Abstract
Mycobacterium abscessus comprises three subspecies: M. abscessus subsp. abscessus , M. abscessus subsp. bolletii , and M. abscessus subsp. massiliense . These closely related strains are typically multi-drug-resistant and can cause difficult-to-treat infections. Dominant clusters of isolates with increased pathogenic potential have been demonstrated in pulmonary infections in the global cystic fibrosis (CF) population. An investigation was performed on isolates cultured from an Asian, predominantly non-CF population to explore the phylogenomic relationships within our population and compare it to global M. abscessus isolates. Whole-genome-sequencing was performed on M. abscessus isolates between 2017 and 2019. Bioinformatic analysis was performed to determine multi-locus-sequence-type, to establish the phylogenetic relationships between isolates, and to identify virulence and resistance determinants in these isolates. A total of 210 isolates were included, of which 68.5 % (144/210) were respiratory samples. These isolates consisted of 140 (66.6 %) M . abscessus subsp. massiliense , 67 (31.9 %) M . abscessus subsp. abscessus, and three (1.4 %) M . abscessus subsp. bolletii . Dominant sequence-types in our population were similar to those of global CF isolates, but SNP differences in our population were comparatively wider despite the isolates being from the same geographical region. ESX (ESAT-6 secretory) cluster three appeared to occur most commonly in ST4 and ST6 M. abscessus subsp. massiliense , but other virulence factors did not demonstrate an association with isolate subspecies or sample source. We demonstrate that although similar predominant sequence-types are seen in our patient population, cross-transmission is absent. The risk of patient-to-patient transmission appears to be largely limited to the vulnerable CF population, indicating infection from environmental sources remains more common than human-to-human transmission. Resistance and virulence factors are largely consistent across the subspecies with the exception of clarithromycin susceptibility and ESX-3.
Keywords: genomic epidemiology, Mycobacterium abscessus complex, Mycobacterium bolletii , Mycobacterium massiliense, whole genome sequencing
Data Summary
Raw sequence reads and assemblies all M. abscessus subspecies in this study have been submitted to GenBank under project accession number PRJNA734660.
Impact Statement.
M. abscessus cultured from our Asian population were dominated by the same sequence type (ST) profiles seen in global cystic fibrosis (CF) populations. However, unlike in some CF centres cross-transmission with clonal isolates were not demonstrable. The infection control risks appear to be largely limited to the vulnerable CF population, indicating that infection from environmental sources is the most likely route rather than human-to-human transmission in our setting.
Background
Non-tuberculous-mycobacteria (NTM) are environmental organisms that may result in human infections in vulnerable patient groups. Mycobacterium abscessus comprises three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. bolletii , and M. abscessus subsp. massiliense . Inoculation of the bacteria following trauma or surgery may result in skin and soft tissue infections [1]. Respiratory infections usually occur in patients with underlying lung disease such as bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis [1].
Treatment of infections of M. abscessus is fraught with difficulties due to significant antimicrobial resistance. Two guidelines are available for treatment of pulmonary infections with M. abscessus : one by the British Thoracic Society, and a joint recommendation made by the American Thoracic Society (ATS), European Respiratory Society (ERS), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and the Infectious Diseases Society of America (IDSA) [2, 3]. Multidrug treatment regimens are usually recommended, with macrolides (clarithromycin or azithromycin) and amikacin being some of the key drugs used. Clarithromycin susceptibility and administration of clarithromycin has a significant impact on treatment outcomes. The impact of other antibiotics on outcome is less clear. Treatment outcomes remain poor despite long periods of treatment. It should also be noted that current guidelines and clinical data is largely based on data from pulmonary M. abscessus infections [2, 3]. Antimicrobials typically recommended for pulmonary infections may have different pharmacokinetic profiles for other infection sites and additional clinical data is required to guide management of extrapulmonary infections.
Unlike tuberculosis, M. abscessus (and NTM in general) are environmental organisms, and human-to-human spread is thought to be limited. However, there is increasing evidence of potential human-to-human transmission, with whole-genome-sequencing data used demonstrating closely-related M. abscesuss isolates within CF centres [4–6]. In conjunction with epidemiological links, these likely represent transmission between patients. CF is the most common predisposing risk factor for pulmonary M. abscessus infections in Caucasian populations [4, 7]. Consequently, genomic analyses of clinical M. abscessus isolates have largely centred on isolates from CF patients [4]. Transmission is postulated to have occurred via generation of infectious aerosols by infected patients, and fomites [4]. Bryant et al. demonstrated that among global collection of M. abscessus isolates from CF patients, the majority of isolates formed three major clusters: two M. abscessus subsp. abscessus and one M. abscessus subsp. massiliense clusters [4]. Higher rates of phagocytosis and intracellular survival were demonstrated among clustered isolates when compared against unclustered isolates, suggesting higher pathogenic potential [4]. Murine models also demonstrated higher intracellular bacterial survival, higher bacterial burdens, and worse inflammation when infected with clustered isolates [4].
The pathology and virulence factors of M. abscessus is an under-studied field. Emerging evidence that intracellular virulence factors, namely the type VII secretion systems encoded by different ESX (ESAT-6 secretory) clusters, in particular ESX-3 and ESX-4 which appear to be unique to M. abscessus subsp. abscessus [8], the glycopeptidolipid (gpl) locus which encompasses a large set of lipid membrane transport proteins (MmpL-MmpS), and phospholipase C are important for pathogenesis [9].
Compared to Caucasian populations, CF is rare in Asians [10]. The findings of previous studies thus may not be applicable to Asian populations, particularly as comprehensive and systematic genomic analyses of M. abscessus from Asian populations are limited. Several questions remain unanswered such as whether there are also clustered clinical M. abscessus isolates in Asian populations, and whether there is similar pathogenic potential in these isolates. These could provide clues to further elucidate potential channels of transmission and pathogenicity in different patient populations. Whole-genome-sequencing of a collection of isolates from an Asian patient population was performed to explore the phylogenomic relationships within our population and compared to global M. abscessus isolates. Potential resistance mutations were also explored.
Methods
Clinical M. abscessus isolates cultured between 1 January 2017 and 31 December 2019 for which susceptibility testing was performed were included in this study. The isolates were identified previously by Bruker MALDI Biotyper (Bruker, Billerica, Massachusetts, US). Susceptibility testing was performed during this period if microbiological criteria for pulmonary samples were fulfilled (>1 positive respiratory culture from the same patients, sample positive from a bronchoalveolar lavage). All non-pulmonary samples had susceptibility testing performed. Only the first sample for which susceptibility testing was performed was included from each patient.
The phenotypic susceptibility testing results of these isolates have been previously performed and reported [11]. This include routinely tested antimicrobials (RAPMYCO plate, Sensititre, Thermo Fisher, Waltham, Massachusetts US: trimethoprim-sulfamethoxazole ciprofloxacin, moxifloxacin, cefoxitin, amikacin, doxycycline, tigecycline, clarithromycin, linezolid, imipenem, minocycline, and tobramycin), and an extended antimicrobial panel using a customized antibiotic panel (SGPNUHS1 plate, Sensititre : vancomycin, oritavancin, dalbavancin, telavancin, rifabutin, eravacycline, delafloxacin, clofazimine, and bedaquiline). In brief, testing was performed as per manufacturer instructions and incubated at 30 °C (ambient conditions). Plates were read at 3–5 days’ incubation based on whether sufficient growth was seen in the control wells. RAPMYCO plates were further incubated to 14 days if initial reading indicated clarithromycin susceptibility to exclude presence of inducible clarithromycin resistance. Where available, the MIC results were interpreted based on CLSI breakpoints [12].
Whole-genome sequencing and bioinformatic analysis
Total genomic DNA was extracted from plate cultures using the QIAamp DNA Mini Kit (Hilden, Germany). Sequencing libraries were prepared using the NexteraXT kit (Illumina Inc., San Diego, CA, USA) and sequenced on the Illumina platform (HiSeq). Raw reads were trimmed using Trimmomatic v. 0.38 [13] then assembled with SPAdes version 3.9.0 [14]. Genome annotation was carried out using Prokka [15]. ABRicate using ResFinder database was used for genetic prediction of both acquired and chromosomal antibiotic resistance determinants. Multilocus sequence typing (MLST) was based on the PubMLST (https://pubmlst.org/mabscessus/) scheme using seven genes (argH, cya, gnd, murC, pta, purH, and rpoB) and performed using the MLST software available at https://githubcom/tseemann/mlst. Clustered isolates from a global CF M. abscessus collection (European Nucleotide Archive under project accession ERP001039) were also obtained and analysed [4]. The isolates used in our analysis are listed on Table S1 (available in the online version of this article). As Bryant et al. [4] did not employ the seven gene MLST scheme, we took the assembled genomes described in Table S1 in order to obtain the sequence types.
For species identification, average nucleotide identity (ANI) values were calculated using the Pyani package (https://github.com/widdowquinn/pyani). The following reference genomes were used for ANI comparisons M. abscessus subsp. abscessus ATCC 19977 (GenBank:GCA_000069185.1), M. abscessus subsp. bolletii BD (GenBank:GCA_003609715.1) and M. abscessus subsp. massiliense str. GO 06 (GenBank:GCA_000277775.2).
For the detection of variants in drug-resistance associated genes (Table 1) Snippy v4.3.0 (https://github.com/tseemann/snippy) was used while the presence and absence of putative virulence factors were determined using custom database coupled with ABRicate. FastTree [16] was used to generate phylogenetic tree based on core genome-SNPs obtained from alignment of the draft genomes using Snippy pipeline. The phylogenetic tree was visualized and annotated using iTOL [17].
Table 1.
List of resistance loci screened in the study genomes
|
Phenotypic resistance to |
Gene |
Product |
M. abscessus subsp. abscessus ATCC 19977 locus tag |
|---|---|---|---|
|
Inducible macrolide resistance |
erm(41) |
23S rRNA methyltransferase |
MAB_2297 |
|
Constitutive macrolide resistance |
rrl |
23S ribosomal RNA |
MAB_r5052 |
|
Aminoglycoside |
rpsL |
30S ribosomal protein S12 |
MAB_3851c |
|
Aminoglycoside |
rrs |
16S ribosomal RNA |
MAB_r5051 |
|
Amikacin |
eis1 |
Gcn5-related N-acetyltransferase |
MAB_4124 |
|
Amikacin |
eis2 |
Gcn5-related N-acetyltransferase |
MAB_4532c |
|
Clofazimine and bedaquiline |
MAB_2299c |
Transcriptional regulatory protein |
MAB_2299c |
|
Clofazimine and bedaquiline |
MmpS-MmpL |
Membrane protein |
MAB_2300 – MAB_2301 |
|
Clofazimine and bedaquiline |
MmpS-MmpL |
Membrane protein |
MAB_1135c – MAB_1134c |
|
Tetracycline |
tetX |
FAD-binding monooxygenase |
MAB_1496c |
|
Tetracycline |
tetR |
TetR regulatory protein |
MAB_1497c |
|
Tigecycline |
whiB7 |
Transcriptional regulator |
MAB_3508c |
|
Rifampicin |
MAB_0591 |
Rifampin adp-ribosyl transferase |
MAB_0591 |
|
Multi-drug |
MAB_2780c |
Transporter |
MAB_2780c |
|
Multi-drug |
MAB_2958 |
Putative transmembrane-transport protein |
MAB_2958 |
|
Multi-drug |
MAB_1935 |
Putative drug resistance transporter |
MAB_1935 |
Data availability
Raw sequence reads and assemblies all M. abscessus in this study have been submitted to GenBank under project accession number PRJNA734660.
Results
Between 1 January 2017 and 31 December 2019, a total of 819 M. abscessus isolates were cultured from 506 patients. Susceptibility testing was performed on 268 isolates from 218 patients. A total of 210 non-duplicate isolates were included, of which 68.5 % (144/210) were respiratory samples (bronchoalveolar lavage, sputum, lung and tracheal aspirates) (Fig. 1). Only two isolates were from patients with cystic fibrosis.
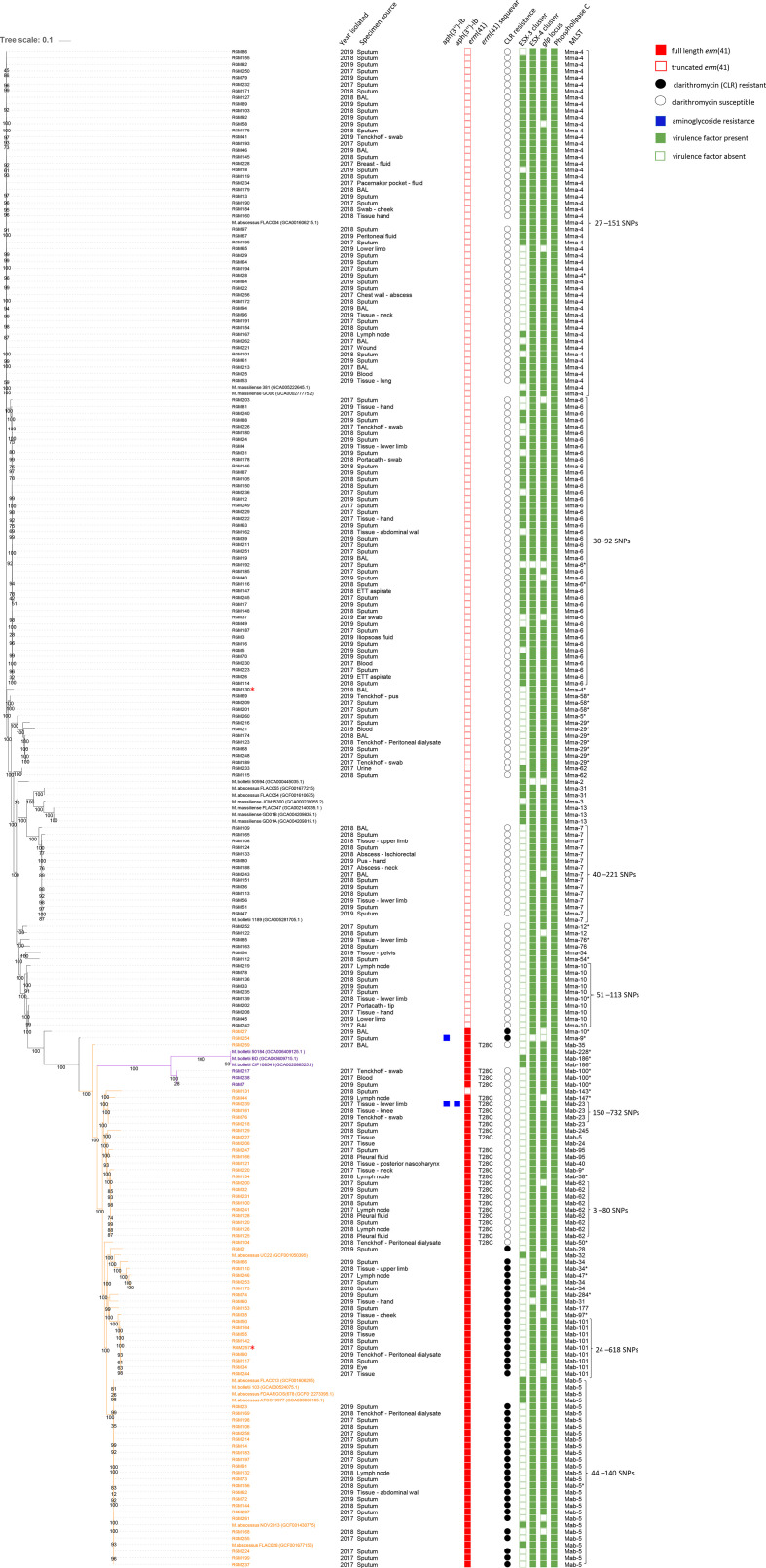
Fig. 1.
Core SNP phylogenetic tree of 210 isolates of Mycobacterium abscessus . The metadata includes specimen source, resistance determinants, virulence factors and multi-locus sequence type (ST). The black branch labels belong to M. abscessus subsp. massiliense, the purple labels to M. abscessus subsp. bolletii and the orange labels to M. abscessus subsp. massiliense. Two isolates marked with red asterisks were from cystic fibrosis (CF) patients. glp; glycopeptidolipid, ESX; ESAT-6 secretion system. The bootstrap values are indicated on the nodes.
An average sequencing depth of 150× was achieved for the genomes. Phylogenetic analysis classified 140 (66.6 %) as M. abscessus subsp. massiliense , 67 (31.9 %) as M. abscessus subsp. abscessus and three (1.4 %) as M. abscessus subsp. bolletii (Fig. 1). The species identification was also supported by ANI values which were typically >99 % when compared to their species reference genome (Table 2).
Table 2.
Average nucleotide identity (ANI) values of Mycobacterium abscessus subspecies
|
Study isolates |
Reference genomes |
|||||
|---|---|---|---|---|---|---|
|
M. abscessus subsp. abscessus (n=69) |
M. abscessus subsp . massiliense (n=138) |
M. abscessus subsp. bolletii (n=3) |
M. abscessus subsp. abscessus ATCC19977 (ASM6918v1) |
M. abscessus subsp. massiliense GO06 (ASM27777v2) |
M. abscessus subsp . bolletii BD (ASM360971v1) |
|
|
M. abscessus ATCC19977 (ASM6918v1) |
99.3–99.9 |
97.4–97.5 |
97.4–97.6 |
100 |
97.4 |
97.4 |
|
M. abscessus subsp. massiliense GO06 (ASM27777v2) |
97.3–97.5 |
99.0–99.9 |
97.1 |
96.9 |
100 |
97.4 |
|
M. abscessus subsp. bolletii BD (ASM360971v1) |
98.2 |
96.9–97.2 |
98.6 |
97.4 |
96.9 |
100 |
GenBank assembly accession numbers are provided for the reference genomes
n, number of study isolates.
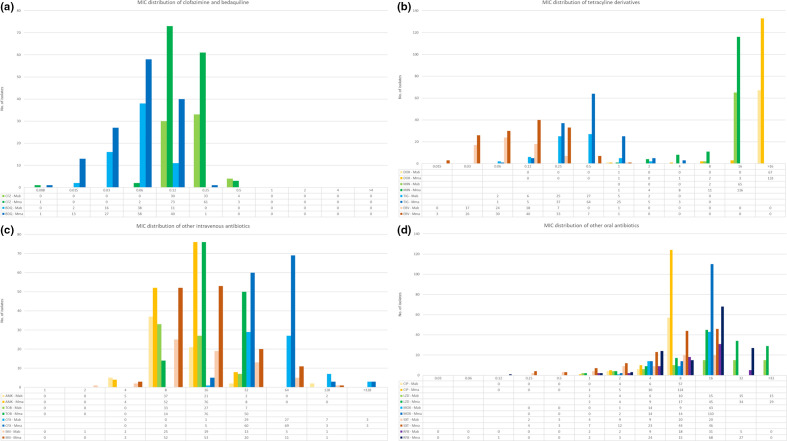
The overall susceptibility testing results have been previously reported without differentiating into the subspecies [11]. With the exception of clarithromycin (Fig. 1), the MIC results did not differ between the two predominant subspecies, M. abscessus subsp. abscessus and M. abscessus subsp. massiliense. The susceptibility profiles are summarized in Table 3 with MIC distributions presented in Fig. 2, stratified by subspecies level identification. M. abscessus subsp. bolletii was not included in this comparison due to lower numbers. Analyses of resistance mechanisms were performed for M. abscessus as a whole given the overlapping MIC range of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense.
Table 3.
Minimum inhibitory concentration of Mycobacterium abscessus subsp. abscessus (n=67) and Mycobacterium abscessus subsp. massiliense (n=140)
|
Antibiotic |
Organism |
MIC50 |
MIC90 |
Sensitive |
Intermediate |
Resistant |
|---|---|---|---|---|---|---|
|
Amikacin |
Mab |
16 |
16 |
94.0 % |
3.0% |
3.0% |
|
Mma |
16 |
32 |
94.3 % |
5.7% |
0.0% |
|
|
Cefoxitin |
Mab |
64 |
64 |
1.5 % |
83.6% |
14.9% |
|
Mma |
64 |
64 |
3.6 % |
92.1% |
4.3% |
|
|
Ciprofloxacin |
Mab |
>4 |
>4 |
0.0 % |
6.0% |
94.0% |
|
Mma |
>4 |
>4 |
0.7 % |
3.6% |
95.7% |
|
|
Doxycycline |
Mab |
>16 |
>16 |
0.0 % |
0.0% |
100.0% |
|
Mma |
>16 |
>16 |
0.7 % |
0.7% |
98.6% |
|
|
Imipenem |
Mab |
16 |
32 |
4.5 % |
66.7% |
28.8% |
|
Mma |
15 |
32 |
2.1 % |
75.0% |
22.9% |
|
|
Linezolid |
Mab |
16 |
>32 |
32.8% |
22.4% |
44.8% |
|
Mma |
16 |
>32 |
22.9% |
32.1% |
45.0% |
|
|
Moxifloxacin |
Mab |
>8 |
>8 |
0.0% |
1.5% |
98.5% |
|
Mma |
>8 |
>8 |
0.0% |
1.4% |
98.6% |
|
|
Trimethoprim-sulphamethoxazole |
Mab |
8 |
>8 |
26.9% |
n/a |
73.1% |
|
Mma |
8 |
>8 |
18.7% |
n/a |
81.3% |
|
|
Tobramycin |
Mab |
16 |
>16 |
0.0% |
0.0% |
100.0% |
|
Mma |
16 |
>16 |
0.0% |
0.0% |
100.0% |
|
|
Minocycline |
Mab |
>8 |
>8 |
0.0% |
0.0% |
100.0% |
|
Mma |
>8 |
>8 |
0.7% |
8.6% |
90.7% |
|
|
Tigecycline |
Mab |
0.5 |
1 |
n/a |
n/a |
n/a |
|
Mma |
0.5 |
1 |
n/a |
n/a |
n/a |
|
|
Clofazimine |
Mab |
0.25 |
0.25 |
n/a |
n/a |
n/a |
|
Mma |
0.12 |
0.25 |
n/a |
n/a |
n/a |
|
|
Bedaquiline |
Mab |
0.06 |
0.12 |
n/a |
n/a |
n/a |
|
Mma |
0.06 |
0.12 |
n/a |
n/a |
n/a |
|
|
Eravacycline |
Mab |
0.06 |
0.25 |
n/a |
n/a |
n/a |
|
Mma |
0.12 |
0.25 |
n/a |
n/a |
n/a |
|
|
Rifabutin |
Mab |
16 |
16 |
n/a |
n/a |
n/a |
|
Mma |
16 |
32 |
n/a |
n/a |
n/a |
MIC distribution in mg l−1; MIC50: MIC required to inhibit the growth of 50 % of included isolates; MIC90: MIC required to inhibit the growth of 90 % of included isolates. Mab: M. abscessus subsp. abscessus; Mma: M. abscessus subsp. massiliense. N/A, CLSI and EUCAST interpretive breakpoints not available.
n, number of study isolates.
Fig. 2.
MIC distribution of tested antimicrobials stratified by subspecies. The figures beneath the histograms indicate the number of isolates with a particular MIC, with the right-most figure indicating no inhibition within the tested MIC range. Blank results indicate MICs outside of the tested ranged. MICs presented in mg l−1; AMK: Amikacin; CFX: Cefoxitin; CIP: Ciprofloxacin; DOX: Doxycycline; IMI: Imipenem; LZD: Linezolid; MOX: Moxifloxacin; SXT: Co-trimoxazole; TOB: Tobramycin; MIN: Minocycline; TIG: Tigecycline; CFZ: Clofazimine; BDQ: Bedaquiline; ERV: Eravacycline; RFB: Rifabutin; Mab: Mycobacterium abscessus subsp. abscessus; Mma: Mycobacterium abscessus subsp. massiliense .
Multi-locus-sequence-typing (MLST)
For M. abscessus subsp. massiliense , a total of 18 sequence types (STs) were detected amongst the 140 isolates. This included ten novel STs. On average, the SNP range for each M. abscessus subsp. massiliense cluster ranged from five to 200 SNPs (Fig. 1). The most common STs were ST4, ST6, ST7 and ST10 (35, 30, 10 and 6.4 %, respectively). ST4 and ST6 belonged to clonal complex four whilst ST7 and ST10 belonged to clonal complex six and two, respectively. ST4, ST6, ST7, and ST10 all formed clusters identified in M. abscessus subsp. massiliense in the global CF population, with ST4 being the most common (18.8%, 48/256) [4]. M. abscessus subsp. abscessus (n=67) isolates appeared to have greater ST diversity with 24 different STs detected including ten novel STs. ST5, ST101, ST62, ST23 (32.8, 13.4, 13.4 and 5.9 %, respectively) were amongst the most common with none belonging to the same clonal complex. ST5 was the dominant clone (16.3%, 119/730) found in the previous study on global isolates [4].
Overall, we did not observe an association between specific STs and specimen sources. No predilection of dominant clones for pulmonary infections was seen (Fig. 1). There were four blood culture isolates which belonged to different STs ( M. abscessus subsp. abscessus n=1, M. abscessus subsp. massiliense n=3). There were also ten peritoneal dialysate peritonitis isolates ( M. abscessus subsp. abscessus n=5, M. abscessus subsp. massiliense n=5) which also had unique STs, indicating they were not clonally related (Fig. 1).
SNP analysis
Analysis of isolates from same patients by Bryant et al. [4] used 20 SNPs as the cut-off for ‘probable’ patient-patient transmission, and 38 SNPs as the cut-off for ‘possible’ recent transmission. Using these criteria, some of the isolates in our population may meet criteria for probable/possible transmission. These include M. abscessus subsp. massiliense ST4 (8–151 SNPs), ST6 (12–92 SNPs), and Mycobacterium abscessus ST62 (3–80 SNPs), ST101 (24–618 SNPs). Although some appear to be closely related, acquisition from the same source due to geographical proximity cannot be excluded. M. abscessus subsp. massiliense ST7 (40–221 SNPs) ST10 (51–113 SNPs), M. abscessus subsp . abscessus ST23 (150–732 SNPs), and ST5 (44–140 SNPs) did not meet the criteria for possible recent transmission.
All isolates with <20 SNPs were reviewed for possible links, including four ST62 M. abscessus subsp. abscessus, and twelve M. abscessus subsp. massiliense (seven ST6, three ST7, two ST4). The two ST4 M. abscessus subsp. massiliense were cultured from samples received from two separate external hospitals, 22 months apart. The three ST7 M. abscessus subsp. massiliense were cultured from internally received samples (n=2), and one external sample, received over 2 years. The seven ST6 M. abscessus subsp. massiliense were cultured from internally received samples (n=4), and another external hospital (n=3). These were received over 2 years, with the shortest interval being 15 days apart. The four ST62 M. abscessus subsp. abscessus were cultured from one internally received sample, and two other external hospitals (n=3). These samples were received within twelve days. As the isolates were received from different hospitals epidemiological links between these isolates were unlikely and the close relationship (SNPs <20) may not represent transmission events whether between individuals. Transmission from a single point-source may be possible but cannot be confirmed.
Distribution of virulence factors
Virulence genes ESX-3 and ESX-4, the glp locus and phospholipase C were sought in the genomes of our isolates. Complete modules of the ESX-3 system were not detected in M. abscessus subsp. abscessus genomes as determined by blast of all the loci (data not shown), and present in only 68 M. abscessus subsp. massiliense. Of note, ESX-3 was most commonly identified in ST4 and ST6 M. abscessus subsp . massiliense isolates. ESX-4 and gpl were not identified in three and eight isolates, respectively, while phospholipase C was ubiquitous in all isolates (Fig. 1). We did not observe a correlation between the distribution of virulence genes and subspecies or isolates from particular specimen sites (Fig. 1).
Resistance determinants in M. abscessus
Clarithromycin resistance in the M. abscessus subsp. abscessus can be constitutive or inducible. Constitutive clarithromycin resistance attributed to rrl mutations (typically point mutations at positions 2058 and 2059 [18]) were not observed in any isolate. All M. abscessus subsp. massiliense isolates had truncated erm(41) gene concordant with their susceptible phenotypes (MICs 0.06–1 mg l−1), which is characteristic of this subspecies [19]. All M. abscessus subsp. abscessus isolates carried the full-length erm(41) gene of which 26 (38.8 %, 26/67) isolates were of the C28 sequevar resulting in a non-functional erm(41) producing a clarithromycin-sensitive phenotype (Fig. 1). Other previously described sequevars were not observed [20]. All three M. abscessus subsp. bolletii isolates carried the full-length erm(41) with C28 sequevar, with phenotypic susceptibility to clarithromycin.
Resistance to aminoglycosides is conferred by several mechanisms, including target mutation, drug modification, and reduced uptake and/or increased efflux [9]. Aminoglycosides-modifying enzymes are found in M. abscessus these include acetyltransferases - AAC(2′), phosphotransferase - APH(3″) and N‐acetyltransferase Eis2 [9]. Out of the 210 genomes, phosphotransferases were detected in only two M. abscessus subsp. abscessus isolates. These were aph(3'')-Ic and aph (6)--Id, in RGM254 and RGM239, respectively. Both had amikacin MICs of 8 mg l−1 and the phosphotransferases did not appear to confer significant aminoglycoside resistance. Target site mutations of rrs and rpsL are responsible for high-level amikacin resistance in M. abscessus subsp. abscessus. Two isolates RGM25 and RGM172 had amikacin MICs of 128 mg l−1 however no mutations observed in rrs and rpsL indicating the possibility of other resistance mechanisms.
Overexpression of the eis2 and the multidrug efflux transporter gene (tap) and transcriptional regulator gene whiB7 have been demonstrated to be involved in the amikacin resistance in M. abscessus subsp. abscessus [21] although overexpression analysis was not investigated in this study.
Loci contributing to resistance in clofazimine, bedaquiline, tetracycline, and rifamycins (Table 1) were examined [22]. MAB_2299c which encode a putative TetR transcriptional regulator controls the expression of two separate two separate MmpS – MmpL efflux pumps (MAB_2300 – MAB_2301 and MAB_1135c-MAB_1134 c) [23, 24] (Table 1). Point mutations or deletion in MAB_2299 c were commonly associated with clofazimine resistance as well as cross-resistance to bedaquiline. High levels of tetracycline and doxycycline resistance typically seen in M. abscessus are conferred by a monooxygenase, TetX (MAB_1496 c), whose expression is induced by the same antibiotics [25]. ADP-ribosyltransferase MAB_0591 is recognized as the major determinant of innate high-level rifamycin resistance in M. abscessus [26]. Overall, no meaningful SNPs (Table S2) were detected in these loci and this was reflected in the antibiograms.
Discussion
The proportion of subspecies observed here mirrored our previous study where M. abscessus subsp. massiliense was the dominant subspecies among M. abscessus identified in our laboratory [27]. Even though the prevalence of each subspecies varies geographically, in most institutions M. abscessus subsp. abscessus is usually predominant and accounted for 51–78 % of the M. abscessus , followed by M. abscessus subsp. massiliense and M. abscessus subsp. bolletii [28, 29]. M. abscessus subsp. massiliense is associated with higher treatment success rates which has been attributed to clarithromycin susceptibility. In addition, the C28 sequevar was seen in a significant proportion (38.8 %) of our M. abscessus subsp. abscessus . A number of Asian studies have now shown that the C28 sequevar form a sizeable portion of isolates carrying erm(41). Studies from South Korean and Taiwan demonstrate that 20 and 37.5 % of their respective M. abscessus isolates had the C28 erm(41) variant [30, 31]. In Japan, the geographical distribution and regional differences of the M. abscessus group indicated that amongst the M. abscessus subsp. abscessus, the proportions of C28 sequevar was highly variable, with some regions having 0 % and in other areas as high as 61.5 % [32]. Although presumed to have a positive impact on treatment outcomes, there is limited clinical data on the correlation of the C28 sequevar with outcomes in M. abscessus subsp. abscessus. Constitutive clarithromycin resistance was not identified in our collection of isolates and parallels our previous observation that constitutive resistance involving rrl mutations was rare and seen in 2.2 % isolates (2/90 isolates) [27]. Similarly, in another study, none of the 42 M. abscessus isolates exhibited point mutations in the rrl gene [33]. Treatment with clarithromycin may select for constitutive mutants over a prolonged treatment duration [34]. Although antibiotic consumption data was not studied here, this may reflect that development of rrl mutations are uncommon in the absence of antibiotic selection pressure. There was otherwise no difference in terms of other drug classes’ susceptibility profiles of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense. The choice of companion antibiotics therefore would be minimally affected by subspecies identification. In vitro data suggest potential for clofazimine, bedaquiline, and eravacycline as new antimicrobial options in treating M. abscessus infections [11].
The dominant sequence types seen in our population ( M. abscessus subsp . abscessus ST5; M. abscessus subsp . massiliense ST4, ST6, ST7, ST10) had been previously identified as clustered STs within the global cystic fibrosis (CF) patient community [4]. The study had involved a large-scale whole genome analysis of a global collection of 1080 clinical M. abscessus isolates from 517 CF patients [4]. The predominance of these STs also suggest that these were also locally dominant circulating clones within the community. Closely-related M. abscessus was demonstrated, which in conjunction with epidemiological and contact links indicates potential infection control risks present when vulnerable patients come into contact with each other in healthcare settings. To investigate whether the same phenomena occurred in Asian populations and in non-pulmonary infections, we investigated virulence factors and SNPs in our population. Of note, ESX3 appears to be most common in the dominant M. abscessus subsp. massiliense ST4 and ST6, but otherwise absent from ST7, ST10, and all M. abscessus subsp. abscessus isolates. There was no predilection to sample type demonstrated. As for other virulence factors investigated, they also appear to be prevalent throughout all subspecies. In addition to ESX3, there may be other virulence factors yet to be characterized, which could contribute to the increased virulence potential seen in clustered M. abscessus isolates.
Despite belonging to the same MLST profile and being from the same geographical region, SNP differences in our population were comparatively wider in our patients. Clusters of isolates did not occur in our isolates as seen in CF patients. Investigations of isolates with <20 SNP differences did not demonstrate any clear links between these patients. The sample sources for these isolates were also quite diverse and included pulmonary and non-pulmonary samples, including one blood culture isolate. While healthcare-associated epidemiological links could not be established, a common exposure in the community cannot be excluded. Comparison of genomes with isolates from environmental sources may provide more clarity in the transmission of M. abscessus in the community.
We also explored isolates from specific invasive infections which may represent a common infection source. Isolates from patients with bacteraemia and patients with peritoneal-dialysis-associated-peritonitis were reviewed and were demonstrated to be from diverse sequence-types with no clonal infections (Fig. 1). There was only one identified infection of a cardiac device (RGM234; pacemaker infection). Again, these results do not suggest clonal M. abscessus infections in our population, and supports these infections have so far been sporadic unrelated events. However, it is important to note that common exposures and risk factors that may still predispose to these infections even when no clear links are established.
We demonstrate that M. abscessus cultured from our Asian population were dominated by the same ST profiles seen in global CF populations and that cross-transmission is absent. The infection control risks appear to be largely limited to the vulnerable CF population, indicating infection from environmental sources remains more common than human-to-human transmission. Virulence factors are largely consistent across the subspecies with the exception of clarithromycin susceptibility and ESX-3. Genomic resistance profiling also demonstrates that clarithromycin susceptibility remains the primary distinguishing phenotype between M. abscessus subsp. abscessus and M. abscessus subsp. massiliense, and wild-type susceptibility profiles of other antibiotics were similar between the two predominant subspecies.
Supplementary Data
Funding information
This study was supported by the National Medical Research Council (NMRC, Singapore) via the Collaborative Solutions Targeting Antimicrobial Resistance Threats in Health System Antimicrobial Resistance Research Grant (CoSTAR-HS/ARGSeedGrant/2019/03), and by the NUS Yong Loo Lin School of Medicine Pitch For Funds Grant.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
This project was reviewed and approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB reference number: 2019/00792).
Footnotes
Abbreviations: ANI, average nucleotide identity; ATCC, American Type Culture Collection; CF, cystic fibrosis; MIC, minimum inhibitory concentration; MLST, multi-locus sequence typing; NTM, non-tuberculous mycobactera; SNP, single-nucleotide polymorphism; ST, sequence type.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Two supplementary tables are available with the online version of this article.
References
- 1.Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139–1154. doi: 10.1080/14787210.2016.1238304. [DOI] [PubMed] [Google Scholar]
- 2.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, et al. British Thoracic Society Guidelines for the Management of Non-tuberculous Mycobacterial Pulmonary Disease (NTM-PD) Thorax. 2017;72:ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 3.Daley CL, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS / ERS / ESCMID / IDSA clinical practice guideline. Clin Infect Dis. 2020;71:1–36. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science. 2016;354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipworth S, Hough N, Weston N, Muller-Pebody B, Phin N, et al. Epidemiology of Mycobacterium abscessus in England: an observational study. The Lancet Microbe. 2021;2:e498–e507. doi: 10.1016/S2666-5247(21)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan J, Kevat A, Martinez E, Teese N, Johnson K, et al. Investigating transmission of Mycobacterium abscessus amongst children in an Australian cystic fibrosis centre. J Cyst Fibros. 2020;19:219–224. doi: 10.1016/j.jcf.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Redondo N, Mok S, Montgomery L, Flanagan PR, McNamara E, et al. Genomic analysis of Mycobacterium abscessus complex isolates collected in Ireland between 2006 and 2017. J Clin Microbiol. 2020;58:e00295-20. doi: 10.1128/JCM.00295-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus . PLoS ONE. 2009;4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus . Nat Rev Microbiol. 2020;18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Rebordosa C, Bernholz J, Sharma N. Epidemiology and genetics of cystic fibrosis in Asia: In preparation for the next-generation treatments. Respirology. 2015;20:1172–1181. doi: 10.1111/resp.12656. [DOI] [PubMed] [Google Scholar]
- 11.Chew KL, Octavia S, Go J, Ng S, Tang YE, et al. In vitro susceptibility of Mycobacterium abscessus complex and feasibility of standardizing treatment regimens. J Antimicrob Chemother. 2021;76:973–978. doi: 10.1093/jac/dkaa520. [DOI] [PubMed] [Google Scholar]
- 12.CLSI Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 3rd ed. CLSI Standard M24. 2018. [PubMed]
- 13.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 16.Price MN, Dehal PS, Arkin AP. FastTree: Computing Large Minimum Evolution Trees with Profiles instead of a Distance Matrix. Mol Biol Evol. 2009:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–5. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastian S, Veziris N, Roux A-L, Brossier F, Gaillard J-L, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm (41) and rrl sequencing. Antimicrob Agents Chemother. 2011;55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm (41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae . Antimicrob Agents Chemother. 2009;53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown-Elliott BA, Vasireddy S, Vasireddy R, Iakhiaeva E, Howard ST, et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015;53:1211–1215. doi: 10.1128/JCM.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M, Li B, Guo Q, Xu L, Zou Y, et al. Detection and molecular characterisation of amikacin-resistant Mycobacterium abscessus isolated from patients with pulmonary disease. J Glob Antimicrob Resist. 2019;19:188–191. doi: 10.1016/j.jgar.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Luthra S, Rominski A, Sander P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol. 2018;9:2179. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutiérrez AV, Richard M, Roquet-Banères F, Viljoen A, Kremer L. The TetR family transcription factor MAB_2299c regulates the expression of two distinct MmpS-MmpL efflux pumps involved in cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus . Antimicrob Agents Chemother. 2019;63:e01000-19. doi: 10.1128/AAC.01000-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Chen J, Zhang S, Shi W, Zhang W, et al. Novel mutations associated with clofazimine resistance in Mycobacterium abscessus . Antimicrob Agents Chemother. 2018;62:e00544-18. doi: 10.1099/jmm.0.000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudra P, Hurst-Hess K, Lappierre P, Ghosh P. High levels of intrinsic tetracycline resistance in Mycobacterium abscessus are conferred by a tetracycline-modifying monooxygenase. Antimicrob Agents Chemother. 2018;62:e00119–18. doi: 10.1128/AAC.00119-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rominski A, Roditscheff A, Selchow P, Böttger EC, Sander P. Intrinsic rifamycin resistance of Mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J Antimicrob Chemother. 2017;72:376–384. doi: 10.1093/jac/dkw466. [DOI] [PubMed] [Google Scholar]
- 27.Chew KL, Cheng JWS, Hudaa Osman N, Lin RTP, Teo JWP. Predominance of clarithromycin-susceptible Mycobacterium massiliense subspecies: Characterization of the Mycobacterium abscessus complex at a tertiary acute care hospital. J Med Microbiol. 2017;66:1443–1447. doi: 10.1099/jmm.0.000576. [DOI] [PubMed] [Google Scholar]
- 28.Kim H-Y, Kook Y, Yun Y-J, Park CG, Lee NY, et al. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J Clin Microbiol. 2008;46:3384–3390. doi: 10.1128/JCM.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chua KYL, Bustamante A, Jelfs P, Chen S-A, Sintchenko V. Antibiotic susceptibility of diverse Mycobacterium abscessus complex strains in New South Wales, Australia. Pathology. 2015;47:678–682. doi: 10.1097/PAT.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 30.Lee M-C, Sun P-L, Wu T-L, Wang L-H, Yang C-H, et al. Antimicrobial resistance in Mycobacterium abscessus complex isolated from patients with skin and soft tissue infections at a tertiary teaching hospital in Taiwan. J Antimicrob Chemother. 2017;72:2782–2786. doi: 10.1093/jac/dkx212. [DOI] [PubMed] [Google Scholar]
- 31.Jeong SH, Kim S-Y, Huh HJ, Ki C-S, Lee NY, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49–56. doi: 10.1016/j.ijid.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Kamada K, Yoshida A, Iguchi S, Arai Y, Uzawa Y, et al. Geographical distribution and regional differences in 532 clinical isolates of rapidly growing mycobacterial species in Japan. Sci Rep. 2021;11:4960. doi: 10.1038/s41598-021-84537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.dos Santos Carneiro M, de Souza Nunes L, Martini de David SM, Barth AL. Lack of association between rrl and erm(41) mutations and clarithromycin resistance in Mycobacterium abscessus complex. Mem Inst Oswaldo Cruz. 2017;112:775–778. doi: 10.1590/0074-02760170080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurer FP, Rüegger V, Ritter C, Bloemberg GV, Böttger EC. Acquisition of clarithromycin resistance mutations in the 23S rRNA gene of Mycobacterium abscessus in the presence of inducible erm(41. J Antimicrob Chemother. 2012;67:2606–2611. doi: 10.1093/jac/dks279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence reads and assemblies all M. abscessus in this study have been submitted to GenBank under project accession number PRJNA734660.