Abstract
Salmonella enterica serovar Pullorum (S. Pullorum) causes pullorum disease (PD), which is an acute systemic disease, in chickens, and leads to serious economic losses in many developing countries because of its high morbidity and mortality rate in young chicks. The live-attenuated vaccine is considered to be an effective measure to control the Salmonella infection. In addition, the DIVA (differentiation of infected and vaccinated animals) feature without the interference of serological monitoring of Salmonella infection is an important consideration in the development of the Salmonella vaccine. In this study, we evaluated the immunogenicity and protective efficacy of a S. Pullorum rough mutant S06004ΔspiCΔrfaH as a live attenuated DIVA vaccine candidate in chickens. The S06004ΔspiCΔrfaH exhibited a significant rough lipopolysaccharides (LPS) phenotype which was agglutinated with the acriflavine, not with the O9 mono antibody. Compared to the wild-type, 50% lethal dose (LD50) of the rough mutant increased 100-fold confirmed its attenuation. The mutant strain also showed a decreased bacterial colonization in the spleen and liver. The immunization with the mutant strain had no effect on the body weight and no tissue lesions were observed in the liver and spleen. The high level of the S. Pullorum-specific IgG titers in the serum indicated that significant humoral immune responses were induced in the immunization group. The cellular immune responses were also elicited from the analysis of lymphocyte proliferation and expression of cytokines in the spleen. In addition, the S06004ΔspiCΔrfaH immunized group exhibited a negative response for the serological test, while the wild-type S06004 infection group was strongly positive for the serological test showing a DIVA capability. The survival rates in the vaccinated chickens were 87% after intramuscular challenge with wild-type S. Pullorum, while the survival rates were 20% in the control groups. Overall, these results have demonstrated that the rough mutant S06004ΔspiCΔrfaH strain can be developed as an efficient live attenuated DIVA vaccine candidate to control the systemic S. Pullorum infection without the interference of salmonellosis monitoring program in poultry.
Key words: Salmonella Pullorum, spiC, rfaH; live attenuated vaccine; DIVA
INTRODUCTION
Salmonella enterica serovar Pullorum (S. Pullorum) is highly adapted to avian species, and is the causative agent of the pullorum disease (PD) (Barrow et al., 2012). PD is an acute systemic disease and can cause high morbidity and mortality in young chicks that are less than 2 to 3 wk old. The adult chickens are often asymptomatic carriers of the bacteria throughout their lives. Additionally, S. Pullorum infection can transmit vertically to chicks through eggs (Barrow and Freitas Neto, 2011; Lu et al., 2020). The PD has been eradicated from commercial poultry in many developed countries, owing to the introduction of pullorum-typhoid programs based on detection and elimination of the affected birds (Barrow and Freitas Neto, 2011). However, the PD remains a big threat to the poultry industry in developing countries and causes serious economic loss every year (Eriksson et al., 2018; Zhou et al., 2020).
Although identification and culling of infected birds were effective measures for the eradication of the PD, the expensiveness of these measures restricts their applications in many developing countries (Penha Filho et al., 2010). In addition, considering the emergence of the multidrug-resistant problem accompanied with antimicrobial therapy strategy, it is an urgent need to search an efficient approach to control the S. Pullorum infection (Pan et al., 2009; Jibril et al., 2021). Vaccination of chickens is an alternative effective and economic strategy to control the Salmonella infections in poultry (Desin et al., 2013). Both humoral and cellular immunity are required for ideal Salmonella vaccines (Mastroeni et al., 2001; Zhao et al., 2021). Attenuated live vaccine can effectively induce strong humoral and cellular immunity (Penha Filho et al., 2010). Vaccination should not interfere with the salmonellosis monitoring program. To distinguish vaccinated animals from naturally infected ones, the DIVA (differentiating infected from vaccinated animals) strategy is considered in vaccine development (Gil et al., 2020). To date, some S. Pullorum vaccines have been developed and used in poultry (Silva et al., 1981; Griffin and Barrow, 1993; Shah et al., 2007). For example, the administration of the Salmonella gallinarum 9R vaccine has decreased the incidence of S. Pullorum infection in poultry (Wigley, 2017). However, PD remains endemic in many parts of the world. New vaccines are still needed to be developed and prevent the infection and the spread of S. Pullorum in the poultry industry.
SpiC was the first SPI2-encoded protein identified and secreted by the Spi/Ssa T3SS2 system into the cytosol of macrophage (Uchiya et al., 1999). Our previous studies confirmed that the deletion of the spiC gene has significantly decreased the Salmonella virulence in chickens (Geng et al., 2014; Cheng et al., 2016; Wang et al., 2021). And, the spiC gene deleted S. Pullorum mutant strain has been evaluated as a potential vaccine candidate in chickens (Geng et al., 2014; Wang et al., 2021). However, the spiC gene deleted S. Pullorum vaccine candidate could not efficiently differentiate the vaccinated chickens from the naturally infected chickens and interfered with the salmonellosis monitoring program. A transcriptional antiterminator, RfaH, is required for the expression of O antigen and core sugar components of the lipopolysaccharides (LPS) (Santangelo and Roberts, 2002). The deletion of the rfaH gene produced truncated LPS and modified the smooth LPS to rough LPS (Lindberg and Hellerqvist, 1980). The rough LPS did not react with the antibodies against the O antigen and therefore, it can be used as the DIVA strategy with the available diagnostic serological tests (Bearson et al., 2014). Therefore, the rfaH gene can be chosen as a target gene for the construction of DIVA vaccine candidates based on spiC gene deleted S. Pullorum mutant strain.
In the present study, we aimed to construct the spiC and rfaH deletion mutant of the S. Pullorum (S06004ΔspiCΔrfaH) and determine its biological characteristics including growth characteristics, biochemical properties, and the LPS phenotype. Then, we planned to evaluate the safety and protective efficacy of the S06004ΔspiCΔrfaH as a live attenuated vaccine for the PD by virulence analysis, monitoring the changes in the body weight and clinical symptoms, as well as determining bacterial persistence, immune response, and protective effects in chickens. In addition, we proposed to evaluate the DIVA capability of S06004ΔspiCΔrfaH using serological methods, slide agglutination test or a commercial Biocheck Salmonella group D Antibody ELISA test to detect the LPS-specific serum antibody.
MATERIALS AND METHODS
Chickens and Ethics Statement
The specific-pathogen free (SPF) Hyline White chickens were bought from Jinan Spafas Poultry CO., Ltd. (Jinan, Shandong, China). Three-day-old chicks were confirmed free from Salmonella infection by serum detection using O9 Dc-ELISA (Xia et al., 2020) and the bacteriological examination. All chickens were housed in separate rearing isolators by group and fed with a pathogen-free drinking water and commercial diet. All the animal experiments were approved by the Animal Welfare and Ethics Committees of Yangzhou University and complied with the Ethics Committee of Laboratory Animals and the guidelines of the Institutional Administrative Committee (SYXK[Su]2016-0020).
Bacterial Strains and Construction of Salmonella Pullorum Mutant Strain S06004ΔspiCΔrfaH
The S. Pullorum S06004 strain, a nalidixic acid-resistant (NalR) clinical isolate was obtained from the chickens with pullorum disease in the Jiangsu Province of China (Geng et al., 2014). It is a wild-type virulent strain and is used as the challenge strain. The S. Pullorum S06004ΔspiC strain, a spiC gene deletion mutant strain (Geng et al., 2014), was used as the background strain for the construction of the S06004ΔspiCΔrfaH. The plasmid pGMB152 and the bacterial Escherichia coli X7213 used for gene deletion were stored in our lab (Geng et al., 2016). The S. Pullorum S06004ΔspiCΔrfaH, an LPS rough mutant, was constructed by the deletion of the rfaH gene in the S06004ΔspiC strain using the suicide vector pGMB152 based on the homologous recombination as described previously (Geng et al., 2016). Briefly, the upstream fragment of the rfaH gene was amplified using PCR with primers: forward primer, 5′-CCCCCCCTGCAGGTCGACCCAGGTTTTGCCGTTCTTTG-3′; reverse primer, 5′-CAGATGCCAACGCCAGAACCTGACTCTTATCCGCTTGTTC-3′. The downstream fragment of the rfaH gene was amplified using primers: forward primer, 5′-GAACAAGCGGATAAGAGTCAGGTTCTGGCGTTGGCATCTG-3′; reverse primer, 5′-CTTATCGATACCGTCGACGTCGGGGCATTCATTGTGGG-3′. The PCR products were purified using the TaKaRa MiniBEST Agarose Gel DNA Extraction Kit Ver 4.0 (TaKaRa Biotechnology Co. Ltd., Dalian, Liaoning, China). The pGMB152 plasmid was digested by the restriction endonucleases Sal Ⅰ (TaKaRa) and purified. The purified plasmid and upstream and downstream fragments were fused using the ClonExpress MultiS One Step Cloning Kit (Vazyme Biotechnology Co., Ltd., Nanjing, Jiangsu, China). The recombinant plasmids were transferred into the X7213 cells and sequenced. The single-crossover mutants were obtained by conjugal transfer of the recombinant suicide plasmids into the S06004ΔspiC strain. The rfaH gene deletion mutant was screened on 10% sucrose Luria-Bertani (LB) plates. The open reading frame (ORF) of the rfaH gene was completely deleted, and this was confirmed by sequencing and PCR analysis (primers: forward primer, 5′-ATGCAATCCTGGTATTTACTG-3′; reverse primer, 5′- CTAAATCTTGCGAAAACCGG-3′). Subsequently, the S. Pullorum S06004ΔspiCΔrfaH strain was used as the DIVA vaccine candidate in this study.
Biological Characteristics Test of the S06004ΔspiCΔrfaH In Vitro
The biochemical properties of S. Pullorum mutant S06004ΔspiCΔrfaH were tested using an API20E plate (BioMérieux, Marcy-l'etoile, France) according to the manufacturer's instructions, including glucose, maltose, sucrose, mannose, mannitol, lactose, dulcitol, adonitol, sorbitol, malonate, lysine decarboxylase, ornithine decarboxylase, urea, H2S, and so on. These results were compared with wild type S. Pullorum S06004. The in vitro growth characteristics analysis of the mutant and wild-type strains was performed by measuring the optical density (OD600) of each strain cultured in 20 mL of LB broth at 37°C with shaking at 180 rpm as previously described (Jiao et al., 2017). The OD600 was determined every hour and the monitoring was continued for 16 h. The LPS rough phenotype of the S06004ΔspiCΔrfaH was identified by slide agglutination test using O9 monoclonal antibody (O9 MAb) developed in our laboratory and acriflavine (Sigma-Aldrich, MO, St. Louis, MO) agglutination test (Jiao et al., 2018).
Assessment of Bacterial Virulence
The virulence of S. Pullorum S06004ΔspiCΔrfaH and S06004 was evaluated in chickens by determining the 50% lethal doses (LD50). One hundred and thirty 3-day old chickens were used in this experiment. Sixty chickens for each strain were randomly assigned into 6 groups (n = 10). Each group was injected intramuscularly with a 10-fold dilution (1 × 105 to 1 × 1010 CFU) dose of the S06004ΔspiCΔrfaH or S06004. Ten chickens were inoculated with 100 μL of phosphate-buffered saline (PBS) via the same route as the control group. Chicken deaths were monitored daily for 3 wk postinfection. The LD50 was calculated using the Reed–Muench method (Reed and Muench, 1938).
Changes of Body Weight and Clinical Symptoms After Immunization
A total of seventy-five 3-day old chickens were randomly divided into 3 groups (n = 25) and administered intramuscularly with 1 × 107 CFU S06004ΔspiCΔrfaH, 1 × 107 CFU S06004 as the positive control, and 100 μL of PBS as blank control. The body weights of these chickens were measured at 1, 7, 14, 21, and 28 d postimmunization (DPI). Mortality and clinical signs including anorexia, diarrhea, and depression were monitored daily after the administration.
Bacterial Colonization and Persistence Assay
Bacterial colonization and persistence in the internal organs of the chickens were evaluated. The liver and spleen samples from 5 chickens in each group were aseptically collected at 1, 7, 14, 21, and 28 DPI which immunized as above description. Then, the samples were weighed and homogenized in 1 mL of PBS. The homogenates were diluted 10-fold serially and subsequently inoculated onto the LB agar plates (containing 40 μg/mL Nal) at 37 °C for 12 to 16 h. The bacterial colonies were calculated as log10 CFU/g.
Histological Analysis
The sections of the spleen, liver, and cecum were collected from the chickens immunized with the S06004ΔspiCΔrfaH or PBS at 14 DPI. Then, these tissue samples were fixed in 10% neutral-buffered formalin. After fixation, the tissues were embedded in paraffin using the conventional method and then stained with H&E as previously described (Kang et al., 2016). Histopathological analyses were performed under a light microscope (Nikon Eclipse Ci-L, Nikon, Tokyo, Japan).
Serum IgG Test
The S. Pullorum-specific IgG antibody titers in the serum were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (Wang et al., 2021). The heat-killed whole bacteria (S06004 strain) were suspended to a density of approximately 1.0 × 108 CFU/mL with carbonate coating buffer and used as coating antigen on 96-well plates (50 μL per well). The serum samples were collected from the immunized chickens at 7, 14, 21, and 28 DPI and then serially 2-fold diluted (starting from 1:50) for the primary antibody. The horseradish peroxidase (HRP)-labeled goat antichicken IgG antibody (1:10,000 dilution, Sigma-Aldrich) was used as the secondary antibody. The HRP activity was determined using 3,3′,5,5′-tetramethylbenzidine substrate solution (Solarbio, Beijing, China). The reaction was stopped by 2 M H2SO4, and the OD450 was measured using an automated microplate reader (BioTek Instruments, Winooski, VT).
Lymphocyte Proliferation Assay
The peripheral blood mononuclear cells (PBMCs) were used for the proliferation assay as previously described (Rana and Kulshreshtha, 2006). The PBMCs were isolated from the whole chicken blood at 7 and 14 DPI using Histopaque-1083 (Sigma-Aldrich) as per the manufacturer's instructions. The soluble antigen was prepared from the wild type S. Pullorum strain S06004 and used as a specific stimulator. The PBMCs (1 × 106 cells/100 μL/well) were seeded onto 96-well plates and stimulated with 10 μg/mL soluble antigen at 41°C for 72 h. The cell proliferation was evaluated using an ELISA-BrdU kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. The cell proliferation was expressed as the stimulation index (SI) and calculated using the following equation: SI = (OD450−OD690 of the antigen-stimulated cells)/(OD450−OD690 of the unstimulated cells) (Song et al., 2018).
The Expression of Cytokines in the Spleen
The mRNA levels of the splenic cytokines IL-2, IL-4, and IFN-γ were evaluated at 3, 7, and 14 DPI using the quantitative real-time PCR (qRT-PCR). The total RNA was extracted from the spleen by using the total RNeasy Plus Mini kit (Qiagen, Hilden, Germany), and the cDNA was synthesized using the PrimeScript RT reagent Kit (TaKaRa, Dalian, China) according to the manufacturer's instructions. The qRT-PCR was performed using the Fsu SYBR Green Master (Roche) in an ABI PRISM 7500 Real-Time PCR System (Applied Biosystems, Foster, CA). The primers used for the qRT-PCR are shown in Table 1. The expression level of cytokines was normalized to the internal control GAPDH and calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Table 1.
Primers used for the qRT-PCR in this study.
| Amplified genes | Gene accession | Primer name | Primer sequences (5’-3’) | Size (bp) |
|---|---|---|---|---|
| GAPDH | NM_204305.2 | GAPDH-F | GGTGGTGCTAAGCGTGTTAT | 264 |
| GAPDH-R | ACCTCTGTCATCTCTCCACA | |||
| IL-2 | AF000631.1 | IL-2-F | ATCTTTGGCTGTATTTCGGTAG | 163 |
| IL-2-R | ACTCCTGGGTCTCAGTTGGTG | |||
| IL-4 | AJ621249.1 | IL-4-F | CCAGCACTGCCACAAGAA | 169 |
| IL-4-R | AGCTAGTTGGTGGAAGAAGG | |||
| IFN-γ | NM_205149.1 | IFN-γ-F | AGCTGACGGTGGACCTATTATT | 259 |
| IFN-γ-R | GGCTTTGCGCTGGATTC |
DIVA Capability Assessment for the S06004ΔspiCΔrfaH
The DIVA capability of the S06004ΔspiCΔrfaH was evaluated using the serological method to detect the LPS-specific serum antibody by a slide agglutination test or a commercial ELISA Kit. The serum was collected from the chicken immunized with the S06004ΔspiCΔrfaH, S06004, or PBS at 14 DPI, and used for the detection of the LPS antibody. The slide agglutination test was performed using the S. Pullorum antigens obtained from Zhonghai Biotech Co., Ltd. (Beijing, China) according to the manufacturer's instructions. The ELISA was performed using the Salmonella group D Antibody test kit (BioCheck, San Francisco, CA) according to the manufacturer's instructions.
Immune Protection Assessment for the S06004ΔspiCΔrfaH
The protective efficacy of the S06004ΔspiCΔrfaH was evaluated in chickens by intramuscular vaccination. Thirty 3-day-old chicks were randomly divided into 2 groups (n = 15) and designated as vaccinated group and nonvaccinated group. They were immunized intramuscularly with 1 × 107 CFU of S06004ΔspiCΔrfaH or 100 μL of PBS. In addition, another 10 chicks without vaccination and challenge were used as the blank group. At 14 DPI, the vaccinated group and nonvaccinated group were challenged with 2 × 109 CFU of the wild-type strain S06004 by intramuscular injections. Deaths and clinical symptoms were recorded daily for 21 d after the challenge.
Statistical Analysis
All experimental data were analyzed by an unpaired Student's t-test using Prism 5.0 software (GraphPad Inc., San Diego, CA). The values were expressed as the mean± SEM, and the significant differences were assigned to P values < 0.05, < 0.01 and < 0.001 denoted by *, **, and ***, respectively.
RESULTS
S06004ΔspiCΔrfaH Construction and Biological Characteristics
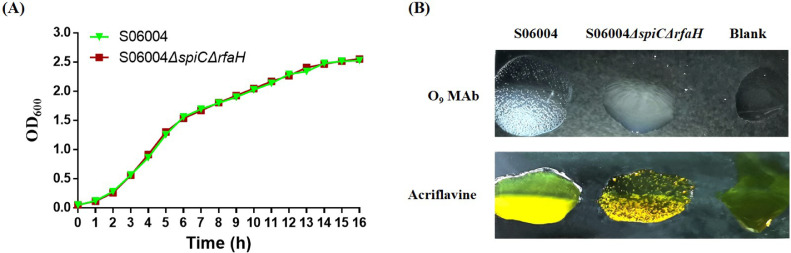
The S06004ΔspiCΔrfaH mutant was constructed using the homologous recombination method. Our PCR results showed that both spiC and rfaH gene were deleted in the S06004ΔspiCΔrfaH mutant (Figure S1). The biological properties of the S06004ΔspiCΔrfaH were analyzed. Growth curve analysis revealed no significant differences between the S06004ΔspiCΔrfaH mutant and wild-type S06004 when cultured in the LB liquid medium at 37°C (Figure 1A). The biochemical test results showed that all tested biochemical indicators were the same between the mutant and wild-type strains (Table S1). Both S06004ΔspiCΔrfaH and S06004 decarboxylated the amino acids lysine and ornithine, and fermented glucose and mannose (Table S1). The slide agglutination result showed that the mutant S06004ΔspiCΔrfaH was not agglutinated with the O9 Mab, however, the S06004 was agglutinated with the O9 Mab (Figure 1B). The acriflavine agglutination result showed that the acriflavine was strongly agglutinated with the S06004ΔspiCΔrfaH strain rather than the wild-type strain (Figure 1B). Both results of slide agglutination and acriflavine agglutination suggested that the mutant strain displayed a rough-phenotype whereas the wild-type strain displayed a smooth-phenotype.
Figure 1.
Biological characteristics test of the S06004ΔspiCΔrfaH. (A) Growth curves of the S06004ΔspiCΔrfaH mutant. The S06004ΔspiCΔrfaH and S06004 were grown in the LB broth at 37°C with shaking at 180 rpm for 16 h, and the OD600 values were determined every hour. (B) Rough phenotype characteristics of the S06004ΔspiCΔrfaH mutant. Agglutination assay was performed using the O9 MAb and acriflavine. Images were taken within 5 min.
The S06004ΔspiCΔrfaH Exhibits Reduced Virulence in a Chicken Model
The virulence of the S06004ΔspiCΔrfaH and S06004 strains was evaluated in 3-day-old Hyline White chickens after intramuscular immunization. As shown in Table 2, the LD50 of S06004ΔspiCΔrfaH was 2.0 × 109 CFU, which was 100-fold higher than that of the wild-type S06004 (2.0 × 107 CFU). The result indicated that the virulence of the S06004ΔspiCΔrfaH was attenuated compared to the wild-type strain.
Table 2.
The LD50 of the S. Pullorum S06004 and S06004ΔspiCΔrfaH in chickens.
| Strains | Challenge dose (CFU) | Number of deaths/Total number of chickens | LD50 (CFU) |
|---|---|---|---|
| S06004 | 1 × 1010 | 10/10 | 2.0 × 107 |
| 1 × 109 | 10/10 | ||
| 1 × 108 | 7/10 | ||
| 1 × 107 | 4/10 | ||
| 1 × 106 | 1/10 | ||
| 1 × 105 | 0/10 | ||
| S06004ΔspiCΔrfaH | 1 × 1010 | 8/10 | 2.0 × 109 |
| 1 × 109 | 4/10 | ||
| 1 × 108 | 0/10 | ||
| 1 × 107 | 0/10 | ||
| 1 × 106 | 0/10 | ||
| 1 × 105 | 0/10 | ||
| Blank | PBS | 0/10 | / |
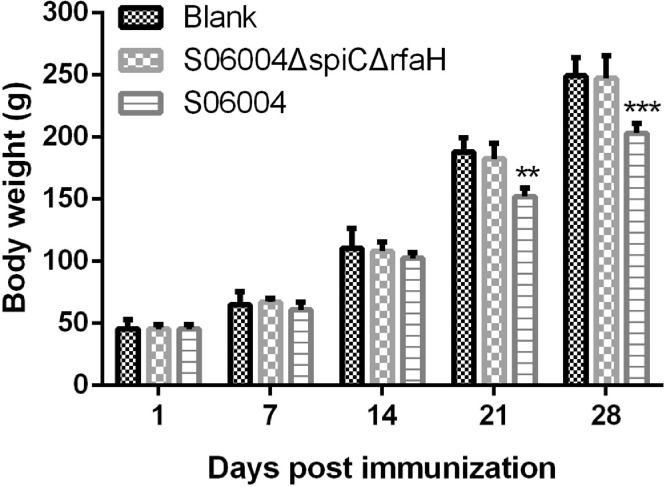
Changes in Body Weight and Clinical Symptoms After Vaccination
The changes in body weight of chickens immunized with the S06004ΔspiCΔrfaH, S06004 (positive control), and PBS (blank control) are shown in Figure 2. No significant difference in the body weight was observed between the S06004ΔspiCΔrfaH immunized group and the blank control. The body weight of the chickens in the S06004 group was significantly decreased compared to that of the S06004ΔspiCΔrfaH group and blank control at 21, and 28 DPI. No clinical symptom changes were observed in the S06004ΔspiCΔrfaH group and the blank control. But the S06004 group showed severe clinical signs, including slight and temporary lethargy, anorexia, white diarrhea, and mortality.
Figure 2.
The bodyweight of chickens after the immunization. Groups of 3-day-old chickens were intramuscularly immunized with 1 × 107 CFU S06004ΔspiCΔrfaH, S06004, and the blank control group received 100 μL of PBS. The body weights of these chickens were recorded at 1, 7, 14, 21, and 28 DPI. Data are presented as mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 compared with the body weight of the blank control group chickens.
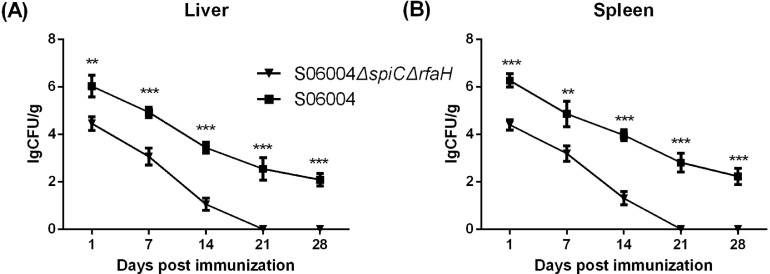
Colonization and Persistence of the S06004ΔspiCΔrfaH in Liver and Spleen
The results of bacteria colonization and persistence in the liver and spleen are shown in Figure 3. All the liver and spleen samples from the blank control group were negative for Salmonella recovery. The S06004ΔspiCΔrfaH colonization was significantly decreased compared to the S06004 both in the liver and spleen at 1, 7, 14, 21, and 28 DPI. The persistence of the strains declined gradually over the course of the experiment. The viable counts in the liver and spleen from the S06004ΔspiCΔrfaH group were negative starting from 21 DPI. But, the samples from the S06004 group still detected the Salmonella at 28 DPI.
Figure 3.
Salmonella colonization and persistence in the liver and spleen after the immunization. The bacterial colonization and persistence in the liver (A) and the spleen (B) were determined at 1, 7, 14, 21, and 28 DPI. The number of bacteria was expressed as log10CFU/g. Data are presented as mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 compared with S06004 immunized group chickens.
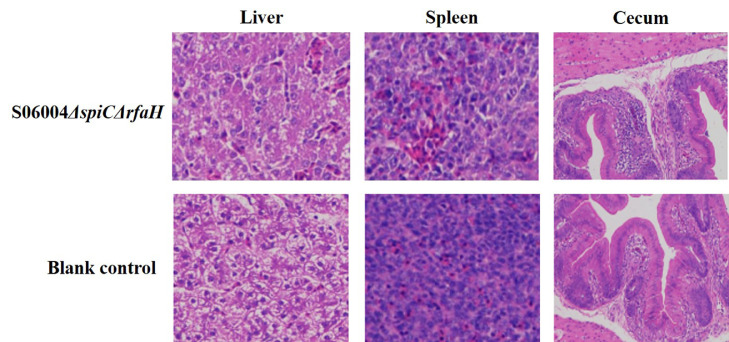
Histological Analysis After Immunization With the S06004ΔspiCΔrfaH
The histological analysis of the spleen, liver, and cecum was performed using the H&E staining at 14 DPI. The histological examination result showed that no obvious lesions were detected in the spleen, liver, and cecum from the S06004ΔspiCΔrfaH group than those in the blank control group (Figure 4).
Figure 4.
The histological analysis after the S06004ΔspiCΔrfaH immunization. The histopathological changes in the liver, spleen, and cecum of chickens were examined by H&E staining at 14 DPI. The results were observed at 200 × magnification using an optical microscope.
Immune Response Induced by the S06004ΔspiCΔrfaH Immunization
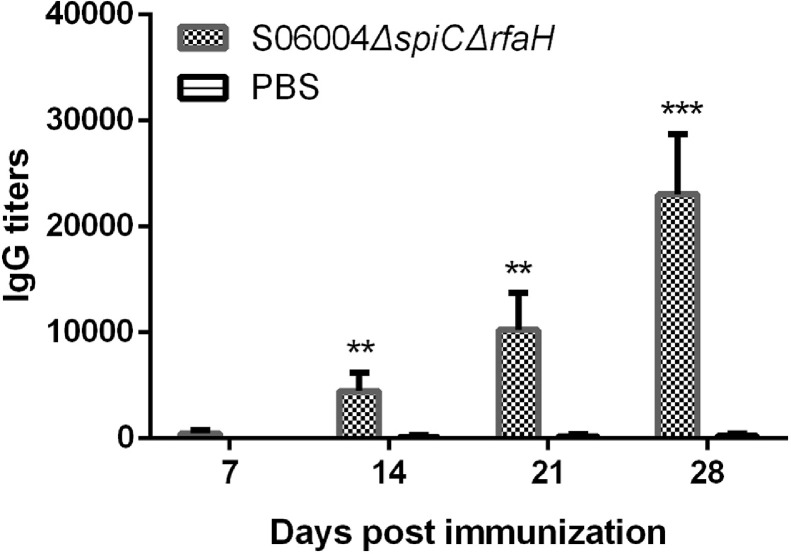
The humoral immune response in chickens following the S06004ΔspiCΔrfaH immunization was evaluated by determining the serum S. Pullorum-specific IgG antibody. As shown in Figure 5, the IgG antibody against the S. Pullorum in the S06004ΔspiCΔrfaH immunized group was detected at 7 DPI and increased dramatically at 14, 21, and 28 DPI. The S06004ΔspiCΔrfaH group had significantly higher S. Pullorum specific IgG antibody titers than those of blank control group at 14, 21, and 28 DPI.
Figure 5.
Determination of the serum IgG antibody titers. The IgG antibody against the S. Pullorum in the serum of chickens at 7, 14, 21, and 28 DPI were measured by ELISA. Data are presented as mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 compared with blank control group chickens.
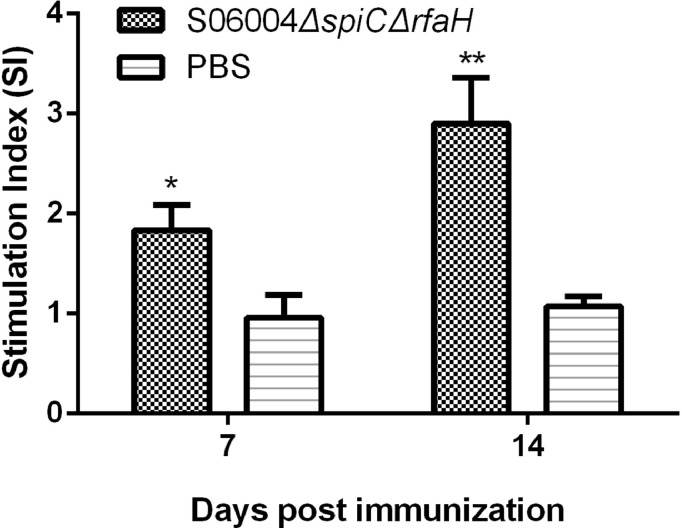
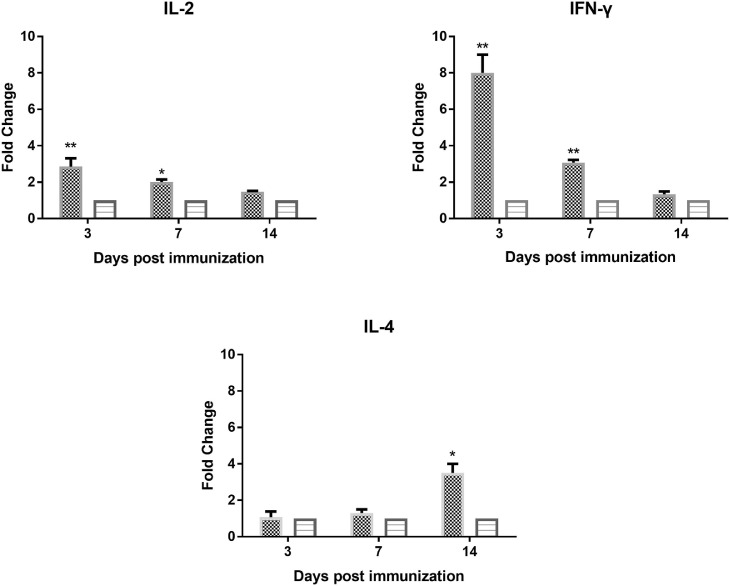
The cellular immune response induced by the S06004ΔspiCΔrfaH was evaluated by peripheral lymphocyte proliferation assay and the determination of the cytokines’ expression in the spleen. The SI values of the PBMCs from S06004ΔspiCΔrfaH group were significantly higher than those of the control blank group after the stimulation with the S. Pullorum soluble antigen at 7 and 14 DPI (Figure 6). The qRT-PCR analysis of the cytokine expression levels in the spleen is shown in Figure 7. The S06004ΔspiCΔrfaH group has significantly higher IL-2 and IFN-γ levels than the blank control group at 3, and 7 DPI. The expression of IL-4 in the S06004ΔspiCΔrfaH group was higher than that in the blank control group at 14 DPI.
Figure 6.
The stimulation index (SI) of the PBMCs proliferation assay. The cell proliferation was determined by ELISA-BrdU at 7 and 14 DPI. The SI was calculated using the following equation: SI = (OD450−OD690 of the antigen-stimulated cells) / (OD450−OD690 of the unstimulated cells). Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, compared with the blank control group chickens.
Figure 7.
The expression of cytokines in the spleen after the immunization. The mRNA expression of IL-2, IL-4, and IFN-γ was measured by the qRT-PCR at 3, 7, and 14 DPI. And the fold change referred to the data compared with the blank control group. Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01.
The DIVA Capability of the S06004ΔspiCΔrfaH
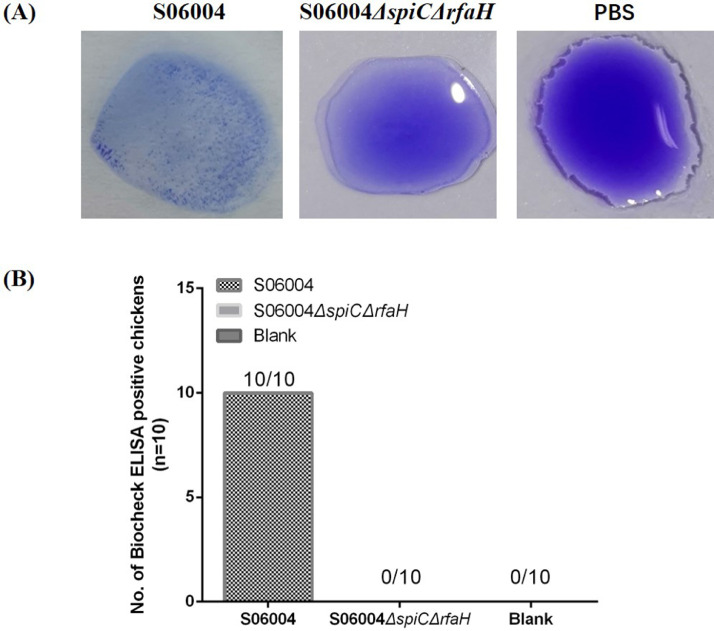
The DIVA capability of the S06004ΔspiCΔrfaH was evaluated using slide agglutination test or Biocheck Salmonella group D Antibody ELISA test to detect the LPS-specific serum antibodies. The slide agglutination test showed that the serum samples from the chicken immunized with the S06004ΔspiCΔrfaH was failed to agglutinate with the commercial S. Pullorum antigens at 14 DPI (Figure 8A). However, the serum samples from the chicken infected with the wild-type S06004 strain were obvious agglutinated with the S. Pullorum antigens (Figure 8A). In addition, the Biocheck Salmonella group D Antibody ELISA test showed that all the serum samples (10/10) from the chicken infected with the wild-type strain were positive, while all the serum samples (10/10) from the chicken immunized with the S06004ΔspiCΔrfaH were negative (Figure 8B).
Figure 8.
The DIVA capability of the S06004ΔspiCΔrfaH. The serum was collected from the chicken immunized with the S06004ΔspiCΔrfaH, S06004, or PBS at 14 DPI and used for the detection of the LPS antibody. (A) Agglutination assay was performed using commercial S. Pullorum antigens. Images were taken within 5 min. (B) Salmonella LPS antibody was detected using the Biocheck Salmonella group D Antibody ELISA test. The relative amounts of antibodies in serum were expressed as S/P ratio (Sample to Positive Ratio). The S/P ratio was calculated using the following equation: S/P = (OD405 of test sample−OD405 of negative control)/(OD405 of positive control−OD405 of negative control). The sample's S/P ratio of ≥0.5 was considered positive and < 0.5 was considered negative.
Immune Protection by the S06004ΔspiCΔrfaH Vaccination Against Virulent S. Pullorum Challenge
The survival percentages in the chickens vaccinated intramuscularly with the S06004ΔspiCΔrfaH followed by the challenge with the virulent S. Pullorum are shown in Table 3. Two chickens died in the vaccinated group, whereas 12 out of 15 chickens in the control group died after the challenge. The clinical symptoms including high morbidity and mortality, anorexia, diarrhea, and depression in the vaccinated group were slight and temporary after challenged compared to the nonvaccinated group.
Table 3.
The protective efficacy of the S06004ΔspiCΔrfaH after intramuscular vaccination.
| Vaccination |
Number | Challenge |
Survivors/Total | Survival rate (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Strain | Route | Dose (CFU) | Strain | Route | Dose (CFU) | |||
| S06004ΔspiCΔrfaH | Intramuscularly | 1 × 107 | 15 | S06004 | Intramuscularly | 2 × 109 | 13/15 | 87 |
| PBS | Intramuscularly | - | 15 | S06004 | Intramuscularly | 2 × 109 | 3/15 | 20 |
| PBS | Intramuscularly | - | 10 | PBS | Intramuscularly | - | 10/10 | 100 |
DISCUSSION
Pullorum disease, avian systemic salmonellosis, caused by S. Pullorum, causes high morbidity and mortality in chickens. It still remains responsible for the huge economic losses in the poultry industry of the developing countries (Zhang et al., 2018). In addition to good farming practices, appropriate management, and strict biosecurity measures, the vaccination of chickens is a useful strategy to control and prevent Salmonella infections in poultry flocks (Revolledo and Ferreira, 2012). The live attenuated vaccines are more effective than the killed or subunit vaccines in inducing an immune response against Salmonella infection (Barrow, 2007). A live Salmonella vaccine should be attenuated, immunogenicity, protective while the vaccinated chickens can be differentiated from the naturally infected flocks (Adriaensen et al., 2007). In this study, we evaluated the safety, protective efficacy, and the DIVA capability of the spiC and rfaH deleted rough mutant of the S. Pullorum S06004 strain as a live attenuated DIVA vaccine candidate.
For the live attenuated Salmonella vaccine, it should be avirulent. Our previous study has confirmed that a single deletion of the spiC gene in the S. Pullorum can raise about 126-fold LD50 compared with that of the wild-type strain and significantly decrease the bacterial virulence (Wang et al., 2021). In this study, we constructed a double-gene deletion mutant of S. Pullorum, which combined the spiC-deleted strain with the rfaH deletion (S06004ΔspiCΔrfaH). The LD50 of the S06004ΔspiCΔrfaH was 100-fold higher than that of the wild-type S06004 suggesting that the virulence of the S06004ΔspiCΔrfaH was significantly attenuated compared to that of the wild-type strain S06004. It indicated that the deletion of the rfaH in the S06004ΔspiC did not decrease its virulence. The result is consistent with a study reporting that the modified expression of the rfaH did not affect the virulence of the S. Gallinarum (Mitra et al., 2013). Despite the attenuation, the S06004ΔspiCΔrfaH maintained its capacity to invade the host organism (Figure 3). The S06004ΔspiCΔrfaH mutant strain was recovered from the liver and spleen until 14 DPI, while the wild-type strain was recovered from these organs during the whole sampling period. Moreover, the mutant strain was recovered in lower numbers throughout the sampling period, compared to the wild-type strain. The result is in accordance with the previous reports on the deletion of the spiC gene in the S. Pullorum or S. Enteritidis (Li et al., 2019; Wang et al., 2021). In addition to being attenuated, the live attenuated Salmonella vaccine should have no side effects in the poultry flocks (Yin et al., 2015). Here, the body weight of the chicken immunized with the S06004ΔspiCΔrfaH was not significantly different from that of the control blank group and no clinical symptoms and histopathological changes were observed after the immunization. These results indicate that the S06004ΔspiCΔrfaH has a very good safety profile and is attenuated to a sufficient degree to enable its use as a live Salmonella vaccine.
The live-attenuated vaccine strain should maintain its immunogenicity while reducing its virulence (Revolledo and Ferreira, 2012). We measured the levels of Salmonella specific antibodies in the serum and monitor the humoral immune responses. The chickens from the immunized group had significantly higher serum IgG titers compared with the blank control group. This result indicated that the S06004ΔspiCΔrfaH could induce a strong humoral immune response and have good immunogenicity in chickens. However, the cellular immune response is more important than the humoral response to eradicate the intracellular Salmonella (Mastroeni et al., 1993). The cellular immune response corresponds to the protection against Salmonella challenge (Rana and Kulshreshtha, 2006). The cellular immune response is especially important for the clearance of S. Pullorum because the S. Pullorum induces a response that is more Th2-like than the Th1-type response, which is more normally associated with the S. Typhimurium or S. Enteritidis (Tang et al., 2018). In this study, the cellular immune response was examined by a lymphocyte proliferation assay (Rana and Kulshreshtha, 2006). The significant increases of the SI values in the mutant immunized group indicated that the S. Pullorum-specific lymphocyte proliferation was produced in the immunized chickens. The activation of the cellular immune response could induce the Th1/Th2 T lymphocytes to secrete cytokines, such as IFN-γ and IL-4 (Mastroeni and Rossi, 2020). In the present study, the immunized groups exhibited high IL-2 and IFN-γ (Th1 cytokines) mRNA expression levels in the spleen at 3, and 7 DPI, and high IL-4 (Th2 cytokines) mRNA expression levels in the spleen at 14 DPI. The result indicated that S06004ΔspiCΔrfaH vaccination could induce Th1 (cellular immune) and Th2 (humoral immune) responses, which is consistent with the results of the lymphocyte proliferation assay and serum antibody measurement. Overall, the S06004ΔspiCΔrfaH induced a robust humoral immune response as well as an effective cellular immune response.
If a Salmonella vaccine cannot differentiate the vaccinated and infected animals, it will interfere with the established serological monitoring programs (Latasa et al., 2016). Thus, the Salmonella DIVA vaccine has more prospects for use in chickens. The DIVA rationale is that the lack of specific antigens or epitopes allows the use of a serological test to discriminate the infected birds from the vaccinated birds. The LPS is a major virulence factor, and the O-antigen is the immunodominant antigen in the serological diagnosis tests (Kong et al., 2011). Truncating the LPS is a method for the use of Salmonella as a DIVA vaccine candidate. Few Salmonella DIVA vaccines based on the LPS have been constructed including Salmonella Typhimurium and Pullorum (Leyman et al., 2011; Bearson et al., 2016; Guo et al., 2017). In this study, the S06004ΔspiCΔrfaH showed an LPS rough phenotype after the deletion of the rfaH gene which is involved in the LPS synthesis. This result is consistent with a report on the rfaH deletion in the Salmonella Typhimurium (Leyman et al., 2011). The rfaH deletion in the Salmonella Typhimurium mutant has been evaluated as a DIVA vaccine in the pig. Here, the slide agglutination test or Biocheck Salmonella group D Antibody ELISA test, the most common sero-diagnostic test for the detection of anti-Salmonella antibodies, was used to evaluate the DIVA capability of the S06004ΔspiCΔrfaH. The S06004ΔspiCΔrfaH immunized group exhibited negative for the serological test, while the wild-type S06004 infection group showed strong positive for the serological test. These results indicated that the S06004ΔspiCΔrfaH vaccination can discriminate the infected birds from the vaccinated birds, and has the potential to use as a DIVA-strategy vaccine.
S. Pullorum deleted with one or several effectors encoding genes provided the efficacious protection against the wild-type S. Pullorum challenge in chickens (Geng et al., 2014; Yin et al., 2015; Guo et al., 2017). In our study, we evaluated the protective efficacy of the candidate vaccine against intramuscular challenge with the wild-type S. Pullorum. The survival rates in the vaccinated chickens were 87% after the challenge, while the survival rates were 20% in the control groups. The result is consistent with our previous study reporting that survival rates of chickens immunized with the S. Pullorum SpiC mutant were 90% followed by challenge with the parent strain (Geng et al., 2014). These results showed that the S06004ΔspiCΔrfaH can afford effective protection for acute systemic PD and can be used as a live attenuated vaccine candidate.
In conclusion, we constructed a rough S. Pullorum mutant S06004ΔspiCΔrfaH. The data demonstrated that the vaccination of the rough mutant had a very good safety profile, and strongly elicited both humoral and cellular immune responses. The rough mutant could provide efficient protection for the systemic S. Pullorum infection by intramuscular vaccination. In addition, the vaccination of S06004ΔspiCΔrfaH could discriminate the infected birds from the vaccinated birds by the serological test. Thus, the S. Pullorum S06004ΔspiCΔrfaH has the potential of being a safe, immunogenic, and DIVA vaccine candidate to control Salmonella infection without the interference of the salmonellosis monitoring program in poultry.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31902278, 31972685, 31730094), the China Postdoctoral Science Foundation (2018M642333), Jiangsu Province Policy Guidance Program (International Science and Technology Cooperation) (BZ2020013), the Research and Development Program of Jiangsu (BE2021354), the Yangzhou University Science and Technology Innovation Team (2018), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
DISCLOSURES
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101655.
Appendix. Supplementary materials
Figure S1. The construction of the S06004ΔspiCΔrfaH mutant was confirmed by the PCR. The rfaH (A) and spiC (B) genes deletion in the mutant confirmed by the PCR. Lane M, DL2000 DNA marker; lane 1: S06004 genomic DNA; lane 2: S06004ΔspiCΔrfaH genomic DNA. The wild type strain S06004 harbors the complete rfaH and spiC genes, with a PCR product length of 489 bp, and 384 bp, respectively, whereas the deletion mutant has no PCR band.
REFERENCES
- Adriaensen C., De Greve H., Tian J.Q., De Craeye S., Gubbels E., Eeckhaut V., Van Immerseel F., Ducatelle R., Kumar M., Hernalsteens J.P. A live Salmonella enterica serovar Enteritidis vaccine allows serological differentiation between vaccinated and infected animals. Infect. Immun. 2007;75:2461–2468. doi: 10.1128/IAI.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P.A. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathol. 2007;36:1–13. doi: 10.1080/03079450601113167. [DOI] [PubMed] [Google Scholar]
- Barrow P.A., Freitas Neto O.C. Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathol. 2011;40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- Barrow P.A., Jones M.A., Smith A.L., Wigley P. The long view: Salmonella–the last forty years. Avian Pathol. 2012;41:413–420. doi: 10.1080/03079457.2012.718071. [DOI] [PubMed] [Google Scholar]
- Bearson B.L., Bearson S.M., Kich J.D. A DIVA vaccine for cross-protection against Salmonella. Vaccine. 2016;34:1241–1246. doi: 10.1016/j.vaccine.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Bearson B.L., Bearson S.M., Kich J.D., Lee I.S. An rfaH mutant of Salmonella enterica serovar Typhimurium is attenuated in swine and reduces intestinal colonization, fecal shedding, and disease severity due to virulent Salmonella Typhimurium. Front. Vet. Sci. 2014;1:9. doi: 10.3389/fvets.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Yin J., Kang X., Geng S., Hu M., Pan Z., Jiao X. Safety and protective efficacy of a spiC and crp deletion mutant of Salmonella Gallinarum as a live attenuated vaccine for fowl typhoid. Res. Vet. Sci. 2016;107:50–54. doi: 10.1016/j.rvsc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Desin T.S., Koster W., Potter A.A. Salmonella vaccines in poultry: past, present and future. Expert. Rev. Vaccines. 2013;12:87–96. doi: 10.1586/erv.12.138. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Soderlund R., Ernholm L., Melin L., Jansson D.S. Diagnostics, epidemiological observations and genomic subtyping in an outbreak of pullorum disease in non-commercial chickens. Vet. Microbiol. 2018;217:47–52. doi: 10.1016/j.vetmic.2018.02.025. [DOI] [PubMed] [Google Scholar]
- Geng S., Tian Q., An S., Pan Z., Chen X., Jiao X. High-efficiency, two-step scarless-markerless genome genetic modification in Salmonella enterica. Curr. Microbiol. 2016;72:700–706. doi: 10.1007/s00284-016-1002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S., Jiao X., Barrow P., Pan Z., Chen X. Virulence determinants of Salmonella Gallinarum biovar Pullorum identified by PCR signature-tagged mutagenesis and the spiC mutant as a candidate live attenuated vaccine. Vet. Microbiol. 2014;168:388–394. doi: 10.1016/j.vetmic.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Gil C., Latasa C., Garcia-Ona E., Lazaro I., Labairu J., Echeverz M., Burgui S., Garcia B., Lasa I., Solano C. A DIVA vaccine strain lacking RpoS and the secondary messenger c-di-GMP for protection against salmonellosis in pigs. Vet. Res. 2020;51:3. doi: 10.1186/s13567-019-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin H.G., Barrow P.A. Construction of an aroA mutant of Salmonella serotype Gallinarum: its effectiveness in immunization against experimental fowl typhoid. Vaccine. 1993;11:457–462. doi: 10.1016/0264-410x(93)90288-9. [DOI] [PubMed] [Google Scholar]
- Guo R., Jiao Y., Li Z., Zhu S., Fei X., Geng S., Pan Z., Chen X., Li Q., Jiao X. Safety, protective immunity, and DIVA capability of a rough mutant Salmonella Pullorum vaccine candidate in broilers. Front. Microbiol. 2017;8:547. doi: 10.3389/fmicb.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Guo R., Tang P., Kang X., Yin J., Wu K., Geng S., Li Q., Sun J., Xu X., Zhou X., Gan J., Jiao X., Liu X., Pan Z. Signature-tagged mutagenesis screening revealed a novel smooth-to-rough transition determinant of Salmonella enterica serovar Enteritidis. BMC Microbiol. 2017;17:48. doi: 10.1186/s12866-017-0951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Xia Z., Zhou X., Guo Y., Guo R., Kang X., Wu K., Sun J., Xu X., Jiao X., Pan Z., Liu X. Signature-tagged mutagenesis screening revealed the role of lipopolysaccharide biosynthesis gene rfbH in smooth-to-rough transition in Salmonella Enteritidis. Microbiol. Res. 2018;212-213:75–79. doi: 10.1016/j.micres.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Jibril A.H., Okeke I.N., Dalsgaard A., Olsen J.E. Association between antimicrobial usage and resistance in Salmonella from poultry farms in Nigeria. BMC Vet. Res. 2021;17:234. doi: 10.1186/s12917-021-02938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X., Yang Y., Jiao Y., Song H., Song L., Xiong D., Wu L., Pan Z., Jiao X. HA1-2-fljB vaccine induces immune responses against pandemic swine-origin H1N1 influenza virus in mice. J. Mol. Microbiol. Biotechnol. 2016;26:422–432. doi: 10.1159/000448895. [DOI] [PubMed] [Google Scholar]
- Kong Q., Yang J., Liu Q., Alamuri P., Roland K.L., Curtiss R., 3rd Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect. Immun. 2011;79:4227–4239. doi: 10.1128/IAI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latasa C., Echeverz M., Garcia B., Gil C., Garcia-Ona E., Burgui S., Casares N., Hervas-Stubbs S., Lasarte J.J., Lasa I., Solano C. Evaluation of a Salmonella Strain lacking the secondary messenger C-di-GMP and RpoS as a live oral vaccine. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman B., Boyen F., Van Parys A., Verbrugghe E., Haesebrouck F., Pasmans F. Salmonella Typhimurium LPS mutations for use in vaccines allowing differentiation of infected and vaccinated pigs. Vaccine. 2011;29:3679–3685. doi: 10.1016/j.vaccine.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhu Y., Ren J., Qiao Z., Yin C., Xian H., Yuan Y., Geng S., Jiao X. Evaluation of the safety and protection efficacy of spiC and nmpC or rfaL deletion mutants of Salmonella Enteritidis as live vaccine candidates for poultry non-typhoidal salmonellosis. Vaccines. 2019;7:202. doi: 10.3390/vaccines7040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A.A., Hellerqvist C.G. Rough mutants of Salmonella Typhimurium: immunochemical and structural analysis of lipopolysaccharides from rfaH mutants. J. Gen. Microbiol. 1980;116:25–32. doi: 10.1099/00221287-116-1-25. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J., Li L., Pan F., Zuo G., Yu D., Liu R., Fan H., Ma Z. PagC is involved in Salmonella Pullorum OMVs production and affects biofilm production. Vet. Microbiol. 2020;247 doi: 10.1016/j.vetmic.2020.108778. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Chabalgoity J.A., Dunstan S.J., Maskell D.J., Dougan G. Salmonella: immune responses and vaccines. Vet. J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Rossi O. Antibodies and protection in systemic Salmonella infections: do we still have more questions than answers. Infect. Immun. 2020;88:e00219–e00220. doi: 10.1128/IAI.00219-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Demarco de Hormaeche R., Hormaeche C.E. Delayed (footpad) hypersensitivity and Arthus reactivity using protein-rich antigens and LPS in mice immunized with live attenuated aroA Salmonella vaccines. Microb. Pathog. 1993;14:369–379. doi: 10.1006/mpat.1993.1036. [DOI] [PubMed] [Google Scholar]
- Mitra A., Loh A., Gonzales A., Laniewski P., Willingham C., Curtiss Iii R., Roland K.L. Safety and protective efficacy of live attenuated Salmonella Gallinarum mutants in Rhode Island Red chickens. Vaccine. 2013;31:1094–1099. doi: 10.1016/j.vaccine.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Pan Z., Wang X., Zhang X., Geng S., Chen X., Pan W., Cong Q., Liu X., Jiao X., Liu X. Changes in antimicrobial resistance among Salmonella enterica subspecies enterica serovar Pullorum isolates in China from 1962 to 2007. Vet. Microbiol. 2009;136:387–392. doi: 10.1016/j.vetmic.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Penha Filho R.A., de Paiva J.B., da Silva M.D., de Almeida A.M., Berchieri A., Jr. Control of Salmonella Enteritidis and Salmonella Gallinarum in birds by using live vaccine candidate containing attenuated Salmonella Gallinarum mutant strain. Vaccine. 2010;28:2853–2859. doi: 10.1016/j.vaccine.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Rana N., Kulshreshtha R.C. Cell-mediated and humoral immune responses to a virulent plasmid-cured mutant strain of Salmonella enterica serotype Gallinarum in broiler chickens. Vet. Microbiol. 2006;115:156–162. doi: 10.1016/j.vetmic.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Revolledo L., Ferreira A.J.P. Current perspectives in avian salmonellosis: vaccines and immune mechanisms of protection. J. Appl. Poultry Res. 2012;21 717-717. [Google Scholar]
- Santangelo T.J., Roberts J.W. RfaH, a bacterial transcription antiterminator. Mol. Cell. 2002;9:698–700. doi: 10.1016/s1097-2765(02)00516-6. [DOI] [PubMed] [Google Scholar]
- Shah D.H., Shringi S., Desai A.R., Heo E.J., Park J.H., Chae J.S. Effect of metC mutation on Salmonella Gallinarum virulence and invasiveness in 1-day-old White Leghorn chickens. Vet. Microbiol. 2007;119:352–357. doi: 10.1016/j.vetmic.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Silva E.N., Snoeyenbos G.H., Weinack O.M., Smyser C.F. Studies on the use of 9R strain of Salmonella Gallinarum as a vaccine in chickens. Avian Dis. 1981;25:38–52. [PubMed] [Google Scholar]
- Song L., Xiong D., Hu M., Jiao X., Pan Z. Enhanced Th1/Th2 mixed immune responses elicited by polyethyleneimine adjuvanted influenza A (H7N9) antigen HA1-2 in chickens. Poult. Sci. 2018;97:4245–4251. doi: 10.3382/ps/pey313. [DOI] [PubMed] [Google Scholar]
- Tang Y., Foster N., Jones M.A., Barrow P.A. Model of persistent Salmonella infection: Salmonella enterica serovar Pullorum modulates the immune response of the chicken from a Th17-type response towards a Th2-type response. Infect. Immun. 2018;86:e00307–e00318. doi: 10.1128/IAI.00307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiya K., Barbieri M.A., Funato K., Shah A.H., Stahl P.D., Groisman E.A. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huang C., Tang J., Liu G., Hu M., Kang X., Zhang J., Zhang Y., Pan Z., Jiao X., Geng S. Salmonella Pullorum spiC mutant is a desirable LASV candidate with proper virulence, high immune protection and easy-to-use oral administration. Vaccine. 2021;39:1383–1391. doi: 10.1016/j.vaccine.2021.01.059. [DOI] [PubMed] [Google Scholar]
- Wigley P. Salmonella enterica serovar Gallinarum: addressing fundamental questions in bacteriology sixty years on from the 9R vaccine. Avian Pathol. 2017;46:119–124. doi: 10.1080/03079457.2016.1240866. [DOI] [PubMed] [Google Scholar]
- Xia Z., Geng H., Cai Y., Wang Y., Sun D., Zhang J., Pan Z., Jiao X., Geng S. A MCAB-based direct competitive ELISA to detect O:9 Salmonella infection in chicken. Front. Vet. Sci. 2020;7:324. doi: 10.3389/fvets.2020.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Cheng Z., Xu L., Li Q., Geng S., Pan Z., Jiao X. Immunogenicity and protective efficacy of Salmonella enterica serovar Pullorum pathogenicity island 2 mutant as a live attenuated vaccine candidate. BMC Vet. Res. 2015;11:162. doi: 10.1186/s12917-015-0497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhuang L., Wang C., Zhang P., Zhang T., Shao H., Han X., Gong J. Virulence gene distribution of Salmonella Pullorum isolates recovered from chickens in China (1953-2015) Avian Dis. 2018;62:431–436. doi: 10.1637/11927-071318-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zeng X., Dai Q., Hou Y., Zhu D., Wang M., Jia R., Chen S., Liu M., Yang Q., Wu Y., Zhang S., Huang J., Ou X., Mao S., Gao Q., Zhang L., Liu Y., Yu Y., Cheng A. Immunogenicity and protection efficacy of a Salmonella enterica serovar Typhimurium fnr, arcA and fliC mutant. Vaccine. 2021;39:588–595. doi: 10.1016/j.vaccine.2020.12.002. [DOI] [PubMed] [Google Scholar]
- Zhou C., Liang J., Jiang W., He X., Liu S., Wei P. The effect of a selected yeast fraction on the prevention of pullorum disease and fowl typhoid in commercial breeder chickens. Poult. Sci. 2020;99:101–110. doi: 10.3382/ps/pez567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The construction of the S06004ΔspiCΔrfaH mutant was confirmed by the PCR. The rfaH (A) and spiC (B) genes deletion in the mutant confirmed by the PCR. Lane M, DL2000 DNA marker; lane 1: S06004 genomic DNA; lane 2: S06004ΔspiCΔrfaH genomic DNA. The wild type strain S06004 harbors the complete rfaH and spiC genes, with a PCR product length of 489 bp, and 384 bp, respectively, whereas the deletion mutant has no PCR band.