Abstract
Background
With the introduction of Xpert MTB/RIF assay (Xpert), its incorporation into tuberculosis (TB) diagnostic algorithm has become an important issue. The aim of this study was to evaluate the performance of the Xpert assay in comparison with a commercial polymerase chain reaction (PCR) assay.
Methods
Medical records of patients having results of both Xpert and AdvanSure TB/NTM real-time PCR (AdvanSure) assays using the same bronchial washing specimens were retrospectively reviewed.
Results
Of the 1,297 patients included in this study, 205 (15.8%) were diagnosed with pulmonary TB. Using mycobacterial culture as the reference method, sensitivity of the Xpert assay using smear-positive specimens was 97.5%, which was comparable to that of the AdvanSure assay (96.3%, p=0.193). However, the sensitivity of the Xpert assay using smear-negative specimens was 70.6%, which was significantly higher than that of the AdvanSure assay (52.9%, p=0.018). Usng phenotypic drug susceptibility testing as the reference method, sensitivity and specificity for detecting rifampicin resistance were 100% and 99.1%, respectively. Moreover, a median turnaround time of the Xpert assay was 1 day, which was significantly shorter than 3 days of the AdvanSure assay (p<0.001).
Conclusion
In comparison with the AdvanSure assay, the Xpert assay had a higher sensitivity using smear-negative specimens, a shorter turnaround time, and could reliably predict rifampin resistance. Therefore, the Xpert assay might be preferentially recommended over TB-PCR in Korean TB diagnostic algorithm.
Keywords: Tuberculosis, Polymerase Chain Reaction, Molecular Diagnostic Techniques
Introduction
Rapid and accurate diagnosis of tuberculosis (TB) and drug resistance are essential for timely and proper patient management. In response, introduction of molecular diagnostic tests has become a major step forward in TB diagnosis [1]. In particular, polymerase chain reaction (PCR) developed in the early 1980s and was subsequently incorporated into the TB diagnostic algorithm [2]. However, TB-PCR requires high levels of infrastructure and trained personnel. In addition, it may present contamination and biosafety issues.
On the other hand, the Xpert MTB/RIF assay (Xpert) is a new generation molecular diagnostic platform. It is a fully automated cartridge-based real-time PCR test that can simultaneously detect both TB and rifampicin resistance in less than 2 hours [3]. Notably, numerous studies have shown that the Xpert assay has a high diagnostic accuracy [4,5], thus contributing to improved patient management [6,7]. With implementation of the Xpert assay in routine TB services, its incorporation into the existing TB diagnostic algorithm has emerged as an important issue [8]. Currently, the World Health Organization (WHO) recommends the Xpert assay as an initial diagnostic test for pulmonary tuberculosis (PTB), replacing smear microscopy for patients of all ages [9].
In Korea, the Xpert assay was first recommended as a rapid drug susceptibility testing (DST) for high-risk groups in 2014 [10], which was then further recommended for early TB detection in 2017 [11]. In contrast, TB-PCR has been recommended as an initial test for early TB detection since 2014 [10]. As a result of these guideline updates, both assays have been recommended as initial TB diagnostics in Korea, without specific prioritization of one over the other.
However, how to incorporate the Xpert assay into the TB diagnostic algorithm in Korea and whether it should replace TB-PCR remain unclear. Thus, the aim of this study was to evaluate the performance of the Xpert assay as compared to a commercial TB-PCR assay in terms of diagnostic accuracy and turnaround time.
Materials and Methods
1. Study population
Patients who had both Xpert and TB-PCR assay results using the same bronchial washing specimens between January 2019 and October 2020 at Pusan National University Yangsan Hospital were included. Those who started anti-TB treatment before bronchoscopy were excluded. Medical records of included patients were retrospectively reviewed to collect their demographic, clinical, and laboratory data. The present study protocol was reviewed and approved by the Institutional Review Board (IRB) of Pusan National University Yangsan Hospital (IRB approval number: 05-2021-129). The requirement for obtaining informed consent was waived due to its retrospective nature.
2. Bronchoscopy procedures
Bronchoscopy was performed by full-time faculty staff of Pulmonology Division at our institution. After inspecting all visible bronchial trees, bronchial washing of the targeted segment showing abnormal chest computed tomography lesions suggestive of active PTB or other diseases was performed. Bronchial washing fluid was obtained by repeatedly instilling and aspiring 10 mL of normal saline until 20–30 mL was obtained in a trap bottle.
3. Laboratory specimen processing and examination
The Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) was performed and interpreted following the manufacturer’s instructions. For the Xpert assay, 2–3 mL of bronchial washing fluid without decontamination or concentration was used. The bronchial washing fluid and the Xpert sample reagent were mixed at 1:2 ratio and incubated at room temperature for 15 minutes. Then 2 mL of the mixture was transferred into an Xpert cartridge.
Remaining bronchial washing fluid was pretreated with equal volumes of 4% sodium hydroxide and centrifuged at 3,000 ×g for 20 minutes. A TB-PCR assay was performed using an AdvanSure TB/NTM real-time PCR kit (AdvanSure, LG Life Science, Daejeon, Korea), following the manufacturer’s protocols. Acid fast Bacilli smears were performed using auramine-rhodamine fluorescent staining and confirmed using Ziehl-Neelsen staining. Sediments were inoculated for culture on 3% Ogawa medium (Eiken Chemical, Tokyo, Japan) and in Mycobacteria Growth Indicator Tube medium (Becton Dickinson, Franklin Lakes, NJ, USA).
4. Definitions
PTB was defined and classified as follows. Culture-confirmed PTB was defined by Mycobacterium tuberculosis growth in liquid or solid culture media. Histologically confirmed PTB was defined as the presence of caseating or noncaseating granulomatous lung tissue inflammation. Patients with a high index of suspicion for PTB based on symptoms and radiographic findings whose symptoms and radiographic findings were improved after anti-TB treatment were defined as having clinically diagnosed PTB. Turnaround time was defined as the time from specimen submission to the reporting of results. It was collected through electronic medical records for each patient.
5. Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using 95% confidence intervals (CI) to determine diagnostic accuracies of Xpert and AdvanSure assays. McNemar’s test was used to compare their sensitivities and specificities. Clinical data are presented as medians with interquartile ranges (IQRs) for continuous variables and as numbers with percentages for categorical variables. Continuous variables were compared using the Mann-Whitney U-test. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. A p-value<0.05 was considered significant. All statistical analyses were performed using MedCalc for Windows, version 20.008 (MedCalc Software, Ostend, Belgium).
Results
1. Patient characteristics
Results of both Xpert and AdvanSure assays obtained using the same bronchial washing specimen, were available for a total of 1,334 patients during the study period. Of them, 37 patients were excluded due to initiation of anti-TB treatment before bronchoscopy. Thus, 1,297 patients were included in the final analysis (Figure 1). Of these 1,297 patients, 205 (15.8%) were diagnosed with PTB. Of these 205 patients with PTB, 166 were culture-confirmed, 12 were histologically confirmed, and 27 were clinically diagnosed. Baseline characteristics of included patients are summarized in Table 1.
Fig. 1.
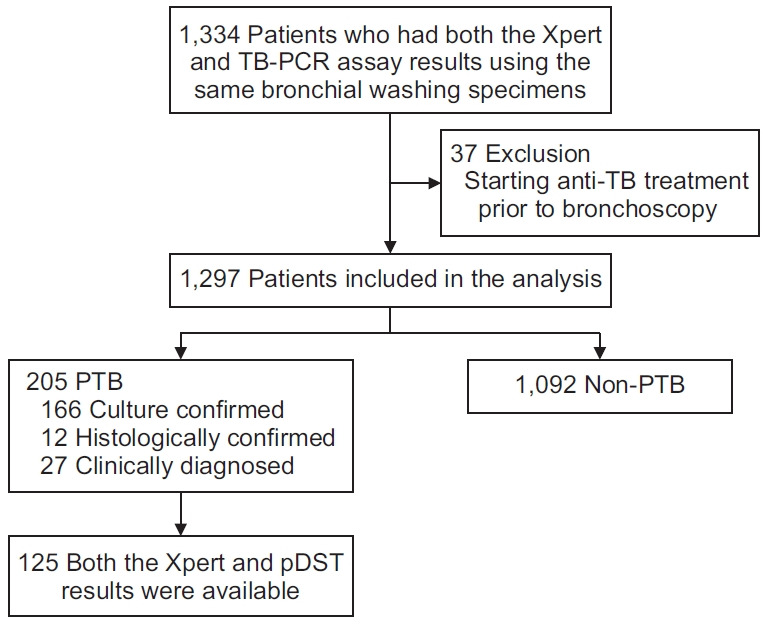
Flow diagram showing the selection of patients for this study. TB: tuberculosis; PCR: polymerase chain reaction; PTB: pulmonary tuberculosis; pDST: phenotypic drug susceptibility testing.
Table 1.
Baseline characteristics of 1,297 included patients
| Characteristic | No. (%) |
|---|---|
| Age, median (IQR), yr | 63 (56–72) |
| Male sex | 795 (61.2) |
| Final diagnosis | |
| Pulmonary tuberculosis | 205 |
| By treatment history | |
| New case | 180 (87.8) |
| Retreatment | 25 (12.2) |
| Relapse | 21 |
| Failed | 1 |
| Lost to follow up | 3 |
| By diagnostic method | |
| Smear positive | 81 (39.5) |
| Culture confirmed | 166 (81.0) |
| Histologically confirmed | 12 (5.8) |
| Clinically diagnosed | 27 (13.2) |
| Other than pulmonary tuberculosis | 1,092 |
| Pneumonia | 331 (30.3) |
| Lung cancer | 200 (18.3) |
| Bronchiectasis | 190 (17.4) |
| Nontuberculous mycobacteria isolation | 132 (12.1) |
| Interstitial lung disease | 122 (11.2) |
| Benign pulmonary nodule | 59 (5.4) |
| Inactive tuberculosis | 45 (4.1) |
| Other | 13 (1.2) |
IQR: interquartile range.
2. Detection of Mycobacterium tuberculosis
Using mycobacterial culture as the reference method, overall sensitivity, specificity, PPV, and NPV of the Xpert assay were 83.7% (95% CI, 77.2–89), 99.4% (95% CI, 98.7–99.8), 95.2% (95% CI, 90.4–97.7), and 97.7% (95% CI, 96.7–98.3), respectively, while those of the AdvanSure assay were 74.1% (95% CI, 66.7–80.6), 99.6% (95% CI, 99.0–99.9), 96.1% (95% CI, 91.1–98.3), and 96.3% (95% CI, 95.3–97.1), respectively (Table 2). There was no significant difference in sensitivity between Xpert and AdvanSure assays for smear-positive samples, whereas the Xpert assay had significantly higher sensitivity than the AdvanSure assay (70.6% [95% CI, 59.7–80.0] vs. 52.9% [95% CI, 41.8–63.9], p=0.018) for smear-negative specimens.
Table 2.
Diagnostic performances of Xpert MTB/RIF and AdvanSure MTB/NTM PCR assay using Mycobacterium tuberculosis culture as the gold standard
| M. tuberculosis culture |
Performance (95% confidence interval, %) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n=166) |
Negative (n=1,131) |
Sensitivity | Specificity | PPV | NPV | ||||
| PCR+ | PCR– | PCR+ | PCR– | ||||||
| Overall | p=0.032 | p=0.500 | |||||||
| Xpert | 139 | 27 | 7 | 1,124 | 83.7 (77.2–89) | 99.4 (98.7–99.8) | 95.2 (90.4–97.7) | 97.7 (96.7–98.3) | |
| AdvanSure | 123 | 43 | 5 | 1,126 | 74.1 (66.7–80.6) | 99.6 (99.0–99.9) | 96.1 (91.1–98.3) | 96.3 (95.3–97.1) | |
| Smear positive | p=0.193 | p>0.99 | |||||||
| Xpert | 79 | 2 | 0 | 0 | 97.5 (91.4–99.7) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | |
| AdvanSure | 78 | 3 | 0 | 0 | 96.3 (89.5–99.2) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | |
| Smear negative | p=0.018 | p=0.500 | |||||||
| Xpert | 60 | 25 | 7 | 1,124 | 70.6 (59.7–80.0) | 99.4 (98.7–99.8) | 89.6 (80.2–94.8) | 97.8 (97.0–98.4) | |
| AdvanSure | 45 | 40 | 5 | 1,126 | 52.9 (41.8–63.9) | 99.6 (99.0–99.9) | 90.0 (78.6–95.7) | 96.6 (95.7–97.2) | |
PCR: polymerase chain reaction; PPV: positive predictive value; NPV: negative predictive value.
Upon addition of histologically confirmed and clinically diagnosed PTB to culture-confirmed PTB as a combined reference standard, overall sensitivity of the Xpert assay was 69.8% (95% CI, 63.0–76.0), which was not significant different from that of the AdvanSure assay (61.5% [95% CI, 54.4–68.2]; p=0.077) (Table 3). Furthermore, for smear-negative specimens, sensitivity of the Xpert assay was significantly higher than that of the AdvanSure assay (51.6% [95% CI, 42.5–60.7] vs. 38.7% [95% CI, 30.1–47.9], p=0.042). Among 1,092 patients diagnosed with other than PTB, two (0.2%) and three (0.3%) patients presented positive results in AdvanSure and Xpert tests, respectively. Clinical characteristics of these false-positive cases are shown in Supplementary Table S1.
Table 3.
Diagnostic performances of Xpert MTB/RIF and AdvanSure MTB/NTM RT-PCR assay using clinical PTB as the combined gold standard
| Clinical PTB |
Performance (95% confidence interval, %) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PTB (n=205) |
Not PTB (n=1,092) |
Sensitivity | Specificity | PPV | NPV | ||||
| PCR+ | PCR– | PCR+ | PCR– | ||||||
| Overall | p=0.078 | p=0.640 | |||||||
| Xpert | 143 | 62 | 3 | 1,089 | 69.8 (63.0–76.0) | 99.7 (99.2–99.9) | 98.0 (93.9–99.3) | 94.6 (93.5–95.6) | |
| AdvanSure | 126 | 79 | 2 | 1,090 | 61.5 (54.4–68.2) | 99.8 (99.3–100.0) | 98.4 (94.0–99.6) | 93.2 (92.1–94.3) | |
| Smear positive | p=0.193 | p>0.99 | |||||||
| Xpert | 79 | 2 | 0 | 0 | 97.5 (91.4–99.7) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | |
| AdvanSure | 78 | 3 | 0 | 0 | 96.3 (89.6–99.2) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | |
| Smear negative | p=0.042 | p=0.640 | |||||||
| Xpert | 64 | 60 | 3 | 1,089 | 51.6 (42.5–60.7) | 99.7 (99.2–99.9) | 95.5 (87.2–98.5) | 94.8 (93.8–95.6) | |
| AdvanSure | 48 | 76 | 2 | 1,090 | 38.7 (30.1–47.9) | 99.8 (99.3–100.0) | 96.0 (85.5–99.0) | 93.5 (92.6–94.3) | |
RT-PCR: reverse transcription polymerase chain reaction; PTB: pulmonary tuberculosis; PPV: positive predictive value; NPV: negative predictive value.
3. Detection of rifampicin resistance and turnaround time
Of 166 culture-confirmed patients, 145 (87%) had phenotypic DST results. Of these 145 patients with phenotypic DST results, 20 presented negative Xpert test results. Therefore, the diagnostic accuracy of Xpert assay for detecting rifampin resistance was evaluated among 125 patients with both Xpert and phenotypic DST results available. Using phenotypic DST as the gold standard, the sensitivity, specificity, PPV, and NPV of the Xpert assay were found to be 100% (95% CI, 69.2–100), 99.1% (95% CI, 95.3–100), 90.9% (95% CI, 58.7–99.8), and 100% (95% CI, 96.8–100), respectively (Table 4). Additionally, the median turnaround time from submitting samples to obtaining results was 1 day (IQR, 0–1 day) for the Xpert assay and 3 days (IQR, 2–5 days) (p<0.001) for the AdvanSure assays.
Table 4.
Diagnostic performance of Xpert MTB/RIF assay for detecting rifampicin resistance using pDST as the gold standard
| Xpert | pDST |
Performance (95% confidence interval, %) |
||||
|---|---|---|---|---|---|---|
| Resistance | Susceptible | Sensitivity | Specificity | PPV | NPV | |
| Resistance | 10 | 1 | 100 (69.2–100) | 99.1 (95.3–100) | 90.9 (58.7–99.8) | 100 (96.8–100) |
| Susceptible | 0 | 114 | ||||
pDST: phenotypic drug susceptibility testing; PPV: positive predictive value; NPV: negative predictive value.
Discussion
In this study, diagnostic performances of Xpert and TB-PCR assays were comparable to those reported in previous meta-analyses [4,12]. For smear-positive samples, the sensitivity and specificity of both assays were comparable, with results above 95%. However, the Xpert assay was superior to the TB-PCR assay as it had higher sensitivity for smear-negative specimens, shorter turnaround times, and determining rifampin resistance prediction with high accuracy.
The main finding of our study was the higher sensitivity of the Xpert assay for smear-negative specimens than the TB-PCR assay. Compared to the TB-PCR, Xpert sensitivity for smear-negative specimens was increased by 17.7% with culture as the gold standard and by 12.9% with clinical diagnosis as the gold standard. This result might possibly be due to difference in decontamination and concentration steps [13,14]. More specifically, the Xpert assay was performed using direct specimens, whereas the TB-PCR assay used decontamination and concentrated sediments, which might have led to the loss of M. tuberculosis samples during the process.
Several studies have already evaluated the performance of Xpert as compared to various TB-PCRs [13-20]. Although some studies have reported higher sensitivities for the TB-PCR assay [15,16], these studies have certain limitations, including the use of frozen or decontaminated samples, heterogeneity in samples including non-respiratory samples, and a small sample size. On the other hand, a study using bronchial washing specimens in real practice, similar to our study, has shown a higher Xpert assay sensitivity than the TB-PCR assay for smear-negative specimens [14]. Compared to the two studies using bronchial washing specimens [14,17], the overall sensitivity of the Xpert and TB-PCR tests was lower. In this study, the sensitivity of both tests in the smear-positive group was as high as approximately 97%, thus the group with lower sensitivity than the two studies [14,17] is likely to be the smear-negative group. This suggests that differences in sensitivity are more likely to be due to differences in the study population than due to the differences in the laboratory. The test sensitivity is usually affected by the degree of clinical suspicion in the study population. Our study might have included more patients with less clinical suspicion than those two previous studies [14,17].
Despite TB-PCR’s high specificity, its modest sensitivity in paucibacillary samples is a major limitation. Although the Xpert assay also has suboptimal sensitivity for smear-negative specimens, recent studies have shown that the Gene Xpert platform is evolving further. The Xpert Ultra assay has significantly improved sensitivity for TB detection [21], and the Xpert MTB/XDR assay has extended the detection of resistance to more key drugs [22].
Moreover, our study showed that the Xpert assay had a high diagnostic accuracy for diagnosing rifampin resistance. Based on phenotypic DST results, the sensitivity and specificity of the Xpert assay for rifampin resistance detection were 100% and 99.1%, respectively. Only 1 of 125 cases showed a discrepancy between Xpert and phenotypic DST results in detecting rifampin resistance. Although not confirmed by rpoB sequencing in our study, one of the reasons for this discrepancy might be disputed mutation. In a Korean study, the frequency of rpoB disputed mutations was reported to be 6.9% [23]. Compared to line probe assay, the Xpert test was equally efficient in detecting rifampin resistance but showed higher sensitivity for smear-negative samples [24], making it more suitable as an initial test for TB. In other countries, there has been a concern regarding the Xpert assay’s high false-negative or false-positive rates, depending on genetic mutation frequency [25]. However, our study demonstrated that the Xpert assay reliably predicted rifampicin resistance in Korea. Additionally, the turnaround time of the Xpert assay was significantly shorter than that of the TB-PCR assay.
Based on findings of our study, the Xpert assay outperformed TB-PCR as an initial test for TB diagnosis. The Xpert assay can detect more patients earlier than the TB-PCR assay. It also predicted rifampin resistance with high accuracy. Furthermore, it requires minimal hands-on technical time and training with fewer biosafety concerns. Taken together, these diagnostic advantages of the Xpert assay can contribute to the improvement of both individual patient management and the TB control program.
Since the endorsement of the Xpert assay by WHO in 2010, significant additional evidence has been generated on its use as an initial test for the diagnosis of TB and rifampicin-resistant TB [9]. Molecular TB diagnostics have also been rapidly evolving into sputum-based integrated assays for simultaneous identification of TB and drug resistance [1]. With these developments, many countries are rapidly adapting and updating their TB diagnostic algorithms [8,26,27]. Although various molecular diagnostic methods are available in Korea, the prioritization and optimal positioning in terms of Xpert and TB-PCR assays have not been properly integrated into the diagnostic algorithm. Therefore, our diagnostic algorithm needs to be updated in line with advancements in molecular diagnostics. Findings of our study provide some evidence needed for this change, although further studies are needed.
Compared to laboratory-based studies, using frozen or decontaminated specimens, our study was conducted in real practice. Thus our results could reflect real-world performances of both tests. Moreover, we only collected bronchial washing specimens, reducing the heterogeneity of specimens and leading to a better diagnostic yield without any sample bias. Additionally, the sample size in our study was larger than those in most studies. Despite these findings, our study had major limitations regarding the generalization of our study results since the AdvanSure TB/NTM real-time PCR kit was the only tested assay from various commercial TB-PCR kits. In addition, our study did not present a cost-effectiveness analysis, including analysis of direct or indirect costs.
In conclusion, the Xpert assay was more sensitive for smear-negative samples and had a shorter turnaround time than the AdvanSure assay. Moreover, it could reliably predict rifampin resistance. Thus, the Xpert test might be preferentially recommended over TB-PCR in the Korean TB diagnostic algorithm.
Footnotes
Authors’ Contributions
Conceptualization: Jeon D. Methodology: Yoon SH, Lee SE, Jeon D. Formal analysis: Son E, Jang J, Kim T, Yeo HJ. Data curation: Jang JH, Chung JH, Seol HY, Yeo HJ, Yoon SH, Lee SE. Validation: Kim YS, Cho WH. Writing - original draft preparation: Son E, Jang J. Writing - review and editing: Kim YS, Cho WH, Jeon D. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Clinical characteristics of patients with false positivity in either Xpert MTB/RIF or AdvanSure MTB/NTM RT-PCR assay.
REFERENCES
- 1.MacLean E, Kohli M, Weber SF, Suresh A, Schumacher SG, Denkinger CM, et al. Advances in molecular diagnosis of tuberculosis. J Clin Microbiol. 2020;58:e01582–19. doi: 10.1128/JCM.01582-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Nucleic acid amplification tests for tuberculosis. MMWR Morb Mortal Wkly Rep. 1996;45:950–2. [PubMed] [Google Scholar]
- 3.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;2014:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Liu B, Peng M, Chen M, Yin W, Tang H, et al. Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: a systematic review and meta-analysis. PLoS One. 2017;12:e0180725. doi: 10.1371/journal.pone.0180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agizew T, Boyd R, Auld AF, Payton L, Pals SL, Lekone P, et al. Treatment outcomes, diagnostic and therapeutic impact: Xpert vs. smear: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23:82–92. doi: 10.5588/ijtld.18.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Tanna GL, Khaki AR, Theron G, McCarthy K, Cox H, Mupfumi L, et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob Health. 2019;7:e191–9. doi: 10.1016/S2214-109X(18)30458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazabon D, Suresh A, Oghor C, Qin ZZ, Kik SV, Denkinger CM, et al. Implementation of Xpert MTB/RIF in 22 high tuberculosis burden countries: are we making progress? Eur Respir J. 2017;50:1700918. doi: 10.1183/13993003.00918-2017. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Policy update [Internet] Geneva: World Health Organization; 2020. Molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary TB and rifampicin resistance: rapid communication. [cited 2021 Jun 10]. Available from: https://www.who.int/publications/i/item/9789240000339. [Google Scholar]
- 10.Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korean Centers for Disease Control and Prevention . Seoul and Cheongju: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention; 2014. Korean guidelines for tuberculosis. 2nd ed. [Internet] [cited 2021 Jun 10]. Available from: https://www.lungkorea.org/bbs/index.html?code=guide&category=&gubun=&page=4&number=3480&mode=view&keyfield=&key= [Google Scholar]
- 11.Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korean Centers for Disease Control and Prevention . Seoul and Cheongju: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention; 2017. Korean guidelines for tuberculosis. 3rd ed. [Internet] [cited 2021 Jun 10]. Available from: https://www.lungkorea.org/bbs/index.html?code=guide&category=&gubun=&page=2&number=7563&mode=view&keyfield=&key= [Google Scholar]
- 12.Greco S, Girardi E, Navarra A, Saltini C. Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax. 2006;61:783–90. doi: 10.1136/thx.2005.054908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park KS, Kim JY, Lee JW, Hwang YY, Jeon K, Koh WJ, et al. Comparison of the Xpert MTB/RIF and Cobas TaqMan MTB assays for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol. 2013;51:3225–7. doi: 10.1128/JCM.01335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko Y, Lee HK, Lee YS, Kim MY, Shin JH, Shim EJ, et al. Accuracy of Xpert((R)) MTB/RIF assay compared with AdvanSure TB/NTM real-time PCR using bronchoscopy specimens. Int J Tuberc Lung Dis. 2016;20:115–20. doi: 10.5588/ijtld.15.0227. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Nam YS, Cho SY, Park TS, Lee HJ. Comparison of the Xpert MTB/RIF assay and real-time PCR for the detection of Mycobacterium tuberculosis. Ann Clin Lab Sci. 2015;45:327–32. [PubMed] [Google Scholar]
- 16.Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium Tuberculosis Direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011;49:3659–62. doi: 10.1128/JCM.00211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo YS, Park JH, Lee JK, Heo EY, Chung HS, Kim DK. Discordance between MTB/RIF and real-time tuberculosisspecific polymerase chain reaction assay in bronchial washing specimen and its clinical implications. PLoS One. 2016;11:e0164923. doi: 10.1371/journal.pone.0164923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011;49:3458–62. doi: 10.1128/JCM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, et al. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med. 2011;8:e1001061. doi: 10.1371/journal.pmed.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CH, Woo H, Hyun IG, Kim C, Choi JH, Jang SH, et al. A comparison between the efficiency of the Xpert MTB/RIF assay and nested PCR in identifying Mycobacterium tuberculosis during routine clinical practice. J Thorac Dis. 2014;6:625–31. doi: 10.3978/j.issn.2072-1439.2014.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021;2:CD009593. doi: 10.1002/14651858.CD009593.pub5. [DOI] [PubMed] [Google Scholar]
- 22.Xie YL, Chakravorty S, Armstrong DT, Hall SL, Via LE, Song T, et al. Evaluation of a rapid molecular drug-susceptibility test for tuberculosis. N Engl J Med. 2017;377:1043–54. doi: 10.1056/NEJMoa1614915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo KW, Lee S, Kang MR, Sung H, Kim MN, Shim TS. Frequency and type of disputed rpoB mutations in Mycobacterium tuberculosis isolates from South Korea. Tuberc Respir Dis. 2017;80:270–6. doi: 10.4046/trd.2017.80.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav RN, Kumar Singh B, Sharma R, Chaubey J, Sinha S, Jorwal P. Comparative performance of Line Probe Assay (Version 2) and Xpert MTB/RIF Assay for early diagnosis of rifampicin-resistant pulmonary tuberculosis. Tuberc Respir Dis. 2021;84:237–44. doi: 10.4046/trd.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zong K, Luo C, Zhou H, Jiang Y, Li S. Xpert MTB/RIF assay for the diagnosis of rifampicin resistance in different regions: a meta-analysis. BMC Microbiol. 2019;19:177. doi: 10.1186/s12866-019-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar SU, Kumar AM, Venkateshmurthy NS, Nair D, Kingsbury R, R P, et al. Implementation of the new integrated algorithm for diagnosis of drug-resistant tuberculosis in Karnataka State, India: How well are we doing? PLoS One. 2021;16:e0244785. doi: 10.1371/journal.pone.0244785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang TY, Fan SY, Jou R. Performance of an Xpert-based diagnostic algorithm for the rapid detection of drug-resistant tuberculosis among high-risk populations in a low-incidence setting. PLoS One. 2018;13:e0200755. doi: 10.1371/journal.pone.0200755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of patients with false positivity in either Xpert MTB/RIF or AdvanSure MTB/NTM RT-PCR assay.


