Abstract
Retinal angiogenesis is a critical process for normal retinal function. However, uncontrolled angiogenesis can lead to pathological neovascularization (NV), which is closely related to most irreversible blindness-causing retinal diseases. Understanding the molecular basis behind pathological NV is important for the treatment of related diseases. Twist-related protein 1 (TWIST1) is a well-known transcription factor and principal inducer of epithelial-mesenchymal transition (EMT) in many human cancers. Our previous study showed that Twist1 expression is elevated in pathological retinal NV. To date, however, the role of TWIST1 in retinal pathological angiogenesis remains to be elucidated. To study the role of TWIST1 in pathological retinal NV and identify specific molecular targets for antagonizing pathological NV, we generated an inducible vascular endothelial cell (EC)-specific Twist1 transgenic mouse model (Tg-Twist1iEC+). Whole-mount retinas from Tg-Twist1iEC+ mice showed retarded vascular progression and increased vascular density in the front end of the growing retinal vasculature, as well as aneurysm-like pathological retinal NV. Furthermore, overexpression of Twist1 in the ECs promoted cell proliferation but disturbed cell polarity, thus leading to uncontrolled retinal angiogenesis. TWIST1 promoted pathological NV by activating the Wnt/β-catenin signaling pathway and inducing the expression of NV formation-related genes, thereby acting as a ‘valve’ in the regulation of pathological angiogenesis. This study identified the critical role of TWIST1 in retinal pathological NV, thus providing a potential therapeutic target for pathological NV.
Keywords: Pathological angiogenesis, TWIST1, Molecular markers, Mouse model, Retinal neovascularization
INTRODUCTION
Retinal angiogenesis is a fundamental biological process involved in normal retinal development. Multiple signaling pathways, including the Wnt/β-catenin (Ye et al, 2009; Zhou et al, 2014), Notch (Benedito et al, 2009; Zhang et al, 2020), and Hippo signaling pathways, act together to coordinate this process (Kim et al, 2017; Park & Kwon, 2018). However, uncontrolled and dysregulated angiogenesis, also known as pathological angiogenesis, can cause detrimental effects in the retina, many of which are related to irreversible blindness-causing diseases in individuals of all ages, such as newborns (retinopathy of prematurity, ROP), middle-aged adults (proliferative diabetic retinopathy, PDR), and the elderly (exudative age-related macular degeneration, wet AMD) (Capitão & Soares, 2016; Cheung et al, 2010; Hartnett & Penn, 2012; Liu et al, 2017; Mitchell et al, 2018). Pathological neovascularization (NV) is a common feature of these diseases, and retinas are characterized by incompetent, leaky retinal vessels that can bleed and contract, leading to hemorrhage or detachment and eventually to blindness (Liu et al, 2017; Penn et al, 2008). Current treatment strategies for these diseases include anti-vascular endothelial growth factor (VEGF) intravitreal injections and retinal photocoagulation. As VEGF is critical for both physiological and pathological angiogenesis, anti-VEGF treatment will also affect normal retinal NV (Krohne et al, 2018; Usui-Ouchi & Friedlander, 2019). Thus, defining the different molecular mechanisms underlying pathological and physiological retinal angiogenesis is critical for the treatment of retinal NV-related diseases.
Twist1, a basic helix-loop-helix (bHLH) transcription factor, is characterized by a basic DNA binding domain that targets the consensus E-box sequence 5'-CANNTG-3' (Schmidt et al, 2015; Thisse et al, 1987). Originally identified in Drosophila as an essential regulator during embryogenesis, particularly in mesoderm formation, specification, and differentiation (Zhao et al, 2017), this molecule is now known as a principal inducer of epithelial-mesenchymal transition (EMT) in a variety of human mammary cancers (Wang et al, 2017; Yang et al, 2012; Zhao et al, 2017). Beyond its functions in EMT, TWIST1 also plays an important role in regulating normal and tumor angiogenesis through multiple mechanisms (Chen & Wu, 2016; Koo et al, 2017; Tseng et al, 2015). Twist1 knockdown in Xenopus impacts embryonic vascular growth (Rodrigues et al, 2008), and TWIST1 is implicated in the up-regulation of macrophage recruitment and VEGF secretion to promote tumor angiogenesis (Low-Marchelli et al, 2013; Sossey-Alaoui et al, 2014). Previous research has also indicated that endothelial TWIST1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering Tie2 expression (Mammoto et al, 2013). Impaired vascular integrity and increased permeability are important features of pathological retinal NV (Campochiaro, 2015).
We previously showed that TWIST1 is highly enriched in pathological neovessels in oxygen-induced retinopathy (OIR) retinas (Li et al, 2014). Conditional Tie2-driven depletion of Twist1 significantly suppresses pathological neovessels in OIR (Li et al, 2014). Twist1 deficiency also results in significantly smaller lesions with decreased vascular leakage in laser-induced choroidal NV models. In vitro, knockdown of TWIST1 in human endothelial cells (ECs) decreases cell proliferation (Li et al, 2014). Thus, TWIST1 may be a novel marker for pathological retinal neovessels, and may promote pathological ocular NV, in part, by modulating EC proliferation. However, the specific roles and mechanisms of TWIST1 enrichment in pathological retinal NV remain to be elucidated.
In the current study, to clarify the mechanism by which TWIST1 functions in retinal pathological angiogenesis, we generated an inducible vascular EC-specific Twist1 transgenic mouse model (Tg-Twist1iEC+) and showed that overexpression of Twist1 in ECs leads to retinal pathological NV without induction of hypoxia. We also showed that TWIST1 promotes EC proliferation but disturbs EC polarity, thus leading to uncontrolled retinal angiogenesis. In terms of mechanism, we found that TWIST1 promotes pathological NV by enhancing the Wnt/β-catenin signaling pathway, increasing retinal gliosis, and inducing the expression of NV-related genes, thereby acting as a "valve" in the regulation of pathological angiogenesis. We developed another mouse model for further research on pathological NV-related retinal diseases, revealing the critical functions of theTwist1 gene in retinal pathological NV and identifying a potential therapeutic target for pathological NV.
MATERIALS AND METHODS
Cell culture and overexpression of TWIST1
Primary cultured human retinal ECs (HRECs) and human umbilical vein ECs (HUVECs) were purchased from Cell Systems (USA) and cultured in EGM2 medium (Lonza, Switzerland) at 37 °C in a 5% CO2 incubator. For overexpression of the TWIST1 gene, HRECs were transfected with a lentivirus carrying a TWIST1 expression plasmid. HRECs and HUVECs at passages 3–7 were used in this study. The use of lentiviruses was approved by the Institutional Biosafety Committee of Sichuan Provincial People’s Hospital on 1 March 2017 (approval No. 201783).
Generation of OIR mouse model
The OIR mouse model was established as reported previously (Smith et al, 1994). Briefly, to induce retinopathy, mouse pups with nursing mothers were exposed to 75% oxygen from postnatal day (P) 7 to P12 (phase I: vaso-obliteration) and returned to room air until P17 (phase II: NV).
Mouse strains and genotyping
All animal research protocols were approved by the Animal Protection and Use Committee of Sichuan Provincial People’s Hospital on 1 March 2017 (approval No. L201783). All experimental procedures and methods were carried out in accordance with the approved research protocols and relevant regulations. The mice were raised under standard light conditions (12 h light:12 h dark cycle) and provided with unrestricted access to food and water. The B6-Gt(ROSA)26Sorem1(CAG-Twist1)Hze/cyagen mice (conditional knock-in mice with a “CAG-loxP-STOP-loxP-kozak-mouse Twist1 CDS-poly A” cassette inserted into the Rosa-26 gene, named Tg-Rosa26-Twist1, driven by a specific Cre to induce Twist1 expression in specific cells or organs, and Pdgfb-CreER mice (inducible EC-specific Cre induced by tamoxifen) (Zhang et al, 2019) were bred to generate inducible Twist1 EC overexpression mice (Tg-Rosa26-Twist1,Pdgfb-CreER). Mouse genotyping was performed using a polymerase chain reaction (PCR)-based method with the primers listed in Supplementary Table S1.
Tamoxifen treatment
Tamoxifen treatment was performed as described previously (Sun et al, 2021). In brief, 100 mg of tamoxifen salt (Sigma, USA) was dissolved in 10 mL of ethanol as a stock solution. On the day of injection, a 1 mg/mL working solution was prepared by well mixing 10 mg/mL stock solution with corn oil (Sigma, USA) at a 1:9 ratio. Mice were intraperitoneally injected with a daily dose of 25 mg/kg body weight for 3 consecutive days.
Immunohistochemistry and ethynyl-2’-deoxyuridine (EdU) labeling of retinal ECs
Retinal dissection was carried out as described previously (Pitulescu et al, 2010). Briefly, eyeballs were enucleated and fixed in 4% paraformaldehyde (PFA) for 20 min at room temperature. After preparation from the eyeballs, whole-mounted retinas were preserved in 0.4% PFA. Enucleated eyes were fixed with 4% PFA and embedded in Tissue-Tek optimal cutting-temperature (OCT) compound. Before immunostaining, whole-mounted retinas and cryosections were rinsed in phosphate buffered-saline (PBS) three times (5 min/rinse) and blocked in PBS containing 5% fetal bovine serum (FBS) and 0.2% Triton X-100 for 30 min at room temperature, followed by incubation with primary antibodies at 4 °C overnight. Primary antibodies were diluted in blocking buffer as follows: Isolectin GS-IB4 (1:200 dilution; Invitrogen, USA), rat anti-mouse Ter-119 (1:20 dilution; BD Biosciences, USA), rabbit anti-ERG (1:200 dilution; Abcam, USA), and rabbit anti-GFAP (1:100 dilution, Cell Signaling Technology, USA). The sections were then washed three times with PBS and labeled for 2 h with Alexa Fluor™-488- or Alexa Fluor™-594-labeled goat anti-rat or anti-rabbit IgG or donkey anti-goat IgG secondary antibodies (1:500 dilution; Invitrogen, USA).
For detection of EC proliferation in the retinas, 200 μg of EdU (Invitrogen, USA) was injected intraperitoneally in each pup 3 h before sacrifice. EdU-positive cells were visible by subsequent staining with a Click-iT EDU Alexa Fluor-488 Imaging Kit (C10337; Invitrogen, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
After extraction of total RNA from cell and tissue samples using TRIzol reagent, 1 μg of total RNA was used to synthesize cDNA using a Superscript cDNA Synthesis Kit (Invitrogen, USA). The cDNA was then used as a template for PCR with different primers (listed in Supplementary Table S2).
RNA sequencing (RNA-seq) analysis
After extraction of total RNA from HRECs using RNeasy Mini kits (Qiagen, USA), RNA purity was assessed using a K5500 spectrophotometer (Kaiao, China). RNA integrity and concentration were assessed using an RNA Nano 6000 Assay Kit with the Bioanalyzer 2100 system (Agilent Technologies, USA). For library preparation, 2 μg of RNA per sample was used as input material for RNA sample preparations. Sequencing libraries were generated using a NEB Next Ultra RNA Library Prep Kit for Illumina (Cat. No. E7530L, USA), following the manufacturer’s recommendations. Index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under an elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5×). First-strand cDNA was synthesized using random hexamer primers and RNase H. Second-strand cDNA synthesis was subsequently performed using deoxynucleoside triphosphate (dNTP), DNA polymerase I, and RNase H. The library fragments were purified with QiaQuick PCR kits (Qiagen, USA) and eluted with elution buffer, and then terminal repair, A-tailing, and adapter addition were implemented. The RNA concentration of the library was measured using a Qubit RNA Assay Kit in Qubit v3.0 to preliminarily quantify the sample, which was then diluted to 1 ng/μL. Insert size was assessed using the Agilent Bioanalyzer 2100 system (Agilent Technologies, USA), and the qualified insert size was accurately quantified using the StepOnePlus™ Real-Time PCR System (ThermoFisher, USA, valid library concentration >10 nmol/L). Clustering of the index-coded samples was performed on the cBot Cluster Generation System using a HiSeq PE Cluster Kit v4-cBot-HS (Illumina, USA) according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on the Illumina HiSeq 2500 platform, and 150 bp paired-end reads were generated. All gene expression values from RNA-seq were changed to a log2 value for further analysis. Gene sets with a nominal P<0.05 were called.
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from cell and tissue samples using an RNeasy Mini Kit (Qiagen, USA) according to the manufacturer’s protocols. A total of 1 μg of extracted RNA was transcribed into cDNA using EasyScript One-Step RT-PCR SuperMix (TransGen Biotech, China). The cDNA was mixed with primers and TransStart Tip Green qPCR SuperMix (TransGen Biotech, China), and mRNA expression levels were measured using a 7500 Real-Time PCR System (Applied Biosystems, USA). The primers were designed using Primer-BLAST and are listed in Supplementary Table S2.
Luciferase assays
HEK293 cells stably harboring the Wnt/β-catenin reporter SuperTOPFlash (HEK293STF) were seeded into a 24-well plate and cotransfected with 500 ng of pcDNA3.1-TWIST1 or pcDNA3.1 control vector, and 100 ng of Renilla luciferase plasmid (pRL-TK) control vector (Promega, USA) were cotransfected for each well using Lipofectamine™ 3000 Transfection Reagent (Invitrogen, USA) according to the manufacturer’s instructions. A TransDetect Double-Luciferase Reporter Assay Kit (TransGen Biotech, China) was used to determine luciferase activity in the transfected cells. Normalized firefly/Renilla values were used to calculate relative luciferase activity. Each assay was performed in triplicate at the same time and repeated three times.
Western blotting
Cells and tissues from mice were lysed in sodium dodecyl sulfate (SDS) lysis buffer (2% SDS and 62.5 mmol/L Tris-HCl, pH 6.8, containing protease inhibitor cocktail tablets ordered from Roche, Switzerland.) and sonicated three times for 5 s. Equal amounts of protein (20 μg) were loaded onto a 10% polyacrylamide gel and analyzed by immunoblotting. The antibodies used for western blotting included GAPDH (Cat. No. 60004-1-Ig, 1:3 000 dilution; Proteintech, China), TWIST1 (1:2 000 dilution; Santa Cruz, USA), FLAG (1:2 000 dilution; Thermo Fisher, USA), Cyclin D1 (Cat. No. MA5-14512, 1:2 000 dilution; Thermo Fisher, USA), GSK3β (Cat. No. 12456, 1:2 000 dilution; Cell Signaling Technology, USA), p-GSK3β (Cat. No. 5558, 1:2 000 dilution; Cell Signaling Technology, USA), and HRP-conjugated goat anti-rabbit (Cat. No. 7074, 1:5 000 dilution; Cell Signaling Technology, USA) and goat anti-mouse secondary antibodies (Cat. No. 7076, 1:5 000 dilution; Cell Signaling Technology, USA).
RESULTS
TWIST1 is a specific marker of pathological NV
We previously found that deletion of the Twist1 gene in ECs significantly inhibits hypoxia-induced retinal NV but does not affect normal retinal vascular development in mice (Li et al, 2014). Here, to study the specific functions of TWIST1 in physiological and pathological NV, we first determined TWIST1 mRNA expression levels in HRECs and HUVECs. Based on RT-PCR, we found that TWIST1 was not significantly expressed in either HRECs or HUVECs (Figure 1A), with western blotting of the TWIST1 protein verifying these results (Figure 1B). We sorted retinal ECs from OIR mice (retinal whole-mounts of OIR mice are shown in Supplementary Figure S1) and normal control mice by flow cytometry and analyzed TWIST1 mRNA expression in the ECs using RT-PCR. Results showed that TWIST1 mRNA was barely expressed in normal retinal ECs but was significantly enhanced in OIR retinal ECs (Figure 1C). Thus, our results indicate that TWIST1 is a specific biomarker of pathological retinal NV.
Figure 1.
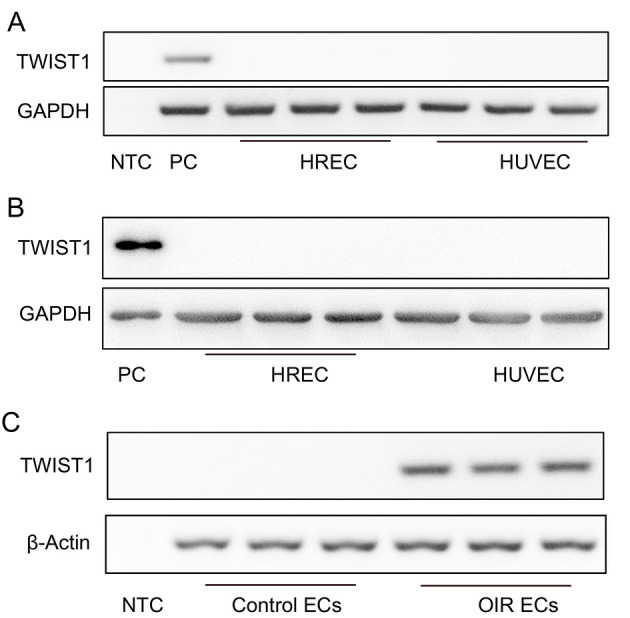
Endothelial expression of Twist1 under physiological and pathological conditions
A: RT-PCR showed no obviousTwist1 expression in HRECs and HUVECs. GAPDH was used as a loading control. B: Western blotting showed no obvious Twist1 expression in HRECs and HUVECs. GAPDH was used as a loading control. C: RT-PCR showed that Twist1 expression increased in retinal ECs from OIR mice. β-actin was used as a loading control. NTC: No template control; PC: Positive control.
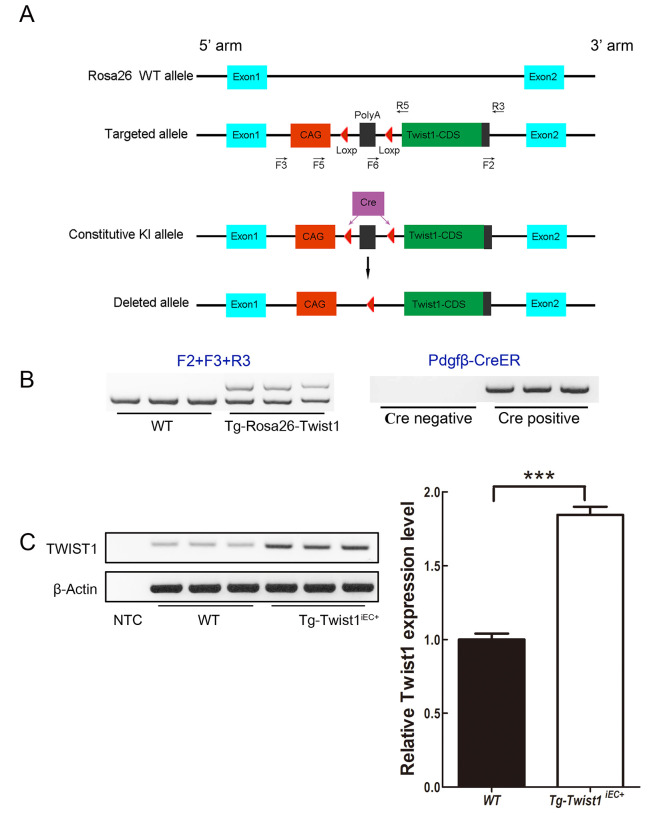
Generation of inducible vascular EC-specific Twist1 overexpression mice
As TWIST1 is a specific biomarker of pathological NV, we hypothesized that overexpression of Twist1 in ECs may also lead to pathological NV without hypoxia induction. To verify this hypothesis and study the specific role of Twist1 in retinal pathological angiogenesis, we generated an EC-specific Twist1 overexpression transgenic mouse model. We first established Twist1 conditional knock-in mice by inserting a CAG-loxP-Poly A (STOP)-loxP-Twist1 CDS element into the first intron of the Rosa26 gene (Figure 2A), namedTg-Rosa26-Twist1 mice, which can be mated with different Cre mice to achieve specific overexpression of the Twist1 gene in different cell types and organs. To achieve vascular EC-specific overexpression of Twist1, we mated Tg-Rosa26-Twist1 mice with Tie2-Cre mice (Kisanuki et al, 2001), however, the target Tg-Rosa26-Twist1-Tie2-Cre mice were embryonically lethal, suggesting that overexpression of Twist1 in ECs may impair early embryonic vasculogenesis. Thus, we replaced Tie2-Cre with Pdgfb-CreER (which can only be expressed when induced by tamoxifen) and mated these mice with Tg-Rosa26-Twist1 mice to obtain an inducible EC-specific Twist1 overexpression mouse model. Mice heterozygous for Tg-Rosa26-Twist1 alleles (Tg-Rosa26-Twist1) were mated with Pdgfb-CreER mice (Claxton et al, 2008) to generate Tg-Rosa26-Twist1-Pdgfb-CreER mice. After 3 consecutive days of tamoxifen induction from postnatal day (P) 2 to P4, we obtained Twist1 EC-specific overexpression mice (named Tg-Twist1iEC+ mice hereafter). PCR was used to identify offspring genotypes: when using the F2, F3, and R3 primers for amplification, the Tg-Rosa26-Twist1 mice showed two bands and wild-type (WT) mice showed one band; when using the Pdgfb-CreER primer for amplification, mice carrying Pdgfb-CreER showed one band (Figure 2B). Specificity and efficacy of Cre-mediated recombination have been evaluated using the tdTomato reporter gene in previous studies (Zhang et al, 2019). In addition, to exclude the influence of alcohol on retinal vascular development during tamoxifen induction, we injected corn oil and corn oil containing 10% ethanol in WT mice, respectively, but found no retinal vascular development at P7 under 10% ethanol exposure (Supplementary Figure S2). To verify whether Tg-Twist1iEC+ cells were successfully constructed, we carried out RT-PCR and RT-qPCR to analyze the expression of Twist1 in the lungs of Tg-Twist1iEC+ mice and their littermate controls. Results showed that TWIST1 mRNA expression was significantly higher (~85%) in the Tg-Twist1iEC+ mice than in the control mice (Figure 2C). Thus, we successfully generated an inducible EC-specific Twist1 overexpression transgenic mouse model, which was used for further study.
Figure 2.
Generation of inducible vascular EC-specific Twist1 overexpression mice
A: Targeting strategy for generating Tg-Twist1iEC+ mice. B: Genotyping of mouse models using PCR methods. C: Analyses of Twist1 mRNA expression in lungs from WT and Tg-Twist1iEC+ mice by RT-PCR. GAPDH was used as a loading control. D: Analyses of Twist1 mRNA expression in lungs from WT and Tg-Twist1iEC+ mice by RT-qPCR, showing that Twist1 was overexpressed in Tg-Twist1iEC+ mice. n=6; two-tailed t-test; ***: P<0.001.
Overexpression of Twist1in ECs causes defective retinal angiogenesis and pathological NV
Mouse retinas are avascular at birth, with a single superficial layer of blood vessels growing progressively from the center toward the periphery from P1 to P7, then subsequently growing into the deep retinal layers (Zhang et al, 2019). After 3 days of tamoxifen injection from P2 to P4 (Figure 3A), retinas were harvested at P6 from the Tg-Twist1iEC+ and littermate control mice (Tg-Rosa26-Twist1). Isolectin B4 (IB4) staining of retinal whole mounts showed that superficial vessel progression was delayed in the Tg-Twist1iEC+ mice compared with the control mice (Figure 3B–D), i.e., approximately 45% completed versus 60% completed. However, careful observation of the retinal vascular morphology of Tg-Twist1iEC+ and control mice showed that the vascular density of the former was greater than that of the latter (Figure 3B–F), and aneurysmal nodules were detected, i.e., pathological NV (Figure 3E, arrows indicated). Pathological NV is often accompanied by leakage, and Ter119 staining of red blood cells (RBCs) showed that leakage indeed occurred in the retinas of the Tg-Twist1iEC+ mice. Unlike normal control mice, where RBCs were confined to the blood vessels, many RBCs in Tg-Twist1iEC+ mice escaped the blood vessels and gathered at the front end of the retinal vessels (Figure 3F).
Figure 3.
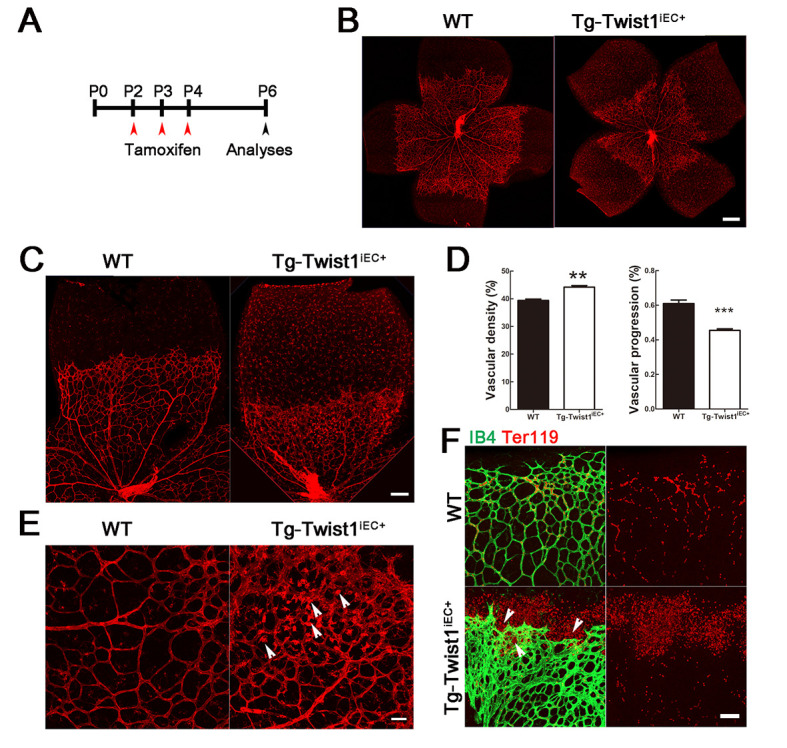
Defective retinal angiogenesis and pathological NV in Tg-Twist1iEC+ mice at P6
A: Tg-Twist1iEC+ and control mice were intraperitoneally injected with tamoxifen at P2 to P4 and analyzed at P6. B–D: Isolectin B4 staining of retinal whole mounts showed delayed superficial vascular progression and increased vascular density in retinas of Tg-Twist1iEC+ mice compared with littermate WT mice. Scale bar: 250 μm for B; 100 μm for C; E: Arrows indicate aneurysmal-like pathological NV in Tg-Twist1iEC+ retinas. Scale bar: 50 μm. F: Red blood cells stained with Ter119 antibody show vessel leakage in Tg-Twist1iEC+ retinas, indicated by arrows. **: P<0.01; ***: P<0.001; two-tailed t-test. Scale bars: 50 μm.
TWIST1 promotes EC proliferation and disrupts EC polarization
Proper EC proliferation and orientation are critical for normal retinal angiogenesis. To assess whether TWIST1 affects the proliferation of ECs, we carried out EdU staining experiments. Results showed that EdU-labeled proliferating ECs were significantly increased in the Tg-Twist1iEC+ retinas compared with the WT retinas (Figure 4A, B), indicating that TWIST1 promotes EC proliferation. This conclusion seems contradictory to the observed slower progression of retinal angiogenesis in Tg-Twist1iEC+ cells (Figure 3B–D). Combined with the increased vascular density in Tg-Twist1iEC+ retinas, we considered that TWIST1 may disrupt the direction of retinal angiogenesis. Thus, we examined whether TWIST1 regulates endothelial polarization during sprout elongation. We analyzed the shapes and locations of EC nuclei in the vascular front by visualizing ETS-related genes (ERGs, marking endothelial nuclei); these nuclei are elliptical in normal proliferating and migrating ECs (Coxam et al, 2014; Kim et al, 2019). Consistent with previous research (Potente & Carmeliet, 2017), the EC nuclei were largely elliptical in the WT mice (Figure 4C, D) but were more spherical and not directed to the avascular area in the Tg-Twist1iEC+ mice (Figure 4C, D). In addition, many EC ERG+ nuclei overlapped in the Tg-Twist1iEC+ mice but were positioned in a planar fashion in the WT mice (Figure 4C). These results indicate that overexpression of Twist1 can disrupt endothelial polarization during sprouting angiogenesis.
Figure 4.
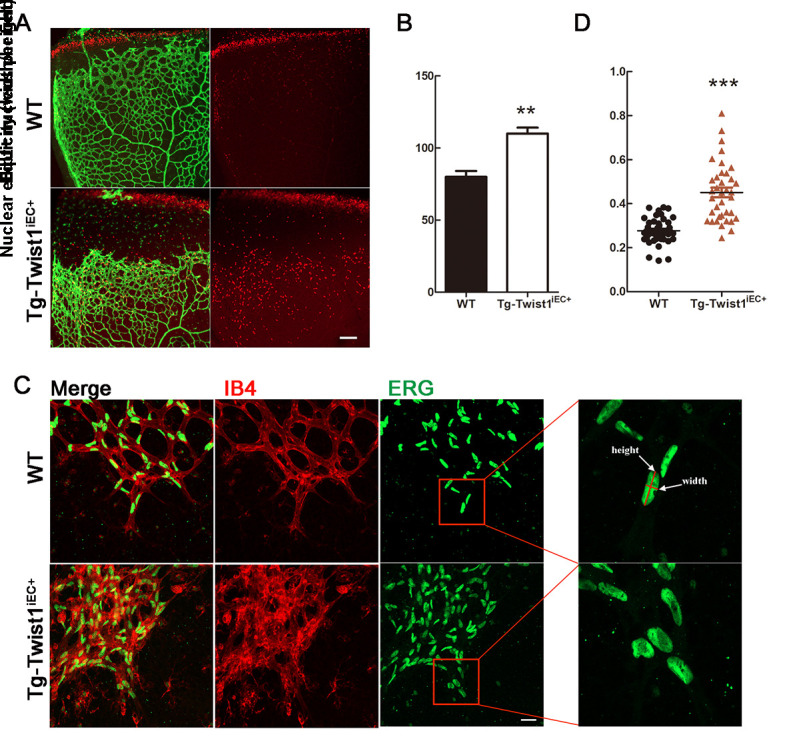
Increased EC proliferation and disrupted EC polarization in Tg-Twist1iEC+ mice at P6
A, B: EdU labeling (red) of retinal whole mounts from WT and Tg-Twist1iEC+ mice at P6 and corresponding statistical results. C, D: IB4 and ERG+ EC nuclei at vascular front in WT and Tg-Twist1iEC+ mice and corresponding statistical results. **: P<0.01, two-tailed t-test. Scale bar: 50 μm.
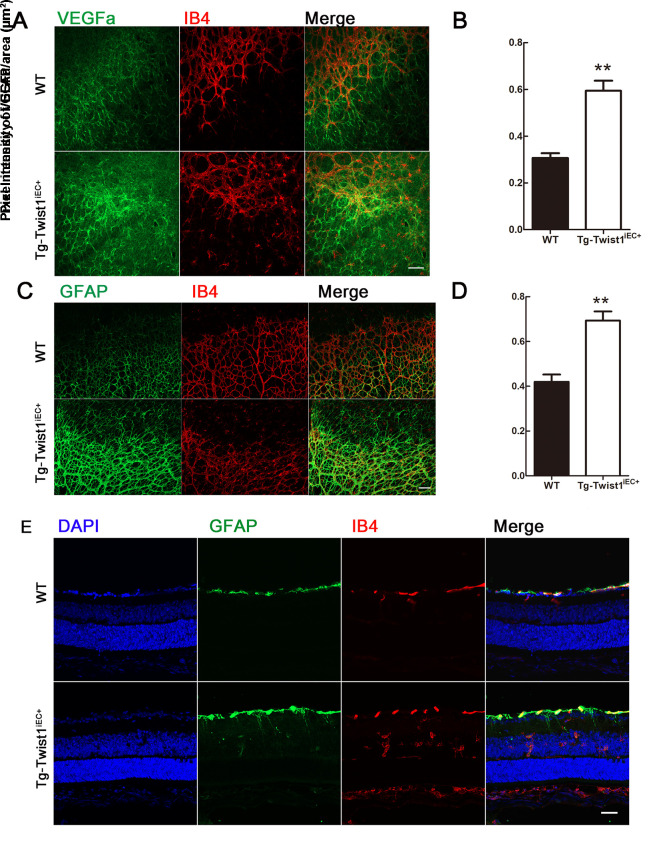
Overexpression of Twist1in ECs increases VEGFA secretion and enhances gliosis
As VEGF is a key factor in both physiological and pathological NV, we stained retinal whole mounts from Tg-Twist1iEC+ mice with a VEGFA antibody. Results showed that VEGFA secretion was increased in the Tg-Twist1iEC+ mice compared to that in the WT mice at P6 (Figure 5A, B). VEGFA is secreted by retinal glial cells. Overexpression of Twist1 in the ECs increased VEGFA secretion and glial fibrillary acidic protein (GFAP) staining (indicating gliosis) showed that the expression of GFAP was enhanced in the Tg-Twist1iEC+ retinas (Figure 5C, D). Moreover, unlike the WT retinas, GFAP only existed in the retinal ganglion cell (RGC) layer, and the GFAP protein of the Tg-Twist1iEC+ retinas stretched to the deep retina and reached the inner nuclear layer (Figure 5E). Thus, enhanced gliosis may lead to increased secretion of VEGFA, which may further induce pathological angiogenesis.
Figure 5.
Increased VEGFA secretion and enhanced gliosis inTg-Twist1iEC+ mice at P8
A–D: VEGFA (A, B), GFAP (C, D) and IB4 staining at vascular front in WT and Tg-Twist1iEC+ mice. VEGFA, green; GFAP, green; IB4, red. Scale bars: 50 μm. **: P<0.01, two-tailed t-test. E: Retinal section staining of GFAP expression to deep retina and INL. RGC: Retinal ganglion cell; INL: Inner nuclear layer; ONL, Outer nuclear layer. Scale bar: 25 μm.
TWIST1 regulates expression of a series of signals related to NV
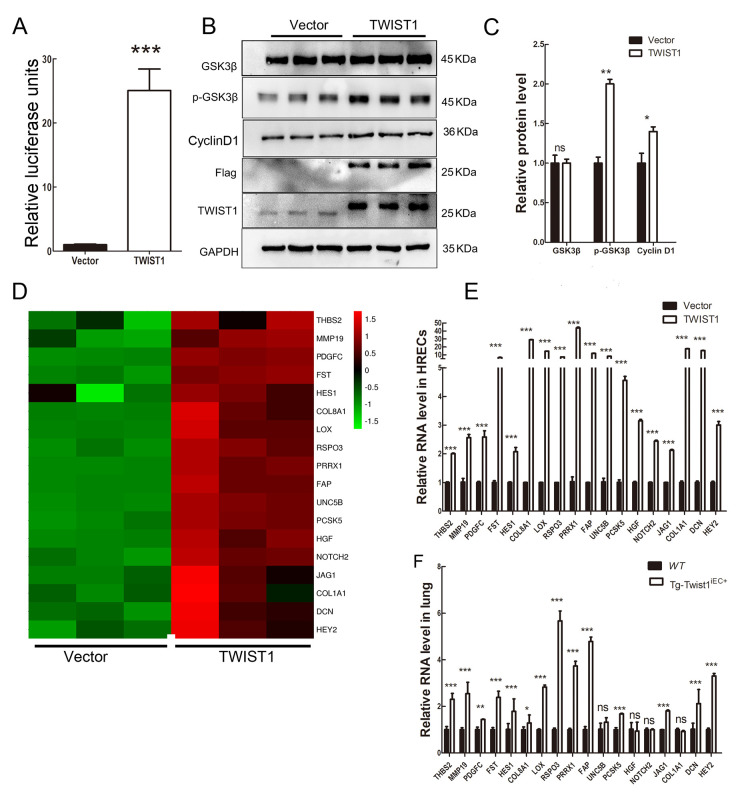
Wnt/β-catenin signaling is one of the most important signaling pathways in the regulation of retinal angiogenesis. Previous research has also suggested an interaction between TWIST1 and Wnt/β-catenin signaling in tumors (Pan et al, 2020). Thus, we studied whether TWIST1 affects Wnt/β-catenin signaling. Luciferase analysis showed that when TWIST1 was transfected in HEK293STF cells, Wnt/β-catenin signaling increased significantly (Figure 6A). Western blotting also showed that the expression levels of CYCLIN D1 and phosphate-GSK3β (p-GSK3β) were significantly increased (Figure 6B, C), suggesting that the Wnt/β-catenin signaling pathway was activated. Further RT-qPCR analysis of RNA expression of downstream genes of the Wnt/β-catenin signaling pathway showed that the expression levels of C-JUN, CLDN5, LEF1, CYCLIN D1, and AXIN2 were also significantly increased (Supplementary Figure S3), verifying activation of the Wnt/β-catenin signaling pathway. This finding is consistent with recent research showing that overactivation of Wnt/β-catenin signaling leads to abnormal retinal angiogenesis (Zhu et al, 2021).
Figure 6.
TWIST1 enhanced Wnt/β-catenin signaling and regulated expression of vascularization-related genes
A: Luciferase analysis showed that Wnt/β-catenin signaling activity was increased in HEK293STF cells transfected with TWIST1 plasmids. B, C: Western blotting showed that cyclin D1 and phosphorylation of GSK3β proteins increased when TWIST1 was overexpressed in HRECs. Western blotting with anti-Flag and TWIST1 showed successful transfection of TWIST1 plasmids. GAPDH was used as the loading control. Data are presented as mean±standard error of the mean (SEM). Sample size n=4. Data are from one typical experiment of three independent experiments. **: P<0.01; *: P<0.05, two-tailed t-test. D: RNA-seq showed that vascularization-related genes were up-regulated when TWIST1 was overexpressed in HRECs. E: RT-qPCR verified RNA-seq results in HRECs for indicated genes. ***: P<0.001. Sample size n=4. F: RT-qPCR verified that expression of most of TWIST1-targeting genes identified by RNA-seq were increased in the lungs of Tg-Twist1iEC+ mice. ***: P<0.001;**: P<0.01; *: P<0.05; ns, no significant difference. Sample size n=4.
Wnt/β-catenin signaling is not the only factor that regulates retinal angiogenesis. To further explore the mechanism underlying pathological NV caused by overexpression of TWIST1, we used a lentivirus carrying TWIST1 plasmids to transfect HRECs. We then analyzed the transcriptome of the HRECs overexpressing Twist1 plasmids and control vectors using RNA-seq. Results showed that the expression levels of various NV-related genes (including THBS2, MMP19, PDGFC, FST, HES1, COL8A1, LOX, RSPO3, PRRX1, FAP, UNC5B, PCSK5, HGF, NOTCH2, JAG1, COL1A1, DCN, and HEY2) were elevated in the HRECs overexpressing TWIST1 (Figure 6D). These results were confirmed by RT-PCR (Figure 6E). The RNA-seq results were also verified in vivo by detecting RNA expression in lung tissue from WT and Tg-Twist1iEC+ mice, which showed that the expression patterns of most target genes were similar to the results obtained in HRECs (Figure 6F). Thus, overexpression of TWIST1 appears to induce the expression of a series of complex NV-related genes and pathological retinal NV, with TWIST1 acting as a “valve” in this process.
DISCUSSION
Pathological retinal NV is the main cause of many sight-threatening eye diseases (Campochiaro, 2015; Chan-Ling et al, 2018; Selvam et al, 2018; Wong et al, 2016; Zhang et al, 2020). Exploring the molecular mechanisms and specific molecular markers for pathological retinal NV should shed light on disease treatment. The TWIST1 transcription factor can be induced in hypoxic conditions and is positively associated with normal tissue and tumor angiogenesis (Chen et al, 2014; Koo et al, 2017; Low-Marchelli et al, 2013; Rodrigues et al, 2008; Tseng et al, 2015; Yang et al, 2008).
By analyzing TWIST1 mRNA expression under physiological and pathological conditions, we identified TWIST1 as a specific biomarker for pathological retinal NV. To mimic Twist1 expression in the ECs of OIR mice and clarify the specific role of Twist1 overexpression in retinal angiogenesis, we generated an inducible transgenic mouse model (Tg-Twist1iEC+) with ectopic overexpression of Twist1 in ECs. The Tg-Twist1iEC+ retina showed defective angiogenesis with pathological NV, indicating that pathological NV could be induced by overexpressing Twist1 in ECs without hypoxic conditions or other external interventions. Compared with the OIR model, the genetically modified mouse model was more stable, which may be of benefit for further therapeutic studies targeting pathological NV-related retinal diseases.
Interestingly, vascular progression was much slower in the Tg-Twist1iEC+ mice compared to the control mice (Figure 3B–C), although EdU staining showed increased EC proliferation in Tg-Twist1iEC+ mice (Figure 4A), which seems paradoxical.
TWIST1 can promote EC proliferation through various mechanisms. Recent study revealed that TWIST1 can regulate endothelial gene transcription, positively of proangiogenic KDR and negatively of antiangiogenic SFRP4. TWIST1 reprogramming enhanced the endothelial lineage commitment of mesenchymal stromal cells and increased the vasculogenic potential (Kaushik & Das, 2020). TWIST1 can also induce endothelial differentiation of tumor cells through the Jagged1-KLF4 axis and Wnt5a (Chen et al, 2014). Wnt5a is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving Wnt/β-catenin (Yang et al, 2009). In vitro, our study showed that overexpression ofTWIST1 in HEK293STF cells activated the Wnt/β-catenin signaling pathway (Figure 6A-C). Similar to our Tg-Twist1iEC+ mice, overactivation of Wnt/β-catenin signaling by Ctnna1 mutation can lead to defective retinal angiogenesis, including slower vascular progression, increased EC proliferation, and greater pathological NV (Zhu et al, 2021). Overactivation of Wnt/β-catenin signaling can promote cell proliferation and is well established in multiple cell types (Li et al, 2020; Yang et al, 2021). Why did the progression of retinal vasculature from the optic nerve to the peripheral retina seem slowed under the condition of TWIST1 promotes EC proliferation?
In tumor studies, TWIST1 has long been known as a principal inducer of EMT, in which epithelial cells lose their polarity (Wang et al, 2017; Yang et al, 2004). Similarly, whether overexpression of Twist1 in ECs disturbs cell polarity remains unclear. Loss of EC polarity could result in disorganized angiogenesis under cell proliferation (Coxam et al, 2014; Kim et al, 2019; Potente & Carmeliet, 2017). Here, we found that EC polarity was indeed disturbed in the Tg-Twist1iEC+ retinas, which may cause angiogenesis to fail to reach the peripheral retina in a predetermined direction, and thereby increase vascular density in the middle peripheral area.
To further explain the morphological changes in Tg-Twist1iEC+ mice, we tested GFAP and soluble VEGFA and found that the expression levels of both were increased. GFAP is a well-known sensitive biomarker for retinal gliosis. Under normal conditions, GFAP is kept in a static state in the retina. However, retinal GFAP expression is up-regulated in astrocytes and Müller cell bodies in OIR models and represents an important response to retinal injury (Lee et al, 2020; Villacampa et al, 2017). In the current study, we observed diffuse vascular leakage in the frontier of retinal vascular in Tg-Twist1iEC+ mice, indicating severe pathological damage to retinal vessels. These pathological changes led to glial cell activation, as indicated by increased GFAP protein expression, which led to greater VEGFA secretion, another inducer of pathological NV.
Using RNA-seq, we also identified a group of NV-related genes regulated by TWIST1, indicating that TWIST1 acts as a ‘valve’ in the regulation of pathological angiogenesis. These findings suggest that inhibiting Twist1 expression may antagonize pathological NV, which is worthy of further study.
In conclusion, we showed that ectopic overexpression of endothelialTwist1 leads to defective retinal angiogenesis and uncontrolled pathological NV in transgenic mice, partly by promoting EC proliferation and disturbing EC polarity. In terms of mechanism, we found that TWIST1 promotes pathological NV by enhancing the Wnt/β-catenin signaling pathway, promoting VEGFA secretion through retinal gliosis, and up-regulating the expression of a group of NV-related genes, thereby acting as a ‘valve’ in the regulation of pathological angiogenesis. Our study provides a new mouse model for the study of retinal diseases associated with pathological NV and may provide a potential therapeutic target for pathological NV.
DATA AVAILABILITY
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request. The RNA-seq data was submitted to the National Center for Biotechnology Information (NCBI, BioProjectID PRJNA778270). The annotation is available upon request.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
J.L., L.Z., and X.J.Z. designed and supervised the study. L.Z., J.L., and S.S.Z. wrote the manuscript. S.S.Z., K.F.W., Y.H.L., K.X.S., and H.J.X. performed animal and immunohistochemical analyses. H.M.L., S.Z.C., W.J.J., and S.M. conducted animal breeding and genotyping. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
Jie Li gratefully acknowledges Dr. Jing Chen, associate professor of Ophthalmology, Boston Children's Hospital/Harvard Medical School for her careful guidance on scientific research during Jie’s visit and study in Boston.
Funding Statement
This study was supported by the National Natural Science Foundation of China (82071009, 81700841) and by the Grant from Chinese Academy of Medical Sciences (2019-I2M-5-032)
References
- 1.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, et al The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA Molecular pathogenesis of retinal and choroidal vascular diseases. Progress in Retinal and Eye Research. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capitão M, Soares R Angiogenesis and inflammation crosstalk in diabetic retinopathy. Journal of Cellular Biochemistry. 2016;117(11):2443–2453. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 4.Chan-Ling T, Gole GA, Quinn GE, Adamson SJ, Darlow BA Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Progress in Retinal and Eye Research. 2018;62:77–119. doi: 10.1016/j.preteyeres.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Chen HF, Huang CH, Liu CJ, Hung JJ, Hsu CC, Teng SC, et al Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nature Communications. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- 6.Chen HF, Wu KJ Endothelial transdifferentiation of tumor cells triggered by the Twist1-Jagged1-KLF4 Axis: relationship between cancer stemness and angiogenesis. Stem Cells International. 2016;2016:6439864. doi: 10.1155/2016/6439864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung N, Mitchell PP, Wong TY Diabetic retinopathy. The Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 8.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46(2):74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 9.Coxam B, Sabine A, Bower NI, Smith KA, Pichol-Thievend C, Skoczylas R, et al Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Reports. 2014;7(3):623–633. doi: 10.1016/j.celrep.2014.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartnett ME, Penn JS Mechanisms and management of retinopathy of prematurity. The New England Journal of Medicine. 2012;367(26):2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik K, Das A TWIST1-reprogrammed endothelial cell transplantation potentiates neovascularization-mediated diabetic wound tissue regeneration . Diabetes. 2020;69(6):1232–1247. doi: 10.2337/db20-0138. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee DH, et al YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. The Journal of Clinical Investigation. 2017;127(9):3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YH, Choi J, Yang MJ, Hong SP, Lee CK, Kubota Y, et al A MST1-FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nature Communications. 2019;10(1):838. doi: 10.1038/s41467-019-08773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kisanuki YY, Hammer RE, Miyazaki JI, Williams SC, Richardson JA, Yanagisawa M. 2001. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental Biology, 230(2): 230–242.
- 15.Koo YJ, Kim TJ, Min KJ, So KA, Jung US, Hong JH. 2017. CXCL11 mediates TWIST1-induced angiogenesis in epithelial ovarian cancer. Tumor Biology, 39(5): 1010428317706226.
- 16.Krohne TU, Müller A, Larsen PP, Holz FG Long-term effects of anti-VEGF therapy for retinopathy of prematurity. Der Ophthalmologe. 2018;115(6):464–468. doi: 10.1007/s00347-018-0700-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Surbeck JW, Drake M, Saunders A, Jin HD, Shah VA, et al Increased glial fibrillary acid protein and vimentin in vitreous fluid as a biomarker for proliferative vitreoretinopathy. Investigative Ophthalmology & Visual Science. 2020;61(5):22. doi: 10.1167/iovs.61.5.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Liu CH, Sun Y, Gong Y, Fu Z, Evans LP, et al Endothelial TWIST1 promotes pathological ocular angiogenesis. Investigative Ophthalmology & Visual Science. 2014;55(12):8267–8277. doi: 10.1167/iovs.14-15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K, Zhang JW, Tian YH, He YQ, Xu XL, Pan WT, et al The Wnt/β-catenin/VASP positive feedback loop drives cell proliferation and migration in breast cancer. Oncogene. 2020;39(11):2258–2274. doi: 10.1038/s41388-019-1145-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu ZP, Yan SY, Wang JJ, Xu YM, Wang Y, Zhang SY, et al Endothelial adenosine A2a receptor-mediated glycolysis is essential for pathological retinal angiogenesis. Nature Communications. 2017;8(1):584. doi: 10.1038/s41467-017-00551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low-Marchelli JM, Ardi VC, Vizcarra EA, Van Rooijen N, Quigley JP, Yang J Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Research. 2013;73(2):662–671. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammoto T, Jiang E, Jiang AMD, Lu YB, Juan AM, Chen J, et al Twist1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering Tie2 expression. PLoS One. 2013;8(9):e73407. doi: 10.1371/journal.pone.0073407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell P, Liew G, Gopinath B, Wong TY Age-related macular degeneration. The Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 24.Pan JC, Fang S, Tian HH, Zhou CW, Zhao XD, Tian H, et al lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β-catenin signaling. Molecular Cancer. 2020;19(1):9. doi: 10.1186/s12943-020-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JA, Kwon YG Hippo-YAP/TAZ signaling in angiogenesis. BMB Reports. 2018;51(3):157–162. doi: 10.5483/BMBRep.2018.51.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME Vascular endothelial growth factor in eye disease. Progress in Retinal and Eye Research. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitulescu ME, Schmidt I, Benedito R, Adams RH Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nature Protocols. 2010;5(9):1518–1534. doi: 10.1038/nprot.2010.113. [DOI] [PubMed] [Google Scholar]
- 28.Potente M, Carmeliet P The link between angiogenesis and endothelial metabolism. Annual Review of Physiology. 2017;79:43–66. doi: 10.1146/annurev-physiol-021115-105134. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues CO, Nerlick ST, White EL, Cleveland JL, King ML A Myc-Slug (Snail2)/Twist regulatory circuit directs vascular development. Development. 2008;135(11):1903–1911. doi: 10.1242/dev.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt JM, Panzilius E, Bartsch HS, Irmler M, Beckers J, Kari V, et al Stem-cell-like properties and epithelial plasticity arise as stable traits after transient Twist1 activation. Cell Reports. 2015;10(2):131–139. doi: 10.1016/j.celrep.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Selvam S, Kumar T, Fruttiger M Retinal vasculature development in health and disease. Progress in Retinal and Eye Research. 2018;63:1–19. doi: 10.1016/j.preteyeres.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'amato R, Sullivan R, et al Oxygen-induced retinopathy in the mouse. Investigative Ophthalmology & Visual Science. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 33.Sossey-Alaoui K, Pluskota E, Davuluri G, Bialkowska K, Das M, Szpak D, et al Kindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesis. The FASEB Journal. 2014;28(5):2260–2271. doi: 10.1096/fj.13-244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun KX, Jiang XY, Li X, Su YJ, Wang JL, Zhang L, et al Deletion of phosphatidylserine flippase β-subunit Tmem30a in satellite cells leads to delayed skeletal muscle regeneration . Zoological Research. 2021;42(5):650–659. doi: 10.24272/j.issn.2095-8137.2021.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thisse B, El Messal M, Perrin-Schmitt F The twist gene: isolation of a Drosophila zygotle gene necessary for the establishment of dorsoventral pattern . Nucleic Acids Research. 1987;15(8):3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng JC, Chen HF, Wu KJ A twist tale of cancer metastasis and tumor angiogenesis. Histology and Histopathology. 2015;30(11):1283–1294. doi: 10.14670/HH-11-638. [DOI] [PubMed] [Google Scholar]
- 37.Usui-Ouchi A, Friedlander M Anti-VEGF therapy: higher potency and long-lasting antagonism are not necessarily better. The Journal of Clinical Investigation. 2019;129(8):3032–3034. doi: 10.1172/JCI129862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villacampa P, Menger KE, Abelleira L, Ribeiro J, Duran Y, Smith AJ, et al Accelerated oxygen-induced retinopathy is a reliable model of ischemia-induced retinal neovascularization. PLoS One. 2017;12(6):e0179759. doi: 10.1371/journal.pone.0179759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LT, Chiou SS, Chai CY, Hsi E, Chiang CM, Huang SK, et al Transcription factor SPZ1 promotes TWIST-mediated epithelial-mesenchymal transition and oncogenesis in human liver cancer. Oncogene. 2017;36(31):4405–4414. doi: 10.1038/onc.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R Diabetic retinopathy. Nature Reviews Disease Primers. 2016;2(1):16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 41.Yang DH, Yoon JY, Lee SH, Bryja V, Andersson ER, Arenas E, et al Wnt5a is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving both Wnt/β-catenin and protein kinase Cα. Circulation Research. 2009;104(3):372–379. doi: 10.1161/CIRCRESAHA.108.185405. [DOI] [PubMed] [Google Scholar]
- 42.Yang F, Guo ZF, He C, Qing L, Wang H, Wu JW, et al Cancer-associated fibroblasts promote cell proliferation and invasion via paracrine Wnt/IL1β signaling pathway in human bladder cancer. Neoplasma. 2021;68(1):79–86. doi: 10.4149/neo_2020_200202N101. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al Direct regulation of TWIST by HIF-1α promotes metastasis. Nature Cell Biology. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 45.Yang WH, Lan HY, Huang CH, Tai SK, Tzeng CH, Kao SY, et al RAC1 activation mediates Twist1-induced cancer cell migration. Nature Cell Biology. 2012;14(4):366–374. doi: 10.1038/ncb2455. [DOI] [PubMed] [Google Scholar]
- 46.Ye X, Wang YS, Cahill H, Yu MZ, Badea TC, Smallwood PM, et al Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Zhang X, Xu HJ, Huang LL, Zhang SS, Liu WJ, et al Exome sequencing revealed Notch ligand JAG1 as a novel candidate gene for familial exudative vitreoretinopathy . Genetics in Medicine. 2020;22(1):77–84. doi: 10.1038/s41436-019-0571-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang SS, Liu WJ, Yang YM, Sun KX, Li SJ, Xu HJ, et al TMEM30A deficiency in endothelial cells impairs cell proliferation and angiogenesis. Journal of Cell Science. 2019;132(7):jcs225052. doi: 10.1242/jcs.225052. [DOI] [PubMed] [Google Scholar]
- 49.Zhao ZX, Rahman MA, Chen ZG, Shin DM Multiple biological functions of Twist1 in various cancers. Oncotarget. 2017;8(12):20380–20393. doi: 10.18632/oncotarget.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou YL, Wang YS, Tischfield M, Williams J, Smallwood PM, Rattner A, et al Canonical WNT signaling components in vascular development and barrier formation. The Journal of Clinical Investigation. 2014;124(9):3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu XJ, Yang M, Zhao PQ, Li SJ, Zhang L, Huang LL, et al Catenin α 1 mutations cause familial exudative vitreoretinopathy by overactivating Norrin/β-catenin signaling. The Journal of Clinical Investigation. 2021;131(6):e139869. doi: 10.1172/JCI139869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request. The RNA-seq data was submitted to the National Center for Biotechnology Information (NCBI, BioProjectID PRJNA778270). The annotation is available upon request.