Abstract
Purpose
Many regulators offered new ways of working to help combat the COVID-19 pandemic, and the rolling review procedure is an important and successful example. In rolling reviews, data are submitted and reviewed as they become available before the full data package is available. This approach is resource intensive but faster than standard review processes and therefore of benefit to society and patients during a health emergency. In this study, we analyze the European Medicines Agency (EMA) rolling review process and extract learning, based on the vaccines and treatments that have been approved to date (November 2021), and formulate 3 suggestions for the future.
Methods
Data and information on rolling reviews and similar related processes were collected from health authority websites across the globe with a focus on the EMA. Literature searches in PubMed and checking company websites for additional information were conducted to complement and corroborate findings as required.
Findings
The duration of a rolling review cycle and the number of cycles before a conditional marketing authorization differ among different applications. Through the rolling review process, COVID-19 vaccines could be approved in record times, ranging from 17 to 36 days. The rolling review process is not limited to vaccines but is applied to promising treatments as well.
Implications
This study indicates that rolling reviews can be successfully conducted during a health emergency, such as the COVID-19 pandemic, to meet an unmet medical need. Other critical conditions or life-threatening diseases with unmet needs exist and may be suitable to be addressed by a rolling review process to accelerate patient access to life-changing treatments. Indeed, we call for an evaluation of the rolling review process, its use, and its efficiency to capture learning with the aim of building a new, lean, and effective expedited review procedure that could be institutionalized and added to the regulatory toolbox.
Key words: COVID-19, dynamic regulatory assessment, expedited approval pathways, pandemic, rolling review, vaccines
Introduction
In 2020 the world was shocked by a pandemic. Unprecedented public measures were introduced, countries went into lockdown, and hospital emergency wards became overloaded with critically ill patients. The pandemic originated in Wuhan, China, which went into lockdown after the spreading of an unknown virus was confirmed and the World Health Organization (WHO) warned.1 Although the Western world was convinced that they were prepared for the virus, it turned out that they were not. COVID-19 started to spread across the globe, and the fight against the virus started. Strategies were put in place and ranged from stopping the transmission and preventing it from spreading to studying repurposed drugs in clinical trials to developing de novo medicines and vaccines.
According to the WHO, a pandemic is declared when a new disease for which people do not have immunity spreads across the globe beyond expectations,1 and in March 2020, the WHO declared the COVID-19 outbreak as a pandemic.2 This declaration prompted health authorities and regulators around the globe to introduce extraordinary measures. A commitment to quickly address the public health emergency led to a (complete) rethinking of the medicine and vaccine review processes and precipitated unprecedented regulatory ways of working, including included fast-track regulatory procedures, measures to ensure uninterrupted supply of medicines, and the accelerated adoption of electronic collaborative platforms, to name but a few examples.3
Across the globe, regulatory agencies adopted agile and flexible ways of working, resulting in new or adjusted policies and agency collaborations.4 Although most of the procedures already existed in different jurisdictions, the pharmaceutical sector had never before experienced the way these procedures were applied and adapted as during the pandemic. 3 One of the regulatory agilities offered during the pandemic was the rolling review procedure. In rolling reviews, data are submitted and reviewed as they become available before the full data package is available. This approach requires a closer collaboration and more intense interaction between the sponsor and the health authority. Although different regulatory jurisdictions approach and define rolling reviews differently, these reviews are a variation of a common theme and have become a reality for a number health agencies.3 , 5 The concept, however, is not new but has been offered by the US Food and Drug Administration (FDA) to fast-track designated products since 1988 and was used for vaccine reviews during the 2009 H1N1 pandemic by the European Medicines Agency (EMA).6 , 7
Indeed, the EMA developed a health threat management plan with 4 levels of emergency stages in the wake of the H1N1 pandemic, which defines the way of working in case of an emerging health threat.8 The plan allows the agency to respond rapidly and efficiently to challenges caused by an outbreak even before a pandemic is officially declared by the WHO. Once a pandemic is declared, the EMA can implement the highest level of the plan in response to the health crisis.
In the health threat management plan, the rolling review procedure is a regulatory tools that can be deployed to accelerate the assessment of a promising vaccine or treatment. Normally, the data on a medicine's effectiveness, tolerability, and quality must be collected and then submitted as a complete dossier before the review for approval of a marketing application can start. In the case of a rolling review, EMA's Committee for Medicinal Products for Human Use (CHMP) reviews data as they become available, before the full dossier is submitted. Once the CHMP decides that sufficient data are available, the complete dossier can be submitted by the company. By reviewing the data as they become available during the development process, the CHMP can reach its final opinion sooner on whether the application could be authorized or not.9
In this study, we investigated the EMA rolling review process and analyzed the European Public Assessment Reports (EPARs) of the different vaccines that have been approved to date (November 2021). This approach allows us to deduce learning and propose ways on how to push the regulatory science envelope forward.
Methods
General data and information on rolling reviews and similar or related processes were collected from the official websites of the FDA,10 the UK Medicines and Healthcare products Regulatory Agency (MHRA),11 Swissmedic in Switzerland,12 the EMA, 13,14 Health Canada,15 the Health Science Authority (HSA) in Singapore, 16,17 the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, the Chinese National Medical Products Administration (NMPA), the WHO, and the Brazilian Agencia Nacional de Vigilancia Sanitaria (ANVISA). In addition, specific data on the approval of COVID-19 vaccines were collected from official documents published by the EMA after approval, in particular the corresponding EPARs. Internal subject matter experts were consulted for input and experience. Searches in PubMed and checking company websites for additional information were conducted to complement and corroborate findings as required.
Results
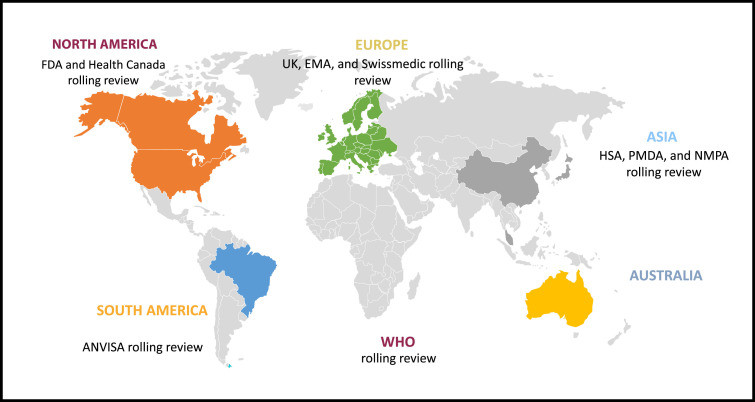
Figure 1 gives an overview of the different agencies around the globe that we could establish already used or introduced the rolling review concept as a result of the pandemic. Definitions and processes may differ among different regulatory jurisdictions, but they all have the same underlaying objectives; they aim to address an emergency health threat and meet an unmet medical need, offer procedural and timeline flexibilities, and stimulate the swift development of new treatments and vaccines. Agencies that have introduced rolling reviews are the FDA,10 MHRA,11 Swissmedic,12 EMA,13 , 14 Health Canada,15 the HSA,16 , 17 PMDA, NMPA, WHO, and ANVISA (Figure 1).
Figure 1.
Pictogram showing the health authorities around the globe that introduced or offered a rolling review process to applicants for the evaluation of COVID-19 treatments and vaccines. ANVISA = Brazilian Agencia Nacional de Vigilancia Sanitaria; EMA = European Medicines Agency; FDA = US Food and Drug Administration; HAS = Health Science Authority; MHRA = UK Medicines and Healthcare Products Regulatory Agency; NMPA = Chinese National Medical Products Administration; PMDA = Pharmaceuticals and Medical Devices Agency; WHO = World Health Organization.
The EMA has communicated how the rolling review process differs from accelerated assessment—a procedure that has been used for many years.18 They stated that “[the rolling review] allows EMA to begin assessing data as they become available during the development process, to expedite the subsequent formal marketing authorization application assessment even further.”18
According to the EU pharmaceutical legislation, the standard timeline for the evaluation of a medicine is a maximum of 210 active days. However, for COVID-19 treatments, the EMA handled marketing authorization applications in an expedited manner, reducing review timelines to <150 working days.
A rolling review process consists of 1 or more review cycles during which each cycle is preagreed on between EMA and the applicant and questions from previous cycles must be addressed before the next cycle can begin.19 In each of the rolling review cycles, the EMA assesses the data and invites the applicant to submit the dossier for a conditional marketing authorization (CMA) once they judge that the data package qualifies for a (final) review.
Continuous dialogue and iterative reviews are integral elements of the rolling review process that require significant mobilization of resources at both agency and sponsor levels. We found that early and continuous dialogue among health authorities, scientific experts, and vaccine developers increased during the COVID-19 pandemic.3 , 18 Together with the strict timelines, this dialogue affected resources on both sides. Working around the clock to assess the data, responding to outstanding questions, and meeting pre–agreed-on timelines led to additional workload for all stakeholders involved in the process.20
On the basis of an analysis of the EPARs of COVID-19 vaccines from Pfizer,21 AstraZeneca,22 Moderna,23 and Janssen Pharmaceuticals,24 we were able to deduce the rolling review cycle timelines and identify what data were submitted and assessed during the different review cycles. The EMA has evaluated a total of 9 vaccines and 5 treatments to combat COVID-19 using the rolling review process. Of the 9 vaccines, 5 applications have resulted in a submission for a CMA of which 4 have been approved to date (November 2021).
Even though rolling reviews are essential to accelerate the assessment, the data available at the beginning remain limited. To ensure a successful rolling review cycle, mature data packages should be submitted with each cycle. Inherent to a CMA, additional data can be submitted after the marketing authorization to further corroborate the tolerability, efficacy, or quality of the product.25
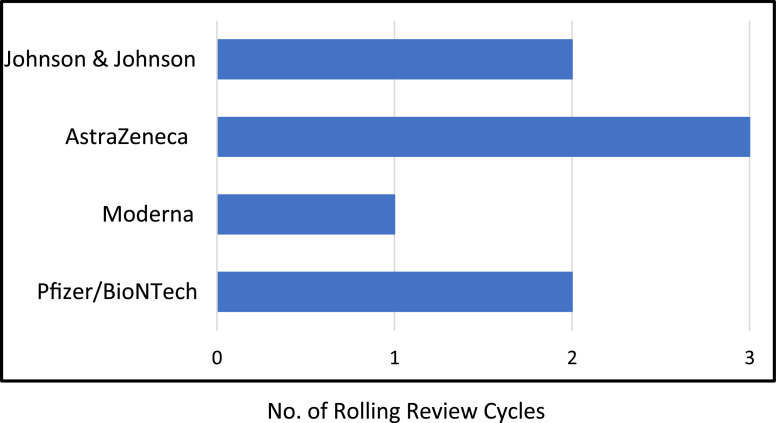
The number of review cycles required by the COVID-19 vaccines approved so far are shown in Figure 2 and vary from 1 to 3. For the vaccines that still are under the rolling review or have submitted a CMA application, the number of cycles are unknown, except for CureVac, which announced its withdrawal on October 12 shortly after the start of the sixth rolling review cycle.26
Figure 2.
The number of rolling review cycles used for the vaccines that have been approved by the European Medicines Agency to date (November 2021).
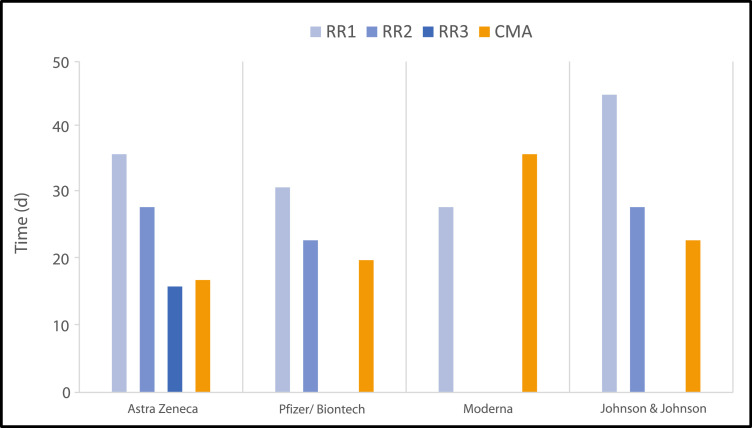
Not only do the number of cycles vary among the different vaccines but so do the length and duration of each individual cycle (Figure 3 ). Cycle timelines vary from 16 to 45 days. Ensuing CMA timelines hover around the 20-day mark, with 1 outlier at 36 days.
Figure 3.
Number of cycles, days per rolling review (RR) cycle, and timelines of the conditional marketing authorization (CMA) review for the vaccines that have been approved by the European Medicines Agency to date (November 2021).
Review cycles also differed in terms of the data submitted (Table 1 ). There is no clear pattern, but nonclinical data seem to be submitted earlier, whereas clinical and quality data are shared later, possibly reflecting inherent development processes of when the data type is generated. The number of extraordinary meetings range from 1 to 5, with Pfizer having the most, which most likely is related to the Pfizer vaccine being the first to go through the rolling review process. Extraordinary meetings are used outside the planned schedule to review emergency measures, prepare the agency opinion, discuss the evaluation of an application, or issue a recommendation. The Pharmacovigilance Risk Assessment Committee (PRAC) or CHMP call for such meetings when deemed necessary.27, 28, 29
Table 1.
Summary of the nature of the data package submitted and the number of extraordinary meetings (before the conditional marketing authorization was granted) for the different COVID-19 vaccines approved by the European Medicines Agency to date (November 2021).
| Sponsor | RR Cycle 1 (Submission Date and Data Package Content) | RR Cycle 2 (Submission Date and Data Package Content) | RR Cycle 3 (Submission Date and Data Package Content) | CMA | No. of Extraordinary Meetings |
|---|---|---|---|---|---|
| AstraZeneca | 10/1/2020 Nonclinical |
12/12/2020 Nonclinical Quality |
12/242020 | 1/122021 | 2 |
| Pfizer | 10/6/2020 Nonclinical |
11/7/2020 Quality |
12/12020 | 5 | |
| Moderna | 11/16/2020 Nonclinical |
12/1/2020 | 2 | ||
| Janssen | 12/1/2020 Nonclinical Clinical |
1/25/2021 Nonclinical |
2/16/2021 | 1 |
CMA = conditional marketing authorization; RR = rolling review.
Next to the approved vaccines with a CMA, 5 more vaccines to date (November 2021) have entered the rolling review process. Submission dates for these vaccines are between February and July 2021, with 1 withdrawal in the beginning of October 202130 and 1 submission for a CMA in November 2021.31 The first monoclonal antibodies evaluated through a rolling review process were approved with a CMA in November 2021, but there is no information on the number of cycles that were used.32 One more COVID-19 treatment started the rolling review process in October, and 4 other treatments have been submitted for CMA after completing the rolling review cycles with start dates between February and May 2021.33
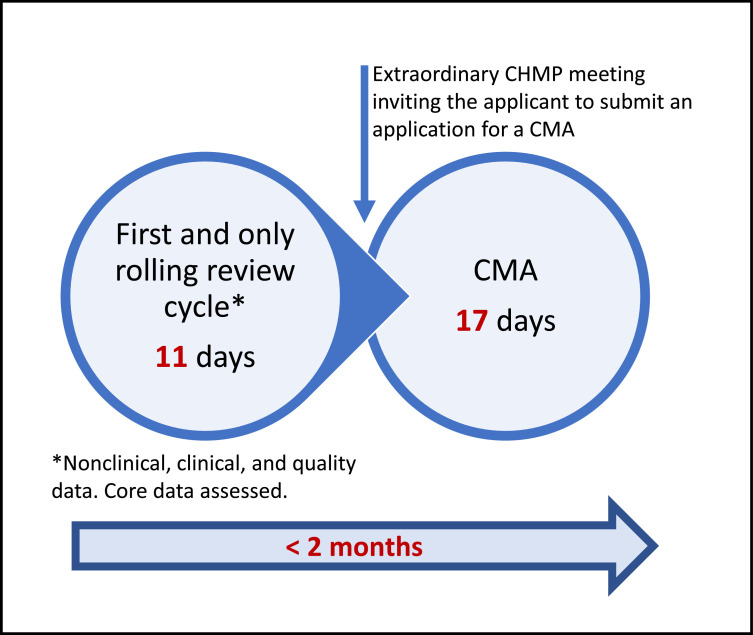
Next to de novo applications, rolling reviews can also be used for lifecycle management activities. Rolling reviews have been used for safety updates and for the line extension of remdesivir.34 , 35 The processing of the line extension took <2 months and only 1 rolling review cycle (Figure 4 ).
Figure 4.
Infographic on the rolling review steps and associated timelines of the nonvaccine treatment remdesivir line extension. CHMP = Committee for Medicinal Products for Human Use.
In October 2021, Eli Lilly informed the EMA about their decision to no longer pursue a CMA with their potential COVID-19 treatment, although it had been under rolling review since March. The withdrawal was based on the requirement by the CHMP for additional validation data, which could only be generated by producing new batches of active substance, but this was not in line with the company's manufacturing plans.36
From the start and throughout the pandemic, the EMA has communicated extensively by issuing guidelines and hosting press briefings and public stakeholder meetings on the approvals, safety monitoring, and effect of COVID-19 vaccines. This proactive and transparent way of communicating was also observed in the publication of the vaccine EPARs, which were published within 7 days of the approval of the COVID-19 vaccines instead of the normal 20 days. Other standard publication timelines were also significantly shortened, including timelines for scientific advice, compassionate use opinion, product information, the risk management plan, the monthly safety updates for vaccines, and the assessment of safety signals among others.
Discussion
According to a joint Heads of Medicines Agency (HMA) and EMA statement on the approval of vaccines, “[M]ost COVID-19 vaccines fall under the scope of the centralised procedure since they are produced by biotechnological processes for which the centralised procedure is mandatory. For other types of vaccines currently under development, such as those composed of whole-inactivated virus or live attenuated virus, the EMA and the Heads of Medicines Agencies (HMA) network encourages marketing authorisation holders to also submit their applications through the centralised procedure to ensure that those vaccines reach all Member States at the same time, with no unfair access in the Union.”37 We could not establish any evidence of vaccines being filed under any other EU procedure, such as the mutual or decentralized procedures or any national procedure.
As part of the health threat management plan, the EMA COVID-19 Pandemic Task Force (ETF) was established. The purpose of the taskforce is to help EU member states and the European Commission (EC) to take accelerated and coordinated regulatory actions on the development, authorization, and safety monitoring of treatments and vaccines for COVID-19 by reviewing available scientific data of potential medicines and to identify promising candidates.38 , 39 The ETF is an expert group charged with assisting the CHMP, PRAC, and Paediatric Committee of the EMA and to take part in early scientific discussions and reviews of development plans, identify promising candidates, approve the start of the rolling review process if scientific data are sufficient, and interact with stakeholders and other regulators in and outside the EU as required. The ETF is accountable to the CHMP for all its activities.40
The possibility for a rolling review with its cycles during the development of a treatment or vaccine is a prominent and important example of an exceptional regulatory agility offered in the EU during the pandemic. Compared with the standard evaluation process, in which the applicant does not submit the full dossier before all the required data are gathered, the rolling review allows the applicant to submit different data packages as soon as they become available. Depending on the nature of the data packages submitted, the number of rolling review cycles as well as the duration of each cycle can differ. To ensure the efficient use of each rolling review cycle, mature quality assured data packages should be submitted, and any outstanding questions from previous cycles can be fully addressed. Once the EMA finds that the totality of data are sufficient, the applicant can submit a formal CMA application that the agency can assess. Altogether, this leads to a significantly reduction of timelines leading up to and including the final CMA approval. After this process, the EMA reaches its conclusion of the assessment and issues their recommendation. Then the final decision on the marketing authorization is legalized and officially communicated by the EC. The vaccines we report on in this study were officially legalized by the EC within a day of the EMA recommendation, which contrasts with the 30 to 60 days it normally takes.41 , 42
These important agilities effectively respond to the shortened development timelines that have been found with the COVID-19 vaccines. An analysis of new drugs developed since 2000 found that the mean development timeline from the start of clinical testing in Phase I to approval of the final product is almost 10 years, contrasting with the development timelines of COVID-19 vaccines, which all were <1 year.43 Some clinical development phases were started before the prior phases had been fully completed. For instance, a Phase II clinical development was started based on an interim readout from a Phase I clinical trial by 1 sponsor.43 Such strategies may be justified in extraordinary circumstances, such as a pandemic of the COVID-19 magnitude, but we believe that they are unlikely to become a benchmark modus operandi in everyday drug development programs. Notwithstanding this, innovative measured risk taking could be considered also for other areas of medical need, such as for oncology indications or for rare diseases for which there are no treatments available.
Regulators took extraordinary steps on several fronts during the public health emergency, including increasing the frequency and intensity of sponsor engagement. Although this level of engagement would likely be difficult to sustain and replicate for every product being developed, the concept of regulatory collaboration has been applied by many agencies to facilitate alternate pathways in drug and medical device development in the past decade. Examples include the FDA's breakthrough therapy designation, fast-track designation program, and the Real-Time Oncology Review pilot.44, 45, 46 Similarly, the EMA created its accelerated assessment timetable and guidelines,47 and Japan's PMDA developed the Sakigake program.48 The expedited medical product development that has occurred during the COVID-19 pandemic may provide fresh inspiration for the next generation of such programs.
Continuous and early dialogue is an integral component of the rolling review process and constitutes a key element of any expedited review program. Because of the bolstered dialogue, the understanding of the benefits, risks, and possible adverse reactions increases in concert on both sides. This dialogue allowed the agency (EMA) to make swift, timely, and well-informed decisions. The EMA also accelerated the procedure for the mandatory submission of the Paediatric Investigation Plan (PIP) for all COVID-19–related marketing authorization applications. The rapid agreement and decision on the PIPs became as important as the rolling reviews themselves.49
Rolling review and accelerated assessment were also used in the postapproval setting (eg, for the extension of COVID-19 vaccine indications). For example, the EMA said, “CHMP will carry out an accelerated assessment of data submitted by the company that markets Comirnaty in order to decide whether to recommend the extension of indication.”50 This decision resulted in an approval in 25 days, similar to the approval of the new indication of the Moderna vaccine (Spikevax) in young people 12 to 17 years of age.51 , 52
According to the EC,53 2 aspects are addressed by the rolling review in a postapproval setting: the accelerated regulatory process and the ramping up of the production of vaccines. First, the regulatory procedure is adjusted to accelerate the approval of COVID-19 vaccines to the new variants, as is currently done with human influenza vaccines.54 This process enables the swift approval of a modified vaccine with a smaller set of additional data submitted to the EMA on a rolling basis. Second, early involvement of the regulatory authority in the certification process of the new production line is also essential. The early and rapid development of the necessary process control, validation, and stability data by companies is key to enable the review by the EMA on a rolling basis and rapid authorization of new production facilities and lines.53
The EMA published a report on the use of the rolling review after the H1N1 2009 influenza pandemic.55 One recommendation put forward was to review the rolling review process from a logistics and resource perspective. Indeed, we believe that learning from the COVID-19 rolling review process should be discussed by the vested parties in the pharmaceutical sector (regulators and industry) with the objective to agree on an optimized process that is less resource intensive, pragmatic, and robust but still nimble and fast while upholding normal scientific and regulatory standards. This goal is aligned with recommendations put forward in the WHO and International Coalition of Medicines Regulatory Authorities report on regulatory agilities and flexibilities in which the organizations call for an increased implementation of rolling submission procedures.4
A foundation for such a dialogue has been put in place by a recent publication on dynamic regulatory assessment (DRA)—a concept that seeks to reimagine the regulatory review interactions across a product life cycle that calls for an iterative regulatory dialogue, data submission, and evidence assessment, enabled by contemporary information technology and bringing significant efficiencies to the pharmaceutical sector for all product types.56 As discussed in the DRA publication, data for regulatory decision making would be uploaded to a common (cloud-based) platform as they becomes available as opposed to being submitted as a complete and validated dossier, which is similar to what was observed in the situation of rolling reviews. Access (uploading and review) to the platform(s) can easily be granted to sponsors and regulators as adequate and on a need basis. Such a cloud-based platform lends itself and is conducive to international collaboration across multiple regulatory jurisdictions—a feature that we believe would be welcome, especially when global collaboration is required to mitigate the effects of a pandemic.
The mobilization of extraordinary resources to assess data packages in a limited time window, such as during the COVID-19 pandemic, is acknowledged and understood by all parties concerned. However, digitalization, operational excellence, and innovation could seamlessly improve the rolling review procedure, taking into account and preserving the beneficial aspects of it. This process would not only serve the pharma sector but be of benefit to society in general and patients in particular.
Conclusion
The pandemic is by no means over yet, but the pharmaceutical sector is starting to debate which regulatory agilities added the most value and extract lessons learned.57 , 58 Pharmaceutical companies, health authorities, and other vested stakeholders have an obligation to leverage this learning and produce transformations that will bring life-changing therapies much faster to patients. Learning from this pandemic should not only be carried over to the next pandemic but should, where appropriate, already be applied for other life-threatening diseases and conditions with unmet medical needs.
We suggest 3 areas that the pharmaceutical sector in the EU jointly should discuss and carry forward regarding rolling reviews. We propose that rolling reviews should become (1) institutionalized and be available as a regulatory tool beyond health emergencies, (2) open for therapies beyond pandemic viruses, and (3) be process optimized to become less resource intensive and hence manageable under normal conditions. The health care system has demonstrated how fast it can respond to an unmedical need. Should this not become a benchmark beyond the COVID-19 pandemic?
Disclosure
All authors are Sanofi employees and may hold shares and/or stock options in the company. All authors hold positions within the pharmaceutical industry, but they have not received any grant, honoraria, or other compensation to author this paper. The study was conceived of, executed on, and written during the authors’ daily job. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
Acknowledgments
All authors designed the study, analyzed the data, and wrote the manuscript.
Disclaimer
The views expressed in this article are the independent views of the authors and should not be understood or quoted as being made on behalf of or reflecting of the position of their company or any other affiliation.
References
- 1.WHO, Timeline: WHO's COVID-19 response https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline#event-0 Last accessed 29 October 2021
- 2.Domenico Cucinotta, Maurizio Vanelli, WHO Declares COVID-19 a Pandemic, https://pubmed.ncbi.nlm.nih.gov/32191675/ Last accessed 29 October 2021 [DOI] [PMC free article] [PubMed]
- 3.Winona Rei Bolislis Maria Lucia de Lucia, Felipe Dolz Runyi Mo Makoto Nagaoka Heraclio Rodriguez May Li Woon Wei Yu Thomas C. Kühler, Regulatory Agilities in the Time of COVID-19: Overview, Trends, and Opportunities https://www.sciencedirect.com/science/article/pii/S0149291820305257 Last accessed 29 October 2021 [DOI] [PMC free article] [PubMed]
- 4.WHO and ICMRA joint Report on the review of regulatory flexibilities/agilities as implemented by National Regulatory Authorities during Covid-19 pandemic –December 2020 https://www.icmra.info/drupal/sites/default/files/2021-12/Regulatory_Flexibilities_during_COVID-19_Report.pdf Last accessed 2 December 2021
- 5.Bruno Speder:“ Rolling Reviews – a useful tool to speed up the regulatory review process” https://hvivo.com/wp-content/uploads/2021/04/Speder-Bruno-Rolling-Reviews-Feature-May-2021.pdf Last accessed 26 November 2021
- 6.FDA, Fast Track, https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track Last accessed 29 October 2021
- 7.EMA, Pandemic report and lessons learned Outcome of the European Medicines Agency's activities during the 2009 (H1N1) flu pandemic, https://www.ema.europa.eu/en/documents/report/pandemic-report-lessons-learned-outcome-european-medicines-agencys-activities-during-2009-h1n1-flu_en.pdf Last accessed 29 October 2021
- 8.EMA, EMA plan for emerging health threats, https://www.ema.europa.eu/en/documents/other/ema-plan-emerging-health-threats_en.pdf Last accessed 29 October 2021
- 9.EMA, EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines, https://www.ema.europa.eu/en/documents/other/ema-initiatives-acceleration-development-support-evaluation-procedures-covid-19-treatments-vaccines_en.pdf Last accessed 29 October 2021
- 10.FDA, Emergency Use Authorization for Vaccines to Prevent COVID-19 Guidance for Industry, https://www.fda.gov/media/142749/download Last accessed 29 October 2021
- 11.UK MHRA, Guidance Rolling review for marketing authorisation applications, https://www.gov.uk/guidance/rolling-review-for-marketing-authorisation-applications Last accessed 29 October 2021
- 12.Swissmedic, Guidance document Authorisation procedures for Covid-19 medicinal products during a pandemic HMV4, https://www.swissmedic.ch/dam/swissmedic/en/dokumente/zulassung/zl_hmv_iv/zl000_00_044d_wl_zulassungsverfahren_covid-19.pdf.download.pdf/ZL000_00_044d_WL_Zulassungsverfahren_f%C3%BCr_Covid_19_Arzneimittel_im_Pandemiefall.pdf Last accessed 26 October 2021
- 13.EMA, EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines, https://www.ema.europa.eu/en/documents/other/ema-initiatives-acceleration-development-support-evaluation-procedures-covid-19-treatments-vaccines_en.pdf Last accessed 26 October 2021
- 14.EMA, EMA considerations on COVID-19 vaccine approval, https://www.ema.europa.eu/en/documents/other/ema-considerations-covid-19-vaccine-approval_en.pdf Last accessed 26 October 2021
- 15.Health Canada, Guidance for market authorization requirements for COVID-19 vaccines: Rolling submissions, non-clinical and clinical requirements, https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/guidance-market-authorization-vaccines/rolling-submissions-non-clinical-requirements.html Last accessed 26 October 2021
- 16.HSA, Pandemic Special Access Route Facilitates Singapore's access to critical novel vaccines, medicines and medical devices, https://www.hsa.gov.sg/hsa-psar Last accessed 26 October 2021
- 17.HSA, guidance note on psar for supply of emergency therapeutic products february 2021, https://www.hsa.gov.sg/docs/default-source/hprg-tpb/guidances/annex-a-to-guidance-note-on-psar-for-emergency-tp_feb2021_final.pdf Last accessed 26 October 2021
- 18.EMA, COVID-19 vaccines: development, evaluation, approval and monitoring, https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring Last accessed 26 October 2021
- 19.EMA, COVID-19 guidance: evaluation and marketing authorization https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-developers-companies/covid-19-guidance-evaluation-marketing-authorisation Last accessed 25 November 2021
- 20.Pharmaintelligence, EMA's Emer Cooke on the Future of Rolling Reviews & the End of Remote Meetings, https://pink.pharmaintelligence.informa.com/PS144983/EMAs-Emer-Cooke-On-The-Future-Of-Rolling-Reviews–The-End-Of-Remote-Meetings Last accessed 30 November 2021
- 21.European Medicine Agency, Comirnaty European Public assessment report, https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty Last accessed 26 October 2021
- 22.European Medicine Agency, Vaxzevria European Public Assessment report, https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca Last accessed 26 October 2021
- 23.European medicine agency, Spikevax European Public assessment report, https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax-previously-covid-19-vaccine-moderna Last accessed 26 October 2021
- 24.European Medicine Agency, Janssen covid-19 vaccine european public assessment report, https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-janssen-epar-public-assessment-report_en.pdf Last accessed 26 October 2021
- 25.EMA, Questions and Answers: Conditional Marketing Authorisation of COVID-19 Vaccines in the EU l What is the EU doing to accelerate the authorisation process for the COVID-19 vaccines? https://ec.europa.eu/commission/presscorner/detail/en/qanda_20_2390 Last accessed 26 October 2021
- 26.EMA, “Announcement of withdrawal of CureVac, https://www.ema.europa.eu/en/news/ema-ends-rolling-review-cvncov-covid-19-vaccine-following-withdrawal-curevac-ag Last accessed 30 November 2021
- 27.EMA, “Extraordinary meeting of the Pharmacovigilance Risk Assessment Committee (PRAC): 20 April 2021” https://www.ema.europa.eu/en/events/extraordinary-meeting-pharmacovigilance-risk-assessment-committee-prac-20-april-2021 Last accessed 30 November 2021
- 28.EMA, “Extraordinary meeting of the Committee for Medicinal Products for Human Use (CHMP): 29 December 2020” https://www.ema.europa.eu/en/events/extraordinary-meeting-committee-medicinal-products-human-use-chmp-29-december-2020 Last accessed 30 November 2021
- 29.EMA, “Extraordinary meeting of the Committee for Medicinal Products for Human Use (CHMP): 25 November 2021” https://www.ema.europa.eu/en/events/extraordinary-meeting-committee-medicinal-products-human-use-chmp-25-november-2021 Last accessed 30 November 2021
- 30.EMA, “EMA ends rolling review of CVnCoV COVID-19 vaccine following withdrawal by CureVac AG” https://www.ema.europa.eu/en/news/ema-ends-rolling-review-cvncov-covid-19-vaccine-following-withdrawal-curevac-ag Last accessed 26 November 2021
- 31.EMA, “EMA receives application for conditional marketing authorisation of Novavax's COVID-19 vaccine, Nuvaxovid” https://www.ema.europa.eu/en/news/ema-receives-application-conditional-marketing-authorisation-novavaxs-covid-19-vaccine-nuvaxovid Last accessed 26 November 2021
- 32.EMA, “COVID-19: EMA recommends authorisation of two monoclonal antibody medicines”, https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-two-monoclonal-antibody-medicines Last accessed 26 November 2021
- 33.EMA, “COVID treatments” https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments Last accessed 26 November 2021
- 34.EMA, “Assessment report, Veklury” https://www.ema.europa.eu/en/documents/assessment-report/veklury-epar-public-assessment-report_en.pdf Last accessed 2 November 2021
- 35.FDA, “Frequently Asked Questions for Velklury (remdesivir)”https://www.fda.gov/media/137574/download. Last accessed 2 November 2021
- 36.EMA, “EMA ends rolling review of the antibodies bamlanivimab and etesevimab for COVID-19 following withdrawal by Lilly” https://www.ema.europa.eu/en/news/ema-ends-rolling-review-antibodies-bamlanivimab-etesevimab-covid-19-following-withdrawal-lilly. Last accessed 22 December 2021
- 37.EMA, HMA/EMA statement on approval of vaccines, https://www.ema.europa.eu/en/news/hmaema-statement-approval-vaccines Last accessed 26 October 2021
- 38.EMA, Mandate, objectives and rules of procedure of the COVID-19 EMA pandemic Task Force (COVID-ETF), https://www.ema.europa.eu/en/documents/other/mandate-objectives-rules-procedure-covid-19-ema-pandemic-task-force-covid-etf_en.pdf Last accessed 26 October 2021
- 39.EMA, COVID-19 EMA pandemic Task Force, https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/emas-governance-during-covid-19-pandemic#covid-19-ema-pandemic-task-force-section Last accessed 26 October 2021
- 40.EMA, “Mandate, objectives and rules of procedure of the COVID-19 EMA pandemic Task Force (COVID-ETF)” https://www.ema.europa.eu/en/documents/other/mandate-objectives-rules-procedure-covid-19-ema-pandemic-task-force-covid-etf_en.pdf Last accessed 25 November 2021
- 41.European Commission, Questions and Answers: Conditional Marketing Authorisation of COVID-19 Vaccines in the EU https://ec.europa.eu/commission/presscorner/detail/en/qanda_20_2390 Last accessed 25 November 2021
- 42.White paper Every Day Counts on Oncology treatments https://www.vintura.com/wp-content/uploads/2020/08/White-paper-every-day-counts-improving-time-to-patient-access-to-innovative-oncology-therapies-in-europe_from-EFPIA_and_Vintura.pdf Last accessed 11 December 2021
- 43.Mckinsey & company, Fast-forward: Will the speed of COVID-19 vaccine development reset industry norms?, https://www.mckinsey.com/industries/life-sciences/our-insights/fast-forward-will-the-speed-of-covid-19-vaccine-development-reset-industry-norms Last accessed 25 November 2021
- 44.FDA, “Frequently Asked Questions: Breakthrough Therapies” https://www.fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/frequently-asked-questions-breakthrough-therapies Last accessed 26 October 2021
- 45.FDA, “Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review” https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review Last accessed 26 October 2021
- 46.FDA, “Real-Time Oncology Review” https://www.fda.gov/about-fda/oncology-center-excellence/real-time-oncology-review Last accessed 26 October 2021
- 47.EMA, “Accelerated assessment” https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/accelerated-assessment Last accessed 26 October 2021
- 48.Minister of Health, Labour and Welfare l Japan l Strategy of Sakigake https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/140729-01.html Last accessed 26 October 2021
- 49.EMA Paediatric Investigation Plans, https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans Last accessed 26 November 2021
- 50.EMA, EMA starts evaluating use of COVID-19 vaccine Comirnaty in young people aged 12 to 15, https://www.ema.europa.eu/en/news/ema-starts-evaluating-use-covid-19-vaccine-comirnaty-young-people-aged-12-15 Last accessed 26 October 2021
- 51.EMA, EMA evaluating the use of COVID-19 Vaccine Moderna in young people aged 12 to 17, https://www.ema.europa.eu/en/news/ema-evaluating-use-covid-19-vaccine-moderna-young-people-aged-12-17 Last accessed 26 October 2021
- 52.EMA, COVID-19 vaccine Spikevax approved for children aged 12 to 17 in EU, https://www.ema.europa.eu/en/news/covid-19-vaccine-spikevax-approved-children-aged-12-17-eu Last accessed 26 October 2021
- 53.European Commission, communication from the commission to the european parliament, the european council and the council hera Incubator: Anticipating together the threat of COVID-19 variants, https://ec.europa.eu/info/sites/default/files/communication-hera-incubator-anticipating-threat-covid-19-variants_en.pdf Last accessed 26 October 2021
- 54.European Commission, “Coronavirus: new procedure to facilitate and speed up approval of adapted vaccines against COVID-19 variants” https://ec.europa.eu/commission/presscorner/detail/en/ip_21_1088 Last accessed 25 November 2021
- 55.European Medicine Agency, Pandemic report and lessons learned Outcome of the European Medicines Agency's activities during the 2009 (H1N1) flu pandemic, https://www.ema.europa.eu/en/documents/report/pandemic-report-lessons-learned-outcome-european-medicines-agencys-activities-during-2009-h1n1-flu_en.pdf Last accessed 26 October 2021
- 56.Science Direct, Esteban Herrero-Martinez, Nasir Hussain, Nadege Le Roux, Judith MacDonald, Mark Mayer, Rodrigo Palacios and Thomas C. Kühler. Dynamic Regulatory Assessment: evolving the European Regulatory Framework for the benefit of patients and public health – An EFPIA view. In press in Clinical Therapeutics. https://www.sciencedirect.com/science/article/pii/S0149291821004562 Last accessed 1 December 2021 [DOI] [PMC free article] [PubMed]
- 57.IFPMA EFPIA PhRMA BIO ABPI Statement, Global COVID-19 Summit: Ending the Pandemic and Building Back Better, https://www.ifpma.org/resource-centre/ifpma-efpia-phrma-bio-abpi-statement-global-covid-19-summit-ending-the-pandemic-and-building-back-better/ Last accessed 26 November 2021
- 58.EFPIA, Policy proposals to minimize medicine supply shortages in Europe Lessons from COVID-19 crisis, COVID-19 Drug shortages EFPIA position paper Last accessed 26 November 2021