Abstract
Long noncoding RNAs (lncRNAs) are crucial regulators of gene expression in many biological processes, but their biological functions remain largely unknown, especially in fungi. Fusarium graminearum is an important pathogen that causes the destructive disease Fusarium head blight (FHB) or head scab disease on wheat and barley. In our previous RNA sequencing (RNA‐Seq) study, we discovered that lncRsp1 is an lncRNA that is located +99 bp upstream of a putative sugar transporter gene, Fgsp1, with the same transcription direction. Functional studies revealed that ΔlncRsp1 and ΔFgsp1 were normal in growth and conidiation but had defects in ascospore discharge and virulence on wheat coleoptiles. Moreover, lncRsp1 and Fgsp1 were shown to negatively regulate the expression of several deoxynivalenol (DON) biosynthesis genes, TRI4, TRI5, TRI6, and TRI13, as well as DON production. Further analysis showed that the overexpression of lncRsp1 enhanced the ability of ascospore release and increased the mRNA expression level of the Fgsp1 gene, while lncRsp1‐silenced strains reduced ascospore discharge and inhibited Fgsp1 expression during the sexual reproduction stage. In addition, the lncRsp1 complementary strains lncRsp1‐LC‐1 and lncRsp1‐LC‐2 restored ascospore discharge to the level of the wild‐type strain PH‐1. Taken together, our results reveal the distinct and specific functions of lncRsp1 and Fgsp1 in F. graminearum and principally demonstrate that lncRsp1 can affect the release of ascospores by regulating the expression of Fgsp1.
Keywords: DON production, Fgsp1, Fusarium graminearum, long noncoding RNA, sexual reproduction
lncRsp1, a long noncoding RNA located +99 bp upstream of a putative sugar transporter gene Fgsp1, can affect the release of ascospores by regulating the expression of Fgsp1 in Fusarium graminearum.

1. INTRODUCTION
The regulatory roles of long noncoding RNAs (lncRNAs) have been well described in animals, plants, and fungi, where they serve as crucial regulators of transcription in a wide range of biological processes (Ponting et al., 2009; Rinn et al., 2007; Swiezewski et al., 2009; Yamashita et al., 2016). lncRNAs can participate in controlling gene expression via various molecular mechanisms. For example, the DHFR (human dihydrofolate reductase) gene contains two promoters. The minor promoter upstream of DHFR encodes an lncRNA that forms a triplex structure with the sequences of the major promoter and interacts directly with transcription factor IIB (TFIIB), resulting in the dissociation of the transcription initiation complex from the major promoter and inhibitor of DHFR (Martianov et al., 2007; Quan et al., 2015). COLDAIR, an Arabidopsis intronic lncRNA induced by cold treatment, has been shown to recruit Polycomb Repressive Complex 2 (PRC2) to suppress the FLOWER LOCUS C (FLC) after vernalization (Heo & Sung, 2011). Saccharomyces cerevisiae SER3 is involved in serine biosynthesis, the 3′ end of lncRNA SRG1 overlaps with the promoter of SER3, and the elongation of SRG1 inhibits the initiation of SER3 transcription by transcription interference under serine‐rich conditions (Martens et al., 2004).
In recent years, several studies have identified or predicted the lncRNAs involved in the growth and development of filamentous fungi such as Neurospora crassa (Arthanari et al., 2014; Cemel et al., 2017), Ustilago maydis (Donaldson & Saville, 2013; Ostrowski & Saville, 2017), Fusarium graminearum (Kim et al., 2018; Wang et al., 2021), Trichoderma reesei (Till, Pucher, et al., 2018), and Ustilaginoidea virens (Tang et al., 2021). However, there are only a few surveys regarding the functional identification of lncRNAs in filamentous fungi. One of the best known lncRNAs is qrf in N. crassa. qrf is a long noncoding antisense RNA of the circadian clock core regulatory frq gene and inhibits the expression of frq by mediating chromatin modification (Belden et al., 2011; Li et al., 2015; Xue et al., 2014). The lncRNA HAX1 interacts with a transcriptional activator Xylanase regulator 1 (Xyr1), interferes with the negative feedback regulatory loop of Xyr1, and ultimately promotes cellulase expression and activity in T. reesei (Till, Mach, et al., 2018; Till, Pucher, et al., 2018). Our recent studies suggest that a novel antisense lncRNA, GzmetE‐AS, is involved in asexual and sexual reproduction by regulating its antisense gene GzmetE through the RNAi pathway in F. graminearum (Wang et al., 2021). These data reveal that lncRNAs also play important regulatory roles in filamentous fungi.
F. graminearum (teleomorph Gibberella zeae) is a globally distributed causal agent of Fusarium head blight (FHB) of cereal crops worldwide, especially of wheat (Goswami & Kistler, 2004; Leslie & Summerell, 2006). In addition to the yield losses caused by the infection of cereal spikelets, F. graminearum can also produce harmful mycotoxins, including deoxynivalenol (DON) and zearalenone (ZEA), which are extremely toxic to humans and livestock (Audenaert et al., 2014; Desjardins, 1996; Zhang et al., 2016). In the disease cycle of FHB, ascospores (sexual spores) and conidia (asexual spores) play a critical role in primary and secondary infection, respectively (Guenther & Trail, 2005; Trail, 2007). Under favourable environmental conditions, F. graminearum spreads rapidly and causes severe grain yield losses. Thus, perithecium formation, ascospore production, and ascospore discharge play crucial roles in the infection cycle of F. graminearum. Several genes are involved in the process of sexual reproduction, including conserved signal transduction pathways, protein kinases, transcription factors, and other genes with diverse functions (Geng et al., 2014; Hou et al., 2002; Hu et al., 2014; Son et al., 2011, 2013; Wang et al., 2011; Yun et al., 2015). In most cases, the disruption of these genes causes sexual development to be stopped or delayed at certain specific stages. For instance, mgv1 and Gpmk1 deletion mutants are female sterile and fail to reproduce perithecia, whereas GEA1 and MID1 are known to be important for ascospore release in F. graminearum (Cavinder et al., 2011; Hou et al., 2002; Jenczmionka et al., 2003; Son et al., 2013). However, lncRNAs involved in the regulation of sexual reproduction have rarely been studied. The identification and functional characterization of lncRNA mutants would provide comprehensive insight into the regulatory mechanisms underlying the sexual development of filamentous fungi.
The major facilitator superfamily (MFS) transport proteins are a large protein family with diverse physiological functions in all living organisms (Yen et al., 2010). The sugar porter (SP) family is the largest branch of the MFS (Pao et al., 1998). In phytopathogenic fungi, MFS proteins have been demonstrated to be involved in the regulation of multidrug resistance and toxin accumulation (Alexander et al., 1999; Choquer et al., 2007; Coleman & Mylonakis, 2009; Kretschmer et al., 2009). In the present study, we discovered an lncRNA, lncRsp1, located +99 bp upstream of a putative sugar porter gene, Fgsp1. Functional studies showed that the disruption of Fgsp1 and lncRsp1 had pleiotropic defects, including on sexual development, virulence, and DON production. Subsequently, silencing and overexpression assays of lncRsp1 were performed to explore their biological functions. Our results suggest that lncRsp1 plays an important role in sexual reproduction and regulates the expression of Fgsp1.
2. RESULTS
2.1. Identification of lncRsp1 and Fgsp1
Based on the sequence analysis of the full‐length FGSG_05042 mRNA, the open reading frame (ORF) was determined to encode a protein of 534 amino acids (GenBank accession no. XP_011323525). NCBI domain analysis indicated that the protein contains a sugar transport domain and two MFS conserved domains. Therefore, FGSG_05042 was designated as Fgsp1 (Figure 1a). Transmembrane domain prediction (http://www.cbs.dtu.dk/services/TMHMM/) indicated that Fgsp1 possesses 12 transmembrane helixes (TM) (Figure 1a), suggesting that Fgsp1 is a member of the 12‐TM group of MFS transporters. An lncRNA, referred to as lncRsp1 here, was detected in the RNA sequencing (RNA‐Seq) data. It was located +99 bp upstream of Fgsp1 and had the same direction as the Fgsp1 gene. The full‐length sequences of Fgsp1 and lncRsp1 were determined by performing the rapid amplification of cDNA ends (RACE) and directional sequencing (Figure S1a–c). The results indicated that Fgsp1 had two transcript isoforms, Fgsp1‐1 and Fgsp1‐2, which were transcribed from different transcription initiation sites (TISs) and shared the same 3ʹ ends with poly(A) tails (Figure 1c). The Fgsp1‐1 transcript isoform was 1975 nucleotides (nt) in length, overlapping with lncRsp1‐2 by 338 bases, while the Fgsp1‐2 transcript isoform was 2798 nt in length. The TIS of Fgsp1‐2 was located upstream of Fgsp1‐1 and overlapped with the entire sequence of Fgsp1‐1 (Figure 1b). The Fgsp1‐2 TIS was only 72 nt apart from that of lncRsp1. The 3ʹ RACE sequences included lncRsp1‐1, 1089 nt in length, whose transcription termination site (TTS) was adjacent to the second exon of Fgsp1‐1. lncRsp1‐2, another 607 nt‐lncRNA transcript isoform sharing the same TIS as lncRsp1‐1, was also located upstream of Fgsp1‐1 (Figure 1b). Although 5′ RACE results indicated that Fgsp1 has two transcript isoforms, Fgsp1‐1 and Fgsp1‐2, according to our previous RNA‐Seq data of the wild‐type strain PH‐1, few reads could be mapped onto the 5′ end of the Fgsp1‐2 transcript isoform in IGV Sashimi plots at different developmental stages, including sexual development stages (0, 7, and 10 days postfertilization [dpf]), conidial stages (3 [mycelial stage] and 12 h after incubation in CMC liquid medium [sporulation stage]) (Figure S2). This suggested that the Fgsp1 gene was mainly transcribed as the Fgsp1‐1 transcript isoform at all the different stages. Therefore, we did not consider the effect of Fgsp1‐2 transcript on the other transcript isoforms. Using the reverse transcription quantitative PCR (RT‐qPCR) method, the expression levels of the two isoforms of Fgsp1 and lncRsp1 were analysed at different developmental stages. The results showed that the expression levels of Fgsp1 and lncRsp1 during sporulation were both higher than those during vegetative growth (Figure 1c). During sexual development, both Fgsp1 and lncRsp1 were significantly up‐regulated at 7 dpf compared with their expression at 0 dpf (Figure 1d).
FIGURE 1.

The expression pattern of lncRsp1 and Fgsp1 during the asexual and sexual stages. (a) The Fgsp1 amino acid sequence contains a sugar transporter domain (green), two major facilitator superfamily domains (MFS, blue), and 12 transmembrane helixes (TM, orange), identified using the SMART protein database (http://smart.embl‐heidelberg.de) and the NCBI protein database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The transmembrane domain was predicted with the TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/).(b) Schematic representation of lncRsp1 and Fgsp1 transcript regions detected by RACE‐PCR analysis. The position of gene‐specific primers (GSP) used to assay the expression levels of lncRsp1 and Fgsp1 are marked with arrows. nt, nucleotide; F, forward primer; R, reverse primer. (c) Expression levels of lncRsp1 and Fgsp1 during asexual stages were measured by reverse transcription quantitative PCR (RT‐qPCR). Cultures were collected from YEPD (black) and CMC (red) liquid medium after 3 and 24 h of inoculation as mycelium and sporulation samples, respectively. The expression levels in the mycelium sample were set as the control. The F. graminearum β‐tubulin gene was used as the internal standard. (d) Expression levels of lncRsp1 and Fgsp1 in the sexual stage were detected by RT‐qPCR. The mycelium collected from a carrot agar plate at 7 days postinoculation was used as the 0 days postfertilization (dpf) sample (black). Perithecia were collected from a carrot agar plate after 7 days of self‐crossing and used as the 7 dpf sample (red). The expression levels in the wild‐type strain PH‐1 at 0 dpf were set as the control. Results shown represent means ± SD. Error bars indicate SD calculated from three replicated data points. The asterisks indicate significant differences (p < 0.01)
To determine their biological functions, Fgsp1 and lncRsp1 deletion mutants (ΔFgsp1 and ΔlncRsp1) were generated using a homology recombination strategy. The entire lncRsp1 transcriptional region (−977,720 to −978,636 of chromosome 3) was deleted in the ΔlncRsp1 mutant, while the ΔFgsp1 mutant was generated by deletion of the Fgsp1 transcriptional region (−978,706 to −980,551 of chromosome 3). The mutants were confirmed by PCR amplification and Southern blotting analysis (Figure S3a–c). Meanwhile, the complementary strains of Fgsp1, ΔFgsp1‐C, were constructed to confirm that the defects observed in ΔFgsp1 were derived from the deletion of Fgsp1. In the asexual stage, no obvious differences in hyphal growth, conidiation, or conidial morphology were observed (Figure S4a–c, Table S1). Therefore, neither Fgsp1 nor lncRsp1 is essential for asexual reproduction and hyphal growth in F. graminearum.
2.2. Fgsp1 and lncRsp1 are involved in sexual reproduction
When we assayed sexual reproduction on carrot agar medium, normal‐shaped perithecia and ascospore cirri were produced from all mutants at 7 and 14 dpf, respectively (Figure 2a). However, compared to that of PH‐1, the number of discharged ascospores of the ΔlncRsp1 mutant was significantly decreased. Additionally, a more pronounced defect in ascospore discharge was observed in the ΔFgsp1 mutant (Figure 2b). Subsequently, we examined the ascospores and asci in perithecia sampled at 6, 7, and 10 dpf. At 7 dpf, less than 20% and 40% of asci produced by ΔFgsp1 and ΔlncRsp1 were type I asci, which contain eight spindle‐shaped ascospores, while 65% of asci were type I in PH‐1 (Figure 2c,d). In ΔFgsp1 and ΔlncRsp1, most of the asci were defective, such as abnormal asci with fewer ascospores (type II) or failed to form asci (type III) (Figure 2c,d). Moreover, ΔFgsp1 and ΔlncRsp1 asci showed significantly reduced glycogen accumulation in comparison with those of the wild type after KI‐I2 staining (Figure 2c). These results indicate that Fgsp1 and lncRsp1 are involved in glycogen accumulation, ascus development, and ascospore discharge in F. graminearum.
FIGURE 2.

Defects of the ΔlncRsp1 and ΔFgsp1 mutants in sexual reproduction. (a) Morphology of perithecia and cirri of the wild‐type PH‐1, ΔlncRsp1, and ΔFgsp1 strains. Perithecia (upper panel) and perithecia with cirri (lower panel) were observed on carrot agar medium. PH‐1 strain was used as the control. Photographs were taken at 7 and 14 days postfertilization (dpf) on carrot agar. Bar = 500 µm. (b) Ascospore discharge was examined using 7‐day‐old perithecia. Photographs were taken after being released for 18 h. The white cloud is the accumulation of discharged ascospores. (c) Asci morphology and glycogen accumulation from cracked perithecia. The samples were stained with KI‐I2 solution. Bar = 20 μm. (d) Morphology and statistical analysis of asci rosettes of 7‐day‐old perithecia. The morphology of type I asci rosettes was selected from the wild‐type strain PH‐1; type II and type III were selected from ΔFgsp1. The statistical analysis for each type of ascus morphology was conducted at 7 dpf. A total of 50 asci rosettes were counted and classified in each strain under light microscopy. Line bars indicate standard errors from three repeated experiments
2.3. Fgsp1 and lncRsp1 play crucial roles in the pathogenicity of F. graminearum
To investigate the roles of Fgsp1 and lncRsp1 in fungal virulence, pathogenicity assays on flowering wheat heads and wheat coleoptiles were conducted. At 14 days postinoculation (dpi), spikes infected with the ΔFgsp1 and ΔlncRsp1 strains displayed similar symptoms to spikes infected by the wild‐type strain (Figure 3a,b). However, when a pathogenicity assay was performed on wheat coleoptiles, the average lengths of the brown lesions caused by the ΔFgsp1 and ΔlncRsp1 mutants were 0.59 ± 0.26 and 0.54 ± 0.26 cm, respectively, whereas those infected by the wild‐type strain showed an average lesion length of 1.03 ± 0.09 cm (Figure 3c,d), indicating a significant reduction in the virulence of the ΔFgsp1 and ΔlncRsp1 mutants. Thus, Fgsp1 and lncRsp1 are required for the virulence of F. graminearum to wheat coleoptiles.
FIGURE 3.

Roles of the lncRsp1 and Fgsp1 in fungal pathogenicity. (a) Pathogenicity assays on wheat spikelets (marked with black dots) with a conidial suspension of the wild‐type PH‐1, ΔlncRsp1, ΔFgsp1 mutants, and ΔFgsp1‐C1/C2 strains. The photographs were taken 14 days after inoculation. The white arrows indicate the inoculation sites on wheat head. (b) The disease index calculated 14 days after inoculation. (c) Wheat coleoptiles infected with each strain were observed and photographed 7 days after inoculation. The black arrows indicate the lesion. (d) The length of the brown lesions on wheat coleoptiles. Mean and standard deviation were calculated from three repeated experiments. Means of the column with different letters represent a statistically significant difference at p = 0.05
2.4. Fgsp1 and lncRsp1 negatively regulate DON production
To detect the production of DON, the mycotoxin produced in infected wheat kernels with scab symptoms at 14 dpi was measured in this study. The DON production (over 1200 ng/kernel) was increased significantly in the ΔFgsp1 and ΔlncRsp1 mutants compared with the 435 ng/kernel DON produced by the wild‐type PH‐1 strain (Figure 4a). We further assayed the DON production in rice medium, as described by Seo et al. (1996). Similar to the results of the wheat grain inoculation assay, more DON production was synthesized in rice medium by ΔFgsp1 and ΔlncRsp1 mutants than by the wild‐type strain PH‐1 (Figure 4a). These results indicate that Fgsp1 and lncRsp1 negatively regulate DON biosynthesis. To further confirm the results, the expression levels of the DON synthesis genes TRI4, TRI5, TRI6, TRI13, TRI101, and TRI12 were measured in DON‐inducing cultures by a RT‐qPCR assay. The results demonstrated that the expression levels of TRI4, TRI5, TRI6, and TRI13 were significantly up‐regulated in the ΔFgsp1 and ΔlncRsp1 mutants compared to those in the wild type (Figure 4b), which is consistent with the fact that the deletion of Fgsp1 and lncRsp1 produced more DON than the wild‐type strain in both wheat kernels and rice medium (Figure 4a). Interestingly, the expression levels of TRI101 and TRI12 were reduced in the Fgsp1 mutant (Figure 4c). As these two genes are not located in the same TRI cluster, Fgsp1 probably modulates them through different pathways. Previous research has suggested that hyphal bulbous structures at the toxigenic stage are related to DON production (Jiang et al., 2016). After being incubated in trichothecene biosynthesis induction (TBI) cultures for 5 days, abundant bulbous hyphal structures were observed in PH‐1 and the ΔlncRsp1 mutant. However, bulbous structures were rarely observed in the ΔFgsp1 mutant under the same conditions (Figure 4d). Together, these results showed that Fgsp1 and lncRsp1 function as negative regulators in DON biosynthesis by suppressing the expression of four genes relevant to DON synthesis, TRI4, TRI5, TRI6, and TRI13. Furthermore, Fgsp1 is also required for DON production‐related cellular differentiation.
FIGURE 4.
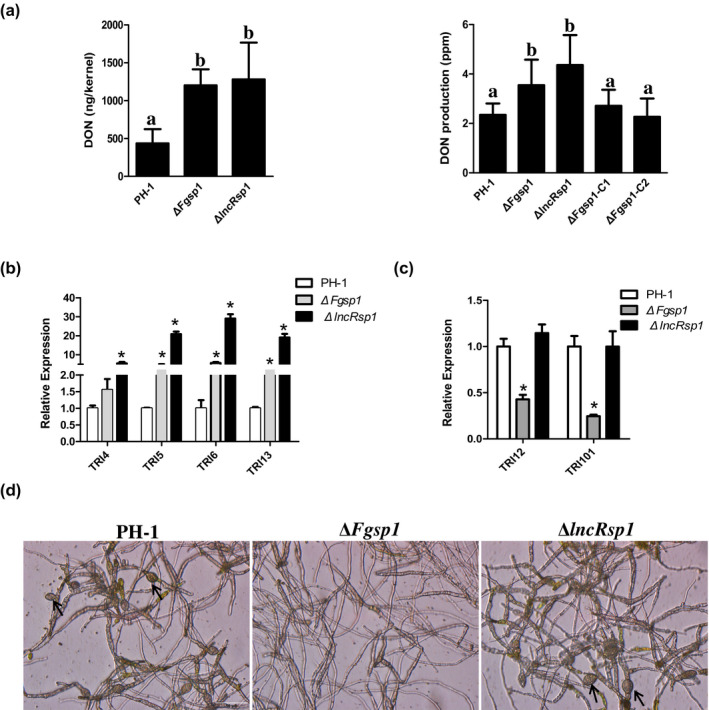
lncRsp1 and Fgsp1 regulate deoxynivalenol (DON) production and TRI gene expression. (a) DON production in 14‐day‐old infected wheat kernels (left) and 21‐day‐old rice grain cultures (right) was measured. Data with three replicates were analysed using the protected Fisher's least significant difference (LSD) test. Different letters on the bars indicate statistically significant differences (p = 0.05). (b) Relative expression levels of TRI4, TRI5, TRI6, and TRI13 in the wild‐type strain PH‐1, ΔlncRsp1, and ΔFgsp1 were measured by reverse transcription quantitative PCR (RT‐qPCR). (c) Relative expression levels of TRI12 and TRI101 genes in PH‐1, ΔlncRsp1, and ΔFgsp1 mutants. The expression level in PH‐1 was set as 1. Error bars indicate the standard deviation from three biological replicates. The asterisks indicate significant differences (p < 0.01). (d) Five‐day‐old trichothecene biosynthesis induction cultures of the wild‐type PH‐1 and the ΔlncRsp1 and ΔFgsp1 mutants were examined to observe bulbous structures (labelled with arrows). Bar = 20 µm
2.5. lncRsp1 positively regulates Fgsp1 transcript levels
The above results indicate that the expression patterns of Fgsp1 and lncRsp1 are similar. Their deletion caused similar defects in ascospore discharge, virulence, and DON biosynthesis. Previous studies have shown that lncRNAs could affect the expression of their neighbouring genes (Mercer et al., 2009; Rinn & Chang, 2012). To investigate the regulatory relationship between lncRsp1 and Fgsp1, the Fgsp1 and lncRsp1 transcript level in the wild type, the ΔlncRsp1 mutant, and the ΔFgsp1 mutant were measured during sexual stages (cultures grown on carrot agar at 0 and 7 dpf). The Fgsp1 expression level in the wild‐type strain was 105 times higher at 7 dpf than that at 0 dpf, and only six times higher in the ΔlncRsp1 mutant. Thus, the deletion of lncRsp1 significantly decreased the expression level of Fgsp1 at 7 dpf. Similarly, the expression level of lncRsp1 was also significantly down‐regulated by the deletion of Fgsp1 at 7 dpf (Figure 5a). However, the physical location of lncRsp1 raised the concern that the lncRsp1 transcript could be part of the Fgsp1 transcript and the deletion of lncRsp1 could cause the disruption of Fgsp1 itself. To address this concern, lncRsp1 silencing and overexpression vectors were randomly integrated into the wild‐type strain to generate lncRsp1‐silenced (Si) and ‐overexpressing (OE) mutant strains, respectively (Figure S5a–d). The expression levels of both lncRsp1 and Fgsp1 were significantly up‐regulated in the lncRsp1‐overexpressing strains lncRsp1OE‐1 and lncRsp1OE‐3 (Figure 5b). In contrast, the expression levels of both lncRsp1 and Fgsp1 in the lncRsp1‐silenced mutants lncRsp1Si‐1, lncRsp1Si‐2, and lncRsp1Si‐5, were significantly down‐regulated compared with the wild‐type strain PH‐1 (Figure 5c). These results demonstrate that lncRsp1 is a positive regulator of the Fgsp1 gene.
FIGURE 5.

lncRsp1 positively regulates the expression of Fgsp1. (a) Relative expression levels of lncRsp1 and Fgsp1 in different strains were measured by reverse transcription quantitative PCR (RT‐qPCR) during the sexual stage. The expression of Fgsp1 in the wild‐type strain PH‐1 at 0 days postfertilization (dpf) was set as the control. (b) Relative expression levels of Fgsp1 and IncRsp1 in PH‐1 and lncRsp1‐overexpressing (OE) strains were measured by RT‐qPCR. (c) Relative expression levels of Fgsp1 and IncRsp1 in PH‐1 and lncRsp1‐silenced (Si) strains. In all panels, the results shown represent means ± SD. Bars indicate the SD of three replicates. The expression of Fgsp1 in the wild‐type strain PH‐1 at 7 dpf was set as the control. (d) Perithecia and cirrhi of lncRsp1Si and lncRsp1OE transformants. Bar = 500 μm. (e) The ascospores discharged from 7‐day‐old mature perithecia. Pictures were taken 18 h after the experiment was initiated. (f) The number of the discharged ascospores. Error bars represent the standard deviations of three repeated experiments. Different letters on the bars indicate statistically significant differences (p = 0.01)
To further analyse the function of lncRsp1 in sexual reproduction, the perithecia and discharged ascospores of lncRsp1‐silenced and ‐overexpressiing strains were investigated. During sexual differentiation, normal perithecium development and perithecia with cirri were observed at 7 or 14 dpf, respectively (Figure 5d). However, compared with the wild type, the number of discharged ascospores was significantly decreased in lncRsp1‐silenced strains (Figure 5e,f). In contrast, strains with lncRsp1 overexpression exhibited a significantly increased ascospore discharge under the same conditions (Figure 5e,f). These results indicate that lncRsp1 could enhance ascospore discharge by promoting the expression of Fgsp1.
Finally, to confirm that lncRsp1 indeed plays a direct role in sexual reproduction, the complementary strains of ΔlncRsp1, ΔlncRsp1‐LC (lncRsp1‐LC‐1 and lncRsp1‐LC‐2), were constructed in situ (Figure S6a). The two complementary strains, lncRsp1‐LC‐1 and lncRsp1‐LC‐2, had a similar sexual reproduction ability to that of the wild‐type strain (Figure S6b–d). These results further indicate that lncRsp1 can affect ascospore discharge via regulating the expression of Fgsp1.
3. DISCUSSION
lncRNAs play crucial regulatory roles in many biological processes in several eukaryotes (Quinn & Chang, 2016; Rinn & Chang, 2012; Sun et al., 2018; Till, Mach, et al., 2018). In filamentous fungi, studies of lncRNAs have been reported in N. crassa, F. graminearum, U. maydis, and T. reesei (Arthanari et al., 2014; Cemel et al., 2017; Donaldson et al., 2017; Kim et al., 2018). However, to date the biological functions and molecular basis of lncRNA‐mediated gene regulation have rarely been studied. Transcriptomes studies have shown a surprising complexity of lncRNAs that are often overlapping with, or interspersed within, multiple coding and noncoding transcripts (Carninci et al., 2005; Ding et al., 2012; Kapranov, 2005). In this study, we discovered and functionally characterized the lncRsp1 located immediately upstream of Fgsp1 and transcribed in the same direction as Fgsp1. RACE was performed to determine the transcription start and end sites of lncRsp1 and Fgsp1. Transcriptional analysis revealed two transcripts of lncRsp1 (lncRsp1‐1 and lncRsp1‐2) that were transcribed from the same strand and overlapped with each other (Figure 1b). They were defined as lncRNAs because none of them was predicted to encode a protein. The Fgsp1 gene exhibited two different transcripts of varying lengths (Fgsp1‐1 and Fgsp1‐2), of which Fgsp1‐2 transcription initiated 72 nt upstream of the lncRsp1 TIS (Figure 1b). According to our RNA‐Seq data of the wild‐type strain PH‐1, the expression of Fgsp1‐2 was almost undetectable at all stages (Figure S2). Therefore, we thought the main transcript of the gene Fgsp1 was Fgsp1‐1 isoform. The expression level of lncRsp1 and Fgsp1 was up‐regulated during sexual reproduction, implying that they were involved in sexual development processes (Figure 1d). Indeed, the number of discharged ascospores of ΔlncRsp1 and ΔFgsp1 mutants was less than that of the wild‐type strain (Figure 2b). In addition, lncRsp1 and Fgsp1 were also found to be involved in regulating the virulence and DON production of F. graminearum.
The deletion of lncRsp1 also disrupted the expression of Fgsp1‐2. However, because the expression level of Fgsp1‐2 isoform was almost undetectable at sexual and asexual stages, we did not consider the effect of lncRsp1 deletion on the expression of Fgsp1. In the lncRsp1 deletion mutant only the transcriptional region of lncRsp1 excluding the transcription start site of Fgsp1‐1 was deleted. To further clarify whether deletion of lncRsp1 disrupted the promoter of Fgsp1‐1, the complementary mutant strains of lncRsp1 were constructed in situ, as shown in Figure S6a. The complementary mutants lncRsp1‐LC1/LC2 fully restored the ascospore discharge defects of ΔlncRsp1 mutants (Figure S6c), indicating that deletion of lncRsp1 did not disrupt the function of the promoter of Fgsp1‐1. Given the modest effect on the Fgsp1‐1 transcript level by the disruption of lncRsp1, we also constructed the lncRsp1‐silenced strains lncRsp1Si and overexpression strains lncRsp1OE by random integration of the vectors into the wild‐type strain (Figure S5a,c). The expression of Fgsp1 was significantly reduced in the lncRsp1Si strains, while it was increased dramatically in the lncRsp1OE strains at the sexual stage (Figure 5b,c). In terms of sexual reproduction, the number of discharged ascospores in lncRsp1Si strains was slightly lower than that of the wild‐type strain PH‐1 (Figure 5d,f). Consistent with expectations, lncRsp1OE strains enhanced the ability of the ascospore discharge (Figure 5e,f). Although silencing of lncRsp1 might also result in the silence of Fgsp1‐2, considering that the expression level of Fgsp1‐2 isoform was very low at both sexual and sporulation stages, we did not analyse the effect of the lncRsp1 silencing on Fgsp1‐2 expression. At the same time, random integration of the lncRsp1 overexpression vector also avoids affecting the promoter of Fgsp1 due to the close distance between lncRsp1 and Fgsp1. Therefore, the above results suggest that lncRsp1 can affect the sexual development of F. graminearum by regulating the expression of Fgsp1. Lots of studies have confirmed that lncRNAs can regulate the expression of their neighbouring coding genes via trans or cis regulation (Engreitz et al., 2016; Yin et al., 2015). In our study, considering exogenous lncRsp1 was randomly integrated into the F. graminearum genome, we concluded that lncRsp1 is a crucial trans‐acting regulator of Fgsp1 transcription.
In F. graminearum, DON was wildly considered as an important virulence effector (Chen et al., 2019). In this study, the deletion of Fgsp1 and lncRsp1 caused a significant increase in DON production, which indicated that Fgsp1 and lncRsp1 negatively regulate DON production (Figure 4a). Previous work has found that the expression level of TRI genes was associated with DON production (Chen et al., 2019). In this study, the expression level of four TRI genes (TRI4, TRI5, TRI6, and TRI13) was significantly up‐regulated in ΔFgsp1 and ΔlncRsp1 mutants, which was consistent with the results of the DON production assay. However, TRI12 and TRI101, known as self‐defence genes for mycotoxin in Fusarium spp. (Alexander et al., 1999; Garvey et al., 2008; Menke et al., 2012), reduced expression levels in ΔFgsp1 mutant (Figure 4c). This result indicated that Fgsp1 might play divergent roles in the DON synthesis pathway. Furthermore, the deletion of Fgsp1 affected the normal hyphal bulbous structure formation in DON‐inducing medium, but no obvious difference was observed between the ΔlncRsp1 mutant and wild‐type strains (Figure 4d), suggesting that the functional relationship between Fgsp1 and lncRsp1 varies in regulating DON biosynthesis. It is worth noting that although DON biosynthesis was increased in the Fgsp1 deletion mutant, it rarely formed the hyphal bulbous structures associated with DON production. Previous research has found that, unlike Fgsp1, the tri6 pde2 mutant fails to produce DON but has increased bulbous structures (Jiang et al., 2016). As the hyphal bulbous structures formed before TRI gene transcription are closely related to DON production in F. graminearum (Jonkers et al., 2012), the absence of bulbous structures caused by the deletion of Fgsp1 seemed to be independent of TRI genes. However, further studies are needed to identify the underlying regulatory mechanisms.
Sexual reproduction is an important developmental process in the disease cycle of FHB. Ascospores, which are forcibly discharged into air from perithecia, play a crucial role in fungal survival and disease propagation (Guenther & Trail, 2005; Trail et al., 2002). Our data showed that the ΔlncRsp1 and ΔFgsp1 mutants produced more abnormal asci and significantly reduced ascospore discharge and glycogen accumulation (Figure 2b,c), indicating that lncRsp1 and Fgsp1 are essential for sexual reproduction in F. graminearum. Previous work has demonstrated that the turgor pressure within the extended asci is necessary for the forcible discharge of ascospores (Trail, 2007). The build‐up of ion fluxes, especially in K+, Na+, Cl−, and Ca2+ ion channels and glycogen accumulation, is tightly related to the generation of turgor pressure (Min et al., 2010; Trail et al., 2002, 2005). Thus, it is likely that the reduced number of discharged ascospores observed in lncRsp1 and Fgsp1 deletion mutants is due to the developmental defects of asci and the decrease in glycogen accumulation.
In conclusion, our results indicate that lncRsp1 and Fgsp1 are important for the sexual reproduction, DON production, and pathogenesis of F. graminearum, while lncRsp1 plays a crucial regulatory role in the expression of Fgsp1. Although the underlying mechanism remains undefined, our findings not only help to further understand the molecular mechanism of the sexual reproduction of F. graminearum but also promote the understanding of the role of lncRNA in plant pathogenic fungi.
4. EXPERIMENTAL PROCEDURES
4.1. Fungal strains and culture conditions
The F. graminearum strain PH‐1 (NRRL 31084) (Trail & Common, 2000) and all mutant strains were cultured on potato dextrose agar (PDA) plates at 25°C in the dark. The colony morphology, growth rate, and conidial germination were measured on PDA, minimum medium (MM), and complete medium (CM) plates incubated at 25°C for 3 days. For conidiation tests, the conidia were induced in carboxymethylcellulose (CMC) liquid culture medium (1 g NH4NO3, 1 g KH2PO3, 0.5 g MgSO4.7H2O, 1 g yeast extract, 15 g CMC, and 1 L water). The 5‐mm mycelial agar blocks were incubated in 4 ml of CMC medium at 25°C for 5 days in a rotary shaker (200 rpm). Conidia were collected in distilled water by filtration. The conidial morphology was observed under a fluorescence microscope. The number of conidia produced by each strain was counted using a haemocytometer (Cappellini & Peterson, 1965). Conidial germination rates were assayed as described previously (Zhou et al., 2010). The conidial suspension (106 conidia/ml) was harvested and then inoculated into yeast extract peptone dextrose (YEPD) (0.3% yeast extract, 1% peptone, 2% dextrose) liquid medium at 25°C in a shaker (200 rpm). The conidial germination was observed after 6 h of incubation. The sexual development, including perithecium formation, cirrus production, ascus development, and ascospore discharge, was performed as previously described (Wang et al., 2021; Zeng et al., 2018). All strains were grown on carrot agar plates under near‐ultraviolet light at 25°C for 7 days. Perithecium formation and cirrus production were observed 1–2 weeks after fertilization. Perithecia and cirri samples were observed with an Olympus IX71 microscope and Olympus cellsens standard camera software. The glycogen staining of asci was performed as described previously (da Rocha Campos & Costa, 2010). Ascospore discharge assays were also performed as previously described (Trail et al., 2002). Carrot agar (10 mm in diameter) covered with mature perithecia cultures was cut in half and placed inverted on a coverslip in a humidity box for 18 h, and the images were captured using a camera. The number of discharged ascospores was counted as follows. Ascospores were collected by placing the carrot agar upside down for 10 h and allowing the mature perithecia to discharge ascospores on Petri dish covers. Ascospores were completely harvested from the Petri dish lids in distilled water and the number of ascospores was counted under a microscope.
4.2. Plant infection, DON production assays, and expression levels of TRI genes
Pathogenicity assays on wheat spikes and coleoptiles were conducted as described previously (Hou et al., 2002; Seong et al., 2005; Wu et al., 2005). A 2‐µl aliquot of conidial suspension (5 × 105 conidia/ml in sterile distilled water) of each strain was used to inoculate each of 20 coleoptiles of 3‐day‐old wheat seedlings with three replicates in a growth chamber. Two microlitres of 0.01% Tween 20 solution was inoculated as a control. The lesion size of each strain was counted and analysed at 7 dpi. Virulence assays on flowering wheat heads were performed as previously described (Hou et al., 2002). To this end, 10 µl of 105 conidia/ml suspension, collected from 5‐day‐old CMC cultures, was inoculated onto the fifth flowering spikelet of wheat variety Zhengmai 9023. The experiment was carried out with three independent replicates. The disease index for each strain was calculated as described by Luo et al. (2014). The number of symptomatic spikelets was counted and images were captured 14 days after inoculation.
To measure the DON production, the infected wheat kernels with typical FHB symptoms were harvested for DON assays as previously described (Tian et al., 2020). DON production in rice cultures was assayed as described (Bluhm et al., 2007; Seo et al., 1996). To determine the expression levels of TRI genes (TRI4, TRI5, TRI6, TRI12, TRI13, TRI101), conidia of each strain were inoculated into TBI medium (Gardiner et al., 2009) and cultured for 3 days at 25°C in a shaker (200 rpm). Mycelia of each sample were harvested and total RNA was extracted. The expression levels of TRI genes were determined by RT‐qPCR assays with the primers listed in Table S2. The experiment was repeated three times independently.
4.3. RT‐qPCR detection of lncRsp1 and Fgsp1
Total RNA was isolated using the TRIzol reagent (Invitrogen) and the first‐strand cDNA synthesis was carried out by the EasyScript One‐step gDNA removal and cDNA Synthesis SuperMix (Transgen Biotech), according to the manufacturer's instructions. qPCR was performed on a CFX96 Real‐Time PCR Detection System (Bio‐Rad) with the iTaq Universal SYBR Green Supermix (Bio‐Rad). In the conidia stage, the total RNA samples of the wild type were extracted at 3 h (mycelial stage) and 24 h (sporulation stage) after incubation in CMC liquid medium. During sexual development, the RNA samples were isolated from 7‐day‐old carrot agar plate hyphae (0 dpf) and 7‐day‐old perithecia (7 dpf). The F. graminearum β‐tubulin gene (FGSG_09530) was used as an endogenous reference and gene‐specific primers (GSP) were used to detect mRNA and lncRNA, respectively. The relative expression of each gene was calculated with the 2–ΔΔ C t method. RT‐qPCR data from three biological replicates for each sample were calculated from the mean ± SD (Livak & Schmittgen, 2001). All the primer sequences are provided in Table S2.
4.4. RACE‐PCR assays
Using 5ʹ‐ and 3ʹ‐RACE experiments analysing the transcriptional start and end sites of lncRsp1 and Fgsp1, the sequence was determined by RACE‐PCR using the SMARTer RACE 5ʹ/3ʹ Kit (Clontech). All the RACE primers are provided in Table S2.
4.5. Construction of the Fgsp1 and lncRsp1 deletion mutants and complementation strains
The strategy for Fgsp1 and lncRsp1 deletion is illustrated in Figure S3. We used a split‐marker system, replacing the two genes with the hygromycin resistance gene (hph) and the geneticin resistance cassette (gen), respectively (Catlett et al., 2003; Zeng et al., 2018). Transformants were selected using the PDA plates amended with 225 μg/ml hygromycin B (Sigma‐Aldrich) or 300 μg/ml geneticin (Sigma‐Aldrich). The Fgsp1 and lncRsp1 deletion mutants were identified by PCR with the relevant primers listed in Table S2 and further confirmed by Southern blot analysis (Leslie & Summerell, 2006).
For Southern blotting, the genomic DNA of PH‐1 and mutants was extracted according to the Fusarium laboratory manual and then digested with XhoI. Southern blotting was performed using the Amersham AlkPhos Direct Labeling and Detection System (GE Healthcare). The probe primers are listed in Table S2 and the probes were labelled with alkaline phosphatase.
For complementation assays, the Fgsp1 fragment including its promoter region was amplified with primers Fgsp1‐CF and Fgsp1‐CR, and cloned into the same sites of vector neoP3300 to generate the Fgsp1 complementary vector (Yang et al., 2016). The vector was transformed into the ΔFgsp1 mutant by random integration, and the resultant strain was designated as ΔFgsp1‐C. The complementation strategy of lncRsp1 is illustrated in Figure S6a. The complementary fragments were constructed by fusing the hygromycin resistance gene and full‐length lncRsp1 by double‐joint PCR according to a previously described method (Liu et al., 2020). The fusion constructs were transformed into the ΔlncRsp1 mutant, as described above, except that hygromycin was used as screening agent.
4.6. Generation of the lncRsp1Si and lncRsp1OE transformants
The lncRsp1 RNAi vector construction was carried out as described previously (Yu et al., 2012; Zhu et al., 2013). To amplify the sense and antisense fragments of lncRsp1, the primers lncRsp1Si1F/lncRsp1Si1R (sense fragment) and lncRsp1Si2F/lncRsp1Si2R (antisense fragment) were used. The amplified lncRsp1 sense and antisense fragments were subsequently ligated into pCIT to generate a new vector. The newly constructed vector was then digested with XhoI and SacI to obtain the repeat fragment, then subsequently ligated with the pCH vector to construct pSi lncRsp1 silencing vector, which was then transformed into the wild‐type strain PH‐1 by random integration, as previously reported (Yu et al., 2012). lncRsp1‐overexpressing strains were generated using the pCETH2 vector. The PCR amplification and ligation of the full‐length lncRsp1 into the pCETH2 vector was carried out. Then, the vector was transformed into the wild‐type strain PH‐1 using the Agrobacterium‐mediated transformation system by random integration, as described by Yu et al. (2012). The lncRsp1 transcript level in silenced strains and overexpressing strains was determined by RT‐qPCR.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
FIGURE S1 Identification of the 5ʹ and 3ʹ ends of lncRsp1 and Fgsp1 transcript. (a) Schematic illustration shows the 5ʹ and 3ʹ RACE assay of lncRsp1 and Fgsp1 using the primers of 5ʹ RACE and 3ʹ RACE (labelled with arrows). The black arrows illustrate the orientation of transcription. The red and blue arrows indicate primer locations. (b) Amplification products of 5ʹ and 3ʹ RACE inner primer with GSP (presented in A) of lncRsp1 and Fgsp1 using 5ʹ and 3ʹ RACE cDNA fragments to obtain the 5ʹ and 3ʹ end products, respectively. F, forward primer; R, reverse primer; M, DL 2000 marker. (c) The sequences of the predicted lncRsp1 and Fgsp1
FIGURE S2 IGV Sashimi plots showing the read coverages of Fgsp1 transcripts in RNA‐Seq data. In the conidia stage, total RNA samples of the wild‐type strain were harvested at 3 h (CMC‐3h) and 12 hr (CMC‐12h) after incubation in carboxymethyl cellulose (CMC) liquid medium. During sexual development, the RNA samples were extracted from cultures grown on carrot plat at different days postfertilization (0, 7, 10 dpf). The coverage of each alignment track is plotted as a bar chart. The numbers in brackets represent the coverage threshold. Arcs indicate splicing junction connect exons. Arcs show the number of reads split across the link. The two alternative transcripts are displayed below the junction track. The RNA‐Seq data used were generated by our laboratory previously
FIGURE S3 Verification of lncRsp1 and Fgsp1 deletion mutants using Southern blotting and PCR. (a) Schematic diagram of lncRsp1 and Fgsp1 replacement strategy. genr, geneticin resistance gene cassette; hygr, hygromycin B resistance gene cassette; P1 (primer pair 1F1/1R), the fragment P1, which was used to identify the upstream homologous recombination of the deletion mutant; P2 (primer pair 2F/2R1), the fragment P2, which was used to identify the downstream homologous recombination of the deletion mutant; P3 (primer pair F/R), the fragment P3, which was used to identify deletion mutant. The red lines indicate the region of PCR amplification with primers. (b) Verification of lncRsp1 and Fgsp1 deletion mutants using PCR. P1, P2, and P3 indicate fragments P1, P2, and P3, respectively. M, DL5000 marker. (c) Confirmation of ΔlncRsp1 and ΔFgsp1 by Southern blot. Genomic DNA from all strains was digested by XhoI. Probe 1 (from geneticin resistance gene) and probe 2 (from hygromycin B resistance gene) were labelled with alkaline phosphatase according to the manual. The sizes of the markers are indicated on the left of the blot. M, λ‐HindIII digest DNA marker
FIGURE S4 Assays of the growth, conidial morphology, and conidial germination of the wild‐type strain PH‐1, ΔlncRsp1, and ΔFgsp1, as well as complemented strains. (a) Colony morphology of the indicated strains on potato dextrose agar (PDA), minimal medium (MM), and complete medium (CM) plates, respectively. Strains were cultured at 25°C for 3 days and photographed. (b) Conidial morphology of the different strains. Conidia were harvested from carboxymethyl cellulose (CMC) medium at 5 days and stained with calcofluor white (CFW). The images were captured under epifluorescence microscopy. Bar = 20 μm. (c) Conidial germination of the indicated strains in yeast extract peptone dextrose (YEPD) medium at 25°C for 3 and 6 h. Bar = 20 μm
FIGURE S5 Schematic diagram of the overexpression (OE) and silencing (Si) strategy for lncRsp1. (a) Schematic diagram of the lncRsp1Si. Upper panels, schematic diagram of the lncRsp1 silenced strategy; lower panels, the silenced fragment of the lncRsp1 gene was amplified with primer pair Si‐1F/Si‐1R, Si‐2F/Si‐2R. (b) Transcript levels of lncRsp1 in the wild‐type PH‐1 and lncRsp1Si strains were analysed by reverse transcription quantitative PCR (RT‐qPCR); lncRsp1 transcript levels were presented relative to that in the wild‐type strain PH‐1, which was set as 1. (c) Schematic diagram shows the strategy of lncRsp1 overexpression. Upper panels, schematic diagram of the lncRsp1 overexpression strategy; lower panels, the overexpression fragment of the lncRsp1 gene was amplified with primer pair OE‐F/OE‐R. The lncRsp1 promoter was replaced with the EF1α promoter of Sclerotinia sclerotiorum. hygr, hygromycin B resistance gene cassette. (d) Transcript levels of lncRsp1 in PH‐1 and lncRsp1OE strains were analysed by RT‐qPCR
FIGURE S6 Construction and sexual development of lncRsp1 complementation (LC) strains. (a) Schematic diagram of the lncRsp1 complementation strategy. An in situ complementation method was used for lncRsp1 complement, and ∆lncRsp1 was used as the original strain. (b) Perithecial morphology of the wild‐type strain PH‐1, ΔlncRsp1, and ΔlncRsp1‐LC1/LC2 strains. Bar = 500 µm. (c) Ascospore discharge was examined at 7 days postfertilization (dpf). (d) Statistical analysis of the discharged ascospores
TABLE S1 Growth rate and conidiation of different Fusarium graminearum strains
TABLE S2 Primers used in this study
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (31301614 and 31901840).
Wang, J. , Zeng, W. , Cheng, J. , Xie, J. , Fu, Y. , Jiang, D. &et al (2022) lncRsp1, a long noncoding RNA, influences Fgsp1 expression and sexual reproduction in Fusarium graminearum . Molecular Plant Pathology, 23, 265–277. 10.1111/mpp.13160
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alexander, N.J. , McCormick, S.P. & Hohn, T.M. (1999) TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: gene isolation and expression in yeast. Molecular and General Genetics, 261, 977–984. [DOI] [PubMed] [Google Scholar]
- Arthanari, Y. , Heintzen, C. , Griffiths‐Jones, S. & Crosthwaite, S.K. (2014) Natural antisense transcripts and long non‐coding RNA in Neurospora crassa . PLoS One, 9, e91353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert, K. , Vanheule, A. , Hofte, M. & Haesaert, G. (2014) Deoxynivalenol: a major player in the multifaceted response of Fusarium to its environment. Toxins, 6, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden, W.J. , Lewis, Z.A. , Selker, E.U. , Loros, J.J. & Dunlap, J.C. (2011) CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genetics, 7, e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm, B.H. , Zhao, X. , Flaherty, J.E. , Xu, J.R. & Dunkle, L.D. (2007) RAS2 regulates growth and pathogenesis in Fusarium graminearum . Molecular Plant‐Microbe Interactions, 20, 627–636. [DOI] [PubMed] [Google Scholar]
- Cappellini, R.A. & Peterson, J.L. (1965) Macroconidium formation in submerged cultures by a non‐sporulating strain of Gibberella zeae . Mycologia, 57, 962–966. [Google Scholar]
- Carninci, P. , Kasukawa, T. , Katayama, S. , Gough, J. , Frith, M.C. , Maeda, N. et al. (2005) The transcriptional landscape of the mammalian genome. Science, 309, 1559–1563. [DOI] [PubMed] [Google Scholar]
- Catlett, N.L. , Lee, B.‐N. , Yoder, O.C. & Turgeon, B.G. (2003) Split‐marker recombination for efficient targeted deletion of fungal genes. Fungal Genetics Reports, 50, 9–11. [Google Scholar]
- Cavinder, B. , Hamam, A. , Lew, R.R. & Trail, F. (2011) Mid1, a mechanosensitive calcium ion channel, affects growth, development, and ascospore discharge in the filamentous fungus Gibberella zeae . Eukaryotic Cell, 10, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemel, I.A. , Ha, N. , Schermann, G. , Yonekawa, S. & Brunner, M. (2017) The coding and noncoding transcriptome of Neurospora crassa . BMC Genomics, 18, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Kistler, H.C. & Ma, Z. (2019) Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annual Review of Phytopathology, 57, 15–39. [DOI] [PubMed] [Google Scholar]
- Choquer, M. , Lee, M.H. , Bau, H.J. & Chung, K.R. (2007) Deletion of a MFS transporter‐like gene in Cercospora nicotianae reduces cercosporin toxin accumulation and fungal virulence. FEBS Letters, 581, 489–494. [DOI] [PubMed] [Google Scholar]
- Coleman, J.J. & Mylonakis, E. (2009) Efflux in fungi: la piece de resistance. PLoS Pathogens, 5, e1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins, A.E. (1996) Reduced virulence of trichothecene‐nonproducing mutants of Gibberella zeae in wheat field tests. Molecular Plant‐Microbe Interactions, 9, 775–781. [Google Scholar]
- Ding, J. , Lu, Q. , Ouyang, Y. , Mao, H. , Zhang, P. , Yao, J. et al. (2012) A long noncoding RNA regulates photoperiod‐sensitive male sterility, an essential component of hybrid rice. Proceedings of the National Academy of Sciences of the United States of America, 109, 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, M.E. , Ostrowski, L.A. , Goulet, K.M. & Saville, B.J. (2017) Transcriptome analysis of smut fungi reveals widespread intergenic transcription and conserved antisense transcript expression. BMC Genomics, 18, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, M.E. & Saville, B.J. (2013) Ustilago maydis natural antisense transcript expression alters mRNA stability and pathogenesis. Molecular Microbiology, 89, 29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz, J.M. , Haines, J.E. , Perez, E.M. , Munson, G. , Chen, J. , Kane, M. et al. (2016) Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature, 539, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, D.M. , Kazan, K. & Manners, J.M. (2009) Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum . Fungal Genetics and Biology, 46, 604–613. [DOI] [PubMed] [Google Scholar]
- Garvey, G.S. , McCormick, S.P. & Rayment, I. (2008) Structural and functional characterization of the TRI101 trichothecene 3‐O‐acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum: kinetic insights to combating Fusarium head blight. Journal of Biological Chemistry, 283, 1660–1669. [DOI] [PubMed] [Google Scholar]
- Geng, Z. , Zhu, W. , Su, H. , Zhao, Y. , Zhang, K.Q. & Yang, J. (2014) Recent advances in genes involved in secondary metabolite synthesis, hyphal development, energy metabolism and pathogenicity in Fusarium graminearum (teleomorph Gibberella zeae). Biotechnology Advances, 32, 390–402. [DOI] [PubMed] [Google Scholar]
- Goswami, R.S. & Kistler, H.C. (2004) Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology, 5, 515–525. [DOI] [PubMed] [Google Scholar]
- Guenther, J.C. & Trail, F. (2005) The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat. Mycologia, 97, 229–237. [DOI] [PubMed] [Google Scholar]
- Heo, J.B. & Sung, S. (2011) Vernalization‐mediated epigenetic silencing by a long intronic noncoding RNA. Science, 331, 76–79. [DOI] [PubMed] [Google Scholar]
- Hou, Z. , Xue, C. , Peng, Y. , Katan, T. , Kistler, H.C. & Xu, J.R. (2002) A mitogen‐activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Molecular Plant‐Microbe Interactions, 15, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hu, S. , Zhou, X. , Gu, X. , Cao, S. , Wang, C. & Xu, J.R. (2014) The cAMP‐PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum . Molecular Plant‐Microbe Interactions, 27, 557–566. [DOI] [PubMed] [Google Scholar]
- Jenczmionka, N.J. , Maier, F.J. , Losch, A.P. & Schafer, W. (2003) Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head‐blight disease of wheat, are regulated by the MAP kinase gpmk1. Current Genetics, 43, 87–95. [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Zhang, C. , Wu, C. , Sun, P. , Hou, R. , Liu, H. et al. (2016) TRI6 and TRI10 play different roles in the regulation of deoxynivalenol (DON) production by cAMP signalling in Fusarium graminearum . Environmental Microbiology, 18, 3689–3701. [DOI] [PubMed] [Google Scholar]
- Jonkers, W. , Dong, Y. , Broz, K. & Kistler, H.C. (2012) The Wor1‐like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum . PLoS Pathogens, 8, e1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranov, P. (2005) Examples of the complex architecture of the human transcriptome revealed by RACE and high‐density tiling arrays. Genome Research, 15, 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W. , Miguel‐Rojas, C. , Wang, J. , Townsend, J.P. & Trail, F. (2018) Developmental dynamics of long noncoding RNA expression during sexual fruiting body formation in Fusarium graminearum . mBio, 9, e01292‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer, M. , Leroch, M. , Mosbach, A. , Walker, A.‐S. , Fillinger, S. , Mernke, D. et al. (2009) Fungicide‐driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea . PLoS Pathogens, 5, e1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, J.F. & Summerell, B.A. (2006) The Fusarium Laboratory Manual. Ames, IA, USA: Blackwell Publishing. [Google Scholar]
- Li, N. , Joska, T.M. , Ruesch, C.E. , Coster, S.J. & Belden, W.J. (2015) The frequency natural antisense transcript first promotes, then represses, frequency gene expression via facultative heterochromatin. Proceedings of the National Academy of Sciences of the United States of America, 112, 4357–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Xie, J. , Fu, Y. , Jiang, D. , Chen, T. & Cheng, J. (2020) The subtilisin‐like protease Bcser2 affects the sclerotial formation, conidiation and virulence of Botrytis cinerea. International Journal of Molecular Sciences, 21, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. & Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2–ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luo, Y. , Zhang, H. , Qi, L. , Zhang, S. , Zhou, X. , Zhang, Y. et al. (2014) FgKin1 kinase localizes to the septal pore and plays a role in hyphal growth, ascospore germination, pathogenesis, and localization of Tub1 β‐tubulins in Fusarium graminearum . New Phytologist, 204, 943–954. [DOI] [PubMed] [Google Scholar]
- Martens, J.A. , Laprade, L. & Winston, F. (2004) Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature, 429, 571–574. [DOI] [PubMed] [Google Scholar]
- Martianov, I. , Ramadass, A. , Serra Barros, A. , Chow, N. & Akoulitchev, A. (2007) Repression of the human dihydrofolate reductase gene by a non‐coding interfering transcript. Nature, 445, 666–670. [DOI] [PubMed] [Google Scholar]
- Menke, J. , Dong, Y. & Kistler, H.C. (2012) Fusarium graminearum Tri12p influences virulence to wheat and trichothecene accumulation. Molecular Plant‐Microbe Interactions, 25, 1408–1418. [DOI] [PubMed] [Google Scholar]
- Mercer, T.R. , Dinger, M.E. & Mattick, J.S. (2009) Long non‐coding RNAs: insights into functions. Nature Reviews Genetics, 10, 155–159. [DOI] [PubMed] [Google Scholar]
- Min, K. , Lee, J. , Kim, J.C. , Kim, S.G. , Kim, Y.H. , Vogel, S. et al. (2010) A novel gene, ROA, is required for normal morphogenesis and discharge of ascospores in Gibberella zeae . Eukaryotic Cell, 9, 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski, L.A. & Saville, B.J. (2017) Natural antisense transcripts are linked to the modulation of mitochondrial function and teliospore dormancy in Ustilago maydis . Molecular Microbiology, 103, 745–763. [DOI] [PubMed] [Google Scholar]
- Pao, S.S. , Paulsen, I.T. & Saier, M.H. (1998) Major facilitator superfamily. Microbiology and Molecular Biology Reviews, 62, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting, C.P. , Oliver, P.L. & Reik, W. (2009) Evolution and functions of long noncoding RNAs. Cell, 136, 629–641. [DOI] [PubMed] [Google Scholar]
- Quan, M. , Chen, J. & Zhang, D. (2015) Exploring the secrets of long noncoding RNAs. International Journal of Molecular Sciences, 16, 5467–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, J.J. & Chang, H.Y. (2016) Unique features of long non‐coding RNA biogenesis and function. Nature Reviews Genetics, 17, 47–62. [DOI] [PubMed] [Google Scholar]
- Rinn, J.L. & Chang, H.Y. (2012) Genome regulation by long noncoding RNAs. Annual Review of Biochemistry, 81, 145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J.L. , Kertesz, M. , Wang, J.K. , Squazzo, S.L. , Xu, X. , Brugmann, S.A. et al. (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell, 129, 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha Campos, A.N. & Costa, M.D. (2010) Histochemistry and storage of organic compounds during basidiosporogenesis in the ectomycorrhizal fungus Pisolithus microcarpus . World Journal of Microbiology and Biotechnology, 26, 1745–1753. [Google Scholar]
- Seo, J.A. , Kim, J.C. , Lee, D.H. & Lee, Y.W. (1996) Variation in 8‐ketotrichothecenes and zearalenone production by Fusarium graminearum isolates from corn and barley in Korea. Mycopathologia, 134, 31–37. [DOI] [PubMed] [Google Scholar]
- Seong, K. , Hou, Z. , Tracy, M. , Kistler, H.C. & Xu, J.R. (2005) Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum . Phytopathology, 95, 744–750. [DOI] [PubMed] [Google Scholar]
- Son, H. , Lee, J. & Lee, Y.W. (2013) A novel gene, GEA1, is required for ascus cell‐wall development in the ascomycete fungus Fusarium graminearum . Microbiology, 159, 1077–1085. [DOI] [PubMed] [Google Scholar]
- Son, H. , Seo, Y.‐S. , Min, K. , Park, A.R. , Lee, J. , Jin, J.‐M. et al. (2011) A phenome‐based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum . PLoS Pathogens, 7, e1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Zheng, H. & Sui, N. (2018) Regulation mechanism of long non‐coding RNA in plant response to stress. Biochemical and Biophysical Research Communications, 503, 402–407. [DOI] [PubMed] [Google Scholar]
- Swiezewski, S. , Liu, F. , Magusin, A. & Dean, C. (2009) Cold‐induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature, 462, 799–802. [DOI] [PubMed] [Google Scholar]
- Tang, J. , Chen, X. , Yan, Y. , Huang, J. , Luo, C. , Tom, H. et al. (2021) Comprehensive transcriptome profiling reveals abundant long non‐coding RNAs associated with development of the rice false smut fungus, Ustilaginoidea virens . Environmental Microbiology, 23, 4998–5013. [DOI] [PubMed] [Google Scholar]
- Tian, B. , Xie, J. , Fu, Y. , Cheng, J. , Li, B.O. , Chen, T. et al. (2020) A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. The ISME Journal, 14, 3120–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till, P. , Mach, R.L. & Mach‐Aigner, A.R. (2018) A current view on long noncoding RNAs in yeast and filamentous fungi. Applied Microbiology and Biotechnology, 102, 7319–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till, P. , Pucher, M.E. , Mach, R.L. & Mach‐Aigner, A.R. (2018) A long noncoding RNA promotes cellulase expression in Trichoderma reesei . Biotechnology for Biofuels, 11, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trail, F. (2007) Fungal cannons: explosive spore discharge in the Ascomycota. FEMS Microbiology Letters, 276, 12–18. [DOI] [PubMed] [Google Scholar]
- Trail, F. & Common, R. (2000) Perithecial development by Gibberella zeae: a light microscopy study. Mycologia, 92, 130–138. [Google Scholar]
- Trail, F. , Gaffoor, I. & Vogel, S. (2005) Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fuarium graminearum). Fungal Genetics and Biology, 42, 528–533. [DOI] [PubMed] [Google Scholar]
- Trail, F. , Xu, H. , Loranger, R. & Gadoury, D. (2002) Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia, 94, 181–189. [PubMed] [Google Scholar]
- Wang, C. , Zhang, S. , Hou, R. , Zhao, Z. , Zheng, Q. , Xu, Q. et al. (2011) Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum . PLoS Pathogens, 7, e1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zeng, W. , Xie, J. , Fu, Y. , Jiang, D. , Lin, Y. et al. (2021) A novel antisense long noncoding RNA participates in asexual and sexual reproduction by regulating the expression of GzmetE in Fusarium graminearum . Environmental Microbiology, 23, 4939–4955. [DOI] [PubMed] [Google Scholar]
- Wu, A.B. , Li, H.P. , Zhao, C.S. & Liao, Y.C. (2005) Comparative pathogenicity of Fusarium graminearum isolates from China revealed by wheat coleoptile and floret inoculations. Mycopathologia, 160, 75–83. [DOI] [PubMed] [Google Scholar]
- Xue, Z. , Ye, Q. , Anson, S.R. , Yang, J. , Xiao, G. , Kowbel, D. et al. (2014) Transcriptional interference by antisense RNA is required for circadian clock function. Nature, 514, 650–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, A. , Shichino, Y. & Yamamoto, M. (2016) The long non‐coding RNA world in yeasts. Biochimica et Biophysica Acta, 1859, 147–154. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Cui, H. , Cheng, J. , Xie, J. , Jiang, D. , Hsiang, T. et al. (2016) A HOPS protein, CmVps39, is required for vacuolar morphology, autophagy, growth, conidiogenesis and mycoparasitic functions of Coniothyrium minitans . Environmental Microbiology, 18, 3785–3797. [DOI] [PubMed] [Google Scholar]
- Yen, M.R. , Chen, J.S. , Marquez, J.L. , Sun, E.I. & Saier, M.H. (2010) Multidrug resistance: phylogenetic characterization of superfamilies of secondary carriers that include drug exporters. Methods in Molecular Biology, 637, 47–64. [DOI] [PubMed] [Google Scholar]
- Yin, Y. , Yan, P. , Lu, J. , Song, G. , Zhu, Y. , Li, Z. et al. (2015) Opposing roles for the lncRNA haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. Cell Stem Cell, 16, 504–516. [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Jiang, D. , Xie, J. , Cheng, J. , Li, G. , Yi, X. et al. (2012) Ss‐Sl2, a novel cell wall protein with PAN modules, is essential for sclerotial development and cellular integrity of Sclerotinia sclerotiorum . PLoS One, 7, e34962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, Y. , Liu, Z. , Yin, Y. , Jiang, J. , Chen, Y. , Xu, J.R. et al. (2015) Functional analysis of the Fusarium graminearum phosphatome. New Phytologist, 207, 119–134. [DOI] [PubMed] [Google Scholar]
- Zeng, W. , Wang, J. , Wang, Y. , Lin, J. , Fu, Y. , Xie, J. et al. (2018) Dicer‐like proteins regulate sexual development via the biogenesis of perithecium‐specific microRNAs in a plant pathogenic fungus Fusarium graminearum . Frontiers in Microbiology, 9, 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Li, B. , Zhang, Y. , Jia, X. & Zhou, M. (2016) Hexokinase plays a critical role in deoxynivalenol (DON) production and fungal development in Fusarium graminearum . Molecular Plant Pathology, 17, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Heyer, C. , Choi, Y.E. , Mehrabi, R. & Xu, J.R. (2010) The CID1 cyclin C‐like gene is important for plant infection in Fusarium graminearum . Fungal Genetics and Biology, 47, 143–151. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Wei, W. , Fu, Y. , Cheng, J. , Xie, J. , Li, G. et al. (2013) A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance. PLoS One, 8, e53901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Identification of the 5ʹ and 3ʹ ends of lncRsp1 and Fgsp1 transcript. (a) Schematic illustration shows the 5ʹ and 3ʹ RACE assay of lncRsp1 and Fgsp1 using the primers of 5ʹ RACE and 3ʹ RACE (labelled with arrows). The black arrows illustrate the orientation of transcription. The red and blue arrows indicate primer locations. (b) Amplification products of 5ʹ and 3ʹ RACE inner primer with GSP (presented in A) of lncRsp1 and Fgsp1 using 5ʹ and 3ʹ RACE cDNA fragments to obtain the 5ʹ and 3ʹ end products, respectively. F, forward primer; R, reverse primer; M, DL 2000 marker. (c) The sequences of the predicted lncRsp1 and Fgsp1
FIGURE S2 IGV Sashimi plots showing the read coverages of Fgsp1 transcripts in RNA‐Seq data. In the conidia stage, total RNA samples of the wild‐type strain were harvested at 3 h (CMC‐3h) and 12 hr (CMC‐12h) after incubation in carboxymethyl cellulose (CMC) liquid medium. During sexual development, the RNA samples were extracted from cultures grown on carrot plat at different days postfertilization (0, 7, 10 dpf). The coverage of each alignment track is plotted as a bar chart. The numbers in brackets represent the coverage threshold. Arcs indicate splicing junction connect exons. Arcs show the number of reads split across the link. The two alternative transcripts are displayed below the junction track. The RNA‐Seq data used were generated by our laboratory previously
FIGURE S3 Verification of lncRsp1 and Fgsp1 deletion mutants using Southern blotting and PCR. (a) Schematic diagram of lncRsp1 and Fgsp1 replacement strategy. genr, geneticin resistance gene cassette; hygr, hygromycin B resistance gene cassette; P1 (primer pair 1F1/1R), the fragment P1, which was used to identify the upstream homologous recombination of the deletion mutant; P2 (primer pair 2F/2R1), the fragment P2, which was used to identify the downstream homologous recombination of the deletion mutant; P3 (primer pair F/R), the fragment P3, which was used to identify deletion mutant. The red lines indicate the region of PCR amplification with primers. (b) Verification of lncRsp1 and Fgsp1 deletion mutants using PCR. P1, P2, and P3 indicate fragments P1, P2, and P3, respectively. M, DL5000 marker. (c) Confirmation of ΔlncRsp1 and ΔFgsp1 by Southern blot. Genomic DNA from all strains was digested by XhoI. Probe 1 (from geneticin resistance gene) and probe 2 (from hygromycin B resistance gene) were labelled with alkaline phosphatase according to the manual. The sizes of the markers are indicated on the left of the blot. M, λ‐HindIII digest DNA marker
FIGURE S4 Assays of the growth, conidial morphology, and conidial germination of the wild‐type strain PH‐1, ΔlncRsp1, and ΔFgsp1, as well as complemented strains. (a) Colony morphology of the indicated strains on potato dextrose agar (PDA), minimal medium (MM), and complete medium (CM) plates, respectively. Strains were cultured at 25°C for 3 days and photographed. (b) Conidial morphology of the different strains. Conidia were harvested from carboxymethyl cellulose (CMC) medium at 5 days and stained with calcofluor white (CFW). The images were captured under epifluorescence microscopy. Bar = 20 μm. (c) Conidial germination of the indicated strains in yeast extract peptone dextrose (YEPD) medium at 25°C for 3 and 6 h. Bar = 20 μm
FIGURE S5 Schematic diagram of the overexpression (OE) and silencing (Si) strategy for lncRsp1. (a) Schematic diagram of the lncRsp1Si. Upper panels, schematic diagram of the lncRsp1 silenced strategy; lower panels, the silenced fragment of the lncRsp1 gene was amplified with primer pair Si‐1F/Si‐1R, Si‐2F/Si‐2R. (b) Transcript levels of lncRsp1 in the wild‐type PH‐1 and lncRsp1Si strains were analysed by reverse transcription quantitative PCR (RT‐qPCR); lncRsp1 transcript levels were presented relative to that in the wild‐type strain PH‐1, which was set as 1. (c) Schematic diagram shows the strategy of lncRsp1 overexpression. Upper panels, schematic diagram of the lncRsp1 overexpression strategy; lower panels, the overexpression fragment of the lncRsp1 gene was amplified with primer pair OE‐F/OE‐R. The lncRsp1 promoter was replaced with the EF1α promoter of Sclerotinia sclerotiorum. hygr, hygromycin B resistance gene cassette. (d) Transcript levels of lncRsp1 in PH‐1 and lncRsp1OE strains were analysed by RT‐qPCR
FIGURE S6 Construction and sexual development of lncRsp1 complementation (LC) strains. (a) Schematic diagram of the lncRsp1 complementation strategy. An in situ complementation method was used for lncRsp1 complement, and ∆lncRsp1 was used as the original strain. (b) Perithecial morphology of the wild‐type strain PH‐1, ΔlncRsp1, and ΔlncRsp1‐LC1/LC2 strains. Bar = 500 µm. (c) Ascospore discharge was examined at 7 days postfertilization (dpf). (d) Statistical analysis of the discharged ascospores
TABLE S1 Growth rate and conidiation of different Fusarium graminearum strains
TABLE S2 Primers used in this study
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


