Summary
Background
Acute meningitis or encephalitis (AME) results from a neurological infection causing high case fatality and severe sequelae. AME lacked comprehensive surveillance in China.
Methods
Nation-wide surveillance of all-age patients with AME syndromes was conducted in 144 sentinel hospitals of 29 provinces in China. Eleven AME-causative viral and bacterial pathogens were tested with multiple diagnostic methods.
Findings
Between 2009 and 2018, 20,454 AME patients were recruited for tests. Based on 9,079 patients with all-four-virus tested, 28.43% (95% CI: 27.50%‒29.36%) of them had at least one virus-positive detection. Enterovirus was the most frequently determined virus in children <18 years, herpes simplex virus and Japanese encephalitis virus were the most frequently determined in 18−59 and ≥60 years age groups, respectively. Based on 6,802 patients with all-seven-bacteria tested, 4.43% (95% CI: 3.94%‒4.91%) had at least one bacteria-positive detection, Streptococcus pneumoniae and Neisseria meningitidis were the leading bacterium in children aged <5 years and 5−17 years, respectively. Staphylococcus aureus was the most frequently detected in adults aged 18−59 and ≥60 years. The pathogen spectrum also differed statistically significantly between northern and southern China. Joinpoint analysis revealed age-specific positive rates, with enterovirus, herpes simplex virus and mumps virus peaking at 3−6 years old, while Japanese encephalitis virus peaked in the ≥60 years old. As age increased, the positive rate for Streptococcus pneumoniae and Escherichia coli statistically significantly decreased, while for Staphylococcus aureus and Streptococcus suis it increased.
Interpretation
The current findings allow enhanced identification of the predominant AME-related pathogen candidates for diagnosis in clinical practice and more targeted application of prevention and control measures in China, and a possible reassessment of vaccination strategy.
Funding
China Mega-Project on Infectious Disease Prevention and the National Natural Science Funds
Keywords: meningitis, encephalitis, sentinel surveillance, etiology, China
Research in context.
Evidence before this study
In Aug 2021, we did a search of PubMed for papers reporting acute meningitis or encephalitis (AME) from Jan 1, 2009 to Aug 5, 2021, using search terms “viral/virus/viruses” or “bacterial/bacteria/bacterium” and “meningitis” or “encephalitis” and “China”, with no data or language restrictions. Among the 463 papers from the preliminary screening, 27 papers have reported the etiological detection with or without the clinical and epidemiological investigation of AME in China during 2009‒2021. Thirteen papers reported single pathogen detection including five papers on Japanese encephalitis virus (JEV), four on enterovirus (EV), one on tick-borne encephalitis virus, one on cytomegalovirus, one on Neisseria meningitidis (N. meningitidis), and one on Klebsiella pneumoniae. Fourteen papers reported multiple AME pathogens detection, including three papers focusing on viral infections, six papers only investigating bacterial infections and five papers investigating both viral and bacterial pathogens. No studies were performed on a nationwide scale and over a long time.
Added value of this study
To the best of our knowledge, this study provided an unprecedented and comprehensive analysis of the etiological and epidemiological features of patients with AME in the mainland of China. Based on the detection of 11 commonly seen pathogens on 20,454 all-age patients that were recruited from 144 sentinel hospitals in 29 provinces of the Chinese mainland from 2009 to 2018, the prevalence and pathogen spectrum of AME patients were disclosed for the first time. The important findings included:
1. The viral detection rates of EV>JEV>HSV>MuV and bacterial detection rates of N. meningitidis>S. pneumoniae>S. aureus>E. coli>S. suis>Hib>M. tuberculosis were revealed respectively.
2. The pathogen spectrum differed among age groups, with children aged 5‒17 years old having the highest viral detection rate with EV as the leading pathogen. The children aged <5 years old had the highest bacterial detection rate, with S. pneumoniae as the leading pathogen. The joinpoint regressions demonstrated different turnaround points at specific age or age range that marked increased or decreased positive rate for each of the detected pathogens.
Implications of all the available evidence
Our findings fill crucial gaps of how the distributions of AME-related pathogens change across China in AME patients. This allows enhanced identification of the predominant AME-related pathogen candidates for diagnosis in clinical practice and more targeted application of prevention and control measures in China, as well as, a possible reassessment of vaccination strategy.
Alt-text: Unlabelled box
Background
Infection of the central nervous system, often presenting as acute meningitis or encephalitis (AME), is associated with substantial mortality and long-term neurological sequelae worldwide.1, 2, 3 A wide variety of infectious agents, including viruses, bacteria, fungi and parasites are causative of AME.1 The incidence of infectious encephalitis is estimated at 1.5–7 cases/100,000 inhabitants/year, with the highest incidence reported in children.2,4 Over one hundred viral and bacterial species have been shown to cause AME,5 and with viral pathogens composing the most part of them.6,7 For example, in the USA (1993‒2008), 31% of visits in emergency department admissions for all-cause meningitis were viral meningitis.8 Furthermore, in England and Wales, the reporting number of viral meningitis or encephalitis increased seven times from 2004 to 2013.9 The studies usually focused on the viral infections in AME patients, while few studies reported both viral and bacterial etiologies of them in China.10,11
In China, high case fatality and severe sequelae are associated with AME.12, 13, 14 A nationwide vaccination program that included vaccines against serogroup A and serogroup A&C meningococcal, conjugate vaccines against Haemophilus influenzae type b (Hib) and Japanese encephalitis virus (JEV), resulted in pronounced declines in the invasive diseases caused by these pathogens across all age groups.15, 16, 17 It is not mandatory to notify the public health authorities about infectious encephalitis. Neisseria meningitidis (N. meningitidis) and JEV are the only two causative agents of AME included in the National Infectious Diseases Surveillance System. In recent years, an increasing body of work has shown a wider range of other common pathogens in encephalitis patients in China, resulting in a much larger list of potential causative pathogens. For example, in Guangxi, enterovirus (EV) was the most frequently detected pathogen, accounting for more than 31.5% of all confirmed viral meningitis cases.18 Moreover, in a study by Li et al., Streptococcus pneumoniae (S. pneumoniae) accounted for 46.9% of bacterial meningitis cases in children in China during 2014‒2016, substantially more than the rate derived from previous studies in other countries.19, 20, 21 Although interesting and suggestive, these studies were usually limited by small specimen sizes, limited coverage of geographic range and population types, short surveillance duration and restricted testing of pathogens, making it difficult to generalize to the overall burden of meningitis/encephalitis. Here we present the results of a nationwide surveillance for patients with AME that overcomes many of these limitations and identify the etiological and epidemiological features of AME in all-age population for a decade in China.
Methods
Hospital based surveillance network
Between 2009 and 2018, an active surveillance was conducted on patients with AME at national level in China. The surveillance was administered under the tertiary network system, managed by Chinese Center for Disease Control and Prevention (China CDC). The number of sentinel hospitals was determined in proportion to the total population size at the scale of ecological region, and the surveillance hospitals were chosen after full consideration of the surveillance and laboratory testing capacities and representativeness of their geographical locations (Supplementary Figure 1). In all participating hospitals, a standard operating procedure (SOP) was applied to recruit and test AME patients, that regulated and standardized patient enrollment, specimen collection and storage, laboratory testing and data collection.22 Pre-study training, whole-procedure supervision, monthly enrollment reports, data audits, and annual study-site visits were also conducted to ensure uniform procedures were employed as guided among the study sites and across the years of the study. The SOP was approved by the institutional review board at each institution and at the CDC (2015-025) and remained unchanged across the reported surveillance period.
Patients’ enrollment, specimen collection and laboratory procedures
Case definitions of AME were created prior to study initiation and clinically based: having acute onset of fever, headache or vomiting, accompanied with at least one of neural paralysis, disturbance of consciousness, meningeal irritation sign or changes in mental status.22 In order to exclude nosocomial infection, patients who developed AME-like symptoms after hospitalization were not enrolled in this study. For patients who met the inclusion criteria and who were willing to participate in the study, samples were collected on admission and before therapy was administered. The samples were required to be tested within 24 hours after collection, and if this was not possible, were stored at -70°C until tested. The sample size was determined by the time window and presenting population.
Cerebrospinal fluid (CSF) and blood samples were collected for detection of four viruses, including EV, JEV, mumps virus (MuV), herpes simplex virus (HSV). Throat swabs were additionally collected from patients who were suspected with viral infection for the test of EV, MuV and HSV, while stool samples were additionally collected for the test of EV from patients who were suspected to have hand, foot, and mouth disease (Supplementary Figure 2). For the laboratory test for viruses, polymerase chain reaction (PCR) or reverse transcription-polymerase chain reaction (RT-PCR) or enzyme-linked immunosorbent assay (ELISA) was performed (Supplementary Table 1). Bacterial culture mainly included culture of samples collected from sterile sites, such as CSF and whole blood, for the isolation of bacteria, including N. meningitidis, S. pneumoniae, Staphylococcus aureus (S. aureus), Hib, Streptococcus suis (S. suis), Mycobacterium tuberculosis (M. tuberculosis) and Escherichia coli (E. coli). For isolated bacteria, morphological identification according to the colony morphology on different media and gram stain microscopic examination were conducted. After morphological identification, biochemical assays specific to different bacteria were conducted. Latex agglutination, PCR and real-time PCR were used to detect negative bacterial culture specimens (Supplementary Tables 2 and 3). All steps of the nucleic acid extraction and PCR/RT-PCR/real-time PCR test were conducted in parallel with positive and negative controls. If any targeted pathogen was detected in any of the specimens, the patient was considered as pathogen-positive (detailed in supplemental material and methods).
Data management and statistical analysis
Data on individual demographic (age, sex), case type (inpatient or outpatient), location, clinical data and medication use were collected by reviewing medical records of the patients. All data were entered into a standardized database by trained clinicians and uploaded to the online management system generated by the China CDC. Data collection was considered as public health surveillance by the National Health Commission of the People's Republic of China and verbal informed consent was obtained from patients or their legal guardians. The project and the above procedure for obtaining consent were approved by the ethical review committee of China CDC. The location of southern or northern China was defined according to latitude (Supplementary Figure 1).
Data were sorted to remove the redundant and the incomplete before analysis. Descriptive statistics were performed for categorical variables to estimate frequency, proportion and rate, for continuous variables to estimate medians and interquartile ranges (IQR). The nonparametric test with Mann-Whitney U test method was used to perform two-group comparison for continuous variables. Pearson chi-square test or Fisher exact test was applied to perform comparison for categorical variables. No allowance for multiplicity was made in the analyses. The Cochran-Armitage trend test was used for the trend analysis.23 Joinpoint regression analysis was used to identify the best-fitting points where a statistically significant change in the log-linear slope of trend occurred.24 The associations between positive detection rate and meteorological and socioeconomic factors were explored by using a negative binomial regression at the city level (detailed in the supplementary appendix). Statistical analysis was performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), ArcGIS version 10.5 (ESRI, ArcGIS, Redlands, CA, USA) and Join-Point Trend Analysis Software version 4.7.0.0 (Statistical Research and Applications Branch, National Cancer Institute, USA). A two-sided P value of <0.05 was statistically significant.
Role of the funding source
The funders for this study had no role in study design, data collection, data analysis, data interpretation, writing of this report, or decision to submit this paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
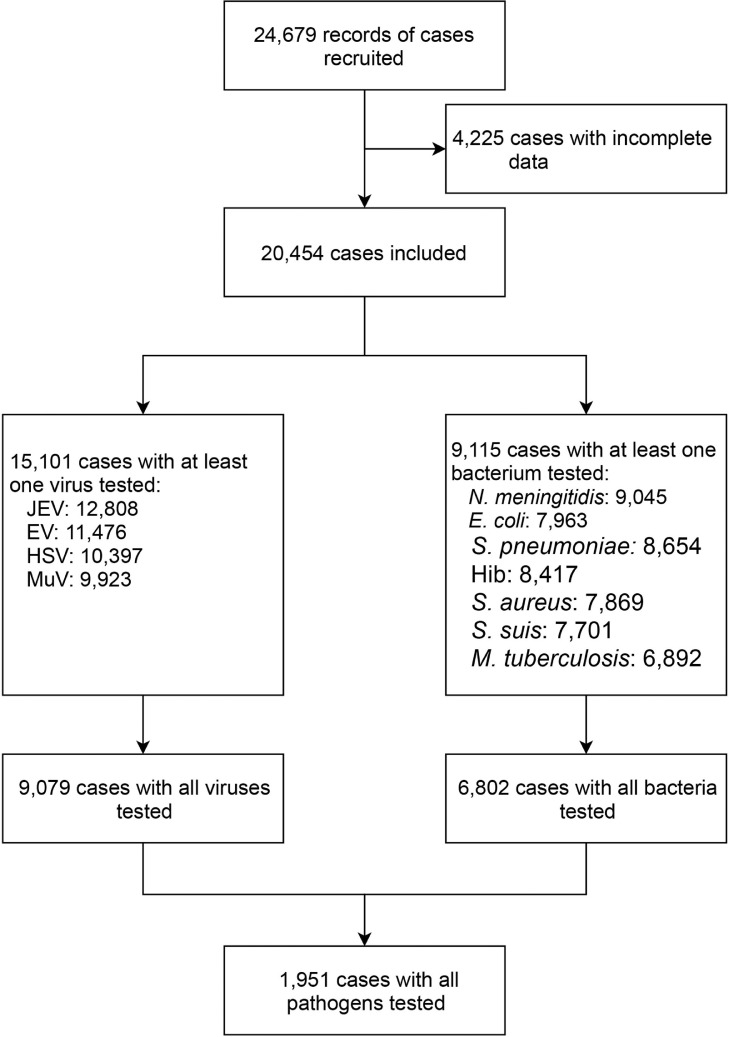
From January 2009 to December 2018, a total of 24,679 patients with clinical diagnosis of AME were enrolled from 144 sentinel hospitals in 29 provinces of the mainland of China (Supplementary Figure 1). Among those patients, 4,225 were excluded due to incomplete data (Supplementary Table 4), and the remaining 20,454 patients were included for further analysis (Figure 1). Among those recruited, 12,956 (63.34%) were male, 7,666 (37.48%) were children <5 years old, 6,506 (31.81%) cases were 5‒17 years old, 5,123 (25.05%) were 18‒59 years old and 1,159 (5.67%) were ≥60 years old. The median interval from disease onset to clinic visit was 3 (IQR: 1‒6) days. The majority of the recruits were hospitalized patients (81.79%) (Table 1). A higher proportion of patients (60.23%) were recruited from sentinel hospitals in southern China. There were 15,101 patients tested for at least one virus, among them 9,079 patients were tested for all four viruses. There were 9,115 patients who were tested for at least one bacterium, and 6,802 patients were tested for all seven bacteria. There were 1,951 patients who were tested for all 11 pathogens (Figure 1).
Figure 1.
The flowchart of patient recruit and sorting procedures. This flow diagram summarizes the number of AME patients subject to each analysis in this study.
Table 1.
Demographic and epidemiological characteristics of patients with acute meningitis and encephalitis in China during 2009‒2018.
| Characteristics | All patients (N=20,454) | At least one virus tested (n=15,101) | At least one bacterium tested (n=9,115) | All viruses tested (n=9,079) | All bacteria tested (n=6,802) | All pathogens tested (n=1,951) |
|---|---|---|---|---|---|---|
| Sex, male, n (%) | 12,956 (63.34) | 9,493 (62.86) | 5,793 (63.55) | 5,704 (62.83) | 4,369 (64.23) | 1,229 (62.99) |
| Age, year, median (IQR) | 7 (3-26) | 6 (3-18) | 15 (4-42) | 6 (3-23) | 17 (3-43) | 25 (6-48) |
| Age group, n (%) | ||||||
| Children (<5 years) | 7,666 (37.48) | 5,820 (38.54) | 2,593 (28.45) | 3,467 (38.19) | 2,003 (29.45) | 373 (19.12) |
| <6 months | 1,122 (5.49) | 559 (3.70) | 713 (7.82) | 363 (4.00) | 567 (8.34) | 79 (4.05) |
| 6‒12 months | 736 (3.60) | 507 (3.36) | 316 (3.47) | 333 (3.67) | 251 (3.69) | 43 (2.20) |
| 1‒ years | 1,855 (9.07) | 1,460 (9.67) | 544 (5.97) | 853 (9.40) | 426 (6.26) | 75 (3.84) |
| 2‒ years | 1,395 (6.82) | 1,122 (7.43) | 387 (4.25) | 624 (6.87) | 295 (4.34) | 51 (2.61) |
| 3‒ years | 1,307 (6.39) | 1,103 (7.30) | 310 (3.40) | 648 (7.14) | 235 (3.45) | 62 (3.18) |
| 4‒ years | 1,251 (6.12) | 1,069 (7.08) | 323 (3.54) | 646 (7.12) | 229 (3.37) | 63 (3.23) |
| 5‒17 years old | 6,506 (31.81) | 5,445 (36.06) | 2,158 (23.68) | 3,063 (33.74) | 1,459 (21.45) | 478 (24.50) |
| 18‒59 years old | 5,123 (25.05) | 3,077 (20.38) | 3,598 (39.47) | 2,099 (23.12) | 2,751 (40.44) | 870 (44.59) |
| ≥60 years old | 1,159 (5.67) | 759 (5.03) | 766 (8.40) | 450 (4.96) | 589 (8.66) | 230 (11.79) |
| Days from disease onset to clinic visit, median (IQR) | 3 (1-6) | 3 (1-6) | 2 (0-6) | 3 (1-6) | 2 (0-6) | 3 (1-6) |
| Case type, n (%) | ||||||
| Inpatient | 16,729 (81.79) | 12,286 (81.36) | 7,376 (80.92) | 7,344 (80.89) | 5,399 (79.37) | 1,406 (72.07) |
| Outpatient | 3,725 (18.21) | 2,815 (18.64) | 1,739 (19.08) | 1,735 (19.11) | 1,403 (20.63) | 545 (27.93) |
| Surveillance location, n (%) | ||||||
| Northern China | 8,134 (39.77) | 7,178 (47.53) | 2,554 (28.02) | 4,170 (45.93) | 1,524 (22.41) | 563 (28.86) |
| Southern China | 12,320 (60.23) | 7,923 (52.47) | 6,561 (71.98) | 4,909 (54.07) | 5,278 (77.59) | 1,388 (71.14) |
Data are n (percentage %) unless otherwise indicated. Percentages may not total 100 because of rounding. IQR, interquartile range. Provinces located in southern and northern China were defined according to latitude.
The virus spectrum based on patients with all-four-virus test
Overall, 28.43% (95% CI: 27.50%‒29.36%) of the patients had at least one virus-positive detection. EV was the most common virus, accounting for 48.89% of all positive test (1,504/3,076), followed by HSV (27.34%), MuV (12.97%) and JEV (10.79%) (Supplementary Figure 3a, Table 2). EV was the most commonly identified virus in the CSF samples as well (Supplementary Figure 4a). The positive rate and pathogen spectrum differed by age. The patients aged 5‒17 years old and children <5 years old had comparable positive rates (36.96% [95% CI: 35.25%‒38.67%] and 30.57% [95% CI: 29.04%‒32.11%]) and the same viral ranking EV>HSV>MuV>JEV. Lower positive rates were obtained from both adult groups aged 18‒59 years old (15.20% [95% CI: 13.66%‒16.73%]) and ≥60 years old (15.56% [95% CI: 12.21%‒18.90%]). These groups also had a different viral ranking, i.e., HSV>EV>JEV>MuV for patients aged 18‒59 years old, while JEV>HSV>EV>MuV for patients aged ≥60 years old. Geographical differences were observed, with positive rate from northern China higher than southern China (37.75% vs. 20.51%, rate difference 17.23%, 95% CI: 15.38%‒19.09%). In both regions, EV was the top-ranking virus, although JEV attained a higher overall ranking in southern China. The overall positive rates were comparable between female and male (28.65% vs. 28.30%, rate difference 0.36%, 95% CI: -1.57%‒2.28%) and case types (28.53% in inpatients and 28.01% in outpatients, rate difference 0.52%, 95% CI: -1.84%‒2.87%).
Table 2.
Pathogen spectrum of patients with acute meningitis or encephalitis in China during 2009‒2018.
| Virus |
Bacterium |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive rate | Proportion (Total positive=3,076) |
Positive rate | Proportion (Total positive=311) |
||||||||||
| EV | HSV | MuV | JEV | S. pneumoniae | S. aureus | N. meningitidis | E. coli | S. suis | Hib | M. tuberculosis | |||
| Overall cases | 2,581 (28.43) | 1,504 (48.89) | 841 (27.34) | 399 (12.97) | 332 (10.79) | 301 (4.43) | 85 (27.33) | 77 (24.76) | 69 (22.19) | 52 (16.72) | 18 (5.79) | 7 (2.25) | 3 (0.96) |
| Sex | |||||||||||||
| Female | 967 (28.65) | 537 (46.41) | 350 (30.25) | 141 (12.19) | 129 (11.15) | 85 (3.49) | 26 (29.89) | 21 (24.14) | 20 (22.99) | 16 (18.39) | 2 (2.30) | 1 (1.15) | 1 (1.15) |
| Male | 1,614 (28.30) | 967 (50.39) | 491 (25.59) | 258 (13.44) | 203 (10.58) | 216 (4.94) | 59 (26.34) | 56 (25.00) | 49 (21.88) | 36 (16.07) | 16 (7.14) | 6 (2.68) | 2 (0.89) |
| P value | 0.072 | 0.027 | 0.005 | 0.697 | |||||||||
| Age | |||||||||||||
| <5 years old | 1,060 (30.57) | 719 (56.48) | 321 (25.22) | 136 (10.68) | 97 (7.62) | 99 (4.94) | 49 (48.51) | 8 (7.92) | 14 (13.86) | 23 (22.77) | 0 (0) | 6 (5.94) | 1 (0.99) |
| 5-17 years old | 1,132 (36.96) | 687 (50.78) | 351 (25.94) | 189 (13.97) | 126 (9.31) | 64 (4.39) | 12 (17.39) | 13 (18.84) | 30 (43.48) | 14 (20.29) | 0 (0) | 0 (0) | 0 (0) |
| 18-59 years old | 319 (15.20) | 86 (23.06) | 147 (39.41) | 62 (16.62) | 78 (20.91) | 110 (4.00) | 21 (18.58) | 47 (41.59) | 17 (15.04) | 13 (11.50) | 12 (10.62) | 1 (0.88) | 2 (1.77) |
| ≥60 years old | 70 (15.56) | 12 (15.58) | 22 (28.57) | 12 (15.58) | 31 (40.26) | 28 (4.75) | 3 (10.71) | 9 (32.14) | 8 (28.57) | 2 (7.14) | 6 (21.43) | 0 (0) | 0 (0) |
| P value | <0.001 | <0.001 | 0.456 | <0.001 | |||||||||
| Case type | |||||||||||||
| Inpatient | 2,095 (28.53) | 1,272 (50.70) | 708 (28.22) | 295 (11.76) | 234 (9.33) | 244 (4.52) | 79 (31.10) | 75 (29.53) | 22 (8.66) | 50 (19.69) | 18 (7.09) | 7 (2.76) | 3 (1.18) |
| Outpatient | 486 (28.01) | 232 (40.92) | 133 (23.46) | 104 (18.34) | 98 (17.28) | 57 (4.06) | 6 (10.53) | 2 (3.51) | 47 (82.46) | 2 (3.51) | 0 (0) | 0 (0) | 0 (0) |
| P value | 0.669 | <0.001 | 0.459 | <0.001 | |||||||||
| Geographic location | |||||||||||||
| Northern | 1,574 (37.75) | 875 (43.77) | 651 (32.57) | 346 (17.31) | 127 (6.35) | 124 (8.14) | 52 (39.69) | 6 (4.58) | 36 (27.48) | 29 (22.14) | 0 (0) | 6 (4.58) | 2 (1.53) |
| Southern | 1,007 (20.51) | 629 (58.40) | 190 (17.64) | 53 (4.92) | 205 (19.03) | 177 (3.35) | 33 (18.33) | 71 (39.44) | 33 (18.33) | 23 (12.78) | 18 (10.00) | 1 (0.56) | 1 (0.56) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||
The viral positive rate was estimated based on 9,079 cases with all four viruses tested and the bacterial positive rate was estimated based on 6,802 cases with all seven bacteria tested. Pathogen spectrum was presented as Number of positive test (proportion of positive %) unless otherwise indicated. The total positive counts for viruses and bacteria were 3,076 and 311, which were larger than the number of positive patients because of the coinfection. Proportions may not total 100 because of rounding. Chi-square test or Fisher's exact test were used for comparisons among different groups. EV, enterovirus; JEV, Japanese encephalitis virus; HSV, herpes simplex virus; MuV, mumps virus; N. meningitidis, Neisseria meningitidis; S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; Hib, Haemophilus influenzae type b; S. suis, Streptococcus suis; M. tuberculosis, Mycobacterium tuberculosis; E. coli, Escherichia coli.
The bacterial spectrum based on patients with all-seven-bacteria test
Overall, 4.43% (95% CI: 3.94%‒4.91%) of the patients had at least one bacteria-positive detection. S. pneumoniae was the most common bacterium, accounting for 27.33% of all the bacterial-positively patients (85/311), followed by S. aureus (24.76%), N. meningitidis (21.19%), and E. coli (16.72%) (Supplementary Figure 3b, Table 2). The same ranking was also observed when the test results from CSF samples were used (Supplementary Figure 4b). The positive rate was comparable across four age groups, while the bacteria ranking differed. For children <5 years old, the top three bacteria were S. pneumonia>E. coli>N. meningitidis, which were switched to N. meningitidis>E. coli>S. aureus in patients aged 5‒17 years old. In a more divergent pattern still, both adult groups had S. aureus up ranked to the first place, followed by S. pneumonia and N. meningitidis in 18‒59 years old, N. meningitidis and S. suis in ≥60 years old, respectively. Geographical differences were observed, showing higher positive rate from northern than from southern China (8.14% vs. 3.35%, rate difference 4.78%, 95% CI: 3.33%‒6.24%). S. pneumoniae was the top listing bacteria in northern China, while S. aureus was the highest ranking in southern China. S. pneumonia and S. aureus were more frequently seen among inpatients, while N. meningitidis was more frequently seen among outpatients (P<0.001) (Supplementary Figure 3b).
Age and sex specific pattern of viral pathogen
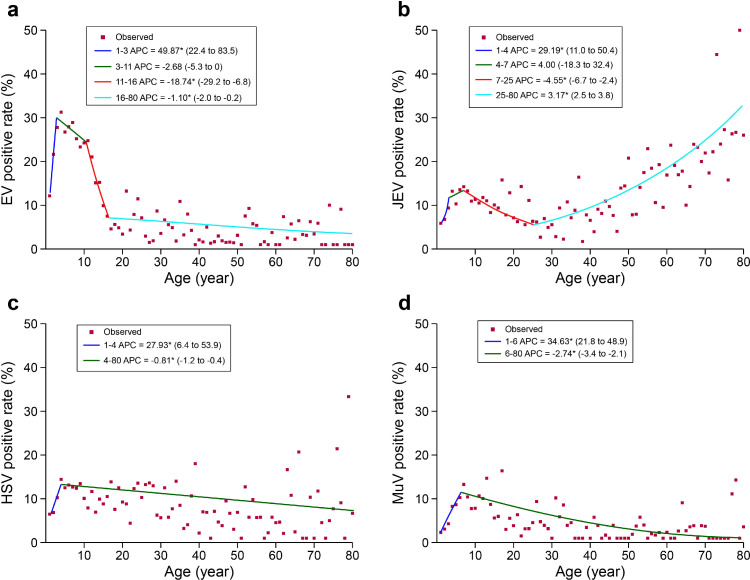
The age pattern of positive detection for the four viruses differed. EV, HSV and MuV were more frequently identified from children <5 years old and 5‒17 years old, while JEV had the highest positive rate in the ≥60 years old (Supplementary Figure 5a, Table 3). By applying joinpoint model, EV had shown three descending turnpoints at three, 11 and 16 years old, with the greatest drop of positive rate seen at 11 years old (APC=-18.74 [95% CI: -29.2‒-6.8]). Both HSV and MuV had shown one descending turnpoint at four and six years old, respectively (Figure 2). For JEV, a descending turnpoint at seven years old (APC=-4.55 [95% CI: -6.7‒-2.4]) and an ascending turnpoint at 25 years (APC=3.17 [95% CI: 2.5‒3.8]) were observed, leading to a different age pattern from those of the other three viruses. The positive rates also differed between sex in an age-dependent manner. For example, JEV was detected more often in females (Supplementary Figure 5a), but only among the ≥60 years old. HSV was more often detected in females, but only among the children <5 years and adults aged 18‒59 years old (Table 4). While MuV was statistically significantly more frequent in males, which was only among patients aged 5‒17 years old. Moreover, higher positive rates for EV and HSV were observed among inpatients, and with a reversed manner for JEV and MuV. Higher rates of EV (24.10% vs. 13.21%, rate difference 10.89%, 95% CI: 9.47%‒12.31%), HSV (13.48% vs. 6.13%, rate difference 7.34%, 95% CI: 6.21%‒8.48%) and MuV (8.79% vs. 4.28%, rate difference 4.51%, 95% CI: 3.52%‒5.51%) were observed in patients from northern China than patients from southern China (Table 3). EV had the highest positive rate in the samples of CSF and throat swabs, and HSV was more frequently identified from blood samples (Supplementary Table 5).
Table 3.
Positive detection rate of viral or bacterial pathogen for patients with acute meningitis or encephalitis in China during 2009‒2018.
| Characteristics | EV | JEV | HSV | MuV |
|---|---|---|---|---|
| Overall cases | 18.44 (2116/11,476) | 11.02 (1,412/12,808) | 9.79 (1,018/1,0397) | 6.25 (620/9,923) |
| Sex | ||||
| Male | 18.72 (1,353/7,228) | 10.40 (831/7,991) | 9.14 (596/6,519) | 6.84 (428/6,259) |
| Female | 17.96 (763/4,248) | 12.06 (581/4,817) | 10.88 (422/3,878) | 5.24 (192/3,664) |
| Rate difference (95% CI) | 0.76 (-0.71, 2.22) | -1.66 (-2.80, -0.52) | -1.74 (-2.94, -0.54) | 1.60 (0.64, 2.55) |
| Age | ||||
| Children (0‒5 years) | 23.66 (1,110/4,692) | 8.97 (411/4,580) | 9.86 (384/3,894) | 5.17 (193/3,734) |
| <6 months | 10.49 (49/467) | 5.58 (26/466) | 7.21 (30/416) | 3.10 (12/387) |
| 6‒12 months | 13.95 (60/430) | 6.19 (26/420) | 5.66 (21/371) | 1.42 (5/353) |
| 1‒ years | 21.65 (265/1,224) | 6.76 (75/1,109) | 6.86 (65/947) | 3.08 (28/909) |
| 2‒ years | 27.79 (259/932) | 9.37 (77/822) | 10.26 (72/702) | 4.28 (29/677) |
| 3‒ years | 31.26 (267/854) | 13.20 (116/879) | 14.43 (103/714) | 8.25 (58/703) |
| 4‒ years | 26.75 (210/785) | 10.29 (91/884) | 12.50 (93/744) | 8.65 (61/705) |
| P value* | <0.001 | <0.001 | <0.001 | <0.001 |
| 5‒17 years old | 23.29 (885/3,800) | 12.21 (555/4,547) | 11.49 (418/3,637) | 10.16 (348/3,425) |
| 18‒59 years old | 4.16 (102/2,454) | 9.69 (286/2,950) | 7.66 (181/2,363) | 2.94 (67/2,278) |
| ≥60 years old | 3.58 (19/530) | 21.89 (160/731) | 6.96 (35/503) | 2.47 (12/486) |
| P value# | <0.001 | <0.001 | 0.002 | <0.001 |
| Case type | ||||
| Inpatient | 18.86 (1,736/9,206) | 10.63 (1,115/10,492) | 10.25 (877/8,558) | 5.90 (477/8,085) |
| Outpatient | 16.74 (380/2,270) | 12.82 (297/2,316) | 7.67 (141/1,839) | 7.78 (143/1,838) |
| Rate difference (95% CI) | 2.12 (0.39, 3.85) | -2.20 (-3.68, -0.71) | 2.58 (1.21, 3.96) | -1.88 (-3.21, -0.55) |
| Geographic location | ||||
| Northern | 24.10 (1,328/5,511) | 11.25 (654/5,812) | 13.48 (698/5,179) | 8.79 (381/4,334) |
| Southern | 13.21 (788/5,965) | 10.83 (758/6,996) | 6.13 (320/5,218) | 4.28 (239/5,589) |
| Rate difference (95% CI) | 10.89 (9.47, 12.31) | 0.42 (-0.67, 1.51) | 7.34 (6.21, 8.48) | 4.51 (3.52, 5.51) |
The data were presented as positive rate (positive number/tested number) unless otherwise indicated. * indicates comparison among six age groups of children by performing Cochran-Armitage trend test. # indicates comparison among children <5 years old, patients aged 5‒17 years old, 18‒59 years old and ≥60 years old by performing Cochran-Armitage trend test. JEV, Japanese encephalitis virus; EV, Enterovirus; MuV, Mumps virus; HSV, Herpes simplex virus.
Figure 2.
Joinpoint regression analysis of the positive rates by age of the patient. (a) EV; (b) JEV; (c) HSV; (d) MuV. EV, Enterovirus; JEV, Japanese encephalitis virus; HSV, Herpes simplex virus; MuV, Mumps virus. Red points indicate the mean positive rate of patients in terms of age and the colored curves indicate fitted patterns by age groups. The annual percent change (APC) value of each fitted curves is given for each virus. *indicates that the APC is statistically significantly different from zero at P<0.05.
Table 4.
Positive detection rate for tested viruses and bacteria by age and sex in patients with acute meningitis or encephalitis in China during 2009‒2018.
| <5 years old |
5‒17 years old |
18‒59 years old |
≥60 years old |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Rate difference (95% CI) |
Female | Male | Rate difference (95% CI) |
Female | Male | Rate difference (95% CI) |
Female | Male | Rate difference (95% CI) |
|
| EV | 23.86 (424/1,777) |
23.53 (686/2,915) |
0.33 (-2.18, 2.84) |
22.52 (290/1,288) |
23.69 (595/2,512) |
-1.17 (-3.99, 1.65) |
4.51 (44/975) |
3.92 (58/1,479) |
0.59 (-1.04, 2.23) |
2.40 (5/208) |
4.35 (14/322) |
-1.94 (-4.99, 1.10) |
| JEV | 9.28 (161/1,735) |
8.79 (250/2,845) |
0.49 (-1.22, 2.21) |
12.37 (193/1,560) |
12.12 (362/2,987) |
0.25 (-1.76, 2.26) |
10.50 (126/1,200) |
9.14 (160/1,750) |
1.36 (-0.84, 3.56) |
31.37 (101/322) |
14.43 (59/409) |
16.94 (10.84, 23.05) |
| HSV | 11.27 (169/1,500) |
8.98 (215/2,394) |
2.29 (0.32, 4.25) |
12.35 (154/1,247) |
11.05 (264/2,390) |
1.30 (-0.91, 3.52) |
9.42 (88/934) |
6.51 (93/1,429) |
2.91 (0.65, 5.18) |
5.58 (11/197) |
7.84 (24/306) |
-2.26 (-6.66, 2.14) |
| MuV | 4.41 (63/1,428) |
5.64 (130/2,306) |
-1.23 (-2.65, 0.20) |
8.36 (96/1,149) |
11.07 (252/2,276) |
-2.72 (-4.77, -0.66) |
3.44 (31/900) |
2.61 (36/1,378) |
0.83 (-0.63, 2.29) |
1.07 (2/187) |
3.34 (10/299) |
-2.27 (-4.79, 0.24) |
| N. meningitidis | 1.33 (13/974) |
1.87 (30/1,606) |
-0.53 (-1.51, 0.45) |
3.59 (26/724) |
3.15 (45/1,427) |
0.44 (-1.19, 2.07) |
1.28 (17/1,327) |
1.75 (39/2,225) |
-0.47 (-1.29, 0.34) |
1.47 (4/273) |
1.84 (9/489) |
-0.38 (-2.23, 1.48) |
| S. pneumoniae | 2.26 (21/929) |
2.36 (36/1,524) |
-0.10 (-1.32, 1.12) |
0.59 (4/674) |
1.66 (22/1,328) |
-1.06 (-1.96, -0.16) |
1.01 (13/1,291) |
1.43 (31/2,170) |
-0.42 (-1.16, 0.32) |
1.53 (4/262) |
0.63 (3/476) |
0.90 (-0.75, 2.54) |
| S. aureus | 0.23 (2/879) |
0.49 (7/1,436) |
-0.26 (-0.74, 0.22) |
1.48 (9/607) |
0.51 (6/1,184) |
0.98 (-0.07, 2.02) |
0.88 (10/1,142) |
2.14 (42/1,962) |
-1.27 (-2.10, -0.43) |
1.74 (4/230) |
1.40 (6/429) |
0.34 (-1.68, 2.36) |
| E. coli | 0.93 (8/863) |
1.19 (17/1,427) |
-0.26 (-1.12, 0.59) |
0.97 (6/620) |
0.67 (8/1,203) |
0.30 (-0.59, 1.20) |
0.17 (2/1,163) |
0.64 (13/2,019) |
-0.47 (-0.89, -0.05) |
0 (0/235) |
0.46 (2/433) |
-0.46 (-1.10, 0.18) |
| S. suis | 0.12 (1/855) |
0 (0/1,402) |
0.12 (-0.11, 0.35) |
0 (0/583) |
0 (0/1,143) |
0 (0, 0) |
0.09 (1/1,114) |
0.82 (16/1,950) |
-0.73 (-1.17, -0.29) |
0.88 (2/228) |
1.41 (6/426) |
-0.53 (-2.18, 1.12) |
| Hib | 0.22 (2/913) |
0.47 (7/1,498) |
-0.25 (-0.71, 0.21) |
0 (0/659) |
0.08 (1/1,302) |
-0.08 (-0.23, 0.07) |
0.16 (2/1,239) |
0.19 (4/2,101) |
-0.03 (-0.32, 0.26) |
0 (0/248) |
0 (0/457) |
0 (0, 0) |
| M. tuberculosis | 0.13 (1/776) |
0.08 (1/1,264) |
0.05 (-0.25, 0.35) |
0 (0/493) |
0 (0/986) |
0 (0, 0) |
0.10 (1/1,002) |
0.17 (3/1,778) |
-0.07 (-0.34, 0.20) |
0.49 (1/204) |
0 (0/389) |
0.49 (-0.47, 1.45) |
The data were presented as positive rate (positive number/tested number) unless otherwise indicated. Chi square test or Fisher's exact test were used for comparisons between male and female in different age groups. EV, enterovirus; JEV, Japanese encephalitis virus; HSV, herpes simplex virus; MuV, mumps virus; N. meningitidis, Neisseria meningitidis; S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; Hib, Haemophilus influenzae type b; S. suis, Streptococcus suis; M. tuberculosis, Mycobacterium tuberculosis; E. coli, Escherichia coli. .
Age and sex specific pattern of bacterial pathogen
The age pattern of positive detection for the seven bacteria differed. The positive rates for S. pneumoniae and E. coli statistically significantly decreased with age (Chi-square for trend, P<0.001 and P=0.003; Supplementary Figure 5b, Table 5), while increased positive rates were observed for S. aureus and S. suis (Chi-square for trend, both P<0.001). Notably, the N. meningitidis was detected with a remarkably higher rate in patients aged 5‒17 years old. The positive rates for S. pneumonia, S. aureus, E. coli and S. suis were higher in inpatients than in outpatients, while for N. meningitidis and Hib, the positive rates were higher in outpatients. Geographic differences were observed for S. pneumoniae, N. meningitidis, E. coli and Hib, which were more frequently identified among patients in northern China than in southern China (Table 5). S. pneumoniae had the highest positive rate in the CSF specimens, and N. meningitidis was more frequently identified from blood specimens (Supplementary Table 5).
Table 5.
Positive detection rate of viral or bacterial pathogen for patients with acute meningitis or encephalitis in China during 2009‒2018.
| Characteristics | N. meningitidis | S. pneumoniae | S. aureus | E. coli | S. suis | Hib | M. tuberculosis |
|---|---|---|---|---|---|---|---|
| Overall cases | 2.02 (183/9,045) | 1.55 (134/8,654) | 1.09 (86/7,869) | 0.70 (56/7,963) | 0.34 (26/7,701) | 0.19 (16/8,417) | 0.10 (7/6,892) |
| Sex | |||||||
| Male | 2.14 (123/5,747) | 1.67 (92/5,498) | 1.22 (61/5,011) | 0.79 (40/5,082) | 0.45 (22/4,921) | 0.22 (12/5,358) | 0.09 (4/4,417) |
| Female | 1.82 (60/3,298) | 1.33 (42/3,156) | 0.87 (25/2,858) | 0.56 (16/2,881) | 0.14 (4/2,780) | 0.13 (4/3,059) | 0.12 (3/2,475) |
| Rate difference (95% CI) | 0.32 (-0.27, 0.91) | 0.34 (-0.18, 0.87) | 0.34 (-0.11, 0.80) | 0.23 (-0.13, 0.60) | 0.30 (0.07, 0.54) | 0.09 (-0.09, 0.27) | -0.03 (-0.19, 0.13) |
| Age | |||||||
| Children (0‒5 years) | 1.67 (43/2,580) | 2.32 (57/2,453) | 0.39 (9/2,315) | 1.09 (25/2,290) | 0.04 (1/2,257) | 0.37 (9/2,411) | 0.10 (2/2,040) |
| <6 months | 0.99 (7/709) | 1.71 (12/700) | 0.74 (5/672) | 2.32 (15/646) | 0 (0/650) | 0.15 (1/689) | 0.17 (1/578) |
| 6‒12 months | 1.59 (5/315) | 4.04 (12/297) | 0.34 (1/290) | 0 (0/285) | 0 (0/279) | 0.68 (2/294) | 0 (0/257) |
| 1‒ years | 1.29 (7/542) | 2.88 (15/520) | 0.20 (1/491) | 0.21 (1/481) | 0 (0/477) | 0.59 (3/511) | 0 (0/434) |
| 2‒ years | 1.82 (7/385) | 2.15 (8/372) | 0.30 (1/333) | 0.59 (2/340) | 0.31 (1/325) | 0.55 (2/366) | 0 (0/301) |
| 3‒ years | 3.55 (11/310) | 1.44 (4/278) | 0.38 (1/262) | 1.88 (5/266) | 0 (0/258) | 0 (0/272) | 0.42 (1/239) |
| 4‒ years | 1.88 (6/319) | 2.10 (6/286) | 0 (0/267) | 0.74 (2/272) | 0 (0/268) | 0.36 (1/279) | 0 (0/231) |
| P value* | 0.031 | 0.749 | 0.114 | 0.121 | 0.572 | 0.888 | 0.981 |
| 5‒17 years old | 3.30 (71/2,151) | 1.30 (26/2,002) | 0.84 (15/1,791) | 0.77 (14/1,823) | 0 (0/1,726) | 0.05 (1/1,961) | 0 (0/1,479) |
| 18‒59 years old | 1.58 (56/3,552) | 1.27 (44/3,461) | 1.68 (52/3,104) | 0.47 (15/3,182) | 0.55 (17/3,064) | 0.18 (6/3,340) | 0.14 (4/2,780) |
| ≥60 years old | 1.71 (13/762) | 0.95 (7/738) | 1.52 (10/659) | 0.30 (2/668) | 1.22 (8/654) | 0 (0/705) | 0.17 (1/593) |
| P value# | 0.375 | <0.001 | <0.001 | 0.003 | <0.001 | 0.055 | 0.432 |
| Case type | |||||||
| Inpatient | 1.47 (108/7,332) | 1.71 (120/7,033) | 1.31 (84/6,398) | 0.84 (54/6,450) | 0.42 (26/6,245) | 0.13 (9/6,800) | 0.09 (5/5,478) |
| Outpatient | 4.38 (75/1,713) | 0.86 (14/1,621) | 0.14 (2/1,471) | 0.13 (2/1,513) | 0 (0/1,456) | 0.43 (7/1,617) | 0.14 (2/1,414) |
| Rate difference (95% CI) | -2.91 (-3.91, -1.90) | 0.84 (0.30, 1.39) | 1.18 (0.84, 1.51) | 0.71 (0.42, 0.99) | 0.42 (0.26, 0.58) | -0.30 (-0.63, 0.03) | -0.05 (-0.26, 0.16) |
| Geographic location | |||||||
| Northern | 3.24 (81/2,498) | 3.75 (88/2,346) | 0.53 (10/1,880) | 1.54 (31/2,008) | 0 (0/1,742) | 0.52 (12/2,292) | 0.25 (4/1,571) |
| Southern | 1.56 (102/6,547) | 0.73 (46/6,308) | 1.27 (76/5,989) | 0.42 (25/5,955) | 0.44 (26/5,959) | 0.07 (4/6,125) | 0.06 (3/5,321) |
| Rate difference (95% CI) | 1.68 (0.93, 2.44) | 3.02 (2.22, 3.82) | -0.74 (-1.17, -0.30) | 1.12 (0.56, 1.69) | -0.44 (-0.60, -0.27) | 0.46 (0.16, 0.76) | 0.20 (-0.06, 0.46) |
The data were presented as positive rate (positive number/tested number) unless otherwise indicated. * indicates comparison among six age groups of children by performing Cochran-Armitage trend test. # indicates comparison among children <5 years old, patients aged 5‒17 years old, 18‒59 years old and ≥60 years old by performing Cochran-Armitage trend test. N. meningitidis, Neisseria meningitidis; S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; Hib, Haemophilus influenzae type b; S. suis, Streptococcus suis; M. tuberculosis, Mycobacterium tuberculosis; E. coli, Escherichia coli.
Temporal and spatial pattern
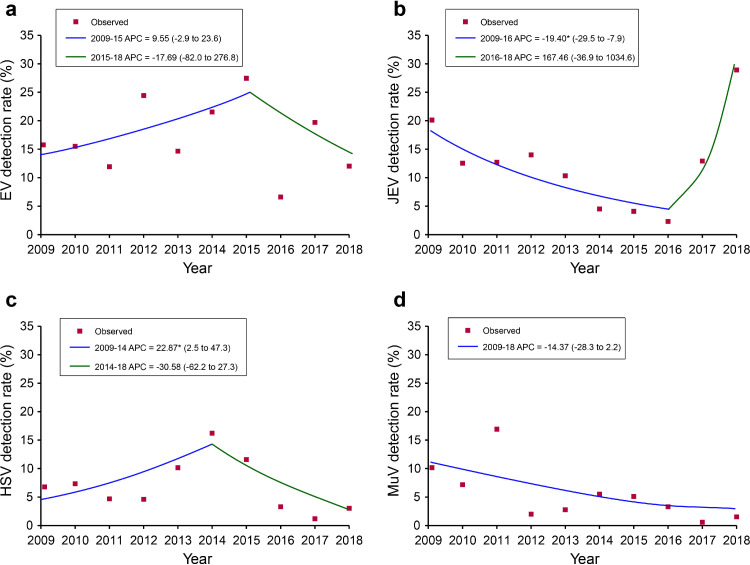
Joinpoint analysis along the study years revealed decreased positive rate of JEV during 2009‒2016 (APC=-19.40 [95% CI: -29.5‒-7.9]), but a statistically significant increase in positive rate of HSV was found during 2009‒2014 (APC=22.87 [95% CI: 2.5‒47.3], Figure 3). No autocorrelations were observed (Supplementary Figure 6). Seasonal pattern for tested viruses was observed based on the monthly positive rate for each pathogen. Both EV and HSV peaked during April‒September, while JEV and MuV peaked during July‒September and April-June, respectively (Supplementary Figure 7). No obvious seasonal trend was shown for any of the tested bacteria.
Figure 3.
Joinpoint regression analysis of the annual positive rates for four viruses. (a) EV; (b) JEV; (c) HSV; (d) MuV. EV, Enterovirus; JEV, Japanese encephalitis virus; HSV, Herpes simplex virus; MuV, Mumps virus. Red points indicate the mean positive rate of tested viruses in terms of year. The colored curves indicate fitted patterns by the red points. Legends give the annual percent change (APC) value of each fitted curve for each virus. *indicates that the APC is statistically significantly different from zero at P<0.05.
The tested pathogens with positive rates greater than 1% were further investigates for their relationship with meteorological and socioeconomic factors at the city level. The weekly average temperature had statistically significantly affected most of the tested agents, however with different directions of effects between viruses and bacteria. The weekly incidence rate ratios (IRRs) for viruses ranged from 1.08 (95% CI: 1.00‒1.17) to 1.74 (95% CI: 1.59‒1.92), indicating a positive association between temperature and viral activity, while IRRs for S. pneumoniae was 0.86 (95% CI: 0.76‒0.98), indicating a negative association between temperature and bacterial activity (Supplementary Table 6). Relative humidity also displayed a bidirectional effect, with the negative association shown for MuV, HSV and N. meningitidis, while the positive association for S. aureus. The weekly precipitation affected JEV and S. aureus statistically significantly, but with different directions of effects. Longer sunshine hours were additionally related to the increased circulation of JEV and S. pneumoniae. GDP per capita was statistically significant for viruses and bacteria, with the IRRs ranging from 0.89 (95% CI: 0.79‒0.99) to 1.21 (95% CI: 1.04‒1.41). The population density was statistically significant for only few tested viruses.
Discussion
The longitudinal surveillance described herein spanned a decade and has provided the first comprehensive understanding of etiological and epidemiological features of AME across China. Age was the most important factor that can impact on the pathogen spectrum. Viral pathogens were the main cause among pediatric patients with AME (usually EV or HSV). This was consistent with previous findings in Chinese children with AME from 2009‒2012.25 Vaccines against mumps and JEV had been included in the expanded program on immunization (EPI) in China since the year of 2007, with the first vaccination given at eight months (Supplementary Table 7). The vaccines were related to the obvious decline of risk of infection among young children. For JEV, a further obvious increase of positive detection was observed after 25 years, indicating increased burden of disease in older adults with potential greater exposure, and also likely related to the decay of vaccine elicited immunity as children age because of the first introduction of JEV vaccine in China in the 1960s. The peak in activity of EV, MuV and HSV was observed among young children, commensurate with likely increases in transmission due to close contacts among school aged populations.26,27
We found the main pathogens of bacterial meningitis or encephalitis in China were S. pneumoniae, S. aureus and N. meningitidis, with each of them responsible for the bacterial meningitis or encephalitis in different age groups. In the studies from other countries, such as Spain and France, the reduction of pneumococcal meningitis was likely to be achieved through immunization with 13-valent pneumococcal conjugated vaccine (PCV 13) in children less than 18 years.28,29 In China, PCV 13 in children were not included in the EPI, leaving S. pneumoniae as the leading cause of pediatric bacterial meningitis or encephalitis. It would clearly be advantageous to supplement PCV 13 vaccines into the EPI schedule to reduce the burden of S. pneumoniae related meningitis or encephalitis in China. Similarly, decrease in N. meningitidis infections has been observed after introduction of the meningitis b vaccine.30
By analyzing aggregated nationwide data over a decade, in combination with meteorological and socio-economic factors, we identified a consistent temperature effect that positively affected viral and negatively affected bacterial activity. Longer sunshine hours and lower relative humidity also enhanced viral activity. These findings were also reflected and corroborated by the seasonal pattern of the tested pathogen in the current study, showing the peak of viral detection in summer and bacterial detection in winter. Further research to narrow the geographic scope or pathogen transmission,31, 32, 33, 34, 35 may assist the specific diagnosis and prevention of the most frequently identified infectious pathogens, and targeting of vaccination.
Annually, the activity of most tested viruses decreased throughout the study period, with exception of JEV, which showed elevated activity in 2017‒2018. The year to year increase reflected the increased trend of the disease that might be attributed to the expansion of the competent vectors as a result of warming climate in recent years.36 According to the current study, other factors involving population density and GDP also affected the viral and bacterial circulation in a significant manner, supporting the hypothesis that crowded conditions and lower living standards also favored infection.37
The joinpoint analysis showed the age threshold that clearly defined the descending turnpoint at 6‒7 years old for JEV and MuV, and after 25 years old for JEV increasing. Therefore, immunization strategies should be accordingly adjusted for JEV and MuV. A booster immunization is therefore advocated according to the age turnpoint that mark these highly vulnerable populations. For example, booster immunity JEV vaccines at around 25 years, might be recommended, in addition to the first shot at 6 years (Supplementary Table 7). PCR-based results were of value in providing pathogen-specific and serotype/group data for the timely prevention and treatment of AME disease. These data also help to warn against the antimicrobial resistance for some bacteria causing AME, which might be a major source of nosocomial infection.
Our study was subject to several limitations. First, we failed to identify any of the candidate etiology in more than 70% of cases. This limitation is common to surveillance studies with similar study design.18,38 Only pre-specified pathogens were tested, thus those negative patients might be infected with organisms not included in our diagnostic algorithms. Previous studies indicated that about 11.8%, 7.2%, 18.9%, and 0.1% of AEM patients were caused by Listeria monocytogenes, group B streptococcus, Cryptococcus neoformans, varicella zoster virus respectively in China, which might account for the undetermined organisms in the current AME patients.39, 40, 41, 42 In addition, autoimmune meningitis or encephalitis are increasingly recognized as accounting for large amounts of AME illness of non-infectious cause,43,44 which was not taken into account in our study. Second, only part of the recruited patients has been tested for all four viruses and seven bacteria tested, which may lead to the selection bias in the analysis of clinical differential among viral and bacterial infections. Third, some of the current positive detection was obtained from a non-sterile site, which confers less diagnostic weight than detection in CSF. This is especially important as the pathogenesis role of the organism causing AME, cannot be determined merely from positive detection, especially for those from samples of unsterile sites (i.e., stool samples), which are less likely to indicate an invasive infection. Identification of organisms outside CNS indeed occurs with high frequency, however, particularly for viruses that cause acute encephalitis through an immune-mediated pathogenesis.45 Finally, the clinical definition of meningitis and encephalitis were used in combination for the recruit of patients, which made the separate analysis for their pathogen spectrum unlikely to be performed.
Despite of these concerns, the determination of the pathogen spectrum, as well as their specific pattern in terms of age and gender might assist in identifying predominant pathogen candidates for applying clinical diagnosis, prevention control, and even vaccination strategy. Long-lasting surveillance is advocated not only for the purpose of establishing a baseline for AME related pathogens in China, but also for the sake of evaluation of public health measures, for example, the nonpharmaceutical interventions (NPI) used to combat the COVID-19 in the mainland of China may have broad impact on the occurrence of meningitis and encephalitis, which warrant to be investigated in the future.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
Acknowledgments
The authors would like to thank all the subjects, their families, and collaborating clinicians for the participation. We thank staff members of the Department of Science and Education of the National Health Commission, and the AME surveillance network laboratories and sentinel hospitals in the 29 participating provinces of China for assistance with administration, field investigation, and data collection. This work was supported by China Mega-Project on Infectious Disease Prevention [No. 2018ZX10713001, 2018ZX10713002, 2018ZX10201001, 2018ZX10101003 and 2017ZX10103004] and the National Natural Science Funds [No. 81825019 and 91846302]
Contributors
GFG, WZY, JGW, SIH, LQF, WL and ZJL conceived, designed and supervised the study. WZY, JL, MFL, SWZ, ZGY, ZHY, XC, YC, ZSY, LM, XHW, YLL, JGW, LYH, HQJ, XW, ALC, SJL and WBX formulated the protocols, guideline and SOP of the active sentinel pathogenic surveillance. YY, QBL, SXZ, HYZ, XAZ, MYL, LSS, XR, YFW, SHL, CHZ, MJG and YLZ collected, cleaned, and analyzed the data. LQF, WL, LPW, QBL and YY wrote the drafts of the manuscript. LQF, WL, ZJL, LPW, YY, QBL and YLL interpreted the findings. GFG, WZY, JGW, SIH, LQF, WL, ZJL, MFL, ZHY and YC commented on and revised drafts of the manuscript. All authors read and approved the final report.
Availability of data and materials
The raw data analyzed during the current study is available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2021.100361.
Contributor Information
Zhong-Jie Li, Email: lizj@chinacdc.cn.
Wei Liu, Email: liuwei@bmi.ac.cn.
Li-Qun Fang, Email: fang_lq@163.com.
Appendix. Supplementary materials
References
- 1.Chaudhuri A, Kennedy PGE. Diagnosis and treatment of viral encephalitis. Postgrad Med J. 2002;78:575–583. doi: 10.1136/pmj.78.924.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher A, Herrmann JL, Morand P, et al. Epidemiology of infectious encephalitis causes in 2016. Med Mal Infect. 2017;47:221–235. doi: 10.1016/j.medmal.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Zunt JR, Kassebaum NJ, Blake N, et al. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:1061–1082. doi: 10.1016/S1474-4422(18)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatesan A, Michael BD, Probasco JC, Geocadin RG, Solomon T. Acute encephalitis in immunocompetent adults. Lancet. 2019;393:702–716. doi: 10.1016/S0140-6736(18)32526-1. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RT. Acute encephalitis. Clin Infect Dis. 1996;23:216–219. doi: 10.1093/clinids/23.2.219. [DOI] [PubMed] [Google Scholar]
- 6.McGill F, Griffiths MJ, Solomon T. Viral meningitis: Current issues in diagnosis and treatment. Curr Opin Infect Dis. 2017;30:248–256. doi: 10.1097/QCO.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 7.Hasbun R, Rosenthal N, Balada-Llasat JM, et al. Epidemiology of Meningitis and Encephalitis in the United States, 2011-2014. Clin Infect Dis. 2017;65:359–363. doi: 10.1093/cid/cix319. [DOI] [PubMed] [Google Scholar]
- 8.Takhar SS, Ting SA, Camargo CA. Pallin DJ. U.S. emergency department visits for meningitis, 1993-2008. Acad Emerg Med. 2012;19:632–639. doi: 10.1111/j.1553-2712.2012.01377.x. [DOI] [PubMed] [Google Scholar]
- 9.Kadambari S, Okike I, Ribeiro S, et al. Seven-fold increase in viral meningo-encephalitis reports in England and Wales during 2004-2013. J Infect. 2014;69:326–332. doi: 10.1016/j.jinf.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Yin Z, Shao Z, et al. Population-based surveillance for bacterial meningitis in China, September 2006-December 2009. Emerg Infect Dis. 2014;20:61–69. doi: 10.3201/eid2001.120375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Z, Wang H, Li Y, et al. Molecular epidemiology of human enterovirus associated with aseptic meningitis in Shandong Province, China, 2006-2012. PLoS One. 2014;9:e89766. doi: 10.1371/journal.pone.0089766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zhang W, Ding F, et al. Deaths associated with Japanese encephalitis, China, 2005-2010. Clin Infect Dis. 2013;56:752. doi: 10.1093/cid/cis1012. [DOI] [PubMed] [Google Scholar]
- 13.Yin Z, Wang X, Li L, et al. Neurological sequelae of hospitalized Japanese encephalitis cases in Gansu province, China. Am J Trop Med Hyg. 2015;92:1125–1129. doi: 10.4269/ajtmh.14-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Zhou M, Wang B, Guo J, Chen N, He L. Clinical characteristics and outcome of clinically diagnosed viral encephalitis in southwest China. Neurol Sci. 2015;36:2191–2197. doi: 10.1007/s10072-015-2333-8. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Hu L, Huang H, et al. Etiology and Clinical Features of Full-Term Neonatal Bacterial Meningitis: A Multicenter Retrospective Cohort Study. Front Pediatr. 2019;7:31. doi: 10.3389/fped.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Li X, Li M, et al. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951-2011. PLoS Negl Trop Dis. 2014;8:e3015. doi: 10.1371/journal.pntd.0003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Li Y, Shao Z, et al. Prevalence of meningococcal meningitis in China from 2005 to 2010. Vaccine. 2015;33:1092–1097. doi: 10.1016/j.vaccine.2014.10.072. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, Tan Y, Chongsuvivatwong V, et al. A Population-Based Acute Meningitis and Encephalitis Syndromes Surveillance in Guangxi, China, May 2007- June 2012. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Feng W ya, wei Lin A, et al. Clinical characteristics and etiology of bacterial meningitis in Chinese children >28 days of age, January 2014–December 2016: A multicenter retrospective study. Int J Infect Dis. 2018;74:47–53. doi: 10.1016/j.ijid.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Okike IO, Ribeiro S, Ramsay ME, Heath PT, Sharland M, Ladhani SN. Trends in bacterial, mycobacterial, and fungal meningitis in England and Wales 2004-11: an observational study. Lancet Infect Dis. 2014;14:301–307. doi: 10.1016/S1473-3099(13)70332-3. [DOI] [PubMed] [Google Scholar]
- 21.Ceyhan M, Ozsurekci Y, Gürler N, et al. Bacterial agents causing meningitis during 2013-2014 in Turkey: A multi-center hospital-based prospective surveillance study. Hum Vaccin Immunother. 2016;12:2940–2945. doi: 10.1080/21645515.2016.1209278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Wang X. 1 ed. Sun Yat-sen University Press; 2016. Pathogen surveillance and detection techniques: encephalitis and meningitis syndrome. [Google Scholar]
- 23.Musicha P, Cornick JE, Bar-Zeev N, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis. 2017;17:1042–1052. doi: 10.1016/S1473-3099(17)30394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Ai J, Xie Z, Liu G, et al. Etiology and prognosis of acute viral encephalitis and meningitis in Chinese children: a multicentre prospective study. BMC Infect Dis. 2017;17:494. doi: 10.1186/s12879-017-2572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall HS, Plotkin S. The changing epidemiology of mumps in a high vaccination era. Lancet Infect Dis. 2019;19:118–119. doi: 10.1016/S1473-3099(18)30541-3. [DOI] [PubMed] [Google Scholar]
- 27.Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. bioRxiv. 2017:1–11. doi: 10.1126/scitranslmed.aao5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Contreras J, Picazo J, Casado-Flores J, et al. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in children. Vaccine. 2017;35:4646–4651. doi: 10.1016/j.vaccine.2017.06.070. [DOI] [PubMed] [Google Scholar]
- 29.Cohen R, Varon E, Béchet S, Bonacorsi S, Levy C. Comparative impact of pneumococcal conjugate vaccines on pneumococcal meningitis according to underlying conditions. Vaccine. 2016;34:4850–4856. doi: 10.1016/j.vaccine.2016.08.069. [DOI] [PubMed] [Google Scholar]
- 30.Chung GS, Hutton DW. Epidemiological impact and cost-effectiveness of universal meningitis b vaccination among college students prior to college entry. PLoS One. 2020;15:1–13. doi: 10.1371/journal.pone.0239926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsay AP, Hope V, Marshall RJ, Salinger J. Meningococcal disease and meteorological conditions in Auckland, New Zealand. Aust N Z J Public Health. 2002;26:212–218. doi: 10.1111/j.1467-842x.2002.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 32.Dowell SF, Whitney CG, Wright C, Rose CE, Schuchatt A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwood B. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–353. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 34.Kumar Pant D, Tenzin T, Chand R, Kumar Sharma B, Raj Bist P. Spatio-temporal epidemiology of Japanese encephalitis in Nepal, 2007-2015. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paireau J, Chen A, Broutin H, Grenfell B, Basta NE. Seasonal dynamics of bacterial meningitis: a time-series analysis. Lancet Glob Heal. 2016;4:e370–e377. doi: 10.1016/S2214-109X(16)30064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis poverty. 2019;8:51. doi: 10.1186/s40249-019-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omoleke SA, Alabi O, Shuaib F, et al. Environmental, economic and socio-cultural risk factors of recurrent seasonal epidemics of cerebrospinal meningitis in Kebbi state, northwestern Nigeria: a qualitative approach. BMC Public Health. 2018;18:1318. doi: 10.1186/s12889-018-6196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L-P, Zhou S, Wang X, et al. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12:2464. doi: 10.1038/s41467-021-22551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang A, Xy G, Meng X, Zhang Q, Li G. Epidemiological analysis of 127 of bacterial encephalitis and meningitis syndrome. Henan J Prev Med. 2020:31. [Google Scholar]
- 40.Jiang H, Su M, Kui L, et al. Prevalence and antibiotic resistance profiles of cerebrospinal fluid pathogens in children with acute bacterial meningitis in Yunnan province, China, 2012-2015. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Zhang Z, Sun Y, Guo J, Zhen S, Wang Y. Epidemic Analysis of 266 Cases With Bacterial Meningitis or Encephalitis. J Prev Med Inf. 2012;28:334–336. [Google Scholar]
- 42.Wu J, Liu X, Li C, Zhang X, Song K, Zhao X. Pathogen spectrum of acute meningitis and encephalitis syndrome cases in Jinan city during 2012-2016 [in Chinese] Chinese J Vaccines Immun. 2019;25:45–48. [Google Scholar]
- 43.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the california encephalitis project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83:166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granerod J, Cunningham R, Zuckerman M, et al. Causality in acute encephalitis: Defining aetiologies. Epidemiol Infect. 2010;138:783–800. doi: 10.1017/S0950268810000725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data analyzed during the current study is available from the corresponding author on reasonable request.