Abstract
Muscle fiber characteristics had beneficial effects on meat masses and meat quality in broilers. Its number is determined at birth and directly affects the growth and development of muscle fibers after birth. However, whether the muscle fiber characteristics in different types of chickens are different at birth has not been fully documented. In this study, the 1-day-old Xueshan chicken (slow-growing broiler) and Ross 308 broiler (fast-growing broiler) were selected, respectively, and the fiber type distribution, fiber density, and fiber size in the breast (pectoralis major, PM) and leg (gastrocnemius, GAS) muscles were detected. The results showed that the PM only made up of type IIB fibers regardless of breed, and that few type I fibers (approximately 17.55%) was identified in GAS of Ross 308 broiler. The PM muscles had significantly higher fiber density, lower cross-sectional area and diameter than those of GAS muscles in both 2 breeds (P < 0.05). The highest fiber density was observed in PM of Xueshan chicken. Furthermore, the muscle fiber characteristics were partly controlled by glycolytic potential (GP), and the GP was also invesgated. The GP in PM and GAS of Ross 308 broiler were higher than in Xueshan chicken (P < 0.05). Taken together, 1-day-old Xueshan chicken exhibited higher fiber density and lower GP compared to 1-day-old Ross 308 broiler, especially in PM, which could not only reveal the differences of muscle characteristics among different types of chickens, but also explore a new way to improve the masses and quality of poultry meat.
Key words: slow-growing broiler, fast-growing broiler, muscle fiber characteristic, glycolytic potential
INTRODUCTION
Chicken, a key source of protein in the human diet, is the most widely consumed poultry meat in the world. Over the past few decades, the selection based on growth rate, management practices, and improved nutrition have been very successful, and significantly associated with higher breast and leg weights (Remignon et al., 1995; Cui et al., 2012; Chang et al., 2016). However, consumers are now increasingly interested in meat from indigenous and local birds because of unique taste and texture, rich flavor, redder meat, and excellent image (Berri et al., 2001). Numerous studies identified that many aspects of both muscle masses and meat quality are closely related with muscle fiber characteristics that are represented by their total number of fibers, cross-sectional area (CSA) of fibers, and fiber type composition in muscle (Joo et al., 2013; Ismail and Joo, 2017; Weng et al., 2021). In order to increase both muscle masses and meat quality of chicken to support increasing consumers' demand, further studies are required to know exactly what has been change in the muscle, both quantitative (muscle fiber number and size) and qualitative (muscle fiber types and metabolic enzyme activities) characteristics of fast- and slow-growing commercial broilers.
In general, fast-growing birds have more muscle fibers than do slower growing birds (Scheuermann et al., 2004; Petracci et al., 2013). The researchers also found that the fast-growing chickens have larger diameter fibers and a higher proportion of glycolytic fibers (fast twitch, glycolytic; type II fibers) in the muscles than slow-growing breeds (Dransfield and Sosnicki, 1999; Verdiglione and Cassandro, 2013; Du et al., 2017). The number of muscle fibers formed before birth and the size of those fibers after birth determine ultimate muscle masses (Picard et al., 2006; Rehfeldt et al., 2008) and relative composition of fiber types in the different muscles influences predominance of muscle's metabolic properties, and therefore meat quality of slaughter animals (Ryu and Kim, 2005; Choi et al., 2007; Joo et al., 2013). According to the reports, type II fibers were found to have higher glycogen contents, and therefore, higher glycolytic potential (GP), than other fiber types in pork (Monin et al., 1987; Choi et al., 2007). Many previous studies have shown the growth performance, carcass characteristics, and meat quality of fast- and slow-growing commercial broilers (Choo et al., 2014; Zhao et al., 2019; Biesek et al., 2020). However, there are only a few studies conducted on the comparison of muscle fiber characteristics and GP of breast and thigh muscles from fast- and slow-growing commercial broilers (Zhao et al., 2012; Verdiglione and Cassandro, 2013; Shu et al., 2017; Mazzoni et al., 2020; Weng et al., 2022).
The Xueshan chicken is a slow-growing chicken that represents an important meat-type yellow-feathered breed in China (Wang et al., 2021). In contrast, Ross 308 broilers, a commonly used commercial breed have been selected for rapid growth, high feed efficiency and meat yields, which may have had consequences on quality traits. Our previous study has been investigated the fiber characteristics and meat quality of different muscular tissues from Xueshan chicken and Ross 308 broilers at market age (Weng et al., 2022). Nonetheless, there is evidence suggesting that the differences in muscle fiber characteristics among beef cattle breeds and between beef and dairy cattle are obvious already at birth (Wegner et al., 2000). Hence, we further compared fiber type distribution, fiber density, and fiber size and GP of breast (pectoralis major, PM) and leg (gastrocnemius, GAS) muscles from 1-day-old Xueshan chicken and Ross 308 broiler to explore whether the muscle fiber characteristics and GP were consistent at birth. These results might provide some evidence for further exploring whether muscle fiber and/or differences observed could be exploited to manage the muscle masses and meat quality in the production of chicken.
MATERIALS AND METHODS
Ethics Statement
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the School of Animal Science and Technology, Yangzhou University (Permit Number: YZUDWSY, Government of Jiangsu Province, China).
Birds, Slaughter Procedures, and Sample Collection
A total of 40emale slow-growing local (Xueshan chicken at 1 day of age with average weight 37.9 g, n = 20) and commercial (Ross 308 broiler at 1 day of age with average weight 43.3 g one day-old, n = 20) chickens were obtained from LiHua Farming Co., Jiangsu, China. Individuals within each breed had the same genetic background. Chickens were not transported, anesthetized with sodium pentobarbital, and killed by neck cut. After killing, carcasses were cooled in a chilling room (4°C), and the muscles (PM and GAS) were dissected from the carcasses. For histological and immunohistochemistry analysis, 3 birds from each breed were randomly selected. All specimens were taken from the central portion of the widest part of the GAS muscle, and in the ventral surface of the PM muscle (facing the skin), excluding the most superficial 3 mm. These samples were cut into 3 pieces (0.5 × 0.5 × 1.0 cm in size), and stored at 4% paraformaldehyde. The rest muscles from the carcasses were excised, frozen in liquid nitrogen and stored at –80°C. For mRNA expression and GP analysis, the PM and GAS muscle samples from the total of 18 birds in each breed were chosen. The muscle samples were divided into 6 pools (∼0.1−0.2 g per pool) containing the muscle from 3 birds per pool. Each of the 6 pools was measured separately.
Histological and Immunohistochemistry Analysis
Muscle samples were fixed in 4% paraformaldehyde for 24 h at room temperature, embedded into paraffin wax, and sectioned to 5 µm. The slides were for stained with hematoxylin and eosin according to routine protocols. Briefly, the first step in hematoxylin and eosin staining was deparaffinization with xylene. After thorough de-waxing, the slides were passed through several changes of alcohol to remove the xylene, and thoroughly rinsed in water. Then, the slides were stained with hematoxylin for 3 to 5 min. Unbound hematoxylin is removed with water rinses followed by an optional differentiation step using acid alcohol. Another water rinse follows to remove the alkaline bluing solution from the slide before staining in Eosin solution for 30 s to 1 min. Following the eosin stain, the slides were passed through several changes of alcohol to remove all traces of water, and rinsed in several baths of xylene solution for 30 s to 1 min each. A thin layer of polystyrene mountant was applied, followed by a glass coverslip. Representative areas were photographed under a Nikon 90i microscope (Nikon, Tokyo, Japan) at a magnification of 800 ×. For immunohistochemistry staining, 2 mouse monoclonal whose specificities for fast myosin skeletal heavy chain MYH1A (1:1,000, #ab51263) and slow myosin skeletal heavy chain MYH7B (1:4,000, #ab11083) were purchased from Abcam (Cambridge, UK). The specificity of the antibodies was assessed by Western blot. After this, the immunohistochemistry staining protocol was performed as previously described (Kim et al., 2016) with minor modifications. In brief, 5-µm thick serial sections were cut and then the sections were blocked with 10% normal goat serum (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and incubated with 2 primary antibodies for 60 min at room temperature. Tissue sections were then incubated with 1:5,000 dilution of secondary goat anti mouse-HRP antibody (Abcam) for 30 min at room temperature. The sections were visualized by incubating with Liquid DAB Plus Substrate Kit (Life Technologies, Carlsbad, CA) according to manufacturer's instructions. Images were acquired and analyzed to determine 3 traits of the muscle fibers, using an image analysis system (Image-Pro plus 5.1, Media Cybernetics Inc., Rockville, MD). Approximately 200 fibers without signs of tissue disruption from a random field in each muscle sample section stained were counted. Fiber diameter (μm), CSAs (μm2), density and fiber area percentages were determined as previously described (Huo et al., 2021). In brief, Muscle fiber density (number/mm2) was presented as the fiber number distributed in 1 mm2 of muscle and muscle fiber size (µm2) was defined as the mean CSA of counted fibers. The area percentage of muscle fiber (%) was defined as the ratio between the total CSA of each fiber type and the total measured fiber area.
mRNA Expression Analysis
Total RNAs were isolated from the samples using the TRIZOL reagent procedure (Invitrogen Corp, Carlsbad, CA) with DNase I (Takara Biotechnology Co. Ltd., Dalian, China) to remove DNA. RNA quality and concentration were determined using a spectrophotometer at 260 and 280 nm. RNA with a 260/280 ratio between 1.9 and 2.1 was used in this study. Each RNA extract was assayed in triplicate and an average value was determined. Reverse transcription was performed using a PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd.) according to the manufacturer's instructions. Quantitative real-time PCR (qRT-PCR) was carried out using ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Premix Ex Taq Kits (Takara Biotechnology Co. Ltd.). MYH7B, MYH1A, MYH1B and 18SrRNA primer sequences used for qRT-PCR were presented in Table 1 and synthesized by Nanjing Qingke bioengineering company. The reaction volume was 20 μL, consisted of 1 μL of each primer (0.5 μL upper primer and 0.5 μL lower primer, 10 nM), 10 μL SYBR Green I Master, 5.0 μL cDNA synthetic products and 4.0 μL doubled-distilled water. The qRT-PCR program consisted of 5 min for 95°C, and then 40 cycles of 95°C for 10 s, 60°C for 30 s. All results were normalized to the internal control gene (18SrRNA) levels and relative expression levels of the target mRNAs were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The experiment was repeated in triplicate.
Table 1.
Primers for qRT-PCR of MyHC-related genes.
| Gene | GenBank accession | Primer sequence (5′→3′) | Length (bp) | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|---|
| MYH7B | NM_204587.2 | F: ACGGGCCTGATCAACCAAAA | 20 | 114 | 60 |
| R: GGCCTTCTTGGCTTTCTCCT | 20 | ||||
| MYH1A | NM_001013396.1 | F: GGTCAACAAGCTCCGAGTGA | 20 | 92 | 60 |
| R: CAGGCCACTTTACTGCCTCA | 20 | ||||
| MYH1B | NM_204228.3 | F: GGGAGACCTGAATGAAATGGAG | 22 | 140 | 60 |
| R: CTTCCTGTGACCTGAGAGCATC | 22 | ||||
| 18SrRNA | AF173612.1 | F: GCGGCTTTGGTGACTCTA | 18 | 194 | 60 |
| R: CTGCCTTCCTTGGATGTG | 18 |
Abbreviations: MyHC, myosin heavy-chain. MYH7B, (type I) slow-twitch myosin heavy-chain; MYH1A, (type IIB) fast-twitch myosin heavy-chain; MYH1B, (type IIA) fast-twitch myosin heavy-chain.
Determination of Glycogen, Glucose, Glucose 6 Phosphate and Lactic Acid Content, and GP
The glycogen, glucose, glucose 6 phosphate, and lactic acid content were measured by spectrophotometry using standard commercial kits from Beijing Solarbio Science & Technology Co., China (Glucogen Content Assay Kit, BC0345; Glucose Content Assay Kit, BC2505; Lactic Acid Content Assay Kit, BC2230). The glucose 6 phosphate content was tested using Glucose 6 Phosphate Assay Kit (Colorimetric, ab83426), which was provided by Abcam (Cambridge). The glycogen, glucose-6-phosphate, glucose, and lactic acid concentrations were measured according to according to the manufacturer's manual from muscle tissue (∼0.4 g). Every sample was detected at least 3 times by utilizing a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA). Compound contents were expressed as µmol/g wet weight and GP values were calculated according to the following formula proposed by (Monin and Sellier, 1985): GP = 2*(Glycogen + Glucose + Glucose 6 phosphate) + Lactic acid.
Statistical Analysis
All experimental data were evaluated as means ± standard error. All of the data were analyzed through the two-way ANOVA using SPSS statistical software (version 18.0, SPSS, Chicago IL). The main effects of breeds and muscular tissues, as well as their interactions, were assessed for all variables. Mean values were separated by applying the parametric Tukey-honestly significant difference (HSD) test. P < 0.05 was considered to be a statistically significant difference.
RESULTS
Comparison of Myosin Heavy Chain-Based Fiber Characteristics
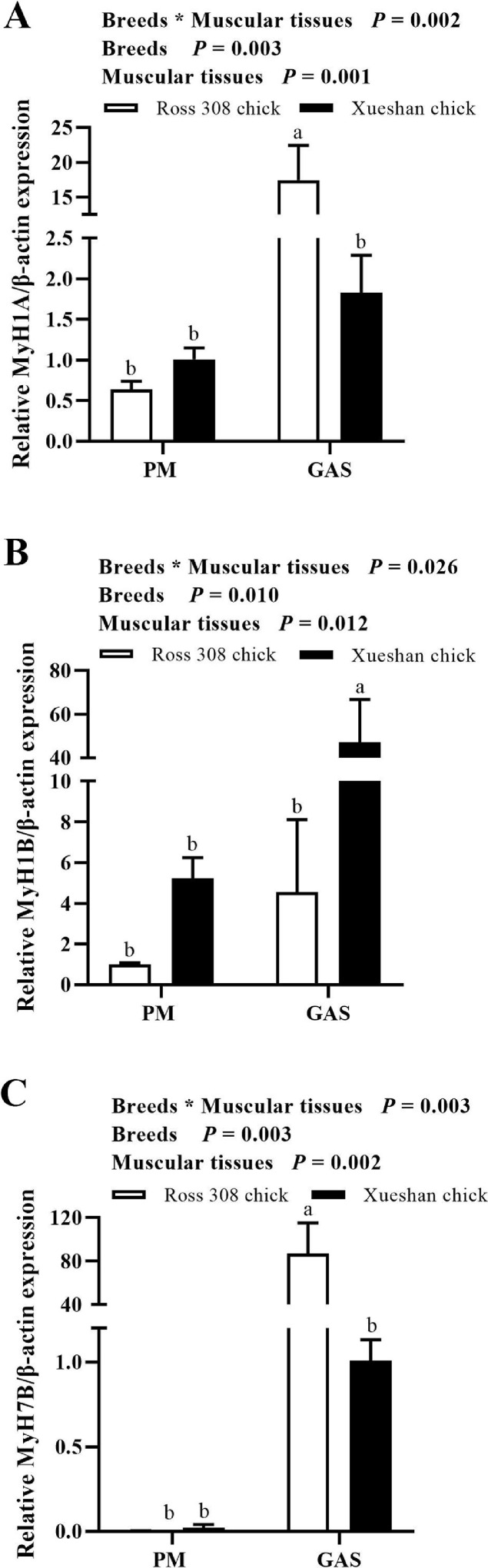
To investigate the myosin heavy chain (MyHC) fiber-type composition of Ross 308 and Xueshan chicken breeds at breast and leg muscles, relative expression of MyHC isoform types I (MyH7B), IIa (MyH1B), and IIb (MyH1A) were detected by qRT-PCR. There was a significant interaction between the breeds and muscular tissues for the relative expression of MyH1A (Figure 1A, P < 0.01), MyH1B (Figure 1B, P < 0.05), and MyH7B (Figure 1C, P < 0.01). When MyHC isoforms were compared, we found that the expression level of MyH1A and MyH7B were significantly higher in GAS of Ross 308 broiler (P < 0.05), whereas the expression levels of MyH1B were significantly lower (P < 0.05). No significant difference in the expression level of MyH1A between PM in both Ross 308 broiler and Xueshan chicken (P > 0.05, Figure 1A). In addition, Ross 308 broiler exhibited lower expression level of MyH1B compared to Xueshan chicken (P < 0.05), except PM (P > 0.05, Figure 1B). Interestingly, Ross 308 broiler exhibited higher expression levels of MyH7B in GAS compared to Xueshan chicken (P < 0.05, Figure 1C).
Figure 1.
Relative mRNA expression of myosin heavy chain (MyHC) isoform genes in the m. pectoralis major (PM) and m.gastrocnemius (GAS) from one-day-old Ross 308 and Xueshan chicken. The given values are reported as means ± standard error (n = 6). Statistically significant differences are indicated by different letters (P < 0.05).
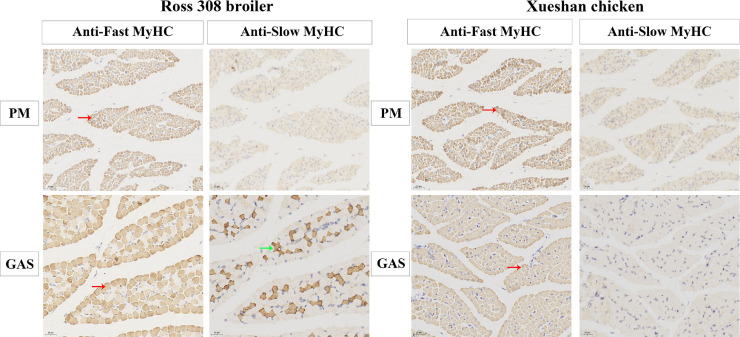
To confirm the distribution of MHCs at the protein level, transverse sections of muscles stained with 2 monoclonal antibodies against specific MyHC isoforms was detected. As shown in Figure 2, two MHC-based fiber types were classified in muscles by immunohistochemistry and two monoclonal antibodies. Anti-fast MyHC (MYH1A), which corresponds to type IIB, was found in the four skeletal muscles from Ross 308 broiler and Xueshan chicken. Anti-slow MyHC (MYH7B), which corresponds to type I, did not stain PM muscles from Ross 308 broiler and Xueshan chicken. In addition, the 2 fiber types (type I and type IIB) were observed in GAS of Ross 308 broiler, whereas pure type (type IIB) was only found in GAS of Xueshan chicken. Numerically counted representative characteristics such as fiber diameter and fiber area percentage were presented in Table 2. When comparing fiber diameter within each muscle, fiber diameter of type IIB was higher in GAS of Ross 308 broiler as compared to fiber type I (12.0 μm vs. 11.0 μm). Similarly, fiber area percentage of type IIB fibers was lower in GAS of Ross 308 broiler than those in Xueshan chicken (82.5% vs. 100.0%). Together, the fiber compositions of PM muscles only consisted of type IIB fibers irrespective of the breeds, while little type I fibers could be identified in GAS from Ross 308 broiler.
Figure 2.
Transverse sections of m. pectoralis major (PM), and m. gastrocnemius (GAS) stained with two monoclonal antibodies against specific myosin heavy chain (MyHC) isoforms. Red arrows point to examples of fibers with positive immunostaining for type IIB fibers; Green arrows point to examples of fibers with positive immunostaining for type I fibers. Magnification of 800 × was used (Bar = 20 μm).
Table 2.
Muscle fiber type composition of the m. pectoralis major (PM) and m. gastrocnemius (GAS) in Ross 308 and Xueshan chicken.
| Items | Ross 308 |
Xueshan chicken |
||
|---|---|---|---|---|
| PM | GAS | PM | GAS | |
| Fiber diameter (μm) | ||||
| Type I fiber | n.d. | 11.0 ± 0.2 | n.d. | n.d. |
| Type II fiber | 6.6 ± 0.7 | 12.0 ± 0.7 | 6.6 ± 0.5 | 10.6 ± 0.7 |
| Fiber area percentage (%) | ||||
| Type I fiber | n.d. | 17.5 | n.d. | n.d. |
| Type II fiber | 100.0 | 82.5 | 100.0 | 100.0 |
Note: n.d., not detected.
Comparison of GP
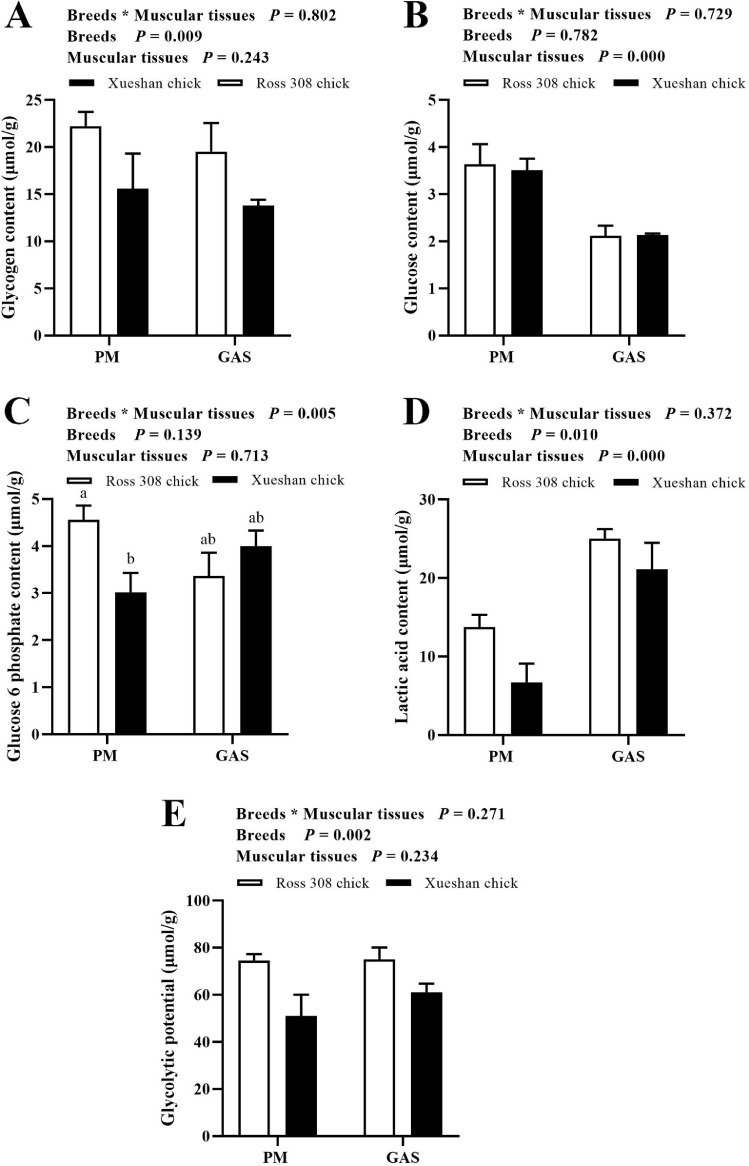
To determine the GP, glycogen, glucose, glucose 6 phosphate, and lactic acid content were measured. As shown in Figure 3 and Table S1, a significant interaction between factors (breeds and muscular tissues) was observed on the contents of glucose 6 phosphate (P < 0.01). The highest glucose 6 phosphate content was detected in PM of Ross 308 broiler (P < 0.05), while the lowest glucose 6 phosphate content could be identified in PM of Xueshan chicken (P < 0.05). Some significant main effects (P < 0.05) were observed for different breeds or muscular tissues on the contents of glycogen, glucose, lactic acid and GP. The higher content of glucose and lactic acid were found in PM and GAS, respectively (P < 0.05). The higher content of glycogen was found in Ross 308 broiler (P < 0.05). The GP in PM and GAS of Ross 308 chicks were higher than in Xueshan chicken (P < 0.05).
Figure 3.
Glycolytic potential in m. pectoralis major (PM) and m. gastrocnemius (GAS) from Ross 308 and Xueshan chicken. (A–D) The content of glycogen (A), glucose (B), glucose 6 phosphate (C), and lactic acid (D). (E): Glycolytic potential. a-c Means ± standard error (n = 6) with different superscripts are significantly different (P < 0.05).
Comparison of Muscle Fiber Morphology Traits Among Breeds
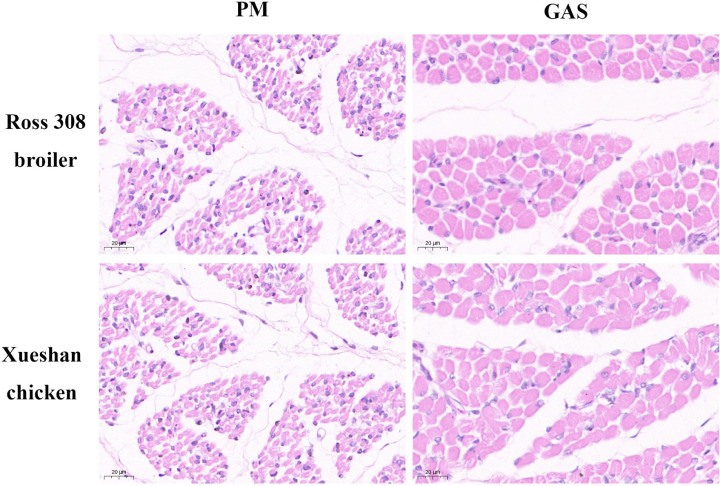
To compare the morphology of muscle fibers in chickens, representative characteristics of 4 muscular tissues were investigated (Figure 4). The results of fiber diameter, CSA, and fiber density of muscles from Ross 308 broiler and Xueshan chicken were presented in Table 3. Significant main effects of breeds or muscular tissues on the fiber diameter, CSA and density were observed (P < 0.05). The higher fiber diameter and CSA were observed in GAS than those of the PM in the Ross 308 broiler and Xueshan chicken (P < 0.05). The fiber density showed an opposite trend from the fiber diameter and CSA. The fiber density was higher in Xueshan chicken than in Ross 308 broiler, without statistical differences (P > 0.05). Fiber density was significantly higher (P < 0.05) in PM than in GAS. The highest fiber density was in PM muscle of the Xueshan chicken (46338.08 fiber number/mm2) and the lowest fiber density was in GAS muscle of the Xueshan chicken (7541.11 fiber number/mm2).
Figure 4.
Hematoxylin and eosin staining in m. pectoralis major (PM) and m. gastrocnemius (GAS) from Ross 308 and Xueshan chicken. Magnification of 800 × was used (Bar = 20 μm).
Table 3.
Muscle fiber morphology traits in m. pectoralis major (PM) and m. gastrocnemius (GAS) from Ross 308 and Xueshan chicks.
| Items | Fiber diameter1 | Fiber cross-sectional area2 | Fiber density3 |
|---|---|---|---|
| Ross 308 broiler | |||
| PM | 6.56 ± 0.15 | 29.44 ± 4.65 | 34.95 ± 6.21 |
| GAS | 11.19 ± 0.64 | 108.59 ± 25.66 | 9.68 ± 1.99 |
| Xueshan chicken | |||
| PM | 6.55 ± 0.49 | 21.72 ± 1.77 | 46.34 ± 3.68 |
| GAS | 10.59 ± 0.65 | 132.86 ± 5.79 | 7.54 ± 0.32 |
| Breeds | |||
| Ross 308 broiler | 8.88 ± 2.42 | 69.01 ± 43.66 | 22.31 ± 13.45 |
| Xueshan chicken | 8.57 ± 2.10 | 77.29 ± 55.73 | 26.94 ± 19.57 |
| Muscular tissues | |||
| PM | 6.56 ± 0.63b | 25.58 ± 5.22b | 40.65 ± 7.65a |
| GAS | 10.89 ± 0.71a | 120.73 ± 22.21a | 8.61 ± 1.78b |
| P-value (Two-Way ANOVA) | |||
| Breeds | 0.187 | 0.407 | 0.034 |
| Muscular tissues | 0.000 | 0.000 | 0.000 |
| Breeds ×* Muscular tissues | 0.197 | 0.130 | 0.119 |
Results are reported as means ± SE (n = 6).
Superscripted letters within a row indicate significant differences (P < 0.05).
Unit of muscle fiber diameter: μm;
Unit of muscle fiber cross-sectional area: μm2.
Definition of muscle fiber density: fiber number/mm2, × 103.
DISCUSSION
Muscle fiber characteristics strongly influence muscle yield and meat quality because skeletal muscles mainly consist of muscle fibers that can be characterized by their morphological traits, contractile, and metabolic properties (Lee et al., 2010). In cattle, the differences in muscle fiber characteristics among beef cattle breeds and between beef and dairy cattle are obvious already at birth (Wegner et al., 2000). As reviewed by Picard et al. (2006) and Luff and Goldspink (1970), contractile and metabolic properties of muscle fibers are subjected to changes, especially shortly after birth, whereas the muscle fiber number in cattle is already fixed at birth. Comparatively, the differences in muscle fiber number, size, and composition according to morphological characteristics of muscle fiber with metabolic properties between slow- and fast-growing broilers at birth have not been clearly established yet. For the first time in fast- and slow-growing commercial broilers, this study compared muscle fiber characteristics and GP of breast and thigh muscles to explore whether the muscle fiber characteristics and GP were consistent at birth.
It has been documented that fiber type composition can profoundly influence postnatal muscle growth and meat quality livestock and fish species (Pette and Staron, 2001; Wu et al., 2015; Kim et al., 2016; Listrat et al., 2016). At birth, muscle is composed of oxidative fibers and the proportion of oxidative fibers decreases while the proportion of glycolytic fibers increases during growth. As reported by Choi et al. (2013), the PM is composed of type IIB fiber, and type I and IIA fibers are not found in the superficial region of the PM from broiler-type chickens harboring a greater muscle masses, which was confirmed by our findings that type I fibers are not found at the protein level in the PM from Xueshan chicken and Ross 308 broiler. Although the percentage of fiber I was higher when Ross 308 broiler compared with the Xueshan chicken in GAS, the proportion of fiber I was low (approximately 17.55%). The findings of the present study seem to suggest that the fiber type composition should only play a minor role in muscle growth and meat quality after hatch in fast- and slow-growing commercial broilers, as previously described by Sams and Janky (1990).
In general, muscles containing higher MyHC I fibers proportion evidenced a higher level of oxidative enzyme activity and a lower level of glycolytic enzyme activity than did muscles containing lower MyHC I fibers proportion (Gil et al., 2003). Here, we investigated the glycolytic enzyme activity of 4 skeletal muscles from Ross 308 broiler and Xueshan chicken. In the present study, PM muscles were found to have higher glycogen contents and higher GP than GAS muscles in fast- and slow-growing commercial broilers. And GAS from Ross 308 broiler had higher MyHC I fibers, which was found to have more glycogen contents, and a higher GP than GAS from Xueshan chicken. As previously described by Zhao et al. (2012) in chickens, higher ratios of type IIb fibers in Arbor Acres broilers (a commercial line) was associated with decreased glycogen reserves of the skeletal muscle and lower GP. These results differed somewhat from our present findings, mainly because of their different genetic backgrounds and age of the experimental animal. There is no direct evidence of an important link between GP and muscle fiber type. In addition, although the mechanism underlying the results remains unclear, it can be inferred that the variation in meat quantity and quality from fast- and slow-growing commercial broilers, might be caused by GP.
Numerous studies identified that many aspects of both muscle masses and meat quality are related with muscle fiber characteristics that are represented by their TNF, CSA, and FTC in muscle (Joo et al., 2013; Ismail and Joo, 2017). It has been proved that the differences in muscle fiber among beef cattle breeds and between beef and dairy cattle are obvious already at birth (Wegner et al., 2000). In the present study, the microscopic evaluations, whatever the muscle fiber diameter or fiber CSA, there was no significant difference between chickens of the 2 breeds at hatch. These observations are in agreement with those of (Remignon et al., 1995), who compared muscles selected from a fast growing line and a slow growing line. As previously shown, the total number of fibers remained unchanged after birth in the pig (Wigmore and Stickland, 1983). Our results also showed that PM muscle had significantly lower CSA and diameter than those of GAS muscle from both breeds. This is in agreement with a recent study carried out on goose skeletal muscles, which has reported the muscle fiber CSA of GAS was significantly higher than that did PM (Weng et al., 2021). Thus, the findings of the present study together with earlier surveys seem to support this point. There are various factors affecting CSA of fibers, including muscle location and muscle function within an animal (Ismail and Joo, 2017), which may be the reason for the higher fiber diameter and CSA in GAS compared to the PM. In the current study, the PM muscles had significantly higher fiber density than those of GAS muscles from Ross 308 broiler and Xueshan chicken. This result supports the findings that during postnatal development, when the number of muscle fibers is high, fibers generally grow more slowly; conversely, fibers grow more rapidly when the number of fibers is low in poultry (Remignon et al., 1995; Dransfield and Sosnicki, 1999). Studies have been well documented that higher growth rates are generally related to an increase in the total number and in the CSA of fibers (Picard et al., 2006; Choi et al., 2013). It could be inferred that the differences in fast- and slow-growing broilers may have been due to the differences in the total number of fibers at birth, especially in PM.
CONCLUSIONS
In conclusion, results of this study provided a first characterization of the muscle fiber characteristics and GP of both the Xueshan chicken and Ross 308 broiler. The results confirmed that muscle fiber characteristics and GP were inconsistent in slow- and fast-growing broilers at birth. At birth, Xueshan chicken exhibited higher fiber density and lower GP compared to Ross 308 broiler, especially in PM, which might in-depth affect the muscle masses and meat quality variations between slow- and fast-growing broilers. Our continued researches will be required to ascertain the fiber characteristics and GP of muscle in slow-growing and fast-growing broilers of different ages, and the effect of muscle fibers and GP on muscle masses and meat quality.
Acknowledgments
ACKNOWLEDGMENTS
This work was financially supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-42-3), the earmarked fund for Jiangsu Agricultural Industry Technology System (JATS(2020)6) and the Plant and Animal Breeding Project of Jiangsu province (PZCZ201735)
DISCLOSURES
The authors declare there was no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101649.
Appendix. Supplementary materials
REFERENCES
- Berri C., Wacrenier N., Millet N., Le Bihan-Duval E. Effect of selection for improved body composition on muscle and meat characteristics of broilers from experimental and commercial lines. Poult. Sci. 2001;80:833–838. doi: 10.1093/ps/80.7.833. [DOI] [PubMed] [Google Scholar]
- Biesek J., Kuźniacka J., Banaszak M., Kaczmarek S., Adamski M., Rutkowski A., Zmudzińska A., Perz K., Hejdysz M. Growth performance and Carcass quality in broiler chickens fed on legume seeds and rapeseed meal. Animals (Basel) 2020;10:846. doi: 10.3390/ani10050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Halley J., Silva M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016;56:1254–1262. [Google Scholar]
- Choi Y.M., Ryu Y.C., Kim B.C. Influence of myosin heavy- and light chain isoforms on early postmortem glycolytic rate and pork quality. Meat Sci. 2007;76:281–288. doi: 10.1016/j.meatsci.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Shin S., Wick M.P., Choe J.H., Lee K. Muscle fiber characteristics of pectoralis major muscle as related to muscle mass in different Japanese quail lines. Animal. 2013;7:1665–1670. doi: 10.1017/S1751731113001298. [DOI] [PubMed] [Google Scholar]
- Choo Y.K., Oh S.T., Lee K.W., Kang C.W., Kim H.W., Kim C.J., Kim C.J., Kim E.J., Kim H.S., An B.K. The growth performance, carcass characteristics, and meat quality of egg-type male growing chicken and white-mini broiler in comparison with commercial broiler (Ross 308) Korean J. Food Sci. Anim. Resour. 2014;34:622–629. doi: 10.5851/kosfa.2014.34.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H.X., Liu R.R., Zhao G.P., Zheng M.Q., Chen J.L., Wen J. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics. 2012;13:213. doi: 10.1186/1471-2164-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Du Y.F., Ding Q.L., Li Y.M., Fang W.R. Identification of differentially expressed genes and pathways for myofiber characteristics in soleus muscles between chicken breeds differing in meat quality. Anim. Biotechnol. 2017;28:83–93. doi: 10.1080/10495398.2016.1206555. [DOI] [PubMed] [Google Scholar]
- Gil M., Oliver M., Gispert M., Diestre A., Sosnicki A.A., Lacoste A., Carrión D. The relationship between pig genetics, myosin heavy chain I, biochemical traits and quality of M. longissimus thoracis. Meat Sci. 2003;65:1063–1070. doi: 10.1016/S0309-1740(02)00324-8. [DOI] [PubMed] [Google Scholar]
- Huo W., Weng K., Gu T., Zhang Y., Zhang Y., Chen G., Xu Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I., Joo S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Kim G.D., Yang H.S., Jeong J.Y. Comparison of characteristics of myosin heavy chain-based fiber and meat quality among four bovine skeletal muscles. Korean J. Food Sci. Anim. Resour. 2016;36:819–828. doi: 10.5851/kosfa.2016.36.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Joo S.T., Ryu Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Picard B., Bugeon J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016;2016 doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luff R.A., Goldspink G. Total number of fibers in muscles of several strains of mice. J. Anim. Sci. 1970;30:891–893. doi: 10.2527/jas1970.306891x. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., Soglia F., Petracci M., Sirri F., Lattanzio G., Clavenzani P. Fiber metabolism, procollagen and collagen Type III immunoreactivity in broiler pectoralis major affected by muscle abnormalities. Animals (Basel) 2020;10:1081. doi: 10.3390/ani10061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin G., Mejenes-Quijano A., Talmant A., Sellier P. Influence of breed and muscle metabolic type on muscle glycolytic potential and meat pH in pigs. Meat. Sci. 1987;20:149–158. doi: 10.1016/0309-1740(87)90034-9. [DOI] [PubMed] [Google Scholar]
- Monin G., Sellier P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: the case of the Hampshire breed. Meat Sci. 1985;13:49–63. doi: 10.1016/S0309-1740(85)80004-8. [DOI] [PubMed] [Google Scholar]
- Petracci M., Sirri F., Mazzoni M., Meluzzi A. Comparison of breast muscle traits and meat quality characteristics in 2 commercial chicken hybrids. Poult Sci. 2013;92:2438–2447. doi: 10.3382/ps.2013-03087. [DOI] [PubMed] [Google Scholar]
- Pette D., Staron R.S. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- Picard B., Jurie C., Duris M.P., Renand G. Consequences of selection for higher growth rate on muscle fibre development in cattle. Livest. Sci. 2006;102:107–120. [Google Scholar]
- Rehfeldt C., Tuchscherer A., Hartung M., Kuhn G. A second look at the influence of birth weight on carcass and meat quality in pigs. Meat. Sci. 2008;78:170–175. doi: 10.1016/j.meatsci.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Remignon H., Gardahaut M.F., Marche G., Ricard F.H. Selection for rapid growth increases the number and the size of muscle fibres without changing their typing in chickens. J. Muscle Res. Cell Motil. 1995;16:95–102. doi: 10.1007/BF00122527. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Sams A.R., Janky D.M. Research note: Simultaneous histochemical determination of three fiber types in single sections of broiler skeletal muscles. Poult. Sci. 1990;69:1433–1436. doi: 10.3382/ps.0691433. [DOI] [PubMed] [Google Scholar]
- Scheuermann G.N., Bilgili S.F., Tuzun S., Mulvaney D.R. Comparison of chicken genotypes: myofiber number in pectoralis muscle and myostatin ontogeny. Poult. Sci. 2004;83:1404–1412. doi: 10.1093/ps/83.8.1404. [DOI] [PubMed] [Google Scholar]
- Shu J.T., Xiao Q., Shan Y.J., Zhang M., Tu Y.J., Ji G.G., Sheng Z.W., Zou J.M. Oxidative and glycolytic skeletal muscles show marked differences in gene expression profile in Chinese Qingyuan partridge chickens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdiglione R., Cassandro M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013;92:2433–2437. doi: 10.3382/ps.2013-03013. [DOI] [PubMed] [Google Scholar]
- Wang L., Kong L., Hu X., Bai H., Wang Z., Jiang Y., Bi Y., Chang G., Chen G. Effect of stocking density on performance, meat quality and cecal bacterial communities of yellow feather broilers. Anim. Biotechnol. 2021;22:1–11. doi: 10.1080/10495398.2021.1898413. [DOI] [PubMed] [Google Scholar]
- Wegner J., Albrecht E., Fiedler I., Teuscher F., Papstein H.J., Ender K. Growth- and breed-related changes of muscle fiber characteristics in cattle. J. Anim. Sci. 2000;78:1485–1496. doi: 10.2527/2000.7861485x. [DOI] [PubMed] [Google Scholar]
- Weng K., Huo W., Gu T., Bao Q., Hou L., Zhang Y., Zhang Y., Xu Q., Chen G. Effects of marketable ages on meat quality through fiber characteristics in the goose. Poult. Sci. 2021;100:728–737. doi: 10.1016/j.psj.2020.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng K., Huo W., Li Y., Zhang Y., Zhang Y., Chen G., Xu Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore P.M., Stickland N.C. Muscle development in large and small pig fetuses. J. Anat. 1983;137:235–245. [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zuo J.J., Yu Q.P., Zou S.G., Tan H.Z., Xiao J., Liu Y.H., Feng D.Y. Effect of skeletal muscle fibers on porcine meat quality at different stages of growth. Genet. Mol. Res. 2015;14:7873–7882. doi: 10.4238/2015.July.14.13. [DOI] [PubMed] [Google Scholar]
- Zhao J.P., Zhao G.P., Jiang R.R., Zheng M.Q., Chen J.L., Liu R.R., Wen J. Effects of diet-induced differences in growth rate on metabolic, histological, and meat-quality properties of 2 muscles in male chickens of 2 distinct broiler breeds. Poult. Sci. 2012;91:237–247. doi: 10.3382/ps.2011-01667. [DOI] [PubMed] [Google Scholar]
- Zhao J.S., Deng W., Liu H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019;98:3040–3049. doi: 10.3382/ps/pez081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.