Abstract
Pancytopenia is a condition when a person has a low count of all three types of blood cells, causing a triage of anaemia, leukopenia and thrombocytopenia. It should not be considered a disease in itself but rather a sign of a disease that needs to be further evaluated. Among the various causes, viral infections like the human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus and parvovirus B19 have been implicated. Pancytopenia is a rare complication and is not commonly seen in patients with COVID-19 disease. Here, we report a case of pancytopenia in a previously immunocompetent elderly male patient with SARS-CoV-2 infection.
Keywords: Pancytopenia, SARS-CoV-2 infection, COVID 19 disease
Introduction
The most common laboratory findings of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are elevated inflammatory markers, hypercoagulable state and lymphopenia. Pancytopenia is a rare complication and is not commonly seen in immunocompetent patients with COVID-19 disease. The common causes of pancytopenia in clinical practise are megaloblastic anaemia, hypersplenism, drug-induced bone marrow toxicity, leukaemia, radiation therapy, chemotherapy, immunosuppressive medications, connective tissue diseases and infections [1]. Pancytopenia as a result of direct bone marrow suppression has been previously reported in viral infections like human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus and parvovirus B19. Here, we report a case of pancytopenia in a previously immunocompetent elderly male patient with SARS-CoV-2 infection.
Case Presentation
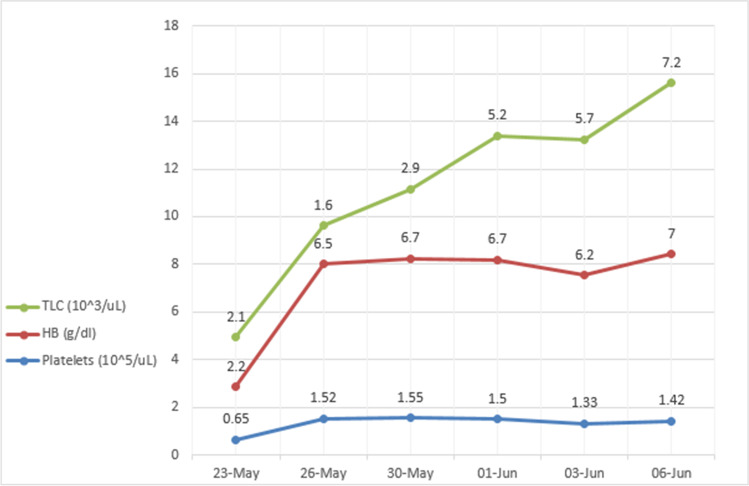
A 69‐year‐old male presented to the hospital emergency department with complaints of fever, dry cough, fatigue and progressive breathlessness for 15 days. He gave history of hypertension but was not on any regular treatment and follow-up. On examination, he had a high blood pressure recording (150/90 mm Hg right arm supine) with tachycardia and tachypnoea and was not maintaining oxygen saturation at room air (SpO2 80% on room air). He was febrile (101°F) and had pallor. Initial laboratory workup on admission showed pancytopenia and deranged serum creatinine of 2.4 mg/dL (findings are summarised in Table 1). It showed total white blood cell count (WBC) 2,100/μL, haemoglobin 2.2gm/dL, platelet count 65,000/μL, urea 46 mg/dL, creatinine 2.4 mg/dL, total bilirubin 0.8 mg/dL, lactate dehydrogenases (LDH) 353 IU/L, aspartate aminotransferase (AST) 32U/L and alanine aminotransferase (ALT) 84U/L. His nasopharyngeal swab test for SARS-CoV-2 PCR was positive. The chest radiograph showed non-homogenous opacities involving left mid and lower zones (Fig. 1). He was diagnosed as a case of severe COVID-19 disease with pancytopenia and was transferred to COVID-19 intensive care unit. He was managed with oxygen therapy, parenteral broad-spectrum antibiotics, packed red blood cell transfusion, dexamethasone (6 mg IV once a day), antihypertensives and anticoagulant therapy (renal-modified dose of injection low molecular weight heparin). His inflammatory markers were raised (C‐reactive protein 20.5 mg/L, D‐dimer 0.80 µg/L, ferritin 900 ng/mL, lactate dehydrogenase 353 IU/L). He was evaluated for the possible causes of pancytopenia which included leukaemia, myelodysplastic syndrome, megaloblastic anaemia, infections, drug-induced bone marrow toxicity, radiation therapy, immunosuppressive medications and connective tissue diseases. He was not on any immunosuppressive medications previously. He also denied history of any radiation therapy and consumption of drugs known to cause bone marrow suppression. His serum antinuclear antibody test was negative which ruled out connective tissue diseases. His serum folate and vitamin B12 were within normal range. Peripheral blood microscopic examination showed pancytopenia and normocytic normochromic anaemia with moderate anisocytosis. Peripheral blood microscopic examination was not suggestive of megaloblastic anaemia, and there was no evidence of haemolysis, sepsis, or atypia (Fig. 2). He underwent bone marrow aspiration and biopsy which showed hypocellular marrow with cellularity less than 20% and increase in fat spaces (Fig. 3). There were focal areas of marrow elements showing trilineage haematopoiesis with markedly diminished myeloid, erythroid and megakaryocytic series. There was no evidence of leukaemia, myelodysplastic syndrome (MDS), infections or metastatic deposits. Peripheral blood smear and bone marrow microscopic examination were suggestive of pancytopenia with hypocellular bone marrow (Figs. 2 and 3). He also underwent ultrasound of abdomen, which showed features of bilateral medical renal disease with no organomegaly. During the hospital course, three units of packed red blood cells were transfused to the patient. The patient's clinical condition and haematology parameters improved with therapy (Fig. 4). He was discharged on day 15 with WBC 7,200/μL, haemoglobin 7gm/dL and platelet count of 1,42,000/μL. His throat swab for SARS-CoV-2 PCR was negative at the time of discharge.
Table 1.
Laboratory findings
| Test name | Result |
|---|---|
| Haemoglobin | 2.2 g/dL |
| Total leucocyte count | 2,100/µL |
| Differential leucocyte count | N-68%,L-29%,M-2%,E-1% |
| Platelet count | 65,000/µL |
| INR | 1.2 |
| Urea | 46 mg/dL |
| Creatinine | 2.4 mg/dL |
| Uric acid | 8.7 mg/dL |
| Albumin | 2.1 gm/dL |
| Bilirubin total | 0.3 mg/dL |
| Serum proteins | 6.0 gm/dL |
| AST | 32 U/L |
| ALT | 84 U/L |
| Albumin | 2.6 g/dL |
| Globulin | 3.4 g/dL |
| Sodium | 134 meq/L |
| Potassium | 5.1 meq/L |
| LDH | 353 U/L |
| D-dimer | 0.8 |
| Ferritin | 900 ng/mL |
| CRP | 20.3 mg/dL |
| Procalcitonin | < 0.5 ng/mL |
INR international normalised ratio, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, CRP-C reactive protein
Fig. 1.
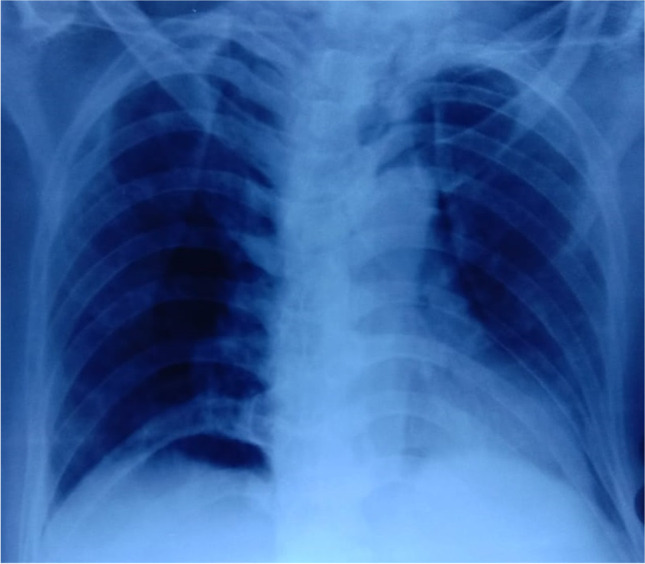
The chest radiograph showing non homogenous opacities involving left mid and lower zones
Fig. 2.
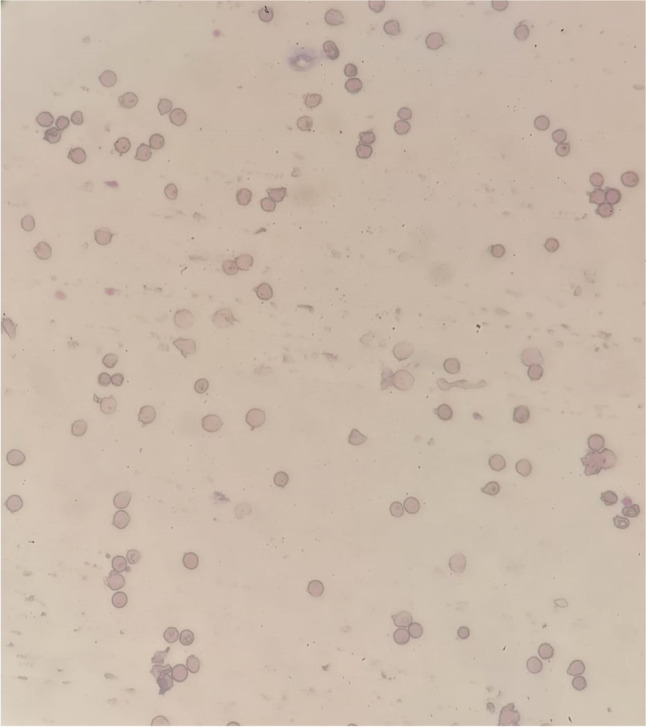
Peripheral blood smear
Fig. 3.
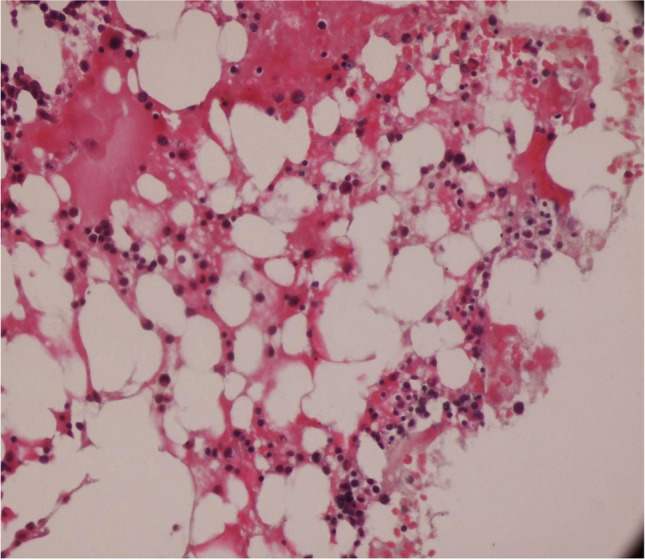
Bone marrow biopsy showed hypocellular marrow and increase in fat spaces
Fig. 4.
Haematological parameters during hospital stay
Discussion
The novel SARS-CoV-2 virus is the etiological agent for COVID-19 disease, and the respiratory system is involved in majority of patients. The clinical presentation of the disease is variable, including asymptomatic infection, mild upper respiratory infection and severe pneumonia with respiratory failure [2]. Recent studies have shown that COVID-19 disease has significant effect on the haematopoietic system and haemostasis. The various haematological abnormalities reported in COVID-19 disease are lymphopenia, thrombocytopenia, leukopenia and hypercoagulability [3]. Lymphopenia is the most common reported finding of all [3, 4]. Pancytopenia is a rare complication and is not commonly seen in patients with COVID-19 disease. Hypercoagulability and thrombotic complications are frequently encountered among COVID-19 patients with severe disease, and these are believed to be due to a hyperinflammatory response caused by the virus [5]. There are several thrombotic complications described in the literature. These include pulmonary thromboembolism, thoracic and abdominal aortic thrombosis, mesenteric ischemia, myocardial infarction, acute cerebrovascular accident and disseminated intravascular coagulation [6].
Pancytopenia is a condition when person has low count of all three types of blood cells causing a triage of anaemia, leukopenia and thrombocytopenia. It should not be considered as a disease in itself but rather the sign of a disease that needs to be further evaluated. The possible causes are nutritional deficiencies, megaloblastic anaemia, hypersplenism, malignancies, radiation therapy, chemotherapy drug-induced bone marrow toxicity, connective tissue diseases and immunosuppressive medications [1]. All these conditions were ruled out in our patient during hospital stay. Pancytopenia as a result of bone marrow suppression has been reported in viral infections and commonly implicated viruses are human immunodeficiency virus, parvovirus B19, Epstein-Barr virus and ytomegalovirus [7]. The decreased myeloid, erythroid and megakaryocytic series was observed in bone marrow biopsy of our patient indicating bone marrow suppression. There was no evidence of lymphoma, fibrosis and myelodysplasia in bone marrow biopsy. There are very few case reports on SARS-CoV2-induced pancytopenia [8]. Issa N et al. reported the first case of persistent pancytopenia associated with SARS-CoV2 bone marrow infiltration in an immunocompromised patient [9]. However, our patient was immunocompetent. He was detected to have chronic kidney disease during evaluation which alone could not explain the severe anaemia and associated pancytopenia. Ufuk F et al. reported a case of COVID-19-associated pancytopenia which was complicated by neutropenic enterocolitis [10]. Our patient responded well to the supportive care, and there were no complications during hospital stay. Once the patient’s infection resolved, his blood counts improved, and at the time of hospital discharge, his leucocyte and platelet count had normalized and only anaemia remained. Hersby DS et al. also reported a similar self-limiting clinical course of patient with COVID-19-induced pancytopenia [8].
The possible pathophysiology of pancytopenia secondary to SARS CoV2 infection could be linked to the angiotensin converting enzyme 2 receptor (ACE 2 receptor), which is present in bone marrow in lower levels [11]. It is possible that direct infection of myelocytes by SARS CoV-2 virus could lead to bone marrow suppression as seen in other viral infections like HIV, parvovirus B19, Epstein-Barr virus and cytomegalovirus. Other possibility is that after viral infection, an antigenic epitope on myelocytes could be exposed which can lead to the production of autoantibody and destruction of blood cells. Also, hyperinflammatory state is a key feature of severe COVID‐19 disease and It is well known that certain cytokines, such as the interferons and tumour‐necrosis factor‐α can affect haematopoietic stem cells and thus impair hematopoiesis [12]. The lung is a site for platelet biogenesis and a reservoir for haematopoietic progenitors and with SARS‐CoV-2 infection leading to lung injury; it is possible that the destruction of lung haematopoietic progenitors could also contribute to the pancytopenia [13].
Conclusion
SARS-CoV-2 infection leading to pancytopenia is rare. We reported a case of pancytopenia associated with COVID‐19 disease likely caused by bone marrow suppression.
Author Contribution
All the authors have been involved in the review of the case report.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Taken from patient.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Neeraj Sharma, Email: dmt18sharma@gmail.com.
Rajat Shukla, Email: docrajatshukla@gmail.com.
Rachna Warrier, Email: rachnawarrier@gmail.com.
Kunal Kumar, Email: kunalgutgutia@gmail.com.
Nalin Singh, Email: meetnalin@gmail.com.
Sourav Ghose, Email: netildoc@gmail.com.
Vivek Kumar, Email: dr.vivafgms@gmail.com.
References
- 1.Jain A, Naniwadekar M. An etiological reappraisal of pancytopenia - largest series reported to date from a single tertiary care teaching hospital. BMC Hematol. 2013;13(1):10. doi: 10.1186/2052-1839-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang I, Pranata R. Lymphopenia in severe coronavirus disease- 2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Samkari H, Karp Leaf RS, DzikWH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. [DOI] [PMC free article] [PubMed]
- 6.Avila J, Long B, Holladay D, Gottlieb M. Thrombotic complications of COVID-19 Am J Emerg Med., 39 (2021), pp. 213–218 [DOI] [PMC free article] [PubMed]
- 7.Pascutti MF, Erkelens MN, Nolte MA. Impact of viral infections on hematopoiesis: from beneficial to detrimental effects on bone marrow output. Front Immunol. 2016;7:364. doi: 10.3389/fimmu.2016.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hersby DS, Do TH, Gang AO, Nielsen TH. COVID-19-associated pancytopenia can be self-limiting and does not necessarily warrant bone marrow biopsy for the purposes of SARS-CoV-2 diagnostics. Ann Oncol. 2021;32(1):121–123. doi: 10.1016/j.annonc.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Issa N, Lacassin F, Camou F. First case of persistent pancytopenia associated with SARS-CoV-2 bone marrow infiltration in an immunocompromised patient. Ann Oncol. 2020;31(10):1418–1419. doi: 10.1016/j.annonc.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ufuk F, Bulgurcu E, Sari T. COVID-19-associated pancytopenia and typhlitis. Am J Emerg Med. 2021. [DOI] [PMC free article] [PubMed]
- 11.LiMY, Li L, Zhang Y,Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Pov. 2020;9(1). 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed]
- 12.Clapes T, Lefkopoulos S, Trompouki E. Stress and non-stress roles of inflammatory signals during HSC emergence and maintenance. Front Immunol. 2016;7:487. doi: 10.3389/fimmu.2016.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.