Abstract
Background
Chemotherapy is the treatment of choice in patients with advanced or metastatic colorectal cancer (CRC) where surgical resection of metastases is not an option. Both irinotecan (IRI) and fluoropyrimidines are often included in first‐ or second‐ line chemotherapy treatment regimens in such patients. However, it is not clear whether combining these agents is superior to irinotecan alone.
Objectives
To compare the efficacy and safety of two chemotherapeutic regimens, irinotecan monotherapy or irinotecan in combination with fluoropyrimidines, for patients with advanced CRC when administered in the first or second‐line settings.
Search methods
We searched the following electronic databases to identify randomized controlled trials: Cochrane Colorectal Cancer Group Specialised Register (January 13, 2016), Cochrane Central Register of Controlled Trials (CENTRAL)(The Cochrane Library Issue 12, 2016), Ovid MEDLINE (1950 to January 13, 2016), Ovid EMBASE (1974 to January 13, 2016), registers of controlled trials in progress, references cited in relevant publications and conference proceedings in related fields (BioMed Central and Medscape's Conference). The key authors or investigators of all eligible studies, and professionals in the field were contacted when necessary. The search from January 2016 identified one eligible study, an ongoing trial currently presented as an abstract, to be considered in an update of this review.
Selection criteria
Randomized controlled trials (RCTs) investigating the efficacy and safety of IRI chemotherapy combined with fluoropyrimidine compared with IRI alone for the treatment of patients with advanced CRC, regardless of treatment line settings.
Data collection and analysis
Study eligibility and methodological quality were assessed independently by the two authors, and any disagreement was solved by a third author. The data collected from the studies were reviewed qualitatively and quantitatively using the Cochrane Collaboration statistical software RevMan 5.3.
Main results
Five studies were included in this review with a total of 1,726 patients. The top‐up search resulted in an additional ongoing trial, the results of which have not been incorporated in this review. Among five included studies, no reduction in all‐cause mortality was observed in the combination arm, with a summary hazard ratio (HR) of 0.91 (95% CI: 0.81‐1.02). Longer progression‐free survival was observed in those treated with the combination chemotherapy (HR: 0.68, 95% CI: 0.53‐0.87), however, this result may have been driven by findings from the single first‐line treatment setting study. The quality of evidence for overall survival was low and for progression‐free survival was moderate, mainly due to study limitation from the lack of information on randomisation methods and allocation concealment. There were higher risks of toxicity outcomes grade 3 or 4 diarrhoea and grade 1 or 2 alopecia, and a lower risk of grade 3 or 4 neutropenia in controls compared to the invervention group. Evidence for toxicity has been assessed to be low to moderate quality.
Authors' conclusions
There was no overall survival benefit of the irinotecan and fluoropyrimidine treatment over irinotecan alone, thus both regimens remain reasonable options in treating patients with advanced or metastatic CRC. Given the low and moderate quality of the evidence, future studies with sufficient numbers of patients in each treatment arms are needed to clarify the benefit observed in progression‐free survival with combination irinotecan and fluoropyrimidines.
Plain language summary
Irinotecan chemotherapy combined with fluoropyrimidines versus irinotecan alone for overall survival and progression‐free survival in patients with advanced and/or metastatic colorectal cancer
Background:
Patients with inoperable colorectal cancer (CRC) are likely to receive chemotherapy drugs as their primary treatment. Irinotecan (IRI) and fluoropyrimidines are two such drugs widely used in this setting, either alone or as part of multi‐drug chemotherapy treatments.
Objectives:
Currently, there is lack of evidence comparing the combination of IRI and fluoropyrimidine with IRI alone. Therefore it was the aim of this review to compare the two treatments for patients with inoperable advanced or metastatic CRC.
Investigation and study characteristics:
We searched the literature on January 13, 2016. We identified five randomised controlled trials with a total of 1,726 patients comparing the combination of IRI and fluoropyrimidine with IRI alone. The search in January 2016 resulted in an additional ongoing trial, the results of which have not been incorporated in this review. This review compared IRI and fluoropyrimidine with IRI alone in terms of overall survival, progression‐free survival, toxicity, response rates and quality of life.
Main results:
There is no evidence to suggest any superiority of the combination of IRI and fluoropyrimidine over IRI alone, but our results on overall survival are limited by the number of studies available to date. Longer progression‐free survival was seen from adding fluoropyrimidines to IRI. Based on current evidence, both the combination regimens and IRI alone seem equally effective for treating advanced or metastatic patients. Patients in the intervention arm were less likely to develop grade 3 or 4 diarrhea and grade 1 or 2 alopecia, and more likely to have grade 3 or 4 neutropenia, compared to patients receiving IRI alone.
Quality of the evidence:
There was moderate quality evidence from these studies suggesting longer progression‐free survival from adding fluoropyrimidines to IRI. However, findings need to be confirmed by larger, high‐quality randomised clinical trials.
Summary of findings
Summary of findings for the main comparison. IRI with fluoropyrimidines versus single agent IRI for advanced and/or metastatic colorectal cancer.
| IRI with fluoropyrimidines versus single agent IRI for advanced and/or metastatic colorectal cancer | |||||
|
Patient or population: patients with advanced and/or metastatic colorectal cancer
Settings: first‐ and second‐ line treatments
Intervention: IRI with fluoropyrimidines Control: Single agent IRI. | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | IRI with fluoropyrimidines | ||||
| Overall survival (Death) Follow‐up: 12 months | 47 per 100 | 44 per 100 (40 to 48) | HR 0.91 (0.81 to 1.02) | 1728 (5 studies) | ⊕⊕⊝⊝ low1 |
| Progression‐free survival (Disease progression) Follow‐up: 12 months | 92 per 100 | 81 per 100 (74 to 88) | HR 0.68 (0.53 to 0.83) | 600 (3 studies) | ⊕⊕⊕⊝ moderate2 |
|
Grade 3/4 diarrhea Follow‐up: |
Study Population | RR 0.66 (0.51 to 0.85) | 1179 (5 studies) | ⊕⊕⊕⊝ moderate3 | |
| 213 per 1000 | 140 per 1000 (109 to 181) | ||||
| Moderate | |||||
| 185 per 1000 | 122 per 1000 (94 to 157) | ||||
|
Grade 3/4 nausea Follow‐up: |
Study Population | RR 0.33 (0.07 to 1.58) | 209 (3 studies) | ⊕⊕⊝⊝ low4 | |
| 49 per 1000 | 16 per 1000 (3 to 77) | ||||
| Moderate | |||||
| 33 per 1000 | 11 per 1000 (2 to 52) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded one level for study limitations (Allocation concealment was only clear for one of the five studies, open‐label intervention) and one level for imprecision (Lack of sufficient number of samples may have reduced the statistical power of the analysis) 2 Downgraded one level for study limitations (Allocation concealment was only clear for one of the five studies, open‐label intervention)
3 Downgraded one level for study limitations (Allocation concealment was only clear for one of the five studies, open‐label intervention)
4 Downgraded one level for study limitations (Allocation concealment was only clear for one of the five studies, open‐label intervention) and one level for imprecision (Lack of sufficient number of samples may have reduced the statistical power of the analysis)
Background
Description of the condition
Colorectal cancer (CRC) is currently the third most commonly diagnosed cancer for men and the second most common for women, as well as a leading cause of death worldwide (Ferlay 2010). In the United States, it was estimated that 136,830 people (71,830 men and 65,000 women) were diagnosed with CRC and 50,310 died from the disease in 2014 (Siegel 2014). Despite the higher incidence and mortality rates of CRC in more developed countries , these rates have been decreasing over the last two decades, particularly in the United States (Jemal 2011; Siegel 2013). Conversely, both CRC incidence and mortality have increased in less developed countries, largely owing to limited resources and inadequate healthcare infrastructure (Center 2009).
Early stage CRC is potentially curable by surgery (Kuhry 2008). However, 20‐25% of CRC patients are first diagnosed with metastatic disease, where curative surgical resection is unlikely to be carried out (Siegel 2014; Van Cutsem 2014), and many patients relapse with metastatic disease after potentially curative resections. For these patients systemic chemotherapy is often the treatment of choice, with the objectives of relieving symptoms, increasing survival and improving quality of life (Simmonds 2000; Ragnhammar 2001).
Description of the intervention
Antimetabolite fluoropyrimidines have been the backbone of CRC chemotherapy for the past 40 years. For decades, treatment efficacy from fluorouracil (FU) monotherapy has been limited. The subsequent combination of 5‐fluorouracil (5‐FU) with leucovorin (LV), a reduced folate that increases thymidylate synthetase inhibition, was used to modulate effects of 5‐FU and improve its efficacy (ACCMAP 1992). This combination has resulted in better response rates than 5‐FU alone for advanced CRC (Thirion 2004) and remains the main component of most chemotherapy regimens in CRC, either as an intravenous (IV) bolus injection, infusion, or both (Chau 2005; Maiello 2005).
Irinotecan is a semisynthetic derivative of the natural alkaloid camptothecin which inhibits topoisomerase I, thus impeding DNA uncoiling and leading to double‐stranded DNA breaks (Hsiang 1985). This drug was shown to have antitumour activity against CRC when administered intravenously alone in a first‐line setting, or as a second‐line regimen for patients with advanced CRC that is refractory to FU (Conti 1996; Pitot 1997; Rothenberg 1999; Rougier 1997; Rougier 1998)
In more recent years, a number of oral fluoropyrimidines such as capecitabine have become available. In addition to its more convenient use as an oral agent, capecitabine has been shown in clinical trials to have superior safety profiles compared to IV 5‐FU/LV with similar (non‐inferior) overall survival (OS), progression‐free survival (PFS) and time to progression (TTP) for patients with metastatic CRC (Petrelli 2012; Van Cutsem 2004). These encouraging results suggest that oral fluoropyrimidine agents may serve as a suitable alternative to IV agents for CRC chemotherapy treatment. However, when both treatment arms were combined with irinotecan (IRI), IV 5‐FU/LV regimens demonstrated longer PFS and less toxicity in metastatic CRC compared to capecitabine (Montagnani 2010), and the combination of capecitabine and IRI is used less commonly now.
How the intervention might work
As a first‐line chemotherapeutic regimen for CRC, IV IRI alone was demonstrated to have comparable antitumour activity to 5‐FU/LV (Cao 2000, Saltz 2000). Besides a different mechanism of action from 5‐FU, the lack of cross‐resistance of IRI to previous 5‐FU/LV treatment, as shown by its similar activity against untreated and 5‐FU‐pretreated CRC, is the rationale for combining it with fluoropyrimidines as first‐line therapy for this disease (Rougier 1997). The synergistic effects between IRI and fluoropyrimidines have been suggested to be comparable with that of IRI and oxaliplatin despite difference different toxicity profiles (Colucci 2005; Tournigand 2004). As the second‐line treatment for advanced CRC, two phase III studies have shown modest benefits in survival with IRI of 2.3 months and 2.7 months compared to IV 5‐FU and best supportive care, respectively (Rougier 1998; Cunningham 1998). Until now, the superiority of IRI combined with fluoropyrimidines over fluoropyrimidines alone has been assumed and the combination regimen is now widely used for advanced CRC patients in clinical practice (Douillard 2000; Maiello 2000; Folprecht 2008; Giessen 2011; Muro 2010).
Why it is important to do this review
Several randomized controlled trials (RCTs) comparing IRI in combination with fluoropyrimidines against IRI alone suggested that the combination regimen leads to better outcomes in OS and TTP for advanced CRC (Saltz 2000; Seymour 2007), while the results of another trial and a meta‐analysis indicated that IRI monotherapy had equivalent efficacy and toxicity (Clarke 2011; Graeven 2007). However, the meta‐analysis comparing IRI and IV 5‐FU/LV combination regimen (FOLFIRI) for second‐line treatment of CRC was not specific for trials concurrently including both treatment arms (Clarke 2011). Thus the benefit of IRI and fluoropyrimidines over IRI monotherapy remains unclear. Taking their efficacy and toxicity into account, we therefore undertook this study to systematically compare the combination regimen with IRI alone to determine which regimen is more suitable for advanced CRC patients, either as a first‐line or a second‐line therapy.
Objectives
The aim of this systematic review was to compare the efficacy and safety of two chemotherapeutic regimens, IRI monotherapy or IRI in combination with fluoropyrimidines, for patients with advanced CRC when administered in the first or second‐line setting.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) investigating the efficacy and safety of chemotherapeutic regimens that compared IRI combined with fluoropyrimidine against IRI alone for the treatment of patients with advanced CRC, regardless of treatment line settings, were eligible for the inclusion. If trials enrolled more than two groups, we only extracted data that related to the two regimens. Relevant cluster RCTs were eligible for this review.
Types of participants
Studies involving patients diagnosed histologically or cytologically with locally advanced and/or metastatic CRC were included.
Types of interventions
The experimental group received the combination regimen, namely IRI with fluoropyrimidines administered intravenously or orally; the control group received single agent IRI. Other agents were acceptable as long as they were common to both treatment arms, except LV, which is specific to IV 5‐FU.
Types of outcome measures
Primary outcomes
The primary outcome measures were:
Overall survival (OS),
Time to progression (TTP) or progression‐free survival (PFS)
All outcomes were analysed on an intention to treat (ITT) basis. Studies which reported survival outcomes either directly or by curves were included, if the relevant data could be obtained by using Parmar methods (Parmar 1998; Tierney 2007).
Secondary outcomes
The secondary outcome measures were:
toxicity, classified according to the National Cancer Institute Common Toxicity Criteria (NCI‐CTC version 2.0),
response rates, classified according to the RECIST criteria (see below),
quality of life, measured by the European Organisation for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ‐C30)
Response rates were classified according to Response Evaluation Criteria In Solid Tumors (RECIST version 1.0) (Therasse 2000), where measurable target and non‐target lesions were determined at baseline and evaluated during follow up. A complete response (CR) was defined as disappearance of full lesion, while partial response (PR) referred to a decrease of at least 30% of lesion and progressive disease (PD) an increase of at least 20% of the lesion. Those with insufficient changes to be categorized as either PR or PD were classified as stable disease (SD). CR and PR were used as outcomes in this review.
Search methods for identification of studies
We performed an updated search in January 2016, resulting in identification of an ongoing trial added to ‘Studies awaiting classification’.
Electronic searches
Published or unpublished trials eligible for inclusion were identified by performing searches in the following databases:
Cochrane Colorectal Cancer Group Specialized Register (December 2014);
Cochrane Central Register of Controlled Trials (CENTRAL)(The Cochrane Library Issue 12, 2014) (Appendix 1);
Ovid MEDLINE from 1950 to 8 December 2014 (Appendix 2)
Ovid EMBASE from 1974 to 8 December 2014 (Appendix 3)
Science Citation Index from 1900 to 8 December 2014 (Appendix 4)
In each database, both medical subject headings and free‐text searching were performed in order to improve the sensitivity of the searches. All above databases were searched from the beginning of electronic records to the time at which the search was conducted and eligible studies in both English and non‐English languages were identified without any publication date or publication status limitations.
Searching other resources
Published meta‐analyses and relevant reviews, registers of controlled trials in progress (World Health Organization's International Clinical Trials Registry and ClinicalTrials.gov), references cited in relevant publications and conference proceedings in related fields were also searched (BioMed Central and Medscape's Conference). The key authors or investigators of all eligible studies, and professionals in the field were contacted if necessary in order to obtain other relevant information on the topic. In addition, bibliographies of identified trials and relevant references were hand‐searched.
Data collection and analysis
Selection of studies
The title, abstract and keywords of every record from retrieved studies obtained by applying the above search strategies were checked independently against the inclusion criteria by two reviewers (AW and NY). All eligible studies were included irrespective of whether measured outcome data were reported on. Disagreements were resolved by a third reviewer (WW). Potentially eligible trials were retrieved in full for further assessment. Where more than one publication of a single trial existed, only the publication with the most complete data was included unless the relevant outcomes were only published in earlier versions.
Data extraction and management
Data was extracted from published papers independently by two reviewers (WW and NY). Any disagreement was resolved by a third reviewer (MVH). Data for overall survival and progression‐free survival was extracted from the publications or estimated from survival curves where necessary. The following data of each study was requested: response rates (complete and partial), toxicity, the outcomes of quality life measurements, if any, the schedule and dosing of IRI or fluoropyrimidines, and baseline characteristics including age, sex, performance status, site of metastatic disease, whether or not patients had received previous adjuvant chemotherapy, site of primary tumor (rectum versus colon). If a study did not include one of the comparators of interest, only available results on the other interventions were included. The investigators of included studies were asked to supply updated data where possible.
Assessment of risk of bias in included studies
The methodological quality of the included studies was evaluated independently by two reviewers (WW and AW) with disagreements resolved by a third reviewer (MVH) according to the Cochrane Handbook. For each study, the following domains were assessed (Higgins 2011):
Selection bias: the generation of allocation schedule (truly random, quasi random, systematic) & concealment of treatment allocation.
Performance bias: blinding of study participants and personnel.
Detection bias: blinding of outcome assessors.
Attrition bias: completeness of follow‐up, withdrawal and drop‐out rates and whether analyses were performed by ITT.
Selective outcome reporting: evidence that outcome data have been reported based on the nature of the results.
Other biases, such as deviation from the study protocol in a way that does not reflect clinical practice.
The methods and procedures within each domain were judged as low, high or unclear risk of bias based on criteria specified in the Cochrane Risk of Bias tool (see Appendix 5)(Higgins 2011). Any disagreements were resolved by discussion between the reviewers. Investigators were contacted where this information could not be extracted from the publication.
Measures of treatment effect
The absolute effects of treatment at different time points were obtained from publication data or read from simple (non‐stratified) Kaplan Meier curves of included trials. Median survivals and TTP (or PFS) were also estimated from Kaplan‐Meier curves.
The information on survival and progress from each study was summarised as a log hazard ratio (HR). When HRs were not reported, observed (O) and the log‐rank expected (E) number of events and variance (V) were calculated from the numbers of events and the numbers at risk at each time interval in published Kaplan‐Meier survival curves (Parmar 1998; Tierney 2007). These numbers were used to estimate the HRs for all time intervals. When estimates from Cox regression were reported, the HRs were included in the analysis instead of those manually derived from the log‐rank method (O‐E/V). The general inverse variance method was used to obtain summary log HRs from combined studies.
All time to event analyses were performed by ITT.
Unit of analysis issues
For individual trials, the unit of analysis used was individual patients. For any eligible cluster RCTs, meta‐analysis was conducted based on results from analysis that took into account clustering design. For studies in which control of clustering was not performed or reported, and individual patient data was not available, the intervention effects of cluster RCT were corrected by reducing the size of each trial to its 'effective sample size', which is the number of original sample size divided by the 'design effect'. The design effect were calculated as 1 + (M‐1)* ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient (Higgins 2011b).
Dealing with missing data
All principal investigators of the selected trials were contacted and asked to provide data that were missing or information which could not be extracted from the publication. Among five authors contacted, two immediately complied, another two no longer had any data at hand, one did not respond. Fortunately, most of the authors whose additional information was unavailable have provided detailed survival data on their published papers.
Assessment of heterogeneity
The studies were evaluated clinically and methodologically to assess if it was reasonable to consider combining data. Statistical heterogeneity was measured by the visual inspection of the forest plots and statistically through an assessment of homogeneity based on the Chi2 test for which a p‐value of less than 0.10 was considered an indication of substantial heterogeneity. The I2 measurement was calculated as an indicator of the amount of statistical variation not attributable to sampling error. A value of more than 50% was considered to represent substantial heterogeneity. Where necessary, further investigations were undertaken to determine the source of the observed heterogeneity and in particular, whether there were any outlying studies driving this heterogeneity. Analyses were then conducted both with and without the outlying studies as part of a sensitivity analysis.
Assessment of reporting biases
Funnel plots were visually inspected to assess publication bias. The presence of publication bias was indicated by an asymmetrical distribution of data points derived from HR estimates and standard errors of log HRs from individual studies in relation to the pooled estimate effect.
Data synthesis
The statistical package Review Manager 5.3 (RevMan 5.3) provided by the Cochrane Collaboration was used for analysing data. For primary outcomes, pooled results on overall survival or progression‐free survival were expressed as HRs with 95% confidence intervals (CI) by calculating the overall HRs and its variance across the trials. A random effects model was used to address potential statistical heterogeneity among included studies. All time‐to‐event analyses were performed by ITT.
For secondary outcomes, data on toxicity, response rates and quality of life, where available, were analysed as dichotomous data and the outcomes were reported as relative risks (RRs) with 95% CI. A Mantel‐Haenszel test was employed to obtain pooled estimates across studies under a random effects model.
Subgroup analysis and investigation of heterogeneity
Where different routes of administration were used, studies were grouped according to whether fluoropyrimidines were administered orally or intravenously. A second subgroup analysis was performed according to the different settings (first‐ or second‐line) under which individual trials were categorised. We also explored possible interactions between different methods of administration of 5‐FU in combination with IRI (infusion versus bolus) in order to investigate possible sources of heterogeneity. The Chi2 test for interaction was used to test for consistency of effects across these subsets of trials.
Sensitivity analysis
Sensitivity analyses were performed in order to assess the robustness of our results to heterogeneity, different assumptions or methodological approaches:
Removing studies at a high risk of bias in all domains assessed using the Cochrane Risk of Bias Tool
Exclusion of studies that used other agents (in both study arms) that may affect treatment effects of study regimen.
Summary of findings
We evaluated the quality of evidence of the two primary outcomes (Overall survival and Progression‐free survival), and one secondary outcome (Toxicity) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and presented it in 'Summary of Findings' tables.
The GRADE system classifies the quality of evidence in one of four grades:
High: Further research is very unlikely to change our confidence in the estimate of effect;
Moderate: Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate;
Low: Further research is very likely to have an important impact on our confidence on the estimate og effect and is likely to change the estimate; or
Very low: Any estimate of effect is very uncertain.
The quality of evidence were to be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes) and imprecision (wide confidence intervald, single trial). The quality could also be upgraded by one level due to large summary effect.
Results
Description of studies
Results of the search
We identified a total of 3,117 records through the electronic searches. After removing duplicates, a total of 2,006 records were left to be checked for eligibility, of which 1,997 studies were clearly irrelevant and thus excluded. The updated search in January 2016 resulted in one ongoing trial, which has been added to ‘Studies awaiting classification’ (Bendell 2014). We retrieved full text of the remaining 8 records for further assessment (Bécouarn 2001; Clarke 2011; Graeven 2007; Saltz 2000; Seymour 2007; Fiorentini 2012; Mitchell 2011; Popov 2006). We excluded 3 studies for reasons listed in the Characteristics of excluded studies. In total, 5 RCTs fulfilled the inclusion criteria (Bécouarn 2001, Clarke 2011, Graeven 2007,Saltz 2000, Seymour 2007). All included studies were individual trials and no relevant cluster RCTs were identified. The study flow diagram is shown in Figure 1.
1.
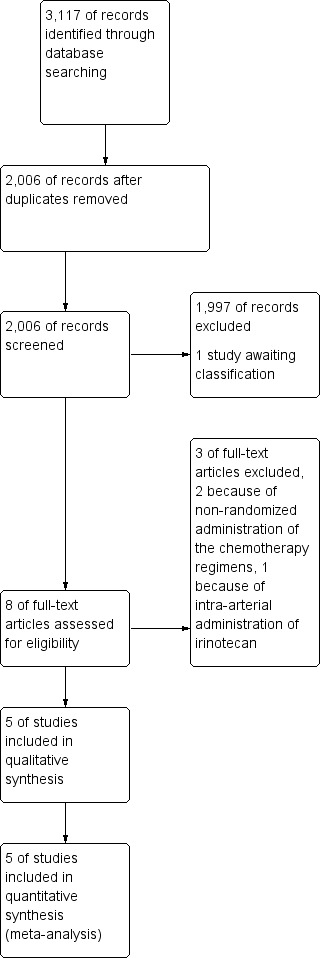
Study flow diagram
Included studies
Five studies were included in this review, and details on IRI and fluoropyrimidine regimens are presented in Table 2. A total of 1,726 patients were randomised: 686 in the IRI‐fluoropyrimidine combination group and 1,040 in the control group. Four of the studies administered IRI and the combination of IRI with fluoropyrimidine as a second‐line treatment (Bécouarn 2001; Clarke 2011; Graeven 2007; Seymour 2007) and one study as a first‐line treatment (Saltz 2000); all used 5‐FU IV as the fluoropyrimidine of choice in the combination arm. No additional chemotherapeutic agents apart from LV in the 5‐FU arm were used except in Bécouarn 2001, where oxaliplatin was administered in both treatment arms in addition to IRI and 5FU/LV. Seymour 2007 compared three strategies of sequential chemotherapy, where a second‐line treatment with IRI was administered in the control group and the IRI and 5‐FU combination was used as a second‐line treatment in one of the intervention groups, both groups had previously been treated with 5‐FU as the first‐line drug of choice. Randomisation occurred prior to first‐line treatments and therefore, the numbers of patients actually assigned to the combination IRI + 5‐FU and IRI groups (185 and 356 patients, respectively) were lower than those at randomisation (365 and 710 patients, respectively). All randomised patients were included when combining results from time‐to‐event analyses. One study (Saltz 2000) administered three treatment arms: IRI alone, 5‐FU/LV alone, and a combination of IRI and 5‐FU‐LV, but a comparison was only made between the latter two. ITT analyses were conducted in all studies.
1. Characteristics of studies.
| Study | Group | Chemotherapy agent(s) | Additional agent(s) | Cycle interval | Median overall survival (months) |
| Bécouarn 2001 | Intervention | IRI 180 mg/m2 IV 90 min on Day 1 5‐FU 400 mg/m2 IV bolus and 600 mg/m2 IV 22 hrs on Day 1,2,15,16 |
Oxaliplatin on Day 1 | 4 weeks | 9.8 (6.4‐13) |
| Control | IRI 200 mg/m2 IV 30 min on Day 1 | Oxaliplatin on Day 15 | 3 weeks | 12.3 (9.8‐14.8) | |
| Clarke 2011 | Intervention | IRI 180 mg/m2 IV 90 min on Day 1 5‐FU 400 mg/m2 IV bolus and 2400 mg/m2 IV 46 hrs on Day 1 |
2 weeks | 15.4 (8.1‐18) | |
| Control | IRI 300‐350 mg/m2 IV 90 min on Day 1 | 3 weeks | 11.2 (8.3‐13.3) | ||
| Graeven 2007 | Intervention | IRI 80 mg/m2 IV 60 min on Day 1,8,15,22,29,36 5‐FU 2000 mg/m2 IV 24 hrs on Day 1,8,15,22,29,36 |
7 weeks | 9.5 (6.5‐13) | |
| Control | IRI 125 mg/m2 IV 30‐60 min on Day 1,8,15,22 | 6 weeks | 10.7 (8‐12.9) | ||
| Saltz 2000 | Intervention | IRI 125 mg/m2 IV 90 min on Day 1,8,15,22 5‐FU 500 mg/m2 IV bolus on Day 1,8,15,22 and 5‐FU 425 mg/m2 IV bolus on Day 1‐5 |
6 weeks | 14.8 | |
| Control | IRI 125 mg/m2 IV 90 min on Day 1,8,15,22 | 6 weeks | 12 | ||
| Seymour 2007 | Intervention | IRI 180 mg/m2 IV 30 min on Day 1 5‐FU 400 mg/m2 IV bolus and 2400 mg/m2 IV 46 hrs on Day 1 |
2 weeks | 15 | |
| Control | IRI 350 mg/m2 IV 30‐90 min on Day 1 | 3 weeks | 13.9 |
Excluded studies
Among the remaining three studies from the search, two trials did not have randomized allocations of IRI regimens and thus were excluded (Mitchell 2011; Popov 2006). One study was excluded because it used intra‐arterial administration of IRI‐loaded drug‐eluting beads (DEBIRI) instead of an IV or oral route (Fiorentini 2012).
Risk of bias in included studies
Risk of bias was assessed from the available information reported by the authors and summarised in the Characteristics of included studies section. This assessment is also presented as the risk of bias graph (Figure 2) and risk of bias summary (Figure 3). Randomisation technique was one of the main components of the assessment. All studies randomised their patients when allocating treatments. Except for Seymour 2007, randomisation took place prior to treatment with experimental and control regimens. Further details on risk of bias in included studies are as following.
2.
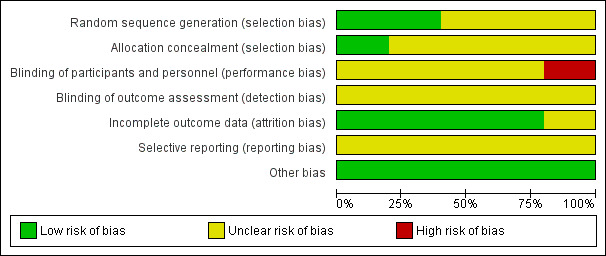
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
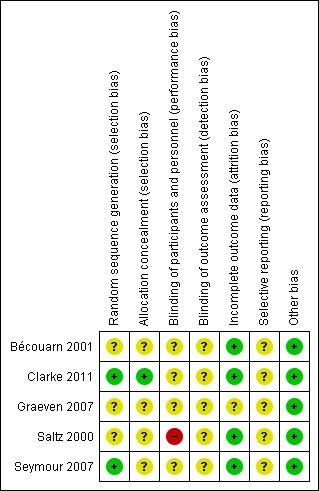
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods used for randomisation and allocation concealment were less explicit. Only two RCTs described the technique used for randomization, minimisation (Clarke 2011; Seymour 2007). Allocation concealment was only clear for one study (Clarke 2011), which conducted central randomisation by telephone, resulting in 20% of the studies to have shown low risk of selection bias based on allocation concealment and 40% based on randomisation methods (Figure 2).
Blinding
One trial was open label and therefore judged to have had high risk of bias (Saltz 2000). Blinding of personnel or study participants was not reported in any of other included studies. No mention of blinding of outcome assessors was made in any publication. Hence, 20% of all studies had high risk of bias from the lack of blinding (Figure 2).
Incomplete outcome data
A total of 15 patients withdrew from the studies Bécouarn 2001; Clarke 2011; Graeven 2007; Saltz 2000: 8 in the experimental group, 7 in the control group, and 2 had no mention of their allocated group. In Seymour 2007, 154 patients who failed first‐line treatment with 5‐FU did not receive the allocated combination of IRI + fluoropyrimidines and 302 did not receive allocated IRI as second‐line treatment. Except for two patients withdrawing from the study in Graeven 2007 and one patient that refused the use of their data after withdrawing from the study in Clarke 2011, all patients were included in the analysis. No reason was reported for patient withdrawal in the Graeven 2007 study, hence we regarded this study to have unclear risk of attrition bias, Overall, this showed 80% of included trials to have low risk of attrition bias (Figure 2).
Selective reporting
Limited information on pre‐specified outcomes was found when assessing reporting bias in individual studies, however, most studies reported both significant and insignificant findings in their publications. Figure 4 shows the funnel plot for results on OS. From visual inspection, all data points representing the estimates and precision of individual studies fell within the triangle comprising 95% confidence interval of the pooled effect estimate, with larger and more precise studies occupying the top of the inverted funnel and smaller and less precise studies more diversely scattered at the bottom. Most smaller and less precise studies (Bécouarn 2001; Graeven 2007) reported positive results. Although an assymetrical funnel plot was shown, interpretation is hampered given the small number of studies. Therefore, more studies are needed to determine a true reporting bias.
4.
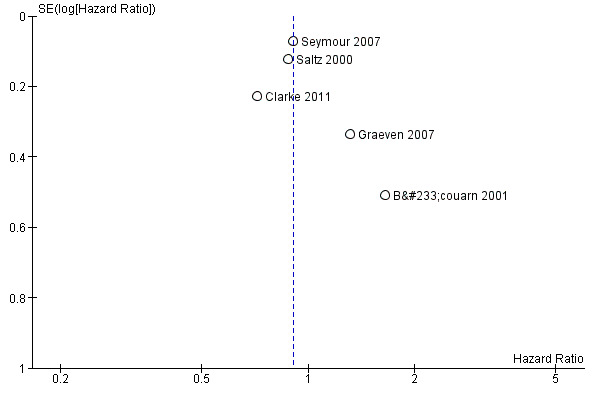
Funnel plot for comparisons of overall survival
Other potential sources of bias
No other potential sources of bias were identified from the studies and thus we assessed all studies (100%) to have low risk of other bias.
Effects of interventions
See: Table 1
1. Primary outcomes
The primary outcomes of this review were Overall Survival (OS) and Progression‐free Survival (PFS)
1.1 Overall survival
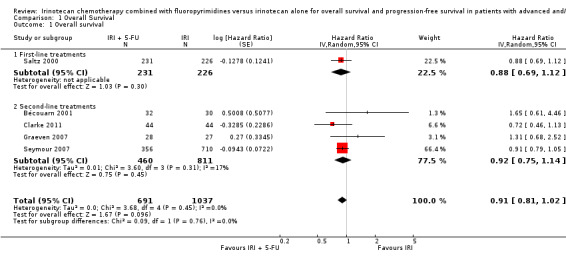
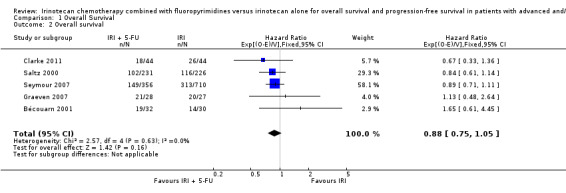
As seen in Table 2, median OS ranged between 9.5‐15.4 months in the intervention arms across all studies, and between 10.7‐13.9 months in the control arms. When comparing efficacy, subgroup analyses was performed according to the line of treatment, although only one study was found for the first‐line treatment setting (Saltz 2000). For OS, analysis of both subgroups combined failed to show any statistically significant difference in overall mortality risk between the combination IRI + 5‐FU regime and IRI monotherapy arms (Figure 5) (HR 0.91, with 95% CI of 0.81 to 1.02). No heterogeneity within and between subgroups was observed. Results were downgraded from high to low due to study limitations (Allocation concealment was only clear for one of the five studies, open‐label intervention) and imprecision (Lack of sufficient number of samples may have reduced the statistical power of the analysis) (Table 1).
5.
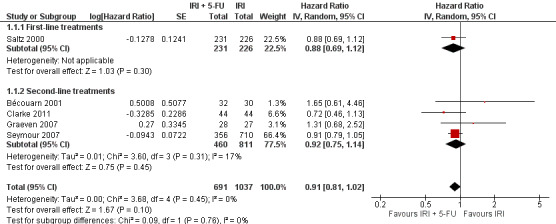
Overall survival.
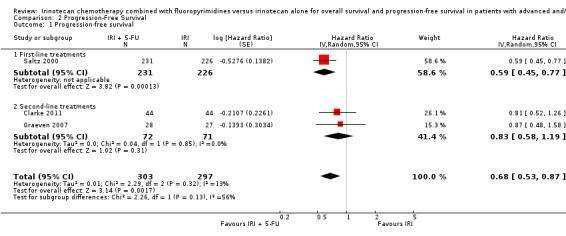
1.2 Progression‐free survival
Three studies provided data on PFS (Clarke 2011; Graeven 2007; Saltz 2000). An increase in progression‐free survival was seen in the combination IRI + 5‐FU arm overall, with a hazard ratio of 0.68 (95% CI: 0. 53‐0.87). Overall, no substantial heterogeneity was found (I2 = 13%), and the difference between subgroups failed to reach significance (p=0.13). Nevertheless, results may have been largely driven by the study with first‐line setting (Saltz 2000), and the summary HR for second‐line treatments showed no difference in risk of disease progression between the experimental and control group (Figure 6). This finding was downgraded from high to moderate due to study limitations (Allocation concealment was only clear for one of the five studies, open‐label intervention) (Table 1).
6.
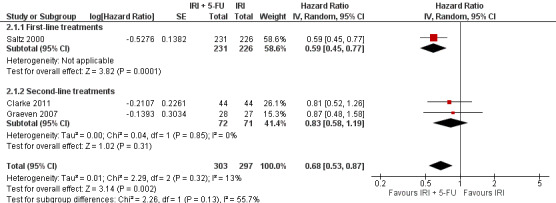
Progression‐free survival
2. Secondary outcomes
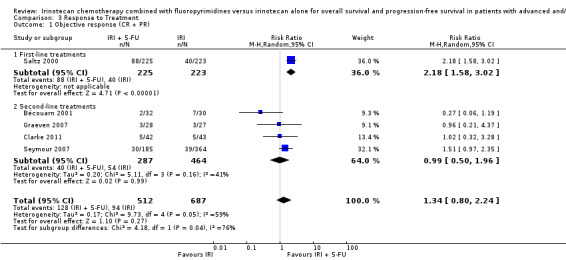
2.1 Response to treatment
Response rates to treatment in the two arms were compared, which included CR and PR as the outcome of interest. No difference was observed with all studies combined. Individually or second‐line treatments by themselves (Figure 7), first‐line treatment with the combination IRI + 5‐FU chemotherapy were shown to result in higher response rates (RR for CR and PR: 2.18 (95% CI: 1.50‐3.02)) compared to the controls. However, there was substantial heterogeneity overall (I2 > 50%). We therefore performed a sensitivity analysis in which we excluded a study with an additional chemotherapeutic agent in both arms (Bécouarn 2001), which may have introduced bias. Re‐running the random effects model with this study excluded eliminated the overall heterogeneity and yielded a summary RR of 1.77 (95% CI: 1.32‐2.39; results not shown in figures), indicating better responses to treatment in the intervention arm compared to controls.
7.
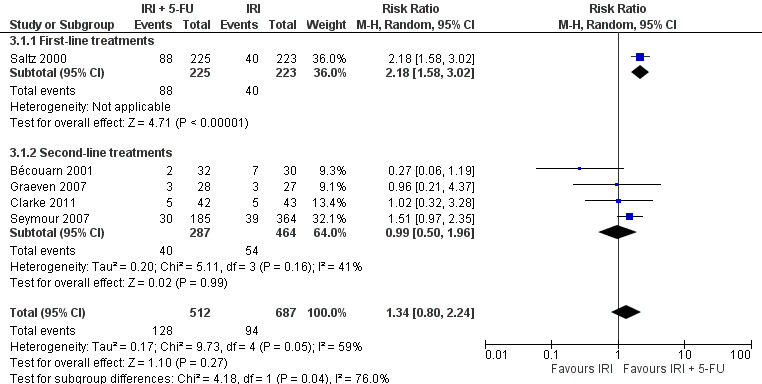
Response to treatment (CR + PR)
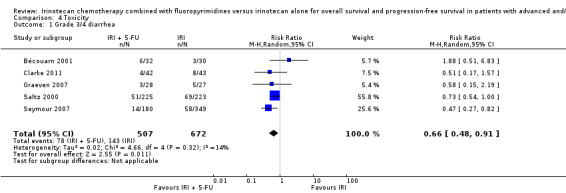
2.2 Toxicity
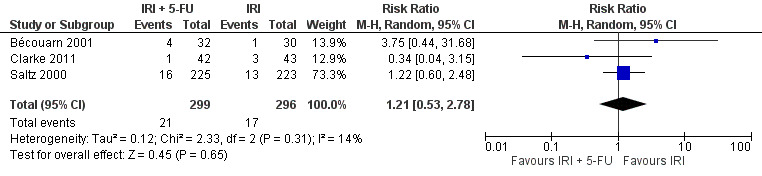
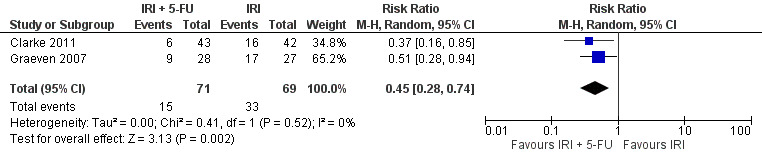
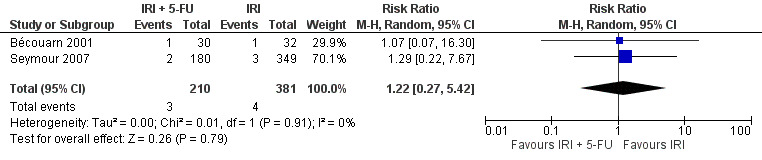
Toxicity profiles were reported in both first‐ and second‐line treatments and combined, where information on individual toxicities was available. For grade 3/4 diarrhoea, a reduced overall risk was observed in the combination IRI + 5‐FU arm (RR: 0.66 (95% CI: 0.48‐0.91) compared to the controls (Figure 8). A similar risk reduction was seen for grade 1/2 alopecia (RR: 0.45 (95% CI: 0.28‐0.74), while an increased risk of grade 3/4 neutropenia was observed in the experimental group (RR: 1.98 (95% CI: 1.48‐2.67) compared to the IRI arm. No marked difference was seen for other toxicities including grade 3 or 4 mucositis, nausea, and vomiting, and neuropathy (Figure 9; Figure 10; Figure 11; Figure 12; Figure 13; Figure 14; Figure 15). For grade 1 or 2 alopecia and neuropathy, only estimates from 2 studies were available. However, a decision to pool the results was made based on similar treatment arms. No marked heterogeneity was found unless for analysis of grade 3 or 4 neutropenia (I2: 48%). A sensitivity analysis for grade 3 or 4 neutropenia excluding a study by Bécouarn 2001, which included oxaliplatin in both treatment arms, revealed less heterogeneity without altering the findings (RR: 1.79 (95% CI: 1.49‐2.28); I2: 28%, results not shown in figures). Summary of findings table 2showed downgrading of evidence for toxicity outcomes grade 3/4 diarrhea to moderate due to study limitation, and grade 3/4 nausea to low due to study limitations and imprecision.
8.
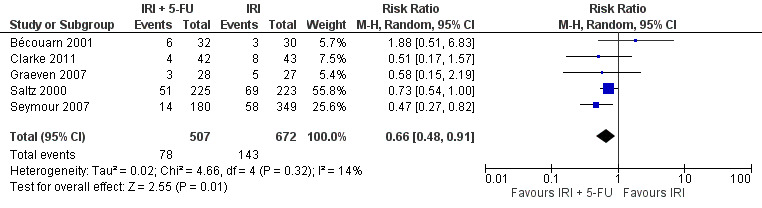
Grade 3/4 diarrhea
9.
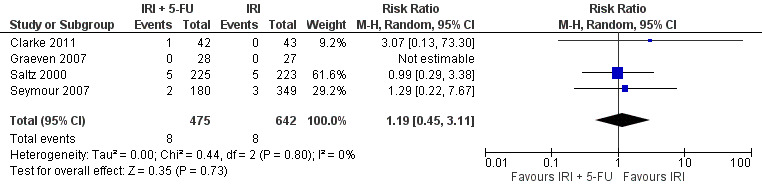
Grade 3/4 mucositis
10.
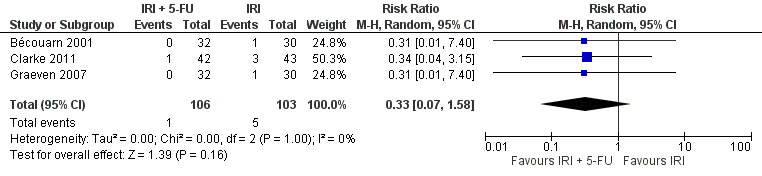
Grade 3/4 nausea
11.
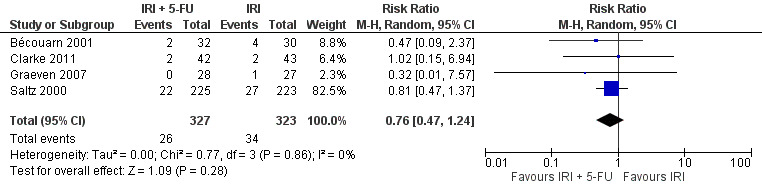
Grade 3/4 vomiting
12.
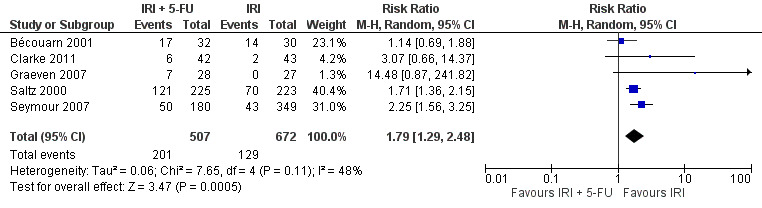
Grade 3/4 neutropenia
13.
Febrile neutropenia
14.
Grade 1/2 alopecia
15.
Neuropathy
2.3 Quality of life
Quality of life was assessed in three studies (Clarke 2011; Saltz 2000; Seymour 2007). However, no detailed results were reported to enable a meta‐analysis. Findings varied for comparisons of overall quality of life between the experimental and control arms. Saltz 2000 reported higher global health status for the combination IRI and 5‐FU regimen, although these results were not statistically significant. On the contrary, Clarke 2011 showed a statistically significant lower overall quality of life with the combination chemotherapy. No benefit or disadvantage was observed between the two groups in Seymour 2007.
Discussion
Summary of main results
Five studies fulfilled the inclusion criteria and were included in this review. High risk of bias due to the lack of blinding was seen with the Saltz 2000 study. Low risk of selection bias based on allocation concealment was shown in one study (Clarke 2011), whereas low risk based on randomisation techniques was seen in two studies (Clarke 2011; Seymour 2007). There was a lack of sufficient information to fully assess other sources of bias. Overall, no OS benefit was seen by combining IRI with fluoropyrimidine for treating advanced or metastatic CRC compared to using IRI alone. Longer PFS and higher response rates were seen in the combination IRI + 5‐FU arms. However, these results may have been driven by the single first‐line treatment study, and for treatment response rates there was substantial heterogeneity. Toxicity profiles were different, with higher risks of grade 3 or 4 diarrhoea and grade 1 or 2 alopecia and a lower risk of grade 3 or 4 neutropenia in the control group compared to the combination arm. No conclusive results were available for quality of life.
Overall completeness and applicability of evidence
Overall, our findings indicate no clinical advantage of the combination of IRI and fluoropyrimidine treatment over IRI alone for patients with advanced or metastatic CRC, but our results were limited by the number of studies available for each treatment line. The significant heterogeneity between first‐ and second‐ line groups indicate that results may only be interpreted with respect to treatment settings.
Quality of the evidence
The quality of evidence in this review was classified as low and moderate (Table 1). This was mostly due to high risk of bias assessed through the meta‐analysis and imprecision of results due to a lack of statistical power. When substantial heterogeneity was found such as in the assessment of response rates, lthough it was likely that the line of treatment is the major determinant of this heterogeneity, the plausible mechanism underlying the different effects on survival with respect to treatment setting is unclear. It is possible that treatment setting is a proxy of other prognostic factors in advanced or metastatic CRC, and therefore the observed heterogeneity reflects the difference in population characteristics rather than the interventions. Additionally, the small numbers of participants and a lack of studies with first‐line settings as mentioned above may indicate the necessity of confirming our findings through larger clinical studies sufficiently addressing each line of treatment.
Potential biases in the review process
The inclusion of randomised studies comprising first‐ and second‐line treatments strengthened this current review. However, even though similar drugs and routes of administration were used in all the included studies, there was variation in dosage and timings that may have affected the overall findings. Nevertheless, several studies suggested similar efficacy in treating advanced and metastatic CRC across IRI‐based regimens with different intervals of IRI administration (Aranda 2009; Bouzid 2003), although further investigations are needed to delineate the impact of administration routes and other agents in combination with IRI. Additionally, only 5‐FU‐based regimens represented the fluoropyrimidine arm in this review. As differences in clinical outcomes have been reported with different fluoropyrimidine agents in IRI‐based regimens (Fuchs 2007), this may limit the generalisability of our findings. In the study conducted by Seymour and colleagues (Seymour 2007), a sequential strategy was employed in which allocation to both first‐ and second‐line chemotherapy was performed at the start of the study. Patient eligibility was assessed prior to first‐line of treatment and this may have explained the high proportions of patients who did not receive the allocated second‐line treatments after failure in the first‐line setting. Insufficient patients may also have limited the results of this review, since a number of analyses had hazard ratios with wide CI. However, this is not likely the case for OS since a consistency between subgroups with first‐ and second‐line treatments was observed. For PFS and response rates, the benefit in the combination group was affected by inclusion of the first‐line treatment setting study (Saltz 2000). Since marked heterogeneity between subgroups was found, this signified the importance of conducting subgroup analyses based on first‐ or second‐line treatments. Such individualised interpretation may also be more useful when translating these findings to clinical context. Through the sensitivity analyses, we observed that including studies with an additional agent in both arms (oxaliplatin in this case) (Bécouarn 2001) increased heterogeneity in the final analyses. The different associations observed in presence of common additional agents, i.e. oxaliplatin, suggest that great care should be made when designing studies and choosing statistical methods to compare IRI with and without fluoropyrimidine where additional chemotherapeutic or biological agents are used. Finally, the fact that one ongoing study from the additional search have not yet been incorporated may be a source of potential bias.
Agreements and disagreements with other studies or reviews
A similar review was published in 2011, focusing on the use of IRI and 5‐FU compared to IRI alone as a second‐line treatment for advanced or metastatic CRC (Clarke 2011), which included three studies that were also selected here (Clarke 2011; Graeven 2007; Seymour 2007). In this previous review, there was no significant benefit or disadvantage in OS or PFS by adding 5‐FU to IRI, which was similar to what we found for the second‐line treatment setting. In the current review, comparisons of toxicities were performed only for studies providing toxicity profiles of both experimental and control arms in both first‐ and second‐line treatments instead of combining results from single‐arm trials. Interestingly, for grade 3 or 4 diarrhea and grade 1 or 2 alopecia, the findings presented here were similar to that obtained from the single‐arm studies in Clarke 2011, with higher risks observed in the single IRI arm. We also observed an increased risk of grade 3 or 4 neutropenia in the experimental arm. This may occur because neutropenia is a well‐known adverse effect of both IRI and 5‐FU. o Nevertheless, no difference in febrile neutropenia was observed between treatment arms.
Authors' conclusions
Implications for practice.
Given available data from clinical trials and large heterogeneity in reported findings, there is no evidence to suggest any superiority in OS of the combination of IRI and fluoropyrimidine over IRI alone. Patients in the combination arm were shown to have longer PFS, but the moderate quality of the evidence indicates the necessity to confirm findings from this review in clinical studies with adequate sample size to address potential subgroup effects. Risks of grade 3 or 4 diarrhea and grade 1 or 2 alopecia were higher in the intervention arm compared to in controls, whereas the risk of grade 3 or 4 neutropenia was higher in the control group. These different toxicity profiles indicate the need for further consideration when selecting a suitable treatment based on individual characteristics of the patients at baseline.
Implications for research.
Despite the emergence of targeted therapies, IRI and fluoropyrimidine remain an important components of the regimens used in treating advanced and metastatic CRC. Therefore, more clinical trials with sufficient numbers of patients in each line of treatment are needed to confirm any benefit seen with regimens containing the combination of IRI and fluoropyrimidine for CRC treatment compared to those containing IRI alone. It would be of interest for trialists to assess both regimens in combination with more recent biological agents, and include other administration routes, in order to achieve optimal clinical benefits. The ongoing study in ‘Studies awaiting classification’ may alter the conclusions of the review once results are available and incorporated.This also calls for designs of RCT protocols that allow for an unbiased comparison between groups of patients receiving IRI with and without the addition of fluoropyrimidine.
History
Protocol first published: Issue 7, 2010 Review first published: Issue 2, 2016
| Date | Event | Description |
|---|---|---|
| 14 December 2014 | New search has been performed | New search performed. 945 additional studies found in initial search, but no new trials identified in full‐text search and checked for inclusion |
| 24 June 2013 | New search has been performed | Updated protocol with new author team |
Acknowledgements
The authors wish to thank the Managing Editor, Trial Search Coordinator and the editorial team from the Cochrane Colorectal Cancer Group for the support and advice provided throughout the preparation of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Colorectal Neoplasms] explode all trees #2 ((colorect* or colon or colonic or rect* or anal* or anus* or intestin* or bowel*) near/3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or metastas*)):ti,ab,kw #3 (#1 or #2) #4 MeSH descriptor: [Camptothecin] explode all trees #5 (irinotecan* or camptothecin* or biotecan or Camptosar or camptothecin‐11 or CPT‐11 or SN‐38):ti,ab,kw #6 (#4 or #5) #7 (#3 and #6)
Appendix 2. MEDLINE search strategy
1. *Colorectal Neoplasms/ 2. ((colorect* or colon or colonic or rect* or anal* or anus* or intestin* or bowel*) and (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or metastas*)).m_titl. 3. 1 or 2 4. exp Camptothecin/ 5. (irinotecan* or camptothecin* or biotecan or Camptosar or camptothecin‐11 or CPT‐11 or SN‐38).m_titl. 6. 4 or 5 7. 3 and 6 8. randomized controlled trial.pt. 9. controlled clinical trial.pt. 10. randomized.ab. 11. placebo.ab. 12. clinical trial as topic.sh. 13. randomly.ab. 14. trial.ti. 15. 8 or 9 or 10 or 11 or 12 or 13 or 14 16. Exp animals/ not humans.sh. 17. 15 not 16 18. 7 and 17
Appendix 3. EMBASE search strategy
1. *colorectal cancer/ 2. ((colorect* or colon or colonic or rect* or anal* or anus* or intestin* or bowel*) and (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or metastas*)).m_titl. 3. 1 or 2 4. *irinotecan/ 5. (irinotecan* or camptothecin* or biotecan or Camptosar or camptothecin‐11 or CPT‐11 or SN‐38).m_titl. 6. 4 or 5 7. 3 and 6 8. CROSSOVER PROCEDURE.sh. 9. DOUBLE‐BLIND PROCEDURE.sh. 10. SINGLE‐BLIND PROCEDURE.sh. 11. (crossover* or cross over*).ti,ab. 12. placebo*.ti,ab. 13. (doubl* adj blind*).ti,ab. 14. allocat*.ti,ab. 15. trial.ti. 16. RANDOMIZED CONTROLLED TRIAL.sh. 17. random*.ti,ab. 18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.) 20. 18 not 19 21. 7 and 20
Appendix 4. Science Citation Index search strategy
#1 Title: ((colorect* or colon or colonic or rect* or anal* or anus* or intestin* or bowel*) near/3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or metastas*)) #2 Title: (irinotecan* or camptothecin* or biotecan or Camptosar or camptothecin‐11 or CPT‐11 or SN‐38) #3 TOPIC: (controlled trial or controlled clinical trial or placebo or clinical trial or random* or trial or cct or rct) #4 (#3 AND #2 AND #1)
Appendix 5. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of ‘Low risk’ of bias. | The investigators describe a random component in the sequence generation process such as: · Referring to a random number table; · Using a computer random number generator; · Coin tossing; · Shuffling cards or envelopes; · Throwing dice; · Drawing of lots; · Minimization*. *Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘High risk’ of bias. | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · Sequence generated by odd or even date of birth; · Sequence generated by some rule based on date (or day) of admission; · Sequence generated by some rule based on hospital or clinic record number. · Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example: · Allocation by judgement of the clinician; · Allocation by preference of the participant; · Allocation based on the results of a laboratory test or a series of tests; · Allocation by availability of the intervention. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · Central allocation (including telephone, web‐based and pharmacy‐controlled randomization); · Sequentially numbered drug containers of identical appearance; · Sequentially numbered, opaque, sealed envelopes. |
| Criteria for the judgement of ‘High risk’ of bias. | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · Using an open random allocation schedule (e.g. a list of random numbers); · Assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); · Alternation or rotation; · Date of birth; · Case record number; · Any other explicitly unconcealed procedure. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; · Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; · Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following: · Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; · The study did not address this outcome. |
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; · Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; · Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following: · Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; · The study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · No missing outcome data; · Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; · Missing data have been imputed using appropriate methods. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; · For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; · Potentially inappropriate application of simple imputation. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following: · Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomized not stated, no reasons for missing data provided); · The study did not address this outcome. |
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any of the following: · The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; · The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · Not all of the study’s pre‐specified primary outcomes have been reported; · One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; · One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; · The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of ‘Low risk’ of bias. | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘High risk’ of bias. | There is at least one important risk of bias. For example, the study: · Had a potential source of bias related to the specific study design used; or · Has been claimed to have been fraudulent; or · Had some other problem. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | There may be a risk of bias, but there is either: · Insufficient information to assess whether an important risk of bias exists; or · Insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Overall Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 5 | 1728 | Hazard Ratio (Random, 95% CI) | 0.91 [0.81, 1.02] |
| 1.1 First‐line treatments | 1 | 457 | Hazard Ratio (Random, 95% CI) | 0.88 [0.69, 1.12] |
| 1.2 Second‐line treatments | 4 | 1271 | Hazard Ratio (Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2 Overall survival | 5 | 1728 | Hazard Ratio (95% CI) | 0.88 [0.75, 1.05] |
1.1. Analysis.
Comparison 1 Overall Survival, Outcome 1 Overall survival.
1.2. Analysis.
Comparison 1 Overall Survival, Outcome 2 Overall survival.
Comparison 2. Progression‐Free Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression‐free survival | 3 | 600 | Hazard Ratio (Random, 95% CI) | 0.68 [0.53, 0.87] |
| 1.1 First‐line treatments | 1 | 457 | Hazard Ratio (Random, 95% CI) | 0.59 [0.45, 0.77] |
| 1.2 Second‐line treatments | 2 | 143 | Hazard Ratio (Random, 95% CI) | 0.83 [0.58, 1.19] |
2.1. Analysis.
Comparison 2 Progression‐Free Survival, Outcome 1 Progression‐free survival.
Comparison 3. Response to Treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Objective response (CR + PR) | 5 | 1199 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.80, 2.24] |
| 1.1 First‐line treatments | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.58, 3.02] |
| 1.2 Second‐line treatments | 4 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.50, 1.96] |
3.1. Analysis.
Comparison 3 Response to Treatment, Outcome 1 Objective response (CR + PR).
Comparison 4. Toxicity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Grade 3/4 diarrhea | 5 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.91] |
| 2 Grade 3/4 mucositis | 4 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.45, 3.11] |
| 3 Grade 3/4 nausea | 3 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.58] |
| 4 Grade 3/4 vomiting | 4 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.47, 1.24] |
| 5 Grade 3/4 neutropenia | 5 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.29, 2.48] |
| 6 Febrile neutropenia | 3 | 595 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.53, 2.78] |
| 7 Grade 1/2 alopecia | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.28, 0.74] |
| 8 Neuropathy | 2 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.27, 5.42] |
| 9 Grade 3/4 anemia | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.08, 5.19] |
4.1. Analysis.
Comparison 4 Toxicity, Outcome 1 Grade 3/4 diarrhea.
4.2. Analysis.
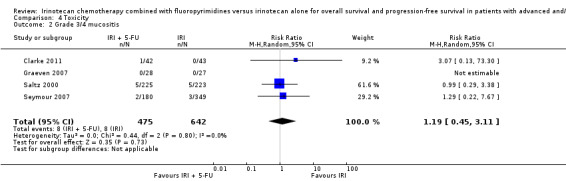
Comparison 4 Toxicity, Outcome 2 Grade 3/4 mucositis.
4.3. Analysis.
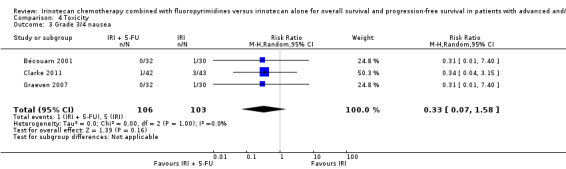
Comparison 4 Toxicity, Outcome 3 Grade 3/4 nausea.
4.4. Analysis.
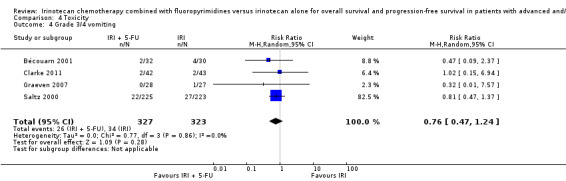
Comparison 4 Toxicity, Outcome 4 Grade 3/4 vomiting.
4.5. Analysis.
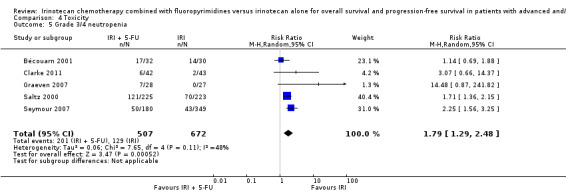
Comparison 4 Toxicity, Outcome 5 Grade 3/4 neutropenia.
4.6. Analysis.
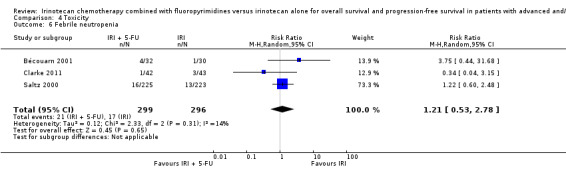
Comparison 4 Toxicity, Outcome 6 Febrile neutropenia.
4.7. Analysis.
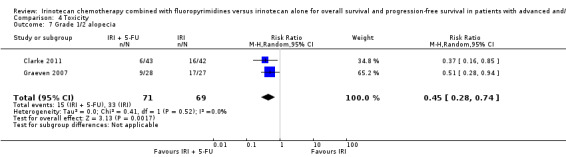
Comparison 4 Toxicity, Outcome 7 Grade 1/2 alopecia.
4.8. Analysis.
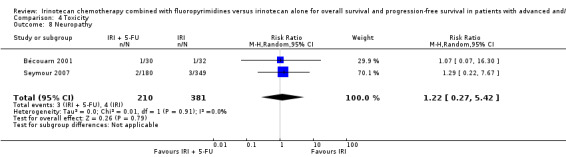
Comparison 4 Toxicity, Outcome 8 Neuropathy.
4.9. Analysis.
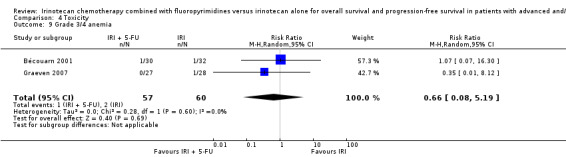
Comparison 4 Toxicity, Outcome 9 Grade 3/4 anemia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bécouarn 2001.
| Methods | Randomized controlled trial Phase II, multicenter |
|
| Participants | CRC with progressive disease after no more than one regimen of optimal 5‐FU FA‐based chemotherapy for metastatic disease and/or no more than one line of 5‐FU–containing treatment after prior adjuvant chemotherapy if discontinued less than 6 months | |
| Interventions | IRI + 5‐FU/LV + oxaliplatin vs IRI + oxaliplatin | |
| Outcomes | Overall survival Progression‐free survival Toxicity |
|
| Notes | Oxaliplatin IV was administered in both treatment arms IR + oxaliplatin had longer survivals and better toxicity profile |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomization sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No mention of any allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients in the control arm did not received any treatment due to a move to a nonparticipating study site in one case and worsening of general status in the other. However they were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No reported pre‐specified outcomes |
| Other bias | Low risk | None |
Clarke 2011.
| Methods | Randomized controlled trial Phase II, multicenter |
|
| Participants | Incurable locally advanced or metastatic CRC, progressive disease after one prior chemotherapy regimen for advanced disease and/or after prior adjuvant therapy, provided that relapse had occurred within 6 months of that treatment | |
| Interventions | IRI + 5‐FU/LV vs IRI | |
| Outcomes | Overall survival Progression‐free survival Toxicity |
|
| Notes | Trial was terminated due to slow recruitment. Similar efficacy between the two arms but IRI + 5‐FU/LV had slightly less toxicities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation |
| Allocation concealment (selection bias) | Low risk | Central randomization/allocation by telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients withdrew early (2 in the intervention and 2 in the control arm). Three withdrew before baseline tumour assessment and did not receive any treatment. The fourth one opted 3 days after consent to receive off‐study irinotecan and cetuximab. These patients were included in the analysis, except one who explicitly refused. |
| Selective reporting (reporting bias) | Unclear risk | No reported pre‐specified outcomes |
| Other bias | Low risk | None |
Graeven 2007.
| Methods | Randomized controlled trial Phase II, multicenter |
|
| Participants | Metastatic CRC after failure of a first‐line chemotherapy, pretreatment was to consist of either 5‐FU/LV, capecitabine or 5‐FU/LV in combination with oxaliplatin, whereas irinotecan‐containing regimens were not allowed | |
| Interventions | IRI + 5‐FU/LV vs IRI | |
| Outcomes | Overall survival Progression‐free survival Toxicity |
|
| Notes | No marked difference was observed between the two groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomization sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No mention of any allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two patients were withdrawn prior to the first administration, but reasons for this were not reported and there was no mention of which treatment arm they were randomized to. Both patients were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No reported pre‐specified outcomes |
| Other bias | Low risk | None |
Saltz 2000.
| Methods | Randomized controlled trial Phase III, multicenter |
|
| Participants | Metastatic CRC, no prior therapy for metastatic disease; patients who had received adjuvant 5‐FU‐based therapy were eligible if they had remained free of disease for at least one year after the completion of adjuvant therapy | |
| Interventions | IRI + 5‐FU/LV vs IRI | |
| Outcomes | Overall survival Progression‐free survival Toxicity |
|
| Notes | The study had another treatment arm with 5‐FU/LV alone. IRI + 5‐FU/LV was superior to FU/LV alone but not directly compared with IRI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomization sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No mention of any allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, thus no blinding of participants and personnel was performed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six patients in the intervention group and three patients in the control arm either did not receive any treatment or received the wrong treatment. However, they were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No reported pre‐specified outcomes |
| Other bias | Low risk | None |
Seymour 2007.
| Methods | Randomized controlled trial | |
| Participants | Inoperable metastatic or locoregional CRC | |
| Interventions | IRI + 5‐FU/LV vs IRI | |
| Outcomes | Overall survival Progression‐free survival Toxicity |
|
| Notes | The intervention arms were part of sequential treatments with three strategies. Two among these administered 5‐FU as the first‐line treatment, followed by a second‐line treatment with IRI in the control group and either IRI + 5‐FU or oxaliplatin in the intervention group. The third strategy used the combination of IRI or oxaliplatin with 5‐FU as the first‐line treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation |
| Allocation concealment (selection bias) | Unclear risk | No mention of any allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Among 339 patients in the intervention group who failed first‐line treatment, 110 died or progressed to terminal care prior to administration and 44 received alternative second‐line regimen. Among 666 patients in the control group who failed first‐line treatment, 251 died or progressed to terminal care prior to administration and 51 received alternative second‐line regimen. However, these patients were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No reported pre‐specified outcomes |
| Other bias | Low risk | None |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fiorentini 2012 | IRI was not administered via an IV or oral route |
| Mitchell 2011 | Assignments to IRI or IRI + 5‐FU were not randomized |
| Popov 2006 | Assignments to IRI or IRI + 5‐FU were not randomized |
Characteristics of studies awaiting assessment [ordered by study ID]
Bendell 2014.
| Methods | Open label RCT / three arm study |
| Participants | 280 adult patients (both gender), aged 18‐75, with a histologically confirmed colorectal cancer with at least on measurable metastatic lesion. |
| Interventions | Exp A – FOLFOXFIRI + Bevacizumab; Exp B – sequential FOLFOXFIRI + Bevacizumab; Exp C – FOLFOX + Bevacizumab |
| Outcomes | Primary – Overall response rate (ORR1); and progression‐free survival (PFS1) during first line therapy. Secondary – Overall response rate during second line therapy (ORR2); progression‐free survival during second line therapy (PFS2); Time to PFS2; Overall survival (OS); Liver resection rate; Rates of conversion from unresectable to resectable disease; Adverse events. |
| Notes | The study is ongoing but not recruiting. Estimated primary completion December 2016. |
Differences between protocol and review
In the protocol, we specified that only English publications would be included in the review. However, during initial search and selection of studies, we included trials in all languages and when limiting to studies in English in the latter stage, this made no difference in studies selected. Therefore, in the current review we no longer limited the studies to publications in English.
The method used to pool estimates from binary outcomes was not specified in the protocol. The use of a Mantel‐Haenszel test has now been included in the review.
In the protocol, we specified that random effects model will be used when heterogeneity is indicated. However, in the review, random effects model selection was performed for all analyses, and investigations of sources of any results inconsistency by sensitivity analyses were performed when heterogeneity was indicated.
Contributions of authors
| TASKS | WHO WILL UNDERTAKE TASKS? |
| Draft the protocol | Wahyu Wulaningsih and Mieke Van Hemelrijck |
| Develop a search strategy | Wahyu Wulaningsih and Mieke Van Hemelrijck |
| Search for trials | Ardyan Wardhana and Naomi Yoshuantari |
| Select which trials to include | Wahyu Wulaningsih and Ardyan Wardhana |
| Extract data from trials | Wahyu Wulaningsih and Naomi Yoshuantari |
| Enter data into RevMan | Wahyu Wulaningsih and Ardyan Wardhana |
| Carry out the analysis | Wahyu Wulaningsih, Ardyan Wardhana, Johnathan Watkins |
| Interpret the analysis | All authors |
| Draft the final review | All authors |
Sources of support
Internal sources
No sources of support supplied, Other.
External sources
No sources of support supplied, Other.
Declarations of interest
None declared.
New
References
References to studies included in this review
Bécouarn 2001 {published data only}
- Bécouarn Y, Gamelin E, Coudert B, Ne´grier S, Pierga JY, Raoul JL, et al. Randomized multicenter phase II study comparing a combination of fluorouracil and folinic acid and alternating irinotecan and oxaliplatin with oxaliplatin and irinotecan in fluorouracil‐pretreated metastatic colorectal cancer patients. Journal of Clinical Oncology 2001;19(22):4195‐201. [DOI] [PubMed] [Google Scholar]
Clarke 2011 {published data only}
- Clarke SJ, Yip S, Brown C, Hazel GA, Ransom DT, Goldstein D, et al. Single‐agent irinotecan or FOLFIRI as second‐line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta‐analysis [corrected]. European Journal of Cancer 2011;47(12):1826‐36. [DOI] [PubMed] [Google Scholar]
Graeven 2007 {published data only}
- Graeven U, Arnold D, Reinacher‐Schick A, Heuer T, Nusch A, Porschen R, et al. A randomised phase II study of irinotecan in combination with 5‐FU/FA compared with irinotecan alone as second‐line treatment of patients with metastatic colorectal carcinoma. Onkologie 2007;30(4):169‐74. [DOI] [PubMed] [Google Scholar]
Saltz 2000 {published data only}
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. New England Journal of Medicine 2000;343(13):905‐14. [DOI] [PubMed] [Google Scholar]
Seymour 2007 {published data only}
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;30(9582):143‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fiorentini 2012 {published data only}
- Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, et al. Intra‐arterial infusion of irinotecan‐loaded drug‐eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Research 2013;33(11):5211. [PubMed] [Google Scholar]
Mitchell 2011 {published data only}
- Mitchell EP, Piperdi B, Lacouture ME, Shearer H, Iannotti N, Pillai MV, et al. The efficacy and safety of panitumumab administered concomitantly with FOLFIRI or Irinotecan in second‐linetherapy for metastatic colorectal cancer: the secondary analysis from STEPP (Skin Toxicity Evaluation ProtocolWith Panitumumab) by KRAS status. Clinical Colorectal Cancer 2011;10(4):333‐9. [DOI] [PubMed] [Google Scholar]
Popov 2006 {published data only}
- Popov I, Jelic S, Krivokapic Z, Micev M, Babic D, Zdrale Z. What is the best sequence of chemotherapy in advanced colorectal cancer? Final results of a five‐arm study. Chemotherapy 2006;52:20–2. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bendell 2014 {published data only}
- Bendell JC, Tan BR, Reeves JA, Xiong HQ, Laeufle R, Byrtek M, et al. STEAM: A randomized, open‐label, phase 2 trial of sequential and concurrent FOLFOXIRI‐bevacizumab (BEV) versus FOLFOX‐BEV for the first‐line (1L) treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC).. Journal of Clinical Oncology 2014;32:5s:suppl; abstr TPS3652. [Google Scholar]
Additional references
ACCMAP 1992
- Advanced Colorectal Cancer Meta‐Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Journal of Clinical Oncology 1992;10(6):896‐903. [DOI] [PubMed] [Google Scholar]
Aranda 2009
- Aranda E, Valladares M, Martinez‐Villacampa M, Benavides M, Gomez A, Massutti B, et al. Randomized study of weekly irinotecan plus high‐dose 5‐fluorouracil (FUIRI) versus biweekly irinotecan plus 5‐fluorouracil/leucovorin (FOLFIRI) as first‐line chemotherapy for patients with metastatic colorectal cancer: a Spanish Cooperative Group for the Treatment of Digestive Tumors Study. Annals of Oncology 2009;20(2):251‐7. [DOI] [PubMed] [Google Scholar]
Bouzid 2003
- Bouzid K, Khalfallah S, Tujakowski J, Piko B, Purkalne G, Plate S, et al. A randomized phase II trial of irinotecan in combination with infusional or two different bolus 5‐fluorouracil and folinic acid regimens as first‐line therapy for advanced colorectal cancer. Annals of Oncology 2003;14(7):1106‐14. [DOI] [PubMed] [Google Scholar]
Cao 2000
- Cao S, Rustum YM. Synergistic antitumor activity of irinotecan in combination with 5‐fluorouracil in rats bearing advanced colorectal cancer: role of drug sequence and dose. Cancer Research 2000;60(14):3717‐21. [PubMed] [Google Scholar]
Center 2009
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA: a cancer journal for clinicians 2009;59(6):366‐78. [DOI] [PubMed] [Google Scholar]
Chau 2005
- Chau I, Norman AR, Cunningham D, Iveson T, Hill M, Hickish T, et al. Longitudinal quality of life and quality adjusted survival in a randomised controlled trial comparing six months of bolus fluorouracil/leucovorin vs twelve weeks of protracted venous infusion fluorouracil as adjuvant chemotherapy for colorectal cancer. European Journal of Cancer 2005;41(11):1551‐9. [DOI] [PubMed] [Google Scholar]
Colucci 2005
- Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Gruppo Oncologico Dell'Italia Meridionale. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia. Journal of Clinical Oncology 2005;23:4866‐75. [DOI] [PubMed] [Google Scholar]
Conti 1996
- Conti JA, Kemeny NE, Saltz LB, Huang Y, Tong WP, Chou TC, et al. Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. Journal of Clinical Oncology 1996;14(3):709‐15. [DOI] [PubMed] [Google Scholar]
Cunningham 1998
- Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352(9138):1413‐8. [PUBMED: 9807987] [DOI] [PubMed] [Google Scholar]
Douillard 2000
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355(9209):1041‐7. [DOI] [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. [DOI] [PubMed] [Google Scholar]
Folprecht 2008
- Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, et al. Irinotecan/fluorouracil combination in first‐line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. Journal of Clinical Oncology 2008;26(9):1443‐51. [DOI] [PubMed] [Google Scholar]
Fuchs 2007
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first‐line treatment of metastatic colorectal cancer: results from the BICC‐C Study. Journal of Clinical Oncology 2007;25(30):4779‐86. [DOI] [PubMed] [Google Scholar]
Giessen 2011
- Giessen C, Weikersthal LF, Hinke A, Stintzing S, Kullmann F, Vehling‐Kaiser U, et al. A randomized, phase III trial of capecitabine plus bevacizumab (Cape‐Bev) versus capecitabine plus irinotecan plus bevacizumab (CAPIRI‐Bev) in first‐line treatment of metastatic colorectal cancer: the AIO KRK 0110 trial/ML22011 trial. BMC cancer 2011;11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. British Medical Journal 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, March 2011. [Google Scholar]
Hsiang 1985
- Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein‐linked DNA breaks via mammalian DNA topoisomerase I. Journal of Biological Chemistry 1985;260(27):14873‐8. [PubMed] [Google Scholar]
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians 2011;61(2):69‐90. [DOI] [PubMed] [Google Scholar]
Kuhry 2008
- Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long‐term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treatment Reviews 2008;34(6):498‐504. [DOI] [PubMed] [Google Scholar]
Maiello 2000
- Maiello E, Gebbia V, Giuliani F, Paoletti G, Gebbia N, Cigolari S, et al. 5‐Fluorouracil and folinic acid with or without CPT‐11 in advanced colorectal cancer patients: a multicenter randomised phase II study of the Southern Italy Oncology Group. Annals of Oncology 2000;11(8):1045‐51. [DOI] [PubMed] [Google Scholar]
Maiello 2005
- Maiello E, Gebbia V, Giuliani F, Paoletti G, Gebbia N, Borsellino N, et al. FOLFIRI regimen in advanced colorectal cancer: the experience of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO 2005;16 Suppl 4:iv56‐60. [DOI] [PubMed] [Google Scholar]
Montagnani 2010
- Montagnani F, Chiriatti A, Licitra S, Aliberti C, Fiorentini G. Differences in efficacy and safety between capecitabine and infusional 5‐fluorouracil when combined with irinotecan for the treatment of metastatic colorectal cancer. Clinical Colorectal Cancer 2010;9(4):243‐7. [DOI] [PubMed] [Google Scholar]
Muro 2010
- Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. Irinotecan plus S‐1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second‐line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non‐inferiority study (FIRIS study). The Lancet Oncology 2010;11(9):853‐60. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Petrelli 2012
- Petrelli F, Cabiddu M, Barni S. 5‐Fluorouracil or capecitabine in the treatment of advanced colorectal cancer: a pooled‐analysis of randomized trials. Medical Oncology 2012;29(2):1020‐9. [DOI] [PubMed] [Google Scholar]
Pitot 1997
- Pitot HC, Wender DB, O'Connell MJ, Schroeder G, Goldberg RM, Rubin J, et al. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. Journal of Clinical Oncology 1997;15(8):2910‐9. [DOI] [PubMed] [Google Scholar]
Ragnhammar 2001
- Ragnhammar P, Hafström L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncologica 2001;40(2‐3):282‐308. [DOI] [PubMed] [Google Scholar]
Rothenberg 1999
- Rothenberg ML, Cox JV, DeVore RF, Hainsworth JD, Pazdur R, Rivkin SE, et al. A multicenter, phase II trial of weekly irinotecan (CPT‐11) in patients with previously treated colorectal carcinoma. Cancer 1999;85(4):786‐95. [PubMed] [Google Scholar]
Rougier 1997
- Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P, et al. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy‐naive patients and patients pretreated with fluorouracil‐based chemotherapy. Journal of Clinical Oncology 1997;15(1):251‐60. [DOI] [PubMed] [Google Scholar]
Rougier 1998
- Rougier P, Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998;352(9138):1407‐12. [DOI] [PubMed] [Google Scholar]
Siegel 2013
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians 2013;63(1):11‐30. [DOI] [PubMed] [Google Scholar]
Siegel 2014
- Siegel R, DeSantis S, Jemal A. Colorectal Cancer Statistics, 2014. CA: A Cancer Journal for Clinicians 2014;64:104‐17. [DOI] [PubMed] [Google Scholar]
Simmonds 2000
- Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta‐analysis. Colorectal Cancer Collaborative Group. British Medical Journal 2000;321(7260):531‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
Thirion 2004
- Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta‐analysis. Journal of Clinical Oncology 2004;22(18):3766‐75. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tournigand 2004
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery‐Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. Journal of Clinical Oncology 2004;22:229‐37. [DOI] [PubMed] [Google Scholar]
Van Cutsem 2004
- Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, et al. Oral capecitabine vs intravenous 5‐fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. British Journal of Cancer 2004;90(6):1190‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Cutsem 2014
- Cutsem E, Cervantes A, Nordlinger B, Arnold D, on behalf of the ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2014;25 (Supplement 3):iii1–iii9. [DOI] [PubMed] [Google Scholar]


