Abstract
Background & Aims:
To compare the effectiveness of glucose-like peptide-1 receptor agonists (GLP-1RA) with dipeptidyl peptidase-4 inhibitors (DPP-4i), sulfonylureas or sodium-glucose cotransporter 2 inhibitors (SGLT-2i) in reducing decompensation events, among patients with cirrhosis and type 2 diabetes.
Methods:
This population-based, retrospective cohort study included patients with type 2 diabetes and cirrhosis, in a commercial healthcare database (IBM Marketscan). We constructed three pairwise, 1:1 propensity score (PS)-matched cohorts of adults initiating GLP-1RA or a comparator medication (i.e. DPP-4i [2006-2020], sulfonylurea [2005-2020] or SGLT-2i [2013-2020]). Patients were followed in an astreated approach for decompensation events (i.e. ascites, spontaneous bacterial peritonitis [SBP], hepatorenal syndrome [HRS], hepatic encephalopathy [HE] or esophageal variceal hemorrhage [EVH]). Within each PS-matched cohort, we estimated HRs and 95%CIs, controlling for >90 baseline characteristics.
Results:
Over 132 days of median follow-up (interquartile range=73, 290 days), PS-matched rates of any decompensation were significantly lower among GLP-1RA initiators, versus DPP-4i initiators (105.2 vs. 144.0/1000 person-years[PY]; HR=0.68 [95%CI=0.53-0.88]; n=1,431 pairs), and versus sulfonylureas (97.3 vs 144.0/1000PY; HR=0.64 [0.48-0.84]; n=1,246 pairs). Similar, inverse associations were found for individual decompensation events, including ascites/SBP/HRS (HRs=0.66 [0.45-0.97] and 0.66 [0.46-0.94], respectively), EVH (HRs=0.62 [0.41-0.92] and 0.59 [0.37-0.92], respectively), and HE (HRs=0.76 [0.55-1.06] and 0.60 [0.39-0.92], respectively). Results persisted in subgroups of patients with and without previously decompensated cirrhosis. In contrast, decompensation rates were similar, when GLP-1RA and SGLT-2i were directly compared (103.5 vs. 112.8/1000PY; HR=0.89 [0.62-1.28]).
Conclusion:
Among cirrhotic patients with type 2 diabetes, we find high rates of decompensation, consistent with previous reports; these rates were substantially lower among GLP-1RA initiators, compared to DPP-4i or sulfonylureas.
Keywords: decompensated cirrhosis, antidiabetic therapy, comparative effectiveness, pharmacoepidemiology
Introduction
Cirrhosis is responsible for over 40,000 deaths each year in the U.S., and nearly 1.3 million deaths, worldwide1 2, and cirrhosis-related mortality is increasing at an alarming pace3. Diabetes disproportionately affects over one-third of patients with cirrhosis, and contributes to substantial morbidity and mortality4–6. Specifically, hyperglycemia promotes the development of hepatocellular carcinoma (HCC)5, and contributes to both hospitalizations and death from decompensation events, including ascites and spontaneous bacterial peritonitis (SBP), bleeding esophageal varices, hepatic encephalopathy (HE) and hepatorenal syndrome (HRS)6–8. Yet, for patients with cirrhosis, the benefit of intensive glucose control in patients remains controversial, and evidence regarding the optimal antidiabetic strategy is scarce, particularly for patients who do not tolerate metformin or require intensification of therapy. Thus, defining the safety and effectiveness of second-line antidiabetic strategies in this high-risk population remains an important unmet need.
Glucagon-like peptide-1 receptor agonists (GLP-1RA) exert numerous beneficial effects beyond reducing blood glucose and improving insulin sensitivity9, including weight loss, improved blood pressure and lipid profiles, and reduced circulating inflammatory markers and adipokines10. In preclinical models, GLP-1RA therapy improves hepatic lipid oxidation, and in humans with non-cirrhotic nonalcoholic steatohepatitis (NASH), GLP-1RAs have demonstrated efficacy for reducing liver fat and lipotoxicity11 12, and reversing NASH13. Despite this, clinical evidence regarding the safety and effectiveness of these medications in cirrhosis is scarce. Moreover, GLP-1RAs are associated with modest but significant increases in heart rate, which could increase the risk of variceal bleeding14.
To date, randomized controlled trials (RCTs) of GLP-1RAs have excluded cirrhotic populations. Furthermore, as these trials did not perform head-to-head comparisons across antidiabetic drug classes, they cannot provide information regarding the comparative effectiveness of these medications. Thus, we directly compared GLP-1RAs to three comparable antidiabetic drug classes, with regard to risk of hepatic decompensation events, in a population-based cohort with established cirrhosis and type 2 diabetes.
Methods
Data Source
Data were collected from a large, nationwide U.S. commercial claims dataset (IBM MarketScan). MarketScan includes data for individuals who are commercially insured or who have primary traditional (part A & B but not D) Medicare insurance plus a supplemental health plan with a pharmacy benefit. For each insured individual, MarketScan includes demographic information, enrollment status and longitudinal patient-level information on all reimbursed medical services, including inpatient and outpatient diagnoses and procedures, and records of all dispensed prescription medications, including medication start date, number of refills, strength, quantity, and days’ supply. This study was approved by the Brigham and Women’s Hospital Institutional Review Board (IRB#2011P002580).
Study Population
Figure 1 outlines the study schema. We identified patients aged 18 or older with established cirrhosis and type 2 diabetes, who initiated GLP-1RA therapy (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide or semaglutide), or one of 3 comparator classes: (1) dipeptidyl peptidase-4 inhibitors (DPP-4i: alogliptin, linagliptin, saxagliptin, or sitagliptin), between 19 October, 2006 (consistent with the marketing of sitagliptin, the first approved DPP-4i) and 17 June, 2020; or (2) sodium-glucose cotransporter 2 inhibitor (SGLT2i: canagliflozin, dapagliflozin, empagliflozin or ertugliflozin), between 29 March, 2013 (consistent with the marketing of canagliflozin, the first approved SGLT2i) and 17 June, 2020; or (3) second or third generation sulfonylureas (glimepiride, glipizide or glyburide), between 28 April 2005 (consistent with the marketing of exenatide, the first approved GLP-1RA) and 17 June 2020. The cohort entry date was the first filled prescription for GLP-1RA or comparator, after at least 180 days of continuous enrollment and no recorded use of either GLP-1RA or comparator during that period.
Figure 1.
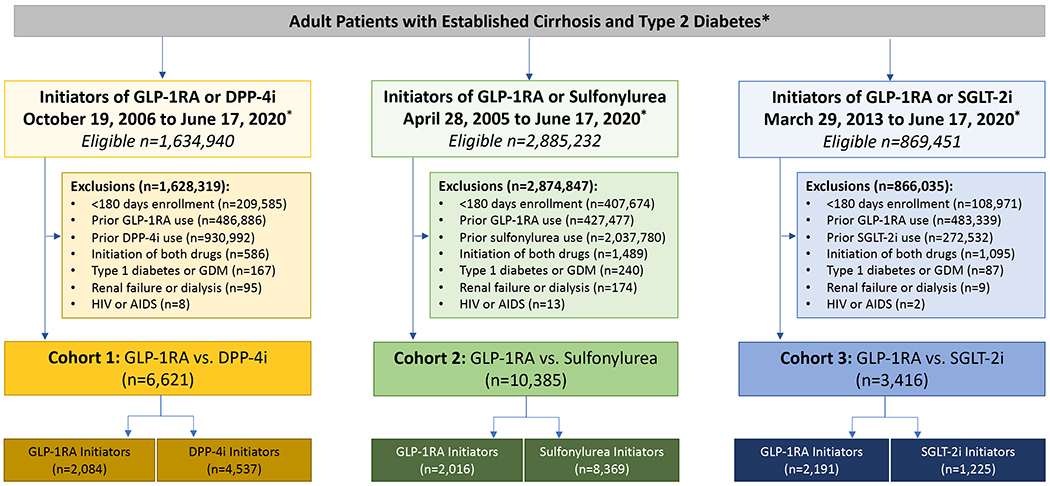
Cohort Construction
Abbreviations: GLP-1 RA, glucagon-like peptide receptor agonist; DPP-4, Dipeptidyl Peptidase-4; SGLT-2, sodium-glucose cotransporter-2; GDM, gestational diabetes
*included patients were adults over age 18 years with diagnoses of both type 2 diabetes and cirrhosis, as outlined in the Methods. Cohort 1 included new initiators of either GLP-1RA or DPP-4i starting on October 19, 2006 (consistent with the marketing of sitagliptin, the first approved DPP-4i); Cohort 2 included new initiators of either GLP-1RA or sulfonylureas, starting on April 28, 2005 (consistent with the marketing of exenatide, the first approved GLP-1RA); Cohort 3 included new initiators of either GLP-1RA or SGLT-2i, starting on March 29, 2013 (consistent with the marketing of canagliflozin, the first approved SGLT-2i). For details, see Methods.
Patients were required to have a diagnosis of both type 2 diabetes (i.e. at least one inpatient or outpatient ICD-9 code of 250.x0 or 250.x2, or ICD-10 code E11.x) and cirrhosis (i.e. at least one inpatient or at least two outpatient ICD-9 codes [571.2, 571.5, 571.6] or ICD-10 code [K70.3, K74, K74.3, K74.4, K74.5, K74.6, K74.60, K74.69]), before the cohort entry date (Table S1). In previous validation studies, analogous ICD-9 and ICD-10 definitions of cirrhosis yielded positive predictive values (PPV) >85-90%15–18. We excluded anyone with diagnoses of type 1 diabetes, gestational diabetes, end stage renal disease or HIV. Patients meeting inclusion criteria contributed only once to each cohort, but they could contribute simultaneously to multiple cohorts. Figure S1 outlines the study schema for the 3 pairwise comparisons.
Outcomes and Patient Characteristics
The primary outcome was a hepatic decompensation event, a composite endpoint defined by hospitalization for ascites, SBP, HRS, bleeding esophageal varices or HE, using claims-based algorithms with demonstrated PPVs >85-95%15–18 (Table S1). Secondary outcomes included individual decompensation events. Because rates of SBP and HRS were relatively low, these outcomes were categorized with ascites.
Follow-up began the day after cohort entry (i.e. drug initiation) and continued in an “as-treated” approach until the first occurrence of: discontinuation or switch to a comparator medication, study outcome, death, end of continuous health plan enrollment, or end of the study period. In cases of treatment interruption or discontinuation, we extended the exposure effect window until 45 days after the end of the last prescription’s supply. Pre-exposure patient characteristics ascertained during the 180-day period before cohort entry are outlined in Tables S2A–B. We defined all comorbidities using ICD-9, ICD-10 and CPT codes.
Statistical analysis
Within the three cohorts, we compared baseline characteristics between initiators of GLP-1RA and each comparator. To address imbalances, we calculated propensity scores (PS) using three multivariable logistic regression models (i.e. one for each pairwise cohort), that predicted the probability of initiating medication (i.e. GLP-1RA or comparator), conditional on >90 baseline characteristics (outlined in Table S2A–B). Within each pairwise cohort, exposure groups were 1:1 PS-matched using the nearest neighbor approach with a maximum caliper width equal to 0.05 of the standard deviation of the logit of the PS19. Covariate balance between groups before and after PS matching was assessed using standardized mean differences, with significant imbalances defined as differences >0.1.20
For each comparison and for all outcomes, we calculated PS-matched incidence rates and corresponding hazard ratios (HRs) with 95% confidence intervals (CIs). Within each PS-matched cohort, we also estimated the cumulative incidence of study outcomes, accounting for the competing risk of all-cause mortality, using the Fine-Gray approach21. To assess the proportional hazards assumption, we tested the significance of the interaction term between exposure and time and confirmed it was not violated. In subgroups, we evaluated patients separately by sex, underlying nonalcoholic fatty liver disease (NAFLD), HBV- or HCV-related cirrhosis, alcohol-related liver disease (ALD), concurrent use of metformin, statins or non-selective beta-blockers (NSBB), insurance status and previous hepatic decompensation events (i.e. prior ascites, SBP, HRS, or bleeding esophageal varices or HE), during the initial 180-day period. For each subgroup, the PS was re-calculated and the PS-matching procedure was repeated22.
We conducted several sensitivity analyses. First, we applied an alternative, strict definition of cirrhosis, that required at least two inpatient or outpatient diagnoses16 17 23–25. Second, to address informative censoring, we carried forward the exposure to an initiated drug for 365 days without censoring for drug discontinuation or switching, to mimic an intention-to-treat approach26. Third, because hepatic decompensation event rates were similar and low in SGLT2i initiators, we directly compared SGLT2i to DPP-4i initiators, after repeating the PS-matching procedure. Fourth, we examined the association between GLP-1RA initiation and a negative control outcome with an expected null finding (i.e. incident fractures). Next, to assess the generalizability of our findings and minimize potential residual confounding due to variable insurance status and missing race/ethnicity data in MarketScan, we repeated our primary analysis in an independent commercial database (Optum Clinformatics Datamart). Optum uses proprietary algorithms to define race, a derived ethnicity constructed from the member’s name and geography. Once ethnicity is determined, the member is mapped to one of four categories (Asian, Black, Hispanic, White). For each of the aforementioned sensitivity analyses, we recalculated the PS and repeated the PS-matching procedure. Finally, we used an established, rule-out approach to test the sensitivity of our results to unmeasured confounding27.
All analyses were performed using Aetion Evidence Platform28, version 4.11, and SAS 9.4 Statistical Software (SAS Institute Inc, Cary, NC).
Results
We identified three unmatched, paired cohorts comprised of new initiators of: (1) either GLP-1RA (n=2,084) or DPP-4i (n=4,537); (2) either GLP-1RA (n=2,016) or a second-generation sulfonylurea (n=8,369); and (3) either GLP-1RA (n=2,191) or SGLT-2i (n=1,225)(Figure 1). From those unmatched cohorts, we then constructed three pairwise, 1:1 PS-matched cohorts, composed of new initiators of either: (1) GLP-1RA or DPP-4i (n=1,431 PS-matched pairs); (2) GLP-1RA vs. sulfonylureas (n=1,246 PS-matched pairs); and (3) GLP-1RA vs. SGLT-2i (n=845 PS-matched pairs).
Table S2A outlines the complete baseline characteristics of each treatment group, in the three unmatched cohorts. Compared to initiators of comparator medications, GLP-1RA initiators were younger, with fewer complications of cirrhosis or individual comorbidities, but they were more likely to use insulin and to have obesity. After PS-matching, all characteristics were well-balanced (Tables 1, S2B).
Table 1.
Selected Baseline Characteristics in Propensity Score Matched Cohorts of Patients with Cirrhosis and Diabetes Initiating GLP-1 Receptor Agonists or a Comparator Medication
| Cohort 1 GLP-1RA vs. DPP-4i |
Cohort 2 GLP-1RA vs. Sulfonylurea |
Cohort 3 GLP-1RA vs. SGLT2i |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline Characteristic | GLP-1RA N=1,431 |
DPP-4i N=1,431 |
GLP-1RA N=1,246 |
Sulfonylurea N=1,246 |
GLP-1RA N=845 |
SGLT2i N=845 |
| Male, % | 778 (54.2) | 785 (54.7) | 704 (56.5) | 693 (55.6) | 502 (52.0) | 498 (51.6) |
| Age, years - mean (SD) | 57.6 (9.1) | 57.6 (9.5) | 57.5 (9.1) | 57.6 (9.6) | 58.0 (8.7) | 58.1 (8.4) |
|
| ||||||
| Etiology of Cirrhosis | ||||||
|
| ||||||
| Viral Hepatitis | 111 (7.7) | 107 (7.5) | 90 (7.2) | 92 (7.4) | 78 (9.2) | 78 (9.2) |
| Alcoholic Liver Disease | 128 (8.9) | 125 (8.7) | 114 (9.2) | 127 (10.2) | 85 (10.1) | 94 (11.1) |
| Nonalcoholic fatty liver disease | 922 (64.3) | 927 (64.6) | 788 (63.2) | 773 (62.0) | 511 (60.5) | 501 (59.3) |
| Other / Unspecified | 274 (19.1) | 276 (19.2) | 255 (20.4) | 255 (20.4) | 171 (20.2) | 172 (20.4) |
|
| ||||||
| Cirrhosis Severity | ||||||
|
| ||||||
| Hospitalization for hepatic decompensation1 | 117 (8.2) | 115 (8.0) | 89 (7.1) | 97 (7.8) | 66 (7.8) | 63 (7.5) |
| Ascites / SBP | 68 (4.7) | 66 (4.6) | 56 (4.5) | 64 (5.1) | 42 (5.0) | 42 (5.0) |
| Hepatic encephalopathy | 58 (4.0) | 60 (4.2) | 55 (4.4) | 54 (4.3) | 37 (4.4) | 33 (3.9) |
| Esophageal varices (any) | 53 (3.7) | 46 (3.2) | 36 (2.9) | 36 (2.9) | 30 (3.6) | 27 (3.2) |
| Bleeding esophageal varices | 17 (1.2) | 14 (1.0) | 10 (0.8) | 5 (0.4) | 7 (0.8) | 6 (0.7) |
| Hepatocellular carcinoma | 36 (2.5) | 37 (2.6) | 28 (2.2) | 29 (2.3) | 24 (2.8) | 23 (2.7) |
|
| ||||||
| Diabetes Severity | ||||||
|
| ||||||
| Nephropathy | 42 (2.9) | 45 (3.1) | 37 (3.0) | 35 (2.8) | 22 (2.6) | 22 (2.6) |
| Neuropathy | 126 (8.8) | 128 (8.9) | 106 (8.5) | 108 (8.7) | 67 (7.9) | 70 (8.3) |
| Retinopathy | 26 (1.8) | 26 (1.8) | 21 (1.7) | 17 (1.4) | 5 (0.6) | 7 (0.8) |
| Diabetic foot | 25 (1.7) | 27 (1.9) | 17 (1.4) | 20 (1.6) | 11 (1.3) | 12 (1.4) |
| Dorsopathies | 240 (16.7) | 237 (16.5) | 195 (15.6) | 186 (14.9) | 102 (12.1) | 104 (12.3) |
| Hypoglycemia | 41 (2.9) | 34 (2.4) | 25 (2.0) | 21 (1.7) | 17 (2.0) | 16 (1.9) |
| Skin & soft tissue infections | 97 (6.8) | 91 (6.3) | 74 (5.9) | 73 (5.9) | 35 (4.1) | 40 (4.7) |
| Diabetes without complications | 978 (68.2) | 971 (67.7) | 810 (65.0) | 797 (63.9) | 408 (48.3) | 407 (48.2) |
|
| ||||||
| Diabetes Medication Use | ||||||
|
| ||||||
| Metformin | 724 (50.5) | 724 (50.5) | 581 (46.6) | 605 (48.5) | 449 (53.1) | 453 (53.6) |
| Glitazone | 150 (10.5) | 136 (9.5) | 114 (9.1) | 126 (10.1) | 45 (5.3) | 48 (5.7) |
| Insulin | 294 (20.5) | 300 (20.9) | 125 (10.0) | 139 (11.1) | 83 (9.8) | 77 (9.1) |
| SGLT2i | 87 (6.1) | 86 (6.0) | 81 (6.5) | 85 (6.8) | -- | -- |
| DPP-4i | -- | -- | 76 (6.1) | 70 (5.6) | 201 (23.8) | 196 (23.2) |
| Sulfonylurea | 463 (32.3) | 459 (32.0) | -- | -- | 270 (32.0) | 254 (30.1) |
| Meglinitides | 9 (0.6) | 8 (0.6) | 11 (0.9) | 11 (0.9) | 4 (0.5) | 3 (0.4) |
|
| ||||||
| Comorbidities | ||||||
|
| ||||||
| Alcohol abuse / dependence | 83 (5.8) | 82 (5.7) | 65 (5.2) | 59 (4.7) | 34 (4.0) | 32 (3.8) |
| Cancer (any) | 105 (7.3) | 104 (7.2) | 76 (6.1) | 78 (6.3) | 47 (5.6) | 48 (5.7) |
| Drug abuse / dependence | 10 (0.7) | 9 (0.6) | 10 (0.8) | 10 (0.8) | 5 (0.6) | 2 (0.2) |
| Obesity | 219 (15.3) | 228 (15.9) | 200 (16.0) | 203 (16.3) | 120 (14.2) | 117 (13.8) |
| Smoking (ever) | 98 (6.8) | 98 (6.8) | 75 (6.0) | 83 (6.7) | 73 (8.6) | 80 (9.5) |
| Hypertension | 617 (43.0) | 630 (43.9) | 503 (40.3) | 495 (39.7) | 292 (34.6) | 275 (32.5) |
| Dyslipidemia | 215 (15.0) | 215 (15.0) | 187 (15.0) | 180 (14.4) | 78 (9.2) | 76 (9.0) |
| Ischemic heart disease | 144 (10.0) | 149 (10.4) | 104 (8.3) | 107 (8.6) | 67 (7.9) | 60 (7.1) |
| Congestive heart failure | 78 (5.4) | 77 (5.4) | 62 (5.0) | 60 (4.8) | 29 (3.4) | 25 (3.0) |
| Cerebrovascular disease | 33 (2.3) | 20 (1.4) | 26 (2.1) | 24 (1.9) | 13 (1.5) | 11 (1.3) |
| Chronic obstructive pulmonary disease | 61 (4.3) | 66 (4.6) | 43 (3.4) | 33 (2.6) | 26 (3.1) | 23 (2.7) |
| Non-diabetes chronic kidney disease | 62 (4.3) | 65 (4.5) | 57 (4.6) | 50 (4.0) | 28 (3.3) | 23 (2.7) |
| Depression | 125 (8.7) | 133 (9.3) | 112 (9.0) | 106 (8.5) | 59 (7.0) | 55 (6.5) |
| Dementia | 14 (1.0) | 16 (1.1) | 13 (1.0) | 14 (1.1) | 4 (0.5) | 4 (0.5) |
| Osteoporosis | 52 (3.6) | 55 (3.8) | 51 (4.1) | 52 (4.2) | 21 (2.5) | 24 (2.8) |
| Falls | 24 (1.7) | 14 (1.7) | 22 (1.8) | 20 (1.6) | 26 (3.1) | 21 (2.5) |
| Fractures | 29 (2.0) | 29 (2.0) | 23 (1.8) | 18 (1.4) | 14 (1.7) | 15 (1.8) |
|
| ||||||
| Medication Use | ||||||
|
| ||||||
| Number of prescription drugs, mean (SD) | 11.2 (5.7) | 11.2 (5.9) | 11.2 (5.9) | 11.2 (6.2) | 12.0 (5.9) | 11.8 (5.6) |
| ACE inhibitors | 475 (33.1) | 466 (32.5) | 380 (30.5) | 405 (32.5) | 285 (33.7) | 279 (33.0) |
| Angiotensin II receptor blockers | 321 (22.4) | 345 (24.0) | 275 (22.1) | 265 (21.3) | 200 (23.7) | 195 (23.1) |
| Anticoagulants | 70 (4.9) | 80 (5.6) | 58 (4.7) | 64 (5.1) | 36 (4.3) | 34 (4.0) |
| Antiplatelets | 82 (5.7) | 75 (5.2) | 75 (6.0) | 73 (5.9) | 52 (6.2) | 54 (6.4) |
| Non-selective beta blockers | 389 (27.1) | 409 (28.5) | 334 (26.8) | 343 (27.5) | 255 (30.2) | 244 (28.9) |
| Bisphosphonates | 27 (1.9) | 27 (1.9) | 27 (2.2) | 31 (2.5) | 12 (1.4) | 11 (1.3) |
| Loop diuretics | 356 (24.8) | 356 (24.8) | 307 (24.6) | 289 (23.2) | 217 (25.7) | 208 (24.6) |
| Other diuretics | 261 (18.2) | 248 (17.3) | 229 (18.4) | 228 (18.3) | 153 (18.1) | 144 (17.0) |
| Statins | 539 (37.6) | 523 (36.4) | 464 (37.2) | 477 (38.3) | 357 (42.2) | 362 (42.8) |
| Non-statin lipid-lowering drugs | 176 (12.3) | 177 (12.3) | 166 (13.3) | 165 (13.2) | 114 (13.5) | 104 (12.3) |
| Antibiotics (any) | 755 (52.6) | 742 (51.7) | 628 (50.4) | 626 (50.2) | 447 (52.9) | 432 (51.1) |
| Antibiotics-fluoroquinolones | 276 (19.2) | 270 (18.8) | 226 (18.1) | 229 (18.4) | 154 (18.2) | 160 (18.9) |
| Anticonvulsants | 261 (18.2) | 248 (17.3) | 229 (18.4) | 228 (18.3) | 166 (19.6) | 163 (19.3) |
| Antidepressants | 514 (35.8) | 514 (35.8) | 468 (37.5) | 460 (36.9) | 311 (36.8) | 314 (37.2) |
| Benzodiazepines | 245 (17.1) | 241 (16.8) | 221 (17.7) | 209 (16.8) | 154 (18.2) | 148 (17.4) |
| Opioids | 608 (42.4) | 582 (40.6) | 519 (41.6) | 492 (39.5) | 343 (40.6) | 329 (38.9) |
| Rifaximin and/or Lactulose | 164 (11.4) | 173 (12.1) | 153 (12.3) | 151 (12.1) | 112 (13.3) | 103 (12.2) |
|
| ||||||
| Healthcare Utilization in Prior 180 Days | ||||||
|
| ||||||
| Any hospitalization | 300 (20.9) | 289 (20.1) | 225 (18.0) | 231 (18.5) | 155 (18.30 | 143 (16.9) |
| Any ED visit | 460 (32.1) | 474 (33.0) | 366 (29.4) | 384 (30.8) | 267 (31.6) | 249 (29.5) |
| Any abdominal ultrasound | 650 (45.3) | 639 (44.5) | 560 (44.9) | 569 (45.6) | 385 (45.6) | 393 (46.5) |
| Any paracentesis | 33 (2.3) | 32 (2.2) | 28 (2.2) | 36 (2.9) | 20 (2.4) | 18 (2.1) |
| Any upper endoscopy | 429 (29.9) | 431 (30.0) | 365 (29.3) | 355 (28.5) | 259 (30.7) | 263 (31.1) |
| Any variceal banding | 74 (5.2) | 69 (4.8) | 58 (4.7) | 65 (5.2) | 45 (5.3) | 47 (5.6) |
| Any visit to gastroenterologist | 775 (54.0) | 749 (52.2) | 691 (55.4) | 701 (56.2) | 479 (56.7) | 486 (57.5) |
|
| ||||||
| Preventative Care in Prior 180 Days | ||||||
|
| ||||||
| Receipt of pneumonia vaccine | 148 (10.3) | 160 (11.1) | 139 (11.1) | 147 (11.8) | 121 (4.3) | 125 (14.8) |
| Receipt of flu vaccine | 238 (16.6) | 256 (17.8) | 225 (18.0) | 235 (18.8) | 147 (17.4) | 152 (18.0) |
| Colonoscopy | 198 (13.8) | 193 (13.4) | 165 (13.2) | 160 (12.8) | 120 (14.2) | 132 (15.6) |
| Mammogram | 170 (11.8) | 169 (11.8) | 170 (13.6) | 168 (13.5) | 99 (11.7) | 103 (12.2) |
| PSA or DRE | 149 (10.4) | 161 (11.2) | 139 (11.1) | 157 (12.6) | 95 (11.2) | 99 (11.7) |
| HbA1c testing | 1,069 (74.5) | 1,075 (74.9) | 925 (74.2) | 918 (73.6) | 650 (76.9) | 654 (77.4) |
| Liver function testing | 541 (37.7) | 553 (38.5) | 466 (37.4) | 492 (39.5) | 282 (33.4) | 281 (33.3) |
Abbreviations: GLP-1RA, glucose-like peptide 1 receptor agonist; DPP-4 inhibitor, dipeptidyl peptidase-4 inhibitor; SGLT-2 inhibitor, sodium-glucose cotransporter-2 inhibitor; SD, standard deviation; SBP, spontaneous bacterial peritonitis; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ED, emergency department; PSA, prostate-specific antigen; DRE, digital rectal examination; HbA1c, hemoglobin A1c).
Hepatic decompensation events included any hospitalization for which primary cause included ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, hepatic encephalopathy or bleeding esophageal varices. For details see Methods.
Hepatic Decompensation Events
Figure 2 depicts the cumulative incidence of the primary outcome (i.e. hospitalization for hepatic decompensation events), comparing PS-matched initiators of GLP-1RA to each of the three comparator groups, after accounting for the competing risk of death. Table 2 outlines absolute incidence rates and relative hazards of the primary outcome and the individual decompensation outcomes, after PS-matching. Median overall follow-up was 132 days (interquartile range=73, 290 days). Overall, we documented 96 decompensation events among GLP-1RA initiators, and 155 events among DPP-4i initiators (cohort 1; incidence rates, 105.2 vs. 144.0/1000 person-years [PY]), corresponding to HR=0.68 (95%CI=0.53-0.88)(Table 2). GLP-1RA initiators also had significantly lower rates of developing ascites, SBP or HRS (HR=0.66, 95%CI=0.45-0.97), or bleeding esophageal varices (HR=0.62, 95%CI=0.41-0.92), and a non-significant trend towards lower rates of HE (HR=0.76, 95%CI=0.55-1.06), compared to DPP-4i initiators.
Figure 2.
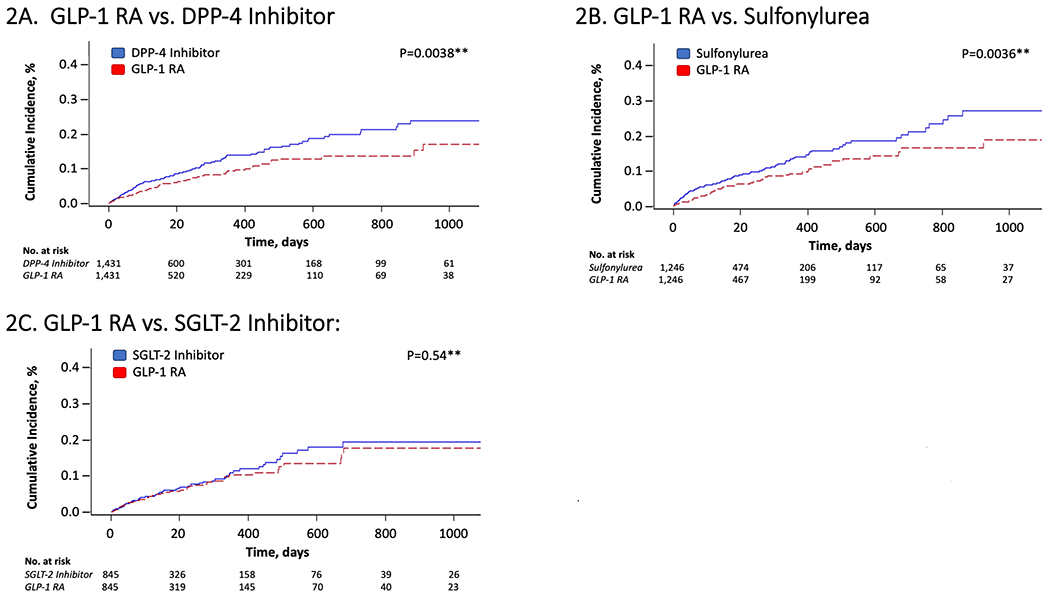
Cumulative Incidence of Hepatic Decompensation* among Propensity Score-Matched Patients with Cirrhosis Initiating GLP-1 RA Therapy or Alternative Antidiabetic Medications
Abbreviations: GLP-1 RA, glucagon-like peptide receptor agonist; DPP-4, Dipeptidyl Peptidase-4; SGLT-2, sodium-glucose cotransporter-2; No., number
*Hepatic decompensation was defined as the first hospitalization for ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, bleeding esophageal varices or hepatic encephalopathy after the cohort entry date (i.e. date of drug initiation). For details, see Methods.
**P-values were obtained using Gray’s test for equality of the cumulative incidence functions between each exposure group after propensity score-matching, accounting for the competing risk of all-cause mortality.
Table 2.
Risk of Hepatic Decompensation among Propensity Score-Matched Patients with Cirrhosis and Type 2 Diabetes Initiating GLP-1 Receptor Agonists or Comparator Antidiabetic Medications
| Cohort 1 (n=1,431 pairs) | Cohort 2 (n=1,246 pairs) | Cohort 3 (n=845 pairs) | ||||
|---|---|---|---|---|---|---|
| GLP-1RA N=1,431 |
DPP-4i N=1,431 |
GLP-1RA N=1,246 |
Sulfonylurea N=1,246 |
GLP-1RA N=845 |
SGLT-2 Inhibitor N=845 |
|
| Median [IQR] follow-up, days | 133 [73, 277] | 136 [73, 342] | 127 [71, 246] | 133 [73, 286] | 133 [73, 288] | 152 [73, 360] |
|
| ||||||
| Hepatic Decompensation events1 | ||||||
|
| ||||||
| • No. of events | 96 | 155 | 78 | 124 | 56 | 64 |
| • Incidence rate, per 1,000 PY | 105.2 | 144.0 | 97.3 | 147.7 | 103.5 | 112.8 |
| • Hazard ratio (95% CI) | 0.68 (0.53-0.88) | 1 (ref.) | 0.64 (0.48-0.84) | 1 (ref.) | 0.89 (0.62-1.28) | 1 (ref.) |
|
| ||||||
| Ascites, SBP or Hepatorenal Syndrome | ||||||
|
| ||||||
| • No. of events | 56 | 87 | 48 | 76 | 38 | 37 |
| • Incidence rate, per 1,000 PY | 62.2 | 82.0 | 58.8 | 87.3 | 69.5 | 63.0 |
| • Hazard ratio (95% CI) | 0.66 (0.45-0.97) | 1 (ref.) | 0.66 (0.46-0.94) | 1 (ref.) | 1.07 (0.68-1.68) | 1 (ref.) |
|
| ||||||
| Bleed from esophageal varices | ||||||
|
| ||||||
| • No. of events | 38 | 69 | 29 | 51 | 28 | 37 |
| • Incidence rate, per 1,000 PY | 40.5 | 61.0 | 35.4 | 58.6 | 50.7 | 63.5 |
| • Hazard ratio (95% CI) | 0.62 (0.41-0.92) | 1 (ref.) | 0.59 (0.37-0.92) | 1 (ref.) | 0.78 (0.48-1.27) | 1 (ref.) |
|
| ||||||
| Hepatic encephalopathy | ||||||
|
| ||||||
| • No. of events | 59 | 87 | 46 | 77 | 35 | 34 |
| • Incidence rate, per 1,000 PY | 64.0 | 77.6 | 58.4 | 88.4 | 64.0 | 58.8 |
| • Hazard ratio (95% CI) | 0.76 (0.55-1.06) | 1 (ref.) | 0.60 (0.39-0.92) | 1 (ref.) | 1.07 (0.67-1.71) | 1 (ref.) |
Abbreviations: GLP-1RA, glucose-like peptide 1 receptor agonist; DPP-4 inhibitor, dipeptidyl peptidase-4 inhibitor; SGLT-2 inhibitor, sodium-glucose cotransporter-2 inhibitor; IQR, interquartile range; PY, person-years; CI, confidence interval; SBP, spontaneous bacterial peritonitis
Hepatic decompensation was defined as the first recorded hospitalization for which the primary cause included ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, bleeding esophageal varices or hepatic encephalopathy. For details see Methods.
Rates of decompensation events were also significantly lower among GLP-1RA initiators, compared to sulfonylurea initiators (cohort 2; 78 vs. 124 events; 97.3 vs. 144.0/1000PY, respectively), translating to HR=0.64 (95%CI=0.48-0.84). Similarly, initiators of GLP-1RAs had significantly lower rates of ascites, SBP or HRS (HR=0.66, 95%CI=0.46-0.94), bleeding esophageal varices (HR=0.59, 95%CI=0.37-0.92) and HE (HR=0.60, 95%CI=0.39-0.92), compared to sulfonylureas (Table 2). In contrast, when GLP-1RA and SGLT-2i initiators were directly compared, no significant differences were observed in rates of hepatic decompensation (cohort 3; 56 vs. 64 events; 103.5 vs. 112.8/1000PY, respectively; HR=0.89, 95%CI=0.62-1.28), or in individual decompensation endpoints (Table 2).
We repeated our primary analysis separately in PS-matched subgroups with compensated or decompensated cirrhosis, and our findings were consistent (Table 3). Similarly, among PS-matched patients with NAFLD cirrhosis, GLP-1RA initiators again demonstrated significantly lower rates of decompensation events, compared with DPP-4i (HR=0.63, 95%CI=0.47-0.85) or sulfonylureas (HR=0.71, 95%CI=0.51-0.98), but rates were similar when compared with SGLT-2i (HR=0.88, 95%CI=0.55-1.41)(Table S3). In further subgroup analyses, the observed benefits of GLP1RAs compared to DPP-4i or sulfonylureas did not differ substantially by gender, HBV/HCV status, underlying ALD, insurance status or concurrent use of metformin, statins or NSBB(Table S4). However, due to the small size of cohort 3 (GLP-1RA vs. SGLT-2i), the PS-matching procedure for these subgroup analyses could only be performed for comparisons of GLP-1RA with DPP-4i and sulfonylureas.
Table 3.
Risk of Hepatic Decompensation Events1 among Propensity Score-Matched Patients with Compensated or Decompensated Cirrhosis* and Type 2 Diabetes Initiating GLP-1 Receptor Agonists or Comparator Antidiabetic Medications
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| GLP-1RA | DPP-4 Inhibitor | GLP-1RA | Sulfonylurea | GLP-1RA | SGLT-2 Inhibitor | |
| Compensated Cirrhosis1, N. | 1,294 | 1,294 | 1,167 | 1,167 | 769 | 769 |
|
| ||||||
| • Median [IQR] follow-up, days | 133 [73, 286] | 160 [73, 382] | 132 [71, 251] | 135 [73, 314] | 135 [73, 290] | 157 [73, 363] |
| • No. of events | 69 | 111 | 53 | 86 | 38 | 44 |
| • Incidence rate, per 1,000 PY | 81.9 | 116.3 | 71.1 | 109.7 | 76.9 | 81.7 |
| • Hazard ratio (95% CI) | 0.68 (0.51-0.93) | 1 (ref.) | 0.63 (0.44-0.88) | 1 (ref.) | 0.93 (0.60-1.43) | 1 (ref.) |
|
| ||||||
| Decompensated Cirrhosis1, N. | 173 | 173 | 161 | 161 | 64 | 64 |
|
| ||||||
| • Median [IQR] follow-up, days | 88 [44, 182] | 83 [36, 204] | 88 [40, 160] | 77 [36, 179] | 98 [54, 217] | 104 [53, 183] |
| • No. of events | 43 | 67 | 40 | 62 | 19 | 19 |
| • Incidence rate, per 1,000 PY | 521.8 | 702.9 | 572.2 | 884.0 | 654.7 | 556.4 |
| • Hazard ratio (95% CI) | 0.68 (0.46-0.98) | 1 (ref.) | 0.65 (0.44-0.97) | 1 (ref.) | 1.15 (0.61-2.18) | 1 (ref.) |
Abbreviations: GLP-1RA, glucose-like peptide 1 receptor agonist; DPP-4 inhibitor, dipeptidyl peptidase-4 inhibitor; SGLT-2 inhibitor, sodium-glucose cotransporter-2 inhibitor; IQR, interquartile range; PY, person-years; CI, confidence interval; SBP, spontaneous bacterial peritonitis
For each subgroup (i.e. compensated cirrhosis and decompensated cirrhosis), the propensity score-matching procedure was repeated to ensure appropriate balance between drug exposure groups. Thus, the total number of patients and the total number of recorded events differs from that shown in Table 2.
Hepatic decompensation was defined as the first recorded hospitalization for which the primary cause included ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, hepatic encephalopathy or bleeding esophageal varices. For details see Methods.
Sensitivity Analyses
Our findings were robust across all sensitivity analyses (Tables S5–S8). We also evaluated incident fractures as a negative control outcome and observed the expected null associations (Table S9). Additionally, we repeated our primary analysis within an independent study population (Optum), after PS-matching for the same covariates plus race/ethnicity (Table S10), and our results again remained similar (Table S11). Finally, we found that an unmeasured confounder would need to have an implausibly strong association with both exposure (i.e. OR=3.95 or stronger) and outcome (i.e. OR=0.1 or less) simultaneously, to move the point estimate to 1.0 (Tables S12A–B).
Discussion:
In a large, population-based cohort of U.S. adults with cirrhosis and type 2 diabetes, initiators of GLP-1RA therapy had lower rates of hepatic decompensation events, compared to initiators of two comparable and commonly-prescribed second-line antidiabetic drugs, DPP-4i and sulfonylureas. GLP-1RA initiators also demonstrated consistently lower rates of individual decompensation events, including ascites, SBP or HRS, bleeding esophageal varices and HE. The observed benefits of GLP-1RAs persisted after accounting for known and putative risk factors for adverse hepatic events, and they were robust in both men and women, in patients with compensated and decompensated cirrhosis, and in an independent study population. Importantly for this sick population, the benefits of GLP-1RA therapy were evident early, within 4 months of treatment initiation. In contrast, rates of major hepatic decompensation events were similarly low when GLP-1RA and SGLT-2i initiators were directly compared, suggesting that SGLT-2i may also confer important hepatoprotective effects, in patients with cirrhosis.
Emerging evidence supports a role for GLP-1RAs in cirrhosis and type 2 diabetes. Chronic hyperglycemia contributes to accelerated hepatic decompensation6–8, and on preclinical studies, GLP-1RA treatment improves hepatic lipid oxidation29, promotes hepatic stellate cell quiescence, diminishes cellular proliferation and improves microvascular function30. GLP-1RAs may also modulate cholangiocyte activation by inhibiting mitochondrial apoptosis31, and by influencing de novo biosynthesis of primary bile acids32. Furthermore, in patients with non-cirrhotic NAFLD, RCTs demonstrate that GLP-1RAs can reduce liver fat, inflammation and lipotoxicity11 12, and reverse NASH13. Collectively, these findings have led to the hypothesis that GLP-1RAs may offer unique benefits for patients with cirrhosis and diabetes. However, evidence to support this is scarce, as cirrhotic patients were previously excluded from all major RCTs of GLP-1RAs, and robust observational data is lacking. Thus, by performing direct, head-to-head comparisons across comparable antidiabetic drug classes in a large, nationwide cohort, the current study provides compelling real-world evidence that supports a role for GLP-1RAs in the setting of cirrhosis and diabetes.
Notably, both GLP-1RA and SGLT-2i initiators had similar and low decompensation event rates, suggesting that SGLT-2i medications may also provide hepatoprotective benefits. This is biologically plausible, for SGLT-2i inhibit renal sodium reabsorption, reducing salt and water retention and attenuating the renin-angiotensin-aldosterone-system33, which may improve fluid balance and correct the maladaptive neurohormonal signaling and pro-inflammatory responses that promote hepatic decompensation. However, SGLT-2i also represent the newest class of approved antidiabetic drugs, and their safety and efficacy in cirrhosis is unknown. Accordingly, we had relatively small numbers of SGLT-2i initiators, thus some of our secondary analyses were underpowered and should be interpreted cautiously. To that end, future large-scale studies are needed that directly compare SGLT-2i with comparable antidiabetic drug classes, and which also compare individual medications.
To our knowledge, this is the first study to evaluate the potential benefits of GLP-1RA use for preventing decompensation events in cirrhotic patients with type 2 diabetes, as directly compared with relevant treatment alternatives. It is strengthened by the inclusion of a large, nationwide population with validated definitions of cirrhosis and study outcomes, and comprehensive prescription medication use data. Careful PS-matching minimized both indication bias and residual confounding. Applying a new-user, active comparator design addressed selection and detection biases, while also aligning subjects at a uniform time (i.e. treatment initiation date), and minimizing potential healthy user bias. Moreover, we conducted numerous sensitivity and subgroup analyses to address potential misclassification and residual confounding, and our findings were consistent in an independent population.
We acknowledge several limitations. First, this was a retrospective study, and prospective studies including RCTs are needed. However, such studies are inherently costly, time-consuming and often exclude vulnerable populations, like patients with advanced cirrhosis. For this reason, comparative effectiveness research using administrative databases represents an innovative approach to efficiently generate robust data to help guide patient care, with findings that have been shown to recapitulate results from RCTs34. Second, although cirrhosis and outcomes were defined using validated algorithms with PPVs >85-90%16 17 23–25, misclassification is still possible. Yet, our findings were robust in an independent cohort, across numerous sensitivity analyses, and after applying alternate definitions of cirrhosis. Third, despite careful PS-matching, confounding may nevertheless persist. This is particularly relevant for the comparison of GLP-1RAs with sulfonylureas, as sulfonylureas are older, less expensive and may be prescribed more frequently to patients with more advanced liver disease. We also lacked data regarding specific indications for other prescriptions, like NSBB. However, our findings were consistent after PS-matching for >90 covariates, including established markers of decompensation and other medication use. Further, our findings were consistent when GLP-1RAs were compared to DPP-4is, a medication class that is similarly new, costly and under-studied in cirrhosis. We also lacked more detailed data regarding indices of cirrhosis severity, laboratory data, the timing of initial cirrhosis diagnoses relative to initial diabetes diagnoses, regular alcohol use, body mass index, use of antiviral therapy during follow-up and duration of diabetes; however, our findings were robust across sensitivity analyses, and we demonstrated that it would be highly unlikely for an unmeasured confounder to fully explain our results. Fourth, because the marketing of GLP-1RAs, DPP-4i and SGLT-2i is relatively new — and cirrhotic patients were excluded from the original RCTs — some of our subgroups were small, and we lacked sufficient numbers to estimate risks in patients with and without previous ascites or esophageal varices, or to directly compare GLP-1RA to thiazolidinediones. Thus, large-scale, prospective studies are needed both to validate our findings and to directly compare the safety and effectiveness of individual antidiabetic medications, according to the adequacy of glycemic control, and also in relation to mortality and HCC. Finally, our results may not be generalizable to patients with different insurance types or those lacking insurance coverage, given differences in demographics, risk factors, socioeconomic status and drug adherence.
In conclusion, within a nationwide population of adults with cirrhosis and type 2 diabetes, initiators of GLP-1RA therapy had meaningfully reduced rates of decompensation events compared to two commonly prescribed, second-line antidiabetic alternatives, DPP-4i and sulfonylureas, consistent with preclinical data29 30 and clinical studies of non-cirrhotic NAFLD11–13. Thus, our findings suggest substantial potential benefits of GLP-1RA therapy in patients with cirrhosis who are engaged in routine clinical care.
Supplementary Material
Grant Support:
NIH K23 DK122104 (TGS)
Harvard University Center for AIDS Research (CFAR) Career Development Award (TGS) Dana-Farber/Harvard Cancer Center GI SPORE Career Enhancement Award (TGS)
Dr. Patorno was supported by a career development grant (K08AG055670) from the National Institute on Aging.
Role of the Funding Source:
The funding sources did not participate in the design or conduct of this study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Disclosures and conflicts of interest:
Dr. Simon has served as a consultant to Aetion and has received grants to the institution from Amgen, for work unrelated to this manuscript.
Dr. Schneeweiss (ORCID# 0000-0003-2575-467X) is participating in investigator-initiated grants to the Brigham and Women’s Hospital from Boehringer Ingelheim unrelated to the topic of this study. He is a consultant to Aetion Inc., a software manufacturer of which he owns equity. His interests were declared, reviewed, and approved by the Brigham and Women’s Hospital and Partners Healthcare System in accordance with their institutional compliance policies.
Dr. Patorno is investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not directly related to the topic of the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Transparency statement:
The lead author (TGS) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and if relevant, registered) have been explained.
Data sharing: No additional data are available
Ethical Approval:
The use of this dataset for research was approved by the institutional review board (#2011P002580) of the Brigham and Women’s Hospital, Boston, MA, and a data use agreement is in place.
REFERENCES:
- 1.Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70(1):151–71. doi: 10.1016/j.jhep.2018.09.014 [published Online First: 2018/09/30] [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1151–210. doi: 10.1016/S0140-6736(17)32152-9 [published Online First: 2017/09/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(1):123–33. doi: 10.1002/hep.29466 [published Online First: 2017/08/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wlazlo N, Beijers HJ, Schoon EJ, et al. High prevalence of diabetes mellitus in patients with liver cirrhosis. Diabet Med 2010;27(11):1308–11. doi: 10.1111/j.1464-5491.2010.03093.x [published Online First: 2010/10/26] [DOI] [PubMed] [Google Scholar]
- 5.Simon TG, King LY, Chong DQ, et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology 2018;67(5):1797–806. doi: 10.1002/hep.29660 [published Online First: 2017/11/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology 2002;122(7):1822–8. doi: 10.1053/gast.2002.33650 [published Online First: 2002/06/11] [DOI] [PubMed] [Google Scholar]
- 7.Butt Z, Jadoon NA, Salaria ON, et al. Diabetes mellitus and decompensated cirrhosis: risk of hepatic encephalopathy in different age groups. J Diabetes 2013;5(4):449–55. doi: 10.1111/1753-0407.12067 [published Online First: 2013/06/05] [DOI] [PubMed] [Google Scholar]
- 8.Quintana JO, Garcia-Compean D, Gonzalez JA, et al. The impact of diabetes mellitus in mortality of patients with compensated liver cirrhosis-a prospective study. Ann Hepatol 2011;10(1):56–62. [published Online First: 2011/02/09] [PubMed] [Google Scholar]
- 9.Fisher M Glucagon-like peptide 1 receptor agonists and cardiovascular risk in type 2 diabetes: a clinical perspective. Diabetes Obes Metab 2015;17(4):335–42. doi: 10.1111/dom.12380 [published Online First: 2014/08/27] [DOI] [PubMed] [Google Scholar]
- 10.Smilowitz NR, Donnino R, Schwartzbard A. Glucagon-like peptide-1 receptor agonists for diabetes mellitus: a role in cardiovascular disease. Circulation 2014;129(22):2305–12. doi: 10.1161/CIRCULATIONAHA.113.006985 [published Online First: 2014/06/04] [DOI] [PubMed] [Google Scholar]
- 11.Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol 2016;64(2):399–408. doi: 10.1016/j.jhep.2015.08.038 [published Online First: 2015/09/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X [published Online First: 2015/11/27] [DOI] [PubMed] [Google Scholar]
- 13.Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med 2021;384(12):1113–24. doi: 10.1056/NEJMoa2028395 [published Online First: 2020/11/14] [DOI] [PubMed] [Google Scholar]
- 14.Vukotic R, Raimondi F, Brodosi L, et al. The Effect of Liraglutide on beta-Blockade for Preventing Variceal Bleeding: A Case Series. Annals of internal medicine 2020;173(5):404–05. doi: 10.7326/L20-0041 [published Online First: 2020/05/26] [DOI] [PubMed] [Google Scholar]
- 15.Bengtsson B, Askling J, Ludvigsson JF, et al. Validity of administrative codes associated with cirrhosis in Sweden. Scand J Gastroenterol 2020:1–6. doi: 10.1080/00365521.2020.1820566 [published Online First: 2020/09/23] [DOI] [PubMed] [Google Scholar]
- 16.Goldberg D, Lewis J, Halpern S, et al. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf 2012;21(7):765–69. doi: 10.1002/pds.3290 [published Online First: 2012/06/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mapakshi S, Kramer JR, Richardson P, et al. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clin Gastroenterol Hepatol 2018;16(10):1677–78. doi: 10.1016/j.cgh.2018.01.042 [published Online First: 2018/02/08] [DOI] [PubMed] [Google Scholar]
- 18.Tapper EB, Korovaichuk S, Baki J, et al. Identifying Patients With Hepatic Encephalopathy Using Administrative Data in the ICD-10 Era. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.12.017 [published Online First: 2019/12/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10(2):150–61. doi: 10.1002/pst.433 [published Online First: 2010/10/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28(25):3083–107. doi: 10.1002/sim.3697 [published Online First: 2009/09/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, and Robert J. Gray. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 22.Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. Bmj 2018;360:k119. doi: 10.1136/bmj.k119 [published Online First: 2018/02/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol 2013;47(5):e50–4. doi: 10.1097/MCG.0b013e3182688d2f [published Online First: 2012/10/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther 2008;27(3):274–82. doi: 10.1111/j.1365-2036.2007.03572.x [published Online First: 2007/11/13] [DOI] [PubMed] [Google Scholar]
- 25.Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018;68(3):872–82. doi: 10.1002/hep.29887 [published Online First: 2018/03/27] [DOI] [PubMed] [Google Scholar]
- 26.Schneeweiss S A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf 2010;19(8):858–68. doi: 10.1002/pds.1926 [published Online First: 2010/08/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneeweiss S Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15(5):291–303. doi: 10.1002/pds.1200 [published Online First: 2006/02/01] [DOI] [PubMed] [Google Scholar]
- 28.Wang SV, Verpillat P, Rassen JA, et al. Transparency and Reproducibility of Observational Cohort Studies Using Large Healthcare Databases. Clin Pharmacol Ther 2016;99(3):325–32. doi: 10.1002/cpt.329 [published Online First: 2015/12/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trevaskis JL, Griffin PS, Wittmer C, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 2012;302(8):G762–72. doi: 10.1152/ajpgi.00476.2011 [published Online First: 2012/01/24] [DOI] [PubMed] [Google Scholar]
- 30.de Mesquita FC, Guixe-Muntet S, Fernandez-Iglesias A, et al. Liraglutide improves liver microvascular dysfunction in cirrhosis: Evidence from translational studies. Sci Rep 2017;7(1):3255. doi: 10.1038/s41598-017-02866-y [published Online First: 2017/06/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzioni M, Alpini G, Saccomanno S, et al. Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 2007;133(1):244–55. doi: 10.1053/j.gastro.2007.04.007 [published Online First: 2007/07/17] [DOI] [PubMed] [Google Scholar]
- 32.Wiest R, Albillos A, Trauner M, et al. Targeting the gut-liver axis in liver disease. J Hepatol 2017;67(5):1084–103. doi: 10.1016/j.jhep.2017.05.007 [published Online First: 2017/05/21] [DOI] [PubMed] [Google Scholar]
- 33.Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016;134(10):752–72. doi: 10.1161/CIRCULATIONAHA.n6.021887 [published Online First: 2016/07/30] [DOI] [PubMed] [Google Scholar]
- 34.Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care. Circulation 2019;139(25):2822–30. doi: 10.1161/CIRCULATIONAHA.n8.039177 [published Online First: 2019/04/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


