Abstract
Background
COVID-19 causes fatal cardiac damages. Despite many overwhelming meta-analysis related to cardiac complications following COVID-19 disease, no umbrella meta-analysis study has been conducted.
Objectives
We aimed to report the summarized pooled incidences of cardiac complications in the overall, critically ill, and deceased patients, compare the cardiac complications between the severe/non-severe or deceased/non-deceased patients, and also compare poor outcomes between patients with/without acute myocardial injury (AMI).
Methods
PubMed, Scopus, web of science, Cochrane, ProQuest, Springer, Sage journals were searched before April 2021. After assessing the quality and duplicate data, data were run by the random/fixed-effect models, I2 heterogeneity index, Egger's test, and sensitivity analysis.
Results
After removing duplicate data, in the overall COVID-19 patients, the pooled incidence of AMI, heart failure, arrhythmia, cardiac arrest, and acute coronary syndrome (ACS) were 21%, 14%, 16%, 3.46%, and 1.3%, respectively. In the patients with severe disease, the pooled incidence of AMI and shock were 33 and 35%, respectively. Similarly, in the deceased COVID-19 patients, the pooled incidence rate of AMI and arrhythmia were 56% and 47.5%, respectively. The patients with severe disease were at higher risk of AMI (RR = 5.27) and shock (OR = 20.18) compared with the non-severe cases. Incidence of AMI was associated with transfer to the intensive care units (ICU) (RR = 2.92) and mortality (RR = 2.57, OR = 8.36), significantly.
Conclusion
Cardiac complications were found to be increased alarmingly in COVID-19 patients. Baseline and during hospitalization checking with electrocardiography, echocardiography, and measuring of cardiac biomarkers should be applied.
Keywords: Cardiac complications, Cardiac injury, Incidence, COVID-19, Umbrella review, Meta-analysis
Abbreviations: ACS, acute coronary syndrome; AMI, acute myocardial injury; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; AHI, acute hepatic injury; MI, myocardial infarction; ACE2, angiotensin-converting enzyme 2; HF, heart failure; OR, odds ratio; RR, risk ratio; ICU, intensive care unit; JBI, joanna briggs institute; CI, confidence interval; PRISMA, preferred reporting items for systematic reviews and meta-analyses; AMSTAR, assessment of multiple systematic reviews tool; DIC, disseminated intravascular coagulation; CRS, cytokine release syndrome
Introduction
COVID-19 usually presents with symptoms of a respiratory infection, but extra-pulmonary symptoms, including neurological, respiratory, renal, and cardiac complications, are also common.1 Cardiac diseases are more prevalent in hospitalized patients with COVID-19.2 Studies have reported different incidence rates of cardiac injuries from 4.2 to 25.3., 4., 5., 6., 7. The incidence is higher in COVID-19 patients with severe disease.7
Diverse cardiac complications, including acute myocardial injury (AMI), myocarditis, heart failure (HF), arrhythmia, pericardial effusion, cardiomyopathy, myocardial infarction (MI), cardiogenic shock, and cardiac biomarkers elevation have been addressed in different studies.1 , 8., 9., 10., 11. A wide range of cardiac involvement, from increased blood biomarkers with no clinical symptoms (such as abnormalities on cardiac imaging or asymptomatic cardiac arrhythmias) to severe cardiac injuries, has been reported in COVID-19 patients.12 In some studies, recovery occurred within a few days,13., 14., 15. whereas in others, fulminant myocarditis resulted in a prolonged recovery.13 , 16 In patients with severe disease, the newly developed cardiac complications often lead to poor outcomes such as admission to the intensive care unit (ICU) or mortality.17 , 18 Direct myocardial injury from hemodynamic disorders or hypoxemia, inflammatory myocarditis, stress cardiomyopathy, thrombosis due to hypercoagulability, or systemic inflammation, are addressed as a potential mechanism of cardiac injury.19
Several systematic reviews and meta-analysis studies related to diverse cardiovascular complications following COVID-19 disease have been published so far.20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32. However, as the number of individual review studies increases, clinical practitioners may find it difficult to keep track of information.33 Additionally, different incidence rates were reported for each cardiac complication in every individual review study. Also, some primary studies included in some reviews were mostly with case report design, which due to their small sample size, the accuracy of their reported effect measure became questionable. In addition, only a few reviews reported cardiac complications based on the disease severity. Additionally, some previous studies have not specified the exact number of cardiac complications and poor outcomes associated with them. Therefore, this umbrella review study attempts to fill the mentioned gaps with three contributions to the existing literature. As a first contribution, we tried to provide a summarized pooled incidence of the cardiac complications based on non-severe, severe cases, and deceased COVID-19 patients, which can facilitate data understanding for clinicians. Our next contributions are to determine which one of the incidences of cardiac complications is more than others or which one of them has a strong association with poor outcomes, which have remained unclear so far. Therefore, to fill these gaps, the authors aimed to conduct the present study.
Methods
This umbrella meta-analysis study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for systematic reviews and meta-analyses34 and Joanna Briggs Institute (JBI) approach for performing umbrella meta-analysis study.33 The protocol of this study was registered in PROSPERO with the registration number CRD42021277992.
Search strategy
PubMed, Scopus, web of science, Cochrane Database of Systematic Reviews, ProQuest, Springer, Sage journal, and the internal database such as Magiran, SID, Irandoc, and elmnet were searched for systematic reviews and meta-analysis studies on cardiac complications following COVID-19 infection from December 2019 to April 2021. Google and Google Scholar were used as a searching engines as well. Furthermore, the reference lists of included articles and gray literature were screened to find more relevant studies. Our syntax was formed with Mesh terms of COVID-19, "2019-nCoV Diseases", "SARS-CoV-2 Infections", coronavirus, cardiac, cardiovascular, myocardial, myocarditis, myocardium, heart, hypotension, arrhythmia, hypertension, “blood pressure”, "systematic review", meta*, pooled effect, and pooled estimate using operators of “OR”, “AND”, “NOT”, and “*”. A sample of search syntax for Scopus was presented in Supplementary Table S1. Two authors (M.J-O), (M.D), searched the databases independently. Any conflict and discrepancy were resolved by the consensus of all authors.
Inclusion and exclusion criteria
We included articles published as a systematic meta-analysis study within peer-reviewed journals by full text in English or Persian languages from December 2019 to April 2021. Articles must also have a clear literature search strategy, a standard quality assessment tool, and report any cardiac complications after COVID-19 infection using pooled estimated approaches (incidence rate, odds ratio (OR), risk ratio (RR)) in adult patients (≥ 18 years). The meta-analysis studies with unnecessary data, narrative reviews, or studies in specific groups such as children or neonates/pregnant were excluded.
Data extraction
After developing a data extraction form using the JBI data extraction tool for systematic reviews and research synthesis,33 primary data were extracted by two authors. The developed data form included items such as the objectives, types of the review articles, year of publication, names of searched databases, the timeframe of literature search, country of studies, sample characteristics, and key findings regarding cardiac complications in COVID-19 patients.
Statistical analysis
To summarize data, quantitative meta-analysis approaches were used. For the effect sizes (incidence rate or RR/OR) were reported in more than one study, the summarized pooled was calculated using a random/fixed-effects model regarding the level of heterogeneity. The Higgin's I2-indices of 0–25, 26–75%, and 75–100% indicate low, moderate, and high between studies heterogeneity levels, respectively.35 We used both the fixed-effect model and DerSimonian-Laird random-effects model for homogeneous (I 〈 50% and P 〉 0.05) and heterogeneous (I ≥ 50% or P ≤ 0.05) data, respectively.36 The DerSimonian-Laird random-effects model considers both within-study and between-studies variations.37 According to the number of included studies, we assessed publication bias with Egger's test for AMI incidence. Assessment of publication bias of the rest pooled estimates was not possible because of an insufficient number of studies, since at least 10 studies are required to assess publication bias.38 A sensitivity analysis with the leave-one-out method was implemented to evaluate the influence of one single study effect on overall pooled estimation. The sub-group analysis was based on the severity of the disease that included the patients with severe disease (ICU admitted, critically ill, or under ventilator patients), the non-severe group (non-admitted to the ICU, non-critically ill, or non-under ventilator patients), and the deceased patients (deceased patients) accordingly. We also evaluated the primary studies for overlapping or duplicate data, as recommended.39 The primary studies in the included meta-analysis studies were reviewed for duplicate data, then any existing overlapping data concerning estimated incidence rates was excluded. To run the forest plot, after removing all duplicate data, we first selected a study with larger or more accessible data and then added other non-duplicate study data to get the summarized effect sizes. Stata version 16.0 software (Stata Corp, TX USA) was used for the quantitative analyses. The p-values for statistical significance was set at 0.05.
The methodological quality of included studies
The Assessment of Multiple Systematic Reviews (AMSTAR) tool was used to assess the methodological quality of the included studies.40 This 11-item tool is scored as 8–11, 4–7, and < 3, showing high, intermediate, and low qualities, respectively. Two authors (M.J-O and T.M) scored the quality of studies. The third author (A.E) who has more competence in review studies resolved any discrepancy.
Ethical consideration
Although no ethical approval is required for conducting a review study, our institute required an ethical code for review studies in Covid-19 related studies. Therefore, to conduct this study, the ethical code IR.BMSU.REC.1399.146 was obtained from Baqiyatallah University of Medical Sciences (BMSU).
Result
Literature search output
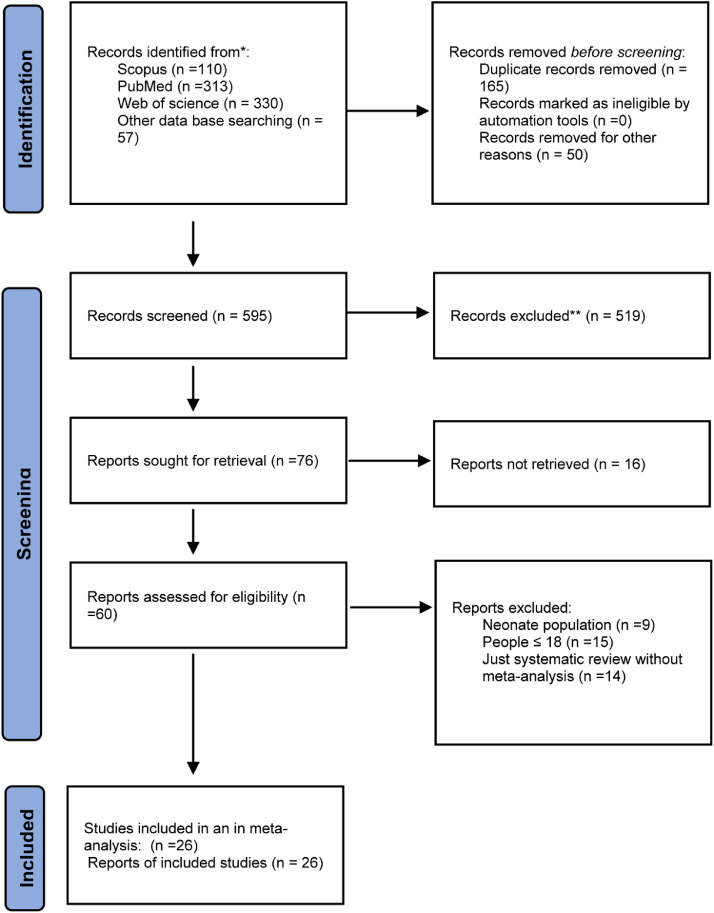
The literature search provided 810 reviews, 40 of which were eligible for full-text review. After reviewing the full text, 26 meta-analysis studies were eligible for inclusion.20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32. , 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51. The study selection process is shown in Fig. 1 .
Fig. 1.
PRISMA flow-chart of study screening and selection process.
Characteristics of included studies
In all included meta-analysis articles, overall, 916 observational studies with more than 180,148 COVID-19 patients were analyzed. The sample sizes of the two studies by Pellicori et al.27 and Momtazmanesh et al.43 with 220 and 54 primary studies, respectively, were not available. The studies of Tondas et al. (24) and Pellicori et al. (14) had the lowest and highest number of studies, with two and 220 primary articles, respectively. All studies were published during the current COVID-19 outbreak. Studies were limited mainly to China,20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31. then the USA22 , 27 , 31 , 32 followed by Italy.31 , 32 The general characteristics of the studies are shown in Table 1 .
Table 1.
The basic characteristic of the included studies.
| Row | Study | Objectives of the reviews | Name and timeframe of databases searched | No. and types of primary studies | Country | Quality score | Sample size and characteristics |
|---|---|---|---|---|---|---|---|
| 1 | Gavriatopoulou et al.1 | Assessing of cardiac complications between the survivors and non-survivours |
PubMed, Scopus, and Web of Science; December 1, 2019 - April 16, 2020. |
22 studies; case-series (18), cohort(4) |
China (22) | High | 4157 patients. |
| 2 | Shafi et al.2 | Incidence of AMI in COVID-19 patients | MEDLINE, EMBASE, and Cochrane; The onset of the outbreak- August 2020 |
N/R | China (22), Italy(1), Iran (2), South Korea(1), USA(1) | High | 8971 patients |
| 3 | Wang et al.3 | Exploring the clinical characteristics of patients with COVID-19 | PubMed, Embase, WanFang, Chinese Biomedical Literature Database and China National Knowledge Infrastructure databases; January 1, 2020 - April 12, 2020, |
40 studies, all retrospective | England(25),Chinese(15), America(2) | High | 2459 patients |
| 4 | Madjid et al.4 | Determining potential risk factors of cardiac injury in COVID-19-infected patients | Pubmed, Embase, and CNKI databases. December 1, 2019 - May 2, 2020 |
17 studies, all retrospective | All China | High | 5726 patients. |
| 5 | Qiu et al.5 | Risk factors for the COVID-19 related death | PubMed, Embase, medRxiv, and Cochrane Library; January 1, 2020 - April 13, 2020. |
15 articles/N/R | N/R | Moderate | 2401survivours and 904 non-survivours |
| 6 | Zhou et al.6 | To identify the symptoms, comorbidities, radiological features, and outcomes in COVID-19 patients. |
MEDLINE, EMBASE, and OVID; the onset of the outbreak - 26 April 2020 |
45 studies, 40 retrospective cohort studies, 2 prospective cohort studies, 1 cross-sectional study, and 2 cohort studies of unclear design. |
All from China except for two studies from the United States and one multicentre European (Belgium, France, Italy, and Spain) |
High | 14 358 patients. |
| 7 |
Chen et al.7 |
Evaluating the clinical characteristics of patients with severe disease and non-severe cases with COVID-19. | PubMed, Web of Science, MEDLINE, CNKI (China Knowledge Resource Integrated Database), and the Cochrane; December 1, 2019 - February 12, 2020 |
5 Descriptive Studies |
All China |
High | 5328 patients |
| 8 | Shi et al.8 | N/R | N/R | 12 cohort studies | N/R | Low | 2445 patients |
| 9 | Huang et al.9 | Exploring the incidence of cardiac injury and the association between cardiac injury poor outcomes in patients with COVID-19 | PubMed, the Cochrane Library, Embase, and MedRxiv databases; December 2, 2019 - June 5, 2020 |
43 studies, 27 were retrospective cohort studies and the remaining 16 were cross-sectional |
40 in China, one in Korea, and two in the USA |
High | 9475 patients |
| 10 | Babapoor-Farrokhran et al.10 | Comparing of disease outcomes between the severe (ICU) group and non-severe (non-ICU) | PubMed, Embase, and Cochrane; December 5, 2019 - April 14, 2020. |
12 cohort studies | All in China | High | 2445 patients |
| 11 | Tajbakhsh et al.11 | Assessing of subsequent cardiovascular complications and clinical events |
Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, COVID-19, ClinicalTrials.gov, and EU Clinical Trial Register; December 2019 - July 2020 |
220 unique publications; retrospective (197), randomized controlled trials (13), and prospective(20) | China (47.7%) or the USA (20.9%); 9.5% were from Italy | High | – |
| 12 | Cheng et al.12 | Assessing of cardiac complications in COVID-19 patients between “deceased” and “recovered” patients | PubMed and Embase; December 2019 - April 2020 |
54 studies; 19 records were case reports, case series, or pathological reports |
N/R | High | N/R |
| 13 | Fried et al.13 | Assessing of the association between COVID-19 and cardiovascular complications. |
PubMed and Embase; December 1, 2019 – November 30, 2020 |
12 studies | N/R | Moderate | 3044 patients |
| 14 | Tavazzi et al.14 | N/R | MEDLINE, Embase, Cochrane Database of Systematic Reviews, Scopus, and Web of Science; December 2019 - April 2020 |
13 observational studies | N/R | Moderate | 49,076 patients; 10,009 severe cases and 7773 non-severe cases |
| 15 | Salamanca et al.15 | Investigating the relationship between AMI and mortality risk in COVID-19 patients |
MEDLINE, Scopus and Web of Science; up to 10 April 2020 |
9 studies, N/R | N/R | Moderate | 1686 patients |
| 16 | Zeng et al.16 | Investigating the incidence, comorbidities, outcomes, and possible mechanisms of acute miyocardial injury in COVID-19 patients. |
PubMed and Embase; January 1, 2020, - May 30, 2020 |
16 studies, Prospective(2), Retrospective (14) |
China(14), Italy(1), and the United States (1) |
High | 2224 patients |
| 17 | Sun et al.17 | Evaluating arrhythmia in patients with severe and non-severe COVID-19 |
PubMed, Embase, Web of Science, and the Cochrane Library; December 2019 - July 25, 2020 |
5 studies, (N/R) | N/R | Moderate | 1553 patients, 349 severely ill and 1204 non-severely ill |
| 18 | Stefanini et al.18 | Analyzing the effect of COVID-19 on acute miyocardial injury |
Medline/PubMed, Scopus, and Google Scholar; December 2019 - March 25, 2020 |
11 studies, all cross-sectional designs |
All China | High |
1394 patients |
| 19 | Tondas et al.19 | Assessing electrocardiographic (ECG) ventricular repolarization indices in patients with COVID-19. | PubMed, EuropePMC, SCOPUS, Cochrane Central Database, and Google Scholar; N/R |
2 studies, two case-control | N/R |
moderate |
241 patients |
| 20 | Libby et al20 | Assessing the prevalence of cardiac complications and the resulting mortality rate in COVID-19 patients |
Medline (using PubMed), Embase, Scopus, and Web of Science, Google and Google scholar; 2019 until April 30th, 2020 |
40 studies, Two: One: Ambispective, four: prospective, 35 studies were retrospective |
the United States, one: Spain, one: Italy, the Rest: China |
High | 15,616 patients |
| 21 | Prasitlumkum et al.21 | Assessing cmorbidities in COVID-19 patients. | PubMed, Embase, medRxiv, and SSRN; Dec 1, 2019 - October 14, 2020, | 29 studies | China 10, Iran 1, German 1, Italy 6, turkey 1, us 5, France 1, Israel 1, spine 1 | High | 3508 patients |
| 22 | Zhong et al.22 | Evaluating the incidence of cardiac arrhythmias in patients with COVID-19 |
PubMed, SCOPUS, Europe PMC, Cochrane Central Databases, and Google Scholar þ Preprint Servers; N/R |
4 studies/ all Observational Retrospective |
N/R | Moderate | 784 patients |
| 23 | Zeng et al.23 | Assessing incidence rate of arrhythmia |
PubMed and Embase; July 25, 2020 - September 15, 2020, |
56 studies, Retrospective (46), Cross-sectional(2), Prospective (5) | N/R | Moderate | 17,435 patients |
| 24 | Bennett et al.24 | N/R | MEDLINE, Embase, and The Cochrane library;2019 - 27 May 2020 |
Seventeen retrospective cohort studies | N/R | Moderate | 5815 patients |
| 25 | Huang et al.25 | Prevalence of cardiovascular complications in ICU-admitted COVID-19 patients |
PubMed and Web of Science; up to November 25, 2020 | 29 studies, 5 prospective cohort studies, 1 prospective cross-sectional study, and 20 retrospective cohort studies |
China 15, USA 7, Italy 2, Spain 1, Thailand1, South Korea1, Denmark1 |
High | 4381 patients |
| 26 | Sinclair et al.32 | Comparing cardiac injuries among different viruses | PubMed and EMBASE; December 2019 - August 29, 2020 |
57 studies including | N/R | Moderate | 34,072 patients |
N/R= not reported.
Quality of studies, publication bias, and sensitivity analysis
All included studies had moderate to high methodological quality. Egger's test discovered no publication bias in the reported pooled incidences of AMI (P = 0.260), (Supplementary Fig. 2). The sensitivity analysis showed no significant single study effect for the total summarized pooled incidence of ACS, AMI, cardiac arrest, myocarditis, arrhythmia, shock, elevated CK, CK-MB, and CTnI, and also for total summarized pooled OR/RR. Supplementary Fig. 3 illustrates an example of the leave-one-out analysis for incidence of AMI.
Type of cardiac complications
A wide range of cardiac complications were identified in COVID-19 patients as follows: arrhythmia,20 , 23 , 27 , 43 , 44 , 47 , 49., 50., 51. HF,20 , 27 , 30 , 43., 44., 45. , 51 pericardial effusion,20 , 30 , 43 valve involvements,20 AMI,20 , 21 , 24 , 26 , 28., 29., 30., 31. , 43 , 44 , 46 , 51 shock,22 , 25 , 31 , 41., 42., 43. acute myocarditis,30 , 43 hypertension,43 , 47 , 49 acute MI43,30 cardiac tamponade,43 cardiac arrest,30 , 51 cardiomyopathy,30 , 43 right ventricular dysfunction,43 and acute coronary syndrome (ACS).44 , 51 Mainly clinical cardiac manifestations of the COVID-19 infected patient were chest pain, chase tightness,43 and palpitation.43
Incidence of cardiac complications in the overall COVID-19 patients
Table 2 indicates the reported (estimated by previous studies) and summarized pooled incidences (with and without duplicate data) of newly developed cardiac complications and clinical manifestations in the overall COVID-19 patients. Since the pooled incidences of ACS,44 , 51 AMI,20., 21., 22. , 24 , 28., 29., 30., 31. , 43 , 44 , 46 , 51 CA,30 , 51 HF,20 , 30 , 43 , 44 , 51 arrhythmia,20 , 31 , 43 , 44 , 49., 50., 51. and shock24 , 42 were reported in more than one study, we were able to estimate their summarized pooled incidence. In 11 studies, the pooled incidence of AMI was ranged from 15% (95% CI = 11–20%)29 to 24.4 (95% CI = 21.4–27.4).28 To calculate the overall AMI incidence in COVID-19 patients, two studies were excluded since their 95% CIs were not available.24 , 46 Also, after removing duplicate data, the summarized pooled incidence of AMI was estimated as 21% (95% CI = 15 - 26, I2 = 80.33). In addition, the summarized pooled incidences of ACS, cardiac arrest, HF, arrhythmia and shock were estimated as 1.3% (95% CI = 0–2, I2 = 55), 3.46% (95% CI = 3.1–3.83, I2 = 35.70), 14% (95% CI = 0–29, I2 = 86.66), 16% (95% CI = 9–22, I2 = 93.99), 6.1 (95% CI = −3 −15, I2 = 96.21), respectively. Further, the summarized pooled incidences of the raised CK20 , 43 CK-MB,20 , 43 cardiac Troponin I (CTnI)20 , 29 , 30 , 43 with their I2 index are indicated in Table 2. The forest plots of these estimations by considering the duplicate data are shown in Supplementary Fig. 2. The incidences of cardiac arrest and the elevated CK were run with a fixed-effect model because their heterogeneity level was lower than 50%, and the others were done with a random effect model. In Table 2, we present the summarized incidences with two forms of with and without duplicate data. We observed that after we removed the duplicate data, the summarised pooled effect size (incidence rate, OR, RR) mostly decreased and their 95% CI widened.
Table 2.
The pooled incidence of cardiac complication in total, non-severe, severe, and deceased COVID-19 patients.
| Variable or indicator | Reference | No. of Studies | Sample Size (n/N) | Reported incidence |
Summarized Incidence a (with duplicate data) |
Summarized Incidence (without duplicate data) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Pooled incidence (%) (95% CI) | I2 (%) | ES (95%CI), type of model | I2 (%) | ES (95%CI), type of model | I2 (%) | ||||
| Chest pain/ tightness | Cheng et al.12 | 6 | (387/1599) | 21.8 (8.5- 35.0) | 99 | 21.8 (8.5- 35.0) | 99 | – | – |
| Palpitation | Cheng et al.12 | 2 | (34/362) | 9.1 (6.2–12.1) | 19 | 9.1 (6.2–12.1) | 19 | – | – |
| ACS | Fried et al.13 | 4 | (18/1484) | 1.0 (0.5–1.5) | 0 | 2.92 (−2.00–7.83) |
73.22 |
1.3 (0.00–0.02) | 55 |
| Bennett et al.24 | 2 | (N/R /101) | 6.2% (1.8–12.3) | N/R | |||||
| AMI* |
Gavriatopoulou et al.1 | 13 | (356/1912) | 17.85 (13.18–23.72) | 86.84 | 20.52 (18.06–22.99) Random-effects model |
77.35 |
21 (15–26) | 80.33 |
| Shafi et al.2 | 27 | (N/R / 8971) | 20.0 (16.1–23.8). | 94.9 | |||||
| Zhong et al.3 | 15 | (N/R / 1118) | 37.1 (27.4–47.4) | 91.2 | |||||
| Stefanini et al.18 | 7 | (162/ 970) | 15 (11- 20). | 63 | |||||
| Sahranavard et al.20 | 26 | (N/R /3941) | 19.46(18.2- 20.72) | 0 | |||||
| Sinclair et al.32 | 57 | (N/R /34,072) | 21 (18–26) | ||||||
| Huang et al.25 | 14 | (N/R /927) | 30 (19–42) | 91 | |||||
| Bennett et al.24 | 11 | (N/R /2028) | 16.3 (11.8- 21.3) | 87 | |||||
| Li et al.6 | 4 | (N/R / 1096) | 16.2 (N/R) | N/R | |||||
| Cheng et al.12 | 16 | (671/2647) | 25.3 (19.5, 31.1) | 93 | |||||
| Fried et al.13 | 8 | (269/1199) | 21.2 (12.3–30.0) | 95.3 | |||||
| Salamanca et al.15 | 9 | (387/1668) | 23.9 (N/R) | 82 | |||||
| Zeng et al.16 | N/R | (542/2224) | 24.4 (21.4–27.4) | (N/R) | |||||
| CA | Sahranavard et al.20 | 3 | (N/R / 10,093) | 3.44 (3.08, 3.82) | (N/R) | 3.46(3.10–3.83) | 35.07 | 3.46(3.10–3.83) | 35.07 |
| Bennett et al.24 | 2 | (N/R /187) | 5.7 (2.7–9.6) | (N/R) | |||||
| Myocarditis | Sahranavard et al.20 | 2 | (N/R /132) | 3.66 (0.88, 7.82) | (N/R) | 3.66 (0.88, 7.82) | (N/R) | – | – |
| HF |
Gavriatopoulou et al.1 | 4 | (108/ 530) | 22.34 (14.05–33.60 | 79.44 |
19.70(17.60–21.79) |
37.01 | 0.14 (−0.00 - 0.29) | 86.66 |
| Cheng et al.12 | 2 | (87/367) | 23.7 (19.3, 28.0) | 0 | |||||
| Bennett et al.24 | 4 | (N/R /3962) | 17.6 (14.2–21.2) | 32 | |||||
| Fried et al.13 | 5 | (180/1718)) | 14.4 (5.7–23,1) | 96.7 | |||||
| Toloui et al.20 | 4 | (N/R /429) | 19.07 (15.38- 23.04) | (N/R) | |||||
| Arrhythmia |
Sahranavard et al.1 | 3 | (40 /378) | 10.11 (5.12–19.00) | 75.21 | 15.1 (11.15–19.05) | 51.2 | 0.16 (0.09–0.22) | 93.99 |
| Momtazmanesh et al12 | 4 | (93/4440 | 26.1 (5.9–46.4) | 97 | |||||
| Zhao et al13 | 6 | (191/1760) | 15.3 (8.4–22.3) | 96.5 | |||||
| Pranata et al22 | 4 | (280 / 784) | 19 (9 - 28) | 91.45 | |||||
| Koeppen et al25 | 6 | (N/R / 504) | 24 (14–36) | 84.8 | |||||
| Kunutsor et al24 | 6 | (N/R / 867) | 9.3 (5.1–14.6) | 90 | |||||
| Liao et al23 | 56 | (N/R / 17,435) | 16.8(12.8–21.2) | 98 | |||||
| Shock | Bennett et al.6 | 12 | (282 / 3191) | 11 (7–14) | N/R |
6.1(−3–15) |
96.21 |
6.1(−3–15) |
96.21 |
| Giri et al.8 | 5 | (29 / 1693) | 1.3 (0.1–2.5) | 75 | |||||
| Pericardial effusion | Sahranavard et al.20 | 2 | (N/R /175) | 2.62 (0.58- 5.73) | N/R | 2.62 (0.58- 5.73) | N/R | – | – |
| Cardiac insufficiency | Sahranavard et al.20 | 2 | (N/R /541) | 15.06 (12.15- 18.22) | N/R | 15.06 (12.15- 18.22) | N/R | – | – |
| Cardiomyopathy | Sahranavard et al.20 | 1 | (N/R /21) | 33.33 (17.19- 54.63) | N/R | 33.33 (17.19–54.63) | N/R | – | – |
| Myocardial infarction | Sahranavard et al.20 | 1 | (N/R /92) | 6.52 (3.02- 13.51) | N/R | 6.52 (3.02- 13.51) | N/R | – | – |
| Elevated CK ** |
Sahranavard et al.1 | 9 | (221/1701) | 12.99 (10.61–15.82) | 52.01 | 13.54(11.19–15.89) | 0 | 13.52 (10.80–16.25) | 0 |
| Momtazmanesh et al12 | 10 | (230/1617) | 15.9 (10.5- 21.3) | 90 | |||||
| Elevated CK-MB *** | Gavriatopoulou et al.1 | 5 | (78/723) | 10.92 (5.36–20.96) | 87.85 | 33.60 (−19.69. –86.9056) | 81.02 | 33.60 (−19.69. –86.9056) | 81.02 |
| Cheng et al.12 | 2 | (302/382) | 66.2 (6.9–100.0) | 99 | |||||
| Elevated cardiac Troponin I (CTnI) **** |
Gavriatopoulou et al.1 | 9 | (274 /1617) | 15.1 (10.79–20.93). | 85.09 | 19.76(9.80–20.72) | 78.54 | 17.94 (15.18–20.71) | 42.16 |
| Cheng et al.12 | 10 | (366/1718) | 25.3 (17.6–33.1) | 94 | |||||
| Stefanini et al.18 | 3 | (28/203) | 3.20(1.84- 4.56) | 0 | |||||
| Sahranavard et al.20 | 19 | (N/R /2474) | 22.86 (21.19–24.56) | 0 | |||||
| Elevated NT-pro BNP | Cheng et al.12 | 7 | (311/1047) | 46.5 (28.9–64.2) | 98 | 46.5 (28.9, 64.2) | 98 | – | – |
| AMI in non-severe cases | Stefanin et al.18 | N/R | 5 (1–12) | N/R | N/R | N/R | – | – | |
| AMI in non-survivors. | Fox et al.9 | 12 | N/R | 48(30–66) | 97.1 | 61(57.89–64.11) |
13.37 |
56 (40–71) | 68.36 |
| Fried et al.13 | 4 | 688 | 61.7(46.8–76.6) | 0 | |||||
| Salamanca et al.15 | 9 | 551, | 61.6(58.1–64.6) | 0 | |||||
| Stefanini et al.18 | 2 | 86 | 44(16–74) | 87 | |||||
| Heart failure in non-survivors. | Fried et al.13 | 3 | 60,336 | 47.8(41.4–54.2) | 87.6 | 47.80 (41.4–54.2) | 87.6 | – | – |
| Arrhythmia in non-survivors. | Fried et al.13 | 2 | 223 | 40.3(1.6–78.9) | 96.6 | 47.56 (38.33–56.78) | 0 | 47.56 (38.33–56.78) | 0 |
| Zhong et al.22 | 4 | 784 | 48(38–57) | 48.08 | |||||
| AMI in patients with severe disease* | Fox et al.9 | 15 | N/R | 36(25- 47) | 90.4 | 29.67 (17.92 – 41.41) | 61.81 | 33 (23–42) | 89.75 |
| Stefanini et al.18 | 4 | N = 87 | 24(15–34) | 0 | |||||
| Shock in severe patients | Wang et al.3 | 11 | (N/R / 899) | 32 (0.164,0.501) | 97.1 | 35 (22–48) | 00 | 35 (22–48) | 00 |
| Huang et al.25 | 6 | (N/R /751) | 39 (20–59) | 95.6 | |||||
Abbreviations: ACS: acute coronary syndrome; AMI: acute myocardial injury.
*troponin I above the 99th percentile upper reference limit(>28 pg/ml), or new abnormalities in electrocardiography and echocardiography; **Elevated CK (upper limit of normal of 170,200,185,310, U/L); ***Elevated Creatine kinase-MB(CK-MB) ((more than 5,18,24 ng/ml); ****Elevated cardiac Troponin I (CTnI) (more than 28,40,15.6 ng/L), *defined as a pneumonia condition with a respiratory rate more or equal than 30 times/min or oxygen saturation at resting state less or equal than 93% or partial pressure of arterial oxygen to fraction of inspired oxygen ratio less than 30,018.
Incidence of cardiac complications in the patients with severe disease and deceased patients
Among cardiac complications, the pooled incidences of AMI, shock, and HF were reported in the severe and deceased COVID-19 patients. Among patients with severe disease, the summarized incidences of AMI,29 , 52 and shock22 , 31 were computed as 29.67% (95% CI = 17.95–41.41) and 35.2 (95% CI = 22.21–47.84), respectively. We did not estimate the summarized pooled incidence of HF, because it was reported only in one study44 (47.8%, 95% CI = 41.4–54.2) .44 Additionally, the summarized pooled incidences of AMI29 , 44 , 46 , 52 and arrhythmia44 , 49 in the deceased COVID-19 patients were 61% (95% CI = 57.89–64.11) and 47.56 (95% CI = 38.33–56.78), respectively (Table 2). The forest plots are shown in Supplementary Fig. 5.
Comparison of risk/odds of cardiac complications in patients with severe disease and non-severe cases or deceased/non-deceased cases
The summarized pooled RR of AMI in patients with severe disease compared with non-severe ones was 5.27 (95% CI = 2.42–8.11, I2 = 0, P < 0.001), showing that in the absence of duplicate data, patients with severe disease suffered from AMI approximately 5.3-fold more than the non-severe group. Regarding the shock, two studies have reported OR in patients with severe disease and non-severe ones. The summarized pooled OR showed that patients with severe disease experienced shock 44 times (95% CI = −12.90–101.20, I2 = 0, P < 0.001) more than non-severe ones. However, after removing duplicate data, OR of shock decreased to 20.18 (95% CI = −44.93–85.30). The pooled OR/RR of HF, arrhythmia, and CAD were reported per a study in both survivors/non-survivors and severe/non-severe COVID-19 patients, so we were unable to estimate their summarized pooled measures (Table 3 ). The forest plot of each summarized estimate is shown in Supplementary Fig. 6.
Table 3.
Reported pooled incidence of cardiac complications between patients with severe disease and non-severe cases or deceased /non-deceased cases.
| Variable or indicator | Pooled estimated of Sever patients/non Sever patientsOR/RR(%)(95%CI) | I2 (%) | Pooled estimated of deceased Patients /non deceased PatientsOR/RR (%)(95%CI) | I2 (%) |
|---|---|---|---|---|
| AMI | OR = 13.48, 3.60 −50.477 | 0 | OR = 40.47, 11.00–69.895 | 78 |
| RR = 4.74, 2.30–9.7818 | 18 | RR = 6.91, 3.19–14.9513 | 18 | |
| RR = 5.99, 3.04–11.8010 | 83 | N/R | – | |
| Shock | OR = 53.17, 12.54–225.47 | 0 | OR = 96.60, 23.80–392.145 | 80 |
| OR = 40.47, 11.18–146.4510 | N/R | – | ||
| Heart failure | OR = 9.77, 5.36–17.7914 | 0 | OR = 5.13, 2.46–10.713 | 75.3 |
| CAD | OR = 6.85, 3.81–12.314 | 48.1 | N/R | – |
| Arrhythmia | OR = 17.97, 11.30, 28.5517 | N/R | N/R | – |
Abbreviations: CAD: coronary artery diseases.
Risk or odds of poor outcomes in COVID-19 patients with/without AMI
The pooled RR of admission to ICU and mortality in COVID-19 patients were reported in two23 , 52 and three studies,23 , 26 , 52 respectively. As the summarized pooled RR shows, patients with AMI hospitalized in ICU or were deceased by 3.33-fold (95% CI = 1.97–4.69, p < 0.001) and 4.76–fold (95% CI = 3.82–5.69, p < 0.001), respectively, more than patients without it. After removing duplicate data, the RR of hospitalization and mortality decreased to 2.92 (95% CI = 0.27–6.12, p < 0.001) and 2.57 (95% CI = 1.99–3.15, p < 0.001), respectively. Also, the summarized pooled OR for three studies30 , 43 , 46 showed that patients with AMI deceased 16.85 times (95% CI = 1.10–22.60, P < 0.001) more than COVID-19 patients without AMI (Table 4 ). Afterwards removing duplicates, OR of mortality dropped to 8.36 (95% CI = 4–12.72, P < 0.001). The forest plots of risk or odds of poor outcomes in COVID-19 patients with/without AMI are shown in Supplementary Fig. 7.
Table 4.
Risk of getting COVID- 19 severity disease, ICU admission, mortality, and other organ injuries in COVID-19 patients with AMI.
| Variable or indicator | Pooled OR(%) (95% CI) | I2 (%) | Pooled RR(%) (95% CI) | I2 (%) | |
|---|---|---|---|---|---|
| COVID-19 severity | N/R | N/R | 3.54 (2.25–5.58)9 | 80.3 | |
| ICU admission | 13.5, 3.61– 50.5212 | 0 | 2.99, 1.85–4.834 | 928 | |
| N/R | N/R | 5.03, 2.69–9.399 | 87.2 | ||
| mortality |
21.6, 95% CI: 8.6–54.15 | 82 | 4.89, 3.84–6.224 | 60. | |
| 15.77, 10.49 to 23.6920 | 45.5 | 4.99, 3.38–7.379 | 91.4 | ||
| 19.64,10.28–37.5312 | N/R | 3.85, 2.13 to 6.9610 | 86.6 | ||
| Complication | AKI | N/R | N/R | 10.09, 3.06–33.294 | 71.2 |
| ARDS | N/R | N/R | 5.89, 3.30–10.534 | 64.4 | |
| AHI | N/R | N/R | 2.24, 1.13–4.47,4 | 72.3 | |
| electrolyte disturbance | N/R | N/R | 3.35, 2.11–5.314 | 0 | |
Abbreviations: AKI: acute kidney injury, ARDS: Acute respiratory distress syndrome; AHI: acute hepatic injury.
Discussion
This study is the first umbrella review that synthesized the current meta-analysis studies about the incidence of cardiac complications following COVID-19 infection. We estimated the summarized pooled incidence of cardiac complications in the sub-groups of overall, severe, and deceased COVID-19 patients. Our findings demonstrated that the deceased patients or patients with severe COVID-19 disease have a higher incidence of cardiac complications compared to the non-severe groups. Similarly, the literature has shown that a higher incidence of newly developed cardiac complications was significantly associated with poor prognosis and mortality.53 , 54 Although the risk of cardiac complications is high in all patients with COVID-19 such as severe and non-severe ones, however, critically ill patients have a much higher incidence of these complications.12
Our findings showed that in the overall COVID-19 patients, the incidence rate of cardiomyopathy, shock, AMI, HF, CA, myocarditis, MI, and pericardial effusion ranged from the highest (33.33%) to the lowest (2.62), respectively. The cardiac injury was marked with the raised cardiac blood markers of NT-pro BNP, CK-MB, cTnI, and CK, and manifested mainly with chest pain/tightness and palpitation, respectively. Different studies revealed that increased cardiac blood markers are indicators of cardiac damage during COVID-19 infection.55 , 56 Evaluation of dynamic changes in the cardiac biomarkers shows that these indicators of heart damage increase abnormally from the middle of hospitalization and peak immediately before the patient's death.57 Cardiac biomarkers can be systematically measured over hospitalization to assist in the early detection of cardiac complications, which in turn can reduce mortality with preliminary intervention.
Another finding of this study was the incidence of other organ injuries such as liver, kidney, or lung in COVID-19 patients who suffered from cardiac complications. Due to the localization of angiotensin converting enzyme II (ACE2) protein in the human organs such as lung tissue, liver, kidney, and digestive system, COVID-19 simultaneously damages all organs by direct binding to ACE2 or by triggering a cytokine storm which results in cytokine release syndrome (CRS) .58 Cytokine storm is characterized by the secretion of pro-inflammatory mediators, such as IL-6, and an invasion of monocytes and macrophages that can cause multi-organ damage.59 CRS response has been also reported in patients infected with SARS-CoV and MERS-CoV 16.60
Our summarized meta-analysis demonstrated that COVID-19 patients with cardiac complications were several times more likely to develop severe disease, or need to be admitted to the intensive care unit, or die than those without it. In accordance with our findings, a study showed that COVID-19 patients with cardiac damage are more likely to develop a severe form of disease or to be transferred to the intensive care unit, or to die.52 In addition, the researchers showed that there was a significant increase in TnI in patients with severe disease compared to non-severe individuals.11 , 61 Further, one study showed that from the tenth day of hospitalization, a rapid increase in troponin levels was reported in the deceased patients, which was not the same as in the survivors.5 Similarly, according to several other studies, patients with severe cardiac injuries who have a high level of TnI have significantly worse outcomes than those who have low or moderate levels of TnI.3 , 62 In future studies, researchers should pay more attention to evaluating the correlation between myocardial injury and mortality in COVID-19-approved patients.
Several possible mechanisms of cardiac complications have been suggested, including direct cardiac toxicity, systematic inflammation response, an imbalance between myocardial supply and demand, systemic thromboembolism, side effects of prescribed medicines, sepsis, disseminated intravascular coagulation (DIC), and electrolyte imbalance.51 As COVID-19 disease progresses and worsens, the likelihood of any of these mechanisms of cardiac damage also increases. Therefore, critically ill patients are at a high risk of cardiac complications.
As mentioned in our study, COVID-19 can cause a variety of complications that have been observed in other viral diseases such as influenza,63 SARS,64., 65., 66. and MERS.67 It was suggested that like COVID-19, the other viruses with the capability of binding to ACE2, such as H1N1, SARS, and H7N9, may lead to a higher incidence of cardiac complications.12 The pooled incidence rates of cardiac injury regarding H1N1, SARS, and H7N9 were reported 39%, 27%, and 40%, respectively; In contrast, the non-ACE2-binding respiratory viruses have a much lower incidence rate of cardiac injury (12%) in critically ill patients.12 ACE2 accelerates the conversion of angiotensin II to angiotensin 1–7, which has a protective effect on the cardiac system. Subsequently, by binding to ACE2, viruses block the release of angiotensin 1–7, in this way, they cause myocardial damage. Viruses also damage the cardiac system directly by binding to ACE2 and initiating the inflammatory response process.68 , 69
Among our reported effect sizes, mostly overlapping data were found. After removing duplicate data, most effect sizes were reduced slightly and their 95% confidence intervals increased. In an umbrella meta-analysis study, the use of initial study data repeatedly reduces the 95% confidence interval and increases the effect size,39 which also was reflected in our study.
Limitation
Our study has limitations that must be disclosed to readers. Since, compared to other countries, most of the initial studies were mainly from China, therefore, the findings of the present study should be interpreted with caution. In addition, our study did not examine the prevalence of cardiac diseases and was limited to their incidence in COVID-19 patients. While the prevalence of cardiac disease in COVID-19 patients can be higher than its incidence. Prevalence can also show the importance of the issue more seriously. We were unable to estimate a summarized pooled incidence for some cardiac complications, such as pericardial effusion, cardiac insufficiency, cardiomyopathy, and MI, therefore, additional umbrella meta-analysis studies are required with new upcoming review reports on these cardiac complications.
Based on the mentioned limitations, we suggest some recommendations for future research. The exact mechanism of death in COVID-19 patients with cardiac complications must be precisely determined by answering the question of whether the death of COVID-19 patients is solely due to cardiac complications or whether they die from other causes such as multiple organ failure? Additionally, in addition to measuring the incidence, measuring the prevalence of cardiac disease in COVID-19 patients can provide more important information about the number of COVID-19 patients who have previously had a cardiac disease and the number of patients who have recently developed cardiac complications. Further, the incidence rates of some cardiac complications, such as cardiomyopathy and cardiac insufficiency, have been less studied, and by more assessing them, a wider range of data regarding cardiac complications will be available. Our study also has an important clinical recommendation, which is that regular cardiac examinations and check-ups should be carried out daily on COVID-19 patients. Because as our findings showed, a high incidence of diverse cardiac complications have prevailed among COVID-19 patients and both the incidence rate and the poor outcomes increase with the progression of the disease.
Conclusion
Our study demonstrated widespread newly developed cardiac complications following COVID-19 infection. Cardiac complications were associated with increased disease severity and mortality. The main signs and symptoms of cardiac injury in COVID-19 patients were chest pain, palpitation, and raised cardiac biomarkers, respectively. Although a wide incidence of cardiac complications has been reported in COVID-19 patients, however, the incidence rates of HF, shock, AMI, arrhythmia, cardiomyopathy, and cardiac insufficiency were more than others. Also, the incidence rates of cardiac complications, such as AMI, HF, and arrhythmia in severe and deceased cases were several times of non-severe non-deceased cases. Furthermore, the severity of disease, ICU admission, mortality, and other organ injuries in the COVID-19 patients with cardiac injuries were several-fold higher than the infected patients without them. Based on our findings, the authors strictly recommend that the physicians and nurses should check and track for signs and symptoms related to cardiac complications, especially in critically ill COVID-19 patients.
CRediT authorship contribution statement
Mehdi Jafari-Oori: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Seyed Tayeb Moradian: Formal analysis, Writing – original draft, Project administration. Abbas Ebadi: Validation, Supervision, Methodology. Mojtaba jafari: Formal analysis, Writing – original draft. Manijeh Dehi: Investigation, Writing – original draft.
Acknowledgments
Acknowledgments
The authors are grateful to the Baqiyatallah University of Medical Sciences for approving and supervising the project. We give appreciations for guidance and assistance from the Clinical Research Development Unit of Baqiyatallah Hospital.
Funding
This work was supported by Baqiyatallah University of Medical Sciences (BUMS).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2022.01.001.
Appendix A
Supplementary data
Appendix B. Supplementary materials
References
- 1.Gavriatopoulou M., Korompoki E., Fotiou D., et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20(4):493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shafi A.M.A., Shaikh S.A., Shirke M.M., Iddawela S., Harky A. Cardiac manifestations in COVID-19 patients-a systematic review. J Card Surg. 2020;35(8):1988–2008. doi: 10.1111/jocs.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox S.E., Lameira F.S., Rinker E.B. Vander heide rs. cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020;173(12):1025–1027. doi: 10.7326/l20-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 12.Cheng M.P., Cau A., Lee T.C., et al. Acute cardiac injury in coronavirus disease 2019 and other viral infections-a systematic review and meta-analysis. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005026. Publish Ah. doi:10.1097/ccm.0000000000005026. [DOI] [PubMed] [Google Scholar]
- 13.Fried J.A., Ramasubbu K., Bhatt R., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/circulationaha.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Hear Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salamanca J., Díez-Villanueva P., Martínez P., et al. COVID-19 “fulminant myocarditis” successfully treated with temporary mechanical circulatory support. JACC Cardiovasc Imaging. 2020;13(11):2457–2459. doi: 10.1016/j.jcmg.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J.H., Liu Y.X., Yuan J., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48(5):773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefanini G.G., Chiarito M., Ferrante G., et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106(19):1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 19.Libby P., Loscalzo J., Ridker P.M., et al. Inflammation, immunity, and infection in atherothrombosis: jacc review topic of the week. J Am Coll Cardiol. 2018;72(17):2071–2081. doi: 10.1016/j.jacc.2018.08.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahranavard M., Rezayat A.A., Bidary M.Z., et al. Cardiac complications in COVID-19: a systematic review and meta-analysis. Arch Iran Med. 2021;24(2):152–163. doi: 10.34172/AIM.2021.24. [DOI] [PubMed] [Google Scholar]
- 21.Prasitlumkum N., Chokesuwattanaskul R., Thongprayoon C., Bathini T., Vallabhajosyula S., Cheungpasitporn W. Incidence of myocardial injury in covid-19-infected patients: a systematic review and meta-analysis. Diseases. 2020;8(4):40. doi: 10.3390/diseases8040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Z., Li H., Zhu J., et al. Clinical characteristics of 2,459 severe or critically ill COVID-19 patients: a meta-analysis. Medicine. 2021;100(5):e23781. doi: 10.1097/MD.0000000000023781. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L., Wang S., Cai J., et al. Clinical characteristics of COVID-19 with cardiac injury: a systematic review and meta-analysis. Epidemiol Infect. 2020 doi: 10.1017/S0950268820002587. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett S., Tafuro J., Mayer J., et al. Clinical features and outcomes of adults with coronavirus disease 2019: a systematic review and pooled analysis of the literature. Int J Clin Pract. 2021;75(3) doi: 10.1111/ijcp.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang D., Lian X., Song F., et al. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta-analysis. Ann Transl Med. 2020;8(9):576. doi: 10.21037/atm-20-2124. 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., He X., Yuan Yuan, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2021;49(1):82–89. doi: 10.1016/j.ajic.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellicori P., Doolub G., Wong C.M., et al. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev. 2021;(3) doi: 10.1002/14651858.CD013879. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou F., Qian Z., Wang Y., Zhao Y., Bai J. Cardiac Injury and COVID-19: a systematic review and meta-analysis. CJC Open. 2020;2(5):386–394. doi: 10.1016/j.cjco.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vakhshoori M., Heidarpour M., Shafie D., Taheri M., Rezaei N., Sarrafzadegan N. Acute cardiac injury in COVID-19: a systematic review and meta-analysis. Arch Iran Med. 2020;23(11):801–812. doi: 10.34172/AIM.2020.107. [DOI] [PubMed] [Google Scholar]
- 30.Toloui A., Moshrefiaraghi D., Neishaboori A.M., Safari S., Yousefifard M., Aghajani M.H. Cardiac complications and pertaining mortality rate in COVID-19 patients; a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;9(1):e18. doi: 10.21203/rs.3.rs-122109/v1. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeppen M., Rosenberger P., Magunia H. COVID-19 related cardiovascular comorbidities and complications in critically Ill patients: a systematic review and meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2021;15 doi: 10.1177/1179548421992327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinclair J.E., Zhu Y., Xu G., et al. A meta-analysis on the role of pre-existing chronic disease in the cardiac complications of SARS-CoV-2 infection. iScience. 2021;24(4) doi: 10.1016/j.isci.2021.102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015 doi: 10.1097/XEB.0000000000000055. Published online. [DOI] [PubMed] [Google Scholar]
- 34.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009 doi: 10.1136/bmj.b2700. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: q statistic or I 2 Index? Psychol Methods. 2006 doi: 10.1037/1082-989X.11.2.193. Published online. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 doi: 10.1002/sim.1186. Published online. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 doi: 10.1016/0197-2456(86)90046-2. Published online. [DOI] [PubMed] [Google Scholar]
- 38.Valentine J.C., Pigott T.D., Rothstein H.R. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010 doi: 10.3102/1076998609346961. Published online. [DOI] [Google Scholar]
- 39.Lunny C., Pieper D., Thabet P., Kanji S. Managing overlap of primary study results across systematic reviews: practical considerations for authors of overviews of reviews. BMC Med Res Methodol. 2021;21(1) doi: 10.1186/s12874-021-01269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shea B.J., Grimshaw J.M., Wells G.A., et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007 doi: 10.1186/1471-2288-7-10. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu P., Zhou Y., Wang F., et al. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32(9):1869–1878. doi: 10.1007/s40520-020-01664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giri M., Puri A., Wang T., Guo S. Clinical features, comorbidities, complications and treatment options in severe and non-severe COVID-19 patients: a systemic review and meta-analysis. Nurs Open. 2021;8(3):1077–1088. doi: 10.1002/nop2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Momtazmanesh S., Shobeiri P., Hanaei S., Mahmoud-Elsayed H., Dalvi B. Malakan Rad E. cardiovascular disease in COVID-19: a systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt Hear J. 2020;72(1) doi: 10.1186/s43044-020-00075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y.-.H., Zhao L., Yang X.-.C., Wang P. Cardiovascular complications of SARS-CoV-2 infection (COVID-19): a systematic review and meta-analysis. Rev Cardiovasc Med. 2021;22(1):159. doi: 10.31083/j.rcm.2021.01.238. [DOI] [PubMed] [Google Scholar]
- 45.Krittanawong C., Virk H.U.H., Narasimhan B., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular risk: a meta-analysis. Prog Cardiovasc Dis. 2020;63(4):527–528. doi: 10.1016/j.pcad.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuin M., Rigatelli G., Zuliani G., Bilato C., Zonzin P., Roncon L. Incidence and mortality risk in coronavirus disease 2019 patients complicated by acute cardiac injury: systematic review and meta-analysis. J Cardiovasc Med. 2020;21(10):759–764. doi: 10.2459/JCM.0000000000001064. (Hagerstown) [DOI] [PubMed] [Google Scholar]
- 47.Wen W., Zhang H., Zhou M., et al. Arrhythmia in patients with severe coronavirus disease (COVID-19): a meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24(21):11395–11401. doi: 10.26355/eurrev_202011_23632. [DOI] [PubMed] [Google Scholar]
- 48.Tondas A.E., Mulawarman R., Trifitriana M., Nurmaini S. Irfannuddin. Arrhythmia risk profile and ventricular repolarization indices in COVID-19 patients: a systematic review and meta-analysis. J Infect Dev Ctries. 2021;15(2):224–229. doi: 10.3855/jidc.13922. [DOI] [PubMed] [Google Scholar]
- 49.Pranata R., Huang I., Raharjo S.B. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2020;20(5):193–198. doi: 10.1016/j.ipej.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao S.C., Shao S.C., Cheng C.W., Chen Y.C., Hung M.J. Incidence rate and clinical impacts of arrhythmia following COVID-19: a systematic review and meta-analysis of 17,435 patients. Crit Care. 2020;24(1):1–7. doi: 10.1186/s13054-020-03368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunutsor S.K., Laukkanen J.A. Cardiovascular complications in COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e139–e141. doi: 10.1016/j.jinf.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Z., Huang P., Du B., et al. Prevalence and clinical outcomes of cardiac injury in patients with COVID-19: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31(1):2–13. doi: 10.1016/j.numecd.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hua A., O'Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa253. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J.W., Han T.W., Woodward M., et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta-analysis. Prog Cardiovasc Dis. 2020;63(4):518–524. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estrada E. Protein-driven mechanism of multiorgan damage in COVID-19. Med Drug Discov. 2020 doi: 10.1016/j.medidd.2020.100069. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020 doi: 10.1126/science.abb8925. (80-)Published online. [DOI] [PubMed] [Google Scholar]
- 60.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0968-3. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han H., Xie L., Liu R., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25809. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estabragh Z.R., Mamas M.A. The cardiovascular manifestations of influenza: a systematic review. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2013.01.274. Published online. [DOI] [PubMed] [Google Scholar]
- 64.Yu C.M., Wong R.S.M., Wu E.B., et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006 doi: 10.1136/pgmj.2005.037515. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander L.K., David Small J., Edwards S., Baric R.S. An experimental model for dilated cardiomyopathy after rabbit coronavirus infection. J Infect Dis. 1992 doi: 10.1093/infdis/166.5.978. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y.C., Huang L.M., Chan C.C., et al. SARS in hospital emergency room. Emerg Infect Dis. 2004 doi: 10.3201/eid1005.030579. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saad M., Omrani A.S., Baig K., et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014 doi: 10.1016/j.ijid.2014.09.003. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.307708. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang F., Yang J., Zhang Y., et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol. 2014 doi: 10.1038/nrcardio.2014.59. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.