the colon, as opposed to the small intestine, is a surprising and unique feature of this time in life.
Peripherally derived RORγt Tregs
Tregs developing early in life have been shown to have distinct roles in restraining inflammatory responses88, and much attention has been focused on a specialized subset of RORγt expressing Foxp3+ Tregs arising in the colon and driven by the microbiota89–91. Indeed early life antibiotics been shown to reduce the development of Tregs92,93. RORγt+ Foxp3+ Tregs have been shown to control a variety of immune responses89,90 and support the development of future tolerogenic responses against dietary antigens85. Clinical data has found a reduction of RORγt+ Foxp3+ Tregs in individuals with food allergy7 revealing the importance of RORγt+ Foxp3+ Tregs in humans. Intriguingly expansion of RORγt+ Foxp3+ Tregs appears to be maternally regulated with a setpoint determined in early life94 and peripherally derived Tregs may have age-dependent fates95 further implicating the importance of early life in the induction of oral tolerance. While pediatricians recommend exposing infants to a wide variety of dietary proteins starting at 6 months old, early life exposure to every food product one would ever encounter to develop dietary antigen specific pTregs to all possible components of the diet is simply unachievable. Given the potent suppressive nature of these RORγt+ Foxp3+ Tregs, their expansion during early life in response to the microbiota may help explain why this phase in life is so critical to lifelong immune balance and tolerance. The multifaceted nature of this population of Tregs in suppressing inflammatory responses suggest presence of these Tregs sets the stage for tolerance96 and disruption of this “window of tolerance” can result in allergy.
Distinct T cell responses induced in various periods of life suggest T cells have the capacity for differential phenotypes depending on the phase of life in which the response is initiated. One potential difference between the colon lamina propria prior to and following weaning still to be explored is the antigen presenting cell population. Dendritic cells necessary for oral tolerance are imprinted by the microbiota in early life97,98, and may have reduced capacity to induce effector T cell responses99,100. Prior to weaning, resident colonic macrophages are derived from yolk sac macrophages, and are replaced by bone-marrow derived macrophages which respond to antigens differently101,102. Thus unique components of the developing immune system might also define this ‘window of tolerance’.
Disrupting the “window of tolerance”
The fine control of the “window of tolerance” relies on input from maternal ligands from the breastmilk and bacterial products from the commensal microbiota to define this phase (Figure 2). Disruption of synchronous breastfeeding by cross-fostering newborn pups to dams delivering litters two weeks prior, resulted in reduced EGF concentrations in the pups’ lumen, reflecting the reduced concentrations of EGF found in formula-fed children’s stool. Asynchronously cross-fostered offspring had significantly reduced RORγt+ Foxp3+ pTregs, and lacked the ability to induce oral tolerance to dietary antigens85. Furthermore, pups asynchronously cross-fostered in the reversed combination, older pups to dams having recently delivered, had a profound inability to induce oral tolerance following exposure to antigens both in prior to weaning, and as adults. Following immunization against antigens that had first been introduced orally, asynchronously cross-fostered mice developed strong Th2 immune responses and became lethargic and hypothermic resembling anaphylaxis85. The increased EGF concentrations in these mice resulted in significantly reduced antigen delivery via GAPs, highlighting the importance of early life exposure to antigens. Thus, the combination of multiple breastmilk proteins with oral antigens support oral tolerance by opening the “window of tolerance” at the proper time and regulating antigen delivery to the colon lamina propria for the induction of long-lived pTregs prior to weaning85.
Figure 2:
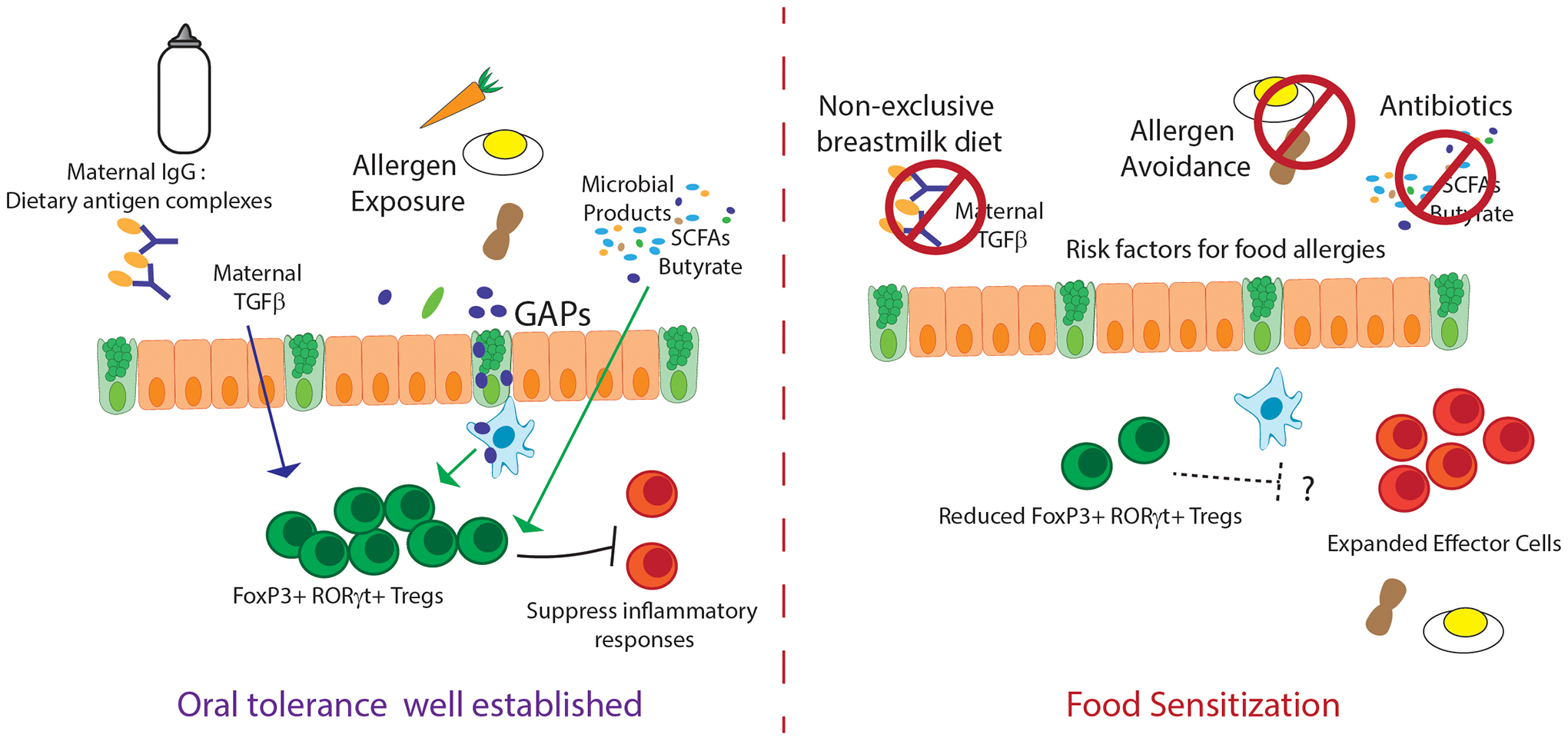
Multiple factors contribute to establishing oral tolerance during early life. Absence of any one of these factors increases the risk of food sensitization and food allergies later in life.
Similar to the first week of murine life being a poor time for the induction of oral tolerance86,87, a defect in induction of oral tolerance was noted at the time of weaning103. This time in life has been associated with a brief inflammatory state coined as a “weaning reaction”93. This inflammatory state requires the developing microbiota and potentially imprints the pTregs to protect from intestinal inflammation later in life93. The presence of the microbiota at the time of weaning also is required to inhibit GAP formation and antigen delivery to the colon following weaning75,76. Extension of antigen exposure past weaning by allowing colonic GAP formation resulted in reduced development of antigen specific Tregs in favor of an expanded effector response specific to commensal bacterial antigens76. Together these data suggest the closing of the “window of tolerance” is equally as important for the stable development of long lived Tregs.
Future Directions: Prevention and Treatment of Food Allergies
Risk factors for food allergy can be described by alterations in this window of tolerance: missing this window, as in the case of delayed allergen exposure, disrupting this window, as in the case of formula-feeding9,104,105, or dysbiosis of the microbiota during this window, as in the case of oral antibiotics, all can contribute to improper development of Tregs and oral tolerance. The necessity of antibiotics to treat bacterial infections and formula for parents unable to breastfeed stresses the need for future work focusing on optimizing these therapies and diets for minimal disruption to oral tolerance. Clinically used antibiotics should be vetted for those that are least disruptive to microbiota, the development of RORγt+ Foxp3+ Tregs, and the induction of tolerance during early life. Additionally, work has long been underway to modulate infant formula through the inclusion of tolerogenic factors found in breastmilk24. With the improvement of these practices, prevention of food sensitization could be simpler than treatment of existing food allergies.
Oral Immunotherapy
Strategies to relieve food allergy symptoms concentrate on oral tolerance, similar to strategies to prevent food allergies. Much attention has been paid to oral immunotherapy, where patients ingest small doses of allergens, particularly peanuts, in an attempt to reduce adverse reactions by modifying allergen specific IgE concentrations106–108. Such trials have shown moderate success and have resulted in “sustained unresponsiveness” or a lack of an allergic reaction towards the allergen in about 1/3–1/2 of the treated group106,109. Patients receiving oral immunotherapy (OIT) treatment for peanut allergy had increased antigen-specific Treg induction, with increased Foxp3 transcripts, along with decreased methylation of the Foxp3 locus consistent with an increased production of Tregs110. Importantly, successful OIT marked by “sustained unresponsiveness” appears to require “maintenance” doses of allergens to prevent regaining reactivity to peanut107. Intriguingly these data parallel the requirement for continued dietary antigen in mice for the maintenance of RORγt+ Foxp3+ Tregs developing in the small intestine after weaning53. Thus, the limitations how oral tolerance is induced and maintained after the “window of tolerance” has important ramifications into how difficult it may be to shift an allergic response towards a tolerant one later in life.
Targeting early life for prevention
Attempting to utilize the potent Treg-inducing nature of some bacterial species, groups have turned to bacteriotherapy to relieve food allergy symptoms, with some modest success in animal models7. General probiotic administration in early life has shown some improvement in allergic diseases, such as eczema and wheeze111,112, but appear to have limited to no effect in food sensitization113. Probiotic bacteria, such as Lactobacillus rhamnosus, has been included along with peanut allergens during oral immunotherapy in clinical trials, again with modest success114,115. However, more research is needed to understand how to utilize the microbiota both to prevent and treat food allergies, to understand how the microbiota is disrupted in allergic individuals, and to understand how reversing dysbiosis may affect allergic outcomes116,117.
To further improve oral tolerance-based therapies to treat food allergies, attention should be given to the components driving the long-lasting oral tolerance initiated during early life. If the desired goal is to recapitulate the RORγt+ Foxp3+ Tregs developing prior to weaning, understanding what drives expansion of this specialized population during early life may be the pathway forward. The concerted effort undertaken to deliver antigens to the colonic lamina propria prior to weaning suggests unique factors present within the colonic environment. As described above, antigens introduced through the small intestine following weaning induce tolerance, but in a manner that requires frequent antigen exposure to maintain these Tregs53. Delivering dietary antigens to the colon following weaning to induce T cell responses may have minimal effect as antigen delivery across the colonic epithelium following weaning in minimized75 and limited to the distal colon77. Allowing GAPs to form in the proximal colon in adults resulted in inflammatory responses toward the commensal microbiota118, possibly due to the complexity and immunogenicity of the adult microbiota. Therefore, directing antigens to the colon following weaning without the context of the infant microbiota and/or other unique factors to this time of life could have deleterious consequences. These observations suggest that recapitulating the unique events surrounding oral tolerance induction to develop therapies for treatment of allergies may be complicated.
In conclusion, the benefit of early introduction of allergens in protection from food allergies later in life, may be due to multiple contributions that regulate oral tolerance in early life. Within the intestinal lumen proteins from the maternal breastmilk and the microbiota synergize to direct dietary antigens and food allergens to the colon prior to weaning. These proteins may also contribute to the immune environment in the infant colon lamina propria which, along with include factors yet unknown, to induce long-lasting Tregs that provide suppression of allergic responses throughout life. Such concepts help clarify what is unique about early life oral tolerance, why complimentary early introduction of food allergens with breastfeeding is protective of food allergy, and what factors may define potential therapeutics for future food allergies treatments.
Funding:
Supported by grants: DK097317-RDN, AI131342-RDN, AI1407551-RDN, AI136515-RDN, DK109006-KAK, AI144721-KAK, DK122187-KAK.
Footnotes
Conflict of Interest: RDN and KAK are inventors on U.S. Nonprovisional Application Serial No. 15/880,658 Compositions And Methods For Modulation Of Dietary And Microbial Exposure.
References
- 1.Iweala OI, Burks AW. Food Allergy: Our Evolving Understanding of Its Pathogenesis, Prevention, and Treatment. Curr Allergy Asthma Rep. 2016;16(5):37. [DOI] [PubMed] [Google Scholar]
- 2.Tordesillas L, Berin MC, Sampson HA. Immunology of Food Allergy. Immunity. 2017;47(1):32–50. [DOI] [PubMed] [Google Scholar]
- 3.Gupta RS, Warren CM, Smith BM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Network Open. 2019;2(1):e185630–e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ober C, Yao T-C. The genetics of asthma and allergic disease: a 21st century perspective. Immunological reviews. 2011;242(1):10–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabieses B, Uphoff E, Pinart M, Antó JM, Wright J. A systematic review on the development of asthma and allergic diseases in relation to international immigration: the leading role of the environment confirmed. PloS one. 2014;9(8):e105347–e105347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stelmaszczyk-Emmel A, Zawadzka-Krajewska A, Szypowska A, Kulus M, Demkow U. Frequency and Activation of CD4+CD25high FoxP3+ Regulatory T Cells in Peripheral Blood from Children with Atopic Allergy. International archives of allergy and immunology. 2013;162(1):16–24. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Gadir A, Stephen-Victor E, Gerber GK, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nature medicine. 2019;25(7):1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhasselt V Neonatal tolerance under breastfeeding influence. Current opinion in immunology. 2010;22(5):623–630. [DOI] [PubMed] [Google Scholar]
- 10.Fewtrell M, Wilson DC, Booth I, Lucas A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2011;342:c5955. [DOI] [PubMed] [Google Scholar]
- 11.Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. The Journal of allergy and clinical immunology. 2008;122(5):984–991. [DOI] [PubMed] [Google Scholar]
- 12.Du Toit G, Sayre PH, Roberts G, et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med. 2016;374(15):1435–1443. [DOI] [PubMed] [Google Scholar]
- 13.Du Toit G, Roberts G, Sayre PH, et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. New England Journal of Medicine. 2015;372(9):803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkin MR, Logan K, Tseng A, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. New England Journal of Medicine. 2016;374(18):1733–1743. [DOI] [PubMed] [Google Scholar]
- 15.Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis. Jama. 2016;316(11):1181–1192. [DOI] [PubMed] [Google Scholar]
- 16.Poole JA, Barriga K, Leung DY, et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006;117(6):2175–2182. [DOI] [PubMed] [Google Scholar]
- 17.Nwaru BI, Erkkola M, Ahonen S, et al. Age at the introduction of solid foods during the first year and allergic sensitization at age 5 years. Pediatrics. 2010;125(1):50–59. [DOI] [PubMed] [Google Scholar]
- 18.Koplin JJ, Osborne NJ, Wake M, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. The Journal of allergy and clinical immunology. 2010;126(4):807–813. [DOI] [PubMed] [Google Scholar]
- 19.Koplin JJ, Dharmage SC, Ponsonby AL, et al. Environmental and demographic risk factors for egg allergy in a population-based study of infants. Allergy. 2012;67(11):1415–1422. [DOI] [PubMed] [Google Scholar]
- 20.Pitt TJ, Becker AB, Chan-Yeung M, et al. Reduced risk of peanut sensitization following exposure through breast-feeding and early peanut introduction. Journal of Allergy and Clinical Immunology. 2018;141(2):620–625.e621. [DOI] [PubMed] [Google Scholar]
- 21.Katz Y, Rajuan N, Goldberg MR, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. The Journal of allergy and clinical immunology. 2010;126(1):77–82.e71. [DOI] [PubMed] [Google Scholar]
- 22.Mosconi E, Rekima A, Seitz-Polski B, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010;3(5):461–474. [DOI] [PubMed] [Google Scholar]
- 23.Ohsaki A, Venturelli N, Buccigrosso TM, et al. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. The Journal of experimental medicine. 2018;215(1):91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rekima A, Macchiaverni P, Turfkruyer M, et al. Long-term reduction in food allergy susceptibility in mice by combining breastfeeding-induced tolerance and TGF-β-enriched formula after weaning. Clinical & Experimental Allergy. 2017;47(4):565–576. [DOI] [PubMed] [Google Scholar]
- 25.Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta paediatrica. 2015;104(467):38–53. [DOI] [PubMed] [Google Scholar]
- 26.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008(10):1–8. [PubMed] [Google Scholar]
- 27.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709. [DOI] [PubMed] [Google Scholar]
- 28.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst T, Sichelstiel A, Schär C, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. American journal of respiratory and critical care medicine. 2011;184(2):198–205. [DOI] [PubMed] [Google Scholar]
- 31.Hill DA, Siracusa MC, Abt MC, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nature medicine. 2012;18(4):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefka AT, Feehley T, Tripathi P, et al. Commensal bacteria protect against food allergen sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(36):13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feehley T, Plunkett CH, Bao R, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nature medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netea SA, Messina NL, Curtis N. Early-life antibiotic exposure and childhood food allergy: A systematic review. The Journal of allergy and clinical immunology. 2019;144(5):1445–1448. [DOI] [PubMed] [Google Scholar]
- 35.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Mother’s and offspring’s use of antibiotics and infant allergy to cow’s milk. Epidemiology. 2013;24(2):303–309. [DOI] [PubMed] [Google Scholar]
- 36.Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science translational medicine. 2015;7(307):307ra152. [DOI] [PubMed] [Google Scholar]
- 37.Wegienka G, Havstad S, Kim H, et al. Subgroup differences in the associations between dog exposure during the first year of life and early life allergic outcomes. Clin Exp Allergy. 2017;47(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesselmar B, Hicke-Roberts A, Lundell AC, et al. Pet-keeping in early life reduces the risk of allergy in a dose-dependent fashion. PLoS One. 2018;13(12):e0208472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tun HM, Konya T, Takaro TK, et al. Exposure to household furry pets influences the gut microbiota of infants at 3–4 months following various birth scenarios. Microbiome. 2017;5(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plunkett CH, Nagler CR. The Influence of the Microbiome on Allergic Sensitization to Food. Journal of immunology (Baltimore, Md : 1950). 2017;198(2):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantoja-Feliciano IG, Clemente JC, Costello EK, et al. Biphasic assembly of the murine intestinal microbiota during early development. The ISME journal. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogier EW, Frantz AL, Bruno MEC, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proceedings of the National Academy of Sciences. 2014;111(8):3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barile D, Rastall RA. Human milk and related oligosaccharides as prebiotics. Curr Opin Biotechnol. 2013;24(2):214–219. [DOI] [PubMed] [Google Scholar]
- 45.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunology. 2012;5:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agace W Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunology Letters. 2010;128(1):21–23. [DOI] [PubMed] [Google Scholar]
- 48.Cording S, Wahl B, Kulkarni D, et al. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 2014;7(2):359–368. [DOI] [PubMed] [Google Scholar]
- 49.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. Journal of immunology. 2007;179(6):3724–3733. [DOI] [PubMed] [Google Scholar]
- 50.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204(8):1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. [DOI] [PubMed] [Google Scholar]
- 53.Kim KS, Hong S-W, Han D, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351(6275):858–863. [DOI] [PubMed] [Google Scholar]
- 54.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annual Review of Physiology. 2011;73(1):283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knoop KA, Miller MJ, Newberry RD. Transepithelial antigen delivery in the small intestine: different paths, different outcomes. Curr Opin Gastroenterol. 2013;29(2):112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hathaway LJ, Kraehenbuhl JP. The role of M cells in mucosal immunity. Cellular and molecular life sciences : CMLS. 2000;57(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6(4):666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spahn TW, Fontana A, Faria AM, et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer’s patches. Eur J Immunol. 2001;31(4):1278–1287. [DOI] [PubMed] [Google Scholar]
- 59.Spahn TW, Weiner HL, Rennert PD, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol. 2002;32(4):1109–1113. [DOI] [PubMed] [Google Scholar]
- 60.Rios D, Wood MB, Li J, Chassaing B, Gewirtz AT, Williams IR. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016;9(4):907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janzi M, Kull I, Sjöberg R, et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133(1):78–85. [DOI] [PubMed] [Google Scholar]
- 62.Tuano KS, Orange JS, Sullivan K, Cunningham-Rundles C, Bonilla FA, Davis CM. Food allergy in patients with primary immunodeficiency diseases: prevalence within the US Immunodeficiency Network (USIDNET). The Journal of allergy and clinical immunology. 2015;135(1):273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kukkonen K, Kuitunen M, Haahtela T, Korpela R, Poussa T, Savilahti E. High intestinal IgA associates with reduced risk of IgE‐associated allergic diseases. Pediatric allergy and immunology. 2010;21(1‐Part‐I):67–73. [DOI] [PubMed] [Google Scholar]
- 64.Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal immunology. 2011;4(6):603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi A, Donaldson DS, Erridge C, et al. The functional maturation of M cells is dramatically reduced in the Peyer’s patches of aged mice. Mucosal Immunol. 2013;6(5):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donaldson DS, Pollock J, Vohra P, Stevens MP, Mabbott NA. Microbial Stimulation Reverses the Age-Related Decline in M Cells in Aged Mice. iScience. 2020;23(6):101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature immunology. 2001;2(4):361–367. [DOI] [PubMed] [Google Scholar]
- 68.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–258. [DOI] [PubMed] [Google Scholar]
- 69.Morita N, Umemoto E, Fujita S, et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature. 2019. [DOI] [PubMed] [Google Scholar]
- 70.Hadis U, Wahl B, Schulz O, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–246. [DOI] [PubMed] [Google Scholar]
- 71.Schulz O, Jaensson E, Persson EK, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. The Journal of experimental medicine. 2009;206(13):3101–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. Journal of immunology (Baltimore, Md : 1950). 2006;176(4):2465–2469. [DOI] [PubMed] [Google Scholar]
- 73.Kim YS, Ho SB. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Current Gastroenterology Reports. 2010;12(5):319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015;8(1):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knoop KA, Gustafsson JK, McDonald KG, et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol. 2017;2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kulkarni DH, Gustafsson JK, Knoop KA, et al. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunology. 2020;13(2):271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kulkarni DH, McDonald KG, Knoop KA, et al. Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal Immunology. 2018;11(4):1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hand TW, Dos Santos LM, Bouladoux N, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337(6101):1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bouziat R, Hinterleitner R, Brown JJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356(6333):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouziat R, Biering SB, Kouame E, et al. Murine Norovirus Infection Induces TH1 Inflammatory Responses to Dietary Antigens. Cell host & microbe. 2018;24(5):677–688.e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang X, Liu H, Yang S, Li Z, Zhong J, Fang R. Epidermal Growth Factor and Intestinal Barrier Function. Mediators of inflammation. 2016;2016:1927348–1927348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee DN, Kuo TY, Chen MC, Tang TY, Liu FH, Weng CF. Expression of porcine epidermal growth factor in Pichia pastoris and its biology activity in early-weaned piglets. Life sciences. 2006;78(6):649–654. [DOI] [PubMed] [Google Scholar]
- 84.Kobata R, Tsukahara H, Ohshima Y, et al. High levels of growth factors in human breast milk. Early human development. 2008;84(1):67–69. [DOI] [PubMed] [Google Scholar]
- 85.Knoop KA, McDonald KG, Coughlin PE, et al. Synchronization of mothers and offspring promotes tolerance and limits allergy. JCI Insight. 2020;5(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanson D Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. The Journal of Immunology. 1981;127(4):1518–1524. [PubMed] [Google Scholar]
- 87.Strobel S, Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatric research. 1984;18(7):588–594. [DOI] [PubMed] [Google Scholar]
- 88.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348(6234):589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohnmacht C, Park J-H, Cording S, et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015. [DOI] [PubMed] [Google Scholar]
- 90.Sefik E, Geva-Zatorsky N, Oh S, et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 2015;349(6251):993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell. 2017;168(5):928–943.e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adami AJ, Bracken SJ, Guernsey LA, et al. Early-life antibiotics attenuate regulatory T cell generation and increase the severity of murine house dust mite-induced asthma. Pediatric research. 2018;84(3):426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al Nabhani Z, Dulauroy S, Marques R, et al. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity. 2019;50(5):1276–1288.e1275. [DOI] [PubMed] [Google Scholar]
- 94.Ramanan D, Sefik E, Galván-Peña S, et al. An Immunologic Mode of Multigenerational Transmission Governs a Gut Treg Setpoint. Cell. 2020;181(6):1276–1290.e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pratama A, Schnell A, Mathis D, Benoist C. Developmental and cellular age direct conversion of CD4+ T cells into RORγ+ or Helios+ colon Treg cells. Journal of Experimental Medicine. 2019;217(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al Nabhani Z, Eberl G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunology. 2020;13(2):183–189. [DOI] [PubMed] [Google Scholar]
- 97.Stern A, Wold AE, Östman S. Neonatal mucosal immune stimulation by microbial superantigen improves the tolerogenic capacity of CD103(+) dendritic cells. PLoS One. 2013;8(9):e75594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pezoldt J, Pasztoi M, Zou M, et al. Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes. Nat Commun. 2018;9(1):3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torow N, Yu K, Hassani K, et al. Active suppression of intestinal CD4(+)TCRαβ(+) T-lymphocyte maturation during the postnatal period. Nature communications. 2015;6:7725–7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papaioannou NE, Pasztoi M, Schraml BU. Understanding the Functional Properties of Neonatal Dendritic Cells: A Doorway to Enhance Vaccine Effectiveness? Frontiers in immunology. 2019;9:3123–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in adult intestine. Nature immunology. 2014;15(10):929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lakhdari O, Yamamura A, Hernandez GE, et al. Differential Immune Activation in Fetal Macrophage Populations. Scientific reports. 2019;9(1):7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller B, Newby T, Stokes C, Hampson D, Brown P, Bourne F. The importance of dietary antigen in the cause of postweaning diarrhea in pigs. American journal of veterinary research. 1984;45(9):1730–1733. [PubMed] [Google Scholar]
- 104.de Silva D, Geromi M, Halken S, et al. Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69(5):581–589. [DOI] [PubMed] [Google Scholar]
- 105.Mathias JG, Zhang H, Soto-Ramirez N, Karmaus W. The association of infant feeding patterns with food allergy symptoms and food allergy in early childhood. International breastfeeding journal. 2019;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. The Journal of allergy and clinical immunology. 2014;133(2):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chinthrajah RS, Purington N, Andorf S, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394(10207):1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. The Lancet. 2019;393(10187):2222–2232. [DOI] [PubMed] [Google Scholar]
- 109.Nagakura KI, Yanagida N, Sato S, et al. Low-dose oral immunotherapy for children with anaphylactic peanut allergy in Japan. Pediatr Allergy Immunol. 2018;29(5):512–518. [DOI] [PubMed] [Google Scholar]
- 110.Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). The Journal of allergy and clinical immunology. 2014;133(2):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–1079. [DOI] [PubMed] [Google Scholar]
- 112.Wickens K, Barthow C, Mitchell EA, et al. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol. 2018;29(8):808–814. [DOI] [PubMed] [Google Scholar]
- 113.Loo EX, Llanora GV, Lu Q, Aw MM, Lee BW, Shek LP. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: a 5-year follow-up. International archives of allergy and immunology. 2014;163(1):25–28. [DOI] [PubMed] [Google Scholar]
- 114.Tang ML, Ponsonby AL, Orsini F, et al. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. The Journal of allergy and clinical immunology. 2015;135(3):737–744.e738. [DOI] [PubMed] [Google Scholar]
- 115.Dunn Galvin A, McMahon S, Ponsonby AL, Hsiao KC, Tang MLK. The longitudinal impact of probiotic and peanut oral immunotherapy on health-related quality of life. Allergy. 2018;73(3):560–568. [DOI] [PubMed] [Google Scholar]
- 116.Aitoro R, Paparo L, Amoroso A, et al. Gut Microbiota as a Target for Preventive and Therapeutic Intervention against Food Allergy. Nutrients. 2017;9(7):672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berni Canani R, Paparo L, Nocerino R, et al. Gut Microbiome as Target for Innovative Strategies Against Food Allergy. Frontiers in immunology. 2019;10:191–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knoop KA, McDonald KG, Kulkarni DH, Newberry RD. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut. 2016;65(7):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]


