Abstract
RNA-based therapy is a promising and potential strategy for disease treatment by introducing exogenous nucleic acids such as messenger RNA (mRNA), small interfering RNA (siRNA), microRNA (miRNA) or antisense oligonucleotides (ASO) to modulate gene expression in specific cells. It is exciting that mRNA encoding the spike protein of COVID-19 (coronavirus disease 2019) delivered by lipid nanoparticles (LNPs) exhibits the efficient protection of lungs infection against the virus. In this review, we introduce the biological barriers to RNA delivery in vivo and discuss recent advances in non-viral delivery systems, such as lipid-based nanoparticles, polymeric nanoparticles, N-acetylgalactosamine (GalNAc)-siRNA conjugate, and biomimetic nanovectors, which can protect RNAs against degradation by ribonucleases, accumulate in specific tissue, facilitate cell internalization, and allow for the controlled release of the encapsulated therapeutics.
Keywords: RNA drugs, Non-viral vector, Biological barrier, Control release, Gene therapy
Graphical abstract
1. Introduction
Among human disease treatment, traditional small molecule drugs and antibody drugs generally modulate disease pathology by targeting the downstream proteins of a gene-caused disease. Unfortunately, lots of pathogenic proteins cannot be targeted by current small molecule drugs and antibody drugs. In recent two decades, gene therapy, a more accurate and efficient treatment strategy, is currently emerging in clinic [[1], [2], [3]].
In 2018, the first therapeutic small interfering RNA (siRNA) (ONPATTRO™) was approved by United States Food and Drug Administration (FDA) [4]. A rapidly expanding market is being promising, as many emerging biopharmaceutical and biotech companies are developing RNA-based therapies. Several RNA-based products have successfully been approved for use in clinic, with many more in varying stages of the drug development pipeline [[4], [5], [6], [7], [8]]. The advent of RNA drugs has brought light to those diseases that has no drug treatment, including messenger RNA (mRNA), microRNA (miRNA), siRNA, antisense oligonucleotides (ASOs), RNA aptamers and so on. The scope of RNA application includes encoding disease-related proteins, silencing protein expression of specific genes, regulating protein function, mediating transcriptional activation of genes, etc.
However, unlike many small molecule and protein drugs, RNA molecules are negatively charged and sensitive to ubiquitous RNases, and their action sites are mostly intracellular. Therefore, the main difficulty in the development and application of RNA drugs lies in the delivery technology. The development of delivery systems will solve many problems existing in RNA delivery in vivo, which is the key for improving the efficacy of RNA drugs. It is now clear that mRNA encoding the spike protein of COVID-19 (coronavirus disease 2019) delivered by lipid nanoparticles (LNPs) exhibits the efficient protection of lungs infection against the virus [9,10]. Apart from LNPs, other non-viral nanocarriers such as lipid-based nanoparticles (NPs) [11,12], polymeric NPs [13,14] N-acetylgalactosamine (GalNAc)-siRNA conjugate [15] and biomimetic nanovectors [16], could also provide the nucleic acids with protection against degradation by nucleases, facilitate their uptake by cells and allow for the controlled release of the encapsulated therapeutic [14,17]. This review mainly focuses on the research progress of non-viral vectors for RNA delivery on the stage of pre-clinic, clinical trials and market. Currently available non-viral RNA delivery vectors generally include four categories: lipid-based NPs, polymeric NPs, inorganic NPs and biomimetic NPs [[18], [19], [20]]. However, many carriers on the stage of pre-clinic and clinical trials only stay at the levels of study due to the toxicity and effectiveness in vivo.
2. RNA drugs approved on the market
Since 1998 the first ASO drug Fomivirsen was marketed for the treatment of eye diseases (later withdrawn due to reduced clinical needs) [21], RNA drugs began to appear on the stage. In 2001, RNA interference (RNAi) technology was rated as one of the top ten scientific advances by Science magazine. However, due to the difficulty of delivering intact RNAs to tissue cells of interest, naked RNA drugs did not make much progress. With the development of chemical modification and delivery systems for nucleic acid, RNA drugs had been gradually gaining momentum. Since the first siRNA drug Patisiran was approved on the market in 2018 [22], RNA drugs have finally entered a stage of rapid development. At the end of 2019, the second siRNA drug Givosiran was approved [4,22,23]. More exciting, it is now clear that mRNA encoding the spike protein of COVID-19 delivered by LNPs exhibits the efficient protection of lungs from infection by the virus [9,10,24]. The commercial potential and clinical value of RNA drugs have finally been proved after 20 years. At present, nine ASOs, four siRNAs, two mRNAs, and one aptamer drugs are on the market (Table 1 ), among which about 80% were launched after 2015, and most of the indications are genetic diseases. There are many other types of RNA drugs are in clinical trials (Table 2 ), including the relatively new miRNA mimic, antimiRNA, small activating RNAs (saRNA), etc. It is believed that more and more RNA drugs will achieve clinical translation in the next few years. Among the RNA drugs approved or clinical trial, the non-viral delivery strategies involved mainly include chemical modification of oligonucleotides, LNPs and covalent coupling of GalNAc. Also, various typical LNPs and GalNAc delivery technologies will be introduced and analyzed here.
Table 1.
Marketed RNA products.
| Type | Product | Gene Target | Indication | Approval Year | Company |
|---|---|---|---|---|---|
| ASO | Vitravene (Fomivirsen) | Cytomegalovirus gene (UL123) | Cytomegalovirus infection | 1998 | Ionis Pharmaceuticals |
| Kynamro (Mipomersen) | ApoB-100 | Hypercholesterolemia | 2013 | Ionis Pharmaceuticals | |
| Exondys 51 (Eteplirsen) | Dystrophin (exon 51) |
Duchenne muscular dystrophy | 2016 | Sarepta Therapeutics | |
| Spinraza (Nusinersen) | SMN2 | Spinal muscular atrophy | 2016 | Ionis Pharmaceuticals | |
| Tegsedi (inotersen) | TTR | TTR-mediated amyloidosis | 2018 | Ionis Pharmaceuticals | |
| Waylivra (Volanesorsen) | ApoCIII | Familial chylomicronemia syndrome | 2019 | Ionis Pharmaceuticals/Akcea | |
| Vyondy 53 (Golodirsen) | Dystrophin (exon 53) |
Duchenne muscular dystrophy | 2019 | Sarepta Therapeutics | |
| Viltepso (viltolarsen) | Dystrophin (exon 53) |
Duchenne muscular dystrophy | 2020 | Nippon Shinyaku | |
| AMONDYS 45 (casimersen) | Dystrophin (exon 45) |
Duchenne muscular dystrophy | 2021 | Sarepta Therapeutics | |
| siRNA | Onpattro (patisiran) | TTR | TTR-mediated amyloidosis | 2018 | Alnylam Pharmaceuticals |
| Givlaari (givosiran) | ALAS1 | Acute hepatic porphyrias | 2019 | Alnylam Pharmaceuticals | |
| OXLUMO (lumasiran) | Glycolate oxidase | Primary hyperoxaluria type 1 | 2020 | Alnylam Pharmaceuticals | |
| Inclisiran (Leqvio) | PCSK9 | Hypercholesterolemia | 2020 | Novartis/ Alnylam Pharmaceuticals | |
| Aptamer | Macugen (Pegaptanib) | VEGF-165 | Age-related macular degeneration and diabetic macular edema | 2004 | Eyetech Pfizer |
| mRNA | Comirnaty (tozinameran) | SARS-CoV-2 spike protein mRNA | COVID-19 | 2020 | BioNTech/Pfizer |
| mRNA-1273 | SARS-CoV-2 spike protein mRNA | COVID-19 | 2020 | Moderna/NIAID/BARDA |
Abbreviation: Apo: Apolipoprotein; SMN2: Survival of motor neuron 2, TTR: Transthyretin, ALAS1: Aminolevulinate synthase 1, PCSK9: Proprotein convertase subtilisin/kexin type 9, VEGF: Vascular endothelial growth factor, COVID-19: Coronavirus disease 2019.
Table 2.
RNA drugs in clinical trials.
| Drug | Target | Vector | Conditions | Stage | Date | NCT Number |
|---|---|---|---|---|---|---|
| siRNA | ||||||
| Mesenchymal Stromal Cells-derived Exosomes with KRAS G12D siRNA | KrasG12D | Exosome | KRAS NP_004976.2:p.G12D Metastatic Pancreatic Adenocarcinoma Pancreatic Ductal Adenocarcinoma Stage IV Pancreatic Cancer AJCC v8 |
Phase 1 | January 27, 2021-March 31, 2022 | NCT03608631 |
| BMS-986263 | HSP47 | LNP | NASH | Phase 2 | March 17, 2021-January 9, 2024 | NCT04267393 |
| NBF-006 | GSTP | LNP | NSCLC; Colorectal Cancer; | Phase 1 | March 18, 2019-March 2023 | NCT03819387 |
| siG12D-LODER | KRAS | LODER Polymer | Pancreatic Ductal Adenocarcinoma; Pancreatic Cancer |
Phase 2 | March 7, 2018-August 2023 | NCT01676259 |
| STP705 | TGF-β1 COX-2 |
Histidine-lysine copolymer PNPs | Keloid | Phase 2 | April 29, 2021-April 29, 2021 | NCT04844840 |
| ALN-AGT01 | AGT | GalNAc conjugated | Hypertension | Phase 2 | June 25, 2021-December 2024 | NCT04936035 |
| ALN-VSP02 | KSP VEGF |
SNALP | Solid Tumors | Phase 1 | July 2010–September 2012 | NCT01158079 |
| Atu027 | PKN3 | Cationic lipoplex | Carcinoma, Pancreatic Ductal | Phase 1/2 | March 2013–January 2016 | NCT01808638 |
| mRNA | ||||||
| BI 1361849 | NY-ESO-1 MAGE-C2 MAGE-C1 Survivin 5 T4 MUC1 |
Cationic protein protamine | Metastatic NSCLC; NSCLC | Phase 1/2 | December 20, 2017-December 2024 | NCT03164772 |
| BNT162b2 | Membrane-anchored prefusion-stabilized spike protein of SARS-CoV-2 | LNP | SARS-CoV-2 Infection, COVID-19 | Phase 2/3 | March 24, 2021-September 27, 2023 | NCT04816643 |
| mRNA-2416 | OX40L | LNP | Relapsed/Refractory Solid Tumor Malignancies or Lymphoma; Ovarian Cancer | Phase 1/2 | August 9, 2017-September 20, 2022 | NCT03323398 |
| BNT113 | HPV16+ | Liposome | Unresectable Head and Neck Squamous Cell Carcinoma; Metastatic Head and Neck Cancer; Recurrent Head and Neck Cancer | Phase 2 | January 7, 2021-May 2025 | NCT04534205 |
| BNT112 | RBL038 RBL039 RBL-040 RBL-041 RBL-045 |
LPX | Prostate Cancer | Phase 1/2 | December 19, 2019-July 2023 | NCT04382898 |
| pp65-shLAMP DC with GM-CSF | pp65 | DCs | Glioblastoma Multiforme; Glioblastoma; Malignant Glioma; Astrocytoma, Grade IV; GBM | Phase 2 | August 2016–June 2024 | NCT03291002 |
| CV7202 | RABV-G | LNP | Rabies | Phase 1 | October 12, 2018-January 2023 | NCT03713086 |
| SARS-CoV-2 mRNA Vaccine | RBD of the SARS-CoV-2 spike protein | LNP | COVID-19; SARS-CoV-2 | Phase 3 | May 28, 2021-May 30, 2023 | NCT04847102 |
| mRNA-4157 | 34 neoantigens | LNP | Melanoma | Phase 2 | July 18, 2019-June 30, 2024 | NCT03897881 |
| miRNA | ||||||
| Remlarsen (MRG-201) | miR-29 | cholesterol-conjugated | Keloid | Phase 2 | June 11, 2018-June 24, 2020 | NCT03601052 |
| TargomiRs | miR-16 mimic | EDVs | Malignant Pleural Mesothelioma; NSCLC | Phase 1 | September 2014–January 4, 2017 | NCT02369198 |
| MRX34 | miR-34 | LNP | Primary Liver Cancer; SCLC; Lymphoma; Melanoma; Multiple Myeloma; Renal Cell Carcinoma; NSCLC | Phase 1 | April 2013–May 2017 | NCT02862145 |
| allogenic mesenchymal stem cells derived exosome enriched by miR-124 | miR-124 | Exosome | Cerebrovascular Disorders | Phase 1/2 | April 17, 2019-December 17, 2021 | NCT03384433 |
| INT-1B3 | miR-193a-3p | LNP | Solid Tumor | Phase 1 | December 18, 2020-December 2024 | NCT04675996 |
| ASO | ||||||
| Pelacarsen (TQJ230) |
Apo(a) | GalNAc conjugated | Cardiovascular Disease and Lipoprotein(a) | Phase 3 | December 12, 2019-June 27, 2024 | NCT04023552 |
| AKCEA-APOCIII-LRx | ApoC-III | GalNAc conjugated | Familial Chylomicronemia Syndrome | Phase 3 | November 18, 2020-June 2023 | NCT04568434 |
| IONIS-FB-LRx | Factor B | GalNAc conjugated | Primary IgA Nephropathy | Phase 2 | December 4, 2019-December 2023 | NCT04014335 |
| CiVi007 | PCSK9 | Locked nucleic acid (LNA) | Hypercholesterolemia | Phase 1 | February 7, 2018-August 18, 2020 | NCT03427710 |
| BP1001-A (Liposomal Grb2 Antisense Oligonucleotide) | GRB2 | Liposome | Solid Tumor, Adult; Carcinoma, Ovarian Epithelial; Fallopian Tube Neoplasms; Endometrial Cancer; Peritoneal Cancer; Solid Tumor | Phase 1 | August 2021–October 2022 | NCT04196257 |
| TGF-β2 antisense oligonucleotide | TGF-β2 | LNA | Primary Open Angle Glaucoma | Phase 1 | April 2015–August 2017 | NCT02406833 |
| Alicaforsen | ICAM1 | 2′-H modified | Pouchitis | Phase 3 | December 3, 2015-October 29, 2018 | NCT02525523 |
| OGX-427 | HSP27 | 2′-MOE modified | Urologic Neoplasms; Metastatic Bladder Cancer; Urinary Tract Neoplasms | Phase 2 | October 2011–November 2014 | NCT01454089 |
| Miravirsen | miR-122 | LNA | Hepatitis C | Phase 2 | November 2012–April 2014 | NCT01727934 |
All the information from https://clinicaltrials.gov/.
Abbreviation: HSP47: heat shock proteins 47, NASH: Nonalcoholic Steatohepatitis, GSTP: Glutathione S-transferase Pi, NSCLC: non-small cell lung cancer, LODER: Local Drug EluteR, TGF-β: transforming growth factor-beta, COX-2: cyclooxygenase-2, PNPs: polypeptide Nanoparticles, AGT: Angiotensinogen, KSP: kinesin spindle protein, PKN3: protein kinase N3, NY-ESO-1: cancer/testis antigen 1B, MAGE-C2: melanoma-associated antigen family C2, 5 T4: oncofetal antigen, MUC1: mucin 1, OX40L: OX40 ligand, HPV: Human papillomavirus, RABV-G: rabies virus glycoprotein, SCLC: small cell lung cancer, EDVs: EnGeneIC Dream Vectors (nonliving bacterial minicells), PCSK9: Proprotein convertase subtilisin/Kexin type 9, GRB-2: growth factor receptor-bound protein-2, ICAM1: intercellular adhesion molecule 1.
2.1. LNP-based RNA drugs
LNP-based delivery platform has been regarded as one of the advanced and promising non-viral delivery systems of RNAs for treating a range of diseases up to date. LNPs are composed of ionizable or cationic lipid, phospholipid, polyethylene glycol (PEG)-lipid and cholesterol. Recently, numerous ionizable lipids have been developed as a critical component of LNPs to overcome the limitation of cationic lipids and further improve the RNA delivery efficiency. These ionizable lipids usually contain ionizable amino head groups with an acid dissociation constant (pKa) less than 7. Hence, ionizable amino heads of lipids are protonated and positively charged at pH < 6.0, which allow high encapsulation efficiencies on RNAs at acidic pH, while they are neutral at physiological condition (pH = 7.4). In this way, the resulted LNPs with neutral surface charge reduced toxicity associated with the positive charge and prolonged the circulation lifetime as compared with cationic delivery systems after systemic administration. After reaching the acidic endosomes of cells, ionizable lipids were ionized, which promoted endosomal escape and the release of encapsulated RNAs into cytoplasm, thus improved the RNA effects.
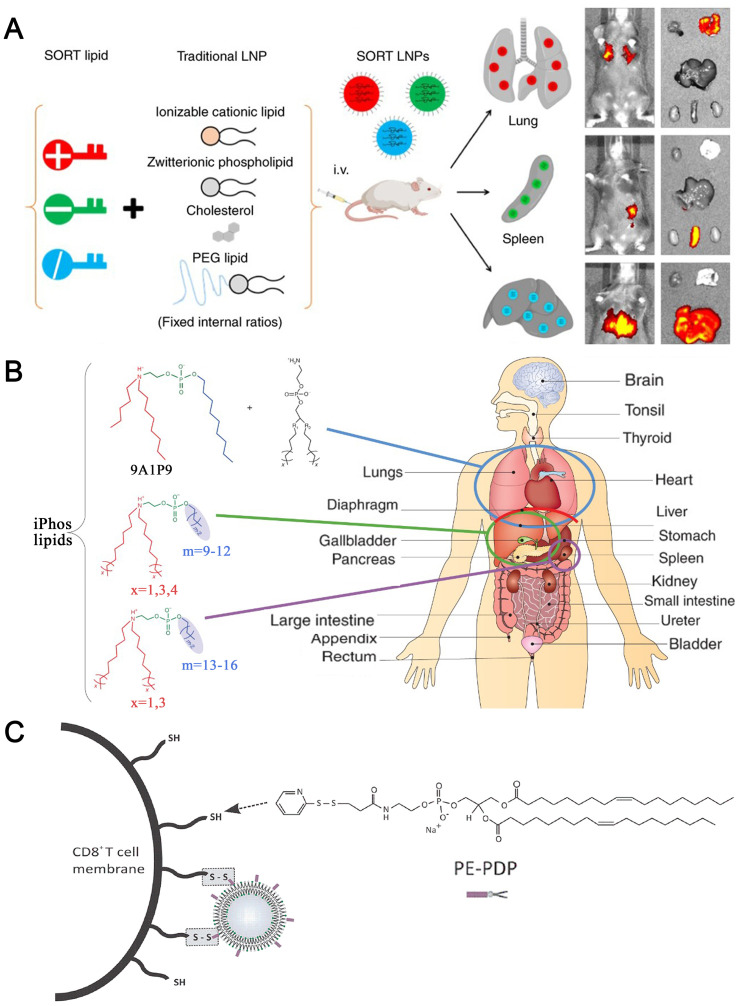
Now, three LNP-based RNA drugs have been approved on the market, including Alnylam's Patisiran (ONPATTRO™), Pfizer's BNT162b2 and Moderna's mRNA-1273. Among them, the former is siRNA drug and the latter two are mRNA vaccines for COVID-19. The specific components of these formulations are shown in Table 3 and Fig. 1 [25,26]. Patisiran, as the first siRNA drug, was approved in 2018, which is the first clinical application of LNP and the first non-viral delivery system for gene drug delivery on the market. The launch of this drug marks the arrival of the era of nucleic acid nanomedicine.
Table 3.
Prescription components of Patisiran, BNT162b2 and mRNA-1273.
| Patisiran | BNT162b2 | mRNA-1273 |
|---|---|---|
| siRNA that specifically silences hATTR mRNA | Nucleoside-modified mRNA that codes for the viral spike (S) glycoprotein of SARS-CoV-2 | Nucleoside-modified mRNA that codes for the viral spike (S) glycoprotein of SARS-CoV-2 |
| DLin-MC3-DMA | ALC-0315: (4-hydroxybutyl) azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) | SM-102: heptadecan-9-yl-8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate |
| PEG2000-C-DMG: 1,2-dimyristoyl-rac-glycero-3‑carbonylaminoethyl-ω-methoxypolyethylene glycol-2000 | ALC-0159: 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide | PEG2000-DMG: 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 |
| DSPC | DSPC | DSPC |
| Cholesterol | Cholesterol | Cholesterol |
| Monobasic potassium phosphate | Monobasic potassium phosphate | Tromethamine hydrochloride |
| Sodium chloride | Sodium chloride | Acetic acid |
| Sodium phosphate dibasic heptahydrate | Sodium phosphate dihydrate dibasic | Sodium acetate |
| Potassium chloride | Tromethamine | |
| Sucrose | Sucrose |
Fig. 1.
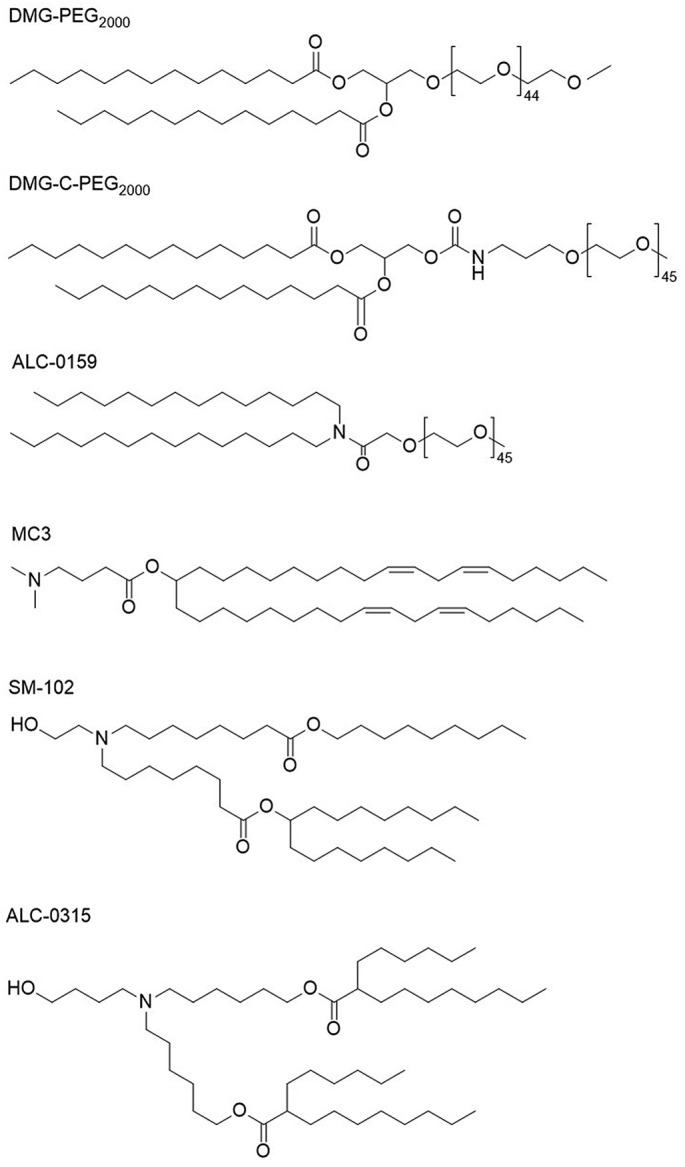
PEG-lipids and lonizable cationic lipids of Patisiran, BNT162b2 and mRNA-1273.
During development, Alnylam found that the functional activity of the LNP/RNA system is strongly correlated with pKa of ionizable lipids used. The pKa value of ionizable amino head of lipids is 6.2–6.5, which displayed an ideal balance between biocompatible neutral charge during circulation to maintain stability and enough positive charge at acidic pH to ensure high encapsulation efficiency on RNAs. Among the reported ionizable lipids, diolemethyl-4-dimethylaminobutyrate (DLin-MC3-DMA, Patent NO. US8158601B2)-based LNP is one of the most effective siRNA delivery systems. The application of DLin-MC3-DMA greatly reduces the dose of siRNA, and at the same time increases the gene silencing efficiency of siRNA in liver tissues.
In preparation of LNP, DLin-MC3-DMA is positively charged under the acidic condition (pH = 4), which can effectively encapsulate negatively charged siRNA. Subsequently, as the pH of solvent system is increased to physiological value (pH = 7.4), the LNP surface charge become near neutral. The introduction of cholesterol and 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) can further increase the stability of LNP [27]. Also, the introduction of PEGylated lipids can increase the storage stability by avoiding particle aggregation. Although the PEGylated nanoparticles can indirectly affect the interaction of LNP with cell surface, the PEG lipid (PEG-C14) containing short acyl chains used in Patisiran can gradually dissociate from the LNP [28,29] during blood circulation. After intravenous administration, Patisiran is adsorbed on endogenous Apolipoprotein E (ApoE) [29]; The removal of surface PEG layer can enhance the binding of ApoE to the surface of LNP. After accumulating in the targeted liver tissues, the uptake of LNP is mediated by ApoE receptors (such as low-density lipoprotein receptors) on the surface of hepatocytes [29]. After entering hepatocytes, the DLin-MC3-DMA is protonated at low pH value in the endosomes, and further interacts with negatively charged endogenous lipids, resulting in instability of endosomal membrane and realizing the release of loaded siRNA [29]. For Patisiran, the siRNA released to the cytoplasm can silence the expression of hereditary transthyretin amyloidosis (hATTR) mRNA by forming the RNA-induced silencing complex (RISC), reducing the production of misfolded TTR protein [30].
The primary adverse effect of Patisiran was mild-to-moderate infusion-related reactions, the incidence of which could decrease over time. In the phase 3 trial, approximately 20% of the patients (225 people in total) receiving Patisiran had mild or moderate infusion-related reactions, while for those who received placebo the rate was 10%; the overall incidence and types of adverse reactions were similar [31,32] .
2.2. GalNAc-conjugated siRNA
Among the RNA drugs currently approved on the market, three of them use GalNAc coupling technology for siRNA delivery. Givosiran (Alnylam), Lumasiran (Alnylam) and Inclisiran (Novartis) have significant advantages in the treatment of liver-related diseases. The GalNAc-conjugated RNA drugs on the market and in clinical trials are listed in Table 4 .
Table 4.
GalNAc-conjugated RNA drugs.
| On the market | ||||||
|---|---|---|---|---|---|---|
| RNA type | Product | Target | Indications | Company | Study start year | |
| siRNA | Givlaari (givosiran) | ALAS1 | Acute hepatic porphyrias | Alnylam Pharmaceuticals | 2019 | |
| siRNA | OXLUMO (lumasiran) | Glycolate oxidase | Primary hyperoxaluria type 1 | Alnylam Pharmaceuticals | 2020 | |
| siRNA | Inclisiran(Leqvio) | PCSK9 | Hypercholesterolemia | Novartis/Alnylam Pharmaceuticals | 2020 | |
| In clinical trials | ||||||
| RNA type | Drug | Target | Conditions | Phase | Identifier | Start-Complete |
| siRNA | SLN124 | TMPRSS6 | Non-transfusion-dependent Thalassemia, Low Risk Myelodysplastic Syndrome | I | NCT04176653 | August 20, 2019-October 14, 2021 |
| siRNA | Lumasiran (ALN-GO1) |
HAO1 | Primary Hyperoxaluria Type 1 | II | NCT03350451 | April 4, 2018-June 2023 |
| siRNA | ALN-PCSSC | PCSK9 | Homozygous Familial Hypercholesterolemia | II | NCT02963311 | December 13, 2016-October 8, 2018 |
| siRNA | ALNAT3SC (Fitusiran, SAR439774) | AT | Hemophilia A; Hemophilia B | I/II | NCT02554773 | September 18, 2015-August 2023 |
| siRNA | ALN-AGT01 | AGT | Hypertension | II | NCT04936035 | June 25, 2021-December 2024 |
| ASO | Pelacarsen (TQJ230) |
Apo(a) | Cardiovascular Disease and Lipoprotein(a) | III | NCT04023552 | December 12, 2019-June 27, 2024 |
| ASO | IONIS-FB-LRx | Factor B | Primary IgA Nephropathy | II | NCT03313778 | December 4, 2019-December 2023 |
| ASO | ApoC-III | Familial Chylomicronemia Syndrome | III | NCT04568434 | November 18, 2020-June 2023 | |
All the information from https://clinicaltrials.gov/.
Abbreviation: TMPRSS6: transmembrane serine protease 6, HAO1: hydroxyacid oxidase 1, AT: antithrombin.
GalNAc is a high-affinity targeting ligand of Asialoglycoprotein receptor (ASGPR) [33]. ASGPR is specifically highly expressed on the surface of hepatocytes – approximately 106 per hepatocytes [34], while the expression of other receptors on cell surface is only 104–105 or even lower. The selection of ASGPR receptor underwent the selection of sugar molecule type, number of antennae, spatial distance and other factors. The triantennary GalNAc was optimized and used as a specific ligand targeting to ASGPR with high affinity. In addition to the advantages of quantity, ASGPR is also an extremely efficient endocytic-circulating receptor with a circulation rate of about 15 min [34], while the circulation time of other cell surface recyclable receptors is usually 90 min. In summary, the two characteristics of the large number of receptors and short circulation time determine that GalNAc-siRNA conjugates can achieve efficient cell internalization.
When GalNAc binds to ASGPR, it can enter the endosome through clathrin-mediated endocytosis. After entering early endosomes, ASGPR dissociates from GalNAc-siRNA conjugate at low pH and circulates back to the surface of hepatocytes. As the early endosomes acidify and mature, they transform into late endosomes or multivesicular body (MVBs). It is estimated that only <0.01% of siRNA can escape from late endosomes or MVBs to the cytoplasm [35]. However, the high-efficiency uptake mentioned above allows nearly one million siRNA to enter the early endosomes every 15 min. Therefore, the amount of siRNA reaching the cytoplasm far exceeds the threshold of RNAi response, which can well meet the dosing needs, making short-term drug efficacy possible. As for the siRNA molecules retained in acidic compartments, it is essential for the maintenance of long-term efficacy. Studies have shown that long-term effect of GalNAc-siRNA conjugates is due to the enhanced metabolic stability of siRNA after chemical modification, which can achieve greatly improvement of siRNA survival rate in acidic compartment and form intracellular siRNA reservoirs [36]. These retained siRNA can be slowly released from acidic compartments to the cytoplasm, and then loaded into the RISC, extending the pharmacodynamic durability of GalNAc–siRNA conjugates.
Although both LNP and GalNAc have excellent accumulation and uptake in the liver, the delivery strategy based on GalNAc is more advantageous than LNP. First of all, LNP intravenous injection can cause infusion-related reactions, so it needs to be used in combination with antihistamine, acetaminophen and dexamethasone; while GalNAc-conjugated nucleic acid drugs can be administered subcutaneously and avoid the safety problems caused by the immunogenicity of lipid molecules and PEG when using LNP. In addition, GalNAc-based products are easier to scale up than LNP-based products, and they are also superior in terms of dosage and frequency of administration. Currently, a large number of RNA drugs in clinical trials are based on GalNAc technology.
3. Synthetic non-viral vectors for RNA delivery
3.1. Challenges and strategies
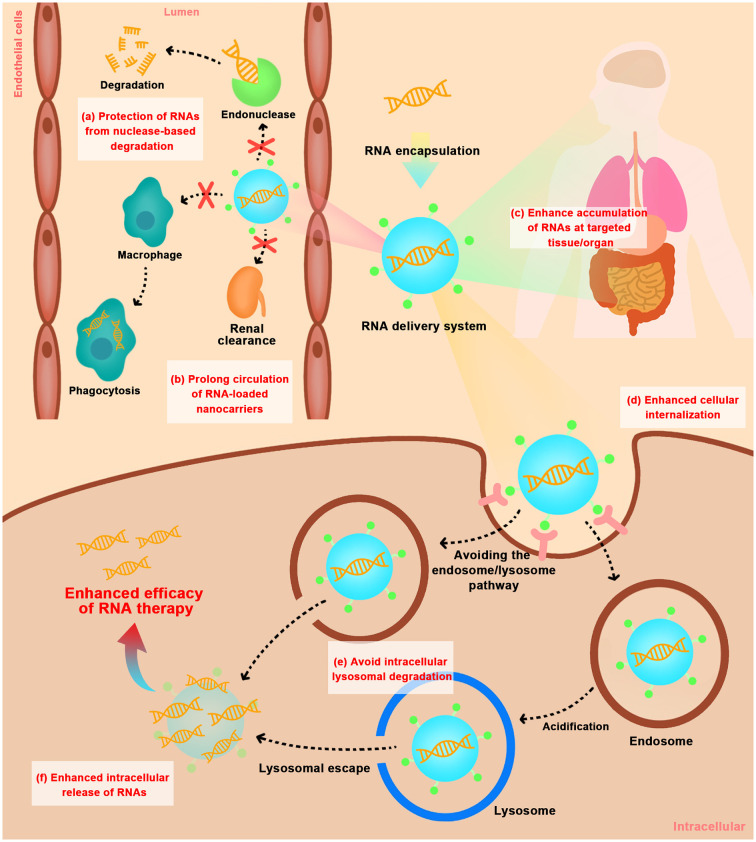
Actually, RNAs are always subjected to both systemic and cellular barriers that hinder their access to intracellular targets. Firstly, RNAs are highly susceptible to the destruction by nucleases or hydrolases in blood or body fluids, and rapid clearance by the kidney. As reported, unprotected RNAs have an extremely short metabolic or systemic half-life (e.g., less than 7 min) [37]. Secondly, RNAs with the physicochemical properties of hydrophilic, negatively charged and high molecular weight is difficult to cross cell membrane into cytoplasm. Thirdly, after cell internalization, internalized RNAs were often trafficked through early/late endosomes and acidic lysosomes microenvironment and destroyed by enzyme degradation, which limits integral RNAs to exert gene effects (Fig. 2 ). Therefore, for efficient delivery of RNAs, especially systemic delivery, one of the most attractive approaches is the development of non-viral vectors to overcome the above hurdles. The ideal delivery system for RNAs should: 1) have efficient encapsulation and protection on RNAs from nuclease-based degradation during systematic delivery; 2) prolong blood circulation time, prevent rapid clearance by kidney and phagocytosis by liver or spleen; 3) enhance targeted tissue/organ penetration and accumulation; 4) facilitate targeted cell internalization; 5) avoid lysosomal degradation during intracellular trafficking pathway; 6) enhance release of RNAs in the cytoplasm to exert gene effects.
Fig. 2.
Extracellular and intracellular barriers for in vivo delivery of RNAs using non-viral vectors. (a) protection of RNAs from nuclease-based degradation; (b) prolong circulation of RNA-loaded nanocarriers by avoiding phagocytosis by mononuclear phagocytic system and rapid kidney clearance; (c) enhance tissue/organ-selective accumulation of RNAs; (d) enhance cellular internalization; (e) avoid intracellular lysosomal degradation; (f) enhance intracellular release of RNAs.
So far, to overcome the limitations of in vivo RNA drugs application, many synthetic non-viral vectors and biomimetic vectors have been employed for RNA delivery. In the following sections, different approaches in the preclinical studies or clinical trials in terms of each significant hurdles for safe and efficient delivery of RNAs are discussed. Synthetic non-viral vectors are constructed by designing many types of natural or synthetic materials and formulations for delivering RNAs, including lipid-based NPs, polymer-based NPs, inorganic NPs, nucleic acid-based nanostructures and others.
3.2. Protection of RNAs from nuclease-based degradation
Due to the instability and negatively charged characters of RNAs, different delivery systems have been widely developed to achieve efficient encapsulation and protection from degradation by RNase. Among the NPs formulation, different structure of materials, method of preparation, and incorporation of excipients can greatly affect the protection efficiency and therapeutic outcome. The strategies for encapsulation of RNAs can be classified into several types: electrostatic adsorption to the surface of pre-formed NPs, incorporation into the core of NPs (core-shell encapsulation), electrostatic interaction-based “layer-by-layer” approach, and non-electrostatic interaction-based encapsulation. Examples of NPs and strategies for RNAs loading are briefly summarized in Table 5 .
Table 5.
Examples of NPs and methods used for RNAs loading.
| Method of Incorporation | Type of RNA | Nanocarriers components | Encapsulation efficiency | Reference |
|---|---|---|---|---|
| Electrostatic Adsorption | siRNA | Branched PEI 25 kDa | Complexed with siRNA above the N/P ratio of 2.5 | [38] |
| siRNA | Linear PEI | N/P ratio of 5 | [39] | |
| siRNA | BPAE-SS | ~100% at polymer/siRNA weight ratios higher than 10 | [40] | |
| siRNA | ABP | ∼95% condensation of siRNA at ABP/siRNA weight ratios of greater than 1.5 | [41] | |
| siRNA | PLGA-PEI | Encapsulation efficiency of 81 ± 2%, loading siRNA of 198 ± 5 μg/30 mg | [42] | |
| siRNA | CS, γ-PGA | CS/siRNA complexes at N/P ratios ranging from 50/1 to 200/1; CS/siRNA/γ-PGA complexes at N/P/C ratio ranging from 100/1/0 to 100/1/50 | [43] | |
| miRNA | PCL-PEI, PCL-PEG | Efficiently condense miRNA at an N:P ratio of 8:1 | [44] | |
| siRNA | PAMAM dendrimers G7 (Gn (n: dendrimer generation number)) | Completely entrap siRNA at N/P ratios >2.5 | [45] | |
| siRNA | Amphiphilic PAMAM dendrimers G5 bearing different alkyl chain length and dendron size | Only the combined effect of the hydrophobic alkyl chain and the cationic hydrophilic PAMAM dendron was able to complexed with siRNA completely at N/P ratios over 2.5 | [46] | |
| siRNA | PCL-g-PDMAEMA, PGA-g-mPEG (or PGA-g-PEG-folate) | Totally encapsulated even at N/P = 3:1 | [47] | |
| siRNA | PAMAM-PEG-PLL | Complexed completely with siRNA at the N/P ratio of 1 and above | [48] | |
| siRNA | Cationic lipid DC-6-14, cholesterol, dioleoylphosphatidylethanolamine, vitamin A | Entrapment efficiencies were 95.6 ± 3.0% | [49] | |
| siRNA | Cationic lipid RPR209120 (2-(3-[Bis-(3-amino-propyl)-amino]-propylamino)-N-ditetradecylcarbamoylme-thyl-acetamide), 1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine (DOPE), | 6 nmoles of RPR209120/μg siRNA | [50] | |
| siRNA | DOTAP | The DOTAP siRNA ratio was 2:1 (vol/wt) | [51,52], | |
| miRNA | mPEG-PLGA-PLL | encapsulation efficiency of 89.4% | [53] | |
| Core-shell encapsulation | siRNA | PEG-P(TMC-DTC)-PEI, cNGQ-PEG-P(TMC-DTC) | siRNA was completely loaded into the polymersomes at siRNA/polymer ratio of 80/100 (w/w) (N/P ratio of 0.45) | [54] |
| miRNA | G4 PAMAM, humanized Archaeoglobus ferritin (HumFt) | > 90% | [55] | |
| siRNA | Acrylate guanidine, N,N′-bis(acryloyl) cystamine containing disulfide bonds, polyethylene glycol with acylate and succinate functional end groups, Angiopep-2 (Ang) peptide | One nanocapsule with single siRNA inside | [56] | |
| siRNA | PCL-PEI, DOPE, cholesterol, DSPE-PEG | Encapsulation efficiency >98% at N/P = 5 | [57] | |
| siRNA | Cationic lipid-like compound (PEI-C12), PLGA, lecithin, DSPE-PEG, peptide H7K(R2)2 | Entrapment efficiency of 87.11 ± 1.79%, drug loading was 322.96 ± 6.66 pmol of siRNA per mg of H7K(R2)2-PSNPs | [58] | |
| siRNA | mPEG5K-PLA25K, BHEM-Chol | Encapsulation efficiency of siRNA could be above 90% and the siRNA loading weight ratio was up to 4.47% | [59] | |
| siRNA | Lanthanum phosphate, CS | Efficient encapsulation of siRNAs in CS/LaP/siRNA NPs and protection from enzymatic degradation in intestinal fluid up to 72 h | [60] | |
| siRNA | Bioreducible cholesterol-grafted poly(amidoamine) (rPAA-Chol polymer), DOTAP, DOPE, cholesterol, DSPE-PEG (or DSPE-PEG-T7) | – | [61] | |
| mRNA | PBAE polymer, EDOPC, DOPE, DSPE-PEG2000 | mRNA was fully encapsulated at PBAE/mRNA ratio (w/w) of 20 or beyond | [62] | |
| siRNA | PLGA, lecithin, cationic lipid G0-C14, DSPE-PEG5K | siRNA encapsulation efficiency at ∼80% and a loading of ∼640 pmol siRNA/mg PLGA | [63] | |
| mRNA | Cationic compound SW-01, ionizable lipid, DOPE, PEG-lipid | Encapsulation efficiency of ~100% remained consistent over a 6-week duration | [64] | |
| siRNA | Mesoporous silica nanoparticles (MSN), DOTAP, block copolymer 454 | siRNA loading capacities of up to 380 μg per mg MSN | [65] | |
| siRNA | PEG77-XPLG*LAGr9X–PCL17 | Complete siRNA binding at N/P ratio of 3:1 | [66] | |
| shRNA | Fe3O4 NPs, FITC-SiO2, PEI-FA | Completely complexed with shRNA at NPs/shRNA weight ratio of 15:1 | [67] | |
| Electrostatic interaction-based layer-by-layer encapsulation | siRNA | Se NPs, CS | – | [68] |
| siRNA | Au NPs, thiolated LPEI, PEGylated LPEI | Total siRNA loading at N/P 2 and above | [69] | |
| siRNA | carboxyl-modified polystyrene latex NPs (CML), poly-L-Arg | a single bilayer on the NP surface could load up to 3500 siRNAs | [70] | |
| mRNA | Dextran sulfate, poly-L-arginine, CaCO3 | loading efficiency of (39.9 ± 6.3)% | [71] | |
| shRNA | DOTAP, HA, CMO, HAase | – | [72] | |
| shRNA | Au-PEI, CS-Aco, PEI | Complete loading at (Au-PEI/CS-Aco/PEI)/shRNA mass ratio of 5/1 | [73] | |
| siRNA | PLGA, poly-L-Arg, HA-methyltetrazine conjugate, CD20 antibody | – | [74] | |
| siRNA | Au NPs, PEI, polymer SS37, polymer 447 | Encapsulation efficiency of ~94%–100%, layering efficiencies of 80 ± 3% | [75] | |
| siRNA | Au NPs, PLL | Total 4 layers of PLL and 3 layers of siRNA | [76] | |
| siRNA | Au NPs, 11-mercaptoundecanoic acid (MUA), PEI 25 kDa | Around 780 siRNA molecules per PEI/siRNA/PEI-AuNP | [77] | |
| miRNA | PLGA, PLL | Loading efficiency of 99%, such that 0.25 mg of PLGA NPs contained 10 nmoles miRNA | [78] | |
| miRNA | mesoporous titania NPs (MTNst), PLL, silica, PEG-block-poly-(l-aspartic acid) (PEG-b-PLD), paclitaxel (PTX) | miR708 was efficiently loaded at an N/P ratio of 2:1 | [79] | |
| Non-electrostatic interaction-based encapsulation | siRNA | CpG-g-PCL, siSTAT3 linker | Nearly 100% loading efficiency of siRNAs | [80] |
| siRNA | DNA tetrahedron with tails (tailed-TET) | The ratio of tailed-TET to siRNA linker at 1: 1.8 | [81] | |
| siRNA | DOX·HCl, PEG-b-PLA | Hydrophobic [siRNA&DOX] with an encapsulation efficacy of 41.16 ± 0.47% | [82] | |
| siRNA | Zinc(II)-bis(dipicolylamine) (Zn2BDPA) lipid derivatives with different fatty acids, GMO, pluronic F108 | Complexed with siRNA formed at Z/P ratio of 10 | [83] | |
| siRNA | DOPG, DOPE, calcium ions | 100% siRNA loading at Ca2+/siRNA molar charge ratio of 2.5/1 | [84] | |
| siRNA | Calcium phosphate, AHA | – | [85] | |
| miRNA | GOA prodrugs | The molar ratio of GOA/miR at 120:1 | [86] | |
| siRNA | Amphiphilic HA conjugate bearing 5β-cholanic acids and ZnII–dipicolylamine complexes (Zn–DPA) | 15 pmol siRNA was binded with 100 μg HADPA-Zn-NPs | [87] | |
| siRNA | PEG-b-poly(benzoxaborole) (PEG-PBO), calcium phosphate | The incorporation efficacy of siRNA was about 94% at the PEG-PBO to siRNA weight ratio of 20 | [88] | |
| siRNA | HA | – | [89] | |
| siRNA | Mesoporous silica NPs (MSNs), calcium ion | The siRNA loading capacity per MSN particle was estimated to be ∼1.25 pmol/μg | [90] | |
| AS1411 or single-stranded RNAs (122S) or antisense oligonucleotide (G3139) | Nucleobase-lipids DXBAs (DOTA, DNTA, DOCA or DNCA) | Unmodified and peptide-conjugated single-stranded oligonucleotides (including 122S, P122S, AS1411 and N-G3139) could be effectively encapsulated at a base ratio of 5:1 (>80%) | [91] |
Abbreviation: SS37: 1-(3-aminopropyl)-4-methylpiperazine end-modified poly(N,N′-bis(acryloyl)cystamine-co-3-amino-1-propanol), 447: 1-(3-aminopropyl)-4-methylpiperazine end-modified poly(1,4-butanediol diacrylate-co-4-amino-1-butanol), BPAE-SS: branched poly(β-amino ester)s containing disulfide linkages, PBAE: poly (β-amino ester), PEG-b-PLA: poly(ethylene glycol)-block-poly(D,l-lactide), DOPG: 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol), PEI: polyethyleneimine, mPEG: methoxy poly (ethylene glycol), PLGA: Poly (lactic-co-glycolic acid), PCL: poly(ε-caprolactone), ABP: arginine grafted bioreducible poly (disulfide amine) polymer, γ-PGA: poly(γ-glutamic acid), PAMAM: polyamidoamine, PDMAEMA: poly(2-dimethylaminoethyl methacrylate), PLL: poly-l-lysine, poly-L-Arg: poly-L-arginine, CS: chitosan, CS-Aco: chitosan-aconitic anhydride, AHA: alendronate-hyaluronan graft polymer, GOA: gemcitabine-oleic acid prodrugs, GMO: glycerol monooleate, HA: hyaluronic acid, HAase: hyaluronidase, CMO: Chitosan with an oleic acid tail.
3.2.1. Protection by electrostatic adsorption
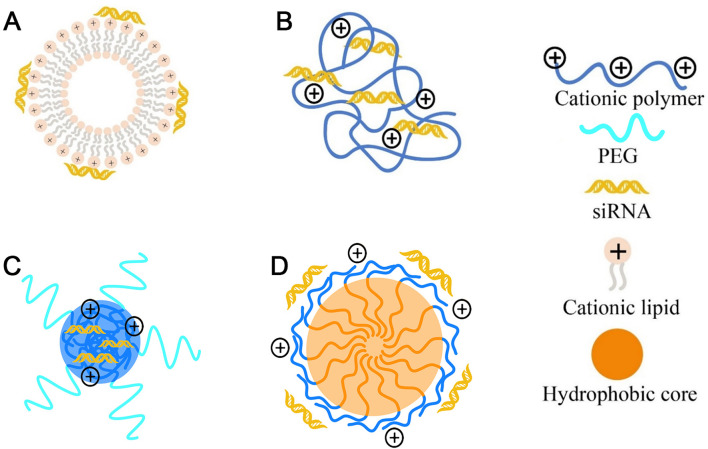
The most common strategy was simply electrostatic adsorption of RNAs onto the surface of pre-formed cationic NPs or combination RNAs with cationic components of NPs to form lipoplexes or polyplexes in a relatively facile manner. Cationic lipids like DOTAP, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), et al. are always used as the major component combining with other helper lipids (DOPC, DOPE, DSPC and cholesterol) to self-assemble into cationic liposomes with bilayer membrane via hydrophobic interactions, and thereby facilitating RNAs adsorption (named lipoplexes) ( Fig. 3A).
Fig. 3.
Protection of RNAs from nuclease degradation by electrostatic adsorption. The common cationic nanovectors for RNAs delivery including (A) cationic lipids-based lipoplexes, (B) cationic polymers-based polyplexes, (C) PEG-based cationic block copolymer formed NPs, and (D) cationic amphipathic-based block copolymer formed NPs.
Cationic polymeric materials such as PEI, PLL, poly-arginine or PAMAM dendrimers, can usually form polyplexes upon complexation with RNAs (Fig. 3B). As reported, branched PEI with molecular weight of 25 kDa could complex with siRNA above the N/P ratio of 2.5 [92]. PAMAM dendrimers composed of the triethanolamine core and branching units starting 10 successive bonds from the center amine were referred to as Gn (n: dendrimer generation number). From generation 1 to 7, the corresponding dendrimers G1–G7 have 6, 12, 24, 48, 96, 192, and 384 terminal amine groups, respectively [93]. PAMAM dendrimers G7 were able to completely entrap siRNA at N/P ratios >2.5 [45]. Besides, to further improve the biostability of polyelectrolyte complexes, A–B-type block copolymer with PEG (A block) and polycations (B block) had been widely used in the construction of RNA nanocarriers (Fig. 3C), like PLL-PEG, PEI-PEG et al. Another strategy to improve the biostability and encapsulation efficiency of polycations/RNAs complexes is to construct amphipathic-based polymer systems via introducing the hydrophobic moieties (Fig. 3D) such as PLA, PLGA or PCL and so on. For example, amphiphilic PAMAM dendrimers G5 bearing hydrophobic alkyl chain was able to form stable self-assembled complexes with siRNA and completely retard the migration of siRNA in a gel at N/P ratios over 2.5, while dendrimer G5 devoid of the alkyl chain could not completely retard siRNA migration even at the N/P ratio of 10 [46].
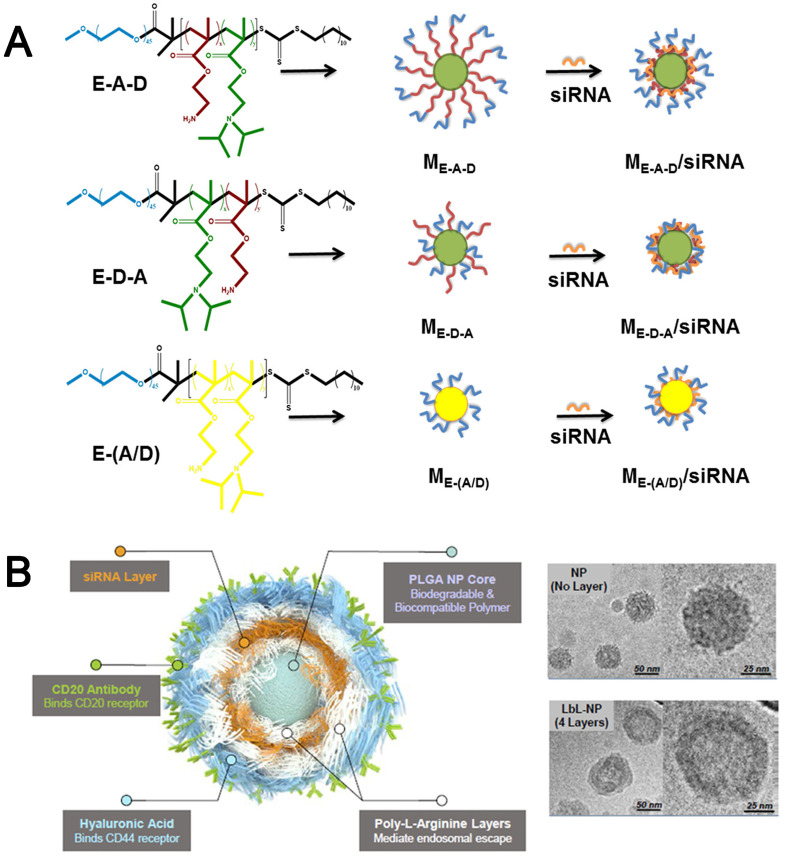
However, these polymeric vectors also exhibited limited encapsulation efficiency, positive charge-associated cytotoxicity, and inevitably interfered by negatively charged biomacromolecules under biological conditions, which were of limited interest for clinical application. To ameliorate these drawbacks, the combination of the above two strategies is proposed by integrating hydrophobic block (C) randomly into the polycationic segments to construct A–(B/C)-type copolymer or by introducing hydrophobic block (C) to construct A–B–C and A–C–B types of triblock copolymers. Thus, these obtained polymeric vectors had better self-assembling capability and protection ability with the help of hydrophobic interaction and PEGylation. For example, Dong et al. [94] focused on the effect of distribution of hydrophobic segments in the chains of amphiphilic cationic polymers on siRNA delivery. In this study, PEG–PAM–PDP (E–A–D), PEG–PDP–PAM (E–D–A) and PE–P(AM/DP) (E–(A/D)) were self-assembled into micelles for siRNA delivery (Fig. 4A), among which aminoethyl methacrylate (AM) was used as the cationic moieties to bind siRNA, and 2-(diisopropylamino)ethyl methacrylate (DP) was used as the pH-sensitive hydrophobic core moieties. The results showed that ME–A–D could completely bind siRNA at N/P = 2, but N/P = 5 was needed for ME–D–A or ME–(A/D), besides, ME–A–D/siRNA and ME–D–A/siRNA had better stability in size with time than that of ME–(A/D)/siRNA micelles. Thus, the distribution of hydrophobic moieties in the polymer chains might affect the RNA binding and the stability of the formed NPs.
Fig. 4.
(A) Molecular Structures of siRNA-loaded micelles of E–A–D, E–D–A, and E–(A/D) [95]. Reproduced with the permission from Ref. 95. Copyright © 2017 American Chemical Society. (B) Schematic illustration of a CD20/CD44 dual-targeted LbL-NP for precision siRNA therapeutics and Cryogenic TEM images of PLGA NPs (upper) and 4-layer LbL-NPs (down) [74]. Reproduced with the permission from Ref. 74. Copyright © 2019 John Wiley and Sons.
In addition to the micelles formed by triblock copolymers, polymeric hybrid micelles (PHMs) consisting of different amphiphilic diblock copolymers were also constructed for RNAs adsorption encapsulation. Simply adjusting the ratio of the two-diblock copolymers instead of altering the copolymer architecture allows easy regulations of the proportion of cationic segments or hydrophobic segments in PHMs. For instance, PHMs with different ratios of PCL-PEG and PCL-PEI were constructed for miRNA delivery. By adjusting the ratio of PCL-PEI to achieve efficient condense of miRNA at an N/P ratio of 8/1, the PHMs avoided the need for extensively and time-consuming resynthesis of copolymer materials to adjust the properties of polymeric nanocarriers [94].
However, electrostatic adsorption-based RNA nanocarriers are generally susceptible to cause leakage of RNAs induced by replacement of anionic substances in the blood or form polyelectrolyte aggregates with biomacromolecules under biological conditions, and easy to induce excessive positive charge-associated cytotoxicity and non-specific interactions with serum or plasm proteins.
3.2.2. Protection by electrostatic interaction-based layer-by-layer encapsulation
Given the disadvantage of surface adsorption-based RNA delivery systems, the alternative protection strategy of RNAs is Layer-by-Layer (LbL)-based nanocarriers, which can encapsulate RNAs into the multi-layered NPs by sequential electrostatic interaction. This strategy has facile preparation by simple adsorption and multiple alternate layers to increase RNA loading to the NPs, while also expand the variety of NP types (like polymer-lipid hybrid NPs, or inorganic-organic hybrid NPs) and functionality of different layers. The stable core of NPs and material choice for each layer are essential to ensure layer attachment for each step. For instance, this method had been successfully used to attach siRNA to PLGA NPs surrounded by layers of alternating polyelectrolyte nanolayers. poly-L-arginine (poly-L-Arg) was layered onto the negatively charged PLGA NPs substrate first to create a positively charged surface. siRNA molecules were then layered onto the poly-L-Arg layers as a negative polyelectrolyte layer, onto which an additional poly-L-Arg layer was added. Finally, HA, a negatively charged natural polysaccharide, was deposited as an outer layer. Moreover, anti-CD20 antibodies (CD20-Ab), which specifically bind with the lymphoma-specific receptor protein CD20, were chemically conjugated on the outer HA layer of the LbL-NPs (Fig. 4B). Cryo-TEM images clearly displayed the uniform nanolayer coating on the spherical PLGA NPs core. In addition, gold NPs (AuNPs) also can be used as the core for LbL coated approach by depositing the 11-mercaptoundecanoic acid (MUA) on the surface of Au NPs surface Thereafter, the NPs were consecutively added to oppositely charged PEI25 kDa and siRNA. It was calculated to be around 780 siRNA molecules per PEI/siRNA/PEI-AuNP [77]. Paula T et al. [70] developed a single NP platform through the modular and controlled layer-by-layer process to co-deliver siRNA and a chemotherapy drug. The uniformly-sized, negatively charged carboxyl-modified polystyrene latex NPs (CML) were used as a model NP core. Several polycations were screened for the construction of siRNA LbL thin films, such as polypeptides, PEI, CS and PBAE, and poly-L-Arg were identified as the promising candidates for in vivo applications due to their high siRNA loading efficiency, film stability and low cytotoxicity. The LbL film of poly-L-Arg was able to load approximately 3500 siRNA molecules per NP per layer, implying a conformal coating of siRNA on the NP with greater than 95% surface coverage. In comparison, this loading was substantially greater than the PEI/siRNA LbL NPs with approximately 500 siRNA molecules per layer. Besides, a lower N/P ratio in the poly-L-Arg/siRNA LbL NPs was found to be 1.7, which led to less toxic effects.
Taken together, such LbL method could shield RNAs from enzymes in the bloodstream and enables complexation of RNAs in a highly compact form without using a large excess of positively charged polymer. Besides, the negatively charged final outer adsorbed has the dual role of preventing opsonization of serum proteins and presenting specific ligands that can target cells of interest. To obtain stable NPs, uniformly-sized and easily modified core is essential (e.g., Au NPs, polystyrene latex NPs, PLGA NPs) onto which the polyelectrolyte can be adsorbed with high yield and to avoid interparticle bridging and flocculation. It is also important to choose the appropriate polyelectrolyte concentration and ionic strength; otherwise, random, self-assembled aggregates of polycations and nucleic acids may suffer from severe aggregation when subjected to the high ion strength of biological fluids. Hence, the complex methodology and formulation ratio of LbL approach may limit the translatability and reproducibility of this approach.
3.2.3. Protection by core-shell encapsulation
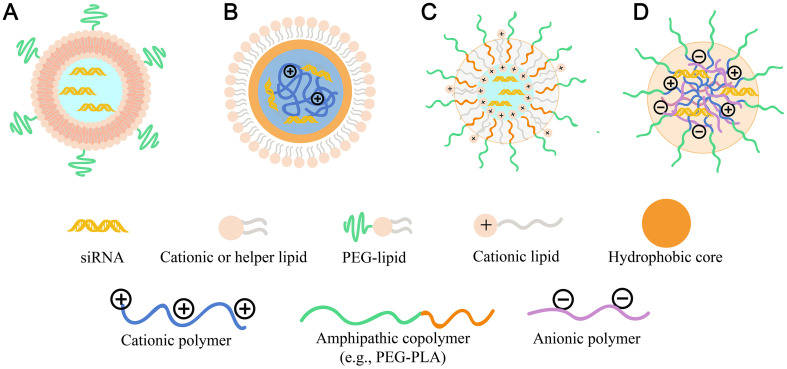
Another alternative protection strategy is using core-shell encapsulation based nanocarriers. One of the critical developments is the introduction of Stable Nucleic-Acid Lipid Particle (SNALP). In general, SNALPs consist of modified RNAs, which is entrapped inside the bilayer membrane made up of cationic–zwitterionic lipids (helper lipid) with an outermost shield of PEG (Fig. 5A). The introduction of cholesterol or DOPE increased stability of NPs and PEG lipid improved the biosafety and pharmacokinetic characters, thus achieving the enhanced protection effects on RNAs during the systematic delivery.
Fig. 5.
Protection of RNAs from nuclease degradation by electrostatic interaction-based layer-by-layer encapsulation and core-shell encapsulation. Illustration of (A) SNALP nanostructure; (B)lipid-polymer hybrid nanostructure (reverse micelle inner core); (C) polymer-lipid hybrid nanostructure (named as “CLAN”); and (D)PIC nanostructure.
To increase the RNAs entrapment inside the core of lipid NPs, siRNA was mixed with calf thymus DNA before complexing with protamine, and then coated with cationic liposomes, consisting of DOTAP, cholesterol and DSPE-PEG-anisamide to obtain Liposome-polycation-DNA (LPD) particles [96]. The addition of calf thymus DNA and protamine in the formulation reduced the particle size by 10–30% and increased delivery efficiency by 20–80%. Besides, the increase in the overall negative charges on the RNAs with the simultaneous increase in the positive charges could provide better electrostatic interaction and encapsulation efficiency. Moreover, hyaluronic acid was also used to replace the calf thymus DNA into the formulations that provided multivalent charges to enhance the particle condensation, forming negatively charged complexes. Then, cationic liposomes were coated as shell to prepare the LPH NPs, which showed approximately 90% siRNA encapsulation efficiency [97]. Besides, the core-shell encapsulation strategy also puts forward advanced requirements for the preparation process. Wang group [98] used amphiphilic block copolymer of mPEG-PLA and the amphiphilic cationic lipid BHEM-Chol to fabricate a NP delivery system with siRNA encapsulation (Fig. 5C). The siRNA was encapsulated in the core by a non-condensation process and a double-emulsion solvent evaporation technique. This process and formulation achieved high siRNA encapsulation efficiency above 90% and the siRNA loading weight ratio was up to 4.47% [98].
Additionally, given the advantage of adjustable chemical structure, strong self-assembly ability and “all-in-one” function, polyionic complex (PIC) micelle (PM) consist of two or more water-soluble copolymer with different charges is also an alternative core-shell encapsulation platform for RNA (Fig. 5D). For instance, the anionic double-hydrophilic block copolymers, composed of PEG and a degradable carboxylic acid-functionalized polyanionic PCL block (PEG43-b-PCL12(COOH)6.5) (DHBC), and the counter-polycation PLL were formed into PIC micelles for stably incorporating siRNA [99]. In this structure, the polycation block PLL complexed with siRNA to form a core, while the DHBC complexed with PLL/siRNA to form an outer shell that decreased the overall cytotoxicity by masking the excessive positive charges. In this way, RNAs could be encapsulated in the core of the PM after the spontaneous formation of an electrostatic complex between the polyanionic block and the polycation block. These resulting micelles showed good encapsulation efficiency on siRNA of 75% at a charge ratio R = 1, great biodegradability and biosafety.
Considering that polyelectrolytes under physiological salt levels might disrupt the stability of PM/RNA NPs by interfering with the charge-charge interaction among PIC, or between PM and RNAs, strategies to promote the stability of polymeric vectors-based micelles have been further proposed. (i) Controlling the chain rigidity of catiomers (eg. introducing hydrophobic moieties in polycation segments [100] or RNAs [101]. Horacio et al. [102] reported that the PIC micelles self-assembled by relatively flexible PEG-poly(glycidyl butylamine) (PEG-PGBA) block copolymer allowed more than 50-fold stronger binding to mRNA than those by the relatively more rigid PEG-PLL block copolymer, resulting in enhanced protection on mRNAs against enzymatic attacks. (ii) Preserving the micelle structure by cross-linking the core with stimuli-responsive covalent bonds. Kataoka et al. [103] introduced the disulphide bonds as the crosslink moieties into the PEG–PLL copolymers, thus attained tighter mRNA packaging in the PM core and improved mRNA nuclease tolerability in serum and intracellular spaces compared with non-crosslinked PMs. (iii) Compressing RNAs into the inner hydrophilic core of reverse micelles or vesicles stabilized by bilayer membrane core-shell NPs (Fig. 5B). Wei et al. [104] compressed siRNA into the inner hydrophilic core of reverse PCL-PEI micelles at a low N/P ratio of 5, followed by coating a neutral lipid membrane (DOPE, cholesterol, DSPE-PEG) to form negatively-charged core-shell NPs by microfluidic technology. These new Lipid/Polymer hybrid nanoassemblies exhibited stronger protection on siRNA locked in core and better stability in circulation with reduced usage of cationic PCL-PEI materials compared with traditional lipid/micelle/siRNA (LMS) NPs.
The core-shell type accounts for a large number of nanocarriers for RNA delivery and has been extended to the broader structural feature (e.g., ionizable cationic lipids-based LNP, as discussed in the section of 3.2.5). More efforts have focused on the structure optimization of materials and usage of facile preparation process to stably entrap RNAs in the core of nanocarriers. Moreover, controlled release at the targeted sites is also of great importance for core-shell type-based RNA delivery systems.
3.2.4. Protection by non-electrostatic interaction-based encapsulation
Although the electrostatic interaction-based encapsulation has been the mainstream for RNA delivery nanocarriers and exhibited great transfection efficiency since their positively-charged surface, these cationic NPs with excess positive charges are susceptible to rapid clearance due to non-specific interaction with anionic components in the blood and uptake by the mononuclear phagocyte system (MPS), and also tend to induce acute cytotoxicity, thereby limiting their therapeutic value. Recently, non-electrostatic interaction-based encapsulation strategies have been employed for RNA delivery, including complementary base pairing-based, hydrogen bonds forces-based, and coordination bond-based RNA delivery systems.
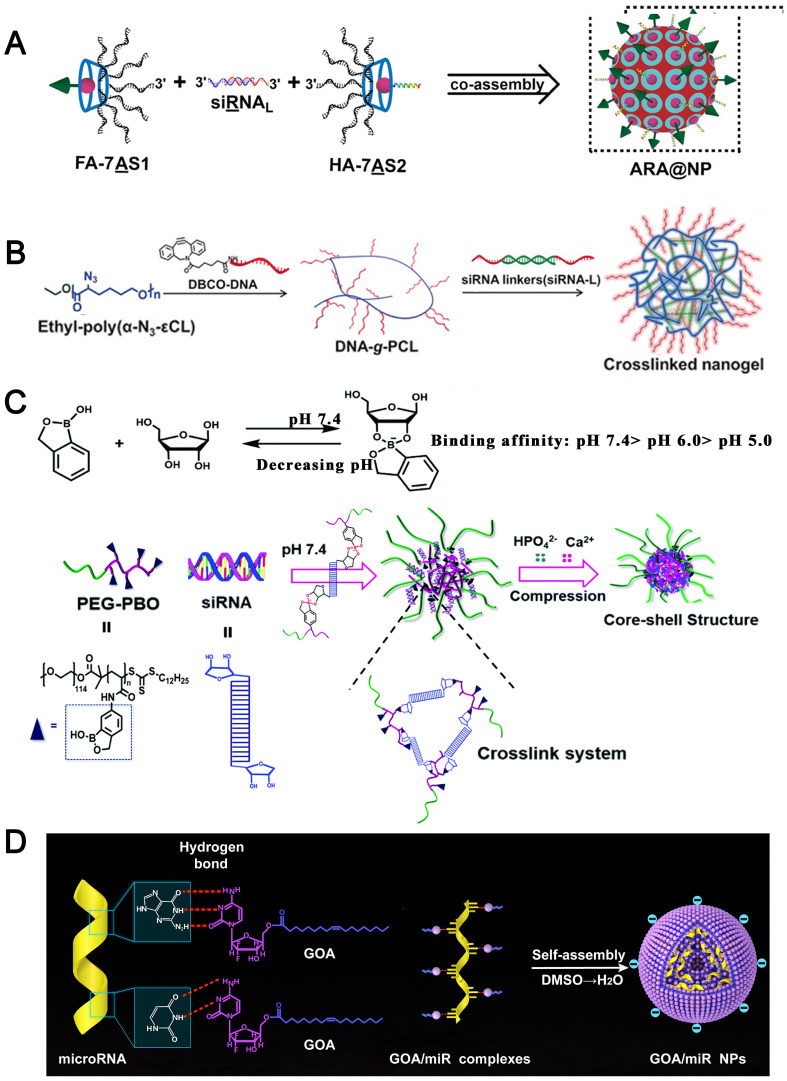
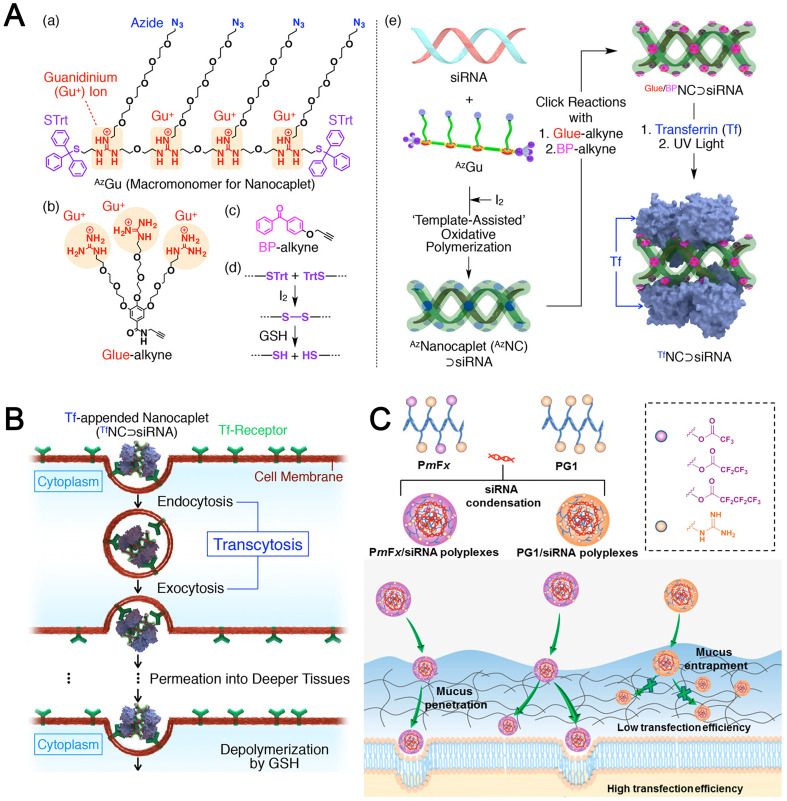
For example, nucleic acid drugs themselves can efficiently co-assemble with RNAs for co-delivery through complementary base pairing. Ding et al. [105] constructed the branched antisense DNA and siRNA co-assembled nucleic acid nanostructures, in which branched antisense (7AS) was synthesized through a copper-free click reaction between azide-modified β-cyclodextrin and DBCO-modified antisense. A siRNA with the 3′ overhangs as the linker (siRNAL) was then recognized by one pair of branched antisenses with seven arms (7AS1 and 7AS2), through DNA–RNA hybridization, to form the 7AS1/siRNAL/7AS2 complex. Based on the host–guest interaction, an adamantane-modified folate molecule and an adamantane-modified influenza hemagglutinin peptide were included. This multifunctional nucleic acid nanostructure could function as both delivery carrier and therapeutic cargo to be released by intracellular RNase H digestion, avoiding the risk of systemic toxicity caused by cationic nanocarriers (Fig. 6A).
Fig. 6.
(A) Schematic illustration of the co-assembly of branched antisense and siRNA for combined gene silencing and tumor therapy in vitro and in vivo. FA: folate for targeting; HA: influenza hemagglutinin peptide for endosomal escape; 7AS1 or 7AS2: branched antisenses covalently cross-linked by β-CD; siRNAL: 3′ terminal extended siRNA; and ARA@NP: FA-7AS1/siRNAL/HA-7AS2 co-assembled by hybridization between functionalized branched antisenses and siRNAL [105]. Reproduced with the permission from Ref. 105. Copyright © 2021 John Wiley and Sons. (B) Illustration of crosslinked nanogel formation and the siRNA delivery in vivo [106]. Reproduced with the permission from Ref. 106. Copyright © 2018 John Wiley and Sons. (C) Assembly of PEG-PBO/siRNA/CaP hybrid nanoparticles and its pH responsive disassembly [88]. Reproduced with the permission from Ref. 88. Copyright © 2018 Royal Society of Chemistry. (D) The nucleobase head of GOA prodrug was proposed to bind to nucleobase of miRNAs with hydrogen-bond interaction, and the oleic acid tail chains could provide hydrophobic forces to self-assemble into GOA/miR nanoparticles in aqueous solution [86]. Reproduced with the permission from Ref. 86. Copyright © 2019 Elsevier.
Similarly, Zhang et al. [107] constructed a spherical nucleic acid (SNA)-like nanogel assembled by a DNA-grafted polycaprolactone (DNA-g-PCL) brush and siRNAs as crosslinker via nucleic acid hybridization in which siRNAs were fully embedded and protected for systemic delivery (Fig. 6B).
In addition, the mild reaction of phenylboronic acid with 1,2- or 1,3-cis-diols to form esters also offers a facile route for binding siRNA to the PBA groups via the ribose ring at the 3′ end of RNAs. Shen et al. [88] designed a block polymer PEG-b-poly (benzoxaborole) (PEG-PBO), which could complex siRNA by forming pH-responsive boronic ester bonds with ribose rings of siRNA and adhere to the hydroxyapatite surface of CaP. The PEG-PBO/siRNA/CaP nanocomposites exhibited high siRNA loading efficiency (about 94% at the PEG-PBO to siRNA weight ratio of 20), low cell cytotoxicity and excellent colloidal stability at neutral pH (Fig. 6C).
The coordination interaction between metal (e.g., Ca2+, Zn2+, Fe3+) and phosphate of RNAs is also widely used for RNA encapsulation. Based on ZnII–dipicolylamine complexes (Zn–DPA) that are highly selective for phosphodiester groups of siRNA, HA conjugate bearing 5β-cholanic acids and Zn–DPA was synthesized and complexed with siRNA to form HADPA-Zn-NPs, and it was demonstrated that 100 μg HADPA-Zn-NPs contained 15 pmol siRNA [87].
Taking advantage of hydrogen bonding and hydrophobic interactions for loading RNAs, Wang et al. [86] synthesized amphiphilic gemcitabine prodrug (GOA conjugate), and used the cytosine of gemcitabine to bind with miRNAs through hydrogen bond interaction of nucleobases. Then the GOA/miRNA complexes were further self-assembled into NPs with a hydrophobic interaction of tail chains. These NPs had a stable encapsulation on miRNAs with non-sequence selectivity and exhibited great biosafety after systematic administration (Fig. 6D).
Unlike most reported RNAs delivery systems, non-electrostatic interaction-based formulations do not need cationic derivatives to complex with RNAs. Usually, they are regarded as lower toxicity and less nonspecific accumulation than polycationic-based formulations. Whereas, compared with cationic nanocarriers, transfection efficiency is limited due to the less cell uptake or endosomal escape, besides, a detailed mechanism for the interaction of these RNA delivery systems with components in the blood circulation and cells needs to be verified, including formation of protein corona, cell internalization, and endosomal escape process. Furthermore, advanced evaluation methods are needed to be exploited for the non-cationic associated toxicology. In summary, the optimum balance between the safety and effectiveness is essential for the success of nanocarriers in RNAs delivery.
3.2.5. Protection by LNPs
Recently, numerous LNP-based nanocarriers have been developed for RNAs delivery and have exhibited great potential in the clinical translation, since their high delivery efficiency and low toxicity. More and more research interesting are focused on the design of various lipids and lipid derivatives, including primary and secondary amino lipids, tertiary amino lipids, lipidoids, lipid-derived lipopeptides and so on [108].
The great protection of ionizable cationic lipids-based LNPs toward RNAs depends on the morphology and encapsulation mechanism of LNPs, yet their structural features remain unclear. Pieter R. Cullis et al. [109] provided an understanding of the structure of LNP-siRNA systems containing DLinKC2-DMA, phospholipid, cholesterol and PEG lipid formed using a rapid microfluidic mixing process. The experimental results showed that these LNP siRNA systems had an interior lipid core containing siRNA duplexes complexed to cationic lipid, phospholipid and cholesterol, which exhibited an electron-dense core (in contrast to bilayer vesicle systems). Meanwhile, along with the increasing siRNA concentration, more cationic lipid would be transferred from external lipid monolayer to the cavity to form into complex with siRNA, suggesting that the siRNA contained in the inverted micelle was surrounded by an inner monolayer of cationic lipid. Consistently, molecular modeling also demonstrated that these LNPs had a nanostructured core consisting of periodic arrangement of aqueous compartments. This structure contained siRNAs and the polar moiety of the lipid to form inverted micelle, followed by association with “empty” inverted micelles (formed from excess ionizable lipid) to form a hydrophobic core which was further surrounded by helper lipids. Additionally, the PEG layer was presented in the outer layer to provide further shielding and protection. Such organization could account for the complete protection of encapsulated siRNA from external RNase.
Whereas, Pieter R. Cullis et al. [110] proposed another potential mechanism of LNPs formation by investigating the LNPs morphology changes during the siRNAs encapsulation process with pH changes. The initial event was formation of small vesicles which contained siRNA between closely apposed lipid monolayers. As the pH was raised, neutralization of the ionizable lipid induced fusion between various particles and deposition in the interior core of the LNPs. Then PEG-lipid and DSPC/cholesterol were deposited in a surface monolayer that inhibited further fusion. It should be noted that, different from the structure proposed previously, siRNAs were not encapsulated in the LNPs interior in a “currant bun” pattern, but rather was associated with closely apposed lipid bilayers sandwiching siRNA molecules that segregate toward the periphery of the LNPs and excess lipids formed an amorphous oil-droplet phase in the cavity.
Although the certain mechanism of formation and structural features of LNPs remain obscure, optimized LNP-RNA delivery system relies on the physiochemical characterization of formulations. Recently, various LNPs have been designed by the adjustment of pKa of ionizable lipid [111], the choice of helper lipids and the proportions of each lipid components [112] to improve stability and encapsulation efficiency on RNAs. It is worth noting that the structural-activity relationship of each lipid in different formulations is still needed to be studied in future.
With the breakthrough of LNPs in the success of mRNA vaccines against COVID-19, LNPs have received more and more attention. However, the obstacles like durability of vaccine efficacy were still remained. Moreover, LNPs can be designed to broaden application across other RNA therapeutics and diseases by improvement of targeting ability, enhancing loading capability, cell uptake, endosomal escape and ultimately delivery efficiency of RNAs.
3.3. Prolong circulation of RNA-loaded nanocarriers
In order to achieve effective targeted tissue accumulation and improve pharmacokinetic characters of RNAs, prolonged blood circulation time is necessary for RNA delivery system, and thus reduce dosing frequency and maintain effective concentrations over the desired period. The main challenge for long blood circulation time is capture of NPs by phagocytic cells of the MPS and rapid kidney clearance. Since phagocytic clearance of NPs is mediated by opsonization, which is attributed to the adsorption of plasma components (i.e., opsonin) onto the surface of a foreign NPs. The exact nature of the opsonin that adsorb onto NPs varies according to the size and surface characteristics of the NPs.
3.3.1. Surface modification of PEG (PEGylation) and its alternatives
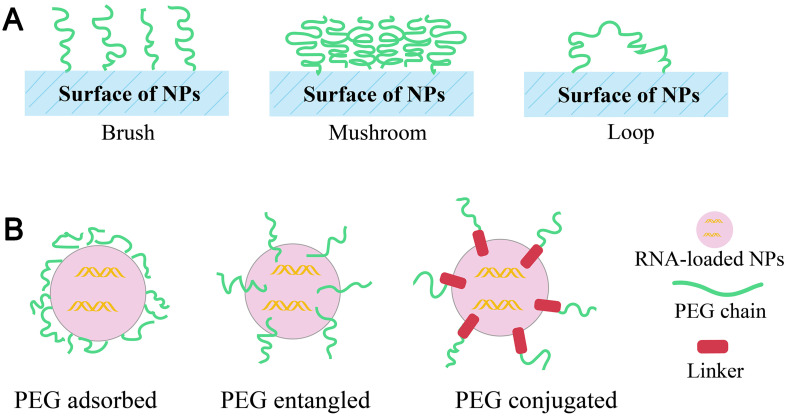
PEGylation is a common strategy to prolong the blood circulation time of NPs. Hydrophilic PEG layer can provide a ‘stealth’ effect to NPs in vivo through shielding the surface to limit the adsorption of serum proteins, thereby prevent opsonization, and ensure protection NPs against mononuclear phagocyte capture and thus contributing to increased circulation time. PEG exists in surface of NPs with different conformations (Fig. 7A) depending upon the surface-grafting density, which ultimately determines the fate of nanocarriers [113]. Generally, higher PEG density on the surface of nanocarriers facilitates better steric barriers through the formation of brush-like conformations [114]. Besides, PEG can be grafted chemically or adsorbed physically on to the NPs or directly used during preparation of NPs (Fig. 7B). Adsorption of pre-formed PEG derivatives through non-covalent interactions or physical adsorption onto the surface of NPs generally weak to ensure complete PEG coverage [113]. Alternatively, they can also added as additives with amphiphilic structures, such as PEG-lipids conjugate or PEG block copolymers introduced during the preparation of NPs [115]. For traditional liposomes, PEG1000 to PEG2000 constituting 5–10%mol of total lipid is sufficient for PEGylation of liposomes [116].
Fig. 7.
(A) Types of PEG conformations (B) techniques of linking PEG to NPs.
Shi et al. [63] developed a lipid-polymer hybrid NPs composed of a cationic lipid (G0-C14)/siRNA complex-containing PLGA polymer core and a lecithin/lipid-PEG shell. The results showed that DSPE-PEG5K NPs, DSPE-PEG3K NPs and ceramide-PEG5K NPs exhibited the prolonged circulation of siRNA with a half-life (t1/2) of ~8.1 h, ~7.1 h and ~ 30 min, respectively. Whereas, the naked siRNA was rapidly cleared from blood within 30 min. Besides, both DSPE-PEG NPs demonstrated ~100-fold greater measurement for area under the curve (AUC) than that of naked siRNA, which demonstrated that the incorporation of PEG on the surface of NPs could efficiently improve the pharmacokinetic characters of RNAs.
Although a higher ratio of PEG enhances the circulation times, it hampers the efficient tissue penetration, cellular uptake and endosomal escape, which is usually regarded as the “PEG dilemma”. To circumvent this problem, cleavable PEG-lipids or polymers are introduced onto NPs, where PEGs are cleaved from NPs surface after PEGylated NPs reach targeted tissues (these contents would be discussed in the section of “3.5.1”). On the other hand, repeated administration of PEGylated NPs, especially liposomes, tends to result in accelerated blood clearance (ABC) phenomenon, which severely hinders the therapeutic efficacy. Briefly, upon the first injection of PEGylated liposomes, IgM antibodies are produced by activated B cells in the splenic marginal zone; the subsequently injected PEGylated liposomes then interact with the residual IgM antibodies in the serum, activate the complement and are finally taken up by macrophages [117].
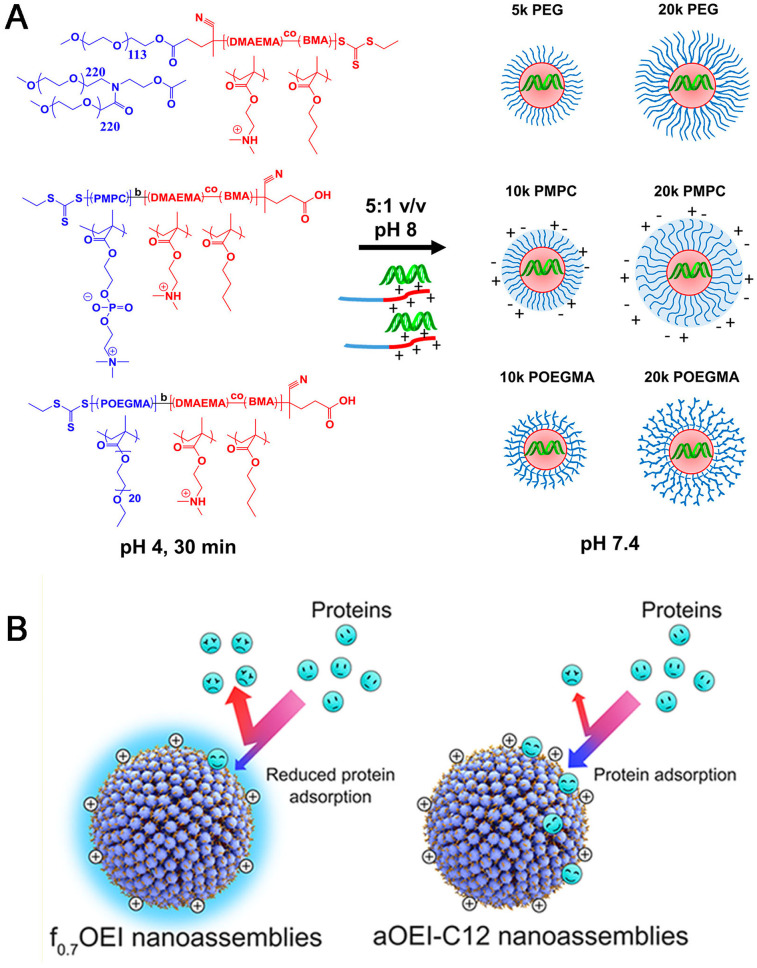
To avoid the drawback induced by PEGylation, synthetic alternatives to PEG have been widely investigated, which mainly includes poly(glycerol) (PG), poly(2-oxazoline)s, poly(N-(2-hydroxypropyl)methacrylamide) (PHPMA), poly(vinylpyrrolidone) (PVP), biodegradable poly(amino acid)s (e.g. PGA, poly(hydroxyethyl-L-asparagine) (PHEA)) [118], and zwitterion (e.g. phosphorylcholine) [119] (Table 6 ). Duvall's group [120] synthesized a library of diblock polymers containing the same pH-responsive, endosomolytic polyplex core-forming block but different corona blocks: 5 kDa (benchmark) and 20 kDa linear PEG, 10 kDa and 20 kDa brush-like poly(oligo ethylene glycol), and 10 kDa and 20 kDa zwitterionic phosphorylcholine-based polymers (PMPC) for siRNA delivery (Fig. 8A). It was found that 20 kDa PEG and 20 kDa PMPC had the highest stability and were the most effective at blocking protein adsorption. Following intravenous delivery, 20 kDa PEG and PMPC coronas both extended circulation half-life 5-fold compared to 5 kDa PEG. Moreover, zwitterionic PMPC-based polyplexes showed highest in vivo luciferase silencing (>75% knockdown) and 3-fold higher average tumor cell uptake than 5 kDa PEG polyplexes (20 kDa PEG polyplexes were only 2-fold higher than 5 kDa PEG).
Table 6.
The examples of synthetic alternatives to PEG.
| Types of alternatives | Name | Structure | Characters |
|---|---|---|---|
| Poly(amino acid)s | PGA | 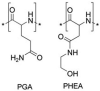 |
decreased ABC clearance, biodegradability, complement activation |
| poly(hydroxyethyl-L-asparagine) (PHEA) | |||
| Polymers with Heteroatoms in the Main Chain | Poly(glycerol) (PG), hyperbranched PG (HPG) | 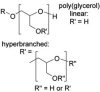 |
non-biodegradability, high degree of branching is advantageous for the circulation time and have low intrinsic viscosity |
| poly(2-methyl-2-oxazoline) (PMeOx), poly(2-ethyl-2-oxazoline) (PEtOx) | 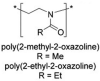 |
show a behavior comparable to PEG in terms of blood circulation time, opsonization, and organ distribution | |
| Vinyl Polymers | poly(acrylamide) (PAAm) | 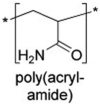 |
the monomer shows toxic side effects produced during thermal and photolytic degradation of the polymer |
| poly(N-(2-hydroxypropyl)methacrylamide) (PHPMA) | 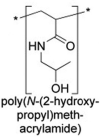 |
PHPMA conjugates (e.g. PHPMA-doxorubicin copolymer) have already entered clinical trials | |
| Zwitterionic polymers | Poly(carboxybetaine) (pCB), poly(sulfobetaine) (pSB)-based polymers | 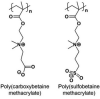 |
strong hydration, high resistance to nonspecific protein, low immunogenicity, Difficult synthesis |
Fig. 8.
(A) siRNA polyplexes containing varied corona architectures. All polymers contain the same polyplex core-forming block consisting of equimolar DMAEMA and BMA. The corona-forming blocks comprise either linear PEG, zwitterionic PMPC, or brush PEG structures (POEGMA), as pictured. Polymer structures are displayed on the left, with the core-forming block in red and corona-forming block in blue. Polymers are complexed with siRNA at low pH, triggering spontaneous assembly of polyplexes before the pH is raised to physiological pH [120]. Reproduced with the permission from Ref 120. Copyright © 2017 American Chemical Society. (B) Schematic illustration of the adsorption efficacy of serum proteins onto the surface of aOEI-C12 NAs and f0.7OEI NAs [121]. Reproduced with the permission from Ref 121. Copyright © 2018 American Chemical Society.
Although many alternatives to PEGylation have been proposed for several years, there are still difficulties to translate into clinical practice. Consequently, it is still in demand that search for other potential alternatives and further proper evaluation and comparison with PEG.
3.3.2. Altering physiochemical properties of the NPs
The physicochemical properties of NPs (including size [[122], [123], [124], [125]] shape [126,127], elasticity [128], hydrophobic surface [121] and charge [129]) can also influence protein adsorption and blood circulation time [124].
It has been reported that for long-circulation of NPs, the size of NPs should be large enough to avoid renal filtration but small enough to minimize opsonization and MPS clearance, thus spherical particles of diameter between 10 and 200 nm appear to fit this description [124]. Wan et al. [125] successfully prepared a small orderly curled silica nanosheet (OCSN) (∼42 nm particle size) with large continuous channels (∼13.4 nm) for efficient siRNA loading. Because the diameter of the OCSNs was small enough, they exhibited a long blood circulation halftime (0.97 h) and low blockade efficacy in a mononuclear phagocytic system.
In addition, hydrophobic surface of NPs is another parameter for long-circulation properties, which could mediate NPs resistance against serum protein adsorption. Considering hydrophobicity of fluorinated chain, Liang et al. [121] fabricated a library of perfluorooctanoyl fluoride-fluorinated (PFF-fluorinated) oligoethylenimines (fxOEIs, x is the PFF:OEI feeding ratio), which can readily form nanoassemblies (fxOEI NAs) capable of efficient siRNA delivery in cells cultured in medium supplemented with fetal bovine serum (FBS). The results demonstrated that the f0.7OEI NAs had the stronger protein adsorption resistance and persisted in the bloodstream for longer times in comparison with alkylated NAs due to the presence of fluorine atoms (Fig. 8B).
Surface charge is also a key determinant for in vivo fate of NPs. Cationic NPs tend to be rapidly removed from circulation through a combination of non-specific cellular interactions (i.e., adsorption to the anionic surface of the blood vessel walls), and/or clearance via the MPS. On the contrary, anionic and neutral NPs are generally taken up sparingly by non-MPS cell types, especially neutral nanocarriers are relatively stable and the circulation time is longer than that of charged nanocarriers [129]. Landen et al. [130] prepared neutral RNA nanocarriers by lyophilizing siRNA/lipid mixture followed by hydration with aqueous buffer to realize high siRNA loading in DOPC-containing liposomes. These NPs showed lower scavenging rate by macrophages in contrast to naked siRNA or cationic DOTAP/siRNA liposomes. Similarly, Halden et al. [131] applied DOPC liposomes to load FAK siRNA, which was effective in reducing FAK expression in vivo up to 4 days.
In addition, the shape of NPs is also important for in vivo pharmacokinetic profile because NPs with different shape can interact differently with the in vivo environment [132]. Especially, the protein corona is drastically changed by the shape of NPs, which will affect the elimination pathways of NPs after systematic administration. Guo et al. [127] used three-way junction (3WJ) motif composed of 3WJ-a, 3WJ-b, and 3WJ-c three component strands for construction of different shape RNA NPs (triangles, squares, and pentagons) with identical size, and showed that triangular NPs appeared to clear the fastest.
Hence, detailed understanding of interactions between NPs and the biological milieu is of great importance for optimizing the characteristics of NPs to prolong circulation time. Moreover, taking advantage of appropriate combinations of available strategies, such as regulation of size, surface modification, charges and shape et al. are required to obtain optimized nanocarriers with ideal pharmacokinetic profile of RNAs.
3.3.3. CD47 modification of NPs
As well known, the CD47 marker on red blood cells (RBCs) prevents RBCs from phagocytosis by macrophages via interactions with the inhibitory receptor SIRPα on macrophage membranes. Inspired by natural CD47–SIRPα pathway, which CD47 serves as a “don't eat me” signal and a “marker of self” to achieve phagocytosis evasion by macrophage, CD47-contained delivery systems are developed for improve circulation time by reducing MPS clearance [133]. For example, a PLGA NP conjugated with CD47 extracellular domain via reactive oxygen species (ROS)-responsive phenylborate ester bond was developed. The experimental results showed that the NPs efficiently increased half-life of payload in blood circulation by preventing engulfment of NPs via phagocytes [134]. Although the above nanocarrier is not used for RNA delivery, the CD47-based strategy of preventing MPS phagocytes may be universal. Moreover, CD47-contained biomembrane was also used to camouflage NPs for prolonging circulation time.
3.3.4. Nanoprimer pre-treatment
As reported, the MPS, previously known as the reticuloendothelial system (RES) [135], are mainly present in liver tissues, and in particular Kupffer cells (KCs) and liver sinusoidal endothelial cells (LSEC) usually take up a significant part of LNPs administered systemically, leading to the decreased circulation time of NPs.
To avoid this capture, a Nanoprimer was administered to transiently occupy MPS prior to the RNA nanocarriers was employed [136]. The Nanoprimer is a liposome designed with specific physicochemical properties to transiently occupy the KC and LESC, which does not contain or encapsulate any drug nor have any moieties attached to its surface. Besides, the Nanoprimer was optimized to be larger than the fenestrae of the liver capillaries (hydrodynamic diameter over 230 nm) to prevent it from passing through the Space of Disse, thus hindering its ability to interact with hepatocytes. The study demonstrated that the pretreatment of mice with the Nanoprimer decreased the LNPs' uptake by the MPS and prolonged the circulation time of LNPs. By accumulating rapidly in the liver cells, the Nanoprimer improved the bioavailability of the LNPs encapsulating human erythropoietin (hEPO) mRNA or factor VII (FVII) siRNA, leading respectively to more hEPO production (by 32%) or FVII silencing (by 49%).
3.4. Enhance accumulation of RNAs at targeted tissue/organ
To achieve high therapeutic efficiency of RNAs, precise delivery and accumulation of RNAs at targeted tissue/organ are of importance for delivery systems. Although the approvals of Patisiran and GalNAc-siRNA have made great progress in the liver-targeting therapy field, other tissues or organs accumulation for RNA delivery systems remain challenges in clinical application. There are a large number of non-targeted RNA-loaded NPs in clinical trials have failed due to insufficient delivery to the target sites [137].
3.4.1. Ligands modification of NPs
To enhance tissue/organ accumulation of RNAs, the most commonly used method is targeting ligands modification onto the RNA-loaded nanocarriers, which can achieve receptors-ligands binding-mediated accumulation strategy at targeted tissue/organ according to the expression of specific receptors on targeted tissues of different disease states. Currently, different types of ligands are applied to the delivery systems, and generally contains peptides, antibodies, and other biologically active small molecules (like lactobionic acid, folate and mannose). The examples of usage of ligands onto the NPs are exhibited in Table 7 .
Table 7.
Modification of targeting ligands onto the NPs.
| Targeted tissue/organ | Nanocarriers components | Ligands | Ref |
|---|---|---|---|
| Mantle cell lymphoma (MCL) | Dlin-MC3-DMA, cholesterol, DSPC, DMG-PEG, and DSPE-PEG-maleimide, siRNA | Anti-CD38 monoclonal antibodies | [138] |
| Lymphoma | PLGA, HA, siRNA, PLA | CD20 antibodies | [74] |
| Acute myeloid leukemia | siRNA, DOTAP, DOPE, cholesterol, albumin NPs, all-trans retinoic acid (ATRA) | Folate | [139] |
| Liver | siRNA, D SPE-PEG-pPB, DlinMC3, PEG-DMG, DSPC, cholesterol | pPB peptide (C*SRNLIDC*) | [140] |
| Brain, Neurons | siRNA, NL4 peptide and ApoA-I modified dendrigraft PLL (DGL) (generation 3) | NL4 peptide and ApoA-I | [141] |
| Brain, Neurons | DOTAP, siRNA, cholesterol, human transferrin | Tf | [142] |
| Colorectal cancer | SeNPs, siRNA, RGDfC peptide | RGDfC | [143] |
| Prostate cancer | HPAA-PEG-APT, miRNA | Anti-PSMA aptamer | [144] |
| Malignant melanomas | DC-Chol, DOPE, siRNA, AS1411-PEG- DOPE | Aptamer AS1411 | [145] |
| Prostate cancer | siRNA, Adamantane-PEG, Dilysine-cyclodextin | Anisamide | [146] |
| Liver | Epoxide-Based Lipidoids (C12-SPM), DSPC, cholesterol, C16-PEG2000-ceramide, α-galactosyl ceramide, siRNA | galactosylated ceramide | [147] |
Abbreviation: RGDfC: Cyclo (Arg-Gly-Asp-D-Phe-Cys).
Kataoka et al. [148] used a glucosylated-polyion complex micelle (Glu-PIC/Ms) self-assembled from glucosylated-PEG-b-PLL modified with 3-mercaptopropyl amidine and 2-thiolaneimine (PEG-PLL(MPA/IM)) block copolymer and ASO through electrostatic interaction as the platform structure for the BBB-crossing nanocarrier. This glucose-coated nanocarrier could be bound by glucose transporter-1 (GLUT1) expressed on the brain capillary endothelial cells and then could be delivered to the brain by crossing the BBB using glycemic control as an external trigger. The results showed that this nanocarrier efficiently accumulated in the brain tissue 1 h after intravenous administration and exhibited significant knockdown of a target long non-coding RNA (lncRNA) as high as 40%.
Different from traditional receptor-ligand recognition strategies, Dan Peer et al. [11] employed a conformation-sensitive targeting strategy that only recognized a specific protein conformation, namely the high-affinity conformation. Since α4β7 integrin expressed in leukocytes always change conformation and dramatically increase the affinity for their ligands (MAdCAM-1) when it is stimulated, a recombinant fusion protein, which fused the integrin binding domains D1 and D2 of the MAdCAM-1 to an IgG–Fc, was generated and conjugated to the surface of LNPs as a targeting moiety. These LNPs achieved in vivo gene silencing in a selective subset of leukocytes and showed potential therapeutic applications in a murine model of colitis. Since the relationship between conformational state and protein functionality is a commonly seen phenomenon, targeting specific conformations of proteins opens new avenues in LNP targeting strategies. However, on a molecular level, little is known about the protein-driven, inside-out signaling processes that result in such conformational changes. In addition, the environment in vivo is more complex, and the study of conformation dynamics is more limited.
3.4.2. SORT molecular-based LNPs
Since the tissue selectivity and organ tropism of delivery systems can be also influenced by the surface potentials and/or internal charges of nanocarriers, altering the surface property (like charge) of NPs to specifically accumulation into the targeted the organ or tissues have been extensively investigated. For example, Daniel J.'s group [149] reported a strategy termed Selective Organ Targeting (SORT) that allowed accurate delivery of diverse cargoes including mRNA, Cas9 mRNA/single guide RNA (sgRNA) and Cas9 ribonucleoprotein (RNP) complexes to the lungs, spleens and livers following intravenous (i.v.) administration through incorporation of differentially charged phospholipids into the LNP formulation. (Fig. 9A). The results found that with an increasing molar percentage of permanent cationic lipid SORT moleculSes DOTAP, the resulting LNP moved progressively from liver to spleen, and then to lung, demonstrating a clear and precise organ-specific delivery trend. When added 50% of DOTAP into the formulations, 80% of the LNPs accumulated in lung. Whereas with the incorporation of 10–40% anionic lipid SORT molecules (1,2-dioleoyl-sn-glycero-3-phosphate, 18PA), LNPs mediated completely selective delivery to the spleen. With the addition of ionizable cationic lipid (1,2-dioleoyl-3-dimethylammonium-propane, DODAP), the LNPs significantly enhanced liver delivery > ten-fold at 20% incorporation and transfected ~93% of hepatocytes. The results suggested that altering the internal and/or external charges of NPs may be a key and universal strategy in tuning tissue tropism. In addition, determining the precise mechanisms that explain how SORT enables tissue targeting will be the next research point.
Fig. 9.
(A) Addition of a supplemental component (termed a SORT molecule) to traditional LNPs systematically alters the in vivo delivery profile and mediates tissue-specific delivery as a function of the percentage and biophysical property of the SORT molecule [150]. Reproduced with the permission from Ref 151. Copyright © 2021 Springer Nature. (B) iPhos lipid design directs LNP tissue targeting. Zwitterion iPhos lipids contain two lipid tails on the positively charged tertiary amine and one lipid tail on the negatively charged phosphate group. Varying the alkyl lipid chain length impacts tissue targeting to liver, lung and spleen, which has been coined selective organ targeting (SORT) [151]. Reproduced with the permission from Ref 151. Copyright © 2021 Springer Nature. (C) Schematic representation of the formation of a disulfide bond between PDP-functionalized lipids that are incorporated into the liposome bilayer and reduced exofacial thiol groups, present at the cell surface [152]. Reproduced with the permission from Ref 152. Copyright © 2016 Elsevier.
In a follow-up to this study, Daniel J. et al. [153] synthesized a library of zwitterion ionizable phospholipids, termed “iPhos”, which consist of one ionizable amino group and one phosphate head group with three hydrophobic lipid tails. Then iPhos lipids synergistically functioned with various helper lipids to formulate mRNA-LNPs (called iPLNPs), and structure–activity relationships revealed that iPhos chemical structure could control in vivo efficacy and organ selectivity. The results showed that alkyl length next to the phosphate group influenced organ selectivity: Shorter chains (9–12 carbons) showed mRNA translation in liver, while longer chains (13–16 carbons) would transfer protein expression to spleen. The detailed mechanistic studies still need to be performed (Fig. 9B).
Therefore, the rationally designed nanocarriers with optimized characters by detailed in vivo structure-activity relationship study will provide translational potential for RNA targeted delivery to realize clinical applications.
3.4.3. Immune cell-mediated hitchhiking NPs
Considering that massive immune cells infiltration and accumulation is usually a key feature for lesion tissue, taking advantages of oriented migratory properties of immune cells to design hitchhiking NPs is another tissue/organ targeting and accumulation strategy. For example, certain cell types have the intrinsic ability to cross the endothelial barrier and infiltrate the tumor tissue, like T cells can identify and migrate to the tumor tissue, which makes them attractive carriers for NP delivery. Stefaan group [154] developed the lipid-enveloped nanogels (siNGs) consist of siRNA-loaded cationic nanogel (NG) core and pyridyldithiopropionate (PDP)-functionalized liposomes as the outer shell, and subsequent attached the siNGs to the surface of cytotoxic CD8+ T lymphocytes (CTLs) based on disulfide bone formation between exofacial thiols present on the CTL plasma membrane and thiol-reactive liposomes (Fig. 9C). Then CD8+ T cells-carried siNGs would transport to the tumors and enhance the accumulation/infiltration of NPs based on intrinsic targeting ability of T cells to migrate to the tumors. Nevertheless, the T Cell-mediated targeting delivery capability are still to be investigated in future research.
Recently, increasing tendency in research works have focused on developing hitchhiking NPs by chemistry conjugation with immune cells, especially for small molecular therapeutics delivery, and few study for macromolecular therapeutics like RNA have been reported. Moreover, the coupling stability, potential influence on cell functionalities, detachment of nanocarriers from cell surface and subsequent intracellular release, and in vivo efficacy evaluation still need to be considered in the future.
3.4.4. Tuning the physicochemical characteristics of NPs
As well known, the physicochemical characteristics (size [155], surface charge [156], particle shape and rigidity/flexibility [157] of structures) play critical roles in tissue penetration and accumulation capability. Generally, small-size NPs have good tissues (e.g., tumor) permeability, while large-size NPs have good tissue accumulation and retention ability. Additionally, negative or neutral charged surface benefits the long blood circulation of the nanocarriers and achieves a high tumor accumulation. While the positive charge will promote the tissue penetration and cell uptake of the nanocarriers. To address the above conflicting need and enhance tissue penetration and accumulation, stimuli-responsive size and charge changeable NPs were developed for RNA delivery. For example, Chen et al. [156] designed a charge conversional multifunctional nanoplatform based on a pH-sensitive copolymer mPEG-PLL-2,3-dimethylmaleic anhydride (DMA) (PLM) and PAMAM dendrimers. The small-sized PAM/siRNA complexes built via electrostatic interaction were as the inner core and then PLM was applied as an outer layer with negative charges. Under a physiological environment, the PLM/PAM/siRNA NPs showed prolonged circulation time and enhanced tumor accumulation. While in the tumor microenvironment, the pH-triggered PLM changed to positive charge due to the cleavable amide bond between mPEG-PLL and DMA, leading to large-size NPs with a negative charge that turned into a positive charge and small NPs with a high tumor-penetrating ability.
In addition, for the deep tissue permeation and accumulation of nanocarriers, paracellular and transcellular pathways have also been considered for the development of RNA delivery systems. Whereas paracellular RNA delivery may be unlikely since intercellular gaps (5–10 nm) [155] are not large enough for large molecular RNA (like siRNA∼5 nm) to pass through. For the purpose of achieving transcellular accumulation of RNAs into deep tissues, the nanocarrier is preferred to be as small as possible and needs to introduce ligands with transcytosis (consecutive endocytosis/exocytosis events) activity like transferrin (Tf). Takuzo et al. [158] developed a small-sized Tf-appended nanocaplet (TfNC ⊃ siRNA) for the purpose of realizing siRNA delivery into deep tissues. For obtaining TfNC ⊃ siRNA, a macromonomer (AzGu) bearing multiple guanidinium (Gu+) ion units, azide (N3) groups, and trityl (Trt)-protected thiol groups in the main chain, side chains, and termini, respectively, was designed and adhered to siRNA strand based on a multivalent Gu+–phosphate salt-bridge interaction. After I2 was added to the preincubated mixture of AzGu and siRNA, oxidative polymerization of AzGu took place along the siRNA strand to obtain the AzNC ⊃ siRNA, and this conjugate was converted into Glue/BPNC ⊃ siRNA by the click reaction with a Gu+-appended bioadhesive dendron (Glue) followed by a benzophenone derivative (BP). Then, Tf was covalently immobilized onto Glue/BPNC ⊃ siRNA by Gu+-mediated adhesion followed by photochemical reaction with BP. The resulting TfNC ⊃ siRNA nanocaplet showed a hydrodynamic diameter (Dh) of 15.8 ± 5.0 nm and permeated deeply into a cancer spheroid at a depth of up to nearly 70 μm with the help of Tf-induced transcytosis (Fig. 10 ).
Fig. 10.
(A) Synthesis of a siRNA-containing nanocaplet appended with transferrin (Tf) units (TfNC⊃siRNA) [158]. Reproduced with the permission from Ref. 158. Copyright © 2019 American Chemical Society. (B) Permeation of TfNC⊃siRNA into cells located in a deep tissue via Tf-mediated transcytosis. Once TfNC⊃siRNA escapes from the endosomes in a cell, glutathione (GSH), abundantly present in the cytoplasm, liberates siRNA to cause RNAi by reductive depolymerization of the nanocaplet part (TfNC) [158]. Reproduced with the permission from Ref. 158. Copyright © 2019 American Chemical Society. (C) Illustration of transmucus and transmembrane siTNF-α delivery mediated by fluorinated and guanidinated bifunctional helical polypeptides [159]. Reproduced with the permission from Ref. 159. Copyright © 2020 American Chemical Society.
In addition, the rigidity/flexibility of NPs is also a key factor for tissues permeability and accumulation. The rigid structures of the nanocarriers are considered to prevent penetration into deeper regions within tumor tissue [157]. While a flexible nanocarrier can be deformed during the paracellular transport, facilitating the intratumoral accumulation. For example, a siRNA-loaded non-cationic soft polyphenol nanocapsule called Nanosac was developed by sequential coating of MSN with siRNA and polydopamine, followed by removal of the sacrificial MSN core to produce a hollow structure [157]. The results of intravital confocal microscopy showed that the mean fluorescence intensity (MFI) of soft Nanosac in the tumor spheroid was 2-fold higher than that of hard NPs (MSNa-cy5/pD), indicating that the soft NPs with flexible structure could improve penetration and accumulation of RNAs at tumors compared to hard counterpart.
3.4.5. Exogenous helper assisted tissue penetration
For enhance accumulation of nanocarriers at targeted tissue/organ, especially tumors, although the enhanced permeability and retention effect (EPR) of solid tumors was proposed and used for varieties of nanocarriers. However, it had been reported that the median delivery efficiency of NPs by EPR in solid tumors was only 0.7% by surveying the literature from 2006 to 2016 [160]. Thus, exogenous helper assisted tissue penetration approaches such as high-intensity focused ultrasound (HIFU) and continuous wavelength laser-induced photothermal effect, as well as photoacoustic (PA) technique have been proposed to enhance the vascular permeability of NPs. For example, Kai et al. [161] developed a NIR-absorbing semiconducting polymer-based nanocarrier (SP/miNP) consist of 4-(4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo [1,2-b:4,5-b’]dithiophene-2-yl)-6,7-bis(3-octyloxy)phenyl)- [1,2,5] thiadiazolo [3,4-g]quinoxaline (BBT-TQP), DSPE-PEG2000, and DSPE-PEG-miRNA. Upon NIR pulse laser irradiation, the nanocarriers could absorb energy and get activated immediately, generating photoacoustic force to enhance their vascular permeability and facilitate efficient miRNA transportation into tumor tissues via both active trans-endothelial and passive inter-endothelial gap pathways. As a result, SP/miNPs boosted the delivery efficiency to tumors by a 5-fold increase, in comparison to the traditional EPR approach.
In addition, HAase, a permeation promoter, has been used to increase the drug penetration of the malignancy by decreasing the intratumoral pressure and loosening the intercellular connective matrix. For instance, CS with a hydrophobic oleic acid tail (CMO) connected by disulfide bonds was synthesized, and then post-inserted into the DOTAP/shRNA lipoplex (LR) to obtain a CS periphery, which could bind with HAase. After coating with HA, the HA/HAase/CS/liposome/shRNA (HCLR) was obtained. The results demonstrated that much more fluorescence could be found in the deep region of HCLR treated tumor in contrast to HLR and LR, suggesting that the released HAase could decrease the density of the extracellular matrix (ECM) and accelerate the diffusion of nanocarriers in tumor, which was responsible for the better tumor penetration ability [72].
3.5. Enhanced cellular internalization
As well known, effective cell internalization is the prerequisite for RNAs to exert gene therapy effects. Since the repulsion between the negatively charged RNAs and cell membrane hinders the efficient cell uptake of RNAs, different strategies were used to enhance cellular internalization.
3.5.1. Stimuli-responsive detachable PEG shell
One of the most widely used strategies is to incorporate cationic materials such as cationic polymers (CS, dendrimers, PEI, PLL, and et al.) and lipids (DOTAP, DOTMA, DOSC and et al.) in the delivery system to construct cationic nanocarriers, which can facilitate cellular internalization via electrostatic interaction. However, they always exhibit high levels of cytotoxicity due to their high positive charge density. Therefore, PEG-shielding strategy was widely applied in the cationic nanocarriers to mask the excessive positive charges on the surface of NPs, but the high PEG density would suppress the cellular uptake of nanocarriers. As such, stimuli-responsive detachable PEG shell with cleavable linkers was introduced to the nanocarriers for improving cell internalization. Generally, acid [162,163]or MMP enzymes [162,164,165] responsive-sheddable PEG shell were used. For example, Wang et al. [166] reported an acid-degradable amide bond (Dlinkm) linked PEG-Dlinkm-PLGA block copolymer and used to encapsulate siRNA into NPs (dPEGNPPLGA/siRNA) through the cationic lipid-assisted double emulsion method. The siRNA-encapsulating NPs prepared by PEG-b-PLA (NPPLA/siRNA) and PEG-b-PLGA (NPPLGA/siRNA) were used as the control formulations. When the dPEGNPPLGA/siRNA achieved accumulation in tumor sites, the PEG surface layer was detached in response to the tumor acidic microenvironment, leading to the changes of surface potential from neutral to positive with the exposure of cationic amino groups (Fig. 11A). The results demonstrated the increased cellular uptake by ~2 fold in contrast to NPPLA/siRNA and NPPLGA/siRNA. Similarly, the PEG derivative O′-mPEG conjugated with lipid by pH-sensitive imine bond was used as a cleavable PEG-lipid to coat on the miRNA-loaded solid LNPs (SLNs) modified with cell-penetrating peptides. After the cleavable PEG layer was responded to low extracellular pH, the CPPs were exposed to improve the cell uptake [167].
Fig. 11.
(A) Scheme illustration showing the preparation of NPPLA/siRNA, NPPLGA/siRNA, and dPEGNPPLGA/siRNA with PEG5K-b-PLA11K, PEG5K-b-PLGA11K and the tumor pH-labile linkage-bridged block copolymer PEG5K-Dlinkm-PLGA11K. Compared with NPPLA/siRNA and NPPLGA/siRNA, dPEGNPPLGA/siRNA can enhance tumor cell uptake by detaching the PEG layer and accelerate intracellular siRNA release with hydrophobic PLGA layer [166]. Reproduced with the permission from Ref. 166. Copyright 2016 ELSEVIER. (B) Schematic illustration of constructing the Nb-modified nanogel (Nb-nanogel) [168]. Reproduced with the permission from Ref. 168. Copyright 2020 ELSEVIER.
3.5.2. “Armchair” structure constrained lipid-based LNPs
In addition to conventional strategy of enhancing cell internalization by increasing positive charge of nanocarriers, “armchair” structure based conformationally constrained lipids, which contained adamantane tail and formed stable constrained LNPs (cLNPs), was also found to enhance siRNA delivery to T cells [169]. The results showed that adamantane-containing LNPs could enable the LNPs massive accumulation in splenic T cells in vivo.
3.5.3. Cell membrane fusogenic functional NPs
Cell membrane fusogenic functional NPs can directly delivery RNAs into cytoplasm via fusing with the cellular membrane, which enhance cellular internalization and avoid endocytosis. Most synthetic carriers that can directly reach the cytoplasm through membrane fusion are preparations containing lipids. For example, some researchers combined fusogenic lipids (DMPC) with solid porous silicon nanoparticles (pSiNP) cores, loaded siRNA into pSiNPs via calcium ion precipitation, and showed that the uptake mechanism could be engineered to be independent of common receptor-mediated endocytosis pathways [170]. Marco et al. [171] also analyzed the composition and proportion of lipids and concluded that factors such as surface charge and particle size would affect the occurrence of membrane fusion, thus affecting the transfer of various nucleic acids. Since most membrane fusion is mainly a process driven by physicochemical properties of particles and plasm membrane, the fusion efficiency is basically irrelevant to cell types. However, it has also been suggested that there is a receptor-mediated membrane fusion process, allowing membrane fusion to act in an active-targeting manner as well.
3.5.4. Ligands functionalized NPs
Ligand modification is commonly used to promote cellular uptake along with cell targeting via ligand-receptor mediated mechanism. The ligands can be the antibodies (e.g., EGFR, VEGF), aptamers (e.g., AS1411), peptides (e.g., Cyclo (Arg-Gly-Asp) (cRGD), cell-penetrating peptides NRP-1), and bioactive small moleculars (e.g., mannose, folate, anisidine) etc. For example, Lee et al. [172] reported the engineered LNP formulations for the targeted delivery of RNA into different types of cells in the liver by screening the content of incorporation of active targeting ligands. The results showed that incorporation of mannose to LNPs with high PEG-lipid content (3%) allowed selective delivery of mRNA/siRNA only to liver sinusoidal endothelial cells (LSECs) through mannose-CD206 interaction.
Additionally, incorporation of peptides that specifically bind to a receptor that is highly expressed on the target cell is commonly used for enhancing cellular internalization. Besides, cell-penetrating peptides (CPPs) are oligopeptides consisting of 10–30 amino acid residues that have excellent membrane permeability [173]. CPPs typically have a large number of arginine (Arg) residues in their primary structures, and the guanidinium groups of the Arg residues are crucial to the penetration efficiencies of CPPs by forming bidentate hydrogen bonds with negatively charged carboxylic, sulfate, and phosphate groups of lipids, glycoproteins (GAGs) or other cell membrane constituents [174]. Kim et al. [175] constructed a CPP with nine arginines (with positive charge) and a macrophage-targeted TKPR (Thr-Lys-Pro-Arg) sequence specifically binding to the neuropilin-1 (NRP-1) receptor on the macrophage surface, and used this peptide to bind with siRNA to form cationic complexes for macrophages-targeted delivery. In addition to the critical roles of guanidine groups of CPPs, peptide conformation and hydrophobic content also have significant effects on CPP's penetration efficiencies. Several well-known CPPs, such as Pep-1, MPG, TP10, and melittin, either adopt inherent helical structures or form helices in the cell membranes, presenting a rigid amphiphilic structure to interact with the lipid bilayers to promote membrane permeation [176]. Yin et al. [159] developed a series of guanidinated and hydrophobic fluorinated bifunctional polypeptides with stable α-helical conformation for the pulmonary delivery of siRNA (Fig. 10C). The α-helical polypeptides were synthesized by modifying the side chains of the polypeptide PPOBLG with azido guanidine and different azido fluorocarbons via click chemistry. Similar to the structure of natural CPPs, the guanidine groups and α-helix could render strong cell membrane penetration, and fluorocarbon modification of the polypeptides further enabled transmucus penetration, which dramatically enhanced the mucus permeation capability and cell internalization by ∼240 folds.
Additionally, the wide availability and selectivity of monoclonal antibodies (mAbs) makes them a desirable strategy. The functional moieties upon the mAbs were commonly covalent conjugated with functional groups on the surface of the nanocarriers to provide the desired targeting features. In this way, several studies have demonstrated the targeted delivering RNAs preferentially to the desired cell subsets including cancer cells and notoriously hard to transfect leukocytes [177]. In addition, Camelid-derived single domain antibody fragments, commonly known as nanobodies (Nbs), which is the smallest naturally occurred antibody with recognition capacity against diverse antigen epitopes in nanomolar range. Due to their small size and unique structure, Nbs exhibit great advantages including flexibility to engineer, better solubility, chemical stability, low immunogenicity, and easy preparation. Zhu et al. [168] constructed a miRNA-embedded nucleic acid nanogel consist of DNA-g-PCL brush and crosslinkers miRNA-34a, and synthesized a short DNA segment with complementary sequence conjugated to the cysteine of nanobody (7D12) against EGFR at its C-terminal (Fig. 11B). By simply mixing the nanogel and Nb-DNA conjugates, the Nb-functionalized nanogel (Nb-nanogel) was formed and greatly promoted the accumulation at the tumor site and cellular uptake efficiency both in vitro and in vivo experiments.
Nevertheless, these chemical conjugation methods easily lead to damaged antibody functionality. Distribution of the antibodies' functional groups, both at the variable and the conserved regions, can cause a random orientation of mAbs upon the nanocarriers. These limitations cause a significant reduction in targeted delivery capabilities [178]. Besides, their clinical translation was also hindered because of the high batch-to-batch variability of current technologies, which rely on chemical conjugation. In addition to chemical conjugation, Dan et al. [179] developed a recombinant membrane-anchored lipoprotein (anchored secondary scFv enabling targeting, named ASSET), which was incorporated into siRNA-loaded LNPs and interacted with the antibody crystallizable fragment (Fc) domain. ASSET was composed of two functional domains—an N-terminal signal sequence followed by a short CDQSSS peptide NlpA motif that undergoes lipidation in bacteria and the scFv of a monoclonal antibody (clone RG7/1.30) that binds to the Fc constant region of Rat IgG2a antibodies.
However, several reports indicated that the use of ligands usually does not prevent liver and spleen uptake of NPs. Proteins from the plasma or serum were reported to absorb onto the surface of NPs, forming a protein corona that might mask the ligand attached to the NPs [180]. In the future, the development and expansion of the protein database, peptide and aptamer libraries could be useful for the RNAs targeting intracellular delivery. Furthermore, the influence of these ligands modification on the in vivo fate of nanocarriers still need to be investigated.
3.6. Avoid intracellular lysosomal degradation
Avoiding RNA drugs degradation by lysosomes is one of the most important responsibilities of the RNA nanocarriers. After RNA nanocarriers are up-taken by cells, intracellular pathways will be different according to cell types, nanocarrier properties, particle concentration, etc., and the entry mode of particles will also affect the subsequent intracellular pathways to a certain extent. Endosomal-lysosomal pathway is the most common intracellular transport pathway for NPs after entering cells. Since the low pH and enzymatic environment of lysosomes have strong damaging effects on RNA, Therefore, avoiding the degradation of RNA drugs by lysosomes is particularly important to ensure that RNA drugs reach the cytoplasm to exert their efficacy. At present, the existing design strategies for increasing the arrival of RNA to the subcellular target site can be roughly divided into two categories: One is the escape from the degradative pathway–endosomal/lysosomal escape strategy; the other is the bypass of the degradation pathway (non-degradable pathway) – the strategy of changing intracellular trafficking pathways.
3.6.1. Rapid escape from endosomes/lysosomes
Commonly used endosomal/lysosomal escape mechanisms can be roughly summarized as follows: membrane rupture, membrane destabilization and formation of membrane pores, membrane fusion, etc. Different mechanisms can be implemented through carrier materials with different characteristics.
Most classic and widely accepted hypothesis of endosomal/lysosomal escape by membrane rupture is the “proton sponge effect”, which is caused by the imbalanced osmotic pressure between endosomal/lysosomal lumen and cytoplasm. The endosomal/lysosomal escape behaviors of nanocarriers containing cationic lipids such as DOTAP, cationic polymers such as PEI and PAMAM, and calcium phosphates can be explained by “proton sponge effect”, which usually have ionizable amine groups and strong buffering capacities in the pH range of 5 to 7. However, the exact mechanism of “proton sponge effect” is still being debated [181]. Besides “proton sponge effect”, Vesicles containing gas that can release gas in acidic lumen triggered by ultrasound, laser or acid are proposed to break the endosomal compartments as well through membrane rupture [182]. To enhance bioavailability and improve endo/lysosomal escape of siRNA, Xu et al. [183] developed pH-activated NPs for augmented cytosolic delivery of POLR2A siRNA (siPol2). The core material of designed NPs was CS with a side chain of guanidinate (CG). The guanidinate could be reversibly combined with CO2 and the guanidine groups and amino groups on CS could bind with negatively charged siRNA. Under neutral conditions, it combined with CO2 to form carbonate, which could then be released under acidic conditions, thus inducing endosomal membrane rupture.
The occurrence of membrane destabilization and formation of membrane pores mostly involves CPP components. CPPs with amphiphilic and high positive charge characteristics [184], such as TAT peptides and other arginine-rich CPPs, can achieve endosomal/lysosomal escape of RNA drugs through membrane destabilization and membrane perforation. In the acidic endosomal compartment, the perforated peptide undergoes a conformational change by forming the α-helical structure, and then inserts into the hydrophobic region of the endosomal membrane and induces the formation of pores. The electrostatic interaction between positively charged CPPs and negatively charged endosomal membranes caused the instability of the membrane, achieving the endosomal/lysosomal escape of RNAs. The membrane destabilization ability of CPPs can be enhanced by screening and optimizing peptides, which is influenced by the sequence and length of amino acids [185].
The nanocarriers that can achieve endosomal/lysosomal escape through membrane fusion mostly mediated by fusion lipids together with cationic lipids. After the nanocarrier reaches the endosome, the cationic lipid can interact with the endosomal membrane rich in anionic lipids through electrostatic interaction. After that, the fusion lipid (such as DOPE) makes the lipid phase transform into an inverted hexagonal conformation [186], so that the fusion lipid is inserted into the endosomal membrane to realize the membrane fusion process.
3.6.2. Adjusting intracellular trafficking pathways of NPs
Another approach to avoid lysosomal degradation of RNA drugs is changing intracellular trafficking pathways. Some researchers believe that NPs entering cells via caveolin or macropinocytosis can bypass lysosomes and be directly transported to the endoplasmic reticulum (ER) or golgi apparatus. However, this statement has been questioned by some scholars. It is believed that the intracellular pathway of particles after entering the cell is related to a variety of factors. The current research in this field is still relatively scarce, and differences in particle types and cell lines will affect the conclusion as well. Therefore, it cannot be directly affirmed or denied such relevance. Studies have used ER retention signal motif KDXX or KDEL peptide to modify carriers to realize cargos' transport to ER [187,188]. Our group previously designed ER membrane-decorated hybrid nanoplexes that altered the intracellular pathway of siRNA. ER isolated from cancer cells was used to fabricate an integrative nanoplexes to alter the traditional intracellular pathway of vectors based on cationic lipid. After entering cells, siRNA was transported through endosome-Golgi apparatus-ER pathway. In this process, functional proteins on the ER membrane such as SNARE proteins and proteins bearing KDEL signal peptide played important roles on altering intracellular trafficking pathway of siRNA. This EM-decorated hybrid lipoplexes significantly improved gene silencing effect of siRNA and inhibited tumor cell growth in vitro and in vivo, which may become a promising strategy for nucleic acid drug delivery in the future [189].
A more thorough understanding of the causes of different intracellular pathways is conducive to better rational and on-demand design of vectors, so as to meet more precise subcellular localization and achieve better drug efficacy. Although there is still a lack of strategies on changing intracellular transport pathway in the field of RNA delivery, it can be regarded as a good direction for future development, provided that a better correlation between the change of pathways and RNA effect is established.
Endosomal/lysosomal escape strategies and intracellular pathway alteration strategies have great application prospects in the design of RNA delivery vectors. This analysis is based on the following aspects. 1) Most vectors enter the cell through endocytosis. After being endocytosed into cells, the early endosome is the first to reach, which is a very important sorting compartment and determines the path of RNA vectors. Therefore, proper vector design can either directly increase the proportion of RNA escaping from endosomal/lysosomal pathway to cytoplasm, or make RNA bypass or less pass through endosomal/lysosomal pathway to the site of effect through non-lysosomal pathway. 2) A very important factor that restricts the efficacy of RNA drugs on the market and in clinical trials is the low endosomal/lysosomal escape efficiency–only less than 1% RNA can escape to cytoplasm. So even a very small increase in escape rate can be very helpful in enhancing RNA effect or lowering administration dosage.
3.7. Enhanced intracellular release of RNAs
The activity of RNA therapeutics relies on the efficient delivery of the RNAs to the cytosol of the cells at the target site, where it is translated into the active protein or exert gene editing/silencing effects. Meanwhile, released RNAs from nanocarriers in its stable and active form into cytosol is essential to fully accomplish the genomic therapy potential.
So far, nanocarriers can achieve this release process either by introducing stimuli-sensitive chemical moieties that break the backbone of delivery system in response to endogenous stimuli (GSH, ROS, pH, enzymes) or through self-immolative charge alteration, which terminates the electrostatic attraction between the vector and RNAs.
In general, distinct characters between targeted tissue/cells and normal tissue/cells (e.g., cancer cells or tumor microenvironment vs normal tissue environment) act as a stimulus of the RNA release. Nanocarriers made for such a stimulus carry the RNAs in physiological environment while release RNAs when such nanocarriers enter in the distinct environment of the targeted cells and generate structural change/destruction in response to the certain stimuli. The stimuli usually include intrinsic (redox potential, enzymes, cofactors, enzymatic products, and pH) or extrinsic stimuli (ultrasound, external magnetic field, temperature, and light). Examples of different nanocarriers used for RNAs release are listed in the Table 8 .
Table 8.
Examples of different nanocarriers used for RNAs release.
| Types and mechanism of RNA release | Stimuli-sensitive chemical groups | Nanocarriers components | Release profile | Reference |
|---|---|---|---|---|
| pH-responsive PIC micelles disassembly | Polyester carboxylic groups | PEG43-b-PCL12 (COOH)6.5, PLL, siRNA | The twice higher fluorescence intensity of released Alexa488-siRNA at pH 5.0 compared to pH 7.4 | [99] |
| Acid-responsive “shrinking”-degradation of shell | Diamine linker A | Chol-PAA, DODAP, DOPC, siRNA | At pH 5.5, ~60% of the total amount of encapsulated dsDNA is released from the A-cross-linked PCLs within 12 h and near-quantitative release is observed within 48 h. | [190] |
| pH-sensitive separation of the shell and core of micelles | Hydrazone bond | cRGD-PEG-Hz-CS-HA2, octyl-Lys-9R, siRNA | Declined FRET effect between CdSeQDs and Cy3-siRNA with incubation time from 2–6 h | [191] |
| GSH-responsive degradation of polymer | Disulfide bond | Ad-PEG, β-CD-SS-PDMAEMA, siRNA | The spatial confocal microscopic images showed that green dots (FITC-siRNA) and red dots (RITC-CD-SS-P) were observed separately after internalization | [192] |
| GSH-responsive separation of hydrophilic and hydrophobic block | Disulfide linkages | mPEG-PCL-g-SS-PDMAEMA, siRNA | Micelleplexes treated with 10 mM DTT yielded siRNA migration identical to free siRNA | [193] |
| GSH-responsive degradation of polymer | Disulfide bonds | A single linear PBAE with disulfide bonds along the polymer backbone, siRNA | Complete siRNA release after incubated NPs with 5 mM glutathione for 15 min | [193] |
| Hydrazide can react with the α-keto acid structure of anionic pyruvate to form α-oxohydrazone, resulting in the reduction of the cationic net charge of the cationic polymer | Hydrazide | PGlu [diethylenetriamine (DET)/hydrazide], siRNA | The siRNA release ratio was 25% when the pyruvate concentration was 3.0 mM | [194] |
| ROS-responsive hydrophobic-to-hydrophilic shift and production of carboxyl groups to interfere with electrostatic interactions | Phenylboronic ester | Ang-PEG-b-PGu, PEG-b-P(Gu/Hb), siRNA | Efficient siRNA release upon treatment NPs with H2O2 at concentrations above 0.1 × 10−3 M | [195] |
| ATP-responsive charge reversal | Borate ester bond | Chol-Dopa, PEI-FPBA, siRNA | The FRET ratios of cy3- and cy5-siRNA co-loaded polyplexes decreased sharply to about 20% with ATP treatment | [196] |
| Self-immolative degradation of oligo(carbonate-β–α-amino ester) into a neutral diketopiperazine through an ester–amide cyclization reaction | Oligo(carbonate-β–α-amino ester) | Oligo(carbonate-β–α-amino ester), mRNA | Self-immolative rearrangement (t1/2 = 2 min) | [197] |
| Self-catalyzed hydrolysis of PDMAEA, which degrades from a polycation into polyanionic pAA in the presence of water releasing DMAE | PDMAEA | PDMAEA-PImPAA-PBA, siRNA | – | [198] |
Abbreviation: pAA: poly(acrylic acid), DMAE: dimethylaminoethanol, PEI-FPBA: 3-fluoro-4-carboxyphenylboronic acid-grafted PEI, PDMAEA-PImPAA-PBA: poly(dimethylaminoethyl) acrylate-P(N-(3-(1H-imidazol-1-yl)propyl)acrylamide-poly(butyl acrylate).
3.7.1. Nanocarrier disassembling by electrostatic repulsion
Since most of nanocarriers encapsulated RNA by electrostatic interaction using cationic segments, undesired intracellular RNAs binding with cationic segments and insufficient RNAs release would hinder the effectiveness of RNA. Thus, stimuli-responsive charge reversal of cations from positive charge to negative charge was used to achieve RNAs release by electrostatic repulsion. In this strategy, Sun et al. [196] synthesized PEI-FPBA as the polycations binding with siRNA, and dopamine (with diol-containing moiety) conjugated with cholesterol as the hydrophobic part (Chol-Dopa). The ultimate micelles (PFCDM) were assembled by the reaction between FPBA and Dopa via borate esters. Upon cell internalization, the intracellular negatively charged ATP could compete with Dopa to bind with FPBA, then effectively triggered the disassembly of micelles and reversed the charge of PEI-FPBA from positive to negative, forcing the release of siRNA by electrostatic repulsion (Fig. 12A).
Fig. 12.
(A) ATP-Triggered Breakage of FPBA-Dopa and ATP-Activated Charge Reversal of PEI-FPBA [196]. Reproduced with the permission from Ref. 196. Copyright © 2018 American Chemical Society. (B) Schematic illustration of formation of Ang-3I-NM@siRNA stabilized by the three “triple-interaction” forces, namely, electrostatic, hydrogen bond, and hydrophobic interactions, and mechanisms of ROS triggered siRNA release. In the presence of tumoral ROS, the hydrophobic phenylboronic ester is converted to the hydrophilic counterpart bearing carboxyl groups. This process first reduces the hydrophobic stabilization force and subsequently interferes with the electrostatic and hydrogen bond interactions resulting in effective siRNA release [195]. Reproduced with the permission from Ref. 195. Copyright © 2019 John Wiley and Sons.
Similarly, the combination of hydrophobic-hydrophilic transition and charge reversal of nanocarriers was also used for RNAs release. Shi et al. [195] used PEG-block-poly [(N-(3-methacrylamidopropyl) guanidinium-co-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl acrylate)] (PEG-b-P(Gu/Hb)), Ang-PEG-block-poly(N-(3-methacrylamidopropyl) guanidinium) (Ang-PEG-b-PGu) and siRNA to construct a ROS-responsive nanomedicine. This “triple-interaction”-stabilized Ang-3I-NM@siRNA nanomedicine was based on the electrostatic and hydrogen bond interactions of the Gu+/PO3 4− bridge together with a hydrophobic interaction of phenylboronic ester. When encountered the reactive oxygen species enriched in cancer cells (ROS concentration in cancer cells up to 100 μM), the hydrophobic phenylboronic ester was converted to its hydrophilic counterpart with carboxyl groups. This hydrophobic-to-hydrophilic shift initially depleted the hydrophobic stabilization force and subsequently the newly produced carboxyl groups interfered with electrostatic and hydrogen bond interactions. This sequential “self-destruct” process enabled effective siRNA release (Fig. 12B).
3.7.2. Stimuli-responsive cleavage of nanocarrier backbone
In additional to electrostatic repulsion-based intracellular release of RNAs, introducing stimuli-sensitive chemical groups into the nanostructure to realize the cleavage of backbone and disassembly of nanocarriers in the specific intracellular environment is another strategy to facilitate rapid RNAs release.
Farokhzad et al. [199] developed a hypoxia-responsive NP (HRNP) by self-assembly of the hypoxia-responsive polypeptide, mPEG-block-poly(l-glutamide-graft-2-nitroimidazole) (mPEG-b-(PLG-g-NI)), and cationic lipid-like compound (G0-C14) for delivery of CDC20 siRNA. Under the hypoxic tumor environment, conversion of the hydrophobic NI group to a hydrophilic 2-aminoimidazole (AI) occured as a result of a series of nitroreductases that catalyzed a single-electron reduction, leading to the disassembly of HRNP and a subsequent rapid release of cargo in tumor cells. Yan et al. [200] synthesized the furoxans-grafted PEI polymer (FDP) with caspase-3 responsive cleavable DEVD linker for siRNA delivery. After GSH-triggered NO release, and then increase the activity of caspase-3, the DEVD peptide sequence was cleaved to facilitate the disassembly of FDP/siRNA nanoplexes, thereby resulting in increased siRNAs of ~40% were released at 48 h and enhanced gene downregulation effects by ~2 fold compared with the caspase-3 non-responsive FDnP/siRNA nanoplexes.
Additionally, introducing the self-degradable moieties into the nanocarriers to achieve charge reversal via sequential self-immolative process have taken the above two strategies together, which may offer another route for RNA release. In this mannner, not only the nanocarriers can achieve biodegradable, but also the RNAs can be effectively released. Waymouth et al. [197] reported a highly effective mRNA delivery system comprising charge-altering releasable transporters (CARTs). The oligo(carbonate-b-α-amino ester)s were synthesized by facile organocatalytic ring-opening polymerization (OROP) and global deprotection, and then functioned as polycations that noncovalently complexed, protected, and delivered polyanionic mRNA in to the cytosol. As the pH was raised toward basic conditions, the oligo(α-amino ester)s rapidly degraded in <5 min through intramolecular rearrangement of ester-to-amide isomerizations and controlled self-immolative degradation, during which cationic amines were converted to neutral amides. This charge alteration reduced or eliminated the electrostatic anion-binding ability of the originally cationic material, thereby facilitating decomplexation and release of anionic mRNA into the cytosol for translation.
Another common change is the self-catalyzed hydrolysis of PDMAEA (pKa ~ 7.1), which degrades from a polycation into negatively charged and non-toxic PAA in the presence of water releasing DMAE [201]. Moreover, the degradation time to form PAA is independent of pH (as tested between pH 5.5 and 10.1) and the molecular weight of PDMAEA. Monteiro et al. [202] reported a polymer carrier consisted of a diblock copolymer with a first block of PDMAEA binding to siRNA. A second block consisting of PImPAA and PBA was designed to induce fusion with the endosome membrane that resulted in escape of the polymer/siRNA complex to the cytosol where release of the siRNA could occur after degradation to PAA (Fig. 13 ). The results showed the full release of siRNA from the nanocarriers at pH 7.6 after 17 h in water.
Fig. 13.
(A) Polymerization and hydrolysis process of PDMAEA [203]. Reproduced with the permission from Ref. 203. Copyright 2011 American Chemical Society. (B) Mechanism for polymer assembly, binding with siRNA and release of siRNA through a self-catalyzed degradation of PDMAEA [198]. Reproduced with the permission from Ref. 198. Copyright 2013 Springer Nature.
Overall, the stimuli-responsive RNAs platforms are highly desired for RNAs delivery and enhanced therapeutic effects of RNAs. It was worth noting that the response sensitivity of nanocarriers to above endogenous stimuli in specific disease states is an important factor for effective release of RNAs. The levels of these triggers can vary between cell lines/ cell environments and even within the same tissue or organ, resulting in variable rates of degradation. This can produce inconsistent results, especially when going from in vitro to in vivo experiments. Furthermore, after degradation, the materials should form biologically benign components avoiding toxic buildup in the tissues.
Currently, the complication arises in that the process of how RNAs are released from the nanocarriers in spatiotemporally programmable manners, including the detailed released kinetics, intracellular bioavailability and structural integrity of RNAs, are still unclear and lack of advanced evaluation methods.
3.8. The balance consideration for safety and efficacy of RNA delivery systems
Considering for clinical translation, the excipients or materials used for RNA formulation should be biocompatible and tolerable to ensure the safety of therapeutic RNA products and avoid excipient-induced immune activation. Additionally, the functional synthetic non-viral vectors that could protect RNA against degradation by ribonucleases, accumulate in specific tissue, facilitate cell internalization, and allow for the controlled release of the encapsulated therapeutic, were usually comprehensive nanosystems associated with unexpected adverse action. Therefore, the balance consideration for safety and efficacy of RNA delivery systems is to be necessary. The stable encapsulation and tissue/cell-specific targeting delivery systems would be beneficial to avoid biological effects of RNA drugs inducing unwanted toxicities in non-target tissues and cells. Meanwhile, efficient endosomal escape and controlled release of RNAs would also provide advantage for increasing the intracellular concentration of drugs, leading to reduction of concentration-dependent side effects such as off-target effects and immune stimulation.
The problems of positive charge-associated plasma protein aggregation, immune stimulation and nonspecific tissue accumulation are often concerned. In lipid-based RNA delivery, cationic lipids were usually used an important materials for condensate RNA molecules in positive-charged nanovectors, which can interact with several proteins, lipoproteins, leading to the formation of the aggregates or premature release of the RNAs leading to the systematic toxicity [204]. In addition, cationic lipids can inhibit the protein kinase c activity and induce lung inflammations. They can also induce non-specific hepatic toxicity [205]. Moreover, PEG-lipid have reported to induce “accelerated blood clearance” phenomenon (ABC) attributed to the formation of PEG-specific antibodies and generate a potent IgM antibody response to PEG after repeated doses of PEGylated liposomes. For example, rare events of anaphylaxis have been reported after vaccination with mRNA-1273 and BNT162b2, with an incidence currently estimated at approximately 1 in 100,000. It has not been elucidated which component of the vaccine is responsible for allergic reactions, but there might be a possible role for the PEGylated lipid and a pre-existence of anti-PEG antibodies (IgEs and/or IgGs), as recently discussed in other reviews [206]. Meanwhile, polymer-based delivery systems also hold the drawback of undefined structural composition-associated potential toxicities, non-biocompatibility and non-biodegradability, and even unknown degradable fragments-induced metabolic toxicity in vivo.
Moreover, Nonspecific immune response to the therapeutic RNAs may initiate the unwanted side effects are also concerned. Although RNAs has specific intracellular target, unintended effects of the other genes having partial complementary regions can cause severe side effects (“off-target” effects). In addition to this, immune activation is another severe issue related to RNAs. Generally, free siRNAs in circulation could be recognized by toll-like receptors (TLRs), such as TLR3, TLR7, and TLR8 [207], to provoke innate immune responses or cytokine release syndrome, which are related to the dose, sequence, size, and folding of RNA agent. There is also growing interest in developing recombinant RNAs made and folded in living cells in vivo [208].
Up to now, several non-viral RNAs delivery systems were failed in clinical trials due to toxicity. The CALAA-01 (Calando Pharmaceuticals-01) is the first targeted, polymer-based NP-carrying siRNA to be systemically administered to humans for the treatment of solid tumors in 2008. CALAA-01 is a four-component system that is manufactured as a two-vial formulation for clinical dosing (Fig. 14 ). Vial 1 contains siRNA designed to reduce the expression of the M2 subunit of ribonucleotide reductase (RRM2). Vial 2 contains a mixture of three delivery components: (i) a linear, cationic cyclodextrin-based polymer (CDP), (ii) a hydrophilic polymer [adamantane PEG (AD-PEG)] used to promote NP stability in biological fluids, and (iii) a human transferrin protein (hTf)-targeting ligand (AD-PEG-hTf) displayed on the exterior of the NP to engage Tf receptors (hTfR) on the surface of the cancer cells. Vials 1 and 2 are mixed at the bedside, and the components self-assemble into ∼75 nm NPs [209]. Although CALAA-01 formulations had a good pharmaceutical quality, it was terminated at phase Ib study due to the dose-limiting toxic events (DLTs) [210,211]. Additionally, The SNALP is a first-generation LNP developed by Tekmira that is designed to deliver the siRNA to the targeted tissue by IV injection. The first drug (TKM-ApoB or PRO-040201) is an siRNA that targets the mRNA of ApoB and is designed to indirectly reduce the uptake of cholesterol in cells. A total of 17 patients received TKM-ApoB and one of the two that received the highest dosage of the drug exhibited flu-like symptoms that were consistent with siRNA induced immune stimulation. Although the drug did not show evidence of toxicity in the liver, the Phase I clinical study (NCT00927459) was terminated because patients exhibited only transient reductions in cholesterol levels [212] .
Fig. 14.
Components and formulation of targeted NP-containing siRNA. (A) The delivery components are (i) a water-soluble, linear cyclodextrin-containing polymer (CDP), (ii) an adamantane (AD)-PEG conjugate (PEG MW of 5000) (AD-PEG), and (iii) the targeting component that is an adamantane conjugate of PEG (PEG MW of 5000) that has human transferrin (Tf) conjugated at the end opposite to the adamantane (Tf-PEG-AD). (B) The formulation contains two vials, one with siRNA and the other with the delivery components. When the two vials are mixed together, the targeted NPs form via self-assembly of the four components [213]. Reproduced with the permission from Ref. 213. Copyright © 2009 American Chemical Society.
Whereas the successful RNA-based therapies have been approved on the market, there are still certain challenges for systemic administration of RNA-based therapies using non-viral vectors. The challenges of stability are greater for mRNA-based therapy than for siRNA-based therapy, as the chemical modifications that confer stability on siRNA duplexes can often render mRNAs ineffective. The structure-activity/toxicity relationship of nanocarriers, especially the influence of each component within the NPs, as well as the in vivo spatiotemporal fate of RNAs during the systematic delivery are also of great importance. Exploring the proper and advanced technologies for more detailed studies of toxicities in vivo may become the research focus.
Overall, to push forward RNAs from bench to clinic, improvement and balance in safety, delivery strategy, pharmacokinetics, and pharmacodynamics is required. Several hurdles mentioned above like endosomal escape, lower cellular uptake, rapid excretion, degradation by nucleases, and immune stimulation need to be overcome to achieve the maximum potential of the therapeutic RNAs. Combining all the solutions in one delivery system could lead to the development of the ideal nanocarrier, which, however, is a difficult task. Combining all the elements to get in one single vector would increase the complexity of the delivery system. Hence, it is indispensable to study the overall physiochemical aspects of each component, how they complement each other's activity and, at the same time, perform their function independently. However, it is generally difficult for synthetic RNA delivery system to overcome all the hurdles despite the development of complicated structure with several functions. Nowadays, biomimetic delivery systems have attracted more and more interest on RNAs delivery, which exhibited improved balance of toxicity and efficacy.
4. Biomimetic vectors for RNAs delivery
The biomimetic vector is a new type of drug delivery system that has rapidly emerged in recent years. These biomimetic nanocarriers are usually constructed by taking advantage of endogenous substances, the function of the inherent protein, the endogenous processes, or certain biological structures. Endogenous substances include cells, biomembranes, proteins and organelles, etc.; endogenous processes have a wide range, such as exosomes-mediated signal transduction or substance transmission [[214], [215], [216], [217]]), T cell killing [[218], [219], [220], [221]], antigen presentation [222,223], blood coagulation [[224], [225], [226]], virus invasion [227], etc. Naturally occurring structures refer to virus structures [[228], [229], [230], [231]], exosome structures [232,233], cell structures [231,[234], [235], [236]], and so on. “Mimetic” refers to the imitation of “bio”, either by mimicking their basic functions by in vitro recombination to reproduce endogenous process or mode of action, or by using endogenous cells to achieve the direct production of drug-loading carriers, in order to make the drug delivery system obtain good targeting, low immunogenicity and other complex functional characteristics suitable for drug efficacy. Currently, different biomembrane originated from various types of cells, such as stem cells [[237], [238], [239]], human embryonic kidney (HEK)-293 cells [240,241]), RBCs [242,243]), platelet [244], endothelial cells [245], T cells [246] and cancer cells [247,248], have been developed as non-viral vectors for RNA delivery ( Table 9 ) ( Fig. 15 ). The emergence of the biomimetic drug delivery system can complement the advantages of synthetic carriers and realize functional integration to jointly solve the problems existing in each step of RNA delivery, which is a promising drug delivery system.
Table 9.
Examples of biomimetic vectors for RNAs delivery.
| Biomimetic types | Endogenous components | RNA Type | RNA loading materials | The function of endogenous components | Reference |
|---|---|---|---|---|---|
| Cell membrane | Cell membrane of H1299 cells (a human NSCLC cell line) | siRNA | PBAE |
|
[262] |
| HeLa cell membranes | siRNA | PLGA |
|
[247] | |
| MSCs membrane | miRNA | Mesoporous silica |
|
[239] | |
| MSCs membrane | siRNA | Polydopamine (PDA) |
|
[249] | |
| RGD decorated macrophage membrane (anchored by click chemistry) | siRNA | Fe3O4 magnetic nanoclusters (PEI as a surfactant) |
|
[250] | |
| cRGD modified red blood cells (RBCs) membrane (lipid insertion) | siRNA | Bovine serum albumin (BSA) |
|
[16] | |
| Angiopep-2 peptide-modified RBCs membrane (lipid insertion) | siRNA | PEI |
|
[242] | |
| Platelet membrane | siRNA | Zeolitic imidazolate framework-8 (ZIF-8) metal-organic framework (MOF) | Tumor-targeting capability | [244] | |
| Whole blood cells | mRNA | Blood cells |
|
[284] | |
| Extracellular vesicles | Rabies virus glycoprotein (RVG) modified exosomes from self-derived dendritic cells (plasmid) | siRNA | Exosomes |
|
[263] |
| RVG modified exosomes derived from murine dendritic cells | siRNA | Exosomes | The ability to cross the blood-brain barrier (BBB) | [251] | |
| AS1411 aptamer-modified EVs (cholesterol conjugation) | miRNA /siRNA | EVs |
|
[252] | |
| EVs from M1 macrophages | siRNA | EVs |
|
[285] | |
| Exosomes from human embryonic kidney (HEK) 293 cells modified with GE11 peptide or EGF (plasmid) | miRNA | Exosomes | Targeting delivery of therapeutic molecules to EGFR-expressing tumors in vivo | [241] | |
| exosomes from HEK 293 cells | miRNA | Exosomes | The ability to deliver miRNA | [286] | |
| anti-HER2 single-chain variable fragment modified EVs from HEK 293 cells | mRNA | EVs |
|
[287] | |
| EVs from HEK 293 T cells and human fibroblast | siRNA (integration in the pre-miR-451 backbone) | EVs | Reduction of therapeutic dose of siRNA | [288] | |
| Tetrahedral DNA nanostructures (TDNs) modified EVs from HEK 293 T cells | gRNA | EVs | The ability to transfer biomolecules | [280] | |
| RVG modified exosomes from HEK 293 T cells (plasmid) | siRNA | Exosomes |
|
[289] | |
| microvesicles from HEK 293 T cells | mRNA | Microvesicles | The ability to carry therapeutic mRNA/protein for cancer treatment | [290] | |
| Exosomes from HEK 293 T cells | mRNA | Exosomes |
|
[291] | |
| EVs from HEK 293 T cells and MCF-7 cells | siRNA | EVs | The ability to deliver small RNA cargo | [292] | |
| Exosomes derived from MSCs | miRNA | Exosomes |
|
[293] | |
| Exosomes from fibroblast-like mesenchymal cells | siRNA or shRNA | Exosomes |
|
[294] | |
| EVs from MSCs | miRNA | EVs |
|
[295] | |
| EVs from adipose tissue-derived MSCs | miRNA | EVs |
|
[296] | |
| Exosomes from bone marrow MSCs | siRNA | Exosomes |
|
[297] | |
| Exosomes from bone marrow MSCs | siRNA | Exosomes | Fine histocompatibility | [298] | |
| exosomes derived from marrow stromal cells | miRNA | Exosomes | Delivery vehicles for anti-tumor miRNA therapy | [299] | |
| RVG modified exosomes from bone marrow MSC (plasmid) | miRNA | Exosomes | The ability to cross the BBB | [300] | |
| brain endothelial cell-derived exosomes | siRNA | Exosomes | The ability to cross the BBB | [301] | |
| exosomes derived from primary endothelial cells | siRNA | Exosomes | The capability to accommodate and deliver short foreign nucleic acids into endothelial cells | [245] | |
| bioengineered bacterial outer membrane vesicles (OMVs) (genetically fused ClyA with an HER2-specific affibody) | siRNA | OMVs |
|
[302] | |
| exosomes originating from HeLa and ascites | siRNA | Exosomes | A carrier for siRNA delivery in vitro | [303] | |
| EVs from Hs578Ts(i)8 cells | miRNA | EVs | The ability to deliver miR-134 in vitro | [304] | |
| mouse fibroblast L929 cell-derived microvesicles | siRNA | Microvesicles |
|
[305] | |
| microvesicles derived from human adult liver stem cells (HLSC) | miRNA mimics or miRNA inhibitors | Microvesicles | The ability to transfer cargos | [306] | |
| Plasma exosomes | siRNA | Exosomes | The ability to deliver heterologous siRNAs to human blood mononuclear cells and lymphocytes in vitro | [307] | |
| EVs from RBCs | ASO, sgRNA, mRNA | EVs |
|
[281] | |
| Exosomes secreted from B16F0 melanoma cells | lncRNA | Exosomes | The ability to transfer Gm26809 lncRNA to fibroblasts | [308] | |
| EVs from endothelial progenitor cells | lncRNA | EVs | The ability to transfer TUG1 lncRNA to macrophages | [309] | |
| EVs from HEK293 cells | lncRNA | EVs | The ability to package and deliver of H19 lncRNA | [310] | |
| EVs from hepatocellular carcinoma | lncRNA | EVs | The ability to delivery PART1 lncRNA to macrophages | [311] | |
| Exosomes from oxaliplatin-resistant colorectal cancer cells and HEK 293 T cells | circRNA | Exosomes |
|
[312] | |
| Exosomes from synovial mesenchymal cells | circRNA | Exosomes | The ability to transfer circEDIL3 into fibroblast-like synoviocytes with intact functional activity | [313] | |
| Exosomes from human malignant glioma cell lines U251 | circRNA | Exosomes | The ability to transfer circ_0042003 to U251 cells | [314] |
Fig. 15.
Biomimetic vectors for RNAs delivery. Many kinds of cells can be used as a source for biomimetic vectors including RBC, white blood cell (WBC), platelet, mesenchymal stem cell (MSC), cancer cell, et al. cell membrane originated from these cells (a) can be directly used to coat RNA loaded nanoparticles to produce biomimetic nanoparticles inheriting donor cells' features. Or by transfecting plasmids into cells (b) to realize active RNA loading process with the help of endogenous mechanism of parent cells. Or by first isolating EVs and then loading RNA drugs (c) to construct RNA loaded EVs.
In designing of synthetic vectors, there is often a contradiction among giving the vector long circulation, low immunogenicity and high uptake properties simultaneously, while the composition is too complicated. For biomimetic vectors, they can fulfill the requirements at the same time due to the introduction of unmodified endogenous substances [239,[247], [248], [249]] or engineered biological components [242,[250], [251], [252]].
Firstly, the biomimetic carrier well inherits the natural multifunctional characteristics of endogenous substances, such as long circulation, biocompatibility and homologous targeting ability (Table 9) [239,248]). The long circulation and application safety in vivo of biomimetic carriers lie in the body's self-recognition mechanism of endogenous substances. For example, CD47, a “don't eat me” signaling molecule, is expressed on surface of all cells in the body [253,254]. Membrane-bound complement regulatory proteins such as CD55 can inhibit the activation of complement system [255]. In addition, the surface potential of endogenous protein and membrane is negative, and the negative surface charge can avoid the dilemma of coagulation, hemolysis and rapid clearance of cationic carriers during intravenous injection, achieving long circulation [[256], [257], [258], [259]]. Therefore, the use of endogenous substances to camouflage nanocarriers can help them evade clearance by the immune system, prolong circulation time, and improve biocompatibility. The homologous targeting ability also relies on the inherent targeting properties of proteins on endogenous membranes [[260], [261], [262]].
Considering of long circulation, low immunogenicity and innate targeting ability of biomimetic vectors with inherent protein on their membrane, the easy programming/modification characteristics of cells or cell membranes can be exploited to construct engineered cells or cell membranes, endowing subsequently constructed biomimetic vectors with specific or enhanced targeting ability. Methods mainly involve: 1) plasmid introduction to construct fusion proteins in order to endue membrane components with targeting ability [263]; 2) avidin-biotin interaction [264]; 3) biorthogonal functionalization strategy [250,[265], [266], [267]]; 4) linking targeting peptides to lipid molecules to introduce exogenous ligands by membrane insertion [16,242]. All these methods, which endow carriers with targeting function by introducing endogenous substances, avoid the cumbersome chemical targeting modification and the potential influence of uncontrollable factors. It is beneficial to vectors' construction and production and has a certain application prospect. Recently, Xue's group [16] designed and constructed a targeted worm-like biomimetic gene vector and studied its application in tumor therapy ( Fig. 16A ). The targeting modules, cRGD (targeting overexpressing αvβ3 integrin on the membrane surface of B16F10 cells), was introduced in RBCs membrane by lipid inserting of cRGD-PEG-DSPE spontaneously. The vector (RGD-RBC-RP) is obtained by wrapping the targeted erythrocyte membrane around a charge-reversible gene-protein core (worm-like), in which bovine serum albumin (BSA) is used to concentrate and protect siRNA. At the same time, pharmacokinetics and biological tissue distribution experiments were used to study the effect of worm-like nanoerythrocyte on circulation in vivo. The results indicated that RGD-RBC-RP had longer circulation time and more tumor accumulation. Intravenous injection of RGD-RBC-RP significantly knocked down survivin expression level and enhanced anti-tumor efficacy against B16F10 tumors. Therefore, modified erythrocyte membrane encapsulation is expected to achieve immune escape, long blood circulation and enhanced targeting capacity of gene carriers, thereby improving the bioavailability of siRNA.
Fig. 16.
(A) Schematic illustration of preparation and charge-reversible profile of RGD-RBC-RP [16]. Reproduced with the permission from Ref. 16. Copyright © 2018 John Wiley and Sons. (B) Schematic description of the architecture of the genetic circuit [268]. Reproduced with the permission from Ref. 268. Copyright © 2021 Springer Nature. (C) Schematic summarization of the exosomes production by packaging cells via the RNA-HuR recognition [269]. Reproduced with the permission from Ref. 269. Copyright © 2019 American Chemical Society.
In addition, the development of biomimetic drug delivery systems has also put forward insights into the loading methods of RNA. The new RNA loading modes brought by the development of biomimetic carriers are mainly active loading modes related to extracellular vesicles (EVs) ( Fig. 15 ), which are naturally secreted nanoscale vesicles and used for intercellular communication or intracellular waste discharge [[270], [271], [272], [273]]. EVs contains various types of RNA, including miRNA, lncRNA, mRNA, ribosomal RNA (rRNA) and circular RNA (circRNA), etc. [274,275], which are ideal RNA delivery carriers of natural origin. Up to now, EVs have been widely used as non-viral vectors for RNA delivery [276], including mRNA [8], siRNA [277,278], miRNA [279], sgRNA [280], ASO [281], etc. There are about 1014 EVs in the human body. Such a huge number of EVs can exist in body fluids and perform different functions in an orderly way, which must have particular modes of regulation. And, perhaps the answer to non-liver targeting is also hidden here. Therefore, how to better understand EVs as a natural signal or substance delivery carrier in the body, how to rationally modify the circulatory system of EVs naturally present in the body instead of treating them simply as ordinary lipid vesicles to exert drug-carrying capacity, and how to truly achieve “biomimetic” is the core point we need to think deeply about.
It is a well-known cell-mediated and active RNA self-packaging mode that cells express specific fusion proteins through plasmid or lentiviral transfection to give exosomes the ability to actively sort and encapsulate RNA [282]. This approach cleverly borrows the natural intracellular biosynthesis pathway of exosomes. Active RNA loading and release during exosome biogenesis is realized by the fusion of exosomal membrane proteins and different types of RNA binding proteins [274]. Li et al. [269] fused exosomal membrane protein CD9 with RNA-binding protein HuR (human antigen R) ( Fig. 16C). HuR locating inside the vesicles could bind to miRNA or mRNA either natively harbors or is engineered with HuR recognition motif (AU rich element). The loading efficiency of RNAs of interest was improved in engineered exosomes and the enriched RNAs could be functional in the recipient cells. However, the release mechanism in target cells still needs to be further refined.
In 2021, Fu et al. [268] designed a novel approach to generate vectors containing therapeutic siRNA, moving the production site of RNA delivery system from in vitro to in vivo ( Fig. 16B). Plasmid DNA (pDNA) encoding an optimized siRNA expression part and a tissue-targeting tag fused to the N-terminus of Lamp2b was designed and synthesized in vitro. After intravenous injection of pDNA, host liver cells were reprogrammed by the genetic circuit. Driven by the promoter part, specific siRNA was transcribed and assembled into secreted exosomes anchored by guidance tags, which were delivered to desired tissues through blood circulation. Controllable RNA self-assembly and targeted delivery were induced in a dynamic environment in vivo and this therapeutic strategy was validated in three disease models. By transferring the model of in vitro cell factory to in vivo application and constructing in vivo cell factory to realize the “self-production and self-marketing” of RNA drug delivery system, it can not only avoid the tedious steps of large-scale extraction and purification of exosomes, but also reduce cost, which is beneficial to quality control and clinical translation. Currently, two exosomes-based RNA delivery vectors are in the clinical trials. The mesenchymal stromal cell-derived exosomes to deliver KrasG12D siRNA is in phase I clinical trial (NCT03608631), which is used for the treatment of metastatic pancreatic ductal adenocarcinoma (PDAC) with KrasG12D mutation [294]. Another biomimetic vectors-based RNA drug, allogenic mesenchymal stem cell-derived exosomes to deliver miR-124, is used for acute ischemic stroke and currently in phase I/II clinical trials (NCT03384433).
5. Summary and prospect
Since the RNA drugs have been successfully marketed, especially the mRNA vaccines, which have been approved most recently to combat against the unprecedented COVID-19 pandemic, a new era of RNAs has been opened up and witnessed. As RNAs provide a high degree of gene selectivity, which is difficult to achieve with the current treatment options, they hold massive potential to be used as a therapeutic tool for genetic disorders. With significant development in the nanocarriers and simultaneous advancement in the chemical modification, more and more RNA drugs translation from bench to bed will have good feasibility. Moreover, it can be believed that with the booming of RNA-based therapies, wider range of therapeutic targets and more diversity of diseases treatable from rare genetic disorders to common ones, such as infection and cancer, will be expanded in the future.
Although enormous improvement of non-viral vector has been achieved over the past few years, only a few RNA therapeutics have been successfully marketed. Part of the reason could be attributed to the numerous biological obstacles in delivery, difficulty of quality control, and the balance of the efficacy and safety of nanomedicine in vivo. Continuous effort is required to improve and develop a mature and sophisticated strategy for delivery system design. Especially strategies for the endosomal escape, cell and tissue targeting, and development of the novel biomaterials are crucial for the translation of RNAs from bench to bed. Moreover, the limitations of cost-effectiveness of RNAs, complexity of production, and standard of quality control are also vital. While great progress in the development microfluidic systems and RNA synthesis procedures, providing scaling up feasibility, reproducibility and reduced immunogenicity for production of RNA nanocarriers, there is still a major technological gap limiting efficient in vivo transfection in specific cells. Besides, it is necessary to reduce the batch-to-batch variation of synthetic nanocarriers and build up quality evaluation standards for RNAs delivery systems.
The advantages of biomimetic vectors provide new insights into the delivery mode of RNA in vivo. Compared with the complexity of the synthesis non-viral vectors with integration of multi-functional modules, biomimetic carriers can obtain composite functional characteristics through introduction of a single endogenous module. The biomimetic carrier not only has excellent circulation and biocompatibility, but also is easy to obtain different targeting properties through innate acquisition or acquired modification. However, there is no biomimetic vectors-based RNA drug approved in the market currently. The reasons why the biomimetic vectors has not yet achieved clinical translation mainly include batch-to-batch variation, difficulties of large scale production and immunogenicity and side effects of long-term application due to the complexity of heterologous components [283]. The solution of these problems and the expansion of available exogenous biomaterials will promote the further development of biomimetic carriers and RNAs delivery; so that the valuable biomimetic carriers will be truly benefit clinical patients.
Declaration of Competing Interest
The authors stated that they have no competing interests.
Acknowledgments
The authors acknowledge financial support from the projects of National Natural Science Foundation of China (Grant No. 81773650, 81690264 and 81973259), Beijing Natural Science Foundation (No. J200018), National Key New Drug Creation and Manufacturing Program, Ministry of Science and Technology of China (Grant No. 2018ZX09721003-004).
References
- 1.Anguela X.M., High K.A. Entering the modern era of gene therapy. Annu. Rev. Med. 2019;70:273–288. doi: 10.1146/annurev-med-012017-043332. [DOI] [PubMed] [Google Scholar]
- 2.Jeon Y.H., Jung Y.T. Production of a replicating retroviral vector expressing Reovirus fast protein for cancer gene therapy. J. Virol. Methods. 2022;299:114332. doi: 10.1016/j.jviromet.2021.114332. [DOI] [PubMed] [Google Scholar]
- 3.Miname M.H., Rocha V.Z., Santos R.D. The role of RNA-targeted therapeutics to reduce ASCVD risk: what have we learned recently? Curr. Atheroscler. Rep. 2021;23(8):40. doi: 10.1007/s11883-021-00936-1. [DOI] [PubMed] [Google Scholar]
- 4.https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation
- 5.Kulkarni J.A., Witzigmann D., Thomson S.B., Chen S., Leavitt B.R., Cullis P.R., van der Meel R. Author correction: the current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021;16(7):841. doi: 10.1038/s41565-021-00937-w. [DOI] [PubMed] [Google Scholar]
- 6.Bai J., Zhang Y., Zheng X., Huang M., Cheng W., Shan H., Gao X., Zhang M., Sheng L., Dai J., Deng Y., Zhang H., Zhou X. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11(9):763. doi: 10.1038/s41419-020-02945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Wu S., Hou L., Zhu D., Yin S., Yang G., Wang Y. Therapeutic effects of simultaneous delivery of nerve growth factor mRNA and protein via exosomes on cerebral ischemia. Molecular Therapy. Nucleic Acids. 2020;21:512–522. doi: 10.1016/j.omtn.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z., Shi J., Xie J., Wang Y., Sun J., Liu T., Zhao Y., Zhao X., Wang X., Ma Y., Malkoc V., Chiang C., Deng W., Chen Y., Fu Y., Kwak K.J., Fan Y., Kang C., Yin C., Rhee J., Bertani P., Otero J., Lu W., Yun K., Lee A.S., Jiang W., Teng L., Kim B.Y.S., Lee L.J. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nature Biomed. Eng. 2020;4(1):69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., Southern J., Swerdlow D.L., Jodar L., Levy Y., Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Baz I., Trobajo-Sanmartin C., Miqueleiz A., Guevara M., Fernandez-Huerta M., Burgui C., Casado I., Portillo M.E., Navascues A., Ezpeleta C., Castilla J., Navarre W.G.S.C. Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Eurosurveillance. 2021;26(39):2100894. doi: 10.2807/1560-7917.ES.2021.26.39.2100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dammes N., Goldsmith M., Ramishetti S., Dearling J.L.J., Veiga N., Packard A.B., Peer D. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 2021;16(9):1030–1038. doi: 10.1038/s41565-021-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball R.L., Hajj K.A., Vizelman J., Bajaj P., Whitehead K.A. Lipid nanoparticle formulations for enhanced co-delivery of siRNA and mRNA. Nano Lett. 2018;18(6):3814–3822. doi: 10.1021/acs.nanolett.8b01101. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.J., Wang J.C., Zhao E.Y., Gao L.Y., Feng Q., Liu X.Y., Zhao Z.X., Ma X.F., Hou W.J., Zhang L.R., Lu W.L., Zhang Q. Self-assembly cationic nanoparticles based on cholesterol-grafted bioreducible poly(amidoamine) for siRNA delivery. Biomaterials. 2013;34(21):5303–5316. doi: 10.1016/j.biomaterials.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Luong D., Kesharwani P., Deshmukh R., Mohd Amin M.C.I., Gupta U., Greish K., Iyer A.K. PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016;43:14–29. doi: 10.1016/j.actbio.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann T.S., Karsten V., Chan A., Chiesa J., Boyce M., Bettencourt B.R., Hutabarat R., Nochur S., Vaishnaw A., Gollob J. Clinical proof of concept for a novel hepatocyte-targeting GalNAc-siRNA conjugate. Mol. Ther. 2017;25(1):71–78. doi: 10.1016/j.ymthe.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Ji X., Ruan M., Liu W., Song R., Dai J., Xue W. Worm-like biomimetic nanoerythrocyte carrying siRNA for melanoma gene therapy. Small. 2018;14(47) doi: 10.1002/smll.201803002. [DOI] [PubMed] [Google Scholar]
- 17.Dammes N., Goldsmith M., Ramishetti S., Dearling J.L.J., Veiga N., Packard A.B., Peer D. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 2021;16(9):1030–1038. doi: 10.1038/s41565-021-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B., Park J.H., Sailor M.J. Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Adv. Mater. 2019;31(49):e1903637. doi: 10.1002/adma.201903637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020;154:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y.Z., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fomivirsen approved for CMV retinitis: first antisense drugAIDS Treat News. 1998;302:7. [PubMed] [Google Scholar]
- 22.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-givosiran-acute-hepatic-porphyria
- 23.Markov O., Oshchepkova A., Mironova N. Immunotherapy based on dendritic cell-targeted/-derived extracellular vesicles-a novel strategy for enhancement of the anti-tumor immune response. Front. Pharmacol. 2019;10:1152. doi: 10.3389/fphar.2019.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., Southern J., Swerdlow D.L., Jodar L., Levy Y., Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana A., Allawadhi P., Khurana I., Allwadhi S., Weiskirchen R., Banothu A.K., Chhabra D., Joshi K., Bharani K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H., Zhang C.L., Yang S.P., Xiao W., Zheng Q., Song X.R. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J. Control. Release. 2021;335:449–456. doi: 10.1016/j.jconrel.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ermilova I., Swenson J. DOPC versus DOPE as a helper lipid for gene-therapies: molecular dynamics simulations with DLin-MC3-DMA. Phys. Chem. Chem. Phys. 2020;22(48):28256–28268. doi: 10.1039/d0cp05111j. [DOI] [PubMed] [Google Scholar]
- 28.Mui B.L., Tam Y.K., Jayaraman M., Ansell S.M., Du X.Y., Tam Y.Y.C., Lin P.J.C., Chen S., Narayanannair J.K., Rajeev K.G., Manoharan M., Akinc A., Maier M.A., Cullis P., Madden T.D., Hope M.J. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA Lipid nanoparticles. Mol. Ther-Nucl. Acids. 2013;2(12):e139. doi: 10.1038/mtna.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X.P., Goel V., Robbie G.J. Pharmacokinetics of patisiran, the first approved RNA interference therapy in patients with hereditary transthyretin-mediated amyloidosis. J. Clin. Pharmacol. 2020;60(5):573–585. doi: 10.1002/jcph.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X.Y., Hope M.J., Madden T.D., Mui B.L., Semple S.C., Tam Y.K., Ciufolini M., Witzigmann D., Kulkarni J.A., van der Meel R., Cullis P.R. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14(12):1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita T., Ueda M., Koike H., Sekijima Y., Yoshinaga T., Kodaira M., Katsuno M., Sobue G., Zhang X.P., White M.T., Sweetser M.T., Wang J.J., Ando Y. Patisiran, an RNAi therapeutic for patients with hereditary transthyretin-mediated amyloidosis: Sub-analysis in Japanese patients from the APOLLO study. Neurol. Clin. Neurosci. 2020;8(5):251–260. [Google Scholar]
- 32.Zhang X.P., Goel V., Attarwala H., Sweetser M.T., Clausen V.A., Robbie G.J. Patisiran pharmacokinetics, pharmacodynamics, and exposure-response analyses in the phase 3 apollo trial in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. J. Clin. Pharmacol. 2020;60(1):37–49. doi: 10.1002/jcph.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springer A.D., Dowdy S.F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic. Acid Ther. 2018;28(3):109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada Y., Sato Y., Nakamura T., Harashima H. Evolution of drug delivery system from viewpoint of controlled intracellular trafficking and selective tissue targeting toward future nanomedicine. J. Control. Release. 2020;327:533–545. doi: 10.1016/j.jconrel.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35(3):222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 36.Brown C.R., Gupta S., Qin J., Racie T., He G., Lentini S., Malone R., Yu M., Matsuda S., Shulga-Morskaya S., Nair A.V., Theile C.S., Schmidt K., Shahraz A., Goel V., Parmar R.G., Zlatev I., Schlegel M.K., Nair J.K., Jayaraman M., Manoharan M., Brown D., Maier M.A., Jadhav V. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 2020;48(21):11827–11844. doi: 10.1093/nar/gkaa670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soutschek J., Akinc A., Bramlage B., Charisse K., Constien R., Donoghue M., Elbashir S., Geick A., Hadwiger P., Harborth J., John M., Kesavan V., Lavine G., Pandey R.K., Racie T., Rajeev K.G., Rohl I., Toudjarska I., Wang G., Wuschko S., Bumcrot D., Koteliansky V., Limmer S., Manoharan M., Vornlocher H.P. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 38.Jeong J.H., Kim S.H., Lee M., Kim W.J., Park T.G., Ko K.S., Kim S.W. Non-viral systemic delivery of Fas siRNA suppresses cyclophosphamide-induced diabetes in NOD mice. J. Control. Release. 2010;143(1):88–94. doi: 10.1016/j.jconrel.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge Q., Filip L., Bai A., Nguyen T., Eisen H.N., Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. U. S. A. 2004;101(23):8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Liang Q., Mao Y., Zhang R., Deng Q., Chen Y., Zhu R., Duan S., Yin L. Bioreducible, branched poly(beta-amino ester)s mediate anti-inflammatory ICAM-1 siRNA delivery against myocardial ischemia reperfusion (IR) injury. Biomater Sci. 2020;8(14):3856–3870. doi: 10.1039/d0bm00631a. [DOI] [PubMed] [Google Scholar]
- 41.Beloor J., Choi C.S., Nam H.Y., Park M., Kim S.H., Jackson A., Lee K.Y., Kim S.W., Kumar P., Lee S.K. Arginine-engrafted biodegradable polymer for the systemic delivery of therapeutic siRNA. Biomaterials. 2012;33(5):1640–1650. doi: 10.1016/j.biomaterials.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Patil Y.B., Swaminathan S.K., Sadhukha T., Ma L.A., Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31(2):358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Z.X., Ho Y.C., Chen H.L., Peng S.F., Hsiao C.W., Sung H.W. Enhancement of efficiencies of the cellular uptake and gene silencing of chitosan/siRNA complexes via the inclusion of a negatively charged poly(gamma-glutamic acid) Biomaterials. 2010;31(33):8780–8788. doi: 10.1016/j.biomaterials.2010.07.086. [DOI] [PubMed] [Google Scholar]
- 44.Li H.M., Fu Y., Zhang T., Li Y.P., Hong X.Y., Jiang J.Y., Gong T., Zhang Z.R., Sun X. Rational design of polymeric hybrid micelles with highly tunable properties to co-deliver MicroRNA-34a and vismodegib for melanoma therapy. Adv. Funct. Mater. 2015;25(48):7457–7469. [Google Scholar]
- 45.Liu X.X., Rocchi P., Qu F.Q., Zheng S.Q., Liang Z.C., Gleave M., Iovanna J., Peng L. PAMAM dendrimers mediate siRNA delivery to target Hsp27 and produce potent antiproliferative effects on prostate cancer cells. Chemmedchem. 2009;4(8):1302–1310. doi: 10.1002/cmdc.200900076. [DOI] [PubMed] [Google Scholar]
- 46.Yu T.Z., Liu X.X., Bolcato-Bellemin A.L., Wang Y., Liu C., Erbacher P., Qu F.Q., Rocchi P., Behr J.P., Peng L. An amphiphilic dendrimer for effective delivery of small interfering rna and gene silencing in vitro and in vivo. Angew. Chem. Int. Edit. 2012;51(34):8478–8484. doi: 10.1002/anie.201203920. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y.Y., Lin D.S., Jiang Q., Zhang W.D., Guo S.T., Xiao P., Zheng S.Q., Wang X.X., Chen H.B., Zhang H.Y., Deng L.D., Xing J.F., Du Q., Dong A.J., Liang Z.C. Binary and ternary complexes based on polycaprolactone-graft-poly (N, N-dimethylaminoethyl methacrylate) for targeted siRNA delivery. Biomaterials. 2012;33(18):4653–4664. doi: 10.1016/j.biomaterials.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 48.Patil M.L., Zhang M., Minko T. Multifunctional triblock nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencing. ACS Nano. 2011;5(3):1877–1887. doi: 10.1021/nn102711d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato Y., Murase K., Kato J., Kobune M., Sato T., Kawano Y., Takimoto R., Takada K., Miyanishi K., Matsunaga T., Takayama T., Niitsu Y. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 2008;26(4):431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 50.Khoury M., Louis-Plence P., Escriou V., Noel D., Largeau C., Cantos C., Scherman D., Jorgensen C., Apparailly F. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006;54(6):1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- 51.Kornek M., Lukacs-Kornek V., Limmer A., Raskopf E., Becker U., Kloeckner M., Sauerbruch T., Schmitz V. 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)-formulated, immune-stimulatory vascular endothelial growth factor a small interfering RNA (siRNA) increases antitumoral efficacy in murine orthotopic hepatocellular. Mol. Med. 2008;14(7–8):365–373. doi: 10.2119/2008-00003.Kornek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kornek M., Lukacs-Kornek V., Limmer A., Raskopf E., Becker U., Klockner M., Sauerbruch T., Schmitz V. 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)-formulated, immune-stimulatory vascular endothelial growth factor a small interfering RNA (siRNA) increases antitumoral efficacy in murine orthotopic hepatocellular carcinoma with liver fibrosis. Mol. Med. 2008;14(7–8):365–373. doi: 10.2119/2008-00003.Kornek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Chen C., Fu H., Yu J., Sun Y., Huang H., Tang Y., Shen N., Duan Y. MicroRNA-125a-loaded polymeric nanoparticles alleviate systemic lupus erythematosus by restoring effector/regulatory T cells balance. ACS Nano. 2020;14(4):4414–4429. doi: 10.1021/acsnano.9b09998. [DOI] [PubMed] [Google Scholar]
- 54.Zou Y., Zheng M., Yang W., Meng F., Miyata K., Kim H.J., Kataoka K., Zhong Z. Virus-mimicking chimaeric polymersomes boost targeted cancer siRNA therapy in vivo. Adv. Mater. 2017;29(42) doi: 10.1002/adma.201703285. [DOI] [PubMed] [Google Scholar]
- 55.Palombarini F., Masciarelli S., Incocciati A., Liccardo F., Di Fabio E., Iazzetti A., Fabrizi G., Fazi F., Macone A., Bonamore A., Boffi A. Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J. Nanobiotechnol. 2021;19(1):172. doi: 10.1186/s12951-021-00921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou Y., Sun X., Wang Y., Yan C., Liu Y., Li J., Zhang D., Zheng M., Chung R.S., Shi B. Single siRNA nanocapsules for effective siRNA brain delivery and glioblastoma treatment. Adv. Mater. 2020;32(24) doi: 10.1002/adma.202000416. [DOI] [PubMed] [Google Scholar]
- 57.Wei W., Sun J., Guo X.Y., Chen X., Wang R., Qiu C., Zhang H.T., Pang W.H., Wang J.C., Zhang Q. Microfluidic-based holonomic constraints of siRNA in the Kernel of lipid/polymer hybrid nanoassemblies for improving stable and safe in vivo delivery. ACS Appl. Mater. Interfaces. 2020;12(13):14839–14854. doi: 10.1021/acsami.9b22781. [DOI] [PubMed] [Google Scholar]
- 58.Zhou M., Jiang N., Fan J., Fu S., Luo H., Su P., Zhang M., Shi H., Zeng J., Huang Y., Li Y., Shen H., Zhang A., Li R. H7K(R2)2-modified pH-sensitive self-assembled nanoparticles delivering small interfering RNA targeting hepatoma-derived growth factor for malignant glioma treatment. J. Control. Release. 2019;310:24–35. doi: 10.1016/j.jconrel.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Yang X.Z., Dou S., Sun T.M., Mao C.Q., Wang H.X., Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J. Control. Release. 2011;156(2):203–211. doi: 10.1016/j.jconrel.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 60.Li P.P., Yan Y., Zhang H.T., Cui S.H., Wang C.H., Wei W., Qian H.G., Wang J.C., Zhang Q. Biological activities of siRNA-loaded lanthanum phosphate nanoparticles on colorectal cancer. J. Control. Release. 2020;328:45–58. doi: 10.1016/j.jconrel.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 61.Gao L.Y., Liu X.Y., Chen C.J., Wang J.C., Feng Q., Yu M.Z., Ma X.F., Pei X.W., Niu Y.J., Qiu C., Pang W.H., Zhang Q. Core-shell type lipid/rPAA-chol polymer hybrid nanoparticles for in vivo siRNA delivery. Biomaterials. 2014;35(6):2066–2078. doi: 10.1016/j.biomaterials.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 62.Persano S., Guevara M.L., Li Z., Mai J., Ferrari M., Pompa P.P., Shen H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials. 2017;125:81–89. doi: 10.1016/j.biomaterials.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X., Xu Y., Solis L.M., Tao W., Wang L., Behrens C., Xu X., Zhao L., Liu D., Wu J., Zhang N., Wistuba O.C., II, Farokhzad B.R., Zetter J. Shi. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl. Acad. Sci. U. S. A. 2015;112(25):7779–7784. doi: 10.1073/pnas.1505629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang R., Deng Y., Huang B., Huang L., Lin A., Li Y., Wang W., Liu J., Lu S., Zhan Z., Wang Y., Wang W., Niu P., Zhao L., Li S., Ma X., Zhang L., Zhang Y., Yao W., Liang X., Zhao J., Liu Z., Peng X., Li H., Tan W. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Transduct. Target Ther. 2021;6(1):213. doi: 10.1038/s41392-021-00634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moller K., Muller K., Engelke H., Brauchle C., Wagner E., Bein T. Highly efficient siRNA delivery from core-shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale. 2016;8(7):4007–4019. doi: 10.1039/c5nr06246b. [DOI] [PubMed] [Google Scholar]
- 66.Wang H.X., Yang X.Z., Sun C.Y., Mao C.Q., Zhu Y.H., Wang J. Matrix metalloproteinase 2-responsive micelle for siRNA delivery. Biomaterials. 2014;35(26):7622–7634. doi: 10.1016/j.biomaterials.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 67.Yang H., Li Y., Li T.T., Xu M., Chen Y., Wu C.H., Dang X.T., Liu Y.Y. Multifunctional core/shell nanoparticles cross-linked polyetherimide-folic acid as efficient notch-1 siRNA carrier for targeted killing of breast cancer. Sci. Rep.-UK. 2014;4:7072. doi: 10.1038/srep07072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharifiaghdam M., Shaabani E., Sharifiaghdam Z., De Keersmaecker H., De Rycke R., De Smedt S., Faridi-Majidi R., Braeckmans K., Fraire J.C. Enhanced siRNA delivery and selective apoptosis induction in H1299 cancer cells by layer-by-layer-assembled se nanocomplexes: toward more efficient cancer therapy. Front. Mol. Biosci. 2021;8:639184. doi: 10.3389/fmolb.2021.639184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taschauer A., Polzer W., Poschl S., Metz S., Tepe N., Decker S., Cyran N., Scholda J., Maier J., Bloss H., Anton M., Hofmann T., Ogris M., Sami H. Combined chemisorption and complexation generate siRNA nanocarriers with biophysics optimized for efficient gene knockdown and air-blood barrier crossing. ACS Appl. Mater. Interfaces. 2020;12(27):30095–30111. doi: 10.1021/acsami.0c06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng Z.J., Morton S.W., Ben-Akiva E., Dreaden E.C., Shopsowitz K.E., Hammond P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano. 2013;7(11):9571–9584. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakran M., Muratani M., Tng W.J., Liang H.Q., Trushina D.B., Sukhorukov G.B., Ng H.H., Antipina M.N. Layered polymeric capsules inhibiting the activity of RNases for intracellular delivery of messenger RNA. J. Mater. Chem. B. 2015;3(28):5842–5848. doi: 10.1039/c5tb00615e. [DOI] [PubMed] [Google Scholar]
- 72.Chen H.Y., Fan X.F., Zhao Y.N., Zhi D.F., Cui S.H., Zhang E.X., Lan H.M., Du J.J., Zhang Z., Zhen Y.H., Zhang S.B. Stimuli-responsive polysaccharide enveloped liposome for targeting and penetrating delivery of survivin-shRNA into breast tumor. ACS Appl. Mater. Interfaces. 2020;12(19):22074–22087. doi: 10.1021/acsami.9b22440. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z.Z., Zhang L.F., He Y.L., Shen Y.Q., Li Y.F. Enhanced shRNA delivery and ABCG2 silencing by charge-reversible layered nanocarriers. Small. 2015;11(8):952–962. doi: 10.1002/smll.201401397. [DOI] [PubMed] [Google Scholar]
- 74.Choi K.Y., Correa S., Min J., Li J.H., Roy S., Laccetti K.H., Dreaden E., Kong S., Heo R., Roh Y.H., Lawson E.C., Palmer P.A., Hammond P.T. Binary targeting of siRNA to hematologic cancer cells in vivo using layer-by-layer nanoparticles. Adv. Funct. Mater. 2019;29(20):1900018. doi: 10.1002/adfm.201900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bishop C.J., Tzeng S.Y., Green J.J. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015;11:393–403. doi: 10.1016/j.actbio.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S.K., Han M.S., Asokan S., Tung C.H. Effective gene silencing by multilayered siRNA-coated gold nanoparticles. Small. 2011;7(3):364–370. doi: 10.1002/smll.201001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaabani E., Sharifiaghdam M., De Keersmaecker H., De Rycke R., De Smedt S., Faridi-Majidi R., Braeckmans K., Fraire J.C. Layer by layer assembled chitosan-coated gold nanoparticles for enhanced siRNA delivery and silencing. Int. J. Mol. Sci. 2021;22(2):831. doi: 10.3390/ijms22020831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kapadia C.H., Ioele S.A., Day E.S. Layer-by-layer assembled PLGA nanoparticles carrying miR-34a cargo inhibit the proliferation and cell cycle progression of triple-negative breast cancer cells. J. Biomed. Mater. Res. A. 2020;108(3):601–613. doi: 10.1002/jbm.a.36840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta B., Ruttala H.B., Poudel B.K., Pathak S., Regmi S., Gautam M., Poudel K., Sung M.H., Ou W., Jin S.G., Jeong J.H., Ku S.K., Choi H.G., Yong C.S., Kim J.O. Polyamino acid layer-by-layer (LbL) constructed silica-supported mesoporous titania nanocarriers for stimuli-responsive delivery of microRNA 708 and paclitaxel for combined chemotherapy. ACS Appl. Mater. Interfaces. 2018;10(29):24392–24405. doi: 10.1021/acsami.8b06642. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Q.S., Guo Y.Y., Zhu L.J., Liu X.L., Yang J.P., Li Y.H., Zhu X.Y., Zhang C. A nucleic acid nanogel dually bears siRNA and CpG motifs for synergistic tumor immunotherapy. Biomater. Sci. UK. 2021;9(13):4755–4764. doi: 10.1039/d1bm00531f. [DOI] [PubMed] [Google Scholar]
- 81.Xue H., Ding F., Zhang J., Guo Y.Y., Gao X.H., Feng J., Zhu X.Y., Zhang C. DNA tetrahedron-based nanogels for siRNA delivery and gene silencing. Chem. Commun. 2019;55(29):4222–4225. doi: 10.1039/c9cc00175a. [DOI] [PubMed] [Google Scholar]
- 82.Xu C.F., Li D.D., Cao Z.T., Xiong M.H., Yang X.Z., Wang J. Facile hydrophobization of siRNA with anticancer drug for non-cationic nanocarrier-mediated systemic delivery. Nano Lett. 2019;19(4):2688–2693. doi: 10.1021/acs.nanolett.9b00657. [DOI] [PubMed] [Google Scholar]
- 83.Tajik-Ahmadabad B., Chollet L., White J., Separovic F., Polyzos A. Metallo-cubosomes: zinc-functionalized cubic nanoparticles for therapeutic nucleotide delivery. Mol. Pharm. 2019;16(3):978–986. doi: 10.1021/acs.molpharmaceut.8b00890. [DOI] [PubMed] [Google Scholar]
- 84.Kapoor M., Burgess D.J. Physicochemical characterization of anionic lipid-based ternary siRNA complexes. Bba-Biomembranes. 2012;1818(7):1603–1612. doi: 10.1016/j.bbamem.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Qiu C., Wei W., Sun J., Zhang H.T., Ding J.S., Wang J.C., Zhang Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8(26):13033–13044. doi: 10.1039/c6nr04034a. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H.T., Sun J., Yan Y., Cui S.H., Wang H., Wang C.H., Qiu C., Chen X., Ding J.S., Qian H.G., Wang J.C., Zhang Q. Encapsulated microRNA by gemcitabine prodrug for cancer treatment. J. Control. Release. 2019;316:317–330. doi: 10.1016/j.jconrel.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Liu G., Choi K.Y., Bhirde A., Swierczewska M., Yin J., Lee S.W., Park J.H., Hong J.I., Xie J., Niu G., Kiesewetter D.O., Lee S., Chen X.Y. Sticky nanoparticles: a platform for siRNA delivery by a bis(zinc(II) dipicolylamine)-functionalized, self-assembled nanoconjugate. Angew. Chem. Int. Edit. 2012;51(2):445–449. doi: 10.1002/anie.201105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Q., Wang Y., Xiang J.J., Piao Y., Zhou Z.X., Tang J.B., Liu X.R., Shen Y.Q. Stabilized calcium phosphate hybrid nanocomposite using a benzoxaborole-containing polymer for pH-responsive siRNA delivery. Biomater. Sci. UK. 2018;6(12):3178–3188. doi: 10.1039/c8bm00575c. [DOI] [PubMed] [Google Scholar]
- 89.Paidikondala M., Rangasami V.K., Nawale G.N., Casalini T., Perale G., Kadekar S., Mohanty G., Salminen T., Oommen O.P., Varghese O.P. An unexpected role of hyaluronic acid in trafficking siRNA across the cellular barrier: the first biomimetic, anionic, non-viral transfection method. Angew. Chem. Int. Edit. 2019;58(9):2815–2819. doi: 10.1002/anie.201900099. [DOI] [PubMed] [Google Scholar]
- 90.Choi E., Lee J., Kwon I.C., Lim D.K., Kim S. Cumulative directional calcium gluing between phosphate and silicate: a facile, robust and biocompatible strategy for siRNA delivery by amine-free non-positive vector. Biomaterials. 2019;209:126–137. doi: 10.1016/j.biomaterials.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Ma Y., Zhu Y.J., Wang C., Pan D.L., Liu S., Yang M.Y., Xiao Z.P., Yang X.T., Zhao W.T., Zhou X.Y., Li Y.D., Pan Y.F., Sun J., Wang S.H., Guan Z., Zhang L.H., Yang Z.J. Annealing novel nucleobase-lipids with oligonucleotides or plasmid DNA based on H-bonding or pi-pi interaction: assemblies and transfections. Biomaterials. 2018;178:147–157. doi: 10.1016/j.biomaterials.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 92.Jeong J.H., Kim S.H., Lee M., Kim W.J., Park T.G., Ko K.S., Kim S.W. Non-viral systemic delivery of Fas siRNA suppresses cyclophosphamide-induced diabetes in NOD mice. J. Control. Release. 2010;143(1):88–94. doi: 10.1016/j.jconrel.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou J.H., Wu J.Y., Hafdi N., Behr J.P., Erbacher P., Peng L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006;22:2362–2364. doi: 10.1039/b601381c. [DOI] [PubMed] [Google Scholar]
- 94.Wang C.R., Du L.L., Zhou J.H., Meng L.W., Cheng Q., Wang C., Wang X.X., Zhao D.Y., Huang Y.Y., Zheng S.Q., Cao H.Q., Zhang J.H., Deng L.D., Liang Z.C., Dong A.J. Elaboration on the distribution of hydrophobic segments in the chains of amphiphilic cationic polymers for small interfering RNA delivery. ACS Appl. Mater. Interfaces. 2017;9(38):32463–32474. doi: 10.1021/acsami.7b07337. [DOI] [PubMed] [Google Scholar]
- 95.Wang C., Du L., Zhou J., Meng L., Cheng Q., Wang C., Wang X., Zhao D., Huang Y., Zheng S., Cao H., Zhang J., Deng L., Liang Z., Dong A. Elaboration on the Distribution of Hydrophobic Segments in the Chains of Amphiphilic Cationic Polymers for Small Interfering RNA Delivery. ACS Appl. Mater. Interfaces. 2017;9(38):32463–32474. doi: 10.1021/acsami.7b07337. [DOI] [PubMed] [Google Scholar]
- 96.Li S.D., Chen Y.C., Hackett M.J., Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol. Ther. 2008;16(1):163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chono S., Li S.D., Conwell C.C., Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J. Control. Release. 2008;131(1):64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang X.Z., Dou S., Sun T.M., Mao C.Q., Wang H.X., Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J. Control. Release. 2011;156(2):203–211. doi: 10.1016/j.jconrel.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 99.El Jundi A., Morille M., Bettache N., Bethry A., Berthelot J., Salvador J., Hunger S., Bakkour Y., Belamie E., Nottelet B. Degradable double hydrophilic block copolymers and tripartite polyionic complex micelles thereof for small interfering ribonucleic acids (siRNA) delivery. J. Colloid Interface Sci. 2020;580:449–459. doi: 10.1016/j.jcis.2020.07.057. [DOI] [PubMed] [Google Scholar]
- 100.Uchida S., Kinoh H., Ishii T., Matsui A., Tockary T.A., Takeda K.M., Uchida H., Osada K., Itaka K., Kataoka K. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials. 2016;82:221–228. doi: 10.1016/j.biomaterials.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 101.Yoshinaga N., Uchida S., Naito M., Osada K., Cabral H., Kataoka K. Induced packaging of mRNA into polyplex micelles by regulated hybridization with a small number of cholesteryl RNA oligonucleotides directed enhanced in vivo transfection. Biomaterials. 2019;197:255–267. doi: 10.1016/j.biomaterials.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 102.Miyazaki T., Uchida S., Nagatoishi S., Koji K., Hong T., Fukushima S., Tsumoto K., Ishihara K., Kataoka K., Cabral H. Polymeric nanocarriers with controlled chain flexibility boost mRNA delivery in vivo through enhanced structural fastening. Adv. Healthc. Mater. 2020;9(16) doi: 10.1002/adhm.202000538. [DOI] [PubMed] [Google Scholar]
- 103.Dirisala A., Uchida S., Tockary T.A., Yoshinaga N., Li J.J., Osawa S., Gorantla L., Fukushima S., Osada K., Kataoka K. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J. Drug Target. 2019;27(5–6):670–680. doi: 10.1080/1061186X.2018.1550646. [DOI] [PubMed] [Google Scholar]
- 104.Wei W., Sun J., Guo X.Y., Chen X., Wang R., Qiu C., Zhang H.T., Pang W.H., Wang J.C., Zhang Q. Microfluidic-based holonomic constraints of siRNA in the Kernel of lipid/polymer hybrid nanoassemblies for improving stable and safe in vivo delivery. ACS Appl. Mater. Interfaces. 2020;12(13):14839–14854. doi: 10.1021/acsami.9b22781. [DOI] [PubMed] [Google Scholar]
- 105.Liu J.B., Lu X.H., Wu T.T., Wu X.H., Han L., Ding B.Q. Branched antisense and siRNA co-assembled nanoplatform for combined gene silencing and tumor therapy. Angew. Chem. Int. Edit. 2021;60(4):1853–1860. doi: 10.1002/anie.202011174. [DOI] [PubMed] [Google Scholar]
- 106.Ding F., Mou Q.B., Ma Y., Pan G.F., Guo Y.Y., Tong G.S., Choi C.H.J., Zhu X.Y., Zhang C. A crosslinked nucleic acid nanogel for effective sirna delivery and antitumor therapy. Angew. Chem. Int. Edit. 2018;57(12):3064–3068. doi: 10.1002/anie.201711242. [DOI] [PubMed] [Google Scholar]
- 107.Ding F., Mou Q., Ma Y., Pan G., Guo Y., Tong G., Choi C.H.J., Zhu X., Zhang C. A crosslinked nucleic acid nanogel for effective siRNA delivery and antitumor therapy. Angew. Chem. Int. Ed. Eng. 2018;57(12):3064–3068. doi: 10.1002/anie.201711242. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y.B., Sun C.Z., Wang C., Jankovic K.E., Dong Y.Z. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 2021;121(20):12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leung A.K.K., Hafez I.M., Baoukina S., Belliveau N.M., Zhigaltsev I.V., Afshinmanesh E., Tieleman D.P., Hansen C.L., Hope M.J., Cullis P.R. Lipid nanoparticles containing siRNA synthesized by microfluidic mixing exhibit an electron-dense nanostructured core (vol 116, pg 18440, 2012) J. Phys. Chem. C. 2012;116(41):22104. doi: 10.1021/jp303267y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kulkarni J.A., Darjuan M.M., Mercer J.E., Chen S., van der Meel R., Thewalt J.L., Tam Y.Y.C., Cullis P.R. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano. 2018;12(5):4787–4795. doi: 10.1021/acsnano.8b01516. [DOI] [PubMed] [Google Scholar]
- 111.Lou G., Anderluzzi G., Schmidt S.T., Woods S., Gallorini S., Brazzoli M., Giusti F., Ferlenghi I., Johnson R.N., Roberts C.W., O'Hagan D.T., Baudner B.C., Perrie Y. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: the impact of cationic lipid selection. J. Control. Release. 2020;325:370–379. doi: 10.1016/j.jconrel.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 112.Kulkarni J.A., Witzigmann D., Leung J., Tam Y.Y.C., Cullis P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11(45):21733–21739. doi: 10.1039/c9nr09347h. [DOI] [PubMed] [Google Scholar]
- 113.D'souza A.A., Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin. Drug. Del. 2016;13(9):1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- 114.Xia Y.Q., Tian J., Chen X.Y. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016;79:56–68. doi: 10.1016/j.biomaterials.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klibanov A.L., Maruyama K., Torchilin V.P., Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 116.Kraft J.C., Freeling J.P., Wang Z., Ho R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014;103(1):29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abu Lila A.S., Kiwada H., Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J. Control. Release. 2013;172(1):38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 118.Knop K., Hoogenboom R., Fischer D., Schubert U.S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Edit. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 119.Garcia K.P., Zarschler K., Barbaro L., Barreto J.A., O'Malley W., Spiccia L., Stephan H., Graham B. Zwitterionic-coated "stealth" nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10(13):2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 120.Jackson M.A., Werfel T.A., Curvino E.J., Yu F., Kavanaugh T.E., Sarett S.M., Dockery M.D., Kilchrist K.V., Jackson A.N., Giorgio T.D., Duvall C.L. Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS Nano. 2017;11(6):5680–5696. doi: 10.1021/acsnano.7b01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang T., Huang Y., Ma X., Gong N., Liu X., Liu L., Ye X., Hu B., Li C., Tian J.H., Magrini A., Zhang J., Guo W., Xing J.F., Bottini M., Liang X.J. Fluorinated oligoethylenimine nanoassemblies for efficient siRNA-mediated gene silencing in serum-containing media by effective endosomal escape. Nano Lett. 2018;18(10):6301–6311. doi: 10.1021/acs.nanolett.8b02553. [DOI] [PubMed] [Google Scholar]
- 122.Fang C., Shi B., Pei Y.Y., Hong M.H., Wu J., Chen H.Z. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006;27(1):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Walkey C.D., Olsen J.B., Guo H.B., Emili A., Chan W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012;134(4):2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 124.Yoo J.W., Chambers E., Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr. Pharm. Design. 2010;16(21):2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y.L., Teng Z.G., Ni Q.Q., Tao J., Cao X.F., Wen Y.T., Wu L.Q., Fang C., Wan B., Zhang X.W., Lu G.M. Orderly curled silica nanosheets with a small size and macromolecular loading pores: synthesis and delivery of macromolecules to eradicate drug-resistant cancer. ACS Appl. Mater. Interfaces. 2020;12(52):57810–57820. doi: 10.1021/acsami.0c19497. [DOI] [PubMed] [Google Scholar]
- 126.Jiang X., Qu W., Pan D., Ren Y., Williford J.M., Cui H.G., Luijten E., Mao H.Q. Plasmid-templated shape control of condensed DNA-block copolymer nanoparticles. Adv. Mater. 2013;25(2):227–232. doi: 10.1002/adma.201202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jasinski D.L., Li H., Guo P.X. The effect of size and shape of RNA nanoparticles on biodistribution. Mol. Ther. 2018;26(3):784–792. doi: 10.1016/j.ymthe.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anselmo A.C., Zhang M., Kumar S., Vogus D.R., Menegatti S., Helgeson M.E., Mitragotri S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9(3):3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 129.Ernsting M.J., Murakami M., Roy A., Li S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release. 2013;172(3):782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Landen C.N., Chavez-Reyes A., Bucana C., Schmandt R., Deavers M.T., Lopez-Berestein G., Sood A.K. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 131.Halder J., Kamat A.A., Landen C.N., Han L.Y., Lutgendorf S.K., Lin Y.G., Merritt W.M., Jennings N.B., Chavez-Reyes A., Coleman R.L., Gershenson D.M., Schmandt R., Cole S.W., Lopez-Berestein G., Sood A.K. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy (vol 12, pg 4916, 2006) Clin. Cancer Res. 2019;25(10):3194. doi: 10.1158/1078-0432.CCR-19-1132. [DOI] [PubMed] [Google Scholar]
- 132.Canton I., Battaglia G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012;41(7):2718–2739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- 133.Das A., Ganesh K., Chaffee S., Khanna S., Sen C.K., Roy S. Engulfment of apoptotic cells induces microrna-21 expression and switches human macrophages from a pro-inflammatory to anti-inflammatory phenotype. Wound Repair Regen. 2013;21(2):A19. [Google Scholar]
- 134.Zhang L., Liu X.G., Liu D.Q., Yu X.L., Zhang L.X., Zhu J., Lu S., Liu R.T. A conditionally releasable “do not eat me” CD47 signal facilitates microglia-targeted drug delivery for the treatment of Alzheimer’s disease. Adv. Funct. Mater. 2020;30(24):1910691. [Google Scholar]
- 135.Chow A., Brown B.D., Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat. Rev. Immunol. 2011;11(11):788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 136.Saunders N.R.M., Paolini M.S., Fenton O.S., Poul L., Devalliere J., Mpambani F., Darmon A., Bergere M., Jibault O., Germain M., Langer R. A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 2020;20(6):4264–4269. doi: 10.1021/acs.nanolett.0c00752. [DOI] [PubMed] [Google Scholar]
- 137.Herrera V.L.M., Colby A.H., Ruiz-Opazo N., Coleman D.G., Grinstaff M.W. Nucleic acid nanomedicines in Phase II/III clinical trials: translation of nucleic acid therapies for reprogramming cells. Nanomedicine-Uk. 2018;13(16):2083–2098. doi: 10.2217/nnm-2018-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weinstein S., Toker I.A., Emmanuel R., Ramishetti S., Hazan-Halevy I., Rosenblum D., Goldsmith M., Abraham A., Benjamini O., Bairey O., Raanani P., Nagler A., Lieberman J., Peer D. Harnessing RNAi-based nanomedicines for therapeutic gene silencing in B-cell malignancies. P Natl. Acad. Sci. USA. 2016;113(1):E16–E22. doi: 10.1073/pnas.1519273113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang D.D., Li H., Chen W.L., Yang H., Liu Y., You B.G., Zhang X.N. Efficient tumor-targeting delivery of siRNA via folate-receptor mediated biomimetic albumin nanoparticles enhanced by all-trans retinoic acid. Mat. Sci. Eng. C-Mater. 2021;119:111583. doi: 10.1016/j.msec.2020.111583. [DOI] [PubMed] [Google Scholar]
- 140.Zhang J.F., Shen H.W., Xu J.J., Liu L., Tan J.W., Li M.H., Xu N., Luo S.G., Wang J., Yang F., Tang J., Li Q.H., Wang Y.T., Yu L., Yan Z.Q. Liver-targeted siRNA lipid nanoparticles treat hepatic cirrhosis by dual antifibrotic and anti-inflammatory activities. ACS Nano. 2020;14(5):6305–6322. doi: 10.1021/acsnano.0c02633. [DOI] [PubMed] [Google Scholar]
- 141.Zhang C., Gu Z.C., Shen L., Liu X.Y., Lin H.W. A dual targeting drug delivery system for penetrating blood-brain barrier and selectively delivering siRNA to neurons for Alzheimer's disease treatment. Curr. Pharm. Biotechnol. 2017;18(14):1124–1131. doi: 10.2174/1389201019666180226152542. [DOI] [PubMed] [Google Scholar]
- 142.Cardoso A.L.C., Simoes S., de Almeida L.P., Plesnila N., de Lima M.C.P., Wagner E., Culmsee C. Tf-lipoplexes for neuronal siRNA delivery: a promising system to mediate gene silencing in the CNS. J. Control. Release. 2008;132(2):113–123. doi: 10.1016/j.jconrel.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 143.Xia Y., Tang G.Y., Guo M., Xu T.T., Chen H.Y., Lin Z.F., Li Y.H., Chen Y., Zhu B., Liu H.S., Cao J. Silencing KLK12 expression via RGDfC-decorated selenium nanoparticles for the treatment of colorectal cancer in vitro and in vivo. Mat. Sci. Eng. C-Mater. 2020;110:110594. doi: 10.1016/j.msec.2019.110594. [DOI] [PubMed] [Google Scholar]
- 144.Ye Y.H., Zhang L.L., Dai Y.H., Wang Z., Li C.E., Peng Y., Ma D., He P.H. PSMA-targeting reduction-cleavable hyperbranched polyamide-amine gene delivery system to treat the bone metastases of prostate cancer. Int. J. Nanomedicine. 2020;15:7173–7184. doi: 10.2147/IJN.S268398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li L.Y., Hou J.J., Liu X.J., Guo Y.J., Wu Y., Zhang L.H., Yang Z.J. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials. 2014;35(12):3840–3850. doi: 10.1016/j.biomaterials.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 146.Fitzgerald K.A., Malhotra M., Gooding M., Sallas F., Evans J.C., Darcy R., O’Driscoll C.M. A novel, anisamide-targeted cyclodextrin nanoformulation for siRNA delivery to prostate cancer cells expressing the sigma-1 receptor. Int. J. Pharm. 2016;499(1–2):131–145. doi: 10.1016/j.ijpharm.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 147.Park H.J., Jeon E.J., Lee J.S., Hong S.H., Cho A.N., Lee J., Moon J.S., Jung K.E., Oh J.W., Lee H., Cho S.W. Galactosylated lipidoid nanoparticles for delivery of small interfering RNA to inhibit hepatitis C viral replication in vivo. Adv. Healthc. Mater. 2016;5(22):2931–2941. doi: 10.1002/adhm.201600416. [DOI] [PubMed] [Google Scholar]
- 148.Min H.S., Kim H.J., Naito M., Ogura S., Toh K., Hayashi K., Kim B.S., Fukushima S., Anraku Y., Miyata K., Kataoka K. Systemic brain delivery of antisense oligonucleotides across the blood-brain barrier with a glucose-coated polymeric nanocarrier. Angew. Chem. Int. Ed. Eng. 2020;59(21):8173–8180. doi: 10.1002/anie.201914751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15(4):313–320. doi: 10.1038/s41565-020-0669-6. s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15(4):313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jadhav S.G., Dowdy S.F. Overcoming delivery barriers with LNPs. Nat. Mater. 2021;20(5):575–577. doi: 10.1038/s41563-021-00988-3. [DOI] [PubMed] [Google Scholar]
- 152.Wayteck L., Dewitte H., De Backer L., Breckpot K., Demeester J., De Smedt S.C., Raemdonck K. Hitchhiking nanoparticles: reversible coupling of lipid-based nanoparticles to cytotoxic T lymphocytes. Biomaterials. 2016;77:243–254. doi: 10.1016/j.biomaterials.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 153.Meng N., Grimm D. Membrane-destabilizing ionizable phospholipids: Novel components for organ-selective mRNA delivery and CRISPR-Cas gene editing. Signal Transduct. Tar. 2021;6(1):206. doi: 10.1038/s41392-021-00642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wayteck L., Dewitte H., De Backer L., Breckpot K., Demeester J., De Smedt S.C., Raemdonck K. Hitchhiking nanoparticles: reversible coupling of lipid-based nanoparticles to cytotoxic T lymphocytes. Biomaterials. 2016;77:243–254. doi: 10.1016/j.biomaterials.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 155.Friend D.S., Gilula N.B. Variations in tight and gap junctions in mammalian tissues. J. Cell Biol. 1972;53(3):758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shi M.H., Wang Y., Zhao X.F., Zhang J.L., Hu H.Y., Qiao M.X., Zhao X.L., Chen D.W. Stimuli-responsive and highly penetrable nanoparticles as a multifunctional nanoplatform for boosting nonsmall cell lung cancer siRNA therapy. Acs Biomater Sci Eng. 2021;7(7):3141–3155. doi: 10.1021/acsbiomaterials.1c00582. [DOI] [PubMed] [Google Scholar]
- 157.Jung H., Shimatani Y., Hasan M., Uno K., Hama S., Kogure K. Development of flexible nanocarriers for siRNA delivery into tumor tissue. Int. J. Pharm. 2017;516(1–2):258–265. doi: 10.1016/j.ijpharm.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 158.Kohata A., Hashim P.K., Okuro K., Aida T. Transferrin-appended nanocaplet for transcellular siRNA delivery into deep tissues. J. Am. Chem. Soc. 2019;141(7):2862–2866. doi: 10.1021/jacs.8b12501. [DOI] [PubMed] [Google Scholar]
- 159.Ge C., Yang J., Duan S., Liu Y., Meng F., Yin L. Fluorinated alpha-helical polypeptides synchronize mucus permeation and cell penetration toward highly efficient pulmonary siRNA delivery against acute lung injury. Nano Lett. 2020;20(3):1738–1746. doi: 10.1021/acs.nanolett.9b04957. [DOI] [PubMed] [Google Scholar]
- 160.Cheng Y.H., He C.L., Riviere J.E., Monteiro-Riviere N.A., Lin Z.M. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano. 2020;14(3):3075–3095. doi: 10.1021/acsnano.9b08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kang T.Y., Ni J.S., Li T.T., Wang J., Li Z.S., Li Y.X., Zha M.L., Zhang C., Wu X., Guo H., Xi L., Li K. Efficient and precise delivery of microRNA by photoacoustic force generated from semiconducting polymer-based nanocarriers. Biomaterials. 2021;275:120907. doi: 10.1016/j.biomaterials.2021.120907. [DOI] [PubMed] [Google Scholar]
- 162.Li H.M., Miteva M., Kirkbride K.C., Cheng M.J., Nelson C.E., Simpson E.M., Gupta M.K., Duvall C.L., Giorgio T.D. Dual MMP7-proximity-activated and folate receptor-targeted nanoparticles for siRNA delivery. Biomacromolecules. 2015;16(1):192–201. doi: 10.1021/bm501394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang X.Z., Du J.Z., Dou S., Mao C.Q., Long H.Y., Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS Nano. 2012;6(1):771–781. doi: 10.1021/nn204240b. [DOI] [PubMed] [Google Scholar]
- 164.Li J.J., Ge Z.S., Liu S.Y. PEG-sheddable polyplex micelles as smart gene carriers based on MMP-cleavable peptide-linked block copolymers. Chem. Commun. 2013;49(62):6974–6976. doi: 10.1039/c3cc43576h. [DOI] [PubMed] [Google Scholar]
- 165.Li H.M., Yu S.S., Miteva M., Nelson C.E., Werfel T., Giorgio T.D., Duvall C.L. Matrix metalloproteinase responsive, proximity-activated polymeric nanoparticles for siRNA delivery. Adv. Funct. Mater. 2013;23(24):3040–3052. doi: 10.1002/adfm.201202215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu C.F., Zhang H.B., Sun C.Y., Liu Y., Shen S., Yang X.Z., Zhu Y.H., Wang J. Tumor acidity-sensitive linkage-bridged block copolymer for therapeutic siRNA delivery. Biomaterials. 2016;88:48–59. doi: 10.1016/j.biomaterials.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 167.Lo Y.L., Chang C.H., Wang C.S., Yang M.H., Lin A.M.Y., Hong C.J., Tseng W.H. PEG-coated nanoparticles detachable in acidic microenvironments for the tumor-directed delivery of chemo- and gene therapies for head and neck cancer. Theranostics. 2020;10(15):6695–6714. doi: 10.7150/thno.45164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Q., Ding F., Liu X., Shen J., Su Y., Qian J., Zhu X., Zhang C. Nanobody-guided targeted delivery of microRNA via nucleic acid nanogel to inhibit the tumor growth. J. Control. Release. 2020;328:425–434. doi: 10.1016/j.jconrel.2020.08.058. [DOI] [PubMed] [Google Scholar]
- 169.Lokugamage M.P., Sago C.D., Can Z.B., Krupczak B.R., Dahlman J.E. Constrained nanoparticles deliver siRNA and sgRNA to T cells in vivo without targeting ligands. Adv. Mater. 2019;31(41):e1902251. doi: 10.1002/adma.201902251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim B., Sun S., Varner J.A., Howell S.B., Ruoslahti E., Sailor M.J. Securing the payload, finding the cell, and avoiding the endosome: peptide-targeted, fusogenic porous silicon nanoparticles for delivery of siRNA. Adv. Mater. 2019;31(35):e1902952. doi: 10.1002/adma.201902952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hoffmann M., Hersch N., Gerlach S., Dreissen G., Springer R., Merkel R., Csiszar A., Hoffmann B. Complex size and surface charge determine nucleic acid transfer by fusogenic liposomes. Int. J. Mol. Sci. 2020;21(6):2244. doi: 10.3390/ijms21062244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim M., Jeong M., Hur S., Cho Y., Park J., Jung H., Seo Y., Woo H.A., Nam K.T., Lee K., Lee H. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021;7(9):eabf4398. doi: 10.1126/sciadv.abf4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fonseca S.B., Pereira M.P., Kelley S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009;61(11):953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 174.Wender P.A., Galliher W.C., Goun E.A., Jones L.R., Pillow T.H. The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug Deliv. Rev. 2008;60(4–5):452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee J., Son W., Hong J., Song Y., Yang C.S., Kim Y.H. Down-regulation of TNF-alpha via macrophage-targeted RNAi system for the treatment of acute inflammatory sepsis. J. Control. Release. 2021;336:344–353. doi: 10.1016/j.jconrel.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 176.Tang H., Yin L., Kim K.H., Cheng J. Helical poly(arginine) mimics with superior cell-penetrating and molecular transporting properties. Chem. Sci. 2013;4(10):3839–3844. doi: 10.1039/C3SC51328A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weinstein S., Toker I.A., Emmanuel R., Ramishetti S., Hazan-Halevy I., Rosenblum D., Goldsmith M., Abraham A., Benjamini O., Bairey O., Raanani P., Nagler A., Lieberman J., Peer D. Harnessing RNAi-based nanomedicines for therapeutic gene silencing in B-cell malignancies. Proc. Natl. Acad. Sci. U. S. A. 2016;113(1):E16–E22. doi: 10.1073/pnas.1519273113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Veiga N., Diesendruck Y., Peer D. Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Adv. Drug Deliv. Rev. 2020;159:364–376. doi: 10.1016/j.addr.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 179.Kedmi R., Veiga N., Ramishetti S., Goldsmith M., Rosenblum D., Dammes N., Hazan-Halevy I., Nahary L., Leviatan-Ben-Arye S., Harlev M., Behlke M., Benhar I., Lieberman J., Peer D. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 2018;13(3):214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 180.Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R., Kelly P.M., Aberg C., Mahon E., Dawson K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8(2):137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 181.Liu Z., Wang S., Tapeinos C., Torrieri G., Kankanen V., El-Sayed N., Python A., Hirvonen J.T., Santos H.A. Non-viral nanoparticles for RNA interference: Principles of design and practical guidelines. Adv. Drug Deliv. Rev. 2021;174:576–612. doi: 10.1016/j.addr.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 182.Xu J.S., Liu Y.H., Li Y.J., Wang H., Stewart S., Van der Jeught K., Agarwal P., Zhang Y.T., Liu S., Zhao G., Wan J., Lu X.B., He X.M. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer (vol, pg, 2019) Nat. Nanotechnol. 2020;15(4):342. doi: 10.1038/s41565-020-0635-3. [DOI] [PubMed] [Google Scholar]
- 183.Xu J., Liu Y., Li Y., Wang H., Stewart S., Van der Jeught K., Agarwal P., Zhang Y., Liu S., Zhao G., Wan J., Lu X., He X. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019;14(4):388–397. doi: 10.1038/s41565-019-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schach D.K., Rock W., Franz J., Bonn M., Parekh S.H., Weidner T. Reversible activation of a cell-penetrating peptide in a membrane environment. J. Am. Chem. Soc. 2015;137(38):12199–12202. doi: 10.1021/jacs.5b06720. [DOI] [PubMed] [Google Scholar]
- 185.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov. Today. 2012;17(15–16):850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 186.Mundra V., Mahato R.I. Design of nanocarriers for efficient cellular uptake and endosomal release of small molecule and nucleic acid drugs: learning from virus. Front. Chem. Sci. Eng. 2014;8(4):387–404. [Google Scholar]
- 187.Wang G.K., Norton A.S., Pokharel D., Song Y., Hill R.A. KDEL peptide gold nanoconstructs: promising nanoplatforms for drug delivery. Nanomed-Nanotechnol. 2013;9(3):366–374. doi: 10.1016/j.nano.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 188.Sneh-Edri H., Likhtenshtein D., Stepensky D. Intracellular targeting of PLGA nanoparticles encapsulating antigenic peptide to the endoplasmic reticulum of dendritic cells and its effect on antigen cross-presentation in vitro. Mol. Pharm. 2011;8(4):1266–1275. doi: 10.1021/mp200198c. [DOI] [PubMed] [Google Scholar]
- 189.Qiu C., Han H.H., Sun J., Zhang H.T., Wei W., Cui S.H., Chen X., Wang J.C., Zhang Q. Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat. Commun. 2019;10(1):2702. doi: 10.1038/s41467-019-10562-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hong B.J., Chipre A.J., Nguyen S.T. Acid-degradable polymer-caged lipoplex (PCL) platform for siRNA delivery: facile cellular triggered release of siRNA. J. Am. Chem. Soc. 2013;135(47):17655–17658. doi: 10.1021/ja404491r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang S.Y., Gan Y., Shao L.L., Liu T.Q., Wei D.Y., Yu Y.Y., Guo H.W., Zhu H.Y. Virus mimetic shell-sheddable chitosan micelles for siVEGF delivery and FRET-traceable acid-triggered release. ACS Appl. Mater. Interfaces. 2020;12(48):53598–53614. doi: 10.1021/acsami.0c13023. [DOI] [PubMed] [Google Scholar]
- 192.Wen Y.T., Bai H.Z., Zhu J.L., Song X., Tang G.P., Li J. A supramolecular platform for controlling and optimizing molecular architectures of siRNA targeted delivery vehicles. Sci. Adv. 2020;6(31):eabc2148. doi: 10.1126/sciadv.abc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin D.S., Jiang Q., Cheng Q., Huang Y.Y., Huang P.S., Han S.C., Guo S.T., Liang Z.C., Dong A.J. Polycation-detachable nanoparticles self-assembled from mPEG-PCL-g-SS-PDMAEMA for in vitro and in vivo siRNA delivery. Acta Biomater. 2013;9(8):7746–7757. doi: 10.1016/j.actbio.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 194.Takemoto H., Wang C.L., Nomoto T., Matsui M., Tomoda K., Nishiyama N. Pyruvate responsiveness based on alpha-oxohydrazone formation for intracellular siRNA release from polyion complex-based carriers. Biomacromolecules. 2019;20(6):2305–2314. doi: 10.1021/acs.biomac.9b00261. [DOI] [PubMed] [Google Scholar]
- 195.Zheng M., Liu Y., Wang Y., Zhang D., Zou Y., Ruan W., Yin J., Tao W., Park J.B., Shi B. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv. Mater. 2019;31(37) doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- 196.Zhou Z., Li C., Zhang M., Zhang Q., Qian C., Oupicky D., Sun M. Charge and assembly reversible micelles fueled by intracellular ATP for improved siRNA transfection. ACS Appl. Mater. Interfaces. 2018;10(38):32026–32037. doi: 10.1021/acsami.8b13300. [DOI] [PubMed] [Google Scholar]
- 197.McKinlay C.J., Vargas J.R., Blake T.R., Hardy J.W., Kanada M., Contag C.H., Wender P.A., Waymouth R.M. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl. Acad. Sci. U. S. A. 2017;114(4):E448–E456. doi: 10.1073/pnas.1614193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Truong N.P., Gu W., Prasadam I., Jia Z., Crawford R., Xiao Y., Monteiro M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013;4:1902. doi: 10.1038/ncomms2905. [DOI] [PubMed] [Google Scholar]
- 199.Li Y., Ding J., Xu X., Shi R., Saw P.E., Wang J., Chung S., Li W., Aljaeid B.M., Lee R.J., Tao W., Teng L., Farokhzad O.C., Shi J. Dual hypoxia-targeting RNAi nanomedicine for precision cancer therapy. Nano Lett. 2020;20(7):4857–4863. doi: 10.1021/acs.nanolett.0c00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yan Y., Wang C.H., Cui S.H., Zhai L., Sun J., Liu X.Y., Chen X., Sun Y., Qian H.G., Gao X., Tang Y.D., Zhu Y.J., Shi Y.J., Zhang Q., Wang J.C. Enhanced cancer therapeutic efficiency of NO combined with siRNA by caspase-3 responsive polymers. J. Control. Release. 2021;339:506–520. doi: 10.1016/j.jconrel.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 201.Gurnani P., Blakney A.K., Terracciano R., Petch J.E., Blok A.J., Bouton C.R., McKay P.F., Shattock R.J., Alexander C. The in vitro, ex vivo, and in vivo effect of polymer hydrophobicity on charge-reversible vectors for self-amplifying RNA. Biomacromolecules. 2020;21(8):3242–3253. doi: 10.1021/acs.biomac.0c00698. [DOI] [PubMed] [Google Scholar]
- 202.Truong N.P., Gu W.Y., Prasadam I., Jia Z.F., Crawford R., Xiao Y., Monteiro M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013;4 doi: 10.1038/ncomms2905. [DOI] [PubMed] [Google Scholar]
- 203.Truong N.P., Jia Z., Burges M., McMillan N.A., Monteiro M.J. Self-catalyzed degradation of linear cationic poly(2-dimethylaminoethyl acrylate) in water. Biomacromolecules. 2011;12(5):1876–1882. doi: 10.1021/bm200219e. [DOI] [PubMed] [Google Scholar]
- 204.Dokka S., Toledo D., Shi X.G., Castranova V., Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm. Res. 2000;17(5):521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 205.Charbe N.B., Amnerkar N.D., Ramesh B., Tambuwala M.M., Bakshi H.A., Aljabali A.A.A., Khadse S.C., Satheeshkumar R., Satija S., Metha M., Chellappan D.K., Shrivastava G., Gupta G., Negi P., Dua K., Zacconi F.C. Small interfering RNA for cancer treatment: overcoming hurdles in delivery. Acta Pharm. Sin. B. 2020;10(11):2075–2109. doi: 10.1016/j.apsb.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J. Control. Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 208.Yu A.M., Batra N., Tu M.J., Sweeney C. Novel approaches for efficient in vivo fermentation production of noncoding RNAs. Appl. Microbiol. Biotechnol. 2020;104(5):1927–1937. doi: 10.1007/s00253-020-10350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 210.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. P Natl. Acad. Sci. USA. 2014;111(31):11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liang X., Liu L., Wei Y.Q., Gao G.P., Wei X.W. Clinical evaluations of toxicity and efficacy of nanoparticle-mediated gene therapy. Hum. Gene Ther. 2018;29(11):1227–1234. doi: 10.1089/hum.2018.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Burnett J.C., Rossi J.J. RNA-based therapeutics: current progress and future prospects. Chem. Biol. 2012;19(1):60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 214.Makarova J., Turchinovich A., Shkurnikov M., Tonevitsky A. Extracellular miRNAs and Cell-Cell communication: problems and prospects. Trends Biochem. Sci. 2021;46(8):640–651. doi: 10.1016/j.tibs.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 215.Kooijmans S.A.A., de Jong O.G., Schiffelers R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev. 2021;173:252–278. doi: 10.1016/j.addr.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 216.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Desrochers L.M., Antonyak M.A., Cerione R.A. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev. Cell. 2016;37(4):301–309. doi: 10.1016/j.devcel.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.West E.E., Kolev M., Kemper C. Complement and the regulation of T Cell responses. Annu. Rev. Immunol. 2018;36:309–338. doi: 10.1146/annurev-immunol-042617-053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pipkin M.E., Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr. Opin. Immunol. 2007;19(3):301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Clynes R.A., Desjarlais J.R. Redirected T cell cytotoxicity in cancer therapy. Annu. Rev. Med. 2019;70:437–450. doi: 10.1146/annurev-med-062617-035821. [DOI] [PubMed] [Google Scholar]
- 221.Masopust D., Schenkel J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013;13(5):309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 222.Jhunjhunwala S., Hammer C., Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer. 2021;21(5):298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 223.Mpakali A., Stratikos E. The role of antigen processing and presentation in cancer and the efficacy of immune checkpoint inhibitor immunotherapy. Cancers. 2021;13(1):134. doi: 10.3390/cancers13010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat. Rev. Cardiol. 2019;16(3):166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 225.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: an update. Eur. Heart J. 2017;38(11):785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zifkos K., Dubois C., Schafer K. Extracellular vesicles and thrombosis: update on the clinical and experimental evidence. Int. J. Mol. Sci. 2021;22(17):9317. doi: 10.3390/ijms22179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van Dongen H.M., Masoumi N., Witwer K.W., Pegtel D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. R. 2016;80(2):369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Somiya M., Kuroda S. Development of a virus-mimicking nanocarrier for drug delivery systems: the bio-nanocapsule. Adv. Drug Deliv. Rev. 2015;95:77–89. doi: 10.1016/j.addr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 229.van Rijn P., Schirhagl R. Viruses, artificial viruses and virus-based structures for biomedical applications. Adv. Healthc. Mater. 2016;5(12):1386–1400. doi: 10.1002/adhm.201501000. [DOI] [PubMed] [Google Scholar]
- 230.Gao X.H., Ding J.Q., Long Q.Q., Zhan C.Y. Virus-mimetic systems for cancer diagnosis and therapy. Wires Nanomed. Nanobi. 2021;13(3):e1692. doi: 10.1002/wnan.1692. [DOI] [PubMed] [Google Scholar]
- 231.Parodi A., Molinaro R., Sushnitha M., Evangelopoulos M., Martinez J.O., Arrighetti N., Corbo C., Tasciotti E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–168. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 232.Lu M., Huang Y.Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242:119925. doi: 10.1016/j.biomaterials.2020.119925. [DOI] [PubMed] [Google Scholar]
- 233.Fernandes M., Lopes I., Teixeira J., Botelho C., Gomes A.C. Exosome-like nanoparticles: a new type of nanocarrier. Curr. Med. Chem. 2020;27(23):3888–3905. doi: 10.2174/0929867326666190129142604. [DOI] [PubMed] [Google Scholar]
- 234.Xie J., Shen Q., Huang K.X., Zheng T.Y., Cheng L.T., Zhang Z., Yu Y., Liao G.J., Wang X.Y., Li C. Oriented assembly of cell-mimicking nanoparticles via a molecular affinity strategy for targeted drug delivery. ACS Nano. 2019;13(5):5268–5277. doi: 10.1021/acsnano.8b09681. [DOI] [PubMed] [Google Scholar]
- 235.Kang M., Hong J., Jung M., Kwon S.P., Song S.Y., Kim H.Y., Lee J.R., Kong S., Han J., Koo J.H., Ryu J.H., Lim S., Sohn H.S., Choi J.M., Doh J., Kim B.S. T-cell-mimicking nanoparticles for cancer immunotherapy. Adv. Mater. 2020;32(39):e2003368. doi: 10.1002/adma.202003368. [DOI] [PubMed] [Google Scholar]
- 236.Nie D., Dai Z., Li J.L., Yang Y.W., Xi Z.Y., Wang J., Zhang W., Qian K., Guo S.Y., Zhu C.L., Wang R., Li Y.M., Yu M.R., Zhang X.X., Shi X.H., Gan Y. Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett. 2020;20(2):936–946. doi: 10.1021/acs.nanolett.9b03817. [DOI] [PubMed] [Google Scholar]
- 237.Yao C., Wu W., Tang H., Jia X., Tang J., Ruan X., Li F., Leong D.T., Luo D., Yang D. Self-assembly of stem cell membrane-camouflaged nanocomplex for microRNA-mediated repair of myocardial infarction injury. Biomaterials. 2020;257:120256. doi: 10.1016/j.biomaterials.2020.120256. [DOI] [PubMed] [Google Scholar]
- 238.Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yao C., Wu W.J., Tang H., Jia X.M., Tang J.P., Ruan X.H., Li F., Leong D.T., Luo D., Yang D.Y. Self-assembly of stem cell membrane-camouflaged nanocomplex for microRNA-mediated repair of myocardial infarction injury. Biomaterials. 2020;257:120256. doi: 10.1016/j.biomaterials.2020.120256. [DOI] [PubMed] [Google Scholar]
- 240.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., Gotoh N., Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Therapy: J. Am. Soc. Gene Therapy. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., Gotoh N., Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol. Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu Y.J., Zou Y., Feng C., Lee A., Yin J.L., Chung R., Park J.B., Rizos H., Tao W., Zheng M., Farokhzad O.C., Shi B.Y. Charge conversional biomimetic nanocomplexes as a multifunctional platform for boosting orthotopic glioblastoma RNAi therapy. Nano Lett. 2020;20(3):1637–1646. doi: 10.1021/acs.nanolett.9b04683. [DOI] [PubMed] [Google Scholar]
- 243.Wang Y.M., Ji X., Ruan M.L., Liu W., Song R.G., Dai J., Xue W. Worm-like biomimetic nanoerythrocyte carrying siRNA for melanoma gene therapy. Small. 2018;14(47):e1803002. doi: 10.1002/smll.201803002. [DOI] [PubMed] [Google Scholar]
- 244.Zhuang J., Gong H., Zhou J.R., Zhang Q.Z., Gao W.W., Fang R.H., Zhang L.F. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci. Adv. 2020;6(13):eaaz6108. doi: 10.1126/sciadv.aaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Banizs A.B., Huang T., Dryden K., Berr S.S., Stone J.R., Nakamoto R.K., Shi W.B., He J. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int. J. Nanomedicine. 2014;9:4223–4230. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bryniarski K., Ptak W., Jayakumar A., Pullmann K., Caplan M.J., Chairoungdua A., Lu J., Adams B.D., Sikora E., Nazimek K., Marquez S., Kleinstein S.H., Sangwung P., Iwakiri Y., Delgato E., Redegeld F., Blokhuis B.R., Wojcikowski J., Daniel A.W., Groot Kormelink T., Askenase P.W. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 2013;132(1):170–181. doi: 10.1016/j.jaci.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xu C., Liu W., Hu Y., Li W.P., Di W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics. 2020;10(7):3325–3339. doi: 10.7150/thno.41228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang L.M., Deng S., Zhang Y.F., Peng Q.S., Li H., Wang P., Fu X.M., Lei X.P., Qin A.P., Yu X.Y. Homotypic targeting delivery of siRNA with artificial cancer cells. Adv. Healthc. Mater. 2020;9(9):e1900772. doi: 10.1002/adhm.201900772. [DOI] [PubMed] [Google Scholar]
- 249.Mu X.P., Li J., Yan S.H., Zhang H.M., Zhang W.J., Zhang F.Q., Jiang J.L. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. Acs Biomater Sci Eng. 2018;4(11):3895–3905. doi: 10.1021/acsbiomaterials.8b00858. [DOI] [PubMed] [Google Scholar]
- 250.Zhang F., Zhao L.J., Wang S.M., Yang J., Lu G.H., Luo N.N., Gao X.Y., Ma G.H., Xie H.Y., Wei W. Construction of a biomimetic magnetosome and its application as a SiRNA carrier for high-performance anticancer therapy. Adv. Funct. Mater. 2018;28(1):1703326. [Google Scholar]
- 251.Cooper J.M., Wiklander P.B.O., Nordin J.Z., Al-Shawi R., Wood M.J., Vithlani M., Schapira A.H.V., Simons J.P., El-Andaloussi S., Alvarez-Erviti L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014;29(12):1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang Y.Y., Chen X., Tian B.Q., Liu J.F., Yang L., Zeng L.L., Chen T.F., Hong A., Wang X.G. Nucleolin-targeted extracellular vesicles as a versatile platform for biologics delivery to breast cancer. Theranostics. 2017;7(5):1360–1372. doi: 10.7150/thno.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Feng M.Y., Jiang W., Kim B.Y.S., Zhang C.C., Fu Y.X., Weissman I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer. 2019;19(10):568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li Z., Li Y., Gao J., Fu Y., Hua P., Jing Y., Cai M., Wang H., Tong T. The role of CD47-SIRPalpha immune checkpoint in tumor immune evasion and innate immunotherapy. Life Sci. 2021;273:119150. doi: 10.1016/j.lfs.2021.119150. [DOI] [PubMed] [Google Scholar]
- 255.Geller A., Yan J. The role of membrane bound complement regulatory proteins in tumor development and cancer immunotherapy. Front. Immunol. 2019;10:1074. doi: 10.3389/fimmu.2019.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lv H., Zhang S., Wang B., Cui S., Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 257.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sakurai H., Kawabata K., Sakurai F., Nakagawa S., Mizuguchi H. Innate immune response induced by gene delivery vectors. Int. J. Pharm. 2008;354(1–2):9–15. doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 259.Barros S.A., Gollob J.A. Safety profile of RNAi nanomedicines. Adv. Drug Deliv. Rev. 2012;64(15):1730–1737. doi: 10.1016/j.addr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 260.Zhao L., Gu C., Gan Y., Shao L., Chen H., Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release Off. J. Controll. Release Soc. 2020;318:1–15. doi: 10.1016/j.jconrel.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 261.Chen J., Wu Z., Ding W., Xiao C., Zhang Y., Gao S., Gao Y., Cai W. SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer. Theranostics. 2020;10(4):1619–1632. doi: 10.7150/thno.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang L., Deng S., Zhang Y., Peng Q., Li H., Wang P., Fu X., Lei X., Qin A., Yu X. Homotypic targeting delivery of siRNA with artificial cancer cells. Adv. Healthc. Mater. 2020;9(9) doi: 10.1002/adhm.201900772. [DOI] [PubMed] [Google Scholar]
- 263.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 264.Chai Z., Ran D., Lu L., Zhan C., Ruan H., Hu X., Xie C., Jiang K., Li J., Zhou J., Wang J., Zhang Y., Fang R.H., Zhang L., Lu W. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13(5):5591–5601. doi: 10.1021/acsnano.9b00661. [DOI] [PubMed] [Google Scholar]
- 265.Yoon H.Y., Shin M.L., Shim M.K., Lee S., Na J.H., Koo H., Lee H., Kim J.H., Lee K.Y., Kim K., Kwon I.C. Artificial chemical reporter targeting strategy using bioorthogonal click reaction for improving active-targeting efficiency of tumor. Mol. Pharm. 2017;14(5):1558–1570. doi: 10.1021/acs.molpharmaceut.6b01083. [DOI] [PubMed] [Google Scholar]
- 266.Wang H., Su D., Huang R., Shu F., Cheng F., Zheng G. Cellular nanovesicles with bioorthogonal targeting enhance photodynamic/photothermal therapy in psoriasis. Acta Biomater. 2021;134:674–685. doi: 10.1016/j.actbio.2021.07.068. [DOI] [PubMed] [Google Scholar]
- 267.Han Y., Pan H., Li W., Chen Z., Ma A., Yin T., Liang R., Chen F., Ma Y., Jin Y., Zheng M., Li B., Cai L. T cell membrane mimicking nanoparticles with bioorthogonal targeting and immune recognition for enhanced photothermal therapy. Adv. Sci. (Weinh). 2019;6(15):1900251. doi: 10.1002/advs.201900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fu Z., Zhang X., Zhou X., Ur-Rehman U., Yu M., Liang H., Guo H., Guo X., Kong Y., Su Y., Ye Y., Hu X., Cheng W., Wu J., Wang Y., Gu Y., Lu S.F., Wu D., Zen K., Li J., Yan C., Zhang C.Y., Chen X. In vivo self-assembled small RNAs as a new generation of RNAi therapeutics. Cell Res. 2021;31(6):631–648. doi: 10.1038/s41422-021-00491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., Sun W., Duan Y., Yang G., Yuan L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19(1):19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 270.Teng F., Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv. Sci. 2021;8(1):2003505. doi: 10.1002/advs.202003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Bi. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 272.Tosar J.P., Witwer K., Cayota A. Revisiting extracellular RNA release, processing, and function. Trends Biochem. Sci. 2021;46(6):438–445. doi: 10.1016/j.tibs.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ratajczak M.Z., Ratajczak J. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia. 2020;34(12):3126–3135. doi: 10.1038/s41375-020-01041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fabbiano F., Corsi J., Gurrieri E., Trevisan C., Notarangelo M., D’Agostino V.G. RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J. Extracell Vesicles. 2020;10(2):e12043. doi: 10.1002/jev2.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fitz N.F., Wang J.B., Kamboh M.I., Koldamova R., Lefterov I. Vol. 159. Neurobiol Dis; 2021. Small nucleolar RNAs in plasma extracellular vesicles and their discriminatory power as diagnostic biomarkers of Alzheimer’s disease. p. 105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Michaela S., Aigner A. Nucleic acid delivery with extracellular vesicles. Adv. Drug Deliv. Rev. 2021;173:89–111. doi: 10.1016/j.addr.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 277.Wahlgren J., De L.K.T., Brisslert M., Vaziri Sani F., Telemo E., Sunnerhagen P., Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17) doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Cooper J.M., Wiklander P.B., Nordin J.Z., Al-Shawi R., Wood M.J., Vithlani M., Schapira A.H., Simons J.P., El-Andaloussi S., Alvarez-Erviti L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Movement Disorders: Off. J. Movement Disorder Soc. 2014;29(12):1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kang J.Y., Park H., Kim H., Mun D., Park H., Yun N., Joung B. Human peripheral bloodderived exosomes for microRNA delivery. Int. J. Mol. Med. 2019;43(6):2319–2328. doi: 10.3892/ijmm.2019.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhuang J.L., Tan J.Z., Wu C.L., Zhang J., Liu T., Fan C.H., Li J.P., Zhang Y.Q. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 2020;48(16):8870–8882. doi: 10.1093/nar/gkaa683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B.Y., San Chan Y., Wei L.K., Chin S.M., Azad A., He A.B.L., Leung A.Y.H., Yang M.S., Shyh-Chang N., Cho W.C., Shi J.H., Le M.T.N. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9(1):2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sutaria D.S., Jiang J.M., Elgamala O.A., Pomeroy S.M., Badawi M., Zhu X.H., Pavlovicz R., Azevedo-Pouly A.C.P., Chalmers J., Li C.L., Phelps M.A., Schmittgen T.D. Low active loading of cargo into engineered extracellular vesicles results in inefficient miRNA mimic delivery. J. Extracell Vesicles. 2017;6(1):1333882. doi: 10.1080/20013078.2017.1333882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 284.Phua K.K.L., Boczkowski D., Dannull J., Pruitt S., Leong K.W., Nair S.K. Whole blood cells loaded with messenger RNA as an anti-tumor vaccine. Adv. Healthc. Mater. 2014;3(6):837–842. doi: 10.1002/adhm.201300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu H.L., Huang L.L., Mao M.C., Ding J.J., Wu G.H., Fan W.L., Yang T.R., Zhang M.J., Huang Y.Y., Xie H.Y. Viral protein-pseudotyped and siRNA-electroporated extracellular vesicles for cancer immunotherapy. Adv. Funct. Mater. 2020;30(52):2006515. [Google Scholar]
- 286.Lee S.T., Im W., Ban J.J., Lee M., Jung K.H., Lee S.K., Chu K., Kim M. Exosome-based delivery of miR-124 in a Huntington's disease model. J. Mov. Disord. 2017;10(1):45–52. doi: 10.14802/jmd.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Forterre A.V., Wang J.H., Delcayre A., Kim K., Green C., Pegram M.D., Jeffrey S.S., Matin A.C. Extracellular vesicle-mediated in vitro transcribed mRNA delivery for treatment of HER2(+) breast cancer xenografts in mice by prodrug CB1954 without general toxicity. Mol. Cancer Ther. 2020;19(3):858–867. doi: 10.1158/1535-7163.MCT-19-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Reshke R., Taylor J.A., Savard A., Guo H.S., Rhym L.H., Kowalski P.S., Trung M.T., Campbell C., Little W., Anderson D.G., Gibbings D. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat. Biomed. Eng. 2020;4(1):52–68. doi: 10.1038/s41551-019-0502-4. [DOI] [PubMed] [Google Scholar]
- 289.Liu Y.C., Li D.M., Liu Z.Y., Zhou Y., Chu D.P., Li X.H., Jiang X.H., Hou D.X., Chen X., Chen Y.D., Yang Z.Z., Jin L., Jiang W., Tian C.F., Zhou G.Y., Zen K., Zhang J.F., Zhang Y.J., Li J., Zhang C.Y. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep.-UK. 2015;5:17543. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mizrak A., Bolukbasi M.F., Ozdener G.B., Brenner G.J., Madlener S., Erkan E.P., Strobel T., Breakefield X.O., Saydam O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013;21(1):101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bu T., Li Z.L., Hou Y., Sun W.Q., Zhang R.X., Zhao L.B., Wei M.Y., Yang G.D., Yuan L.J. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics. 2021;11(20):9988–10000. doi: 10.7150/thno.64229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lamichhane T.N., Jeyaram A., Patel D.B., Parajuli B., Livingston N.K., Arumugasaamy N., Schardt J.S., Jay S.M. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell. Mol. Bioeng. 2016;9(3):315–324. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang B., Yao K., Huuskes B.M., Shen H.H., Zhuang J.L., Godson C., Brennan E.P., Wilkinson-Berka J.L., Wise A.F., Ricardo S.D. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 2016;24(7):1290–1301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S.J., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.O'Brien K.P., Khan S., Gilligan K.E., Zafar H., Lalor P., Glynn C., O'Flatharta C., Ingoldsby H., Dockery P., De Bhulbh A., Schweber J.R., John K. St, Leahy M., Murphy J.M., Gallagher W.M., O'Brien T., Kerin M.J., Dwyer R.M. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 296.Zhang K.L., Dong C., Chen M., Yang T.T., Wang X., Gao Y.H., Wang L.J., Wen Y.H., Chen G.Y., Wang X.L., Yu X.C., Zhang Y.L., Wang P.S., Shang M.F., Han K., Zhou Y. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411–425. doi: 10.7150/thno.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhou W.X., Zhou Y., Chen X.L., Ning T.T., Chen H.Y., Guo Q., Zhang Y.W., Liu P.X., Zhang Y.J., Li C., Chu Y.C., Sun T., Jiang C. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. doi: 10.1016/j.biomaterials.2020.120546. [DOI] [PubMed] [Google Scholar]
- 298.Li H.D., Yang C., Shi Y.J., Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J. Nanobiotechnol. 2018;16(1):103. doi: 10.1186/s12951-018-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Katakowski M., Buller B., Zheng X.G., Lu Y., Rogers T., Osobamiro O., Shu W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yang J.L., Zhang X.F., Chen X.J., Wang L., Yang G.D. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Ther-Nucl. Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yang T.Z., Fogarty B., LaForge B., Aziz S., Pham T., Lai L.N., Bai S.H. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19(2):475–486. doi: 10.1208/s12248-016-0015-y. [DOI] [PubMed] [Google Scholar]
- 302.Gujrati V., Kim S., Kim S.H., Min J.J., Choy H.E., Kim S.C., Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525–1537. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 303.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.O'Brien K., Lowry M.C., Corcoran C., Martinez V.G., Daly M., Rani S., Gallagher W.M., Radomski M.W., MacLeod R.A.F., O'Driscoll L. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6(32):32774–32789. doi: 10.18632/oncotarget.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhang Y., Li L., Yu J., Zhu D., Zhang Y., Li X., Gu H., Zhang C.Y., Zen K. Microvesicle-mediated delivery of transforming growth factor beta1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014;35(14):4390–4400. doi: 10.1016/j.biomaterials.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 306.Fonsato V., Collino F., Herrera M.B., Cavallari C., Deregibus M.C., Cisterna B., Bruno S., Romagnoli R., Salizzoni M., Tetta C., Camussi G. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30(9):1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wahlgren J., Karlson T.D., Brisslert M., Sani F.V., Telemo E., Sunnerhagen P., Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Hu T.R., Hu J.C. Melanoma-derived exosomes induce reprogramming fibroblasts into cancer-associated fibroblasts via Gm26809 delivery. Cell Cycle. 2019;18(22):3085–3094. doi: 10.1080/15384101.2019.1669380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ma W.T., Zhang W.H., Cui B., Gao J., Liu Q.H., Yao M.Y., Ning H.B., Xing L.H. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in sepsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death Dis. 2021;12(11):1056. doi: 10.1038/s41419-021-04117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tao S.C., Rui B.Y., Wang Q.Y., Zhou D., Zhang Y., Guo S.C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25(1):241–255. doi: 10.1080/10717544.2018.1425774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhou J., Che J., Xu L., Yang W., Zhou W., Zhou C. Tumor-derived extracellular vesicles containing long noncoding RNA PART1 exert oncogenic effect in hepatocellular carcinoma by polarizing macrophages into M2. Dig. Liver Dis. 2021 doi: 10.1016/j.dld.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 312.Wang X.Y., Zhang H.Y., Yang H.O., Bai M., Ning T., Deng T., Liu R., Fan Q., Zhu K.G., Li J.L., Zhan Y., Ying G.G., Ba Y. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol. Oncol. 2020;14(3):539–555. doi: 10.1002/1878-0261.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang J., Zhang Y., Ma Y., Luo L., Chu M., Zhang Z. Therapeutic potential of exosomal circRNA derived from synovial mesenchymal cells via targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF functional module in rheumatoid arthritis. Int. J. Nanomedicine. 2021;16:7977–7994. doi: 10.2147/IJN.S333465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Si J., Li W., Li X., Cao L., Chen Z., Jiang Z. Heparanase confers temozolomide resistance by regulation of exosome secretion and circular RNA composition in glioma. Cancer Sci. 2021;112(9):3491–3506. doi: 10.1111/cas.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]