Abstract
Tsaoko Fructus, the dried ripe fruit of Amomum tsao-ko Crevost & Lemarié, is used as both medicinal material and food additive. This review summarized the traditional uses, botany, phytochemistry, and pharmacological progress on Tsaoko Fructus. One classical prescription and the other 11 representative prescriptions containing Tsaoko Fructus were reviewed. The indications of these prescriptions are major in treating spleen and stomach disorders and epidemic febrile diseases including malaria. At least 209 compounds have been isolated and identified from Tsaoko Fructus, most of which belong to terpenoids, phenylpropanoids, and organic acids. Essential oil, crude extract, and some compounds were observed to have pharmacological activities such as anti-biotics, anti-inflammation, antioxidant, mostly via in vitro experiments. However, the mechanism of its medicinal uses remains unclear. This review provides a comprehensive understanding of Tsaoko Fructus, which will be beneficial to exploring the mechanism and potential medicinal applications of Tsaoko Fructus, as well as developing a rational quality control system for Tsaoko Fructus as a medicinal material in the future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11101-021-09793-x.
Keywords: Amomum tsao-ko, Traditional use, Botany, Phytochemistry, Pharmacological and biological activity
Introduction
Amomum tsao-ko Crevost & Lemarié is a perennial Zingiberaceae herb, mainly growing in the warm and humid southwestern China and northern Vietnam. Its dried ripe fruit, called Tsaoko Fructus (Caoguo in Chinese), smells aromatic and spicy and has been used as both folk medicine and food additive. The earliest record of the medicinal application of Tsaoko Fructus can be dated back to Official Prescription of the Royal Medical Prescriptions (Taiping Huimin Heji Ju Fang, Ju Fang in brief) and Summary of Medicinal Herbs in Baoqing (Baoqing Bencao Zhezhong) in the Song Dynasty (Chen 2007; Gao and Wang 2007). Since then, the actions, compatibility, and prescriptions of Tsaoko Fructus had been developed and recorded in successive ancient medical books such as Yanshi Ji Sheng Fang (Ji Sheng Fang in brief) in the South Song dynasty, Wen Yi Lun in the Ming dynasty, and Wen Bing Tiao Bian in the Qing dynasty (Yao 2002; Shi et al. 2013). Five prescriptions consisting of Tsaoko Fructus were included in Pharmacopoeia of the People’s Republic of China (China Pharmacopoeia in brief) (Chinese Pharmacopoeia Commission 2015).
A number of prescriptions composed of Tsaoko Fructus were recorded in ancient medicinal books and China Pharmacopoeia. Those prescriptions were major in treatment of abdominal pain, diarrhea, hemorrhoids, throat infections, and malaria (Gao and Wang 2007). In recent years, Tsaoko Fructus-containing prescriptions have been developed and used to treat Hepatitis B, influenza, the Severe Acute Respiratory Syndromes (SARS), and the Coronavirus Disease 2019 (COVID-19) (Hu 1993; Yao 2002; Zhang and Chen 2008; Ding et al. 2020; Shen et al. 2020; Zong et al. 2020). Phytochemical research revealed at least 209 compounds belonging to terpenoids, flavonoids, diarylheptanoids, and organic acids were present in Tsaoko Fructus (Hong et al. 2015; Lee et al. 2019; He et al. 2020d). Some of them have anti-biotic, anti-inflammatory, anti-tumor, anti-diabetic, and neuroprotective activities (Zhang et al. 2014, 2015; Kim et al. 2016; Lee et al. 2019; He et al. 2020a, d).
However, the mechanism of the medicinal uses of Tsaoko Fructus has not been elucidated. As a result, there are some unreasonable points in the present quality control system of Tsaoko Fructus. In fact, its value as a medicinal material had received less attention than as a condiment (Gao and Wang 2007), although it has a long history of clinical applications.
In this review, the information related different aspects of A. tsao-ko was collected from reviewing peer-reviewed journals covering 1981–2021. Pubmed, SciFinder, Web of Science, EBSCO Medline, Baidu Scholar, and CNKI were used for electronic retrieval of the information. Based on this information, we gave a comprehensive review of A. tsao-ko, aiming to provide information for better understanding its pharmacological mechanism and the potential medicinal applications, and for developing a rational quality control system of Tsaoko Fructus as medicinal material in the future.
Traditional uses of Tsaoko Fructus
According to the theory of traditional Chinese medicine (TCM), Tsaoko Fructus has a mild property and has effects on removing dampness and warming the spleen and stomach (Yuan et al. 2000). It is indicated to treat interior obstruction of cold-dampness, distending pain in the epigastrium and abdomen, vomiting, malaria with cold and fever, and pestilence fever (Chen 2007; Gao and Wang 2007; Chinese Pharmacopoeia Commission 2015).
In traditional uses, Tsaoko Fructus or the seed of Tsaoko Fructus was commonly used in combination with other medicinal materials. One classical prescription and the other 11 representative ones were listed in Table 1. The main function and indication of these prescriptions covers two aspects. One is digestive system disorders resulted from cold and dampness of spleen and stomach, and the other is epidemic diseases such as malaria caused by epidemic pathogen infection (Table 1). Among the 12 prescriptions, Caoguo decoction (Caoguo Yin) from Ju Fang, Guofu decoction (Guofu Tang), and Qingpi decoction (Qingpi Yin) from Ji Sheng Fang, Dayuan decoction (Dayuan Yin) from Wen Yi Lun, Caoguo Zhimu decoction (Caoguo Zhimu Tang) from Wen Bin Tiao Bian had effects on both digestive system disorders and epidemic febrile diseases. Changshan decoction (Changshan Yin) from Ju Fang was intended for curing malaria. Suopi decoction (Supi Yin) from Ju Fang and four prescriptions from China Pharmacopoeia (Jiebai Pills, Lige Pills, Piweishu Pills, and Piwei Xiaozhi Pills) were adopted in treating disorders of digestive system. Although it seems some prescriptions had similar functions, their specific applicable indications were not exactly the same. For example, regarding to the treatment of malaria, Dayuan Yin was used to treat early malaria, Guofu Tang could prevent attack of malaria, and Changshan Yin suited for curing all types of malaria including the chronic one. It was worth mentioning that Ershiwuwei Zhenzhu Pills (Ershiwuwei Zhenzhu Wan) documented in China Pharmacopoeia had different indications from the other 11 ones. It was effective for the treatment of apoplexy manifested as hemiplegia, deviated eyes and mouth, coma, disordered consciousness, delirious speech, and so on. Information of these prescriptions including ingredients, functions, indications and others was listed in Table 1.
Table 1.
Twelve representative prescriptions composed of Tsaoko Fructus recorded in literatures
| Prescriptions | Ingredients* | Functions and Indications | Sources |
|---|---|---|---|
| Changshan Yin# | Anemarrhenae Rhizoma, Dichroae Radix, Tsaoko Fructus, Glycyrrhizae Radix Et Rhizoma (stir-baked with liquid), Alpiniae Officinarum Rhizoma, Mume Fructus (without core) (In the mass ratio of 10:10:10:10:6:5, 9 g a dose, dipped in 150 ml water and boiled to 100 ml, one dose a day) | Functions and indications: Treating all types of malaria including chronic malaria | Taiping Huimin Heji Ju Fang, Vol. 8, Song dynasty, 1151 |
| Caoguo Yin# | Perillae Folium, Seeds of Tsaoko Fructus, Chuanxiong Rhizoma, Angelicae Dahuricae Radix, Alpiniae Officinarum Rhizoma (stir-baked), Citri Reticulatae Pericarpium Viride (without flesh, stir-baked), Glycyrrhizae Radix Et Rhizoma (stir-baked) (In the mass ratio of 1:1:1:1:1:1:1, 6 g a dose, dipped in 150 ml water and boiled to 100 ml, three doses on the first day, then one dose a day) | Functions and indications: Warming spleen and stomach, dispersing cold and heat, regulating qi, treating cold spleen and preventing attack of malaria | Taiping Huimin Heji Ju Fang, Vol. 3, Song dynasty, 1151 |
| Suopi Yin# | Amomi Fructus, Mume Fructus (without core), seeds of Tsaoko Fructus, Glycyrrhizae Radix Et Rhizoma (stir-baked with liquid), Puerariae Lobatae Radix, Lablab Semen Album (stir-baked, without cortex) (In the mass ratio of 2:2:2:2:1:1, 12 g a dose, dipped in 250 ml water and boiled to 200 ml, taken as frequently as tea) | Functions and indications: Reducing fever and fidgetiness after cholera, treating heat- and dampness-resulted vomit | Taiping Huimin Heji Ju Fang, Vol. 2, Song dynasty, 1151 |
| Guofu Tang# | Seeds of Tsaoko Fructus, Aconiti Lateralis Radix Praeparata (Processed, without cortex) (In the mass ratio of 1:1, 25 g a dose, dipped in a bottle of water and boiled with 7 pieces of ginger and one Jujubae Fructus, taken anytime) | Functions and indications: Warming spleen and stomach, preventing attack of malaria | Yanshi Ji Sheng Fang, Vol. 18, South Song dynasty, 1253 |
| Qingpi Tang# | Citri Reticulatae Pericarpium Viride (without flesh), Magnoliae Officinalis Cortex (stir-baked with ginger), Atractylodis Macrocephalae Rhizoma, Seeds of Tsaoko Fructus, Bupleuri Radix (without stem), Poria (without cortex), Pinelliae Rhizoma (Soaking in the water seven times), Scutellariae Radix, Glycyrrhizae Radix Et Rhizoma (stir-baked with liquid) (Equal proportion, 12–20 g a dose, dipped in 220 ml water and boiled with five pieces of ginger to 150 ml, taken anytime) | Functions and indications: Expelling phlegm, removing dampness, harmonizing stomach, and preventing attack of malaria | Yanshi Ji Sheng Fang, Vol. 18, South Song dynasty, 1253 |
| Dayuan Yin#** | Arecae Semen, Magnoliae Officinalis Cortex, Seeds of Tsaoko Fructus, Anemarrhenae Rhizoma, Paeoniae Radix Alba, Scutellariae Radix, Glycyrrhizae Radix Et Rhizoma (In the mass ratio of 4:2:1:2:2:2:1, 21 g a dose, dipped in 200 ml water and boiled to about 160 ml, one dose a day) | Functions: Eliminating pathogens between interior and exterior, eliminating fetid and turbid-transmission. Indications: Pathogens, early malaria, pathogens between interior and exterior, aversion to cold and high fever, fullness in the chest, vomiting, headache, and restlessness | Wen Yi Lun, Ming dynasty, 1642 |
| Caoguo Zhimu Tang# | Tsaoko Fructus, Anemarrhenae Rhizoma, Pinelliae Rhizoma, Magnoliae Officinalis Cortex, Scutellariae Radix, Mume Fructus, Pollen (In the mass ratio of 3:4:6:4:3:3:3, 39 g a dose, dipped in 1000 ml of water and boiled with 25 ml of ginger juice to 400 ml, divided into two parts and taken twice a day) | Functions: Relieving cold in the back, fullness and discomfort of qi in the chest, preventing attack of malaria | Wen Bing Tiao Bian, Vol. 2, Qing dynasty, 1798 |
| Jiebai Wan$ | Chebulae Fructus, Calcitum, Pterocephali Herba, Trogopteri Faeces Extract, Inulae Radix, Punicae Granati Fructus, Chaenomelis Fructus, Aquilariae Lignurn Resinatum, Caryophylli Flos, Pulveratum Calx, Carthami Flos, Myristicae Semen, Alpiniae Katsumadai Semen, Seeds of Tsaoko Fructus (In the mass ratio of 60:35:14:30:4:4:4:3:3:2:1:2:2:2) | Functions: Fortifying the spleen, harmonizing the stomach, relieving epigastric pain and vomiting, separating the clear and excrete the turbid. Indications: Distension and fullness in the chest and the abdomen, indigestion, hiccup, diarrhea, and inhibited urination | Pharmacopoeia of the People’s Republic of China, 2015 |
| Lige Wan$ | Raphani Semen (stir-baked), Arecae Semen, Rhei Radixet Rhizoma (processed with wine), Magnoliae Officinalis Cortex (baked with ginger), Crataegi Fructus, Massa Medicata Fermentata (stir-baked), Amomi Fructus, Platycodonis Radix, Citri Reticulatae Pericarpium Viride (processed with vinegar), Aurantii Fructus (stir-baked with bran), Hordei Fructus Germinatus (stir-baked with bran), Aucklandiae Radix, Citri Reticulatae Pericarpium, Atractylodis Rhizoma (stir-baked with bran), Pogostemonis Herba; Seeds of Tsaoko Fructus; Glycyrrhizae Radixet Rhizoma (In the mass ratio of 4:4:4:2:2:2:1:2:2:2:2:2:2:2:2:2:2) | Functions: Soothing the chest and diaphragm, eliminating accumulation and relieving pain. Indications: Qi stagnation and constraint, distension and fullness in the chest and the diaphragm, pain in the epigastrium and abdomen, and retained fluid | Pharmacopoeia of the People’s Republic of China, 2015 |
| Piweishu Wan$ | Trionycis Carapax (processed), Astragali Radix Praeparata, Citri Pericarpium Reticulatae, Aurantii Immaturus Fructus, Paeoniae Radix Alba, Macrocephalae Rhizoma (stir-fried with bran), Cyperi Rhizoma (processed with vinegar), Tsaoko Fructus, Mume Fructus (stir-baked), Chuanxiong Rhizoma, Arecae Semen Tostum, Magnoliae Officinalis Cortex (Equal proportion) | Functions: Soothing the liver, regulate qi, fortifying the spleen, harmonizing the stomach, eliminating accumulation, and promoting digestion. Indications: Indigestion, poor appetite, epigastric upset, abdominal distention, borborygmus, nausea, vomiting, sloppy stool, distending pain in the hypochondrium, irritability, insomnia and dream-disturbed sleep; Chronic gastritis, chronic hepatitis and early stage liver cirrhosis with the symptoms described above | Pharmacopoeia of the People’s Republic of China, 2015 |
| Tiaowei Xiaozhi Wan$ | Officinalis Cortex Magnoliae (stir-baked with ginger juice), Notopterygii Rhizoma et Radix, Guangdong Shenqu, Aurantii Fructus, Cyperi Rhizoma (processed), Pinelliae Rhizoma (stir-baking with ginger juice), Saposhniloviae Radix, Peucedani Radix, Chuanxiong Rhizoma (stemming with distillate spirits), Angelicae Dahuricae Radix, Menthae Haplocalycis Herba, AmomiFructus, Tsaoko Fructus, Aucklandiae Radix, Amomi Rotundus Fructus, Poria, Atractylodis Rhizorna (macerate), Pogostemonis Herba, Linderae Radix (steaming with vinegar), Glycyrrhizae Radix (et Rhizoma), Perillae Folium, Citri Reticulatae Pericarpium (In the mass ratio of 10:10:10:5:1:10:10:10:1:10:10:10:5:1:10:10:10:1:10:5:10:10) | Functions: Dispersing wind, releasing the exterior, dissipate cold, resolving dampness, invigorating the stomach, and promoting digestion. Indications: Common cold due to wind-cold with dampness and internal food stagnation, manifested as chills, fever, headache, body heaviness with difficult movement, reduced food intake, fetid belching, acid reflux, abdominal pain, and diarrhea | Pharmacopoeia of the People’s Republic of China, 2015 |
| Ershiwuwei Zhenzhu Wan$ | Margarita, Margaritifera Concha, Myristicae Semen, Calx Pulveraturn, Carthami Flos, Tsaoko Fructus, Caryophylli Flos, Dalbergiae Odoriferae Lignum, Amomi Fructus Rotundus, Chebulae Fructus, Santali Albi Lignum, Phyllanthi Fructus, Aquilariae Lignum Resinatum, Cinnamomi Cortex, Terminaliae Billericae Fructus, Eriocheir seu Potamon, Aucklandiae Radix, Malvae Fructus, Piperis Longi Fructus, Fragariae Herba, Micae Lapis Aureus, Bovis Calculus Sativus, Cumni Cymini Fructus, Croci Stigma, Nigellae Semen, Moschus Artifactus, Bubali Cornu. (Proportion unavailable) | Functions: Tranquilizing the mind and opening the orifices. Indications: Apoplexy manifested as hemiplegia, deviated eyes and mouth, coma, disordered consciousness, delirious speech, and mania etc | Pharmacopoeia of the People’s Republic of China, 2015 |
*Tsaoko Fructus and the related information in these prescriptions is highlighted in bold. The medicinal material names are referred in China Pharmacopoeia (Chinese Pharmacopoeia Commission 2015). The sources of these medicinal material are listed in Table S1. The mass ratio of the ingredients and the usage of the decoction are indicated in brackets
**Dayuan Yin is one of the classical prescriptions to treat malaria.
# To prepare the decoction, a certain amount medicinal material was precisely weighted and firstly dipped into appropriate volume of water, then boiled to a certain volume. The filtered solution was taken when it was warm
$ The preparation and usage of these prescription can be retrieved from China Pharmacopoeia
In most of the prescriptions, Tsaoko Fructus acts as the main medicine due to its efficacy on invigorating the spleen and stomach, promoting qi to disperse stagnation, and eliminating pathogens (Gao and Wang 2007). For instance, Dayuan Yin is the classic prescription formulated by Wu Youke in the Ming dynasty to treat malaria (Fang and Yue 2021). In this prescription, Tsaoko Fructus acts as one of the “minister” medicines to cooperate with Arecae Semen, the “monarch”, and Magnoliae Officinalis Cortex, the other “minister”, to eliminate the pathogens between interior and exterior, according to the “monarch, minister, assistant, and guide” formula theory of TCM (Fang and Yue 2021; Li 2021). The other four ingredients of the prescription, Anemarrhenae Rhizoma, Paeoniae Radix Alba, Scutellariae Radix, and Glycyrrhizae Radix Et Rhizoma do not directly clear away the pathogens but serve as reconciliation agents, the “assistant” and the “guide”, to recover the balance of the body (Fang and Yue 2021; Li 2021). To prepare this decoction, a total of 21 g of the medicinal materials at a ratio of 4:2:1:2:2:2:1 are firstly dipped in 200 ml water and then boiled to about 160 ml. Then all the debris are discarded and the filtered solution is taken as medicine once a day.
Recently, Dayuan Yin has been used to prevent and treat SARS and the COVID-19, two epidemic diseases that cause severe damage to the respiratory system. When it was used to treat 112 confirmed SARS cases during 2003, more than 93.7% of patients had experienced noticeable symptom relief and recovery (Ren et al. 2021). When used for mild and common cases of COVID-19 combined with antiviral drugs, it could relieve symptoms of cough, asthma, and dry throat, improve prognosis of COVID-19 patients, and shorten disease progression (Ren et al. 2021). The volatile oil of Tsaoko Fructus was speculated as one of the effective ingredients to treat these epidemic diseases due to their anti-inflammatory and antibacterial properties (Zhang et al. 2020).
Botany of A. tsao-ko
A. tsao-ko is of forest understory habitat in the tropical and subtropical regions (Fig. 1a) and mainly distributes in the southwestern of China including Yunnan, Guangxi, and Guizhou provinces, and the northern Vietnam. A. tsao-ko herb typically grows about 2–2.5 m high. Its leaves are green, smooth, slightly sharp, and oval-shaped, approximately 40–70 cm in length and about 10–20 cm in width (Fig. 1a). Its anthotaxy is spica, and the yellow or white flowers are serried inserted on the thick rachis (Fig. 1b). Its fruits are oval-shaped red capsules, densely packed together when fresh (Fig. 1c). The dried ripe capsules are roughly 2.5–4.5 cm in length, the pericarps of which are grayish-brown to brown with longitudinal furrows and ribs without hair or spikes (Fig. 1d). The seeds grow in clusters in the capsule, generally divided into three parts by rows, and are wrapped by pulp. Seeds are conical polyhedral, reddish-brown, covered with grayish-white membranous aril (Wu et al. 2014). The ripe capsules are harvested before crack usually during September to November when becoming grey to brown. The harvested capsules are dried into brown Tsaoko Fructus (Fig. 1d) in the sun or in a thermostat.
Fig. 1.
Amomum tsao-ko plant and its growing environment, flowers, and fruits. a A. tsao-ko plant and its growing environment. b flowers. c fresh fruits. d dried ripe fruits
A. tsao-ko is a cultivated herb. It has different cultivated populations that have varied phenotypes. According to the morphological characteristics, for example, the shape of the capsules, A. tsao-ko has at least five cultivars, the spheroidal-, the near spheroidal-, the spindle-, the ellipsoid-, and the cone-shaped fruit groups (Fig. 1d) (Zhang et al. 2011; Lu et al. 2019; Wei et al. 2019). Tsaoko Fructus of different shape have different chemical profiles. Taken A. tsao-ko cultivated in Xishuangbanna as an example, the ellipsoid shape Tsaoko Fructus contained 3.55 mL/100 g of essential oil (EO) with 20.33% of geraniol (5), the spindle-shaped ones had 2.75 mL/100 g of EO with 14.40% of geraniol (5), and the spheroidal-, the near spheroidal-shaped ones had 4.00 mL/100 g of EO with 17.86% of geraniol (5) and 3.33 mL/100 g of EO with 16.87% of geraniol (5), respectively (Ma et al. 2008). The varied phenotypes suggest that A. tsao-ko has morphologic and genetic diversity among populations.
Genetic diversity assay based on phenotypic traits revealed that A. tsao-ko cultivars clustered into a number of large groups and sub-groups, indicating A. tsao-ko germplasm has high genetic diversity (Yan 2012; Yang et al. 2014; Ma et al. 2017b, a, 2020; Hu et al. 2018a, 2019a, b; Xie et al. 2018; Lu et al. 2019; Ma and Lu 2020). As mentioned above, the concentration of EO varied in different shape fruits (Ma et al. 2008), which indicated that the chemical profiles may be related to morphological characteristics such as fruit forms. Sim et al. (2019) also found that A. tsao-ko fruits distilled for EO A showed a more conical shape, while pods used for EO B had an elliptic form. The composition of main ingredients in EO A/B had significant variations in concentration, e.g., eucalyptol (10), 4-indanecarbaldehyde (16), and (2E)-decenal (200) in EO A/B was 28.1%/22.6%, 4.3%/2.3%, and 3.0%/6.1%, respectively (Sim et al. 2019). However, the relationship between the morphological characteristics such as fruit forms and the chemical profiles, especially the characteristics of active ingredients, is still unclear. Such work is important for selecting high quality Tsaoko Fructus germplasm resources, and worth exploring in the future. There are 23 novel microsatellite markers found in A. tsao-ko (Lu et al. 2021) and several site variations in matK, psbA-trnH and ycf1 sequences of A. tsao-ko cultivars (Hu et al. 2019b). These DNA markers may help develop molecular tools for the germplasm characterization, and the selection and breeding of good germplasm A. tsao-ko.
Some Zingiberacea plants have similar capsules to that of A. tsao-ko and also have overlaps in the distribution. As a result, they are often mixed up with A. tsao-ko and incorporated into Tsaoko Fructus containing medicines (Shi et al. 2013), thus adding difficulty to its regular use as medicinal material. The commonly confused species include other Amomum genera such as A. paratsao-ko, A. Koenigii, A. kravanh, A. subulatum, and A. xanthoides, the Alpinia genera such as A. galanga, A. katsumadai, and A. zerumbet, and Elettaria Maton specie like E. cardamomum (Shi et al. 2013; Wu et al. 2014). The species of Zingiberaceae was usually identified by the seed and fruit features, according to the macroscopic morphological characteristics and the microscopic features of their seeds and fruits (Table S2, Table S3) (Shi et al. 2013; Wu et al. 2014). The chemical profiles of EO of A. tsao-ko fruits are different from that of other species like A. paratsaoko. For example, the dried fruit of A. tsao-ko is rich in 1,8-cineole (10) and citral, which are 19.50% and 14.95%, respectively, whereas the dried fruit of A. paratsaoko only contains 0.25% of 1,8-cineole and undetectable citral (Huang et al. 2014). Thus, chemical profiles can also be used to distinguish Tsaoko Fructus from other easily-confused species.
In these years, DNA molecule labeling technology has been explored to study the genetic property of A. tsao-ko and the related plant species. The Internal Transcribed Spacer (ITS), Random Amplified Polymorphic DNA Markers (RAPD), Simple Sequence Repeat (SSR) or Microsatellite sequence (MS), and complete chloroplast genome of A. Tsaoko have been explored (Yan 2012; Yang et al. 2014; Ma et al. 2017b, a, 2020; Hu et al. 2018a, 2019a, b; Xie et al. 2018; Lu et al. 2019, 2021; Ma and Lu 2020). DNA barcoding sequence analysis revealed that ITS, matK, psbA-trnH and ycf1 could accurately distinguish A. tsao-ko from 18 other Amomum genus (Hu et al. 2019b). Chloroplast genome was also workable, as revealed by phylogenetic analysis using complete chloroplast genome of A. tsao-ko and 16 other related species (Ma and Lu 2020).
Phytochemistry
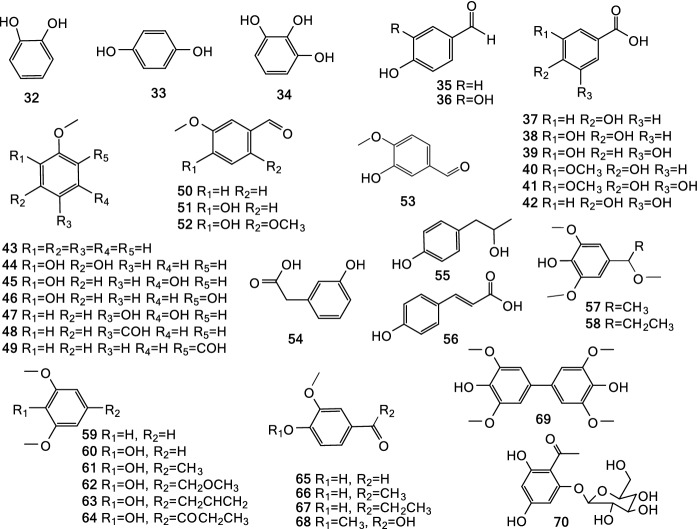
Phytochemicals are the medicinal basis substances of medicinal plants, as well as an important reservoir for candidate drug development. To date, more than 300 compounds have been detected in Tsaoko Fructus (Tables 2, S4), at least 209 of which have been isolated and identified (Table 2, Figs. 2, 3, 4, 5, 6, 7, 8). According to the characteristics of core structure, these compounds can be classified as terpenoids, phenylpropanoids, organic acids, and other compounds (Table 2). Overall, there are 32 terpenoids (1–31), 157 phenylpropanoids (32–188), 19 organic acids (189–208), and one pyrrole (209). Since Tsaoko Fructus has an aromatic and spicy odor, its volatile oil, also called essential oil (EO), has attracted much attention (Yang et al. 2008; Feng et al. 2010; Min et al. 2010; He et al. 2013; Cui et al. 2017; Sim et al. 2019). A. tsao ko EO contains terpenoids, phenolic acids, and organic acids.
Table 2.
Isolated and identified compounds from A. tsao-ko fruits
| Compd. no. | Chemical name | Molecular formula | References |
|---|---|---|---|
| Terpenoids | |||
| Monoterpene hydrocarbons | |||
| 1 | Limonene | C10H16 | Wang et al. (2014) |
| Oxygenated monoterpenes | |||
| 2 | Myrcenol | C10H18O | Wang et al. (2014) |
| 3 | 8-hydroxy-2,6-dimethyl-1,6-octadien-3-one | C10H16O2 | Lee et al. (2019) |
| 4 | (2E,6E)-8-(acetyloxy)-2,6-dimethyl-2,6-octadienal | C12H18O3 | Lee et al. (2019) |
| 5 | Geraniol | C10H18O | Dai et al. (2016a) |
| 6 | Geraniol acetate | C12H20O2 | Yang et al. (2009) |
| 7 | (2E,6E)-8-hydroxy-2,6-dimethyl-2,6-octadienal acetate | C12H18O3 | Yang et al. (2009) |
| 8 | (2E,6E)-8-hydroxy-2,6-dimethyl-2,6-octadienal | C10H16O2 | Yang et al. (2009) |
| 9 | 8-oxogeraniol | C10H16O2 | Lee et al. (2008) |
| 10 | 1,8-cineole (eucalyptol) | C10H18O | Wang et al. (2014), Dai et al. (2016b) |
| 11 | p-menth-1-ene-5,6-diol | C10H18O2 | Lee et al. (2008) |
| 12 | 3α-hydroxycarvotagenone | C10H16O2 | Lee et al. (2008) |
| 13 | Tsaokoin | C10H14O2 | Moon et al. (2004), Yang et al. (2009), Kim et al. (2019b) |
| 14 | Isotsaokoin | C10H14O2 | Moon et al. (2004) |
| 15 | 5-indanecarbaldehyde (5-Indancarboxaldehyde)* | C10H10O | Jin et al. (2013), Sim et al. (2019) |
| 16 | 4-indanecarbaldehyde* | C10H10O | Jin et al. (2013), Wang et al. (2014), Dai et al. (2016b), Sim et al. (2019) |
| 17 | 6-hydroxyindan-4-carbaldehyde (6-hydroxy-4-aldehydeindene) | C10H10O2 | Lee et al. (2008), Yang et al. (2009), Jin et al. (2013) |
| 18 | 6,7-dihydroxy-indan-4-carbaldehyde | C10H10O3 | Lee et al. (2008), Jin et al. (2013) |
| 19 | (1RS,5SR,6RS)-5-hydroxybicyclo[4.3.0]non-2-ene-2-carbaldehyde | C10H14O2 | Yang et al. (2009) |
| 20 | trans-2,3,3a,7a-tetrahydro-1H-indene-4-carbaldehyde (trans-dihydroindane-4-carboxylaldehyde)* | C10H10O | Starkenmann et al. (2007), Sim et al. (2019) |
| 21 | trans-2,3,3a,7a-tetrahydro-1H-indene-5-carbaldehyde | C10H10O | Sim et al. (2019) |
| 22 | cis-2,3,3a,7a-tetrahydro-1H-indene-4-carbaldehyde (cis-dihydroindane-4-carboxylaldehyde)* | C10H10O | Starkenmann et al. (2007), Sim et al. (2019) |
| 23 | cis-2,3,3a,7a-tetrahydro-1H-indene-5-carbaldehyde | C10H10O | Sim et al. (2019) |
| Sesquiterpenoids | |||
| 24 | (3S,6E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol (trans-nerolidol) | C15H26O | Hong et al. (2015), Lee et al. (2019) |
| Diterpenoids | |||
| 25 | Coronadiene | C17H26O2 | Liu et al. (2018) |
| 26 | (3E)-4-[(1S,4aS,8aS)-decahydro-5,5,8a-trimethyl-2-methylene-1-naphthalenyl]-3-buten-2-one | C18H28O | Lee et al. (2019) |
| 27 | Amotsaokonal A | C20H30O | Hong et al. (2015) |
| 28 | Amotsaokonal B | C20H32O | Hong et al. (2015) |
| 29 | Amotsaokonal C | C20H32O | Hong et al. (2015) |
| Steroids | |||
| 30 | β-sitosterol | C29H50O | Martin et al. (2000), Zhang et al. (2014) |
| 31 | β-sitosterol-3-O-glucoside (daucosterol) | C35H60O6 | Martin et al. (2000), He et al. (2020c) |
| Phenylpropanoids | |||
| Phenolic acids | |||
| 32 | Catechol | C6H6O2 | Wang et al. (2009), Jin et al. (2013), Zhang et al. (2014) |
| 33 | Hydroquinone | C6H6O2 | Jin et al. (2013) |
| 34 | Pyrogallic acid | C6H6O3 | Wang et al. (2009) |
| 35 | 4-hydroxy-benzaldehyde | C7H6O2 | Yang et al. (2009) |
| 36 | Protocatechualdehyde | C7H6O3 | Martin et al. (2000), Liu et al. (2018), Choi et al. (2018) |
| 37 | p-hydroxybenzoic acid | C7H6O3 | Martin et al. (2000), Wang et al. (2009) |
| 38 | Protocatechuic acid | C7H6O4 | Martin et al. (2000), Wang et al. (2009) |
| 39 | 3,5-dihydroxybenzoic acid | C7H6O4 | Jin et al. (2013) |
| 40 | Vanillic acid (4-hydroxy-3-methoxy-benzoic acid) | C8H8O4 | Martin et al. (2000), Wang et al. (2009), Liu et al. (2018), Choi et al. (2018) |
| 41 | 3-O-methylgallic acid | C8H8O5 | Liu et al. (2018) |
| 42 | 3,4-dihydroxybenzoic acid | C7H6O4 | Liu et al. (2018) |
| 43 | Anisole | C7H8O | Jin et al. (2013) |
| 44 | 3-methoxy-catechol | C7H8O3 | Jin et al. (2013) |
| 45 | 2-methoxy-hydroquinone | C7H8O3 | Jin et al. (2013) |
| 46 | 2-methoxy-resorcinol | C7H8O3 | Jin et al. (2013) |
| 47 | 4-methoxy-catechol | C7H8O3 | Jin et al. (2013) |
| 48 | 4-methoxybenzaldehyde | C8H8O2 | Jin et al. (2013) |
| 49 | 2-methoxy-benzaldehyde | C8H8O2 | Jin et al. (2013) |
| 50 | 3-methoxy-benzaldehyde | C8H8O2 | Jin et al. (2013) |
| 51 | 3-methoxy-4-hydroxy-benzaldehyde | C8H8O3 | Jin et al. (2013) |
| 52 | 4-hydroxy-2,5- dimethoxy-benzaldehyde | C9H10O4 | Jin et al. (2013) |
| 53 | 4-methoxy-3-hydroxy-benzaldehyde | C8H8O3 | Yang et al. (2009), Jin et al. (2013) |
| 54 | 3-hydroxybenzoic acid | C8H8O3 | Jin et al. (2013) |
| 55 | 4-(2-hydroxypropyl)phenol | C9H12O2 | Jin et al. (2013) |
| 56 | (E)-p-coumaric acid | C9H8O3 | Liu et al. (2018), Choi et al. (2018) |
| 57 | 2,6-dimethoxy-4-[(1R)-1-methoxyethyl]-phenol | C11H16O4 | Lee et al. (2019) |
| 58 | 2,6-dimethoxy-4-[(1R)-1-methoxypropyl]-phenol | C12H18O4 | Lee et al. (2019) |
| 59 | 1,3-dimethoxybenzene | C8H10O2 | Jin et al. (2013) |
| 60 | 2,6-dimethoxy-phenol | C8H10O3 | Lee et al. (2019) |
| 61 | 2,6-dimethoxy-4-methyl-phenol | C9H12O3 | Lee et al. (2019) |
| 62 | 2,6-dimethoxy-4-(methoxymethyl)-phenol | C10H14O4 | Lee et al. (2019) |
| 63 | 2,6-dimethoxy-4-(2-propen-1-yl)-phenol | C10H14O4 | Lee et al. (2019) |
| 64 | 1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone | C11H14O4 | Lee et al. (2019) |
| 65 | 4-hydroxy-3-methoxy-benzaldehyde | C8H8O3 | Lee et al. (2019) |
| 66 | 1-(4-hydroxy-3-methoxyphenyl)-ethanone | C9H10O3 | Lee et al. (2019) |
| 67 | 1-(4-hydroxy-3-methoxyphenyl)-1-propanone | C10H12O3 | Lee et al. (2019) |
| 68 | 3,4-dimethoxy-benzoic acid | C9H10O4 | Lee et al. (2019) |
| 69 | 3,3’,5,5’-tetramethoxy-[1,1’-biphenyl]-4,4’-diol | C16H18O6 | Lee et al. (2019) |
| 70 | Myrciaphenone A | C14H18O9 | (Choi et al. 2018) |
| Flavonoids | |||
| 71 | ( +)-afzelechin | C15H14O5 | He et al. (2021) |
| 72 | 8-aldehyde-catechin | C16H14O7 | He et al. (2021) |
| 73 | (-)-catechin | C15H14O6 | Martin et al. (2000), Jin et al. (2013), Choi et al. (2018), He et al. (2021) |
| 74 | (-)-epi-afzelechin | C15H14O5 | He et al. (2021) |
| 75 | ( +)-epicatechin | C15H14O6 | Martin et al. (2000), Zhang et al. (2014), Choi et al. (2018), He et al. (2021) |
| 76 | (2R,3R,4R)-3',5'-dimethoxy-3,4,7,4'-tetrahydroxy-flavan | C17H18O7 | Jin et al. (2013) |
| 77 | Quercetin | C15H10O7 | Zhang et al. (2014) |
| 78 | quercetin-3-O-β-D-glucopyranoside | C21H20O12 | Wang et al. (2009), Zhang et al. 2014), Rahman et al. 2017) |
| 79 | quercetin-7-O-β-glucoside | C21H20O12 | Zhang et al. (2014), Rahman et al. (2017) |
| 80 | Rutin | C27H30O16 | Wang et al. (2009), Dai and Peng 2011) |
| 81 | 3',7-dihydroxy-4'-methoxy-flavan | C16H16O4 | Jin et al. (2013) |
| 82 | Abyssinoflavanone VII | C25H28O6 | Jin et al. (2013) |
| 83 | Alpinetin | C16H14O4 | Kim et al. (2019b) |
| 84 | Naringenin-5-O-methyl ether | C16H14O5 | Kim et al. (2019b) |
| 85 | Naringenin | C15H12O5 | Kim et al. (2019b) |
| 86 | Hesperetin | C16H14O6 | Kim et al. (2019b) |
| 87 | 4',7-dihydroxy-3',6-diprenylflavone | C25H26O4 | Jin et al. (2013) |
| 88–91 | Geranylated pyranoflavanones | - | Kim et al. (2019a) |
| 92–95 | Farnesylated pyranoflavanones | - | Kim et al. (2019a) |
| 96 | 4'-hydroxy-2'-methoxychalcone | C16H14O3 | Jin et al. (2013) |
| 97 | 4-hydroxy-4'-methoxychalcone | C16H14O3 | Jin et al. (2013) |
| 98 | 2',4',6'-trihydroxy-4-methoxy chalcone | C16H14O5 | Kim et al. (2019b) |
| 99 | 4-hydroxy-2'-methoxychalcone | C16H14O3 | Jin et al. (2013) |
| 100 | 4'-hydroxy-4-methoxychalcone | C16H14O3 | Jin et al. (2013) |
| 101 | 2',4'-dihydroxy-4-methoxy-chalcone | C16H14O4 | Jin et al. (2013) |
| 102 | 4,4'-dimethoxychalcone | C17H16O3 | Jin et al. (2013) |
| 103 | 2',4,4'-trimethoxychalcone | C18H18O4 | Jin et al. (2013) |
| 104 | Boesenbergin B | C26H28O4 | Kim et al. (2019b) |
| 105 | 4-hydroxyboesenbergin B | C26H28O5 | Kim et al. (2019b) |
| 106,107 | Farnesylated pyranochalcones | - | Kim et al. (2019a) |
| 108 | 3’,5’-di-C-β-D-glucopyranosylphloretin | C21H26O9 | Wang et al. (2009), Hussain et al. (2018) |
| 109 | 2-(4-hydroxy-3-methoxybenzoyl)-4-methoxy-benzaldehyde | C15H14O5 | Jin et al. (2013) |
| 110 | Flavanocoumarin | C18H14O7 | He et al. (2021) |
| 111 | Sappanone B | C16H14O6 | He et al. (2021) |
| 112 | Brazilin | C16H14O5 | He et al. (2021) |
| 113 | Epi-catechin-(4β → 8,2β → O → 7)-epi-afzelechin | C30H24O11 | He et al. (2021) |
| 114 | Proanthocyanidin A-2 | C30H24O12 | He et al. (2021) |
| Flavanol-menthane conjugates | |||
| 115 | Amomutsaokin A | C25H28O6 | He et al. (2021) |
| 116 | Amomutsaokin B | C25H28O6 | He et al. (2021) |
| 117 | Amomutsaokin C | C25H28O6 | He et al. (2021) |
| 118 | Amomutsaokin D | C25H28O6 | He et al. (2021) |
| 119 | Amomutsaokin E | C25H30O7 | He et al. (2021) |
| 120 | Amomutsaokin F | C25H30O7 | He et al. (2021) |
| 121 | Amomutsaokin G | C25H30O7 | He et al. (2021) |
| 122 | Amomutsaokin H | C25H30O7 | He et al. (2021) |
| Flavanol-fatty alcohol hybrids | |||
| 123 | Tsaokoflavanol A | C23H28O7 | He et al. (2020a) |
| 124 | Tsaokoflavanol B | C25H32O7 | He et al. (2020a) |
| 125 | Tsaokoflavanol C | C25H32O7 | He et al. (2020a) |
| 126 | Tsaokoflavanol D | C25H30O8 | He et al. (2020a) |
| 127 | Tsaokoflavanol E | C27H32O9 | He et al. (2020a) |
| 128 | Tsaokoflavanol F | C27H36O7 | He et al. (2020a) |
| 129 | Tsaokoflavanol G | C23H28O7 | He et al. (2020a) |
| 130 | Tsaokoflavanol H | C23H28O7 | He et al. (2020a) |
| 131 | Tsaokoflavanol I | C23H28O7 | He et al. (2020a) |
| 132 | Tsaokoflavanol J | C25H32O7 | He et al. (2020a) |
| 133 | Tsaokoflavanol K | C25H32O7 | He et al. (2020a) |
| 134 | Tsaokoflavanol L | C25H32O7 | He et al. (2020a) |
| 135 | Tsaokoflavanol M | C25H32O7 | He et al. (2020a) |
| 136 | Tsaokoflavanol N | C25H30O8 | He et al. (2020a) |
| 137 | Tsaokoflavanol O | C25H30O8 | He et al. (2020a) |
| 138 | Tsaokoflavanol P | C27H32O9 | He et al. (2020a) |
| 139 | Tsaokoflavanol Q | C27H32O9 | He et al. (2020a) |
| 140 | Tsaokoflavanol R | C27H36O7 | He et al. (2020a) |
| 141 | Tsaokoflavanol S | C27H36O7 | He et al. (2020a) |
| Flavanol-monoterpenoid hybrids | |||
| 142 | Tsaokol A | C25H26O7 | He et al. (2020b) |
| 143 | Tsaokol B | C25H26O7 | He et al. (2020b) |
| Diarylheptanoids | |||
| 144 | ( +)-hannokinol | C19H24O4 | Martin et al. (2000), Lee et al. (2008), Liu et al. 2018), Choi et al. 2018) |
| 145 | (3R,5R)-3-acetoxy-5- hydroxy-1,7-bis(4-hydroxyphenyl) heptane | C21H26O5 | He et al. (2020d) |
| 146 | (3R,5R)-3,5-dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane | C19H24O5 | He et al. (2020d) |
| 147 | (3R,5R)-3,5-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)heptane | C20H26O5 | He et al. (2020d) |
| 148 | meso-hannokinol | C19H24O4 | Martin et al. (2000), Lee et al. (2008), Zhang et al. (2014), Liu et al. (2018), Choi et al. (2018), He et al. (2020d) |
| 149 | rel-(3R,5S)- 3,5-dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane | C19H24O5 | He et al. (2020d) |
| 150 | (3R,5S)-3,5-dihydroxy-1-(4-hydroxy-3-methox- yphenyl)-7-(4-hydroxy phenyl)heptane | C20H26O5 | He et al. (2020d) |
| 151 | rel-(3R,5S)-3,5-dihydroxy-1-(4-hydroxy-3-methoxy phenyl)-7-(3,4-dihy- droxyphenyl)heptane | C21H26O5 | He et al. (2020d) |
| 152 | (4E,6E)-1,7-bis(4-hydroxyphenyl)hepta-4,6-dien-3-one | C19H18O3 | Zong et al. (2020), He et al. (2020d) |
| 153 | Tsaokoarylone | C20H20O4 | Lee et al. (2008), Yang et al. (2009), Jin et al. (2013), Kim et al. (2019b), He et al. (2020d) |
| 154 | (4E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-4,6-dien-3-one/1,7-bis(4-hydroxy-3-methoxyphenyl)-4,6-hepta-dien-3one | C21H22O5 | Lee et al. (2008), He et al. (2020d) |
| 155 | 1,7-bis(3,4-dihydroxyphenyl)-hepta-4E,6E-dien-3-one | C19H18O5 | Hussain et al. (2018), He et al. (2020d) |
| 156 | 4-[(3S,5E)-3-hydroxy-7-(4-hydroxyphenyl)hept-5-en-1-yl]-2-methoxyphenol | C20H24O4 | He et al. (2020d) |
| 157 | Amomutsaokol G | C19H22O4 | He et al. (2020d) |
| 158 | Amomutsaokol H | C20H24O5 | He et al. (2020d) |
| 159 | Amomutsaokol I | C22H26O5 | He et al. (2020d) |
| 160 |
1,7-bis(4-hydroxyphenyl)-3-hepten-5-one (1,7-bis(4-hydroxyphenyl)-4(E)-hepten-3-one) |
C19H20O3 | Jin et al. (2013), He et al. (2020d) |
| 161 | hannokinin | C19H22O4 | Lee et al. (2008) |
| 162 | Amomutsaokol J | C20H20O4 | He et al. (2020d) |
| 163 | Amomutsaokol K | C20H24O5 | He et al. (2020d) |
| 164 | Amomutsaokol C | C20H22O6 | He et al. (2020d) |
| 165 | Amomutsaokol D | C20H18O6 | He et al. (2020d) |
| 166 | Amomutsaokol E | C20H20O6 | He et al. (2020d) |
| 167 | Amomutsaokol A | C31H34O11 | He et al. (2020d) |
| 168 | Amomutsaokol B | C29H32O6 | He et al. (2020d) |
| 169 | Amomutsaokol F | C22H26O8 | He et al. (2020d) |
| 170 | 2,3-dihydro-2-(4′-hydroxy-phenylethyl)-6-[(3″,4″-dihydroxy-5″-methoxy)phenyl]-4-pyrone | C20H20O6 | Zhang et al. (2015, 2016) |
| 171 | 4-dihydro-2-(4′-hydroxy-phenylmethyl)-6-[(3″,4″-dihydroxy-5″-methoxyphenyl)methylene]-pyran-3,5-dione | C20H18O7 | Zhang et al. (2015, 2016) |
| 172 | Tsaokopyranol A | C28H32O9 | He et al. (2020c) |
| 173 | Tsaokopyranol B | C29H34O10 | He et al. (2020c) |
| 174 | Tsaokopyranol C | C21H26O8 | He et al. (2020c) |
| 175 | Tsaokopyranol D | C22H28O9 | He et al. (2020c) |
| 176 | Tsaokopyranol E | C20H24O6 | He et al. (2020c) |
| 177 | Tsaokopyranol F | C20H24O6 | He et al. (2020c) |
| 178 | Tsaokopyranol G | C21H26O8 | He et al. (2020c) |
| 179 | Tsaokopyranol H | C20H24O5 | He et al. (2020c) |
| 180 | Tsaokopyranol I | C20H24O6 | He et al. (2020c) |
| 181 | Tsaokopyranol J | C21H24O7 | He et al. (2020c) |
| 182 | Tsaokopyranol K | C20H24O6 | He et al. (2020c) |
| 183 | Tsaokopyranol L | C20H22O4 | He et al. (2020c) |
| 184 | (2R,6R)-3,4-dehydro-4'-de-O-methyl centrolobin | C19H22O3 | He et al. (2020c) |
| 185 | Tsaokopyranol M | C20H22O4 | He et al. (2020c) |
| 186 | (2R,6S)-3,4-dehydro-1,7-bis(4-hydroxy phenyl)-4'-de-O-methyl centrolobine | C19H22O3 | He et al. (2020c) |
| 187 | Phaeoheptanoxide | C19H22O5 | He et al. (2020c) |
| 188 | Engelheptanoxides C | C20H24O5 | He et al. (2020c) |
| Phenylethanoid glycosides | |||
| 189 | 2-methoxy-1,4-biphenol-1-O-[6-O-(3-methoxy-4-hydroxybenzoyl)]-β-d-glucopyranoside | C21H24O11 | Wang et al. (2009) |
| Organic acid | |||
| Fatty acids | |||
| 190 | 6,7-dihydroxy-3,7-dimethyloct-2-enoic acid | C10H18O4 | Lee et al. (2008) |
| 191 | (2E,7Z,10Z,13Z)-hexadeca-2,7,10,13-tetraenoic acid | C16H24O2 | Liu et al. (2018) |
| 192 | (2E,7Z)-tetradeca-2,7-dienoic acid | C14H24O2 | Liu et al. (2018) |
| 193 | (E)-dodec-2-enoic acid ((2E)-2-dodecenoic acid) | C12H22O2 | Liu et al. (2018), Lee et al. (2019) |
| 194 | (E)-tetradec-2-enoic acid ((2E)-2-tetradecenoic acid) | C14H26O2 | Liu et al. (2018), Lee et al. (2019) |
| 195 | (11R)-hydroxyhexadeca-(2E,7Z,9E)-trienoic acid | C16H26O3 | Lee et al. (2019) |
| 196 | (9S,10E,12Z)-9-hydroxy-10,12-octadecadienoic acid | C18H32O3 | Lee et al. (2019) |
| 197 | (9S,6Z,10E,12Z)-9-hydroxy-6,10,12-octadecatrienoic acid | C18H30O3 | Lee et al. (2019) |
| 198 | (2E)-dodecenoic acid | C12H22O2 | Hong et al. (2015) |
| Aliphatic aldehydes | |||
| 199 | 2E-decenal | C10H18O | Yang et al. (2009) |
| 200 | Hexadecanal | C16H32O | Yang et al. (2009) |
| 201 | (2E,8E)-10-hydroxy-decadienal | C10H16O2 | Yang et al. (2009) |
| Aliphatic alcohols | |||
| 202 | (E)-2-decen-1-ol/(2E)-decenol | C10H20O | Yang et al. (2009) |
| 203 | 2,8-decadiene-1,10-diol (DDO) | C10H18O2 | Kim et al. (2016) |
| 204 | (2E,8E)-2,8-decadiene-1,10-diol | C10H18O2 | Lee et al. (2019) |
| 205 | 1-acetate 2,8-decadiene-10-ol | C12H20O3 | Lee et al. (2019) |
| 206 | (2E)-1-acetate 2-dodecen-1-ol | C14H26O3 | Lee et al. (2019) |
| Aliphatic esters | |||
| 207 | (2E)-dodecenyl acetate | C14H26O2 | Hong et al. (2015) |
| 208 | 1,10-diacetate-2,8-decadiene-1,10-diol (acetoxytsaokol A) | C14H22O4 | Lee et al. (2019) |
| Other Compounds | |||
| Pyrroles | |||
| 209 | Pyrrole-2-carboxylic acid | C5H5NO2 | Hong et al. (2015) |
The compounds labeled with an asterisk (15, 16, 20, 22) are species-specific components in A. tsao-ko. Those with certificated biological activities are highlighted in bold
Fig. 2.
Terpenoids isolated and identified from A. tsao-ko fruits
Fig. 3.
Phenolic acids isolated and identified from A. tsao-ko fruits
Fig. 4.
Flavonoids isolated and identified from A. tsao-ko fruits
Fig. 5.
Flavanol-menthane, -fatty alcohol, and -monoterpenoid hybrids isolated and identified from A. tsao-ko fruits
Fig. 6.
Diarylheptanoids (144–171) isolated and identified from A. tsao-ko fruits
Fig. 7.
Diarylheptanoids (172–188) and phenylethanoid glycoside (189) isolated and identified from A. tsao-ko fruits
Fig. 8.
Fatty acids, aliphatic ketones, and hydrocarbons isolated and identified from A. tsao-ko fruits
Terpenoids
Terpenoids are abundant in A. tsao-ko EO (Tables 2, S4, Fig. 2). There was one monoterpene hydrocarbon (1), 22 oxygenated monoterpenes (2–23), one sesquiterpenoid (24), five diterpenoids (25–29), and two sterols (30, 31) isolated and identified from A. tsao-ko. All these compounds are present in Tsaoko Fructus. Limonene (1) and 1,8-cineole (10) also exist in A. tsao-ko stems and leaves (Yang 2019).
Among the 23 monoterpenes, 1,8-cineole (eucalyptol, 10) accounts for the highest proportion, taking 34.6%-45.24% (Feng et al. 2010; Cui et al. 2017; Rahman et al. 2017; Gu et al. 2018; Liu et al. 2018; Sun et al. 2018; Sim et al. 2019). Specially, there were four indanecarbaldehydes, 5-indanecarbaldehyde (15), 4-indanecarbaldehyde (16), trans-dihydroindane-4-carboxylaldehydes (20), and cis-dihydroindane-4-carboxylaldehydes (22). These compounds have not been found in any other species, but only in A. tsao-ko from both China and Vietnam, thus could be used as chemical marker of A. tsao-ko species probably regardless of the growing regions (Sim et al. 2019).
Sesquiterpenoids are the condensation products of three isopentenyl pyrophosphate molecules. one linear sesquiterpenoid, (3S,6E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol, also named trans-nerolidol (24), has been isolated from Tsaoko Fructus (Hong et al. 2015; Lee et al. 2019).
The five diterpenoids included two labdane-type trinorditerpenes, namely coronadiene (25) and (3E)-4-[(1S,4aS,8aS)-decahydro-5,5,8a-trimethyl-2-methylene-1-naphthalenyl]-3-buten-2-one (26) (Liu et al. 2018; Lee et al. 2019), two cycloterpenals, namely amotsaokonal B (28) and amotsaokonal C (29), and one benzaldehyde, amotsaokonal A (27) (Hong et al. 2015). It should be noted that amotsaokonal A (27) may be formed through the dehydrogenation of 29 from the perspective of biosynthetic pathway, so it is regarded as diterpenoids here.
There were two steroids isolated from Tsaoko Fructus. They were β-sitosterol (30) and its glycosylation product, β-sitosterol-3-O-glucoside (daucosterol, 31).
Besides the 31 terpenoids mentioned above, there were other 85 terpenoids detected by LC–MS or GC–MS in the extracts of A. tsao-ko fruits (Table S4) (Feng et al. 2010; Hong et al. 2015; Hu et al. 2018b; Sim et al. 2019).
Phenylpropanoids
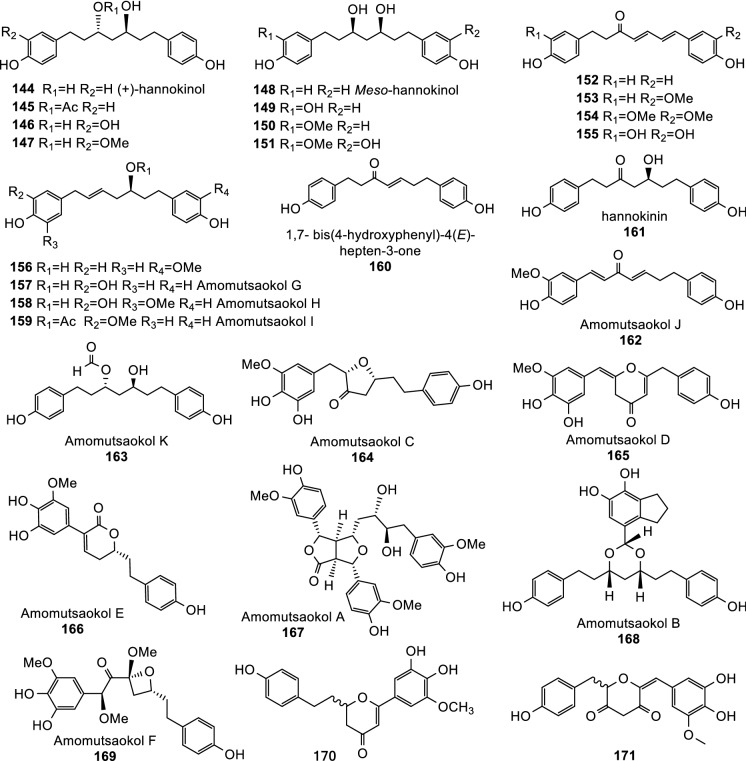
Phenylpropanoids are the large group of secondary metabolites in plants. At least 157 phenylpropanoids have been isolated and identified from Tsaoko Fructus (Table 2). These phenylpropanoids included simple phenolic acids (32–70, Fig. 3), typical flavonoids (71–114, Fig. 4), and flavonoid derivatives including flavanol-menthane conjugates (115–122, Fig. 5) (He et al. 2020c), flavanol-fatty alcohol hybrids (123–141, Fig. 5) (He et al. 2020a), flavanol-monoterpenoid hybrids (142, 143, Fig. 5) (He et al. 2020b), diarylheptanoids (144–188, Fig. 6, Fig. 7), and phenylethanoid glycoside (189, Fig. 7). Six other phenolic acids have also been detected but not separated from Tsaoko Fructus (Table S4).
Out of the phenolic acids from Tsaoko Fructus, 40 are simple phenolic acids that have one aromatic ring with hydroxyl-, aldehyde-, carbonyl-, methoxy-, or carboxyl-groups attached to it (32–70, Fig. 3).
The flavonoids (71–114) isolated from Tsaoko Fructus included flavan-3-ols (71–75), flavan-3,4-diol (76), flavonols and their corresponding glycosylated derivatives (77–80), flavan (81), flavanones (82–95), chalcones (96–107), dihydrochalcone (108), and flavanol conjugates with other groups such as flavanocoumarin (110), epi-catechin-(4β → 8,2β → O → 7)-epi-afzelechin (113), proanthocyanidin A-2 (114) (He et al. 2021). Other flavonoid derivatives like sappanone B (112), brazilin (113), flavanol-menthane conjugates (115–122), flavanol-fatty alcohol hybrids (123–141), and flavanol-monoterpenoid hybrids (142, 143) were also reported (He et al. 2020a, b, 2021) (Fig. 4, Fig. 5). In particular, there were nine flavonoids that were geranylated or farnesylated at the A ring of the skeleton, including geranylated pyranoflavanones (88–91), farnesylated pyranoflavanones (92–95), and farnesylated pyranochalcones (106, 107) (Kim et al. 2019a).
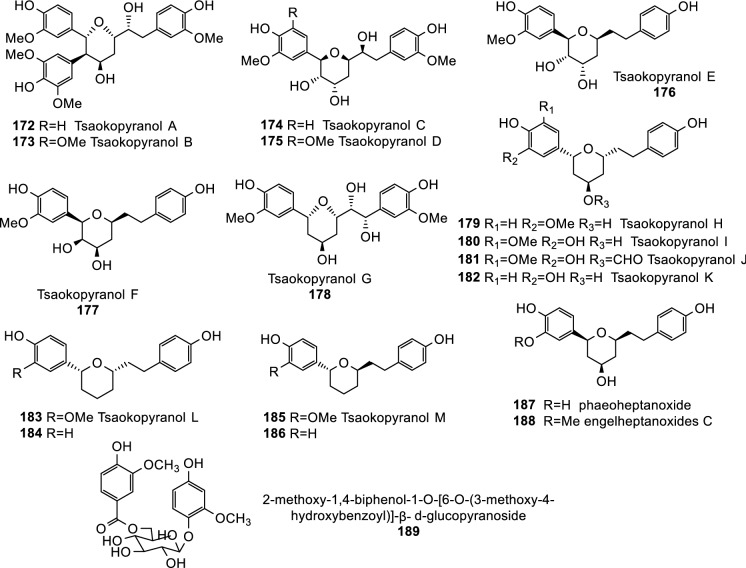
Diarylheptanoids are another type phenylpropanoids, which are characterized with a 1,7-diphenylheptane core Lee et al. (2008; He et al. 2020c, d). These compounds are widespread in Zingiberaceae. Twenty linear diarylheptanoids (144–163; Fig. 6) and 25 cyclic diarylheptanoids (164–188; Fig. 6, Fig. 7) have been isolated and identified from Tsaoko Fructus Lee et al. (2008; He et al. 2020c, d).
One phenylethanoid glycoside, 2-methoxy-1,4-biphenol-1-O-[6-O-(3-methoxy-4-hydroxybenzoyl)]-β-d-glucopyranoside (189), has also been identified from the dried fruits of A. tsao-ko(Wang et al. 2009).
Organic acids
Organic acids from Tsaoko Fructus included fatty acids (190–198), aliphatic aldehydes (199–201), aliphatic alcohols (202–206), and aliphatic esters (207–208) (Fig. 8)(Yang et al. 2008; Min et al. 2010; He et al. 2013; Hu et al. 2018b; Liu et al. 2018; Lee et al. 2019; Sim et al. 2019; Xu et al. 2019). These compounds were also rich in A. tsao-ko EO (Li et al. 1998; Ma et al. 2008). For example, 2E-decenal (199) accounts for 3.41%-10.92% of A. Tsaoko EO (Ma et al. 2008). In addition, 49 other organic acids were also detected in A. tsao-ko EO (Table S4).
Other compounds
Besides the main constituents of Tsaoko Fructus listed above, pyrrole-2-carboxylic acid (209, Table 2, Fig. 8) (Hong et al. 2015), alicyclic compounds, furan compounds, and heterocyclic compound were also reported (Table S4).
Pharmacological and biological activities
Biological activities of EO, extracts, and isolated compounds of A. tsao-ko fruits have been investigated by several research groups. Most of the bioactivities were evaluated by in vitro experiments, including antibiotic, anti-tumor and anti-cancer, anti-inflammatory, anti-diabetes, neuroprotective, plasma and liver triacylglycerol decreasing activities (Table 3).
Table 3.
The pharmacological activities of extract, essential oil, and isolated compounds of A. tsao-ko fruits*
| Activity | Extract/EO/ compound | Measure of activity | Positive control and activity | Cell line/strain/Model | Method | References |
|---|---|---|---|---|---|---|
| Antibiotic activity | EtOH Ex | MIC: 1, 2, and 2 mg/mL for S. aureus, S. Typhimurium, and P. aeruginosa, respectively |
Vanillin: MIC: 250 μg/mL |
Staphylococcus aureus ATCC 6538 Salmonella Typhimurium ATCC 50,013 Pseudomonas aeruginosa ATCC 9027 |
Agar disk diffusion method Flask incubation assay |
Rahman et al. (2017) |
|
EtOH Ex., EtOAc Ex., 25, 40–42, 191–194 |
MIC: 5, 5, 5, 5, 10, 10, 10, 50, 50, 50 μg/mL, respectively |
Chloramphenicol: MIC: 5 μg/mL |
Klebsiella pneumoniae | Broth-dilution method | Liu et al. (2018) | |
| EO |
In vitro: MIC: 22.49 to 1438.91 μg/mL In vivo: 0.92 g/kg/d (intramuscularly): 100.00% survival rate for S. aureus and E. coli infected mice 1.84 g/kg/d (intragastrically): 100.00% and 70% survival rates for S. aureus and E. coli infected mice, respectively |
Cefradine for mice infected with S. aureus Cefminox for mice infected with E. coli |
Reference strains: E. coli ATCC 25922, S. aureus ATCC 25923, Pseudomonas aeruginosa NCTC 10662, E. coli CMCCB 44102, S. aureus CMCCB 26003, S. pneumoniae ATCC 49619 Clinical isolated strains: 85 S. aureus, E. coli, P. aeruginosa, Proteus vulgaris, Shigella flexneri, Enterobacter cloacae Mouse peritonitis model: Infected with S. aureus or E. coli |
Agar dilution method In vivo anti-infectious efficacy |
Dai et al. (2016b) | |
| EO |
MLC = 44.97 μg/mL; IC50 = 22.49 μg /mL for T. vaginalis isolate Tv1; MLC = 89.93 μg/mL; IC50 = 44.97 μg/mL for Tv2 |
Metronidazole: MLC = 4.88 μg/mL; IC50 = 2.44 μg /mL for Tv1; MLC = 9.77 μg/mL; IC50 = 4.88 μg /mL for Tv2; Ornidazol: MLC = 2.44 μg/mL; IC50 = 1.22 μg /mL for Tv1; MLC = 4.88 μg/mL; IC50 = 2.44 μg /mL for Tv2; |
Clinically isolated strains: Trichomonas vaginalis isolates Tv1, Tv2 |
Liquid dilution method TEM |
Dai et al. (2016a) | |
| 5 | MLC = 342.96 μg/mL; IC50 = 171.48 μg/mL for both Tv1 and Tv2 | |||||
| 14 | The inhibition zone was 1.5 and 2.0 mm at 20 and 40 µg/disk, respectively |
Amphotericin B: The inhibition zone was 2.5 and 3.0 mm at 20 and 40 µg/disk, respectively |
Trycophyton mentagrophytes KCTC 6085 | Paper-disk agar diffusion method | Moon et al. (2004) | |
| EO |
LD50 = 16.52 μg/adult; LC50 = 5.85 mg/L air against T. castaneum LD50 = 6.14 μg/adult; LC50 = 8.70 mg/L air against L. serricorne |
Pyrethrins: LD50 = 0.26 μg/adult against T. castaneum Pyrethrins: LD50 = 0.24 μg/adult against L. serricorne Methyl bromide: LC50 = 1.75 mg/L air against T. castaneum Phosphine: LC50 = 9.23 × 10–3 mg/L air against L. serricorne |
Tribolium castaneum (Herbst) Lasioderma serricorne (Fabricius) |
Contact toxicity bioassay Fumigant toxicity bioassay |
Wang et al. (2014) | |
| 1 |
LD50 = 14.97 μg/adult; LC50 = 6.21 mg/L air against T. castaneum LD50 = 13.66 μg/adult; LC50 = 14.07 mg/L air against L. serricorne |
|||||
| 10 |
LD50 = 18.83 μg/adult; LC50 = 5.47 mg/L air against T. castaneum LD50 = 15.58 μg/adult; LC50 = 5.18 mg/L air against L. serricorne |
|||||
|
Anti-inflammatory activity |
EtOH Ex. (seeds) |
No cytotoxic effect below 400 μg/mL IC50 = 194.92 μg/mL for iNOS IC50 = 151.00 μg/mL for COX-2 |
- | LPS-induced RAW264.7 macrophages |
MTT assay, Nitrite assay using the Griess reaction ELISA, Western Blot, Immunofluorescence Microscopy |
(Li et al. 2014) |
| 203 |
No cytotoxic effect on up to 300 μM IC50 = 136.66 μM for NO production Inhibition on iNOS, COX-2, IL-6, NF-κB, and MAKPs at 200 μM, and TNF-α at 100 μM |
NG-methyl-L-arginine (L-NMMA, 100 μM) | LPS-induced RAW264.7 macrophages | MTT assay, Nitrite assay using the Griess reaction ELISA, Western blot, Immunofluorescence Microscopy | Kim et al. (2016) | |
| EtOH Ex | IC50 = 59.5 μg/mL for NO production and MTT > 100% | N-Monomethyl-L-arginine: IC50 = 27.3 μM for NO production and MTT > 100% | LPS-induced RAW264.7 macrophages | MTT assay, Nitrite assay using the Griess reaction ELISA, Western blot | (Choi et al. 2018) | |
|
73 75 |
IC50 = 73.32 μM for NO production and MTT > 100% IC50 = 70.57 μM for NO production and MTT > 100% |
N-Monomethyl-L-arginine: IC50 = 25.29 μM for NO production and MTT > 100% | ||||
| 75 | Inhibition on iNOS, TNF-α, IL-1β, IL-10 at 25–100 μM |
Dexa: Inhibition on iNOS, TNF-α, IL-1β, IL-10 at 10 μM |
||||
| 170, 171 |
Cell viability: 80.34% and 69.82% at 50 μg/mL, respectively NO inhibition: 60.46% and 48.62% at 100 µg/mL, respectively |
Vitamin C: Cell viability: 84.80% at 50 μg/mL |
LPS-stimulated macrophage RAW 264.7 cells H2O2-treated PC-12 cells |
MTT assay, Nitrite assay using the Griess reaction | (Zhang et al. 2016) | |
| 9, 11, 13, 14, 17, 18, 144, 148, 153, 154, 161, 190 | 68.8% to 1.1% NO inhibition at a concentration ranging from 1 μM to 100 μM | NAME (ω-nitro-L-arginine methyl ester): 58.5% to 11.2% for NO inhibition at a concentration ranging from 1 μM to 100 μM | LPS-induced BV2 microglial cells | MTT assay, Nitrite assay using the Griess reaction | Lee et al. (2008) | |
| 98, 104, 105, 153 | IC50 = 10.9 to 22.5 µM |
Aminoguanidine: IC50 = 21.4 µM |
LPS-induced RAW 264.7 macrophages | MTT assay, Nitrite assay using the Griess reaction | Kim et al. (2019b) | |
| 88–95, 106, 107 | IC50 = 10.6 to 41.5 μM |
Aminoguanidine: IC50 = 21.7 µM |
LPS-induced RAW 264.7 macrophages | MTT assay, Nitrite assay using the Griess reaction | Kim et al. (2019a) | |
| Anti-tumor and anti-cancer | Ethyl acetate fraction of EtOH Ex |
Inhibition rate at 400 μg/mL: 71.4% against SMMC-7721; About 60% against HepG-2, Hela and A549 |
5-fluorouracil: Inhibition rate at 400 μg/mL: About 70% against SMMC-7721 and A549; About 60% against HepG-2 and Hela |
HepG-2, SMMC-7721, Hela and A549 human cancer cells | MTT assay | Zhang et al. (2015) |
| Petroleum ether fraction of EtOH Ex |
Inhibition rate at 400 μg/mL: About 70% against SMMC-7721 and A549; About 60% against HepG-2 and Hela |
|||||
| 170 | IC50 = 91.23, 89.08, 117.83, 79.77 μg/mL to SMMC-7721, HepG-2, Hela, A549 cells, respectively |
5-fluorouracil: IC50 = 59.83, 73.89, 65.89, and 72.29 μg/mL to SMMC-7721, HepG-2, Hela, and A549 cells, respectively |
||||
| 171 | IC50 = 44.66, 97.18, 71.71, and 80.95 μg/mL to SMMC-7721, HepG-2, Hela, A549 cells, respectively | |||||
| 14 | IC50 = 72.14 μg/mL to Hela cells | |||||
| Hexane fraction of EtOH Ex. (seeds) | SPHK1 and SPHK2 inhibition: inhibited 39% and 67% of the control, respectively, at 100 μg/mL | - | BV2 microglial cells |
MTT assay SPHK1/2 activity inhibition assay |
Lee et al. (2019) | |
| 61–63, 68, 194, 197, 204–206 |
No significant cell death at 10 μM SPHK1 inhibition: 59.75% (205) to 77.51% of the control |
Resveratrol for SPHK1 inhibition: 85.43% of the control SKI-II for SPHK2 inhibition: 75.35% of the control |
||||
| 3, 57, 58, 65, 193, 204, 205 |
No significant cell death at 10 μM SPHK2 inhibition: 22.75% (3), 25.40% (205) to 58.20% of the control |
|||||
| EO |
IC50 = 31.80 μg/mL (for HepG2)—600 μg/mL (for A549, no obvious cytotoxicity) IC50 = 163.91 and 272.41 μg/mL for HUVEC and HL-7702, respectively |
Mitomycin: IC50 = 5.93 μg/mL for HepG2 IC50 = 2.54 and 16.04 μg/mL for HUVEC and HL-7702, respectively |
Carcinoma cell lines: Human HepG2, Hela, Bel-7402, SGC-7901, PC-3 Normal cell lines: Human HUVEC and HL-7702 |
MTT assay, DNA content and cell cycle analysis | Yang et al. (2010) | |
| Antioxidant activity | Ethyl acetate fraction of 95% EtOH Ex | > 90% DPPH radicals inhibition rate at 200 μg/mL | Vitamin C: > 90% DPPH radicals inhibition rate at 200 μg/mL | HepG-2, SMMC-7721, Hela, and A549 human cancer cells | DPPH radical- scavenging activity assay | Zhang et al. (2015) |
| 171 | About 80% DPPH radical inhibition rate at 100 μg/mL | Vitamin C: > 80% DPPH radicals inhibition rate at 100 μg/mL | ||||
| 40–42, 144, 148, 191, 192 |
MIC: 5, 100, 100, 100, 100, 100, 100 μg/mL; > 80%, > 80%, > 80%, 77.08%, > 70%, > 70%, 60.83% |
Vitamin C: MIC: 5 μg/mL, > 90% |
- | DPPH radical scavenging activity assay | Liu et al. (2018) | |
| 36, 60, 73, 75 | IC50 = 12.55, 12.66, 15.89, 14.39 µM, respectively |
α-Tocopherol: IC50 = 12.57 µM |
- | DPPH radical scavenging activity assay | Martin et al. (2000) | |
| EO (obtained by M-SFME) |
IC50 = 5.27 mg/mL for DPPH assay IC50 = 0.63 mg/mL for β-carotene/linoleic acid bleaching assay |
Vitamin C for DPPH assay: IC50 = 0.046 mg/mL BHT for β-carotene/linoleic acid bleaching assay: IC50 = 0.02 mg/mL |
- |
DPPH radical scavenging activity assay, β-carotene/linoleic acid bleaching assay |
Cui et al. (2017) | |
| EO |
IC50 = 5.12 mg/mL for DPPH assay IC50 = 0.04 = mg/mL for TAB test FRAP = 24.27 µM Fe2+/mg |
L-ascorbic acid for DPPH: IC50 = 2.17 µg/mL BHT for TBA test: IC50 = 0.05 µg/mL L-ascorbic acid: FRAP = 10.33 mM Fe2+/mg |
Human HepG2, Bel-7402, Hela, A549, SGC-7901, and PC-3 cancer cell lines, and HL-7702, HUVEC normal cell lines | DPPH radical scavenging activity assay, TBA test (Lipid peroxidation inhibition assay), FRAP assay | Yang et al. (2010) | |
| Anti-diabetic activity | Aqueous Ex. of A. tsao-ko seeds |
IC50 = 1.04 mg/mL for α-amylase IC50 = 1.4 mg/mL for α-glucosidase |
Acarbose: IC50 = 2.1 mg/mL for α-amylase IC50 = 1.90 mg/mL for α-glucosidase |
- |
α-amylase inhibition assay α-glucosidase inhibition assay |
Hussain et al. (2018) |
| Aqueous Ex. of A. tsao-ko rinds |
IC50 = 1.24 mg/mL for α-amylase IC50 = 2.4 mg/mL for α-glucosidase |
|||||
|
MeX Polar fraction of MeX |
IC50 = 0.02 mg/mL for α-glucosidase No IC50 for α-amylase and lipase Plasma glucose: About 100 mg/dL of polar fraction |
Diet control: Plasma glucose: About 200 mg/dL |
Male mice of the Crlj:CD-1 (ICR) strain |
In vitro: α-amylase, α-glucosidase, and lipase activity assay In vivo: Plasma glucose assay |
Yu et al. (2010) | |
| 50% EtOH Ex | IC50 = 38.6 μg/mL |
Acarbose: IC50 = 219.0 μM |
- | α-glucosidase inhibitory assay | He et al. (2020c) | |
| 176, 179–182, 187 | IC50 = 59.4 to 97.0 μM | |||||
| 172, 173, 175, 177, 188 | IC50 = 100.1 to 179.5 μM | |||||
| 110, 114, 116, 117, 120 | IC50 = 201.45 to 317.51 μM | Suramin sodium: IC50:199.39 μM | - |
PTP1B inhibitory assay TCPTP assay |
He et al. (2021) | |
| 71, 112, 114–117, 119–122 | IC50 = 3.73 to 76.23 μM |
Acarbose: IC50 = 193.77 μM |
- | α-glucosidase inhibitory assay | He et al. (2021) | |
| 123, 124, 128, 133, 140 | IC50 = 5.2 to 9.0 μM, |
Acarbose: IC50 = 180.0 μM |
- | α-glucosidase inhibitory assay | He et al. (2020a) | |
| 128, 132–134, 141 | IC50 = 56.4 to 80.4 μM |
Suramin sodium: IC50 = 200.5 μM |
- |
PTP1B inhibitory assay TCPTP assay |
He et al. (2020a) | |
|
142 143 |
IC50 = 18.8 μM IC50 = 38.6 μM |
Acarbose: IC50 = 213 μM |
- | α-glucosidase inhibitory assay | He et al. (2020b) | |
| Lipid reducing activity |
MeX polar fraction of MeX |
Body lipid: About 12.5% |
Diet control: Body lipid: about 20% |
Male mice of the Crlj:CD-1 (ICR) strain |
Plasma and liver lipid analysis Plasma TBARS concentration assay |
(Yu et al. 2010) |
| 191–194 | MIC: 50 μg/mL, 50.07%; MIC: 50 μg/mL, 61.56%; MIC: 50 μg/mL, 59.37%; MIC: 50 μg/mL, 49.32% |
Orlistat: MIC: 5 μg/mL, 58.78% |
- | Lipase inhibition assay | Liu et al. (2018) | |
| Neuroprotective activity |
170 171 |
80.34% cell viability at 50 µg/mL 69.82% cell viability at 50 µg/mL |
Vitamin C: 84.80% cell viability at 50 µg/mL |
LPS-stimulated macrophage RAW 264.7 cells H2O2-treated PC-12 cells |
MTT assay, Nitrite assay using the Griess reaction | Zhang et al. (2016) |
| 77 |
up to 78.9% cell viability at 50 µg/mL DPPH radical-scavenging activity at 100 µg/mL |
Hydrogen peroxide: > 50% cell viability |
H2O2-treated PC-12 cells |
MTT assay, Nitrite assay using Griess reaction, DPPH radical scavenging activity assay |
Zhang et al. (2014) | |
| Anti-complementary activity |
33 160 |
CH50: 0.55 mM; AP50: 0.53 mM CH50: 0.42 mM; AP50: 0.66 mM |
Heparin: CH50: 40 mM; AP50: 97 mM |
Sheep erythrocytes | In vitro test for complement- inhibitory properties against CP and AP, In vitro hemolytic assays | Jin et al. (2013) |
*All the extracts, EO, and isolated compounds were from the dried fruits of A. tsao-ko, except where specified
ELISA Enzyme-Linked Immunosorbent Assay, EtOAc Ex. Ethyl Acetate Extracts, EtOH Ex. Ethanol Extracts, IC50 50% inhibitory concentration, LC50 50% Lethal Concentration, LD50 50% Lethal Dose, LPS Lipopolysaccharide, MAPK Mitogen-Activated Protein Kinase, MBC Minimum Bactericidal Concentration, MeX Methanol Extracts, DPPH 2,2-Diphe- Nyl-1-Picrylhydrazyl, MIC Minimum Inhibitory Concentration, MLC Minimum Lethal Concentration, MLD Minimal Lethal Dose, MTT 3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2-H-Tetrazolium Bromide, TBARS Thiobarbitutic Acid Reactive Substances, TCPTP T-Cell Protein Tyrosine Phosphatase, TEM Transmission Electron Microscopy
Antibiotic activity
Antibiotic activity of Tsaoko Fructus is extensively studied through the inhibition of various microbes such as fungi, protozoa, and both Gram-positive and Gram-negative bacteria, and against insects like Tribolium castaneum, mainly using agar dilution/diffusion or liquid/broth dilution methods in vitro.
Both the ethanol extracts (EtOH Ex.) and the ethyl acetate extracts (EtOAc Ex.) of A. tsaoko fruits showed inhibitory activities against Staphylococcus aureus, Salmonella Typhimurium, Pseudomonas aeruginosa, and Klebsiella pneumonia, a gram-negative bacterium caused pneumonia with high morbidity and mortality (Rahman et al. 2017; Liu et al. 2018). The Minimum Inhibitory Concentration (MIC) of EtOH Ex. for S. aureus, S. Typhimurium, and P. aeruginosa was 1, 2, and 2 mg/mL, which was 25%, 25%, and 12.5% of the positive control (vanillin), respectively (Rahman et al. 2017). The MIC for K. pneumonia was 5 μg/mL, equal to the chloramphenicol positive control (Liu et al. 2018). Investigation with purified compounds showed that MIC values of fatty acids (191–194), phenolic acids (40–42), and coronadiene (25) for K. pneumonia ranged from 5 to 50 μg/mL, 100% to 10% of the positive control, proving that the anti-microbial activity of EtOH Ex. and EtOAc Ex. was most likely contributed to the synergistical effects of these components (Liu et al. 2018).
A. tsao-ko EO also showed antibiotic activity in vitro. It had an inhibitory effect on a broad spectrum microbial organisms, including gram-positive and gram-negative bacteria such as E. coli, S. aureus, and Pseudomonas aeruginosa, with MIC ranging from 22.49 to 1438.91 μg/mL (Dai et al. 2016b). It could also suppress the growth of Trichomonas vaginalis Tv1 and Tv2, with IC50 values of 22.49 μg/mL and 44.97 μg/mL, respectively; the IC50 values of metronidazole positive control were 2.44 μg/mL and 4.88 μg/mL, respectively (Dai et al. 2016a). Observation under transmission electron microscopy (TEM) showed the anti-T. vaginalis activity was possibly due to the damage of membrane structure, reduction or disappearance of ribosomes, organelles disintegration, cell disintegration and necrosis (Dai et al. 2016a). Geraniol (5) was thought to be the most effective substance due to its high content in A. tsao-ko EO (13.69%) and its inhibitory activity against T. vaginali (IC50 = 171.48 μg/mL for both Tv1 and Tv2) (Dai et al. 2016a).
A. tsao-ko EO had considerable toxicity on stored-product insects, T. castaneum (Herbst) and Lasioderma serricorne (Fabricius) (Wang et al. 2014). Further isolation led to two components, limonene (1) and eucalyptol (10). Both compounds showed pronounced contact toxicity against T. castaneum and L. serricorne. The LD50 values of 1 for T. castaneum and L. serricorne were 14.97 μg/adult and 13.66 μg/adult, respectively; those of 10 were 18.83 μg/adult and 15.58 μg/adult, respectively (Wang et al. 2014). These two components also possessed strong fumigant toxicity against both insect species. The LC50 values of 10 for T. castaneum and L. serricorne were 5.47 mg/L air and 5.18 mg/L air, and those of 1 were 6.21 mg/L air and 14.07 mg/L, respectively (Wang et al. 2014).
Isotsaokoin (14), another A. tsao-ko EO component, showed antifungal activity against Trycophyton mentagrophytes a little better than the positive control (amphotericin B) (Moon et al. 2004). The inhibition zone of 14 was 1.5 and 2.0 mm at 20 and 40 µg/disk, respectively, whereas that of amphotericin B was 2.5 and 3.0 mm at 20 and 40 µg/disk, respectively (Moon et al. 2004).
In vivo experiment also demonstrated that A. tsao-ko EO had antibiotic activity. When intramuscularly supplied 0.92 g/kg/d, A. tsao-ko EO could protect the mice from the infection of S. aureus or Escherichia coli, showing 100.00% survival rates (Dai et al. 2016b).
The broad-spectrum antibiotic activities of Tsaoko Fructus against microorganisms especially pathogenic microbes makes Tsaoko Fructus a promising and potential natural source for developing broad-spectrum antibiotics, which also gives a hint to the probable mechanism of its clinical application such as curing malaria and diarrhea.
Anti-inflammatory activity
It was proved that EtOH Ex. and some purified compounds from Tsaoko Fructus had anti-inflammatory activities. Lipopolysaccharide (LPS)-treated RAW 264.7 macrophage cells and BV2 microglial cells are commonly used to evaluate the effects and to explore the possible molecular mechanism of anti-inflammatory activities.
NNMBS227, the 70% EtOH Ex. of A. tsao-ko seeds (At-EE), was reported to suppress the expression of two pro-inflammatory mediators, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), in the LPS-induced inflammatory responses in RAW264.7 cells and exhibited IC50 values of 194.92 ± 1.38 μg/mL and 151.00 ± 1.06 μg/mL, respectively (Li et al. 2014). Another research also revealed that the 80% EtOH Ex. of A. tsao-ko decreased LPS-induced NO production in RAW 264.7 cells with an IC50 value of 59.5 ± 1.8 μg/mL, and the N-Monomethyl-L-arginine positive control had an IC50 value of 27.3 ± 0.3 μM (Choi et al. 2018). Bioassay guided purification and inhibitory effect assay led to the finding of two active compounds, (-)-catechin (73) and ( +)-epicatechin (75) (Choi et al. 2018). Both showed high activity (IC50 = 70.6 μM and IC50 = 73.3 μM, respectively) against NO production without cytotoxicity (Choi et al. 2018). Pharmacological research with purified compounds revealed that diarylheptanoids (170, 171) and aliphatic alcohol (2,8-decadiene-1,10-diol, DDO, 203) from the ethanol extracts, and oxygenated monoterpenes (9, 11–14, 17, 18), flavonoids (88–95, 98, 104–107), diarylheptanoids (144, 148, 153, 154, 161), and fatty acid (190) from the methanol extracts also had considerable inhibitory effect against LPS-induced inflammatory response, with IC50 values or the inhibition effects equivalent to that of the positive control Lee et al. (2008) (Table 3).
Western blot, RT-PCR, and ELISA analysis proved that (-)-catechin (73) and (+)-epicatechin (75) inhibited the NO production in LPS-stimulated RAW 264.7 cells through suppressing the expression of iNOS and the translocation of nuclear factor kappa-B (NF-κB) and reducing the production of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 (Choi et al. 2018). For 203, besides reducing NO production and iNOS transcription, it could also inhibit the production of prostaglandin E2 (PGE2) and pro-inflammatory cytokines such as IL-6 and TNF-α. Such biological effect was resulted from the inactivation of the MAPKs such as extracellular signal-regulated kinase, c-Jun-N-terminal kinase and p38MAPK and the suppression of the NF-κB pathway such as degradation of κB-α and NF-κB inhibitors (Kim et al. 2016). It appeared that the anti-inflammatory activities of Tsaoko Fructus extracts and the isolated compounds were possibly achieved through the downregulation of the mitogen-activated protein kinase (MAPK) pathway and the NF-κB pathway.
The anti-inflammatory activity of Tsaoko Fructus indicates that it could be used as a potent therapeutic agent for the treatment of inflammatory disorders. It also implies that the medicinal value of Tsaoko Fructus such as its treatment on spleen and stomach disorders might be at least partially due to its anti-inflammatory activity.
Anti-tumor/cancer and antioxidant activity
The anti-tumor and anti-cancer activities were usually evaluated by MTT assay against human cancer cells such as hepatoma cells HepG-2 and SMMC-7721, cervical cancer cell Hela, and lung cancer cell A549 in vitro. Besides, since oxidative stress is among the main causes of cancer-related death and the chemoprevention is defined as the use of antioxidants to prevent cancer formation or cancer progress, antioxidative activity assay such as DPPH radical scavenging ability assay was also used to screen potential anti-tumor/cancer chemicals (Zhang et al. 2015).
It was reported that the ethyl acetate fraction and the petroleum ether fraction of 95% ethanol extracts of A. tsao-ko fruits showed > 60% inhibition rate at 400 μg/mL against several cancer cell lines including Hela, HepG-2, SMMC-7721, and A549 (Zhang et al. 2015). Bioactivity-guided separation led to the isolation of isotsaokoin (14) and two diarylheptanoids (170 and 171) (Zhang et al. 2015). 170 and 171 inhibited the proliferation of HepG-2, SMMC-7721, Hela and A549 cells with IC50 ranging from 44.66 μg/mL to 117.83 μg/mL, nearly equal to that of the positive control (5-fluorouracil: 59.83–73.89 μg/mL), while 14 only had inhibitory activity against Hela cells (IC50 = 72.14 μg/mL) (Zhang et al. 2015). 171 also showed DPPH scavenging ability, equivalent to vitamin C (Vc) (Zhang et al. 2015).
Sphingosine kinases 1 and 2 (SPHK1/2) are considered rate limiting enzymes for the formation of sphingosine 1 phosphate (S1P), which serves an important function in cellular and physiological processes Lee et al. (2019). So, the inhibition of SPHK1/2 may induce cell cycle arrest and apoptosis, exerting anticancer effects Lee et al. (2019). Research revealed the hexane fraction of 50% EtOH Ex. of A. tsao-ko seeds showed inhibitory effect on SPHK1 and SPHK2 by 39% and 67% of the control, respectively Lee et al. (2019). Under the guidance of this assay, 25 compounds had been isolated, of which phenolic acids 61, 62, 63 and 68, fatty acids 194 and 197, aliphatic alcohols 204, 205 and 206 showed inhibition against SPHK1 activity up to 20%, and monoterpene 3, phenolic acids 57, 58 and 65, fatty acid 193, aliphatic alcohols 204 and 205 had inhibition against SPHK2 activity up to 40% compared with the control Lee et al. (2019) (Table 3). Compound 205 had the highest potency to inhibit the activity of SPHK1, by 59.75%, and compound 3 showed the highest potency in suppressing SPHK2 activity, by 22.75%, in comparison with the control, where both exhibited higher inhibitory effect than the corresponding positive control (Resveratrol for SPHK1: 85.43%; SKI-II for SPHK2: 75.35%) Lee et al. (2019). Docking modeling analysis indicated that 205 and 3 bind into the hydrophobic substrate-binding pocket of SPHK1 and SPHK2, respectively, suggesting they might act as substrate-competitive inhibitors of SPHK1/2 enzymes Lee et al. (2019).
Compounds from the 95% EtOH extract or 70% acetone fraction of dichloromethane extracts of Tsaoko Fructus had antioxidative activity. These compounds included phenolic acids 36, 40, 41 and 42, flavonoids 60, 74 and 76, diarylheptanoids 145 and 149, and fatty acids 192 and 193 (Martin et al. 2000; Liu et al. 2018). Among them, 36, 60, 73 and 75 had the IC50 values of 12.55–15.89 µM, equivalent to that of the positive control (α-tocopherol: IC50 = 12.57 µM) (Martin et al. 2000), and 42 exhibited almost excellent DPPH scavenging activity at a concentration of 100 μg/mL (DPPH radical inhibition rate > 90%), which was very close to that of vitamin C at the same concentration (DPPH radical inhibition rate about 95%) (Liu et al. 2018).
A. tsao-ko EO was also proved to have anti-oxidative and anti-tumor activities. Cytotoxicity analysis by MTT assay showed that A. tsao-ko EO was cytotoxic to HepG2, Hela, Bel-7402, SGC-7901 and PC-3 cell lines. And the lowest IC50 of 31.80 ± 1.18 μg/mL was obtained for HepG2 carcinoma cell lines, compared to 5.93 ± 0.30 μg/mL of the positive control (mitomycin) (Yang et al. 2010). It had weak antioxidant activity as measured by DPPH radical assay, thiobarbituric acid (TBA) test, and ferric reducing antioxidant power (FRAP) assay (Yang et al. 2010).
The strong antioxidant and anti-tumor activity against tested tumor cell lines of 171 indicates it is worthy of further study as a potential nutraceutical compound and chemotherapeutic drug. The studies of 205 and 3 on the inhibition of SPHK1 and SPHK2 enzymatic activities also suggest that these compounds could be developed as potential anti-tumor drugs.
Anti-diabetic activity
The anti-diabetic activities of extracts, EO, and isolated compounds of A. tsao-ko are usually assessed by assaying the inhibition activity of enzymes such as α-amylase, α-glucosidase, protein tyrosine phosphatase 1B (PTP1B), and T-Cell protein tyrosine phosphatase (TCPTP) through in vitro and in vivo experiments.
The aqueous extracts of A. tsao-ko seeds showed obvious inhibitory activities against α-amylase and α-glucosidase in vitro, with IC50 of 1.04 mg/mL and 1.4 mg/mL, in contrast to 2.1 mg/mL and 1.90 mg/mL of the positive control (acarbose), respectively (Hussain et al. 2018). The methanol extracts (MeX) and the polar fraction of MeX of A. tsao-ko fruits inhibited α-glucosidase activity with an IC50 of 0.02 mg/mL in vitro (Yu et al. 2010). Dietary feeding experiments in mice proved that feeding the polar fraction of MeX can reduce plasma glucose to about 50% of the negative control, indicating that the polar fraction of MeX had effective hypoglycemic activity in vivo (Yu et al. 2010).
Recently, it has been demonstrated that 50% ethanol–water extract of A. tsao-ko dried fruits had significant α-glucosidase inhibitory activity (IC50 = 38.6 μg/mL) (He et al. 2020c). Bioactivity-guided isolation on the active fraction afforded seventeen 2,6-epoxy diarylheptanoids (172–188)(He et al. 2020c) Among them, tsaokopyranols E, H, I, J, K (176, 179–182) and phaeoheptanoxide (187) showed obvious α-glucosidase inhibitory activity with IC50 below 100 μM, much lower than the positive control (acarbose: IC50 = 219.0 μM). Tsaokopyranols A, B, D, F (172, 173, 175, 177) and engelheptanoxide C (188) exhibited moderate activity with IC50 ranging from 100.1 to 179.5 μM, comparable to the positive control (He et al. 2020c). Applying similar approaches, the same research group also isolated a series of flavonoids and flavonoid derivatives from the EtOH extract of A. tsao-ko fruits and demonstrated that some of them had anti-diabetic activities (He et al. 2020a, b, 2021). These compounds included flavonoids, namely ( +)-afzelechin (71), flavanocoumarin (110), sappanone B (111), brazilin (112) and proanthocyanidin A-2 (114), rare flavanol-menthane conjugates, namely amomutsaokins A-C and E–H (115–117, 119–122), new flavanol-fatty alcohol hybrids, namely tsaokoflavanols A, B, F, J-L, R and S (123, 124, 128, 132–134, 140, 141), and two unusual flavanol-monoterpenoid hybrids, tsaokols A (142) and B (143) (He et al. 2020a, b, 2021) (Table 3). Tsaokols A (142) and B (143) showed significant α-glucosidase inhibitory effect with IC50 values of 18.8 and 38.6 μmol/L (He et al. 2020b). Compounds 110, 114, 116, 117 and 120 exhibited PTP1B selective inhibition with IC50 values of 201.45–317.51 μM, and 71, 111, 112, 114–117 and 119–122 displayed α-glucosidase inhibitory effect with IC50 values ranging from 3.73 to 76.23 μM (He et al. 2021). Tsaokoflavanols A, B, F, K and R (123, 124, 128, 140) exhibited inhibitory activity against α-glucosidase with IC50 values of 5.2–9.0 μM, 20–35 times stronger than the positive control (acarbose: IC50 = 180.0 μM). And tsaokoflavanols F, J-L and S (128, 132–134, 141) were PTP1B/TCPTP selective inhibitors with IC50 values of 56.4–80.4 μM, 2–4 times stronger than the positive control (suramin sodium: IC50 = 200.5 μM)(He et al. 2020a). Enzyme kinetics study indicated that compounds 123, 124, 128 and 133 were α-glucosidase and PTP1B mixed-type inhibitors with Ki values ranging from 2.9 to 13.0 μM and 39.2 to 142.3 μM, respectively (He et al. 2020a). Using docking simulation they proved that the hemiacetal hydroxy, the orientation of 3,4-dihydroxyphenyl, and the length of alkyl were essential in binding with α-glucosidase and PTP1B (He et al. 2020a).
Lipid reducing activity
Methanol extracts (MeX) of A. tsao-ko fruits could reduce the body lipid in mice at about 50% of the control, and ( +)-epicatechin (75) was believed to be the main active component (Yu et al. 2008, 2010). Through in vitro assay, Liu et al. (2018) proved that fatty acids 191, 192, 193 and 194 exhibited inhibition effects on lipase activity, and when the concentrations were at 50 μg/mL, their inhibition rates were 50.07%, 61.56%, 59.37% and 49.32%, respectively. The inhibition effects of (2E,7Z)-tetradeca-2,7-dienoic acid (192) and (E)-tetradec-2-enoic acid (193) on lipase were even better than the positive control (orlistat: 58.78%) at a concentration of 50 μg/mL (Liu et al. 2018).
Neuroprotective activity
The neuroprotective effect is closely in correlation with the antioxidant activity, just as anti-inflammatory and anti-tumor activities (Zhang et al. 2015, 2016). H2O2 induced nerve injury of PC-12 cells were commonly used to assay the neuroprotective activity.
Besides the activity against inflammation, diarylheptanoids 170 and 171 also showed significant neuroprotective activity by reversing the loss of cell viability induced by H2O2, with nearly equal activity to the Vc control (Zhang et al. 2016). The 95% EtOH and the ethyl acetate fraction of A. tsao-ko fruits also showed potent protective effect on the damage to PC-12 cells induced by H2O2 (Zhang et al. 2014). Bioactivity-guided separation led to the isolation of six active compounds including quercetin (77), daucosterol (31), ( +)-epicatechin (75), quercetin-7-O-β-glucoside (79), quercetin-3-O-β-D-glucopyranoside (78), meso-hannokinol (148). Quercetin (77) exhibited the strongest neuroprotective effect, and the cell viability was up to 78.9% at a concentration of 50 μg/mL. The other five compounds 31, 75, 78, 79 and 148 also showed protective effects. The cell viability was 75.6%, 70.4%, 68.1%, 68.1% and 63.8% after treatment with these compounds, respectively (Zhang et al. 2014). Quercetin (77) exhibited good DPPH radical-scavenging activity at a concentration of 100 μg/mL (DPPH radical inhibition rate > 80%), very close to Vc at the same concentration (about 83%) (Zhang et al. 2014). But, quercetin (77) is a widespread natural product in plants and can interact with many proteins in vitro (Gertsch 2009). Whether it works as the effective ingredient of Tsaoko Fructus or not requires further exploration.
Anti-complementary activity
In the effort to search for anti-complementary agents under the guidance of bioactivity-directed fractionation and isolation, Jin et al. (2013) obtained 14 compounds (15, 18, 32, 33, 44–47, 55, 96, 99, 101, 153, 160) from the ethanolic extract of A. tsao-ko dried fruits. All the 14 compounds exhibited anti-complementary activities against the classical pathway (CP) and the alternative pathway (AP) through in vitro evaluation (Jin et al. 2013). Among them, hydroquinone (33) and 1,7-bis(4-hydroxyphenyl)-4(E)-hepten-3-one (160) showed the strongest anti-complementary activity. The CH50 and AP50 values of 33 and 160 were 0.55 ± 0.11 mM and 0.53 ± 0.15 mM, 0.42 ± 0.15 mM and 0.66 ± 0.11 mM, respectively; compared to the positive control, heparin, the CH50 and AP50 of which were 40 μg/mL and 97 μg/mL, respectively (Jin et al. 2013). Hemolytic assays indicated that 160 blocked C1q, C2, C3, C4, C5 and C9 in the complement system, and 33 acted on C1q, C2, C3, C5 and C9 (Jin et al. 2013). The anti-complementary activity of A. tsao-ko extracts and the purified compounds, in particular 33 and 160, suggests that they have the potency to be complement inhibitors.
Although most of the pharmacological activities of the extracts and compounds from Tsaoko Fructus were obtained only by in vitro experiments at present, the advanced achievements have provided certain evidences for elucidating the therapeutic mechanism. It also makes Tsaoko Fructus an expected potential health care product and medicinal source such as dietary supplements for reducing blood glucose and lipid levels or as new anti-diabetic drug candidates. Moreover, it has been demonstrated that the ethanol extract of Tsaoko Fructus showed no toxic and no-observed adverse effects in mice when fed with the extract at 2000 mg/kg/day (Park et al. 2015).
Quality control of Tsaoko Fructus as a medicinal material
Quality control of medicinal materials is of great importance to keep the clinical efficacy and safety. There is no international standard of Tsaoko Fructus at present. The current quality control of Tsaoko Fructus is based on the content of eucalyptol (10), besides the normal morphological detection and authentication, according to the newly published Pharmacopoeia of the People’s Republic of China (2020). Yet, eucalyptol (10) universally exists in the volatile oil of many plants, not unique to Tsaoko Fructus. More importantly, besides the anti-biotic activity, eucalyptol (10) has little bioactivities (Table 4), which makes it improper as the “quality” standard index. A more proper standard should be developed to focus on either A. tsao-ko-specific compounds such as 15, 16, 20, and 22 (Sim et al. 2019) (Table 2) or components relevant to its clinical efficacy like 14 that has anti-inflammatory and anti-tumor activities.
Table 4.
Compounds with certificated biological activities from A. tsao-ko fruits
| Pharmacological activity | Compounds isolated and identified from A. tsao-ko fruits |
|---|---|
| Anti-biotic activity | Monoterpenoids (1, 5, 10, 14), Diterpenoids (25), Phenolic acids (40–42), Fatty acids (191–194) |
| Anti-inflammatory activity | Monoterpenoids (9, 11–14, 17, 18), Phenolic acids (73, 75), Flavonoids (88–95, 98, 104–107), Diarylheptanoids (144, 148, 153, 154, 161, 170, 171), Fatty Acids (190), Aliphatic alcohols (203) |
| Anti-tumor and anti-cancer activity | Monoterpenoids (3, 14), Phenolic acids (57, 58, 61–63, 65, 68), Diarylheptanoids (144, 170, 171), Fatty acids (193, 194, 197), Aliphatic alcohols (204–206) |
| Antioxidant activity | Phenolic acids (36, 40–42, 60), Flavonoids (73, 75), Diarylheptanoids (144, 148, 171), Fatty acid (191) |
| Anti-diabetic activity | Flavonoids (71, 110, 112, 114), Flavanol-menthane conjugates (115–117, 119–122), Flavanol-fatty alcohol hybrids (123, 124, 128, 132–134, 140, 141), Flavanol-monoterpenoid hybrids (142, 143), Diarylheptanoids (172, 173, 175–177, 179–182, 187, 188) |
| Lipid-reducing activity | Fatty acids (191–194) |
| Neuroprotective activity | Phenolic acids (77), Diarylheptanoids (170, 171) |
| Anti-complementary properties | Phenolic acid (33), Diarylheptanoid (160) |
Additionally, since Tsaoko Fructus has multiple pharmacological activities (Tables 3, 4) and there are a great variety of chemicals in Tsaoko Fructus (Tables 2, S4), it will be better to study the biological activities of a fraction or extract and establish a specific chemical fingerprint correlated with a certain clinical efficacy or bioactivity, instead of just focusing on one or two particular compounds.
Conclusion and perspective
The dried fruits of A. tsao-ko (Tsaoko Fructus) are valuable medicinal materials that have been used clinically more than one thousand years ago. Its traditional uses in treating malaria have contributed to the successful application of Tsaoko Fructus-containing prescriptions in the treatment and prevention of the current epidemic diseases, SARS and COVID-19. Nowadays, epidemic has become the greatest threat to people’s health and life. Novel viruses and multiple antibiotic-resistant bacteria keep emerging, posing an unprecedented challenge to the health even life security of human. People are in urgent need of safe and effective medicines. The extracts, EO, and isolated compounds of Tsaoko Fructus exhibited a broad-spectrum inhibition against multiple microbes, which makes it a potential source of safe and natural antibiotics, especially in an era that pathogens have become the greatest enemy to us.
Pharmacological studies of the extracts, EO, and isolated compounds of Tsaoko Fructus provided a certain basis for its mechanism of medicinal function and potential application. The inhibition activities on a broad-spectrum of microorganism of Tsaoko Fructus may account for its suppression of many pathogen-related diseases such as malaria, diarrhea, throat infections, pathogen induced fever and other pathogen infections. The relief of abdominal pain and the elimination of phlegm is possible related to its anti-inflammatory activities. The anti-diabetic and lipid-reducing activities of A. tsao-ko make it a potential resource to prevent or relieve age or life-style related diseases such as diabetes, obesity and hypertension.
Behind its various biological activities lies an abundant number of phytochemicals. At least 209 components have been separated and identified including terpenoids, flavonoids, aromatic compounds and a diversity of simple organic molecules, some of which have already been tested for their bioactivities.
However, there still exist some research gaps to date. Firstly, current research has revealed many biological activities of Tsaoko Fructus, but those bioactivities haven’t been well linked to its medical usage. More study on the relationship between the bioactivity and medical uses should be done in the future. Investigation at molecular and cellular levels and in vivo experiments are expected to reveal what exactly are functioning as key components and how they work, considering the bioavailability of the compounds. This can subsequently help us better understand the mechanism to treat disease and make better use of this medicinal material. Secondly, in many phytochemical research, there exist some unidentified signals in the results of LC–MS or GC–MS profiles, which might be due to instrumental accuracy and precision, or some compounds that were not listed in the current database. This can be a point to dig into, which may help to discover some new compounds with biological activities. At last, according to Pharmacopoeia of the People’s Republic of China (2020), a more appropriate quality control system on the basis of the unique components and more relevant to its pharmacological activity or clinical efficacy should be developed for Tsaoko Fructus. Using a specific chemical feature related with a certain pharmacological activity or clinical efficacy may be more rational, because there are a great number of compounds in Tsaoko Fructus and it has multiple bioactivities and clinical applications.
In conclusion, Tsaoko Fructus has a long historical clinical use to treat a number of disorders. The present studies revealed that it contains hundreds of compounds and has multiple biological activities. These achievements indicate a bright future of Tsaoko Fructus as a natural source of next-generation medications, and also lay foundation for further elucidating the therapeutic mechanism, and revealing the relationship between clinical usage, chemical composition and pharmacological activity of Tsaoko Fructus in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We greatly appreciate Prof. Guixin Chou, Prof. Ling Zhao, and Prof. Hailian Shi from Shanghai University of Traditional Chinese Medicine for their assistance in the identification and classification of the compounds, and the interpretation and explanation of Tsaoko Fructus-containing prescriptions.
Authors' contributions
Zhengtao Wang gave the outline of the review and revised the manuscript. Daju Chen retrieved and classified most of the literatures and revised the manuscript. Siyuan Yang drafted and revised the manuscript, draw the chemical structures and made most of the tables. Yafu Xue participated in modifiying the manuscript.
Funding
This work was supported by “Science and Technology Innovation Action Program” of Shanghai Science and Technology Commission (No. 19395800300) and the "Wang Zhengtao" Expert Workstation of Yunnan Province (No. 2018IC146) granted to Professor Zhengtao Wang.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daju Chen, Email: chendaju88@126.com, Email: 67301267@qq.com.
Zhengtao Wang, Email: ztwang@shutcm.edu.cn.
References
- Chen Y. Caoguo-Baoqing Bencao Zhezhong. In: Zheng J, editor. Three Issues of Rare Bencao in Nan Song Dynasty. Beijng: People’s Medical Publishing House; 2007. p. 588. [Google Scholar]
- Chinese Pharmacopoeia Commission (2015) Pharmacopoeia of the People’s Republic of China. China Medical Science Press
- Choi CW, Shin JY, Seo C, et al. In vitro anti-inflammatory activity of the components of Amomum tsao-ko in murine macrophage raw 2647 cells. Afr J Tradit Complement Altern Med. 2018;15:26–34. doi: 10.21010/ajtcam.v15i2.4. [DOI] [Google Scholar]
- Cui Q, Wang LT, Liu JZ, et al. Rapid extraction of Amomum tsao-ko essential oil and determination of its chemical composition, antioxidant and antimicrobial activities. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1061–1062:364–371. doi: 10.1016/j.jchromb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Dai M, Peng C. Research progress on chemical compositions and pharmacological actions of Amomum tsao-ko Crevost et Lemaire. Pharm Clin Chin Mater Medica. 2011;2:55–59. [Google Scholar]
- Dai M, Peng C, Peng F, et al. Anti-Trichomonas vaginalis properties of the oil of Amomum tsao-ko and its major component, geraniol. Pharm Biol. 2016;54:445–450. doi: 10.3109/13880209.2015.1044617. [DOI] [PubMed] [Google Scholar]
- Dai M, Peng C, Sun F. Anti-infectious efficacy of essential oil from Caoguo (Fructus Tsaoko) J Tradit Chin Med. 2016;36:799–804. doi: 10.1016/s0254-6272(17)30018-3. [DOI] [PubMed] [Google Scholar]
- Ding R, Wang F, Lu H (2020) Diagnosis and treatment of COVID-19 from spleen and stomach. Acta Chinese Med 35:
- Fang Z, Yue D. Analysis of the classic prescription Dayuanyin and exploration of its inheritance and application. Jilin J Chin Med. 2021;41:569–571. [Google Scholar]
- Feng X, Jiang ZT, Wang Y, Li R. Composition comparison of essential oils extracted by hydrodistillation and microwave-assisted hydrodistillation from Amomum tsao-ko in China. J Essent Oil-Bearing Plants. 2010;13:286–291. doi: 10.1080/0972060X.2010.10643823. [DOI] [Google Scholar]
- Gao J, Wang H. A survey of traditional and modern prescirions containing Caoguo. Tianjin J Tradit Chin Med. 2007;24:15–18. [Google Scholar]
- Gertsch J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J Ethnopharmacol. 2009;122:177–183. doi: 10.1016/j.jep.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Gu F, Zhang L, Fang Y, et al. Analysis of physical properties, essential oil content and composition of Amomum tsao-ko from different origins of Yunnan. Chinese J Trop Crop. 2018;39:1440–1446. doi: 10.3969/j.issn.1000-2561.2018.07.026. [DOI] [Google Scholar]
- He Q, Qin J, Huang Y, et al. Analysis of volatile oils from the seeds and shells of Amomum tsao-ko by GC-MS. Chin J Exp Tradit Med Formulae. 2013;19:2–7. [Google Scholar]
- He X-F, Chen J-J, Li T-Z, et al. Nineteen new flavanol-fatty alcohol hybrids with α-glucosidase and PTP1B dual inhibition: one unusual type of antidiabetic constituent from Amomum tsao-ko. J Agric Food Chem. 2020;68:11434–11448. doi: 10.1021/acs.jafc.0c04615. [DOI] [PubMed] [Google Scholar]
- He X-F, Chen J-J, Li T-Z, et al. Tsaokols A and B, unusual flavanol-monoterpenoid hybrids as α-glucosidase inhibitors from Amomum tsao-ko. Chin Chem Lett. 2020 doi: 10.1016/j.cclet.2020.08.050. [DOI] [Google Scholar]
- He X-F, Zhang X-K, Geng C-A, et al. Tsaokopyranols A-M, 2,6-epoxydiarylheptanoids from Amomum tsao-ko and their α-glucosidase inhibitory activity. Bioorg Chem. 2020;96:103638. doi: 10.1016/j.bioorg.2020.103638. [DOI] [PubMed] [Google Scholar]
- He X, Wang H, Geng C, et al. Amomutsaokols A-K, diarylheptanoids from Amomum tsao-ko and their α-glucosidase inhibitory activity. Phytochemistry. 2020;177:112418. doi: 10.1016/j.phytochem.2020.112418. [DOI] [PubMed] [Google Scholar]
- He XF, Chen JJ, Huang XY, et al. The antidiabetic potency of Amomum tsao-ko and its active flavanols, as PTP1B selective and α-glucosidase dual inhibitors. Ind Crops Prod. 2021;160:112908. doi: 10.1016/j.indcrop.2020.112908. [DOI] [Google Scholar]
- Hong SS, Lee JH, Choi YH, et al. Amotsaokonal A-C, benzaldehyde and cycloterpenal from Amomum tsao-ko. Tetrahedron Lett. 2015;56:6681–6684. doi: 10.1016/j.tetlet.2015.10.045. [DOI] [Google Scholar]
- Hu Z. 300 cases of Chaigui Caoguo Tang on treatment of influenza. Zhejing J Tradit Chinese Med. 1993;2:65. [Google Scholar]
- Hu Y, Zhang X, Xu S, et al. Analysis of genetic diversity and genetic relationship of Amomum tsao-ko germplasm resources in Yunnan by SSR markers. Chinese Tradit Herb Drugs. 2018;49:5388–5395. doi: 10.7501/j.issn.0253-2670.2018.22.025. [DOI] [Google Scholar]
- Hu Y, Zhang Z, Zhang T, et al. Analysis of volatile oil ingredients from different cultivars of the Amomum tsao-ko by GC-MS. J Wenshan Univ. 2018;31:15–22. [Google Scholar]
- Hu Y, Di Y, Zhang X, Yang Z. Optimization of SSR-PCR system and primers screening of Amomum tsao-ko Crevost et Lemarie in Yunnan. Mol Plant Breed. 2019;17:195–200. [Google Scholar]
- Hu Y, Zhang X, Shi N, Yang Z. DNA barcoding sequence analysis of Amomum tsao-ko germplasm resources in Yunnan province. Chin Tradit Herb Drugs. 2019;50:6091–6097. doi: 10.7501/j.issn.0253-2670.2019.24.025. [DOI] [Google Scholar]
- Huang Y, Qin L, Hu Q, et al. GAS chromatogrphy-mass spectrometry analysis of essential oil from Amomum tsao-ko Crevost et Lemarie and Amomum Paratsao-ko S. Q. Tong et Y. M. Xia grow in Guangxi. Res Pract Chin Med. 2014;28:22–24. [Google Scholar]
- Hussain SA, Hameed A, Fu J, et al. Comparative in vitro analysis of anti-diabetic activity of Indo-Pak black cardamom (Amomum subulatum Roxb) and Chinese black cardamom (Amomum tsao-ko Crevost et Lemaire) Prog Nutr. 2018;20:403–414. doi: 10.23751/pn.v20i3.6196. [DOI] [Google Scholar]
- Jin J, Cheng Z, Chen D. Two new compounds and anti-complementary constituents from Amomum tsao-ko. Nat Prod Commun. 2013;8:1715–1718. doi: 10.1177/1934578x1300801214. [DOI] [PubMed] [Google Scholar]
- Kim MS, Ahn EK, Hong SS, Oh JS. 2,8-decadiene-1,10-diol inhibits lipopolysaccharide-induced inflammatory responses through inactivation of mitogen-activated protein kinase and Nuclear Factor-κb signaling pathway. Inflammation. 2016;39:583–591. doi: 10.1007/s10753-015-0283-1. [DOI] [PubMed] [Google Scholar]
- Kim JG, Jang H, Le TPL, et al. Pyranoflavanones and Pyranochalcones from the Fruits of Amomum tsao-ko. J Nat Prod. 2019;82:1886–1892. doi: 10.1021/acs.jnatprod.9b00155. [DOI] [PubMed] [Google Scholar]
- Kim JG, Le Linh TP, Hong HR, et al. Nitric oxide inhibitory constituents from the fruits of Amomum tsao-ko. Nat Prod Sci. 2019;25:76–80. doi: 10.20307/NPS.2019.25.1.76. [DOI] [Google Scholar]
- Lee KY, Kim SH, Sung SH, Kim YC. Inhibitory constituents of lipopolysaccharide-induced nitric oxide production in BV2 microglia isolated from Amomum tsao-ko. Planta Med. 2008;74:867–869. doi: 10.1055/s-2008-1074552. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee JC, Subedi L, et al. Bioactive compounds from the seeds of Amomum tsao-ko Crevost et Lemaire, a Chinese spice as inhibitors of sphingosine kinases, SPHK1/2. RSC Adv. 2019;9:33957–33968. doi: 10.1039/c9ra07988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Identifying the monarch medicine in formulas. J Nanjung Univ Tradit Chin Med. 2021;37:481–484. [Google Scholar]
- Li Z, Luo L, Dai W, et al. Chemical constituents of the essential oil on Amomum tsao-ko from Yunnan Province. Acta Bot Yunnanica. 1998;20:119–122. [Google Scholar]
- Li B, Choi HJ, Lee DS, et al. Amomum tsao-ko suppresses lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages via Nrf2-dependent heme oxygenase-1 expression. Am J Chin Med. 2014;42:1229–1244. doi: 10.1142/S0192415X14500773. [DOI] [PubMed] [Google Scholar]
- Liu H, Yan Q, Zou D, et al. Identification and bioactivity evaluation of ingredients from the fruits of Amomum tsao-ko Crevost et Lemaire. Phytochem Lett. 2018;28:111–115. doi: 10.1016/j.phytol.2018.10.007. [DOI] [Google Scholar]
- Lu B, Ma M, Wang T, et al. Genetic diversity and genetic relationships of Amomum tsao-ko based on Random Amplified Polymorphic DNA Markers. Int J Agric Biol. 2019 doi: 10.17957/IJAB/15.0729. [DOI] [Google Scholar]
- Lu B, Ma M, Zhang W, et al. Development of 23 novel microsatellite markers of Amomum tsao-ko (Zingiberaceae) based on restriction-site-associated DNA sequencing. Mol Biol Rep. 2021;48:1943–1949. doi: 10.1007/s11033-020-06127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Lu B. The complete chloroplast genome of Amomum tsao-ko. Mitochondrial DNA Part B. 2020;5:848–849. doi: 10.1080/23802359.2020.1717382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang L, Peng J. Study on chemical constituents of introduced and cultivated grass and fruits in Xishuangbanna. Chinese Tradit Pat Med. 2008;30:1192–1194. [Google Scholar]
- Ma M, Lei E, Meng H, et al. Cluster and principal component analysis based on SSR markers of Amomum tsao-ko in Jinping County of Yunnan Province. AIP Conf Proc. 2017 doi: 10.1063/1.4992887. [DOI] [Google Scholar]
- Ma M, Wang T, Lei E, et al. Genetic diversity analysis of Amomum tsao-ko in Jinping County of Yunnan Province using SSR markers. AIP Conf Proc. 2017 doi: 10.1063/1.4992888. [DOI] [Google Scholar]
- Ma M, Wang T, En L, et al. Genetic diversity analysis of Amomum tsao-ko in Jinping based on phenotypic traits and SSR markers. Crops. 2020;2:54–59. doi: 10.16035/j.issn.1001-7283.2020.02.009. [DOI] [Google Scholar]
- Martin TS, Kikuzaki H, Hisamoto M, Nakatani N. Constituents of Amomum tsao-ko and their radical scavenging and antioxidant activities. J Am Oil Chem Soc. 2000;77:667–673. doi: 10.1007/s11746-000-0107-4. [DOI] [Google Scholar]
- Min Y, Zhang W, Yao L, et al. Study on the essential oil ingredients of Amomum tsao-ko from different regions of Yunnan Province. Med Plant. 2010;1:33–35. [Google Scholar]
- Moon SS, Lee JY, Cho SC. Isotsaokoin, an antifungal agent from Amomum tsao-ko. J Nat Prod. 2004;67:889–891. doi: 10.1021/np030464l. [DOI] [PubMed] [Google Scholar]
- Park JH, Cho YR, Ko HJ, et al. Evaluation of 3-week repeated dose oral toxicity on Amomum tsao-ko extract in balb/c mice. J Appl Biol Chem. 2015;58:139–143. doi: 10.3839/jabc.2015.024. [DOI] [Google Scholar]
- Rahman MRT, Lou Z, Yu F, et al. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amomum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J Biol Sci. 2017;24:324–330. doi: 10.1016/j.sjbs.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Liang P, Ma Y, et al. Research progress of Traditional Chinese Medicine against COVID-19. Biomed Pharmacother. 2021;137:111310. doi: 10.1016/j.biopha.2021.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zheng M, Xie S, Sun C. Composition regularities of Chinese Materia Medica Formulus for the Coronavirus Disease 2019. China Pharm. 2020;29:25–28. [Google Scholar]
- Shi Y, Jin H, Yang Y, et al. Medicinal textual researcg on Caoguo. Mordern Chin Med. 2013;15:913–916. [Google Scholar]
- Sim S, Tan SK, Kohlenberg B, Braun NA. Amomum tsao-ko-Chinese black cardamom: detailed oil composition and comparison with two other cardamom species. Nat Prod Commun. 2019 doi: 10.1177/1934578X19857675. [DOI] [Google Scholar]
- Starkenmann C, Mayenzet F, Brauchli R, et al. Structure elucidation of a pungent compound in black cardamom: Amomum tsao-ko Crevost et Lemarié (Zingiberaceae) J Agric Food Chem. 2007;55:10902–10907. doi: 10.1021/jf072707b. [DOI] [PubMed] [Google Scholar]
- Sun WM, Ma YN, Yin YJ, et al. Effects of essential oils from zingiberaceae plants on root-rot disease of Panax notoginseng. Molecules. 2018;23:1–11. doi: 10.3390/molecules23051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang C, Zhang Y. Phenolic constituents from the fruits of Amomum tsao-ko (Zingiberaceae) Acta Bot Yunnanica. 2009;31:284–288. doi: 10.3724/SP.J.1143.2009.09031. [DOI] [Google Scholar]
- Wang Y, You CX, Wang CF, et al. Chemical constituents and insecticidal activities of the essential oil from Amomum tsao-ko against two stored-product insects. J Oleo Sci. 2014;63:1019–1026. doi: 10.5650/jos.ess14087. [DOI] [PubMed] [Google Scholar]
- Wei Z, Bingyue L, Hengling M, et al. Phenotypic diversity analysis of the fruit of Amomum tsao-ko Crevost et Lemarie, an important medicinal plant in Yunnan, China. Genet Resour Crop Evol. 2019;66:1145–1154. doi: 10.1007/s10722-019-00765-x. [DOI] [Google Scholar]
- Wu M, Zhang W, Guo P, Zhao Z. Identification of seven Zingiberaceous species based on comparative anatomy of microscopic characteristics of seeds. Chin Med. 2014;9:10. doi: 10.1186/1749-8546-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Ning D, Zhou J, et al. RAPD primers screen for genetic diversity analysis in Amomum tsao-ko. J West China for Sci. 2018;47:45–50. [Google Scholar]
- Xu S, Bai J, Yang W, et al. Analysis of the key odorants in dried Amomum tsao-ko Crevost et Lemaire. Fine Chem. 2019 doi: 10.13550/j.jxhg.20190538. [DOI] [Google Scholar]
- Yan M (2012) The analysis of genetic diversity of Amomum tsao-ko (Zingiberaceae) and the molecular identification between A. tsao-ko and its related plant (A. paratsaoko). Yunnan University of Chinese Medicine
- Yang Z. Analysis on the content and main chemical components of volatile oils from Amomum tsao-ko Stems and Leaves in Yunnan province. J Chin Med Mater. 2019;42:339–343. [Google Scholar]
- Yang Y, Yan R-W, Cai X-Q, et al. Chemical composition and antimicrobial activity of the essential oil of Amomum tsao-ko. J Sci Food Agric. 2008;88:2111–2116. doi: 10.1002/jsfa.3321. [DOI] [Google Scholar]
- Yang X, Küenzi P, Plitzko I, et al. Bicyclononane aldehydes and antiproliferative constituents from Amomum tsao-ko. Planta Med. 2009;75:543–546. doi: 10.1055/s-0029-1185320. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yue Y, Runwei Y, Guolin Z. Cytotoxic, apoptotic and antioxidant activity of the essential oil of Amomum tsao-ko. Bioresour Technol. 2010;101:4205–4211. doi: 10.1016/j.biortech.2009.12.131. [DOI] [PubMed] [Google Scholar]
- Yang YW, Yang ZY, Yan MR, et al. Isolation and characterization of microsatellite markers for Amomum tsao-ko (Zingiberaceae), an economically important plant in China. Genet Mol Res. 2014;13:8220–8224. doi: 10.4238/2014.October.8.3. [DOI] [PubMed] [Google Scholar]
- Yao M. Observation of Yugan Tang on treatment of Hepatitis B based on 62 clinical cases. Mod J Integr Tradit Chin West Med. 2002;11:1116. [Google Scholar]
- Yu L, Shirai N, Suzuki H, et al. Effect of lipid extracted from Tsao-ko (Amomum tsao-ko Crevost et Lemaire) on digestive enzyme activity, antioxidant activity, plasma and liver lipids, and blood glucose levels of mice. J Nutr Sci Vitaminol (tokyo) 2008;54:378–383. doi: 10.3177/jnsv.54.378. [DOI] [PubMed] [Google Scholar]
- Yu L, Shirai N, Suzuki H, et al. The effect of methanol extracts of tsao-ko (Amomum tsao-ko Crevost et Lemaire) on digestive enzyme and antioxidant activity in vitro, and plasma lipids and glucose and liver lipids in mice. J Nutr Sci Vitaminol (tokyo) 2010;56:171–176. doi: 10.3177/jnsv.56.171. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Ren J, Huang L, Gao G, editors. Caoguo Chinese-English Dictionary of Traditional Chinese Medicine. Beijing: People’s Medical Publishing House; 2000. [Google Scholar]
- Zhang T, Chen D. Anticomplementary principles of a Chinese multiherb remedy for the treatment and prevention of SARS. J Ethnopharmacol. 2008;117:351–361. doi: 10.1016/j.jep.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang S, Wei X, Xie S. Developing status and strategies of Amomum tsao-ko plantation in Yunnan. World Sci Technol Tradit Chin Med Mater Med. 2011;13:899–903. doi: 10.3969/j.issn.1674-3849.2010.04.003. [DOI] [Google Scholar]
- Zhang TT, Lu CL, Jiang JG. Bioactivity evaluation of ingredients identified from the fruits of Amomum tsao-ko Crevost et Lemaire, a Chinese spice. Food Funct. 2014;5:1747–1754. doi: 10.1039/c4fo00169a. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Lu CL, Jiang JG. Antioxidant and anti-tumour evaluation of compounds identified from fruit of Amomum tsao-ko Crevost et Lemaire. J Funct Foods. 2015;18:423–431. doi: 10.1016/j.jff.2015.08.005. [DOI] [Google Scholar]
- Zhang TT, Lu CL, Jiang JG. Neuroprotective and anti-inflammatory effects of diphenylheptanes from the fruits of Amomum tsao-ko, a Chinese spice. Plant Foods Hum Nutr. 2016;71:450–453. doi: 10.1007/s11130-016-0570-5. [DOI] [PubMed] [Google Scholar]
- Zhang XR, Li TN, Ren YY, et al. The important role of volatile components from a Traditional Chinese Medicine Dayuan-Yin Against the COVID-19 Pandemic. Front Pharmacol. 2020;11:1–13. doi: 10.3389/fphar.2020.583651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Ding ML, Jia KK, et al. Exploring active compounds of Da-Yuan-Yin in treatment of COVID-19 based on network pharmacology and molecular docking method. Chin Tradit Herb Drugs. 2020;51:836–844. doi: 10.7501/j.issn.0253-2670.2020.04.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.