Abstract
Purpose
This case was conducted to report the effectiveness and security of a manually made needle to inject triamcinolone acetonide in the suprachoroidal space (SCS) in a 52-year-old female with pseudophakic cystoid macular edema (PCME) in the challenging socio-economical situations in Syria.
Methods
This case report is an interventional case of a 52-year-old female presented with a four-week history of reduced vision secondary to Pseudophakic cystoid macular edema (PCME). The patient attended Marashi Eye Clinic Center for a clinical examination and followed up with Optical Coherence Tomography (OCT) at baseline. The patient was treated by one injection of triamcinolone acetonide and followed up within one week, 4 weeks, 8 weeks, 16 weeks, and 24 weeks in the suprachoroidal space (SCS) using a manually made needle with assessing the efficacy and potential ocular complications.
Results
The best-corrected visual acuity (BCVA) had improved significantly from baseline 20/60 to 20/30 at 24 weeks with a complete anatomical resolution of macular edema at 24 weeks from baseline. No ocular complications were noticed during the study period.
Conclusions and Importance
Injecting triamcinolone acetonide in suprachoroidal space (SCS) using a manually made needle plays an essential role in treating Pseudophakic cystoid macular edema (PCME) without compromising security and efficiency.
Keywords: Pseudophakic cystoid macular edema, Suprachoroidal, Injection, Triamcinolone acetonide, Efficacy, Socio-economical
1. Introduction
Pseudophakic cystoid macular edema (PCME) is a common post-cataract surgery complication that may reduce vision. PCME may associate with or without vitreomacular abnormalities and may be caused by vascular instability or an inflammatory process due to surgical manipulation. Thus, inflammatory mediators can disrupt the retinal blood barrier and increase fluid leakage from the perifoveal microvessels and induce cystoid macular edema. Another mechanism is inducing vitreomacular traction on the macula or exacerbating pre-exciting tangential or anterior-posterior traction. The treatment of PCME can be treated simply by topical non-steroidal anti-inflammatory agents with topical steroids.1 However, in refractory and long-standing cases of PCME, intravitreal injection triamcinolone2 would be considered the treatment of choice. When PCME is combined with an element of vitreomacular abnormalities, then pars plana vitrectomy is the treatment of choice.3
In this case report, we define the potential role of injecting 0.1 ml of triamcinolone acetonide in the suprachoroidal space (SCS) using a manually made needle in a 52-year-old female suffering from Pseudophakic cystoid macular edema (PCME), showing the overall impact of this manually made needle on treating with efficacy and safety.
2. Case report
A 52-year-old diabetic female presented to Marashi Eye Clinic with a complaint of reduced vision in her left eye. On presentation, her best correct visual acuity (BCVA) was 20/80 at performance with refraction −3.25/-1.00 x 18, where her right eye was pseudophakia with BCVA of 20/25. Her anterior segment examination was normal, with no marks of rubeosis iridis and a central intraocular lens.
There was an opened-angle on gonioscopy without any sign of neovascularization, Schlemm's canal was clear, and the intraocular pressure (IOP) was 19 mm Hg. However, slit-lamp examination confirmed the presence of cataract that can significantly compromise visual acuity with nuclear sclerosis stage three. Fundus examination showed no signs of diabetic macular edema with signs of mild Non-Proliferative Diabetic Retinopathy (NPDR).
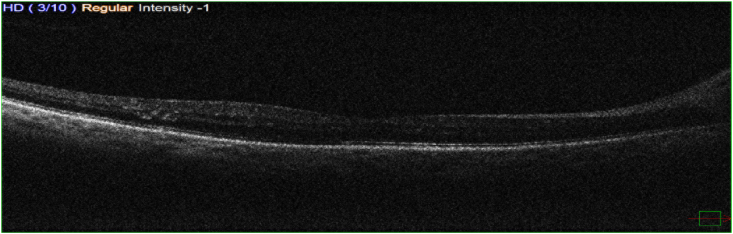
Pre-operatively, Optical Coherence Tomography (OCT) did not show any abnormalities. Specifically, no cystic changes nor increased central macular thinness (the central macular thickness was 232 μm) or vitreomacular abnormalities (Fig. 1).
Fig. 1.
Shows a normal cross-section OCT for the patient before phacoemulsification surgery.
Phacoemulsification was performed uneventfully with the implantation of monofocal aspheric hydrophilic acrylic intraocular lens in the bag with subconjunctival triamcinolone at the end of surgery. Nepafenac 0.1% was prescribed three times daily, along with topical dexamethasone 0.1% q.i.d. two days before surgery, then two weeks of nepafenac, and one week of dexamethasone post-operatively as prophylaxis.
Four weeks later, the patient complained about reduced BCVA to 20/60 with no signs of anterior segment postoperative complications such as synechia, Posterior capsule opacification (PCO), endophthalmitis, or refractive surprise.
The OCT showed a central empty cystic change, increased at the level between the inner and outer nuclear layer with mild to moderate disorganization of inner retinal layers and outer nuclear layer disruption with cystic changes and the case was presented subretinal fluids with increased central macular thickness up to 586 μm. However, the ellipsoid zone and external limiting membrane look intact, but the interdigitation zone is not clearly presented.
A lengthy discussion regarding options of treatment was made with the patient and her sibling. Likely outcomes and potential risks were fully explained to the patient.
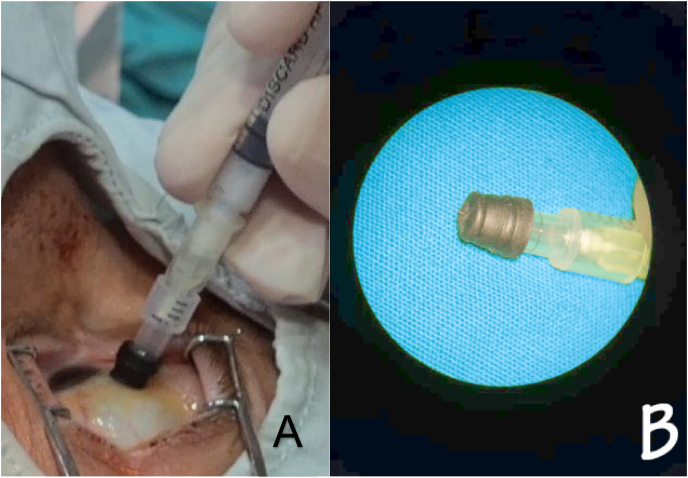
Other options of treatment included intravitreal injection (IVI) of triamcinolone such as intravitreal dexamethasone implant, monthly IVI of Anti-VEGF, or suprachoroidal triamcinolone. The monthly IVI of Anti-VEGF and intravitreal dexamethasone were not probable due to the lack of funds. However, there are concerns about elevated IOP spikes and floaters with IVI of triamcinolone. Therefore, for socio-economical reasons, suprachoroidal triamcinolone was chosen as a treatment of choice for this patient considering Syria's current economic situation and poverty. Informed consent was signed after being discussed. We administered the suprachoroidal injection following sterile circumstances, by sterilizing the epidermis using povidone-iodine 10% and anesthetizing the eye by topical anesthesia, then disinfecting the conjunctiva using povidone-iodine 4%. After finishing the sterilizing process, we carried out the needle and put the lid speculum to isolate eyelashes. We placed the injection site in the superior temporal quadrant and measured 4 mm from the limbus using calipers. Then, we used the manually made needle (30 gauge) with a rubber stopper, which will permit 1000 μm only from the needle to penetrate the sclera (Fig. 2), then we pushed 0.1ml triamcinolone inside the suprachoroidal space (SCS).
Fig. 2.
(A) The injecting process. (B) Shows the manually made needle with a rubber stopper.
After injecting, the intraocular pressure (IOP) was 15 mm. We observed the IOP weekly and continuously persisted without raised spikes until 24 weeks. The BCVA recovered to 20/50 in the 1st week, 20/40 at the 8 weeks, and 20/30 at 24 weeks.
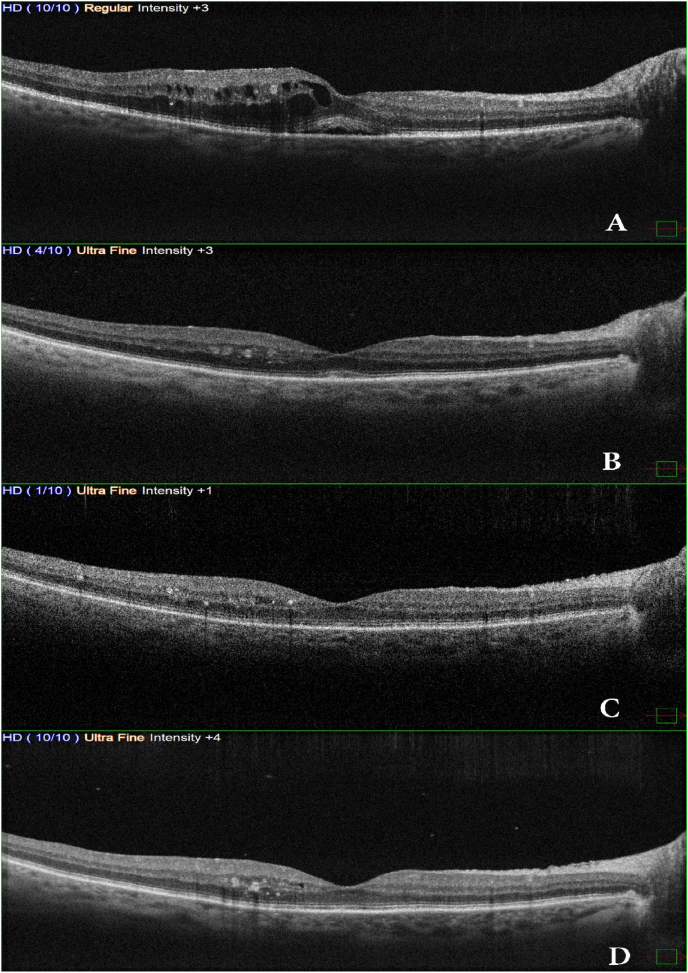
On OCT there was a significant thickness decrease within 24 hours (348 μm); however, after 8 weeks, OCT showed improvement in retinal anatomical changes and the retinal thickness persisted normal (265 μm), while results were reported in the 24 weeks and the central macular thickness was 257 μm with mild disorganization of inner retinal layers and hyperreflective foci and small cystic change at the level of the inner nuclear layer with intact ellipsoid zone and external limiting membrane (Fig. 3).
Fig. 3.
OCT scans show (A) Cystic macular edema with subretinal fluid. (B) 24 hours later, after injecting triamcinolone acetonide in the suprachoroidal space, shows a decrease in central macular thickness and resolved intraretinal cysts. (C, D) 8 weeks and 24 weeks OCT scans show a maintained result of therapy through injecting triamcinolone acetonide in the suprachoroidal space.
3. Discussion
A dilatation of retinal capillaries after cataract extraction surgery may occur, which increases the diffusion of serous fluids in the intra-retinal space forming cystic spaces or/and fluid can accumulate in the subretinal space. This mechanism is regulated by prostaglandins and their products, especially when iris is traumatized during surgery.4
The management of pseudophakic cystoid macular edema (PCME) usually done by topical application of NSAIDs and steroids before and after surgery, and subconjunctival triamcinolone can be considered in diabetic patients,5 in case of treatment failure, then switching to posterior subtenon or intravitreal steroids are recommended.6 However, when the vitreomacular traction is the leading force for cystic macular edema formation, then pars plana vitrectomy is recommended if the spontaneous release does not happen within months, especially in cases of moderate visual loss.7
In our case, topical nepafenac and dexamethasone failed to stop or treat cystoid macular edema, while intravitreal triamcinolone may cause high intraocular pressure (IOP) and floaters; however, intravitreal dexamethasone implant may have a more secured characterization, but the patient cannot reimburse them.
In this case, the suprachoroidal space (SCS) route to deliver triamcinolone was chosen because it is minimally invasive compared to injecting triamcinolone in the posterior subtenon. At the same time, SCS may decrease the hazard of producing spikes of high IOP because triamcinolone is delivered to the choroid and retina, which enhances its effectiveness and may not interpose with the angle of the anterior segment by contrast to the intravitreal route. However, the directed amount of triamcinolone in the retina is 40% only, whereas the rest of the triamcinolone is separated into the crystalline lens and anterior segment angle8 and eliminates the patient's risk seeing irritating floaters.
Corticosteroids can inhibit prostaglandin production and their pathways and restabilize the blood-retinal barrier, thus reducing the macular thickness and resolving the cystic macular edema.9 Since triamcinolone acteonid was injected in the suprachoroidal space (SCS), the concentration of steroids at the macula's level and choroid is much higher than using the intravitreal route. Injecting triamcinolone will be diluted and disseminated in the vitreous cavity with more interaction with the anterior segment angle compared to the SCS triamcinolone acteonid. Thus, it will make the SCS route more robust with fewer complications compared to the intravitreal route for treating PCME.
4. Conclusion
Using a manually made needle for injecting triamcinolone acetonide in the suprachoroidal space (SCS) to manage Pseudophakic cystoid macular edema (PCME) looks a safe and effective method in our case. Nevertheless, we should conduct more extensive randomized trials to estimate persistence and long term security and effectiveness.
Patient consent
Informed consent was obtained from the patient for the purpose of publication.
Funding/financial
The authors have received no funding or grant support related to this article.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Declaration of competing interest
All authors have no financial disclosures; no conflicts were reported.
Acknowledgments
None.
References
- 1.Russo A., Costagliola C., Delcassi L., et al. Topical nonsteroidal anti-inflammatory drugs for macular edema. Mediat Inflamm. 2013;2013 doi: 10.1155/2013/476525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas J.B., Kreissig I., Degenring R.F. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol. 2003;136(2):384–386. doi: 10.1016/s0002-9394(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 3.Grzybowski A., Sikorski B.L., Ascaso F.J., Huerva V. Pseudophakic cystoid macular edema: update 2016. Clin Interv Aging. 2016;11:1221–1229. doi: 10.2147/CIA.S111761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lally DR Md, Shah C.P., Md M.P.H. 2014. Pseudophakic Cystoid Macular Edema Pseudophakic CME remains a common cause of reduced vision after cataract surgery. A look at its causes and treatment. Published online. [Google Scholar]
- 5.Carricondo P.C., Abalem M.F., Machado C.G., Kara-Junior N. Prophylaxis and treatment of cystoid macular edema after cataract surgery. Rev Bras Oftalmol. 2015;74(2):113–118. [Google Scholar]
- 6.Koutsandrea C., Moschos M.M., Brouzas D., Loukianou E., Apostolopoulos M., Moschos M. Intraocular triamcinolone acetonide for pseudophakic cystoid macular edema: optical coherence tomography and multifocal electroretinography study. Retina. 2007;27(2):159–164. doi: 10.1097/IAE.0b013e31802e3e5c. [DOI] [PubMed] [Google Scholar]
- 7.Pendergast S.D., Margherio R.R., Williams G.A., Cox M.S., Jr. Vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol. 1999;128(3):317–323. doi: 10.1016/s0002-9394(99)00158-0. [DOI] [PubMed] [Google Scholar]
- 8.Patel S.R., Berezovsky D.E., McCarey B.E., Zarnitsyn V., Edelhauser H.F., Prausnitz M.R. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson C.A., Berkowitz B.A., Sato Y., Ando N., Handa J.T., de Juan E., Jr. Treatment with intravitreal steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992;110(8):1155–1159. doi: 10.1001/archopht.1992.01080200135041. [DOI] [PubMed] [Google Scholar]