Abstract
This experiment was undertaken to investigate the effects of parental dietary DL-methionine (DL-Met) and DL-methionyl-DL-methionine (DL-Met-Met) supplementation on the intestinal development of young squabs. A total of 108 pairs of breeding pigeons and 432 one-day-old squabs were randomly divided into 3 groups: the control group (CON) was fed a basal diet (CP = 15%) and the experimental groups were fed a basal diet supplemented with 0.3% DL-Met or DL-Met-Met. Each pair of breeding pigeons nourished 4 young squabs, and 8 squabs from each treatment were randomly sampled at the end of the experiment. The results indicated that DL-Met and DL-Met-Met supplementation improved the intestinal morphology and structure in the squabs, as reflected by the increased relative intestinal weight of each small intestinal segment, villus height, and villus to crypt ratio. In addition, DL-Met and DL-Met-Met supplementation significantly increased the protein expression of cell proliferation markers (Ki67 and PCNA) and tight junction proteins (ZO-1 and Claudin-1) in the jejunum and strengthened the fluorescence signal intensity of Ki67, PCNA and Villin. Moreover, the expression of Wnt/β-catenin signaling pathway-related proteins (Frizzled 7 [FZD7], p-GSK-3β, Active β-catenin, β-catenin, TCF4, c-Myc, and Cyclin D1), and intestinal peptide transporter 1 (PepT1) in the jejunum was considerably higher in the treatment group than in the CON group (P < 0.05), with the DL-Met-Met group having the highest expression. Consistently, the molecular docking results predicted the possibility that DL-Met or DL-Met-Met binds to the membrane receptor FZD7, which mediates Wnt/β-catenin signaling. Collectively, the improvement of the intestinal development in squabs after parental dietary 0.3% DL-Met and DL-Met-Met supplementation could be through activation of Wnt/β-catenin signaling pathway, and DL-Met-Met is superior to DL-Met. Our findings may provide basic data for further optimizing the feeding formula of breeding pigeons and improving the growth and development of squabs.
Key words: pigeon, DL-Met-Met, intestinal development, Wnt/β-catenin signaling, PepT1
INTRODUCTION
The mortality of young squabs is as high as 15%, which is critically affected by intestinal development retardation, low utilization of nutrients and a high prevalence of intestinal diseases. Particularly, young pigeons are initially nourished by parents with crop milk containing mainly proteins and lipids (Dong et al., 2013; Chen et al., 2020). As pigeons grow, the crop milk is gradually replaced by grains, a transition characterized by abrupt removal from nurturing by parents and instantaneous exposure to solid feed (Sales and Janssens, 2003; Xie et al., 2019).
As a result, the gut architecture and permeability of the epithelial barrier are altered to adapt to these changes, resulting in excessive multiplication of potential pathogens in the intestinal tract and inducing stress (Spreeuwenberg et al., 2001; Dong et al., 2012). Therefore, it is widely believed that the dietary transition is a stressful event associated with intestinal impairment, most often manifested by villus atrophy and barrier dysfunction, especially in the small intestine (Lalles et al., 2004; Pieper et al., 2008; Wijtten et al., 2011). Nevertheless, the small intestine, especially the jejunum, is a primary site for the absorption of nutrients and it plays a vital role in determining the developmental potential of hatching birds (Uni et al., 1998; Liu et al., 2014).
Dietary amino acids are crucial for the maintenance of gut integrity and function. (Wu et al., 2014). As the first-limiting amino acid in diets for poultry, substantial studies have demonstrated that increased methionine (Met) provision above basal requirements can enhance growth performance and meat quality (Wen et al., 2014; Li et al., 2017; Park et al., 2018; Jiang et al., 2019). In addition, supplementation with DL-Met-Met is able to mitigate pathogenic infections and consequent intestinal cellular damage in broilers infected with Eimeria spp. (Khatlab et al., 2019). However, studies of the effects of DL-methionine (DL-Met) and DL-methionyl-DL-methionine (DL-Met-Met) on intestinal development in pigeons are still scarce.
Extensive evidence indicates the crucial role of Wnt/β-catenin signaling in maintaining the gut architecture and homeostasis. More importantly, it facilitates intestinal renewal by controlling cell proliferation and differentiation (Ootani et al., 2009; Clevers and Nusse, 2012; Schuijers et al., 2015). Interestingly, Met deprivation suppressed intestinal stem cell (ISC) proliferation and marker expression in the Drosophila midgut (Saito et al., 2017; Obata et al., 2018). Consistently, Met-depleted medium inhibits canonical Wnt/β-catenin signaling, while the addition of S-adenosylmethionine (SAM) is sufficient to rescue Wnt signaling (Albrecht et al., 2019). Importantly, Wnt signaling is mediated by the membrane receptor Frizzled7 (FZD7). Nevertheless, further in-depth research is required to reveal the exact mechanism by which DL-Met or DL-Met-Met promotes the intestinal development of pigeons and how Wnt/β-catenin signaling exerts its function in response to DL-Met or DL-Met-Met.
Given the peculiar feeding pattern of breeding pigeons, precise nutrition provision to the parents seems to be an effective method to improve the health status of squabs. Therefore, we explored whether appropriate DL-Met and DL-Met-Met supplementation could improve the intestinal structural morphology and barrier function of squabs and evaluated the effects of DL-Met and DL-Met-Met on the intestinal development of squabs.
MATERIALS AND METHODS
Ethical Statement
All methods and management procedures used in this study were complied with the guidelines established by South China Agricultural University (Guangzhou, China), and the protocol for this experiment was approved by the Animal Ethics Committee of South China Agricultural University (Guangzhou, China).
Animals and Experimental Treatments
DL-Met (purity >99%) and DL-Met-Met (purity >95%) purchased from Evonik Degussa (Frankfurt, Germany) were added to the basal diet at a dose of 0.3% (CP = 15%). The methionine level is the optimal doses based on our previous study (Chen et al., 2020).
White king pigeons and experimental sites were provided by WENS Foodstuff Group Co., Ltd. (Yingde, China). A total of 108 pairs of white king breeding pigeons with similar production performance, reproduction performance, and laying eggs on the same day were selected and randomly allocated to 3 treatments, which consisted of 6 replications of 6 pairs of pigeons each. The three experimental diets were formulated as follows: control group (basal diet), 0.3% DL-Met supplementation group and 0.3% DL-Met-Met supplementation group (Table 1). Each pair of breeding pigeons was tending 4 young squabs. The parent pigeons were fed the experimental diets continually for the entire experimental period (46 d), including during the egg incubation period and the feeding period. The squabs were fed crop milk secreted by the parent pigeons. On the 46th d, eight squabs per treatment were euthanized to collect intestine samples and to measure the unit intestinal weight of each section of the small intestine.
Table 1.
Compositions and nutrient levels of the experimental diets (air-dried basis, %).
| Ingredient (%)1 | Basal diet | 0.30% DL-Met | 0.30% DL-Met-Met |
|---|---|---|---|
| Corn | 51.00 | 51.00 | 51.00 |
| Soybean meal | 17.50 | 17.50 | 17.50 |
| Wheat | 24.00 | 24.00 | 24.00 |
| Sorghum | 3.00 | 3.00 | 3.00 |
| Dicalcium phosphate | 1.20 | 1.20 | 1.20 |
| Shell powder | 1.00 | 1.00 | 1.00 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vitamin and mineral premix2 | 1.00 | 1.00 | 1.00 |
| Lysine (98.5%) | 0.40 | 0.40 | 0.40 |
| DL-Methionine (DL-Met) | 0.00 | 0.30 | 0.00 |
| DL-Methionyl-DL-Methionine (DL-Met-Met) | 0.00 | 0.00 | 0.30 |
| Zeolite powder | 0.60 | 0.30 | 0.30 |
| Total | 100.00 | 100.00 | 100.00 |
| Formulated nutrient composition (%) | |||
| Metabolic energy (MJ/kg) | 12.13 | 12.13 | 12.13 |
| Crude protein | 15.00 | 15.00 | 15.00 |
| Calcium | 0.76 | 0.76 | 0.76 |
| Total phosphorus | 0.56 | 0.56 | 0.56 |
| Nonphytate phosphorus | 0.32 | 0.32 | 0.32 |
| DL-Met | 0.25 | 0.55 | 0.25 |
| DL-Met-Met | - | - | 0.30 |
| Lysine | 0.96 | 0.96 | 0.96 |
Provided per kilogram of diet: vitamin A, 4,000.00 IU; vitamin D3, 1,725.00 IU; vitamin E, 24.00 mg; vitamin K3, 1.00 mg; vitamin B1, 3.00 mg; vitamin B2, 13.00 mg; vitamin B6, 2.00 mg; vitamin B12, 25.00 µg; niacin, 15.00 mg; folic acid, 0.55 mg; pantothenic acid, 7.50 mg; biotin, 0.12 mg; choline chloride, 200.00 mg; copper, 10.00 mg; iron, 35.00 mg; manganese, 55.00 mg; zinc, 35.00 mg; iodine, 0.20 mg; selenium, 0.25 mg.
Hematoxylin and Eosin Staining
After fixing with 4% paraformaldehyde, the jejunum samples were washed with water, dehydrated using graded ethanol, immersed in wax and made into paraffin-embedded tissues for subsequent histological analysis. Then, 5-μm thick serial sections of the paraffin-embedded tissues were cut by a microtome (Microm-HM340E, Thermo Fisher Scientific), attached to glass slides and stained with hematoxylin and eosin (H & E). Finally, the jejunum tissues were observed and photographed under a microscope (Eclipse Ti-S, Nikon, Melville, NY), and the villus height and crypt depth were measured with Image-Pro Plus software.
Western Blotting
Proteins were isolated from jejunum samples and analyzed as described previously (Xie et al., 2020). The expression levels were quantified using ImageJ software (version 1.8.0 112, National Institute of Health, Bethesda, MD). The antibodies used were: anti-ZO-1 (#339100, Thermo Fisher, Waltham, MA), anti-Claudin-1 (#374900, Thermo Fisher), anti-Frizzled7 (FZD7,#bs-5125R, Bioss ANTIBODIES, Beijing, China), anti-p-GSK-3β (#5558, Cell Signaling Technology, Danvers, MA), anti-GSK-3β (#340449, Zen BioScience, Chengdu, Sichuan, China), anti-Active β-Catenin (#19807, CST), anti-β-catenin (#201328, Zen BioScience), anti-T cell factor (TCF4, ab130014, Abcam, Cambridge, MA), anti-CyclinD1 (#SC-450, Santa Cruz Biotechnology, Dallas, TX), anti-c-Myc (#SC-41, Santa Cruz), anti-proliferating cell nuclear antigen (PCNA, #200947, Zen BioScience), anti-PepT1(#SC-373742, Santa Cruz), anti-β-actin (#250132, Zen BioScience), and anti-rabbit IgG (#511203, Zen BioScience) and anti-mouse IgG (#511103, Zen BioScience) secondary antibodies.
Immunofluorescence
The jejunum sections were placed on glass slices as described previously (Chen et al., 2020). Briefly, the samples were blocked in 5% BSA for 2 h and incubated with primary antibodies against ZO-1 (#bs-1329R, Bioss), Ki67 (#NB500-170, Novus Biologicals, Littleton, CO), PCNA (#2586, CST, Danvers, MA), Villin (#SC-58897, Santa Cruz), FZD7 (#bs-5125R, Bioss) and PepT1 (#SC-373742, Santa Cruz) overnight at 4°C and with FITC-(#115-545-003, Jackson Laboratory, Jackson, MS) or Cy3-conjugated secondary antibodies (#111-165-045, Jackson Laboratory) for 2 h at room temperature. The nuclei were stained with DAPI (Sigma-Aldrich, Burlington, MA) for 20 min at room temperature. Fluorescence images were acquired with a microscope (NIS-Elements, Nikon, Tokyo, Japan).
Molecular Docking
The three-position format of DL-Met (Zinc1532529) or DL-Met-Met (ZINC1605259) and the crystal structure of the FZD7 cystine-rich region (10.2210/pdb5T44/pdb) were obtained from ZINC (http://zinc.docking.org/) and Protein Data Bank (https://www.rcsb.org/). Autodock serials software was used for molecular structure optimization, conformation search, and the molecular docking process. Meanwhile, PyMOL was used for the molecular graphic display to screen the binding sites of DL-Met or DL-Met-Met with FZD7 that could be involved in flexible docking.
Statistics
The data were analyzed using one-way ANOVA with SPSS software version 20 (SPSS Inc., Chicago, IL). The t test and LSD multiple range test were used to evaluate the differences between 2 or multiple groups. The results are expressed as the mean ± standard error, and P-values < 0.05 were considered significant.
RESULTS
DL-Met and DL-Met-Met Improve Growth Performance and Epithelial Structural Integrity
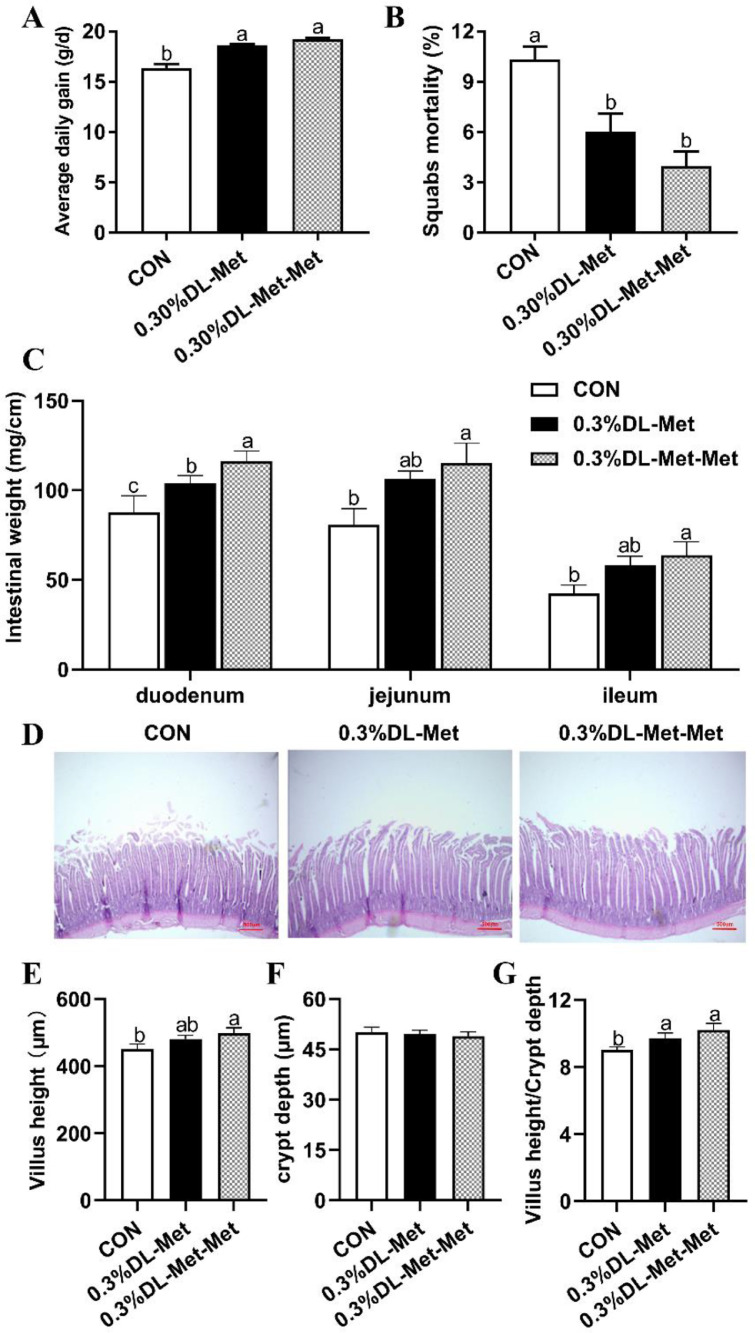
To elucidate the effect of DL-Met and DL-Met-Met on the growth performance and intestinal development of squabs, we recorded related indicators of squab growth performance, measured the length and weight of the duodenum, jejunum, and ileum, and examined the morphology of the villi by H&E staining. The results showed a significant increase in average daily gain and a decrease in the mortality of the squabs after DL-Met or DL-Met-Met supplementation (P < 0.05, Figures 1A and 1B). Moreover, compared with the CON group, DL-Met-Met supplementation significantly increased the small intestinal weight of each section (P < 0.05, Figure 1C), while the DL-Met group only had a significant increase in the duodenum. H&E staining indicated that compared with the CON group, parental supplementation with DL-Met-Met significantly increased the height of the villi and the villous crypt ratio (VCR) (P < 0.05, Figures 1D–1G), although the crypt depth in the jejunum was not significantly different among all groups.
Figure 1.
DL-methionine and DL-methionyl-DL-methionine improve growth performance and epithelial structural integrity. (A, B) Growth performance of squabs. (A) Average daily gain; (B) mortality; (C) unit intestinal weight; (D) representative images of H&E staining of the jejunums are shown (40 ×). Scale bar = 500 μm. (E–G) Statistical analysis of villus height (E), crypt depth (F), and villous crypt ratio (G). “a-c” indicates significant differences (P < 0.05). The values are mean ±S.E.M. with n = 8.
DL-Met and DL-Met-Met Increase Abundance of Intestinal Tight Junction Proteins
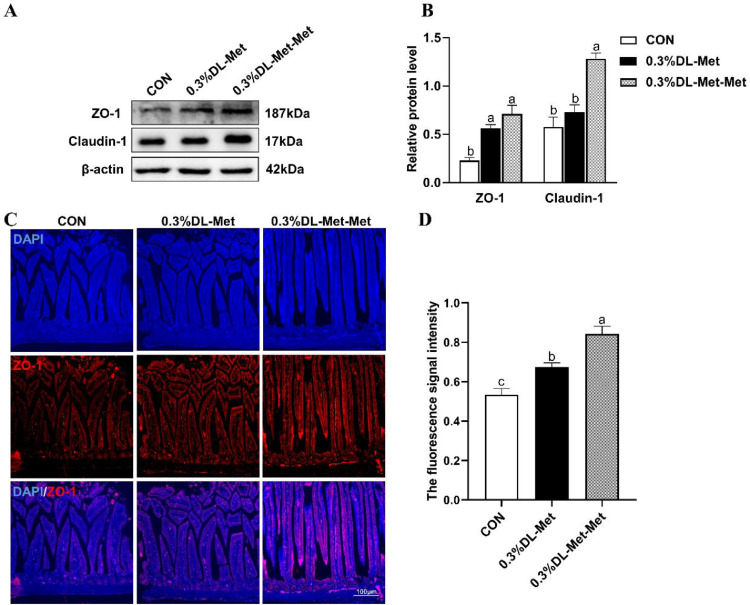
To clarify whether DL-Met and DL-Met-Met supplementation enhances the intestinal development in squabs, we detected the expression of the tight junction proteins ZO-1 and Claudin1. As shown in Figure 2, compared with the CON group, the expression of ZO-1 and Claudin-1 in the jejunum were significantly increased after DL-Met-Met supplementation (P < 0.05, Figures 2A and 2B), as was the fluorescence signal intensity of ZO-1 (Figures 2C and 2D).
Figure 2.
DL-methionine and DL-methionyl-DL-methionine increase abundance of intestinal tight junction proteins. (A) The expression of ZO-1 and Claudin-1 in the jejunum of squabs raised by pigeons supplemented DL-Met or DL-Met-Met. (B) Statistical analysis of the expression of ZO-1 and Claudin-1 based on the images shown in (A). (C) Representative images of IF staining with ZO-1 antibodies of the pigeon jejunum (100 ×). Scale bar = 100 μm. (D) Statistical analysis of the expression of ZO-1 based on the images shown in (C). “a-b” indicates significant differences (P < 0.05). The values are mean ±S.E.M. with n = 3.
DL-Met and DL-Met-Met Promote the Proliferation and Differentiation of Intestinal Cells
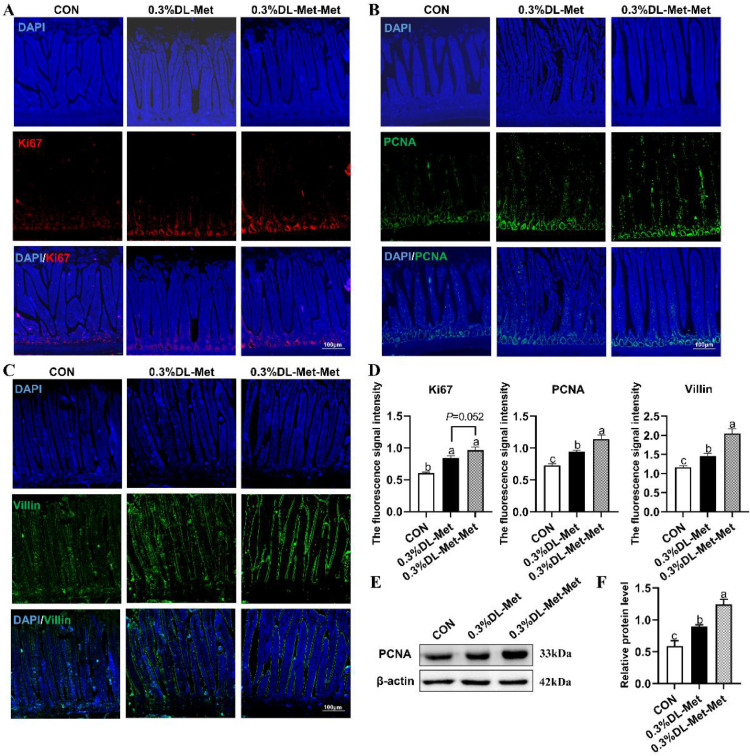
Subsequently, we tested the effects of DL-Met and DL-Met-Met on the proliferation and differentiation of intestinal epithelial cells. A stronger fluorescence signal intensity of Ki67 (a marker of proliferating cells) (P < 0.05, Figures 3A and 3D), PCNA (P < 0.05, Figures 3B and 3D) and Villin (marker of absorptive cells) (P < 0.05, Figures 3C–3D), coupled with increased expression of PCNA (a marker of proliferating cells) (P < 0.05, Figures 3E and 3F), was observed in squab jejunum after parental dietary DL-Met and DL-Met-Met supplementation, suggesting that DL-Met promotes the differentiation of ISCs into epithelial absorptive cells.
Figure 3.
DL-methionine and DL-methionyl-DL-methionine promote the proliferation and differentiation of intestinal cells. (A–C) Representative images of IF staining with Ki67 (A), PCNA (B), and Villin (C) antibodies of the pigeon jejunum (100 ×). Scale bar = 100 μm. (D) Statistical analysis of the expression of Ki67, PCNA and Villin based on the images shown in (A–C). (E) The expression of PCNA in the jejunum of squabs raised by pigeons supplemented with DL-Met or DL-Met-Met. (F) Statistical analysis of the expression of PCNA based on the images shown in (E) “a-c” indicates significant differences (P < 0.05). The values are mean ±S.E.M. with n = 3.
DL-Met and DL-Met-Met Potentiate PepT1 Expression
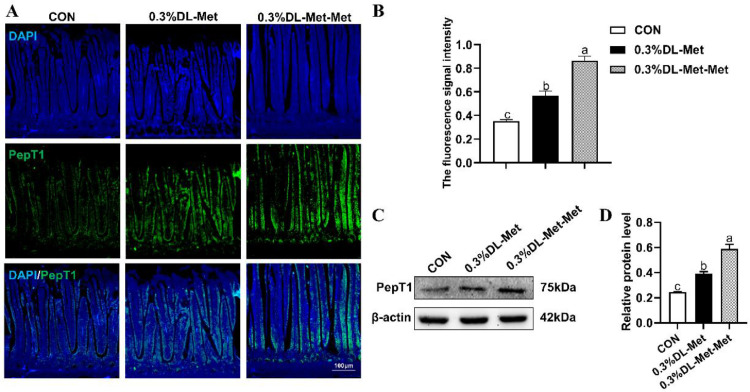
In addition, immunofluorescence (Figures 4A and 4B), together with western blotting (Figures 4C and 4D), showed that the protein expression of intestinal peptide transporter 1 (PepT1) in the treatment group was significantly increased (P < 0.05) in comparison with that in the CON group, and the DL-Met-Met group exhibited the highest expression of PepT1.
Figure 4.
DL-methionine and DL-methionyl-DL-methionine potentiate PepT1 expression. (A) Representative images of IF staining with PepT1 antibody in the pigeon jejunum (100 ×). Scale bar = 100 μm. (B) Statistical analysis of the fluorescence signal intensity based on the images shown in (A). (C) The expression of PepT1 in the jejunum of squabs raised by pigeons supplemented with DL-Met or DL-Met-Met. (D) Statistical analysis of the expression of PepT1 based on the images shown in (C). “a-c” indicates significant differences (P < 0.05). The values are mean ±S.E.M. with n =3.
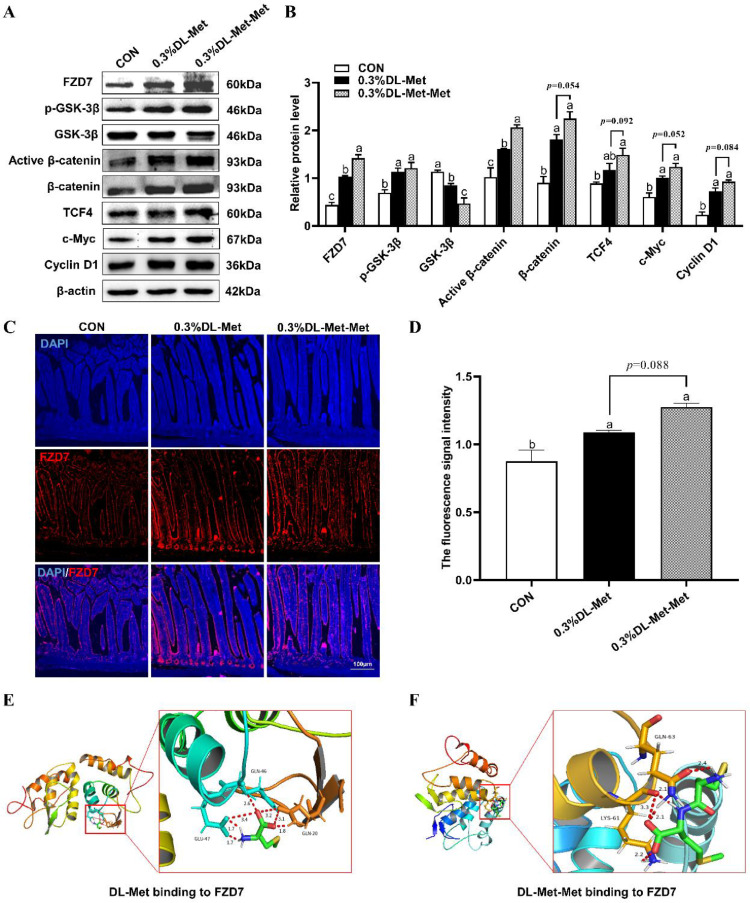
DL-Met and DL-Met-Met Intensify Wnt/β-Catenin Activity by Upregulating the Expression of Membrane Receptor FZD7
Next, we explored the probable mechanism of DL-Met or DL-Met-Met in promoting intestinal development and barrier function. We found that the Wnt/β-catenin pathway was significantly upregulated after DL-Met or DL-Met-Met supplementation. Specifically, the GSK-3β level was decreased, and the levels of FZD7, p-GSK-3β, Active β-catenin, β-catenin, TCF4, c-Myc, and Cyclin D1 were increased (Figures 5A and 5B). Consistently, the fluorescence signal intensity of FZD7 was significantly increased (P < 0.05, Figures 3B and 3C). The docking results predicted that there were 5 or 7 hydrogen bonds between DL-Met or DL-Met-Met and the cysteine-rich region of FZD7, suggesting that DL-Met or DL-Met-Met might bind to the subdomain of FZD7. The docking calculations showed the lowest binding energy conformer, with binding energies of −2.86 and −1.72 kcal/mol, respectively (Figures 5E and 5F).
Figure 5.
DL-methionine and DL-methionyl-DL-methionine increase abundance of FZD7 and activate Wnt/β-catenin signaling pathway in pigeon jejunal tissue. (A) The expression of Wnt/β-catenin signaling pathway-associated proteins in the jejunum of pigeons with DL-Met or DL-Met-Met. (B) Statistical analysis of the expression of target proteins based on the images shown in (A). (C) Representative images of IF staining with FZD7 antibodies of the pigeon jejunum (100 ×). Scale bar = 100 μm. (D) Statistical analysis of the expression of FZD7 based on the images shown in (C). (E, F) Binding mode of DL-Met to FZD7 (E) and DL-Met-Met to FZD7 (F). The protein is shown in cartoon form. Surrounding residues are represented in stick form. The dashed lines indicate the formation of hydrogen bonds. The numbers beside the dash show the distance (Å) of hydrogen bonds. “a-c” indicates significant differences (P < 0.05). The values are mean ±S.E.M. with n = 3.
DISCUSSION
The intestines are the most important organs wherein most nutrients are absorbed (Feng et al., 2019). Desirable dietary interventions targeting parental pigeons are proposed to be a good strategy to facilitate the maintenance of intestinal health status and homeostasis in squabs (Xu et al., 2020a,b). It is now generally accepted that intestinal functions are reflected by the intestinal size and structure, especially the villus height and crypt depth. Our present study demonstrates that parental supplementation of DL-Met and DL-Met-Met resulted in a heavier intestinal weight, especially in the duodenum, compared with that of the CON group. In addition, a significant increase in villus height was observed in the DL-Met and DL-Met-Met groups. Increased intestinal weight and villus height are usually associated with an enhancement of digestive and absorptive capacity by the small intestine, which in turn improves the health status of the animals (Upadhaya et al., 2020). Furthermore, the VCR in the jejunum was significantly increased in squabs by parental dietary DL-Met and DL-Met-Met supplementation. A higher VCR generally equates to a more absorptive area, and fewer intestinal epithelial cells (IECs) are recruited for cell renewal; therefore, more abundant enterocytes are available for the digestion and absorption of nutrients (Qin et al., 2019). Collectively, DL-Met and DL-Met-Met enhanced intestinal development by improving the small intestinal morphology, and significant improvements were observed in the DL-Met-Met group compared with the DL-Met group.
The intestinal barrier is a semipermeable structure that is comprised of tightly attached continuous monolayer IECs and tight junctions (TJs) between enterocytes. The TJs serve as a protective barrier that allows for the uptake of necessary nutrients while restricting pathogenic molecules and bacteria from the external environment from escaping from the intestinal lumen (Chelakkot et al., 2018). It has been confirmed that compromised intestinal barrier function contributes to the risk of a variety of intestinal and systemic diseases (Odenwald and Turner, 2017). Importantly, transmembrane protein complexes (e.g., claudins and occludin) and peripheral membrane proteins (e.g., ZO-1 and ZO-2) together constitute TJs. The assembly and combination of diverse TJs are of premier importance to seal the intercellular space and further regulate the paracellular and transcellular pathways. Interestingly, Claudin-ZO protein interactions, which link claudins to the actin cytoskeleton through scaffold proteins such as ZO-1, are essential for tight junction assembly (Findley and Koval, 2009). Our study demonstrated that parental dietary DL-Met-Met improved the intestinal development of squabs, as shown by significantly increased expression of tight junction proteins in the jejunum (ZO-1 and Claudin-1). Based on the size-selective sieving of molecules by TJs, different claudin isoforms may be differentially expressed to perform their corresponding functions (Peltonen et al., 2007). This might explain the inconsistent expression of claudin-1 and ZO-1 in the DL-Met group.
Enterocytes express tight junction proteins that modulate the permeability of the epithelium and thereby maintain the intestinal integrity (Peterson and Artis, 2014; Ahmad et al., 2017). Moreover, constant self-renewal of IECs depends on vigorous proliferation of ISCs in crypts. Consequently, the proliferation of ISCs is indispensable for sustaining the integrity of the intestinal structure and barrier function. Both Ki67 and PCNA are markers of cell proliferation. In the present study, DL-Met and DL-Met-Met supplementation significantly increased the expression of Ki67 and PCNA in the jejunum, indicating that DL-Met and DL-Met-Met stimulate the prompt renewal of the intestinal epithelium by accelerating cell proliferation. During development, IECs differentiate into firmly cohesive polarized cells, wherein many microvilli could increase the cell surface contact area and favor absorptive and secretory functions (Lhocine et al., 2015). In particular, as a plentiful actin-modifying protein in the epithelial brush border, Villin participates in the formation of the epithelial structure and the function of the microvilli (Delacour et al., 2016). Our study showed that Villin was highly expressed after DL-Met or DL-Met-Met supplementation (especially DL-Met-Met), suggesting that DL-Met and DL-Met-Met could maintain the architecture of the IECs and enhance their absorptive capacity.
The maintenance of stem cells in the intestine involves complex interactions of multiple signal transduction pathways, especially Wnt/β-catenin (van der Flier and Clevers, 2009; Merenda et al., 2020). More importantly, attenuating Wnt/β-catenin leads to decreased cell proliferation capacity and intestinal dysfunction (Hu et al., 2018; Li et al., 2019). Similarly, our previous study demonstrated that Met or HMB ameliorates deoxynivalenol-induced ISC injury through Wnt/β-catenin signaling reactivation, which promotes ISC expansion and further drives intestinal epithelial regeneration (Zhou et al., 2019). Previous study have shown that the methionine metabolite SAM might be activating wnt signaling (Albrecht et al., 2019), However, whether DL-Met can act on the Wnt/β-catenin pathway in other fashion is poorly understood. In the current study, we confirmed that parental dietary DL-Met or DL-Met-Met resulted in a higher level of FZD7 in the squab jejunum. Similarly, molecular docking analysis predicted the possible sites where DL-Met or DL-Met-Met might bind to the membrane receptor FZD7.
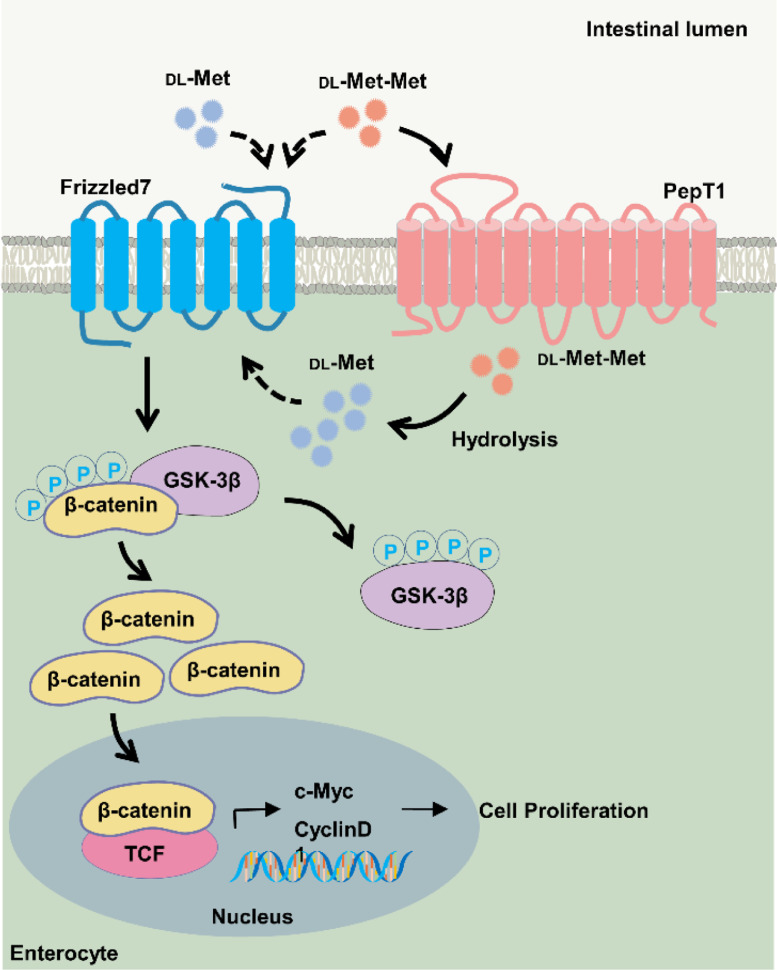
Based on our results, we speculate that the direct binding between DL-Met or DL-Met-Met and FZD7 leads to enhanced activity of the canonical Wnt pathway, which promotes the phosphorylation of GSK-3β and inhibits GSK-3β (Figure 6). As a consequence, the protein complex (including CK1α, GSK3, Axin1, and APC) falls apart, and unphosphorylated β-catenin is stabilized and accumulates, subsequently translocating into the nucleus and binding to TCF to induce the expression of its target genes (Nusse and Clevers, 2017). Interestingly, DL-Met-Met seems to be more efficient than DL-Met, which may be explained by the fact that the high transport capacity of PepT1 activated by DL-Met-Met allows intestinal uptake of DL-Met (Daniel, 2004; Flanagan et al., 2019). Furthermore, DL-Met-Met might bind to FZD7 directly or be transported into the cytoplasm by PepT1 to be hydrolyzed to the corresponding DL-Met, which engages with FZD7, which in turn enhances Wnt/β-catenin signaling activity. Nonetheless, further research should be performed to reveal the exact mechanism by which DL-Met or DL-Met-Met regulates Wnt/β-catenin during intestinal development.
Figure 6.
Integrated model depicts the mechanism by which DL-methionine and DL-methionyl-DL-methionine intensify FZD7-mediated Wnt/β-catenin signaling activity and the pathway involved in the cellular uptake of DL-methionyl-DL-methionine mediated by PepT1 in the brush border membrane of enterocytes.
In the jejunum, PepT1 is abundantly expressed specifically in well-differentiated absorptive epithelial cells in the villi, shedding light on the pivotal role of PepT1 in the absorption process (Nguyen, et al., 2007; Dranse et al., 2018). Through the PepT1 KO mouse model, Zhang et al. (2016) demonstrated that PepT1 critically modulates miRNA and protein expression directly or indirectly along the crypt/villus axis in the jejunum and thus facilitates the maintenance of intestinal homeostasis and proper functions. Consistent with previous results, the immunofluorescence of PepT1 showed that the highest expression was detectable at the villus tip, while little expression was detectable at lower crypt cells (Spanier and Rohm, 2018). It is plausible that the expression gradient of PepT1 along the crypt-villus axis is quite important in intestinal function and the maintenance of normal intestinal epithelial integrity. Notably, the DL-Met-Met group exhibited the highest expression of PepT1, which may be attributed to the fact that Met-Met serves as a PepT1 substrate to activate the PepT1 promoter and enhance the transcriptional activity of PepT1 (Wang et al., 2017). Furthermore, PepT1 activity may be directly regulated by amino acids (Shiraga et al., 1999). However, the regulatory mechanisms by which DL-Met acts on PepT1 still require further investigation.
CONCLUSIONS
Our study found that parental dietary supplementation with DL-Met and DL-Met-Met effectively increases intestinal development in squabs, we speculate that the reason for this might be the reinforcement of PepT1 expression and Wnt/β-catenin signaling activity (Figure 6). Most importantly, DL-Met-Met appears to be more efficient than DL-Met. Our findings support a potential nutritional manipulation strategy to improve the growth and intestinal development of squabs.
Acknowledgments
ACKNOWLEDGMENTS
This work was jointly supported by the National Natural Science Foundation of China (32172746), the Natural Science Foundation of Guangdong Province, China (2019B1515210001) and the Technical System of Poultry Industry of Guangdong Province, China (2021KJ128).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
REFERENCES
- Ahmad R., Sorrell M.F., Batra S.K., Dhawan P., Singh A.B. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal. Immunol. 2017;10:307–317. doi: 10.1038/mi.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht L.V., Bui M.H., De Robertis E.M. Canonical Wnt is inhibited by targeting one-carbon metabolism through methotrexate or methionine deprivation. Proc. Natl. Acad. Sci. U.S.A. 2019;116:2987–2995. doi: 10.1073/pnas.1820161116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018;50:103. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.J., Pan N.X., Wang X.Q., Yan H.C., Gao C.Q. Methionine promotes crop milk protein synthesis through the JAK2-STAT5 signaling during lactation of domestic pigeons (Columba livia) Food Funct. 2020;11:10786–10798. doi: 10.1039/d0fo02257h. [DOI] [PubMed] [Google Scholar]
- Chen M.J., Fu Z., Jiang S.G., Wang X.Q., Yan H.C., Gao C.Q. Targeted disruption of TORC1 retards young squab growth by inhibiting the synthesis of crop milk protein in breeding pigeon (Columba livia) Poult. Sci. 2020;99:416–422. doi: 10.3382/ps/pez513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- Delacour D., Salomon J., Robine S., Louvard D. Plasticity of the brush border - the yin and yang of intestinal homeostasis. Nat. Rev. Gastro. Hepat. 2016;13:161–174. doi: 10.1038/nrgastro.2016.5. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Dai L., Azzam M.M.M., Wang C., Zou X.T. Posthatch development of intestinal morphology and digestive enzyme activities in domestic pigeons (Columba livia) Poult. Sci. 2012;91:1886. doi: 10.3382/ps.2011-02091. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Song H.H., Zou X.T. Effects of in ovo injection of carbohydrate solution on small intestine development in domestic pigeons (Columba livia) J. Anim. Sci. 2013;91:3742–3749. doi: 10.2527/jas.2013-6400. [DOI] [PubMed] [Google Scholar]
- Dranse H.J., Waise T.M.Z., Hamr S.C., Bauer P.V., Abraham M.A., Rasmussen B.A., Lam T.K.T. Physiological and therapeutic regulation of glucose homeostasis by upper small intestinal PepT1-mediated protein sensing. Nat. Commun. 2018;9:1118. doi: 10.1038/s41467-018-03490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Chen S.J., Zhang L.J., Qu W., Chen Z.G. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019;254:112960. doi: 10.1016/j.envpol.2019.112960. [DOI] [PubMed] [Google Scholar]
- Findley M.K., Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61:431–437. doi: 10.1002/iub.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan D.J., Barker N., Costanzo N.S.D., Mason E.A., Gurney A., Meniel V.S., Koushyar S., Austin C.R., Ernst M., Pearson H.B., Boussioutas A., Clevers H., Phesse T.J., Vincan E. Frizzled-7 is required for Wnt signaling in gastric tumors with and without mutations. Cancer. Res. 2019;79:970–981. doi: 10.1158/0008-5472.CAN-18-2095. [DOI] [PubMed] [Google Scholar]
- Hu Y., Yu K., Wang G., Zhang D., Shi C., Ding Y., Hong D., Zhang D., He H., Sun L., Zheng J.N., Sun S., Qian F. Lanatoside C inhibits cell proliferation and induces apoptosis through attenuating Wnt/β-catenin/c-Myc signaling pathway in human gastric cancer cell. Biochem. Pharmacol. 2018;150:280–292. doi: 10.1016/j.bcp.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Jiang S., Pan N., Chen M., Wang X., Yan H., Gao C. Effects of dietary supplementation with dl-Methionine and dl-Methionyl-dl-Methionine in breeding pigeons on the carcass characteristics, meat quality and antioxidant activity of squabs. Antioxidants Basel. 2019;8:435. doi: 10.3390/antiox8100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatlab A.S., Del Vesco A.P., Rodrigues Oliveira Neto A., Almeida F.L.A., Gasparino E. Dietary supplementation with free methionine or methionine dipeptide improves environment intestinal of broilers challenged with Eimeria spp. J. Anim. Sci. 2019;97:4746–4760. doi: 10.1093/jas/skz339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalles J., Boudry G., Favier C., Le Floc'h N., Lurona I., Montagne L., Oswald I., Pie S., Piel C., Seve B. Gut function and dysfunction in young pigs: physiology. Anim. Res. 2004;53:301–316. [Google Scholar]
- Lhocine N., Arena E., Bomme P., Ubelmann F., Prévost M., Robine S., Sansonetti P. Apical invasion of intestinal epithelial cells by Salmonella typhimurium requires villin to remodel the brush border actin cytoskeleton. Cell. Host. Microbe. 2015;17:164–177. doi: 10.1016/j.chom.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Lee C., Cadete M., Zhu H., Koike Y., Hock A., Wu R., Botts S., Minich A., Alganabi M., Chi L., Zani-Ruttenstock E., Miyake H., Chen Y., Mutanen A., Ngan B., Johnson-Henry K., De Coppi P., Eaton S., Määttänen P., Delgado-Olguin P., Sherman P., Zani A., Pierro A.J.C.d. Impaired Wnt/β-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell. Death. Dis. 2019;10:743. doi: 10.1038/s41419-019-1987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang H., Chen Y., Ying Z., Su W., Zhang L., Wang T. Effects of dietary l-methionine supplementation on the growth performance, carcass characteristics, meat quality, and muscular antioxidant capacity and myogenic gene expression in low birth weight pigs. J. Anim. Sci. 2017;95:3972–3983. doi: 10.2527/jas2017.1652. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang J., Zhang S., Yang F., Thacker P., Zhang G., Qiao S., Ma X. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J. Agr. Food. Chem. 2014;62:860–866. doi: 10.1021/jf403288r. [DOI] [PubMed] [Google Scholar]
- Merenda A., Fenderico N., Maurice M.M. Wnt signaling in 3D: recent advances in the applications of intestinal organoids. Trends. Cell. Biol. 2020;30:60–73. doi: 10.1016/j.tcb.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Nguyen H., Charrier-Hisamuddin L., Dalmasso G., Hiol A., Sitaraman S., Merlin D. Association of PepT1 with lipid rafts differently modulates its transport activity in polarized and nonpolarized cells. Am. J. Physiol. Gastr. L. 2007;293:G1155–G1165. doi: 10.1152/ajpgi.00334.2007. [DOI] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Obata F., Tsuda-Sakurai K., Yamazaki T., Nishio R., Nishimura K., Kimura M., Funakoshi M., Miura M. Nutritional control of stem cell division through S-Adenosylmethionine in Drosophila intestine. Dev. Cell. 2018;44:741–751. doi: 10.1016/j.devcel.2018.02.017. e743. [DOI] [PubMed] [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastro. Hepat. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootani A., Li X., Sangiorgi E., Ho Q.T., Ueno H., Toda S., Sugihara H., Fujimoto K., Weissman I.L., Capecchi M.R., Kuo C.J. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Pasquetti T., Malheiros R.D., Ferket P.R., Kim S.W. Effects of supplemental L-methionine on growth performance and redox status of turkey poults compared with the use of DL-methionine. Poult. Sci. 2018;97:102–109. doi: 10.3382/ps/pex259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen S., Riehokainen J., Pummi K., Peltonen J. Tight junction components occludin, ZO-1, and claudin-1,-4 and-5 in active and healing psoriasis. Br. J. Dermatol. 2007;156:466–472. doi: 10.1111/j.1365-2133.2006.07642.x. [DOI] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Pieper R., Janczyk P., Zeyner A., Smidt H., Guiard V., Souffrant W.B. Ecophysiology of the developing total bacterial and lactobacillus communities in the terminal small intestine of weaning piglets. Microb. Eco. 2008;56:474–483. doi: 10.1007/s00248-008-9366-y. [DOI] [PubMed] [Google Scholar]
- Qin L., Ji W., Wang J., Li B., Hu J., Wu X. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food. Funct. 2019;10:2359–2371. doi: 10.1039/c8fo02327a. [DOI] [PubMed] [Google Scholar]
- Saito Y., Iwatsuki K., Hanyu H., Maruyama N., Aihara E., Tadaishi M., Shimizu M., Kobayashi-Hattori K. Effect of essential amino acids on enteroids: methionine deprivation suppresses proliferation and affects differentiation in enteroid stem cells. Biochem. Bioph. Res. Co. 2017;488:171–176. doi: 10.1016/j.bbrc.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Sales J., Janssens G.P.J. Nutrition of the domestic pigeon (Columba livia domestica) World Poult. Sci. J. 2003;59:221–232. doi: 10.1093/ps/82.9.1457. [DOI] [PubMed] [Google Scholar]
- Schuijers J., Junker J.P., Mokry M., Hatzis P., Koo B.K., Sasselli V., van der Flier L.G., Cuppen E., van Oudenaarden A., Clevers H. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell. 2015;16:158–170. doi: 10.1016/j.stem.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Shiraga T., Miyamoto K., Tanaka H., Yamamoto H., Taketani Y., Morita K., Tamai I., Tsuji A., Takeda E. Cellular and molecular mechanisms of dietary regulation on rat intestinal H+/Peptide transporter PepT1. Gastroenterology. 1999;116:354–362. doi: 10.1016/s0016-5085(99)70132-0. [DOI] [PubMed] [Google Scholar]
- Spanier B., Rohm F. Proton coupled oligopeptide transporter 1 (PepT1) function, regulation, and influence on the intestinal homeostasis. Compr. Physiol. 2018;8:843–869. doi: 10.1002/cphy.c170038. [DOI] [PubMed] [Google Scholar]
- Spreeuwenberg M.A., Verdonk J.M., Gaskins H.R., Verstegen M.W. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001;131:1520–1527. doi: 10.1093/jn/131.5.1520. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ganot S., Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult. Sci. 1998;77:75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- Upadhaya S.D., Jiao Y., Kim Y.M., Lee K.Y., Kim I.H. Coated sodium butyrate supplementation to a reduced nutrient diet enhanced the performance and positively impacted villus height and faecal and digesta bacterial composition in weaner pigs. Anim. Feed. Sci. Tech. 2020:265. [Google Scholar]
- van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Liu S., Xie X.N., Tan Z.R. Regulation profile of the intestinal peptide transporter 1 (PepT1) Drug. Des. Dev. Ther. 2017;11:3511–3517. doi: 10.2147/DDDT.S151725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Chen X., Chen G.Y., Wu P., Chen Y.P., Zhou Y.M., Wang T. Methionine improves breast muscle growth and alters myogenic gene expression in broilers. J. Anim. Sci. 2014;92:1068–1073. doi: 10.2527/jas.2013-6485. [DOI] [PubMed] [Google Scholar]
- Wijtten P.J.A., van der Meulen J., Verstegen M.W.A. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Dai Z., Li D., Wang J., Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014;2:387–417. doi: 10.1146/annurev-animal-022513-114113. [DOI] [PubMed] [Google Scholar]
- Xie W.Y., Chen M.J., Jiang S.G., Yan H.C., Wang X.Q., Gao C.Q. The Wnt/β-catenin signaling pathway is involved in regulating feather growth of embryonic chicks. Poult. Sci. 2020;99:2315–2323. doi: 10.1016/j.psj.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.Y., Fu Z., Pan N.X., Yan H.C., Wang X.Q., Gao C.Q. Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the TOR signaling pathway in the domestic pigeon (Columba livia) Poult. Sci. 2019;98:5514–5524. doi: 10.3382/ps/pez296. [DOI] [PubMed] [Google Scholar]
- Xu Q.Q., Ma X.W., Dong X.Y., Tao Z.R., Lu L.Z., Zou X.T. Effects of parental dietary linoleic acid on growth performance, antioxidant capacity, and lipid metabolism in domestic pigeons (Columba livia) Poult. Sci. 2020;99:1471–1482. doi: 10.1016/j.psj.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.Q., Wen J.S., Wang X.M., Zou X.T., Dong X.Y. Maternal dietary linoleic acid altered intestinal barrier function in domestic pigeons (Columba livia) Br. J. Nutr. 2020;126:1003–1016. doi: 10.1017/S0007114520004973. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Viennois E., Zhang M., Xiao B., Han M.K., Walter L., Garg P., Merlin D. PepT1 expression helps maintain intestinal homeostasis by mediating the differential expression of miRNAs along the crypt-villus axis. Sci. Rep. (UK) 2016;6:27119. doi: 10.1038/srep27119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Y., Wang Z., Zhang S.W., Lin H.L., Gao C.Q., Zhao J.C., Yang C., Wang X.Q. Methionine and its hydroxyl analogues improve stem cell activity to eliminate deoxynivalenol-induced intestinal injury by reactivating Wnt/β-Catenin signaling. J. Agr. Food. Chem. 2019;67:11464–11473. doi: 10.1021/acs.jafc.9b04442. [DOI] [PubMed] [Google Scholar]