Abstract
Objectives
Little is known whether differences exist in virus shedding, immune and inflammatory response related to SARS-CoV-2 in people living with human immunodeficiency virus (PLWH). We assessed viral RNA and cytokine profiles of HIV and SARS-CoV-2 coinfection in Hong Kong.
Methods
PLWH hospitalized with SARS-CoV-2 infection in Hong Kong were included, compared with age-matched and disease severity-matched SARS-CoV-2 infected controls (ratio of 1:5) from February 1st 2020 to July 31st 2020. SARS-CoV-2 infection was confirmed by public health laboratory and virus concentration was quantified by an in-house real-time reverse transcription-quantitative polymerase chain reaction. A panel of cytokines and chemokines were performed.
Results
HIV patients had a similar respiratory shedding profile compared to controls. Duration of faecal shedding of patient A, B, C and D were at least 9, 10, 33, and 11 days, respectively. HIV patients had lower plasma levels of IL-10 and NT-pro-BNP. All 4 PLWH cases showed seroconversion to SARS-CoV-2 with anti-SARS-CoV-2 S antibodies detected in serum collected between day 18 and 30 after symptom onset.
Conclusions
PLWH behaves similarly with HIV-negative controls in respiratory viral load, but with decrease in IL-10 and NT-proBNP. PLWH may have a lower risk of immunostimulatory effect due to lower IL-10.
Keywords: Adults, Coinfection, COVID-19, Human immunodeficiency virus, Severe respiratory syndrome coronavirus 2
1. Introduction
US Centers for Disease Control and Prevention has regarded human immunodeficiency virus (HIV) as a condition at increased risk of severe illness from SARS-CoV-2 [1]. There were no difference in outcome of COVID-19 by HIV status in a previous study [2]. However, the reason why some co-infected patients suffered from poorer outcomes remains poorly understood.
2. Materials and methods
2.1. Hospital setting
PLWH hospitalized with SARS-CoV-2 in Hong Kong were included, compared with age-matched and disease severity-matched SARS-CoV-2 infected controls (ratio of 1:5).
2.2. Microbiological investigations
SARS-CoV-2 infection was confirmed by public health laboratory and virus concentration was quantified by in-house real-time reverse transcription-quantitative polymerase chain reaction as described previously [3].
2.3. SARS-CoV-2 viral load quantification using real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR)
Viral RNA was extracted from plasma, respiratory and urine samples using PureLink™ Viral RNA/DNA Mini Kit (Invitrogen, USA) according to manufacturer's protocol. Viral RNA from stool was extracted using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). 1 g of stool was suspended in 1 mL of transport medium. 140 µL of filtrate was used as starting material.
Primer-probe set N1 (2019-nCoV_N1-F: 5’-GAC CCC AAA ATC AGC GAA AT-3’, 2019-nCoV_N1-R: 5’-TCT GGT TAC TGC CAG TTG AAT CTG-3’ and 2019-nCoV_N1-P: 5’-FAM-ACC CCG CAT TAC GTT TGG TGG ACC-BHQ1-3’) designed by US CDC were used.
PCR reaction contained 5 μL of extracted preparation, 4 μL TaqMan™ Fast Virus 1-Step Master Mix in a final reaction volume of 20 μL. Primer and probe concentration were 0.5 μM and 0.125 μM. Cycling conditions, 25 °C for 2 min, 50 °C for 15 min, 95 °C for 2 min, followed by 45 cycles of 95 °C for 15 s, and 55 °C for 30 s, were performed with StepOnePlus Real-Time PCR System (Applied Biosystems, USA).
Ct values of real time RT-PCR were converted into viral RNA copies based on a standard curve prepared from 10-fold serial dilutions of known copies of plasmid containing full N gene (2019-nCoV_N_Positive Control, Integrated DNA Technologies, USA). Samples were considered as negative if Ct values exceeded 39.9 cycles. Detection limit of real-time RT PCR was 694.12 copies/mL.
2.4. Immunological and serological investigations
Concentrations of cytokines and chemokines were measured using MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay (Merck Millipore, MA, USA) on a Bio-Plex 200 System (Bio-Rad Laboratories, CA, USA). Concentrations of NT-proBNP and soluble ACE2 were measured using Human NT-proBNP ELISA kit (Abcam, Cambridge, UK) and Human ACE-2 ELISA Kit (RayBiotech, Inc. GA, USA). SARS-CoV-2 IgG antibodies were detected by an in-house ELISA coated with S protein. (SinoBiological, Beijing, China)
3. Results
3.1. Clinical course
All 4 PLWH were men aged 33–54 with no other HIV-related complications (Table 1 ). HIV viral load at admission of patient A is less than 20 copies per ml with normal CD4: CD8 ratio. Patient D had normal CD4: CD8 ratio (Table 1).
Table 1.
Demographics and clinical course of the four HIV SARS-CoV-2 co-infected cases
| Patient A | Patient B | Patient C | Patient D | |
|---|---|---|---|---|
| General demographics | ||||
| Age (years) | 33 | 54 | 49 | 41 |
| Sex | Male | Male | Male | Male |
| Race | Asian | Asian | Asian | Asian |
| Smoking | Non-smoker | Ex-smoker | Non-smoker | Non-smoker |
| Past Medical history | ||||
| Co-morbidities | Good past health | Hyperlipidaemia | Good past health | Chronic tonsillitis with bilateral tonsillectomy |
| HIV status | ||||
| Year of HIV diagnosis | NA | 2004 | NA | 2020 |
| CD4/CD8 ratio at baseline | 0.79 | NA | NA | 0.68 |
| HIV viral load at or before admission (copies per ml) | <20 copies/ml | NA | NA | NA |
| ART regime before admission | ABC/DTG/3TC | ABC/3TC & NVP | FTC/TDF & DRV/COBI | NA |
| ART regime during admission | Changed to DTG | Changed to ABC/DTG/3TC | FTC/TDF & DRV/COBI | LPV/r & FTC/TDF, changed to BIC/FTC/TAF |
| Clinical presentation at hospital admission | ||||
| Interval between symptom onset and admission to hospital (days) | 7 | 33 | 15 | 7 |
| Symptoms | Sore throat | Fever, malaise | Cough, rhinorrhoea, sore throat, diarrhoea | Fever, cough, rhinorrhoea, diarrhoea |
| Chest radiograph | Clear | Mild right lung filtrate | Mild right lower zone infiltrate | Clear |
| Disease Severityb | Mild | Moderate | Moderate | Mild |
| Antiviral therapy during hospitalization (days of therapy) | LPV/r & Ribavirin (14 days) | Remdesivir (10 days) | No | LPV/r (1 day)a, INF β-1b (5 days) |
| Antibiotic during hospitalization (days of therapy) | No | Augmentin (8 days) | Augmentin (7 days) | Augmentin (8 days) |
| Immunomodulatory agents (corticosteroids or tocilizumab) | No | No | No | No |
| Length of hospital stay | 23 days | 27 days | 30 days | 14 days |
Abbreviations: NA, not available, ART, antiretroviral therapy; ABC/DTG/3TC, Abavacir/dolutegravir/lamuvidine; ABC/3TC, Abacavir/lamuvidine; NVP, Nevirapine; FTC/TDF, Entricitabine/tenofovir disoproxil fumarate; DRV/COBI, Darunavir/cobicistat; LPV/r, Lopinavir/ritonavir; BIC/FTC/TAF, Bictegravir/entricitabine/tenofovir alafenamide; INF β-1b, Interferon beta-1b; DTG, Dolutegravir.
Intolerable to Lopinavir/ritonavir
Disease severity was defined as mild (no imaging signs of pneumonia), moderate (imaging signs of pneumonia), severe (respiratory rate ≥ 30 times/min, oxygen saturation ≤ 93% at rest or PaO2/FiO2 ≤ 300 mmHg) and critically ill (shock, mechanical ventilation, intensive care unit care).
Patient A and B received lopinavir/ritonavir with ribavirin and remdesivir, respectively, for COVID-19. Patient D, diagnosed with HIV on this admission, was given lopinavir/ritonavir and interferon β-1b. Patient D had a co-infection with Klebsiella pneumoniae. All cases recovered uneventfully.
Age of 20 controls (12 male, 8 female) selected ranged from 22 to 61 (mean: 41.4).
3.2. Viral RNA and cytokine profiles
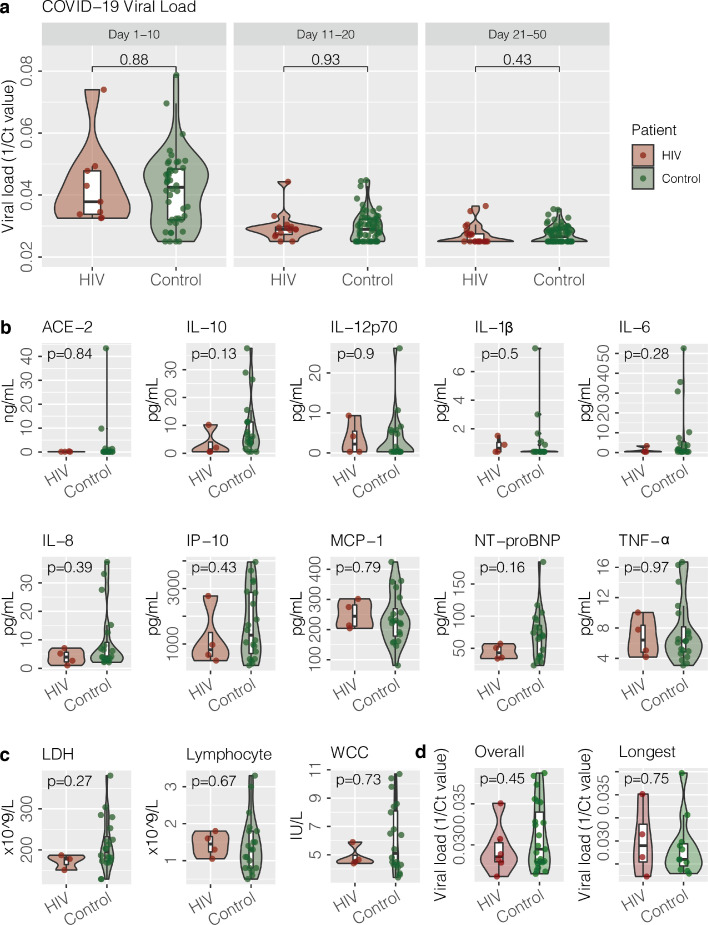
There were no significant differences in virus concentrations detected from upper respiratory samples from cases and controls during different periods after symptom onset (Day 1–10, Day 11–20, Day 21–50) ( Fig. 1A).
Fig. 1.
Upper respiratory viral load, cytokines, inflammatory markers and stool viral load of cases and controls. (A) Viral load in upper respiratory specimens from PLWH cases infected with SARS-CoV-2 and controls. One over cycle threshold (1/Ct) values of N gene of SARS-CoV-2 in early upper respiratory specimens, including nasopharygeal swab (NPS), combined nasopharygeal swab and throat swab (NPSTS) and combined nasopharygeal aspirate and throat swab (NPATS) during different periods from symptom onset (Day 1–10, Day 11–20, Day 21–50). (B). Cytokines in early serum samples after hospital admission from four PLWH patients infected with SARS-CoV-2 and controls. (C). Inflammatory markers (White blood cell, absolute lymphocyte count, lactate dehydrogenase) from four PLWH patients infected with SARS-CoV-2 and controls. (D) Overall stool viral load in stool of & viral load of stool with the longest time period between collection date and symptom onset date from PLWH cases infected with SARS-CoV-2 and controls.
Abbreviations: ACE-2, Angiotensin converting enzyme 2; IL-10, Interleukin 10; IL-12p70, Interleukin-12p70; IL-1β, interleukin-1β; IL-6, Interleukin-6; IL-8, Interleukin-8; IP-10, Interferon gamma-induced protein 10; MCP-1, monocyte chemoattractant protein-1; NP-proBNP, N-terminal prohormone of brain natriuretic peptide (NT-proBNP); TNF-α, tumor necrosis factor-α.
Cytokines and chemokines were performed in the earliest available serum samples after admission. There were no significant differences in proinflammatory cytokine (interleukin-1β, interleukin-6/IL-6 and tumor necrosis factor/TNF-α) and monocyte chemoattractant protein-1 (MCP-1) between cases and controls (Fig. 1B). However, interleukin-10 (IL-10) in PLWH appeared lower with median of IL-10 1.29 pg/mL (25th percentile to 75th percentile: 0.55–4.07 pg/mL). Median of IL-10 in controls was 5.59 pg/mL (25th percentile to 75th percentile: 3.45–11.25 pg/mL). (p value 0.13) Also, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) appeared lower in PLWH with median of NT-proBNP 43.00 pg/mL (25th percentile to 75th percentile: 35.09–52.17 pg/mL). Median of NT-proBNP in controls was 69.37 pg/mL (25th percentile to 75th percentile: 38.28–85.46 pg/mL) (p value 0.16)
All 4 PLWH cases showed seroconversion with anti-SARS-CoV-2 S antibodies detected between day 18–30 after symptom onset.
There were no significant differences in inflammatory markers between cases and controls (Fig. 1C).
Of note, all PLWH patients had SARS-CoV-2 detected from stool. Duration of faecal shedding of patient A, B, C and D were at least 9, 10, 33, and 11 days, respectively. One did not have diarrhea. Patient C's stool PCR remained positive despite symptoms subsided for 3 days. There were no significant differences in stool virus concentrations from cases and controls. When stool with longest viral shedding duration were compared in 4 HIV cases and 11 controls, there were no significant differences between the 2 groups (Fig. 1D).
4. Discussion
Our data showed that PLWH behaves similarly with controls in respiratory viral load, and PLWH had lower IL-10 and NT-proBNP.
Faecal viral shedding in PLWH ranged from 9 to 33 days. A meta-analysis showed a significant portion of COVID-19 patients had positive stool PCR [4]. Our findings suggest potential increased risk of faecal transmission from PLWH.
Our data showed there was a decrease in IL-10 and NT-proBNP in PLWH. The dramatic elevation of IL-10 in critically ill patients is a unique characteristic of COVID-19 cytokine storm. Early induction of IL-10 might represent a negative feedback mechanism to inflammation. However, the increase of endogenous IL-10 might act as an immune activating agent [5]. Compared with non-ICU patients, ICU patients had higher IL-10 [6]. In HIV, IL-10 regulation is impaired [7]. Our data has suggested PLWH with SARS-CoV-2 may have lower risk of immunostimulatory effect due to lower IL-10. Also, elevated NT-pro BNP is associated with increased mortality [8]. The implications of decreased IL-10 and NT-pro BNP in PLWH with COVID-19 warrant further investigation.
In a cohort of PLWH with COVID 19 coinfection, one patient's IgM and IgG were negative from day 9 to day 87 after symptom onset [9]. In contrast, all PLWH in our study had seroconversion by day 30 after symptom onset.
Our study has several limitations. First, our co-infected cases had relatively good CD4 counts. Our data may not necessarily reflect those HIV cases with lower CD4 counts or AIDS-defining illnesses. Second, lopinavir/ritonavir, ribavirin, remdesivir and steroid were given to controls, which could reduce IL-6 and TNF-α [10]. The fact that more controls were given lopinavir/ritonavir and ribavirin may underestimate the difference of proinflammatory cytokines between 2 groups. Thirdly, anti-retroviral therapy (ART) may decrease IL-10 in HIV subjects [11]. Also, higher NT-Pro-BNP were observed in treatment-naïve patients with low CD4 count and high HIV viral load in a study [12], and this association is reversible by ART. Our observation of lower IL-10 and NT-proBNP in co-infected subjects could be due to HIV infection, ART or co-infection, and results require careful interpretation. Lastly, difference of IL-10 and NT-proBNP in cases and controls did not reach statistical significance, and it may be due to the small sample size.
In this study, cases and controls were recruited during early stage of pandemic. Since currently circulating viruses are variants, results should be interpreted with regard to this.
5. Conclusion
As a condition at increased risk of severe illness from SARS-CoV-2, HIV is an important disease to consider in co-infected SARS-CoV-2 patients. PLWH behaves similarly with controls in respiratory viral load, and PLWH had lower IL-10 and NT-proBNP. Our study shall serve as a basis for further research on virus dynamics, cytokine response and immunity of HIV SARS-CoV-2 co-infection, and how they affects COVID-19 related outcomes.
CRediT authorship contribution statement
Rita Wai-Yin Ng: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. Chun-Kwok Wong: Data curation, Writing – review & editing. Grace Chung-Yan Lui: Data curation, Writing – review & editing. Eugene Yuk-Keung Tso: Data curation, Writing – review & editing. Zigui Chen: Data curation, Writing – review & editing, Visualization. Owen Tak-Yin Tsang: Data curation, Writing – review & editing. Siaw Shi Boon: Data curation, Writing – review & editing. Christopher Koon-Chi Lai: Data curation, Writing – review & editing. Kitty Sau-Chun Fung: Data curation, Writing – review & editing. Apple Chung-Man Yeung: Data curation, Writing – review & editing. Wendy Ching-Sze Ho: Data curation, Writing – review & editing. David Shu-Cheong Hui: Data curation, Writing – review & editing. Paul Kay-Sheung Chan: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Methodology, Supervision, Writing – review & editing. Jacky Man-Chun Chan: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
None.
Funding
This work was supported by the Health and Medical Research Fund - Commissioned Research on the Novel Coronavirus Disease (COVID-19) (reference no. COVID190107) from the Food and Health Bureau, Hong Kong SAR Government.
Ethical approval
The study was approved by Joint Chinese University of Hong Kong New Territories East Cluster Clinical Research Ethics Committee.
References
- 1.Centers for Disease Control and Prevention. People with certain medical conditions. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Accessed 11 June 2021
- 2.Sigel K., Swartz T., Golden E., Paranjpe I., Somani S., Richter F. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin. Infect. Dis. 2020;10029:1–6. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lui G., Ling L., Lai C.K.C., Tso E.Y.K., Fung K.S.C., Chan V., et al. Viral dynamics of SARS-CoV-2 across a spectrum of disease severity in COVID-19. J. Infect. 2020;81(2):324–327. doi: 10.1016/j.jinf.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong M.C., Huang J., Lai C., Ng R., Chan F.K., Chan P.K. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Infect. 2020 doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L., Zhang H., Dauphars D.J., He Y.-W. A potential role of interleukin-10 in COVID-19 pathogenesis. Trends Immunol. 2020 doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223) doi: 10.1016/s0140-6736(20)30252-x. (vol 395, pg 497, 2020) 496-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackburn S.D., Wherry E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15(4):143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Pranata R., Huang I., Lukito A.A., Raharjo S.B. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S., Saito M., Nagai E., Toriuchi K., Nagai H., Yotsuyanagi H., et al. Antibody response to SARS-CoV-2 in people living with HIV. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimfarth L., Serafini M.R., Martins-Filho P.R.S., Quintans J.S.S., Júnior L.J.Q. Drug repurposing and cytokine management in response to COVID-19: a review. Int. Immunopharmacol. 2020 doi: 10.1016/j.intimp.2020.106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osuji F.N., Onyenekwe C.C., Akaneku J.E, Ukibe N.R. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. J. Biomed. Sci. 2018 doi: 10.1186/s12929-018-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster C., Binder C., Strassl R., Aichelburg M.C., Blackwell E., Pavo N., et al. Impact of HIV infection and antiretroviral treatment on N-terminal prohormone of brain natriuretic peptide as surrogate of myocardial function. AIDS. 2017 doi: 10.1097/QAD.0000000000001350. [DOI] [PubMed] [Google Scholar]