Abstract
With an ever-increasing number of COVID-19 survivors, providers are tasked with addressing the longer lasting symptoms of COVID-19, or postacute sequelae of SARS-CoV-2 infection (PASC). For critically ill patients, existing knowledge about postintensive care syndrome (PICS) represents a useful structure for understanding PASC. Post-ICU clinics leverage a multidisciplinary team to evaluate and treat the physical, cognitive, and psychological sequelae central to both PICS and PASC in critically ill patients. While management through both pharmacologic and nonpharmacologic modalities can be used, further research into both the optimal treatment and prevention of PASC represents a key public health imperative.
Keywords: Postacute sequelae of SARS-CoV-2, Postintensive care syndrome, Post–acute COVID-19 syndrome, Post-COVID-19 programs, Long COVID
Key points
-
•
Many survivors of COVID-19 critical illness will experience long-term impairments in physical, mental, cognitive, social, and financial health
-
•
The sequelae of COVID-19 critical illness overlap considerably with postintensive care syndrome; existing knowledge of postintensive care syndrome can serve as a useful framework for approaching patients with COVID-19 recovering from critical illness
-
•
Evaluation and management of postintensive care syndrome and postacute sequelae of COVID-19 critical illness require a multidisciplinary approach
-
•
Post-ICU clinics offer opportunities for quality improvement and research that may improve the care of patients while they are in the ICU
Introduction
Coronavirus disease 2019 (COVID-19) has claimed over 4 million deaths worldwide1 and has created an unprecedented burden on intensive care units globally.2 Much of the dialogue surrounding the pandemic has centered on mortality, which has been as high as 50% in critically ill patients.3 However, most patients will survive acute illness from COVID-19, and survival, despite being a desired outcome, is also fraught with challenges. Initial reports from Italy, France, and the United States suggest that 66% to 87% of hospitalized patients with COVID-19 have symptoms that persist after hospital discharge.4, 5, 6 The term “long COVID” has helped to raise awareness of the postacute sequelae of SARS-CoV-2 infection (PASC) and the potentially long-lasting health consequences that can stem from acute illness. Particularly in patients surviving COVID-19 critical illness, survival will not equate to recovery, and understanding and addressing the long-term needs of survivors is a societal imperative. While PASC has been reported even among patients who were not critically ill or hospitalized, this review focuses on PASC in patients surviving COVID-19 critical illness.
Postintensive care syndrome (PICS), defined as new or worsening impairments in mental, cognitive, or physical health following critical illness,7 affects nearly all ICU survivors at the time of hospital discharge, and continues to impact more than half of these patients 1 year after discharge.8 This syndrome has been studied and described for over a decade and can serve as a useful framework for approaching patients surviving COVID-19 critical illness who continue to have impairments in the postacute setting. In this review, we describe PICS—and what this can tell us about PASC in the critically ill.
Clinical manifestations of postintensive care syndrome and postacute sequelae of SARS-CoV-2 infection in the critically ill
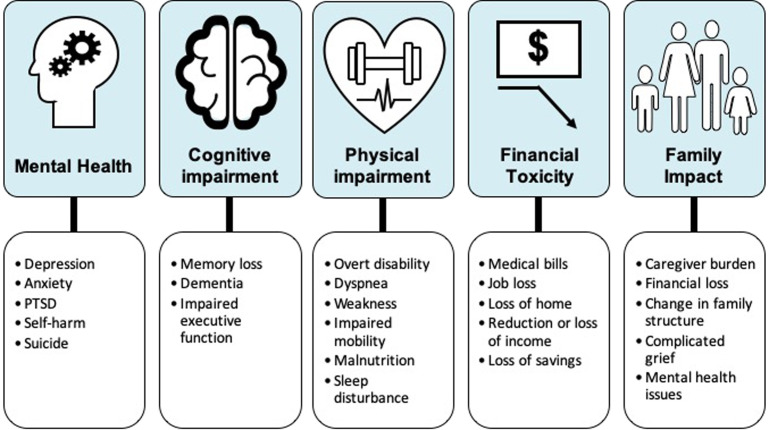
Due to medical, scientific, and technological advances in the last several decades, survival rates of patients with ICU have increased dramatically.9 , 10 This rise in survivorship coupled with an aging population have created a growing cohort of patients suffering from varied long-term consequences of critical care.11 , 12 In recent years, there has been a growing body of literature outlining the long-term sequelae of an intensive care unit (ICU) stay (Fig. 1 ).13 While the 3 major components of PICS—deficits in mental, cognitive, or physical health—are illustrated individually, a complex relationship exists between each domain, with a single impairment in any one domain influencing the others,8 , 14, 15, 16 and often coexisting with the others.17 , 18 The clinical manifestations, incidence, and risk factors for each component are first described, and then compared with our existing knowledge about these symptoms in PASC.
Fig. 1.
Common sequelae of critical illness in both COVID-19 and non–COVID-19 survivors.
Cognitive Impairment
In terms of cognitive functioning, critical illness can lead to new and clinically important cognitive impairments regardless of age, coexisting disease, and preexisting conditions, often mirroring the degree of impairment seen in Alzheimer’s dementia.19 , 20 Patients who have experienced delirium in the ICU are at particularly high risk for long-term cognitive impairment.19 The areas of cognition most commonly affected include attention, concentration, mental processing speed, memory, and executive function, with dysfunction in the latter 2 prevalent in 35% of ICU survivors at 3 months.21 , 22 In turn, this places patients at higher risk for disruptions in medication adherence and appropriate follow-up, and acts as a major obstacle in returning to premorbid levels of socioeconomic functioning.12 , 22 , 23 A number of factors unique to COVID-19 ICU survivors increases their risk for cognitive impairment. Frequently, they are mechanically ventilated and on high amounts of sedation.24 Additionally, they have experienced critical illness with the added burdens of social isolation due to infection control measures; lack of family visitation has been identified as an independent risk factor for ICU delirum.25 Consequently, cognitive deficits have been described as some of the most common and debilitating long-term sequelae in patients with PASC, with decreased concentration, memory concerns, and cognitive impairment reported in a median of 24%, 19%, and 17% of patients, respectively.26
Physical Impairment
The spectrum of physical impairment in patients with PICS is wide, with up to 80% of patients experiencing a new physical dysfunction at the time of discharge.27 , 28 These include critical illness neuropathy (CIN), critical illness myopathy (CIM), cachexia, fatigue, dyspnea, impaired pulmonary function, decreased exercise tolerance, sexual dysfunction, and respiratory failure.29, 30, 31 Functionally, patients are believed to lose as much as a kilogram of lean body mass (LBM) per day, which predisposes to muscle weakness and related physical impairments that can persist for months to years.11 , 32 , 33 ICU-acquired weakness, defined as neuromuscular dysfunction with no plausible cause other than critical illness and its treatments, is thought to originate from CIN, CIM, or a combination of the two.31 , 34 While the prevalence varies widely based on patient population, risk factors, and methods used for diagnosis, it is believed that 43% of patients in the ICU suffer from this complication, which is associated with both hospital mortality and long-term mortality, with decreased survival seen in patients up to 5 years later.35 , 36 Consequently, patients have difficulties performing their daily activities with persistently lower health-related quality of life (HRQL) measures when compared with age matched norms.37 , 38
In areas hit hard by the pandemic, ICU staffing shortages may contribute to limited patient mobilization, a preventative measure known to reduce the risk of ICU-acquired weakness.39 Indeed, the receipt of care in overwhelmed and understaffed hospitals has been associated with adverse outcomes.25 , 40 Other risk factors for long-term physical sequelae of critical illness in COVID-19 survivors include frequent use of prone positioning and arterial line placement, which can each increase the risk of neuropathy.41 Corticosteroids and prolonged used of neuromuscular blocking agents, which are prescribed to treat COVID-19 pneumonia and manage severe acute respiratory distress syndrome, respectively, further increase the risk of CIM when used in combination.42 , 43
Psychological Impairment
The psychological sequelae of PICS are estimated to occur in up to a third of survivors, with PTSD, depression, and anxiety as the predominant conditions.13 , 44 While it can be situational for some, others have symptoms that persist for months to years after discharge, disrupting daily functioning and reducing overall quality of life; ICU survivors also have a higher incidence of suicide and self-harm when compared with hospital survivors who never required ICU admission.45 , 46 The psychological sequelae extend beyond the patient to those in the family as well, collectively known as PICS-Family (PICS-F).7 Having a critically ill family member has been shown to have profound effects on relatives, with over two-thirds reporting anxiety or depression when visiting their loved ones, and 30% suffering from anxiety, depression, or PTSD beyond discharge.47 , 48 Further, the complex interactions of the various domains of PICS, as outlined above, amplify the burden on patient’s families as well as dramatically increase the cost for health care systems.49 , 50 Patients with COVID-19 have similarly been found to have high rates of PTSD, anxiety, depression, and insomnia, likely due to both disease-specific and pandemic-related factors including stigmatization, social isolation, and media sensationalism, among others.51 Existing studies suggest that approximately 30%, 20%, 13%, and 27% of COVID-19 survivors suffer from anxiety, depression, PTSD, and insomnia, respectively.26
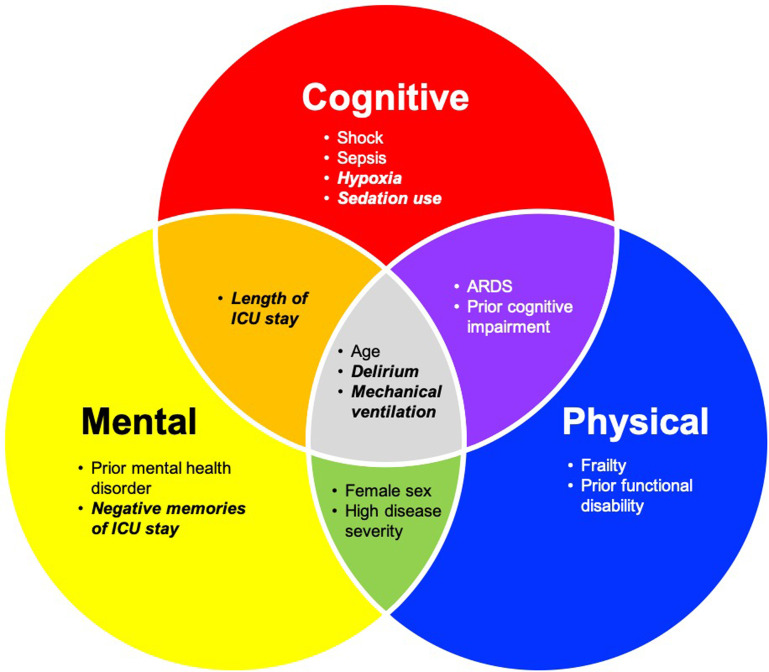
As PICS has profound effects across mental, cognitive, and physical domains, a first step involves recognizing the comorbidities that predispose to developing it in the first place. To date, there have been many studies evaluating the risk factors associated with PICS, although the mechanisms continue to be poorly understood. While certain risk factors are preexisting and thus nonmodifiable, others are ICU-specific, and thus have the potential to be optimized (Fig. 2 ).13 , 52, 53, 54 For example, delirium, which is associated with increased mortality, ICU length of stay, and long-term cognitive impairment, may represent one modifiable risk factor.12 , 55, 56, 57 As such, multicomponent ICU-level strategies, such as the “ABCDEF bundle,” have been used with success.12 , 56 This systematic method of pain assessment, both spontaneous awakening and breathing trials, choosing safe and effective medication regimens for managing pain and agitation, delirium monitoring, exercise/early mobility, and family engagement, has been shown to reduce the amount of sedative use, duration of delirium, and ICU length of stay.58, 59, 60 With the COVID-19 pandemic bringing a growing number of patients to the ICU, it is becoming increasingly imperative to identify additional modifiable risk factors for PICS so that new preventative interventions can be developed in the future.
Fig. 2.
Risk factors associated with PICS. Each circle represents the PICS domain associated with each risk factor. Those in italics represent potentially modifiable risk factors; others are pre-existing.
Health Care Utilization and Disability
While symptoms of PICS may improve over time, survivors of critical illness still face a number of long-term challenges, including increased mortality, rehospitalization, reduced quality of life, and financial loss. A study of US Medicare beneficiaries comparing ICU survivors to age, sex, and race-matched controls from the general population found that ICU survivors have increased mortality at 3 years (39.5% vs 14.9%)61; similar findings were seen in Dutch62 and Scottish63 cohorts. Long-term mortality in mechanically ventilated ICU survivors is markedly increased, with rates of 41% to 58% reported in multicenter cohorts.61 , 64 , 65 Similarly, health care utilization, including hospital readmission, increases after critical illness. In an observational study comparing health care utilization among ICU survivors before and after critical illness, ICU survivors in the year following critical illness were found to have an increase in outpatient visits, emergency department visits, and hospitalizations of 8%, 33%, and 60%, respectively, when compared with the prior year.66 Expectedly, postdischarge health care costs are also greater than costs in the year before critical illness. Less predictably, health care costs can remain increased from baseline for up to 5 years following discharge.63
Functional status and HRQL also suffer after critical illness.67, 68, 69, 70, 71, 72, 73, 74 At least partial disability in activities of daily living is seen in one-fifth of previously independent individuals 1 year after discharge.69 Frailty, which is associated with new-onset disability,75 is also common among survivors of critical illness. In a recent multicenter study, transition to a state of increased frailty occurred in 40% of ICU survivors at 1 year, including 23% of patients who were not frail at baseline.76 Likewise, HRQL is worse in ICU survivors compared with population norms. However, it remains unclear to what degree post-ICU HRQL is a reflection of premorbid quality of life. HRQL has also been found to improve over time, particularly during the first year following ICU discharge.68 , 71, 72, 73
Patients with COVID-19 similarly suffer from increased health care utilization and risk of death, with estimates suggesting a 1 in 5 risk of readmission and a 1 in 10 risk of death among hospitalized COVID-19 patients in the first 60 days after discharge.77 Beyond readmission and death, survivors also have decreased HRQL, increased outpatient health care visits, and increased pharmacotherapy utilization of opioid pain medications, antidepressants, anxiolytics, and more.78 , 79
Social and Financial Considerations
The COVID-19 pandemic has brought increased attention to the role of socioeconomic status in critical illness. Indeed, lower socioeconomic position and social vulnerability are associated with increased risk of critical illness and death from COVID-19 infection.80 , 81 However, an inverse relationship between socioeconomic position and health outcomes in ICU survivors has previously been established, with lower socioeconomic position associated with increased risk of long-term mortality and reduced HRQL.82 , 83
Just as socioeconomic status affects outcomes in the critically ill, critical illness itself has an impact on subsequent social and economic outcomes. Job loss and delayed return to work are common after critical illness, likely a result of post-ICU impairments. Of patients who were previously employed, only 56% to 60% return to work 1 year after critical illness, and one-third remain jobless after 5 years.84 , 85 While the long-term work implications in critically ill COVID-19 survivors are still being investigated, preliminary evidence suggests similar findings, with less than half of patients surviving COVID-19 critical illness returning to work at 3 to 4 months after discharge.79 Consequently, loss of income is common, reported in 71% of ICU survivors in the year following critical illness,86 as are other elements of financial toxicity, such as loss of health care coverage, depletion of savings, and medical bills.86, 87, 88 Family structure and roles may be also altered, as one-quarter of ICU survivors report needing a caregiver 1 year after critical illness. The vast majority of care is provided by family members, half of whom report a resultant negative impact on employment.89 , 90
Evaluation of Postacute Sequelae of SARS-CoV-2 Infection in Survivors Of Critical Illness
While limited evidence exists to inform the optimal evaluation of PASC, significant experience in the post-ICU arena can help guide these efforts.91 Indeed, to evaluate for PICS and the constellation of downstream effects outlined above, post-ICU clinics have been developed.92 Guidelines from the United Kingdom recommend that all adults who have stayed in an ICU for more than 4 days be followed after discharge, though implementation barriers have hampered widespread adoption of this policy in the UK and elsewhere.93 As many patients transfer first to facilities such as skilled nursing facilities or acute rehabilitation units before discharging to home, coordinating the ideal timing of the first post-ICU visit can be challenging. Consensus guidelines recommend an assessment 2 to 4 weeks after hospital discharge.94
Experience from centers specializing in post-ICU care suggests that a discharge navigator can be particularly useful in identifying and recruiting eligible patients,95 , 96 and may be associated with decreased readmission rates and decreased loss to follow-up.96 The navigator role may be filled by one of many different providers, including nurse practitioners, social workers, respiratory therapists, or case managers. In this role, the provider can connect with patients while still hospitalized, schedule and share information about the post-ICU clinic visit, and serve as a point of contact for the patients and their families as they navigate the transition out of the hospital. In settings where access to post-ICU follow-up may be more limited, navigators may choose to screen for risk factors to identify patients at particularly high risk for PICS and prioritize these patients for follow-up.
To address all of the components of PICS, post-ICU clinics are typically composed of a multidisciplinary team. Providers have debated which medical specialty is best equipped to lead these clinics (ie, intensivists vs rehabilitation specialists), yet this debate seems to only further highlight the importance of the interdisciplinary approach.97 , 98 We believe it is important to incorporate an ICU provider in the clinic, as studies suggest that this can facilitate longitudinal care delivery for patients, circle back to improve processes for future patients in the ICU, and reduce ICU staff burnout.92 , 99 , 100 In addition to an intensivist, a number of other clinicians typically comprise the multidisciplinary team, including a specialist to assess for physical debility (eg, a physical therapist, physiatrist, and/or respiratory therapist), psychological sequelae (eg, a psychologist and/or social worker), and cognitive impairment (eg, an occupational therapist, speech/language pathologist, or neurocognitive specialist). Additional team members may include a pharmacist, nutritionist, chaplain, case manager, or palliative care specialist.
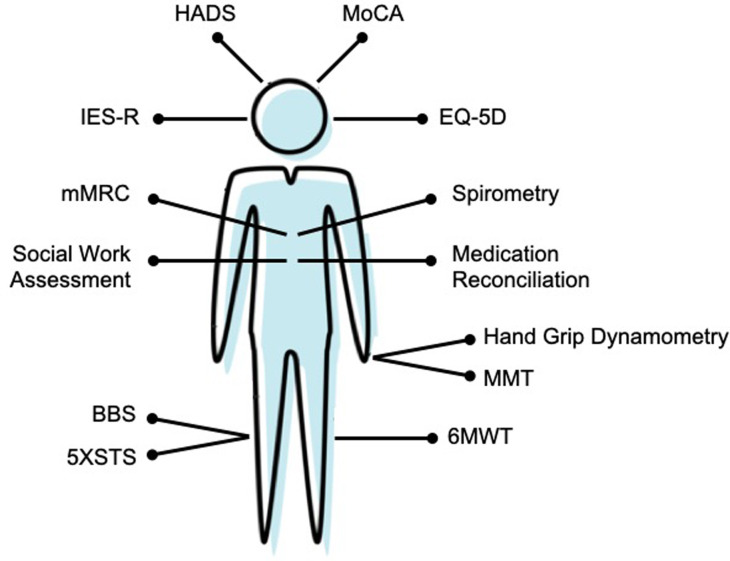
During the clinic visit, standardized tools should be adopted to systemically evaluate PICS and track progression over time (Fig. 3 ). Although further research into the optimal assessment tools are needed, guidelines have been developed based on expert opinion.94 , 101 Current guidelines recommend using the Hospital Anxiety and Depression Scale (HADS) to assess for anxiety and depression, as well as either the Impact of Events Scale-Revised (IES-R) or the shorter IES-6 to evaluate for posttraumatic stress disorder.94 , 101 While expert consensus has not been reached regarding the optimal cognitive screening tool, the Montreal Cognitive Assessment (MOCA) or MOCA-Blind may be used to screen for cognitive impairment.94 To evaluate for physical and pulmonary function, the Society of Critical Care Medicine suggests using the 6-minute walk test94; our center also uses bedside spirometry and the Modified Medical Research Council dyspnea scale as further assessments of pulmonary function. Some experts also suggest further evaluation of ICU-acquired weakness, including CIM and CIN, through the use of manual muscle testing and handgrip dynamometry; our center also uses the Borg Balance Scale (BBS) and Five Times Sit to Stand (5XSTS) instruments for further assessment of physical disability.101 Finally, the EuroQol-5D (EQ-5D) questionnaire can be used to evaluate both HRQL and pain.101
Fig. 3.
Outpatient evaluation of PASC in survivors of critical illness. HADS, Hospital Anxiety and Depression Scale; MoCA, Montreal Cognitive Assessment; IESR, Impact of Event Scale-Revised; EQ-5D, EuroQol-5D; mMRC, Modified Medical Research Council; BBS, Borg Balance Scale; 5XSTS, Five Times Sit-to-Stand; MMT, Manual Muscle Testing; 6MWT, Six-Minute Walk Test
In addition to using these screening assessments, a complete medication reconciliation should be performed to reduce the risk of polypharmacy. Our center’s practice also includes evaluation for new or persistent symptoms, appropriate referrals to further assist with ongoing physical and medical recovery, and screening for health care maintenance gaps such as immunizations. To help educate the patient and family, we summarize the patient’s ICU course, counsel on expected ICU recovery and supports available, and answer any questions. We also include family members in this process, as the adverse psychological effects that an ICU stay can have on family members, or PICS-Family, have become increasingly appreciated.7 Finally, we ask for feedback to help with ongoing quality improvement efforts within the ICU.
While these appointments have traditionally been performed in-person, the expanding role of telemedicine amidst the COVID-19 pandemic has opened up possibilities for expanding the reach of post-ICU clinics.102 This can be particularly useful in the post-ICU population, where limited patient mobility, large geographic distances, financial strain, and reduced access to transportation services can make in-person visits challenging.103
Management of postacute sequelae of SARS-CoV-2 infection in survivors of critical illness
As PICS and PASC remain relatively novel concepts, much of the management rests on expert opinion or extrapolation from other specialties. Similar to the multidisciplinary approach to assessment that is outlined above, a cross-disciplinary approach incorporating both pharmacologic and nonpharmacological domains will often need to be used.
In treating cognitive dysfunction, the provider should first evaluate for and manage any potentially reversible etiologies. This includes psychiatric conditions such as depression that can manifest with cognitive dysfunction, polypharmacy that may occur due to inadvertently-continued ICU medications on discharge (eg, atypical antipsychotics), sleep disorders, and metabolic or nutritional disturbances. Once these have been addressed, other treatment options such as cognitive therapy and exercise can be considered. Cognitive rehabilitation therapy aims to improve thought processes and behavior through multimodal strategies such as memory training exercises and/or the incorporation of organizational devices such as phone reminders.104 This has been evaluated in a limited number of studies on ICU survivors and may lead to improvements in cognitive functioning, and particularly executive function, though further studies are needed.105 For appropriate patients, exercise therapy has been shown to improve cognitive function in patients with mild cognitive impairment, and may similarly be of benefit to patients with PICS-associated cognitive impairment.106
We typically refer patients with ongoing physical limitations to physical therapy and/or occupational therapy for ongoing recovery, with the acknowledgment that there are limited studies for rehabilitation in CIM and CIN at present.107 In addition, randomized trials of rehabilitation-based programs for ICU survivors have not yet shown benefit for HRQL metrics.108, 109, 110 Nevertheless, given its potential to improve functional capacity, we continue to recommend physical and occupational therapy in this patient population. Rehabilitation specialists may also assist with recommendations regarding mobility aides and environmental adjustments. For COVID-19 survivors specifically, providers should avoid prolonging corticosteroid courses in the outpatient setting unless an alternative condition such as organizing pneumonia exists.111 In addition, given parallels between myalgic encephalomyelitis/chronic fatigue syndrome and the fatigue that many recovering patients with COVID-19 describe, patients should be counseled on the importance of graded exercise increase to decrease the risk of setbacks and postexertional malaise.112
Patients with pulmonary limitations due to post-ARDS fibrosis are managed with both supportive and preventative care. The prevalence of post-ARDS fibrosis in patients with and without COVID-19 remains unclear, but can be evaluated with serial pulmonary function testing and imaging.113 Thus far, evidence suggests that the majority of these patients experience improvement in both physiologic testing and radiographic changes over time.67 , 114 , 115 In this population, providers can thus assist with oxygen weaning, radiographic and pulmonary function test follow-up, and pulmonary rehabilitation referrals when indicated. Pulmonary rehabilitation, which involves supervised graded aerobic exercise training, strength training, and education on topics such as breathing techniques, inhaler use, and red flag symptoms, has been shown to improve pulmonary function and HRQL in ARDS survivors.116 We also ensure that vaccinations against Streptococcus pneumoniae, influenza, and COVID-19 are up to date. In spite of these measures, a minority of patients might not improve and may ultimately need to be referred to a center specializing in interstitial lung disease.
Patients with persistent psychiatric impairments after ICU survival benefit from referral to a mental health professional for appropriate management. Treatment frequently involves a combination of pharmacotherapy and psychotherapy. Patients with depression can be treated with either an antidepressant or psychotherapy alone, as each has shown efficacy in randomized trials, though data also suggest that combination therapy may be more efficacious than either treatment individually.117, 118, 119 For anxiety, cognitive behavioral therapy remains the most studied, and thus first-line, psychotherapeutic option, though mindfulness-based therapies are gaining increasing attention and may be more feasible for patients to initiate themselves during the initial recovery period.120 Pharmacotherapy for generalized anxiety disorder may also be considered for patients who meet diagnostic criteria. First-line treatment of posttraumatic stress disorder involves trauma-focused therapies such as cognitive behavioral therapy and exposure-based therapy, with medications reserved for individuals with a strong preference toward this.121 Some post-ICU centers also offer peer support groups through either in-person or virtual platforms, which have been shown to have a myriad of beneficial effects for patients.122 , 123 An additional challenge we have found during the COVID-19 pandemic is that after discharge, recovered patients are frequently hesitant to leave their homes due to fear of contracting the virus again, inadvertently restricting their opportunities for mobilization, which can exacerbate deconditioning and functional limitations, and also lead to worsened quality of life. In these situations, providers should evaluate for anxiety, agoraphobia, and PTSD and refer for treatment when applicable. They can also reinforce masking and social distancing precautions, and assess for appropriate COVID-19 vaccination timing. We have also found that a minority of patients can become consumed with media reports and social medial rabbit holes on long COVID-19, which often focus on outlier patients and can thus paint an overly negative picture of COVID-19 recovery. Similar stressors have been described in survivors of the SARS pandemic.124 Providers can assist by counseling patients on an expected recovery trajectory, normalizing their experience, and validating their progress.125
Beyond COVID-19: the role of post-ICU clinics in quality improvement and research
In response to the COVID-19 pandemic, multidisciplinary post-ICU clinics have been newly created by centers worldwide.40 While these clinics currently serve a crucial role in meeting the needs of patients recovering from COVID-19, they also provide a number of opportunities for improving care for all critically ill patients. On a center level, previously undetected issues can be identified during outpatient follow-up and serve as targets for ICU quality improvement. From the standpoint of clinician education, witnessing a patient’s recovery process may result in greater reflective practice in the ICU, influencing clinical decision-making and improving accuracy in predicting outcomes.126
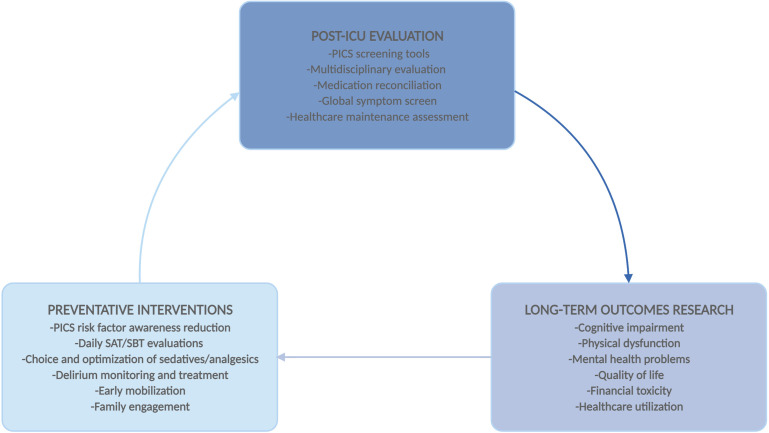
Post-ICU clinics also provide a much-needed avenue for conducting long-term outcomes research, which has been methodologically challenging in critical care. Loss to follow-up is a common limitation in long-term ICU outcomes studies, likely resulting in the exclusion of some of the most severely ill patients, who may have physical or cognitive deficits leading to study withdrawal.127 Such patients may potentially be more likely to present for clinical care than for research follow-up. As more patients survive a critical illness, improving care in the ICU will increasingly need to focus on preventing morbidity, and creating additional opportunities for postdischarge assessment is invaluable. The proliferation of post-ICU clinics during the COVID-19 pandemic may ultimately help improve care for all critically ill patients (Fig. 4 ).
Fig. 4.
Role of PICS clinics in improving ICU care. Multidisciplinary evaluation in post-ICU clinics can provide a basis for long-term research, which in turn can inform future preventative interventions in the ICU. PICS, Post-Intensive Care Syndrome; SAT, Spontaneous Awakening Trial; SBT, Spontaneous Breathing Trial.
Summary
Amidst a growing appreciation of the wide-ranging and long-term public health effects of COVID-19, PICS represents a useful contextual framework for diagnosing and treating PASC in critically ill survivors of COVID-19. While these conditions are not one and the same, there is substantial overlap, and providers can draw on existing knowledge of PICS when treating COVID-19 survivors. Further research into the prevention, diagnosis, and treatment of both PICS and PASC are needed as we move into the new frontier of COVID-19 survivorship.
Clinics care points
-
•
Patients recovering from critical illness after COVID-19 infection are at increased risk of cognitive impairment, ICU-acquired weakness, and psychiatric illness including anxiety, depression, PTSD, and insomnia.
-
•
Multidisciplinary management approaches, including non-pharmacologic options such as cognitive rehabilitation therapy, psychotherapy, and peer support groups, represent cornerstones of treatment in postintensive care syndrome.
-
•
Further research into the optimal treatment of PASC in critically ill patients is needed.
Conflict of interest
All authors report no relevant conflicts of interest.
References
- 1.Center for systems science and Engineering at Johns Hopkins University COVID-19 Dashboard. https://coronavirus.jhu.edu/map.html Available at: Accessed September 15 2021.
- 2.Tan E., Song J., Deane A.M., et al. Global impact of Coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest. 2021;159:524–536. doi: 10.1016/j.chest.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domecq J.P., Lal A., Sheldrick C.R., et al. Outcomes of patients with Coronavirus disease 2019 Receiving organ support therapies: the international viral infection and respiratory illness Universal study Registry. Crit Care Med. 2021;49:437–448. doi: 10.1097/CCM.0000000000004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra V., Flanders S.A., O'Malley M., et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 8.Marra A., Pandharipande P.P., Girard T.D., et al. Co-occurrence of post-intensive care syndrome Problems among 406 survivors of critical illness. Crit Care Med. 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman J.E., Kramer A.A., Knaus W.A. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17:R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin G.S., Mannino D.M., Eaton S., et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 11.Herridge M.S., Moss M., Hough C.L., et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42:725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 12.Jackson J.C., Pandharipande P.P., Girard T.D., et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai S.V., Law T.J., Needham D.M. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 14.Bruck E., Schandl A., Bottai M., et al. The impact of sepsis, delirium, and psychological distress on self-rated cognitive function in ICU survivors-a prospective cohort study. J Intensive Care. 2018;6:2. doi: 10.1186/s40560-017-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikkelsen M.E., Shull W.H., Biester R.C., et al. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology. 2009;14:76–82. doi: 10.1111/j.1440-1843.2008.01419.x. [DOI] [PubMed] [Google Scholar]
- 16.Sukantarat K., Greer S., Brett S., et al. Physical and psychological sequelae of critical illness. Br J Health Psychol. 2007;12:65–74. doi: 10.1348/135910706X94096. [DOI] [PubMed] [Google Scholar]
- 17.Bienvenu O.J., Colantuoni E., Mendez-Tellez P.A., et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642–653. doi: 10.1097/CCM.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra A., Pandharipande P.P., Girard T.D., et al. Co-occurrence of post-intensive care syndrome Problems among 406 survivors of critical illness. Crit Care Med. 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandharipande P.P., Girard T.D., Ely E.W. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370:185–186. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 20.Iwashyna T.J., Ely E.W., Smith D.M., et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukantarat K.T., Burgess P.W., Williamson R.C., et al. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005;60:847–853. doi: 10.1111/j.1365-2044.2005.04148.x. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins R.O., Weaver L.K., Pope D., et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 23.Rothenhausler H.B., Ehrentraut S., Stoll C., et al. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pun B.T., Badenes R., Heras La Calle G., et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groff D., Sun A., Ssentongo A.E., et al. Short-term and long-term rates of Postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey M.A., Davidson J.E. Postintensive care syndrome: Right care, Right Now...and later. Crit Care Med. 2016;44:381–385. doi: 10.1097/CCM.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths J., Hatch R.A., Bishop J., et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17:R100. doi: 10.1186/cc12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtake P.J., Lee A.C., Scott J.C., et al. Physical impairments associated with post-intensive care syndrome: systematic review based on the World health Organization's international Classification of functioning, disability and health framework. Phys Ther. 2018;98:631–645. doi: 10.1093/ptj/pzy059. [DOI] [PubMed] [Google Scholar]
- 30.Le Maguet P., Roquilly A., Lasocki S., et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 31.Latronico N., Bolton C.F. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 32.Stanojcic M., Finnerty C.C., Jeschke M.G. Anabolic and anticatabolic agents in critical care. Curr Opin Crit Care. 2016;22:325–331. doi: 10.1097/MCC.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan E., Dowdy D.W., Colantuoni E., et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens R.D., Marshall S.A., Cornblath D.R., et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37:S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 35.Dinglas V.D., Aronson Friedman L., Colantuoni E., et al. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45:446–453. doi: 10.1097/CCM.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan E., Cheek F., Chlan L., et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190:1437–1446. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 37.Bagshaw S.M., Stelfox H.T., Johnson J.A., et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43:973–982. doi: 10.1097/CCM.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin M.R., Reid M.C., Westlake A.A., et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014;29:401–408. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweickert W.D., Pohlman M.C., Pohlman A.S., et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churpek M.M., Gupta S., Spicer A.B., et al. Hospital-level Variation in death for critically ill patients with COVID-19. Am J Respir Crit Care Med. 2021;204:403–411. doi: 10.1164/rccm.202012-4547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosey M.M., Needham D.M. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6:60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between Administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Jama. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss M., Huang D.T., Brower R.G., et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatch R., Young D., Barber V., et al. Anxiety, depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit Care. 2018;22:310. doi: 10.1186/s13054-018-2223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myhren H., Ekeberg O., Toien K., et al. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14:R14. doi: 10.1186/cc8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernando S.M., Qureshi D., Sood M.M., et al. Suicide and self-harm in adult survivors of critical illness: population based cohort study. BMJ. 2021;373:n973. doi: 10.1136/bmj.n973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zante B., Camenisch S.A., Schefold J.C. Interventions in post-intensive care syndrome-family: a systematic literature review. Crit Care Med. 2020;48:e835–e840. doi: 10.1097/CCM.0000000000004450. [DOI] [PubMed] [Google Scholar]
- 48.Gries C.J., Engelberg R.A., Kross E.K., et al. Predictors of symptoms of posttraumatic stress and depression in family members after patient death in the ICU. Chest. 2010;137:280–287. doi: 10.1378/chest.09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Needham D.M., Feldman D.R., Kho M.E. The functional costs of ICU survivorship. Collaborating to improve post-ICU disability. Am J Respir Crit Care Med. 2011;183:962–964. doi: 10.1164/rccm.201012-2042ED. [DOI] [PubMed] [Google Scholar]
- 50.Dowdy D.W., Eid M.P., Dennison C.R., et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 51.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bienvenu O.J., Colantuoni E., Mendez-Tellez P.A., et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davydow D.S., Gifford J.M., Desai S.V., et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikkelsen M.E., Christie J.D., Lanken P.N., et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ely E.W., Gautam S., Margolin R., et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duggan M.C., Wang L., Wilson J.E., et al. The relationship between executive dysfunction, depression, and mental health-related quality of life in survivors of critical illness: results from the BRAIN-ICU investigation. J Crit Care. 2017;37:72–79. doi: 10.1016/j.jcrc.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ely E.W., Shintani A., Truman B., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 58.Vasilevskis E.E., Ely E.W., Speroff T., et al. Reducing iatrogenic risks: ICU-acquired delirium and weakness--crossing the quality chasm. Chest. 2010;138:1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balas M.C., Vasilevskis E.E., Olsen K.M., et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42:1024–1036. doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsieh S.J., Otusanya O., Gershengorn H.B., et al. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med. 2019;47:885–893. doi: 10.1097/CCM.0000000000003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wunsch H., Guerra C., Barnato A.E., et al. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 62.Brinkman S., de Jonge E., Abu-Hanna A., et al. Mortality after hospital discharge in ICU patients. Crit Care Med. 2013;41:1229–1236. doi: 10.1097/CCM.0b013e31827ca4e1. [DOI] [PubMed] [Google Scholar]
- 63.Lone N.I., Gillies M.A., Haddow C., et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med. 2016;194:198–208. doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C.Y., Calfee C.S., Paul D.W., et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40:388–396. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernando S.M., Qureshi D., Tanuseputro P., et al. Mortality and costs following extracorporeal membrane oxygenation in critically ill adults: a population-based cohort study. Intensive Care Med. 2019;45:1580–1589. doi: 10.1007/s00134-019-05766-z. [DOI] [PubMed] [Google Scholar]
- 66.Hirshberg E.L., Wilson E.L., Stanfield V., et al. Impact of critical illness on Resource utilization: a Comparison of Use in the Year before and after ICU admission. Crit Care Med. 2019;47:1497–1504. doi: 10.1097/CCM.0000000000003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herridge M.S., Cheung A.M., Tansey C.M., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. New Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 68.Cuthbertson B.H., Roughton S., Jenkinson D., et al. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson J.C., Pandharipande P.P., Girard T.D., et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfoh E.R., Wozniak A.W., Colantuoni E., et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–1566. doi: 10.1007/s00134-016-4530-1. [DOI] [PubMed] [Google Scholar]
- 71.Gerth A.M.J., Hatch R.A., Young J.D., et al. Changes in health-related quality of life after discharge from an intensive care unit: a systematic review. Anaesthesia. 2019;74:100–108. doi: 10.1111/anae.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofhuis J.G.M., Schrijvers A.J.P., Schermer T., et al. Health-related quality of life in ICU survivors—10 years later. Scientific Rep. 2021:11. doi: 10.1038/s41598-021-94637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oeyen S.G., Vandijck D.M., Benoit D.D., et al. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38:2386–2400. doi: 10.1097/CCM.0b013e3181f3dec5. [DOI] [PubMed] [Google Scholar]
- 74.Herridge M.S., Tansey C.M., Matté A., et al. Functional disability 5 Years after acute respiratory distress syndrome. New Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 75.Vermeiren S., Vella-Azzopardi R., Beckwee D., et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:1163 e1–e17. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Brummel N.E., Girard T.D., Pandharipande P.P., et al. Prevalence and course of frailty in survivors of critical illness. Crit Care Med. 2020;48:1419–1426. doi: 10.1097/CCM.0000000000004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donnelly J.P., Wang X.Q., Iwashyna T.J., et al. Readmission and death after initial hospital discharge among patients with COVID-19 in a large Multihospital system. Jama. 2021;325:304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 79.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riou J., Panczak R., Althaus C.L., et al. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: a population-based analysis. Lancet Public Health. 2021;6:e683–e691. doi: 10.1016/S2468-2667(21)00160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karmakar M., Lantz P.M., Tipirneni R. Association of social and Demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4:e2036462. doi: 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones J.R.A., Berney S., Connolly B., et al. Socioeconomic position and health outcomes following critical illness. Crit Care Med. 2019;47:e512–e521. doi: 10.1097/CCM.0000000000003727. [DOI] [PubMed] [Google Scholar]
- 83.Bastian K., Hollinger A., Mebazaa A., et al. Association of social deprivation with 1-year outcome of ICU survivors: results from the FROG-ICU study. Intensive Care Med. 2018;44:2025–2037. doi: 10.1007/s00134-018-5412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mcpeake J., Mikkelsen M.E., Quasim T., et al. Return to employment after critical illness and its association with Psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16:1304–1311. doi: 10.1513/AnnalsATS.201903-248OC. [DOI] [PubMed] [Google Scholar]
- 85.Kamdar B.B., Suri R., Suchyta M.R., et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75:17–27. doi: 10.1136/thoraxjnl-2019-213803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamdar B.B., Huang M., Dinglas V.D., et al. Joblessness and Lost Earnings after acute respiratory distress syndrome in a 1-year National multicenter study. Am J Respir Crit Care Med. 2017;196:1012–1020. doi: 10.1164/rccm.201611-2327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauschildt K.E., Seigworth C., Kamphuis L.A., et al. Financial toxicity after acute respiratory distress syndrome: a National Qualitative cohort study. Crit Care Med. 2020;48:1103–1110. doi: 10.1097/CCM.0000000000004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwashyna T.J., Kamphuis L.A., Gundel S.J., et al. Continuing Cardiopulmonary symptoms, disability, and financial toxicity 1 Month after hospitalization for third-Wave COVID-19: early results from a US Nationwide cohort. J Hosp Med. 2021:16. doi: 10.12788/jhm.3660. [DOI] [PubMed] [Google Scholar]
- 89.Griffiths J., Hatch R.A., Bishop J., et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17:R100. doi: 10.1186/cc12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson C.C., Suchyta M.R., Darowski E.S., et al. Psychological sequelae in family caregivers of critically III intensive care Unit patients. A systematic review. Ann Am Thorac Soc. 2019;16:894–909. doi: 10.1513/AnnalsATS.201808-540SR. [DOI] [PubMed] [Google Scholar]
- 91.Parker A.M., Brigham E., Connolly B., et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. Lancet Respir Med. 2021;9(11):1328–1341. doi: 10.1016/S2213-2600(21)00385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sevin C.M., Jackson J.C. Post-ICU clinics should Be staffed by ICU clinicians. Crit Care Med. 2019;47:268–272. doi: 10.1097/CCM.0000000000003535. [DOI] [PubMed] [Google Scholar]
- 93.Connolly B., Douiri A., Steier J., et al. A UK survey of rehabilitation following critical illness: implementation of NICE Clinical Guidance 83 (CG83) following hospital discharge. BMJ Open. 2014;4:e004963. doi: 10.1136/bmjopen-2014-004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mikkelsen M.E., Still M., Anderson B.J., et al. Society of critical care Medicine's international consensus conference on prediction and Identification of long-term impairments after critical illness. Crit Care Med. 2020;48:1670–1679. doi: 10.1097/CCM.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 95.Eaton T.L., McPeake J., Rogan J., et al. Caring for survivors of critical illness: current practices and the role of the nurse in intensive care Unit Aftercare. Am J Crit Care. 2019;28:481–485. doi: 10.4037/ajcc2019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bloom S.L., Stollings J.L., Kirkpatrick O., et al. Randomized clinical trial of an ICU recovery Pilot program for survivors of critical illness. Crit Care Med. 2019;47:1337–1345. doi: 10.1097/CCM.0000000000003909. [DOI] [PubMed] [Google Scholar]
- 97.Meyer J, Brett SJ, Waldmann C. Should ICU clinicians follow patients after ICU discharge? Yes. Intensive Care Med United States 2018;44:1539–41. [DOI] [PubMed]
- 98.Vijayaraghavan BKT, Willaert X, Cuthbertson BH. Should ICU clinicians follow patients after ICU discharge? No. Intensive Care Med United States:1542-1544. [DOI] [PubMed]
- 99.Haines K.J., Sevin C.M., Hibbert E., et al. Key mechanisms by which post-ICU activities can improve in-ICU care: results of the international THRIVE collaboratives. Intensive Care Med. 2019;45:939–947. doi: 10.1007/s00134-019-05647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jarvie L., Robinson C., MacTavish P., et al. Understanding the patient journey: a mechanism to reduce staff burnout? Br J Nurs. 2019;28:396–397. doi: 10.12968/bjon.2019.28.6.396. [DOI] [PubMed] [Google Scholar]
- 101.Needham D.M., Sepulveda K.A., Dinglas V.D., et al. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santhosh L., Block B., Kim S.Y., et al. Rapid Design and implementation of post-COVID-19 clinics. Chest. 2021;160:671–677. doi: 10.1016/j.chest.2021.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jalilian L., Cannesson M., Kamdar N. Post-ICU recovery clinics in the Era of Digital health and Telehealth. Crit Care Med. 2019:e796–e797. doi: 10.1097/CCM.0000000000003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cicerone K.D., Goldin Y., Ganci K., et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil. 2019;100:1515–1533. doi: 10.1016/j.apmr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 105.Muradov O., Petrovskaya O., Papathanassoglou E. Effectiveness of cognitive interventions on cognitive outcomes of adult intensive care unit survivors: a scoping review. Aust Crit Care. 2021;34:473–485. doi: 10.1016/j.aucc.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Petersen R.C., Lopez O., Armstrong M.J., et al. Practice guideline update summary: mild cognitive impairment: report of the guideline Development, Dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mehrholz J., Pohl M., Kugler J., et al. Physical rehabilitation for critical illness myopathy and neuropathy: an abridged version of Cochrane Systematic Review. Eur J Phys Rehabil Med. 2015;51:655–661. [PubMed] [Google Scholar]
- 108.Cuthbertson B.H., Rattray J., Campbell M.K., et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. Bmj. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walsh T.S., Salisbury L.G., Merriweather J.L., et al. Increased hospital-based physical rehabilitation and information Provision after intensive care Unit discharge: the RECOVER randomized clinical trial. JAMA Intern Med. 2015;175:901–910. doi: 10.1001/jamainternmed.2015.0822. [DOI] [PubMed] [Google Scholar]
- 110.McDowell K., O'Neill B., Blackwood B., et al. Effectiveness of an exercise programme on physical function in patients discharged from hospital following critical illness: a randomised controlled trial (the REVIVE trial) Thorax. 2017;72:594–595. doi: 10.1136/thoraxjnl-2016-208723. [DOI] [PubMed] [Google Scholar]
- 111.Myall K.J., Mukherjee B., Castanheira A.M., et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nath A. Long-haul COVID. Neurology. 2020:559–560. doi: 10.1212/WNL.0000000000010640. [DOI] [PubMed] [Google Scholar]
- 113.George P.M., Barratt S.L., Condliffe R., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 114.Han X., Fan Y., Alwalid O., et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299 doi: 10.1148/radiol.2021203153. E177-e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van den Borst B., Peters J.B., Brink M., et al. Comprehensive health assessment 3 Months after recovery from acute Coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021;73:e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsieh M.J., Lee W.C., Cho H.Y., et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza Other Respir Viruses. 2018;12:643–648. doi: 10.1111/irv.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kupfer D.J., Frank E., Phillips M.L. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cuijpers P., Dekker J., Hollon S.D., et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219–1229. doi: 10.4088/JCP.09r05021. [DOI] [PubMed] [Google Scholar]
- 119.Cuijpers P., van Straten A., Warmerdam L., et al. Psychotherapy versus the combination of psychotherapy and pharmacotherapy in the treatment of depression: a meta-analysis. Depress Anxiety. 2009;26:279–288. doi: 10.1002/da.20519. [DOI] [PubMed] [Google Scholar]
- 120.Hoge E.A., Bui E., Marques L., et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74:786–792. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74:596–607. doi: 10.1037/amp0000473. [DOI] [PubMed] [Google Scholar]
- 122.McPeake J., Iwashyna T.J., Boehm L.M., et al. Benefits of peer support for intensive care Unit survivors: Sharing experiences, care Debriefing, and Altruism. Am J Crit Care. 2021;30:145–149. doi: 10.4037/ajcc2021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lassen-Greene C.L., Nordness M., Kiehl A., et al. Peer support group for intensive care Unit survivors: Perceptions on supportive recovery in the Era of social distancing. Ann Am Thorac Soc. 2021;18:177–182. doi: 10.1513/AnnalsATS.202007-799RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tansey C.M., Louie M., Loeb M., et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 125.McPeake J., Boehm L.M., Hibbert E., et al. Key components of ICU recovery programs: what Did patients report provided benefit? Crit Care Explorations. 2020;2:e0088. doi: 10.1097/CCE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haines K.J., Sevin C.M., Hibbert E., et al. Key mechanisms by which post-ICU activities can improve in-ICU care: results of the international THRIVE collaboratives. Intensive Care Med. 2019;45:939–947. doi: 10.1007/s00134-019-05647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilcox M.E., Ely E.W. Challenges in conducting long-term outcomes studies in critical care. Curr Opin Crit Care. 2019;25:473–488. doi: 10.1097/MCC.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]