Summary
Background
Hand, foot, and mouth disease (HFMD) is an important public health problem. A monovalent EV-A71 vaccine was launched in China in 2016. Previous studies showed that inactivated monovalent EV-A71 vaccines were highly efficient against HFMD associated with EV-A71 but not against HFMD with other etiologies, leading to a hypothesis that the introduction of EV-A71 vaccines might change the pathogen spectrum and epidemiological trend of HFMD. In this study, we described for the first time the changing epidemiological characteristics of HFMD after the launch of the EV-A71 vaccine.
Methods
We extracted individual-based epidemiological data on HFMD cases reported to the Chinese Center for Disease Control and Prevention between January 2013 and December 2019. We described the changing epidemiological characteristics of HFMD before and after vaccine launch according to the distribution of diseases characteristics (demographic, temporal, and geographical) and evaluated the potential changes in risk factors of severe patients. All analyses were stratified by the phase before and after vaccine launch, and by enterovirus serotype.
Findings
During 2013−2019, 15,316,710 probable cases of HFMD were reported. Of these, 787,197 (5·1%) were laboratory confirmed and 76,982 (0·5%) were severe. After the launch of the EV-A71 vaccine, the median age of HFMD patients infected with EV-A71 increased from 2·24 years (IQR:1·43, 3·56) to 2·81 years (IQR:1·58, 4·01). The proportion of patients less than 3 years of age decreased while the proportion of patients 3−5 years of age increased. There was a large decrease (60·7%) in the proportion of severe cases as well as a decline (28·3%) in HFMD patients infected with EV-A71. After the launch of the EV-A71 vaccine, the severe illness rate and mortality rate of HFMD patients in all age groups has decreased sharply, 62·20% and 83·78% respectively. The timing of the HFMD epidemic peak was delayed (1-2 months) . After the launch of EV-A71 vaccine, the risk of becoming a severe case for EV-A71 serotype was decreased, whereas that risk was instead increased for CV-A16 (from 0·17 (95% CI:0·16, 0·18) to 0·23 (95% CI:0·21, 0·25)) and other enterovirus compared to EV-A71 (from 0·38 (95% CI:0·37, 0·39) to 0·58 (95% CI:0·56, 0·61)). The longer the time from onset to diagnosis, the higher was the risk of being a severe case, but the effect size was decreased.
Interpretation
The introduction of the EV-A71 vaccine has effectively reduced the proportion of severe HFMD cases and mortality, but changes to the dominant serotypes should be closely monitored. Development of multivalent vaccines to avoid an increased case burden due to other enteroviruses is greatly needed.
Funding
This research was supported by the National Natural Science Foundation of China (81973102, 81773487), Public Health Talents Training Program of Shanghai Municipality (GWV-10.2-XD21), the 5th Three-year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System (GWV-10.1-XK05), the Major Project of Scientific and Technical Winter Olympics from National Key Research and Development Program of China (2021YFF0306000), 13th Five-Year National Science and Technology Major Project for Infectious Diseases (2018ZX10725-509) and Key projects of the PLA logistics Scientific research Program (BHJ17J013).
Keywords: HFMD, Enterovirus 71 vaccine, Changing epidemiology, Comparative study
Research in context.
Evidence before this study
Hand, foot and mouth disease (HFMD) is a common childhood illness caused by serotypes of enteroviruses and has caused a substantial burden throughout East and Southeast Asia. Since 2016, the EV-A71 vaccine has been used in China, but whether and how it has changed the pathogen spectrum of HFMD and epidemiological trend remains unclear. Here, we searched PubMed and preprint archives for articles published with no language or time restrictions on September 7, 2021, using the terms “HFMD”, “EV-A71”, “EV71” “enterovirus71”, and “vaccine”, and “changed epidemiology”, “changing epidemiology”, “epidemiological changes”. A total of 38 articles were found and 4 studies were directly related to the changing epidemiology of HFMD, which were all limited within local areas, providing limited evidence through basic simple description. So, strong evidence from the whole of mainland China illustrating the changing epidemiology of HFMD is lacking.
Added value of this study
Our study described the changing epidemiological characteristics of HFMD in the whole of mainland China between January 1, 2013, and December 31, 2019, including after the launch of the EV-A71 vaccine, focusing on the demographic characteristics, temporal pattern and spatial distribution, and the potential change in risk factors for being a severe patient. To the best of our knowledge, our paper covers the largest area studied in post-vaccine China. Our findings showed that, after the introduction of the EV-A71 vaccine, the severe illness rate and mortality rate of HFMD patients have decreased sharply, the median age of HFMD patients infected with EV-A71 tended to be older (2·81 IQR 1·58, 4·01 years), the peak epidemic timing of HFMD was delayed, after the launch of EV-A71 vaccine, the risk of becoming a severe case for EV-A71 subtype was decreased, whereas that risk was instead increased for CV-A16 and other enterovirus compared to EV-A71, and the longer the time from onset to diagnosis, the higher the risk of being a severe case, but the effect size was decreased.
Implications of all the available evidence
The introduction of the EV-A71 vaccine has effectively reduced HFMD severe illness and mortality rates, but the vaccination of children aged 3−5 years should be strengthened. The substantial HFMD epidemic with co-circulating EV-A71, CV-A16 and other enteroviruses in China has a potential for the EV-71 serotype to be replaced by other enterovirus serotypes. Therefore, to reduce the burden of HFMD, timely efforts are needed to actively develop multivalent vaccines, strengthen comprehensive laboratory monitoring, and to avoid the additional burden of cases caused by other enteroviruses.
Alt-text: Unlabelled box
Introduction
HFMD has the highest mortality rate among class ‘C’ infectious diseases that was implemented in mainland China. As a common infectious disease, children younger than 5 years are especially prone to HFMD. Although in most patients it is a self-limiting illness, in some serious cases neurological and systemic complications can occur that can be fatal.1 Since 2013 Coxsackie virus A6 (CV-A6), Coxsackie virus A10 (CV-A10) and other enteroviruses have also caused a high incidence of HFMD,2 although Enterovirus 71 (EV-A71) and Coxsackie virus A16 (CV-A16) remain the two most common virus serotypes.3,4 Moreover, EV-A71 is the most frequently identified serotype among both severe and fatal cases, and has caused several outbreaks characterized by severe neurological symptoms.5
There is no specific antiviral treatment for HFMD. Vaccination is considered the most effective method to prevent and control HFMD. The monovalent EV-A71 vaccine was launched in China in 2016, and is currently the only vaccine in the world that can prevent HFMD. Since then, more than 45 million doses of HFMD vaccine have been administered in mainland China between 2017 and 2019. The indicated vaccination age range is 6 months to 5 years. Previous research showed that the inactivated monovalent EV-A71 vaccine could prevent 90% of HFMD cases caused by EV-A71,6,7 but provided no cross-protection against CV-A16 or other serotype infections.6,8 This finding prompted concerns about whether the epidemiology of HFMD will change due to vaccination and whether there might be a shift in serotypes. A few local studies9, 10, 11, 12 have been conducted to address EV-A71 vaccination impacts, but the evidence generated has been limited. Among the four studies conducted, only periodicity − without risk factor analysis − has been described. Only one study described the spatial distribution of HFMD, and only one analyzed demographic characteristics. Strong evidence from the whole of mainland China to illustrate the changing epidemiology of HFMD is still lacking.
To bridge this gap in the literature, in this study we used the national surveillance data from 2013 to 2019 to describe the epidemiology of HFMD in mainland China, focusing on the changes in demographic, seasonal and geographical patterns before and after the EV-A71 vaccine launch. Our aim was to provide crucial evidence to inform a timely and effective public health strategy for HFMD in the post EV-A71 vaccine era.
Methods
Data sources
HFMD cases reported between January 1, 2013 and December 31, 2019 with basic demographic information (sex, date of birth), case classification (probable or confirmed), severity (mild or severe), date of symptoms onset, date of diagnosis, date of death (if applicable) and virus serotype (EV-A71, CV-A16 or other enterovirus) were retrieved from the Chinese Center for Disease Control and Prevention. The annual population sizes between January 1, 2013 and December 31, 2019 for all provinces for different age groups (<12 months, 12−23 months, 24−35 months, 36−59 months, 5−9 years, 10−14 years, ≥15 years) were retrieved from the Chinese Center for Disease Control and Prevention.
Case definitions
A probable case of HFMD was defined as a patient with papular or vesicular rash on the hands, feet, mouth or buttocks, with or without fever. A confirmed case was defined as a probable case with laboratory evidence of enterovirus infection (including EV-A71, CV-A16 or other enterovirus) detected by RT-PCR, real-time PCR or virus isolation. HFMD cases, whether probable or confirmed, were classified as severe if the patient had any neurological complications (aseptic meningitis, encephalitis, encephalomyelitis, acute flaccid paralysis or autonomic nervous system dysregulation), or cardiopulmonary complications (pulmonary edema, pulmonary hemorrhage or cardiorespiratory failure) or both; otherwise, HFMD cases were categorized as mild. The sample size of this study was determined by the time window and the above case definitions.
Data analysis
All analyses were stratified by the phase (before versus after the launch of the EV-A71 vaccine, in 2016) and enterovirus serotype. To reflect the changes in HFMD epidemiology over time, the study period was classified into three sub-periods: 1. baseline (2013−2015), prior to implementation of the EV-A71 vaccine; 2. the transition year (2016); and 3. implementation of the EV-A71 vaccination program (2017−2019).
We calculated frequency and proportion of cases for different age groups, sex, severity, and serotypes for each year. We also estimated age-specific rates of incidence, severe illness, and mortality and estimated 95% CIs assuming a Poisson distribution. For rates with a denominator greater than 100 000 and numerator of 20 or more, we calculated 95% CIs assuming a normal distribution.13
To describe seasonal patterns of HFMD by province, we created heat maps. The proportion of cases identified in each week of the year were standardized by the number of annual cases. The median values of the proportion of cases in each week of the year from 2013 to 2019 were plotted as the seasonal distribution of cases of HFMD. This procedure was repeated by stratifying on phase (before versus after the launch of the EV-A71 vaccine) and virus serotype. To further quantify the seasonal pattern of HFMD incidence in each province, we fitted a seasonal multiple linear regression model to the proportion of cases reported each week. We used harmonic terms representing annual and semiannual periodic cycles, and estimated the peak timing and amplitude of the annual and semi-annual periodicities of HFMD activity in each province. The model is as follow (details were described in a previous paper13), where t is reporting week:
The cumulative incidence14, 15, 16 in all provinces over the study period was estimated, and stratified by vaccination phase and virus serotypes to illustrate the changes in the geographical distribution ranges of HFMD in mainland China.
To identify potential differences in predictors for severe outcomes, we applied logistic regression of demographic variables, time from onset to diagnosis, and virus serotypes separately for laboratory-confirmed cases and probable cases, stratified by the vaccination phase.
All analyses were done with R (version 4.0.3) and QGIS (version 3.16.5).
Ethical approval
The collection of data from HFMD cases was part of a continuing public health investigation of an emerging outbreak determined by the National Health and Family Planning Commission. Hence, this study was exempt from institutional review board assessment.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Demographic distribution
Nationally 15,316,710 probable cases of HFMD were reported to the China CDC surveillance system between 2013 and 2019, of which 787,197 (5·1%) were laboratory confirmed and 76,982 (0·5%) were severe. Severe cases accounted for 58·1% and 39·0% of all severe cases before and after the launch of the vaccine, respectively.
There was a large decrease (60·7%) in the proportion of severe cases after the introduction of EV-A71 vaccine. After the launch of the EV-A71 vaccine, the median age of HFMD patients infected with EV-A71 serotype tended to be older (from 2·24 years (IQR:1·43, 3·56) to 2·81 years (IQR:1·58, 4·01)). There was a lower proportion of HFMD patients under 3 years of age, and especially patients under 1 year of age; there was also a large decrease in the proportion of patients under 5 years of age infected by EV-A71 (Appendix Figure A1), but a greater proportion of patients 3−5 years of age (Table 1). For different serotypes, the proportion of HFMD patients infection with the EV-71 serotype decreased (28·3%), while the proportion infected with CV-A16 and other enterovirus serotypes showed an increased trend (Table 1 & Appendix Figure A2). Compared with before the launch of the EV-A71 vaccine, the incidence of patients of under 2 years old decreased, but the decrease was not significant in the other age groups, showing a fluctuating pattern (Table 2). Following the launch of the EV-A71 vaccine, the severe illness rate and mortality rate of HFMD patients in all age groups has decreased sharply, 62·20% and 83·78% respectively(Table 2 & Table A1).
Table 1.
Characteristics of hand, foot, and mouth disease in mainland China by different stages of vaccine launch, 2013–19.
| Before EV-71 vaccine launch |
2016 | After EV-71 vaccine launch |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | Total | 2017 | 2018 | 2019 | Total | ||
| Median age (P25, P75) | 2·00(1·16,3·12) | 2·21(1·43,3·59) | 2·00(1·19,3·24) | 2·00(1·27,3·36) | 2·35(1·46,3·74) | 2·17(1·30,3·60) | 2·28(1·42,3·82) | 2·49(1·49,3·92) | 2·32(1·40,3·79) |
| EV-A71 | 2·09(1·34,3·45) | 2·30(1·49,3·64) | 2·29(1·38,3·52) | 2·24(1·43,3·56) | 2·56(1·57,3·82) | 2·86(1·63,4·04) | 2·58(1·47,3·92) | 2·85(1·64,4·12) | 2·81(1·58,4·01) |
| CV-A16 | 2·61(1·57,3·91) | 2·65(1·63,3·97) | 2·86(1·68,3·99) | 2·70(1·63,3·98) | 2·81(1·71,3·99) | 2·92(1·68,4·01) | 2·94(1·69,4·10) | 3·21(1·99,4·56) | 3·02(1·82,4·31) |
| Other Enterovirus | 1·95(1·10,3·00) | 2·00(1·20,3·17) | 1·99(1·13,3·05) | 1·99(1·14,3·05) | 2·00(1·28,3·34) | 1·99(1·19,3·27) | 2·02(1·33,3·61) | 2·25(1·38,3·64) | 2·04(1·30,3·53) |
| Age group | |||||||||
| <12 months (%) | 358381(19·6) | 432045(15·5) | 394770(19·8) | 1185196(17·9) | 302717(12·4) | 301105(15·6) | 282940(12·0) | 219335(11·0) | 803380(12·8) |
| 12–23 months (%) | 604592(33·1) | 844007(30·4) | 641104(32·1) | 2089703(31·6) | 731322(30·0) | 586768(30·4) | 757699(32·2) | 578856(29·1) | 1923323(30·7) |
| 24–35 months (%) | 362681(19·8) | 5546063(19·7) | 390571(19·6) | 1299315(19·7) | 496171(20·3) | 367789(19·1) | 420566(17·9) | 400949(20·2) | 1189304(19·0) |
| 36–59 months (%) | 369104(20·2) | 699737(25·2) | 416132(20·8) | 1484973(22·5) | 664234(27·2) | 482102(25·0) | 612289(26·0) | 523142(26·3) | 1617533(25·8) |
| 5–9 years (%) | 109491(6·0) | 224223(8·1) | 129823(6·5) | 463537(7·0) | 215547(8·8) | 162799(8·4) | 235814(10·0) | 226072(11·4) | 624685(10·0) |
| 10–14 years (%) | 14085(0·8) | 20697(0·7) | 13725(0·7) | 48507(0·7) | 19464(0·8) | 16435(0·9) | 25257(1·1) | 23213(1·2) | 64905(1·0) |
| ≥15 years (%) | 10043(0·5) | 12089(0·4) | 11246(0·6) | 33378(0·5) | 11537(0·5) | 12552(0·7) | 18745(0·8) | 16683(0·8) | 47980(0·7) |
| Gender | |||||||||
| Male (%) | 1118941(61·2) | 1672498(60·2) | 1206005(60·4) | 3997444(60·5) | 1462219(59·9) | 1151027(59·7) | 1400550(59·5) | 1174283(59·1) | 3725860(59·4) |
| Female (%) | 709436(38·8) | 1106363(39·8) | 791366(39·6) | 2607165(39·5) | 978773(40·1) | 778523(40·3) | 952759(40·5) | 813967(40·9) | 2545249(40·6) |
| Severity | |||||||||
| Mild (%) | 18181119(99·9) | 2754209(99·1) | 1987547(99·5) | 6559867(99·3) | 2426221(99·4) | 1919089(99·5) | 2348632(99·8) | 1985920(99·9) | 6253641(99·7) |
| Severe (%) | 10266(0·1) | 24652(0·9) | 9824(0·5) | 44742(0·7) | 14771(0·6) | 10461(0·5) | 4678(0·2) | 2330(0·1) | 17469(0·3) |
| Enterovirus serotype | |||||||||
| EV-A71 (%) | 32752(37·4) | 56078(44·5) | 29181(29·7) | 118011(37·8) | 52808(39·7) | 46081(41·4) | 21355(17·5) | 17194(15·8) | 84630(24·7) |
| CV-A16 (%) | 12797(14·6) | 36546(29·0) | 18110(18·4) | 67453(21·6) | 35103(26·4) | 13945(12·5) | 28836(23·6) | 38269(35·2) | 81050(23·7) |
| Other Enterovirus (%) | 42022(48·0) | 33336(26·5) | 51100(51·9) | 126458(40·5) | 45262(33·9) | 51183(46·1) | 72034(58·9) | 53205(49·0) | 176422(51·6) |
Table 2.
Estimated rates of incidence, severe illness, and mortality in mainland China by different stages of vaccine launch, 2013–19.
| Before the EV-A71 Vaccines Launch |
2016 | After the EV-A71 Vaccines Launch |
|||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2017 | 2018 | 2019 | ||
| Incidence rates (95% CI) | |||||||
| <12 months | 24881·9 (24801·5,24962·4) | 28865·4 (28780·6,28950·3) | 26028·0 (25947·9,26108·2) | 19474·2 (19405·5,19542·9) | 19096·7 (19029·2,19164·3) | 17944·7 (17879·1,18010·2) | 13910·7 (13852·9,13968·5) |
| 12–23 months | 35810·7 (35722·0,35899·3) | 48395·8 (48295·1,48496·5) | 36279·9 (36192·7,36367·1) | 41494·0 (41400·9,41587·1) | 32842·2 (32759·6,32924·9) | 42409·5 (42316·0,42502·9) | 32399·4 (32317·3,32481·5) |
| 24–35 months | 22594·1 (22521·4,22666·8) | 32585·2 (32500·2,32670·2) | 22957·3 (22886·2,23028·5) | 29235·3 (29155·1,29315·4) | 21353·3 (21285·0,21421·6) | 24417·5 (24344·6,24490·3) | 23278·5 (23207·3,23349·7) |
| 36–59 months | 12401·4 (12361·6,12441·1) | 23571·6 (23517·0,23626·1) | 13866·6 (13824·8,13908·5) | 22199·5 (22146·7,22252·3) | 15904·3 (15859·7,15948·8) | 20199·1 (20149·0,20249·2) | 17258·2 (17211·8,17304·5) |
| 5–9 years | 1360·8 (1352·8,1368·9) | 2756·4 (2745·0,2767·8) | 1578·6 (1570·0,1587·2) | 2630·9 (2619·9,2642) | 1968·4 (1958·8,1977·9) | 2851·2 (2839·7,2862·7) | 2733·4 (2722·1,2744·6) |
| 10–14 years | 212·0 (208·5,215·5) | 321·2 (316·8,325·5) | 210·7 (207·1,214·2) | 299·5 (295·3,303·8) | 250·1 (246·3,253·9) | 384·3 (379·6,389·1) | 353·2 (348·7,357·8) |
| ≥15 years | 8·9 (8·8,9·1) | 10·8 (10·6,11·0) | 9·9 (9·7,10·1) | 10·1 (9·9,10·2) | 10·9 (10·7,11·1) | 16·3 (16·0,16·5) | 14·5 (14·2,14·7) |
| Severe illness rates (95% CI)* | |||||||
| <12 months | 140·3 (134·2,146·4) | 302·3 (293·5,311·1) | 150·9 (144·7,157·1) | 146·5 (140·5,152·6) | 119·1 (113·7,124·5) | 48·6 (45·2,52·1) | 24·1 (21·7,26·5) |
| 12–23 months | 248·9 (241·4,256·4) | 577·9 (566·6,589·2) | 219·5 (212·5,226·4) | 345·8 (337·1,354·4) | 231·2 (224·1,238·2) | 118·5 (113·5,123·6) | 58·3 (54·7,61·8) |
| 24–35 months | 132·4 (126·8,138·1) | 300·4 (292·1,308·7) | 109·7 (104·8,114·7) | 192·9 (186·3,199·5) | 120·1 (114·9,125·3) | 45·3 (42·2,48·5) | 27·5 (25.0,29·9) |
| 36–59 months | 53·1 (50·5,55·7) | 138·0 (133·8,142·3) | 49·0 (46·5,51·5) | 86·1 (82·8,89·5) | 62·4 (59·6,65·2) | 24·9 (23·1,26·6) | 11·5 (10·3,12·7) |
| 5–9 years | 3·9 (3·4,4·3) | 10·5 (9·8,11·2) | 3·6 (3·2,4·0) | 6·3 (5·7,6·8) | 5·6 (5·1,6·1) | 2·8 (2·4,3·2) | 1·0 (0·8,1·2) |
| 10–14 years | 0·3 (0·2,0·5) | 0·7 (0·5,1·0) | 0·3 (0·2,0·4) | 0·5 (0·3,0·6) | 0·4 (0·3,0·6) | 0·3 (0·2,0·5) | 0·1 (0·0,0·2) |
| ≥15 years # | 0·0 (0·0,0·0) | 0·0 (0·0,0·0) | 0·0 (0·0,0·0) | 0·0 (0·0,0·0) | 0·0 (0·0,0·0) | 0·0 (0·0,0·0) | 0·0 (0·0,0·0) |
| Mortality rates (95% CI)* | |||||||
| <12 months | 4·0 (3·0,5·1) | 8·3 (6·8, 9·7) | 2·1 (1·4, 2·8) | 2·7 (1·9, 3·5) | 1·8 (1·2, 2·5) | 0·4 (0·2, 0·9) | 0·4 (0·1, 0·8) |
| 12–23 months | 6·5 (5·2,7·7) | 11·1 (9·6, 12·7) | 2·8 (2·0, 3·6) | 4·5 (3·5, 5·5) | 1·6 (1·0, 2·1) | 1·1 (0·6, 1·6) | 0·3 (0·1, 0·7) |
| 24–35 months | 3·6 (2·6, 4·5) | 5·8 (4·7, 7·0) | 1·6 (1·0, 2·2) | 2·3 (1·6, 3·0) | 1·0 (0·6, 1·6) | 0·2 (0·1, 0·6) | 0·2 (0·1, 0·6) |
| 36–59 months | 0·7 (0·4, 1·0) | 2·6 (2·0, 3·2) | 0·6 (0·3, 0·9) | 1·0 (0·7, 1·4) | 0·5 (0·3, 0·9) | 0·1 (0·0, 0·3) | 0·1 (0·0, 0·2) |
| 5–9 years # | 0·0 (0·0, 0·1) | 0·1 (0·0, 0·2) | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) | 0·1 (0·0, 0·1) | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·1) |
| 10–14 years # | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) | 0·0 (0·0, 0·1) |
| ≥15 years # | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) |
per 1 million.
More detailed information is provided in the supplementary file in Table A4.
Time series
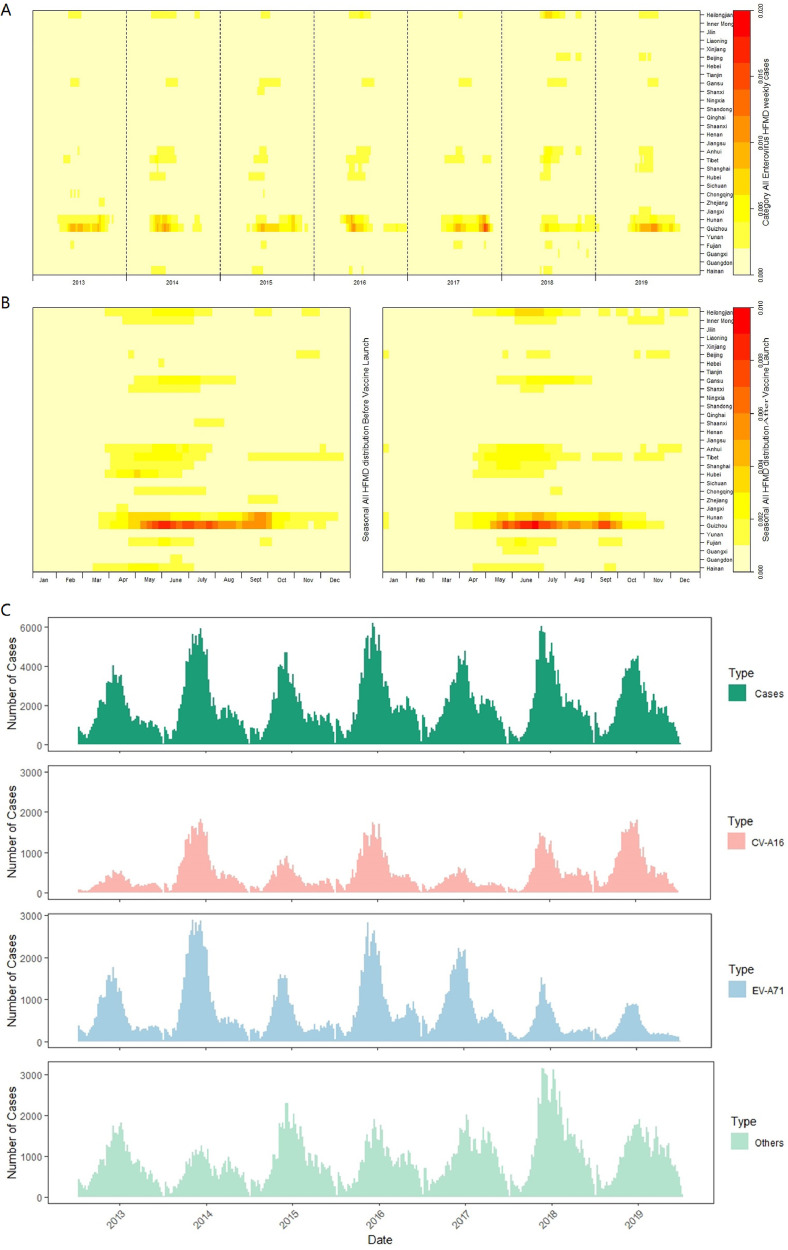
Nationally, the prevalence peaks and periodicity of HFMD varied according to latitude. After the launch of the EV-A71 vaccine, the timing of HFMD epidemic peaks tended to be delayed, and a similar trend was also observed for the other enterovirus serotypes (Figure 1 & Appendix Figures A3-A5).
Figure 1.
Heat map of surveillance data for hand, foot, and mouth disease by mainland Chinese province, 2013–19. A: Time series of monthly probable and laboratory-confirmed cases of HFMD, standardised by the number of annual cases; B: Seasonal distribution of cases of HFMD, plotted as the median value of proportion of cases in each month of the year from 2013 to 2019, the left is seasonal distribution before EV-A71 vaccines, the right is that after the vaccines launch. For A & B, the provinces were ordered by latitude from northernmost (top) to southernmost (bottom). C: Number of cases of HFMD by month of illness onset. EV-A71=enterovirus A71. CV-A16=Coxsackie virus A16.
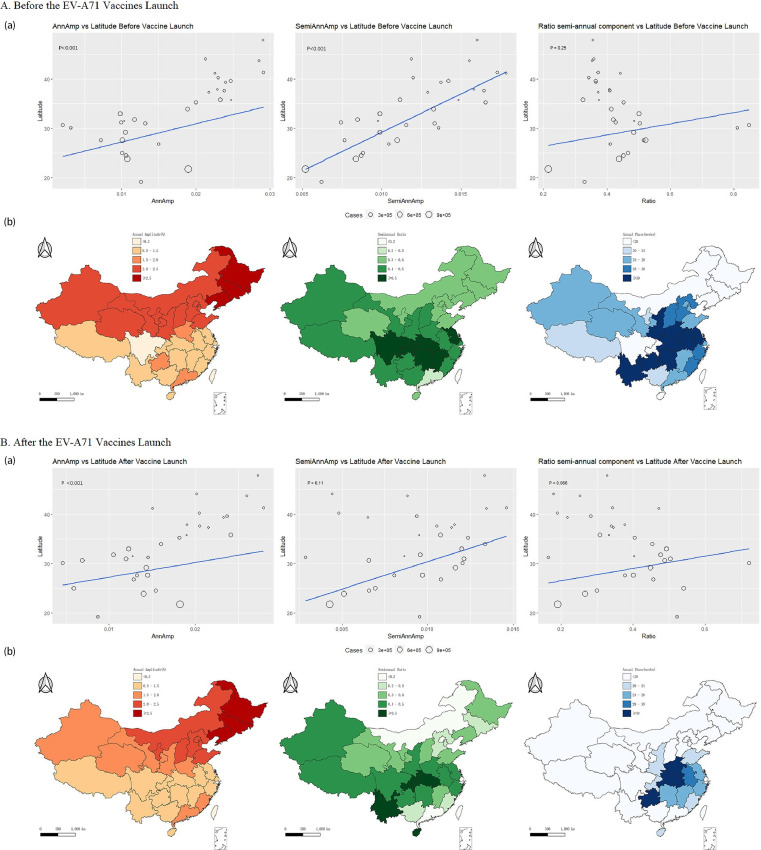
The annual amplitude and semi-annual amplitude of HFMD prevalence overall increased with higher latitude (Figure 2 & Appendix Figures A6-A8). After the launch of the EV-A71 vaccine, the latitude gradient of the semi-annual amplitude of EV-A71 serotype HFMD prevalence increased, but became statistically non-significant (Appendix Figure A7). CV-A16 serotype showed an opposite pattern (Appendix Figure A6). Also, the dominant annual and semi-annual periodicity did not change greatly (Figure 2), but CV-A16 serotype showed a stronger semi-annual periodicity at lower latitudes (Appendix Figure A6).
Figure 2.
Seasonality of hand, foot, and mouth disease. A: Seasonality of hand, foot, and mouth disease before the EV-A71 vaccines launch. B: Seasonality of hand, foot, and mouth disease after the EV-A71 vaccines launch. (a): Latitudinal gradients in seasonality of hand, foot, and mouth disease. Left: Latitudinal gradients in Annual Amplitude. Middle: Latitudinal gradients in Semi-annual Amplitude. Right: Importance of the semiannual periodicity, measured by the ratio of the amplitude of the semiannual cycle to the sum of the amplitudes of annual and semiannual cycles. (b): Periodicity and peak timing of hand, foot, and mouth disease epidemics in China. Left: Amplitude of the annual cycle from pale red (low) to red (high). Middle: Importance of the semiannual periodicity, measured by the ratio of the amplitude of the semiannual cycle to the sum of the amplitudes of annual and semiannual cycles. Pale green shows strongly annual epidemics; dark green shows dominant semiannual activity. Right: Timing of primary annual hand, foot, and mouth disease peak, in weeks from Jan 1. Timing is colour coded from pale blue to dark blue. EV-A71=enterovirus A71. CV-A16=Coxsackievirus A16.
Geographical distribution
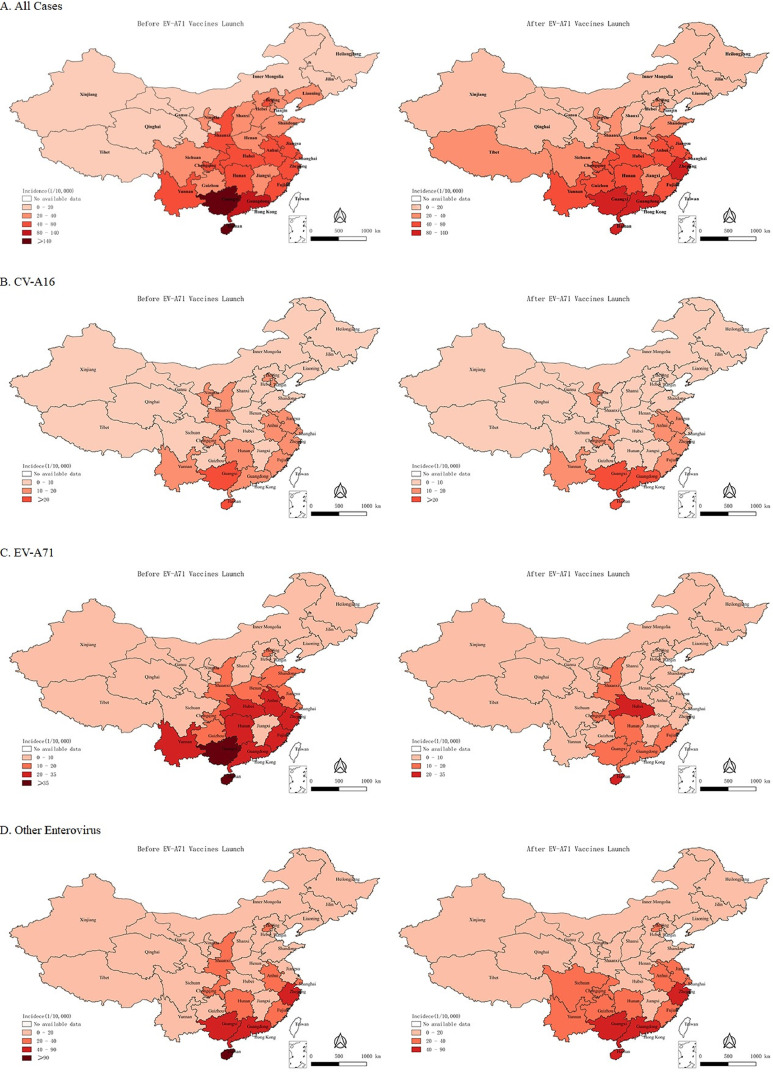
HFMD cases were reported from all provinces of the mainland China throughout the study period. The range of the geographical distribution was mainly concentrated in central, southern and eastern China (Figure 3).
Figure 3.
Hand, foot and mouth disease epidemiological regions and dynamical distritbution following EV71 vaccine lauch. A: Geographical distribution of hand, foot, and mouth disease epidemics in China. B: Geographical distribution of CV-A16 serotype hand, foot, and mouth disease epidemics in China. C: Geographical distribution of EV-A71 serotype hand, foot, and mouth disease epidemics in China. D: Geographical distribution of other enterovirus serotype hand, foot, and mouth disease epidemics in China. Left: Geographical distribution of hand, foot, and mouth disease epidemics in China before the EV-A71 vaccines launch. Right: Geographical distribution of hand, foot, and mouth disease epidemics in China after the EV-A71 vaccines launch. The shades of color indicate the cumulative incidence of hand, foot and mouth disease in each province.
After the launch of the EV-A71 vaccine, changes in the cumulative incidence of HFMD varied across the country, but there were obvious changes in the spatial distribution range (Figure 3). There was a decrease in the geographical distribution range of EV-A71 serotype, in addition to an increased incidence in Hubei province and Chongqing (Figure 3 & Appendix Table A2). The geographical distribution range of other enterovirus serotypes showed a trend of increasing (Figure 3), but with no obvious changes for CV-A16 serotype (Figure 3).
Risk factors analysis
Several consistent risk factors for severe cases were identified, including young age, males, infection with EV-A71, and longer interval between onset and diagnosis (Table 3). For laboratory-confirmed cases, after the launch of the EV-A71 vaccine, children under five years of age and male patients had an increased risk of severe disease. After the launch of EV-A71 vaccine, the risk of becoming a severe case for EV-A71 subtype was decreased, whereas that risk was instead increased for CV-A16 and other enterovirus compared to EV-A71. The longer the time from onset to diagnosis, the higher was the risk of being severe case, but the effect size after the launch of the EV-A71 vaccine decreased (Table 3). Inclusion of probable cases in the analysis yielded similar risk predictors (Appendix Table A3).
Table 3.
Risk factors associated with severe illness in laboratory-confirmed cases of hand, foot, and mouth disease, 2013-19.
| Laboratory-confirmed cases of hand, foot, and mouth disease (n=787197) | ||||||
|---|---|---|---|---|---|---|
| Whole period (2013-19) |
Before EV71 vaccine launch(2013-15) |
After EV71 vaccine launch(2017-19) |
||||
| Number of cases | OR(95% CI) | Number of cases | OR (95% CI) | Number of cases | OR (95% CI) | |
| Age group | ||||||
| >=5 years | 78937 | Ref | 25270 | Ref | 41231 | Ref |
| 36-59 months | 210171 | 1·72(1·63,1·83) | 75708 | 1·60(1·48,1·73) | 96820 | 1·66(1·49,1·85) |
| 24-35 months | 152204 | 2·94(2·77,3·11) | 61742 | 2·45(2·26,2·65) | 63152 | 2·91(2·62,3·25) |
| 12-23 months | 237954 | 3·89(3·69,4·11) | 97060 | 3·22(2·98,3·48) | 100892 | 3·96(3·58,4·39) |
| <12 months | 107931 | 3·35(3·17,3·56) | 52142 | 2·48(2·29,2·69) | 40007 | 4·00(3·59,4·47) |
| Gender | ||||||
| Female | 309881 | Ref | 119385 | Ref | 137414 | Ref |
| Male | 477316 | 1·18(1·16,1·21) | 192537 | 1·15(1·11,1·18) | 204688 | 1·21(1·16,1·27) |
| Enterovirus serotype | ||||||
| EV-A71 | 255449 | Ref | 118011 | Ref | 84630 | Ref |
| CV-A16 | 183606 | 0·19(0·18,0·20) | 67453 | 0·17(0·16,0·18) | 81050 | 0·23(0·21,0·25) |
| Others | 348142 | 0·30(0·39,0·41) | 126458 | 0·38(0·37,0·39) | 176422 | 0·58(0·56,0·61) |
| Time from illness onset to diagnosis | ||||||
| 0 day | 76682 | Ref | 26114 | Ref | 37664 | Ref |
| 1 day | 99947 | 0·96(0·93,0·99) | 37425 | 1·01(0·96,1·07) | 45830 | 0·99(0·93,1·05) |
| 2 days | 65555 | 1·19(1·15,1·23) | 25668 | 1·39(1·32,1·46) | 29404 | 1·08(1·02,1·16) |
| ≥3 days | 138055 | 1,41(1·37,1·45) | 63855 | 1·68(1·61,1·76) | 53777 | 1·11(1·05,1·17) |
Risk factors associated with severe illness in laboratory-confirmed cases of hand, foot, and mouth disease. Results from multivariate logistic regression with backward selection of demographic and geographical variables, time from onset to diagnosis, and virus serotype (p value for exclusion ≥0·01). OR=odds ratio. EV-A71=enterovirus 71. CV-A16= Coxsackie virus A16· Ref=reference.
Discussion
Our study provides the first comprehensive description of the changing epidemiology of laboratory-confirmed cases of HFMD before and after the launch of the EV-A71 vaccine. We have described the demographic characteristics, temporal pattern and spatial distribution of HFMD epidemics throughout mainland China, and assessed the potential change in risk factors for severe patients before and after the launch of the EV-A71 vaccine.
After the launch of the EV-A71 vaccine, the proportion of younger HFMD cases and those infected by EV-A71 decreased, indicating that overall EV-A71 vaccination has had a positive impact on the control of HFMD. We also found that there was a higher proportion of patients 3−5 years of age. Due to the implementation of the vaccine, the HFMD susceptible population might have changed, however this requires further monitoring in the future and is extremely important for the implementation and adjustment of immunization strategies. Also, the vaccination in this age group should be strengthened. After the launch of the EV-A71 vaccine, we found that the median age of HFMD patients infected with EV-A71 tended to be older. One possible reason for this age shift is the waning of vaccine-derived immunity and decline in the risk of infection throughout the whole population after the implementation of EV-A71 vaccine, so that the average age of EV-A71 infection increases, while the age-pattern of infection of non-EV-A71 serotypes remains unchanged.9,17
EV-A71 is the main serotype causing severe or fatal cases of HFMD,5 and the EV-A71 inactivated vaccine has a good protective effect against EV-A71. Because of the use of this vaccine, fewer cases of HFMD due to EV-A71 serotype were reported, resulting in a substantial decrease in the proportion of severe cases (60·96%). Although cases of other enterovirus serotypes have increased in recent years, they are less likely to cause severe disease. Thus, the implementation of EV-A71 vaccination has produced a positive public health impact.
Many factors can influence the seasonal distribution of HFMD, such as accumulation of susceptible populations, progress of prevention and control measures, characteristics of viral serotypes, and climatic and environmental factors. Previous studies found that meteorological factors − including temperature and humidity − can induce EV-A71 and CV-A16 to cause differing epidemic scales.18 After the launch of the EV-A71 vaccine, the timing of the HFMD epidemic peak was delayed, regardless the serotypes of enterovirus. We think that the increased population immunity created by the EV-A71 vaccination program may disrupt the infection cycle.11 With vaccination, the number of susceptible people decreases, and it takes longer for the number of susceptible people to accumulate to a certain threshold to allow an HFMD epidemic. Also, it is possible that different serotypes have different transmission dynamics; with EV-A71 vaccination the relative prevalence of other serotypes has increased and therefore delayed epidemic peaks might have become more apparent. In 2018 there was renewed efforts to control HFMD in China. With the implementation and strengthening of prevention and control measures, the periodicity of HFMD have been affected to some extent. Whether the change in seasonal peak described in this study is related to specific serotypes and other risk factors requires further study. This topic is of great significance for epidemic prediction and the implementation of prevention and control measures.
Previous studies have shown that two inactivated monovalent EV-A71 vaccine doses in children are highly efficacious (94·8−97·4%) against EV-A71-associated HFMD, but not cross-protective against HFMD caused by CV-A16 or other serotypes. This to some extent explains the decrease in the proportion of patients infected with EV-A71 serotype and the increase infection with CV-A16 or other enterovirus serotypes after the launch of the EV-A71 vaccination program, and the risk was instead increased for CV-A16 and other enterovirus compared to EV-A71 after the launch of EV-A71 vaccine. In our study we found that the cumulative incidence of EV-A71 serotype HFMD showed a significant decrease in its geographical distribution, except Hubei province and Chongqing. In the case of Hubei, the EV-A71 virus serotype was used as an indicator for the early identification of severe cases. Because of this local policy, the prevalence in the province might have been overestimated. Studies from several provinces/prefectures reported that a median of 49% (IQR: 43, 56%) of all EV test-positive specimens were due to CV-A6 in 2013 and 2015, indicating widespread emergence of CV-A6 in China since 2013.19, 20, 21, 22, 23, 24, 25, 26, 27 The increasing contribution of other enteroviruses in China since 2013 was also consistent with the worldwide (including Europe) outbreak of CV-A6 in 2013. After the launch of the EV-A71 vaccine, we also found that the spatial distribution range of HFMD caused by other enterovirus serotypes has become larger.
We also believe that the prevalence of the other enterovirus serotypes might have been influenced by the introduction of the vaccine. For example, after the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), increases in the prevalence of non-PCV7 serotypes were broadly observed.28,29 It is widely known that the introduction of a vaccine can induce recombination between circulating serotypes, immune escape among sub genotype strains, and spontaneous mutations in the viral genome, which result in changes in serotype distribution.6,30 Whether after the introduction of the vaccine an increasing epidemic risk from nonvaccine serotypes (serotype replacement) might lead to increased disease burden is unknown. Therefore, further research on HFMD requires ongoing monitoring and strengthening of the monitoring system (including laboratory testing) to fully understand enterovirus epidemics and the role other intestinal viruses have in severe HFMD and clinical manifestations, and to develop multivalent vaccines to further reduce the burden from HFMD.
Several consistent risk factors for severe cases of HFMD were observed including younger age, male, EV-A71 infection, and longer interval from onset to diagnosis. After the vaccine launch, the effect size for severe cases of HFMD for patients aged 0−5 years increased relative to children aged more than 5 years. After the launch of the EV-A71 vaccine, male patients had a higher risk of developing severe illness. Previous research found that male children were more susceptible to HFMD and should be considered as a group at significant risk. The reasons for the significant differences between male and female patients remain unclear, but might be related to the extra outdoor activities of boys, increasing the level of exposure and the risk of infection compared with girls. At present, few studies have focused on the influence of sex on the severe outcome of HFMD and the population sex ratio and implications for population-level impact. Future studies might reveal the pathogenesis of HFMD based on the physiological characteristics of men and women, as well as from the perspective of sociology and behavior. The longer the time from onset to diagnosis, the higher is the risk of being a severe case, but the effect size after the launch of the EV-A71 vaccine was decreased. In recent years, the number of cases of other serotypes of HFMD has increased. Their neurotropism is lower than that of the EV-A71 serotype, which might lead to a slower progression of symptoms. In 2018, China issued new guidelines for the diagnosis and treatment of HFMD, which clarified the early diagnosis and treatment of HFMD. With the gradual improvement in understanding of the disease, as well as national and local HFMD monitoring systems, early detection, early diagnosis and early treatment, more effective measures can be taken to control and prevent the occurrence of HFMD.
This study has several limitations. First, our study was based on a descriptive analysis of surveillance data. Unfortunately, we cannot explain the intrinsic mechanism of some changes described, such as the delay of the timing of HFMD epidemic peak; this is beyond the scope of our study. Second, we did not have data on enterovirus serotypes other than CV-A16 and EV-A71, although some serotypes such as CV-A6 have become more predominant in southern China.31 Third, the vaccination coverage rate was not included in our study because there is no crossover between the surveillance system and immune system for HFMD. Also, it is possible to roughly estimate the vaccine coverage rate for each province in the mainland China from 2016 to 2019, but this data is the unpublished internal data of the Chinese Center for Disease Control and Prevention and has not been made public. Forth, access to and provision of health care and technical capacity varied between and sometimes within provinces, with no formal quality assurance or systematic audit for disease surveillance. Assessment of the criteria for pathogen detection among provinces was beyond the scope of this study, but this might have some biases for this study results, especially few labs will test more EV-A71 serotypes than non-EV-A71 serotypes due to the reason of EV-A71 vaccine research and development, such as Hubei province.
In conclusion, we found substantial HFMD illness associated with co-circulating EV-A71, CV-A16 and other enteroviruses in mainland China, disproportionately affecting children younger than 5 years of age. After the launch of the EV-A71 vaccine, there was a higher proportion of patients aged 3−5 years; increased vaccination in this group is highly recommended. There was a decrease in the geographical distribution range of EV-A71 serotype in almost all provinces. We recorded changes in HFMD seasonality across the study period, which might be associated with climate and geographical distributions across mainland China, and might be associated with vaccination. Patients of younger age, male and infection with non-EV-A71 slightly increased the risk of being a severe case after the EV-A71 vaccine launch.
Overall, our study used population-based, national data to analyze the changes in the epidemiology of HFMD after the launch of EV-A71 vaccine in mainland China. Our results provide a preliminary understanding of the changing epidemic of HFMD after the launch of the EV-A71 vaccine, and provide a direction for preliminary evaluation of the EV-A71 vaccine's effect on the prevention and control of HFMD in recent years. More broadly, further research is needed to quantify the contribution of EV-A71 vaccine to HFMD prevention and control. Factors other than vaccine should also be considered in further studies.
Declaration of Competing Interest
We declare that we have no conflicts of interest.
Acknowledgments
Contributors
HJ designed the study, analyzed the data and wrote the manuscript. FFL, MRR, XR helped with research design, data collection. ZZ, QS helped with data processing. WT, MPW, JQH, XC, and JXL helped with critical revision of manuscript. YH, ZJL, ZRC and ZJZ supervised all aspects of this study. All authors contributed to and approved the final version for submission.
Acknowledgements
We would like to thank the Chinese Center for Disease Control and Prevention for assistance in research design and implementation and data collection. The views expressed are those of the authors alone. This research was supported by the National Natural Science Foundation of China (81973102, 81773487), Public Health Talents Training Program of Shanghai Municipality (GWV-10.2-XD21), the 5th Three-year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System (GWV-10.1-XK05), the Major Project of Scientific and Technical Winter Olympics from National Key Research and Development Program of China (2021YFF0306000), 13th Five-Year National Science and Technology Major Project for Infectious Diseases (2018ZX10725-509) and Key projects of the PLA logistics Scientific research Program (BHJ17J013).
Data availability statement
The data and code used and analyzed during the current study available from the corresponding author on reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2021.100370.
Contributor Information
Zhaorui Chang, Email: changzr@chinacdc.cn.
Zhijie Zhang, Email: epistat@gmail.com.
Appendix. Supplementary materials
Reference
- 1.Wang J, Hu T, Sun D, et al. Epidemiological characteristics of hand, foot, and mouth disease in Shandong, China, 2009-2016. Sci Rep. 2017;7(1):8900. doi: 10.1038/s41598-017-09196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin DQ, Wang CB, Wang CB, Xiao Z, Ji SX. Epidemiology Characteristics of Human Coxsackievirus A16 and Enterovirus 71 Circulating in Linyi, China, from 2009 to 2017. Jpn J Infect Dis. 2018;71(6):470–473. doi: 10.7883/yoken.JJID.2018.035. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14(12):e1076–e1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Chu AC, Greenblatt DT. In: Infectious Diseases (Third Edition) Cohen J, Opal SM, Powderly WG, editors. Mosby; London: 2010. Chapter 12 - Dermatologic manifestations of systemic infections; pp. 140–146. [Google Scholar]
- 5.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9(11):1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi S, Liao Q, Van Boeckel TP, et al. Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination. PLoS Med. 2016;13(2) doi: 10.1371/journal.pmed.1001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370(9):829–837. doi: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 8.Li JX, Song YF, Wang L, et al. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15(1):129–137. doi: 10.1586/14760584.2016.1096782. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Jiang L, Zhang C, He W, Tan Y, Ning C. The changes in the epidemiology of hand, foot, and mouth disease after the introduction of the EV-A71 vaccine. Vaccine. 2021;39(25):3319–3323. doi: 10.1016/j.vaccine.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Zhang Z, Rao Q, et al. The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming, China. Emerg Microbes Infect. 2021;10(1):619–628. doi: 10.1080/22221751.2021.1899772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y, Chen Z, Zheng K, et al. Epidemiology of Hand, Foot, and Mouth Disease Before and After the Introduction of Enterovirus 71 Vaccines in Chengdu, China, 2009-2018. Pediatr Infect Dis J. 2020;39(10):969–978. doi: 10.1097/INF.0000000000002745. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Jiang H, Tian X, Xia X, Huang T. Ongoing Epidemiological Changes in Hand, Foot, and Mouth Disease Following the Introduction of Enterovirus 71 Vaccines in Yunnan Province, China, 2008-2019; 2021.
- 13.Xing W, Liao Q, Viboud C, et al. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis. 2014;14(4):308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian L, Liang F, Xu M, Jia L, Pan X, Clements ACA. Spatio-temporal analysis of the relationship between meteorological factors and hand-foot-mouth disease in Beijing, China. BMC Infect Dis. 2018;18(1):158. doi: 10.1186/s12879-018-3071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong ZM, Wang HH, Wang YJ, Wang WR. Spatiotemporal analysis of hand, foot and mouth disease data using time-lag geographically-weighted regression. Geospat Health. 2020;15(2) doi: 10.4081/gh.2020.849. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Lai Y, Du Z, et al. Spatiotemporal Distribution of Hand, Foot, and Mouth Disease in Guangdong Province, China and Potential Predictors, 2009⁻2012. Int J Environ Res Public Health. 2019;16(7) doi: 10.3390/ijerph16071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang B, Wu P, Wu JT, et al. Seroprevalence of Enterovirus 71 Antibody Among Children in China: A Systematic Review and Meta-analysis. Pediatr Infect Dis J. 2015;34(12):1399–1406. doi: 10.1097/INF.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z, Huang Y, Lawrence WR, et al. Leading Enterovirus Genotypes Causing Hand, Foot, and Mouth Disease in Guangzhou, China: Relationship with Climate and Vaccination against EV71. Int J Environ Res Public Health. 2021;18(1) doi: 10.3390/ijerph18010292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JL, Yuan J, Yang F, et al. Epidemic characteristics of hand, foot, and mouth disease in southern China, 2013: coxsackievirus A6 has emerged as the predominant causative agent. J Infect. 2014;69(3):299–303. doi: 10.1016/j.jinf.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Guo WP, Lin XD, Chen YP, et al. Fourteen types of co-circulating recombinant enterovirus were associated with hand, foot, and mouth disease in children from Wenzhou, China. J Clin Virol. 2015;70:29–38. doi: 10.1016/j.jcv.2015.06.093. [DOI] [PubMed] [Google Scholar]
- 21.Hongyan G, Chengjie M, Qiaozhi Y, et al. Hand, foot and mouth disease caused by coxsackievirus A6, Beijing, 2013. Pediatr Infect Dis J. 2014;33(12):1302–1303. doi: 10.1097/INF.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 22.Han JF, Xu S, Zhang Y, et al. Hand, foot, and mouth disease outbreak caused by coxsackievirus A6, China, 2013. J Infect. 2014;69(3):303–305. doi: 10.1016/j.jinf.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Yuan J, Wang X, et al. Severe hand, foot, and mouth disease and coxsackievirus A6-Shenzhen, China. Clin Infect Dis. 2014;59(10):1504–1505. doi: 10.1093/cid/ciu624. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Zeng H, Zheng H, et al. Hand, foot and mouth disease in Guangdong, China, in 2013: new trends in the continuing epidemic. Clin Microbiol Infect. 2014;20(7):O442–O445. doi: 10.1111/1469-0691.12468. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Sun Y, Du Y, et al. Characterization of Coxsackievirus A6- and Enterovirus 71-Associated Hand Foot and Mouth Disease in Beijing, China, from 2013 to 2015. Front Microbiol. 2016;7:391. doi: 10.3389/fmicb.2016.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160(4):1097–1104. doi: 10.1007/s00705-015-2340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Zhu R, Yang Y, et al. Phylogenetic analysis of the major causative agents of hand, foot and mouth disease in Suzhou City, Jiangsu province, China, in 2012-2013. Emerg Microbes Infect. 2015;4(2):e12. doi: 10.1038/emi.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salleras L, Domínguez A, Ciruela P, et al. Changes in serotypes causing invasive pneumococcal disease (2005-2007 vs. 1997-1999) in children under 2 years of age in a population with intermediate coverage of the 7-valent pneumococcal conjugated vaccine. Clin Microbiol Infect. 2009;15(11):997–1001. doi: 10.1111/j.1469-0691.2009.02938.x. [DOI] [PubMed] [Google Scholar]
- 29.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. Jama. 2007;297(16):1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 30.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He YQ, Chen L, Xu WB, et al. Emergence, circulation, and spatiotemporal phylogenetic analysis of coxsackievirus a6- and coxsackievirus a10-associated hand, foot, and mouth disease infections from 2008 to 2012 in Shenzhen, China. J Clin Microbiol. 2013;51(11):3560–3566. doi: 10.1128/JCM.01231-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and code used and analyzed during the current study available from the corresponding author on reasonable request.