Abstract
Background
The aim of the study was to assess, characterize, and describe the prevalence and predicting factors of patient-reported severe coronavirus disease 2019 (COVID-19) infection and post-acute sequelae of COVID-19 (PASC).
Methods
We prospectively surveyed patients who received care in our outpatient clinic for COVID-19 from March 13, 2020, through August 17, 2020, and then retrospectively reviewed their electronic health records. We collected data for age, sex, and persistence of symptoms and compared data for hospitalized and nonhospitalized patients. Continuous and categorical variables were summarized, including time from COVID-19 onset, time to resuming normal activities, and length of time away from work.
Results
Of those receiving the survey, 437 adult patients with different degrees of severity of COVID-19 illness responded: 77% were between 3 and 6 months from the onset of infection. In total, 34.9% had persistent symptoms, and 11.5% were hospitalized. The most common symptom was fatigue (75.9%), followed by poor sleep quality (60.3%), anosmia (56.8%), dysgeusia (55%), and dyspnea (54.6%). Predicting factors for PASC were female sex and a negative psychological impact of the disease. Age, hospitalization, persistent symptoms, psychological impact (e.g., anxiety and depression), and time missed from work were significantly associated with perception of having severe COVID-19 illness. Hospitalization was not significantly associated with PASC.
Conclusions
Over one-third of patients in our study had PASC. Persistent symptoms correlated with severity of disease and were significantly more common for women, for patients who had psychological symptoms (depression and/or anxiety), and for patients reporting inability to resume normal activities.
Key Indexing Terms: Epidemiology, Fatigue, Infectious diseases, SARS
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected every country in the world. Although most persons recover from the acute disease quickly, others do not and may continue to have symptoms or develop new symptoms after the initial illness.1 , 2 Risk factors for severe disease along with the potential of severe disease to be associated with chronic sequelae, have both been reported,3 , 4 which aligns with the reported symptomatic persistence of other coronavirus infections.5, 6, 7 Long COVID-19 or post–COVID-19 syndrome were the terms originally given to disease that lingered well after patients had recovered from the acute infection. More recently, the terminology has been updated. Post COVID-19 condition is now defined as a condition that persists 3 months from the onset of symptoms and lasts for at least 2 months; post-acute sequelae of COVID-19 (PASC) describes the syndrome of persistent symptoms, generally for more than 4 weeks after recovery from acute COVID-19.8, 9, 10
With PASC, patients usually have either nonspecific symptoms, such as fatigue and shortness of breath, or serious single or multisystem sequelae including cardiopulmonary, thromboembolic, renal, psychiatric, and neurologic complications.11 An initial study reported up to 76% of patients discharged from the hospital had at least 1 remaining symptom 6 months after the onset of COVID-19, with more women affected than men.12 A recent study showed that just 65% of persons had returned to their usual state of health 14 to 21 days after a positive COVID-19 test.4
Understanding the prevalence and predisposing factors for chronic sequelae can help in early identification, intervention, and support of patients at higher risk for developing PASC. However, the more commonly reported symptoms of PASC, such as fatigue, cognitive impairment, and sleep disorders, are difficult to quantitate, making it important to study patient perceptions of illness severity as well as the prevalence and severity of PASC symptoms. Therefore, we prospectively surveyed patients to describe and characterize the prevalence and predictive factors of severe COVID-19. We also assessed the association between patient perceptions of symptom severity and the clinical presentation of PASC. Herein, we report the prevalence of PASC in our patient population and specific predicting factors for development of PASC.
Methods
The survey study was deemed exempt by the Mayo Clinic Institutional Review Board on December 30, 2020 (IRB application # 20–012275).
Study design and patients
We conducted a single-center, prospective survey study with a retrospective review of the electronic health record (EHR) at Mayo Clinic in Jacksonville, Florida. Patients were evaluated at their intake in the COVID-19 virtual clinic; testing results, demographic characteristics, and contact information were obtained. We used the following eligibility criteria for inclusion in the study: (1) every patient who had positive test results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reverse transcription polymerase chain reaction; (2) patients at least 18 years old; and (3) patients who received care in the COVID-19 virtual clinic from March 13, 2020, through August 17, 2020. The patients were then followed up prospectively and surveyed thereafter.
Email invitations for the survey were sent on December 31, 2020, to patients who met the above inclusion criteria. Each patient received a dedicated link to complete the survey, which could not be completed multiple times. A follow-up reminder was sent to those who had not completed the survey on January 13, 2021. The survey was closed 2 weeks after the last reminder was sent. Survey completion served as implied consent per detailed instructions included in the invitation email. The survey was distributed using REDCap Software, Version 10.0.33 (Vanderbilt University).
Survey
The email invitation to complete the survey instructed patients to reflect on their COVID-19 illness experience. The survey included 3 main sections. The first section focused on basic demographic questions about age and sex. The second section included questions focused on the patient's acute infection experience. Patients were asked to rate their perception of the severity of their illness using a 5-point Likert scale (from 1, very mild; to 5, very severe) and to indicate whether they had been hospitalized for COVID-19. Time since initial diagnosis was determined by a question using the following ranges: 1 to 4 weeks, 1 to 3 months, 3 to 6 months, or greater than 9 months. The third section included questions focused on long-term effects, specifically the presence and length of persistent or chronic symptoms from COVID-19 illness. If patients indicated that they still had some residual symptoms of COVID-19 illness, they were prompted with a list of 19 symptoms that have been previously reported.13 The patients were asked to rate these symptoms using a 6-point scale (1, none; 2, very mild; 3, mild; 4, moderate; 5, severe; and 6, very severe). The final symptom option was open-ended to allow patients to add any other unlisted symptoms. The remainder of questions in the third section focused on identifying the length of time patients experienced persistent symptoms; whether patients were symptom free for any period before onset of new symptoms; the length of time before they were able to resume normal activities; and the amount of time missed from work (if applicable). The length of time was divided into the following periods: less than 1 week, 1 to 2 weeks, 3 to 4 weeks, 1 to 3 months, more than 3 to 6 months, and greater than 6 months. We also asked patients to indicate if they experienced any negative psychological issues, specifically anxiety and/or depression from COVID-19 illness and if they were diagnosed with PASC (“yes” or “no” option for both questions).
Aims
Our aim was to characterize the prevalence of PASC and to describe specific predisposing factors in our population. Our secondary aim was to assess the association between patients’ perceptions of severity of COVID-19 disease and PASC. Patient report of hospitalization was used to determine disease severity. We hypothesized that PASC would be more prevalent in patients who had severe acute COVID-19. We also hypothesized that age and sex would be predictable and associated with severe acute COVID-19 and PASC. We also compared self-reported perceptions of severity of acute COVID-19 and the presence of PASC in the patients who were hospitalized (a measure of disease severity) to test our hypothesis that patients who had more severe illness would also have a higher incidence of PASC.
Statistical analysis
Continuous variables were summarized with median and range, and categorical variables were summarized with frequency and percent. Differences among severity levels were evaluated using the Kruskal-Wallis rank sum test for continuous measures and the χ2 test for categorical measures. We used a trend test to evaluate the 3 ordinal variables: (1) How long has it been since you were diagnosed with COVID-19? (2) How long was it before you felt able to resume normal activities? and (3) How much time did you miss from work because of your illness? All tests were 2-sided, and P values less than 0.05 were considered statistically significant. All analysis programming was done in RStudio, version 3.6.2 (R Project).
Results
The invitation for the survey was sent to 1697 patients whose email addresses were documented in the EHR. Of those, 25 invitations were undeliverable because of incorrect email addresses. The invited population included 795 women (47%) and 902 men (53%), and their ages ranged between 18 and 99 years (median, 43 years; mean [SD], 43.5 [18.1] years). A total of 448 anonymized patients responded to the survey. Upon review of the responses, we learned that 11 patients were under the age of 18 years. These 11 patients were excluded, leaving 437 patients included in the study. Demographic and clinical characteristics of the study population are summarized in Table 1 . The median (range) age was 54 (18–99) years. Severity level did not differ for women or men (P = 0.71). Patients with severe/very severe disease were more likely to be hospitalized (48.2%) than patients with mild/very mild disease (1.4%) or moderate disease (4.9%) (P < 0.001). There were significant differences between severity levels for persistent symptoms, time before resuming normal activities, time missed from work, and negative psychological issues from COVID-19 (P < 0.001). Although results were not significant, almost one-third of respondents were symptom free for a period of time before new symptoms started. Of these respondents, the highest percentage was in the milder disease category: 40 of 116 (34.5%).
TABLE 1.
Patient characteristics and baseline variables by degree of severity.
| No. (%)a |
|||||
|---|---|---|---|---|---|
| Characteristic | Mild/very mild (n = 211) | Moderate (n = 142) | Severe/very severe (n = 84) | Total (N = 437) | P valueb |
| Age | <0.001 | ||||
| Median (range) | 51 (18–90) | 54 (19–99) | 59 (23–79) | 54 (18–99) | |
| Sex | 0.71 | ||||
| Female | 123 (58.3) | 89 (62.7) | 51 (60.7) | 263 (60.2) | |
| Male | 88 (41.7) | 53 (37.3) | 33 (39.3) | 174 (39.8) | |
| Were you hospitalized for your illness? | <0.001 | ||||
| No. missing | 0 | 0 | 1 | 1 | |
| No | 208 (98.6) | 135 (95.1) | 43 (51.8) | 386 (88.5) | |
| Yes | 3 (1.4) | 7 (4.9) | 40 (48.2) | 50 (11.5) | |
| How long has it been since you were diagnosed with COVID-19? | 0.20 | ||||
| 1–4 wk | 4 (1.9) | 0 (0.0) | 3 (3.6) | 7 (1.6) | |
| 1–3 mo | 20 (9.5) | 7 (4.9) | 9 (10.7) | 36 (8.2) | |
| 3–6 mo | 163 (77.3) | 115 (81.0) | 60 (71.4) | 338 (77.3) | |
| >9 mo | 24 (11.4) | 20 (14.1) | 12 (14.3) | 56 (12.8) | |
| Do you have any persistent symptoms from COVID-19? | <0.001 | ||||
| No. missing | 1 | 1 | 0 | 2 | |
| No | 165 (78.6) | 77 (54.6) | 41 (48.8) | 283 (65.1) | |
| Yes | 45 (21.4) | 64 (45.4) | 43 (51.2) | 152 (34.9) | |
| Were you symptom free for any period before new symptoms started? | 0.23 | ||||
| No. missing | 95 | 45 | 22 | 162 | |
| No | 76 (65.5) | 70 (72.2) | 48 (77.4) | 194 (70.5) | |
| Yes | 40 (34.5) | 27 (27.8) | 14 (22.6) | 81 (29.5) | |
| How long was it before you felt able to resume normal activities? | <0.001 | ||||
| No. missing | 3 | 2 | 2 | 7 | |
| <1 wk | 88 (42.3) | 4 (2.9) | 2 (2.4) | 94 (21.9) | |
| 1–2 wk | 59 (28.4) | 30 (21.4) | 5 (6.1) | 94 (21.9) | |
| 3–4 wk | 38 (18.3) | 56 (40.0) | 24 (29.3) | 118 (27.4) | |
| 1–3 mo | 15 (7.2) | 39 (27.9) | 32 (39.0) | 86 (20.0) | |
| 3–6 mo | 7 (3.4) | 6 (4.3) | 17 (20.7) | 30 (7.0) | |
| >6 mo | 1 (0.5) | 5 (3.6) | 2 (2.4) | 8 (1.9) | |
| How much work time did you miss because of your infection? | <0.001 | ||||
| No. missing | 68 | 35 | 36 | 139 | |
| <1 wk | 43 (30.1) | 12 (11.2) | 1 (2.1) | 56 (18.8) | |
| 1–2 wk | 68 (47.6) | 42 (39.3) | 9 (18.8) | 119 (39.9) | |
| 3–4 wk | 22 (15.4) | 34 (31.8) | 20 (41.7) | 76 (25.5) | |
| 1–3 mo | 7 (4.9) | 16 (15.0) | 7 (14.6) | 30 (10.1) | |
| 3–6 mo | 3 (2.1) | 3 (2.8) | 6 (12.5) | 12 (4.0) | |
| >6 mo | 0 (0.0) | 0 (0.0) | 5 (10.4) | 5 (1.7) | |
| Did you experience any negative psychological effects of COVID-19, such as anxiety or depression? | <0.001 | ||||
| No. missing | 0 | 2 | 0 | 2 | |
| No | 160 (75.8) | 84 (60.0) | 43 (51.2) | 287 (66.0) | |
| Yes | 51 (24.2) | 56 (40.0) | 41 (48.8) | 148 (34.0) | |
| Have you ever been diagnosed with post-COVID-19 syndrome? | 0.22 | ||||
| No. missing | 1 | 0 | 0 | 1 | |
| No | 207 (98.6) | 139 (97.9) | 80 (95.2) | 426 (97.7) | |
| Yes | 3 (1.4) | 3 (2.1) | 4 (4.8) | 10 (2.3) | |
Data are presented as No. (%) unless otherwise indicated.
The Kruskal-Wallis test was used for numerical variables, the trend test for ordinal variables, and the χ2 test for categorical variables.
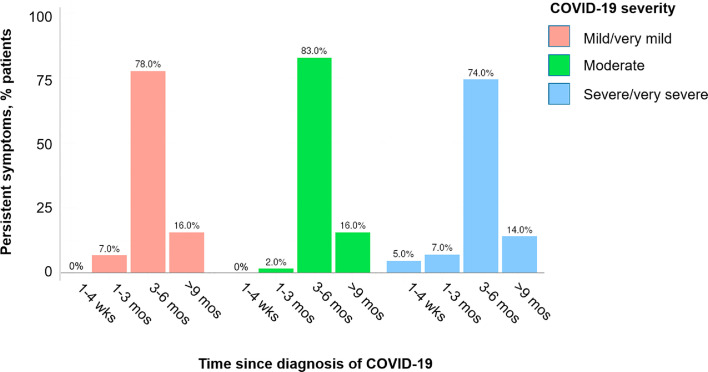
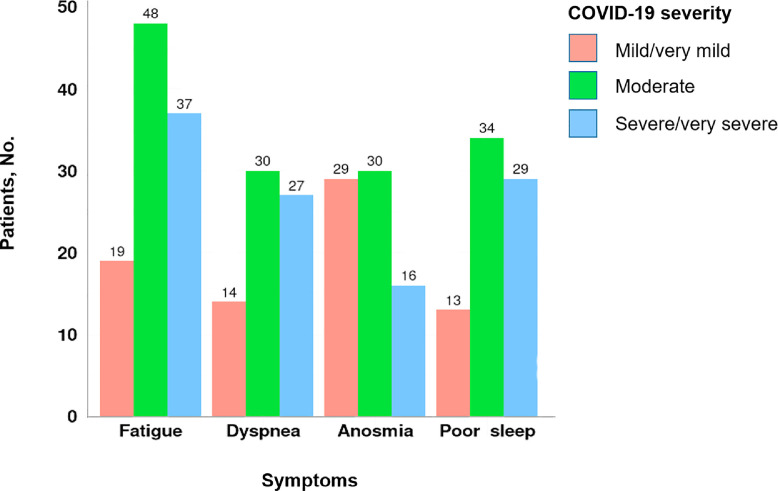
Table 2 summarizes symptoms by severity level. Of illness severity levels, 211 patients (48.3%) had mild or very mild illness, 142 patients (32.5%) had moderate illness, and 84 patients (19.2%) had severe or very severe illness. Most patients had symptoms for at least 3 months (Figure 1 ). Fatigue (75.9%), poor sleep quality (60.3%), anosmia (56.8%), dysgeusia (55%), and dyspnea (54.6%) were the most reported symptoms of patients at all levels of COVID-19 (Figure 2 ).
TABLE 2.
Specific symptoms by degree of severity.
| No. (%) |
||||
|---|---|---|---|---|
| Symptom | Mild/very mild (n = 211) | Moderate (n = 142) | Severe/very severe (n = 84) | Total (N = 437) |
| Fever | ||||
| No. missing | 177 | 89 | 48 | 314 |
| None | 32 (94.1) | 48 (90.6) | 29 (80.6) | 109 (88.6) |
| Mild/very mild | 2 (5.9) | 2 (3.8) | 2 (5.6) | 6 (4.9) |
| Moderate | 0 (0.0) | 3 (5.7) | 2 (5.6) | 5 (4.1) |
| Severe/very severe | 0 (0.0) | 0 (0.0) | 3 (8.3) | 3 (2.4) |
| Fatigue | ||||
| No. missing | 174 | 84 | 42 | 300 |
| None | 18 (48.6) | 10 (17.2) | 5 (11.9) | 33 (24.1) |
| Mild/very mild | 16 (43.2) | 23 (39.7) | 10 (23.8) | 49 (35.8) |
| Moderate | 3 (8.1) | 21 (36.2) | 11 (26.2) | 35 (25.5) |
| Severe/very severe | 0 (0.0) | 4 (6.9) | 16 (38.1) | 20 (14.6) |
| Dyspnea | ||||
| No. missing | 174 | 87 | 46 | 307 |
| None | 23 (62.2) | 25 (45.5) | 11 (28.9) | 59 (45.4) |
| Mild/very mild | 12 (32.4) | 19 (34.5) | 8 (21.1) | 39 (30.0) |
| Moderate | 2 (5.4) | 9 (16.4) | 17 (44.7) | 28 (21.5) |
| Severe/very severe | 0 (0.0) | 2 (3.6) | 2 (5.3) | 4 (3.1) |
| Joint pain | ||||
| No. missing | 177 | 85 | 45 | 307 |
| None | 24 (70.6) | 28 (49.1) | 8 (20.5) | 60 (46.2) |
| Mild/very mild | 4 (11.8) | 17 (29.8) | 12 (30.8) | 33 (25.4) |
| Moderate | 5 (14.7) | 11 (19.3) | 9 (23.1) | 25 (19.2) |
| Severe/very severe | 1 (2.9) | 1 (1.8) | 10 (25.6) | 12 (9.2) |
| Chest pain | ||||
| No. missing | 176 | 88 | 47 | 311 |
| None | 27 (77.1) | 39 (72.2) | 25 (67.6) | 91 (72.2) |
| Mild/very mild | 7 (20.0) | 12 (22.2) | 5 (13.5) | 24 (19.0) |
| Moderate | 1 (2.9) | 2 (3.7) | 5 (13.5) | 8 (6.3) |
| Severe/very severe | 0 (0.0) | 1 (1.9) | 2 (5.4) | 3 (2.4) |
| Cough | ||||
| No. missing | 175 | 86 | 45 | 306 |
| None | 29 (80.6) | 35 (62.5) | 21 (53.8) | 85 (64.9) |
| Mild/very mild | 6 (16.7) | 16 (28.6) | 10 (25.6) | 32 (24.4) |
| Moderate | 0 (0.0) | 4 (7.1) | 4 (10.3) | 8 (6.1) |
| Severe/very severe | 1 (2.8) | 1 (1.8) | 4 (10.3) | 6 (4.6) |
| Anosmia | ||||
| No. missing | 173 | 84 | 48 | 305 |
| None | 9 (23.7) | 28 (48.3) | 20 (55.6) | 57 (43.2) |
| Mild/very mild | 9 (23.7) | 10 (17.2) | 2 (5.6) | 21 (15.9) |
| Moderate | 9 (23.7) | 11 (19.0) | 4 (11.1) | 24 (18.2) |
| Severe/very severe | 11 (28.9) | 9 (15.5) | 10 (27.8) | 30 (22.7) |
| Sicca syndrome | ||||
| No. missing | 177 | 87 | 43 | 307 |
| None | 23 (67.6) | 37 (67.3) | 10 (24.4) | 70 (53.8) |
| Mild/very mild | 9 (26.5) | 10 (18.2) | 10 (24.4) | 29 (22.3) |
| Moderate | 1 (2.9) | 6 (10.9) | 12 (29.3) | 19 (14.6) |
| Severe/very severe | 1 (2.9) | 2 (3.6) | 9 (22.0) | 12 (9.2) |
| Rhinitis | ||||
| No. missing | 177 | 88 | 46 | 311 |
| None | 27 (79.4) | 35 (64.8) | 18 (47.4) | 80 (63.5) |
| Mild/very mild | 6 (17.6) | 16 (29.6) | 13 (34.2) | 35 (27.8) |
| Moderate | 1 (2.9) | 3 (5.6) | 4 (10.5) | 8 (6.3) |
| Severe/very severe | 0 (0.0) | 0 (0.0) | 3 (7.9) | 3 (2.4) |
| Red eyes | ||||
| No. missing | 176 | 88 | 47 | 311 |
| None | 32 (91.4) | 44 (81.5) | 24 (64.9) | 100 (79.4) |
| Mild/very mild | 3 (8.6) | 7 (13.0) | 10 (27.0) | 20 (15.9) |
| Moderate | 0 (0.0) | 3 (5.6) | 1 (2.7) | 4 (3.2) |
| Severe/very severe | 0 (0.0) | 0 (0.0) | 2 (5.4) | 2 (1.6) |
| Dysgeusia | ||||
| No. missing | 173 | 86 | 47 | 306 |
| None | 12 (31.6) | 29 (51.8) | 18 (48.6) | 59 (45.0) |
| Mild/very mild | 11 (28.9) | 13 (23.2) | 7 (18.9) | 31 (23.7) |
| Moderate | 7 (18.4) | 6 (10.7) | 7 (18.9) | 20 (15.3) |
| Severe/very severe | 8 (21.1) | 8 (14.3) | 5 (13.5) | 21 (16.0) |
| Headache | ||||
| No. missing | 176 | 86 | 44 | 306 |
| None | 22 (62.9) | 29 (51.8) | 15 (37.5) | 66 (50.4) |
| Mild/very mild | 8 (22.9) | 14 (25.0) | 5 (12.5) | 27 (20.6) |
| Moderate | 5 (14.3) | 8 (14.3) | 9 (22.5) | 22 (16.8) |
| Severe/very severe | 0 (0.0) | 5 (8.9) | 11 (27.5) | 16 (12.2) |
| Sputum production | ||||
| No. missing | 177 | 89 | 48 | 314 |
| None | 26 (76.5) | 42 (79.2) | 20 (55.6) | 88 (71.5) |
| Mild/very mild | 7 (20.6) | 11 (20.8) | 9 (25.0) | 27 (22.0) |
| Moderate | 1 (2.9) | 0 (0.0) | 5 (13.9) | 6 (4.9) |
| Severe/very severe | 0 (0.0) | 0 (0.0) | 2 (5.6) | 2 (1.6) |
| Lack of appetite | ||||
| No. missing | 177 | 88 | 46 | 311 |
| None | 27 (79.4) | 39 (72.2) | 26 (68.4) | 92 (73.0) |
| Mild/very mild | 4 (11.8) | 9 (16.7) | 4 (10.5) | 17 (13.5) |
| Moderate | 2 (5.9) | 5 (9.3) | 3 (7.9) | 10 (7.9) |
| Severe/very severe | 1 (2.9) | 1 (1.9) | 5 (13.2) | 7 (5.6) |
| Sore throat | ||||
| No. missing | 178 | 89 | 47 | 314 |
| None | 29 (87.9) | 44 (83.0) | 25 (67.6) | 98 (79.7) |
| Mild/very mild | 3 (9.1) | 7 (13.2) | 8 (21.6) | 18 (14.6) |
| Moderate | 1 (3.0) | 2 (3.8) | 2 (5.4) | 5 (4.1) |
| Severe/very severe | 0 (0.0) | 0 (0.0) | 2 (5.4) | 2 (1.6) |
| Vertigo | ||||
| No. missing | 176 | 86 | 46 | 308 |
| None | 26 (74.3) | 30 (53.6) | 19 (50.0) | 75 (58.1) |
| Mild/very mild | 7 (20.0) | 19 (33.9) | 12 (31.6) | 38 (29.5) |
| Moderate | 2 (5.7) | 5 (8.9) | 2 (5.3) | 9 (7.0) |
| Severe/very severe | 0 (0.0) | 2 (3.6) | 5 (13.2) | 7 (5.4) |
| Myalgia | ||||
| No. missing | 177 | 86 | 44 | 307 |
| None | 26 (76.5) | 34 (60.7) | 13 (32.5) | 73 (56.2) |
| Mild/very mild | 6 (17.6) | 12 (21.4) | 10 (25.0) | 28 (21.5) |
| Moderate | 1 (2.9) | 7 (12.5) | 7 (17.5) | 15 (11.5) |
| Severe/very severe | 1 (2.9) | 3 (5.4) | 10 (25.0) | 14 (10.8) |
| Diarrhea | ||||
| No. missing | 178 | 89 | 46 | 313 |
| None | 30 (90.9) | 40 (75.5) | 20 (52.6) | 90 (72.6) |
| Mild/very mild | 1 (3.0) | 9 (17.0) | 7 (18.4) | 17 (13.7) |
| Moderate | 1 (3.0) | 4 (7.5) | 7 (18.4) | 12 (9.7) |
| Severe/very severe | 1 (3.0) | 0 (0.0) | 4 (10.5) | 5 (4.0) |
| Poor sleep | ||||
| No. missing | 177 | 88 | 46 | 311 |
| None | 21 (61.8) | 20 (37.0) | 9 (23.7) | 50 (39.7) |
| Mild/very mild | 4 (11.8) | 24 (44.4) | 12 (31.6) | 40 (31.7) |
| Moderate | 7 (20.6) | 5 (9.3) | 8 (21.1) | 20 (15.9) |
| Severe/very severe | 2 (5.9) | 5 (9.3) | 9 (23.7) | 16 (12.7) |
| Other | ||||
| No. missing | 178 | 93 | 59 | 330 |
| None | 26 (78.8) | 32 (65.3) | 13 (52.0) | 71 (66.4) |
| Mild/very mild | 2 (6.1) | 8 (16.3) | 2 (8.0) | 12 (11.2) |
| Moderate | 2 (6.1) | 1 (2.0) | 4 (16.0) | 7 (6.5) |
| Severe/very severe | 3 (9.1) | 8 (16.3) | 6 (24.0) | 17 (15.9) |
Figure 1.
Persistent symptoms by numbers of patients.
Figure 2.
Severity levels for most common symptoms by time since diagnosis.
Age, sex, and hospitalization were compared between patients with and without persistent symptoms (Table 3 ). Women were significantly more likely to have persistent symptoms than men (P < .001). The association between persistence of symptoms and time to resume normal activities (more than 1 month) was significant: 50.7% for patients with persistent symptoms and 17.5% for patients without persistent symptoms (P < 0.001). Persistence of symptoms was not significantly associated with hospitalization as a clinical measure of disease severity (P = 0.08).
TABLE 3.
Characteristics and baseline variables for patients with and without persistent symptoms.
| No. (%)a |
||||
|---|---|---|---|---|
| Characteristic | Persistent symptoms (n = 152) | No persistent symptoms (n = 283) | Total (N = 435)b | P value |
| Age | 0.50 | |||
| Median (range) | 52 (20–99) | 55 (18–90) | 54 (18–99) | |
| Sex | <0.001 | |||
| Female | 110 (72.4) | 152 (53.7) | 262 (60.2) | |
| Male | 42 (27.6) | 131 (46.3) | 173 (39.8) | |
| Were you hospitalized for your illness? | 0.08 | |||
| No. missing | 1 | 0 | 1 | |
| No | 128 (84.8) | 256 (90.5) | 384 (88.5) | |
| Yes | 23 (15.2) | 27 (9.5) | 50 (11.5) | |
| Did you experience any negative psychological impact, such as anxiety or depression, from COVID-19? | <0.001 | |||
| No. missing | 1 | 1 | 2 | |
| No | 72 (47.7) | 213 (75.5) | 285 (65.8) | |
| Yes | 79 (52.3) | 69 (24.5) | 148 (34.2) | |
| Were you symptom free for any period before new symptoms started? | 0.48 | |||
| No. missing | 20 | 142 | 162 | |
| No | 96 (72.7) | 97 (68.8) | 193 (70.7) | |
| Yes | 36 (27.3) | 44 (31.2) | 80 (29.3) | |
| Was it less or more than 1 month before you felt able to resume normal activities? | <0.001 | |||
| No. missing | 4 | 3 | 7 | |
| Less than 1 month | 73 (49.3) | 231 (82.5) | 304 (71.0) | |
| More than 1 month | 75 (50.7) | 49 (17.5) | 124 (29.0) | |
Data are presented as No. (%) unless otherwise indicated.
Two patients did not record persistent symptoms, leaving 435 patients to be included in this analysis.
Discussion
The results of our study showed a high prevalence of prolonged symptoms (fatigue, dyspnea, anosmia, poor sleep) for women and for patients with self-reported severe illness. Our study also showed that patients with self-reported mild disease at onset may experience prolonged symptoms and later develop new symptoms. Female sex, psychological symptoms (depression and/or anxiety), and inability to resume normal activities at 1 month were predictive factors for PASC. There was a significant association between patient-reported severe illness and hospitalization.
Although most COVID-19 cases are mild, many patients are ill much longer than expected. At the time of this writing, 239 million such cases have been reported.14 The Centers for Disease Control and Prevention has estimated that 10% to 30% of persons who have survived COVID-19 have long-term issues, including psychological,9 with various consequences such as lost workdays, reduced productivity, and increased use of health care resources. However, not much has been published that describes the occurrence and characteristics of prolonged symptoms in patients who do not require hospitalization;15 hence, there is a critical need for further research about this population. For example, in our study, about 35% of patients reported prolonged symptoms, which is comparable to percentages in other US studies16 , 17 and less than that from a study from Wuhan, China, which reported that up to 76% of patients had prolonged symptoms.12 That study and others13 , 18 , 19 included only patients who had been discharged from the hospital. Sudre et al.3 used a mobile phone application in a study that included nonhospitalized patients and showed only 2.3% of patients reported symptoms lasting over 3 months. A recent report by Havervall et al.15 evaluated outcomes of mild cases of COVID-19, but the study was limited to health care professionals only. They showed that 26% of persons had at least 1 moderate to severe symptom that lasted for 2 months, and 15% of patients had a symptom that lasted for 8 months. The most common symptoms lasting for 2 months were anosmia, fatigue, ageusia, and dyspnea.
The more cogent hypotheses on causes of prolonged symptoms from COVID-19 suggest they are due to 1 or more mechanisms, such as a single but severe immunologic response, reactivation of the virus, or persistent immunologic response to the initial viral exposure.11 , 20 It is also possible that prolonged symptoms result from recirculation of viral particles with a subsequent immunologic response that leads to recurrent pathologic effects. Evidence shows that persistent viral shedding can be present for over 3 weeks,21 so if there is smoldering immunologic activity, symptoms could last for at least this amount of time. Prolonged symptoms could also result from any combination of the above hypotheses. Immunity from COVID-19 reinfection has been determined to be present for at least 3 months22 and possibly for up to 5 to 8 months, with 95% of patients showing immune memory in that time frame.23 One study showed protection against reinfection to be approximately 80% after 6 months.24 For some patients with PASC, this immunity could come with a cost of persistent viral shedding and complications of prolonged symptoms. A subset of our patients reported new complications after recovery from the acute infection, suggesting much about the pathophysiology of this virus that remains to be learned.
Little is also known regarding the reasons why some people have a prolonged recovery after COVID-19. Prolonged viremia, absence of antibody response, relapse, reinfection, immune reactions, deconditioning, and mental health factors, such as posttraumatic stress, have all been cited as possible reasons.11 , 25 , 26 Asthma and early signs of ageusia or myalgia have been associated with a longer duration of viral shedding.27 Most of our respondents reported symptoms for 3 to 6 months after infection. Longer term sequelae for this study were limited by the newness of this pandemic; this time frame is also congruent with case surges in our area at the time of study. If data from our study are combined with data from other studies, the results may lead to a clearer prediction for which patients can be expected to have prolonged symptoms. This information would be important for patients, employers, policy makers, and educators. Ordinal scales for COVID-19 functional outcomes have also been proposed to aid in predicting functional status.28 Combined with other studies, our data can also help guide the standardization of such scales to improve their accuracy.
Post-acute COVID-19 symptoms may vary. Symptoms may include cough, low-grade fever, fatigue that varies in intensity, skin rashes, thromboembolic conditions, shortness of breath, headaches, muscle pains and weakness, gastrointestinal symptoms, chest pain, neurocognitive disorders, and mental health disorders such as depression.29 For these reasons, evaluation for PASC should include laboratory work, imaging studies, and consultations for interdisciplinary care.30 , 31 Of the symptoms reported in this and other studies, fatigue was the most common symptom after severe COVID-19 infection,13 , 16 , 17 , 32 with dyspnea second and poor sleep quality third. Most patients hospitalized for COVID-19 (i.e., in the severe disease category) had respiratory complications, which can explain the high response of dyspnea in this group. Fatigue is a nonspecific symptom with multifactorial causes, and further research may help differentiate which aspects of COVID-19 lead to this adverse outcome. It would not be far-fetched to think that other secondary effects of the illness could be causative. The reported psychological impact of COVID-19 was high in our cohort and was as high as 33% in other study cohorts.33 , 34 Could prolonged fatigue be caused by secondary effects such as increased stress, job loss, economic downturn, and isolation? In most cases, it would be reasonable to think so. However, other secondary physical conditions also need to be considered, such as myocarditis, pulmonary complications, venous thromboembolism, renal injuries, and neurologic insults. Central sensitization syndrome has a well-known connection to fatigue and chronic disease. It also likely has a strong role in PASC. Postinfectious inflammation (e.g., prolonged symptoms) has been reported for other diseases such as mononucleosis, herpes zoster, and Borrelia infections. In some ways COVID-19–related fatigue is similar to chronic fatigue syndrome, which has been described after SARS-CoV-2.6
Our study has limitations. Sex of patients and age range were well represented; however, risk factors for mental illness were not included. We studied patient self-reports of illness severity rather than clinical markers, which risks recall bias. However, we believe patient perspectives were important to analyze because most PASC symptoms are self-reported and affect whether persons can function at their normal level of activity after COVID-19. In addition, we had a modest sample size, although it was substantially larger than sample sizes for most studies published about PASC to date. Because the sample was small, we were unable to analyze certain demographic characteristics, including race and socioeconomic status. In addition, our nonresponse rate was 74%, which may have been related to the difficulties patients were having recovering from their illness.
Although the study has limitations, it also has strengths. To our knowledge, this is the first outpatient study in the US with a prospective follow-up design for studying PASC. In addition, we report the largest number of PASC symptoms assessed in outpatients (i.e., 19 distinct symptoms). We believe the study is also the first to report perceptions of illness severity by hospitalized and nonhospitalized patients with PASC, which may facilitate additional research that would identify prognostic factors and strategies useful for monitoring long-term consequences of disease.
Conclusions
We have described the prevalence of patient-reported COVID-19 severity and PASC and associated long-term symptoms and quality-of-life–related outcomes for hospitalized and nonhospitalized patients. Our data showed that age and hospitalization were significant determinants of COVID-19 severity, whereas female sex, psychological symptoms (depression and/or anxiety), and inability to resume normal activities 1 month after illness onset were predictive of PASC. Our results provide a framework for early recognition of patients at increased risk for PASC, highlight certain demographic risk factors for developing PASC, and provide an initial understanding of the natural history of PASC. Further research will lead to improved screening tools and guide the necessary steps in evaluation and treatment of PASC. Further global and collaborative research is needed to address the spectrum of recovery from acute COVID-19 infection, predictors of severity and persistence of symptoms, pathophysiology, and consequences of living with PASC.
Clinical significance
To date, 180 million cases of COVID-19 have been reported worldwide. Over one-third of patients have persistent symptoms.
Age is associated with severity of illness and long-term symptoms.
Women are more likely to have persistent symptoms, fatigue being most common.
Identifying at-risk patients is important because intervention can reduce severity of persistent symptoms.
Authors contributions
D.K., B.M., S.H., and A.D. conceived and designed the project; I.L. collected the data; A.D. contributed to data collection tools; I.L. performed the analysis; D.K., B.M., M.H., and G.M. wrote the manuscript with input from all authors. S.H. supervised the project.
Role of the funding source
None.
Financial support and disclosure
None.
Acknowledgment
The authors would like to thank Yaohua Ma and her colleagues for statistical analysis support. Editing, proofreading, and reference verification were provided by Scientific Publications, Mayo Clinic.
References
- 1.SeyedAlinaghi S., Afsahi A.M., MohsseniPour M., et al. Late complications of COVID-19; a systematic review of current evidence. Arch Acad Emerg Med. 2021;9(1):e14. doi: 10.22037/aaem.v9i1.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhalgh T., Knight M., A’Court C., et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 3.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenforde M.W., Kim S.S., Lindsell C.J., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 6.Lam M.H., Wing Y.K., Yu M.W., et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 7.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins F.S. NIH launches new initiative to study “Long COVID”. Natl Inst Health. 2021 https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid Available at: [Google Scholar]
- 9.Centers for Disease Control and Prevention. Post-COVID conditions. Updated 2021 Apr 8. Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html. Accessed April 22, 2021.
- 10.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed October 21, 2021.
- 11.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carfi A., Bernabei R., Landi F., et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havervall S., Rosell A., Phillipson M., et al. Symptoms and functional impairment assessed 8 months after Mild COVID-19 among health care workers. JAMA. 2021;325:2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenforde M.W., Billig Rose E., Lindsell C.J., et al. Characteristics of adult outpatients and inpatients with COVID-19 - 11 academic medical centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(26):841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logue J.K., Franko N.M., McCulloch D.J., et al. Sequelae in adults at 6 months after COVID-19 Infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold D.T., Hamilton F.W., Milne A., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra V., Flanders S.A., O’Malley M., et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altmann DM, Boyton RJ. Confronting the pathophysiology of long COVID updated 2020, Dec 9. Available at: https://blogs.bmj.com/bmj/2020/12/09/confronting-the-pathophysiology-of-long-covid/. Accessed May 3, 2021.
- 21.Woodruff A., Walsh K.L., Knight D., et al. COVID-19 infection: strategies on when to discontinue isolation, a retrospective study. Am J Infect Control. 2020;48(9):1032–1036. doi: 10.1016/j.ajic.2020.06.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodda L.B., Netland J., Shehata L., et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184(1):169–183. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen C.H., Michlmayr D., Gubbels S.M., et al. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID. Symptom Study. How long does COVID last? Updated 2020 Jun 6. Available at: https://covid.joinzoe.com/post/covid-long-term. Accessed April 22, 2021.
- 26.Dasgupta A., Kalhan A., Kalra S. Long term complications and rehabilitation of COVID-19 patients. J Pak Med Assoc. 2020;70(5):S131–S135. doi: 10.5455/JPMA.32. [DOI] [PubMed] [Google Scholar]
- 27.Knight D., Downes K., Munipalli B., et al. Symptoms and clinical outcomes of coronavirus disease 2019 in the outpatient setting. SN Compr Clin Med. 2021:1–8. doi: 10.1007/s42399-021-00746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klok F.A., Boon G., Barco S., et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assaf G, Davis H, McCorkell L, et al. An analysis of the prolonged COVID-19 symptoms survey by patient-led research team. Patient-led research collaborative. Updated 2020 May 11. Available at: https://patientresearchcovid19.com/research/report-1/. Accessed April 22, 2021.
- 30.National Institute for Health and Care Excellence; 2021. COVID-19 Rapid Guideline: Managing The Long-Term Effects of COVID-19 (NICE Guideline, No. 188)https://www.ncbi.nlm.nih.gov/books/NBK567261/ Updated 2020 Dec 18. Available at: [PubMed] [Google Scholar]
- 31.Gemelli Against C-P-ACSG Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613–1620. doi: 10.1007/s40520-020-01616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang H.J., Nan J., Lv Z.Y., et al. Psychological impacts of the COVID-19 epidemic on Chinese people: exposure, post-traumatic stress symptom, and emotion regulation. Asian Pac J Trop Med. 2020;13(6):252–259. doi: 10.4103/1995-7645.281614. Original Article. [DOI] [Google Scholar]
- 34.Taquet M., Geddes J.R., Husain M., et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]