Abstract
Neurofibromatosis Type 1 (NF1) is a genetic disorder presenting with chronic pain symptoms that has limited treatment options for addressing the pain. The utilization of a mobile application allows for greater reach and scalability when using empirically valid psychosocial self-management treatments for pain. The iCanCope mobile application has been utilized in several different populations dealing with pain symptoms and has demonstrated initial effectiveness. To address the need for this population, we have customized the iCanCope mobile application for the NF1 population and included additional tailored features. We describe the rationale and design of a pilot randomized control study with a sample of 108 adults with NF1, in which two groups will receive access to the mobile application, of which one group will be incentivized to engage in the mobile application and the third group will treatment as usual over the course of 8-week period with a six-week follow-up. Outcomes will focus on the acceptability of the iCanCope-NF mobile application within the NF1 population and the impact of pain related activity on psychometric evaluations to determine if the contingency management will impact the engagement of mobile application, as well as to identify the participants’ experiences in relationship to their treatment satisfaction and perceived support.
Keywords: Neurofibromatosis type 1, iCanCope, Mobile application, Contingency management, Pilot randomized control trial
1. Introduction
Neurofibromatosis Type 1 (NF1) is an autosomal dominant genetic condition affecting 1 in 2500 individuals [1]. Over 50% of individuals with NF1 report significant pain and discomfort [2,3] which can be associated with tumors, but is often not localized to a structural lesion, thus presenting treatment challenges for patients and their medical caregivers [4,5]. Individuals with NF1 suffering from persistent pain have multiple potential barriers to obtaining comprehensive pain specific treatment including: (a) difficulty accessing these services (e.g., no services available in many geographic areas, long wait times); (b) limited availability of trained professionals with disease specific knowledge (e.g. physicians, psychologists), particularly in non-urban centers; and (c) lack of self-management resources [[6], [7], [8], [9], [10]]. Self-management interventions provide individuals with disease-specific knowledge, strategies to manage symptoms such as pain (e.g., cognitive-behavioral therapies; CBT), to promote optimal health outcomes [11].
Cognitive Behavior Therapy (CBT) incorporates training in strategies for managing disease-related symptoms and other stressors, enhancing self-efficacy, as well as guidance on developing and implementing a long-term self-management plan [12,13]. A recent meta-analysis showed that the most successful interventions in improving pain and associated functional impairments in adults with chronic pain are rooted in CBT [14]. Moreover, CBT has been demonstrated effective across multiple various chronic pain conditions [[15], [16], [17], [18]]. To date, there has been little peer reviewed research within the NF1 population utilizing CBT to treat pain symptoms.
Mobile technologies can be applied to enhance the accessibility of pain self-management therapies [19,20]. Smartphones offer an ideal platform to prompt practice of coping skills, disease information, and mindfulness skill in vivo [21]. This technology can be leveraged to build a tailored self-management program that emphasizes empowerment, facilitates the creation and tracking of personalized goals, and offers prompt practice of CBT-based coping skills as needed. Yet, with any mobile application poor engagement is a common problem which can limit the effectiveness of intervention [22,23]. One approach to combat poor engagement and increase patient engagement and retention is contingency management [24,25]. Contingency management (CM) arranges systematic application of behavioral consequences of desired behavior and withholds reinforcement of undesired behaviors [26]. By using points, levels, prizes or other rewards, and well-established reinforcement schedules, individuals are actively engaged [[27], [28], [29]], and CM can encourage individuals to practice behavioral change in a novel and entertaining way [[30], [31], [32]], that can be generalized to the person's natural environment and enhance motivation for change. In evaluating CM conditions versus non-CM conditions, research has consistently shown CM conditions to improve adherence/retention, engagement, and compliance [33,34].
The iCanCope mobile (ICC) was developed to meet the need for pain self-management support among youth with chronic pain by providing a user-centered, iterative design [35]. The ICC mobile application provides personalized goal setting to improve pain and function, CBT-based pain self-management training and rehearsal, and peer-based social support, while providing key health information and coping skills to individuals. It has been empirically evaluated for multiple painful diseases in adolescents and young adults (ages 12–25) [[35], [36], [37], [38]].
1.1. Objectives and specific aims
This project will seek to adapt the ICC mobile application to suit the needs of adults with pain caused by NF1 through qualitative focus groups with individuals with NF1 and providers with experience in NF1. Additionally, the development of a customized contingency management program will be integrated in the ICC mobile application providing an empirically validated treatment in which individuals with NF1 can learn to self-manage their own pain symptoms, while learning new alternative treatments can provide tremendous potential and positive change for those living with persistent pain. Therefore, the objective of this study is to evaluate the feasibility, acceptability and determine the preliminary efficacy of the iCanCope-NF program for adults with NF-1 and chronic pain in a pilot randomized control trial (RCT). In doing so, we will evaluate whether the iCanCope-NF program reduces pain (pain severity and pain interference) in adults with NF1. We hypothesize that by customizing the CBT and the MBAA in the mobile application for adults with NF1, individuals will be motivated to engage daily, thus acquiring a new set of skills to facilitate their own pain self-management, therefore pain will decrease. The specific aims are: Specific Aim 1: To evaluate the acceptability of the iCanCope-NF program. We define acceptability as (1) rates of accrual and dropout, daily log-ins, engagement, and outcome measures completed and (2) perceived acceptability and satisfaction of the intervention; Specific Aim 2: To determine how the iCanCope-NF program compares to the control condition in impact on pain and pain-related activity limitations, sleep functioning, emotional functioning (depression, anxiety), pain catastrophizing, self-efficacy, respondent burden (e.g. Physical Functioning, Vitality, Social Functioning, Role-Emotional, and Mental Health), and psychological flexibility between the timeless admission (T1), completion of the intervention (T2) and follow-up (T3); Specific Aim 3: To determine if the contingency management will increase the engagement of the iCanCope-NF program as compared to iCanCope-NF without CM.
1.2. Study design
The current research study will implement a pilot randomized control trial across the three groups (iCanCope-NF, iCanCope-NF + Contingency Management (CM), Control Group). We will utilize an urn randomization procedure and control for gender. The intervention will last for 8 weeks with outcomes assessments evaluated at admission (T1), completion of intervention (T2) and 6-week post-discharge (T3).
The current study was approved by the Yale Human Research Protection Program and has been registered as a clinical trial at ClinicalTrials.gov.
1.3. Participant eligibility, recruitment, enrollment and randomization
Patients will be included in the study if they meet the following inclusion criteria: (1) adults who are 18 and above with a diagnosis of NF1; (2) able to read and understand English at 5th grade level; (3) permanently reside in the United States and (4) have pain interference aggregate scores of three or more in the last two weeks using the Brief Pain Inventory-Short Form (BPI-SF) scale. Patients will be excluded if they: (1) have an undiagnosed case of NF1; (2) have documented major co-occurring psychiatric disease; (3) have moderate to severe cognitive deficits using the SCID-5; or (4) have depression assessed using the Patient Health Questionnaire (PHQ-9) or anxiety assessed using the Generalized Anxiety Disorder scale (GAD-7) greater than or equal to the appropriate thresholds (10 = mild major depression; 5 = mild severe anxiety).
Participants will be recruited from two NF advocacy organizations: NF Network and NF Northeast. Active registrants across both organizations will be screened by the study investigator's team. Recruitment will consist of social media posts on the advocacy organization's websites and mass email request to active registrants. Individuals will be directed to a Qualtrics link to complete initial screening questions and follow-up would be completed by the research team through email. Consent and intake will take place through a secure video conferencing link. After obtaining consent, participants will be asked to complete pre-treatment assessments. Following completion of pre-treatment measures, participants will be randomized to an intervention condition. Participants within the intervention and control groups will be instructed on the procedures to be followed using standardized manuals.
Participants will be randomized to one of the three conditions using an urn randomization algorithm which modifies ongoing randomization probabilities based on prior group composition groups and controls for other predictive factors. This process greatly reduces experimenter bias and other internal validity threats to integrity [39]. Gender will be controlled for to ensure equal representation across the groups within the urn randomization using stratification.
1.4. Description of study arms
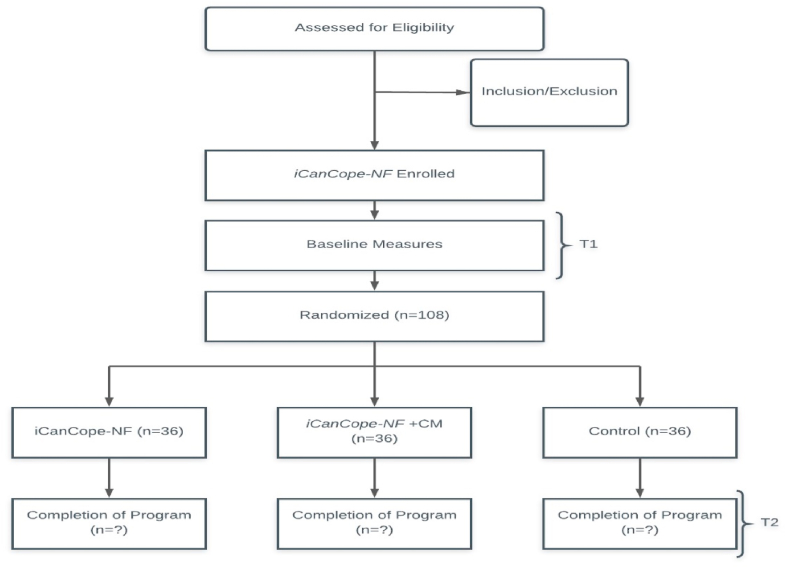
Fig. 1 presents a CONSORT study flow diagram [40]. Potential participants will be required to access an online secure site to learn about the study and to be evaluated for eligibility, provide informed consent, and complete baseline assessments. Eligible participants will be randomly assigned to one of the 3 conditions: ICC-NF, ICC-NF + CM, or the attentional control treatment as usual (TAU). Individuals in the iCanCope groups will receive 8 weeks of 24-h access to the program.
Fig. 1.
CONSORT study flow diagram.
iCanCope Orientation. At study intake, individuals in the two treatment/intervention condition will receive a 10-min video orientation to the iCanCope program. The orientation will encourage daily usage of the CBT treatment platform. Individuals will receive a login and password information, general information and instruction, and contact information for technical problems.
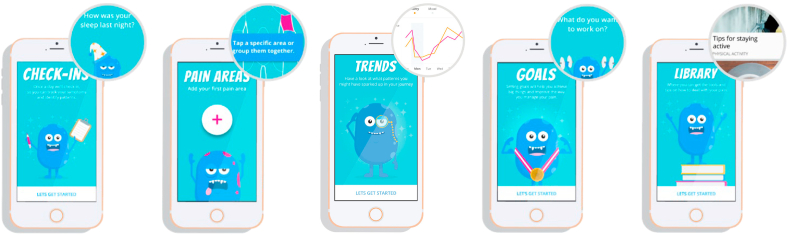
ICC-NF Group: Individuals assigned to this group will have complete access to the ICC-NF mobile application for an 8-week time period and will be asked to check-in daily along with engaging in the different components of the mobile application. As seen on Fig. 2, there are five main components of the ICC-NF mobile application (Check-Ins, Pain Areas, Trends, Goals, and Libraries). Check-ins allow the participant to answer a few short questions about the sleep, pain, mood, and physical activity for the day on a Likert scale that can be answered at any part of the day. The pain area component provides the participant to visually denote where their pain symptoms occur on an anatomically correct figure (anterior and posterior), which is segmented across different regions of their body (e.g., foot, hand, forearm). The trends section allows individuals to visually depict historical check-in data on a line graph over the course of trial. Within this section individuals can plot several values at a time (e.g., pain and sleep) and visually inspect the differences between these values. Participants can establish goals that are designed to support participants in setting and tracking personalized goals and can modify and adjust preestablished goals, or establish new goals based on various categories. The libraries section provides empirically based content to manage chronic pain. Participants have access to over 100 curated articles to learn, manage and live with their NF1. In particular, sections of libraries provide specific content on diagnoses of NF1, treatment of NF1 pain, common causes of pain in NF1, and clinical features of NF1. Additionally, instruction on shortened yoga stances and poses to reduce chronic pain along with two videos on mindfulness and breathing exercises are available.
Fig. 2.
Core Features of the iCanCope-NF1 mobile application.
ICC-NF + CM Group: Participants randomized to this condition will have complete access to the outlined above ICC-NF mobile application. However, participants will be incentivized and rewarded for engaging in the following: 1) Frequency of system use per day (1 point/check-in up to 10 min, double points beyond 10 min); 2) consecutive days of completing assessments (5 points for the first, increasing by 2 points for each day up to a ceiling of 15 points); 3) completion of learning modules and activities (15 points for previously unassessed modules; 5 points for previously accessed modules); 4) consecutive days of completing goals (5 points for the first, increasing by 2 points for each day up to a ceiling of 15 points). Points will be accrued through access to new sections, daily check-ins, and engagement of the mobile application. Based on research, the total amount of money that can be earned by the patient over the course of the two months is 50 dollars USD.
1.5. Control group: The control group is designed to assess for potential effects on
Outcomes of time, attention, during the study. In addition to usual care, participants will be required to complete baseline and follow-up assessments similar to that of the intervention groups. They will be provided patient education through information found from national websites regarding pain management, but no self-management strategies or opportunities for social support will be offered. They will not have access to the mobile application during the trial.
1.6. Study monitoring procedures
The mobile application will have a web-accessible dashboard that will allow us to track adherence. ABSTRACT will track all user-level interactions with the iCanCope app (e.g., articles viewed, check-ins completed, goals set). All other assessments will be completed on Qualtrics (www.qualtrics.com), which is a secure survey site. Microsoft Excel or SQL database administrative tools will be used to download all data for analysis. Data will be hosted on secure servers that collect and store data using web-based applications. All connections to the systems are secured and encrypted and only authorized users will be able to access the system. The system will track participant progression throughout the study, and reports will be generated for monitoring progress and adherence. All data collected in the study can be transferred at any point to the PI or Data Safety and Monitoring Board in the form of electronic data files using the format predetermined by the PI and the investigators. At the request of the PI, all data from its systems including the backups can be erased.
1.7. Measures
In the pilot RCT, we plan to assess a broad range of subject characteristics, acceptability and clinical outcome measures over the course of the study. (See Table 1). Baseline assessments are designed to ensure that patients meet eligibility criteria. The remaining assessments will be categorized by feasibility and clinical measures. Data will be self-reported and will be collected online and stored as per HIPAA privacy legislation. All measures have evidence of reliability and validity in adults in this age range and include target specific measures of NF1 have recommended in previous trials [41,42].
Table 1.
Description of measures.
| Measures | Description of Measures | Time to Complete | T1 | T2 | T3 |
|---|---|---|---|---|---|
| Screening and Background Measures | |||||
| Patient Health Questionnaire (PHQ) | A 9-item self-report scale to evaluate depression, which scores each of the nine DSM-V criteria | 2–3 min | X | X | X |
| Generalized Anxiety Disorder Scale (GAD-7) | A 7-item self-report scale used for screening, diagnosis and severity assessment of anxiety disorder | 2–3 min | X | X | X |
| Brief Pain Inventory-Short Form (BPI-SF) | A 15-item tool that measures pain intensity and impact on functioning | 5 min | X | X | X |
| Background Questionnaire | This questionnaire will assess sociodemographic variables, pain location, number of surgeries, current medications and access/use/comfort with smartphones and internet technology | 5 min | X | ||
| Feasibility Outcome Measures | |||||
| iCanCope Program Engagement31 | App and web usage (e.g., logins, dropout rates, completion of outcome measures and activity, etc.) at user and aggregate level using ABSTRACT. | N/A | X | ||
| The Treatment Services Review (TSR) | A self-report assessment to collect information on the type and amount of health and psychosocial services received outside of the study | 5 min | X | X | |
| A fill-in-the-blank survey will be used to assess overall satisfaction with the iCanCope-NF program. Specific items will assess interest, helpfulness, and ease of use. | 5 min | X | |||
| Clinical Outcome Measures | |||||
| Short Form Survey-20 (SF-20) | A 20-item self-report scale with 8 subscales on respondent burden (Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional, and Mental Health). | 5 min | X | X | X |
| Goal Setting31 | Engagement in goal setting will be tracked on the iCanCope app using the following parameters: number and type of goals created, number of goals tracked, and rate of goal completion. | N/A | X | ||
| Chronic Pain Acceptance Questionnaire-Revised (CPAQ-Revised) | 20-item scale designed to measure acceptance of pain. The acceptance of chronic pain is thought to reduce unsuccessful attempts to avoid or control pain and thus focus on engaging in valued activities and pursuing meaningful goals. | 2–3 min | X | X | X |
| Psychological Inflexibility in Pain Scale (PIPS) | 16-item scale used to assess psychological inflexibility (i.e. avoidance, acceptance, fusion, values orientation, dirty discomfort) in people with chronic pain. | 5 min | X | X | X |
| Pain Catastrophizing Scale (PCS) | 13-item self-report scale, help quantify an individual's pain experience, asking about how they feel and what they think about when they are in pain. | 2–3 min | X | X | X |
| Pain Self-Efficacy Questionnaire (PSEQ) | 10-item scale that requires patients to take their pain into account when rating their self-efficacy belief. | 2–3 min | X | X | X |
1.8. Retention and adherence
We predict the pilot RCT will require 18 months to complete. The patient networks that we are recruiting from have over 25,000 adults across both groups. A conservative estimate of 20% of participants should be within the appropriate age criterions (∼5000). As we are seeking to enroll 36 participants/subjects/patients in each group for a total of 108 for the pilot RCT, we intend to recruit 140 participants to account for 20% attrition. All participants in each group will receive 25-dollar Amazon gift cards for participating and completing the study, and participants who are in the ICC-NF + CM group can earn additional 50-dollars in gift cards based on usage. Participants are encouraged to contact the research team if there are any issues with the mobile application through a specific email. Participants will be reminded of (T2) and (T3) intervals through secure emails or phone calls.
For those who have access to the mobile application, we have added more content specific to that of the NF1, along with resources that will target specific what for the disease. In addition, we have included yoga poses to help reduce target specific pain points by a world-renown yoga instructor. Lastly, for those who have been randomized to the ICC-NF + CM group, we have added gamification elements such as incentives for completing tasks, goals and check ins.
1.9. Sample size
An a priori power analysis conducted through G*Power (Universität Kiel, v. 3.1.9.2) estimated a conservative sample size analysis for the RCT. We are expecting a total of 140 participants, and given the length of the trial, we estimate 20% attrition rate of the final sample, giving us a total sample of (n = 36) per group, for a final sample size of 108. The power analysis is based on previously utilized research in which estimated effect sizes ranged from 20 (small effect) to 40 participants per group (large effect) [43]. We will attempt to enroll equal numbers of male and female participants to evaluate gender differences through urn randomization process.
1.10. Data analysis
We will use α < 0.05 but will use appropriate corrections for multiple tests. Statistical analyses will be conducted in collaboration by the principal investigator and biostatistician using SPSS, and/or SAS, with consultation from the co-investigators. All variables will be examined for outliers, skewness/kurtosis, normality, and linearity prior to analyses being conducted. Using logistic regression for categorical and ANOVA for continuous measures, we will conduct preliminary analyses of the adequacy of the randomization procedure, the comparability of baseline measures across conditions, and the possible need for covariates in the analyses of treatment outcome data.
A general linear model of count data (logins, dropout rates, completion of outcome measures, engagement activity, (e.g., user and aggregate levels) will be used to evaluate the primary first outcome: Can we identify the acceptability of the iCanCope-NF program? Additionally, the proportional changes will be evaluated using multiple ANOVAS. Due to potential missing data assumptions, a Cox regression will evaluate for differences in relationship between pre-and post. For second primary outcome: what are perceptions regarding intervention acceptability and satisfaction. Aggregate values will be summed and standardized through a (z-score analysis) to ensure equal representation of groups. Lastly, for the levels of engagement and number of activities tried/TSN exercises will be examined with linear and non-linear Mixed-effects Models (LMM) with autoregressive 1 correlation structure (AR1) to test between conditions, while allowing for intra-participant serial correlation and unequal variance and covariance structure across time.
Secondary analyses will explore three research questions: (1) Does the smartphone program (iCanCope-NF) compared with the control condition lead to differences in pain and pain-related activity limitations, sleep functioning, emotional functioning (depression, anxiety), pain catastrophizing, self-efficacy, knowledge, psychological flexibility immediately post-treatment (T2)? This will be evaluated by incorporating an estimating equation approach to understand the differences in scores and using a sequential multilevel logistic regression models to estimate the independent and combined effects of proposed time-varying (contextual) variables on the probability of pain levels. We will also test interaction effects between stable/trait-level participant characteristics and contextual variables. (2) Does the ICC-NF + CM increase the usage of the ICC-NF program as compared to ICC-NF without CM, and do their corresponding levels of pain and pain-related activity decrease? This will be evaluated between the two intervention groups using the difference in nominal data sets and simple qualitative analyses. (3) Do individuals with NF1 utilize the MBAA more readily because they are on the mobile application? To assess the moderation effect, we will include an interaction term for each separate moderator variable and key predictor of interest (two-way interaction between moderator and group for cross-sectional analysis and three-way interaction between moderator, group and time for longitudinal analysis) in the regression models and test its significance using Wald t-test.
2. Discussion
There have been only a limited number of clinical trials specifically focused on pain symptoms for individuals with NF1 [44], and to our knowledge, none have focused on complimentary alternative approaches (CAM). The ICC mobile application has been shown initially effective in children and adolescents with various pain disorders such as arthritis [45] and chronic pain [35], and this adaptation will extend the utility and feasibility of the mobile application with the addition of a novel adult pain disorder. By integrating the iCanCope-NF mobile application within future clinical trials, researchers will be able to access real-time data and understanding of pain symptoms throughout potential trials, thereby allowing for clearer understanding of antecedent- and consequence-based reactions to pain symptoms.
A novel integration in conjunction with the ICC mobile application is connecting to a high-performance analytics platform that supports CM. While CM is not integrated into ICC, the Abstract analytics platforms enables CM functionality through analyzing ICC server data in real time to derive engagement behaviors. Integrating contingency management into the research design allows for several unique contributions: 1) a comparison in engagement, clinical and feasibility outcomes between participants receiving the ICC-NF application only to that who receive ICC-NF + CM, 2) An initial understanding if CM increases the use of mind body alternative approaches to reduce pain severity and interference scores, 3) the long-term effects (2 months) of using CM within the NF1 population, and 4) integration of a backend server to provide near-immediate reinforcement to clients when the task(s) has been accomplished, thus allowing the research team to evaluate how the patient(s) progress through the mobile application.
The current protocol will focus on adults with NF1 and emphasize techniques and strategies for combating chronic pain. An emerging body of evidence suggests that as individuals with NF1 age and continue to experience cumulative recurrent chronic pain episodes, central neurobiological mechanisms may become a key etiology [46]. Thus, in our customization of the iCanCope-NF mobile application, we attempted to classify the pain episodes in the patient's ability to graphically denote when the events occurred allowing for visual inspection by both the individuals suffering from the disease and potentially the participant's doctor.
In establishing the specific customizations for an adapted version on the ICC mobile application not all features and specific formats could be addressed. For example, adding a community function would allow participants to engage in meaningful dialogue with each other (development of coping strategies, and build rapport) along with investigators to target specific concepts of pain and techniques to reduce the pain. Additionally, the iCanCope-NF was developed specifically for adults with NF1 and not for adolescents with NF1. Future iterations of the iCanCope-NF mobile application could address these areas, establishing a community function and developing an ICC iteration solely for adolescents with NF. We anticipate the current study will be largely impactful to the NF community. This will be the first developed mobile application for individuals with NF specifically targeting self-management techniques to reduce pain caused by NF1 tumors. Additionally, with COVID restrictions currently in place, the mobile application allows for treatment strategies to be addressed at the participant's home, while incorporating the empirically based treatment of CBT.
3. Conclusions
The current study expects individuals with NF1 utilizing the ICC program to have decreased pain severity and interference if they regularly engage in the program. In addition, due to the added contingency management, we expect to see differences between the intervention groups (ICC–NF and ICC-NF + CM). By adding contingencies in addition to current ICC processes to increase engagement, the CM grouping should show more significantly reduce pain intensity and pain interference. If we demonstrate initial acceptability and preliminary impact, a large-scale RCT will be conducted. In future studies, evaluating the sustainability over the course of 12 months will be critical in reducing chronic pain for individuals with NF1. Thus, following the patients in a 1, 3 and 6-month follow-up post-treatment is necessary, along with extension to other forms of NF (e.g., Neurofibromatosis Type 2 and Schwannamotosis) and adolescents.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the U.S. Army Medical Research Materiel Command endorsed by the U.S. Army, through the Congressionally Directed Medical Research Programs’ Neurofibromatosis Research Program under Award No. W81XWH-19-1-0618.
References
- 1.Allen T.M., Struemph K.L., Toledo-Tamula M.A., Wolters P.L., Baldwin A., Widemann B., et al. The relationship between heart rate variability, psychological flexibility, and pain in neurofibromatosis type 1. Pain Pract. 2018;18(8):969–978. doi: 10.1111/papr.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brems H., Beert E., de Ravel T., Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10(5):508–515. doi: 10.1016/S1470-2045(09)70033-6. [DOI] [PubMed] [Google Scholar]
- 3.Tucker T., Friedman J.M., Friedrich R.E., Wenzel R., Fünsterer C., Mautner V.F. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J. Med. Genet. 2009;46(2):81–85. doi: 10.1136/jmg.2008.061051. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J.L., Gutmann D.H. Neurofibromatosis type 1. Handb. Clin. Neurol. 2015;132:75–86. doi: 10.1016/B978-0-444-62702-5.00004-4. [DOI] [PubMed] [Google Scholar]
- 5.Sanagoo A., Jouybari L., Koohi F., Sayehmiri F. Evaluation of QoL in neurofibromatosis patients: a systematic review and meta-analysis study. BMC Neurol. 2019;19(1):123. doi: 10.1186/s12883-019-1338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsch L.A. Technology-based interventions targeting substance use disorders and related issues: an editorial. Subst. Use Misuse. 2011;46(1):1–3. doi: 10.3109/10826084.2011.521037. [DOI] [PubMed] [Google Scholar]
- 7.Budman S.H. Behavioral health care dot-com and beyond: computer-mediated communications in mental health and substance abuse treatment. Am. Psychol. 2000;55(11):1290–1300. [PubMed] [Google Scholar]
- 8.Nolan L.J., Scagnelli L.M. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst. Use Misuse. 2007;42(10):1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz R.P., Kelly S.M., O'Grady K.E., Gandhi D., Jaffe J.H. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction. 2012;107(5):943–952. doi: 10.1111/j.1360-0443.2011.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. J. Am. Board Fam. Pract. 2001;14(3):211–218. [PubMed] [Google Scholar]
- 11.Spek V., Cuijpers P., Nyklícek I., Riper H., Keyzer J., Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol. Med. 2007;37(3):319–328. doi: 10.1017/S0033291706008944. [DOI] [PubMed] [Google Scholar]
- 12.Connors G.J., Tonigan J.S., Miller W.R. A longitudinal model of intake symptomatology, AA participation and outcome: retrospective study of the project MATCH outpatient and aftercare samples. J. Stud. Alcohol. 2001;62(6):817–825. doi: 10.15288/jsa.2001.62.817. [DOI] [PubMed] [Google Scholar]
- 13.Hall J.A., Huber D.L. Telephone management in substance abuse treatment. Telemed. J. e Health. 2000;6(4):401–407. doi: 10.1089/15305620050503870. [DOI] [PubMed] [Google Scholar]
- 14.Marks I., Shaw S., Parkin R. Computer-aided treatments of mental health problems. Clin. Psychol. Sci. Pract. 1998;5(2):151–170. [Google Scholar]
- 15.Williams A.C., Eccleston C., Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2012;11(11) doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henschke N., Ostelo R.W., van Tulder M.W., Vlaeyen J.W., Morley S., Assendelft W.J., et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst. Rev. 2010;(7) doi: 10.1002/14651858.CD002014.pub3. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richmond H., Hall A.M., Copsey B., Hansen Z., Williamson E., Hoxey-Thomas N., et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0134192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehde D.M., Dillworth T.M., Turner J.A. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am. Psychol. 2014;69(2):153–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 19.10 Facts about Smartphones as the iPhone Turns 10. Pew Research Center; 2017. http://www.pewresearch.org/fact-tank/2017/06/28/10-facts-about-smartphones/ [updated 28 June 2017. Available from: [Google Scholar]
- 20.Toll B.A., O'Malley S.S., Katulak N.A., Wu R., Dubin J.A., Latimer A., et al. Comparing gain- and loss-framed messages for smoking cessation with sustained-release bupropion: a randomized controlled trial. Psychol. Addict. Behav. 2007;21(4):534–544. doi: 10.1037/0893-164X.21.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentice J.L., Dobson K.S. A review of the risks and benefits associated with mobile phone applications for psychological interventions. Can. Psychol./Psychol. can. 2014;55(4):282–290. [Google Scholar]
- 22.O'Connor S., Hanlon P., O'Donnell C.A., Garcia S., Glanville J., Mair F.S. Understanding factors affecting patient and public engagement and recruitment to digital health interventions: a systematic review of qualitative studies. BMC Med. Inf. Decis. Making. 2016;16(1):120. doi: 10.1186/s12911-016-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torous J., Nicholas J., Larsen M.E., Firth J., Christensen H. Clinical review of user engagement with mental health smartphone apps: evidence, theory and improvements. Evid. Base Ment. Health. 2018;21(3):116–119. doi: 10.1136/eb-2018-102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickel W.K., Christensen D.R., Marsch L.A. A review of computer-based interventions used in the assessment, treatment, and research of drug addiction. Subst. Use Misuse. 2011;46(1):4–9. doi: 10.3109/10826084.2011.521066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll K.M., Ball S.A., Martino S., Nich C., Babuscio T.A., Nuro K.F., et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am. J. Psychiatr. 2008;165(7):881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins S.T., Petry N.M. Contingency management. Incentives for sobriety. Alcohol Res. Health. 1999;23(2):122–127. [PMC free article] [PubMed] [Google Scholar]
- 27.Greenfield S.F., Brooks A.J., Gordon S.M., Green C.A., Kropp F., McHugh R.K., et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgins D.C., el-Guebaly N., Addington J. Treatment of substance abusers: single or mixed gender programs? Addiction. 1997;92(7):805–812. [PubMed] [Google Scholar]
- 29.Blum T.C., Roman P.M., Harwood E.M. Employed women with alcohol problems who seek help from employee assistance programs. Description and comparisons. Recent Dev. Alcohol. 1995;12:125–156. doi: 10.1007/0-306-47138-8_7. [DOI] [PubMed] [Google Scholar]
- 30.Copeland J. A qualitative study of barriers to formal treatment among women who self-managed change in addictive behaviours. J. Subst. Abuse Treat. 1997;14(2):183–190. doi: 10.1016/s0740-5472(96)00108-0. [DOI] [PubMed] [Google Scholar]
- 31.Brady K.T., Grice D.E., Dustan L., Randall C. Gender differences in substance use disorders. Am. J. Psychiatr. 1993;150(11):1707–1711. doi: 10.1176/ajp.150.11.1707. [DOI] [PubMed] [Google Scholar]
- 32.The Dasis Report—Facilities Offering Special Programs or Groups for Women: 2005. 2007. Rockville, MD. [Google Scholar]
- 33.Kurti A.N., Davis D.R., Redner R., Jarvis B.P., Zvorsky I., Keith D.R., et al. A review of the literature on remote monitoring technology in incentive-based interventions for health-related behavior change. Transl. Issues Psychol. Sci. 2016;2(2):128–152. doi: 10.1037/tps0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins S.T. Comments on contingency management and conditional cash transfers. Health Econ. 2010;19(10):1255–1258. doi: 10.1002/hec.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinson J.N., Lalloo C., Harris L., Isaac L., Campbell F., Brown S., et al. iCanCope with Pain™: user-centred design of a web- and mobile-based self-management program for youth with chronic pain based on identified health care needs. Pain Res. Manag. 2014;19(5):257–265. doi: 10.1155/2014/935278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birnie K.A., Campbell F., Nguyen C., Lalloo C., Tsimicalis A., Matava C., et al. iCanCope PostOp: user-centered design of a smartphone-based app for self-management of postoperative pain in children and adolescents. JMIR Form. Res. 2019;3(2) doi: 10.2196/12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palermo T.M., Zempsky W.T., Dampier C.D., Lalloo C., Hundert A.S., Murphy L.K., et al. iCanCope with Sickle Cell Pain: design of a randomized controlled trial of a smartphone and web-based pain self-management program for youth with sickle cell disease. Contemp. Clin. Trials. 2018;74:88–96. doi: 10.1016/j.cct.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grasaas E., Fegran L., Helseth S., Stinson J., Martinez S., Lalloo C., et al. iCanCope with pain: cultural adaptation and usability testing of a self-management app for adolescents with persistent pain in Norway. JMIR Res. Protoc. 2019;8(6) doi: 10.2196/12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbons R.D., Hedeker D., Elkin I., Waternaux C., Kraemer H.C., Greenhouse J.B., et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch. Gen. Psychiatr. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 40.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin S., Wolters P.L., Toledo-Tamula M.A., Schmitt S.N., Baldwin A., Starosta A., et al. Acceptance and commitment therapy in youth with neurofibromatosis type 1 (NF1) and chronic pain and their parents: a pilot study of feasibility and preliminary efficacy. Am. J. Med. Genet. 2016;170(6):1462–1470. doi: 10.1002/ajmg.a.37623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolters P.L., Burns K.M., Martin S., Baldwin A., Dombi E., Toledo-Tamula M.A., et al. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. Am. J. Med. Genet. 2015;167a(9):2103–2113. doi: 10.1002/ajmg.a.37123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy K.R. In: The Reviewer's Guide to Quantitative Methods in the Social Sciences. Hancock G.R., Mueller R.O., editors. Routledge.; New York, New York: 2010. Power analysis; pp. 93–115. [Google Scholar]
- 44.Walsh K.S., Janusz J., Wolters P.L., Martin S., Klein-Tasman B.P., Toledo-Tamula M.A., et al. Neurocognitive outcomes in neurofibromatosis clinical trials. Recommend. dom. atten. 2016;87(7 Supplement 1):S21–S30. doi: 10.1212/WNL.0000000000002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lalloo C., Harris L.R., Hundert A.S., Berard R., Cafazzo J., Connelly M., et al. The iCanCope pain self-management application for adolescents with juvenile idiopathic arthritis: a pilot randomized controlled trial. Rheumatology. 2021;60(1):196–206. doi: 10.1093/rheumatology/keaa178. [DOI] [PubMed] [Google Scholar]
- 46.Crawford H.A., Barton B., Wilson M.J., Berman Y., McKelvey-Martin V.J., Morrison P.J., et al. The impact of neurofibromatosis type 1 on the health and wellbeing of Australian adults. J. Genet. Counsel. 2015;24(6):931–944. doi: 10.1007/s10897-015-9829-5. [DOI] [PubMed] [Google Scholar]