Abstract
An association of enterovirus infection with endemic cardiomyopathy (Keshan disease [KD]) and outbreaks of myocarditis in selenium-deficient rural areas of southwestern China has been established. Enteroviruses have been isolated from patients with KD or during outbreaks of myocarditis in last two decades. Six of these isolates grew readily in cell lines (Vero or HEp-2) and were investigated by a novel molecular typing method apart from serotyping and pathogenicity. A neutralization assay identified two isolates from KD as coxsackievirus serotype B2 (CVB2) and two isolates from myocarditis as coxsackievirus serotype B6 (CVB6) but failed to type the remaining two isolates, also from myocarditis. Direct nucleotide sequencing of reverse transcription-PCR products amplified from the 5′ nontranslated region (5′NTR) of these viruses confirmed that they belong to a phylogenetic cluster consisting of coxsackie B-like viruses, including some echovirus serotypes. Sequence analysis of the coding region for viral capsid protein VP1 showed that two isolates serotyped as CVB2 have the highest amino acid sequence homology with CVB2 and that the remaining four isolates, two CVB6 and the two unknown serotypes, are most closely related to the sequence of CVB6. Sequences among these isolates varied from 82.3 to 99% in the 5′NTR and from 69 to 99% in VP1, indicating no cross contamination. The pathogenicity of these viruses in adult and suckling mice was assessed. None caused pathologic changes in the hearts of adult MF-1 or SWR mice, although pancreatitis was evident. However, the four CVB6-like viruses caused death in suckling mice, similar to a virulent coxsackievirus group B3 laboratory strain. In conclusion, the sequence data confirm that coxsackievirus group B serotypes are predominant in the region in which KD is endemic and may be the etiological agents in outbreaks of myocarditis. VP1 genotyping of enteroviruses is accurate and reliable. Animal experiments indicate that isolates may differ in pathogenicity.
Enteroviruses are the most common etiological agents of human viral myocarditis and are associated with some cases of dilated cardiomyopathy (DCM) (5, 26, 38). DCM alone afflicts approximately 5 to 8 persons per 100,000 per year worldwide (25, 36). Results from recent studies have also demonstrated a possible etiological role of enterovirus infection in a particular form of heart muscle disease, selenium deficiency-related endemic cardiomyopathy (Keshan disease [KD]), seen in China (23, 24, 45). Enteroviruses also cause outbreaks in some subpopulations, such as young children within maternity units or nurseries (7). Such outbreaks, occurring in selenium-deficient areas, may affect the general population and are more severe, with high mortality. The incidence of outbreaks of enteroviral myocarditis in selenium-deficient regions of southwestern China has increased in the last decade (15 and our unpublished data). During an outbreak in 1991, 67 cases in patients 2 to 75 years old were reported, with 16 fatalities within 24 h of onset (15).
KD is endemic exclusively in selenium-deficient rural areas of China, from the northeast to the southwest, including 14 provinces and autonomous regions (13). Its clinical features are low body selenium content and acute or chronic episodes of heart disorder characterized by cardiogenic shock, arrythemia, and/or congestive heart failure, with an enlarged heart. Four types of the disease are seen: acute, subacute, chronic, and latent or compensated. Pathologically, it is characterized by multifocal myocardial necrosis and fibrous replacement throughout the myocardium, with various degrees of cellular infiltration and calcification, depending upon the type of disease. In some cases, the pathologic changes are similar to those of myocarditis or DCM (13, 22). Selenium deficiency has been considered a major cause since KD was first reported in 1935. Selenium supplementation has led to a decrease in the incidence of KD in areas of endemicity (11) but has not eliminated KD. In addition, a seasonal and annual fluctuation of KD incidence is seen. In the Chuxiong region of southwestern China, for instance, the prevalence of endemic KD is higher in summer and higher in some years than in others (13). This fact suggests an infectious etiology of KD in southwestern China.
Various enteroviruses have been isolated from patients with KD or during outbreaks of myocarditis in the selenium-deficient Chuxiong region of southwestern China. Most were coxsackievirus group B (CVB) strains, but some of them could not be identified antigenically using traditional serotyping methods (8, 15, and our unpublished data). Selenium deficiency increases the cardiovirulence of CVB3 in animal models by changing the viral genomic sequence (4). However, little is known about the biological properties of enteroviruses prevalent in selenium-deficient areas or areas in which KD is endemic, partly because of the inability to identify some isolates using conventional serotyping methods.
Sixty-six human enterovirus serotypes have been distinguished on the basis of a neutralization assay (28). Although this assay is generally reliable and is used routinely, it is labor-intensive and time-consuming and may fail to identify a clinical isolate because of antigenic variation, recombination, or the presence of multiple serotypes. Nucleotide sequencing of the 5′ nontranslated region (5′NTR) and the capsid protein VP4-VP2 junction has been used as a diagnostic and epidemiologic tool for some enteroviruses. However, the sequence of these regions does not correspond to the serotype (2, 32), which is determined mainly by sequences encoding epitopes on viral capsid protein VP1. Recent studies have found that sequences coding for VP1, particularly the 3′ half, correlate with results of the neutralization assay. This information has been confirmed for prototype strains and for clinical isolates of various serotypes (30, 31) and is likely to be useful for isolates that are difficult or impossible to type using standard immunological reagents.
In the present study, six enteroviruses isolated from patients with subacute KD or during outbreaks of viral myocarditis in selenium-deficient areas were characterized by nucleotide sequencing of the 5′NTR and the VP1 coding region and determination of their pathogenicity in adult and suckling mice.
MATERIALS AND METHODS
Enteroviruses.
Of six virus isolates in this study, two were isolated from cardiac blood obtained at autopsy from patients with subacute KD in 1985 and 1987 in the Chuxiong region of Yunnan Province, southwestern China. Selenium deficiency is evident there in the environment, food chain, and inhabitants. The remaining four isolates were obtained from blood or stool of four patients during outbreaks of acute myocarditis in the same region in the summers of 1991 and 1992 (Table 1), initially by use of primary cultures of monkey kidney cells. All these patients had a history of upper respiratory tract infection and a clinical diagnosis of myocarditis. Patients 3 and 4 had a fourfold increase in neutralizing antibody titer against CVB6 (from 1:80 to 1:320), while patients 5 and 6 had a twofold increase in neutralizing antibody titer against CVB4 and CVB6. These isolates were serotyped in two separate institutions, a virology laboratory in China and the Public Health Laboratory Service in the United Kingdom, using a standard neutralization assay described by Melnick et al. (28, 29). A cardiovirulent laboratory strain of CVB3 was used as a control (40).
TABLE 1.
Clinical data for virus isolates
| Isolate | Source | Date obtained | Sex of patient | Patient age (yr) | Diagnosisa | Serotypeb |
|---|---|---|---|---|---|---|
| 1 | Cardiac blood | 1985 | Male | 4 | SKD | CVB2 |
| 2 | Cardiac blood | 1987 | Male | 3 | SKD | CVB2 |
| 3 | Blood | 1991 | Female | 43 | VMC | CVB6 |
| 4 | Stool | 1991 | Female | 38 | VMC | CVB6 |
| 5 | Stool | 1992 | Female | 16 | VMC | Nontypeable |
| 6 | Blood | 1992 | Male | 3 | VMC | Nontypeable |
SKD, subacute KD; VMC, viral myocarditis.
Determined by a neutralization assay.
Cell culture, plaque assay, and single-step growth.
To adapt these isolates to established cell lines, confluent Vero cell (European Collection of Cell Cultures [ECACC] no. 88020401) or HEp-2 cell (ECACC no. 86030501) monolayers were inoculated with 0.1 PFU of virus per cell. After absorption at room temperature for 1 h, the cultures were washed twice in phosphate-buffered saline (PBS), maintained in Dulbecco's modified Eagle medium (DMEM) with 2% fetal bovine serum, and incubated until there was greater than a 50% cytopathic effect. After three cycles of freezing-thawing from −70°C to room temperature, cell debris was removed by centrifugation and the supernatants were used as virus stock for the following experiments.
Virus infectivity was titrated by a plaque assay as previously described (40). Briefly, cells were grown to confluence in 35-mm culture dishes. After absorption of virus at various dilutions, cells were covered with 2.5 ml of an overlay prepared by mixing equal volumes of 2× complete DMEM and 1.5% (wt/vol) SeaPlaque agarose (Flowgen). After being incubated at 37°C in an atmosphere containing 5% CO2 for 2 to 3 days, the monolayers were fixed with 10% formalin in PBS and stained with 0.03% crystal violet to visualize plaques.
The extent of virus replication was determined by single-step growth as follows. Confluent cells were infected with 5 PFU of virus per cell. After absorption as described above, the monolayers were washed with three changes of PBS, incubated as described above, and frozen at −20°C at specific times (2, 4, 6, 8, and 10 h postinfection). After three cycles of freezing-thawing followed by centrifugation, supernatants were titrated for virus infectivity by a plaque assay.
RNA extraction, RT, and PCR.
Total RNA was extracted from infected cells (25-cm2 flask) using Tri-reagent (Sigma) and dissolved in 50 μl of diethyl pyrocarbonate-treated H2O. The enterovirus-specific primers used for reverse transcription (RT)-PCR and sequencing of the 5′NTR and the VP1 coding region are shown in Table 2. RT was carried out as recommended by the manufacturer (Superscript II RNase H-free reverse transcriptase; GIBCO-BRL) and as described previously (1, 42). PCR was carried out with a 50-μl mixture containing 1 mM deoxynucleoside triphosphates, 0.5 mM forward primer (various), 0.5 mM reverse primer (various), 10 μl of reaction buffer (Promega), 1.0 to 2.5 mM MgCl2, 2.5 U of Taq (Promega), and 5 μl of the RT reaction product. The PCR program was 1 cycle of 2 min at 95°C, 2 min at N°C, and 3 min at 72°C, followed by 30 cycles of 1 min at 95°C, 1 min at N°C, and 2 min at 72°C, where N is 5°C below the annealing temperature of the primer with the lower melting temperature. A negative control containing 45 μl of the reaction mixture plus 5 μl of UV-treated H2O and a positive control containing 45 μl of the reaction mixture plus 5 μl of the RT reaction product from cells infected with the virulent laboratory strain of CVB3 were included in each set of PCRs.
TABLE 2.
Oligonucleotide primers used for RT, PCR, and sequencing
| Primer | Sequence (5′→3′) | Gene | Nucleotide positionsa |
|---|---|---|---|
| F16 | TTAAAACAGCCTGTGGGTTG | 5′NTR | 1–20 |
| F8 | AAACACGGACACCCAAAGTA | 5′NTR | 548–564 |
| 252 | GGCCCCTAGATGCGGCTAA | 5′NTR | 452–470 |
| 253 | GATACYTGAGCNCCCAT | 5′NTR-VP4 | 742–758 |
| 68 | GGGACCTTCCACCACCANCC | VP2 | 1177–1196 |
| 008 | GCRTGCAATGAYTTCTCWGT | VP3 | 2383–2402 |
| 011 | GCICCIGAYTGITGICCRAA | 2A | 3301–3320 |
| 012 | ATGTAYGTICCICCIGGIGG | VP1 | 2874–2893 |
Based on CVB3 (GenBank accession number M33854).
Cycle sequencing and sequence analysis.
The cycle sequencing reaction was carried out using 4 μl of DNA polymerase FS-terminator mix (PE-ABI), 3.2 mM primer, and 5 ng of PCR product per 100-bp length of template DNA, with the volume made up to 10 μl with H2O. Reactions were run for 25 cycles with the following program: 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min. DNA was precipitated, dissolved in loading buffer (16.7% formamide, 73.3% dextran), and separated in a 4.1% denaturing polyacrylamide gel on an ABI model 377 DNA sequencer (1, 42). Sequence alignment and analysis were carried out as previously described (24, 42).
Animal experiments.
Five-week-old male MF-1 and SWR mice were obtained from HARLEN OLAC Ltd. or Charles River and housed in negative-pressure isolators. The animals were inoculated intraperitoneally with 106 PFU of virus and sacrificed by cervical dislocation at 7 days postinfection. The heart and pancreas were removed and fixed in 10% formalin in PBS for histologic study. One- to 2-day-old MF-1 suckling mice were inoculated subcutaneously with 103 PFU of virus and inspected daily for 10 days, and survival or death was recorded. Mice were inoculated similarly with a cardiovirulent laboratory strain of CVB3 or DMEM as positive and negative controls, respectively, for both adult and suckling mice.
Histology.
Paraffin-embedded sections were stained with hematoxylin and eosin by standard procedures. Two slides with two or three sections each, cut 40 μm apart, were prepared from each sample, and histopathologic changes were examined under a light microscope by two investigators independently.
Nucleotide sequence accession numbers.
The sequences reported here have been deposited in the GenBank sequence database under accession numbers AF225467 to AF225473.
RESULTS
Serotype and growth properties of enterovirus isolates.
The serotypes of the isolates were determined by the standard neutralization assay as either CVB2 or CVB6 for four of six strains (Table 1). The remaining two isolates were identified in one laboratory as CVB3 but were reported as CVB4 by another laboratory; these two isolates are therefore regarded as nontypeable by the neutralization assay.
Apart from isolate 2, all isolates grew readily in both Vero and HEp-2 cells. Isolate 2 grew in HEp-2 cells but not in Vero cells, indicating different growth properties for isolate 1 and isolate 2 despite the fact that they were the same serotype (CVB2). The replication efficiency of the isolates was assessed under the same condition with HEp-2 cells. Similar patterns of single-step growth were exhibited by the isolates (Fig. 1), and these patterns were similar to that of the cardiovirulent laboratory strain of CVB3 (data not shown). Isolate 1 showed the highest replication in HEp-2 cells, while other isolates exhibited lower infectivity, but within a factor of 10-fold.
FIG. 1.
Single-step growth curves for enterovirus isolates in HEp-2 cells. Single-step growth of all six isolates (isolate 1 [●], isolate 2 [○], isolate 3 [■], isolate 4 [□], isolate 5 [▴], and isolate 6 [▵]) was measured under the infection and culture conditions described in Materials and Methods. The curves were plotted based on duplicate results for each isolate.
Sequence analysis and molecular typing.
Genomic sequences of the 5′NTR (700 bp) and the 3′ half of or the complete VP1 coding region of all six isolates were determined in both orientations without ambiguity. Isolates 1 and 2 had identical sequences in the 5′NTR (700 of 700 bp) and more than 99% sequence identity in VP1 (359 of 360 bp). Isolates 3, 4, 5, and 6 also had more than 99% sequence identity in the VP1 region, but the 5′NTR sequences of isolates 3 and 4 were different from those of isolates 5 and 6 by 17.8%. The different sequences of the 5′NTR or VP1 among the six isolates indicated no cross contamination during virus propagation or RT-PCR and cycle sequencing procedures.
When compared to the sequences in the GenBank DNA database (Table 3), the 5′NTR sequences of the isolates were similar to those of the CVB or echovirus group by up to 93% but differed from the sequences of prototype strains of either CVB2 (CVB2 strain Ohio, accession no. AF081485) or CVB6 (CVB6 strain Schmitt, accession no. AF039205) by 14 to 17.1%, failing to correlate with the serotype determined by the neutralization test.
TABLE 3.
DNA sequence analysis of the 5′NTR and VP1 of the isolates
| Isolate | Sequenced regiona | No. of identical positions/no. sequenced (%) | Virusb | Accession no. |
|---|---|---|---|---|
| 1 | 5′NTR | 447/480 (93) | Echovirus 6 | U16283 |
| 580/700 (82.9)c | CVB2 (Ohio) | AF081485 | ||
| VP1-bp | 308/359 (85) | CVB2 | AF085363 | |
| 240/360 (66.7)d | Echovirus 30 | AF152887 | ||
| VP1-aa | 119/120 (99.2) | CVB2 | AF081621 | |
| 93/120 (77)d | CVB4 | S39291 | ||
| 2 | 5′NTR | 447/480 (93) | Echovirus 6 | U16283 |
| 580/700 (82.9)c | CVB2 (Ohio) | AF081485 | ||
| VP1-bp | 722/845 (85) | CVB2 | AF085363 | |
| 564/846 (66.7)d | Echovirus 30 | AF152887 | ||
| VP1-aa | 276/282 (97) | CVB2 | AF085363 | |
| 211/281 (75)d | CVB4 | S39291 | ||
| 3 | 5′NTR | 555/599 (92) | Echovirus 30 | E30133661 |
| 602/700 (86)c | CVB6 (Schmitt) | AF039205 | ||
| VP1-bp | 583/734 (79) | CVB6 | AF114384 | |
| 250/381 (65.6)d | CVA9 | AF081614 | ||
| VP1-aa | 264/278 (94) | CVB6 | AF114384 | |
| 213/278 (76)d | CVB5 | AF114383 | ||
| 4 | 5′NTR | 555/599 (92) | Echovirus 30 | E30133661 |
| 602/700 (86)c | CVB6 (Schmitt) | AF039205 | ||
| VP1-bp | 660/846 (78) | CVB6 | AF114384 | |
| 250/381 (65.6)d | CVA9 | AF081614 | ||
| VP1-aa | 267/282 (94) | CVB6 | AF114384 | |
| 216/282 (76)d | CVB5 | AF114383 | ||
| 5 | 5′NTR | 549/601 (91) | CVB5 | AF114383 |
| 583/700 (83.3)c | CVB6 (Schmitt) | AF039205 | ||
| VP1-bp | 298/381 (78.2) | CVB6 | AF114384 | |
| 250/381 (65.6)d | CVA9 | AF081614 | ||
| VP1-aa | 119/127 (93) | CVB6 | AF114384 | |
| 99/127 (77)d | CVB3 | Q66282 | ||
| 6 | 5′NTR | 549/601 (91) | CVB5 | AF114383 |
| 583/700 (83.3)c | CVB6 (Schmitt) | AF039205 | ||
| VP1-bp | 295/378 (78) | CVB6 | AF114384 | |
| 250/378 (66.1)d | CVA9 | AF081614 | ||
| VP1-aa | 118/126 (93) | CVB6 | AF114384 | |
| 96/126 (76)d | Echovirus 11 | AF081642 |
VP1-bp, VP1 gene sequence; VP1-aa, predicted amino acid sequence.
The identified type of the enterovirus isolates is shown in boldface.
Identity with the corresponding prototype strain.
Best sequence similarity to heterologous enteroviruses.
The sequences of the 3′ half of VP1 of the isolates were used for sequence comparison and serotype prediction as recommended in a recent publication (31). Isolates 1 and 2 (CVB2) and isolates 3 and 4 (CVB6) match best to their respective prototype human enterovirus strains (Table 3), with nucleotide sequence homology of 78 to 85% and amino acid similarity of 93 to 99.2%, correlating with their respective serotypes. In contrast, the best sequence similarities of these four isolates to heterologous enteroviruses are less than 66.7% for the nucleotide sequence and 75 to 77% for the amino acid sequence (Table 3). The two unidentified isolates, isolates 5 and 6, have the highest homology to the CVB6 prototype strain (accession no. AF114384) at either the nucleotide (78 to 78.2%) or the amino acid (93%) sequence level; therefore, both are considered to belong to genotype CVB6 and are predicted to be serotype CVB6. As the 5′NTR sequences differed from those of isolates 3 and 4 (CVB6 serotype) by 17.7%, it is believed that isolates 5 and 6 are different strains from isolates 3 and 4 but are of the same serotype.
Pathogenicity of isolates in mice.
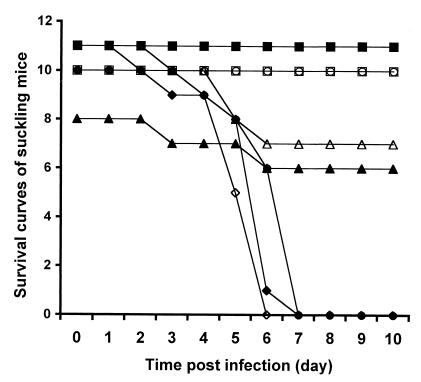
Animal experiments confirmed that the virulent laboratory strain of CVB3 caused typical myocarditis and pancreatitis in adult MF-1 mice, similar to that seen in the characterized SWR mouse model (41). This result validates MF-1 mice as an alternative model for viral myocarditis which could be used to test the pathogenicity of enteroviruses. There were no obvious pathologic changes in the hearts of MF-1 mice 7 days after inoculation with any of the isolates (106 PFU/mouse). Isolate 2 was also inoculated into SWR mice, with similar results. However, pancreatitis was evident in all mice inoculated with isolate 3, 4, 5, or 6, and enterovirus RNA was detected in myocardium by in situ hybridization (data not shown). To further characterize pathogenicity, groups of 10 suckling mice were inoculated with 103 PFU of particular viruses. Isolate 3, 4, 5, or 6 caused death within 6 days after inoculation, comparable to that seen with the virulent laboratory strain of CVB3. No mortality was observed for mice inoculated with isolate 1 or 2 or in negative controls mock infected with culture medium (Fig. 2).
FIG. 2.
Survival curves for suckling mice after infection with enterovirus isolates. Groups of 10 suckling mice were inoculated with isolate 1 (□), 5 (◊), or 6 (⧫) or mock infected with DMEM as negative controls (○); a group of 11 mice was given isolate 2 (■) or 4 (▵) or the cardiovirulent laboratory strain of CVB3 as positive controls (●); and a group of 8 mice was given isolate 3 (▴). For details, see Materials and Methods.
DISCUSSION
KD has been studied for over 60 years within China. One of the key issues has been the complex etiology of this endemic disease, with a focus on environmental-nutritional factors and infectious agents (6, 8, 11, 13). Selenium deficiency in the food chain has been recognized as a major but not exclusive environmental-nutritional factor, and increasing evidence supports an etiological role of enteroviruses in KD (6, 23, 24, 34, 44, 45). In addition, annual summer outbreaks of fulminant enteroviral myocarditis occurring in similar selenium-deficient areas as KD appear to be more severe, with a higher mortality (15 and our unpublished data). It is important to identify the prevalent virus types and strains in these areas and to investigate their biological properties. Rapid and correct serotyping will make it possible to use hyperimmune globulin or type-specific immune globulin for treatment or passive immunization before and during an outbreak (27, 39). Serotyping will also provide information on the spectrum of viruses involved and will be useful in designing specific vaccines (9, 14, 33, 43).
Various enteroviruses have been isolated from patients with KD or during outbreaks of myocarditis in the Chuxiong region. They have been serotyped by a neutralization assay, but some have failed to be typed by this approach. A recent study has shown that certain sequences within the region coding for capsid protein VP1 correlate with serotype. It is known that VP1 contains major neutralizing antigenic sites, the basis of serotyping by the neutralization assay (29), and an enterovirus can be typed genetically by comparison of partial VP1 sequences to a database of sequences of enterovirus prototype strains or other typed strains (31). These sequences are determined by PCR amplification of part of or the entire VP1 gene with generic RT-PCR primers, designed to react with all known human enterovirus serotypes, followed by nucleotide sequencing of the products. This molecular typing approach has been used to discriminate between prototype strains of all human enterovirus serotypes (30, 31), to identify isolates from clinical specimens, or and to identify isolates refractory to antigenic typing and so to identify potential new viruses.
In this study, we determined the sequences of the 5′NTR and the 3′ half of the VP1 coding region of six enterovirus isolates from cases of KD or outbreak myocarditis. Not surprisingly, isolates 1 and 2, from subacute KD cases occurring in the same geographic area, show high nucleotide sequence similarity in both the 5′NTR and the VP1 region (>99%). Similarly, isolates 3 and 4 or isolates 5 and 6, from myocarditis cases occurring in the same geographic area in 1991 or 1992, respectively, have more than 99% nucleotide sequence identity. A partial VP1 sequence alignment correlates with serotype for isolates 1, 2, 3, and 4 and identifies isolates 5 and 6, nontypeable by a conventional neutralization test, as CVB6. However, the 5′NTR sequences of isolates 3 and 4 are dissimilar from those of isolates 5 and 6, indicating that CVB6 strains recovered during outbreaks in 1991 and 1992 are not the same.
CVB6 infection is uncommon in humans. Lau (21) analyzed the level of CVB-specific antibodies in 1,020 normal subjects 1 to 60 years old and from all 18 health districts of New Zealand. CVB6 was the least prevalent, and 970 (95.1%) of the subjects had no antibody to this serotype, compared with 30.4 to 71.3% with antibodies against CVB1 to CVB5. Similar results were obtained from a 5-year clinical and epidemiologic study in France (35), but some studies have suggested that CVB6 is associated with human disease, such as pancreatitis (19) and respiratory tract infection (12), and CVB6 has been isolated from aborted fetal heart, brain, liver, kidney, and spleen tissue (3). Few studies have reported CVB6-induced heart muscle disease, and so it is intriguing to find CVB6 in outbreaks of myocarditis in selenium-deficient areas.
Infection with the same CVB3 strain can cause severe myocarditis and pancreatitis in some strains of mice but can cause only pancreatitis or is avirulent in others because of their different genetic backgrounds (16). SWR mice have been successfully used as a model of CVB3 myocarditis (41, 43), but little is known of their susceptibility to other CVB strains. MF-1 mice have not been used previously as a model for CVB infection. Our study shows the typical pathologic changes of myocarditis and pancreatitis in MF-1 mice after infection with the laboratory strain of CVB3, similar to those seen in SWR mice, suggesting that MF-1 mice could be an alternative model for CVB3-induced myocarditis.
We assessed the pathogenicity of the six isolates in MF-1 mice. Isolate 1 or 2 (CVB2) did not cause pathologic changes in the heart or pancreatitis in adult MF-1 or SWR mice (isolate 2 only) or death in suckling mice (MF-1). A similar result was obtained with a mouse model and a prototype CVB2 strain from the Virus Reference Laboratory, Public Health Laboratory Service, Colindale, London, England, by other authors (20). It is possible that our isolates are not virulent in these mouse strains, although the presence of enterovirus RNA in mouse myocardial tissue suggests virus replication in the heart (17, 37). The remaining four isolates (serotype CVB6) caused pancreatitis in adult MF-1 mice, despite no apparent myocarditis, and caused death in suckling mice, similar to the laboratory strain of CVB3. These results indicate differences in pathogenicity among these isolates and imply that virulence in an experimental mouse model may not reflect pathogenicity in humans (10, 18). Investigation of more isolates from selenium-deficient areas and a search for virulence determinants in viral genomic RNA sequences (4) will provide further information on pathogenicity.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Public Health of the People's Republic of China, (grant 95-397), the Yunnan Scientific Commission, the Wellcome Trust, (grant 052954Z97), and University of London Central Funds and partially by the British Heart Foundation.
REFERENCES
- 1.Archard L C, Khan M A, Soteriou B A, Zhang H, Why H J, Robinson N M, Richardson P J. Characterization of coxsackie B virus RNA in myocardium from patients with dilated cardiomyopathy by nucleotide sequencing of reverse transcription-nested polymerase chain reaction products. Hum Pathol. 1998;29:578–584. doi: 10.1016/s0046-8177(98)80006-3. [DOI] [PubMed] [Google Scholar]
- 2.Arola A, Santti J, Ruuskanen O, Halonen P, Hyypia T. Identification of enteroviruses in clinical specimens by competitive PCR followed by genetic typing using sequence analysis. J Clin Microbiol. 1996;34:313–318. doi: 10.1128/jcm.34.2.313-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso N G, Fonseca M E, Garcia A G, Zuardi J A, Silva M R, Outani H. Enterovirus isolation from foetal and placental tissues. Acta Virol. 1990;34:49–57. [PubMed] [Google Scholar]
- 4.Beck M A, Shi Q, Morris V C. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 5.Bowles N E, Richardson P J, Olsen E J. Detection of coxsackie B virus specific sequences in myocardial biopsies from cases of myocarditis and dilated cardiomyopathy. Lancet. 1986;1:1120–1122. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 6.Department of Microbiology. Investigation of biological aetiology of Keshan disease. J Chongqing Med Coll. 1978;2:1–5. [Google Scholar]
- 7.Druyts-Voets E, Renterghem L V, Gerniers S. Coxsackie B virus epidemiology and neonatal infection in Belgium. J Infect. 1993;27:311–316. doi: 10.1016/0163-4453(93)92329-u. [DOI] [PubMed] [Google Scholar]
- 8.Ecology Study Group. Ecological environment of Keshan disease in Chuxiong region. In: Yu W H, Wang F, editors. Proceedings of comprehensive scientific investigation of KD in Chuxiong region. Beijing, China: People's Health Press; 1988. pp. 48–83. [Google Scholar]
- 9.Fohlman J, Pauksen K, Morein B, Bjare U, Ilback N G, Friman G. High yield production of an inactivated coxsackie B3 adjuvant vaccine with protective effect against experimental myocarditis. Scand J Infect Dis Suppl. 1993;88:103–108. [PubMed] [Google Scholar]
- 10.Gauntt C J, Pallansch M A. Coxsackievirus B3 clinical isolates and murine myocarditis. Virus Res. 1996;41:89–99. doi: 10.1016/0168-1702(95)01250-8. [DOI] [PubMed] [Google Scholar]
- 11.Ge K Y, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr. 1993;57(Suppl. 2):259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- 12.Goldwater P N. Immunoglobulin M capture immunoassay in investigation of coxsackievirus B5 and B6 outbreaks in South Australia. J Clin Microbiol. 1995;33:1628–1631. doi: 10.1128/jcm.33.6.1628-1631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu B Q, Cheng T O. Keshan disease. In: Cheng T O, editor. The international textbook of cardiology. New York, N.Y: Pergamon Press; 1986. pp. 752–765. [Google Scholar]
- 14.Henke A, Wagner E, Whitton J L, Zell R, Stelzner A. Protection of mice against lethal coxsackievirus B3 infection by using DNA immunization. J Virol. 1998;72:8327–8331. doi: 10.1128/jvi.72.10.8327-8331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou L C, Deng X Q, Liang X, Niu C L. An outbreak of acute viral myocarditis in Chuxiong. Chin J Endemiol. 1992;11:373–375. [Google Scholar]
- 16.Huber S A, Stone J E, Wagner D H, Kupperman J, Pfeiffer L, David C, O'Brien R L, Davis G S, Newell M K. γδ+ T cells regulate major histocompatibility complex class II (IA and IE)-dependent susceptibility to coxsackievirus B3-induced autoimmune myocarditis. J Virol. 1999;73:5630–5636. doi: 10.1128/jvi.73.7.5630-5636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber S A. Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the γδ+ T-cell receptor. J Virol. 1996;70:3039–3044. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuno S, Itagaki A, Yamazaki I, Katsumoto T, Kurimura T. Pathogenicity of newly isolated coxsackievirus B4 for mouse pancreas. Acta Virol. 1984;28:433–436. [PubMed] [Google Scholar]
- 19.Lal S M, Fowler D, Losasso C J, Berg G G. Coxsackie virus-induced acute pancreatitis in a long-term dialysis patient. Am J Kidney Dis. 1988;11:434–436. doi: 10.1016/s0272-6386(88)80058-1. [DOI] [PubMed] [Google Scholar]
- 20.Lansdown A B. Pathological changes in the pancreas of mice following infection with coxsackie B viruses. Br J Exp Pathol. 1976;57:331–338. [PMC free article] [PubMed] [Google Scholar]
- 21.Lau R C. Coxsackie B virus infections in New Zealand patients with cardiac and non-cardiac diseases. J Med Virol. 1983;11:131–137. doi: 10.1002/jmv.1890110207. [DOI] [PubMed] [Google Scholar]
- 22.Li G S, Wang F, Kang D R. Keshan disease: an endemic cardiomyopathy in China. Hum Pathol. 1985;16:602–609. doi: 10.1016/s0046-8177(85)80110-6. [DOI] [PubMed] [Google Scholar]
- 23.Li Y W, Yang Y Z, Chen H Z. Detection of enteroviral RNA in paraffin-embedded myocardial tissue from patients with Keshan disease by nested PCR. Natl Med J China. 1995;75:344–345. [PubMed] [Google Scholar]
- 24.Li Y W, Peng T Q, Yang Y Z, Niu C L, Archard L C, Zhang H Y. High prevalence of enteroviral genomic sequences in myocardium from cases of endemic cardiomyopathy (Keshan disease) in China. Heart. 2000;83:696–701. doi: 10.1136/heart.83.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manolio T A, Baughman K L, Rodeheffer R, Pearson T A, Bristow J D, Michels V V, Abelmann W H, Harlan W R. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute workshop) Am J Cardiol. 1992;69:1458–1466. doi: 10.1016/0002-9149(92)90901-a. [DOI] [PubMed] [Google Scholar]
- 26.Martino T P, Liu P, Sole M J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ Res. 1994;74:182–188. doi: 10.1161/01.res.74.2.182. [DOI] [PubMed] [Google Scholar]
- 27.McNamara D M, Rosenblum W D, Janosko K M, Trost M K, Villaneuva F S, Demetris A J, Murali S, Feldman A M. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–2478. doi: 10.1161/01.cir.95.11.2476. [DOI] [PubMed] [Google Scholar]
- 28.Melnick J L, Rennick V, Hampil B, Schmidt N J, Ho H H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull W H O. 1973;48:263–268. [PMC free article] [PubMed] [Google Scholar]
- 29.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, Channock R M, Melnick J L, Monath T P, Roizman B, Stranus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 655–712. [Google Scholar]
- 30.Oberste M S, Maher K, Kilpatrick D R, Pallansch M A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberste M S, Maher K, Kilpatrick D R, Flemister M R, Brown B A, Pallansch M A. Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol. 1999;37:1288–1293. doi: 10.1128/jcm.37.5.1288-1293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberste M S, Maher K, Pallansch M A. Molecular phylogeny of all human enterovirus serotypes based on comparison of sequences at the 5′ end of the region encoding VP2. Virus Res. 1998;58:35–43. doi: 10.1016/s0168-1702(98)00101-4. [DOI] [PubMed] [Google Scholar]
- 33.See D M, Tilles J G. Efficacy of a polyvalent inactivated-virus vaccine in protecting mice from infection with clinical strains of group B coxsackieviruses. Scand J Infect Dis. 1994;26:739–747. doi: 10.3109/00365549409008644. [DOI] [PubMed] [Google Scholar]
- 34.Su C Q, Gong G M, Li Q. Preliminary summary of viral aetiology of Keshan disease. Chin Med J. 1979;59:466–470. [Google Scholar]
- 35.Wattre P, Leroy O, Dewilde A, Thery C. Coxsackie B virus infections in cardiology. Apropos of 66 cases. Pathol Biol (Paris) 1987;35:347–352. [PubMed] [Google Scholar]
- 36.Williams D G, Olsen E G. Prevalence of overt dilated cardiomyopathy in two regions of England. Br Heart J. 1985;54:153–155. doi: 10.1136/hrt.54.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfgram L J, Beisel K W, Herskowitz A, Rose N R. Variations in the susceptibility to coxsackie B3 virus induced myocarditis among different strains of mice. J Immunol. 1986;136:1846–1852. [PubMed] [Google Scholar]
- 38.Woodruff J F. Viral myocarditis: a review. Am J Pathol. 1980;101:425–483. [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida H, Morita M, Kobayashi N, Takeuchi O, Wakita S, Hachisu T, Hara M, Suzuki T. Efficacy of immunized milk for preventing viral infection. Kansenshogaku Zasshi. 1999;73:122–129. doi: 10.11150/kansenshogakuzasshi1970.73.122. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H Y, Yousef G E, Cunningham L, Blake N W, Ouyang X, Bayston T A, Kandolf R, Archard L C. Attenuation of a reactivated cardiovirulent coxsackievirus B3: the 5′-nontranslated region does not contain major attenuation determinants. J Med Virol. 1993;41:129–137. doi: 10.1002/jmv.1890410208. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H Y, Yousef G E, Ouyang X M, Archard L C. Characterization of a murine model of myocarditis induced by a reactivated coxsackievirus B3. Int J Exp Pathol. 1994;75:99–110. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H Y, Soteriou B, Knowlson S, Theodoridou A, Archard L C. Characterisation of genomic RNA of coxsackievirus B3 in murine myocarditis: reliability of direct sequencing of reverse transcription-nested polymerase chain reaction products. J Virol Methods. 1997;69:7–17. doi: 10.1016/s0166-0934(97)00122-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H Y, Morgan-Capner P, Latif N, Pandolfino Y A, Fan W, Dunn M J, Archard L C. Coxsackievirus B3-induced myocarditis: characterization of stable attenuated variants that protect against infection with the cardiovirulent wild-type strain. Am J Pathol. 1997;150:2197–2207. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong X K, Zhou Y C, Yu W H. Detection of coxsackievirus B neutralization antibodies in sera from children with Keshan disease. Chin J Endemiol. 1987;6:356–358. [Google Scholar]
- 45.Zhong X K, Zhang F M, Jiang Z R. Relationship between coxsackie B viruses and subacute Keshan disease in Yunnan Province using in situ hybridization. Chin J Endemiol. 1993;12:193–195. [Google Scholar]