Abstract
Nesidioblastosis is defined as the neoformation of the islets of Langerhans from the pancreatic ductal epithelium and is recognized as the most common cause of hyperinsulinemic hypoglycemia in infants. We herein report an extremely rare case of adult-onset focal nesidioblastosis with the unusual feature of hyperplastic nodular formation. A 55-year-old woman was admitted to our hospital for a tumor detected in the body of the pancreas by magnetic resonance imaging screening. Laboratory examinations showed a high insulin level in the blood. Contrast-enhanced computed tomography and the selective arterial calcium injection test suggested the presence of multiple insulinomas in the body and tail of the pancreas, and, thus, the patient underwent distal pancreatectomy. A histopathological examination of the tumor in the body of the pancreas showed the nodular hyperplasia of islet-like cell clusters. In addition, many small intralobular ductules and islet cells appeared to be budding from the proliferating ductal epithelium, forming “ductuloinsular complexes”. No other abnormal lesion was detected in the remainder of the pancreas. The histopathological diagnosis was focal nesidioblastosis. The patient has remained free of the recurrence of hypoglycemic episodes for more than 31 months. The present case of rare adult-onset focal nesidioblastosis with hyperplastic nodular formation was preoperatively identified as an apparent pancreatic tumor mimicking insulinoma. Nesidioblastosis and insulinoma need to be considered in cases of hyperinsulinemic hypoglycemia, even in adult patients.
Keywords: nesidioblastosis, insulinoma, hyperinsulinemic hypoglycemia, adult-onset, focal type, SACI test
Nesidioblastosis is the most common cause of hyperinsulinemic hypoglycemia in infants and is rarely found in adults [1, 2]. It is defined as the nonneoplastic proliferation of both islet and ductular cells and formation of ductuloinsular complexes, which are characterized by islet cell hyperplasia arising from a proliferating ductal epithelium [3]. Nesidioblastosis was recently reported in adults after gastric bypass surgery [4] and has been described as noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) [5]. We herein describe a rare case of nesidioblastosis in an adult who had had postprandial loss of consciousness for years. Moreover, the present case of focal nesidioblastosis had the unusual feature of hyperplastic nodular formation, which was identified as an apparent pancreatic tumor detected by preoperative imaging mimicking insulinoma.
Case Report
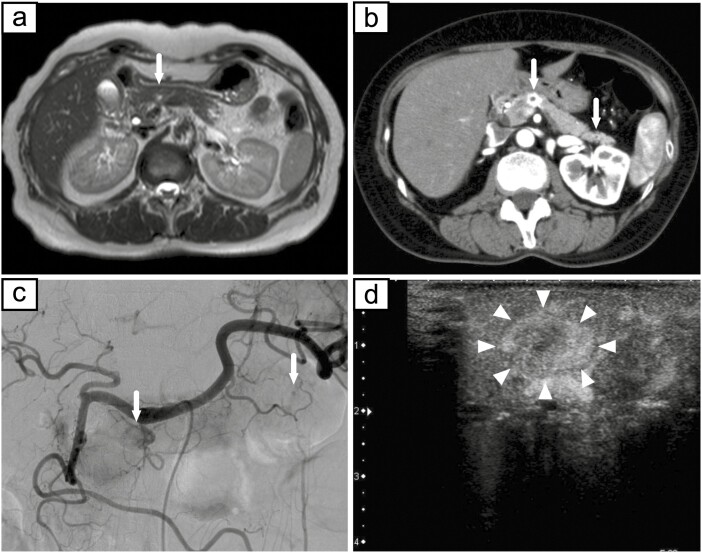
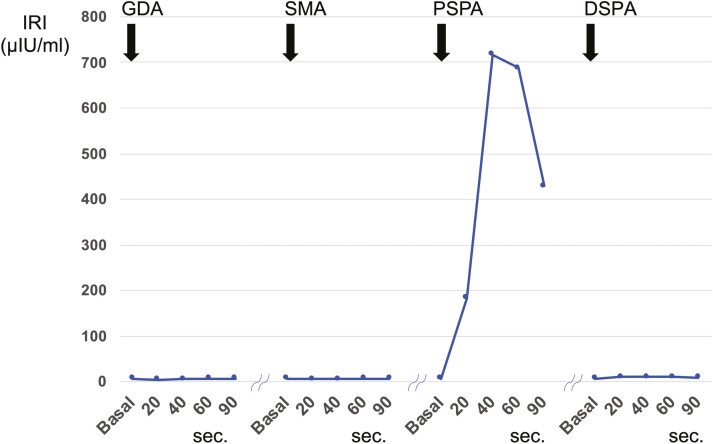
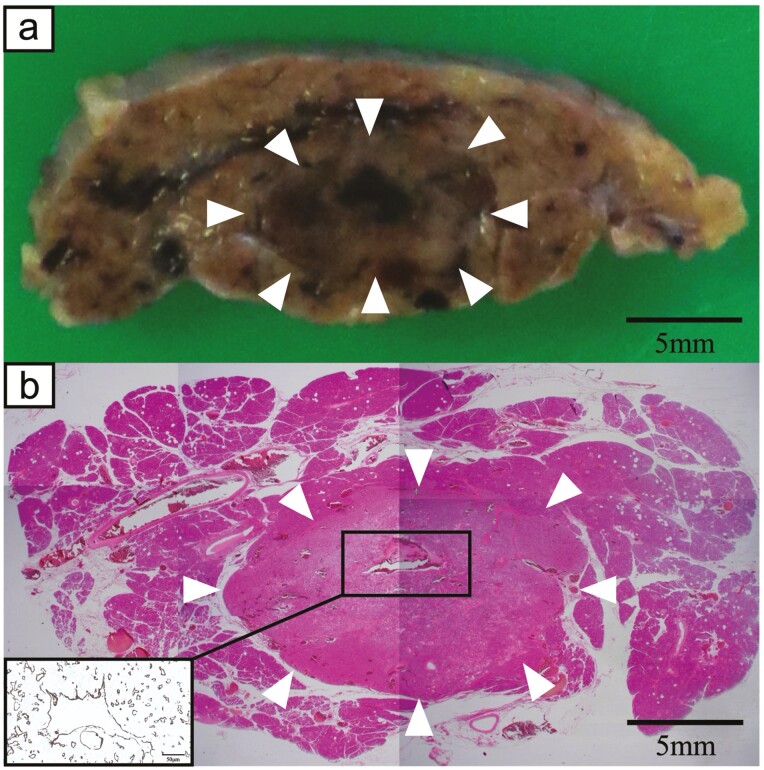
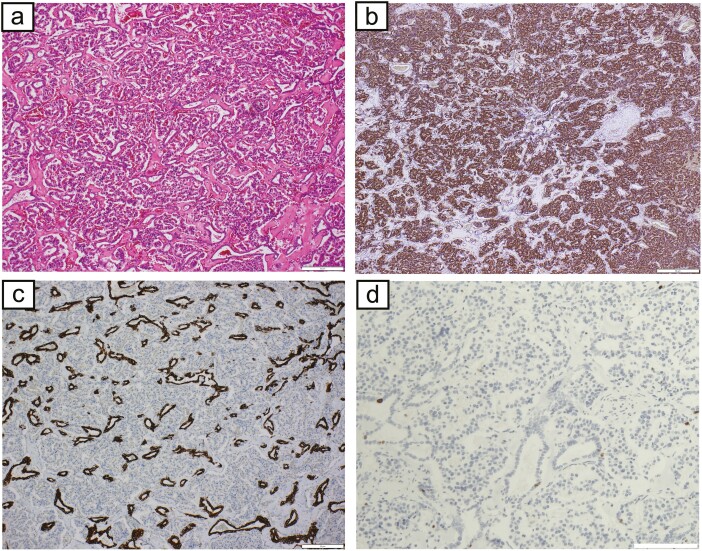
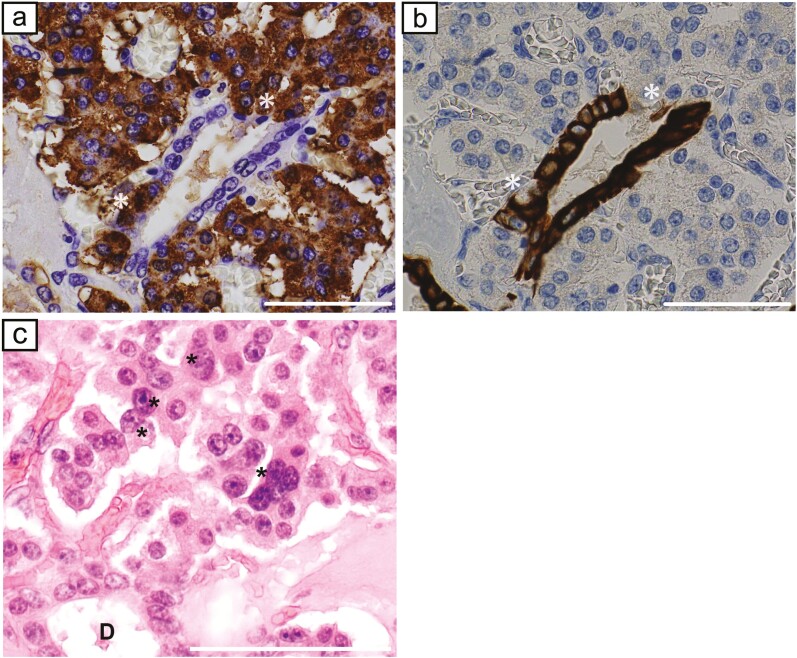
The patient was a 55-year-old woman who was admitted to our hospital for a 15-mm tumor that was incidentally discovered in the body of the pancreas by magnetic resonance imaging (MRI) (Fig. 1A). The tumor had a round and clear shape and a 3-mm cyst-like area in the center. She had prior episodes of postprandial loss of consciousness and falls with fractures many times from the age of 20 years. She had had no blood tests at any point of clinical presentation; thus, the cause had remained unknown. At the time of consultation to our hospital, fasting laboratory examinations showed high level of insulin (31.7 µIU/mL) and normal level of glucose (90 mg/dL) in the blood. Contrast-enhanced computed tomography (CT) showed 2 well-defined tumors with strong enhancement and diameters of 15 and 5 mm in the body and tail of the pancreas, respectively (Fig. 1B). These tumors had a small cystic area without enhancement. Positron emission tomography (PET) and octreotide scintigraphy (Octreoscan) showed no abnormal uptake within the whole pancreas. Although Whipple triad was not fulfilled, neuroendocrine tumors including insulinoma were suspected from image features and the selective arterial calcium injection (SACI) test was performed. Angiography showed 2 tumor stains in the body and tail of the pancreas (Fig. 1C), and blood samples were collected from the hepatic vein via the inferior vena cava 20, 40, 60, and 90 seconds after injecting calcium gluconate into the following arteries: the gastroduodenal artery (GDA), superior mesenteric artery (SMA), proximal splenic artery (PSPA), and distal splenic artery (DSPA). Marked elevations were observed in the level of immunoreactive insulin (IRI) in response to the PSPA stimulation (Fig. 2). The SACI test and enhanced CT suggested the presence of multiple insulinomas in the body and tail of the pancreas, and, thus, the patient underwent distal pancreatectomy. Intraoperative sonazoid-enhanced ultrasonography showed a round and definite tumor in the body of the pancreas (Fig. 1D) but failed to detect any other tumor within the pancreas. The patient underwent distal pancreatectomy under a preoperative diagnosis based on the results of the SACI test and imaging modalities. Macroscopic findings of the resected specimen revealed that the tumor in the body of the pancreas formed a round and well-defined mass (Fig. 3A). No other abnormal lesion was detected in the remainder of the pancreas. Hematoxylin-eosin staining revealed that small lobules of the pancreas had aggregated and exhibited nodular formation (Fig. 3B). A histopathological examination of the tumor showed the nodular hyperplasia of islet-like cell clusters (Fig. 4A). Although enhanced CT and angiography showed a tumor in the pancreatic tail, there were no abnormal findings in the pancreatic tail histopathology. Immunohistochemically, the majority of tumor cells were positive for insulin (Fig. 4B) as well as positive for endocrine cell markers for chromogranin A and synaptophysin (data not shown). CK7 staining revealed many small intralobular ductules evenly distributed throughout the tumor (Fig. 4C), and that the central cyst-like lesion was an expanding pancreatic duct (Fig. 3B inset). The endocrine cells in the tumor were negative for Ki-67 and the ductal cell had low frequency of Ki-67 positivity (Fig. 4D). Insulin positive cells appeared to be budding from the proliferating ductal epithelium, forming “ductuloinsular complexes” (Fig. 5A and 5B). In addition, hematoxylin-eosin staining showed enlarged heterochromatic nuclei in the nodule (Fig. 5C). The histopathological diagnosis was focal nesidioblastosis of the pancreas. After surgery, the patient has remained free of the recurrence of hypoglycemic episodes for more than 31 months.
Figure 1.
a, MRI showed a 15-mm tumor in the body of the pancreas (white arrow). b, c, Contrast-enhanced CT and angiography showed 2 well-defined tumors with strong enhancement and diameters of 15 and 5 mm in the body and tail of the pancreas, respectively (white arrows). d, Intraoperative sonazoid-enhanced ultrasonography showed a round and definite tumor in the body of the pancreas (white arrowheads).
Figure 2.
Results of the SACI test. To assess insulin levels, blood samples were collected from the hepatic vein via the inferior vena cava 20, 40, 60, and 90 seconds after injecting calcium gluconate into the following arteries: the gastroduodenal artery (GDA), superior mesenteric artery (SMA), proximal splenic artery (PSPA), and distal splenic artery (DSPA). Marked elevations were observed in the level of immunoreactive insulin (IRI) in response to the PSPA stimulation.
Figure 3.
a, Macroscopic findings of a resected specimen revealed that the tumor in the body of the pancreas formed a round and well-defined mass (white arrowheads). b, Hematoxylin-eosin staining showed that small lobules of the pancreas aggregated and morphologically exhibited nodular formation similar to a hyperplastic pancreatic lobule (white arrowheads). Inset: CK7 staining revealed that the central cyst-like lesion was an expanding pancreatic duct.
Figure 4.
a, Hematoxylin-eosin staining showed the nodular hyperplasia of islet-like cell clusters b, The majority of cells were positive for insulin. c, CK7 staining revealed many small intralobular ductules evenly distributed throughout the tumor. d, The endocrine cells in the tumor were negative for Ki-67 and the ductal cell had low frequency of Ki-67 positivity. (a, b, c, d: scale bar, 50 μm).
Figure 5.
a, Insulin staining. b, CK7 staining. Insulin positive cells appeared to be budding from the proliferating ductal epithelium, forming “ductuloinsular complexes.” *indicate the same area/cells in both panels. c, Hematoxylin-eosin staining showed enlarged heterochromatic nuclei in the nodule (asterisks). (a, b, c: scale bar, 50 μm).
Discussion
Hyperinsulinemic hypoglycemia in adults is most commonly induced by insulinoma, but is also rarely caused by nesidioblastosis [2, 6, 7]. Nesidioblastosis is defined as the neoformation of the islets of Langerhans from the pancreatic ductal epithelium [8] and is recognized as the most common cause of hyperinsulinemic hypoglycemia in infants [1]. However, nesidioblastosis has been reported in adults after gastric bypass surgery for morbid obesity or Roux-en-Y anastomosis in gastric surgery [4]. Adult-onset nesidioblastosis is increasingly being reported in patients without any previous surgery, as in the present case [9-15] and has been described as noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) [5].
In the present case, her pancreatic tumor was found incidentally on MRI screening. While she had no history of documented hypoglycemia, the presence of the tumor and her loss of consciousness after meals suggested hypoglycemia. The preoperative differentiation of NIPHS from insulinoma is difficult because both diseases exhibit similar clinical features related to hypoglycemia [16]. Fasting hypoglycemia may occur in NIPHS and insulinoma. However, the classical clinical feature of NIPHS is postprandial hypoglycemia, which is absent in patients with insulinoma. Hypoglycemia results in a number of symptoms, including confusion, loss of consciousness, and seizures [17]. The present case had recurrent hypoglycemic attacks after meals but was misdiagnosed with a psychological disorder or epilepsy. A detailed inquiry into the clinical features associated with hypoglycemia needs to be the primary approach, and nesidioblastosis needs to be considered in patients with postprandial hypoglycemia.
Conventional radiographic features by CT and MRI may not necessarily be useful for differentiating NIPHS from insulinoma [16]. Most insulinomas are well-defined hypervascular tumors that show hyperattenuation in the arterial phase of contrast-enhanced CT; however, occult sporadic insulinoma without any detectable pancreatic tumors has also been reported [18]. Nesidioblastosis is classified into focal and diffuse types. The diffuse type is common in adult-onset nesidioblastosis, while only a few studies have reported the focal type.9.10 The focal type is characterized by the nodular hyperplasia of islet-like cell clusters, whereas the diffuse type involves the entire pancreas [19]. Some cases of focal nesidioblastosis were detected as enhanced tumors on contrast-enhanced CT, similar to the present case. In contrast, diffuse nesidioblastosis fails to be identified as a detectable tumor. Intraoperative ultrasonography is useful for detecting tumors such as apparent insulinoma and focal nesidioblastosis, as in the present case. Nevertheless, the SACI test is an indispensable modality for confirming the localization of insulin hypersecretion in the pancreas, particularly for occult sporadic insulinoma and diffuse nesidioblastosis without any detectable pancreatic tumors [20].
A definitive diagnosis of nesidioblastosis may be achieved postoperatively by histopathological findings. Pathological criteria have been established for the diagnosis of diffuse nesidioblastosis in adults [21]. The 4 major criteria are as follows: (1) the exclusion of insulinoma by macroscopic, microscopic, and immunohistochemical examinations; (2) multiple β cells with enlarged and hyperchromatic nuclei and an abundant clear cytoplasm; (3) islets with the normal spatial distribution of various cell types; and (4) no proliferative activity by endocrine cells. The identification of a ductuloinsular complex, which is characterized by the neoformation of islet cells arising from the pancreatic ductal epithelium is also conclusive evidence for nesidioblastosis. However, while there are no clear criteria for the adult focal nesidioblastosis, neonatal focal nesidioblastosis has been characterized by Goosens et al, as a well demarcated compact nodule with tumor-like histology with frequent ductuloinsular complexes with some enlarged beta cells with large nuclei [19].
Histopathological findings in the present case satisfied the above criteria for focal nesidioblastosis, and they revealed a large number of ductuloinsular complexes connected with proliferating pancreatic ducts and enlarged heterochromatic nuclei in the nodule. In the present case, islet-like cell clusters coalesced to form large aggregates, similar to the expanded lobule of a hyperplastic nodule, and small intralobular ductules proliferated in a well-structured state and were evenly distributed in the nodule. We herein described a rare case of focal nesidioblastosis, which morphologically exhibited nodular formation similar to a hyperplastic pancreatic lobule. These findings are characteristic of hyperplastic growth in focal nesidioblastosis, which is different from the neoplastic growth of insulinoma.
On the other hand, previous studies have reported insulinoma with insuloductular differentiation. Shintaku et al reported ductuloinsular pancreatic endocrine tumor with amyloid deposition [22]. The case had high proliferation of both endocrine and ductal compartments with essentially segregated components with the nodular proliferation of small ducts accompanied by desmoplastic stroma. Regitnig et al in reporting the liver metastases with insulinoma of the pancreas [23], showed insular-ductular differentiation; however, in the liver it would be difficult to tell which were pancreatic ducts and bile ducts since both stain for the same cytokeratins. Van Eden et al showed 16 cases of ductuloinsular tumors of the pancreas [24]. They suggested entrapped ductules and some islets in the tumor without ductuloinsular complexes, and the ducts were confined to a more central part of the tumor. Deshpande et al reported that 15 of 77 pancreatic endocrine tumors were actually ductuloinsular pancreatic endocrine tumors, and 11 of these had abundant fibrotic and sclerotic stroma around the ducts [25]. Contrary to these studies, the present case showed the complete integration of ducts and endocrine tissues with very little stroma, and multiple ductal-islet complexes. These are certainly not like the entrapped ductules or even segregated ones described in the previous papers. In particular, this case is different from insulinoma in that an abnormally expanded pancreatic duct was in the center of the tumor and many small intralobular ductules evenly distributed throughout the tumor.
The exact cause and pathological mechanisms of adult-onset nesidioblastosis are unknown. Nesidioblastosis was recently reported in adults after gastric bypass surgery for morbid obesity or Roux-en-Y anastomosis [4]. Some studies suggested that glucagon-like peptide-1 (GLP-1), which is an incretin hormone and secreted after gastric bypass surgery, induces the neoformation of islets from the pancreatic ductal epithelium, leading to nesidioblastosis [4, 26]. However, adult-onset nesidioblastosis is increasingly reported in patients without any previous surgery, as in the present case [9-15]. The histopathological findings of the present case revealed the proliferation of small and large pancreatic ducts and their even distribution in the tumor. These results are identical to those of tissue regeneration after chronic pancreatitis [27]. In addition, hormone positive islet cells appear to bud from the proliferating epithelium of small intralobular ductules. Islet structures coalesced to form large aggregates, which may have an identical appearance to the apparent tumor observed in insulinoma. The majority of cells in nesidioblastic foci are β cells; however, other major cell types are also represented to varying degrees.
Although the pathogenetic pathway of nesidioblastosis remains unclear, the condition appears to be an aberrant regenerative response, and may be induced by a range of pancreatic insults, including partial pancreatectomy, the ectopic autotransplantation of pancreatic tissue, and ductular occlusion. It may also be observed in animals without other apparent pancreatic diseases [28]. Further studies are needed to elucidate the underlying mechanisms of this disease.
The definitive treatment of adult-onset nesidioblastosis is surgical resection; however, a few cases were successfully treated by medical therapy with diazoxide [29]. Medical treatment is more effective for secondary nesidioblastosis after gastric bypass than for idiopathic nesidioblastosis. Previous studies showed that the sufficient reduction of hyperinsulinemia in diffuse nesidioblastosis may require extended resection, such as subtotal pancreatectomy; however, >90% resection frequently results in insulin-dependent diabetes [11]. On the other hand, some cases of focal nesidioblastosis were previously reported to be enucleated [9, 10]. However, cases of multifocal nesidioblastosis or the coexistence of insulinoma and diffuse nesidioblastosis have been reported, for which incomplete or missed surgical resection resulted in recurrence and reoperation on the residual pancreas [30, 31]. Therefore, the preoperative SACI test reliably guides the appropriate extent of resection for the surgical treatment of nesidioblastosis as well as insulinoma. In the present case, a preoperative diagnosis based on the results of the SACI test and radiographic imaging suggested multiple insulinomas in the body and tail of the pancreas; therefore, the patient underwent distal pancreatectomy and has remained free of hypoglycemic symptoms or diabetes mellitus after surgery. Nesidioblastosis needs to be considered in adults with hyperinsulinemic hypoglycemia, and the SACI test is essential for identifying other undetectable tumors, even if an apparent pancreatic tumor is clearly recognized by preoperative images, as in the present case.
Conclusions
We herein described a case of rare adult-onset and focal nesidioblastosis with the unusual feature of hyperplastic nodular formation, which was preoperatively identified as an apparent pancreatic tumor mimicking insulinoma. Nesidioblastosis and insulinoma need to be considered in cases of hyperinsulinemic hypoglycemia, even in adult patients. To prevent overlooking undetectable tumors and the failure to complete surgical resection, the preoperative SACI test is an indispensable modality not only to confirm the localization of insulin hypersecretion, but also to guide the appropriate extent of surgical resection for nesidioblastosis.
Acknowledgments
We thank Dr Gordon C. Weir and Dr Susan Bonner-Weir (Joslin Diabetes Center, Harvard Medical School, Boston, MA) for their kind review of the manuscript and helpful discussion.
Glossary
Abbreviations
- CT
computed tomography
- MRI
magnetic resonance imaging
- NIPHS
noninsulinoma pancreatogenous hypoglycemia syndrome
- PET
positron emission tomography
- PSPA
proximal splenic artery
- SACI
selective arterial calcium injection
Financial Support
Funding information is not applicable.
Disclosure Summary
The authors have nothing to disclose. The authors declare no conflicts of interest.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Goudswaard WB, Houthoff HJ, Koudstaal J, Zwierstra RP. Nesidioblastosis and endocrine hyperplasia of the pancreas: a secondary phenomenon. Hum Pathol. 1986;17(1):46-54. [DOI] [PubMed] [Google Scholar]
- 2. Jabri AL, Bayard C. Nesidioblastosis associated with hyperinsulinemic hypoglycemia in adults: review of the literature. Eur J Intern Med. 2004;15(7):407-410. [DOI] [PubMed] [Google Scholar]
- 3. Wanda MH, Colin GR, Matthew AW.. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. 3rd ed. Amsterdam: Academic Press; 2013. [Google Scholar]
- 4. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254. [DOI] [PubMed] [Google Scholar]
- 5. Service FJ, Natt N, Thompson GB, et al. . Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84(5):1582-1589. [DOI] [PubMed] [Google Scholar]
- 6. Rothmund M, Angelini L, Brunt LM, et al. . Surgery for benign insulinoma: an international review. World J Surg. 1990;14(3):393-398; discussion 398. [DOI] [PubMed] [Google Scholar]
- 7. Okabayashi T, Shima Y, Sumiyoshi T, et al. . Diagnosis and management of insulinoma. World J Gastroenterol. 2013;19(6):829-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laidlaw GF. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol. 1938;14(2):125-134.5. [PMC free article] [PubMed] [Google Scholar]
- 9. Qin H, Li Z, Qu L, et al. . A rare case of focal nesidioblastosis causing adult-onset hypoglycemia. Exp Ther Med. 2015;10(2):723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McElroy MK, Lowy AM, Weidner N. Case report: focal nesidioblastosis (“nesidioblastoma”) in an adult. Hum Pathol. 2010;41(3):447-451. [DOI] [PubMed] [Google Scholar]
- 11. Kowalewski AM, Szylberg Ł, Kasperska A, Marszałek A. The diagnosis and management of congenital and adult-onset hyperinsulinism (nesidioblastosis) - literature review. Pol J Pathol. 2017;68(2):97-101. [DOI] [PubMed] [Google Scholar]
- 12. García-Santos EP, Manzanares-Campillo Mdel C, Padilla-Valverde D, et al. . Nesidioblastosis. A case of hyperplasia of the islets of Langerhans in the adult. Pancreatology. 2013;13(5):544-548. [DOI] [PubMed] [Google Scholar]
- 13. Maeda Y, Yokoyama K, Takeda K, et al. . Adult-onset diffuse nesidioblastosis causing hypoglycemia. Clin J Gastroenterol. 2013;6(1):50-54. [DOI] [PubMed] [Google Scholar]
- 14. Ferrario C, Stoll D, Boubaker A, Matter M, Yan P, Puder JJ. Diffuse nesidioblastosis with hypoglycemia mimicking an insulinoma: a case report. J Med Case Rep. 2012;6:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsujino M, Sugiyama T, Nishida K, et al. . Noninsulinoma pancreatogenous hypoglycemia syndrome: a rare case of adult-onset nesidioblastosis. Intern Med. 2005;44(8):843-847. [DOI] [PubMed] [Google Scholar]
- 16. Starke A, Saddig C, Kirch B, Tschahargane C, Goretzki P. Islet hyperplasia in adults: challenge to preoperatively diagnose non-insulinoma pancreatogenic hypoglycemia syndrome. World J Surg. 2006;30(5):670-679. [DOI] [PubMed] [Google Scholar]
- 17. Levine R. Hypoglycemia. JAMA. 1974;230(3):462-463. [PubMed] [Google Scholar]
- 18. Noone TC, Hosey J, Firat Z, Semelka RC. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19(2):195-211. [DOI] [PubMed] [Google Scholar]
- 19. Goossens A, Gepts W, Saudubray JM, et al. . Diffuse and focal nesidioblastosis. A clinicopathological study of 24 patients with persistent neonatal hyperinsulinemic hypoglycemia. Am J Surg Pathol. 1989;13(9):766-775. [PubMed] [Google Scholar]
- 20. Kenney B, Tormey CA, Qin L, Sosa JA, Jain D, Neto A. Adult nesidioblastosis. Clinicopathologic correlation between pre-operative selective arterial calcium stimulation studies and post-operative pathologic findings. Jop. 2008;9(4):504-511. [PubMed] [Google Scholar]
- 21. Anlauf M, Wieben D, Perren A, et al. . Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol. 2005;29(4):524-533. [DOI] [PubMed] [Google Scholar]
- 22. Shintaku M, Tado H, Inayama K, Murakami T, Suzuki T. Ductulo-insular pancreatic endocrine tumor with amyloid deposition: report of a case. Pathol Int. 2015;65(4):197-201. [DOI] [PubMed] [Google Scholar]
- 23. Regitnig P, Spuller E, Denk H. Insulinoma of the pancreas with insular-ductular differentiation in its liver metastasis–indication of a common stem-cell origin of the exocrine and endocrine components. Virchows Arch. 2001;438(6):624-628. [DOI] [PubMed] [Google Scholar]
- 24. van Eeden S, de Leng WW, Offerhaus GJ, et al. . Ductuloinsular tumors of the pancreas: endocrine tumors with entrapped nonneoplastic ductules. Am J Surg Pathol. 2004;28(6):813-820. [DOI] [PubMed] [Google Scholar]
- 25. Deshpande V, Selig MK, Nielsen GP, Fernandez-del Castillo C, Lauwers GY. Ductulo-insular pancreatic endocrine neoplasms: clinicopathologic analysis of a unique subtype of pancreatic endocrine neoplasms. Am J Surg Pathol. 2003;27(4):461-468. [DOI] [PubMed] [Google Scholar]
- 26. Patti ME, McMahon G, Mun EC, et al. . Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236-2240. [DOI] [PubMed] [Google Scholar]
- 27. Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144(6):1180-1193. [DOI] [PubMed] [Google Scholar]
- 28. Traverso LW, Bockman DE, Pleis SK, Dail DH. Nesidioblastosis as a mechanism to prevent fibrosis-induced diabetes after pancreatic duct obstruction. Pancreas. 1993;8(3):325-329. [DOI] [PubMed] [Google Scholar]
- 29. Arao T, Okada Y, Hirose A, Tanaka Y. A rare case of adult-onset nesidioblastosis treated successfully with diazoxide. Endocr J. 2006;53(1):95-100. [DOI] [PubMed] [Google Scholar]
- 30. Rosman J, Bravo-Vera R, Sheikh A, Gouller A. Metastatic insulinoma in an adult patient with underlying nesidioblastosis. J Endocrinol Invest. 2007;30(6):521-524. [DOI] [PubMed] [Google Scholar]
- 31. Valli V, Blandamura S, Pastorelli D, Merigliano S, Sperti C. Nesidioblastosis coexisting with non-functioning islet cell tumour in an adult. Endokrynol Pol. 2015;66(4):356-360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.