Abstract
Background
Preoperative frailty has been associated with adverse postoperative outcomes. Additionally, low testosterone has been associated with physical frailty and cognitive decline. However, the impact of simultaneous frailty and low testosterone on surgical outcomes is understudied.
Methods
Preoperative frailty status and testosterone levels were obtained in patients undergoing a diverse range of surgical procedures. Preoperative frailty was evaluated independently and in combination with testosterone through the creation of composite risk groups. Relationships between preoperative frailty and composite risk groups with overall survival were determined using Kaplan–Meier and logistic regression analyses. Bivariate analysis was used to determine the associations between frailty and testosterone status on postoperative complications, length of hospital stay, and readmission rates.
Results
Median age of the cohort was 63 years, and the median follow-up time was 105 weeks. Thirty-one patients (23%) were frail, and 36 (27%) had low free testosterone. Bivariate analysis demonstrated a statistically significant relationship between preoperative frailty and overall survival (P = .044). In multivariate analysis, coexisting frailty and low free testosterone were significantly associated with decreased overall survival (hazard ratio 4.93, 95% confidence interval, 1.68–14.46, P = .004).
Conclusion
We observed preoperative frailty, both independently and in combination with low free testosterone levels, to be significantly associated with decreased overall survival across various surgical procedures. Personalizing the surgical risk assessment through the incorporation of preoperative frailty and testosterone status may serve to improve the prognostication of patients undergoing major surgery.
Abbreviations: T, testosterone; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern COoperative Oncology Group; ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; eGFR, estimated Glomerular Filtration Rate; BMI, body mass index; IQR, interquartile range
Highlights
-
•
Both frailty (P = .015) and low free testosterone (P = .005) were independently associated with 1-year mortality.
-
•
After stratifying our cohort into 4 composite groups based on frailty and testosterone status, frail patients with low free T had the shortest overall survival when compared to the reference group, with nearly a 5-fold higher risk of death.
INTRODUCTION
Initially defined by Fried et al as an age-related decline in physical function and emotional reserve, frailty is a multifactorial process that is associated with increased morbidity and mortality in patients managed with surgery [1,2]. Hence, frailty has gained attention as a preoperative screening tool, as it may outperform traditional screening measures such as the Eastern Cooperative Oncology Group (ECOG) and American Society of Anesthesiologists (ASA) scores [1,3,4]. In previous studies, combining frailty with other patient-specific factors, such as cognitive function and comorbidities, has demonstrated value in predicting long-term survival outcomes, surgical complications, and length of hospital stay [1,5,6]. As a patient's frailty status may offer an objective view into his or her ability to tolerate surgery, it is important to clarify the relationship between frailty and postoperative outcomes [1].
Testosterone (T) is an essential hormone involved in the maintenance of physiologic homeostasis and, when deficient, can contribute to decreased muscle strength, bone differentiation, erythropoiesis, and cognitive function [7,8]. The majority of serum T is bound to plasma protein, with 50%–60% bound to sex hormone-binding globulin and 40%–50% bound to albumin, with the remaining 1–2% circulating freely. Collectively, free and albumin-bound T represents the bioactive form [9]. It is well established that androgen levels decline steadily with age, with studies suggesting a potential interplay between T deficiency and frailty [12,13]. However, to our knowledge, no studies have investigated the prognostic utility of combined frailty and T status in patients undergoing major surgery.
We hypothesize that preoperative physical frailty will be associated with T deficiency in male surgical candidates. In addition, we hypothesize that physical frailty, when analyzed independently and in combination with low free T, will be significantly associated with adverse outcomes in patients undergoing major surgery.
MATERIALS AND METHODS
Study Population
The Emory Institutional Review Board approved this prospective study of patients undergoing major surgery at Emory University Hospital (IRB No. 55598). Informed consent was obtained during the preoperative visit. Patients under the care of three urologists were screened before their preoperative visit for evaluation of inclusion and exclusion criteria. A strobe diagram displaying the enrollment and exclusion process is shown in Supplemental Fig 1. Inclusion criteria consisted of adult male patients who were scheduled for urologic surgical procedures requiring overnight hospital admission, including partial and radical nephrectomy, prostatectomy, cystectomy, adrenalectomy, and penectomy. Seventeen patients from our previous prospective studies who underwent general surgery, thoracic surgery, or surgical oncology intervention requiring hospitalization and had valid preoperative T values were also included for analysis [6].
Patients with history or plans to undergo procedures impacting T levels, such as orchiectomy, or with a history of T altering treatment, such as prior history of chemotherapy, radiation, androgen deprivation therapy, and medication such as T supplements, were excluded. Patients who failed to obtain valid T measurements, were unable to ambulate, had poor dexterity, or had an inability to perform the grip strength tests were excluded from this study (Supplemental Fig 1).
Supplement Fig 1.
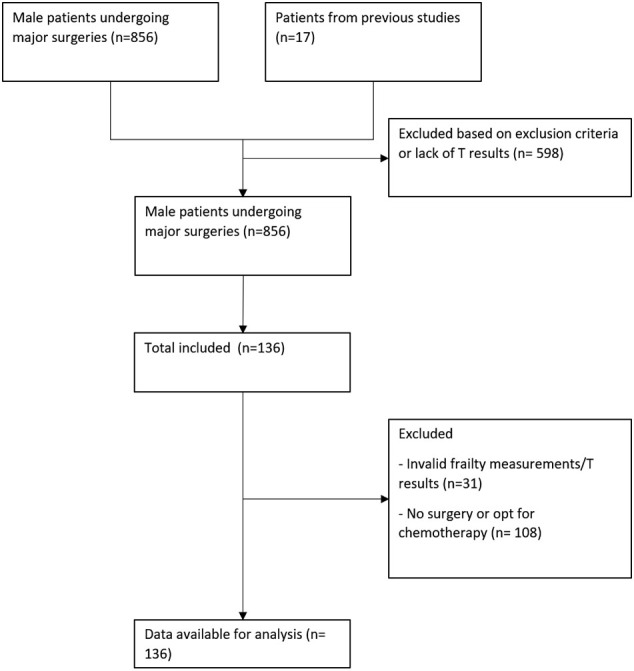
Strobe diagram shows the recruitment process between 2014 and 2019 in Emory University Hospital.
An a priori power analysis was conducted pre-enrollment to determine the appropriate sample size for this study. A sample size of 130 individuals, with a 3:1 ratio of nonfrail to frail patients, was determined to have 80% power for detecting a 30% difference in free T at an α of .05.
Testosterone and Frailty Measurements
T levels have been shown to decline in the afternoon due to diurnal variation, which also decrease with age [10]. As a result, free and total T levels were obtained during the preoperative visit between 8:00 am and noon as recommended by the Endocrine Society [11]. Total T was measured by immunoassay, and free T was measured by liquid chromatography tandem mass spectrometry (Quest Diagnostics, Secaucus, NJ). Both were calibrated against the Centers for Disease Control and Prevention laboratory values as part of the Hormone Standardization Project. Total and free T levels were assessed as normal or low based on the reference ranges provided by the Endocrine Society and Quest Diagnostics (Appendix A).
Patients were assessed for frailty from 5 categories: grip strength, walking speed, shrinking, exhaustion, and low physical activity (Appendix B) [1]. Grip strength of the dominant hand was tested using a hydraulic hand dynamometer (JAMAR Sammons Preston, Bolingbrook, IL). The test was repeated 3 times, and the mean grip strength result was calculated. Each category yielded a dichotomous score of 0 or 1, and the sum of all 5 categories yielded the total score. Following previous protocols, we combined the prefail (score = 2) with the frail group because of small sample size of frail patients (n < 10) [2,6]. Patients with total scores of 0 or 1 were classified as nonfrail, and patients with total scores of 2 to 5 were classified as frail. A 4-level system that combined frailty and age-adjusted free T was developed to further classify patients into one of the following 4 groups: nonfrail with normal free T, nonfrail with low free T, frail with normal free T, and frail with low free T.
Preoperative Covariates
Demographic information including age at time of surgery, sex, race/ethnicity, and body mass index (BMI) were obtained during the preoperative consultation. Additionally, patients were assessed for risk indices, including ASA score, Charlson Comorbidity Index (CCI), and ECOG score [3,12,13]. Serum biochemical measurements (hemoglobin, creatinine, estimated glomerular filtration rate [eGFR], platelets) were also obtained preoperatively and assessed as normal or low based on the reference ranges provided by the Emory Laboratory (Appendix A).
Primary and Secondary Outcomes
The primary outcome of this study was overall survival (OS), defined as the time from surgery to death from any cause. Secondary outcomes included postoperative length of stay, discharge disposition (home versus facility), as well as postoperative major complications and hospital readmissions within 30 and 90 days. Complications were assessed using the Clavien–Dindo classification system, with major complications defined as a Grade ≥ IIIb [14]. Complications with a grade < IIIb were not included in the analysis. Mortality data were acquired through a combination of follow-up phone calls, medical record review, and the Social Security National Death Index.
Statistical Analysis
Descriptive statistics for each variable were reported for the overall population. Bivariate analyses between demographic, clinical, and outcome variables with each composite free T and frailty cohort were reported. Free T levels and frailty scores were tested with all demographic and outcome variables individually. Bivariate associates of categorical outcomes or cohorts were performed using the χ2 or Fisher exact test for categorical covariates and ANOVA for numerical covariates.
Multivariate models were created for outcome variables through logistic regression and a Cox proportional hazard model for overall survival while controlling for age, race, sex, BMI, CCI score, ECOG score, and ASA score. The multiple logistic regression model was built with backward stepwise elimination at α level of .3. All variables were initially included in the multivariate model and were consecutively removed with the largest P value > .3. This was continued one at a time until all variables in the model had a P value < .3. Kaplan–Meier plots were created to evaluate the associations between the composite free T and frailty cohorts and OS. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Demographics
The study included 136 male patients undergoing surgery between 2014 and 2019 at our tertiary care academic medical center. Patient demographics, including the preoperative frailty and laboratory characteristics of our cohort, are presented in Table 1. Sixty-six percent of the cohort were white, the median age at time of surgery was 63 years (interquartile range [IQR] 54–70), and the median BMI of our cohort was 28.8 (IQR 26.4–32.8) kg/m2. The majority of surgeries performed were urological procedures (87.5%). Other surgery divisions included cardiac (3.68%), general surgery (2.21%), neurosurgery (4.41%), thoracic (1.47%), and vascular (0.74%). Among all surgeries, 54 (39.7%) were open procedures, 43 (31.6%) were robotic, and 28 (20.6%) were laparoscopic. Surgeries were classified as oncologic versus non-oncologic procedures, with 118 (86.6%) being oncologic surgeries and 18 (13.4%) non-oncologic surgeries. Patients were not compared by detailed surgical techniques due to high heterogeneity.
Table 1.
Demographic and clinical data
| Variable | Total population (n = 136) | P value with combined frailty & T score |
|---|---|---|
| Age at time of surgery, median (IQR) | 63 (54–70) | .009 |
| Race, n (%) | .892 | |
| White | 90 (66.2) | |
| Nonwhite | 46 (33.8) | |
| BMI (kg/m2), median (IQR) | 28.8 (26.4–32.8) | .077 |
| Date of surgery, n (%) | .282 | |
| 2014–2016 | 37 (27.2) | |
| 2017–2019 | 99 (72.8) | |
| Surgery technique, n (%) | .329 | |
| Open | 54 (39.7) | |
| Robotic | 43 (31.6) | |
| Laparoscopic | 28 (20.6) | |
| Other | 11 (8.09) | |
| Primary surgeon division, n (%) | .819 | |
| Urology | 119 (87.5) | |
| Other | 17 (12.5) | |
| Surgery types, n (%) | ||
| Oncology | 118 (86.6) | .510 |
| Non-oncology | 18 (13.4) | |
| ASA, n (%) | .116 | |
| 1–2 | 35 (25.7) | |
| 3–4 | 101 (74.3) | |
| CCI, n (%) | .070 | |
| 0 | 61 (44.9) | |
| 1–2 | 14 (10.3) | |
| ≥ 3 | 61 (44.9) | |
| ECOG, n (%) | .174 | |
| 0 | 96 (70.6) | |
| 1–2 | 40 (29.4) | |
| Hemoglobin (g/dL) | ||
| Patients with abnormal levels, n (%) | 55 (40.4) | .022 |
| Creatinine (mg/dL) | ||
| Patients with abnormal levels, n (%) | 35 (25.7) | .430 |
| GFR (mL/min/1.73 m2) | ||
| Patients with abnormal levels, n (%) | 38 (27.9) | .661 |
| Platelets (10E3/μL) | ||
| Patients with abnormal levels, n (%) | 21 (15.4) | .065 |
| Free testosterone (pg/mL), median (IQR) | 43.1 (32.5–55.3) | – |
| Normal free T, n (%) | 100 (73.5) | |
| Low free T, n (%) | 36 (26.5) | |
| Total testosterone (ng/dL), median (IQR) | 261 (207–346) | – |
| Normal total T, n (%) | 54 (39.7) | |
| Low total T, n (%) | 82 (60.3) | |
| Low free T and low total T, n (%) | 31 (22.8) | |
| Frailty score, n (%) | – | |
| 0 | 70 (51.5) | |
| 1 | 35 (25.7) | |
| 2 | 22 (16.2) | |
| 3 | 7 (5.2) | |
| 4 | 1 (0.7) | |
| 5 | 1 (0.7) | |
| Frailty, n (%) | – | |
| Nonfrail (score 0–1) | 105 (77.2) | |
| Frail (score 2–5) | 31 (22.8) |
Traditional preoperative risk screening revealed that nearly 75% of our cohort had an ASA score of 3 or 4 (74.3%), whereas nearly 71% had an ECOG score of 0. The median CCI score was 2 (IQR 0–4) (Table 1).
Independent Analysis of Frailty, Testosterone, and Other Serum Studies
After adjusting for age, 36 patients (26.5%) had low free T and 82 (60.3%) had low total T (Table 1). Among them, 31 (22.8%) patients had both low free T and low total T values. The Fried criteria assessment revealed that 31 (22.8%) patients were deemed frail with a frailty score ≥ 2. Free T was associated with physical frailty, as determined by bivariate analysis (P < .001, Table 2). There was no significant association found between total T and physical frailty (P > .05).
Table 2.
Bivariate analysis of free testosterone with frailty
| Free T levels, n (%) | P value | ||
|---|---|---|---|
| Categories of frailty, n (%) | Normal free T | Low free T | <.001 |
| Nonfrail (score 0–1) | 84 (61.8) | 21 (15.4) | |
| Frail (score 2–5) | 15 (11.0) | 16 (11.8) | |
In bivariate analysis, hemoglobin and platelet levels were significantly associated with physical frailty (P = .03 and P = .02, respectively). Except for levels of hemoglobin (P = .02, Table 1), other biochemical measurements did not have a statistically significant association with the combined frailty and free T score. The relationship between serum T and BMI was examined, and the association was not statistically significant (P = .3). The same was found for the association between BMI and the composite frailty and free T score (P = .08). No other serum laboratory measurements were found to be associated with free T (all P > .05).
Composite System and Surgical Outcomes
Eighty-four (61.8%) patients were classified as nonfrail with normal levels of free T, 21 (15.4%) patients were nonfrail but had low levels of free T, 15 (11.0%) patients were frail with normal free T, and 16 (11.8%) patients were both frail and had low free T (Table 2).
In total, 22 deaths occurred (16.2%) over the median follow-up period of 105 weeks. Two of the 22 deaths occurred within 30 days after surgery, and 1 additional death occurred within 90 days after surgery. Causes of death for these 3 patients included progression of disease (1), multiorgan failure (1), and cardiac arrest (1). Among all deaths, 3 were of unknown causes, 6 were disease-related or due to disease progression, and the rest (n = 13) were due to pneumonia, hemorrhagic stroke, heart failure, liver failure, sepsis, or multiorgan failure.
In the bivariate analysis, both low free T (P = .005) and frailty (P = .015) were independently associated with 1-year mortality. Similarly, the composite free T and frailty score was significantly associated with 1-year (P = .003) and 2-year (P = .028) mortality.
Other postoperative outcomes, including 30- or 90-day mortality, presence of major complications, 30- or 90-day readmission, length of stay, and discharge disposition, were not significantly associated with frailty, free T, or the composite score.
Composite System and Overall Survival
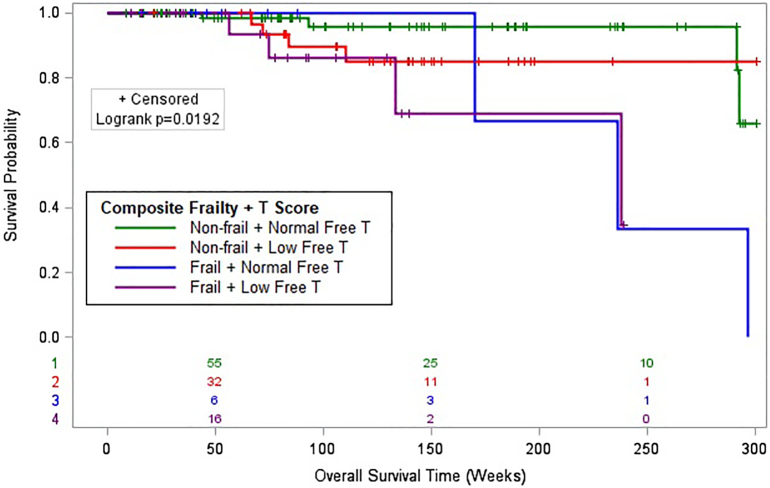
Surgical technique, categorical eGFR levels, and the composite frailty and T score were found to have P values < .3 with OS in univariate analysis. Kaplan–Meier analysis of the 4-level composite system combining frailty and free T revealed significant differences in OS between groups (log rank P = .019, Fig 1). Among the 4 groups, frail patients with low free T had the shortest OS when compared to the reference group. In multivariate analysis, presence of frailty and low free T was significantly associated with decreased overall survival (hazard ratio [HR] 4.93, 95% confidence interval [CI] 1.68–14.46, P = .004; Table 3). Although not found to be significant, nonfrail patients with low levels of free T were also at risk, with a higher risk of mortality compared to the reference group (HR 2.11, 95% CI 0.70–6.37, P = .185).
Fig 1.
Kaplan–Meier curve displays the overall survival of patients following major procedures in all four levels of the composite system with frailty score and free testosterone (T) level combined (n = 136). The log-rank test indicates a significant difference between the survival curves.
Table 3.
Univariate and multivariable analysis of preoperative variables with overall survival (months)
|
Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Frailty & free T score | .019 | .037 | ||
| [1] Nonfrail + normal free T | – | – | – | – |
| [2] Nonfrail + low free T | 2.30 (0.77–6.88) | .136 | 2.11 (0.70–6.37) | .185 |
| [3] Frail + normal free T | 1.71 (0.37–7.95) | .497 | 1.82 (0.39–8.51) | .449 |
| [4] Frail + low free T | 5.52 (1.89–16.12) | .002 | 4.93 (1.68–14.46) | .004 |
| Age (0 to 65 vs > 65) | 1.30 (0.56–3.03) | .542 | ||
| Race (white versus nonwhite) | 1.02 (0.41–2.50) | .973 | ||
| BMI | 0.95 (0.88–1.04) | .262 | ||
| Date of surgery (2014–2016 vs 2017–2019) | 1.39 (0.49–3.93) | .531 | ||
| Surgery technique (robotic versus other) | 2.63 (0.77–8.94) | .121 | 2.32 (0.68–7.95) | .182 |
| Primary surgery division (urology versus other) | 0.66 (0.24–1.82) | .421 | ||
| ASA (1–2 vs 3–4) | 1.09 (0.40–2.98) | .862 | ||
| CCI (0–2 vs ≥ 3) | 1.46 (0.63–3.37) | .381 | ||
| ECOG (0 vs 1–2) | 1.27 (0.54–3.00) | .591 | ||
| Hemoglobin (patients with normal versus abnormal levels) | 1.46 (0.63–3.40) | .376 | ||
| Creatinine (patients with normal versus abnormal levels) | 1.48 (0.60–3.64) | .392 | ||
| GFR (patients with normal versus abnormal levels) | 1.68 (0.70–4.00) | .244 | 1.67 (0.69–4.05) | .261 |
| Platelets (patients with normal versus abnormal levels) | 1.39 (0.47–4.12) | .558 | ||
DISCUSSION
In this prospective study, we evaluated the combined effect of free T levels and physical frailty in predicting postoperative outcomes across various surgical procedures. We found frailty and free T to be independent predictors of 1-year mortality. Moreover, we found that the combination of frailty and free T was more significantly associated with 1-year mortality than frailty alone (HR 4.93, 95% CI 1.68–14.46, P = .004 vs HR 1.82, 95% CI 0.39–8.51, P = .45).
T plays an important role in many physiologic and metabolic pathways, and metabolic syndrome and T deficiency demonstrate a bidirectional causal relationship. In male subjects, T is converted to estradiol by aromatase in the adipose tissue. Increased fat deposition leads to an increased conversion of T to estradiol, which in turn inhibits central gonadotrophin secretion through a negative feedback mechanism [15,16]. The resulting T deficiency contributes to decreased insulin sensitivity and metabolic inflexibility between catabolic and anabolic states, which disrupt normal muscle metabolism and in turn cause reduced lean muscle mass [16]. T is important for stimulating bone formation and muscle strength, whereas T deficiency is associated with decreased bone mineral density, muscle wasting, and cognitive impairment [7,8,17]. T deficiency affects multiple aspects of physical and mental health, leading to the frailty phenotype. Moreover, metabolic imbalance and hypogonadism may introduce comorbidities such as diabetes and cardiovascular disease. These factors combined may lead to increased risks of surgical complications, prolonged recovery periods, and mortality.
There is a paucity of literature delineating the influence of serum T levels on surgical outcomes. One retrospective study suggests that low T level at time of kidney transplant is associated with increased mortality and graft loss, notably with a higher rate of mortality secondary to cardiovascular and septic events [18]. Here, we observed low preoperative T levels to be significantly associated with decreased mortality in several surgical procedures. Furthermore, 26.5% of our cohort had low preoperative free T levels. Given this relatively high prevalence and the observed association between low free T and postoperative mortality, further research is needed to investigate the utility of analyzing T in the preoperative risk assessment.
Current interventions to combat the frailty phenotype involve physical exercise, nutritional supplements, cognitive training, or a combination of these methods [26,27]. Frail patients participating in physical exercise show a greater increase in functional ability and fewer surgical complications when compared to control groups [19,20]. Meanwhile, nutritional intervention shows a large degree of variation in its effect on modifying frailty status. Some studies suggest improvements in malnourished patients, whereas other studies have found no significant improvements, after nutritional intervention [21]. A randomized trial comparing the effectiveness of physical exercise, nutritional supplements, cognitive training, and a combination of the 3 methods demonstrates a similar level of improvement in frailty status for each intervention group [22]. Given the correlation between low T and frailty, T replacement therapy (TRT) has theoretical potential for managing frailty. Currently, a diagnosis of sexual dysfunction or hypogonadism is an indication for TRT. Meanwhile, the use of TRT in late-onset hypogandism is still under debate, as there have been mixed results on whether TRT results in increased cardiovascular risks, and other uses of TRT outside of the indications mentioned above are to be evaluated on a case-to-base basis [[23], [24], [25]]. Benefits of TRT include increased lean body mass, increased bone density, increased hemoglobin levels, improved physical function, and improved mood [26]. Therefore, T supplementation may improve physical functioning and energy levels in frail patients; however, future studies are warranted to investigate the risks and benefits of TRT in this scenario [27].
In our study, most of the deaths occurred outside of the 90-day postoperative period, indicating that surgery may not influence perioperative mortality rates among patients with frailty and low free T. Even if surgery was not a strong contributing factor in the immediate postoperative period, it is still important for surgeons to be cognizant of frailty's potential long-term effects on survival [28]. In the current study, we demonstrated frailty and low free T to be significantly associated with inferior survival outcomes beyond the perioperative period. We also observed that 50% of deaths occurred after 2 years and most were not disease-specific. This may be secondary to the high comorbidity rate in our cohort, as nearly 45% of our patients had a CCI ≥ 3. Higher CCI scores impart increased mortality rates in patients undergoing major surgery, offering a potential rationale for the high percentage of deaths attributed to causes unrelated to their surgically managed pathology [29].
This study is limited by several factors. During screening, a large number of patients were excluded owing to history of medical or surgical treatments that would interfere with T results. This included prior history of chemotherapy, radiation, androgen deprivation therapy, and medication such as T supplements. Additionally, we had a high percentage of exclusion cases due to a large number of invalid or missed T results. This leads to a relatively small sample size and a short follow-up period that could limit the power of our analyses. Our cohort was recruited at a single tertiary medical center, with majority being evaluated for suspected or known neoplasm. Therefore, there may be selection bias, thus limiting the external validity and generalizability of our conclusions to the general population. Furthermore, patients unable to finish the physical frailty assessment due to ambulatory problems or other ailments were excluded, and results may not be applicable in populations with further physical impairment. This study also examined T only once, which is distinct from other studies and guidelines, and therefore is unable to capture the changes of T levels [11]. Additionally, we did not include other hormone measurements, such as luteinizing hormone, albumin, or sex-hormone binding globulin, which may contribute to related physiological processes [30]. Lastly, this is an observational study with no interventions; therefore, we cannot confirm causal relationships between T, frailty, and survival outcomes. Further multicenter prospective trials are warranted to confirm the results.
In conclusion, in this study, we demonstrated that frailty is predictive of overall survival after major surgery. Both free T and frailty were independently associated with 1-year mortality. Furthermore, we demonstrated that the addition of free T with a frailty assessment has greater potential to identify surgical patients at higher risks than frailty measures alone. Frail patients with low free T levels have almost a 5-fold increased risk of long-term mortality when compared to nonfrail patients with normal free T. Further multicenter prospective trials should examine the relationship between frailty and low free T to confirm the results.
The following are the supplementary data related to this article.
Author Contribution
FL drafted the manuscript. FL, GH, SM, and FP completed data acquisition and analysis. FK completed statistical analysis and provided critical revisions of the manuscript. MH, IC, RN, EM, AM, and CR contributed to data analysis and interpretation and provided critical revisions of the manuscript. VAM and KO contributed to conception and design of the study and supervision, and provided critical revisions of the manuscript.
Conflict of Interest
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgment
John Robinson Churchill family foundation support is gratefully acknowledged. Special thanks to Dr Mehrdad Alemozaffar and Dr Benjamin Petrinec who have offered great insight into the study design and manuscript revision.
Appendix A. Interpretation of laboratory results
| Laboratory test | Reference range |
|---|---|
| Free testosterone (1 pg/mL = 3.47 nmol/L) | |
| Men 18–69 y | 35.0–155.0 pg/mL |
| Men > 69 y | 30.0–135.0 pg/mL |
| Total testosterone (1 ng/dL = 0.0347 nmol/L) | |
| Men | > 300 ng/dL |
| Laboratory test | Reference range for men, 18–150 y old |
| Creatinine | 0.7–1.3 mg/dL |
| Hemoglobin | 12.9–16.1 g/dL |
| eGFR | ≤ 60 mL/min/1.73 m2 |
Appendix B. Fried criteria of frailty for adult men
| Weight loss | Unintentional weight loss of ≥ 10 lb in the last year | |
|---|---|---|
| Decreased grip strength | BMI ≤ 24 | Grip strength ≤ 29 kg |
| BMI 24.1–26 | Grip strength ≤ 30 kg | |
| BMI 26.1–28 | Grip strength ≤ 31 kg | |
| BMI > 28 | Grip strength ≤ 32 kg | |
| Exhaustion | ≥ 2 d of exhaustion in the past week | |
| Low activity | < 1602.47 kJ/wk in the past 2 wk | |
| Walking speed (10 m) | Height ≤ 173 cm | Walking time ≥ 7 s |
| Height > 173 cm | Walking time ≥ 6 s | |
References
- 1.Makary M.A., Segev D.L., Pronovost P.J., Syin D., Bandeen-Roche K., Patel P., et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 3.Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T., et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 4.Aronson W.L., McAuliffe M.S., Miller K. Variability in the American Society of Anesthesiologists physical status classification scale. AANA J. 2003;71:265–274. [PubMed] [Google Scholar]
- 5.Li J.L., Henderson M.A., Revenig L.M., Sweeney J.F., Kooby D.A., Maithel S.K., et al. Frailty and one-year mortality in major intra-abdominal operations. J Surg Res. 2016;203:507–512.e1. doi: 10.1016/j.jss.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Makhani S.S., Kim F.Y., Liu Y., Ye Z., Li J.L., Revenig L.M., et al. Cognitive impairment and overall survival in frail surgical patients. J Am Coll Surg. 2017;225:590–600.e1. doi: 10.1016/j.jamcollsurg.2017.07.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder P.J., Kopperdahl D.L., Stephens-Shields A.J., Ellenberg S.S., Cauley J.A., Ensrud K.E., et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177:471. doi: 10.1001/jamainternmed.2016.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv W., Du N., Liu Y., Fan X., Wang Y., Jia X., et al. Low testosterone level and risk of Alzheimer’s disease in the elderly men: a systematic review and meta-analysis. Mol Neurobiol. 2016;53:2679–2684. doi: 10.1007/s12035-015-9315-y. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman J.M., Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla D.J., Matsumoto A.M., Araujo A.B., McKinlay J.B. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhasin S., Brito J.P., Cunningham G.R., Hayes F.J., Hodis H.N., Matsumoto A.M., et al. Testosterone therapy in men with hypogonadism: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–284. doi: 10.1097/00000542-194105000-00004. [DOI] [Google Scholar]
- 14.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 15.Cohen P.G. The hypogonadal–obesity cycle: role of aromatase in modulating the testosterone–estradiol shunt—a major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52:49–51. doi: 10.1054/mehy.1997.0624. [DOI] [PubMed] [Google Scholar]
- 16.Kelly D.M., Jones T.H. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25–R45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 17.Corrigan F.E., Al Mheid I., Eapen D.J., Hayek S.S., Sher S., Martin G.S., et al. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol. 2015;194:94–99. doi: 10.1016/j.ijcard.2015.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoskes D.A., Kerr H., Askar M., Goldfarb D.A., Schold J. Low testosterone at time of transplantation is independently associated with poor patient and graft survival in male renal transplant recipients. J Urol. 2014;192:1168–1171. doi: 10.1016/j.juro.2014.03.102. [DOI] [PubMed] [Google Scholar]
- 19.Apóstolo J., Cooke R., Bobrowicz-Campos E., Santana S., Marcucci M., Cano A., et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep. 2018;16:140–232. doi: 10.11124/JBISRIR-2017-003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theou O., Stathokostas L., Roland K.P., Jakobi J.M., Patterson C., Vandervoort A.A., et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:1–19. doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walston J., Buta B., Xue Q.-L. Frailty screening and interventions. Clin Geriatr Med. 2018;34:25–38. doi: 10.1016/j.cger.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng T.P., Feng L., Nyunt M.S.Z., Feng L., Niti M., Tan B.Y., et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. 2015;128:1225–1236.e1. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Gagliano-Jucá T., Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol. 2019;16:555–574. doi: 10.1038/s41569-019-0211-4. [DOI] [PubMed] [Google Scholar]
- 24.Argalious M.Y., Steib J., Daskalakis N., Mao G., Li M., Armanyous S., et al. Association of testosterone replacement therapy and the incidence of a composite of postoperative in-hospital mortality and cardiovascular events in men undergoing cardiac surgery. Anesth Analg. 2020;130:890–898. doi: 10.1213/ANE.0000000000004115. [DOI] [PubMed] [Google Scholar]
- 25.Tsametis C.P., Isidori A.M. Testosterone replacement therapy: for whom, when and how? Metabolism. 2018;86:69–78. doi: 10.1016/j.metabol.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Snyder P.J., Bhasin S., Cunningham G.R., Matsumoto A.M., Stephens-Shields A.J., Cauley J.A., et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy C.N., Snyder P.J., Stephens-Shields A.J., Artz A.S., Bhasin S., Cohen H.J., et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177:480. doi: 10.1001/jamainternmed.2016.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banning L.B.D., El Moumni M., Visser L., van Leeuwen B.L., Zeebregts C.J., Pol R.A. Frailty leads to poor long-term survival in patients undergoing elective vascular surgery. J Vasc Surg. 2021;73:2132–2139.e2. doi: 10.1016/j.jvs.2020.10.088. [DOI] [PubMed] [Google Scholar]
- 29.Sinha P., Kallogjeri D., Piccirillo J.F. Assessment of comorbidities in surgical oncology outcomes: comorbidity in surgical oncology. J Surg Oncol. 2014;110:629–635. doi: 10.1002/jso.23723. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Maxwell C.A., Yu F. Biological processes and biomarkers related to frailty in older adults: a state-of-the-science literature review. Biol Res Nurs. 2019;21:80–106. doi: 10.1177/1099800418798047. [DOI] [PubMed] [Google Scholar]