Abstract
Background
Recent studies have suggested a blunted immune response to messenger RNA vaccines in solid organ transplant (SOT) recipients. Given the paucity of data on adenovirus vector vaccines use in immunosuppressed SOT recipients, we sought to describe the safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine in a heart transplant population.
Methods
Heart transplant recipients aged 18 to 70 years scheduled to receive 2 doses of the ChAdOx1 nCoV-19 vaccine were enrolled into a prospective study involving serum analysis to define their antibody response. An antibody concentration against the spike protein receptor-binding domain of ≥0.8 U/mL was deemed a detectable antibody response.
Results
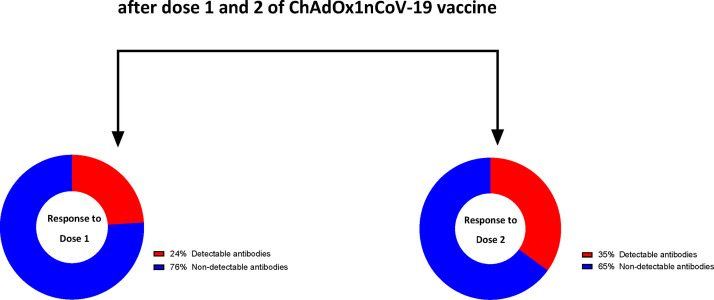
A total of 99 heart transplant recipients (mean age 51 ± 12.5 years, 28% female) were enrolled. No major adverse events were recorded after vaccination; minor symptoms included injection site pain (24%), fatigue (21%) and headache (14%). Of 7 patients with prior SARS-CoV-2 confirmed by PCR testing, all (100%) had detectable antibody responses following first and second vaccine doses. In those with no prior SARS-CoV-2 infection (n = 92), 24% (n = 22) showed an antibody response after dose 1, increasing to 34.8% (n = 32) after dose 2, p < 0.001. Chronic kidney disease (CKD) stage ≥3 (OR 4.7, 95% CI 1.5-15, p = 0.009) and mycophenolate use (OR 4.1, 95% CI 1.2-14, p = 0.02) were independently associated with a nondetectable antibody response.
Conclusions
Almost two-thirds of heart transplant recipients aged 18 to 70 years without a history of prior SARS-CoV-2 infection failed to develop a detectable antibody response following administration of the ChAdOx1 nCoV-19 vaccine. Patient phenotyping may help predict which patients are less likely to develop detectable antibody responses.
KEYWORDS: SARS-CoV-2, heart transplant, solid organ transplant, vaccination, immunosuppressed patients, ChAdOx1 nCoV-19 vaccine
Abbreviations: SOT, solid organ transplant; NIAC, National Immunisation Advisory Committee
Solid organ transplant (SOT) recipients have an excess mortality risk from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 , 2 While vaccines against SARS-CoV-2 have proved highly effective at reducing the risk of hospitalization and death from the virus in the general population, the pivotal SARS-CoV-2 vaccine trials excluded high-risk patient cohorts such as those chronically immunosuppressed after SOT.3, 4, 5
The need to understand the safety and efficacy of protection from SARS-CoV-2 offered by vaccination in this group is underpinned by the increased risk of adverse outcomes from primary viral and secondary bacterial and fungal infections when hospitalized.6 This risk is further increased by the high prevalence of cardiovascular risk factors among SOT recipients that are associated with adverse outcomes after contacting SARS-CoV-2.7 , 8 In addition, there has been concern that infection with SARS-CoV-2 infection in immunocompromised patients is associated with prolonged viral infection, viral shedding and the development of potentially more transmissible or more pathogenic SARS-CoV-2 variants.9 , 10
Preliminary data based on messenger RNA SARS-CoV-2 vaccines suggests that SOT recipients have a suboptimal response to these vaccines11, 12, 13 No published studies to date have examined the immunogenicity of the adenovirus vector vaccines including ChAdOx1 nCoV-19 (AZD1222) (Oxford-AstraZeneca) in SOT recipients.
In March 2021, the Department of Health in Ireland advised 2 doses of the ChAdOx1 nCoV-19 vaccine for high-risk cohorts including heart transplant recipients aged between 18 and 70 years of age. The aim of this study was to examine the safety and humoral response of the ChAdOx1 nCoV-19 vaccine in a well phenotyped heart transplant population.
Materials and methods
Study population
This was a prospective cohort study carried out in the National Heart and Lung Transplant Centre at the Mater University Hospital, Dublin, Ireland. All heart transplant recipients aged 18 to 70 years of age vaccinated with the ChAdOx1 nCoV-19 (AZD1222) (Oxford-AstraZeneca) vaccine in the Republic of Ireland were invited to participate. Major exclusion criteria included active SARS-CoV-2 infection or heart transplantation within the 30 days preceding the first vaccination dose round, active pregnancy, alternate vaccine use, and any medical reason precluding inclusion (as determined by a Transplant Cardiologist). All participants provided written informed consent and completed a questionnaire that assessed individual symptoms after each vaccine dose. Documentation of any previous or interim detectable SARS-CoV-2 PCR tests or possible exposure to the virus was also assessed.
Baseline demographic, clinical, and transplant-specific characteristics were compared among those who had a detectable antibody response after 2 doses of the vaccine and those who did not.
Clinical outcomes including PCR confirmed SARS-CoV-2 infection and all-cause mortality were also recorded following dose 2 administration for 90 days.
Ethical approval for this study was granted by Institutional Review Board at the Mater University Hospital (Reference 1/378/2239) and the study was conducted in compliance with the International Society for Heart and Lung Transplantation (ISHLT) Ethics statement.
Vaccination schedule and blood sampling
The National Immunisation Advisory Committee (NIAC) directs the vaccination program in Ireland. As per NIAC guidance, all transplant recipients aged 18 to 70 years of age received the ChAdOx1 nCoV-19 vaccine with a 12-week interval between the first and second dose. ChAdOx1 nCoV-19 is a vaccine delivered by a nonreplicating adenovirus encoding the SARS-CoV-2 Spike glycoprotein. Vaccination of this patient cohort began in March 2021 with the second dose administered in June 2021.
A serum sample was drawn from each participant at 2 prespecified time points: at week 12 after dose 1 (Time 1) and 4 weeks after dose 2 (Time 2).
SARS-CoV-2 antibodies
All serum samples were tested for total antibodies (IgM and IgG) against the receptor-binding domain (RBD) of the spike (S) protein (antispike antibodies) using the quantitative Elecsys anti-SARS-CoV-2 S electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics, Germany). Demonstrable titers >250 U/mL were diluted 10-fold to provide a value up to 2,500 U/ml (as is feasible according to manufacturer instructions). Our laboratory performed quality and control procedures to verify this assay in accordance with the International Organization for Standardization (ISO 15189). The in-house evaluation of assay performance demonstrated a clinical sensitivity of 97.8% and a specificity of 100%, whist the manufacturer reported a clinical sensitivity and specificity of 98.8% and 99.98% respectively.14
In addition to a screening questionnaire for prior SARS-CoV-2 each patient had their serum sample tested for antinucleocapsid antibodies using the qualitative Elecsys anti-SARS-CoV-2 ECLIA (Roche Diagnostics, Germany).15 A validated index of ≥1.0 was regarded as reactive and an indicator of prior SARS-CoV-2 infection.16 An antispike antibody titer of ≥0.8 U/mL in the absence of symptomatology or prior laboratory confirmed SARS-CoV-2 infection, was regarded as a detectable SARS-CoV-2 vaccine antibody response.
Statistical analysis
All statistical analyses were performed for the study with IBM SPSS Statistics 27. Continuous variables are presented as either mean ± standard deviation (SD) or median [Q1; Q3] depending on the distribution of the datapoints within each variable. Categorical data are presented as frequencies and percentages. Clinical and transplant-specific characteristics were compared across detectable vs nondetectable antibody response groups. A chi square test (X 2) or Fisher's exact test was performed for the analysis of 2 nominal datasets. For the comparison of nominal and continuous data an independent t-test was completed for normally distributed data whilst a Mann Whitney U test was used for nonnormally distributed data. A paired t-test was used to analyze the difference in means for consecutive continuous values within the same patient.
Univariate and multivariate logistic regression analyses were used to determine predictors of a nondetectable antibody response. Predictor variables were included in the multivariate model if the p-value was <0.1 on univariate analysis. Results are presented as odds ratios (OR) and 95% confidence intervals (CI). Model fit was evaluated by the Hosmer-Lemeshow test.
Collinearity between individual univariate parameters was investigated using Pearson's correlation coefficient. To eliminate collinearity (defined as R>0.5), the most clinically relevant parameter in this specific clinical cohort was chosen to be included in the final model. In the case of both parameters being deemed independently clinically relevant to the outcome being studied, model fit was repeated to determine the optimal model.
During the analysis process both mycophenolate mofetil and mycophenolic acid use were combined to produce a single predictor variable titled “mycophenolate-based regimen.” Patients taking mycophenolic acid 360 mg twice daily were therefore recorded as taking 500 mg twice daily and similarly 720 mg twice daily was recorded as 1 g twice daily.
Results
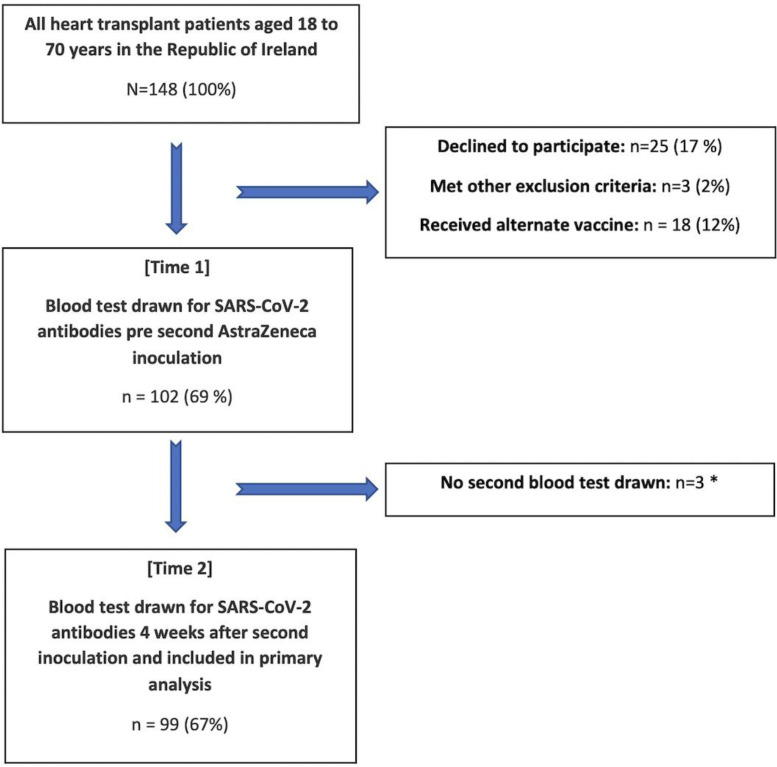
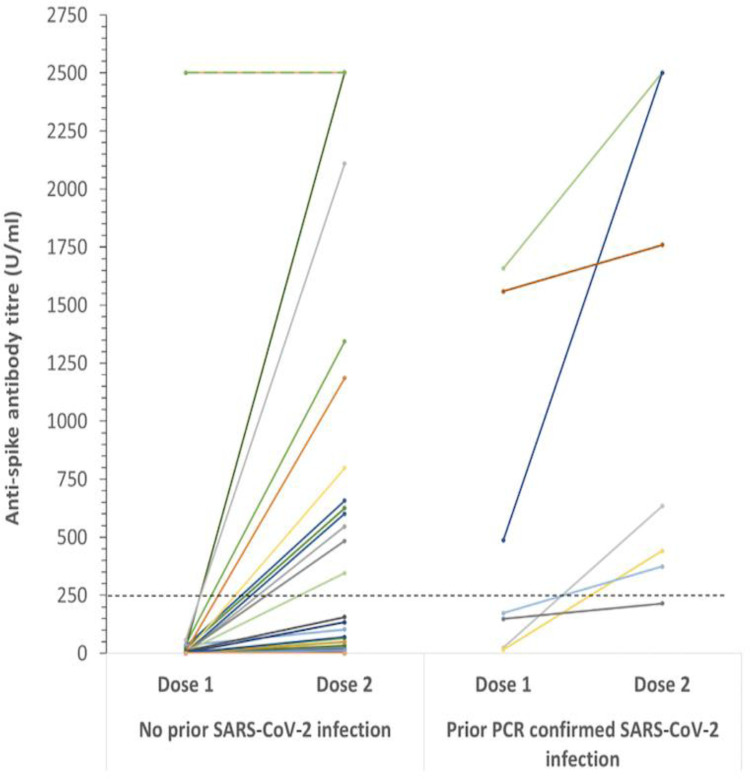
In total, 99 heart transplant recipients were enrolled in the study. Figure 1 outlines the study inclusion protocol. The overall mean age was 51 ± 12.5 years, 28% were female and the median time since transplant was 7 (3.6, 12) years. Patient demographics for the total group are detailed in Table 1 . Of these, 7 patients had prior SARS-CoV-9 confirmed by PCR testing. The median time from PCR positivity to study enrollment was 5.5 (5, 9.3) months and 3 of these patients had positive nucleocapsid antibodies at Time 1. Four weeks later, 2 patients had positive nucleocapsid antibodies. All 7 patients (100%) had detectable antibody levels against the spike protein at Time 1 and Time 2 (Figure 2 ). Median antispike antibody titers following 2 doses of ChAdOx1 nCoV-19 were significantly higher in those with prior SARS-CoV-2 infection compared to those without a history of prior infection (634 U/mL (374, 2252) vs 0.4 U/mL (0.4, 15.7), p < 0.001).
Figure 1.
Flowchart of study population. Transplant patients aged 18 to 70 years in Ireland with study inclusion and exclusion criteria applied. *N = 2 non-covid-19 related deaths, N = 1 did not attend for second blood draw. PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Characteristics of the Total Patient Population Included in the Study
| Total N = 99 | |
|---|---|
| Age (mean ± SD) | 51 ± 12.5 |
| Female n (%) | 28 (28%) |
| Caucasian Race | 97 (98%) |
| Native heart failure etiology | |
| Ischemic, n (%) | 22 (22%) |
| NIDCM, n (%) | 66 (67%) |
| ACHD, n (%) | 7 (7%) |
| Valvular, n (%) | 4 (4%) |
| Years since heart transplantation, Median (IQR) | 7 (3.6, 12) |
| Graft function | |
| CAV/CAD, n (%) | 26 (26%) |
| EF >50%, n (%) | 91 (92%) |
| Second organ transplanted, n (%) | 3 (3%) |
| Hypertension, n (%) | 79 (80%) |
| Renal impairment | |
| CKD ≥3, n (%) | 68 (69%) |
| On dialysis, n (%) | 2 (2%) |
| Diabetes, n (%) | 21 (21%) |
| Nonskin malignancy, n (%) | 6 (6%) |
| Rejection history | |
| Acute cellular rejection ≥2R, n (%) | 51 (52%) |
| Antibody mediated rejection, n (%) | 7 (7.1%) |
| Donor specific antibodies | |
| Negative, n (%) | 70 (71%) |
| Weak, n (%) | 17 (18%) |
| Significant, n (%) | 8 (8%) |
| Panel reactive antibodies at time of transplant (%) | 26 (3, 54) |
| Median (IQR) |
Abbreviations: ACHD, adult congenital heart disease; CAD, coronary artery disease; CAV, cardiac allograft vasculopathy; CKD, Chronic kidney disease; EF, ejection fraction; NIDCM, nonischemic dilated cardiomyopathy; SD, standard deviation.
Figure 2.
Antispike antibody titer levels following the first and second vaccine doses in patients without (left panel) and with (right panel) a prior history of SARS-CoV-2 infection. The wide variability of detectable results is illustrated. Of note, single points may represent multiple patients with the same titre level. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In order to determine the specific effect of the vaccine on antibody response against the spike protein of SARS-CoV-2, subsequent analyses were confined to those without prior documented SARS-CoV-2 infection, supported by a screening questionnaire and negative antinucleocapsid response (N = 92). Within this group, 24% of patients (n = 22) had a detectable antibody response after dose 1, increasing significantly to 34.8% (n = 32) following dose 2, (p < 0.001), Figure 3 . Clinical characteristics (Table 2 ) and immunosuppressant regimes (Table 3 ) were compared based on the presence or absence of detectable antibodies after 2 vaccine doses. Regarding clinical characteristics, the presence of chronic kidney disease (CKD) stage ≥3 was more prevalent in patients with a nondetectable antibody response (p = 0.001). Similarly, when considering immunosuppressive strategies, nondetectable antibody responses were associated with mycophenolate prescription and higher dose usage of the drug (p = 0.009 and p = 0.03 respectively). A similar finding was detected in those taking tacrolimus vs cyclosporine as the prescribed calcineurin-inhibitor (p = 0.02) and also with the use of maintenance prednisolone (p = 0.03).
Figure 3.
Antibody response after vaccination in patients with no history of SARS-CoV-2.
Table 2.
Characteristics of Patients With a Detectable and Nondetectable Antibody Response (Prior SARS-CoV-2 Excluded)
| Total n = 92 | Nondetectable antibody response n = 60 | Detectable antibody response n = 32 | p-value | |
|---|---|---|---|---|
| Age (mean ± SD) | 50.6 ± 12.5 | 50.9 ± 12.7 | 49.4 ± 11.6 | 0.6 |
| Female, n (%) | 27 (29.3%) | 19 (32%) | 8 (25%) | 0.5 |
| Caucasian Race | 90 (97.8%) | 59 (98%) | 31 (97%) | 1 |
| Native heart failure etiology | ||||
| Ischemic, n (%) | 21 (22.8%) | 18 (30%) | 3 (9%) | |
| NIDCM, n (%) | 60 (65.2%) | 35 (58%) | 25 (78%) | |
| ACHD, n (%) | 7 (7.6%) | 4 (7%) | 3 (9%) | |
| Valvular, n (%) | 4 (4.4%) | 3 (5%) | 1 (3%) | |
| Years since heart transplantation, Median (IQR) | 7.1 (3.4, 13.5) | 4.5 (3, 8.6) | 11.2 (7.5,20) | <0.001 |
| Graft function | ||||
| CAV/CAD, n (%) | 22 (23.9%) | 9 (15%) | 13 (41%) | 0.006 |
| EF >50%, n (%) | 84 (91.3%) | 56 (93%) | 28 (88%) | 1 |
| Second organ transplanted, n (%) | 3 (3.3%) | 3 (5%) | 0 | 0.5 |
| Hypertension, n (%) | 74 (80.4%) | 45 (75%) | 29 (91%) | 0.07 |
| Renal impairment | ||||
| CKD ≥3, n (%) | 61 (66%) | 47 (78%) | 14 (44%) | 0.001 |
| On dialysis, n (%) | 1 (1.1%) | 1 (2%) | 0 | 1 |
| Diabetes, n (%) | 18 (19.6%) | 10 (17%) | 8 (25%) | 0.34 |
| Non skin malignancy, n (%) | 5 (5.4%) | 4 (7%) | 1 (3%) | 0.7 |
| Rejection history | ||||
| Acute cellular rejection ≥2R, n (%) | 46 (50%) | 33 (55%) | 13 (41%) | 0.2 |
| Antibody mediated rejection, n (%) | 6 (6.5%) | 6 (10%) | 0 | 0.09 |
| Donor specific antibodies | ||||
| Negative, n (%) | 65 (70.7%) | 43 (72%) | 22 (69%) | |
| Weak, n (%) | 15 (16.3%) | 11 (18%) | 4 (13%) | |
| Significant, n (%) | 8 (8.7%) | 4 (7%) | 4 (13%) | 0.5 |
| Panel reactive antibodies at time of transplant (%), Median (IQR) | 27 (5, 56) | 21 (1, 53) | 35 (7,75) | 0.15 |
Abbreviations: ACHD, adult congenital heart disease; CAD, coronary artery disease; CAV, Cardiac allograft vasculopathy; CKD, chronic kidney disease; EF, ejection fraction; NIDCM, Nonischemic Dilated Cardiomyopathy; PCI, percutaneous coronary intervention; SD, Standard deviation.
Table 3.
Immunosuppressant Regime of Patients With a Detectable and Nondetectable Antibody Response (Prior SARS-CoV-2 Excluded)
| Total n = 92 | Nondetectable antibody response n = 60 | Detectable antibody response n = 32 | p-value | |
|---|---|---|---|---|
| Number of immunosuppressants | 2.6 ± 0.5 | 2.7 ± 0.5 | 2.4 ± 0.5 | 0.06 |
| Antimetabolite Use, n (%) | 79 (85.9%) | 55 (92%) | 24 (75%) | 0.06 |
| Mycophenolate-based regimen, n (%) | 67 (72.8%) | 49 (82%) | 18 (56%) | 0.009 |
| Mycophenolate-based regimen Dose (BD) | 799 ± 276 mg | 842 ± 264 mg | 680 ± 282 mg | 0.03 |
| Azathioprine, n (%) | 12 (13%) | 6 (10%) | 6 (19%) | 0.3 |
| Azathioprine dose (OD) | 79 ± 45 mg | 96 ± 53 mg | 63 ± 31 mg | 0.2 |
| Tacrolimus, n (%) | 80 (87%) | 56 (93%) | 24 (75%) | 0.02 |
| Tacrolimus level | 8.4 ± 2 | 8.5 ± 2.1 | 8.4 ± 2 | 0.9 |
| Cyclosporin, n (%) | 12 (13%) | 4 (7%) | 8 (25%) | 0.02 |
| Cyclosporin level | 115 ± 36 | 125 ± 45 | 110 ± 32 | 0.7 |
| Prednisolone, n (%) | 57 (62%) | 42 (70%) | 15 (47%) | 0.03 |
| Prednisolone dose | 6 ± 2.2 mg | 6.3 ± 2.3 mg | 5.5 ± 1.5 mg | 0.15 |
| Sirolimus, n (%) | 9 (9.8%) | 2 (3%) | 7 (22%) | 0.009 |
| Sirolimus level | 4 ± 2 | 4 ± 0.1 | 3.9 ± 2 | 0.9 |
Abbreviations: BD, twice daily; OD, once daily.
Values are expressed as mean ± standard deviation unless specified.
Clinical characteristics associated with a detectable antibody response included increased years since transplant (p ≤ 0.001), the presence of coronary allograft vasculopathy (CAV) (p = 0.006) and sirolimus use (p = 0.009).
The associates of a nondetectable antibody response were examined and illustrated in Table 4 . Significant correlations were found between the presence of CAV and sirolimus use (R = 0.54, p < 0.001) and cyclosporin vs tacrolimus use (R = -1.0, p < 0.001). Given that sirolimus is predominantly prescribed in the presence of a diagnosis of CAV, it was excluded for the final model. Cyclosporin was excluded and tacrolimus chosen for the final model in its place given that all patients are treated with 1 of these 2 agents, and tacrolimus was the most frequently prescribed calcineurin inhibitor (87%) in this cohort. Although the presence of CAV and the variable “years posttransplant” were found to be significantly correlated with each other (R = 0.6, p < 0.001), given the clinical relevance of each parameters, both included in the final multivariate model (model fit X2 =86.6, p = 0.4). In this final model, the use of a mycophenolate-based regimen (OR 4.1, 95% CI 1.2-14, p = 0.02) and presence of CKD stage ≥3 (OR 4.7, 95% CI 1.5-15, p = 0.009) were found to be independently associated with a nondetectable antibody response.
Table 4.
Logistic Regression Analysis of Associates of a Nondetectable Antibody Response After 2 Doses of the ChAdOx1 nCoV-19 Vaccine
| Univariate analysis OR, 95% CI | p-value | Multivariate analysis OR, 95% CI | p-value | |
|---|---|---|---|---|
| Increasing years Posttransplant | 0.87 (0.8-0.94) | <0.001 | 0.9 (0.84-1.0) | 0.1 |
| CKD ≥Stage 3 | 4.4 (1.7-11.2) | 0.002 | 4.7 (1.5-15) | 0.009 |
| CAV | 0.2 (0.09-0.7) | 0.008 | 0.9 (0.2-4.4) | 0.9 |
| Hypertension | 0.31 (0.08-1.2) | 0.08 | 0.8 (0.2-3.6) | 0.8 |
| Mycophenolate-based regime | 3.5 (1.3-9.0) | 0.01 | 4.1 (1.2-14) | 0.02 |
| Tacrolimus | 4.7 (1.3-17) | 0.02 | 3.5 (0.7-17.2) | 0.13 |
| Prednisolone | 2.6 (1.1-6.4) | 0.03 | 1.1 (0.4-3.5) | 0.8 |
Abbreviations: CAV, cardiac allograft vasculopathy; CKD, chronic kidney disease; CI, confidence interval; EF, ejection fraction; NIDCM, nonischemic dilated cardiomyopathy; OR, odds ratio; SD, standard deviation.
Hosmer-Lemeshow X2 statistic 86.6, p = 0.4. Bold values signifies p < .05.
In total, 38% of the patient cohort without prior documented SARS-CoV-2 infection reported symptoms following the second vaccine dose. The most common symptoms included localized pain at injection site (24%), fatigue (21%) and headache (14%). No patient required hospitalization for a suspected vaccine-associated syndrome.
Regarding follow-up clinical outcomes, 1 patient included in the study developed PCR confirmed SARS-CoV-2 infection 39 days after completing the 2 vaccine doses. This patient did not have a detectable antibody response.
Discussion
This study provides one of the first insights into the humoral response of a replication-deficient adenovirus vector vaccination (ChAdOx1 nCoV-19) in heart transplant recipients. While an acceptable safety profile was demonstrated, a majority of the cohort – 65% - failed to mount a detectable antibody response following the completion of the 2-dose protocol. CKD stage ≥3 and mycophenolate use were both found to be independently associated with a nondetectable antibody response.
In the immunocompetent population, in addition to proven efficacy against clinical outcomes, vaccination with ChAdOx1 nCoV-19 has been shown to promote a detectable antibody response (threshold, >0.8 U/mL) in >99% of vaccine recipients, with the majority reaching an antibody level of >250 U/mL after the second dose.3 , 17 At the time of writing, data on clinical efficacy of this SARS-CoV-2 vaccine subtype in immunosuppressed SOT recipients is yet to emerge. This study, while not designed to assess vaccine efficacy, aimed to investigate the humoral immunological response of the ChAdOx1 nCoV-19 in this population for the first time. Studies in similar populations that utilized mRNA-based vaccine technology have been recently published.11, 12, 13 , 18 , 19 The largest of these found a detectable antispike protein antibody in 54% of a mixed (N = 658) SOT recipient group a median of 29 days following the second dose of either of the 2 available mRNA vaccines.12 Another study in a heart transplant specific population demonstrated detectable antispike antibodies in 18% of recipients, a mean of 21 days after dose 2 of the Pfizer BioNTech vaccine. 19 Although variations between the antibody assays limits direct comparison between studies, our finding of approximately 35% antispike antibody seropositivity (using the Elecsys immunoassay, Roche Diagnostics, Germany) after the second dose of the ChAdOx1 nCoV-19 vaccine appears broadly similar to these and other mRNA-based vaccines studies, overall reporting seropositivity rates of 18% to 58.8%.11, 12, 13 , 18 , 19 In contrast, all patients (n = 7) with prior SARS-CoV-2 infection had detectable antibody responses following vaccination. The absence of detectable nucleocapsid antibodies in 5 of these patients at the time of their second blood test suggests a failure to develop antibodies to SARS-CoV-2 infection or possible waning immunity to the virus over time. Interestingly, not only did all patients with prior SARS-CoV-2 have detectable antispike antibodies after both dose 1 and dose 2, but their antispike antibody titers were significantly higher than those without prior history of infection. However, larger studies are needed in this population to determine if vaccination has a differential impact in transplant recipients with a prior history of SARS-CoV-2 infection.
The ability to review (humoral) immunological responses in the SOT recipient population to >1 type of vaccine modality underscores the likelihood that the suboptimal antibody responses seen across the different vaccine subtypes reflect the innate characteristics of the underlying population, rather than intrinsic vaccine-associated properties. Antimetabolite therapy such as mycophenolate mofetil has been associated with a significant blunted antibody response in SOT recipients across many of the recent mRNA-based vaccine studies.12 , 13 , 18 , 19 In the current study mycophenolate use was also shown to be significantly associated with a nondetectable antibody response in the multivariate model. In contrast to mycophenolate, increasing time interval since transplantation, and the presence of CAV were significantly associated with a detectable antibody response on univariate analysis. It is most likely that CAV is not in itself a primary risk factor for modified vaccine immunogenicity but rather it is indirectly reflecting a modified immunosuppression substrate. As per local practice at our center, sirolimus is combined with lower dose tacrolimus in treating selected patients with CAV, replacing a mycophenolate-based immunosuppressant which may underlie the association between the presence of CAV and a detectable antibody response. Furthermore, using Pearson correlation, years posttransplant and CAV were positively correlated. This is likely related to the increased frequency of CAV with further time out posttransplant, which is itself frequently associated with a greater likelihood of a less intensive immunosuppressant regimen. Notably, the presence of CKD stage ≥3 was also found to be independently associated with a nondetectable antibody response on multivariate analysis. This highlights the usefulness of detailed clinical phenotyping in predicting which SOT recipients may be more or less likely to develop nondetectable antibody responses. All factors combined however underscore the need to consider immunogenicity in immunosuppressed populations as a multifactorial issue rather than the use of single predictors in isolation.
Interestingly, all patients with prior confirmed SARS-CoV-2 demonstrated detectable antispike antibody responses after dose 2 of the vaccine in the present study. A recent study by Havlin et al supports this immunological finding, in which antibody responses in lung transplant recipients with prior SARS CoV 2 infection were compared to those following the mRNA vaccine (Pfizer BioNTech) without a history of SARS-CoV-2 infection.20 While antispike antibodies were detected in 85% of the patients (n = 33 at 90 days) following prior SARS-CoV-2 diagnosis, no patients (n = 21 at 4-6 weeks following dose 2) in the vaccinated group showed a detectable antispike antibody response. Despite similar maintenance immunosuppressive regimens overall, mycophenolate had been temporarily discontinued in 76% of the prior infection group at time of detection of infection, while the dosage in vaccinated patients was unchanged.20 Notwithstanding the fact that observational data suggests that immunosuppressive therapies appear to alter the antibody response to SARS-CoV-2 vaccination, the authors support current recommendations that maintenance immunosuppressive regimes should not routinely be altered for the purpose of promoting an antibody response from the vaccine, given the competing concerns regarding increased risk of organ rejection and/or predisposition to development of donor specific antibodies.21 , 22
Despite studies to date reporting reduced SARS CoV-2 vaccine efficacy at a humoral level, a strong argument for vaccination of transplant recipients remains in this vulnerable patient cohort. Recent real-world data from the National Health Service in the United Kingdom reported clinical efficacy in transplant recipients between December 2020 and June 2021.23 Out of approximately 6,700 unvaccinated transplant recipients, 7% (n = 466) contacted SARS-CoV-2. Ultimately, 40% (n = 189) of this group died within 28 days. This compared to approximately 39,000 fully vaccinated transplant recipients with a subsequent SARS-CoV-2 incidence of less than 1% (n = 76) and a notable lower mortality at 28 days; 8% (n = 6).23 A study of 2,151 SOT recipients in California also found that those fully vaccinated (70% mRNA-1273 vaccine) had a 80% reduction in the incidence of SARS-CoV-2 compared to those partially or unvaccinated.24
Although a SARS CoV-2 “booster” vaccine or third dose is increasingly being considered and/or sanctioned by National health bodies, the optimal timing and type of the booster dose has not been established. A third dose of the Pfizer–BioNTech vaccine was examined in a French cohort of SOT recipients in which 40% had anti-SARS-CoV-2 antibodies after 2 doses.25 The additional inoculation given at a mean of 61 days after the second vaccine resulted in 44% (26/59) of the seronegative patients having detectable antibodies 4 weeks later and a significant increase in antibody concentration for those already serodetectable.25 A smaller study on 30 SOT recipients found an additional 33% seroconversion with a third vaccine dose (50% Janssen [Ad26.COV2.S)], 50% mRNA vaccine) at a median of 67 days (IQR, 54 to 81 days) and a similar increase in the antibody level for those already serodetectable.26 The mixing of vaccines as used in the later study, known as heterologous prime–boost regimens, appears efficacious, safe and offers patients and care providers more flexibility.27 A Pfizer-BioNTech second dose in ChAdOx1-S-primed healthy participants was recently shown to be particularly effective in promoting a robust immune response.28 These studies have prompted the ISHLT and American Society of Transplantation to recommend a third dose of mRNA vaccine to SOT recipients previously vaccinated with a 2-dose mRNA regime.21 No recommendations have been given to date surrounding booster regimens in those fully vaccinated with replication-deficient adenovirus vector vaccines.
Limitations
The assessment of humoral response in isolation has inherent limitations as vaccine induced immunity against viral infections is also mediated by cell mediated immunity. In the study by Havlin et al, 4/12 of the vaccinated lung transplant recipients with a nondetectable antibody response demonstrated a T-cell response to the RBD-antigen.20 The present study did not include investigation of the cellular immune response. Parallel assessment of T-cell response alongside humoral responses in future studies with larger SOT populations will be important to fully elucidate the immunogenicity of vaccination regimens against SARS-CoV-2 in immunosuppressed individuals. The exact protective threshold of SARS-CoV-2 IgG antibodies after infection or vaccination is not yet known. Although the Elecsys anti-SARS-CoV-2 assay can detect antibodies against SARS-CoV-2 triggered by vaccination, and our in-house evaluation of assay performance demonstrated excellent sensitivity and specificity in line with manufacturer reports, the assay has not yet been fully validated for assessing antibody response to vaccination. Whilst the presence of antispike antibodies after SARS-CoV-2 infection has been associated with a substantially reduced risk of SARS-CoV-2 reinfection in the ensuing 6 month29 the current Centers for Disease Control (CDC) guidelines do not recommend antibody testing to assess for immunity to SARS-CoV-2 infection following COVID-19 vaccination as some assays do not detect antibodies induced by the vaccine.30
In this study we employed a testing strategy which targeted both the nucleocapsid and spike proteins to distinguish a specific vaccine induced immune response and therefore establish whether an individual has had an antibody response to the vaccine. The number of patients with prior SARS-CoV-2 may have been underestimated despite a screening questionnaire and testing for nucleocapsid antibodies due to potential asymptomatic infections and waning antibody levels. Although this approach is likely not 100% sensitive for prior SARS-CoV-2, it was felt that this 2-step approach was sufficient to identify the majority of those with prior infection. Finally, we did not assess the clinical efficacy of the vaccine in this study.
Conclusions
While globally scaling SARS-CoV-2 vaccination remains a challenge, tailoring vaccination protocols to niche patient cohorts such as heart transplant recipients must remain a key priority given the disproportionate morbidity and mortality in this cohort with SARS-CoV-2 infection. Although the safety profile was reassuring, our findings of a nondetectable antibody response to the ChAdOx1 nCoV-19 vaccine in almost two-thirds of heart transplant recipients highlights the continued need for adherence to recommended nonpharmacological public health measures to prevent infection and supports further informed discussion around consideration of additional booster vaccine administration. Clinical phenotyping of this cohort may help predict which patients are less likely to develop detectable humoral immune responses.
Authors’ contributions
Richard Tanner: Developed concept for the study, consented patients, collected blood samples, performed literature review and lead author on writing the final manuscript. Neasa Starr: Collected blood samples, performed statistical analysis on blood sample results and contributed to writing the manuscript. Grace Chan: Contributed to the literature review and advised on assay use, also had role in writing the results and editing of the final manuscript before submission. Emma Heffernan: Analyzed all blood samples and provided guidance on interpretation of results, reviewed and edited manuscript before submission. Eimear Dempsey: Coordinated the scheduling of blood sampling and contributed to blood sampling, reviewed and edited manuscript before finalization. Ellen Newman: Primary role in consenting patients and collection of blood samples, reviewed and provided edits for paper before submission. James O'Neill: Analysis and interpretation of study results, reviewed and edited the final manuscript. Breda Lynch: Contributed to analysis and interpretation of results, provided edits for the main manuscript and reviewed it before submission. Margaret Hannan: Senior advisor for the study on assessment of antibody response after vaccination, contributed to planning of study protocol and editing of final manuscript. Emer Joyce: Senior author and provided guidance on all aspects of the study, developed concept for the study, contributed to planning of study protocol, consented patients, drafting and editing of final manuscript.
Disclosure statement
The authors have no conflicts of interest to declare.
This work was supported by the Mater Foundation Advanced Heart Failure Research Fund.
Acknowledgments
The authors thank all the heart transplant recipients who volunteered to participate in this study.
References
- 1.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with Coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottio T, Bagozzi L, Fiocco A, et al. COVID-19 in heart transplant recipients: a multicenter analysis of the Northern Italian Outbreak. JACC Heart Fail. 2021;9:52–61. doi: 10.1016/j.jchf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genuardi MV, Moss N, Najjar SS, et al. Coronavirus disease 2019 in heart transplant recipients: risk factors, immunosuppression, and outcomes. J Heart Lung Transplant. 2021;40:926–935. doi: 10.1016/j.healun.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collard D, Nurmohamed NS, Kaiser Y, et al. Cardiovascular risk factors and COVID-19 outcomes in hospitalised patients: a prospective cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-045482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with Immunosuppression. N Engl J Med. 2021;385:562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of Viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden IK, Bistrup C, Nilsson AC, et al. Immunogenicity of SARS-CoV-2 mRNA vaccine in solid organ transplant recipients. J Intern Med. 2021;290:1264–1267. doi: 10.1111/joim.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients - a prospective cohort study. Eur J Heart Fail. 2021;23:1555–1559. doi: 10.1002/ejhf.2199. [DOI] [PubMed] [Google Scholar]
- 14.Higgins V, Fabros A, Kulasingam V. Quantitative Measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59:e03149–20. doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heffernan E, Hannan MM, Fitzgibbon M. The role of SARS-Cov-2 antibody testing; current and future applications. J Infect Dis Ther. 2021;9:466. [Google Scholar]
- 16.Heffernan E, Kennedy L, Hannan MM, et al. Performance characteristics of five SARS-CoV-2 serological assays: clinical utility in health-care workers. Ann Clin Biochem. 2021;58:496–504. doi: 10.1177/00045632211012728. [DOI] [PubMed] [Google Scholar]
- 17.Shrotri M, Fragaszy E, Geismar C, et al. Spike-antibody responses following first and second doses of ChAdOx1 and BNT162b2 vaccines by age, gender, and clinical factors - a prospective community cohort study (virus watch) medRxiv. 2021 2021.2005.2012.21257102. [Google Scholar]
- 18.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ISHLT. Joint statement about vaccine efficacy in organ transplant recipients. Published 2021. https://ishlt.org/ishlt/media/documents/ISHLT-AST_SARS-CoV-2-Vaccination_8-13-21.pdf. Accessed August 29, 2021.
- 22.Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant. 2021;40:763–766. doi: 10.1016/j.healun.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHS. Latest NHS advice on COVID-19 vaccine for patients and recipients. Published 2021. https://www.organdonation.nhs.uk/get-involved/news/latest-nhs-advice-on-covid-19-vaccine-for-patients-and-recipients/. Accessed August 26, 2021.
- 24.Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13705. doi: 10.1111/tid.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. Overview of testing for SARS-CoV-2 (COVID-19). Published 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html#VaccinationSARSTesting. Accessed August 9, 2021.