Abstract
Human louse-borne relapsing fever occurs in sporadic outbreaks in central and eastern Africa that are characterized by significant morbidity and mortality. Isolates of the causative agent, Borrelia recurrentis, were obtained from the blood of four patients during a recent epidemic of the disease in southern Sudan. The glpQ gene, encoding glycerophosphodiester phosphodiesterase, from these isolates was sequenced and compared with the glpQ sequences obtained from other relapsing-fever spirochetes. Previously we showed that GlpQ of Borrelia hermsii is an immunogenic protein with utility as a serological test antigen for discriminating tick-borne relapsing fever from Lyme disease. In the present work, we cloned and expressed the glpQ gene from B. recurrentis and used recombinant GlpQ in serological tests. Acute- and convalescent-phase serum samples obtained from 42 patients with louse-borne relapsing fever were tested with an indirect immunofluorescence assay (IFA) and an enzyme-linked immunosorbent assay (ELISA) that used whole cells of B. recurrentis and with immunoblotting to whole-cell lysates of the spirochete and Escherichia coli producing recombinant GlpQ. The geometric mean titers of the acute- and convalescent-phase serum samples measured by IFA were 1:83 and 1:575, respectively. The immunoblot analysis identified a high level of reactivity and seroconversion to GlpQ, and the assay was more sensitive than the whole-cell IFA and ELISA using purified, recombinant histidine-tagged GlpQ. Serum antibodies to GlpQ and other antigens persisted for 27 years in one patient. We conclude that assessment of anti-GlpQ antibodies will allow serological confirmation of louse-borne relapsing fever and determination of disease prevalence.
Relapsing fever is reported to have been described by Hippocrates in the 4th century B.C. (30). The name relapsing fever has been attributed by several workers to Craigie (18, 30, 57), who, along with Henderson, described an epidemic fever of humans in Edinburgh, Scotland, in 1843 (17, 35). Jenner also presented a clinical description of the disease in 1850 (39). The spirochetal agent of louse-borne relapsing fever (LBRF) was first observed in the blood of patients by Obermeier during an outbreak of the disease in Berlin, Germany, in 1868 (7). However, the role of the human body louse (Pediculus humanus) in spirochete transmission was not described until 1907 (45). The causative agent of LBRF is now known as Borrelia recurrentis. The organism has no wild animal reservoir and is transmitted solely among humans by the body louse (14). LBRF was once widespread globally when human body lice were much more abundant than they are today (30). In the 19th century, substantial outbreaks occurred in the British Isles (17), Europe (7), and the United States (49). Outbreaks also were documented in some parts of Europe, India (19), China (16), the Andean region of South America, and several African countries in the first half of the 20th century. In recent decades, LBRF has been recorded only in northeastern and central Africa, especially Ethiopia, Somalia, and Sudan, where infestations of human body lice remain prevalent (1–3, 8, 10, 15, 24, 34, 48, 60, 66, 68, 69; P. L. Perine and D. F. Reynolds, Letter, Lancet ii:1324–1325, 1974).
The clinical manifestations of LBRF include the classical recurrence of acute episodes of fever (13, 56), sometimes complicated by bleeding associated with thrombocytopenia (25). Antibiotic treatment can initiate a rapid and fatal Jarisch-Herxheimer reaction (72, 74). Historically, laboratory confirmation of all relapsing fevers, including both louse-borne and tick-borne forms, has relied on the identification of spirochetes in patient blood in the febrile episodes (11). However, spirochetes frequently are not identified because of the cyclic nature of the spirochetemia and low sensitivity of detection by light microscopy. As a consequence, various types of serological tests have been developed to detect antibodies produced during infection with relapsing-fever spirochetes (11, 30, 31) and thereby enhance diagnosis. However, the utility of these assays has been limited by a lack of sensitivity and specificity.
Previously, we reported that the enzyme glycerophosphodiester phosphodiesterase (GlpQ) is absent in Lyme disease spirochetes but is present and immunogenic in the tick-borne relapsing-fever spirochete, Borrelia hermsii (62). We found that GlpQ was recognized by antisera obtained from humans and other animals after infection with tick-borne relapsing-fever spirochetes. In contrast, serum samples taken from humans with a diagnosis of Lyme disease were nonreactive.
Cutler and coworkers (21) demonstrated in 1994 that Kelly's medium (40) supported the continuous growth of B. recurrentis. This ability to culture LBRF spirochetes creates opportunities to perform in vitro studies and to develop new diagnostic tests. Four isolates of B. recurrentis were cultured from the blood of acutely ill, spirochetemic patients in a recent outbreak of LBRF in southern Sudan. The glpQ gene from each of these isolates and those from four other Borrelia species were sequenced. Recombinant B. recurrentis GlpQ protein was used for serological testing of acute- and convalescent-phase serum samples from LBRF patients. Our findings demonstrate the utility of GlpQ to serologically confirm LBRF. This antigen will also be useful for retrospective serological surveys when the presence of LBRF is suspected.
MATERIALS AND METHODS
Borrelia strains and cultivation.
B. recurrentis isolates 107, 115, 119, and 132 were obtained from the blood of four LBRF patients living in Rumbek County of southern Sudan during an epidemiological investigation in April 1999. B. hermsii strain DAH was isolated at Rocky Mountain Laboratories (RML) from the blood of a human with relapsing fever in eastern Washington (62). Borrelia coriaceae CO53 (ATCC 43381) was isolated from Ornithodoros coriaceus collected in California (42). Borrelia parkeri RML, Borrelia turicatae RML, and Borrelia anserina RML were isolated from Ornithodoros parkeri, Ornithodoros turicata, and a domestic chicken, respectively, and are part of the RML bacterial reference collection. Borrelia crocidurae CR2A was provided by Sven Bergström, Umeå University, Umeå Sweden. B. burgdorferi B31 was isolated from Ixodes scapularis collected on Shelter Island, New York (12). Lysates of Treponema pallidum were provided by Steven Norris, University of Texas Health Science Center, Houston.
Borrelia cultures were maintained in BSK-H medium (Sigma Chemical Co., St. Louis, Mo.) at 34°C and passaged twice a week. The isolates of B. recurrentis had been passaged three to six times when examined.
PCR and DNA sequence analysis.
Total genomic DNA was purified from 100-ml cultures of each B. recurrentis isolate or 500-ml cultures of the other Borrelia species, quantified by UV spectroscopy, and diluted to approximately 0.1 μg for use in each 100-μl PCR (50, 51). Taq enzyme and reaction constituents were used as recommended by the manufacturer (Perkin-Elmer, Roche Molecular Systems, Inc., Branchburg, N.J.). Primers used for amplifying glpQ DNA fragments were manufactured by Life Technologies, Baltimore, Md. (Table 1). PCRs were performed under mineral oil for 25 cycles with a Perkin-Elmer thermocycler. Each cycle consisted of denaturation at 94°C for 1 min, annealing at 50°C for 30 s, and extension at 72°C for 2 min. After the 25th cycle, an additional 7-min extension was done at 72°C.
TABLE 1.
Primers used in PCR amplification and sequencing of glpQ
| Primer | Primer sequence (5′-3′) |
|---|---|
| glpQ F+1 | GGGGTTCTGTTACTGCTAGTGCCATTAC |
| glpQ F−1 | CAATTTTAGATATGTCTTTACCTTGTTGTTTATGCC |
| glpQ R−1 | GCACAGGTAGGAATGTTGGAATTTATCCTG |
| glpQ R−2 | CAATACTAAGACCAGTTGCTCCTCCGCC |
| Br-R2 | GTTGCTCCTCCGCCAATTATTATTAAGTC |
| Br-glpQ RBS | GAGAGGATAAATTAATGAAATTCAAATTAACAATG |
| M13 reverse | GGAAACAGCTATGACCATG |
| T7 | GTAATACGACTCACTATAGGGC |
| Br GlpQ fus 5′ | GCCGCTCGAGGAAAAGAAAATGCAAAAATAAATAAAAAATC |
| Br GlpQ fus 3′ | GGCGGATCCGCTTGACCAGTTGCTCCTCCGC |
The amplified products were visualized by examining 10 μl of the reaction mixture by agarose gel electrophoresis. If unwanted secondary bands were present, the remaining reaction mixture was electrophoresed in an agarose gel, the band of interest was excised, and the DNA was purified with Minus ethidium bromide spin columns (Supelco, Inc., Bellefonte, Pa.). Products of PCRs that resulted in a single DNA fragment of the predicted size were purified with a Centricon 100 concentrator (Millipore Corp., Bedford, Mass.). All DNA samples were then quantified by UV spectroscopy and diluted to the appropriate concentration recommended for automated DNA sequencing.
DNA sequencing reactions were performed with a model 373A Stretch Automated DNA Sequencer (Applied Biosystems Inc., Foster City, Calif.) and ABI PRISMTM Dye Terminator Cycle Sequencing Ready Reaction sequencing kits (Applied Biosystems, Inc.) according to the manufacturer's instructions. Nucleotide and deduced amino acid sequences were analyzed with the MacVector version 6.0 software package (Oxford Molecular, Beaverton, Oreg.). Alignments were first constructed with the ClustalV program (36) in the Lasergene (DNASTAR) software package. Phylogenetic trees were constructed with the nearest-neighbor-joining method of Saitou and Nei (59). To confirm these results, alignments were transferred into the PHYLIP Phylogeny Inference Package (J. Felsenstein, PHYLIP—Phylogeny Inference Package, version 3.57c; Department of Genetics, University of Washington, Seattle). A distance matrix computed with the Jukes-Cantor method (DNADIST) was then analyzed with the neighbor-joining method (NEIGHBOR). Alignments for the glpQ genes were bootstrapped (SEQBOOT) and analyzed by distance matrix construction (DNADIST) or parsimony analysis (DNAPARS). The phylogenetic trees were viewed with TreeView (version 1.5) (R. D. M. Page, Treeview, version 1.5; Division of Environmental and Evolutionary Biology, University of Glasgow, Glasgow, United Kingdom).
The glpQ DNA sequences and their inferred amino acid sequences were analyzed with the BLAST set of database search programs (4). Percent identities among the DNA and amino acid sequences were calculated with BestFit (University of Wisconsin Genetics Computer Group-LITE, Madison) (26). The GenBank/LANL accession numbers for the glpQ DNA sequences are as follows: B. recurrentis 107, AF247152; B. recurrentis 115, AF247153; B. recurrentis 119, AF247154; B. recurrentis 132, AF247155; B. crocidurae CR2A, AF247151; B. turicatae RML, AF247157; B. parkeri RML, AF247156; B. coriaceae CO53, AF247158; and B. hermsii DAH, U40762.
PCR amplification and glpQ cloning.
The glpQ gene and presumed ribosomal binding site were amplified by PCR from B. recurrentis isolate 115 with primers formulated on the basis of the glpQ gene region of B. hermsii (Table 1). After amplification, 10 μl of the total 100-μl reaction mixture was examined in a 0.7% agarose electrophoresis gel stained with ethidium bromide. An amplification product of the correct size was cloned into the pCR2.1 vector of the TA Cloning System (Invitrogen Corp., San Diego, Calif.) and transformed into Escherichia coli. The sequence of the entire DNA insert in this recombinant plasmid (pTA-115) was determined with the M13 universal primers and an internal set of primers (Table 1), which confirmed the identity, integrity, and proper orientation of glpQ.
Production of rabbit antiserum.
Antisera to recombinant GlpQ were produced in two rabbits with different antigen preparations. For one preparation, a 100-ml overnight culture of E. coli TA-115 was centrifuged and suspended in 10 ml of phosphate-buffered saline (PBS), and the cells were disrupted by sonication. An equal volume of the lysate was emulsified in Ribi adjuvant (Corixa Montana, Hamilton, Mont.), and 5 ml of the mixture was injected subcutaneously at five sites. For the second preparation, a whole-cell lysate of E. coli TA-115 was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the unfixed gel was stained with a water-based Coomassie blue to visualize the GlpQ band. This band was excised from the gel, triturated in 5 ml of PBS with a mortar and pestle, and injected subcutaneously at five sites. Both rabbits were bled prior to the primary immunizations and boosted subcutaneously on day 28 postimmunization with a preparation identical to that used for the primary immunization. Blood from both rabbits was collected again 33 and 57 days after the first booster injection.
Human serum.
Acute- and convalescent-phase serum samples obtained from 42 patients hospitalized in Addis Ababa, Ethiopia, with fever and documented spirochetemia during the acute phase of illness have been described previously (54). Although the spirochetes infecting these patients were not identified, LBRF is hyperendemic in Ethiopia (8; G. Borgnolo, B. Hailu, and F. Chiabrera, Letter, Lancet 338:827, 1991), and no recent reports of tick-borne relapsing fever are known for this region, strongly suggesting that B. recurrentis was the cause of the infections. These samples were collected from December 1970 to June 1971 (54) and were stored frozen at −20°C at RML until used in this study. The duration between the collection of acute- and convalescent-phase samples ranged from 5 to 17 days (mean of 11 days). No information regarding antibiotic treatment of these patients was available.
Four serum samples obtained from a medical researcher who had been infected twice while working with spirochetemic LBRF patients in Addis Ababa also were studied. The onset of the first illness was noted on 13 February 1973. The infection was confirmed by a positive blood smear, and prompt antibiotic treatment resulted in full recovery. The onset of the second illness was on 19 April 1973. Infection was confirmed by a positive blood smear. The patient again was treated and recovered uneventfully. No subsequent infection with B. recurrentis or any other Borrelia species occurred. Serum samples were collected in 1990, 1993, and 1997 and on 9 February 2000, spanning a range of 17 to 27 years after infection.
Serum samples were also available from patients with a clinical diagnosis of tick-borne relapsing fever that was confirmed by detection of spirochetes with dark-field microscopy, blood smears stained with Giemsa stain, or culture of spirochetes in BSK-H medium. These patients were infected in California, Washington, Idaho, or British Columbia, regions where B. hermsii is endemic (9, 28), or in Texas, where B. turicatae is endemic (58). Serum samples from Lyme disease patients living on Long Island, N.Y., were provided by Alan MacDonald, Southampton Hospital, Southampton, N.Y. These Lyme disease patients were diagnosed on the basis of erythema migrans or an arthritis and positive Western blot. Serum samples from syphilis patients were provided by Brian Kiehl, General Biometrics, Inc., San Diego, Calif. Serum samples from healthy adult controls have been described previously (62).
One-dimensional gel electrophoresis.
Whole-cell lysates of spirochetes were prepared as described previously (61). One-dimensional SDS-PAGE using Laemmli buffer (41) and a vertical gel apparatus (Bethesda Research Laboratories-GIBCO, Gaithersburg, Md.) was used to separate proteins.
Western blot analysis.
Whole-cell lysates were electrophoresed in one-dimensional acrylamide gels and blotted onto nitrocellulose membranes with Towbin buffer (71) and a Trans-Blot cell (Bio-Rad Laboratories, Hercules, Calif.). The membranes were blocked overnight at room temperature with TSE-Tween (50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20) and incubated with either antiflagellin monoclonal antibody H9724 (6), rabbit antisera to the recombinant GlpQ diluted 1:500, or human antisera diluted 1:100. Bound antibodies were detected by 125I-labeled protein A autoradiography (61).
To standardize the human serological reactivity to GlpQ, all membranes were prepared in advance and contained replicate panels made with lysates of B. recurrentis, E. coli TA-115 expressing recombinant GlpQ, and E. coli containing only the vector. Each serum sample was tested for reactivity to these three lysates at the same time and with the same reagents. Film was exposed to the membranes in cassettes with light-intensifying screens at −70°C for the same length of time (18 h) before being developed. The level of reactivity to recombinant GlpQ was scored by measuring the thickness of the band with a transmission dissecting microscope fitted with a calibrated ocular micrometer.
Indirect immunofluorescence assay (IFA).
B. recurrentis 132 was cultured in BSK-H medium, harvested by centrifugation, rinsed twice with PBS, and mixed with fresh, washed sheep red blood cells. These sheep cells provide a negative background for comparison and an internal check for nonspecific binding of conjugate. Thin smears of the cell suspensions were made on glass microscope slides, dried at room temperature, fixed with methanol, wrapped with foil, and stored at −20°C until used. Human serum samples were tested with twofold serial dilutions ranging from 1:16 to 1:2,048. Bound antibodies were detected with a 1:100 dilution of goat anti-human immunoglobulin G (heavy plus light chains)–fluorescein isothiocyanate (Kirkegaard & Perry, Gaithersburg, Md.) and epifluorescence microscopy. The intensity of fluorescence was scored as 1+ to 4+, and the endpoint was defined as the highest dilution that provided reactivity greater than the background with the red blood cells. The geometric mean titers were determined by Perkins's method (55).
ELISA with whole-cell antigen.
A 500-ml culture of B. recurrentis containing approximately 108 cells per ml was centrifuged (12,500 × g), rinsed and suspended twice in PBS, and diluted to an optical density of 0.05 at 600 nm. This suspension was sonicated on ice in 20-ml portions for 2 min at an output setting of 5 using a Branson Sonifier-Cell Disruptor 185 (VWR Scientific, San Francisco, Calif.). The protein concentration of the sonicated suspension was determined with the Bradford assay (Bio-Rad Laboratories). The sonicate was stored at 4°C prior to use. To test sera by enzyme-linked immunosorbent assay (ELISA), Immulon-2 96-well, flat-bottomed microdilution plates (Dynatech Laboratories, Inc., Alexandria, Va.) were coated with 100 μl of the sonicated spirochetal suspension per well (229 μg per ml) and were dried overnight at 37°C. Wells were blocked to inhibit nonspecific binding with 200 μl of diluent (PBS, 5% horse serum, 0.05% Tween 20, 0.001% dextran sulfate) for 1 h at 37°C and then washed once with PBS–0.05% Tween 20. One pair of acute- and convalescent-phase serum samples were tested at eight twofold serial dilutions (1:32 to 1:4,096) by incubating 100 μl of each dilution per well for 1 h at 37°C. After three washes, 100 μl of a 1:2,500 dilution of goat anti-human immunoglobulin G (heavy and light chains) conjugated to horseradish peroxidase (Kirkegaard & Perry Laboratories) was added per well and incubated for 1 h at 37°C. After three washings, a substrate of 50% 2,2′-azino-di-(3-ethyl-benzthiazoline sulfonate) was added and left for 20 min before analysis at 405 nm with a Labsystems Multiskan Plus microtiter plate reader (Fisher Scientific, Pittsburgh, Pa.). This assay showed good discrimination between the negative and positive samples at dilutions above 1:128. A dilution of 1:250 was chosen to test the 84 Ethiopian serum samples and 12 normal serum samples by the procedure described above. Each serum sample was tested in triplicate, and the mean absorbance value was determined. Samples were considered positive if their mean absorbance was greater than the mean plus 3 standard deviations (SDs) of the absorbance of normal control sera tested at the same dilution.
Synthesis and purification of recombinant GlpQ for ELISA.
The glpQ gene of B. recurrentis isolate 115 was amplified with PCR using primers that contained XhoI (Br GlpQ fus 5′) and BamHI (Br GlpQ fus 3′) sites (Table 1) for cloning in frame with an amino-terminal histidine (His) tag in the pET-15b expression vector (Novagen Inc., Madison, Wis.). Total genomic DNA (40 ng) of the spirochete was used as the template in the PCR with an initial heating at 94°C for 2 min; 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min; and a final extension at 72°C for 7 min. The 5′ fusion primer began with the codon for the first amino acid immediately downstream of the lipidated cysteine, and the 3′ primer corresponded to a region of DNA sequence downstream of the B. recurrentis glpQ stop codon. The PCR fragment was ligated in the pCR2.1 vector (Invitrogen) according to the instructions of the manufacturer, and the plasmid was transformed into E. coli. Bacterial colonies were screened with PCR, and plasmid DNA was purified from a positive clone with a miniprep kit (Qiagen Inc., Valencia, Calif.) and digested with BamHI and XhoI. The restricted DNA fragment was purified with a QiaexII gel purification kit (Qiagen) and quantitated by UV spectroscopy. The pET-15b vector was digested with BamHI and XhoI, purified with a Quick Step clean-up kit (Edge BioSystems, Gaithersburg, Md.), quantitated by UV spectroscopy, treated with HK-phosphatase (Epicentre, Madison, Wis.) for 1 h at 30°C, and heat inactivated at 65°C for 15 min. The B. recurrentis glpQ PCR fragment was ligated to the pET-15b vector, transformed into E. coli XL1-Blue cells (Stratagene, La Jolla, Calif.) by electroporation, and grown on Luria broth plates with ampicillin (100 μg/ml). A single recombinant was picked and checked by PCR using the PCR conditions described above and the Br GlpQ fus 5′ and Br GlpQ fus 3′ primers. Vector DNA was purified from this recombinant with a Qiagen miniprep kit, quantitated, and transformed into chemically competent E. coli BLR(DE3) cells. A single recombinant was examined with PCR using the Br GlpQ fus 5′ and Br GlpQ fus 3′ primers and used for expression of the GlpQ fusion protein.
The His-GlpQ fusion protein was purified from E. coli cells following growth in Luria broth with 100 μg of carbenicillin per ml using the procedures described in the pET System Manual (Novagen). Cells were lysed by sonication, and the soluble protein fraction was passed through precharged Ni2+ Quick Columns provided in the HIS-Bind Purification Kit (Novagen), following the instructions of the manufacturer to separate the His-GlpQ fusion protein. The eluted sample was dialyzed with PBS at 4°C for 24 h in a Slide-A-Lyzer Dialysis Cassette (Pierce, Rockford, Ill.) to remove the salts and imidazole and examined by SDS-PAGE for purity, and the protein concentration was determined with the Bradford assay (Bio-Rad Laboratories). The His-GlpQ protein was adsorbed onto microtiter well surfaces of Ni-nitrilotriacetic acid HisSorb plates (Qiagen) by incubating 100 μl of the protein suspension (320 μg/ml) per well for 2 h at room temperature while shaking the plates (120 rpm) and then incubating overnight at 4°C without shaking. The next morning, the antigen solution was removed and the plates were washed. The assays were performed as described above except that the serum samples were tested at a 1:100 dilution and the incubations of the diluent, serum samples, and secondary conjugated antibody were done at room temperature with shaking of the plates.
RESULTS
Identification of B. recurrentis.
The four spirochete isolates cultured from patients in southern Sudan were presumptively identified as B. recurrentis on the basis of clinical and epidemiological history, presence of human body lice, detection of spirochetes in blood smears, ability of modified Kelly's medium to support spirochetal growth, and history of prior LBRF outbreaks in this region. Immunoblot analysis of whole-cell lysates with the genus-specific monoclonal antibody H9724 (6) identified the spirochetes as Borrelia (data not shown). The plasmid profiles of these four isolates were distinct from those obtained from all other species of Borrelia in our collection, were different from those described for Borrelia duttonii (20), and were similar to those described for B. recurrentis (22). The DNA sequences of the 16S rRNA and flagellin genes from the four isolates were identical to B. recurrentis sequences deposited in GenBank (data not shown), thereby confirming the species identification.
Identification and DNA sequence analysis of the glpQ genes of B. recurrentis and other Borrelia species.
Genomic DNA preparations obtained from the four B. recurrentis isolates and single isolates of B. crocidurae, B. turicatae, B. parkeri, and B. coriaceae yielded PCR amplification products of the appropriate size for glpQ. The amplified products were sequenced, and BLAST searches of the eight open reading frames confirmed that the sequences were glpQ.
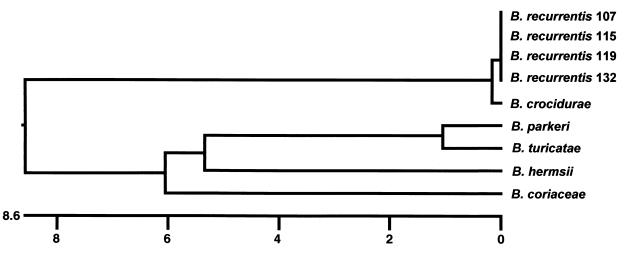
The glpQ gene of the four B. recurrentis isolates were 999 bp, excluding the stop codon. The four sequences were 99.8 to 100% identical. The glpQ gene in B. crocidurae also was 999 bp in length and was nearly (99.4%) identical to the B. recurrentis glpQ sequence. The glpQ sequences in the North American Borrelia species (B. hermsii, B. turicatae, B. parkeri, and B. coriaceae) were less similar, with identities around 82%. The glpQ genes in these species were also slightly larger: 1,011 bp in B. coriaceae, 1,014 bp in B. turicatae and B. parkeri, and 1,020 bp in B. hermsii (62, 64). The dendrogram based on these nine glpQ sequences (Fig. 1) has two deeply branching clusters, one comprised of B. recurrentis and B. crocidurae (Old World species), with nearly identical glpQ sequences, and the other comprised of the North American species, with less similar glpQ sequences.
FIG. 1.
Dendrogram showing the relatedness of the glpQ DNA sequences from B. recurrentis (four isolates) and B. crocidurae, B. parkeri, B. turicatae, B. hermsii, and B. coriaceae (one isolate each). The scale represents the percentage of nucleotide substitutions observed out of the total number of nucleotides compared.
The deduced amino acid sequences of the Borrelia GlpQ proteins were aligned (Fig. 2). All B. recurrentis isolates had the identical 333-amino-acid sequences. The B. crocidurae sequence also had 333 amino acids, but it differed from the B. recurrentis sequence at one amino acid (residue 292) (Fig. 2). At amino acid position 18 or 21 of each sequence, there was a cysteine residue preceded by the tetrapeptide Leu-Ile-Ile-Ser, Leu-Ile-Ile-Ala, or Leu-Ile-Thr-Ser (Fig. 2). These amino acid sequences are consistent with the occurrence of a signal peptide with a signal peptidase II cleavage site located at the cysteine, as described for other bacterial lipoproteins (73).
FIG. 2.
Alignment of the deduced GlpQ amino acid sequences, including those from B. recurrentis 115, B. crocidurae CR2A, B. parkeri RML, B. turicatae RML, B. hermsii DAH, and B. coriaceae CO53. Consensus amino acid residues are framed, and dashes indicate gaps introduced to maximize alignment.
Immunological detection of GlpQ.
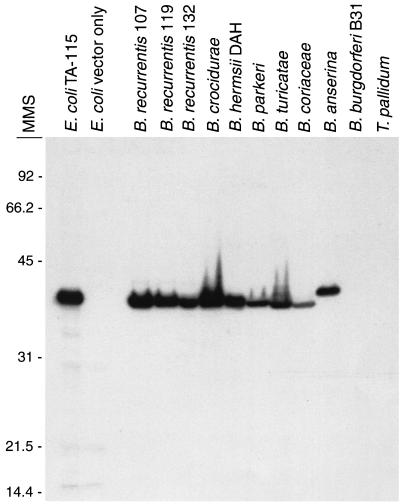
The glpQ gene of B. recurrentis isolate 115 was cloned into the pCR2.1 vector. DNA sequence analysis confirmed that glpQ had been cloned and that no spurious mutations were present. This transformant of E. coli, designated TA-115, was inoculated into a rabbit to produce antibodies to GlpQ. The preinoculation serum was nonreactive, but serum obtained at 28 days after the primary rabbit immunization reacted strongly to a single 39-kDa protein in the B. recurrentis lysate (data not shown). A protein with a slightly greater apparent molecular weight was identified in E. coli TA-115 but was absent in the lysate made from E. coli containing vector only. The difference in electrophoretic mobility between the native and recombinant GlpQ may be due to differential processing in E. coli and B. recurrentis. The rabbit immunized with the suspension of acrylamide containing recombinant GlpQ had very weak serological reactivity 28 days after the primary immunization. However, at 33 days following the booster injection, this serum reacted strongly by immunoblot analysis to GlpQ in lysates of B. recurrentis and E. coli TA-115. In addition, this serum identified the homologous GlpQ protein in B. crocidurae, B. hermsii, B. parkeri, B. turicatae, B. coriaceae, and B. anserina (Fig. 3). As expected, this serum did not react with B. burgdorferi, which lacks GlpQ (32, 62). The serum also did not react with T. pallidum, which has a GlpQ with only 38.7 to 42.9% amino acid identity with GlpQ of B. recurrentis (the variation in identity values is due to the use of different algorithms).
FIG. 3.
Immunoblot analysis with rabbit antiserum collected 4 weeks after booster injection with GlpQ excised from the gel preparation of E. coli TA-115, showing the presence of GlpQ in whole-cell lysates of B. recurrentis and other Borrelia species except B. burgdorferi. No reactivity with GlpQ made by T. pallidum was detectable. Lysates of E. coli TA-115 expressing GlpQ and E. coli with the vector only were used as controls. Molecular mass standards (MMS) are shown on the left in kilodaltons.
Reactivity of LBRF patient serum samples to B. recurrentis and recombinant GlpQ.
IFA is a useful test for the initial serological screening of antibodies resulting from Borrelia infections (5, 63), but the assay has not been applied previously to LBRF. Paired serum samples from 42 human LBRF patients hospitalized with acute fever and spirochetemia in Addis Ababa, Ethiopia, were tested by IFA with whole cells of B. recurrentis isolate 132. The IFA geometric mean titers for the acute- and convalescent-phase serum samples were 1:83 and 1:575, respectively. Thirty-three patients (78.5%) had a fourfold or greater increase in titer in the convalescent-phase sample, five patients (12%) had a twofold increase, and four patients (9.5%) had no increase in titer. None of the serum pairs had an acute-phase titer greater than that of the convalescent-phase sample. Seventeen (40%) of the 42 acute-phase serum samples had an IFA titer equal to or greater than 1:128, suggesting that (i) these patients had seroconverted prior to hospitalization, (ii) the patients were infected prior to the current illness, or (iii) the titers were falsely positive.
Serological reactivity by IFA to two other species of Borrelia was examined for 10 of the higher-titer convalescent-phase serum samples from LBRF patients (Table 2). These samples showed considerable cross-reactivity to B. hermsii and B. burgdorferi, with many of the titers equal to or greater than 1:2,048. Four serum samples collected 17 to 27 years after the illness from one LBRF patient infected in 1973 were also tested. Although these samples were still quite reactive to B. recurrentis, unlike the other samples, there was little or no reactivity to the other species (Table 2).
TABLE 2.
Comparative IFA titers of LBRF patients from Ethiopia with three species of Borrelia used as test antigen
| Sample | Titer with:
|
||
|---|---|---|---|
| B. recurrentis 132 | B. hermsii DAH | B. burgdorferi B31 | |
| Samples from 10 patients | |||
| 16B | 1:2,048 | 1:2,048 | 1:2,048 |
| 17B | 1:512 | 1:256 | 1:128 |
| 19B | 1:2,048 | 1:2,048 | 1:2,048 |
| 24B | 1:2,048 | 1:2,048 | 1:2,048 |
| 28B | 1:2,048 | 1:256 | 1:256 |
| 39B | 1:2,048 | 1:2,048 | 1:256 |
| 46B | 1:2,048 | 1:512 | 1:32 |
| 51B | 1:2,048 | 1:2,048 | 1:2,048 |
| 56B | 1:2,048 | 1:2,048 | 1:2,048 |
| 62B | 1:2,048 | 1:2,048 | 1:64 |
| Samples from the same person | |||
| 73/1990a | 1:1,024 | 1:32 | <1:16 |
| 73/1993 | 1:1,024 | 1:16 | <1:16 |
| 73/1997 | 1:512 | 1:16 | <1:16 |
| 73/2000 | 1:256 | 1:16 | <1:16 |
Year of infection/year of sample collection.
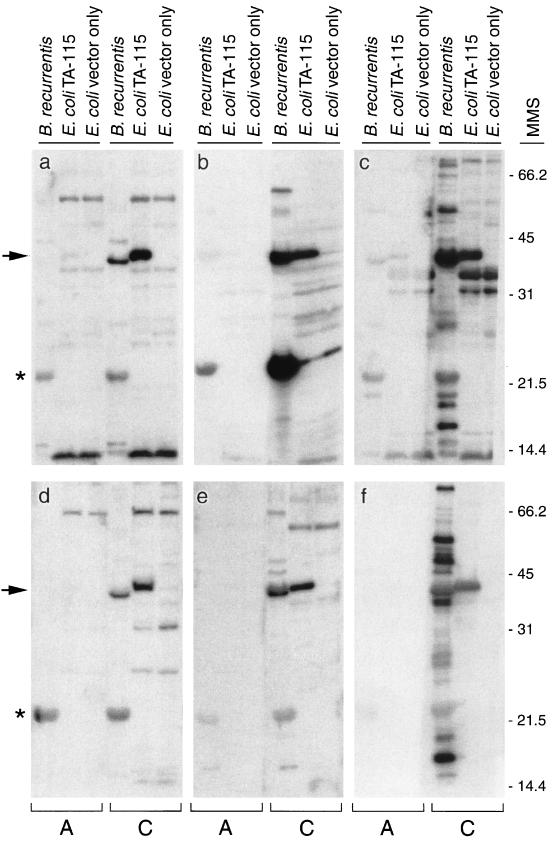
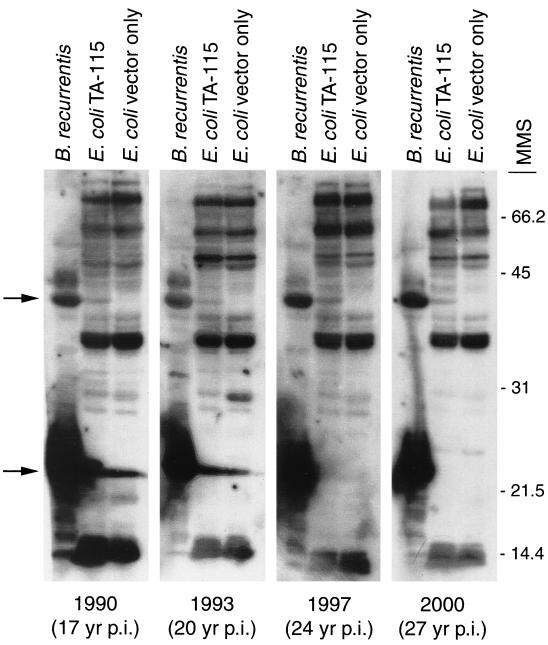
Western blot analysis was conducted to enhance the specificity of the serological reactivity. Serum samples were tested at a dilution of 1:100 for reactivity with GlpQ made by B. recurrentis and E. coli TA-115. Five levels of reactivity to the recombinant GlpQ were assessed for each sample: negative (no reactivity) and 1+ to 4+ (representing increased levels of reactivity observed in the blots determined by the thickness of the band). Fifteen (36%) of the acute-phase samples had no reactivity to GlpQ. However, serum samples obtained 8 to 13 days later from 14 of these 15 patients had anti-GlpQ antibody (Fig. 4). The other 27 acute-phase samples (64%) had various degrees of reactivity to GlpQ. Many samples had quite strong reactivity, suggesting that these patients had been diagnosed after seroconversion or that they had LBRF previously. All but 1 of the 42 convalescent-phase samples (98%) had anti-GlpQ reactivity. The four serum samples obtained from the patient infected in 1973 still had detectable antibodies to recombinant GlpQ (Fig. 5), demonstrating that antibodies specific for this antigen persisted for 27 years.
FIG. 4.
Immunoblots with representative paired acute-phase (lanes A) and convalescent-phase (lanes C) human serum samples from six LBRF patients (a to f) showing seroconversion to GlpQ (arrow). Whole-cell lysates of B. recurrentis, E. coli TA-115 with recombinant GlpQ, and E. coli vector only were used as controls. The asterisk shows reactivity to a putative 22-kDa variable small protein. Convalescent-phase samples were obtained 10 to 13 days after the acute-phase samples were drawn. Molecular mass standards (MMS) are shown on the right in kilodaltons.
FIG. 5.
Immunoblot analysis of four serum samples obtained from the same individual collected 17 to 27 years after documented infection with B. recurrentis. The patient was infected in 1973 in Addis Ababa, Ethiopia. Each serum sample was tested against whole-cell lysates of B. recurrentis, E. coli TA-115 expressing recombinant GlpQ, and E. coli containing the vector only. The top arrow indicates reactivity with GlpQ in E. coli TA-115, which is weak compared to the reactivity shown in Fig. 4 with serum samples obtained from patients only 10 to 13 days after hospitalization. The lower arrow indicates strong reactivity to a putative 22-kDa variable small protein. Molecular mass standards (MMS) are shown on the right in kilodaltons. p.i., postinfection.
The immunoblot analysis also identified reactivity with an abundant 22-kDa protein. We presume that this protein is a member of the family of variable small proteins (Vsps) because of its amount relative to those of other proteins, size, and antigenicity. Many of the LBRF patients (28 of 42 convalescent-phase serum samples [67%]) had antibodies that reacted with this putative Vsp (Fig. 4), as did the patient infected in 1973 (Fig. 5), a result suggesting that this protein may be worthy of further investigation as a serological test antigen.
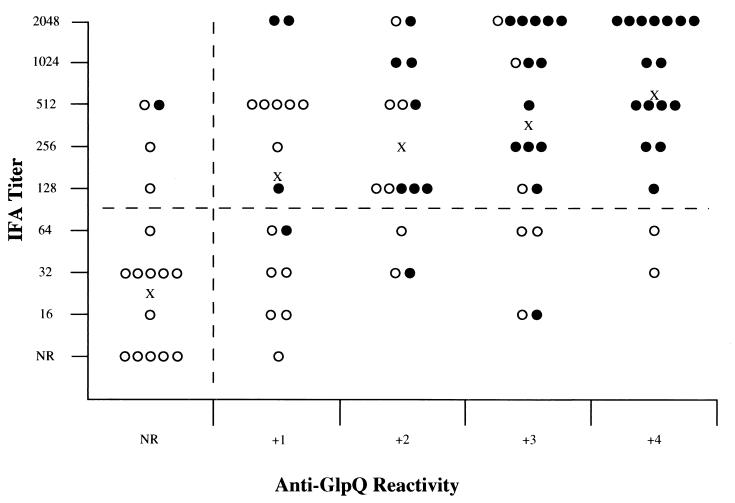
IFA titers and reactivity to GlpQ of the 84 LBRF serum samples were summarized in a scatter plot to compare the performances of the two assays (Fig. 6). The difference in geometric mean titers between the acute- and convalescent-phase serum samples corresponded directly with the reactivity to GlpQ in the two groups. However, if an IFA titer of 1:128 or greater is accepted as positive (a conservative value for this type of assay), the plot shows that 19% (16 of 84) of the samples were negative by IFA but were positive for anti-GlpQ antibodies by immunoblotting. Therefore, the immunoblot assay was more sensitive than the whole-cell IFA for detecting antibodies to the recombinant GlpQ. This result was also obtained when the second rabbit anti-GlpQ antiserum was tested by IFA and had no reactivity greater than the background observed using the preimmunization sample. Thus, anti-GlpQ antibodies appeared to contribute little or nothing to the serum reactivity in IFA.
FIG. 6.
Scatter plot showing reactivity of each sample of acute-phase (○) and convalescent-phase (●) serum determined by IFA to the entire spirochete and by immunoblotting to recombinant GlpQ (n = 84). An IFA titer equal to or greater than 1:128 was considered to be positive. The horizontal dashed line separates values judged to represent positive and negative titers. A positive immunoblot result was considered to be any reactivity to recombinant GlpQ. The vertical dashed line separates positive and negative immunoblot samples. X indicates the IFA geometric mean titer for each group of samples with equal reactivity to recombinant GlpQ. Reactivity to GlpQ was quantified by measuring the thickness of the band in the blot.
Forty additional serum samples from humans not having LBRF were also tested by immunoblotting for reactivity to recombinant GlpQ. No reactivity was observed with serum samples from seven syphilis patients (known to be reactive to T. pallidum by immunoblot analysis at a 1:100 dilution); 15 serum samples from Lyme disease patients residing on Long Island, N.Y.; and serum samples from five healthy controls. Convalescent-phase serum samples from 13 tick-borne relapsing-fever patients from southern British Columbia, Washington, California, and Texas were either nonreactive (n = 7) or weakly reactive (n = 6). The weak cross-reactivity obtained with some of the antisera from tick-borne relapsing-fever patients was anticipated due to the presence of GlpQ in these other spirochetes.
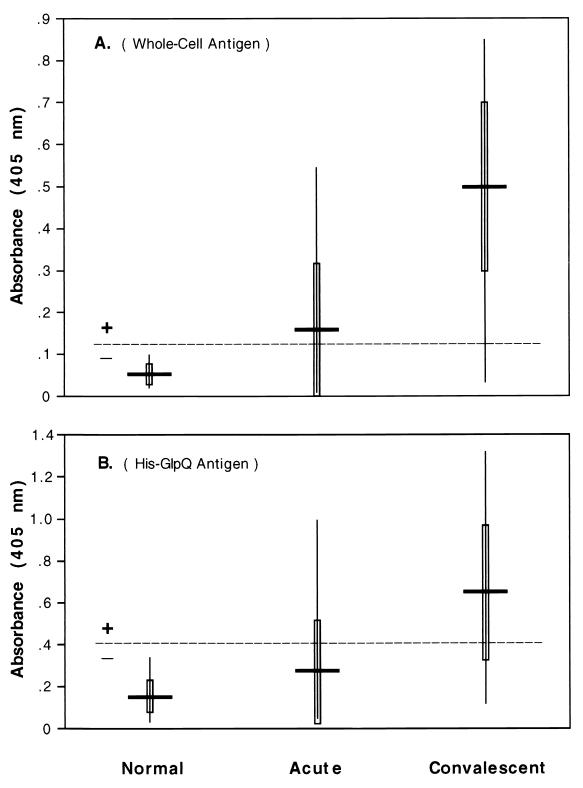
ELISAs with different antigens were also used to test the acute- and convalescent-phase serum samples of the LBRF patients (Fig. 7). Using the B. recurrentis whole-cell sonicated antigen, 43% (18 of 42) of the acute-phase samples and 93% (39 of 42) of the convalescent-phase samples were positive. These results were based on absorbance values greater than 0.129, which was the threshold determined by the mean absorbance plus 3 SDs of the normal serum samples. These results were very close to the values achieved by IFA, which yielded 40 and 93% positivity for the acute- and convalescent-phase samples, respectively. The mean absorbances were 0.164 with the acute-phase samples and 0.499 with the convalescent samples (Fig. 7A). An ELISA using purified, recombinant His-tagged GlpQ as the antigen resulted in 21% of the acute-phase samples and 69% of the convalescent-phase samples being positive (greater than the threshold absorbance of 0.414) (Fig. 7B). Forty-eight percent of the patients seroconverted with this assay, and the mean absorbance values for the acute- and convalescent-phase serum samples were 0.271 and 0.642, respectively. However, this ELISA was less sensitive than the immunoblot analysis in detecting specific anti-GlpQ antibody in the LBRF serum samples.
FIG. 7.
ELISA absorbance values of acute- and convalescent-phase LBRF patient serum samples with two antigen preparations. (A) Whole-cell sonicate of B. recurrentis as antigen. All serum samples were tested in triplicate at 1:250, and the average value was used. The mean absorbance for each group is shown by the horizontal solid bar, 1 SD above and below the mean is shown by the vertical open bar, and the range is shown by the vertical line. The horizontal dashed line represents the threshold for determining a positive sample, determined by the mean absorbance plus 3 SDs of the absorbance values of the normal serum samples. (B) His-GlpQ purified protein as antigen and serum samples tested in triplicate at a 1:100 dilution.
DISCUSSION
Previously, we identified, cloned, and characterized the B. hermsii glpQ gene, made recombinant GlpQ protein, and produced rabbit anti-GlpQ antibody (62). The rabbit antiserum reacted specifically with single proteins of approximately 38 to 42 kDa in B. hermsii, B. parkeri, B. turicatae, the Florida canine borrelia, B. crocidurae, B. coriaceae, B. anserina, and recombinant E. coli. Mice infected with B. hermsii, B. turicatae, the Florida canine borrelia, B. crocidurae, and B. duttonii and chickens infected with B. anserina seroconverted to recombinant B. hermsii GlpQ. Humans with tick-borne relapsing fever in North America also had antibodies to GlpQ, whereas Lyme disease patients did not. These serological data suggest that all species of Borrelia other than those assigned to the B. burgdorferi species complex (sensu lato) make a GlpQ homolog. In the present work, we characterized the glpQ genes in B. recurrentis, B. crocidurae, B. turicatae, B. parkeri, and B. coriaceae. We also confirmed that B. anserina makes a putative GlpQ homolog. We have been unable to amplify glpQ in this species and, as a consequence, have sequenced only about 50% of the gene. The protein appears to be larger than homologs in all other species by immunoblot analysis (Fig. 3). GlpQ was not detected in B. burgdorferi or T. pallidum by immunoblotting with the rabbit anti-B. recurrentis GlpQ antibody, results identical to our earlier observations with antiserum produced to B. hermsii GlpQ (62). The absence of glpQ in B. burgdorferi was confirmed when it was shown to be absent from the genome sequences (32). In contrast, T. pallidum has a glpQ (=gpd) homolog (33, 65, 67). Its lack of reactivity with anti-Borrelia GlpQ antisera is likely due to the low level of identity (38%) between the amino acid sequences.
Glycerophosphodiester phosphodiesterase (GlpQ) was first described for E. coli as the product of a member of the glp regulon (43). The proteins encoded by genes in this regulon participate primarily in the salvage of glycerol released when phospholipids and triglycerides are degraded (44). Specifically, GlpQ in E. coli hydrolyzes deacylated phospholipids to form an alcohol and glycerol-3-phosphate (70). Glycerol-3-phosphate is either used in the synthesis of new phospholipids or converted to dihydroxyacetone phosphate by GlpD and then to glyceraldehyde-3-phosphate by glyceraldehyde-3-phosphate dehydrogenase for use in glycolysis (44). Homologs of glpQ also have been identified in Haemophilus influenzae (38, 52), Bacillus subtilis (53), and several other species by genome sequencing projects.
The function of GlpQ in Borrelia spirochetes is unknown, and its subcellular localization in B. hermsii is uncertain. Previously we found no reduction in the amount of this protein detected following proteinase K treatment of intact B. hermsii, suggesting that GlpQ is not located on the outer surface (62). However, Shang and coworkers (64) reported that GlpQ (=Gpd) was present in outer membrane preparations and therefore may be associated with the inner surface of the outer membrane. Regardless of its location, this putative enzyme in relapsing-fever spirochetes is quite immunogenic.
GlpQ of B. recurrentis rapidly stimulates a strong antibody response in humans that is detectable by immunoblotting 1 to 2 weeks after clinical presentation. However, this protein is produced by many species of Borrelia that achieve significant densities in blood and are transmitted by argasid ticks. In Africa, the tick-borne relapsing-fever spirochetes B. duttonii and B. crocidurae may occur where their respective tick vectors, Ornithodoros moubata and Ornithodoros erraticus sonrai, also occur. Therefore, in geographic regions where the ranges of these infected ticks overlap with the presence of humans infested with body lice, there could be difficulties in serological confirmation of infection with these closely related species. We found that the GlpQ proteins of B. recurrentis and B. crocidurae differ by only one amino acid. Hence, retrospective serological testing with only GlpQ in the absence of a clinical history or epidemiological information will not distinguish serologically between exposure to these two species of spirochetes. The geographical distribution of O. erraticus in Africa suggests possible overlap with regions of LBRF endemicity in central and eastern Africa (23, 29), as is true for O. moubata (27, 37). Although we were unable to investigate B. duttonii, we expect that this species has a GlpQ with high amino acid sequence identity to the B. recurrentis and B. crocidurae GlpQs. Hence, identifying the probable vector as either the human body louse or Ornithodoros ticks will support serological testing for anti-GlpQ antibodies to identify the causative agent.
IFA titers with serum samples from LBRF patients obtained soon after infection were strongly cross-reactive with B. hermsii and B. burgdorferi. We and others have observed this phenomenon with serum samples from tick-borne relapsing-fever and Lyme disease patients (46, 47, 62), emphasizing the importance of immunoblotting with specific recombinant antigens. However, the serum samples obtained from the patient infected with B. recurrentis 17 to 27 years earlier were nearly or completely nonreactive with these other species but were still positive with B. recurrentis. These data, although limited, suggest that serological cross-reactivity by IFA may wane with time after exposure.
In summary, we have characterized the glpQ genes in four isolates of B. recurrentis from Sudan and in single isolates of B. crocidurae, B. turicatae, B. parkeri, and B. coriaceae. The gene from B. recurrentis was cloned and expressed in E. coli, and the recombinant GlpQ protein was used to test sera from human LBRF patients. Paired acute- and convalescent-phase serum samples from these patients demonstrated seroconversion to this antigen 1 to 2 weeks after hospitalization. Immunoblotting with recombinant GlpQ was more sensitive than the ELISA with purified His-tagged GlpQ, although the sensitivity of this assay may be improved by increasing the concentration of antigen. The IFA with fixed, whole spirochetes and the ELISA with sonicated, whole-cell borrelia antigen performed equally but are less specific in their reactivity for borrelioses. Thus, GlpQ will significantly increase the specificity of serological testing for LBRF in regions, like Africa, where patients infested with body lice present with recurrent febrile disease.
ACKNOWLEDGMENTS
We thank members of the joint World Health Organization-U.S. Centers for Disease Control and Prevention epidemic assistance team (D. T. Dennis, D. O'Leary, K. Orloski, M. Ryan, R. Shoo, and P. Tharmaphornpilas) for providing cultures of LBRF spirochetes isolated from patients in southern Sudan in April 1999; J. Plorde, NAMRU-3, Ethiopia Detachment, for providing LBRF patient serum samples; R. Karstens, R. Larson, and C. Rittner for technical assistance; W. Burgdorfer, G. Somerville, and M. Chausse for reviewing the manuscript prior to submission; and G. Hettrick for help with graphic arts. We thank J. M. Musser for editorial assistance.
REFERENCES
- 1.Abdalla R E. Some studies on relapsing fever in the Sudan. J Trop Med Hyg. 1969;72:125–128. [PubMed] [Google Scholar]
- 2.Ahmed M A M, Wahab S M A, Malik M O A, Gadir A M A, Salih S Y, Omer A, Hassan A M A. Louse-borne relapsing fever in the Sudan: a historical review and a clinico-pathological study. Trop Geogr Med. 1980;32:106–111. [PubMed] [Google Scholar]
- 3.Almaviva M, Hailu B, Borgnolo G, Chiabrera F, Tolesse G, Gebre B. Louse-borne relapsing fever epidemic in Arssi region, Ethiopia: a six months survey. Trans R Soc Trop Med Hyg. 1993;87:153. doi: 10.1016/0035-9203(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A G. Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev. 1988;1:399–414. doi: 10.1128/cmr.1.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkhaug K. Relapsing fever in the Americas. Washington, D.C.: American Association for the Advancement of Science; 1942. pp. 7–14. [Google Scholar]
- 8.Borgnolo G, Hailu B, Ciancarelli A, Almaviva M, Woldemariam T. Louse-borne relapsing fever: a clinical and epidemiological study of 389 patients in Asella Hospital, Ethiopia. Trop Geogr Med. 1993;45:66–69. [PubMed] [Google Scholar]
- 9.Boyer K M, Munford R S, Maupin G O, Pattison C P, Fox M D, Barnes A M, Jones W L, Maynard J E. Tick-borne relapsing fever: an interstate outbreak originating at Grand Canyon National Park. Am J Epidemiol. 1977;105:469–479. doi: 10.1093/oxfordjournals.aje.a112406. [DOI] [PubMed] [Google Scholar]
- 10.Brown V, Larouze B, Desve G, Rousset J J, Thibon M, Fourrier A, Schwoebel V. Clinical presentation of louse-borne relapsing fever among Ethiopian refugees in northern Somalia. Ann Trop Med Parasitol. 1988;82:499–502. doi: 10.1080/00034983.1988.11812282. [DOI] [PubMed] [Google Scholar]
- 11.Burgdorfer W. The diagnosis of relapsing fevers. In: Johnson R C, editor. The biology of parasitic spirochetes. New York, N.Y: Academic Press, Inc.; 1976. pp. 225–234. [Google Scholar]
- 12.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 13.Butler T, Hazen P, Wallace C K, Awoke S, Habte-Michael A. Infection with Borrelia recurrentis: pathogenesis of fever and petechiae. J Infect Dis. 1979;140:665–675. doi: 10.1093/infdis/140.5.665. [DOI] [PubMed] [Google Scholar]
- 14.Buxton P A. The louse. An account of the lice which infest man, their medical importance and control. 2nd ed. Baltimore, Md: Williams & Wilkins; 1946. [Google Scholar]
- 15.Charters A D. Relapsing fever in Abyssinia. Trans R Soc Trop Med Hyg. 1942;35:271–279. [Google Scholar]
- 16.Chung H-L, Chang F C. Relapsing fever: clinical and statistical study of 337 cases. Chinese Med J. 1939;55:6–33. [Google Scholar]
- 17.Craigie D. Notice of a febrile disorder which has prevailed at Edinburgh during the summer of 1843. Edinburgh Med Surg J. 1843;60:410–418. [PMC free article] [PubMed] [Google Scholar]
- 18.Creig E D W. An epidemic of relapsing fever in Edinburgh in 1843. Edinburgh Med J. 1943;50:681–685. [Google Scholar]
- 19.Cunningham J. Serological observations on relapsing fever in Madras. Trans R Soc Trop Med Hyg. 1925;19:11–40. [Google Scholar]
- 20.Cutler S J, Akintunde C O K, Moss J, Fukunaga M, Kurtenbach K, Talbert A, Zhang H, Wright D J M, Warrell D A. Successful in vitro cultivation of Borrelia duttonii and its comparison with Borrelia recurrentis. Int J Syst Bacteriol. 1999;49:1793–1799. doi: 10.1099/00207713-49-4-1793. [DOI] [PubMed] [Google Scholar]
- 21.Cutler S J, Fekade D, Hussein K, Knox K A, Melka A, Cann K, Emilianus A R, Warrell D A, Wright D J M. Successful in-vitro cultivation of Borrelia recurrentis. Lancet. 1994;343:242. doi: 10.1016/s0140-6736(94)91032-4. [DOI] [PubMed] [Google Scholar]
- 22.Cutler S J, Moss J, Fukunaga M, Wright D J M, Fekade D, Warrell D. Borrelia recurrentis characterization and comparison with relapsing-fever, Lyme-associated, and other Borrelia spp. Int J Syst Bacteriol. 1997;47:958–968. doi: 10.1099/00207713-47-4-958. [DOI] [PubMed] [Google Scholar]
- 23.Davis G E, Hoogstraal H. The relapsing fevers: a survey of the tick-borne spirochetes of Egypt. J Egyptian Public Health Assoc. 1954;29:139–143. [Google Scholar]
- 24.de Jong J, Wilkinson R J, Schaeffers P, Sondorp H E, Davidson R N. Louse-borne relapsing fever in southern Sudan. Trans R Soc Trop Med Hyg. 1995;89:621. doi: 10.1016/0035-9203(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 25.Dennis D T, Awoke S, Doberstyn E B, Fresh J W. Bleeding in louse-borne relapsing fever: clinical and laboratory features in 29 patients. East Afr Med J. 1976;53:220–225. [PubMed] [Google Scholar]
- 26.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupont H T, La Scola B, Williams R, Raoult D. A focus of tick-borne relapsing fever in southern Zaire. Clin Infect Dis. 1997;25:139–144. doi: 10.1086/514496. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin M S, Anderson D E, Jr, Schwan T G, Shoemaker P C, Banerjee S N, Kassen B O, Burgdorfer W. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- 29.El-Ziady S. The behavior of Ornithodoros erraticus (Lucas, 1849), small form (Ixodoidea, Argasidae), towards certain environmental factors. Ann Entomol Soc Am. 1958;51:317–336. [Google Scholar]
- 30.Felsenfeld O. Strains, vectors, human and animal borreliosis. St. Louis, Mo: Warren H. Green, Inc.; 1971. Borrelia. [Google Scholar]
- 31.Felsenfeld O. Borreliae, human relapsing fevers, and parasite-vector-host relationships. Bacteriol Rev. 1965;29:46–74. doi: 10.1128/br.29.1.46-74.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, Vugt R V, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 33.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McLeod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith H O, Venter J C. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–378. doi: 10.1126/science.281.5375.375. , 387–388. [DOI] [PubMed] [Google Scholar]
- 34.Greaves F C, Gezon H M, Alston W F. Studies on louse-borne relapsing fever in Tunisia. US Naval Med Bull. 1945;45:1029–1048. [PubMed] [Google Scholar]
- 35.Henderson W. On some of the characters which distinguish the fever at present epidemic from typhus fever. Edinburgh Med Surg J. 1844;61:201–225. [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins D G, Bleasby A J, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. CABIOS. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 37.Hoogstraal H. African Ixodoidea. I. Ticks of the Sudan (with special reference to Equatoria province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). Cairo, Egypt: U.S. Naval Medical Research Unit no. 3; 1956. [Google Scholar]
- 38.Janson H, Heden L-O, Grubb A, Ruan M, Forsgren A. Protein D, an immunoglobulin D-binding protein of Haemophilus influenzae: cloning, nucleotide sequence, and expression in Escherichia coli. Infect Immun. 1991;59:119–125. doi: 10.1128/iai.59.1.119-125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenner W. General description of the symptoms and lesions of structure of relapsing fever. Med Times. 1850;22:646–647. [Google Scholar]
- 40.Kelly R. Cultivation of Borrelia hermsi. Science. 1971;173:443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- 41.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 42.Lane R S, Burgdorfer W, Hayes S F, Barbour A G. Isolation of a spirochete from the soft tick, Ornithodoros coriaceus: a possible agent of epizootic bovine abortion. Science. 1985;230:85–87. doi: 10.1126/science.3898367. [DOI] [PubMed] [Google Scholar]
- 43.Larson T J, Ehrmann M, Boos W. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem. 1983;258:5428–5432. [PubMed] [Google Scholar]
- 44.Lin E C C. Dissimilatory pathways for sugars, polyols, and carboxylates. In: Neidhardt F C, Ingraham J L, Magasanik B, Low K B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1987. pp. 244–284. [Google Scholar]
- 45.Mackie F P. The part played by Pediculus corporis in the transmission of relapsing fever. Br Med J. 1907;2:1706–1709. doi: 10.1136/bmj.2.2450.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnarelli L A, Anderson J F, Johnson R C. Cross reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–187. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 47.Magnarelli L A, Meegan J M, Anderson J F, Chappell W A. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J Clin Microbiol. 1984;20:181–184. doi: 10.1128/jcm.20.2.181-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mekasha A, Meharie S. Outbreak of louse-borne relapsing fever in Jimma, southwestern Ethiopia. East Afr Med J. 1996;73:54–58. [PubMed] [Google Scholar]
- 49.Miller P B M. Epidemic relapsing fever among the Chinese at Oroville. Pac Med Surg J. 1875;17:370–375. [Google Scholar]
- 50.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 51.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 52.Munson R S, Jr, Sasaki K. Protein D, a putative immunoglobulin D-binding protein produced by Haemophilus influenzae, is glycerophosphodiester phosphodiesterase. J Bacteriol. 1993;175:4569–4571. doi: 10.1128/jb.175.14.4569-4571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsson R-P, Beijer L, Rutberg B. The glpT and glpQ genes of the glycerol regulon in Bacillus subtilis. Microbiology. 1994;140:723–730. doi: 10.1099/00221287-140-4-723. [DOI] [PubMed] [Google Scholar]
- 54.Ormsbee R, Peacock M, Philip R, Casper E, Plorde J, Gabre-Kidan T, Wright L. Serologic diagnosis of epidemic typhus fever. Am J Epidemiol. 1977;105:261–271. doi: 10.1093/oxfordjournals.aje.a112382. [DOI] [PubMed] [Google Scholar]
- 55.Perkins F T. A ready reckoner for the calculation of geometric mean antibody titres. J Gen Microbiol. 1958;19:540–541. doi: 10.1099/00221287-19-3-540. [DOI] [PubMed] [Google Scholar]
- 56.Rahlenbeck S I, Gebre-Yohannes A. Louse-borne relapsing fever and its treatment. Trop Geogr Med. 1995;47:49–52. [PubMed] [Google Scholar]
- 57.Raoult D, Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis. 1999;29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- 58.Rawlings J A. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Texas Med. 1995;91:56–59. [PubMed] [Google Scholar]
- 59.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 60.Salih S Y, Mustafa D, Wahab S M A, Ahmed M A M, Omer A. Louse-borne relapsing fever. I. A clinical and laboratory study of 363 cases in the Sudan. Trans R Soc Trop Med Hyg. 1977;71:43–48. doi: 10.1016/0035-9203(77)90206-1. [DOI] [PubMed] [Google Scholar]
- 61.Schwan T G, Kime K K, Schrumpf M E, Coe J E, Simpson W J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi) Infect Immun. 1989;57:3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwan T G, Schrumpf M E, Hinnebusch B J, Anderson D E, Konkel M E. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwan T G, Simpson W J, Rosa P A. Laboratory confirmation of Lyme disease. Can J Infect Dis. 1991;2:64–69. doi: 10.1155/1991/637201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang E S, Skare J T, Erdjument-Bromage H, Blanco D R, Tempst P, Miller J N, Lovett M A. Sequence analysis and characterization of a 40-kilodalton Borrelia hermsii glycerophosphodiester phosphodiesterase homolog. J Bacteriol. 1997;179:2238–2246. doi: 10.1128/jb.179.7.2238-2246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shevchenko D V, Akins D R, Robinson E J, Li M, Shevchenko O V, Radolf J D. Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, and glycerophosphodiester phosphodiesterase in outer membrane fractions from Treponema pallidum, the syphilis spirochete. Infect Immun. 1997;65:4179–4189. doi: 10.1128/iai.65.10.4179-4189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sholdt L L, Holloway M L, Fronk W D. The epidemiology of human pediculosis in Ethiopia. Jacksonville, Fla: Navy Disease Vector Ecology and Control Center; 1979. [Google Scholar]
- 67.Stebeck C E, Shaffer J M, Arroll T W, Lukehart S A, Van Voorhis W C. Identification of the Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase homologue. FEMS Microbiol Lett. 1997;154:303–310. doi: 10.1111/j.1574-6968.1997.tb12660.x. [DOI] [PubMed] [Google Scholar]
- 68.Sundnes K O, Haimanot A T. Epidemic of louse-borne relapsing fever in Ethiopia. Lancet. 1993;343:1213–1215. doi: 10.1016/0140-6736(93)92190-5. [DOI] [PubMed] [Google Scholar]
- 69.Tarizzo M L. The control of lice and louse-borne diseases, p. 50–59. Scientific Publication no. 263. Washington, D.C.: Pan American Health Organization; 1973. [Google Scholar]
- 70.Tommassen J, Eiglmeier K, Cole S T, Overduin P, Larson T J, Boos W. Characterization of two genes, glpQ and ugpQ, encoding glycerophosphyl diester phosphodiesterases of Escherichia coli. Mol Gen Genet. 1991;226:321–327. doi: 10.1007/BF00273621. [DOI] [PubMed] [Google Scholar]
- 71.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warrell D A, Perine P L, Krause D W, Bing D H, MacDougal S J. Pathophysiology and immunology of the Jarisch-Herxheimer-like reaction in louse-borne relapsing fever: comparison of tetracycline and slow-release penicillin. J Infect Dis. 1983;147:898–909. doi: 10.1093/infdis/147.5.898. [DOI] [PubMed] [Google Scholar]
- 73.Wu H C, Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- 74.Zein Z A. Louse borne relapsing fever (LBRF): mortality and frequency of Jarisch-Herxheimer reaction. J R Soc Health. 1987;107:146–147. doi: 10.1177/146642408710700410. [DOI] [PubMed] [Google Scholar]