Abstract
Background
Severe asthma (SA) is a common health problem associated with increased morbidity and mortality and high medical costs. Biological therapies have emerged in recent decades as promising treatment options for patients with high type 2 (T2) SA. This retrospective observational study from Saudi Arabia aimed to investigate the effects of additional biologics therapy on reducing oral corticosteroid (OCS) consumption, frequency of asthma exacerbations, improvement in lung function, and asthma control.
Methods
This multicenter observational study enrolled a cohort of 97 patients from March 2019 to February 2021. Outcomes of anti-IgE, anti-IL5/IL5R, and anti-IL4R therapies in severe type 2 asthma were recorded and analyzed in terms of number of exacerbations (emergency visits or hospitalizations required), asthma symptoms, and use of oral corticosteroids, blood eosinophil count, asthma control according to GINA classification, and FEV1 before and during biologic therapy.
Results
Ninety-seven patients were included in the analysis The mean age was 46.7±14.1 years, and 69.1% of them were female. The average duration of biological treatment was 16.4±6.8 months. At the time of data collection, the four biologic therapies reduced the exacerbation rate per year from 82/97 (84.5%) to 14/97 (14.4%) with a percent improvement of 83% from 2.9 per year in the year before biologic treatment to 1.6 per year (p<0.001). OCS was reduced from 75/97 (77.3%) to 10/97 (10.3%) for a percent improvement of 86.7%, and the average OCS dose decreased from 7.12 mg to 6.8 mg. Mean blood eosinophil count also decreased after biologic therapy from 750.5±498.5 to 188.0±122.4 cells/μl, most significant result achieved with benralizumab, and mean FEV1 improved from 59.0±12.9% to 76.0±10.2%, most significant result achieved with omalizumab. ll patients had uncontrolled asthma before biologics therapy, but asthma control improved by 91.8% after treatment.
Conclusions
Biologic as add-on therapy for high T2 SA was found to reduce asthma exacerbations, systemic glucocorticoid doses, and SA symptoms.
Key words: asthma therapies, biologics, eosinophils, monoclonal antibodies, severe asthma
Introduction
Asthma can be a heterogeneous disease characterized by chronic airway limitation and airway hyperresponsiveness (AHR) with a history of recurrent classic respiratory symptoms with variable airway limitation [1,2]. Asthma is considered the most common chronic respiratory disease in Saudi Arabia, with an increase in prevalence in recent decades [3].
Severe asthma (SA) affects 3-10% of asthma patients and is associated with increased mortality, hospitalization, decreased quality of life, and higher health care costs [2]. SA is defined as “asthma that requires treatment with high-dose inhaled corticosteroids (ICS) plus a second controller (e.g., long-acting beta-2 agonist (LABA), long-acting muscarinic antagonist (LAMA), leukotriene modifier) (and/or OCS) to prevent it from becoming “uncontrolled” or that remains uncontrolled despite such therapy” [4,5].
There are different types of asthma, which we call “phenotypes”. These include high and low type 2 phenotypes, which have particular implications for biological therapy [2]. Most asthma patients suffer from T2 high (eosinophilic asthma), which affects 40-70% of asthma patients. A sputum sample of ≥2% leukocytes and a blood sample of ≥150 μl and a fractional exhaled nitric oxide (FeNO) level of ≥20 ppb are the features that respond well to ICS. It is further divided into two types: early-onset allergic eosinophilic (usually begins in childhood and is triggered by an allergen) [2,6,7] and late-onset non-allergic eosinophilic (usually begins in adulthood) airway inflammation and usually has more severe airway limitation and AHR without proven allergies [8]. T2-low (non-eosinophilic) type asthma includes neutrophilic inflammation and paucigranulocytic inflammation, and the patient is usually older, less responsive to corticosteroids, and has fewer allergic symptoms at the time of diagnosis [9,10]. Mixed T2-high and T2- low type asthma (granulocytic asthma) has features of both eosinophilic and neutrophilic inflammation [11].
Biologic therapies have been shown to be effective treatment options for patients with T2 SA because they target specific inflammatory pathways involved in the development of the disease [1,2,12]. Early consideration of biologic therapy may prevent patients from the side effects of OCS such as infections, weight gain, diabetes, osteoporosis, and asthma attacks [2]. One anti-IgE therapy (omalizumab) for allergic asthma, three biologics targeting IL-5/IL5R (mepolizumab, benralizumab, and reslizumab), and one biologic targeting IL-4R (dupilumab), for eosinophilic asthma, have been shown to reduce asthma exacerbations, OCS consumption, improve asthma symptoms, lung function, and quality of life in appropriately selected patients [12,13]. Cost-effectiveness is determined by what can be saved through lower exacerbations, hospitalizations, OCS usage, and work absences.
This retrospective, multicenter Saudi Arabian observational study aimed to evaluate the impact of additional biologics therapy for high T2 SA on reducing asthma exacerbations and OCS use, and to assess improvement in lung function and asthma symptoms.
Methods
Study population
This observational study enrolled a cohort of patients with SA from Almoosa Specialist Hospital in Al Ahsa, King Khaled Hospital in Hail, Al Hayat National Hospital in Jizan, and Almana General Hospital in Hofuf, Saudi Arabia, from March 2019 to February 2021. Ethical approval was obtained from the Institutional Review Board of our hospitals (IRB protocol number: ARC -21.03.3).
The primary objectives were to evaluate the rate of exacerbations and OCS reduction after the 4 biologics. The secondary objectives were to describe the improvement in forced expiratory volume in the first second (FEV1), blood eosinophils, and asthma symptom control.
Inclusion criteria
Adult patients with high T2 SA (older than 14 years) with stage 5 according to the Saudi Initiative for Asthma (SINA) [2] and Global Initiative for Asthma (GINA) [15] guidelines when asthma is not controlled with the maximum dosage of dual therapy ICS and LABA, possibly supplemented with other controllers, after confirmation of asthma diagnosis, treatment of comorbidities (allergic rhinitis, gastroesophageal reflux, obstructive sleep apnea, anxiety and others ), and adequate adherence.
Exclusion criteria
Adult patients whose asthma has been well controlled with conventional medications, patients with SA who do not use or refuse biological asthma therapy, and patients with low T2 SA.
The following data were collected for each patient: demographic data (age, sex, smoking and body mass index (BMI)); concomitant diseases (T2 diabetes, hypertension, allergic rhinitis, gastroesophageal reflux, anxiety, obstructive sleep apnea and obesity); clinical data (asthma symptoms/ week, control medications such as high-dose ICS/ LABA, LAMA, leukotriene receptor antagonists (LTRA), OCS, exacerbations, emergency department visits, and hospitalizations); and lung function tests, asthma control according to the GINA assessment of asthma control; biomarkers (blood eosinophil count before and after the biological therapy); serum total IgE at the beginning. Blood eosinophil count - a simple and inexpensive biomarker - was preferred to sputum eosinophil count (difficult to routinely collect and analyze) in assessing suitability for therapy against IL-5/ IL-5R/ IL-4 [16].
Spirometer function tests were performed at baseline and after starting the biological therapy. Parameters measured included FEV1%, FEV1/forced vital capacity (FVC) ratio, and FEV1% after bronchodilator, mean expiratory flow of 25-75%, and degree of reversibility of FEV1% after bronchodilator. The percentages of FEV1 and FEF25-75% were considered. The degree of positive reversibility was determined by a 12% or 200 ml improvement in FEV1 over the pre-bronchodilator value after use of a 200-400 g salbutamol metered dose inhaler [17].
Chest radiographs were obtained in all patients to detect diagnose or rule out asthma mimic. High-resolution computed tomography (HRCT) was performed in cases with abnormal chest radiographs.
Biologic therapy was started after at least three months of standard drug therapy with at least ICS/LABA to review patient history, confirm compliance and adherence, and control comorbidities such as gastroesophageal reflux, rhinitis, anxiety, and sleep apnea [1].
Medications used
The available biologics were omalizumab and mepolizumab in all hospitals, while benralizumab and dupilumab were available in 2 hospitals.
In 2017, omalizumab (anti-IgE) became available. The dosage of this drug is based on the level of IgE in the blood (from 30 to 700 IU) and the weight of the patient in kilograms (less than 150 kg) [1,2]. Omalizumab was administered subcutaneously / 2-4 weeks. Ten patients received 600 mg omalizumab monthly, 7 patients received 450 mg, and 5 patients received 300 mg.
Mepolizumab: in 2018, mepolizumab (anti-IL5) became available. The recommended dose of mepolizumab is 100 mg subcutaneously every 4 weeks.
Dupilumab: Dupilumab (anti-IL4) became available in 2019. Dupilumab is administered as a subcutaneous loading dose of 600 mg followed by 300 mg every two weeks [1,2].
Benralizumab, a drug targeting IL-5 receptors, became available in 2019. Subcutaneous administration of 30 mg every four weeks is recommended for the first three months and every eight weeks thereafter [1,2].
Several factors should be considered when selecting a biologic, including frequency of administration, cost, side effect profile, age at onset of asthma, and presence of comorbidities such as nasal polyps, previous response, and physician experience with treatment based on appropriate indications and availability [2].
Saudi guidelines for the use of biologics according to SINA 2021: SA uncontrolled at maximum treatment level 4:
For allergic phenotype: anti-IgE therapy (omalizumab) is recommended if IgE level is in the appropriate therapeutic range when allergy test is positive.
For eosinophilic asthma: anti- IL-5 therapy may be considered for uncontrolled eosinophilic asthma or ≥2 attacks in the last 12 months requiring systemic corticosteroids. Dupilumab (anti-IL4) is indicated for severe eosinophilic asthma with blood eosinophils ≥150 μl or FeNO >25 ppb or oral steroiddependent SA, regardless of blood eosinophil count.
Mixed phenotype: to date, there is no evidence that anti-IgE therapy is better than anti-IgE IL-5 or anti-IL4R in patients with proven atopy and high blood eosinophil counts. People with eosinophilic/mixed allergy and eosinophilic SA may have difficulty choosing the best biologics. Due to the lack of direct comparison of biologics, advocating for one biologic's superiority over another via indirect comparisons like meta-analyses and matching-adjusted techniques can be ineffective and misleading [18].
The selection of biologics (omalizumab, mepolizumab, dupilumab, and benralizumab) was based on the availability of biologics, insurance approval, patient preference (frequent or infrequent injection), and physician experience.
Twenty patients with mixed-allergic eosinophilic phenotype were not eligible for omalizumab due to a high IgE level of more than 700 IU/ml (13 patients) and exceeding the recommended dose according to serum IgE level and patient body weight (7 patients).
Asthma control was assessed using the GINA asthma control assessment classification, which classifies asthma as well controlled, partially controlled, or uncontrolled [1].
A monthly follow up was conducted to assess asthma control, adherence, and treatment compliance. For patient safety and to detect early allergic reactions, each asthma biologic was administered in the outpatient clinic, followed by at least 30 min of observation.
Statistical analysis of data
It was conducted using the SPSS version 25.0 computer package (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). For descriptive statistics, mean ± SD was used for quantitative variables and frequency and percentage for qualitative variables. The chi-square test or Fisher’s exact test were used to evaluate the differences in the frequency of the qualitative variables, while the Wilcoxon or the Kruskal-Wallis -tests were used to evaluate the differences in the means of the quantitative nonparametric variables. The statistical methods were checked assuming a significance level of p<0.05 and a highly significant level of p<0.001.
Results
Demographics and concomitant diseases
Ninety-seven patients with SA high T2 phenotype were uncontrolled on standard medications (level 5 GINA/SINA). The mean age of patients was 46.7±14.1 years, 69.1% were female, mean BMI was 32.8±6.9 kg/m2, with obesity found in 66 patients (68%), only 4 patients were active smokers, and about two-thirds were exposed to Bakhoor (indoor air pollution). The most common comorbidities were chronic allergic rhinitis (38 patients; 39.2%), gastroesophageal reflux disease (22 patients; 22.7%), anxiety (16 patients; 16.5%), and obstructive sleep apnea (6 patients; 6.2%). Type 2 diabetes with or without hypertension was found in 52 patients (53.6%). Ten patients (10.3%) had abnormal chest radiographs; 6 patients with cystic changes, 3 patients with alveolar shadows, and one patient with mucinous impaction. Four patients were diagnosed with allergic bronchopulmonary aspergillosis and 2 with chronic eosinophilic pneumonia. The mean baseline lung function test values were FEV1 59.0±12.9%, FVC 75.5±13.2%, FEV1/FVC 65.0±9.4%, reversibility 15.5±6.5 and FEF 25-75% 46.1±14.8%. The inflammatory phenotype was: SA allergic in 10 patients (10.3%), SA eosinophilic in 11 patients (11.3%), and a mixed allergic and eosinophilic phenotype in 76 patients (79.4%). The mean baseline serum total IgE was 318.3±347.8 IU/ml, while the mean blood eosinophil count was 750.5±498.5 cells/μl (Table 1).
Prior to biologics therapy: 58 patients (59.8%) received a high dose of ICS/LABA and montelukast, 22 patients (22.7%) received a high dose of ICS/LABA/ LAMA and montelukast, 12 patients (12.4%) received a high dose of ICS/LABA/ LAMA, and 5 patients (5.2%) received a high dose of ICS/LABA. Before starting treatment with biologics, the average daily OCS dose was 7.1 mg in the anti-IL5/ILR5 group and 4.8 mg in the anti-IgE group, and the frequency of OCS courses was 4.2/year in the anti-IL5/IL5R group and 2.5/year in the anti-IgE group.
OCS were taken by 75 patients (77.3%) either daily (15 patients) or for short oral courses (60 patients), and the exacerbation rate was 84.5%. The mean duration of biologics therapy was 16.4±6.8 months, lasting up to 24 months in some patients. Regarding the biologic therapy used, 53 cases (54.6%) were treated with mepolizumab, 22 cases (22.7%) with omalizumab, 12 cases (12.4%) with dupilumab, and 10 cases (10.3%) with benralizumab (Table 2).
Treatment response and general characteristics of biological therapy
No significant differences in age, sex, BMI, comorbidities including allergic rhinitis, exacerbations per year, and asthma control were found between patients receiving the four biologics, whereas the duration of biologic treatment was significantly lower for benralizumab (new drug). However, the need for OCS was significantly higher with benralizumab and mepolizumab (Table 3).
Before biological therapy: cases treated with omalizumab showed significantly lower blood eosinophil count and FEV1/FVC ratio, non-significantly low FVC%, FEV25-75% and reversibility, and non-significantly higher serum IgE. Benralizumab-treated cases had significantly higher blood eosinophil counts and low FEV1%, non-significantly lower IgE levels and reversibility, and non-significantly high FVC% and FEV25-75% (Table 4).
Outcomes of the biological therapy
The four biological therapies used reduced the exacerbation rate per year from 82/97 (84.5%) to 14/97 (14.4%) with a percentage improvement of 83%, and the average exacerbations per year decreased from 2.9 to 1.6. OCS were reduced from 75/97 (77.3%) to 10/97 (10.3%) with a percentage improvement of 86.7%, and the average OCS doses decreased from 7.12 mg to 6.8 mg. Mean blood eosinophil count also decreased from 750.5±498.5 to 188.0±122.4 cells/μl after biological therapy and mean FEV1 improved from 59.0±12.9% to 76.0±10.2% (Table 5, Figure 1).
Table 1.
Demographics, concomitant diseases, and asthma phenotypes.
| Variables | n=97 (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 46.7±14.1 |
| Min – Max | 15 – 84 |
| Sex | |
| Female | 67 (69.1) |
| Male | 30 (30.9) |
| BMI (kg/m2) | |
| Mean ± SD | 32.8±6.9 |
| Min – Max | 15 – 53 |
| Obese | |
| BMI (kg/m2) >30 | 66 (68.0) |
| Smoking history (active) | 4 (4.1) |
| Indoor air pollution (bakhour) | 66 (68.0) |
| Total serum IgE (IU/ml) | |
| Mean ± SD | 318.3±347.8 |
| Eosinophil count baseline (cells/μl) | |
| Mean ± SD | 750.5±498.5 |
| Comorbidities* | |
| Total | 81 (83.5) |
| GERD | 22 (22.7) |
| Anxiety | 16 (16.5) |
| Allergic rhinitis | 38 (39.2) |
| OSA | 6 (6.2) |
| Type 2 diabetes ± hypertension | 52 (53.6) |
| Chest radiograph° (CXR, HRCT chest) | 10 (10.3) |
| Type 2 phenotypes | |
| Eosinophilic (eosinophil (>150 cells/μl) | 11 (11.3) |
| Allergic (IgE > 30 (IU/ml) | 10 (10.3) |
| Mixed | 76 (79.4) |
| FEV1% | |
| Mean ± SD | 59.0 ± 12.9 |
| FVC% | |
| Mean ± SD | 75.5 ± 13.2 |
| FEV1/FVC ratio | |
| Mean ± SD | 65.0 ± 9.4 |
| FEF 25-75% | |
| Mean ± SD | 46.1 ± 14.8 |
| Reversibility | |
| Mean ± SD | 15.5 ± 6.5 |
| ABPA | 4 (4.1) |
| CEP2 (2.1) |
*One condition in 56 patients, two conditions in 17 patients, and three or more conditions in 8 patients;°cystic changes in 6 patients, alveolar shadows in 3 patients, and mucous impaction in 1 patient; BMI, Body mass index; GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea; ABPA, sllergic bronchopulmonary aspergillosis; CEP, chronic eosinophilic pneumonia.
Patient safety profile
Few patients had concerns about the biologic therapy, but it was safe and no other adverse events were reported during treatment. One patient had transient eosinophilia in the blood (1460 cells/μl) after treatment with dupilumab, but this later returned to normal, while comparing the efficacy of the four biologics, there was a significant decrease in mean exacerbations per year and mean OCS doses after treatment with all four biologics. Benralizumab showed the most significant results in terms of reduction in mean blood eosinophil count (from 966.0±478.3 to 101.5±127.7 cells/μL; p<0.001), exacerbation rate per year and OCS (from 100% to 0%; p<0.001). While omalizumab was associated with a significant improvement in mean FEV1% (from 55.7±11.2 to 77.1±8.0%; p<0.001) (Table 6). All patients were uncontrolled before biologics therapy, but after treatment and at the time of data collection, asthma control improved in 90 cases (92.8%), where 48 cases (49.5%) were well controlled, 42 cases (43.3%) were partially controlled, while 7 cases (7.2%) remained uncontrolled (Figure 2).
Discussion
The current multicenter observational study in Saudi Arabian patients with high T2 SA shows that the four biologics have a very favorable therapeutic effect. Due to the lack of comparative studies, it is difficult to determine which biologic is most effective in T2 SA. There is good evidence of efficacy and safety for all currently available biologics, although they differ in their ability to improve respiratory function and particularly in their ability to spare OCS [17]. The GINA 2021/ SINA 2021 guidelines define SA as asthma that is uncontrolled despite maximal ICS/LABA or requires high-dose combinations to remain controlled [2,5].
In this study, the most common comorbidities associated with SA were chronic allergic rhinitis (38 patients; 39.2%), gastroesophageal reflux disease (22 patients; 22.7%), anxiety (16 patients; 16.5%), obstructive sleep apnea (6 patients; 6.2%), and eosinophilic pneumonia (ABPA/CEP) were 6 patients (6.2%). Type 2 diabetes with or without hypertension was detected in 52 patients (53.6%). This is in agreement with Porsbjerg et al. who reported that allergic rhinosinusitis/nasal polyps (in 50%), vocal cord dysfunction (32%-50%), COPD, bronchiectasis (in 25%-40%), GERD (in 17%-74%), anxiety/depression (in 4%-17%), obstructive sleep apnea (in 31%), and allergic bronchopulmonary aspergillosis (in 1%-2%) were the most common comorbidities associated with SA [19]. Proper diagnosis and treatment of comorbidities can significantly reduce the morbidity of asthma and improve quality of life [19].
Table 2.
Asthma controlling medications and biologics used.
| Variables | n=97 (%) |
|---|---|
| Asthma medications before the biologics | |
| ICS /LABA | 5 (5.2) |
| ICS/LABA/LAMA | 12 (12.4) |
| ICS/LABA/ Montelukast | 58 (59.8) |
| ICS/LABA/LAMA/ Montelukast | 22 (22.7) |
| OCS | 75 (77.3) |
| Exacerbations/year* | 82 (84.5) |
| Duration of biologic therapy | |
| Mean ± SD (months) | 16.4±6.8 |
| Min – Max | 1 – 24 |
| Biologic therapy used | |
| Dupilumab | 12 (12.4) |
| Mepolizumab | 53 (54.6) |
| Omalizumab | 22 (22.7) |
| Benralizumab | 10 (10.3) |
*One/year in 20 patients, two/year in 23 patients, three/year in 19 patients and four or more/year in 20 patients.
Most of the patients in our study were female (69.1%). This is in line with SINA and GINA reports [1,2]. According to Fuseini et al., women are twice as likely to be affected by asthma as men, due to hormones that affect lung cells. Lung inflammation and mucus production can be affected by testosterone [20]. About 68% of patients in our study were obese and 53.6% had chronic diseases such as diabetes and hypertension. Severe asthmatics are very likely to be obese (31%-57%). Asthma becomes uncontrolled in obese individuals due to poor lung capacity, lack of fitness, and sleep apnea, GERD, and small airway dysfunction [21]. In this study, most of patients did not smoke, but one of the cultural habits was to consume bakhoor daily. A variety of respiratory diseases, including asthma, are associated with frankincense exposure, according to Al-Rawas et al. [22].
Table 3.
General characteristics and treatment according to the used biologic drugs.
| Variables | Dupilumab | Mepolizumab | Omalizumab | Benralizumab | p |
|---|---|---|---|---|---|
| n=12 (%) | n=53 (%) | n=22 (%) | n=10 (%) | ||
| Age (years) | 49.8±12.2 | 47.1±14.4 | 43.5±13.9 | 48.2±15.3 | 0.490 |
| BMI | 33.6±6.6 | 32.2±5.8 | 34.3±10.3 | 31.4±2.9 | 0.655 |
| Sex | |||||
| Male | 3 (25.0) | 19 (35.8) | 6 (27.3) | 2 (20.0) | 0.683 |
| Female | 9 (75.0) | 34 (64.2) | 16 (72.7) | 8 (80.0) | |
| Comorbidities | 11 (91.7) | 46 (86.8) | 16 (72.7) | 8 (80.0) | 0.401 |
| Allergic rhinitis | 4 (33.3) | 26 (49.1) | 5 (22.7) | 3 (30.0) | 0.158 |
| Exacerbations/year | 11 (91.7) | 46 (86.8) | 15 (68.2) | 10 (100.0) | 0.072 |
| Oral steroid need | 6 (50.0) | 45 (84.9) | 14 (63.6) | 10 (100.0) | 0.007* |
| Duration of biologic therapy (months) | 18.0±3.6 | 17.4±6.9 | 17.9±4.5 | 5.8±4.4 | <0.001* |
| Asthma control | |||||
| Well-controlled | 5 (41.7) | 26 (49.1) | 11 (50.0) | 6 (60.0) | 0.522 |
| Partly controlled | 5 (41.7) | 25 (47.2) | 8 (36.4) | 4 (40.0) | |
| Uncontrolled | 2 (16.7) | 2 (3.8) | 3 (13.6) | 0 (0.0) |
Values presented as mean ± SD were analyzed by Kruskal Wallis test; values presented as number and percent were analyzed by Chi-square test; *significant.
Table 4.
Laboratory and respiratory characteristics according to the used biologic drugs.
| Variables | Dupilumab | Mepolizumab | Omalizumab | Benralizumab | p |
|---|---|---|---|---|---|
| Eosinophil (cells/μl) | 817.1±456.3 | 768.3±433.6 | 330.2±248.3 | 966.0±478.3 | 0.001* |
| IgE (IU/ml) | 317.0±477.6 | 306.7±370.7 | 374.5±281.5 | 258.0±142.1 | 0.125 |
| FEV1% | 68.7±17.2 | 59.6±12.3 | 55.7±11.2 | 52.0±5.6 | 0.013* |
| FVC% | 80.4±15.3 | 76.1±13.6 | 69.9±11.7 | 78.8±7.1 | 0.064 |
| FEV1/FVC ratio | 73.6±9.2 | 63.7±9.1 | 63.0±8.3 | 65.8±9.1 | 0.007* |
| FEF 25-75% | 50.6±11.9 | 44.0±16.1 | 45.4±15.2 | 53.2±4.3 | 0.203 |
| Reversibility | 18.6±16.4 | 15.3±3.5 | 14.8±3.7 | 14.3±2.7 | 0.864 |
Values presented as mean ± SD were analyzed by Kruskal Wallis test; *significant.
Table 5.
Treatment response before and after biologic therapy.
| Variables | Before the biologic n=97 (%) | After the biologic n=97 (%) |
|---|---|---|
| Exacerbations/year | ||
| Total | 82 (84.5) | 14 (14.4) |
| Average* | 2.9 | 1.6 |
| Oral corticosteroid | ||
| Total | 75 (77.3) | 10 (10.3) |
| Average° | 7.12 | 6.8 |
| Blood eosinophil count (cells/μL) | 750.5±498.5 | 188.0±122.4 |
| FEV % | 59.0±12.9 | 76.0±10.2 |
*Before therapy, the overall exacerbations per year were 238 occurred in 82 patients with average 2.9 while after therapy, the overall exacerbations per year were 22 occurred in 14 patients with average 1.6; °before therapy, the overall doses of OCS were 534 mg given to 75 patients with average 7.12 mg while after therapy, the overall doses of OCS were 68 mg given to 10 patients with average 6.8 mg.
Figure 1.
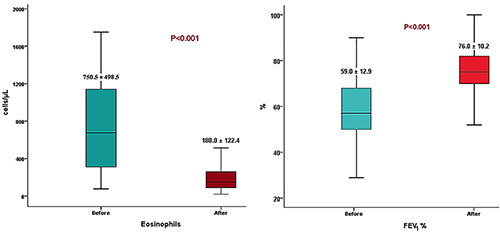
Mean total eosinophils and FEV1% before and after biologic therapy.
Figure 2.
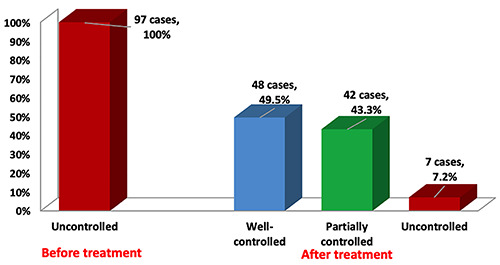
Asthma control before and after biologic therapy.
Table 6.
Treatment response before and after biologic therapy.
| Variables | Dupilumab | Mepolizumab | Omalizumab | Benralizumab | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=12 (%) | n=53 (%) | n=22 (%) | n=10 (%) | |||||||||
| Before | After | Before | After | Before | After | Before | After | |||||
| Eosinophil (cells/μl) | 817.1±456.3 | 222.1±124.6 | 768.3±433.6 | 187.1±114.0 | 330.2±248.3 | 177.3±130.7 | 966.0±478.3 | 101.5±127.7 | ||||
| p | <0.001* | <0.001* | 0.047* | <0.001* | ||||||||
| FEV1% | 68.7±17.2 | 82.5±11.0 | 59.6±12.3 | 75.4±10.7 | 55.7±11.2 | 77.1±8.0 | 52.0±5.6 | 68.0±7.4 | ||||
| p | 0.024* | <0.001* | <0.001* | <0.001* | ||||||||
| Exacerbations / year | ||||||||||||
| Total | 11 | 2 | ||||||||||
| (91.7) | (16.7) | 46 | 7 | 15 | 5 | 10 | 0 | |||||
| p | 0.001* | (86.8) | (13.2) | (68.2) | (22.7) | (100.0) | (0.0) | |||||
| Average | 2.6±1.6 | 0.25±0.62 | <0.001* | 0.006* | <0.001* | |||||||
| p | <0.001* | <0.001* | 2.1±1.72 | 0.12±0.3 | 3.1±2.2 | 0.5±0.91 | 2.5±1.4 | 0 | ||||
| Oral corticosteroid | <0.001* | <0.001* | ||||||||||
| Total | 6 | 0 | <0.001* | |||||||||
| (50.0) | (0.0) | 45 | 5 | 14 | 5 | 10 | 0 | |||||
| p | 0.014* | (84.9) | (9.4) | (63.6) | (22.7) | (100.0) | (0.0) | |||||
| Average | 3.8±2.1 | 0 | <0.001* | 0.014* | <0.001* | |||||||
| p | <0.001* | 5.8±6.2 | 0.75±1.3 | 4.9±3.1 | 1.1±1.8 | 7.1±6.4 | 0 | |||||
| <0.001* | <0.001* | |||||||||||
Values presented as mean ± SD were analyzed by Wilcoxon test; values presented as number and percent were analyzed by Fischer Exact test; *significant.
Asthma phenotypes were classified according to T2 cytokine levels. There were 10 allergic patients (10.3%), 11 eosinophilic patients (11.3%), and 76 patients (79.4%) with a mixed allergic and eosinophilic phenotype. According to many studies, asthma characteristics may overlap, e.g., severe allergic asthma and severe eosinophilic asthma, so there may be overlap in eligibility for biologics therapy [23-27]. In asthma patients, overlapping phenotypes (severe allergic asthma may overlap with a severe eosinophilic asthma) are common and are often associated with clinical and inflammatory profiles. About 73.4% of participants had overlapping phenotypes [28]. The GINA guidelines suggest various combinations of factors to identify T2 inflammation, including blood eosinophil count, FeNO, sputum eosinophil count, need for OCS maintenance therapy, and comorbidities [1]. Most of our participants were obese and had multiple comorbidities that can flare up with long-term OCS, so biologic therapy was preferred over OCS for severe uncontrolled asthma. Early consideration of biologic therapy may spare patients from frequent or chronic use of OCS and reduce its side effects such as infections, weight gain, diabetes and osteoporosis, and asthma attacks [2].
In this study, the four biologics used reduced asthma exacerbation rates by 83%, while OCS was reduced by 86.7%, with benralizumab achieving the most significant results (from 100% to 0%; p<0.001). Overall, all five biologics currently approved for SA appear to reduce exacerbation rates by approximately 50%, with greater effects at higher absolute sputum and blood eosinophil counts [12]. A systematic review of the efficacy and safety of biologic therapy was conducted by Agache et al. who found that each biologic added to asthma treatment reduced exacerbation rates by more than 50% compared with standard treatment (benralizumab >50%, dupilumab 29.4%, mepolizumab >50%, and omalizumab >60%) [29]. GINA analyzed randomized controlled trials (RCTs) of treatment of SA with adjunctive biologic therapy in adults who had at least one exacerbation in the past year, and omalizumab reduced exacerbations by 50-65% and OCS by 40-50%. Mepolizumab, reslizumab, and benralizumab all reduced severe exacerbations by 55% and OCS by 50%. Dupilumab significantly reduced severe exacerbations and OCS by 50% [30]. ERS /ATS Task Force for management of SA (5) reviewed RCTs in which biologics were used to treat SA (3 for mepolizumab, 5 for benralizumab, 3 for dupilumab) and concluded, that exacerbations were reduced by 50% for mepolizumab and benralizumab and 70.5% for dupilumab, and OCS-dependent doses were reduced by 50% for mepolizumab and dupilumab and 75% for benralizumab [5].
The results reported by ERS /ATS were higher than in GINA, due to differences in inclusion criteria and the definition of SA used before the analysis of RCTS. Kotisalmi et al. found that anti- IL5/IL5R significantly reduced OCS doses, as did omalizumab in their real-life study (although not statistically significantly). Anti- IL5/IL5R and omalizumab reduced the need for surgery for chronic rhinosinusitis. In anti-IL5/IL5R and anti-IgE, the frequency of OCS treatments decreased significantly. In the anti-IL5/IL5R group, exacerbations decreased from 7.6 to 3.2 per year [24]. Using a meta-analysis of real-world data, Bousquet et al. found that omalizumab reduced the proportion of patients receiving OCS by 41% at 1 year. Omalizumab reduced hospitalizations and severe exacerbations by 85% and 59%, respectively, over a 12-month period [31]. Other real-world studies also reported a reduction in exacerbations and hospitalizations with omalizumab [32,33]. Many studies reported inconclusive data demonstrating a reduction in OCS in patients treated with omalizumab [34]. A meta-analysis by Li et al. found that mepolizumab was associated with fewer exacerbations and hospitalizations, with a significant reduction in OCS [35]. An analysis of 130 patients with severe eosinophilic asthma treated with benralizumab showed that the rate of acute exacerbations decreased by 72.8% at 48 weeks and 51.4% were able to complete maintenance therapy [36]. In real-world studies, dupilumab reduced asthma exacerbations by 60%, OCS use by 70%, and OCS discontinuation by 50% in patients with T2 SA [37,38]. On the other hand, some studies reported that most RCTs of biologics in patients with uncontrolled SA showed a significant response to placebo with a reduction in exacerbations, improvement in lung function, and improvement in patient-reported outcomes. These results suggest that SA is not inherently severe but is often poorly controlled. Therefore, these studies suggest that although targeting T2 cytokines with biologics may improve asthma control, it may not be necessary for many patients [39,40].
Real-life studies showed better response to biologic therapy than RCTs, which may be due to differences in inclusion criteria and patient selection. The results of this study were consistent with real-life studies and better than RCTs.
The GINA classification to assess asthma control was used. Before biologics therapy, all patients were uncontrolled, but after treatment and at the time of data collection, 92.8% showed improved asthma control. This is consistent with Agache et al. who reported that dupilumab, omalizumab, benralizumab, and mepolizumab can improve asthma control [29]. According to Kotisalmi et al. [24], both anti-IL5/IL5R and anti-IgE drugs significantly improved asthma control test results (ACT). Using real-world data on the efficacy of biologics as add-on therapy for the treatment of SA, omalizumab [31-33], mepolizumab [35,41], benralizumab [36], and dupilumab [37,38] were found to contribute to better asthma control (different questionnaires were used to assess asthma control). However, ERS /ATS reports that the effects of the different biologics on asthma control and quality of life are modest for all drugs and do not reach the MCID threshold [5].
The four biologic therapies used were able to reduce blood eosinophil counts, but benralizumab showed the most marked improved results, with a decrease in mean blood eosinophil count (from 966.0±478.3 to 101.5±127.7 cells/μl; p<0.001). This conclusion is consistent with other studies [5,30]. Ortega et al. reported that eosinophil levels decreased by 83-86 percent in patients receiving mepolizumab [41]. Anti- IL -5 therapy (mepolizumab and reslizumab) effectively reduced the number of circulating eosinophils and the number of eosinophils in sputum, but did not improve airway mucosal eosinophilia, rates of acute exacerbations, lung function, and symptom scores in several studies. These disappointing results may also be due to inappropriate patient selection [41]. Benralizumab results in almost complete clearance of eosinophils in the blood and complete clearance of eosinophils in the airways within 24 hours of administration compared with mepolizumab [42]. The transient increase in blood eosinophil granulocytes associated with dupilumab steadily decreases over time to levels below baseline in some asthma patients taking the drug. This may suggest that eosinophil granulocytes in the blood are not transiently mobilized to the lungs to reduce the production of eosinophil chemokines in the local area of inflammation [43]. Omalizumab reduced the amount of circulating T cells and eosinophils, according to Djukanović et al. [44].
In this study, FEV1% after bronchodilation improved significantly after treatment with the biologics, but omalizumab was associated with the most significant improvement in FEV1, more than the other biologic (from 55.7±11.2 to 77.1±8.0%; p<0.001). This is consistent with the results of Agache et al. who found an increase in FEV1 compared with standard of care (benralizumab MD + 140 ml, mepolizumab MD + 110.9 ml, reslizumab MD + 141.82 ml, and omalizumab mean percent change + 3. %) and that dupilumab can increase FEV1 compared with standard of care (110 to 250 ml) [29]. Clinical trials of novel agents targeting these pathways have demonstrated efficacy and ability to improve FEV1 in patients with SA [45]. Some studies reported significant improvement in FEV1 with omalizumab [23,31,32], mepolizumab [35,41,46,47], benralizumab [36,48,49], and dupilumab [37,38,43], whereas other studies found no significant change in FEV1 with omalizumab [33], mepolizumab [25,50,51], benralizumab [52], and dupilumab [53]. ERS /ATS reported that the effects of the various biologics on FEV1 were modest for all drugs and did not reach the MCID threshold [5]. Dupilumab was most effective in preventing asthma exacerbations and improving FEV1 only when patients had eosinophils ≥150 cells/μL and FeNO ≥25 ppb [54].
No significant side effects were reported, except that one case experienced mild blood eosinophilia (1480 μl) with dupilumab, but this later resolved. Biological medicines are generally safe, but side effects are possible. The side effects of biologic drugs depend on the particular drug and the route of administration [12]. The available five biologics were considered safe with no significant side effects more than placebo [5].
Conclusion
Our real-life experience supports the efficacy of omalizumab, mepolizumab, dupilumab, and benralizumab as an adjunctive biologic treatment for T2 SA in reducing asthma exacerbations and the use of OCS, and in improving asthma symptom control. It also resulted in improved lung function (FEV1%) and a reduction in blood eosinophil count without significant side effects, in appropriately selected patients. These results appear to be consistent with those obtained in previous RCTs and real-life studies. The biologic would be an optimal choice to improve the clinical treatment and management of patients with allergic, eosinophilic, and mixed allergic eosinophilic T2 SA, particularly those who have been uncontrolled with at least the maximum dose of ICS/LABA and other controllers and who can be spared the side effects of long-term OCS.
Study limitations
The retrospective design of this study and the nonblinded selection of the biologic used could be a disadvantage. Not all comorbidities such as food and drug allergies have been investigated and no specific allergy tests (in vivo or in vitro) have been performed. Exhaled fractionated nitric oxide and sputum eosinophil were not measures.
There is a need for more information on the appropriate duration of biologics therapy in patients with SA. It should also be investigated whether temporary interruption of therapy affects outcomes. Further studies with larger populations are needed.
Acknowledgments
We would like to thank the research centers in our hospitals.
Abbreviations
- CXR:
chest X-ray
- FEF:
forced-expiratory flow
- FeNO:
fractionated exhaled nitric oxide
- FEV1:
forced expiratory volume first second
- FVC:
forced vital capacity
- GINA:
global initiative for asthma
- HRCT:
high-resolution computed tomography
- ICS:
inhaled corticosteroids
- Ig:
immunoglobulin
- IL:
interleukin
- IU:
international unit
- LABA:
long-acting beta2 agonist
- LAMA:
long-acting muscarinic antagonist
- OCS:
oral corticosteroids
- RCTS:
randomized controlled trials
- SA:
severe asthma
- SINA:
Saudi initiative for asthma
- T2:
Type 2
References
- 1.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2019. Accessed: 14 Oct 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf [Google Scholar]
- 2.Al-Moamary MS, Alhaider SA, Alangari AA, Idrees MM, Zeitouni MO, Al Ghobain MO, et al. The Saudi Initiative for Asthma - 2021 Update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med 2021;16:4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohamed Hussain S, Ayesha Farhana S, Mohammed Alnasser S. Time trends and regional variation in prevalence of asthma and associated factors in Saudi Arabia: A systematic review and meta-analysis. Biomed Res Int 2018;2018:8102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014. 43:343–73. [DOI] [PubMed] [Google Scholar]
- 5.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020;55:1900588. [DOI] [PubMed] [Google Scholar]
- 6.Del Giacco SR, Bakirtas A, Bel E, Custovic A, Diamant Z, Hamelmann E, et al. Allergy in severe asthma. Allergy 2017;72:207-20. [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015;16:45-56. [DOI] [PubMed] [Google Scholar]
- 8.Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat Med 2013;19:977-9. [DOI] [PubMed] [Google Scholar]
- 9.Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, et al. Three phenotypes of adultonset asthma. Allergy 2013;68:674-80. [DOI] [PubMed] [Google Scholar]
- 10.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 2017;377:965-76. [DOI] [PubMed] [Google Scholar]
- 11.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet 2018;391:783-800. [DOI] [PubMed] [Google Scholar]
- 12.McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med 2019;199:433-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakakos A, Loukides S, Usmani OS, Bakakos P. Biologics in severe asthma: the overlap endotype - opportunities and challenges. Expert Opin Biol Ther 2020;20:1427-34. [DOI] [PubMed] [Google Scholar]
- 14.McQueen RB, Sheehan DN, Whittington MD, van Boven JFM, Campbell JD. Cost-effectiveness of biological asthma treatments: A systematic review and recommendations for future economic evaluations. Pharmacoeconomics 2018;36:957-71. [DOI] [PubMed] [Google Scholar]
- 15.Global Initiative for Asthma (GINA). Pocket guide for asthma management and prevention. Last updated: April 2021. Available from: https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/ [Google Scholar]
- 16.Pouliquen IJ, Kornmann O, Barton SV, Price JA, Ortega HG. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. Int J Clin Pharmacol Ther 2015;53:1015-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention National Institute for Occupational Safety and Health (NIOSH) [Internet]. Spirometry. Available from: https://www.cdc.gov/niosh/topics/spirometry/ [Google Scholar]
- 18.Papaioannou AI, Diamant Z, Bakakos P, Loukides S. Towards precision medicine in severe asthma: Treatment algorithms based on treatable traits. Respir Med 2018;142:15-22. [DOI] [PubMed] [Google Scholar]
- 19.Porsbjerg C, Ulrik C, Skjold T, Backer V, Laerum B, Lehman S, et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J 2018;5:1440868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep 2017;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiroki T, Shore SA. Obesity and severe asthma. Allergol Int 2019;8:135-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Rawas OA, Al-Maniri AA, Al-Riyami BM. Home exposure to Arabian incense (bakhour) and asthma symptoms in children: a community survey in two regions in Oman. BMC Pulm Med 2009;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16. [DOI] [PubMed] [Google Scholar]
- 24.Kotisalmi E, Hakulinen A, Mäkelä M, Toppila-Salmi S, Kauppi P. A comparison of biologicals in the treatment of adults with severe asthma – real-life experiences. Asthma Res Pract 2020;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol 2015;135:299-310. [DOI] [PubMed] [Google Scholar]
- 26.Tran TN, Zeiger RS, Peters SP, Colice G, Newbold P, Goldman M, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol 2016;116:37-42. [DOI] [PubMed] [Google Scholar]
- 27.Albers FC, Müllerová H, Gunsoy NB, Shin JY, Nelsen LM, Bradford ES, et al. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J Asthma 2018;55:152-60. [DOI] [PubMed] [Google Scholar]
- 28.Han YY, Zhang X, Wang J, Wang G, Oliver BG, Zhang HP, et al. Multidimensional assessment of asthma identifies clinically relevant phenotype overlap: A cross-sectional study. J Allergy Clin Immunol Pract 2021;9:349-362.e18. [DOI] [PubMed] [Google Scholar]
- 29.Agache L, Beltran J, Akdis C, Akdis M, Canelo-Aybar C, Canonica GW, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biological in severe asthma. Allergy 2020;75:1023-42. [DOI] [PubMed] [Google Scholar]
- 30.Global Initiative for Asthma (GINA). Diagnosis and management of difficult-to-treat and severe asthma in adolescent and adult patients. Available from: https://ginasthma.org/severeasthma/ [Google Scholar]
- 31.Bousquet J, Humbert M, Gibson PG, Kostikas K, Jaumont X, Pfister P, Nissen F. Real-world effectiveness of omalizumab in severe allergic asthma: A meta-analysis of observational studies. J Allergy Clin Immunol Pract 2021;9:2702-14. [DOI] [PubMed] [Google Scholar]
- 32.Niven RM, Saralaya D, Chaudhuri R, Masoli M, Clifton I, Mansur AH, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open 2016;6:e011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, et al. Omalizumab effectiveness by biomarker status in patients with asthma: Evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract 2019;7:156-164.e1. [DOI] [PubMed] [Google Scholar]
- 34.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes- Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011;154:573-82. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Zhang Q, Wang J, Gao S, Li C, Wang J, et al. Real-world effectiveness of mepolizumab in severe eosinophilic asthma: A systematic review and meta-analysis. Clin Ther 2021;43:e192-e208. [DOI] [PubMed] [Google Scholar]
- 36.Kavanagh JE, Hearn AP, Dhariwal J, d'Ancona G, Douiri A, Roxas C, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2021;159:496-506. [DOI] [PubMed] [Google Scholar]
- 37.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018;378:2486-96. [DOI] [PubMed] [Google Scholar]
- 38.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoiddependent severe asthma. N Engl J Med 2018;378:2475-85. [DOI] [PubMed] [Google Scholar]
- 39.Nair P. Anti-interleukin-5 monoclonal antibody to treat severe eosinophilic asthma. N Engl J Med 2014;371:1249-51. [DOI] [PubMed] [Google Scholar]
- 40.Lacy P, Nair P. The human eosinophil. In: Greer J, Arber D, Glader B, Lost A, Means R, Paraskevas F., et al., editors. Wintrobe’s clinical hematology, 14th ed. Philadelphia: Lippincott Williams & Wilkins; 2018. [Google Scholar]
- 41.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198-207. [DOI] [PubMed] [Google Scholar]
- 42.Roxas C, Fernandes M, Green L, D’Ancona G, Kavanagh J, Kent B, et al. A comparison of the clinical response to mepolizumab and benralizumab at 4 weeks. Thorax 2018;73:A50 [Google Scholar]
- 43.Wechsler M, Klion A, Paggiaro P, Nair P, Staumont-Salle D, Amr Radwan A, et al. Effect of dupilumab treatment on blood eosinophil levels in patients with asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), eosinophilic esophagitis (EoE), or atopic dermatitis (AD). J Allergy Clin Immunol 2021;147:AB140. [DOI] [PubMed] [Google Scholar]
- 44.Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2004;170:583-93. [DOI] [PubMed] [Google Scholar]
- 45.Johnson N, Varughese B, De La Torre MA, Surani SR, Udeani G. A review of respiratory biologic agents in severe asthma. Cureus 2019;11:e5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2018;143:1742-51. [DOI] [PubMed] [Google Scholar]
- 47.Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther 2019;41:2041-56. [DOI] [PubMed] [Google Scholar]
- 48.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomized, multicenter, placebo-controlled phase 3 trial. Lancet 2016;388:2115-27. [DOI] [PubMed] [Google Scholar]
- 49.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128–41. [DOI] [PubMed] [Google Scholar]
- 50.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651-9. [DOI] [PubMed] [Google Scholar]
- 51.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017;9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017;376:2448-58. [DOI] [PubMed] [Google Scholar]
- 53.Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, et al. A. Liberty Asthma QUEST: Phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther 2018;35:737-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busse WW. Biological treatments for severe asthma: A major advance in asthma care. Allergol Int 2019;68:158-66. [DOI] [PubMed] [Google Scholar]


