Abstract
Background
There is an urgent need for highly efficacious antiviral therapies in immunosuppressed hosts who develop coronavirus disease (COVID-19), with special concern for those affected by hematological malignancies.
Case presentation
Here, we report the case of a 75-year-old male with chronic lymphocytic leukemia who was deficient in CD19+CD20+ B-lymphocyte populations due to previous treatment with anti-CD20 monoclonal antibodies. The patient presented with severe COVID-19 pneumonia due to prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and was treated with two courses of the antiviral plitidepsin on a compassionate use basis. The patient subsequently achieved an undetectable viral load, and his pneumonia resolved.
Conclusions
Treatment with plitidepsin was well-tolerated without any further hematological or cardiovascular toxicities. This case further supports plitidepsin as a potential antiviral drug in SARS-CoV-2 patients affected by immune deficiencies and hematological malignancies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-021-01220-0.
Keywords: Plitidepsin, Covid-19, SARS-CoV-2, Anti-CD20 monoclonal antibody, Prolonged viral replication, Chronic lymphocytic leukemia
To the Editor,
Patients affected by hematological malignancies constitute a unique population with regard to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. This population has demonstrated an increased risk of persistent infection with SARS-CoV-2, and severe outcomes and mortality due to coronavirus disease 2019 (COVID-19) [1, 2].
In addition to the malignancy itself, short- and long-term effects on lymphocytic populations can be produced by anticancer treatments. Treatment impact on the immune system is of particular note in the case of anti-CD20 monoclonal antibodies (rituximab, obinutuzumab), which, despite variability among patients, generally lead to the reduction (or depletion) of B lymphocyte subpopulations that persists for months.
Here, we report the clinical and virological course of a prolonged viral SARS-CoV-2 infection in an adult patient previously treated for CLL with obinutuzumab, who was in complete remission of his hematological malignancy. Treatment with plitidepsin was granted for compassionate use as authorized by the Spanish Agency for Medicinal Products (AEMPS) (AUT334100148189/21) [3]. The patient signed informed consent for treatment and before manuscript preparation.
A 75-year-old male with a previous history of CLL arrived to the emergency department (ED) with 1-week of dry cough and tiredness in January 2021. SARS-CoV-2 infection was confirmed by nasopharyngeal (NP) swab samples, and real-time, reverse transcription-polymerase chain reaction (RT-PCR; see Additional file 1: Supplementary material). Additional cardiovascular morbidities were recorded, including permanent atrial fibrillation and stable ischemic heart disease.
After 22 days of continued illness, the patient was admitted to our hospital for persistent fever, fatigue, the appearance of respiratory insufficiency, and ongoing bilateral opacities by chest X-ray. The patient had undetectable levels of antibodies against SARS-CoV-2, and peripheral blood flow cytometry showed undetectable levels of CD19+ and CD20+ B cells.
The patient experienced further clinical and image severity progression 48 days after disease onset, which prompted the compassionate use of plitidepsin 2.5 mg q.d. for three days according to available protocols. The RT-PCR cycle threshold (Ct) value was 23 at the time plitidepsin was initiated (Additional file 1: Fig. 1S-2S, table 1S-4S).
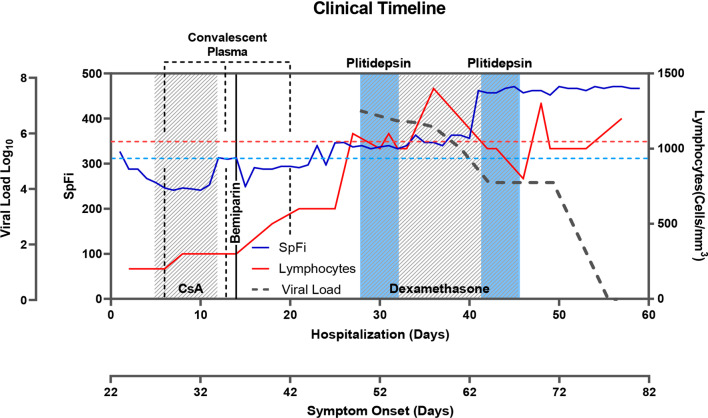
Fig. 1.
Timeline of main laboratory and microbiological parameters during and after plitidepsin therapy. Two timepoints have been taken as reference: the number of days from SARS-CoV-2 symptom onset, and the number of days since hospital admission. Plitidepsin was administered on days 49–51 and 65–67 after symptom onset. Parameters shown in the clinical course are as follows: A Quantitative viral load (log10 copies/ml) using nasopharyngeal swabs samples (grey-dotted line). B SpFI (AU) blue line. C Lymphocyte total count (cells/mm3) (red line). AU arbitrary units, CsA cyclosporine A, SpFi ratio of oxygen saturation in blood (SpO2)/fraction of inspired oxygen (FiO2) at or below 300 AU
Clinical improvement was apparent within one week, which led to the withdrawal of oxygen therapy 7 days after the first dose of plitidepsin. However, RT-PCR continued to show positive values for SARS-CoV-2, with a Ct value of 34 (Fig. 1).
On day 61, a single dose of 20 g of human immunoglobulin was administered intravenously; 24-h later (and two weeks after the first course of plitidepsin), a second equivalent course of plitidepsin was started. On day 74, the patient received his first negative RT-PCR test and was subsequently negative for SARS-CoV-2 in three consecutive samples. The patient was discharged from the hospital on day 82 and did not experience any signs of SARS-CoV-2 relapse during outpatient follow-up.
Prolonged viral replication of SARS-CoV-2 is increasingly being recognized as an emerging clinical problem among immunocompromised individuals. Subacute or chronic COVID-19 pneumonia can cause persistent lung damage and may lead to viral escape phenomena [4–7]. An underlying defect in the immune response is the main reason for ongoing viral replication and defective viral clearance in patients with hematological malignancies due to the absence of B-cell precursors. Furthermore, immune system deficiencies secondary to treatment with anti-CD20 monoclonal antibodies can impair the development of neutralizing antibodies after two doses of mRNA vaccines against SARS-CoV-2 [8].
Plitidepsin is a known inhibitor of SARS-CoV-2 replication, whose antiviral mechanism of action has been recently described using a drug-resistant mutant model [9]. In brief, the antiviral activity of plitidepsin is mediated through inhibition of the host eukaryotic protein elongation factor 1α (eEF1A). Through inhibition of eEF1A, plitidepsin prevents the expression of SARS-CoV-2 nucleocapsid (N) protein, particularly early during the infection, likely by inhibiting viral RNA translation (10).
The current report has obvious limitations. Though it only describes one patient, the successful outcome observed here should be evaluated in the context of the poor prognosis for immunocompromised patients with hematologic malignancies. Another limitation of this study is that we were unable to amplify and sequence viral RNA from swab samples collected, so the precise SARS-CoV-2 variant that infected this patient remains unknown. We were also unable to capture changes to the patient’s cytokine profile during hospital admission and during plitidepsin infusions.
To our knowledge, this is the first evidence of the successful use of a potential SARS-CoV-2 antiviral in a patient with hematological malignancy and depleted B cells B cells due to previous CLL therapy and with prolonged viral replication. The patient was followed up for six months since being discharged from the hospital with no signs of SARS-CoV-2 relapse or reinfection.
Supplementary Information
Additional file 1. Text summarizes methods used, analytical procedures, complications, therapies prescribed during hospitalization and further considerations; Table 1S–4S show analytical parameters that include: absence of immunoresponse against SARS-CoV-2; an increase in lymphocytic populations over time and serum biochemical results during the clinical event. A summary of ISARIC mortality score is also shown. Figure 1S Timeline of qPCR Ct over time, patient shows a reduction in the viral load after the treatment with the second cycle of plitidepsin. Figure 2S Bilateral pneumonia improvement after plitidepsin treatment determined by thorax imagen.
Acknowledgements
We thank the patient, and his relatives, for his participation in this study, Lorena Martin Peña and Margarita Remirez de Esparza Otero, Ph.D., for providing technical support, and Timothy D. Silverstein, Ph.D. for writing and editorial assistance.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- CLL
Chronic lymphocytic leukemia
- NHL
Non-Hodgkin lymphoma
- AEMPS
Spanish Agency for Medicinal Products
- ED
Emergency department
- NP
Nasopharyngeal
- RT-PCR
Real-time, reverse transcription-polymerase chain reaction
- ISARIC 4C mortality score
International Severe Acute Respiratory Infection Consortium Clinical Characterization Protocol
- Ct
Cycle threshold
- Ig
Immunoglobulin
- eEF1A
Eukaryotic protein elongation factor 1α
- N
Nucleocapsid
- REGN-CoV2
Casirivimab and imdevimab
Authors’ contributions
PGV, JAG, JMJ, JALM designed and wrote the first draft of the manuscript. PGV and JAG performed the data analysis. PGV, MMCG, EMB, MDSM, DCR, GSF, JMP, RGV, RBP participated in patient selection, obtained informed consent, developed protocol treatment and procedures, and coordinated the local team. JMFS, PGV, JAG, JMJ, JALM, XELE were involved in study design and in the therapeutic intervention model. MGC was actively involved in the microbiological follow-up of the patient, and swab sample manipulations. All authors approved the final version of the manuscript.
Funding
Research supported by Pharma Mar S.A. (Colmenar Viejo, Spain).
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Additional file 1: Supplementary information files).
Declarations
Ethics approval and consent to participate
We obtained institutional written consent from the patient for publication of his data under anonymized format.
Consent for publication
We obtained the patient’s signed written consent form for the publication of the current case report.
Competing interests
José M. Fernández-Sousa is President and Founder of Pharma Mar, S.A. (Madrid, Spain). José M. Jimeno holds stocks of Pangae Oncology, has a non-remunerated role in the Scientific Advisory Board, and holds stocks of Phosplatin Therapeutics; and is a full-time employee of Pharma Mar, S.A. (Madrid, Spain). José A. López-Martín is an employee and shareholder of Pharma Mar, S.A (Madrid, Spain), and a co-inventor of a patent for Plitidepsin (WO2008135793A1). Xarles Erik Luepke Estefan is an employee and shareholder of Pharma Mar, S.A (Madrid, Spain). All remaining authors have declared no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Corey L, Beyrer C, Cohen MS, et al. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilich T, Roerden M, Maringer Y, et al. Preexisting and post–COVID-19 immune responses to SARS-CoV-2 in patients with cancer. Cancer Discov. 2021;11(8):1982–1995. doi: 10.1158/2159-8290.CD-21-0191. [DOI] [PubMed] [Google Scholar]
- 3.Varona JF, Landete P, Lopez-Martin JA et al. Plitidepsin has a positive therapeutic index in adult patients with COVID-19 requiring hospitalization. medRxiv: the preprint server for health sciences [Internet]. 2021; Available from May 25;2021.05.25.21257505. 10.1101/2021.05.25.21257505.
- 4.Lee CY, Shah MK, Hoyos D et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. medRxiv: the preprint server for health sciences [Internet]. 2021; Available from Aug 29;2021.08.25.2162417. 10.1101/2021.08.25.2162417.
- 5.Reuken PA, Stallmach A, Pletz MW, et al. Severe clinical relapse in an immunocompromised host with persistent SARS-CoV-2 infection. Leukemia. 2021;35(3):920–923. doi: 10.1038/s41375-021-01175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–282. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223(1):23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White KM, Rosales R, Yildiz S, et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371(6532):926–931. doi: 10.1126/science.abf4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuschl AK, Thorne LG, Zuliani-Álvarez L, et al. Host-directed therapies against early-lineage SARS-CoV-2 retain efficacy against B.1.1.7 variant. bioRxiv: the preprint server for health sciences [Internet]. 2021; Available from Feb 4;2021.01.24.427991. 10.1101/2021.01.24.427991.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Text summarizes methods used, analytical procedures, complications, therapies prescribed during hospitalization and further considerations; Table 1S–4S show analytical parameters that include: absence of immunoresponse against SARS-CoV-2; an increase in lymphocytic populations over time and serum biochemical results during the clinical event. A summary of ISARIC mortality score is also shown. Figure 1S Timeline of qPCR Ct over time, patient shows a reduction in the viral load after the treatment with the second cycle of plitidepsin. Figure 2S Bilateral pneumonia improvement after plitidepsin treatment determined by thorax imagen.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Additional file 1: Supplementary information files).