Abstract
Objective
To investigate whether the diagnostic performance of CT angiography (CTA) could be improved by modifying the conventional criterion (anastomosis site abnormality) to diagnose hepatic artery occlusion (HAO) after liver transplantation (LT) in suspected patients with Doppler ultrasound (US) abnormalities.
Materials and Methods
One hundred thirty-four adult LT recipients (88 males and 46 females; mean age, 52.7 years) with suspected HAO on Doppler US (40 HAO and 94 non-HAO according to the reference standards) were included. We evaluated 1) abnormalities in the HA anastomosis, categorized as a cutoff, ≥ 50% stenosis at the anastomotic site, or diffuse stenosis at both graft and recipient sides around the anastomosis, and 2) abnormalities in the distal run-off, including invisibility or irregular, faint, and discontinuous enhancement. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the conventional (considering anastomosis site abnormalities alone) and modified CTA criteria (abnormalities in both the anastomosis site and distal run-off) for the diagnosis of HAO were calculated and compared using the McNemar test.
Results
By using the conventional criterion to diagnose HAO, the sensitivity, specificity, PPV, NPV, and accuracy were 100% (40/40), 74.5% (70/94), 62.5% (40/64), 100% (70/70), and 82.1% (110/134), respectively. The modified criterion for diagnosing HAO showed significantly increased specificity (93.6%, 88/94) and accuracy (93.3%, 125/134) compared to that with the conventional criterion (p = 0.001 and 0.002, respectively), although the sensitivity (92.5%, 37/40) decreased slightly without statistical significance (p = 0.250).
Conclusion
The modified criterion considering abnormalities in both the anastomosis site and distal run-off improved the diagnostic performance of CTA for HAO in suspected patients with Doppler US abnormalities, particularly by increasing the specificity.
Keywords: Hepatic artery, CT angiography, Diagnostic performance, Liver transplantation
INTRODUCTION
Hepatic artery occlusion (HAO) is a critical complication of liver allograft dysfunction and failure after liver transplantation (LT). Although the incidence of such complications has been reduced to less than 5% because of improved surgical techniques [1,2], HAO is still a serious complication because poor or late treatment can cause mortality or graft failure [1,3,4]. If it is diagnosed early and promptly managed during the postoperative period, the outcome is favorable.
Three-dimensional CT angiography (CTA) can complement Doppler ultrasound (US), which has low specificity, and CTA has been considered as a second-line tool in the diagnosis of HAO after LT [5]. Considering the invasiveness and potential complications of hepatic arteriography, which is commonly indicated for a confirmative diagnosis of HAO, an appropriate second-line imaging tool with a reasonable specificity is needed.
Several studies have reported the diagnostic performance of CTA in diagnosing HAO with a three-dimensional reformatting technique, such as maximum intensity projection (MIP) or volume rendering [6,7,8]. CTA showed almost perfect sensitivity for detecting HAO; however, its specificity is somewhat low (83.5%–87.5%) [1,8]. The conventional criterion used to diagnose HAO in previous studies was the degree of luminal narrowing at the anastomotic site (i.e., > 75% as severe and ≥ 50% as moderate stenosis) [1,8]. Therefore, to increase the specificity for the accurate diagnosis of HAO and to reduce or even eliminate the need for invasive angiography, it might be helpful to modify the CTA criteria and consider any ancillary findings, such as distal run-off abnormality at the post-anastomotic HA graft. Therefore, the purpose of our study was to investigate whether the diagnostic performance of CTA for HAO after LT could be improved by modifying the diagnostic criteria by considering both anastomosis site abnormalities and distal run-off abnormalities.
MATERIALS AND METHODS
The Institutional Review Board approved this study, and the need to obtain informed patient consent was waived because of the retrospective nature of the analyses (IRB No. 2017-0313).
Patients
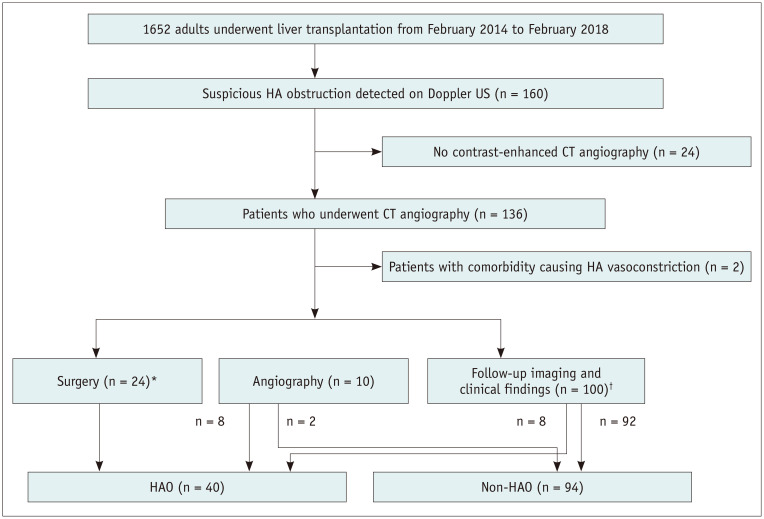
Between February 2014 and February 2018, 1652 adults (19 years old or older) underwent LT at a single medical institution. Among them, 160 patients (9.7%) were suspected of having HAO on Doppler US during the first hospitalization. The US criteria for the diagnosis of HAO on Doppler are as follows: 1) no Doppler signal, 2) tardus parvus waveform (with resistive index < 0.5, systolic acceleration time > 0.08 seconds) at the graft HA, or 3) a focal high-velocity jet > 2 m/s at the anastomosis [9,10]. An extensive radiology-based postoperative complication surveillance program was implemented at the institution where this study was conducted. Doppler US was routinely performed daily during the first 3 days after surgery and, subsequently, once or twice per week during hospitalization. Additional studies were performed based on clinical requirements (i.e., elevation of liver enzymes), as necessary. We excluded 24 patients in whom CTA was not obtained within a week after the detection of an abnormality on Doppler US. We also excluded two patients with comorbidities, such as pancreatitis or septic shock, that led to HA vasoconstriction. The exclusion was because, given the purpose of this study, HAO was defined as an anastomotic complication that might require revision surgery or endovascular intervention. Figure 1 illustrates the flowchart of the study population. Finally, 134 patients (patients with HAO, n = 40; patients without HAO, n = 94) were included. The recipient characteristics in each category (HAO and non-HAO groups) are summarized in Table 1. We reviewed electronic medical records to obtain the liver enzyme levels (i.e., aspartate transaminase [AST]; alanine aminotransferase [ALT]) on the day before and the day of the CT scan.
Fig. 1. Flow diagram describing the flow of the study population.
*The surgery included angioplasty (n = 20) and liver re-transplantation (n = 4), †Follow-up imaging and clinical findings determined HAO when there was persistent no flow or progressive change from the tardus parvus pattern to no flow on Doppler US follow-up studies, associated with non-anastomotic biliary complications, such as bile duct necrosis/biloma, development of multifocal subsegmental ischemia/infarction, and HAO at the anastomotic site with a collateral reconstruction of the intrahepatic artery branches on follow-up CT, and consistent clinical findings of graft dysfunction or even failure. Non-HAO was defined as normalization of Doppler US abnormalities and no association with complications described above within 6 months of follow-up. HA = hepatic artery, HAO = hepatic artery occlusion, US = ultrasound
Table 1. Patient Characteristics.
| Characteristics | Patients with HAO (n = 40) | Patients without HAO (n = 94) | P | ||
|---|---|---|---|---|---|
| Age, year | 55.2 ± 9.7 (21–74) | 51.7 ± 10.6 (19–74) | 0.079 | ||
| Sex, male:female | 24:16 | 64:30 | 0.369 | ||
| Body weight, kg | 65.8 ± 12.7 (48.3–102.0) | 68.5 ± 17.1 (37.0–119.3) | 0.358 | ||
| Body mass index, kg/m2 | 24.6 ± 4.6 (18.7–40.7) | 24.8 ± 4.9 (14.6–39.1) | 0.820 | ||
| Transplantation type | 0.227 | ||||
| DDLT | 9 | 31 | |||
| LDLT (dual grafts) | 31 (4) | 63 (36) | |||
| Doppler abnormality type | < 0.001 | ||||
| No detectable flow | 36 | 35 | |||
| Tardus parvus waveform | 4 | 59 | |||
| Laboratory findings | |||||
| AST, IU/L | |||||
| D (-1) | 201.9 ± 239.8 (11–952) | 240.1 ± 569.1 (11–3482) | 0.687 | ||
| D (0) | 363.5 ± 470.1 (19–2553) | 287.4 ± 557.7 (11–3780) | 0.452 | ||
| AST ratio | 4.7 ± 12.2 (0.2–77.0) | 2.5 ± 3.9 (0.2–20.6) | 0.190 | ||
| ALT, IU/L | |||||
| D (-1) | 180.5 ± 195.0 (6–991) | 215.6 ± 385.7 (6–2403) | 0.591 | ||
| D (0) | 356.8 ± 365.7 (8–1764) | 239.5 ± 362.2 (10–2413) | 0.090 | ||
| ALT ratio | 5.8 ± 17.9 (0.3–113.0) | 2.9 ± 5.3 (0.5–28.8) | 0.153 | ||
Values are mean ± standard deviation with the range in parentheses or the patient number. ALT = alanine aminotransferase, ALT ratio = ALT value of D (0) divided by D (-1) value, AST = aspartate transaminase, AST ratio = AST value of D (0) divided by D (-1) value, DDLT = deceased donor liver transplant, D (0) = the day of CT scan, D (-1) = the day before CT scan, HAO = hepatic artery occlusion, LDLT = living donor liver transplant
Image Analysis
We evaluated anastomotic site abnormalities, categorized as a cutoff, focal stenosis ≥ 50% at the anastomotic site, or diffuse stenosis at both graft and recipient sides around the anastomosis. Distal run-off abnormalities were also evaluated as follows: invisible or irregular, faint, and discontinuous enhancement (Figs. 2, 3, 4). CTAs were anonymized, coded, and loaded onto a picture archiving and communication system folder. Images of each patient were evaluated using MIP in conjunction with multi-planar images that could be evaluated interactively on the picture archiving and communication system by scrolling through them. A consensus review was performed by two reviewers (blinded for a peer reviewer, with more than 15 years of experience in LT imaging and blinded for a peer reviewer, with 6 years of experience in LT imaging).
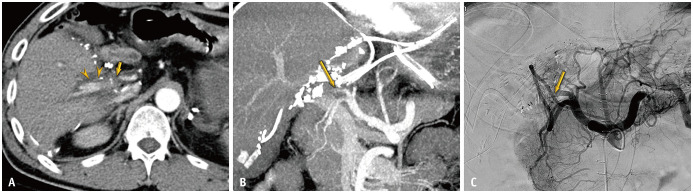
Fig. 2. True-positive diagnosis using the modified criterion of anastomotic site abnormality and distal run-off abnormality in a 49-year-old male after living-donor liver transplantation.
A. Axial CT image shows combination of anastomosis site abnormality (cutoff type, arrow) and distal run-off abnormality (irregular and discontinuous enhancement) (arrowheads). B. Maximal intensity projection image shows cutoff type anastomosis site abnormality (arrow). C. Angiography shows near total occlusion of the hepatic artery around the anastomosis (arrow).
Fig. 3. False-positive diagnosis using the conventional criterion for anastomosis site abnormality in a 46-year-old male who underwent deceased-donor liver transplantation.
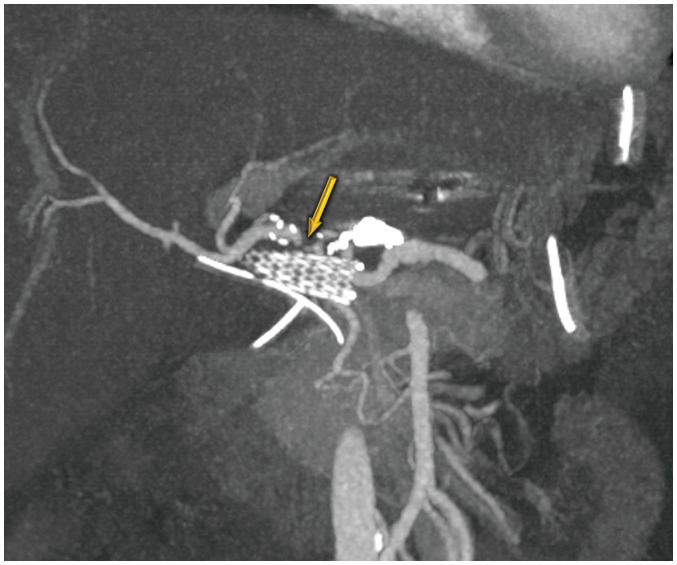
Maximal intensity projection image shows more than 50% focal narrowing (arrow) without distal run-off abnormality because of hepatic artery angulation. Doppler ultrasound abnormalities were normalized after 1 month, and no associated complication was seen in this patient within 6 months of follow-up.
Fig. 4. False-positive diagnosis using the conventional criterion of anastomosis site abnormality without distal run-off abnormality in a 48-year-old female after living-donor liver transplantation.
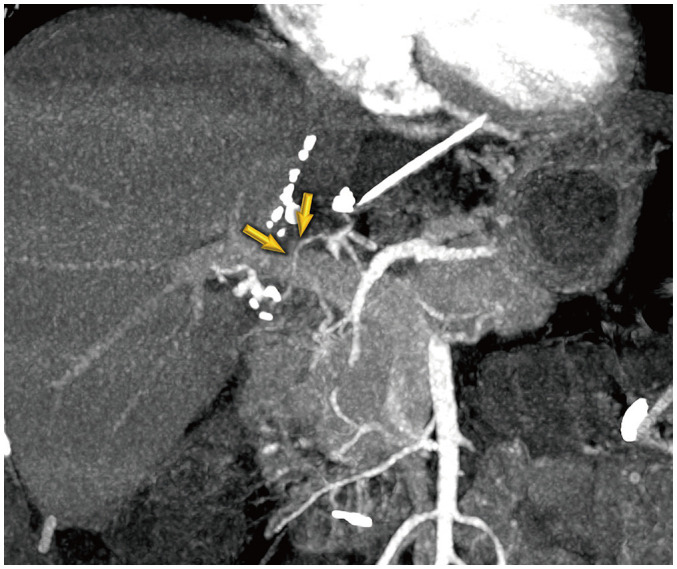
Maximal intensity projection image shows diffuse stenosis at the anastomosis site (arrows). The patient had normal laboratory findings. Doppler ultrasound abnormalities were normalized after 25 days, and no associated complication was seen in this patient within 6 months of follow-up.
Reference Standards
The reference diagnosis of HAO was made as follows: 1) hepatic arteriography showing findings from near-total or total occlusion to luminal diameter < 50% and flow disturbance due to thrombosis or stenosis, 2) surgery requiring HA revision or re-transplantation because of graft failure related to HAO, or 3) follow-up imaging and clinical findings revealing persistent no flow or progressive change from the tardus parvus pattern to no flow on follow-up Doppler US examination accompanied by relevant follow-up CT findings (non-anastomotic biliary complications, such as bile duct necrosis/biloma, development of multifocal subsegmental ischemia/infarction, and HAO at the anastomotic site with collateral reconstruction of the intra-HA branches) and clinical findings of graft dysfunction or even failure. Non-HAO was defined as normalization of Doppler US abnormalities and no development of the complications described above within 6 months of follow-up.
Statistical Analysis
The demographics of the two groups (HAO vs. non-HAO) were compared using the Student’s t test after testing for normality using the Kolmogorov-Smirnov test for continuous variables and chi-square test for discrete variables.
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of CTA for the diagnosis of HAO were calculated using either the conventional criterion (considering anastomosis site abnormalities alone) or the modified criterion (requiring abnormalities in both the anastomosis site and distal run-off, i.e., positive for HAO on CT when both the anastomosis and distal run-off were abnormal). Distal run-off findings were used only to modify the positive anastomotic findings. The diagnostic performance of each criterion was compared using the McNemar’s test.
All statistical analyses were performed using IBM SPSS Statistics for Windows version 26.0 (IBM Corp.) or MedCalc version19.7 (MedCalc). Two-tailed p values less than 0.05 were considered statistically significant.
RESULTS
Figure 1 illustrates the distribution of the study population for each category. Recipients with HAO had the first documented abnormality on Doppler US at a mean ± standard deviation (SD) of 12.6 ± 21.0 days (range, 1–165 days; median, 6 days) after LT. HAO was confirmed by surgery (HA revision [n = 20] or re-transplantation for graft failure related to HAO [n = 4]), angiography (n = 8), and follow-up studies (n = 8). The mean duration ± SD between the CTA and angiography or HA revision was 1.4 ± 2.9 days. HAO occurred more frequently in patients with no detectable flow than in those with a tardus parvus waveform on Doppler US (p < 0.001). There were no significant differences in liver enzymes on the day before or the day of CT scan or their ratios between the HAO and non-HAO groups (p = 0.090–0.687) (Table 1).
Table 2 summarizes the diagnostic performance of the conventional and modified CTA criteria for the diagnosis of HAO in suspected patients with Doppler US abnormalities. Although the conventional criterion led to 100% sensitivity, the PPV was relatively low at 62.5%. Using the modified criterion, although the sensitivity slightly decreased without statistical significance (92.5% vs. 100%, p = 0.250), the specificity and PPV increased compared to those with conventional criteria (specificity, 93.6% vs. 74.5%, p = 0.001; PPV, 86.0% vs. 62.5%). Consequently, the modified criterion showed a higher accuracy than that of the conventional criterion (93.3% vs. 82.1%, p = 0.002).
Table 2. Diagnostic Performance of Conventional and Modified CT Angiography Criteria for Hepatic Artery Occlusion.
| Parameter | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Conventional criterion (anastomosis site abnormalities alone) | 100 (91.2, 100.0) [40/40] |
74.5 (64.4, 82.9) [70/94] |
62.5 [40/64] |
100 [70/70] |
82.1 (74.5, 88.2) [110/134] |
| Modified criterion (both anastomosis site and distal run-off abnormalities) | 92.5 (79.6, 98.4) [37/40] |
93.6 (86.6, 97.6) [88/94] |
86.0 [37/43] |
96.7 [88/91] |
93.3 (87.6, 96.9) [125/134] |
| p * | 0.250 | 0.001 | 0.002 |
Data are presented as percentages with 95% confidence intervals in parentheses and number of patients in brackets. Anastomosis site abnormality was defined as more than 50% stenosis at or around the anastomosis. Distal run-off abnormality means no visible, or irregular, faint, and discontinuous enhancement of post anastomotic hepatic artery. *The p values were by comparison between conventional and modified criteria. NPV = negative predictive value, PPV = positive predictive value
When patients with anastomotic site abnormalities (n = 64) were sub-categorized according to specific findings, including a cutoff, focal stenosis at the anastomotic site, or diffuse stenosis in both graft and recipient HAs around the anastomosis, PPV was 90.3% (28/31) for the cutoff, 30.0% (6/20) for focal stenosis, and 46.2% (6/13) for diffuse stenosis; the modified criterion decreased false positives, particularly for focal stenosis and diffuse stenosis subcategories of the anastomosis site abnormalities (Table 3). This was assumed to be related to insignificant focal HA stenosis and angulation of the HA in the former and low-grade HA dissection in the latter.
Table 3. True Positive and False Positive Diagnosis of Hepatic Artery Occlusion in 64 Patients with Anastomosis Site Abnormality Divided into Subcategories.
| Anastomosis Site Abnormality Subcategories | True Positive | False Positive | |
|---|---|---|---|
| Cutoff (n = 31) | |||
| Cutoff anastomosis abnormality with distal run-off abnormality (n = 29) | 27 (93.1) | 2 (6.9) | |
| Cutoff anastomosis abnormality without distal run-off abnormality (n = 2) | 1 (50.0) | 1 (50.0) | |
| Focal stenosis (n = 20) | |||
| Focal anastomosis abnormality with distal run-off abnormality (n = 9) | 5 (55.6) | 4 (44.4) | |
| Focal anastomosis abnormality without distal run-off abnormality (n = 11) | 1 (9.1) | 10 (90.9) | |
| Diffuse stenosis with recipient hepatic artery stenosis (n = 13) | |||
| Diffuse stenosis with distal run-off abnormality (n = 5) | 5 (100) | 0 (0) | |
| Diffuse stenosis without distal run-off abnormality (n = 8) | 1 (12.5) | 7 (87.5) | |
Data in parentheses are percentages. The majority of false positive occurred when there was anastomosis site abnormality alone, without distal run-off abnormality; therefore, it can be reduced when a combination of both anastomosis site abnormality and distal run-off abnormality is applied as a modified criterion.
DISCUSSION
According to previous studies, the conventional criterion (anastomotic site abnormality alone) has almost perfect sensitivity; however, a specificity of 80% has also been reported [1,8]. In this study, the modified criterion (a combination of both anastomosis site and distal run-off abnormalities) showed significantly higher accuracy and specificity than that of the anastomotic site abnormality alone (82.1% vs. 93.3%, p = 0.002; 74.5% vs. 93.6%, p < 0.001), despite a slight decrease in sensitivity (100% vs. 92.5%, p = 0.250) in patients with suspected HAO on Doppler US. Therefore, in this era of advanced CT, distal run-off HA evaluation is necessary, and unnecessary angiography might be avoided by increasing the specificity of CTA.
HAO is a serious complication that threatens graft survival after LT [11]. CTA is considered a second-line investigation modality for the evaluation of HAO after LT when an HA abnormality is suspected on Doppler US [8]. Considering the invasiveness and potential complications of conventional hepatic arteriography, the accurate evaluation of HAO using a second-line imaging tool is important.
In general, it is assumed that vascular patency and hemodynamic significance can be assessed by evaluating the degree of narrowing at the stenotic point and degree of decrease in the distal run-off of the vessels and parenchymal perfusion. Early in the history of LT from cadaveric donors, the anastomosis was large enough to evaluate, and the conventional criterion used to diagnose HAO was solely based on the degree of luminal narrowing at the site of anastomosis (i.e., > 75% for severe and ≥ 50% for moderate stenosis) [6,12,13,14]. While the distal run-off abnormality was intuitively assessed when there was a high-grade HAO with an abrupt cutoff at the anastomosis, the decreased opacity in the distal run-off was difficult to evaluate in the low-channel CT era, particularly in patients with living-donor LT in whom small HAs were often invisible despite the absence of HAO. However, multidetector row CT has enabled high-speed and high-resolution imaging. The combination of fast helical scanning and image processing three dimensionally has resulted in high image quality and the ability to depict fine vascular structures even after living-donor LT. Therefore, the evaluation of distal run-off at graft HAs is considered a very useful finding for the evaluation of HAO.
In our study, by combining the distal run-off abnormality with the anastomosis site abnormality, the rate of false positives particularly decreased in focal stenosis at the anastomosis site and diffuse stenosis in both graft and recipient HAs around the anastomosis. It was assumed that false positives in focal stenosis at the anastomosis site without distal run-off abnormality may be because of insignificant focal HA stenosis and angulation of the HA. Most false-positive results in diffuse stenosis, in both graft and recipient HAs around the anastomosis without distal run-off abnormality, were considered low-grade HA dissection. Therefore, if these anastomotic site abnormalities are encountered in clinical practice, it would be helpful to evaluate the distal run-off abnormality to reduce the false positives.
According to a previous study that compared the diagnostic performance of CTA (using conventional criteria of ≥ 50% stenosis at the anastomosis as positive) and contrast-enhanced ultrasound (CEUS) for the diagnosis of HAO in patients with a Doppler US abnormality [1], CEUS showed higher specificity and PPV compared to CTA. As modified CTA criteria with a combination of both anastomotic site abnormalities and distal run-off abnormality showed improved specificity and PPV than that with the conventional criteria, it might be comparable to CEUS as a second-line imaging tool for the evaluation of HAO, which should be validated in future studies.
Our study has some limitations. First, there was an inherent limitation because of the retrospective design; hence, some patients who did not undergo CTA were excluded. However, the strength of our study is the relatively large number of HAOs compared to those in previous studies. Second, we included only patients who showed abnormalities on Doppler US. Therefore, patients with significant HAO without abnormalities on Doppler US might have been missed. However, previous studies have reported that Doppler US has a high sensitivity for significant HAO diagnosis [10,15]. Moreover, Doppler US is usually used as a surveillance method for HAO, and CTA is usually performed when there are abnormal findings on Doppler US. Therefore, this study design better reflects the circumstances encountered in daily practice. Third, we did not conjugate laboratory findings as the diagnostic criteria for HAO. Although AST and ALT levels on the day of CT scan and their ratios to those on the day before the scan were slightly higher in the HAO group than in the non-HAO group, the difference was not statistically significant. While we frequently refer to laboratory findings for the diagnosis of HAO in clinical practice, laboratory findings vary according to the graft type, size, and quality, and there is no specific cutoff for the diagnosis of HAO. In addition, in practice, patients with HAO may not exhibit remarkable elevation of liver enzymes if detected early enough, before considerable ischemic damage of the liver graft. Nevertheless, they are frequently indicated for angiography for confirmative diagnosis or exclusion of HAO because of the possible devastating complication of graft ischemia. This may be the reason why radiologic screening is vital for the diagnosis of HAO. Fourth, visualization of the distal run-off of HA could be related to the patient’s total blood flow volume. Considering that the amount of body fluid change after surgery could be significant, a more complex multivariate analytic model combined with clinical factors, such as blood pressure, could offer a better diagnostic criterion. However, our retrospective study could not obtain sufficient records of the clinical factors. Multivariate analysis, involving various clinical factors, should be performed through a prospective design in the future.
In conclusion, the modified criterion, which requires abnormalities in both the anastomosis site and distal run-off, could improve the accuracy and specificity of CTA for the diagnosis of HAO without significantly decreasing the sensitivity.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kyoung Won Kim.
- Data curation: Jin Sil Kim.
- Formal analysis: Jin Sil Kim, Dong Wook Kim.
- Investigation: all authors.
- Methodology: Jin Sil Kim, Kyoung Won Kim.
- Supervision: Kyoung Won Kim, Gi Won Song, Sung Gyu Lee.
- Validation: all authors.
- Writing—original draft: Jin Sil Kim.
- Writing—review & editing: Kyoung Won Kim.
Funding Statement: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. 2017R1E1A1A03070961).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Kim JS, Kim KW, Lee J, Kwon HJ, Kwon JH, Song GW, et al. Diagnostic performance for hepatic artery occlusion after liver transplantation: computed tomography angiography versus contrast-enhanced ultrasound. Liver Transpl. 2019;25:1651–1660. doi: 10.1002/lt.25588. [DOI] [PubMed] [Google Scholar]
- 2.Astarcıoglu I, Egeli T, Gulcu A, Ozbilgin M, Agalar C, Cesmeli EB, et al. Vascular complications after liver transplantation. Exp Clin Transplant. 2019 Mar; doi: 10.6002/ect.2018.0240. [Epub] [DOI] [PubMed] [Google Scholar]
- 3.Steinbrück K, Enne M, Fernandes R, Martinho JM, Balbi E, Agoglia L, et al. Vascular complications after living donor liver transplantation: a Brazilian, single-center experience. Transplant Proc. 2011;43:196–198. doi: 10.1016/j.transproceed.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Khalaf H. Vascular complications after deceased and living donor liver transplantation: a single-center experience. Transplant Proc. 2010;42:865–870. doi: 10.1016/j.transproceed.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 5.Park YS, Kim KW, Lee SJ, Lee J, Jung DH, Song GW, et al. Hepatic arterial stenosis assessed with doppler US after liver transplantation: frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff. Radiology. 2011;260:884–891. doi: 10.1148/radiol.11102257. [DOI] [PubMed] [Google Scholar]
- 6.Brancatelli G, Katyal S, Federle MP, Fontes P. Three-dimensional multislice helical computed tomography with the volume rendering technique in the detection of vascular complications after liver transplantation. Transplantation. 2002;73:237–242. doi: 10.1097/00007890-200201270-00015. [DOI] [PubMed] [Google Scholar]
- 7.Piolanti M, Fabbro E, Pascali E, Rossi C, Caputo M, Varotti G, et al. CT angiography for the evaluation of adult orthotopic liver transplantation arterial complications. Radiol Med. 2001;102:348–356. [PubMed] [Google Scholar]
- 8.Kayahan Ulu EM, Coskun M, Ozbek O, Tutar NU, Ozturk A, Aytekin C, et al. Accuracy of multidetector computed tomographic angiography for detecting hepatic artery complications after liver transplantation. Transplant Proc. 2007;39:3239–3244. doi: 10.1016/j.transproceed.2007.08.097. [DOI] [PubMed] [Google Scholar]
- 9.García-Criado A, Gilabert R, Berzigotti A, Brú C. Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR Am J Roentgenol. 2009;193:128–135. doi: 10.2214/AJR.07.3919. [DOI] [PubMed] [Google Scholar]
- 10.Dodd GD, 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994;192:657–661. doi: 10.1148/radiology.192.3.8058930. [DOI] [PubMed] [Google Scholar]
- 11.Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746–757. doi: 10.1111/j.1600-6143.2008.02541.x. [DOI] [PubMed] [Google Scholar]
- 12.Vignali C, Bargellini I, Cioni R, Petruzzi P, Cicorelli A, Lazzereschi M, et al. Diagnosis and treatment of hepatic artery stenosis after orthotopic liver transplantation. Transplant Proc. 2004;36:2771–2773. doi: 10.1016/j.transproceed.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Katyal S, Oliver JH, 3rd, Buck DG, Federle MP. Detection of vascular complications after liver transplantation: early experience in multislice CT angiography with volume rendering. AJR Am J Roentgenol. 2000;175:1735–1739. doi: 10.2214/ajr.175.6.1751735. [DOI] [PubMed] [Google Scholar]
- 14.Legmann P, Costes V, Tudoret L, Girardot C, Hazebroucq V, Uzan E, et al. Hepatic artery thrombosis after liver transplantation: diagnosis with spiral CT. AJR Am J Roentgenol. 1995;164:97–101. doi: 10.2214/ajr.164.1.7998578. [DOI] [PubMed] [Google Scholar]
- 15.Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, et al. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology. 2006;241:267–274. doi: 10.1148/radiol.2411050597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.