Abstract
Cenozoic tectonic evolution in the Tethyan region has greatly changed the landforms and environment of Eurasia, driving the evolution of animals and greatly affecting the diversity patterns of Eurasian animals. By combining the latest Tethyan paleogeographic models and some recently published Eurasian zoological studies, we systematically summarize how tectonic evolution in the Tethyan region has influenced the evolution and diversity patterns of Eurasian animals. The convergence of continental plates, closure of Tethys Sea, and Tethyan sea-level changes have directly affected the composition and spatial distribution of Eurasian animal diversity. The topographic and environmental changes caused by Tethyan tectonics have determined regional animal diversity in Eurasia by influencing animal origin, dispersal, preservation, diversification, and extinction. The ecological transformations resulted in the emergence of new habitats and niches, which promoted animal adaptive evolution, specialization, speciation, and expansion. We highlight that the Cenozoic tectonic evolution of the Tethyan region has been responsible for much of the alteration in Eurasian animal distribution and has been an essential force in shaping organic evolution. Furthermore, we generalize a general pattern that Tethyan geological events are linked with Eurasian animal evolution and diversity dynamics.
Keywords: Tethys, Mediterranean, Biogeography, Zoogeography, Phylogeny
INTRODUCTION
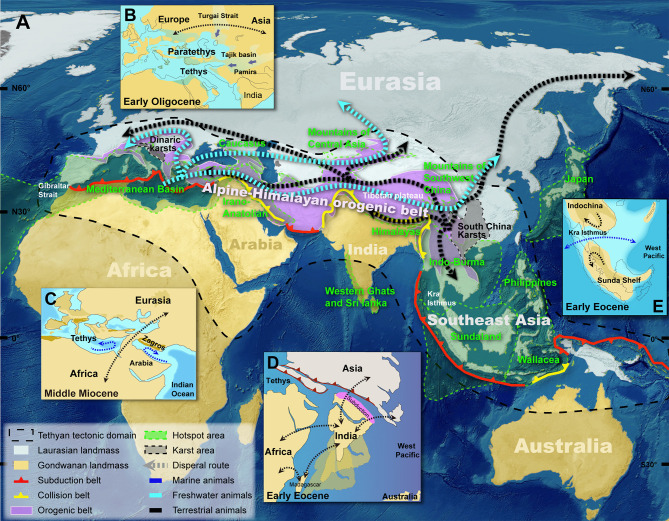
Eurasia is the largest landmass on earth and the richest biologically, hosting 12 of Earth’s 36 recognized biodiversity hotspots (Figure 1A, green area). However, due to a huge population increase, rapid economic growth, and industrial agriculture, Eurasia is considered one of the most biologically vulnerable regions (Mittermeier et al., 2011). Thus, ecologists and zoologists are eager to determine the drivers and rules that underlie Eurasian animal evolution and the current diversity patterns. The answers are extremely valuable in predicting future biodiversity changes and the management of threatened wildlife. In the past decade, zoologists have studied the evolution and diversity of animals in species-rich regions of Eurasia, such as the Mediterranean Basin (Borko et al., 2021), Central Asia (Zhao et al., 2020), Himalaya-Tibetan Plateau (Chang et al., 2008; Price et al., 2014), and Southeast Asia (Ballarin & Li, 2018; Bellwood et al., 2017; Li & Li 2018). These studies have emphasized that evolution and diversification were driven by regional geotectonic and climatic events (e.g., orogeny, regression, and aridification). Few studies have focused on the potential links behind these driven events from a Tethyan perspective, although all above regions are distributed in the Tethyan tectonic domain (Figure 1A, black dashed line area) on the southern margin of Eurasia.
Figure 1.
The Tethyan tectonic domain and the impacts of Eurasian sea-land changes on animal diversity patterns
The present-day landforms of Eurasia were mainly formed in the Cenozoic; prior to this, most of the southern margin of Eurasia was under the sea. Based on the marine animal fossils distributed in the Alps and Himalayas, the Austrian geologist Suess (1893) proposed that there was a long-standing but now vanished sea which once stretched across part of Eurasia, and he named it “Tethys” after both sister and consort of Oceanus, the ancient Greek god of the ocean. The Tethys Sea is the predecessor of the Indian and Atlantic Oceans and has a long history of regression. Tethyan tectonic evolution greatly changed the geomorphology and environment of Eurasia, and the existing Mediterranean Sea, Black Sea, and Caspian Sea are the vestiges of the Tethys Sea (Stow, 2010).
The earlier studies of the Tethyan fauna mainly relied on fossils (Newton, 1988). Recently zoologists have begun to use genetic data to study the spatiotemporal evolution of the diversity of extant fauna in the Tethyan region by combining phylogenetic histories with paleogeography (Hou et al., 2011; Mamos et al., 2016; Zhao et al., 2020). Although current zoological studies of the Eurasian fauna are not about Tethyan tectonic evolution, both paleontological and molecular biological studies have recognized that the origin, dispersal, divergence, adaptation, survival, and extinction of Eurasian animals are closely related to the series of Cenozoic tectonic events in the Tethyan region.
Here, we review recent studies on the evolution and diversification of Eurasian animals in combination with the latest Tethyan paleogeographic reconstructions. We detail how tectonic evolution in the Tethyan region has influenced the evolution and diversity patterns of Eurasian animals. This includes the impact of changing physical connectivity between habitats on the assembly and spatial distribution of animal diversity and the impact on the evolution and diversification of animals by changes in topography, regional climate, and habitat types in Eurasia. Finally, we discuss the general role of Tethyan geological events in the evolution and diversity dynamics of Eurasian animals.
RECONSTRUCTIONS OF TETHYAN TECTONIC EVOLUTION AND EURASIAN SEA-LAND CHANGES
Since the 1980s, more reliable and detailed tectonic hypotheses and models have been put forward, and the tectonic evolution of the Tethyan region has been gradually reconstructed (Barrier et al., 2018; Dercourt et al., 2000; Popov et al., 2004). Recent results indicate that the Tethys Sea mainly existed from 250 to 65 million years ago (Ma), in the Mesozoic. During the Jurassic, the breakup of Pangea into Laurasia to the north and Gondwana to the south resulted in a gradual opening of the Tethys Sea into a dominant marine seaway that became the habitat of many organisms (Hou & Li, 2018; Stow, 2010). In the Cretaceous, the northward drift of the African, Indian, and Australian plates (resulted from the disintegration of Gondwana and the Indian plate moved faster) caused the Tethys Sea to gradually narrow (Figure 1D).
In the Cenozoic, crustal shortening resulted in the formation of the Alpine-Himalayan orogenic belt at the southern margin of the Asian plate (Rosenbaum & Lister, 2002) (Figure 1A), causing further regression of the Tethys Sea from the present-day Tibet and Pamir regions to the Mediterranean Basin (Carrapa et al., 2015; Sun & Jiang, 2013). At the Eocene/Oligocene boundary, the Tethys Sea was separated by this orogenic belt. The huge inland shallow sea to the north of the belt is called the Paratethys Sea, extending from central Europe to inner Asia (Figure 1B). In the Oligocene, the land area of central and western Europe increased because of the regression of the Paratethys Sea. The Paratethys Sea withdrew from the Turgai Strait causing the formation of terrestrial corridors between Europe and Asia (Popov et al., 2004). During the mid-Miocene, the incremental convergence of the Arabian block with Eurasia caused the closure of the Tethys Sea (Figure 1C), blocking global equatorial currents and isolating the Atlantic/Mediterranean Sea and Indo-West Pacific (Hamon et al., 2013). In the Late Miocene (5.96–5.33 Ma), the closure of the Strait of Gibraltar cut off the marine gateways between the Atlantic Ocean and the Mediterranean Sea, and the Tethys Sea almost dried up due to evaporation (Messinian salinity crisis) (Duggen et al., 2003; Garcia-Castellanos & Villaseñor, 2011). In the Pliocene, due to the rise of global sea level, the Atlantic Ocean quickly refilled the Mediterranean Basin (Zanclean flood), forming the present Mediterranean Sea (Garcia-Castellanos et al., 2009).
THE MAJOR TETHYAN GEOLOGICAL EVENTS AND THEIR DIRECT IMPACTS
Continental convergence facilitated exchanges of terrestrial and freshwater faunas
In the Cenozoic, the northward movement of the African, Indian, and Australian plates caused a gradual reduction of the area of the Tethys Sea. This reduction caused the formation of several temporary land bridges, which provided opportunities for faunal exchange, directly affecting the assembly and distribution of Eurasian animal diversity (Figure 1C, D). The Indian plate began to collide with Asia at about 55 Ma and remains ongoing (Zhu et al., 2017). Geological data suggest that the Indian plate experienced a long period of isolation before contact with Asia (Figure 1D), but fossil data from contemporary faunas indicate that during India’s northward journey, the Eurasian fauna maintained exchanges with Gondwanan landmasses (e.g., Africa and Madagascar) via India, because faunal links could be maintained by vagile animals that were able to surmount minor marine barriers (Briggs, 2003). For example, Natatanuran frogs originated in Africa and then dispersed to Asia through India in the Late Cretaceous. In the Early Cenozoic, the Malagasy mantellid frogs originated in Asia and dispersed to Madagascar via India (Yuan et al., 2019). When continents approached, connections also occurred with freshwater fauna. Results of a study including multiple organisms (including freshwater fishes) indicate that the biotic exchange between the Indian subcontinent and mainland Asia has accelerated since the Middle Eocene, pointing to a continuous dispersal corridor since that time and reaching a peak during the Middle Miocene (Klaus et al., 2016).
Likewise, biotic exchange between the Indian subcontinent and Southeast Asia (including Indochina) is found within rhacophorid tree frogs (Li et al., 2013), dragon Lizards (Grismer et al., 2016), and invertebrates, such as fruit flies (Krosch et al., 2012) and spiders (Li et al., 2020). Some researchers suggest that, in addition to geology, the tropical, perhumid climates at that time may have facilitated migration between India and Southeast Asia (Li et al., 2020). With the collision of the Turkish and Arabian plates around 20 Ma, the first land bridge formed between Africa and Eurasia (Figure 1C), promoting biotic exchange between Asia and Africa during the Miocene (Krosch et al., 2012; Yuan et al., 2019). Fossil data have also shown that the mammalian exchange between Africa, Arabia, and Eurasia began at the Oligocene/Miocene boundary (Kappelman et al., 2003).
Narrowing and closure of the Tethys Sea disrupted marine connectivity
Until the end of the Eocene, the Tethys Sea provided a potential migration route between the Atlantic and the Indo-West Pacific, and a considerable degree of overlap in fossils reflects this connectivity (Hou & Li, 2018). The narrowing of the Tethys Sea reduced gene flow between aquatic animals in the Atlantic and the Indo-West Pacific (Figure 1B). For example, many aquatic mollusks began to diverge into Atlantic and Indo-West Pacific groups in the Middle Eocene (Malaquias & Reid, 2009; Uribe et al., 2017 ). The oldest divergence (ca. 37 Ma) occurs between Arabian and Mediterranean Eurasian fresh-water killifishes (Aphanius) corresponding to the separation of the eastern and western Tethys Sea (Hrbek & Meyer, 2003). Additionally, many coral reef fishes were separated into Atlantic/Mediterranean and Indo-West Pacific groups from the Oligocene to the Early Miocene (Cowman & Bellwood, 2013). Similar cases are also found in crustaceans (Page et al., 2008) and bryozoans (Nikulina et al., 2007).
During the Miocene (18–12 Ma), the Tethys Sea closed (Figure 1C) due to the collision of the African-Arabian and Eurasian plates (Hamon et al., 2013). The closure of the Tethys Sea erected physical barriers to the exchange of marine animals near the equator between the Atlantic and the Indo-West Pacific, promoting vicariant divergence. The divergence time and distribution patterns of many extant marine animals clearly reflect the impacts, such as deep-sea squat lobsters (Rodríguez-Flores et al., 2020), intertidal talitrids (Liu et al., 2018), seahorses (Li et al., 2021), and other coral reef fishes (Cowman & Bellwood, 2013). Data from fossil deposits, such as the significant decrease in the similarity between the Mediterranean and Pakistan gastropod faunas during the Early Miocene, also reflect vicariance (Harzhauser et al., 2007). After the closure of the Tethys Sea, faunal exchange between the Atlantic and the Indo-West Pacific tropical fauna could only occur via the Cape of Good Hope (Hou & Li, 2018).
Sea-level changes in the Tethyan region acted as species pumps
Sea-level change is regarded as one of the most important factors affecting animal diversity. Since the Cenozoic, the global sea level has fluctuated dramatically due to changes in seawater temperature, sea-floor spreading, and freeze-thaw cycles of glaciers (Miller et al., 2005). The transgression and regression in the Tethyan region are closely related to changes in global sea level and have had a profound impact on faunal diversity patterns in Southeast Asia (Hanebuth et al., 2011; Lohman et al., 2011). The early divergence between the Indo-Burmese and Sundaic fauna coincides with the formation of inland seaways in the Kra Isthmus during the Early Cenozoic when sea level rose (Figure 1E). This vicariant pattern has also been found in native Althepus spiders and spitting spiders (Li & Li, 2018; Luo & Li, 2018). During periods of low sea level, a land bridge was formed across the Kra Isthmus, and the surrounding islands may have been connected with Indochina through Sundaland. Additionally, changes in Plio-Pleistocene sea levels acted as a “species pump”, promoting genetic divergence and speciation of animals in Southeast Asia, such as in shrews (Esselstyn & Brown, 2009), cats (Luo et al., 2014), and snakes (Inger & Voris, 2001).
In the Quaternary (2.6 Ma–present), glacial cycles caused fluctuation of the sea level in the Paratethyan region, resulting in recurrent geographical and genetic isolation and exchange, which affected the distribution and population genetic structure of European animals (Hewitt, 2000). For example, during the glacial retreat, the European freshwater fish (Vimba vimba) expanded from its refugium at the margins of the Black Sea towards the Baltic Basin when a large amount of water from melting glaciers filled the basin (Hänfling et al., 2009). The Arctic marine crustacean Gammaracanthus colonized the Caspian Sea through the Turgai Strait and Aral Basin when sea level rose ca. 1 Ma, and the Caspian population was isolated by subsequent glaciations (Väinölä et al., 2001). Quaternary sea-level fluctuations connected the Mediterranean islands with the mainland intermittently which maintained a genetic connection between the island and mainland animals. The distribution of widespread diving beetles (Agabus brunneus) from the Balearic Islands, Corsica, and Sardinia is an obvious example of this connectivity (Hidalgo-Galiana et al., 2014).
IMPACTS OF TOPOGRAPHIC AND ENVIRONMENTAL CHANGES CAUSED BY TETHYAN TECTONICS
Formation of the Alpine-Himalayan orogenic belt created diversity hotspots
In the Cenozoic, the collision between plates caused the entire southern margin of Eurasia to be uplifted out of the sea, forming a continuous orogenic belt stretching from Spain to Southeast Asia―the Alpine-Himalayan belt (Rosenbaum & Lister, 2002) (Figure 1A, pink area). East of the belt, the subduction of India beneath Asia generated the Tibetan Plateau, the highest plateau on Earth (Chatterjee et al., 2013). The formation of the Alpine-Himalayan belt accelerated the regression of the Paratethys Sea and resulted in widespread environmental changes in Eurasia that greatly affected the diversity patterns of Eurasian animals by influencing their spatiotemporal evolution. For instance, the Eocene-Oligocene uplift of the Tibetan Plateau caused an early divergence in coelotine spiders and imbalanced diversity on both sides of the plateau. The lineage blocked from the southeast of the plateau comprises a rich species assemblage, while another widespread lineage in the north (Figure 1A, black dashed line) has low species richness caused by a set of early extinction events (Zhao & Li, 2017). The uplift of the Tibetan Plateau changed the paleo-drainage systems which modified the distribution and diversity patterns of freshwater fauna, such as the Gammarus amphipods (Hou et al., 2007) and badid fishes (Rüber et al., 2004). The formation of the Truong Son Mountain Range and several major rivers caused by the India-Asia collision and subsequent orogenesis shaped the diversity patterns of spitting spiders in Southeast Asia (Luo & Li, 2018). The formation of the Ailao Shan-Red River shear zone during the Oligocene/Miocene boundary (26–17 Ma) resulted in genetic divergence and separation of the southern China and Indochina fauna (Ballarin & Li, 2018; Luo & Li, 2018). During the Quaternary glacial episodes, expansions and contractions of ice sheets rendered large areas uninhabitable for most species. However, the southern area of the Alpine-Himalayan belt and the low-altitude areas of the mountains provided glacial refugia that allowed the maintenance of species diversity (Krehenwinkel et al., 2016; Wang et al., 2013). Species that survived usually expanded during postglacial periods, impacting present-day animal distributions. The effects are particularly pronounced in the European fauna (Provan & Bennett, 2008).
The Alpine-Himalayan belt and nearby regions are centers of animal diversification in Eurasia, with extremely high species diversity. The “sky islands” (land archipelagos, suitable alpine habitats separated by dry lowlands) and rate (mountain uplifts cause a high speciation rate) hypotheses are widely accepted mechanisms that are often used to explain the abundant endemicity and high species richness in the Alpine-Himalayan belt (He & Jiang, 2014; Zhao et al., 2020). Steinbauer et al. (2016) highlighted the relationship between topography-driven speciation and the globally consistent pattern of high endemism at high elevations. In the Himalayas, for instance, due to orogenesis and the intensification of the modern South Asian monsoon, there was an increase in both rates of native diversification and neighborhood migration of amphibians and reptiles during the Early to Middle Miocene (Xu et al., 2020). Similar diversification patterns have also been found in invertebrates that are distributed in or nearby the Alpine-Himalayan belt, such as butterflies (Leneveu et al., 2009) and spiders (Zhao & Li, 2017). Spicer (2017) noticed that close proximity niche diversity caused by complex topography and seasonally varying climates is accompanied by repeated episodes of genetic isolation in sky islands, turning mountainous regions in southern Asia into “biodiversity factories”. Some studies imply that the diversification of Himalayan birds is closely related to an increase of available niches and niche filling (Cai et al., 2018; Price et al., 2014). Although glaciation is often seen as destructive for biodiversity, in the Quaternary, glaciation drove transverse alpine allopatric speciation in the Alpine-Himalayan belt (Wallis et al., 2016).
Regression of the Paratethys Sea caused marine extinction
During the middle-late Eocene (47–39 Ma), due to the northward extension of the Pamir Plateau caused by the India-Asia collision, the Paratethys Sea gradually regressed westward from the Tarim Basin via the Tajik Basin (Carrapa et al., 2015; Sun & Jiang, 2013). The Paratethys Sea regressed from the Turgai Strait beginning in the Early Oligocene (Popov et al., 2004), opening up a terrestrial migration corridor for European and Asian animals (Yuan et al., 2016; Zhao et al., 2020) (Figure 1B). During the Oligocene, the Paratethys Sea gradually shrank into the relictual Caspian and Black Seas. With changes in land and sea area, a new ecosystem formed, ranging from brackish to freshwater (Popov et al., 2004; Sun & Jiang, 2013). The desalinization opened a waterway between the Balkans and the Caucasus for freshwater Crustaceans (Esmaeili-Rineh et al., 2015; Sidorov et al., 2018; Zakšek et al., 2007). The marine habitat in the Paratethyan region completely vanished around 11.6 Ma after the closure of the Tethys Sea (Harzhauser & Mandic, 2008). The change from marine to brackish and freshwater ecosystems resulted in the extinction of some marine fauna in the Paratethyan Basin, such as corals, foraminiferans, and mollusks (Harzhauser & Piller, 2007; Studencka & Jasionowski, 2011). The disappearance of marine habitats led to relict distributions of some marine animals, the best-known case being the restriction of thermosbaenacean species to inland subterranean waters and anchialine caves at Mediterranean coastlines and other areas covered by the ancient Tethys Sea (Jaume, 2008).
Aridification in Central Asia caused disjunct distributions and divergence
With the regression of the Paratethys Sea, a restricted, evaporitic marine environment appeared, and desert-like environments were established in the vast interior of Asia beginning in the Late Eocene (ca. 39 Ma) (Carrapa et al., 2015). During the Middle Miocene, global cooling and the rain shadow of the Alpine-Himalayan belt increased aridification in Central Asia (Manafzadeh et al., 2017; Miao et al., 2012). Currently, Central Asia remains mainly composed of the desert, gobi, grassland, arid woodland, and other open and dry habitats, whose origins led to the disjunct distribution of some animals that can only live in humid habitats in Eurasia, such as amphibians (Zheng et al., 2009) and some spiders (Wang et al., 2017). The aridity has promoted the divergence of some Central Asian animals, such as the Miocene diversification of the widespread Eurasian racerunner lizards (Eremias) (Guo et al., 2011). The radiation that occurred within Asian jerboas, specialized desert dwellers, was associated with the expansion of open habitat starting in the Middle Miocene (Zhang et al., 2013). The diversification of ground-dwelling spiders (Pireneitega) relied on invading numerous wet valleys created by the uplift of mountains in arid Central Asia during the Miocene (Zhao et al., 2020). Due to the arid environment, the impact of Quaternary glaciation on the organisms in Central Asia is not as significant as that in other areas of the northern hemisphere (Manafzadeh et al., 2017).
Messinian salinity crisis and Zanclean flood caused homogeneity in European marine fauna
The Messinian salinity crisis (5.96–5.33 Ma) occurred due to the combination of lowered global sea level and the collision between the European and African plates that caused the uplift of the Gibraltar Strait (Figure 1A). These events reduced inflow from the Atlantic Ocean to the Mediterranean Sea, and there was more evaporation than precipitation, creating a large amount of salt and simultaneously lowering the level of the Mediterranean Sea (Duggen et al., 2003; Garcia-Castellanos & Villaseñor, 2011). The crisis extirpated most of the Mediterranean fauna (Harzhauser et al., 2007). The end of the salinity crisis came by means of the Zanclean flood (ca. 5.33 Ma), when Atlantic waters quickly refilled the Mediterranean, driven by tectonic subsidence at the Gibraltar sill, flow incision, and global sea level rise (Garcia-Castellanos et al., 2009). The reopening of the Gibraltar Strait allowed marine species to recolonize the Mediterranean from the Atlantic Ocean, which resulted in little genetic variation between Mediterranean and Atlantic populations of many marine species (Patarnello et al., 2007), and there are fewer marine endemics in the Mediterranean than prior to the crisis (Meynard et al., 2012).
After the crisis, diversity and endemism decreased in the European marine fauna but increased in the freshwater fauna (Reyjol et al., 2007). During the crisis, the dry Mediterranean Basin connected the previously isolated islands with the mainland, promoting the dispersal of freshwater animals to Mediterranean islands. When the marine environment was restored in the Mediterranean, the freshwater animals were separated and experienced rapid speciation due to isolation by seawater, as seen in such animals as freshwater crabs (Potamon) (Jesse et al., 2011), planarians (Dugesia) (Solà et al., 2013) and freshwater subterranean Niphargus amphipods (Delić et al., 2020).
IMPACTS OF EMERGENCE OF NEW HABITATS AND NICHES
Appearance of new freshwater habitats (adaptation, radiation, and endemism)
The Tethyan sea-land changes led to the emergence of new freshwater habitats, promoting the adaptive evolution and rapid diversification of aquatic animals in the Tethyan region. In many cases, the transition only occurred once and led to subsequent rapid diversification in the new habitats. For example, the widely distributed Eurasian freshwater crustaceans Gammarus cluster together phylogenetically and underwent rapid diversification and expansion during the Late Eocene to the Middle Miocene (Figure 1A, light blue dashed line). Such a pattern suggests that diversification in the new freshwater ecosystems followed a single freshwater colonization event associated with Tethyan and Paratethyan changes (Hou et al., 2011). Pufferfishes may have occupied freshwater habitats in Southeast Asia during the Eocene, which is consistent with the India-Asia collision promoting the cohesive zone shift from shallow marine waters to freshwater habitats (Yamanoue et al., 2011). In other cases, such as that of Mediterranean gobioid fishes (Huyse et al., 2004), the transition between marine and freshwater habitats occurred multiple times.
Although the transition from marine to brackish and freshwater ecosystems resulted in the extinction of marine animals in the Paratethyan region, the progressively desalinized habitats drove the formation of rich and highly endemic brackish and freshwater mollusk faunas during the Miocene (Lukeneder et al., 2011; Neubauer et al., 2015). The extant Ponto-Caspian crustaceans (Hou & Sket, 2016) are considered to have originated in the same period. During the Messinian salinity crisis, the origin of the hyposaline sea-sized lake Lago-Mare (Roveri et al., 2014), promoted the adaptation of some marine species to freshwater environment. Following reflooding of the Mediterranean Basin, these freshwater species were isolated from their euryhaline relatives. This probably gave rise to the freshwater endemism seen currently in the Mediterranean region, such as in gobioid fishes (Huyse et al., 2004) and snails (Wilke, 2003).
Emergence of a new archipelago (expansion, speciation, and diversification)
The Indo-Australian Archipelago comprises more than 20 000 islands straddling the equator in Southeast Asia and peninsular Thailand, with extraordinary species richness and endemism (Mittermeier et al., 2011). Over the past 50 million years, the geography of this region has changed considerably. During the Miocene, the Australia-Southeast Asia collision led to the emergence of more land and intermittently emergent islands, as well as the increase in the area of shallow seas throughout Southeast Asia (Lohman et al., 2011). The creation of an extensive tropical shallow sea promoted the proliferation of reef-building corals, the rapid expansion of coral-feeding fishes (Bellwood et al., 2017), and rapid speciation in other marine animals in the Miocene, such as gastropods (Frey & Vermeij, 2008) and ostracods (Yamaguchi et al., 2012). Meanwhile, a significant decrease in diversity occurred in the Mediterranean marine fauna after the closure of the Tethys Sea. Thus, the Indo-West Pacific has gradually become the center of global marine biodiversity since the Early Miocene (Harzhauser et al., 2007; Leprieur et al., 2016; Renema et al., 2008). The increase in the number of islands and the fluctuation of sea level resulted in recurring dispersal and vicariance of terrestrial animals in Southeast Asia, promoting rapid diversification (Li & Li, 2018; Lohman et al., 2011).
Formation of a high plateau environment (specialization, origin, and dispersal)
The Alpine-Himalayan belt is not only a center of species diversification in Eurasia but also the birthplace of high plateau fauna. Some animals, such as schizothoracines, Triplophysa, Tibetan antelopes, and yaks, are highly morphologically adapted to survive in cold temperatures, low oxygen, high radiation, and aridity in the unique high altitude habitat of the Tibetan Plateau (Chang et al., 2008; Wang et al., 2015). Both fossil and molecular data have revealed these animals' evolutionary history on the plateau (Deng et al., 2020). In addition, some of the animals that adapted to the harsh plateau environment dispersed out of Tibet when right conditions were being met. A good example of the “Out of Tibet” hypothesis occurs in the Tibetan woolly rhinoceros (Deng et al., 2011), which showed that cold-tolerant species in Pliocene high Tibet were pre-adapted to conditions that were to become widespread during the subsequent Pleistocene Ice Age, and thus Tibet was likely a cradle for the diversification of Ice Age megafauna. The linkage of an extinct Pliocene Tibetan fox with the extant arctic fox is a subset of the Out of Tibet hypothesis (Wang et al., 2014). Some similar patterns of Tibetan origin and expansion have also been found in other animals, like Himalayan pikas (Wang et al., 2020) and white-bellied rats (Ge et al., 2021).
Development of subterranean habitats (adaptation, isolation, and diversification)
The retreat of the Paratethys Sea and the Tibetan uplift strongly influenced atmospheric circulation in Eurasia. Beginning in the Miocene, the eastern margin of Asia became humid and the dry areas shifted to the vast Asian interior from the east in response to the intensified continentality and strengthened the Asian monsoon system (Bosboom et al., 2011; Miao et al., 2012). In the Miocene, the synchronous rain and heat brought by the subtropical warm monsoon, and the massive uplift caused by the India-Asia collision, led to the restarting of karstification in southeastern Asia (Cui et al., 1996; Xiong, 1996). The new cave systems of the South China Karsts (Figure 1A, grey area) provided unique habitats for a multitude of animals, including cave-dwelling spiders (Li et al., 2019; Wang et al., 2017; Zhang & Li, 2014), amphipods (Hou et al., 2013; Zheng et al., 2018), fishes (Zhang et al., 2019), and many other vertebrates and invertebrates (Ran & Yang, 2015). These new habitats promoted their adaptation (e.g., troglomorphic evolution) and diversification, and drove the formation of permanent subterranean fauna in middle latitudes. Pleistocene glaciations are generally invoked to explain the origin and current distribution of cave-dwelling invertebrates of the South China Karsts, as in Nesticella spiders (Zhang & Li, 2013). However, a recent study on Nesticella with more extensive sampling (including both hypogean and epigean species) showed that the mid-Miocene climatic shift (global cooling) was the major evolutionary force that caused a habitat shift from surface to subterranean (Ballarin & Li, 2018). The karsts of Southeast Asia are considered arks of biodiversity (Clements et al., 2006) and often contain high levels of endemism (Yao et al., 2016).
In Europe, the Dinaric karst region of the Balkan Peninsula (Figure 1A, grey area), the region from which karst derives its name, also hosts a considerable subterranean faunal diversity (Sket, 2018). The geological history (and speleogenesis) of/in the Dinaric karst are the results of lithology, tectonic structures, regional climate, and geomorphic evolution that are closely associated with the history of Tethyan tectonics (Zupan Hajna, 2019). Aquatic animals account for a considerable proportion of European subterranean fauna. Studies show that the Dinaric cave-dwelling shrimp (Troglocaris) diversified about 5 Ma, corresponding to karstification in the Mediterranean that was initiated by active groundwater movement during the Late Miocene (Zakšek et al., 2007). This pattern of diversification time also occurs in other crustaceans, such as Niphargus amphipods (Trontelj et al., 2012). In Niphargus, the emerging subterranean unoccupied habitats triggered adaptive radiation. Typically, the ecomorphological structure of subterranean animals is convergent due to similar selection pressures in extreme environments. However, the different microhabitats within caves caused morphological diversification (Borko et al., 2021; Trontelj et al., 2012). In addition, some anchialine groups, such as anchialine cave shrimps (Atyidae) (Jurado-Rivera et al., 2017) and Thermosbaenacea (Jaume, 2008) seem to have originated directly from the sea, although their non-cavernicolous marine relatives are either extinct or inhabit the deep sea. Their extremely disjunct global distribution was driven by Tethyan changes and precisely matches the area covered by the ancient Tethys Sea or its coastlines.
CONCLUSIONS AND FUTURE PROSPECTS
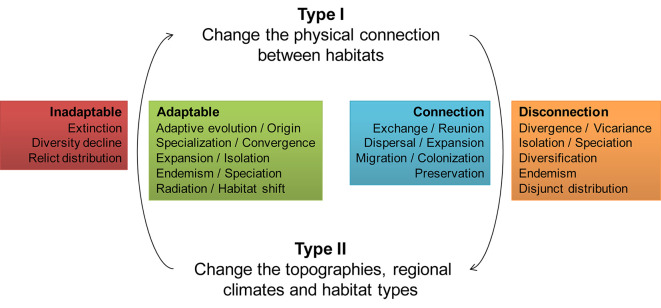
By systematically reviewing recent representative studies of animal distribution, diversity, and evolution in Eurasia, we reveal the potential links between the formation of the current diversity patterns of Eurasian animals and the geological history in the Tethyan region. Though the ways in which geological events change the evolutionary trajectory of animals are myriad (Supplementary Table S1), we find a general pattern: the habitat changes caused by geological events can be roughly divided into physical change (e.g., landbridges and waterway) and qualitative change (e.g., desalinization and aridification). Both types of habitat changes are related to animal evolution and diversification dynamics, but in different aspects (Figure 2). Frequently, both habitat changes occurred simultaneously or in close succession, perpetually driving the speciation, dispersal, extinction and adaptation of animals which are the basic processes of evolution and major drivers behind geological events responsible for the generation of biodiversity patterns.
Figure 2.
A general pattern that Tethyan geological events impact Eurasian animal evolution and diversity dynamics
A commonality found in different animals indicates that areas with active tectonics tend to have more species. Whether in the Alpine-Himalayan orogenic belt or the Indo-West Pacific, all kinds of animals show high species diversity. In many cases, however, the impact of the same geological event on terrestrial, freshwater, and marine animals are different. For example, the convergence of plates has opened corridors for the exchange of terrestrial animals, but it has led to the isolation and divergence of marine animals. Furthermore, a geological event through time produced a series of different circumstances prompting opposing processes sometimes in the same fauna, such as dispersal and vicariance in exchanging periods; extinctions followed by rapid speciation. These impacts of geological events sum over time and results are reflected in nowadays biodiversity patterns of species and phylogenetic richness as well as clades.
A scientific challenge is how to properly reconstruct the impacts of past geological events on organisms. Reconstructions are often limited by the availability of extant and fossil taxa and the extinction of close relatives. This is particularly serious in subterranean relict lineages, which often lack data from relatives (extinction and/or show low levels of fossilization). The lack of ancient basally branching lineages is also a common problem in biogeographic reconstructions. In addition, geographical changes usually deal with longer (multimillion) time scales. The recurring basic evolutionary processes of organisms (e.g., dispersal following vicariance, extinction following speciation) over a smaller (multimillennial or even multidecadal) time scale can obscure the impacts of ancient geological events. Thus, based on extant DNA sequences and meager fossil taxa, can we fully estimate the impact of geological events on organisms' evolution?
As the geological history of the Tethys Sea becomes clearer, it provides an excellent biogeographic background for the study of animal diversity; however, current research in the Tethyan region is of a more regional scale. This will improve with the application of large-scale sampling in animal diversity and zoogeography research. Our review has shown the potential for more large-scale biodiversity and biogeography research under the theme of Tethyan tectonic changes. It is expected that this paper can bring some inspiration and clues to the future study of animal diversity and zoogeography and contribute to the future protection of animal diversity in Eurasia.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
All authors participated in writing the manuscript, then read and approved the final version of the manuscript.
We are indebted to Sarah Crews for invaluable advice and English correction; to Xiao-Qing Zhang for his assistance in the illustrations.
ACKNOWLEDGEMENTS
We are indebted to Sarah Crews for invaluable advice and English correction; to Xiao-Qing Zhang for his assistance in the illustrations.
Funding Statement
This study was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDB31000000), National Natural Sciences Foundation of China (32170447), and program of Youth Innovation Promotion Association of Chinese Academy of Sciences (2019087)
References
- 1.Ballarin F, Li SQ. 2018. Diversification in tropics and subtropics following the mid-Miocene climate change: a case study of the spider genus Nesticella. Global Change Biology, 24(2): e577–e591.
- 2.Barrier E, Vrielynck B, Brouillet JF, Brunet MF. 2018. Paleotectonic reconstruction of the central Tethyan Realm. Tectonono-sedimentary-palinspastic maps from late Permian to Pliocene. Paris: Commission for the Geological Map of the World.
- 3.Bellwood DR, Goatley CHR, Bellwood O The evolution of fishes and corals on reefs: form, function and interdependence. Biological Reviews. 2017;92(2):878–901. doi: 10.1111/brv.12259. [DOI] [PubMed] [Google Scholar]
- 4.Borko Š, Trontelj P, Seehausen O, Moškrič A, Fišer C A subterranean adaptive radiation of amphipods in Europe. Nature Communications. 2021;12:3688. doi: 10.1038/s41467-021-24023-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosboom RE, Dupont-Nivet G, Houben AJP, Brinkhuis H, Villa G, Mandic O, et al. 2011. Late Eocene sea retreat from the Tarim Basin (west China) and concomitant Asian paleoenvironmental change. Palaeogeography, Palaeoclimatology, Palaeoecology, 299(3–4): 385–398.
- 6.Briggs JC The biogeographic and tectonic history of India. Journal of Biogeography. 2003;30(3):381–388. doi: 10.1046/j.1365-2699.2003.00809.x. [DOI] [Google Scholar]
- 7.Cai TL, Fjeldså J, Wu YJ, Shao SM, Chen YH, Quan Q, et al What makes the Sino-Himalayan mountains the major diversity hotspots for pheasants? Journal of Biogeography. 2018;45(3):640–651. doi: 10.1111/jbi.13156. [DOI] [Google Scholar]
- 8.Carrapa B, DeCelles PG, Wang X, Clementz MT, Mancin N, Stoica M, et al Tectono-climatic implications of Eocene Paratethys regression in the Tajik basin of central Asia. Earth and Planetary Science Letters. 2015;424:168–178. doi: 10.1016/j.epsl.2015.05.034. [DOI] [Google Scholar]
- 9.Chang M, Wang XM, Liu HZ, Miao DS, Zhao QH, Wu GX, et al Extraordinarily thick-boned fish linked to the aridification of the Qaidam Basin (northern Tibetan Plateau) Proceedings of the National Academy of Sciences of the United States of America. 2008;105(36):13246–13251. doi: 10.1073/pnas.0805982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee S, Goswami A, Scotese CR The longest voyage: tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Research. 2013;23(1):238–267. doi: 10.1016/j.gr.2012.07.001. [DOI] [Google Scholar]
- 11.Clements R, Sodhi NS, Schilthuizen M, Ng PKL Limestone karsts of Southeast Asia: imperiled arks of biodiversity. BioScience. 2006;56(9):733–742. doi: 10.1641/0006-3568(2006)56[733:LKOSAI]2.0.CO;2. [DOI] [Google Scholar]
- 12.Cowman PF, Bellwood DR Vicariance across major marine biogeographic barriers: temporal concordance and the relative intensity of hard versus soft barriers. Proceedings of the Royal Society B:Biological Sciences. 2013;280(1768):20131541. doi: 10.1098/rspb.2013.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui ZJ, Gao QZ, Liu GN, Pan BT, Chen HL. 1996. Planation surfaces, palaeokarst and uplift of Xizang (Tibet) Plateau. Science in China (Series D), 39(4): 391–400, 449.
- 14.Delić T, Stoch F, Borko Š, Flot JF, Fišer C How did subterranean amphipods cross the Adriatic Sea? Phylogenetic evidence for dispersal–vicariance interplay mediated by marine regression–transgression cycles. Journal of Biogeography. 2020;47(9):1875–1887. doi: 10.1111/jbi.13875. [DOI] [Google Scholar]
- 15.Deng T, Wang XM, Fortelius M, Li Q, Wang Y, Tseng ZJ, et al Out of Tibet: Pliocene woolly rhino suggests high-plateau origin of Ice Age megaherbivores. Science. 2011;333(6047):1285–1288. doi: 10.1126/science.1206594. [DOI] [PubMed] [Google Scholar]
- 16.Deng T, Wu FX, Zhou ZK, Su T Tibetan Plateau: an evolutionary junction for the history of modern biodiversity. Science China Earth Sciences. 2020;63(2):172–187. doi: 10.1007/s11430-019-9507-5. [DOI] [Google Scholar]
- 17.Dercourt J, Gaetani M, Vrielynck B, Barrier E, Biju-Duval B, Brunet MF, et al. 2000. Atlas peri-Tethys. Palaeogeographic maps: 24 maps and explanatory notes. Paris: Commission for the Geological Map of the World.
- 18.Duggen S, Hoernle K, van den Bogaard P, Rüpke L, Morgan JP Deep roots of the Messinian salinity crisis. Nature. 2003;422(6932):602–606. doi: 10.1038/nature01553. [DOI] [PubMed] [Google Scholar]
- 19.Esmaeili-Rineh S, Sari A, Delić T, Moškrič A, Fišer C Molecular phylogeny of the subterranean genus Niphargus (Crustacea: Amphipoda) in the Middle East: a comparison with European Niphargids . Zoological Journal of the Linnean Society. 2015;175(4):812–826. doi: 10.1111/zoj.12296. [DOI] [Google Scholar]
- 20.Esselstyn JA, Brown RM The role of repeated sea-level fluctuations in the generation of shrew (Soricidae: Crocidura) diversity in the Philippine Archipelago . Molecular Phylogenetics and Evolution. 2009;53(1):171–181. doi: 10.1016/j.ympev.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Frey MA, Vermeij GJ Molecular phylogenies and historical biogeography of a circumtropical group of gastropods (Genus: Nerita): implications for regional diversity patterns in the marine tropics . Molecular Phylogenetics and Evolution. 2008;48(3):1067–1086. doi: 10.1016/j.ympev.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Castellanos D, Estrada F, Jiménez-Munt I, Gorini C, Fernàndez M, Vergés J, et al Catastrophic flood of the Mediterranean after the Messinian salinity crisis. Nature. 2009;462(7274):778–781. doi: 10.1038/nature08555. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Castellanos D, Villaseñor A Messinian salinity crisis regulated by competing tectonics and erosion at the Gibraltar arc. Nature. 2011;480(7377):359–363. doi: 10.1038/nature10651. [DOI] [PubMed] [Google Scholar]
- 24.Ge DY, Feijó A, Wen ZX, Abramov AV, Lu L, Cheng JL, et al Demographic history and genomic response to environmental changes in a rapid radiation of wild rats. Molecular Biology and Evolution. 2021;38(5):1905–1923. doi: 10.1093/molbev/msaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grismer JL, Schulte II JA, Alexander A, Wagner P, Travers SL, Buehler MD, et al The Eurasian invasion: phylogenomic data reveal multiple Southeast Asian origins for Indian Dragon Lizards. BMC Evolutionary Biology. 2016;16:43. doi: 10.1186/s12862-016-0611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo XG, Dai X, Chen DL, Papenfuss TJ, Ananjeva NB, Melnikov DA, et al Phylogeny and divergence times of some racerunner lizards (Lacertidae: Eremias) inferred from mitochondrial 16S rRNA gene segments . Molecular Phylogenetics and Evolution. 2011;61(2):400–412. doi: 10.1016/j.ympev.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Hamon N, Sepulchre P, Lefebvre V, Ramstein G The role of eastern Tethys seaway closure in the Middle Miocene Climatic Transition (ca. 14 Ma) Climate of the Past. 2013;9(6):2687–2702. doi: 10.5194/cp-9-2687-2013. [DOI] [Google Scholar]
- 28.Hanebuth TJJ, Voris HK, Yokoyama Y, Saito Y, Okuno J. 2011. Formation and fate of sedimentary depocentres on Southeast Asia's Sunda Shelf over the past sea-level cycle and biogeographic implications. Earth-Science Reviews, 104(1–3): 92–110.
- 29.Hänfling B, Dümpelmann C, Bogutskaya NG, Brandl R, Brändle M Shallow phylogeographic structuring of Vimba vimba across Europe suggests two distinct refugia during the last glaciation . Journal of Fish Biology. 2009;75(9):2269–2286. doi: 10.1111/j.1095-8649.2009.02415.x. [DOI] [PubMed] [Google Scholar]
- 30.Harzhauser M, Kroh A, Mandic O, Piller WE, Göhlich U, Reuter M, et al Biogeographic responses to geodynamics: a key study all around the Oligo-Miocene Tethyan Seaway. Zoologischer Anzeiger - A Journal of Comparative Zoology. 2007;246(4):241–256. doi: 10.1016/j.jcz.2007.05.001. [DOI] [Google Scholar]
- 31.Harzhauser M, Mandic O. 2008. Neogene lake systems of Central and South-Eastern Europe: faunal diversity, gradients and interrelations. Palaeogeography, Palaeoclimatology, Palaeoecology, 260(3–4): 417–434.
- 32.Harzhauser M, Piller WE. 2007. Benchmark data of a changing sea—Palaeogeography, Palaeobiogeography and events in the Central Paratethys during the Miocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 253(1–2): 8–31.
- 33.He K, Jiang XL Sky Islands of southwest China. I: an overview of phylogeographic patterns. Chinese Science Bulletin. 2014;59(7):585–597. doi: 10.1007/s11434-013-0089-1. [DOI] [Google Scholar]
- 34.Hewitt G The genetic legacy of the Quaternary ice ages. Nature. 2000;405(6789):907–913. doi: 10.1038/35016000. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo-Galiana A, Sánchez-Fernández D, Bilton DT, Cieslak A, Ribera I Thermal niche evolution and geographical range expansion in a species complex of western Mediterranean diving beetles. BMC Evolutionary Biology. 2014;14:187. doi: 10.1186/s12862-014-0187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou ZE, Fu JZ, Li SQ A molecular phylogeny of the genus Gammarus (Crustacea: Amphipoda) based on mitochondrial and nuclear gene sequences . Molecular Phylogenetics and Evolution. 2007;45(2):596–611. doi: 10.1016/j.ympev.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Hou ZE, Li JB, Li SQ Ten new Gammarus species (Crustacea: Amphipoda: Gammaridae) from Yunnan-Guizhou Plateau, China . Zootaxa. 2013;3687(1):1–95. doi: 10.11646/zootaxa.3687.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Hou ZE, Li SQ Tethyan changes shaped aquatic diversification. Biological Reviews. 2018;93(2):874–896. doi: 10.1111/brv.12376. [DOI] [PubMed] [Google Scholar]
- 39.Hou ZE, Sket B A review of Gammaridae (Crustacea: Amphipoda): the family extent, its evolutionary history, and taxonomic redefinition of genera. Zoological Journal of the Linnean Society. 2016;176(2):323–348. doi: 10.1111/zoj.12318. [DOI] [Google Scholar]
- 40.Hou ZE, Sket B, Fišer C, Li SQ Eocene habitat shift from saline to freshwater promoted Tethyan amphipod diversification. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14533–14538. doi: 10.1073/pnas.1104636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hrbek T, Meyer A Closing of the Tethys Sea and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae) Journal of Evolutionary Biology. 2003;16(1):17–36. doi: 10.1046/j.1420-9101.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 42.Huyse T, Van Houdt J, Volckaert FAM Paleoclimatic history and vicariant speciation in the “sand goby” group (Gobiidae, Teleostei) Molecular Phylogenetics and Evolution. 2004;32(1):324–336. doi: 10.1016/j.ympev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Inger RF, Voris HK The biogeographical relations of the frogs and snakes of Sundaland. Journal of Biogeography. 2001;28(7):863–891. [Google Scholar]
- 44.Jaume D Global diversity of spelaeogriphaceans & thermosbaenaceans (Crustacea; Spelaeogriphacea & Thermosbaenacea) in freshwater. Hydrobiologia. 2008;595(1):219–224. doi: 10.1007/s10750-007-9017-1. [DOI] [Google Scholar]
- 45.Jesse R, Grudinski M, Klaus S, Streit B, Pfenninger M Evolution of freshwater crab diversity in the Aegean region (Crustacea: Brachyura: Potamidae) Molecular Phylogenetics and Evolution. 2011;59(1):23–33. doi: 10.1016/j.ympev.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Jurado-Rivera JA, Pons J, Alvarez F, Botello A, Humphreys WF, Page TJ, et al Phylogenetic evidence that both ancient vicariance and dispersal have contributed to the biogeographic patterns of anchialine cave shrimps. Scientific Reports. 2017;7:2852. doi: 10.1038/s41598-017-03107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappelman J, Rasmussen DT, Sanders WJ, Feseha M, Bown T, Copeland P, et al Oligocene mammals from Ethiopia and faunal exchange between Afro-Arabia and Eurasia. Nature. 2003;426(6966):549–552. doi: 10.1038/nature02102. [DOI] [PubMed] [Google Scholar]
- 48.Klaus S, Morley RJ, Plath M, Zhang YP, Li JT Biotic interchange between the Indian subcontinent and mainland Asia through time. Nature Communications. 2016;7:12132. doi: 10.1038/ncomms12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krehenwinkel H, Graze M, Rödder D, Tanaka K, Baba YG, Muster C, et al A phylogeographical survey of a highly dispersive spider reveals eastern Asia as a major glacial refugium for Palaearctic fauna. Journal of Biogeography. 2016;43(8):1583–1594. doi: 10.1111/jbi.12742. [DOI] [Google Scholar]
- 50.Krosch MN, Schutze MK, Armstrong KF, Graham GC, Yeates DK, Clarke AR A molecular phylogeny for the Tribe Dacini (Diptera: Tephritidae): systematic and biogeographic implications. Molecular Phylogenetics and Evolution. 2012;64(3):513–523. doi: 10.1016/j.ympev.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Leneveu J, Chichvarkhin A, Wahlberg N Varying rates of diversification in the genus Melitaea (Lepidoptera: Nymphalidae) during the past 20 million years . Biological Journal of the Linnean Society. 2009;97(2):346–361. doi: 10.1111/j.1095-8312.2009.01208.x. [DOI] [Google Scholar]
- 52.Leprieur F, Descombes P, Gaboriau T, Cowman PF, Parravicini V, Kulbicki M, et al Plate tectonics drive tropical reef biodiversity dynamics. Nature Communications. 2016;7:11461. doi: 10.1038/ncomms11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Zhao Z, Zhang CT, Li SQ Troglocoelotes gen. n., a new genus of Coelotinae spiders (Araneae, Agelenidae) from caves in South China . Zootaxa. 2019;4554(1):219–238. doi: 10.11646/zootaxa.4554.1.7. [DOI] [PubMed] [Google Scholar]
- 54.Li CY, Olave M, Hou YL, Qin G, Schneider RF, Gao ZX, et al Genome sequences reveal global dispersal routes and suggest convergent genetic adaptations in seahorse evolution. Nature Communications. 2021;12:1094. doi: 10.1038/s41467-021-21379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li FY, Li SQ Paleocene–Eocene and Plio–Pleistocene sea-level changes as “species pumps” in Southeast Asia: evidence from Althepus spiders . Molecular Phylogenetics and Evolution. 2018;127:545–555. doi: 10.1016/j.ympev.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Li FY, Shao LL, Li SQ Tropical niche conservatism explains the Eocene migration from India to Southeast Asia in ochyroceratid spiders. Systematic Biology. 2020;69(5):987–998. doi: 10.1093/sysbio/syaa006. [DOI] [PubMed] [Google Scholar]
- 57.Li JT, Li Y, Klaus S, Rao DQ, Hillis DM, Zhang YP Diversification of Rhacophorid frogs provides evidence for accelerated faunal exchange between India and Eurasia during the Oligocene. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3441–3446. doi: 10.1073/pnas.1300881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu HG, Li SQ, Ugolini A, Momtazi F, Hou ZE Tethyan closure drove tropical marine biodiversity: vicariant diversification of intertidal crustaceans. Journal of Biogeography. 2018;45(4):941–951. doi: 10.1111/jbi.13183. [DOI] [Google Scholar]
- 59.Lohman DJ, de Bruyn M, Page T, von Rintelen K, Hall R, Ng PKL, et al Biogeography of the Indo-Australian archipelago. Annual Review of Ecology, Evolution, and Systematics. 2011;42:205–226. doi: 10.1146/annurev-ecolsys-102710-145001. [DOI] [Google Scholar]
- 60.Lukeneder S, Zuschin M, Harzhauser M, Mandic O Spatiotemporal signals and palaeoenvironments of endemic molluscan assemblages in the marine system of the Sarmatian Paratethys. Acta Palaeontologica Polonica. 2011;56(4):767–784. doi: 10.4202/app.2010.0046. [DOI] [Google Scholar]
- 61.Luo SJ, Zhang Y, Johnson WE, Miao L, Martelli P, Antunes A, et al Sympatric Asian felid phylogeography reveals a major Indochinese-Sundaic divergence. Molecular Ecology. 2014;23(8):2072–2092. doi: 10.1111/mec.12716. [DOI] [PubMed] [Google Scholar]
- 62.Luo YF, Li SQ Cave Stedocys spitting spiders illuminate the history of the Himalayas and southeast Asia . Ecography. 2018;41(2):414–423. doi: 10.1111/ecog.02908. [DOI] [Google Scholar]
- 63.Malaquias MAE, Reid DG. 2009. Tethyan vicariance, relictualism and speciation: evidence from a global molecular phylogeny of the opisthobranch genus Bulla. Journal of Biogeography, 36(9): 1760–1777.
- 64.Mamos T, Wattier R, Burzyński A, Grabowski M The legacy of a vanished sea: a high level of diversification within a European freshwater amphipod species complex driven by 15 My of Paratethys regression. Molecular Ecology. 2016;25(3):795–810. doi: 10.1111/mec.13499. [DOI] [PubMed] [Google Scholar]
- 65.Manafzadeh S, Staedler YM, Conti E Visions of the past and dreams of the future in the Orient: the Irano-Turanian region from classical botany to evolutionary studies. Biological Reviews. 2017;92(3):1365–1388. doi: 10.1111/brv.12287. [DOI] [PubMed] [Google Scholar]
- 66.Meynard CN, Mouillot D, Mouquet N, Douzery EJP A phylogenetic perspective on the evolution of Mediterranean teleost fishes. PLoS One. 2012;7(5):e36443. doi: 10.1371/journal.pone.0036443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miao YF, Herrmann M, Wu FL, Yan XL, Yang SL. 2012. What controlled Mid-Late Miocene long-term aridification in central Asia?—Global cooling or Tibetan Plateau uplift: a review. Earth-Science Reviews, 112(3–4): 155–172.
- 68.Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, Katz ME, et al The Phanerozoic record of global sea-level change. Science. 2005;310(5752):1293–1298. doi: 10.1126/science.1116412. [DOI] [PubMed] [Google Scholar]
- 69.Mittermeier RA, Turner WR, Larsen FW, Brooks TM, Gascon C. 2011. Global biodiversity conservation: the critical role of hotspots. In: Zachos FE, Habel JC. Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas. Berlin, Heidelberg: Springer, 3–22.
- 70.Neubauer TA, Harzhauser M, Georgopoulou E, Kroh A, Mandic O Tectonics, climate, and the rise and demise of continental aquatic species richness hotspots. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(37):11478–11483. doi: 10.1073/pnas.1503992112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton CR Significance of "Tethyan" fossils in the American Cordillera. Science. 1988;242(4877):385–391. doi: 10.1126/science.242.4877.385. [DOI] [PubMed] [Google Scholar]
- 72.Nikulina EA, Hanel R, Schäfer P Cryptic speciation and paraphyly in the cosmopolitan bryozoan Electra pilosa—impact of the Tethys closing on species evolution . Molecular Phylogenetics and Evolution. 2007;45(3):765–776. doi: 10.1016/j.ympev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 73.Page TJ, Humphreys WF, Hughes JM Shrimps down under: evolutionary relationships of subterranean crustaceans from Western Australia (Decapoda: Atyidae: Stygiocaris) . PLoS One. 2008;3(2):e1618. doi: 10.1371/journal.pone.0001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patarnello T, Volckaert FAMJ, Castilho R Pillars of Hercules: is the Atlantic-Mediterranean transition a phylogeographical break? Molecular Ecology. 2007;16(21):4426–4444. doi: 10.1111/j.1365-294X.2007.03477.x. [DOI] [PubMed] [Google Scholar]
- 75.Popov SV, Rögl F, Rozanov AY, Steininger FF, Shcherba IG, Kovac M. 2004. Lithological-Paleogeographic maps of Paratethys 10 maps Late Eocene to Pliocene. Stuttgart: Schweizerbart Science Publishers, 1–46.
- 76.Price TD, Hooper DM, Buchanan CD, Johansson US, Tietze DT, Alström P, et al Niche filling slows the diversification of Himalayan songbirds. Nature. 2014;509(7499):222–225. doi: 10.1038/nature13272. [DOI] [PubMed] [Google Scholar]
- 77.Provan J, Bennett KD Phylogeographic insights into cryptic glacial refugia. Trends in Ecology & Evolution. 2008;23(10):564–571. doi: 10.1016/j.tree.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Ran JC, Yang WC A review of progress in Chinese troglofauna research. Journal of Resources and Ecology. 2015;6(4):237–246. doi: 10.5814/j.issn.1674-764x.2015.04.007. [DOI] [Google Scholar]
- 79.Renema W, Bellwood DR, Braga JC, Bromfield K, Hall R, Johnson KG, et al Hopping hotspots: global shifts in marine biodiversity. Science. 2008;321(5889):654–657. doi: 10.1126/science.1155674. [DOI] [PubMed] [Google Scholar]
- 80.Reyjol Y, Hugueny B, Pont D, Bianco PG, Beier U, Caiola N, et al Patterns in species richness and endemism of European freshwater fish. Global Ecology and Biogeography. 2007;16(1):65–75. doi: 10.1111/j.1466-8238.2006.00264.x. [DOI] [Google Scholar]
- 81.Rodríguez-Flores PC, Buckley D, Macpherson E, Corbari L, Machordom A Deep-sea squat lobster biogeography (Munidopsidae: Leiogalathea) unveils Tethyan vicariance and evolutionary patterns shared by shallow-water relatives . Zoologica Scripta. 2020;49(3):340–356. doi: 10.1111/zsc.12414. [DOI] [Google Scholar]
- 82.Rosenbaum G, Lister GS Reconstruction of the evolution of the Alpine-Himalayan Orogen - an introduction. Journal of the Virtual Explorer. 2002;8:1–2. [Google Scholar]
- 83.Roveri M, Flecker R, Krijgsman W, Lofi J, Lugli S, Manzi V, et al The Messinian Salinity Crisis: past and future of a great challenge for marine sciences. Marine Geology. 2014;352:25–58. doi: 10.1016/j.margeo.2014.02.002. [DOI] [Google Scholar]
- 84.Rüber L, Britz R, Kullander SO, Zardoya R Evolutionary and biogeographic patterns of the Badidae (Teleostei: Perciformes) inferred from mitochondrial and nuclear DNA sequence data. Molecular Phylogenetics and Evolution. 2004;32(3):1010–1022. doi: 10.1016/j.ympev.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 85.Sidorov D, Taylor SJ, Sharina S, Gontcharov A. 2018. Zenkevitchiidae fam. Nov. (Crustacea: Gammaroidea), with description of new subterranean amphipods from extremely deep cave habitats. Journal of Natural History, 52(23–24): 1509–1535.
- 86.Sket B. 2018. Subterranean (Hypogean) habitats in karst and their fauna. In: Finlayson CM, Milton GR, Prentice RC, Davidson NC. The Wetland Book: II: Distribution, Description, and Conservation. Dordrecht: Springer, 331–344.
- 87.Solà E, Sluys R, Gritzalis K, Riutort M Fluvial basin history in the northeastern Mediterranean region underlies dispersal and speciation patterns in the genus Dugesia (Platyhelminthes, Tricladida, Dugesiidae) . Molecular Phylogenetics and Evolution. 2013;66(3):877–888. doi: 10.1016/j.ympev.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 88.Spicer RA Tibet, the Himalaya, Asian monsoons and biodiversity - In what ways are they related? Plant Diversity. 2017;39(5):233–244. doi: 10.1016/j.pld.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steinbauer MJ, Field R, Grytnes JA, Trigas P, Ah-Peng C, Attorre F, et al Topography-driven isolation, speciation and a global increase of endemism with elevation. Global Ecology and Biogeography. 2016;25(9):1097–1107. doi: 10.1111/geb.12469. [DOI] [Google Scholar]
- 90.Stow D. 2010. Vanished Ocean: How Tethys Reshaped the World. Oxford: Oxford University Press.
- 91.Studencka B, Jasionowski M Bivalves from the Middle Miocene reefs of Poland and Ukraine: a new approach to Badenian/Sarmatian boundary in the Paratethys. Acta Geologica Polonica. 2011;61(1):79–114. [Google Scholar]
- 92.Suess E Are great ocean depths permanent? Natural Science. 1893;2:180–187. [Google Scholar]
- 93.Sun JM, Jiang MS Eocene seawater retreat from the southwest Tarim Basin and implications for early Cenozoic tectonic evolution in the Pamir Plateau. Tectonophysics. 2013;588:27–38. doi: 10.1016/j.tecto.2012.11.031. [DOI] [Google Scholar]
- 94.Trontelj P, Blejec A, Fišer C Ecomorphological convergence of cave communities. Evolution. 2012;66(12):3852–3865. doi: 10.1111/j.1558-5646.2012.01734.x. [DOI] [PubMed] [Google Scholar]
- 95.Uribe JE, Williams ST, Templado J, Buge B, Zardoya R Phylogenetic relationships of Mediterranean and north-east Atlantic Cantharidinae and notes on Stomatellinae (Vetigastropoda: Trochidae) Molecular Phylogenetics and Evolution. 2017;107:64–79. doi: 10.1016/j.ympev.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Väinölä R, Vainio JK, Palo JU Phylogeography of “glacial relict” Gammaracanthus (Crustacea, Amphipoda) from boreal lakes and the Caspian and White seas . Canadian Journal of Fisheries and Aquatic Sciences. 2001;58(11):2247–2257. doi: 10.1139/f01-165. [DOI] [Google Scholar]
- 97.Wallis GP, Waters JM, Upton P, Craw D Transverse alpine speciation driven by glaciation. Trends in Ecology & Evolution. 2016;31(12):916–926. doi: 10.1016/j.tree.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Wang CX, Xu X, Li SQ Integrative taxonomy of Leptonetela spiders (Araneae, Leptonetidae), with descriptions of 46 new species . Zoological Research. 2017;38(6):321–448. doi: 10.24272/j.issn.2095-8137.2017.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang WJ, McKay BD, Dai CY, Zhao N, Zhang RY, Qu YH, et al Glacial expansion and diversification of an East Asian montane bird, the green-backed tit (Parus monticolus) . Journal of Biogeography. 2013;40(6):1156–1169. doi: 10.1111/jbi.12055. [DOI] [Google Scholar]
- 100.Wang XM, Tseng ZJ, Li Q, Takeuchi GT, Xie GP From ‘third pole’ to north pole: a Himalayan origin for the arctic fox. Proceedings of the Royal Society B:Biological Sciences. 2014;281(1787):20140893. doi: 10.1098/rspb.2014.0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang XM, Wang Y, Li Q, Tseng ZJ, Takeuchi GT, Deng T, et al Cenozoic vertebrate evolution and paleoenvironment in Tibetan Plateau: progress and prospects. Gondwana Research. 2015;27(4):1335–1354. doi: 10.1016/j.gr.2014.10.014. [DOI] [Google Scholar]
- 102.Wang XY, Liang D, Jin W, Tang MK, Shalayiwu, Liu SY, et al Out of Tibet: genomic perspectives on the evolutionary history of extant pikas. Molecular Biology and Evolution. 2020;37(6):1577–1592. doi: 10.1093/molbev/msaa026. [DOI] [PubMed] [Google Scholar]
- 103.Wilke T Salenthydrobia gen. nov. (Rissooidea: Hydrobiidae): a potential relict of the Messinian salinity crisis . Zoological Journal of the Linnean Society. 2003;137(2):319–336. doi: 10.1046/j.1096-3642.2003.00049.x. [DOI] [Google Scholar]
- 104.Xiong KN Development of cone karst in response to Neotectonism in Guizhou. Guzhou Geology. 1996;13(2):181–186. [Google Scholar]
- 105.Xu W, Dong WJ, Fu TT, Gao W, Lu CQ, Yan F, et al Herpetological phylogeographic analyses support a Miocene focal point of Himalayan uplift and biological diversification. National Science Review. 2020;8(9):nwaa263. doi: 10.1093/nsr/nwaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi T, Mashiba H, Kamiya T Miocene Ostracodes from the Osaki formation, Kukinaga group, Tanegashima, Southwest Japan, and their significance for the biogeography of the Indo-West Pacific. Paleontological Research. 2012;16(2):107–123. doi: 10.2517/1342-8144-16.2.107. [DOI] [Google Scholar]
- 107.Yamanoue Y, Miya M, Doi H, Mabuchi K, Sakai H, Nishida M Multiple invasions into freshwater by pufferfishes (Teleostei: Tetraodontidae): a mitogenomic perspective. PLoS One. 2011;6(2):e17410. doi: 10.1371/journal.pone.0017410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao ZY, Dong TT, Zheng G, Fu JZ, Li SQ. 2016. High endemism at cave entrances: a case study of spiders of the genus Uthina. Scientific Reports, 6: 35757.
- 109.Yuan ZY, Zhang BL, Raxworthy CJ, Weisrock DW, Hime PM, Jin JQ, et al Natatanuran frogs used the Indian Plate to step-stone disperse and radiate across the Indian Ocean. National Science Review. 2019;6(1):10–14. doi: 10.1093/nsr/nwy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan ZY, Zhou WW, Chen X, Poyarkov NA Jr, Chen HM, Jang-Liaw NH, et al Spatiotemporal diversification of the true frogs (Genus Rana): a historical framework for a widely studied group of model organisms . Systematic Biology. 2016;65(5):824–842. doi: 10.1093/sysbio/syw055. [DOI] [PubMed] [Google Scholar]
- 111.Zakšek V, Sket B, Trontelj P Phylogeny of the cave shrimp Troglocaris: evidence of a young connection between Balkans and Caucasus . Molecular Phylogenetics and Evolution. 2007;42(1):223–235. doi: 10.1016/j.ympev.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 112.Zhang PL, Huang TF, Wu T, Huang XL, Zhang YX, Liu ZX Checklist, distribution and conservation of typical cavefish in China. Carsologica Sinica. 2019;38(6):937–945. [Google Scholar]
- 113.Zhang Q, Xia L, Kimura Y, Shenbrot G, Zhang ZQ, Ge DY, et al Tracing the origin and diversification of dipodoidea (Order: Rodentia): evidence from fossil record and molecular phylogeny. Evolutionary Biology. 2013;40(1):32–44. doi: 10.1007/s11692-012-9167-6. [DOI] [Google Scholar]
- 114.Zhang YY, Li SQ Ancient lineage, young troglobites: recent colonization of caves by Nesticella spiders . BMC Evolutionary Biology. 2013;13:183. doi: 10.1186/1471-2148-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang YY, Li SQ A spider species complex revealed high cryptic diversity in South China caves. Molecular Phylogenetics and Evolution. 2014;79:353–358. doi: 10.1016/j.ympev.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Z, Li SQ Extinction vs. rapid radiation: the juxtaposed evolutionary histories of coelotine spiders support the Eocene–Oligocene orogenesis of the Tibetan Plateau. Systematic Biology. 2017;66(6):988–1006. doi: 10.1093/sysbio/syx042. [DOI] [PubMed] [Google Scholar]
- 117.Zhao Z, Shao LL, Li FY, Zhang XQ, Li SQ Tectonic evolution of the Tethyan region created the Eurasian extratropical biodiversity hotspots: tracing Pireneitega spiders’ diversification history . Ecography. 2020;43(9):1400–1411. doi: 10.1111/ecog.05044. [DOI] [Google Scholar]
- 118.Zheng YC, Fu JZ, Li SQ. 2009. Toward understanding the distribution of Laurasian frogs: a test of Savage's biogeographical hypothesis using the genus Bombina. Molecular Phylogenetics and Evolution, 52(1): 70–83.
- 119.Zheng YM, Hou ZE, Li SQ Bogidiella pingxiangensis, a new species of subterranean Amphipoda from southern China (Bogidiellidae) . ZooKeys. 2018;790:63–75. doi: 10.3897/zookeys.790.28671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu DC, Wang Q, Zhao ZD Constraining quantitatively the timing and process of continent-continent collision using magmatic record: method and examples. Science China Earth Sciences. 2017;60(6):1040–1056. doi: 10.1007/s11430-016-9041-x. [DOI] [Google Scholar]
- 121.Zupan Hajna N. 2019. Dinaric karst—Geography and geology. In: White WB, Culver DC, Pipan T. Encyclopedia of Caves. 3rd ed. London: Academic Press, 353–362.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.