Abstract
OBJECTIVES
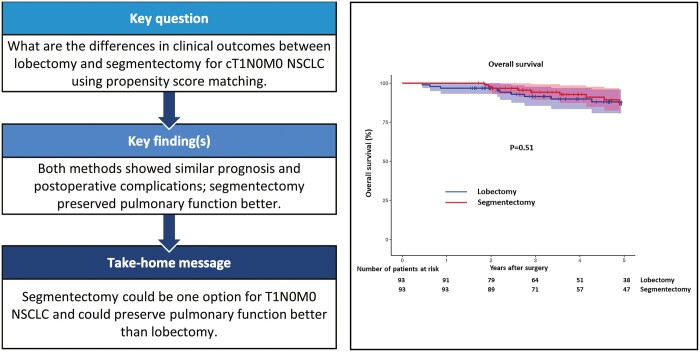
To demonstrate the differences in clinical outcomes between lobectomy and segmentectomy for non-small cell lung cancer using propensity score matching.
METHODS
A single-centre, retrospective, matched cohort study was conducted in clinical T1N0M0 non-small cell lung cancer patients treated by surgery between 2012 and 2019. Differences in freedom from recurrence, overall survival, postoperative complications, chest drainage and preservation of pulmonary function between lobectomy and segmentectomy were evaluated using the propensity score model. Matched variables of patients were age, sex, comorbidity index and pulmonary function. Matched variables of tumours were tumour size, T-stage, fluorodeoxyglucose uptake on positron emission tomography, histopathology, lobe site and tumour distance ratio from the hilum.
RESULTS
Of the 112 patients treated by lobectomy and 233 patients treated by segmentectomy, 93 patients each from both groups were selected after the matching. The median tumour distance ratio from hilum was 0.7 in lobectomy and 0.8 in segmentectomy group (P = 0.59), i.e. almost outer third tumour location. There were no significant differences in freedom from recurrence (P = 0.38), overall survival (P = 0.51), postoperative complications (P = 0.94), drainage period (P = 0.53) and prolonged air leakage (P = 0.82) between the two. Median preservation of pulmonary function was 93.2% after segmentectomy, which was significantly higher than 85.9% after lobectomy (P < 0.001).
CONCLUSIONS
Freedom from recurrence, overall survival, postoperative complications and chest drainage were similar between segmentectomy and lobectomy. Segmentectomy could be one of the options for clinical T1N0M0 non-small cell lung cancer located outer third as well as being able to preserve pulmonary function better than lobectomy.
Clinical trial registration
Name: Retrospective analysis of segmentectomy and lobectomy for cT1N0M0 non-small cell lung cancer
Date of approval: February 2014
Number of IRB approval: 14-003.
Keywords: Lung cancer, Segmentectomy, Lobectomy, Prognosis, Pulmonary function
Pulmonary segmentectomy is expected to change the surgical treatment of clinical (c) T1N0M0 non-small cell lung cancer (NSCLC).
INTRODUCTION
Pulmonary segmentectomy is expected to change the surgical treatment of clinical (c) T1N0M0 non-small cell lung cancer (NSCLC). While several retrospective studies have reported similar prognoses between lobectomy and segmentectomy in patients with cT1N0M0 NSCLC [1–4], the other studies have reported an inferior prognosis after segmentectomy compared to lobectomy [5, 6]. In addition, the superiority of segmentectomy in preserving pulmonary function compared with that of lobectomy remains controversial [1, 7]. A more definitive conclusion is expected from a Japanese randomized control trial between lobectomy and segmentectomy, JCOG0802/WJOG4607L [8], which has recently been reported in the annual meeting of American Association for Thoracic Surgery (AATS) in 2021; however, it has not been published in details as a manuscript. Therefore, at the current moment, propensity score matching as a retrospective analysis will be helpful to compare the 2 procedures.
There have been 3 previous studies that used propensity score matching to compare prognosis in cT1N0M0 NSCLC patients between lobectomy and segmentectomy [1–3]. While these 3 studies showed the similar freedom from recurrence and overall survival (OS) between lobectomy and segmentectomy in cT1N0M0 NSCLC, they did not match the tumour location (central versus peripheral) between the two. When a tumour is close to the lung hilum, a sufficient surgical margin is difficult to be taken with segmentectomy, and the possibility of pathological N1 increases, which makes surgeons select lobectomy rather than segmentectomy. Therefore, the present study added the tumour distance from the lung hilum to the matching variables. We also added the data of fluorodeoxyglucose positron emission tomography (PET).
In this study, we compared not only the prognosis but also postoperative complications, chest drainage and preservation of pulmonary function between the 2 procedures.
MATERIALS AND METHODS
Study design and setting
The study was a single-centre, retrospective, matched cohort study of consecutively acquired data. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [9]. A protocol for segmentectomy for patients with cT1N0M0 NSCLC was adopted in August 2012 by the Lung Cancer Board of the institute. The retrospective analysis protocol for patients treated by lobectomy and segmentectomy was approved by the institutional ethics committee in February 2014 (approval number: 14-003).
Data source
Between 2012 and 2019, 429 patients with cT1N0M0 NSCLC were scheduled to undergo surgery. Comorbidity was assessed using the Charlson comorbidity index [10]. Postoperative complications were assessed using the Clavien-Dindo classification [11]. Maximum tumour size was measured including ground-glass opacity area, which was not taken into account in the T-stage with eighth edition. The consolidation to tumour ratio was also calculated.
Eligibility for segmentectomy
Clinical tumour-node-metastasis (TNM) staging followed the eighth edition of the tumour-node-metastasis Classification [12]. The inclusion criteria for segmentectomy were as follows: (i) peripheral-type [outer third on computed tomography (CT)] cT1N0M0 NSCLC; (ii) a single lesion within a lobe; (iii) sufficient preserved function of the target lobe confirmed by lung perfusion scintigraphy; and (iv) patients requesting segmentectomy rather than lobectomy. The exclusion criteria were as follows: (i) tumours in the right middle lobe; (ii) patients requesting lobectomy rather than segmentectomy; and (iii) surgical margin greater than 2 cm or tumour size could not be obtained. All patients were informed of the following: (i) the standard surgical procedure for cT1N0M0 NSCLC is lobectomy; and (ii) the risk of local recurrence caused by segmentectomy has been reported to be ∼5% [4]. All patients provided informed consent after fully discussing the risks and benefits of segmentectomy with their surgeons.
Surgical procedure
The surgical margin from the tumour to the adjacent segment was measured on CT findings, including axial, coronal, and sagittal images. Segmentectomy was performed via anterolateral or limited lateral thoracotomy in the aim of complete hilar nodal dissection and appropriate surgical margin [13]. After dissecting the segmental bronchus and vessels, the tumour was grasped by a ring-shaped forceps with a diameter of 3 cm. The intersegmental plane was cut by electrocautery in the shallow lung tissue with taking more than 2 cm or tumour size of surgical margin, followed by application of a stapler in the deep tissue. Hilar nodal stations were completely dissected with taping vessels and bronchi [4]. After segmentectomy, the dissected hilar and mediastinal nodes were examined with intraoperative frozen sections [14]. When lymph nodes showed metastasis or the surgical margin was insufficient, a completion lobectomy was performed. The pleura-defective site of the preserved lobe was closed with sutures or covered with polyglycolic acid mesh (Neoveil®; Gunze Ltd Co., Kyoto, Japan) and fibrin glue with more than 2 layers as reported before [15].
Lobectomy was performed by using thoracoscopic surgery or under limited lateral thoracotomy. Since there is no evidence that the difference in these thoracic approaches affects the postoperative prognosis and pulmonary function, we did not include these thoracic approaches in the analysis.
Acquisition of fluorodeoxyglucose positron emission tomography/computed tomography data
A PET/CT device (Discovery ST; GE Medical Systems, Amersham, UK) was used to examine the fluorodeoxyglucose uptake before surgery, which was measured by standardized uptake value (SUV).
Evaluation of postoperative preservation of pulmonary function
Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were measured using a dry rolling-seal spirometer (CHEST AC-8800; CHEST Ltd., Tokyo, Japan). Preoperative pulmonary function tests were conducted within 1 month before surgery. Postoperative pulmonary function tests were conducted 6 months after surgery, since the pulmonary function has been reported to be fully recovered and stable after this period [16]. Percentage of preserved pulmonary function (%PPF) after surgery was calculated using the following formula: [FEV1 after surgery/FEV1 before surgery] × 100 (%). Since smoking history has no evidence of affecting the postoperative pulmonary function, we did not include it in the analysis, whereas FEV1/FVC and %FEV1 were included in the matched variables.
Examination of tumour distance ratio from the lung hilum
To measure the tumour distance from the hilum, the most evaluable cross-section was chosen from the axial, coronal and sagittal views. On the same cross-section, the distance from the lobar bronchus to the tumour centre and the distance from the lobar bronchus to the thoracic wall were measured. The tumour distance ratio from the hilum was evaluated by [distance from the lobar bronchus to the tumour centre/distance from the lobar bronchus to the thoracic wall].
Definition of local recurrence
Local recurrences after segmentectomy were defined as recurrences occurring at the sites of the surgical margin, in the lung within the preserved lobe, and in the hilar lymph nodes, because recurrences at these sites are caused by segmentectomy itself. Local recurrences were histologically diagnosed by needle biopsy.
Follow-up
All patients were followed up with CT scans every 3 months until 3 years after surgery, every 4–6 months between 3 and 5 years after surgery and at least yearly thereafter. Follow-up data were collected from medical records in August 2020. The median follow-up period was 56 months (range: 5–99 months).
Study outcomes
The first outcome was differences in freedom from recurrence and OS between lobectomy and segmentectomy. The secondary outcome was differences in postoperative complications, chest tube drainage and %PPF between the 2 groups.
Statistical analysis
Propensity scores were computed from potentially confounding variables of patient characteristics and tumour characteristics; the former included age, sex, comorbidity index, %FEV1 and FEV1/FVC, and the latter included maximum tumour size, clinical T-stage, SUV on PET, tumour histology, lobe site and tumour distance ratio from the hilum. The clinical T-stage was reclassified for statistical processing as follows: Tis as 0, T1mi or T1a as 1, T1b as 2 and T1c as 3. Clavien-Dindo classification was reclassified for statistical processing as follows: none as 0, grade I as 1, grade II as 2, grade IIIa as 3 and grade IIIb as 4. The consolidation to tumour ratio was not included into the matching variables, because of confounding with clinical T-stage, tumour size and SUV. Because the year of surgery was not significantly different between the 2 procedures [median: 2016, interquartile range (IQR): 2015–2018 in lobectomy; and median: 2015, IQR: 2014–2017 in segmentectomy; P = 0.96], we did not include it in the analysis. Continuous variables were compared using Student’s t-test before matching and Mann–Whitney U-test after matching. Categorical variables were compared using χ2 or Fisher’s exact test before and after matching. Allowable calliper used for the matching was 0.25 multiplied standard deviation of logit transformed propensity scores. Match balance of continuous variables between the groups was assessed with standardized mean differences and was considered appropriate if none of the absolute standardized mean differences exceeded 0.1. Freedom from recurrence and OS were assessed using the Kaplan–Meier method. The matching analysis was performed using SPSS software (IBM Corp. New York, Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
For the matched patients, significant variables for the freedom from recurrence and OS were evaluated with univariable analysis, and the variables demonstrating a significant association were then analysed by multivariable analysis using the Cox regression model without adjustment. Values of P < 0.05 were accepted as significant. Statistical analyses were performed using Microsoft Excel for Windows 10.
RESULTS
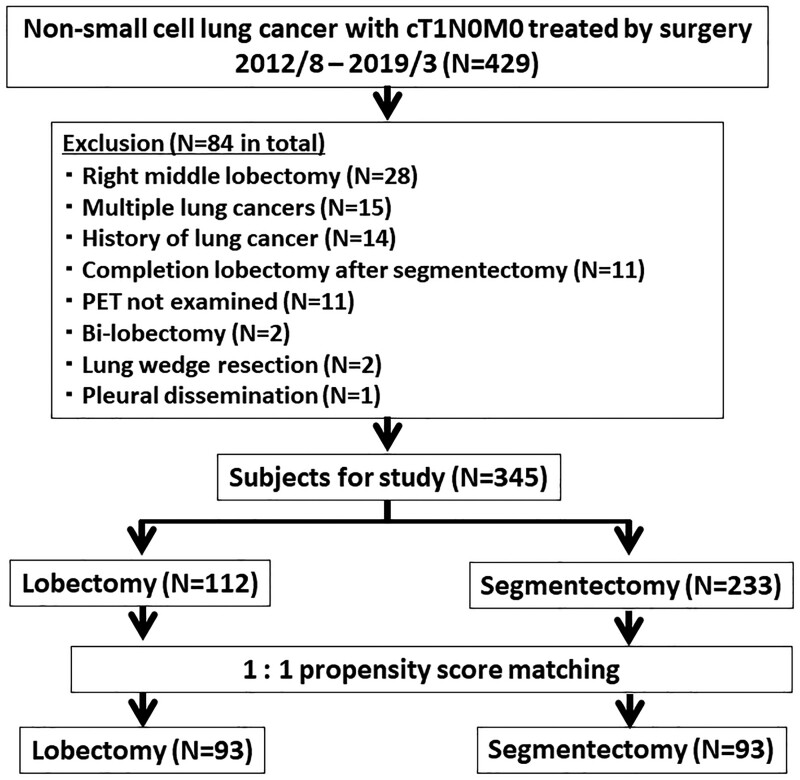
Figure 1 shows the patient algorithm. During the study period, 429 patients with cT1N0M0 NSCLC were treated by surgery. Eighty-four patients were excluded. Of the 84 excluded patients, 15 patients had multiple lung cancers, 14 had history of lung cancer and 11 did not receive PET examination, of which the total was 40. Of these 40 patients, 33 were treated by wedge resection. Two patients not classified into these were treated by wedge resection. Therefore, the total number of patients treated by wedge resection was 35 of the 429 entry patients (8%). Of the remaining 345 patients, 112 patients were treated by lobectomy and 233 underwent segmentectomy. A propensity score matching selected 93 patients each from the lobectomy and segmentectomy groups.
Figure 1:
Patient algorithm for the study. PET: positron emission tomography.
The differences between unmatched and matched patients are shown in Table 1. Before matching, comorbidity index, maximum tumour size, clinical tumour stage, SUV and tumour distance ratio from the hilum were significantly different between the lobectomy and segmentectomy groups (P = 0.02 to <0.001). After matching, all the differences in both the continuous and categorical variables between the 2 groups became non-significant (P = 0.35–0.95). All the standardized mean differences in matched continuous variables were <0.1. While the tumour distance ratio from the hilum before matching was also significantly smaller in the lobectomy group than that in the segmentectomy group (P < 0.001), it became non-significant after matching (P = 0.59), with a median value of 0.7 (IQR: 0.6–0.8) in the lobectomy group and 0.8 (IQR: 0.7–0.8) in the segmentectomy group. After matching, the tumour size >2 cm was present in 38 patients (41%) in the lobectomy group and in 36 patients (39%) in the segmentectomy group. The median consolidation to tumour ratios in the lobectomy and segmentectomy group before matching were 1.0 (IQR: 0.46–1) and 1.0 (IQR: 0.37–1), respectively, of which the difference was not significant (P = 0.13), and those after matching were 1.0 (IQR: 0.50–1) and 1.0 (IQR: 0.54–1), respectively, of which the difference was not significant (P = 0.95). The number of patients whose follow-up did not reach 2 years was 10 in the lobectomy group and 2 in the segmentectomy group.
Table 1:
Variables included in the matching
| Before matching |
After matching |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lobectomy | Segmentectomy | P-value | SMD | Lobectomy | Segmentectomy | P-value | SMD | |
| (n = 112) | (n = 223) | (n = 93) | ||||||
| Patient variable | ||||||||
| Age (years old) | 69 [63–75] | 69 [65–74] | 0.96 | 0.009 | 71 [64–75] | 69 [64–74] | 0.92 | 0.03 |
| Sex = male | 60 | 121 | 0.91 | 50 | 54 | 0.55 | ||
| Comorbidity index | 0 [0–1] | 1 [0–2] | 0.02 | 0.28 | 1 [0–2] | 1 [0–1] | 0.92 | 0.007 |
| FEV1/FVC | 73 [66–77] | 70 [64–77] | 0.2 | 0.14 | 72 [65–77] | 72 [65–77] | 0.98 | 0.02 |
| %FEV1 | 108 [98–120] | 107 [90–122] | 0.5 | 0.10 | 108 [98–120] | 105 [88–121] | 0.22 | 0.09 |
| Tumour variable | ||||||||
| Tumour size (cm) | 2.3 [1.8–2.7] | 1.8 [1.4–2.3] | <0.001 | 0.75 | 2.3 [1.8–2.6] | 2.2 [1.8–2.6] | 0.65 | 0.06 |
| Tumour size | (≤2 cm vs >2 cm | <0.001 | 0.76 | |||||
| ≤2 cm | 42 | 138 | 38 | 36 | ||||
| >2cm | 70 | 85 | 55 | 57 | ||||
| SUV | 2.8 [1.5–4.8] | 1.9 [1.0–3.9] | <0.001 | 0.28 | 2.7 [1.5–4.7 | 2.6 [1.3–5.2] | 0.69 | 0.02 |
| Distance from hilum | 0.7 [0.6–0.8] | 0.8 [0.7–0.9] | <0.001 | 1 | 0.7 [0.6–0.8] | 0.8 [0.7–0.8] | 0.76 | 0.06 |
| Clinical tumour stage | <0.001 | 0.99 | ||||||
| Tis | 7 | 26 | 7 | 5 | ||||
| T1mi/T1aN0M0 | 14 | 51 | 12 | 13 | ||||
| T1bN0M0 | 48 | 94 | 39 | 40 | ||||
| T1cN0M0 | 43 | 52 | 35 | 35 | ||||
| Histology | 0.96 | 0.8 | ||||||
| Ad | 92 | 184 | 76 | 76 | ||||
| Sq | 17 | 35 | 14 | 16 | ||||
| AdSq | 3 | 3 | 3 | 0 | ||||
| La | 0 | 1 | 0 | 1 | ||||
| Lobesite | 0.90 | 0.53 | ||||||
| Right upper lobe | 39 | 75 | 35 | 26 | ||||
| Right lower lobe | 30 | 55 | 25 | 26 | ||||
| Left upper lobe | 20 | 57 | 14 | 24 | ||||
| Left lower lobe | 23 | 36 | 19 | 17 | ||||
Each figure: median [interquartile range].
Ad: adenocarcinoma; AdSq: adenosquamous carcinoma; FEV1: forced expiratory volume in one second; FVC: functional vital capacity; La: large cell carcinoma; SMD: standardized mean difference; Sq: squamous cell carcinoma; SUV: standardized uptake value.
In the matched patients, segmentectomy of more than 5 subsegments was performed in 17 patients (18%). Simple segmentectomy, such as resection of the lower lobe apical segment, basal segment, left upper division and left lingula division, were performed in 31 patients (33%).
Table 2 shows the postoperative complications, drainage period and prolonged air leakage in the lobectomy and segmentectomy groups. No perioperative mortality was observed. There was no significant difference in the Clavien-Dindo grade of postoperative complications between the 2 groups (P = 0.94). Two patients had grade IIIb complications in the lobectomy group; one patient experienced an empyema requiring thoracoplasty and the other experienced postoperative bleeding requiring reoperation. One patient with grade IIIb in the segmentectomy group experienced postoperative bleeding requiring reoperation. The median chest tube drainage period was 2 days (IQR: 2–3) in both groups. Prolonged air leakage, that continued more than 7 days but was not treated (grade I in Clavien-Dindo classification), was seen in 2 patients in both groups, and prolonged air leakage that required chemical-pleurodesis or re-drainage (grade IIIa) was seen in 9 patients (10%) in the lobectomy group and 8 (9%) in the segmentectomy group. Thus, a total of 11 patients (12%) in the lobectomy group and 10 (11%) in the segmentectomy group showed prolonged air leakage, of which the difference was not significant (P = 0.82).
Table 2:
Postoperative complication and drainage period: lobectomy versus segmentectomy
| Outcome | Lobectomy | Segmentectomy | Difference |
|---|---|---|---|
| Total | 93 | 93 | |
| Complications (Clavien-Dindo) | P = 0.94 | ||
| None | 77 | 81 | |
| I | 5 | 1 | |
| II | 0 | 2 | |
| IIIa | 9 | 8 | |
| IIIb | 2 | 1 | |
| Drainage period (days) | |||
| Median | 2 | 2 | |
| Interquartile range | 2–3 | 2–3 | |
| Prolonged air leakage (Clavien-Dindo) | |||
| None | 82 | 83 | P = 0.82 |
| I | 2 | 2 | |
| IIIa | 9 | 8 | |
Prolonged air leakage with grade I: air leakage more than 7 days but not treated.
Prolonged air leakage with grade IIIa: requiring chemo-pleurodesis or re-drainage.
The pathological tumour-node-metastasis was not significantly different between the 2 groups (Supplementary Material, Table S1, P = 0.87). Pathological N1 or N2 stages were seen in 12 patients (13%) in the lobectomy group and 9 (10%) in the segmentectomy group, of which the difference was not significant (P = 0.49). The 9 patients with pathological N1 or N2 in the segmentectomy group were not converted to lobectomy, because of elderly (n = 3), microscopic metastasis in the permanent section (n = 3), and a single #12 metastasis in a case of the lower lobe apical segmentectomy, which could be curable by segmentectomy (n = 3).
Postoperative adjuvant chemotherapy was administered in 12 patients (13%) in the lobectomy group and 6 (6%) in the segmentectomy group, but the difference was not significant (P = 0.14). Tumour recurrences were observed in 17 patients (18%) in the lobectomy group (Supplementary Material, Table S2) and 14 (15%) in the segmentectomy group (Supplementary Material, Table S3), of which difference was not significant (P = 0.56). Intrathoracic recurrences were seen in 8 patients (9%) in the lobectomy group and 10 (11%) in the segmentectomy group. Of the 10 patients with intrathoracic recurrences in the segmentectomy group, 5 patients showed local recurrences, i.e. recurrences at the surgical margin in 3 patients and within the preserved lobe in 2, of which 4 were treated by completion lobectomy, resulting in alive without disease in 3 patients and dead with disease in 1.
Figure 2 shows the freedom from recurrence curves, which showed no significant difference between lobectomy and segmentectomy (P = 0.38, log-rank test). Five-year freedom from recurrence rates were 79% in the lobectomy group and 84% in the segmentectomy group.
Figure 2:
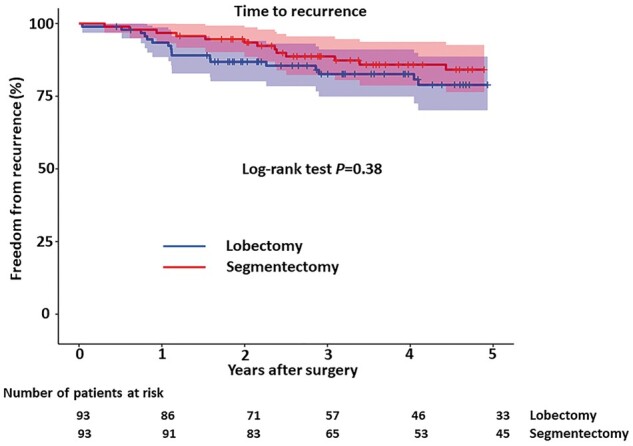
Freedom from recurrence period after lobectomy versus segmentectomy. Dotted lines show 95% confidence limits.
Figure 3 shows the OS curves, which showed no significant difference between the groups (P = 0.51, log-rank test). Five-year OS rates were 86% in both groups.
Figure 3:
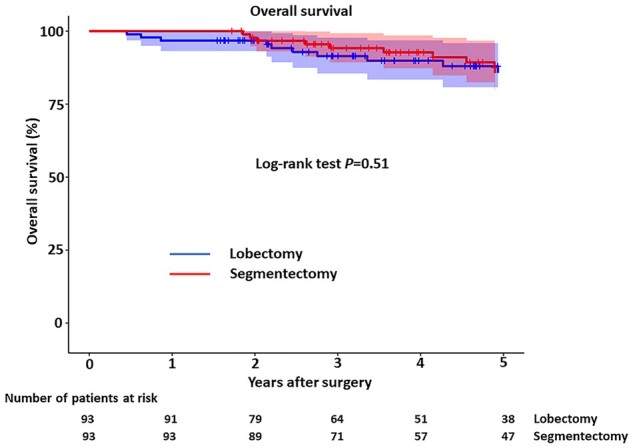
Overall survival after lobectomy versus segmentectomy. Dotted lines show 95% confidence limits.
Postoperative pulmonary function could be examined in all patients, except one patient in the lobectomy group due to recurrence within 6 months after surgery. Figure 4 shows the distribution of %PPF in the remaining 92 matched pairs. The median %PPF in the segmentectomy group was 93.2% (IQR: 82.8–89.1%), which was significantly higher than 85.9% (IQR: 81.0–91.8%) in the lobectomy group (P < 0.001).
Figure 4:
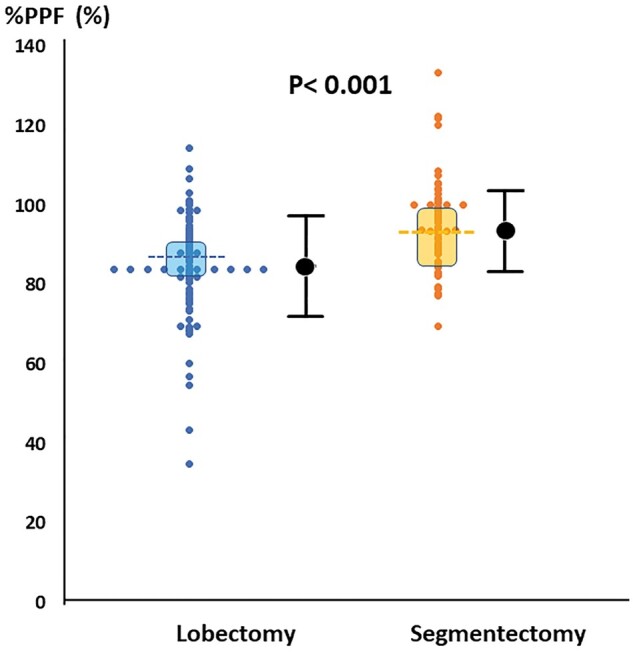
Percentage of postoperative preserved pulmonary function after lobectomy versus segmentectomy. %PPF: percentage of preserved pulmonary function. The dotted line showed the median value. Shadow area showed the first quartile and the third quartile.
Table 3 shows the results of univariable analysis of the variables for freedom from recurrence and OS. For the freedom from recurrence, the clinical tumour stage and SUV were significant (P = 0.01 and 0.002, respectively). For the OS, age, comorbidity index, FEV1/FVC, clinical tumour stage, SUV and tumour histology were significant (P = 0.01, 0.03, 0.02, 0.003, 0.009, and 0.003, respectively).
Table 3:
Univariable univariate analysis of freedom from recurrence and overall survival
| Variables | P-value |
|---|---|
| Freedom from recurrence | |
| Patient variables | |
| Age | 0.86 |
| Sex | 0.51 |
| Comorbidity index | 0.5 |
| FEV1/FVC | 0.11 |
| %FEV1 | 0.18 |
| Tumour variables | |
| Operation procedure | 0.56 |
| Tumour size | 0.27 |
| Clinical tumour stage | 0.01a |
| SUV | 0.002a |
| Histology | 0.54 |
| Tumour distance from hilum | 0.18 |
| Lobe site | 1 |
| Overall survival | |
| Patient variables | |
| Age | 0.01a |
| Sex | 0.16 |
| Comorbidity index | 0.03a |
| FEV1/FVC | 0.02a |
| %FEV1 | 0.09 |
| Tumour variables | |
| Operation procedure | 0.82 |
| Tumour size | 0.07 |
| Clinical tumour stage | 0.003a |
| SUV | 0.009a |
| Histology | 0.003a |
| Tumour distance from hilum | 0.25 |
| Lobe site | 0.94 |
Statistically significant.
FEV1: forced expiratory volume in 1 s; FVC: functional vital capacity; SUV: standardized uptake value.
Table 4 shows the results of multivariable analysis of the variables which were significant in the univariable analysis. Proportionality assumption was confirmed by Shoenfeld residuals. For the freedom from recurrence, clinical tumour stage and SUV were significant (P = 0.04 and 0.005, respectively). For the OS, clinical tumour stage was significant (P = 0.02), but the SUV did not reach the significance (P = 0.09).
Table 4:
Multivariable multivariate analysis of freedom from recurrence and overall survival
| Hazard ratio [95% CI] | P-value | |
|---|---|---|
| Freedom from recurrence | ||
| Clinical tumour stage | 1.75 [1.02, 2.98] | 0.04a |
| SUV | 1.12 [1.03, 1.21] | 0.005a |
| Overall survival | ||
| Age | 1.05 [0.98, 1.12] | 0.18 |
| Comorbidity index | 1.26 [0.82, 1.94] | 0.29 |
| FEV1/FVC | 0.99 [0.95, 1.03] | 0.62 |
| Clinical tumour stage | 2.47 [1.15, 5.29] | 0.02a |
| SUV | 1.11 [0.99, 1.24] | 0.09 |
| Histology | 1.23 [0.64, 2.37] | 0.53 |
Statistically significant.
CI: confidence interval; FEV1: forced expiratory volume in 1 s; FVC: functional vital capacity; SUV: standardized uptake value.
DISCUSSION
The present study showed the following: (i) freedom from recurrence and OS were not significantly different between the lobectomy and segmentectomy groups; (ii) local recurrences specifically caused by segmentectomy were experienced in 5 of 93 patients (5%); (iii) postoperative complications, chest drainage period and prolonged air leakage rate showed no significant differences between the 2 groups; and (iv) postoperative preservation of pulmonary function after segmentectomy was significantly higher than that after lobectomy.
There have been 3 previous studies that examined the difference in prognosis between segmentectomy and lobectomy using propensity score matching [1–3]. Deng et al. [1] reported that the recurrence-free survival and OS were not different between the 2 groups in tumours ≤2 cm, whereas these were worse in the segmentectomy group in tumours that were 2.1–3 cm, with a marginal significance (P = 0.05). In contrast, Landreneau et al. [2], in an analysis of T1 (≤3 cm) and T2 tumours (>3 and ≤5 cm), reported that there were no significant differences in recurrence and OS between segmentectomy and lobectomy, and they also reported that tumour size was not a significant variable for predicting recurrence. The present data, including maximum tumour size >2 cm in ∼40%, showed similar results with that of Landreneau et al.; the maximum tumour size including ground-glass opacity area was not a significant variable for predicting recurrence. However, the tumour stage defined by size of solid part was a significant factor for predicting recurrence.
The present study added the tumour distance ratio from the lung hilum to the matching variables. Before matching, tumours in the lobectomy group were located significantly nearer to the lung hilum than those in the segmentectomy group. The tumour distance ratio from the hilum became non-significant after matching. The Japanese randomized control trial between segmentectomy and lobectomy (JCOG0802/WJOG4607L) set one of the inclusion criteria as ‘center of tumour is located in the outer third of the lung field’ [8], which was a similar tumour location in the present study. It is considered that when the cT1N0M0 NSCLC is located within the outer third of the lung field, the postoperative recurrence and OS would be similar between lobectomy and segmentectomy.
The JCOG0802/WJOG4607L reported that while postoperative complications and median postoperative drainage period were not different between lobectomy and segmentectomy groups, prolonged air leakage was more frequent in the segmentectomy group than in the lobectomy group (P = 0.04) [17]. The present study showed no significant differences in the rate of prolonged air leakage between the 2 groups (P = 0.82). We closed the pleura-defective site of preserved lobe by suturing or covering using polyglycolic acid mesh with more than 2 layers [15], which could make the postoperative air leakage after segmentectomy similar with that after lobectomy.
The only purpose of segmentectomy is to preserve pulmonary function higher than lobectomy. It has been recently reported that the advantage of segmentectomy preserving higher pulmonary function than lobectomy is seen in the resection of <2 segments or 5 subsegments [18, 19]. The propensity score matching study by Deng et al. [1] indicated non-significant difference of postoperative pulmonary function between lobectomy and segmentectomy, whereas they did not show the extent of resection in the segmentectomy group. In the other 2 matching studies by Landreneau et al. [2] and Kamigaichi et al. [3], 131 of 312 patients (42%) and 42/130 patients (32%) in the segmentectomy group were treated by the resection of more than 5 subsegments, respectively; however, they did not show the difference of postoperative pulmonary function. The JCOG0802/WJOG4607L reported that the preservation of pulmonary function after segmentectomy was significantly higher than that after lobectomy (89.6% vs 86.9%) (P < 0.001) [8], whereas the meeting abstract has not clarified the extent of segmentectomy. The present study had patients treated with resection of more than 5 subsegments in only 17/93 (18%) cases, which could make the preservation of pulmonary function in the segmentectomy group overwhelmingly higher than that in the lobectomy group (93.2% vs 85.9% in median value).
Local recurrence specifically caused by segmentectomy was seen in 5 of 93 (5%) patients in the present study, the rate of which was almost similar with that in a previous report, i.e. 4% [4]. It should be kept in mind that, while the freedom of recurrence was similar between lobectomy and segmentectomy, local recurrence occurring at the surgical margin or in the preserved lobe is an inevitable risk of segmentectomy.
We conducted segmentectomy via anterolateral or limited lateral thoracotomy. While lobectomy can be conducted using video-assisted thoracic surgery (VATS) without difficulty because of simple anatomy, segmentectomy is much more difficult than lobectomy when using only monitor guidance. Even when possible, the risk of local recurrence must be considered, because the sufficient surgical margin is sometimes hard to be taken and the complete hilar nodal dissection is difficult under VATS. The VATS also carries an opposite risk of taking excessive surgical margins due to cutting the lung tissue with using only stapler, resulting in decreased pulmonary function. The previous reports evaluating pain scores showed no significant difference of postoperative pain between VATS and limited thoracotomy [13, 20]. We believe that the thoracotomy would be necessary for segmentectomy, because the direct vision can reduce those problems associated with VATS procedures.
Limitations
The limitations of the present study are as follows: (i) The study design was retrospective in nature, (ii) one of the indications for segmentectomy was determined by a predicted function of the preserved lobe, which is not generally examined; and (iii) the numbers of sample and event were too small to show non-inferiority of segmentectomy compared to lobectomy.
In conclusion, segmentectomy could be one of the options for cT1N0M0 NSCLC as well as being able to preserve pulmonary function better than lobectomy.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Author contributions
Hiroaki Nomori: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Ikuo Yamazaki: Data curation. Youichi Machida: Data curation. Ayumu Otsuki: Data curation. Yue Cong: Data curation. Hiroshi Sugimura: Data curation. Yu Oyama: Data curation.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Pierre-Yves Brichon and the other anonymous reviewers for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CT
Computed tomography
- FDG
Fluorodeoxyglucose
- FVC
Forced vital capacity
- FEV1
Forced expiratory volume in 1 s
- IQR
Interquartile range
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PET
Positron emission tomography
- %PPF
Preserved pulmonary function
- SUV
Standardized uptake value
- VATS
Video-assisted thoracic surgery
Date and number of IRB approval: February 2014 (approval number: 14-003).
REFERENCES
- 1. Deng B, Cassivi SD, de Andrade M, Nichols FC, Trastek VF, Wang Y et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landreneau RJ, Normolle DP, Christie NA, Awais O, Wizorek JJ, Abbas G et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamigaichi A, Tsutani Y, Mimae T, Miyata Y, Ito H, Nakayama H et al. Prognosis of segmentectomy and lobectomy for radiologically aggressive small-sized lung cancer. Eur J Cardiothorac Surg 2020;58:1245–53. [DOI] [PubMed] [Google Scholar]
- 4. Nomori H, Mori T, Shiraishi A, Fujino K, Sato Y, Ito T et al. Long-term Prognosis after segmentectomy for cT1N0M0 non-small cell lung cancer. Ann Thorac Surg 2019;107:1500–6. [DOI] [PubMed] [Google Scholar]
- 5. Rao S, Ye L, Min L, Zhao G, Chen Y, Huang Y et al. Meta-analysis of segmentectomy versus lobectomy for radiologically pure solid or solid-component stage IA non-small cell lung cancer. J Cardiothorac Surg 2019;14:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whitson BA, Groth SS, Andrade RS, Maddaus MA, Habermann EB, D'Cunha J. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943–50. [DOI] [PubMed] [Google Scholar]
- 7. Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640–7. [DOI] [PubMed] [Google Scholar]
- 8. Asamura H, Okada M, Saji H, Tsuboi M, Nakajima R. Randomized trial of segmentectomy compared to lobectomy in small-sized peripheral non-small cell lung cancer. 101st Annual Meeting of American Association for Thoracic Surgery 2021. pp. 311–2. [Google Scholar]
- 9. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P et al. ; IASLC Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2017;12:1109–21. [DOI] [PubMed] [Google Scholar]
- 13. Nomori H, Cong Y, Sugimura H. Limited thoracotomy for segmentectomy: a comparison of postoperative pain with thoracoscopic lobectomy. Surg Today 2016;46:1243–8. [DOI] [PubMed] [Google Scholar]
- 14. Nomori H, Cong Y, Sugimura H. Utility and pitfalls of sentinel node identification using indocyanine green during segmentectomy for cT1N0M0 non-small cell lung cancer. Surg Today 2016;46:908–13. [DOI] [PubMed] [Google Scholar]
- 15. Nomori H, Abe M, Sugimura H, Takegawa Y, Oka S, Takeshi A. Triple-layer sealing with absorptive mesh and fibrin glue is effective in preventing air leakage after segmentectomy: results from experiments and clinical study. Eur J Cardiothorac Surg 2014;45:910–3. [DOI] [PubMed] [Google Scholar]
- 16. Nomori H, Horio H, Suemasu K. Anterior limited thoracotomy with intrathoracic illumination for lung cancer: its advantages over anteroaxillary and posterolateral thoracotomy. Chest 1999;115:874–80. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki K, Saji H, Aokage K, Watanabe S-I, Okada M, Mizusawa J et al. ; Japan Clinical Oncology Group. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895–907. [DOI] [PubMed] [Google Scholar]
- 18. Nomori H, Shiraishi A, Yamazaki I, Ohtsuki A, Cong Y, Sugimura H et al. Extent of segmentectomy that achieves greater lung preservation than lobectomy. Ann Thorac Surg 2020. doi:10.1016/j.athoracsur.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 19. Nomori H, Cong Y, Sugimura H. Systemic and regional pulmonary function after segmentectomy. J Thorac Cardiovasc Surg 2016;152:747–53. [DOI] [PubMed] [Google Scholar]
- 20. Rizk NP, Ghanie A, Hsu M, Bains MS, Downey RJ, Sarkaria IS et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg 2014;98:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.