Abstract
Human papillomaviruses (HPVs) are responsible for cutaneous and mucosal lesions. Persistent HPV infection remains a leading cause of uterine cancer in women, but also of cutaneous squamous cell carcinoma in patients with epidermodysplasia verruciformis (EV), and of rare and devastating benign tumors, such as “tree-man” syndrome. HPV infections are usually asymptomatic or benign in the general population. Severe manifestations in otherwise healthy subjects attest to inherited immunodeficiencies. The human genetic dissection of these cases has identified critical components of the immune response to HPVs, including the non-redundant roles of keratinocyte-intrinsic immunity in controlling β-HPVs, and of T cell-dependent adaptive immunity for controlling all HPV types. A key role of the CD28 T-cell costimulation pathway in controlling common warts due to HPVs was recently discovered. This review summarizes the state of the art in the human genetics of HPV infection, focusing on two key affected cell types: keratinocytes and T cells.
Keywords: Papillomavirus, Human, HPV, Genetics, Inborn Error of immunity, IEI, Primary immunodeficiencies, PID, Keratinocyte, T cell, CD28
Introduction
Human papillomaviruses (HPVs) are non-enveloped viruses with ~8 kb double-stranded circular DNA genomes packaged into an icosahedral capsid. They display strict tropism for skin and squamous stratified epithelia. They first infect basal stem cells and complete their cycle during the course of keratinocyte differentiation in successive layers. HPVs have been classified into five genera, including over 200 HPV genotypes, according to the International Human Papillomavirus Reference Center (Papillomavirus Episteme (PaVE); https://pave.niaid.nih.gov/#home) [1]. HPVs of the β, γ, μ and ν genera display strict cutaneous tropism, whereas α-HPVs include both cutaneous and mucosal specific types. β-HPVs generally cause asymptomatic infections, but are responsible for pytiriasis versicolor-like lesions and flat warts, and non-melanoma skin cancer (NMSC) in rare patients with epidermodysplasia verruciformis (EV) [2]. γ-, μ- and ν-HPVs cause benign cutaneous common and plantar warts. Different types of α-HPVs are associated with cutaneous warts, benign mucosal lesions and carcinomas of the anogenital and oropharyngeal tracts [3,4]. The vast majority of individuals within the human population have been exposed to HPVs, as demonstrated by serological data [5–8]. Infection is mostly asymptomatic or benign and self-healing [9]. The onset of HPV-induced cancer can be attributed primarily to persistent lesions, due to a failure to clear replicative infection. For instance, once infected, less than 10–20% of women fail to clear cervical lesions spontaneously within two years, revealing strong interindividual variability in host immunity to HPVs [4,10].
Severe HPV infections of all types are frequent in patients with acquired immunodeficiencies, whether due to human immunodeficiency virus (HIV) infection or immunosuppressive treatment, suggesting an important role for adaptive T-cell immunity, and CD4+ T cells in particular, in the control of HPV infection [11–14]. However, many patients without acquired immunodeficiencies suffer from severe HPV infection, suggesting a congenital immune defect. The characterization of inborn errors of immunity (IEI) in these patients provides an interesting approach for the study of non-redundant immune mechanisms for the effective control of HPV infection. These studies have already revealed an important role for tissue-specific and cell-intrinsic immunity in keratinocytes, and key molecular mechanisms for protective T-cell immunity against HPVs. We review here the knowledge acquired from the study of these IEI, with particular focus on the two key cell types affected: keratinocytes and T cells. Indeed, the underlying defects range from inborn errors of keratinocyte-dependent intrinsic immunity to inborn errors of T cell-dependent adaptive immunity affecting both T cells and antigen-presenting cells (APCs). Neither known disorders of B-cell immunity nor defects of myeloid or lymphoid leukocyte-mediated innate immunity are associated with a predisposition to HPV lesions.
1-. Keratinocyte intrinsic immunity
Viruses generally display cell-specific tropism, and HPVs display a particular tropism for keratinocytes. The ability of target cells to restrict viral replication and assembly directly and/or to signal their “infected” status to immune cells, both of which define cell-intrinsic immunity, is crucial for infection control. Cell-intrinsic immunity can operate in any of the > 400 discernable hematopoietic and non-hematopoietic cell types of the human body. The development and persistence of HPV lesions reflect a two-step mechanism in which keratinocytes are permissive to replicative HPV infections, and T cells fail to clear infected keratinocytes. β-HPVs are frequently found in the skin of healthy individuals, but copy numbers are generally low, and they do not cause visible lesions. By contrast, EV patients have a high viral load and visible lesions [15–17]. Three IEI have been identified in patients with isolated EV: EVER1, EVER2 and CIB1 deficiencies [2,18,19]. EVER1, EVER2 and CIB1 form a complex that restricts β-HPV infection in keratinocytes. This keratinocyte-intrinsic viral restriction accounts for the lack of symptomatic β-HPV infection in healthy individuals, and the occurrence of such infections in EV patients. ZnT1, a zinc transporter, interacts with both EVER proteins, but not with CIB1. Consistent with this finding, abnormal zinc homeostasis is observed only in EVER-deficient cells, suggesting that EVER proteins have functions other than HPV restriction that are CIB1-independent and not involved in EV pathogenesis. Furthermore, CIB1-deficient keratinocytes have no particular abnormal migration, cell growth or adhesion phenotype. The EVER/CIB1 complex is targeted by the viral E5 and E8 proteins expressed by α-HPVs and γ-, μ- and ν-HPVs, respectively, but not by β-HPVs, suggesting that E5 and E8 function, at least partly, to overcome EVER/CIB1-dependent restriction. The precise molecular mechanism by which β-HPV replication is controlled by the EVER/CIB1 complex in keratinocytes remains to be established, but we hypothesize that the lack of a functional EVER/CIB1 complex compensates for the missing E5/E8 proteins, suggesting a role for this complex in a regulatory pathway controlling the division of infected epidermal stem cells.
2-. Defects of APCs and T cells
a. Immune infiltrates in regressing warts
Human and animal studies of regressing or inflamed warts have provided clues to the cells involved in the antiviral effector phase [20–27]. In dogs infected with COPV (canine oral papillomavirus), the lesions start to regress eight weeks post-infection, and this regression is associated with an infiltration of CD4+ and CD8+ T cells, and dendritic cells (DCs) into the infected epithelium and the underlying lamina propria [24]. In rabbits, the regression of skin lesions caused by CRPV (cottontail rabbit virus) is mediated by CD8+ T cells, but not CD4+ T cells [23]. The most detailed study of HPV-induced lesions in remission to date concerned HPV6+ and HPV11+ anogenital warts and was published in 1994. This study showed an infiltrate of leukocytes, predominantly CD4+ and, to a lesser extent, CD8+ cells, in the epidermis and dermis of the regressing warts, and an accumulation of macrophages in the dermis under the lesion [22]. The findings of this study were consistent with those of a 1986 study of planar wart regression, showing a dermal and epidermal infiltrate constituted principally of CD4+ cells [20]. Recent studies of regressing cutaneous warts have reported a major dermal infiltrate of poorly characterized DCs, including a few plasmacytoid DCs (pDCs), and a lymphocytic infiltrate in the dermis below the lesion, consisting mostly of CD8+ T cells, contrasting with the results of previous studies [25,27]. One possible explanation for this discordance may be the expression of CD4 on some myeloid cells undistinguishable from CD4+ T cells by immunohistochemistry. Together, these studies suggest a major role for T cell-mediated immunity in the control of HPV infection.
b. Hypomorphic mutations of severe combined immunodeficiency genes
Given the importance of immunological infiltrates in wart regression, it was no surprise to find that IEI affecting adaptive cell responses were associated with severe HPV infections, provided of course that they were compatible with survival beyond infancy. Indeed, although PCR-based studies detect β- and γ-HPV DNA on skin swabs as soon as the first days/weeks of life [16], it should be borne in mind that HPV-driven lesions are rare in young children. The prevalence of cutaneous warts gradually increases in children of school age, peaking at 10–15 years of age [28]. The prevalence of anogenital warts is highest between 20 and 30 years of age [29]. Diseases such as reticular dysgenesis or severe combined immunodeficiency (SCID), in which T-cell development is abolished, have not, therefore, been found to be associated with HPV infection, because the patients die before the age of one year in the absence of hematopoietic stem cell transplantation (HSCT) [30]. By contrast, hypomorphic mutations of SCID genes allowing some T-cell development and survival into adulthood have been associated with a high degree of susceptibility to HPVs and many other pathogens. The mutations concerned include autosomal recessive (AR) hypomorphic mutations of the ADA [31,32], CORO1A [33–35], DCLRE1C [36,37], JAK3 [38], LIG4 [39,40], RAG1 and RAG2 [41,42], and ZAP70 [43] genes, and X-linked recessive (XLR) mutations of IL2RG [44–46].
c. Other IEI impairing T-cell development or migration
Other IEI partially impairing T-cell development have also been associated with severe cutaneous warts. These IEI include biallelic mutations of the TRAC [47], NHEJ1 [48], ATM [49], LCK [50], IL7 [51,52] and STK4 [53,54] genes, and hemizygous mutations of the SASH3 gene [55]. Deficiencies of TRAC, NHEJ1 and ATM partially impair T-cell receptor (TCR) and/or B-cell receptor (BCR) rearrangement. Deficiency of SASH3 impairs thymocyte survival. LCK is a signal-transducing molecule acting downstream from several T-cell costimulation molecules, such as CD4, CD8 and CD28. IL-7 deficiency partially impairs T-cell development, whereas complete loss-of-function (LOF) mutations of the genes encoding its two coreceptors, IL7R and IL2RG, are associated with a complete absence of T cells. STK4 is important for T-cell development, and studies in mice have shown that peripheral naïve T lymphopenia is due to impaired thymic egress [56,57]. Deficiencies of LCK, IL-7, SASH3 and STK4 lead predominantly to CD4+ T-cell lymphopenia. Two inherited immunodeficiencies with a very high penetrance for severe HPV infection are caused by autosomal dominant (AD) gain-of-function (GOF) CXCR4 [58–60] and AD LOF GATA2 [61–65] mutations. These immunodeficiencies are associated with severe cutaneous or anogenital wart in 80% and 50% of patients, respectively. Unlike the other IEI mentioned above, they simultaneously compromise multiple leukocyte subsets, notably reducing APC and T-cell counts, particularly for CD4+ T cells. The important role of myeloid cells in the susceptibility to HPV of CXCR4-deficient patients was confirmed by the remission of warts in a patient who spontaneously lost the CXCR4 GOF allele in the myeloid compartment [66]. Consistent with the importance of both APCs and T cells, AR DOCK8 deficiency is associated with a combined immunodeficiency characterized by severe viral infections of the skin and mucosae, including warts in >40% of the patients [67–69]. In particular, DOCK8 plays a crucial role in optimal dendritic cell migration to lymph nodes, and in the survival of T cells in collagen-dense tissues [67,70,71]. Its deficiency therefore impairs both priming and effector anti-HPV immune responses. In addition to its role in thymic egression, STK4 promotes T-cell migration via DOCK8 recruitment [57], possibly contributing to skin and mucosal viral infections in STK4 deficient patients. Thus, studies of IEI show that low counts of APCs and T cells, and the impairment of APC and T-cell migration confer a predisposition to severe HPV infection.
d. IEI impairing optimal TCR activation or costimulation
Other IEI underlying HPV lesions have only a modest impact on the counts of T lymphocytes and APCs. These disorders inform us about specific molecular events required for HPV clearance. Mutations impairing signaling downstream from the TCR also confer a predisposition to HPV infection. AR RHOH deficiency is associated with multiple conditions, including EV. RHOH regulates LCK/ZAP70 signaling in the TCR pathway [72]. A biallelic missense mutation of TAOK2 has been reported in two patients with severe warts and normal T-cell counts but impaired TCR activation [73]. TAOK2 activates the MAP kinase pathway and regulates cytoskeleton dynamics, but its role in T cells remains unknown [74]. More recent findings have suggested that some other T-cell coreceptors are more important for the control of HPV infection. Biallelic LOF mutations of CD4 have been reported in a patient with isolated severe warts [75]. CD4 is a coreceptor for human leukocyte antigen II (HLA-II). It increases positive selection in the thymus and the antigen response of CD4+ T cells [76–78]. However, CD4 is also expressed on myeloid cells, and could therefore also play a role in APCs. We recently reported CD28 deficiency in three patients with severe isolated verrucosis, including one patient with “tree man” syndrome [79]. CD28 is a major costimulation molecule in TCR signaling, recognizing CD80 and CD86 on APCs. Our findings suggest that the CD28 costimulation pathway plays an important role in controlling the HPVs responsible for cutaneous common warts. About 30% of patients with AR CARMIL2, AD CARD11 or XLR MAGT1 deficiency have severe cutaneous or anogenital warts, in addition to several other infections [80–85]. In light of the discovery of patients with CD28 deficiency, the susceptibility to HPV of these patients can now be explained by a CD28 signaling defect. CARMIL2 deficiency specifically impairs CD28 signaling in mouse and human T lymphocytes [80,86]. MAGT1 is involved in magnesium regulation and protein glycosylation. Its deficiency results in a glycosylation defect that, among its over effects, impairs CD28 expression on T lymphocytes. Dominant-negative CARD11 mutations impair NF-κB activation upon CD3/CD28 costimulation, and CARD11 is a direct partner of CARMIL2 [87,88]. Together, given the absence of warts in patients with IEI affecting other T-cell costimulation receptors (e.g. CD27, ICOS, CD40L), these recent discoveries suggest a non-redundant role for CD4 and CD28 costimulation in the control of the virus.
Conclusion
IEI have provided important clues to improve our understanding of the immune response to HPVs. First, despite a clear preventive effect of anti-HPV vaccination, inborn errors of adaptive B-cell immunity do not confer a predisposition to severe HPV infection. Likewise, IEI of leukocyte (myeloid or lymphoid) innate immunity (affecting the oxidative burst, for example) are not associated with overt HPV infections. It is now clear that the major contributing branches of immunity are keratinocyte-intrinsic immunity for β-HPVs, and T cell-dependent adaptive immunity for all HPV types. CD4+ T cells clearly play a central role, as highlighted by severe HPV infections in patients with HIV-mediated acquired immunodeficiency or IEI resulting in selective CD4+ T-cell lymphopenia, and the recent discovery of CD4 deficiency in a patient with generalized verrucosis. IEI affecting the Th1 (IL-12/IFN-γ) and Th17 (IL-17) axes of CD4+ T-cell immunity are not associated with HPV disease, and have instead been shown to be associated with susceptibility to mycobacterial and fungal infections, respectively [89,90]. Future studies should decipher the functional component of the CD4+ T-cell response required for the control of papillomaviruses. A key role for the CD28 costimulation pathway has recently emerged, with the implication of CD28, CARD11, CARMIL2 and MAGT1 deficiencies, all of which impair CD28 expression or signaling in T cells. Finally, patients with SCID due to IL2RG or JAK3 deficiencies may develop EV, or other recurrent and persistent warts, despite successful HSCT and full correction of their T-cell immune defect [91–93]. Conversely, patients with SCID due to other IEI (e.g. RAG1 or RAG2 deficiencies) are not prone to the development of HPV infections after HSCT. It is tempting to speculate that the post-transplantation susceptibility to HPV of patients with IL2RG and JAK3 deficiencies is skin-intrinsic, affecting either keratinocytes or Langerhans cells, the only professional APCs in the epidermis, or both [94]. HSCT in patients with SCID will constitute an interesting model in which to extend our knowledge of the skin-intrinsic mechanisms at work in the control of HPV infection.
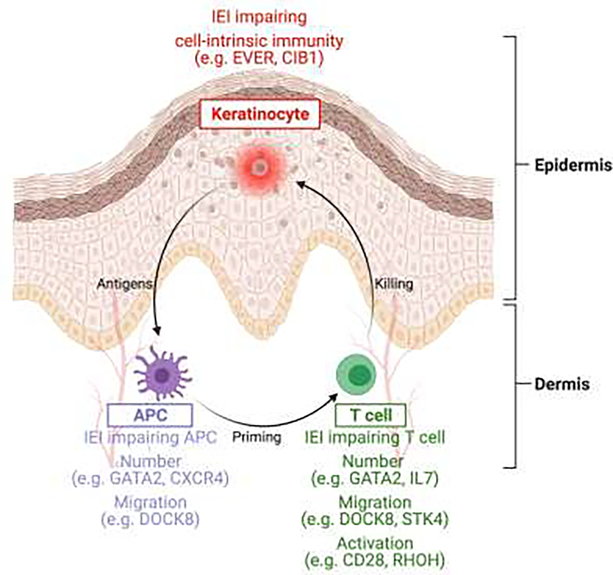
Figure.
Inborn errors of immunity (IEI) conferring predisposition to severe HPV infections impair either keratinocyte-intrinsic immunity or adaptive T-cell immunity at the priming or effector level. APC: antigen presenting cell.
Acknowledgments
We thank all members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions, and Yelena Nemirovskaya and Lazaro Lorenzo for administrative assistance. With financial support from ITMO Cancer of Aviesan and INCa within the framework of the 2021–2030 Cancer Control Strategy, French Primary Immunodeficiency Reference Center (CEREDIH), French National Research Agency (ANR) under the Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID), ANR grant (CARMIL2), the French Foundation for Medical Research (FRM) (EQU201903007798), the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program (UL1TR001866), the National Institute of Allergy and Infectious Diseases (NIAID), NIH (R01AI143810), the Rockefeller University, Institut National de la Santé et de la Recherche Médicale (INSERM), the Howard Hughes Medical Institute, Paris Descartes University, and the St. Giles Foundation.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Doorslaer K, Li Z, Xirasagar S, Maes P, Kaminsky D, Liou D, Sun Q, Kaur R, Huyen Y, McBride AA: The Papillomavirus Episteme: a major update to the papillomavirus sequence database. Nucleic Acids Res 2017, 45:D499–D506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orth G: Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Semin Immunol 2006, 18:362–374. [DOI] [PubMed] [Google Scholar]
- 3.de Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H: Classification of papillomaviruses. Virology 2004, 324:17–27. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, Stanley MA, Franceschi S: Carcinogenic human papillomavirus infection. Nat Rev Dis Primer 2016, 2:16086. [DOI] [PubMed] [Google Scholar]
- 5.Antonsson A, Green AC, Mallitt K, O’Rourke PK, Pandeya N, Pawlita M, Waterboer T, Neale RE: Prevalence and stability of antibodies to 37 human papillomavirus types — A population-based longitudinal study. Virology 2010, 407:26–32. [DOI] [PubMed] [Google Scholar]
- 6.Casabonne D, Waterboer T, Michael KM, Pawlita M, Mitchell L, Newton R, Harwood C, Proby C: The seroprevalence of human papillomavirus by immune status and by ethnicity in London. Infect Agent Cancer 2009, 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michael KM, Waterboer T, Sehr P, Rother A, Reidel U, Boeing H, Bravo IG, Schlehofer J, Gärtner BC, Pawlita M: Seroprevalence of 34 Human Papillomavirus Types in the German General Population. PLOS Pathog 2008, 4:e1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman S, Pierce Campbell CM, Waterboer T, Rollison DE, Ingles DJ, Torres BN, Michel A, Sudenga SL, Pawlita M, Villa LL, et al. : Seroprevalence of cutaneous human papillomaviruses (HPVs) among men in the multinational HPV Infection in Men study. J Gen Virol 2016, 97:3291–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I: Human papillomavirus molecular biology and disease association. Rev Med Virol 2015, 25:2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R, Proyecto Epidemiológico Guanacaste Group: Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008, 100:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gormley RH, Kovarik CL: Human papillomavirus–related genital disease in the immunocompromised host: Part I. J Am Acad Dermatol 2012, 66:867.e1–867.e14. [DOI] [PubMed] [Google Scholar]
- 12.Moerman M, Danielides VG, Nousia C-S, van Wanzeele F, Forsyth R, Vermeersch H: Recurrent Focal Epithelial Hyperplasia due to HPV13 in an HIV-Positive Patient. Dermatology 2001, 203:339–341. [DOI] [PubMed] [Google Scholar]
- 13.Tschachler E, Bergstresser PR, Stingl G: HIV-related skin diseases. The Lancet 1996, 348:659–663. [DOI] [PubMed] [Google Scholar]
- 14.Wieland U, Kreuter A, Pfister H: Human papillomavirus and immunosuppression. Curr Probl Dermatol 2014, 45:154–165. [DOI] [PubMed] [Google Scholar]
- 15.Antonsson A, Erfurt C, Hazard K, Holmgren V, Simon M, Kataoka A, Hossain S, Håkangård C, Hansson BG: Prevalence and type spectrum of human papillomaviruses in healthy skin samples collected in three continents. J Gen Virol 2003, 84:1881–1886. [DOI] [PubMed] [Google Scholar]
- 16.Antonsson A, Karanfilovska S, Lindqvist PG, Hansson BG: General Acquisition of Human Papillomavirus Infections of Skin Occurs in Early Infancy. J Clin Microbiol 2003, 41:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael KM, Waterboer T, Pfister H, Gariglio M, Majewski S, Favre M, Pawlita M: Seroreactivity of 38 Human Papillomavirus Types in Epidermodysplasia Verruciformis Patients, Relatives, and Controls. J Invest Dermatol 2010, 130:841–848. [DOI] [PubMed] [Google Scholar]
- 18.Ramoz N, Rueda L-A, Bouadjar B, Montoya L-S, Orth G, Favre M: Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet 2002, 32:579–581. [DOI] [PubMed] [Google Scholar]
- 19.de Jong SJ, Créquer A, Matos I, Hum D, Gunasekharan V, Lorenzo L, Jabot-Hanin F, Imahorn E, Arias AA, Vahidnezhad H, et al. : The human CIB1–EVER1–EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. J Exp Med 2018, 215:2289–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwatsuki K, Tagami H, Takigawa M, Yamada M: Plane Warts Under Spontaneous Regression: Immunopathologic Study on Cellular Constituents Leading to the Inflammatory Reaction. Arch Dermatol 1986, 122:655–659. [DOI] [PubMed] [Google Scholar]
- 21.Okabayashi M, Angell MG, Christensen ND, Kreider JW: Morphometric analysis and identification of infiltrating leucocytes in regressing and progressing shope rabbit papillomas. Int J Cancer 1991, 49:919–923. [DOI] [PubMed] [Google Scholar]
- 22.Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, Taylor-Robinson D, Stanley MA: Immunological events in regressing genital warts. Am J Clin Pathol 1994, 102:768–774. [DOI] [PubMed] [Google Scholar]
- 23.Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO: Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and persistence of viral DNA after regression. J Virol 1997, 71:5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls PK, Moore PF, Anderson DM, Moore RA, Parry NR, Gough GW, Stanley MA: Regression of Canine Oral Papillomas Is Associated with Infiltration of CD4+ and CD8+ Lymphocytes. Virology 2001, 283:31–39. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama Y, Asagoe K, Yamauchi A, Yamamoto T, Shirafuji Y, Morizane S, Nakanishi G, Iwatsuki K: Dendritic cell subsets and immunological milieu in inflammatory human papilloma virus-related skin lesions. J Dermatol Sci 2011, 63:173–183. [DOI] [PubMed] [Google Scholar]
- 26.Hibma MH: The Immune Response to Papillomavirus During Infection Persistence and Regression. Open Virol J 2012, 6:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saadeh D, Kurban M, Abbas O: Plasmacytoid dendritic cells and type I interferon in the immunological response against warts. Clin Exp Dermatol 2017, 42:857–862. [DOI] [PubMed] [Google Scholar]
- 28.Kilkenny M, Marks R: The descriptive epidemiology of warts in the community. Australas J Dermatol 1996, 37:80–86. [DOI] [PubMed] [Google Scholar]
- 29.Patel H, Wagner M, Singhal P, Kothari S: Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis 2013, 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer A, Notarangelo LD, Neven B, Cavazzana M, Puck JM: Severe combined immunodeficiencies and related disorders. Nat Rev Dis Primer 2015, 1:15061. [DOI] [PubMed] [Google Scholar]
- 31.Antony FC, Webster ADB, Bain M.d, Harland CC: Recalcitrant palmoplantar warts associated with adult-onset adenosine deaminase deficiency. Br J Dermatol 2002, 147:180–195. [DOI] [PubMed] [Google Scholar]
- 32.Shovlin CL, Hughes JMB, Simmonds HA, Fairbanks L, Deacock S, Lechler R, Roberts I, Webster ADB: Adult presentation of adenosine deaminase deficiency. The Lancet 1993, 341:1471. [DOI] [PubMed] [Google Scholar]
- 33.Stray-Pedersen A, Jouanguy E, Crequer A, Bertuch AA, Brown BS, Jhangiani SN, Muzny DM, Gambin T, Sorte H, Sasa G, et al. : Compound Heterozygous CORO1A Mutations in Siblings with a Mucocutaneous-Immunodeficiency Syndrome of Epidermodysplasia Verruciformis-HPV, Molluscum Contagiosum and Granulomatous Tuberculoid Leprosy. J Clin Immunol 2014, 34:871–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Punwani D, Pelz B, Yu J, Arva NC, Schafernak K, Kondratowicz K, Makhija M, Puck JM: Coronin-1A: Immune Deficiency in Humans and Mice. J Clin Immunol 2015, 35:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yee CS, Massaad MJ, Bainter W, Ohsumi TK, Föger N, Chan AC, Akarsu NA, Aytekin C, Ayvaz DÇ, Tezcan I, et al. : Recurrent viral infections associated with a homozygous CORO1A mutation that disrupts oligomerization and cytoskeletal association. J Allergy Clin Immunol 2016, 137:879–888.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodbine L, Grigoriadou S, Goodarzi AA, Riballo E, Tape C, Oliver AW, van Zelm MC, Buckland MS, Davies EG, Pearl LH, et al. : An Artemis polymorphic variant reduces Artemis activity and confers cellular radiosensitivity. DNA Repair 2010, 9:1003–1010. [DOI] [PubMed] [Google Scholar]
- 37.Tahiat A, Badran YR, Chou J, Cangemi B, Lefranc G, Labgaa Z-M, Oussalam S, Kaddouri-Slimani A, Belarbi A, Bendissari-Bouzid K, et al. : Epidermodysplasia verruciformis as a manifestation of ARTEMIS deficiency in a young adult. J Allergy Clin Immunol 2017, 139:372–375.e4. [DOI] [PubMed] [Google Scholar]
- 38.Frucht DM, Gadina M, Jagadeesh GJ, Aksentijevich I, Takada K, Bleesing JJH, Nelson J, Muul LM, Perham G, Morgan G, et al. : Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun 2001, 2:422–432. [DOI] [PubMed] [Google Scholar]
- 39.O’Driscoll M, Cerosaletti KM, Girard P-M, Dai Y, Stumm M, Kysela B, Hirsch B, Gennery A, Palmer SE, Seidel J, et al. : DNA Ligase IV Mutations Identified in Patients Exhibiting Developmental Delay and Immunodeficiency. Mol Cell 2001, 8:1175–1185. [DOI] [PubMed] [Google Scholar]
- 40.Tamura S, Higuchi K, Tamaki M, Inoue C, Awazawa R, Mitsuki N, Nakazawa Y, Mishima H, Takahashi K, Kondo O, et al. : Novel compound heterozygous DNA ligase IV mutations in an adolescent with a slowly-progressing radiosensitive-severe combined immunodeficiency. Clin Immunol 2015, 160:255–260. [DOI] [PubMed] [Google Scholar]
- 41.Delmonte OM, Schuetz C, Notarangelo LD: RAG Deficiency: Two Genes, Many Diseases. J Clin Immunol 2018, 38:646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henrickson SE, Walter JE, Quinn C, Kanakry JA, Bardakjian T, Dimitrova D, Ujhazi B, Csomos K, Bosticardo M, Dobbs K, et al. : Adult onset myopathy in a patient with hypomorphic RAG2 mutations and combined immune deficiency. J Clin Immunol 2018, 38:642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinn IK, Sanders RP, Stray-Pedersen A, Coban-Akdemir ZH, Kim VH-D, Dadi H, Roifman CM, Quigg T, Lupski JR, Orange JS, et al. : Novel Combined Immune Deficiency and Radiation Sensitivity Blended Phenotype in an Adult with Biallelic Variations in ZAP70 and RNF168. Front Immunol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks EG, Schmalstieg FC, Wirt DP, Rosenblatt HM, Adkins LT, Lookingbill DP, Rudloff HE, Rakusan TA, Goldman AS: A novel X-linked combined immunodeficiency disease. J Clin Invest 1990, 86:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmalstieg FC, Leonard WJ, Noguchi M, Berg M, Rudloff HE, Denney RM, Dave SK, Brooks EG, Goldman AS: Missense mutation in exon 7 of the common gamma chain gene causes a moderate form of X-linked combined immunodeficiency. J Clin Invest 1995, 95:1169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamashita M, Wakatsuki R, Kato T, Okano T, Yamanishi S, Mayumi N, Tanaka M, Ogura Y, Kanegane H, Nonoyama S, et al. : A synonymous splice site mutation in IL2RG gene causes late-onset combined immunodeficiency. Int J Hematol 2019, 109:603–611. *This paper illustrates that hypomorphic mutations in SCID genes can underlie severe warts.
- 47. Rawat A, Singh A, Dobbs K, Pala F, Delmonte OM, Vignesh P, Jindal AK, Gupta A, Suri D, Kaur A, et al. : Skewed TCR Alpha, but not Beta, Gene Rearrangements and Lymphoma Associated with a Pathogenic TRAC Variant. J Clin Immunol 2021, doi: 10.1007/s10875-021-01047-x. *This paper reports severe warts in TRAC deficient patients.
- 48.Buck D, Malivert L, de Chasseval R, Barraud A, Fondanèche M-C, Sanal O, Plebani A, Stéphan J-L, Hufnagel M, le Deist F, et al. : Cernunnos, a Novel Nonhomologous End-Joining Factor, Is Mutated in Human Immunodeficiency with Microcephaly. Cell 2006, 124:287–299. [DOI] [PubMed] [Google Scholar]
- 49.Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM: Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr 2004, 144:505–511. [DOI] [PubMed] [Google Scholar]
- 50.Li S-L, Duo L-N, Wang H-J, Dai W, Zhou E-YH, Xu Y-N, Zhao T, Xiao Y-Y, Xia L, Yang Z-H, et al. : Identification of LCK mutation in a family with atypical epidermodysplasia verruciformis with T-cell defects and virus-induced squamous cell carcinoma. Br J Dermatol 2016, 175:1204–1209. [DOI] [PubMed] [Google Scholar]
- 51.Horev L, Unger S, Molho-Pessach V, Meir T, Maly A, Stepensky P, Zamir M, Keller B, Babay S, Warnatz K, et al. : Generalized verrucosis and HPV-3 susceptibility associated with CD4 T-cell lymphopenia caused by inherited human interleukin-7 deficiency. J Am Acad Dermatol 2015, 72:1082–1084. [DOI] [PubMed] [Google Scholar]
- 52. Kosumi H, Natsuga K, Takashima S, Miyauchi T, Huang Y-T, Nomura T, Yanagi T, Huang H-Y, Chiu FP-C, Chen P-C, et al. : Two Cases of Interleukin-7–Deficient Generalized Verrucosis. Clin Infect Dis 2020, doi: 10.1093/cid/ciz1240. *This paper confirmed in a different ethnical background the previous finding from Horev et al (ref 51) that IL7 deficiency underlies isolated severe cutaneous warts.
- 53.Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schäffer AA, Gertz EM, Schambach A, Kreipe HH, Pfeifer D, et al. : The phenotype of human STK4 deficiency. Blood 2012, 119:3450–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crequer A, Picard C, Patin E, D’Amico A, Abhyankar A, Munzer M, Debre M, Zhang SY, de Saint-Basile G, Fischer A, et al. : Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLoS One 2012, 7:e44010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delmonte OM, Bergerson JRE, Kawai T, Kuehn HS, McDermott DH, Cortese I, Zimmermann MT, Dobbs K, Bosticardo M, Fink D, et al. : SASH3 variants cause a novel form of X-linked combined immunodeficiency with immune dysregulation. Blood 2021, doi: 10.1182/blood.2020008629. **First description of SASH3 deficiency in human. SASH3 deficiency impairs thymocyte development and underlies susceptibility to cutaneous warts among other infectious susceptibilities.
- 56.Kurz ARM, Catz SD, Sperandio M: Noncanonical Hippo Signalling in the Regulation of Leukocyte Function. Trends Immunol 2018, 39:656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mou F, Praskova M, Xia F, Van Buren D, Hock H, Avruch J, Zhou D: The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J Exp Med 2012, 209:741–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawai T, Malech H: WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol 2009, 16:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDermott DH, Murphy PM: WHIM syndrome: Immunopathogenesis, treatment and cure strategies. Immunol Rev 2019, 287:91–102. [DOI] [PubMed] [Google Scholar]
- 60.Tarzi MD, Jenner M, Hattotuwa K, Faruqi AZ, Diaz GA, Longhurst HJ: Sporadic case of warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis syndrome. J Allergy Clin Immunol 2005, 116:1101–1105. [DOI] [PubMed] [Google Scholar]
- 61.Hsu A, McReynolds L, Holland S: GATA2 deficiency. Curr Opin Allergy Clin Immunol 2015, 15:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuriyama Y, Hattori M, Mitsui T, Nakano H, Oikawa D, Tokunaga F, Ishikawa O, Shimizu A: Generalized verrucosis caused by various human papillomaviruses in a patient with GATA2 deficiency. J Dermatol 2018, 45:e108–e109. [DOI] [PubMed] [Google Scholar]
- 63.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, et al. : GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014, 123:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toboni MD, Bevis KS: Vulvar Cancer as a Result of GATA2 Deficiency, a Rare Genetic Immunodeficiency Syndrome. Obstet Gynecol 2018, 132:1112–1115. [DOI] [PubMed] [Google Scholar]
- 65.West ES, Kingsbery MY, Mintz EM, Hsu AP, Holland SM, Rady PL, Tyring SK, Grossman ME: Generalized verrucosis in a patient with GATA2 deficiency. Br J Dermatol 2014, 170:1182–1186. [DOI] [PubMed] [Google Scholar]
- 66.McDermott DH, Gao J-L, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, et al. : Chromothriptic cure of WHIM syndrome. Cell 2015, 160:686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, Kostyuchenko L, Genel F, Kütükcüler N, Karaca N, et al. : DOCK8 Deficiency: Clinical and Immunological Phenotype and Treatment Options - a Review of 136 Patients. J Clin Immunol 2015, 35:189–198. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y-Q, Zhang G-L, Mo X-H, Wang B, Wu F, Chen J, Luo H, Zhu L-D, Xu M-Y, Zhou Q, et al. : A novel homozygous DOCK8 mutation associated with unusual coexistence of gross molluscum contagiosum and epidermodysplasia verruciformis in a DOCK8 deficiency patient. J Eur Acad Dermatol Venereol 2017, 31:e504–e505. [DOI] [PubMed] [Google Scholar]
- 69.Sanal O, Jing H, Ozgur T, Ayvaz D, Strauss-Albee DM, Ersoy-Evans S, Tezcan I, Turkkani G, Matthews HF, Haliloglu G, et al. : Additional Diverse Findings Expand the Clinical Presentation of DOCK8 Deficiency. J Clin Immunol 2012, 32:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Dove CG, Hor JL, Murdock HM, Strauss-Albee DM, Garcia JA, Mandl JN, Grodick RA, Jing H, Chandler-Brown DB, et al. : DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med 2014, 211:2549–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, et al. : DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 2012, 119:4451–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, Zeng X, Fan Z, Lim B: RhoH modulates pre-TCR and TCR signalling by regulating LCK. Cell Signal 2011, 23:249–258. [DOI] [PubMed] [Google Scholar]
- 73.Molho-Pessach V, Ramot Y, Mogilevsky M, Cohen-Daniel L, Eisenstein EM, Abu-Libdeh A, Siam I, Berger M, Karni R, Zlotogorski A: Generalized verrucosis and abnormal T cell activation due to homozygous TAOK2 mutation. J Dermatol Sci 2017, 87:123–129. [DOI] [PubMed] [Google Scholar]
- 74.Fang C-Y, Lai T-C, Hsiao M, Chang Y-C: The Diverse Roles of TAO Kinases in Health and Diseases. Int J Mol Sci 2020, 21:7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fernandes RA, Perez-Andres M, Blanco E, Jara-Acevedo M, Criado I, Almeida J, Botafogo V, Coutinho I, Paiva A, van Dongen JJM, et al. : Complete Multilineage CD4 Expression Defect Associated With Warts Due to an Inherited Homozygous CD4 Gene Mutation. Front Immunol 2019, 10. **First report of AR CD4 deficiency. The single patient was susceptible to common warts but was otherwise healthy.
- 76.Glaichenhaus N, Shastri N, Littman DR, Turner JM: Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell 1991, 64:511–520. [DOI] [PubMed] [Google Scholar]
- 77.Marrack P, Endres R, Shimonkevitz R, Zlotnik A, Dialynas D, Fitch F, Kappler J: The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med 1983, 158:1077–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strong J, Wang Q, Killeen N: Impaired survival of T helper cells in the absence of CD4. Proc Natl Acad Sci 2001, 98:2566–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Béziat V, Rapaport F, Hu J, Titeux M, Bonnet des Claustres M, Bourgey M, Griffin H, Bandet É, Ma CS, Sherkat R, et al. : Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell 2021, 184:3812–3828.e30. **First report of AR CD28 deficiency. The patients were susceptible to common warts, and one patient suffered from tree man syndrome. The patients were otherwise suprisingly healthy.
- 80.Wang Y, Ma CS, Ling Y, Bousfiha A, Camcioglu Y, Jacquot S, Payne K, Crestani E, Roncagalli R, Belkadi A, et al. : Dual T cell– and B cell–intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med 2016, 213:2413–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sorte HS, Osnes LT, Fevang B, Aukrust P, Erichsen HC, Backe PH, Abrahamsen TG, Kittang OB, Øverland T, Jhangiani SN, et al. : A potential founder variant in CARMIL2/RLTPR in three Norwegian families with warts, molluscum contagiosum, and T- cell dysfunction. Mol Genet Genomic Med 2016, 4:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schober T, Magg T, Laschinger M, Rohlfs M, Linhares ND, Puchalka J, Weisser T, Fehlner K, Mautner J, Walz C, et al. : A human immunodeficiency syndrome caused by mutations in CARMIL2. Nat Commun 2017, 8:14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li F-Y, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ: Second messenger role for Mg 2+ revealed by human T-cell immunodeficiency. Nature 2011, 475:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ravell JC, Matsuda-Lennikov M, Chauvin SD, Zou J, Biancalana M, Deeb SJ, Price S, Su HC, Notarangelo G, Jiang P, et al. : Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease. J Clin Invest 2020, 130:507–522. *This paper shows that 30% of patients with MAGT1 deficiency suffer from skin or anogenital warts. It also demonstrates that MAGT1 deficiency results in a glycosylation defect associated with impaired expression of several cell surface molecules, notably CD28.
- 85. Dorjbal B, Stinson JR, Ma CA, Weinreich MA, Miraghazadeh B, Hartberger JM, Frey-Jakobs S, Weidinger S, Moebus L, Franke A, et al. : Hypomorphic caspase activation and recruitment domain 11 (CARD11) mutations associated with diverse immunologic phenotypes with or without atopic disease. J Allergy Clin Immunol 2019, 143:1482–1495. *This paper shows that 27% of patients carrying dominant negative mutations in CARD11 suffer from skin warts. CARD11 was previously shown to interact with CARMIL2 downstream CD28.
- 86.Liang Y, Cucchetti M, Roncagalli R, Yokosuka T, Malzac A, Bertosio E, Imbert J, Nijman IJ, Suchanek M, Saito T, et al. : The lymphoid lineage-specific actin-uncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat Immunol 2013, 14:858–866. [DOI] [PubMed] [Google Scholar]
- 87.Roncagalli R, Cucchetti M, Jarmuzynski N, Grégoire C, Bergot E, Audebert S, Baudelet E, Menoita MG, Joachim A, Durand S, et al. : The scaffolding function of the RLTPR protein explains its essential role for CD28 co-stimulation in mouse and human T cells. J Exp Med 2016, 213:2437–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park J, Yang J, Wenzel AT, Ramachandran A, Lee WJ, Daniels JC, Kim J, Martinez-Escala E, Amankulor N, Pro B, et al. : Genomic analysis of 220 CTCLs identifies a novel recurrent gain-of-function alteration in RLTPR (p.Q575E). Blood 2017, doi: 10.1182/blood-2017-02-768234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puel A: Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum Genet 2020, 139:1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bustamante J: Mendelian susceptibility to mycobacterial disease: recent discoveries. Hum Genet 2020, 139:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laffort C, Deist FL, Favre M, Caillat-Zucman S, Radford-Weiss I, Fraitag S, Blanche S, Cavazzana-Calvo M, Basile G de S, de Villartay JP, et al. : Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common γc cytokine receptor subunit or JAK-3 deficiency. The Lancet 2004, 363:2051–2054. [DOI] [PubMed] [Google Scholar]
- 92.Gaspar HB, Harwood C, Leigh I, Thrasher AJ: Severe cutaneous papillomavirus disease after haematopoietic stem-cell transplantation in patients with severe combined immunodeficiency. Br J Haematol 2004, 127:232–233. [DOI] [PubMed] [Google Scholar]
- 93.Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, Mahlaoui N, Debre M, Casanova JL, Dal Cortivo L, et al. : Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood 2009, 113:4114–24. [DOI] [PubMed] [Google Scholar]
- 94.Béziat V: Human genetic dissection of papillomavirus-driven diseases: new insight into their pathogenesis. Hum Genet 2020, 139:919–939. [DOI] [PMC free article] [PubMed] [Google Scholar]