Abstract
Plasma is regularly alluded to as the fourth form of matter. Its bounty presence in nature along with its potential antibacterial properties has made it a widely utilized disinfectant in clinical sciences. Thermal plasma and non-thermal (or cold atmospheric) plasma (NTP) are two types of plasma. Atoms and heavy particles are both available at the same temperature in thermal plasma. Cold atmospheric plasma (CAP) is intended to be non-thermal since its electrons are hotter than the heavier particles at ambient temperature. Direct barrier discharge (DBD), atmospheric plasma pressure jet (APPJ), etc. methods can be used to produce plasma, however, all follow a basic concept in their generation. This review focuses on the anticipated uses of cold atmospheric plasma in dentistry, such as its effectiveness in sterilizing dental instruments by eradicating bacteria, its advantage in dental cavity decontamination over conventional methods, root canal disinfection, its effects on tooth whitening, the benefits of plasma treatment on the success of dental implant placement, and so forth. Moreover, the limitations and probable solutions has also been anticipated. These conceivable outcomes thus have proclaimed the improvement of more up-to-date gadgets, for example, the plasma needle and plasma pen, which are efficient in treating the small areas like root canal bleaching, biofilm disruption, requiring treatment in dentistry.
Keywords: Non-thermal plasma, Dentistry, Sterilization, Tooth disinfection, Cold atmospheric plasma, Plasma pen, Dental implant modification
Graphical abstract
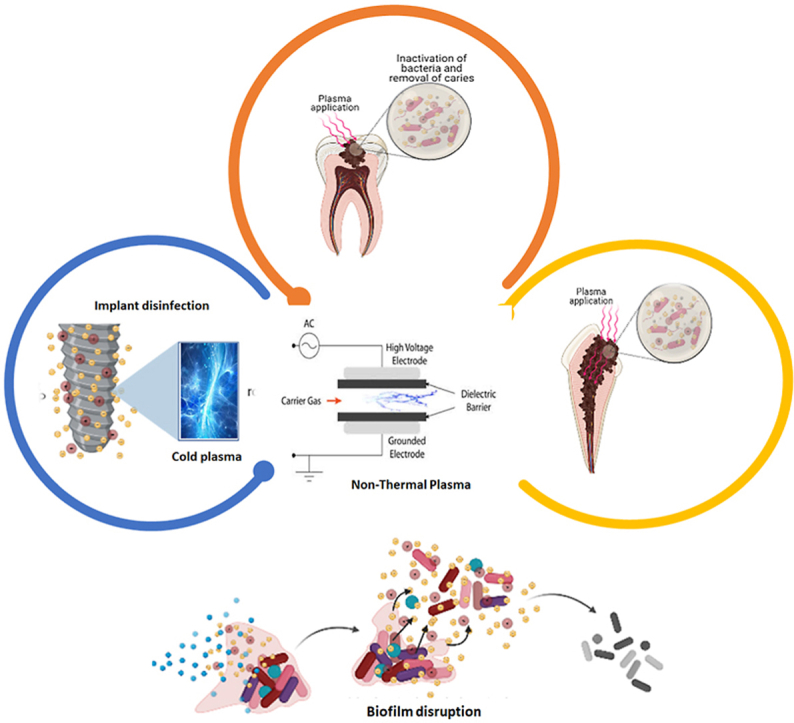
Highlights
-
•
Non-thermal plasma (NTP) has regarded as an important tool for biomedical application especially dental application.
-
•
The surface application of NTP can be used for disinfecting microbial infection in endodontic issues.
-
•
NTP can be used to eradicate the microorganism biofilm responsible for dental caries.
-
•
NTP can also be utilized in would healing, implant modifications and adhesive restoration.
-
•
NTP is potential candidate for clinical application in dentistry based on the experimental proofs.
1. Introduction
What precisely is plasma? Solid, liquid, and gas are the three most well-recognized states of matter. Plasma is often referred to as the fourth state (Fig. 1). Plasma a gas contains particles in plasma state. It can be characterized as a gas containing free electrons, ions, and various other active atomic or molecular radicals such as hydroxyl radicals (OH-). Plasma also contains energetic photons (ultraviolet light) and intense transient electric field. Both play a key role for bond breaking or reactive species generation due to huge reduced electric field in pulsed discharge [[1], [2], [3], [4]]. It is, truth be told, the most spread form of matter, making up about 99% of the noticeable universe. From the northern lights to the core of a star, plasma is available in an assortment of structures, over the universe. Furthermore, we humans have bridling such a different material, be it as neon lights, bright signs, or food handling. Microelectronics, arc welding as two of the most widespread technologies where plasma is used along with other applications in biomedical and dental sciences.
Fig. 1.
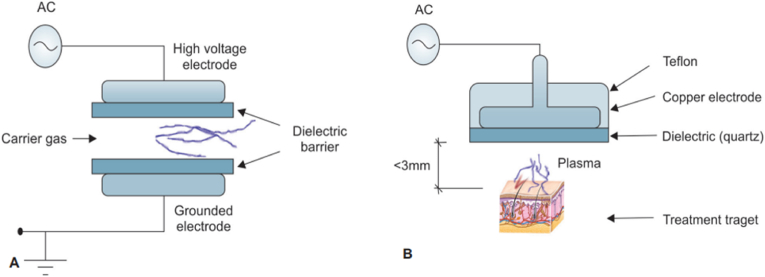
Generation of plasma by different techniques: Dielectric barrier discharge (DBD) and a floating dielectric barrier discharge (FE-DBD). (A) Formation of plasma by DBD; and (B) FE-DBD.
Plasma is categorized as thermal or non-thermal based on the relative temperatures of the electrons, ions, and neutrals. In thermal plasma, the electrons and heavy particles are in thermal equilibrium, but in non-thermal plasma (NTP), the electrons are hotter, but the ions and neutrals are at room temperature, therefore it is also known as cold atmospheric plasma (CAP) [5,6].
Plasma (Ancient Greek πλάσμα) implies mold-able substance Sir William Crookes first identified plasma in 1879 in Crookes tube and Plasma, according to him, is a radiant matter [7]. The word plasma was coined by Irving Langmuir. He depicted plasma as an electrified fluid transporting electrons and ions, like blood plasma, which transports red and white corpuscles [8]. In 1897, Sir J.J. Thomson distinguished the cathode beam's nature (electron beam) in his plum pudding model, wherein he exhibited the dispersion of electrons and protons in an atom with his cathode ray experiment [9]. The Siemens Company in the last part of the 1850s utilized plasma release to create ozone. Bol'shakov et al., in 2004 published the impacts of radiofrequency oxygen plasma on bacteria.
Most of cold plasma for medicine are pulsed (kHz, MHz). However, considering the stability and safety of microwave-derived plasma, it has been considered to be useful for biomedical applications and slowly becoming famous in various medicinal fields [10]. The use of NTP for sterilizing medical equipment [11], food packaging in the food industry, blood coagulation, wound healing, and other applications have recently been reported in the literature. Non-thermal plasma (NTP) is an antimicrobial treatment being explored for use on fruits, vegetables, and other foods with delicate surfaces in the food processing industry. As of late, NTP sources with <40 °C temperature at the point of application have been introduced that offer the likelihood to treat human beings [12,13].
NTP has been recognized for its different types of potential clinical and research applications in dental science (Table 1). The first application of NTP in dentistry isn't clear even though it was likely utilized during the assembling of dental instruments or in their sanitization. Clinically, one of the main apprehensions that patients have towards dental treatment is the potential pain that may cause during dental procedures [14]. Cold plasma has great potential for use in dentistry as it is vibration-free, leading to lesser pain perception by the patient [15]. It can be useful to some extent for the patients who are anxious or fearful of using a drill for cavity preparation before tooth restoration and removal of necrotic, diseased, or non-remineralizable tissues. Due to its capabilities of deactivation of pathogenic bacteria and alteration of non-inflammatory tissue, it can be a useful tool for the treatment of dental cavities and composite restorations [16]. Researchers have anticipated that the Plasma treatment would be a non-destructive, painless, and tissue-saving technique to treat cavities, because of its reliance on chemical based reactions compared to heat or mechanical interactions of conventional techniques. Moreover, the chemical bonding between teeth and fillings created by the plasma treatment is much stronger than drills or laser techniques. Furthermore, NTP is used in dentistry for root canal disinfection, sterilizing dental instrumentation, plaque removal, tooth whitening (bleaching), and improving bond strength at the dentin-composite interface [17].
Table 1.
Dental application of different types of non-thermal plasma on biological models.
| Dental science applications | Source of plasma/plasma devices | Biological models | References |
|---|---|---|---|
| Dental canal disinfection | Plasma jet device/He; He/O2 | Human extracted tooth | [68] |
| Dental canal disinfection | Plasma jet device/Ar/O2 | Human extracted tooth | [108] |
| Improvement of dental structures | Plasma brush/Ar; low-pressure plasma device/O2, Ar, N2, and He + N2; HDBD device/Ar; plasma jet device | Human extracted tooth | [76,80,109] |
| Biofilm reduction | Kinpen MED ® plasma jet/Ar | In vitro (Bacteria, lab condition) | [65] |
| Biofilm reduction on titanium discs | Three different types of CAP devices: (a) kINPen plasma jet/Ar; (b) HDBD device/Ar; (c) VDBD device/Ar | In vitro (Bacteria, lab condition), Extracted tooth | [110] |
2. Generation of NTP
Plasma can be produced by radio frequency, high voltage alternating current (AC) or direct current (DC), microwave frequencies [18,19]. There are many different devised which are used for the production of plasma. One of the specific type is a needle which is being used for medicinal application. A syringe and a needle are included in a medically utilized plasma device. The syringe has a 6 mm diameter, a 200 μm needle diameter, and a 3 cm length. Through a ballast, resistor (R) 60 - kO and capacitor (C) 50 - pF, the needle functions as an electrode and is linked to a high voltage direct current (amplitude up to 10 kV, repetition rate up to 10 kHz, and pulse width variable from 200 ns to DC). The discharged current is restricted to a safe range due to the presence of a resistor and capacitor [20]. When a working gas such as helium/oxygen (20%) is injected into a hollow syringe at a flow rate of 0.4 L/min and a high voltage DC is applied to the needle, plasma is produced [21].
2.1. Direct barrier discharge (DBD)
It was created by Siemens in 1857. Di-electric covering two-level metal terminals is available in DBD (Fig. 1A). One is a high voltage, and another is a grounded electrode. Gas passing between electrodes is ionized and plasma is formed. A high voltage alternative current is required, and power consumption is 10–100W [22]. DBD with input power in the W range have also been often reported for biomedical applications [23].
A variety of electrodes is possible, like dielectric material covering just a single electrode rather than two and a cylindrical electrode is present instead of flat. Another variant, as shown in Fig. 1B, is the floating electrode DBD (Fe - DBD), which was developed by Fridman et al. [24]. It comprises a high voltage electrode and another active electrode like human skin and organs. The powered electrode ought to be put close (<3 mm) to the second electrode to create plasma.
DBD is utilized in the sanitation of living organisms, angiogenesis, surface treatment, and the inactivation of microbes like Bacillus stratosphericus [25]. It may be additionally utilized on melanoma.
2.2. Atmospheric plasma pressure jet (APPJ)
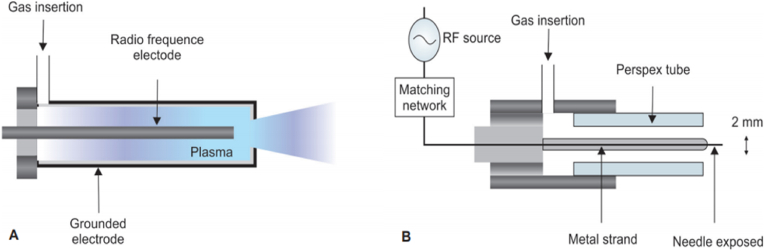
Atmospheric Plasma Pressure Jet (APPJ) are the plasma jets having the gas temperature ranges from 25–200 °C with a charged-particle density of 1011–1012 cm−3 and reactive species at high concentrations, i.e., 10–100 ppm [26]. It can be made up with different setups in which a gas mixture (helium, oxygen, and other gases) is pushed to flow quickly between two electrodes. One of the common setup is shown in Fig. 2A where a radiofrequency of 13.56 MHz at a power of 50–100 W is applied on the discharge creating a central (cathode) electrode, and the outer electrode (anode) is grounded. The cathode is made of tungsten or stainless steel with 1 mm diameter (Fig. 2A) [27].
Fig. 2.
An APPJ and a plasma needle. (A) A schematic representation of the APPJ created by Schűtze in 1998; and (B) A schematic representation of the plasma needle created by Stoffels et al., in 2002.
APPJ devices are not only limited to MHz devices, rather, they have been reported to be based on a dielectric barrier discharge (DBD) reactor powered by either nanosecond or microsecond rise-time high-voltage pulses [27,28].
APPJ is applied in the inactivation of bacteria. It is also noteworthy that the first radiofrequency cold plasma was developed by Koinuma et al., in 1992 [25].
2.3. Plasma needle
Stoffels et al. [29] invented a miniature atmospheric plasma needle in 2002. Fig. 3B shows a schematic representation of the same. In 2004, a new version was developed [30]. The new plasma needle has a 0.3 mm diameter metal support with a perspex tube with a sharpened tip. Helium is the most commonly utilized gas due to low breakdown voltage and the homogenous nature of the discharge. When radiofrequency power ranging from 10 mW to several watts is delivered at 13.05 MHz, microplasma is produced. Microplasma is effective for treating tiny regions in dentistry that requires attention [31,32]. E. coli bacteria can be killed by this process [33].
Fig. 3.
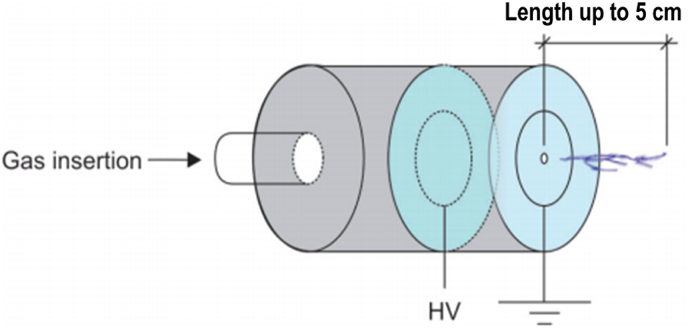
A schematic representation of the plasma needle designed by Laroussi et al.
2.4. Plasma pencil
Laroussi et al. [34], developed the plasma pencil. is made of two electrodes having an identical radius (1.25 mm). A space of 0.3 cm–1.0 cm is available between them. The dielectric disc is connected to a thin copper ring. High voltage sub-microsecond pulses are applied between these terminals to make plasma, while the gas is infused through an electrode hole. When the discharge is created, a plasma plume is propelled through the gap of the external terminal. Because of its low temperature (290 K), the plasma plume (length up to 5 cm) can be touched securely. A schematic representation is shown in Fig. 3.
Plasma pencil can be used to inactivate P. gingivalis and E. coli bacteria. These bacterial strains have been reported to exhibit biofilm formation leading to dental caries. Inactivation of plankton bacteria by plasma pencil also provides opportunity to inhibit the biofilm formation. Moreover, it can also be an approached technique for the curation of leukaemia [35].
3. Application of NTP in dentistry
3.1. Sterilization
Sterilization is the process of killing all microorganisms, including viruses, bacteria, fungus, and bacterial endospores. Sterilization procedures include steam autoclaves, dry heat, and chemical vapours that are not saturated. Therefore, autoclaving is the most frequently used method of sterilizing dental instruments. Under the pressure of 15 pounds at 121 °C for 20 min at 134 °C for 3 min, it produces steam. It penetrates deeply, allowing all instrument surfaces to be exposed to the steam. Sterilizes water-based beverages in a relatively short period. Also available are ovens that use dry heat or chemical vapour sterilizers that use unsaturated chemical vapours. Those microorganisms that are directly exposed to high temperature and dry heat can be killed. Temperatures between 160 °F and 170 °F, sustained for 1 h, are sterilizing. However thermal sterilization methods are not suitable for use in all dental materials whereas the non-thermal methods, like chemical sterilization may cause undesirable changes in the materials and instruments.
The effectiveness of plasma sterilization is affected by gas composition, bacterial stain, and driving frequency. It surpasses the other conventional non-thermal techniques. When compared to conventional approaches, plasma devices have been found to destroy bacteria faster [36]. The plethora of plasma components such as reactive oxygen species (ROS), electromagnetic fields, UV and ions, and electrons is related to the mechanism of plasma sterilization [37]. In the lipid bilayer of the bacterial cell membrane, unsaturated fats and proteins are involved in membrane transport [38]. Bacteria are rendered inactive by hydroxyl radical assaults on unsaturated fatty acids, which are produced by plasma and damage membrane lipids [39]. The region around the site of contact can also be affected by plasma.
Of late, plasma sterilization technique and devices (Sterlink, Germany) are becoming popular in the biomedical applications and on the verge of being used in dentistry [40], however, the utilization of non-thermal plasma for decontamination of surgical instruments is still limited. According to Whittaker et al. using plasma gas cleaning to reduce the absolute number of proteinaceous compounds from pulp that can be transmitted between patients when endodontic tools and files are used might be highly successful [36]. Plasma sterilization, according to Li et al. overcomes the limits of existing sterilizing techniques. It has evolved into a unique sterilizing technology due to its benefits of safety, completeness, speed, and low temperature [41]. Sung et al. investigated the efficacy of the NTP device for sterilizing metal, rubber, and plastic equipment and a variety of instruments [42]. The non-thermal plasma device was shown to be very effective in deactivating Bacillus subtilis and E. coli, and to be more effective in killing E. coli than the UV sterilizer [43].
Independent studies by the likes of Socransky et al. [44] amongst others have found that various types of bacteria like P. aeruginosa [45], Staphylococcus aureus [4], Escherichia Coli [4,46], Enterococcus faecalis [47], can be inactivated by plasma, as mentioned in Table 2.
Table 2.
Types of bacterial strains inactivated by non-thermal plasma.
| S. No. | Species | Strains |
|---|---|---|
| With experimental evidences | ||
| 1. | Streptococci | Streptococcus mutans [111], Streptococcus sobrinus [112], Streptococcus sanguis [113], Streptococcus anginosus [113] |
| 2. | Lactobacilli | Lactobacillus fermentum [114], Lactobacillus plantarum [115] |
| 3. | Actinomyces | Actinomyces israelii [116], Actinomyces odontolyticus [117] |
| Probable stains (in studies) | ||
| 4. | Aerobes | Neisseria mucosa, Haemophilus parainfluenzae |
| 5. | Anaerobes 1 | Fusobacterium nucleatumssnucleatum, Campylobacter rectus, Veillonellaparvula, Capnocytophagagingivalis, Peptostreptococcusasaccharolyticus, Gemellamorbillorum, Prevotellamelaninogenica, Leptotrichiabuccalis, Eubacteriumsaburreum, Corynebacterium matruchotii, Prevotellanigrescens/intermedia |
| 6. | Anaerobes 2 | Porphyromonasgingivalis, Selenomonasnoxia, Micromonas micros |
The review of literature thus reveals that non-thermal plasma is an effective method of sterilization of dental instruments as well as various dental surfaces because of its potent bactericidal properties. When we compare NTP with conventional sterilization protocols, it has been found to not only be more efficient but also a much faster, thorough alternative [48]. The fact that the same method may be used for a variety of instruments ranging from plastics, glasses, to metal and more, eliminates the need to have multiple cumbersome sterilizations and disinfection methods and equipment, thus saving time and investment.
3.2. Dental caries
Mechanical or laser methods can be used to clean and disinfect infected tissue in a tooth cavity or a root canal. Both methods, however, have the potential to heat and destroy healthy tissue. Additionally, the vibrations caused by the drill is another disadvantage as it may lead to patient discomfort. The generation of heat, vibrations and noise may increase the pain perception of patients as well as their anxiety and fear of dental treatment.
When used in dental cavities, cold plasma decontaminates the cavities without the need to drill them (Fig. 4). Despite plasma's surface-level nature, its active plasma species can penetrate deep into the cavity. The clinical procedure can be carried out by disinfecting bacteria, which are in the dentine tubules or close to the pulp, after the removal of most of soft caries tissue with the bur [43]. Thus, CAP, being vibration-free, has found an extraordinary potential for use in dentistry as it can decreases the patient's pain perception [49]. Though the clinical proofs are awaited, it has been in sighted that the it can be very helpful for children or patients who are afraid of using the drill for cavity preparation before tooth restoration and removal of necrotic, diseased, or non-remineralizable tissues because of its indirect use.
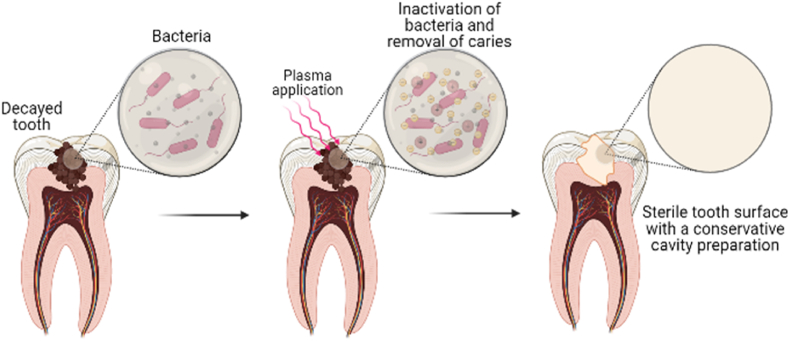
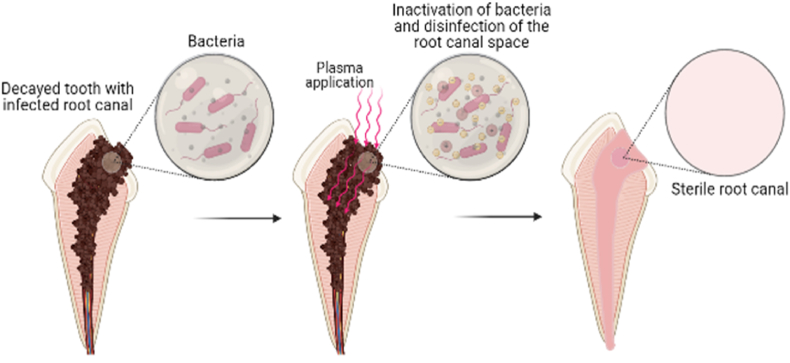
Fig. 4.
Application of Non-thermal plasma in removal of caries in tooth; The prepared cavity in decayed tooth is sterilised through inactivation of bacteria by applying non-thermal plasma.
Raymond et al. studied the interactions of non-thermal plasma with tooth tissue using a plasma needle [43]. For microbial decontamination, plasma needles are an effective source of radicals. Comparatively to lasers or other traditional mechanical methods of dental cavity preparation, because it functions at normal temperature, it does not induce mass tissue pulverization. It has been concluded that plasma therapy is a popular tissue-saving approach for cleaning uneven structures and small channels within the damaged tooth [43]. Short-lived chemical species generated by the plasma needle in the gas phase can interact with the surface of a tooth and dissolve into a liquid. Unlike the antibacterial liquid rinses that remain in the mouth after the operation, the plasma needle generates bactericidal agents locally, allowing them to reach the cavity and fissure areas [50].
The usage of plasma needles was recommended by Eva Stoffels, who pioneered this technique, because of its capacity to kill E. coli [29]. Goree et al. presented compelling evidence that non-atmospheric plasma can kill S. mutans [51]. Yang et al. then presented a low-temperature atmospheric argon plasma brush, which was proven to be very effective in deactivating and decontaminating Streptococcus mutans and Lactobacillus acidophilus [52]. The authors determined that for S. mutans, 100% bacterial eradication was accomplished in 15 s while it took 5 min for Lactobacillus acidophilus.
It can safely be concluded that disinfection of dental cavities using cold plasma is extremely desirable due to its many advantages, the most important being the elimination of the drill. Direct treatment with plasma can disinfect the dental cavities because of their reactive oxygen species, however, it can be said to have a limitation of having the soft dentine tissue left after the treatment. This ultimately helps in reducing the level of the patients’ anxiety and fear, as well as potential pain during the treatment procedures, thereby making them very compliant. Of course, its extreme potency in reducing the bacterial load as well as its conservative approach in preparing dental cavities and the elimination of heat production are added advantages when compared to the conventional methods, as both serve to protect the surrounding healthy living tissues.
3.3. Root canal disinfection
Root canal disinfection is the most important step of root canal therapy. Cleaning and sanitizing the canal's walls and the lumen is critical to achieving a good treatment outcome. Canals with bacterial infection are known to fail. This means germs that remained in a canal after chemical and mechanical preparation must be maintained to a minimum to provide successful therapy and treatment.
It is widely believed that efficient bacterial eradication necessitates first cleaning the canal by removing the smear layer and then breaking up the biofilm, allowing the germs to be exposed to the disinfectant. A variety of items can be used to remove the smear and/or disrupt the biofilm formation. Sodium hypochlorite, EDTA, citric acid, polyacrylic acid, and others are examples. Most doctors now prefer sodium hypochlorite over other irrigants because it has a proteolytic action along with being a disinfectant.
However, sodium hypochlorite's bactericidal efficacy is largely reliant on the length of time the solution is kept in the canal and the usage of large volumes of the solution because the disinfection ingredient is free chlorine, which depletes quickly. A little amount of product applied for a short period will have a limited amount of impact. The hypochlorite is also inefficient against any pathogenic bacteria, notably Enterococcus faecalis, which is related to recalcitrant canals [30].
Toxicity and microbial resistance are important issues with conventional disinfectants, as most of those with efficient bactericidal action is utilized at dosages where normal tissue toxicity becomes a significant concern. This can result in negative tissue responses.
As the concentration of a solution increases, the surface tension of the solution also increases, preventing the solution from effectively moistening canal walls. There is a risk that the biofilm layer on the canal wall will not be sufficiently disrupted, and the solution will not penetrate as far into the lateral canals or dentinal tubules as it should.
Recently a novel disinfecting system has been tested using non-thermal plasma for the disinfection of root canal systems (Fig. 5), which overcomes the drawbacks of the conventional methods, yet has the potential to achieve a greater amount of disinfection.
Fig. 5.
Application of Non-thermal plasma in Root canal disinfection of an infected tooth. Once a root canal is opened, it is treated with non-thermal plasma for a specific time for the disinfection.
Lu et al. used a reliable and easy-to-use plasma jet device that could generate plasma inside the root canal. Without causing any pain, the plasma could be touched with bare hands and manually directed into the root canal for disinfection. Isthmus, deltas, ramifications, specific dentinal tubules, and abnormalities are all part of the root canal system. Bacteria have been reported to enter dentinal tubules as deep as 500–1000 μm [53]. The results showed a rotational temperature of roughly 300 K and a vibrational temperature of approximately 2700 K when NTP comprising Helium/Oxygen (20%) gas was utilized. A peak current discharge of 10 mA is commonly found. at this condition. Preliminary inactivation study results showed plasma produced at this level can completely kill Enterococcus faecalis which is one of the main bacteria responsible for failure of root canal treatment within few minutes [54].
Li et al. performed an in-vitro study and found that 12 min of exposure to non-thermal plasma for three weeks completely kill E. Faecalis [54]. Pan et al. in his in-vitro study checked the feasibility of NTP for disinfection of root canal. NTP, according to the authors, has a high rate of destroying harmful microorganisms in the root canal [55].
In our opinion, the biggest advantage of non-thermal plasma in treating root canal infections that can be depicted is its ability to reach small spaces that cannot be instrumented normally however clinical studies and proof are yet to come. These areas are the niche of the bacterial that cause reinfections, ultimately causing root canal treatment failures. Its gaseous nature gives it an enormous advantage over traditional instrumentation methods, apart from its highly efficient antibacterial properties.
3.4. Tooth bleaching
Teeth that were discoloured and had no pulp were bleached for the first time in 1864 using a range of medicines, including chloride and other hypochlorite-based agents as well as sodium perborate and hydrogen peroxide.
Enamel surfaces can be damaged by bleaching chemicals due to their acidity. The microhardness, structure, and morphology of enamel are affected by the penetration of bleaching chemicals. Surface porosity increases as a result of protein breakdown in the enamel matrix and mineral loss. Hydrogen peroxide produced from bleaching chemicals at higher concentrations might cause larger alterations and perhaps deepen microporosities in the surface enamel structure. The physical and/or chemical characteristics of enamel surfaces can also be affected by tooth whitening methods employing light sources. Because of this, the process of whitening enamel surfaces can induce substantial morphological structural alterations. Dentin becomes rougher and bacterial adherence to the enamel surface increases as a result. Due to an increase in surface roughness and bacterial adherence, dental plaque can develop, which raises the risk of dental caries and periodontitis. To circumvent these disadvantages, a plasma-based tooth bleaching technique has been offered as a solution (Fig. 6).
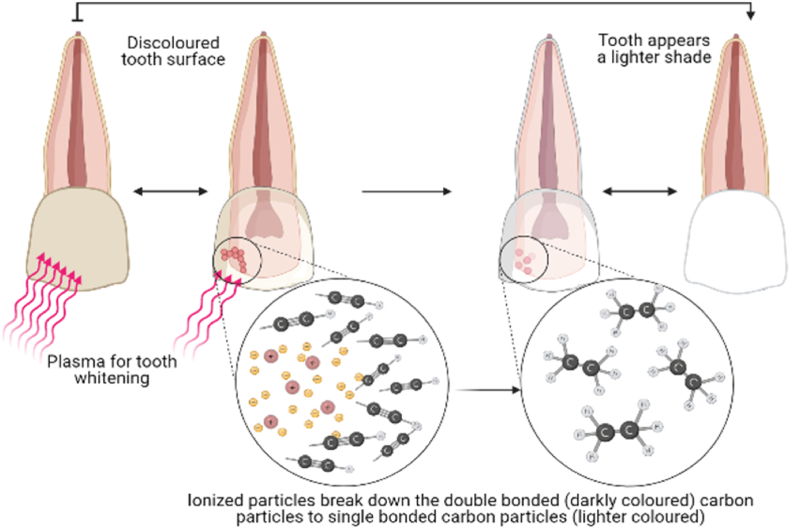
Fig. 6.
Application of Non-thermal plasma for bleaching of tooth. The surface of the tooth is treated with non-thermal plasma which breakdown the carbon particles present as a dirt on the surface. The carbon particles are then easily get washed away.
Lee et al. described the use of NTP for teeth bleaching. When atmospheric pressure plasma is used with hydrogen peroxide, OH radicals are released, and surface proteins are removed [56]. They also claimed that mixing plasma with hydrogen peroxide erased coffee or alcohol staining from extracted teeth [57]. Tooth whitening can also be done with direct current plasma jet and hydrogen peroxide [58]. During teeth whitening, intrinsic stains are always a major concern [57,59]. According to Park et al. a low-frequency plasma source combined with hydrogen peroxide can be employed to eliminate the intrinsic stain [60]. Using a radiofrequency-driven gas-liquid hybrid plasma system, Kim et al. used plasma to generate plasma treated liquid in 2012. In this work, the RF plasma jet was submerged in deionized water with the target tooth. On the treated tooth's surface after 8 min, a color change (bleaching) was noticed. Radical OH was identified as the primary source of bleaching in this study [61]. Nam et al. employed a plasma jet to bleach teeth in in-vitro research. It consisted of forty human molar teeth that had been removed and had their crowns intact. n = 10 teeth were randomly separated into four groups and treated with carbamide peroxide + plasma arc lamp (PAC), carbamide peroxide + CAP, carbamide peroxide + diode laser, or carbamide peroxide by itself (control). The authors discovered that CAP, rather than carbamide peroxide alone or a combination of carbamide peroxide and diode laser, was the most successful in tooth bleaching without causing dental damage [58]. Claiborne et al.'s in-vitro work backs up this conclusion [62]. D. Claiborne et al. used a plasma plume to treat removed human teeth. When CAP +36% hydrogen peroxide gel was used instead of just 36% hydrogen peroxide, they noticed a considerable boost in tooth whitening. According to Jamali et al. extended plasma therapy without bleaching removed just a little amount of blue-stain, while a combination of plasma treatment and bleaching eliminated most of the blue stain [63]. In research by Zhu et al. the initial bond strength of resin-enamel treated with cold plasma was not altered when compared to conventional tooth whitening [64].
We can thus deduce from the above studies and their findings that cold atmospheric plasma has great potential in tooth bleaching procedures and can either be safely used with the conventional methods of tooth whitening to give better and faster results without causing any undesirable toxic damage to the tissues.
3.5. Clinical removal of biofilms
Bacterial biofilms are made up of adhering consortia of microorganisms encased in self-produced polymer matrixes made up of polysaccharides, proteins, lipids, and extracellular DNA. Biofilms may grow on nearly any living or non-living surface, and they are a common occurrence in natural, industrial, and medical environments. Dental biofilms are important etiological components in dental illnesses that are a global public health concern. Microbial succession and maturation occur if the dental biofilm is kept undisturbed. When the biofilm undergoes this transformation, its bacterial makeup shifts from cocci to filamentous organisms to spirochetes, and from gram-positive bacteria to gram-negative bacteria. As a result, biofilms grow on teeth and other surfaces in the mouth, including the tongue. Caries, gingivitis, periodontitis, oral mucositis, and peri-mucositis, and peri-implantitis are all caused by the acquisition of bacteria and the growth of the biofilm microbiota. Caries and periodontitis, which are caused by oral biofilms, are global public health concerns. Several substances and technologies with antibacterial effects have been created to prevent the production of oral biofilms. For oral biofilm management, antimicrobial chemicals can be added to items like dentifrices and mouthwashes or integrated into dental-restorative materials. Bacterial adherence can be decreased by engineering materials and tooth surfaces. Biofilm bacteria, on the other hand, are much more resistant to antibacterial agents than planktonic bacteria. This characteristic of biofilm presents a problem to dentists in their everyday job, and it may explain why many oral preventive medications that are anticipated to be effective in vitro only have a minimal impact in vivo. There is a need for more efficient biofilm eradication because of the prevalence and costs associated with the prevention and treatment of biofilm-induced dental disorders, and NTP offers promising prospects (Fig. 7).
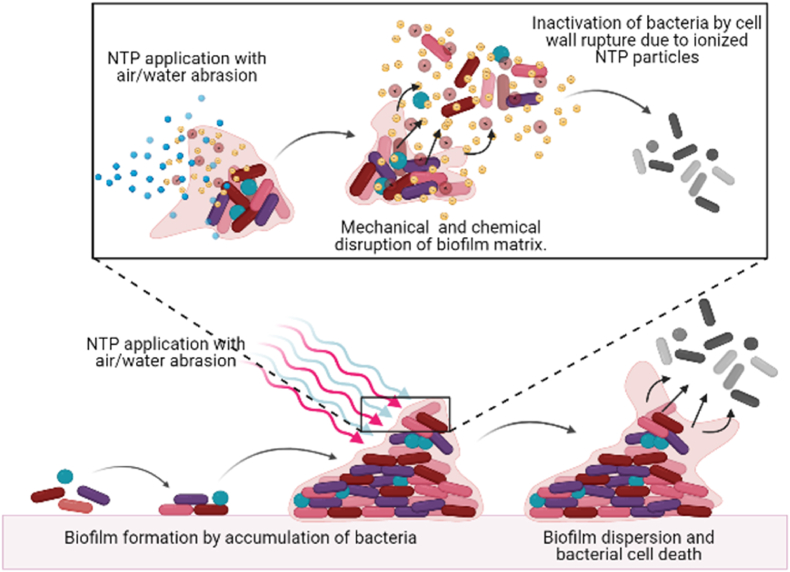
Fig. 7.
Application of Non-thermal plasma for disruption of biofilm on surface of teeth. The wall of the bacteria and extracellular matrix get distorted due to ionized non-thermal plasma eradicating the presence of biofilm on the surface of teeth.
NTP can break down the biofilm matrix without harming the oral tissue [65]. Rupf et al. discovered that combining plasma therapy with a non-abrasive air/water spray is efficient in removing biofilms from dental implants [66]. The in vitro treatment of S. mutans comprising dental biofilms with NTP was shown to be more successful than the treatment with chlorhexidine in another study by Koban et al. [67]. NTP is also efficient for the decontamination of biofilms on root canals and dental slices. Jiang et al. employed a plasma plume to disinfect the root canals of removed human teeth at room temperature in their in-vitro investigation [68]. Plasma disinfected the root canal better than the control, according to the authors. In vivo biofilm removal effectiveness of plasma needle on root canals of removed teeth was studied by Schaudinn et al. [69]. Teeth were separated into three groups: those who received the plasma needle, those who received 6% sodium hypochlorite (an antiseptic), and control. They found that eliminating biofilms from removed teeth with 6% sodium hypochlorite was more effective than using a plasma needle.
The findings reveal that non-thermal plasma may be used in the effective removal of biofilms from both, the surfaces of the tooth, as well as the root canals (i.e. surfaces within the tooth). The removal of biofilms is crucial to a healthy oral cavity, preventing the failure of treatments of various diseases like gingivitis, irreversible pulpitis, periodontitis, mucositis. The application of NTP, causing successful elimination of these biofilms goes a long way in quickening the treatment pace and in resulting in a better prognosis.
3.6. Polymerization
Non-thermal plasma can also help in polymerization [70]. High cross-linking and polymerization levels are caused by plasma exposure [71]. Plasma are curing units, used for curing composite resin because they cure faster than traditional devices [72,73]. Plasma polymerization characteristics, however, have been reported to be less than optimal due to an increase in the development of residual stresses as well as polymerization shrinkage [74]. The non-thermal plasma brush was recently used to successfully polymerize self-etch adhesives [75].
We found that studies have shown various effects of cold atmospheric plasma of polymerization. While there is an increased advantage in terms of the polymerization procedure, there are also drawbacks that cannot be overlooked like increased development of residual stresses. More studies are required to confirm findings and find optimal solutions to these disadvantages.
3.7. Adhesive restorations
Plasma therapy raised bonding strength at the dentin-composite interface by almost 60%, and this enhanced interface bonding improved composite performance, lifespan, and durability. Chemical bonding is less common in clinical practice than mechanical bonding. The main culprit for the failure of mechanical bonding is the smear layer which is composed of type I collagen that develops at the dentin/adhesive junction. The etch techniques create a porous surface for adhesive infiltration. The smear layer inhibits adhesive diffusion throughout the prepared dentin surface. Dentin/adhesive interface is exposed, allowing bacterial enzymes to penetrate and further damage the composite-to-tooth contact due to insufficient bonding [49].
Plasma treatment increases the number of free radicals and ions on tooth substrate creating increased bond strength. A reported increase in durability and longevity of the bond (due to removal of smear layer and better exposure of type I collagen fibers) has made its use more attractive.
Dong et al. examined the effect of NTP on composite restoration. They found that the application of plasma modifies the dentin surface and increases dentin/adhesive interfacial bonding [76]. This was collaborated by Kong et al. who also reported the effects of plasma treatment on dental composite restoration. They investigated that atmospheric cold plasma brush (ACPB) treatment can modify the dentin surface and increase the dentin-adhesive interfacial bonding [13]. Ritts et al. also assessed the effect of NTP brush on composite restoration [77]. They reported that interfacial bonding strength of peripheral dentin surface was increased by plasma treatment, while over 100 s of prolonged treatment decreased the interfacial bonding strength. It was concluded that NTP can alter the surface characteristics of dentin, which results in increased bonding between dentin and adhesive restorations. However, no improvement was observed in the bonding strength of plasma-treated inner dentin probably due to the variation in the composition of dentin [78]. Chen et al. suggested that plasma brush treatment resulted from super - hydrophilic enamel, dentin surface for composite restoration [79]. Yavirach et al. in their study found that plasma treatment of fiber-reinforced composite and resin composite has more tensile shear bond than traditional core build-up [80].
Based on the experimental studies done at lab scale, it can be deduced that NTP treatment alters the surface proteins and thus the surface characteristics of dentin. This results in increased bond strength, it prevents the incidence of microleakage and thus secondary caries. It also leads to a more retentive and longer-lasting restoration.
3.8. Post and core
The shear bond strength between fiber-reinforced composite posts and resin composite for core building was examined by Yavrich et al. According to their findings, plasma treatment enhanced the binding strength between the post and composite [80]. As Costa Dantas et al. observed, plasma treatment enhanced the post's wettability [81]. Argon plasma treatment did not affect adhesion, whereas ethylenediamine plasma treatment resulted in substantially rougher surfaces. Plasma surface therapy, according to studies, has an anti-aging impact [82]. In their study, Ye et al. found that non-thermal plasma therapy caused post-surface aging. After being treated with plasma, the fiber posts were exposed to air for 1 h or more, and the bond strength improved [82]. Non-thermal plasma treatment of posts has thus shown significant advantages in terms of strength and retention. However, the amount of strength is subjective to time and thus NTP treatment of posts appears to be technique sensitive. An added disadvantage is the aging effects of non-thermal plasma treatment of posts that have been noted. Further research is required to assess the viability of NTP treatment on posts.
3.9. Implant modification
Modification focuses upon the interaction of implant to body fluid which helps in bone healing. Plasma increases surface roughness and wettability which help in cell adhesion [83,84]. Chairside application of non-thermal atmospheric pressure plasma (NTAPP) before placement of the implant is recently reported, which helps in reducing contact angle and supporting the spread of osteoblastic cells [85,86]. It is known to aid in osteoblastic proliferation and osseointegration, thus increasing the success rates of implants. One of the advantages of using plasma is that no residue is present after plasma treatment. Some physicochemical characteristics were changed like surface free energy, functional hydroxyl groups, component of hydrocarbon [87].
We conclude that cold plasma treatment immensely increases the success rate of implants as it helps in increasing the integration of the implant within the alveolar socket (Fig. 8). Thus, the implant is accepted by the body, and it forms a stable, non-mobile unit of the human dentition.
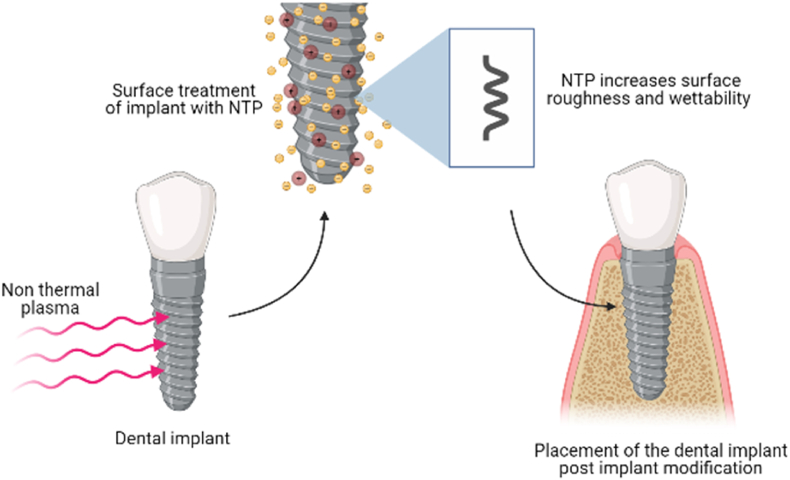
Fig. 8.
Application of Non-thermal plasma for implant modification. Implant surface when treated with non-thermal plasma increases their roughness and wettability, which helps in perfect placement of the implant in sterilised condition.
3.10. Periodontal diseases
It has been shown by Koban et al. that direct application of NTAPP reduces the contact angle of untreated dentin surface, which leads to greater osteoblast proliferation on the dentin. In the future, these discoveries might be used to regenerate periodontal tissue [88]. Miletic et al. established the interaction between NTAPP and human periodontal ligament mesenchymal stem cells [89]. However, NTAPP did not alter cell viability, according to the researchers. In addition, the plasma dramatically reduced the proliferation of the cells, while promoting their osteogenic differentiation [89].
We may derive that cold plasma may be beneficial in the treatment of periodontal diseases like the elimination of pocket. It may also be used to treat and reduce the mobility of teeth and periodontitis, due to its potential of inducing osteogenic differentiation in cells.
3.11. Wound healing
Wounds in the mouth cavity are frequent and can be caused by trauma or surgery. Soft tissue healing in the mouth cavity follows the same principles as soft tissue healing in other parts of the body, such as the skin. Wound healing is a normal biological process in the human body. This process involves a variety of cell types, including leukocytes, lymphocytes, monocytes, neutrophils, fibroblasts, and keratinocytes. Wound healing is a complicated and well-orchestrated system. Wound healing is divided into three stages: inflammation, proliferation, and remodelling. During these overlapping periods, the cells release a variety of cytokines, including growth factors and interleukins, to stimulate the surrounding tissue and aid in wound healing [90]. Despite the enormous amount of data available about wound healing in general, molecular features of healing in different areas of the oral cavity are still emerging. Chronic wounds are extremely difficult to heal. There is a global demand for innovative and more effective treatment options.
The treatment of wounds using atmospheric pressure plasma may be a new alternative [[91], [92], [93], [94]]. Human skin and immunological cells have been demonstrated in vitro to respond in a dose-dependent way following plasma therapy. Long treatment periods cause apoptosis in the cells, whereas brief treatments encourage normal growth. This impact is accompanied by a change in gene expression as well as a change in protein activation or secretion.
ROS, RNS, UV-radiation, and an electric field are all produced by the plasma [95]. The complex mixture of reactive species, which includes H2O2, O3, O2∗, NO, NO2, N2∗, and OH, promotes wound healing. ROS and RNS, electric fields, and UV radiation are all known to hasten healing processes by increasing cytokine production in various cell types. As a result, plasma medicine, particularly wound healing, emerges as a potential study topic.
From the above data, we conclude that plasma generates free radicals well within the physiologic range of the body during natural tissue repair allowing for a higher cell turnover rate. So it has the potential to facilitate the wound-healing procedure, reducing the incidence of complications like dry socket after extraction procedures or infected unhealed tissues after flap surgeries. However, a large number of studies need to be carried out, under varying conditions, to support this hypothesis.
3.12. Intraoral diseases
Candida albicans causes denture stomatitis, angular stomatitis, median rhomboid glossitis, and gingival erythema. Koban et al. and Yamazaki et al. reported that plasma jet can cure these diseases which are caused by C. albicans [96,97].
Therefore, NTP has the potential to treat several intraoral diseases, however, extensive research is required incorrectly determining its effects on a large variety of oral and dental diseases.
3.13. Oncology
The major treatment method for oral cancer is typically decided by the disease's stage, with surgical treatment constituting the backbone of multimodal treatment. Early-stage illness is largely handled with surgery or brachytherapy without functional morbidity, but multimodal treatment is suggested for an advanced-stage disease, not only for better survival but also for improved quality of life. Reconstructive surgery is necessary after the excision of big primary tumors. Postoperative radiation or chemoradiotherapy is suggested for patients with multiple node metastases or extracapsular dissemination, with the lymph nodes located outside the limits of the radical neck dissection, such as the lingual and retropharyngeal nodes, getting special care. Because numerous possible adverse effects have been documented, targeted treatment for oral cancer is still a relatively novel idea, and additional research is needed to establish the clinical efficacy of these medicines. A relatively safer and non-invasive approach with minimal to no side effects has been proposed by using non-thermal plasma therapy as an alternative to chemotherapy and other conventional methods.
In a study by Hoffmann et al. [10], it was reported that CAP induces necrosis, cell detachment, apoptosis, and deterioration of tumour cells by disrupting the 'S' phase of cell division. The effective result of CAP was obtained from in vivo and in vitro studies in oncology. NTP has shown great potential in the removal of infected cells, and preservation of healthy tissue in necrotic areas of teeth. It is no wonder that these findings may be extended beyond the tooth and be applied to the remaining oral cavity, especially in cancerous lesions. Oncology too requires the eradication of the infected cells with the preservation of the healthy tissues. This may be used in adjunct to, or maybe someday, as a replacement of chemotherapy as it has the advantage of not leaving behind toxic residues. However, many more studies are needed for proper technique and implementation.
4. Discussion
Plasma can undoubtedly enter unpredictable pits, fissures, and crevices where files or burs will be unable to reach since it is a vaporous medium. Plasma offers several benefits over laser beams when it comes to treating oral tissues. Due to its target specificity, it exclusively kills bacteria in bacterial plaque. Further, the absence of pain during plasma treatment as thermal damage to tissues is evaded is an added advantage. Additionally, it does not leave behind any toxic residues. It has minimal potential side effects and appears to be extremely biocompatible.
Plasma delivery system in the dental tissue is designed as an opportunity in the future [[98], [99], [100], [101]]. In light of known physical and biological properties of plasma some dental applications are conceivable, yet further examination is needed to know the mechanism of how plasma impacts cells [102,103]. Considering all qualities of plasma, the utilization of plasma in dental health care is a novel procedure [104]. Be that as it may, we are as yet distant from dental and medical plasma application. Further study is required to develop a safe, efficient, and eco-friendly plasma sources.
5. Limitations
The limitations are those that are perhaps associated with any innovation. In addition to cost and availability, other concerns are also being addressed, including marketing and promotion. Maintenance of such expensive devices is an added liability.
Moreover, the direct clinical implication of the Non-Thermal Plasma on oral cavities is also a major concern. An antimicrobial effect of plasma has been proved on agar plates or on a substrate, as they are usually performed in the laboratory. Hence, it is not easily applied to the treatment of caries, where bacteria are hidden in the spongy enamel of the tooth, or that the gas flow cannot reach further depth even if the channels are blocked. Depending on the type of construction, the gas discharge does not occur at the site where the microorganisms are located, which is problematic issue on accessibility with the plasma source to the tooth. One more considerable aspect is also the formation of ozone during the production of plasma, which the patients and the practitioner inhale during clinical procedure. Other limitations also include the release of severity due to the electric current when no anaesthesia is given. The requirement of humidity during the clinical procedure also limits the direct application of plasma. Moreover, the reactive oxygen species (ROS), that is created during the bleaching and other procedures may also show the toxicity to the healthy tissues and can create further complications.
A part from this, the effect of plasma on tooth and oral tissues which has been operated previously should also be taken in to consideration. The released ROS can affect the implant or the operated tooth.
The method is quite important with this technique since it is greatly dependent upon it. In instances when amalgam restorations are present in the oral cavity, it is less effective. NTP is now being studied for its influence on cancer cells, with some promising results. A study of the effects of the drug on regular cells is necessary before it can be used effectively [[105], [106], [107]].
The most important drawback is the lack of availability of the material and equipment, due to which its use in research, as well as clinical practice, is extremely limited. Developing a safe, efficient, and environmentally-friendly plasma technology that can be utilized in clinical settings in a cost-effective, efficient, and predictable way requires more research. Despite its potential, plasma needle technology has a way to go before it can be widely used.
6. Conclusion
The effects of NTP on human and animal cells are not yet well understood. Further research should be conducted in this area. This may be related to the fact that plasma dentistry is still in its development. As plasma dental technology advances, this misunderstanding will certainly be clarified up shortly. As a result of the availability of hand-held equipment designed for clinic use, the method is expected to increase in popularity. Researchers and doctors might potentially benefit from a better knowledge of the cellular and molecular mechanisms involved.
Data availability
All data included in this study are available upon request from the corresponding author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge infrastructure support available through DBT-BUILDER program (BT/INF/22/SP42155/2021) at KIIT UNIVERSITY.
Contributor Information
Suresh K. Verma, Email: suresh.verma@physics.uu.se, sureshverma22@gmail.com.
Mrutyunjay Suar, Email: msuar@kiitbiotech.ac.in.
References
- 1.Robert E., Darny T., Dozias S., Iseni S., Pouvesle J.M. New insights on the propagation of pulsed atmospheric plasma streams: from single jet to multi jet arrays. Phys. Plasmas. 2015;22 doi: 10.1063/1.4934655. [DOI] [Google Scholar]
- 2.Obradović B.M., Ivković S.S., Kuraica M.M. Spectroscopic measurement of electric field in dielectric barrier discharge in helium. Appl. Phys. Lett. 2008;92 doi: 10.1063/1.2927477. [DOI] [Google Scholar]
- 3.Bourdon A., Darny T., Pechereau F., Pouvesle J.M., Viegas P., Iséni S., Robert E. Numerical and experimental study of the dynamics of a μs helium plasma gun discharge with various amounts of N2 admixture. Plasma Sources Sci. Technol. 2016;25 doi: 10.1088/0963-0252/25/3/035002. [DOI] [Google Scholar]
- 4.Maho T., Binois R., Brulé-Morabito F., Demasure M., Douat C., Dozias S., Bocanegra P.E., Goard I., Hocqueloux L., Le Helloco C., Orel I., Pouvesle J.M., Prazuck T., Stancampiano A., Tocaben C., Robert E. Anti-bacterial action of plasma multi-jets in the context of chronic wound healing. Appl. Sci. 2021;11 doi: 10.3390/app11209598. [DOI] [Google Scholar]
- 5.von Woedtke T., Reuter S., Masur K., Weltmann K.D. Plasmas for medicine. Phys. Rep. 2013;530:291–320. doi: 10.1016/j.physrep.2013.05.005. [DOI] [Google Scholar]
- 6.Schulz M. Introduction to plasma theory. Eos, Trans. Am. Geophys. Union. 1986;67:79. doi: 10.1029/eo067i007p00079-02. [DOI] [Google Scholar]
- 7.Crookes W. On radiant matter, a lecture delivered to the British Association for the Advancement of Science. Br. Assoc. Adv. Sci. 1879;August:22. [Google Scholar]
- 8.Langmuir I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. Unit. States Am. 1928;14:627–637. doi: 10.1073/pnas.14.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson J.J. XL. Cathode rays, London, Edinburgh, Dublin. Philos. Mag. J. Sci. 1897;44:293–316. doi: 10.1080/14786449708621070. [DOI] [Google Scholar]
- 10.Hoffmann C., Berganza C., Zhang J. Cold Atmospheric Plasma: methods of production and application in dentistry and oncology. Med. Gas Res. 2013;3 doi: 10.1186/2045-9912-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmke A., Gerling T., Weltmann KD. In: Comprehensive Clinical Plasma Medicine. Springer, Cham. Metelmann HR., von Woedtke T., Weltmann KD., editors. 2018. Plasma sources for biomedical applications. [DOI] [Google Scholar]
- 12.Weltmann K.-D., von Woedtke T. Plasma medicine—current state of research and medical application. Plasma Phys. Contr. Fusion. 2017;59 doi: 10.1088/0741-3335/59/1/014031. [DOI] [Google Scholar]
- 13.Kong M.G., Kroesen G., Morfill G., Nosenko T., Shimizu T., Van Dijk J., Zimmermann J.L. Plasma medicine: an introductory review. New J. Phys. 2009;11 doi: 10.1088/1367-2630/11/11/115012. [DOI] [Google Scholar]
- 14.Kim J.H., Lee M.A., Han G.J., Cho B.H. Plasma in dentistry: a review of basic concepts and applications in dentistry. Acta Odontol. Scand. 2014;72:1–12. doi: 10.3109/00016357.2013.795660. [DOI] [PubMed] [Google Scholar]
- 15.Ranjan R., Krishnamraju P.V., Shankar T., Gowd S. Nonthermal plasma in dentistry: an update. J. Int. Soc. Prev. Community Dent. 2017;7:71–75. doi: 10.4103/jispcd.JISPCD_29_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha S., Park Y.S. Plasma in dentistry. Clin. Plasma Med. 2014;2:4–10. doi: 10.1016/j.cpme.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gherardi M., Tonini R., Colombo V. Plasma in dentistry: brief history and current status. Trends Biotechnol. 2018;36:583–585. doi: 10.1016/j.tibtech.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Robert E., Barbosa E., Dozias S., Vandamme M., Cachoncinlle C., Viladrosa R., Pouvesle J.M. Experimental study of a compact nanosecond plasma gun. Plasma Process. Polym. 2009;6:795–802. doi: 10.1002/ppap.200900078. [DOI] [Google Scholar]
- 19.Weltmann K.D., Kinde E., Von Woedtke T., Hähnel M., Stieber M., Brandenburg R. Atmospheric-pressure plasma sources: prospective tools for plasma medicine. Pure Appl. Chem. 2010;82:1223–1237. doi: 10.1351/PAC-CON-09-10-35. [DOI] [Google Scholar]
- 20.Singh D.S., Chandra D.R., Tripathi D.S., Rahman D.H., Tripathi D.P., Jain D.A., Gupta D.P. The bright future of dentistry with cold plasma – Review. IOSR J. Dent. Med. Sci. 2014;13 doi: 10.9790/0853-131040613. 06–13. [DOI] [Google Scholar]
- 21.Chiper A.S., Chen W., Mejlholm O., Dalgaard P., Stamate E. Atmospheric pressure plasma produced inside a closed package by a dielectric barrier discharge in Ar/CO2 for bacterial inactivation of biological samples. Plasma Sources Sci. Technol. 2011;20 doi: 10.1088/0963-0252/20/2/025008. [DOI] [Google Scholar]
- 22.Chirokov A., Gutsol A., Fridman A. Atmospheric pressure plasma of dielectric barrier discharges. Pure Appl. Chem. 2005;77:487–495. doi: 10.1351/pac200577020487. [DOI] [Google Scholar]
- 23.Omran A.V., Busco G., Ridou L., Dozias S., Grillon C., Pouvesle J.M., Robert E. Cold atmospheric single plasma jet for RONS delivery on large biological surfaces. Plasma Sources Sci. Technol. 2020;29 doi: 10.1088/1361-6595/abaffd. [DOI] [Google Scholar]
- 24.Cooper M., Fridman G., Fridman A., Joshi S.G. Biological responses of Bacillus stratosphericus to floating electrode-dielectric barrier discharge plasma treatment. J. Appl. Microbiol. 2010;109:2039–2048. doi: 10.1111/j.1365-2672.2010.04834.x. [DOI] [PubMed] [Google Scholar]
- 25.Koinuma H., Ohkubo H., Hashimoto T., Inomata K., Shiraishi T., Miyanaga A., Ihayashi S. Development and application of a microbeam plasma generator. Appl. Phys. Lett. 1992;60:816–817. doi: 10.1063/1.106527. [DOI] [Google Scholar]
- 26.Schütze A., Jeong J.Y., Babayan S.E., Park J., Selwyn G.S., Hicks R.F. The atmospheric-pressure plasma jet: a review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998;26:1685–1694. doi: 10.1109/27.747887. [DOI] [Google Scholar]
- 27.Robert E., Sarron V., Riès D., Dozias S., Vandamme M., Pouvesle J.M. Characterization of pulsed atmospheric-pressure plasma streams (PAPS) generated by a plasma gun. Plasma Sources Sci. Technol. 2012;21 doi: 10.1088/0963-0252/21/3/034017. [DOI] [Google Scholar]
- 28.Lu X., Laroussi M., Puech V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012;21 doi: 10.1088/0963-0252/21/3/034005. [DOI] [Google Scholar]
- 29.Stoffels E., Flikweert A.J., Stoffels W.W., Kroesen G.M.W. Plasma needle: a non-destructive atmospheric plasma source for fine surface treatment of (bio)materials. Plasma Sources Sci. Technol. 2002;11:383–388. doi: 10.1088/0963-0252/11/4/304. [DOI] [Google Scholar]
- 30.Kieft I.E., Laan E.P.V.D., Stoffels E. Electrical and optical characterization of the plasma needle. New J. Phys. 2004;6:1–14. doi: 10.1088/1367-2630/6/1/149. [DOI] [Google Scholar]
- 31.Plasma Needle: the Future of Dentistry, (n.d.).
- 32.Jiang C., Gundersen M.A., Schaudinn C., Webster P., Jaramillo D.E., Sedghizadeh P.P., Costerton J.W. 2011. An Atmospheric Pressure Non-thermal Plasma Needle for Endodontic Biofilm Disinfection. 1–1. [DOI] [Google Scholar]
- 33.Sladek R.E.J., Stoffels E. Deactivation of Escherichia coli by the plasma needle. J. Phys. D Appl. Phys. 2005;38:1716–1721. doi: 10.1088/0022-3727/38/11/012. [DOI] [Google Scholar]
- 34.Laroussi M., Lu X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005;87 doi: 10.1063/1.2045549. [DOI] [Google Scholar]
- 35.Laroussi M., Tendero C., Lu X., Alla S., Hynes W.L. Inactivation of bacteria by the plasma pencil. Plasma Process. Polym. 2006;3:470–473. doi: 10.1002/ppap.200600005. [DOI] [Google Scholar]
- 36.Whittaker A.G., Graham E.M., Baxter R.L., Jones A.C., Richardson P.R., Meek G., Campbell G.A., Aitken A., Baxter H.C. Plasma cleaning of dental instruments. J. Hosp. Infect. 2004;56:37–41. doi: 10.1016/j.jhin.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Knoll R.F.Z.Z.W. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process. Polym. 2005;2:245–444. [Google Scholar]
- 38.Verma S.K., Jha E., Panda P.K., Das J.K., Thirumurugan A., Suar M., Parashar S.K.S. Molecular aspects of core-shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine. 2018;13:43–68. doi: 10.2217/nnm-2017-0237. [DOI] [PubMed] [Google Scholar]
- 39.Paul P., Patel P., Verma S.K., Mishra P., Sahu B.R., Panda P.K., Kushwaha G.S., Senapati S., Misra N., Suar M. The Hha–TomB toxin–antitoxin module in Salmonella enterica serovar Typhimurium limits its intracellular survival profile and regulates host immune response. Cell Biol. Toxicol. 2021 doi: 10.1007/s10565-021-09587-z. [DOI] [PubMed] [Google Scholar]
- 40.Mohd Nasir N., Lee B.K., Yap S.S., Thong K.L., Yap S.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016;605:76–85. doi: 10.1016/j.abb.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 41.Yang Hongli H.T., Liu Siming. Application of low-temperature plasma in dental clinical sterilization. Int. J. Stomatol. 2013;40:483–485. http://en.cnki.com.cn/Article_en/CJFDTOTAL-GWKQ201304026.htm [Google Scholar]
- 42.Sung S.J., Huh J.B., Yun M.J., Chang B.M.W., Jeong C.M., Jeon Y.C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont. 2013;5:2–8. doi: 10.4047/jap.2013.5.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sladek R.E.J., Stoffels E., Walraven R., Tielbeek P.J.A., Koolhoven R.A. Plasma treatment of dental cavities: a feasibility study. IEEE Trans. Plasma Sci. 2004;32:1540–1543. doi: 10.1109/TPS.2004.832636. [DOI] [Google Scholar]
- 44.Socransky S.S., Smith C., Martin L., Paster B.J., Dewhirst F.E., Levin A.E. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 45.Ben Belgacem Z., Carre G., Charpentier E., Le-Bras F., Maho T., Robert E., Pouvesle J.M., Polidor F., Gangloff S.C., Boudifa M., Gelle M.P. Innovative non-thermal plasma disinfection process inside sealed bags: assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakudo A., Misawa T. Antibiotic-resistant and non-resistant bacteria display similar susceptibility to dielectric barrier discharge plasma. Int. J. Mol. Sci. 2020;21:1–12. doi: 10.3390/ijms21176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang C., Schaudinn C., Jaramillo D.E., Webster P., Costerton J.W. In vitro antimicrobial effect of a cold plasma jet against Enterococcus faecalis biofilms. ISRN Dent. 2012;2012:1–6. doi: 10.5402/2012/295736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borges A.C., Kostov K.G., Pessoa R.S., de Abreu G.M.A., de Lima G.M.G., Figueira L.W., Koga-Ito C.Y. Applications of cold atmospheric pressure plasma in dentistry. Appl. Sci. 1975;11(2021) doi: 10.3390/app11051975. [DOI] [Google Scholar]
- 49.Smitha T., Chaitanya Babu N. Plasma in dentistry: an update. Indian J. Dent. Adv. 2010;2:1–7. http://linkinghub.elsevier.com/retrieve/pii/S2212816614000055 [Google Scholar]
- 50.V. B L From distant stars to dental chairs: an update on plasma needle. Int. J. Dent. Sci. Res. 2014;2:19–20. doi: 10.12691/ijdsr-2-6b-6. [DOI] [Google Scholar]
- 51.Goree J., Liu B., Drake D., Stoffels E. Killing of S. mutans bacteria using a plasma needle at atmospheric pressure. IEEE Trans. Plasma Sci. 2006;34:1317–1324. doi: 10.1109/TPS.2006.878431. [DOI] [Google Scholar]
- 52.Yang B., Chen J., Yu Q., Li H., Lin M., Mustapha A., Hong L., Wang Y. Oral bacterial deactivation using a low-temperature atmospheric argon plasma brush. J. Dent. 2011;39:48–56. doi: 10.1016/j.jdent.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haapasalo M., Ørstavik D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987;66:1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Sun K., Ye G., Liang Y., Pan H., Wang G., Zhao Y., Pan J., Zhang J., Fang J. Evaluation of cold plasma treatment and safety in disinfecting 3-week root canal Enterococcus faecalis biofilm in vitro. J. Endod. 2015;41:1325–1330. doi: 10.1016/j.joen.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Pan J., Sun K., Liang Y., Sun P., Yang X., Wang J., Zhang J., Zhu W., Fang J., Becker K.H. Cold plasma therapy of a tooth root canal infected with enterococcus faecalis biofilms in vitro. J. Endod. 2013;39:105–110. doi: 10.1016/j.joen.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Lee H.W., Kim G.J., Kim J.M., Park J.K., Lee J.K., Kim G.C. Tooth bleaching with nonthermal atmospheric pressure plasma. J. Endod. 2009;35:587–591. doi: 10.1016/j.joen.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Lee H.W., Nam S.H., Mohamed A.A.H., Kim G.C., Lee J.K. Atmospheric pressure plasma jet composed of three electrodes: application to tooth bleaching. Plasma Process. Polym. 2010;7:274–280. doi: 10.1002/ppap.200900083. [DOI] [Google Scholar]
- 58.Nam S.H., Lee H.W., Cho S.H., Lee J.K., Jeon Y.C., Kim G.C. High-efficiency tooth bleaching using non-thermal atmospheric pressure plasma with low concentration of hydrogen peroxide. J. Appl. Oral Sci. 2013;21:265–270. doi: 10.1590/1679-775720130016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun P., Pan J., Tian Y., Bai N., Wu H., Wang L., Yu C., Zhang J., Zhu W., Becker K.H., Fang J. Tooth whitening with hydrogen peroxide assisted by a direct-current cold atmospheric-pressure air plasma microjet. IEEE Trans. Plasma Sci. 2010;38:1892–1896. doi: 10.1109/TPS.2009.2039585. [DOI] [Google Scholar]
- 60.Park J.K., Nam S.H., Kwon H.C., Mohamed A.A.H., Lee J.K., Kim G.C. Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching. Int. Endod. J. 2011;44:170–175. doi: 10.1111/j.1365-2591.2010.01828.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim M.S., Koo I.G., Choi M.Y., Jung J.C., Eldali F., Lee J.K., Collins G.J. Correlated electrical and optical studies of hybrid argon gas-water plasmas and their application to tooth whitening. Plasma Process. Polym. 2012;9:339–345. doi: 10.1002/ppap.201100141. [DOI] [Google Scholar]
- 62.Claiborne D., McCombs G., Lemaster M., Akman M., Laroussi M. Low-temperature atmospheric pressure plasma enhanced tooth whitening: the next-generation technology. Int. J. Dent. Hyg. 2014;12:108–114. doi: 10.1111/idh.12031. [DOI] [PubMed] [Google Scholar]
- 63.Jamali A., Evans P.D. Plasma treatment and bleaching to remove blue-stain from lodgepole pine sapwood. Eur. J. Wood Wood Prod. 2013;71:675–677. doi: 10.1007/s00107-013-0717-0. [DOI] [Google Scholar]
- 64.meng Zhu M., min Wang G., Sun K., long Li Y., Pan J. Bonding strength of resin and tooth enamel after teeth bleaching with cold plasma. Beijing Da Xue Xue Bao. 2016;48:116–120. [PubMed] [Google Scholar]
- 65.Aparecida Delben J., Evelin Zago C., Tyhovych N., Duarte S., Eduardo Vergani C. Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rupf S., Idlibi A.N., Al Marrawi F., Hannig M., Schubert A., von Mueller L., Spitzer W., Holtmann H., Lehmann A., Rueppell A., Schindler A. Removing biofilms from microstructured titanium Ex Vivo: a novel approach using atmospheric plasma technology. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.K I., H B., H. N O., M R., S R., K E., W K.-D., W A., K A., K T., Koban I., Holtfreter B., Hübner N.-O., Matthes R., Sietmann R., Kindel E., Weltmann K.-D., Welk A., Kramer A., Kocher T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro - proof of principle experiment. J. Clin. Periodontol. 2011;38:956–965. doi: 10.1111/j.1600-051X.2011.01740.x. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L51530769%0Ahttps://doi.org/10.1111/j.1600-051X.2011.01740.x. [DOI] [PubMed] [Google Scholar]
- 68.Jiang C., Chen M.T., Gorur A., Schaudinn C., Jaramillo D.E., Costerton J.W., Sedghizadeh P.P., Vernier P.T., Gundersen M.A. Nanosecond pulsed plasma dental probe. Plasma Process. Polym. 2009;6:479–483. doi: 10.1002/ppap.200800133. [DOI] [Google Scholar]
- 69.Schaudinn C., Jaramillo D., Freire M.O., Sedghizadeh P.P., Nguyen A., Webster P., Costerton J.W., Jiang C. Evaluation of a nonthermal plasma needle to eliminate ex vivo biofilms in root canals of extracted human teeth. Int. Endod. J. 2013;46:930–937. doi: 10.1111/iej.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simionescu B., Leanca M., Ananiescu C., Simionescu C. Plasma-induced polymerization. Polym. Bull. 1980;3:437–440. doi: 10.1007/BF00283818. 3–3. [DOI] [Google Scholar]
- 71.Eisner C.B., Espey M., Ow H., Wang K.W., Wiesner U., Schnermann J. Measurement of plasma volume using nanoparticles in mice. Faseb. J. 2009;23 doi: 10.1096/fasebj.23.1_supplement.804.19. [DOI] [Google Scholar]
- 72.Friedman J., Hassan R. Comparison study of visible curing lights and hardness of light-cured restorative materials. J. Prosthet. Dent. 1984;52:504–506. doi: 10.1016/0022-3913(84)90333-0. [DOI] [PubMed] [Google Scholar]
- 73.Rueggeberg F.A., Caughman W.F., Curtis J.W., Davis H.C. Factors affecting cure at depths within light-activated resin composites. Am. J. Dent. 1993;6:91–95. [PubMed] [Google Scholar]
- 74.Peutzfeldt A., Sahafi A., Asmussen E. Characterization of resin composites polymerized with plasma arc curing units. Dent. Mater. 2000;16:330–336. doi: 10.1016/S0109-5641(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 75.Chen M., Zhang Y., Yao X., Li H., Yu Q., Wang Y. Effect of a non-thermal, atmospheric-pressure, plasma brush on conversion of model self-etch adhesive formulations compared to conventional photo-polymerization. Dent. Mater. 2012;28:1232–1239. doi: 10.1016/j.dental.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong X., Chen M., Wang Y., Yu Q. A mechanistic study of plasma treatment effects on demineralized dentin surfaces for improved adhesive/dentin interface bonding. Clin. Plasma Med. 2014;2:11–16. doi: 10.1016/j.cpme.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.R A.C., L H., Y Q., X C., Y X., H L., W Y., Ritts A.C., Li H., Yu Q., Xu C., Yao X., Hong L., Wang Y. Dentin surface treatment using a non-thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur. J. Oral Sci. 2010;118:510–516. doi: 10.1111/j.1600-0722.2010.00761.x. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L359517462%0Ahttps://doi.org/10.1111/j.1600-0722.2010.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swift E.J., Perdigao J. 1995. Bonding to Enamel and Dentin: a Brief History and State of the Art.https://www.researchgate.net/publication/15629846 [PubMed] [Google Scholar]
- 79.Chen M., Zhang Y., Sky Driver M., Caruso A.N., Yu Q., Wang Y. Surface modification of several dental substrates by non-thermal, atmospheric plasma brush. Dent. Mater. 2013;29:871–880. doi: 10.1016/j.dental.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yavirach P., Chaijareenont P., Boonyawan D., Pattamapun K., Tunma S., Takahashi H., Arksornnukit M. Effects of plasma treatment on the shear bond strength between fiber reinforced composite posts and resin composite for core build-up. Dent. Mater. J. 2009;28:686–692. doi: 10.4012/dmj.28.686. [DOI] [PubMed] [Google Scholar]
- 81.Costa Dantas M.C., Do Prado M., Costa V.S., Gaiotte M.G., Simão R.A., Bastian F.L. Comparison between the effect of plasma and chemical treatments on fiber post surface. J. Endod. 2012;38:215–218. doi: 10.1016/j.joen.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Ren Y., Wang C., Qiu Y. Aging of surface properties of ultra high modulus polyethylene fibers treated with He/O2 atmospheric pressure plasma jet. Surf. Coating. Technol. 2008;202:2670–2676. doi: 10.1016/j.surfcoat.2007.09.043. [DOI] [Google Scholar]
- 83.Feng B., Weng J., Yang B.C., Qu S.X., Zhang X.D. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials. 2003;24:4663–4670. doi: 10.1016/S0142-9612(03)00366-1. [DOI] [PubMed] [Google Scholar]
- 84.Rapuano B.E., Singh H., Boskey A.L., Doty S.B., MacDonald D.E. Heat and radiofrequency plasma glow discharge pretreatment of a titanium alloy: eveidence for enhanced osteoinductive properties. J. Cell. Biochem. 2013;114:1917–1927. doi: 10.1002/jcb.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giro G., Tovar N., Witek L., Marin C., Silva N.R.F., Bonfante E.A., Coelho P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. 2013;101 A:98–103. doi: 10.1002/jbm.a.34304. [DOI] [PubMed] [Google Scholar]
- 86.Duske K., Koban I., Kindel E., Schröder K., Nebe B., Holtfreter B., Jablonowski L., Weltmann K.D., Kocher T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012;39:400–407. doi: 10.1111/j.1600-051X.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 87.Noro A., Kaneko M., Murata I., Yoshinari M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal) J. Biomed. Mater. Res. B Appl. Biomater. 2013;101 B:355–363. doi: 10.1002/jbm.b.32846. [DOI] [PubMed] [Google Scholar]
- 88.Koban I., Duske K., Jablonowski L., Schröder K., Nebe B., Sietmann R., Weltmann K.D., Hübner N.O., Kramer A., Kocher T. Atmospheric plasma enhances wettability and osteoblast spreading on dentin in vitro: proof-of-principle. Plasma Process. Polym. 2011;8:975–982. doi: 10.1002/ppap.201100030. [DOI] [Google Scholar]
- 89.Miletić M., Mojsilović S., Okićorević I., Maletić D., Puač N., Lazović S., Malović G., Milenković P., Petrović Z. Lj, Bugarski D. Effects of non-thermal atmospheric plasma on human periodontal ligament mesenchymal stem cells. J. Phys. D Appl. Phys. 2013;46 doi: 10.1088/0022-3727/46/34/345401. [DOI] [Google Scholar]
- 90.Makkar H., Verma S.K., Panda P.K., Pramanik N., Jha E., Suar M. Molecular insight to size and dose-dependent cellular toxicity exhibited by a green synthesized bioceramic nanohybrid with macrophages for dental applications. Toxicol. Res. (Camb). 2018;7:959–969. doi: 10.1039/C8TX00112J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isbary G., Morfill G., Schmidt H.U., Georgi M., Ramrath K., Heinlin J., Karrer S., Landthaler M., Shimizu T., Steffes B., Bunk W., Monetti R., Zimmermann J.L., Pompl R., Stolz W. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010;163:78–82. doi: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- 92.Nastuta A.V., Topala I., Grigoras C., Pohoata V., Popa G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J. Phys. D Appl. Phys. 2011;44 doi: 10.1088/0022-3727/44/10/105204. [DOI] [Google Scholar]
- 93.Haertel B., von Woedtke T., Weltmann K.D., Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014;22:477–490. doi: 10.4062/biomolther.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.García-Alcantara E., López-Callejas R., Morales-Ramírez P.R., Peña-Eguiluz R., Fajardo-Muñoz R., Mercado-Cabrera A., Barocio S.R., Valencia-Alvarado R., Rodríguez-Méndez B.G., Muñoz-Castro A.E., de la Piedad-Beneitez A., Rojas-Olmedo I.A. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch. Med. Res. 2013;44:169–177. doi: 10.1016/j.arcmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Busco G., Robert E., Chettouh-Hammas N., Pouvesle J.M., Grillon C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020;161:290–304. doi: 10.1016/j.freeradbiomed.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Koban I., Matthes R., Hübner N.O., Welk A., Meisel P., Holtfreter B., Sietmann R., Kindel E., Weltmann K.D., Kramer A., Kocher T. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J. Phys. 2010;12 doi: 10.1088/1367-2630/12/7/073039. [DOI] [Google Scholar]
- 97.Yamazaki H., Ohshima T., Tsubota Y., Yamaguchi H., Jayawardena J.A., Nishimura Y. Microbicidal activities of low frequency atmospheric pressure plasma jets on oral pathogens. Dent. Mater. J. 2011;30:384–391. doi: 10.4012/dmj.2010-190. [DOI] [PubMed] [Google Scholar]
- 98.Diwan R., Debta F., Deoghare A., Ghom S., Khandelwal A., Deb Sikdar S., Ghom A.G. Plasma therapy: an overview. J. Indian Acad. Oral Med. Radiol. 2011;23:120–123. doi: 10.5005/jp-journals-10011-1109. [DOI] [Google Scholar]
- 99.Vijayarangan V., Delalande A., Dozias S., Pouvesle J.M., Robert E., Pichon C. New insights on molecular internalization and drug delivery following plasma jet exposures. Int. J. Pharm. 2020;589 doi: 10.1016/j.ijpharm.2020.119874. [DOI] [PubMed] [Google Scholar]
- 100.Vijayarangan V., Delalande A., Dozias S., Pouvesle J.M., Pichon C., Robert E. Cold atmospheric plasma parameters investigation for efficient drug delivery in HeLa cells. IEEE Trans. Radiat. Plasma Med. Sci. 2018;2:109–115. doi: 10.1109/TRPMS.2017.2759322. [DOI] [Google Scholar]
- 101.Bekeschus S., Favia P., Robert E., von Woedtke T. White paper on plasma for medicine and hygiene: future in plasma health sciences. Plasma Process. Polym. 2019;16 doi: 10.1002/ppap.201800033. [DOI] [Google Scholar]
- 102.Leduc M., Guay D., Leask R.L., Coulombe S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009;11 doi: 10.1088/1367-2630/11/11/115021. [DOI] [Google Scholar]
- 103.Biointerphases. 2015;10(no. 2) (n.d.) [Google Scholar]
- 104.Gherardi M., Tonini R., Colombo V. Plasma in dentistry: brief history and current status. Trends Biotechnol. 2018;36:583–585. doi: 10.1016/j.tibtech.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 105.Vandamme M., Robert E., Lerondel S., Sarron V., Ries D., Dozias S., Sobilo J., Gosset D., Kieda C., Legrain B., Pouvesle J.M., Le Pape A. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer. 2012;130:2185–2194. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 106.Volotskova O., Shashurin A., Stepp M.A., Pal-Ghosh S., Keidar M. Plasma-controlled cell migration: localization of cold plasma-cell interaction region. Plasma Med. 2011;1:85–92. doi: 10.1615/PlasmaMed.v1.i1.70. [DOI] [Google Scholar]
- 107.Lippens S., Denecker G., Ovaer P., Vandenabeele P., Declercq W. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 2005;12:1497–1508. doi: 10.1038/sj.cdd.4401722. [DOI] [PubMed] [Google Scholar]
- 108.Armand A., Khani M., Asnaashari M., AliAhmadi A., Shokri B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagnosis Photodyn. Ther. 2019;26:327–333. doi: 10.1016/j.pdpdt.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 109.Yang Y., Guo J., Zhou X., Liu Z., Wang C., Wang K., Zhang J., Wang Z. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: an in vitro study. Dent. Mater. J. 2018;37:157–166. doi: 10.4012/dmj.2017-030. [DOI] [PubMed] [Google Scholar]
- 110.Koban I., Holtfreter B., Hübner N.O., Matthes R., Sietmann R., Kindel E., Weltmann K.D., Welk A., Kramer A., Kocher T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro - proof of principle experiment. J. Clin. Periodontol. 2011;38:956–965. doi: 10.1111/j.1600-051X.2011.01740.x. [DOI] [PubMed] [Google Scholar]
- 111.Nima G., Harth-Chu E., Hiers R.D., Pecorari V.G.A., Dyer D.W., Khajotia S.S., Giannini M., Florez F.L.E. Antibacterial efficacy of non-thermal atmospheric plasma against Streptococcus mutans biofilm grown on the surfaces of restorative resin composites. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-03192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.H S., M G., H J., I A., B R., R M., H G., H I. Streptococcus mutans and Streptococcus sobrinus biofilm formation and metabolic activity on dental materials. Acta Odontol. Scand. 2012;70:114–121. doi: 10.3109/00016357.2011.600703. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L365183653%0Ahttps://doi.org/10.3109/00016357.2011.600703. [DOI] [PubMed] [Google Scholar]
- 113.Julák J., Scholtz V., Vaňková E. Medically important biofilms and non-thermal plasma. World J. Microbiol. Biotechnol. 2018;34 doi: 10.1007/s11274-018-2560-2. [DOI] [PubMed] [Google Scholar]
- 114.Abonti T.R., Kaku M., Kojima S., Sumi H., Kojima S., Yamamoto T., Yashima Y., Miyahara H., Okino A., Kawata T., Tanne K., Tanimoto K. Irradiation effects of low temperature multi gas plasma jet on oral bacteria. Dent. Mater. J. 2016;35:822–828. doi: 10.4012/dmj.2016-062. [DOI] [PubMed] [Google Scholar]
- 115.Moreau M., Orange N., Feuilloley M.G.J. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol. Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 116.Sarkar A. Cold atmospheric plasma-future of dentistry. IOSR J. Dent. Med. Sci. e-ISSN. 2018;17:15–20. www.iosrjournals.org [Google Scholar]
- 117.Idlibi A.N., Al-Marrawi F., Hannig M., Lehmann A., Rueppell A., Schindler A., Jentsch H., Rupf S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling. 2013;29:369–379. doi: 10.1080/08927014.2013.775255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request from the corresponding author.