Abstract
Background and Objective
The novel coronavirus disease (COVID-19) outbreak is currently ravaging populations worldwide. Many studies were registered and conducted in rapid response to the epidemic, but how to choose the proper design for clinical trials remains the main concern. This study aimed to determine the fundamental characteristics of study design during the COVID-19 pandemic and provide references for other emerging infectious diseases.
Methods
We searched the database of ClinicalTrials.gov with the keyword “COVID-19” and compared the results with the design features of other conventional studies except for COVID-19.
Results
From January 1, 2020 to September 30, 2021, 55,334 trials were registered at ClinicalTrials.gov. Of all the registered trials, 6,408 were related to COVID-19 (11.58%). There were significant differences in the proportion of observational studies between COVID-19 (43.48%) and others (23.27%). The completion rate of observational trials and interventional trials in COVID-19 was 29.04% and 25.84%, respectively. COVID-19 trials showed a higher rate of completion than others (P<0.01). The time distribution and trend of observational studies and interventional studies varied considerably.
Conclusion
Appropriately designed trials can help to improve research efficiency and reduce the possibility of research failure. In addition to randomized controlled trials, observational and single-armed studies are also worth considering.
Keywords: COVID-19, Clinical trials, Trial registration, Trial characteristics, Emerging infectious diseases
Introduction
The novel coronavirus disease (COVID-19) outbreak is currently ravaging populations worldwide. Timely identification and cutting off the route of transmission can control the source of infection (Lotfi et al, 2020, Saran et al, 2020, Ting Wu H, 2021). On this basis, the medical system will face the dilemma of how to treat patients effectively.
The particularity of emerging infectious diseases will bring difficulties to the selection of research and design schemes. Because the disease is threatening, the priority must be empirical symptomatic treatment or exploratory treatment with existing drugs. However, sometimes when we prepare for a long-term large-scale clinical study, the epidemic is over, and there are no patients to perform trials. A report published by a committee of the United States National Academies of Sciences, Engineering, and Medicine, which reviewed clinical researches conducted during the outbreak of Ebola, also expressed similar concerns (National Academies of Sciences et al, 2017). These particularities determined the choice of clinical research design scheme of new infectious diseases to be different from that of general diseases. Therefore, we review the clinical study design relating to COVID-19 registered in ClinicalTrials.gov since January 2020 with the aim of determining fundamental characteristics of study design during the COVID-19 epidemic and providing reference for other emerging infectious diseases.
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. In 1997, the United States Congress mandated the creation of the ClinicalTrials.gov registry to assist persons in gaining access to trials (Califf et al, 2012). In September 2004, the International Committee of Medical Journal Editors (ICMJE) made a statement that only trials registered before the beginning of patient recruitment would be considered for publication (De Angelis et al, 2005). To improve the diagnosis, treatment, and prevention of this disease, many clinical trials have been registered and carried out all over the world since the outbreak of COVID-19. We also noticed that there were several pieces of literature collecting the registered COVID-19 trials, but they mainly focused on interventional trials and the characteristics in terms of participants, interventions, and outcomes (Jones et al, 2020, Wang et al, 2020, Luo et al, 2021). By searching for the characteristics of all clinical research design schemes concerning COVID-19 registered in this database since January 2020 and comparing them with the characteristics of other conventional studies except COVID-19, we can find the clinical research design rules and features of new infectious diseases, which will help us to select more appropriate research design schemes for new infectious diseases in the future. We also aim to provide evidence for improving research efficiency and reducing the possibility of research failure.
2. Materials and methods
2.1. Data sources and search
We searched the database of ClinicalTrials.gov with the keyword “COVID-19,” without restriction on languages and study type. The search time was limited from January 1, 2020 to September 30, 2021. The registration information of all relevant studies was downloaded. At the same time, the details of all clinical trials registered after January 2020 were also collected to compare the characteristics of trials. The search was performed on October 4, 2021.
2.2. Data extraction and analysis
Two researchers independently collected data from the downloaded registration information files, after which a third researcher checked the data. The following information was collected from each study: title, date of registration, current status, locations, study type, study designs, interventions and control, sponsor and collaborators, phases, sample size, outcomes, and recruiting status. The overall status of registered clinical trials on COVID-19 was reviewed and collated.
We reported absolute numbers and percentages for categorical variables and compared them by using χ2 analysis. Analyses were conducted on the design, study type, and time distribution of registered trials.
Results
3.1. Search results
From January 1, 2020 to September 30, 2021, 55,334 trials were registered at ClinicalTrials.gov. Of all the registered trials, 6,408 were related to COVID-19 (11.58%).
3.2. Comparison of study type between COVID-19 and other trials
Overall numbers of identified trials and comparisons by study type of registered clinical trials are presented in Table 1 . Within 6,408 COVID-19 trials, the number of interventional studies and observational studies was 3,622 (56.52%) and 2,786 (43.48%), respectively, whereas patient registry studies accounted for 5.76% in observational studies. For the other 48,926 studies, 37,539 (76.73%) were interventional and 11,387 (23.27%) were observational, whereas there were 1,491 (3.05%) patient registry studies in the observational studies. There was a significant difference in the proportions of interventional studies (P<0.001). On the other hand, the difference in proportions of patient registry studies between COVID-19 trials and the other trials was also significant (P<0.001).
Table 1.
Study design based on ClinicalTrials.gov database compared with COVID-19 and others
| Study type | COVID-19 (n=6408) | Others (n=48926) | ALL (n=55334) | P value |
|---|---|---|---|---|
| Interventional Studies | 3622(56.52%) | 37539(76.73%) | 41161(74.39%) | <0.001 |
| Observational Studies | 2786(43.48%) | 11387(23.27%) | 14173(25.61%) | |
| -Patient Registry Studies | 369(5.76%) | 1491(3.05%) | 1860(3.36%) | <0.001 |
3.3. Comparison of study design between interventional COVID-19 and other trials
All interventional studies were grouped according to the method of allocation and further divided into 5 intervention models: crossover assignment, factorial assignment, parallel assignment, sequential assignment, and single group assignment.
Random allocation was used in 2,673 (73.80%) COVID-19 trials and in 23,970 (63.85%) other trials. Randomized studies accounted for the largest proportion in both COVID-19 trials and the others, and the ratio of randomized studies in COVID-19 trials was significantly higher than in the other trials (P<0.001). Furthermore, the difference of proportion in randomized studies with crossover, parallel, and sequential assignment between COVID-19 trials and the other trials was also significant (P<0.001). For 618 (17.06%) COVID-19 trials and 9,988 (26.61%) other trials in which allocation methods were not applicable, the intervention model of single group assignment was mostly used (n=608, n=9,654).
Non-random allocation was used in 330 (9.11%) COVID-19 trials and in 3,545 (9.44%) other trials, and the difference was not statistically significant. More details are shown in Table 2 .
Table 2.
Study design for interventional studies compared with COVID-19 and others
| Interventional Studies | COVID-19 (n=3622) | Others (n=37539) | P value |
|---|---|---|---|
| Allocation: N/A | 618(17.06%) | 9988(26.61%) | <0.001 |
| Intervention Model: Crossover Assignment | 0(0.00%) | 9(0.02%) | 0.582 |
| Intervention Model: Parallel Assignment | 0(0.00%) | 8(0.02%) | 0.619 |
| Intervention Model: Sequential Assignment | 10(0.28%) | 317(0.84%) | 0.030 |
| Intervention Model: Single Group Assignment | 608(16.79%) | 9654(25.72%) | 0.019 |
| Allocation: Non-Randomized | 330(9.11%) | 3545(9.44%) | 0.513 |
| Intervention Model: Crossover Assignment | 19(0.52%) | 157(0.42%) | 0.268 |
| Intervention Model: Factorial Assignment | 2(0.06%) | 41(0.11%) | 0.523 |
| Intervention Model: Parallel Assignment | 224(6.18%) | 2069(5.51%) | 0.001 |
| Intervention Model: Sequential Assignment | 53(1.46%) | 870(2.32%) | 0.001 |
| Intervention Model: Single Group Assignment | 32(0.88%) | 408(1.09%) | 0.321 |
| Allocation: Randomized | 2673(73.80%) | 23970(63.85%) | <0.001 |
| Intervention Model: Crossover Assignment | 87(2.40%) | 2494(6.64%) | <0.001 |
| Intervention Model: Factorial Assignment | 47(1.30%) | 388(1.03%) | 0.589 |
| Intervention Model: Parallel Assignment | 2381(65.74%) | 20106(53.56%) | <0.001 |
| Intervention Model: Sequential Assignment | 123(3.40%) | 656(1.75%) | <0.001 |
| Intervention Model: Single Group Assignment | 35(0.97%) | 326(0.87%) | 0.830 |
| Allocation: Unknown | 1 (0.03%) | 36(0.10%) | 0.151 |
3.4. Comparison of study design between observational COVID-19 and other trials
Table 3 compares the study design of registered observational COVID-19 studies (n=2,786) and the other studies (n=1,1387). Trials were classified into seven categories: case-control, case-crossover, case-only, cohort, ecologic/community, family-based, and other.
Table 3.
Study design for observational studies compared with COVID-19 and others
| Observational Studies | COVID-19 (n=2786) | Others (n=11387) | Pvalue |
|---|---|---|---|
| Observational Model: Case-Control | 312(10.85%) | 1443(12.67%) | 0.034 |
| Time Perspective: Cross-Sectional | 41(1.43%) | 299(2.63%) | 0.002 |
| Time Perspective: Prospective | 176(6.12%) | 893(7.84%) | 0.072 |
| Time Perspective: Retrospective | 82(2.85%) | 206(1.81%) | <0.001 |
| Time Perspective: Other | 13(0.45%) | 45(0.40%) | 0.348 |
| Observational Model: Case-Crossover | 29(1.01%) | 106(0.93%) | 0.592 |
| Time Perspective: Cross-Sectional | 11(0.38%) | 38(0.33%) | 0.836 |
| Time Perspective: Prospective | 11(0.38%) | 59(0.52%) | 0.090 |
| Time Perspective: Retrospective | 6(0.38%) | 9(0.08%) | 0.070 |
| Time Perspective: Other | 1(0.03%) | 0(0.00%) | 0.215 |
| Observational Model: Case-Only | 296(10.29%) | 1406(12.35%) | 0.012 |
| Time Perspective: Cross-Sectional | 41(1.43%) | 225(1.98%) | 0.354 |
| Time Perspective: Prospective | 156(5.42%) | 881(7.74%) | 0.001 |
| Time Perspective: Retrospective | 72(2.50%) | 242(2.13%) | 0.004 |
| Time Perspective: Other | 27(0.94%) | 58(0.51%) | <0.001 |
| Observational Model: Cohort | 1738(60.43%) | 6937(60.92%) | 0.155 |
| Time Perspective: Cross-Sectional | 143(4.97%) | 497(4.36%) | 0.129 |
| Time Perspective: Prospective | 1141(39.67%) | 5155(45.27) | <0.001 |
| Time Perspective: Retrospective | 342(11.89%) | 1049(9.21%) | <0.001 |
| Time Perspective: Other | 112(3.89%) | 236(2.07%) | <0.001 |
| Observational Model: Ecologic or Community | 95(3.30%) | 139(1.22%) | <0.001 |
| Time Perspective: Cross-Sectional | 37(1.29%) | 73(0.64%) | 0.041 |
| Time Perspective: Prospective | 45(1.56%) | 48(0.42%) | 0.049 |
| Time Perspective: Retrospective | 8(0.28%) | 10(0.09%) | 0.729 |
| Time Perspective: Other | 5(0.17%) | 8(0.07%) | 0.872 |
| Observational Model: Family-Based | 7(0.24%) | 33(0.29%) | 0.731 |
| Time Perspective: Cross-Sectional | 5(0.17%) | 7(0.06%) | 0.017 |
| Time Perspective: Prospective | 2(0.07%) | 20(0.18%) | 0.130 |
| Time Perspective: Retrospective | 0(0.00%) | 2(0.02%) | 0.677 |
| Time Perspective: Other | 0(0.00%) | 4(0.04%) | 0.448 |
| Observational Model: Other | 308(10.71%) | 1319(11.58%) | 0.433 |
| Time Perspective: Cross-Sectional | 80(2.78%) | 286(2.51%) | 0.104 |
| Time Perspective: Prospective | 137(4.76%) | 716(6.29%) | 0.002 |
| Time Perspective: Retrospective | 56(1.95%) | 185(1.62%) | 0.064 |
| Time Perspective: Other | 35(1.22%) | 132(1.16%) | 0.480 |
| Observational Model: Unknown | 1(0.03%) | 4(0.04%) | 0.665 |
Cohort studies (n=1,738, 60.43%) accounted for the largest part of the observational COVID-19 studies, and there were 6,937 (60.92%) cohort studies in the other studies. The proportions of case-control, case-only, and ecologic/community studies in the other observational studies were significantly lower (P<0.05) than in the observational COVID-19 trials.
3.5. Status of registered trials
Table 4 demonstrates the status of registered trials. Of the 3,622 interventional COVID-19 clinical trials, 934 (25.79%) had not yet started recruiting, 1,581 (43.65%) were still recruiting, and 936 (25.84%) were completed. The completion rate of intervention trials related to COVID-19 was significantly higher than that of the other clinical trials (P<0.001).
Table 4.
Status for all studies compared with COVID-19 and others
| Status | COVID-19 (n=6408) | Other (n=48926) | P value |
|---|---|---|---|
| Interventional Studies | 3622 | 37539 | |
| not recruiting | 934(25.79%) | 10482(27.92%) | 0.006 |
| Recruiting | 1581(43.65%) | 22377(59.61%) | <0.001 |
| Completed | 936(25.84%) | 3602(9.60%) | <0.001 |
| Other | 171(4.72%) | 1078(2.87%) | <0.001 |
| Observational Studies | 2786 | 11387 | |
| not recruiting | 530(19.02%) | 3312(29.09%) | <0.001 |
| Recruiting | 1416(50.83%) | 6544(57.47%) | <0.001 |
| Completed | 809(29.04%) | 1318(11.57%) | <0.001 |
| Other | 30(1.08%) | 209(1.84%) | 0.005 |
| Unknown | 1(0.04%) | 4(0.04%) | 0.665 |
| All | 6408 | 48926 | |
| not recruiting | 1464(22.85%) | 13794(28.19%) | <0.001 |
| Recruiting | 2997(46.77%) | 28921(59.11%) | <0.001 |
| Completed | 1745(27.23%) | 4920(10.06%) | <0.001 |
| Other | 201(3.14%) | 1287(2.63%) | 0.019 |
| Unknown | 1(0.02%) | 4(0.01%) | 0.460 |
The analysis of observational studies shows the same result, with completion rates of 29.04% and 11.57% in the COVID-19 trials and the others, respectively (P<0.001).
Statistical analysis was also performed in all identified trials. The ratio of recruiting trials that had not started recruiting was significantly higher (P<0.001) in the other clinical trials and the completion rate still lower (P<0.001) than those of the COVID-19 clinical trials.
3.6. Time distribution of COVID-19 trials
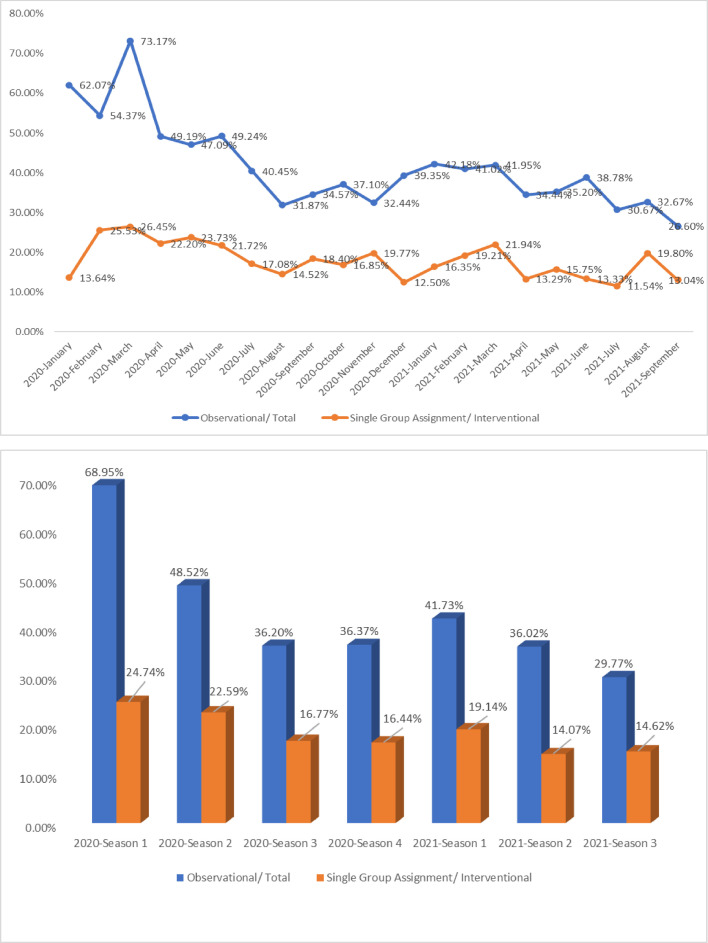
The numbers and proportions of different types of studies were classified by start date and are shown in Table 5 . 4,489 clinical trials related to COVID-19 were launched in 2020, and 1,919 trials started in 2021. In season 1 of 2020, the beginning of the COVID-19 outbreak, only 612 trials were launched, according to the registration information, and the number of clinical trials significantly increased in the next season (n=2,055). As time went by, the overall trend of numbers of registered trials was downward.
Table 5.
Time distribution and trend of COVID-19 study design
| Total | Observational | Observational/ Total | Interventional | Single Group Assignment | Single Group Assignment/ Interventional | |
|---|---|---|---|---|---|---|
| 2020 | 4489 | 2080 | 46.34% | 2409 | 479 | 19.88% |
| Season 1 | 612 | 422 | 68.95% | 190 | 47 | 24.74% |
| January | 58 | 36 | 62.07% | 22 | 3 | 13.64% |
| February | 103 | 56 | 54.37% | 47 | 12 | 25.53% |
| March | 451 | 330 | 73.17% | 121 | 32 | 26.45% |
| Season 2 | 2055 | 997 | 48.52% | 1058 | 239 | 22.59% |
| April | 860 | 423 | 49.19% | 437 | 97 | 22.20% |
| May | 669 | 315 | 47.09% | 354 | 84 | 23.73% |
| June | 526 | 259 | 49.24% | 267 | 58 | 21.72% |
| Season 3 | 1000 | 362 | 36.20% | 638 | 107 | 16.77% |
| July | 403 | 163 | 40.45% | 240 | 41 | 17.08% |
| August | 273 | 87 | 31.87% | 186 | 27 | 14.52% |
| September | 324 | 112 | 34.57% | 212 | 39 | 18.40% |
| Season 4 | 822 | 299 | 36.37% | 523 | 86 | 16.44% |
| October | 283 | 105 | 37.10% | 178 | 30 | 16.85% |
| November | 262 | 85 | 32.44% | 177 | 35 | 19.77% |
| December | 277 | 109 | 39.35% | 168 | 21 | 12.50% |
| 2021 | 1919 | 706 | 36.79% | 1213 | 196 | 16.16% |
| Season 1 | 798 | 333 | 41.73% | 465 | 89 | 19.14% |
| January | 275 | 116 | 42.18% | 159 | 26 | 16.35% |
| February | 256 | 105 | 41.02% | 151 | 29 | 19.21% |
| March | 267 | 112 | 41.95% | 155 | 34 | 21.94% |
| Season 2 | 633 | 228 | 36.02% | 405 | 57 | 14.07% |
| April | 241 | 83 | 34.44% | 158 | 21 | 13.29% |
| May | 196 | 69 | 35.20% | 127 | 20 | 15.75% |
| June | 196 | 76 | 38.78% | 120 | 16 | 13.33% |
| Season 3 | 487 | 145 | 29.77% | 342 | 50 | 14.62% |
| July | 150 | 46 | 30.67% | 104 | 12 | 11.54% |
| August | 150 | 49 | 32.67% | 101 | 20 | 19.80% |
| September | 188 | 50 | 26.60% | 138 | 18 | 13.04% |
| Total | 6408 | 2786 | 43.48% | 3622 | 675 | 18.64% |
As is shown in Table 5, among 612 trials started in season 1 of 2020, 422 were observational. Observational studies accounted for the maximal proportion (68.95%) of clinical trials launched in the first quarter of 2020 and gradually decreased.
For interventional studies, the proportion of trials with single-grouped assignments also showed a decreasing tendency by season in 2020.
Although the total number of observational studies (n=2,786) and interventional studies (n=3,662) showed similarly, the time distribution and trend of quantity varied considerably (Figure 1 ).
Figure 1.
Discussion
Emerging and sudden infectious diseases (such as Ebola, Middle East Respiratory Syndrome, and COVID-19) have brought great harm to human health. Since the first outbreak of COVID-19 in China from an unknown origin, this newly emerging respiratory disease quickly became a global pandemic. Countries around the world have called for the launching of clinical trials and rapidly expanding response activities around emerging infectious diseases to seek new therapeutic strategies and specific medicine.
Among all the clinical trials, there is no doubt that randomized controlled trial (RCT) is the optimal way to evaluate new therapeutic strategies with the highest level of grading quality of evidence (Byar et al, 1976, Bothwell et al, 2016). However, at the beginning of the COVID-19 outbreak, how long the disease will last and how many patients will be infected remained unclear. Limited information on its source of initial infection, pathogenesis, route of transmission, and clinical outcomes in hospitalized patients with COVID-19 also caused difficulty in the design of RCTs. By comparison, the design of observational studies was affected little by these factors.
Statistics in our analysis also showed that the observational studies accounted for the largest part of COVID-19 clinical trials at the beginning of the epidemic, and the ratio of it in COVID-19 trials was significantly higher than in other contemporaneous trials. For newly emerging infectious diseases, there were no proven safe and effective therapeutic products or vaccines when the epidemic began. Selection of treatment in the control group remained difficult, and it was considered unethical to use placebos without adequate knowledge in face of a disease (Ellenberg et al, 2018). It also usually took a long time for interventional trials to be planned, vetted, and initiated. In such cases, it is not an unattractive option to quickly start observational studies in consideration of the emergency of the disease, and evidence from well-designed observational studies can be helpful in filling evidence gaps. Failure to react quickly may lead to a missed opportunity to collect reliable clinical evidence if the outbreak subsides. Furthermore, beyond generally taking less time and money to conduct, observational studies have several strengths, such as reducing potential harms associated with interventional research when equipoise is unclear and capturing diverse patient populations (Gershon et al, 2021). With the further cognition of the disease and the development of research, the proportion of interventional trials increased gradually. However, according to Table 4, the completion rate of observational trials and interventional trials in COVID-19 was 29.04%, and 25.84% until October 4, 2021, respectively. In contrast to interventional trials, observational studies usually started earlier during the outbreak. Also, as the epidemic approached its waning in China, patients eligible for interventional trials became fewer and fewer, and many trials had to be stopped.
The single-arm trials constituted an important part of registered interventional clinical trials during the pandemic of COVID-19. Single-arm designs have the advantages of being easier to employ as pragmatic trials and requiring fewer patients, and all of them receive the experimental treatment (Philip et al, 2014, Ventz et al, 2019). With limitations on time and therapeutic method, an expedited result through a single-arm trial is desirable when feasible, and may enable an efficacious treatment into larger confirmatory studies before the epidemic wanes, or to allow an ineffective approach to be abandoned for trials of other possible treatments (Whitehead et al, 2016, Brueckner et al, 2018).
There were also several limitations in our study. First, ClinicalTrials.gov is not the only clinical trial registry platform. Information on other platforms, such as the Australian New Zealand Clinical Trials Registry, the United Kingdom's International Standard Randomized Controlled Trial Number(ISRCTN )registry, and World Health Organization(WHO) International Clinical Trials Registry Platform, was not included in this study. Second, although the epidemic of COVID-19 is under great control in China, it is continuing in other countries. It is, therefore, likely that COVID-19 trials will be continuously registered.
The design of clinical trials during the outbreak of newly infectious disease has its specificity. Inadequate, insufficient knowledge on the pathophysiology of COVID-19 brought difficulties to the design and conducting of trials, but the emergency of the epidemic required that trial protocols be developed and approved within a very short time frame to seek reliable clinical evidence during the outbreak and to save lives.
Different study designs have their own characteristics and advantages; it is of vital importance to make trade-offs between these methods of research. This work compares the characteristics between COVID-19 trials and other trials registered during the same period and summarizes the time distribution of COVID-19 clinical trials, trying to collect existing evidence and help to identify trial designs most likely to succeed at a particular point during an outbreak.
Conclusion
Our research suggests that the choice of study design is of vital importance in face of emerging infectious diseases. Appropriately designed trials can help to improve research efficiency and reduce the possibility of research failure. RCTs have the highest quality of evidence, but there are various barriers to their effective implementation during the epidemic. Observational and single-armed studies should be accorded importance as well. Further study can focus more on clinical trial design processes.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgments
Acknowledgments
Authors’ contributions: C.H.J., W.M. and H.T.W. were responsible for the concept and design of the study; R.C.D., P.J.H. and J.L. were responsible for data acquisition; H.T.W., Q.S.L., and H.T.L. were responsible for statistical analysis; Q.G., C.H.J. and W.M. were responsible for interpretation of results; and C.H.J. and H.T.W. analyzed the data and drafted the manuscript. All authors critically revised the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work.
Funding Sources
This work was supported by the Key R & D projects from the Department of Science and Technology of Zhejiang Province (No.2020C03126), Health Commission of Zhejiang Province (No.2017KY502), and the Administration of Traditional Chinese Medicine of Zhejiang Province (NO.2017ZZ007), the People's Republic of China.
Ethical Approval Statement
This study is based on a public database and is not suitable for the approval of the ethics committee.
References
- Bothwell LE, Podolsky SH. The Emergence of the Randomized, Controlled Trial. N Engl J Med. 2016;375:501–504. doi: 10.1056/NEJMp1604635. [DOI] [PubMed] [Google Scholar]
- Brueckner M, Titman A, Jaki T, Rojek A, Horby P. Performance of different clinical trial designs to evaluate treatments during an epidemic. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byar DP, Simon RM, Friedewald WT, Schlesselman JJ, DeMets DL, Ellenberg JH, et al. Randomized clinical trials. Perspectives on some recent ideas. N Engl J Med. 1976;295:74–80. doi: 10.1056/NEJM197607082950204. [DOI] [PubMed] [Google Scholar]
- Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307:1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- De Angelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Is this clinical trial fully registered?–A statement from the International Committee of Medical Journal Editors. N Engl J Med. 2005;352:2436–2438. doi: 10.1056/NEJMe058127. [DOI] [PubMed] [Google Scholar]
- Ellenberg SS, Keusch GT, Babiker AG, Edwards KM, Lewis RJ, Lundgren JD, et al. Rigorous Clinical Trial Design in Public Health Emergencies Is Essential. Clin Infect Dis. 2018;66:1467–1469. doi: 10.1093/cid/cix1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AS, Lindenauer PK, Wilson KC, Rose L, Walkey AJ, Sadatsafavi M, et al. Informing Healthcare Decisions with Observational Research Assessing Causal Effect. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2021;203:14–23. doi: 10.1164/rccm.202010-3943ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CW, Woodford AL, Platts-Mills TF. Characteristics of COVID-19 clinical trials registered with ClinicalTrials.gov: cross-sectional analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi M, Hamblin MR, Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Yang M, Tang QL, Hu XY, Willcox ML, Liu JP. Characteristics of registered clinical trials on traditional Chinese medicine for coronavirus disease 2019 (COVID-19): A scoping review. Eur J Integr Med. 2021;41 doi: 10.1016/j.eujim.2020.101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences E, et al. In: Integrating Clinical Research into Epidemic Response: The Ebola Experience. Busta ER, Mancher M, Cuff PA, McAdam K, Keusch G, editors. National Academies Press (US); Washington (DC): 2017. Medicine, Health, Medicine D, Board on Health Sciences P, Board on Global H. [PubMed] [Google Scholar]
- Philip PA, Chansky K, LeBlanc M, Rubinstein L, Seymour L, Ivy SP, et al. Historical controls for metastatic pancreatic cancer: benchmarks for planning and analyzing single-arm phase II trials. Clin Cancer Res. 2014;20:4176–4185. doi: 10.1158/1078-0432.CCR-13-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran S, Gurjar M, Baronia AK, Lohiya A, Azim A, Poddar B, et al. Personal protective equipment during COVID-19 pandemic: a narrative review on technical aspects. Expert Rev Med Devices. 2020;17:1265–1276. doi: 10.1080/17434440.2020.1852079. [DOI] [PubMed] [Google Scholar]
- Ting Wu H SLQ, Chen Dai R, Liu S, Wu L, Mao W. Effects of air-conditioning systems in the public areas of hospitals: A scoping review. Epidemiol Infect. 2021;149:1–11. [Google Scholar]
- Ventz S, Lai A, Cloughesy TF, Wen PY, Trippa L, Alexander BM. Design and Evaluation of an External Control Arm Using Prior Clinical Trials and Real-World Data. Clin Cancer Res. 2019;25:4993–5001. doi: 10.1158/1078-0432.CCR-19-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou Q, Xu M, Kang J, Chen Y. Characteristics of Clinical Trials relating to COVID-19 registered at ClinicalTrials.gov. J Clin Pharm Ther. 2020;45:1357–1362. doi: 10.1111/jcpt.13222. [DOI] [PubMed] [Google Scholar]
- Whitehead J, Olliaro P, Lang T, Horby P. Trial design for evaluating novel treatments during an outbreak of an infectious disease. Clin Trials. 2016;13:31–38. doi: 10.1177/1740774515617740. [DOI] [PMC free article] [PubMed] [Google Scholar]