Endothermy is the ability to generate and conserve metabolic heat to maintain body temperature above that of the surrounding environment. Endothermy enhances the physiological and ecological advantages of mammals, birds, and certain fish species. The opah, Lampris megalopsis (Lampridiformes), is the only known fish to exhibit whole-body endothermy. Currently, however, the underlying molecular mechanism for this remains unclear. Hence, the opah offers an excellent opportunity to study the evolutionary mechanism of whole-body endothermy in aquatic animals. In this study, we assembled a L. megalopsis genome (1.09 Gb in size) and performed comparative genomic analysis with ectothermic fish to reveal the genetic basis of endothermy. Based on analysis of positive selection, rapid evolution, and gene family expansion, we discovered several genes that likely contributed to thermogenesis and heat preservation. As the first reported L. megalopsis genome, our results not only clarify the possible molecular and genetic mechanisms involved in endothermic adaptation but also increase our understanding of endothermic fish biology.
Fish face a massive challenge in maintaining body temperature due to the high thermal conductivity of water, with fewer than 0.1% of fish species able to retain internally produced heat. Endothermic fish exhibit strong swimming efficiency, enhanced cold tolerance, high visual acuity for vertical migration, and competitive advantages in predator-prey interactions and niche expansion (Wegner et al., 2015). Recently, the opah (L. guttatus) was discovered to show whole-body endothermy, which is thought to be unique among fish. Globally distributed opahs were originally described as Lampris guttatus (Brünnich, 1788), but have since been divided into L. incognitus, L. australensis, L. megalopsis, L. guttatus, and L. lauta based on geographical distribution and morphology (Hyde et al., 2014; Underkoffler et al., 2018). The opahs have two thermogenic strategies. They use metabolization of pectoral muscles to generate heat, and they use a specialized intracranial thermogenesis tissue to generate heat. In addition, the opahs have two heat conservation strategies. An extensive of retia mirabilia in intracranial and gill is used for insulation. And thick adipose tissue is another strategy for heat conservation (Franck et al., 2019; Runcie et al., 2009; Wegner et al., 2015).
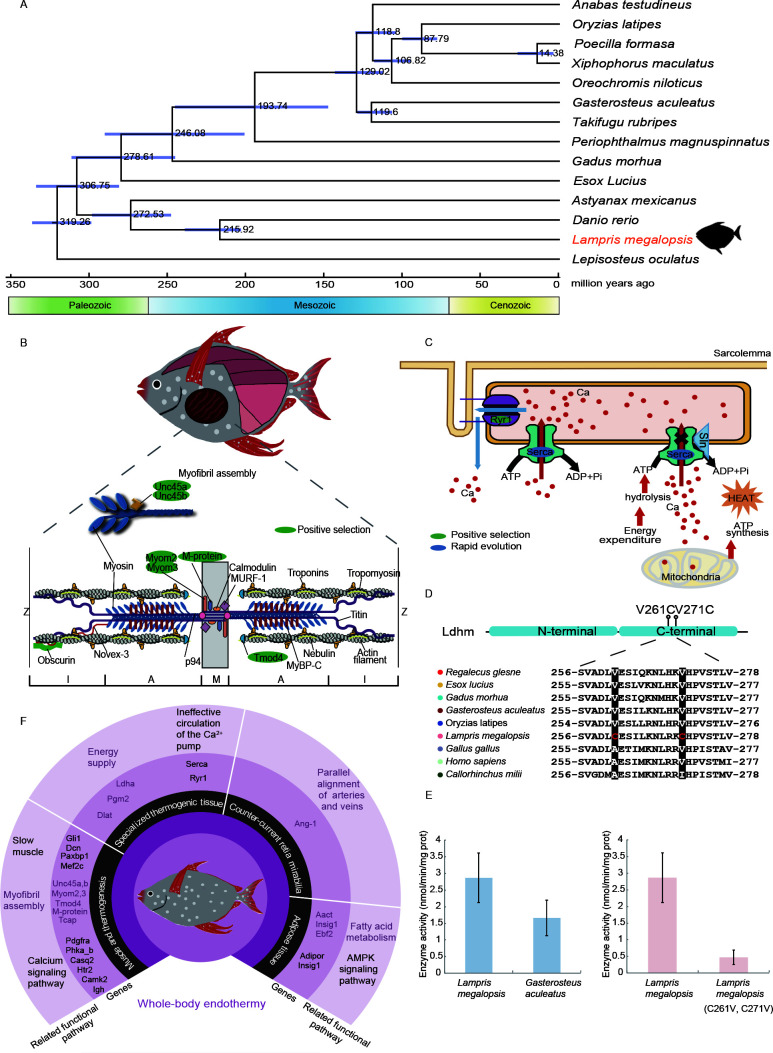
In the current study, an opah specimen was collected from the southern Indian Ocean onboard the Chinese tuna longline vessel “PING TAI RONG 65” and was transported to Zhoushan, China, on 12 September 2018, by “PING TAI RONG LENG 2” for dissection and muscle extraction (Supplementary Figure S1). Mitochondrial genes (cytochrome c oxidase I (COI) and cytochrome b (cytb)) identified the specimen as a bigeye pacific opah (L. megalopsis) (Supplementary Figures S2, S3), which is known to be distributed in the southern Indian Ocean (Underkoffler et al., 2018). We assembled a L. megalopsis genome (1 091 Mb), which consisted of 12 081 contigs, with a contig N50 of 590 kb and N90 of 30 kb (Supplementary Table S1). Compared with 38.8% for the previously released L. guttatus genome (ASM90030254v1), BUSCO assessment of single-copy orthologs showed that the completeness of our genome was 90.5%, indicating high-quality assembly. A total of <number>23755</number> genes were annotated in the opah genome. We constructed a phylogenetic tree with all single-copy genes ( Figure 1A). Among 13 ectothermic fish, opah was closest to the zebrafish, with an estimated divergence time of ~216 million years ago (Ma).
Figure 1.
Figure 1.
Genomic analyses of Lampris megalopsis
Green genes are under positive selection and blue genes exhibit rapid evolution. A: Phylogenetic relationships and divergence time of opah. Purple node bars indicate 95% high posterior density. B: Enrichment of positively selected genes in myofibril assembly. Green genes are under positive selection. C: Sln-mediated heat production pathways in specialized thermogenic tissue. D: Ldha gene coding Ldhm protein. Ldhm sequences of multiple species were aligned, and alignments of adjacent positively selected sites (V261C and V271C) are shown. E: Ldhm enzyme activities in opah compared with stickleback in vitro. Error bars indicate standard deviation. F: Summary of genetic mechanisms of opah in whole-body endothermy, shown in inner black circle. Inner purple circle shows candidate genes based on positively selected gene sets, expanded gene families, and rapidly evolving gene sets. Outer gray violet circle shows related pathways.
The opah uses its dark red aerobic pectoral muscles for continuous swimming and metabolic heat production. On average, the muscle has a temperature that is 4.8±1.2 °C higher than that of the surrounding water (7.8–10.8 °C), and muscle content accounts for 16% of total weight, the highest among reported fish (Wegner et al., 2015). Here, we identified several genes under positive selection (Gli1, Dcn, and Paxbp1) and rapid evolution (Mef2c), which are also known to be involved in muscle development and differentiation (Cheng et al., 2010; Liu et al., 2014; Sun et al., 2013).
Gene Ontology (GO) enrichment analysis identified seven genes (Unc45a, Unc45b, Tmod4, Myom2, Myom3, M-protein, and Tcap) under positive selection that were enriched in “myofibril assembly” (Figure1B). During muscle contraction and relaxation, calcium ions are involved in the regulation of contractile protein activity (Cho et al., 2017). In our study, based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) calcium signaling pathway, three genes (i.e., Pdgfra, Phka_b, and Casq2) were found to be under positive selection, and three gene families (Htr2, Camk2, and Igh) were expanded, which may have contributed to the tireless nature of opah pectoral muscle contraction.
The intracranial tissue of fish is specialized for non-shivering thermogenic activity, which is primarily mediated by Sln (Franck et al., 2019). Here, in the Sln pathway, Ryr1 was found to be under positive selection and Serca was identified as a rapidly evolving gene in opah (Figure1C). Ryr1 is discretely expressed in specialized thermogenic tissues and promotes the release of Ca2+ from the sarcoplasmic reticulum (Maurya & Periasamy, 2015). Serca acts as a calcium pump and binds to Sln, resulting in the release of Ca2+ back into the cytoplasm (Franck et al., 2019). The Ryr1 and Serca of opah may lead to the provision of additional calcium for circulation pathways, further consumption of energy, and greater generation of heat.
In research on thermogenesis of energy supply, Pgm2 and Dlat are positive selected genes participate in gluconeogenesis and the tricarboxylic acid cycle. In addition, positive selection analysis showed Ldha was a positively selected gene. Main LDH isoenzyme of skeletal muscle is the Ldhm, which is coding by Ldha (Read et al., 2001). Through enzymatic activity detection experiments, we found that Ldhm activity was significantly higher in opah than in stickleback. In addition, the positively selected sites significantly affected enzymatic activity (Figure 1D, E).
Opah fish also use two heat preservation mechanisms, including counter-current retia mirabilia (Wegner et al., 2015). Apelin produced from arterial endothelial cells stimulates the expression of the apelin receptor in venous endothelial cells to induce alignment of arteries and veins, which is involved in thermoregulation. And apelin expression is induced by Ang-1 (Kidoya et al., 2015). Ang-1 exhibited gene expansion of opah in this study. Thus, Ang-1 may provide a new way to study the distribution of retia mirabilia in endotherms. However, the mechanism of what causes counter-current exchange retia mirabilia to develop at specific tissues is not yet clear.
Opahs also preserve heat via thick fatty tissue (Wegner et al., 2015), although little attention has been paid to their adipose-related pathways. Regarding fatty acid metabolism in opah, we found that Aact was under positive selection and Insig1 and Ebf2 were rapidly evolving genes. Given their participation in fatty acid metabolism, these three genes may help clarify the position of fatty acids in heat producing areas (Ai et al., 2016; Sun et al., 2013; Wang et al., 2019).
Fat in heat producing areas and gills may not only have a physical insulating effect but may also influence the function of internal blood vessels by releasing adipokines. Adipokines released by perivascular adipose tissue may affect blood vessel function via AMP-activated protein kinase (AMPK)(Wu et al., 2018). Based on KEGG pathway analysis, two rapidly evolving genes (Adipor and Insig1) were enriched in the “AMPK signaling pathway”.
We sequenced and assembled a complete opah genome, which provides information for endothermic research. We conducted comparative genomic analysis of opah and ectothermic fish based on two significant aspects of endothermy: i.e., heat production and preservation. The genetic changes in opah are summarized in Figure 1F and Supplementary Tables S2–S4. Although the changes found in the opah genome require functional confirmation, this study should improve our understanding of endothermic adaptation in opahs and endothermic evolution in fish.
DATA AVAILABILITY
The genomic and raw data of the opah specimen were deposited in the CNGB Sequence Archive (CNSA) of the China National Gene Bank Database (https://db.cngb.org/) under accession No. CNP0001856. The whole-genome sequence data reported in this paper were deposited in the NCBI under BioProjectID PRJNA776990 and GWH at the National Genomics Data Center under accession No. GWHBEIC00000000, which is publicly accessible at https://ngdc.cncb.ac.cn/gwh.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
S.P.H. led the project. L.D.Y. conceived and designed the project. J.B. performed evolutionary analyses. W.Q.L., N.S., C.W., and P.L. performed the sampling. K.W. and C.G.F. assembled and annotated the genome. J.B. and L.D.Y. analyzed the data and wrote and revised the paper. All authors read and approved the final version of the manuscript.
This research was supported by the Wuhan Branch, Supercomputing Center, Chinese Academy of Sciences, China and Institute of Deep-sea Science and Engineering, Supercomputing Center, Chinese Academy of Sciences, China.
ACKNOWLEDGEMENTS
This research was supported by the Wuhan Branch, Supercomputing Center, Chinese Academy of Sciences, China and Institute of Deep-sea Science and Engineering, Supercomputing Center, Chinese Academy of Sciences, China.
Funding Statement
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDB42000000) and National Natural Science Foundation of China (41876179) to S.P.H., and Strategic Priority Research Program of CAS (XDB31040104), National Natural Science Foundation of China (31972866), and Youth Innovation Promotion Association, CAS (http://www.yicas.cn) to L.D.Y.
Contributor Information
Shun-Ping He, Email: clad@ihb.ac.cn.
Lian-Dong Yang, Email: yangld@ihb.ac.cn.
References
- 1.Ai Q, Yan J, Mai K Research progresses of lipids and fatty acids transport in fish. Acta Hydrobiologica Sinica. 2016;40:859–868. [Google Scholar]
- 2.Cheng J, Chu WY, Zhang JS Progresses and perspectives of the studies on fish muscle-related genes and their expression. Life Science Research. 2010;14(4):355–362. [Google Scholar]
- 3.Cho CH, Woo JS, Perez CF, Lee EH A focus on extracellular Ca2+ entry into skeletal muscle . Experimental and Molecular Medicine. 2017;49(9):378. doi: 10.1038/emm.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franck JPC, Slight-Simcoe E, Wegner NC Endothermy in the smalleye opah (Lampris incognitus): a potential role for the uncoupling protein sarcolipin. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology. 2019;233:48–52. doi: 10.1016/j.cbpa.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Hyde JR, Underkoffler KE, Sundberg MA DNA barcoding provides support for a cryptic species complex within the globally distributed and fishery important opah (Lampris guttatus) . Molecular Ecology Resources. 2014;14(6):1239–1247. doi: 10.1111/1755-0998.12268. [DOI] [PubMed] [Google Scholar]
- 6.Kidoya H, Naito H, Muramatsu F, Yamakawa D, Jia WZ, Ikawa M, et al APJ regulates parallel alignment of arteries and veins in the skin. Developmental Cell. 2015;33(3):247–259. doi: 10.1016/j.devcel.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Wang H, Cheng G, Jiang B, Zan L Expression analysis of Gli1 and Gli2 in different tissues and muscle-derived cells of Qinchuan cattle. Genetics and Molecular Research. 2014;13(4):8767–8775. doi: 10.4238/2014.October.27.18. [DOI] [PubMed] [Google Scholar]
- 8.Maurya SK, Periasamy M Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacological research. 2015;102:270–275. doi: 10.1016/j.phrs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read J, Winter V, Eszes C, Sessions R, Brady R Structural basis for altered activity of M‐and H‐isozyme forms of human lactate dehydrogenase. Proteins:Structure, Function, and Bioinformatics. 2001;43:175–185. doi: 10.1002/1097-0134(20010501)43:2<175::AID-PROT1029>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA Evidence for cranial endothermy in the opah (Lampris guttatus) . Journal of Experimental Biology. 2009;212(4):461–470. doi: 10.1242/jeb.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun LL, Liu HH, Wang HH, Si JM, Jin HB, Li XX, et al Molecular cloning of the duck MEF2C gene cDNA coding domain sequence and its expression during fetal muscle tissue development. Genes & Genomics. 2013;35(3):317–325. [Google Scholar]
- 12.Underkoffler KE, Luers MA, Hyde JR, Craig MT A taxonomic review of Lampris guttatus (Brünnich 1788) Lampridiformes; Lampridae) with descriptions of three new species . Zootaxa. 2018;4413(3):551–566. doi: 10.11646/zootaxa.4413.3.9. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Cao Y, Liang Y, Xiao C, Jin H, Jin H Advance in acetyl-CoA acyltransferase 1 gene. Chinese Journal of Animal Science. 2019;55(10):42–46. [Google Scholar]
- 14.Wegner NC, Snodgrass OE, Dewar H, Hyde JR Whole-body endothermy in a mesopelagic fish, the opah. Lampris guttatus. Science. 2015;348(6236):786–789. doi: 10.1126/science.aaa8902. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Zhang L, Li B, Jiang H, Duan Y, Xie Z, et al AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Frontiers in Physiology. 2018;9:122. doi: 10.3389/fphys.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The genomic and raw data of the opah specimen were deposited in the CNGB Sequence Archive (CNSA) of the China National Gene Bank Database (https://db.cngb.org/) under accession No. CNP0001856. The whole-genome sequence data reported in this paper were deposited in the NCBI under BioProjectID PRJNA776990 and GWH at the National Genomics Data Center under accession No. GWHBEIC00000000, which is publicly accessible at https://ngdc.cncb.ac.cn/gwh.