Abstract
Several genetic loci have been utilized to genotype isolates of Mycobacterium tuberculosis. A shortcoming of the most commonly used method, IS6110 fingerprinting, is that it does not adequately discriminate between isolates having few copies of IS6110. This study was undertaken to compare pTBN12 fingerprinting of polymorphic GC-rich repetitive sequence genes and spoligotyping of the direct repeat locus as secondary typing procedures for M. tuberculosis isolates having fewer than six copies of IS6110. A total of 88 isolates (100% of the isolates with fewer than six copies of IS6110 isolated in Arkansas during 1996 and 1997) were included in this study. Among the 88 isolates, 34 different IS6110 patterns were observed, 10 of which were shared by more than 1 isolate, involving a total of 64 isolates. The 64 isolates were subdivided into 13 clusters (containing 37 isolates) and 27 unique isolates based on a combination of IS6110 and pTBN12 fingerprinting and into 11 clusters (containing 51 isolates) and 13 unique isolates based on a combination of IS6110 fingerprinting and spoligotyping. Identical spoligotypes were found among isolates having different IS6110 patterns, as well as among isolates showing different pTBN12 patterns. In contrast, all isolates that had different IS6110 patterns were found to be unique by pTBN12 typing. The clustering rate was 73, 58, and 42%, respectively, for IS6110 fingerprinting alone, IS6110 fingerprinting and spoligotyping combined, and IS6110 and pTBN12 combined fingerprinting. The data indicate that the pTBN12 method has greater discriminating power among low-copy-number isolates than does spoligotyping.
DNA fingerprinting of Mycobacterium tuberculosis strains based on the insertion element IS6110 is an important new tool to differentiate strains and to study the epidemiology of tuberculosis (8, 18, 30). Due to the relative stability of IS6110 within a strain over time and the variable copy number and locations within the genome (9, 20, 30), IS6110 DNA fingerprinting has been beneficial in directing outbreak investigations (12, 13, 21), distinguishing between exogenous reinfection and relapse of cured infection (25, 27), and confirming suspected laboratory cross-contamination (5, 6, 23). The major limitation of the IS6110 fingerprinting method is its low discriminating power for isolates with fewer than six copies of IS6110. In previous large-scale investigations, from one-fifth to one-third of the M. tuberculosis isolates were found to be low-copy-number strains (3, 4, 7, 24, 28, 31). Secondary typing methods to subgroup low-copy-number isolates have been developed based on different short repetitive DNA sequences associated with some degree of genetic diversity, including major polymorphic tandem repeats, direct repeats (DR), polymorphic GC-rich repetitive sequences (PGRS), and variable-number tandem repeats (10, 14, 15, 16, 17, 22). The most popular secondary typing methods are PGRS fingerprinting using the recombinant plasmid pTBN12 and DR spoligotyping. To date, none of the evaluations of genotyping methods have systematically compared the two methods with M. tuberculosis isolates having a low IS6110 copy number.
The DR locus, which is the basis for the spoligotyping method, consists of a series of directly repeated sequence of 36 bp, with each DR being separated by nonrepetitive unique spacer DNA of 34 to 41 bp in length. Most M. tuberculosis strains contain a copy of IS6110 at a site within the DR region. When the DR regions of several strains are compared, the order of spacers appears to be about the same among all strains, but insertions or deletions of spacers and DR do occur (15, 16, 17). The spoligotyping method involves PCR amplification of the DR locus and hybridization to a series of oligonucleotides representing each of the unique spacer sequences in the DR locus. The final result is an array of hybridizing spots that can be readily analyzed in a word processing program. The most attractive aspect of the method is the ability to rapidly fingerprint strains without the need to subculture isolates for DNA isolation. Furthermore, it can be applied to bacilli found in concentrated sputum sediments when such specimens are smear positive, reducing the time required to determine the secondary fingerprint by approximately a month.
The PGRS method is similar to the standardized IS6110 fingerprinting in that it requires purified DNA for Southern blot hybridization and banding pattern analysis. PGRS fingerprinting has proven to be useful for differentiating M. tuberculosis strains with fewer than six copies of IS6110 that could not readily be differentiated by IS6110 fingerprinting (1, 4, 7, 10, 29). A better correlation between DNA fingerprinting data and the results of conventional epidemiology was found when a combination of the IS6110 and PGRS fingerprinting was applied than when the IS6110 was used alone (7, 29). However, the difficulties in computerizing the analysis of PGRS fingerprints due to the complexity of the fingerprint patterns have limited the wide use of PGRS fingerprinting.
In the present study we have tested 88 isolates having fewer than six copies of IS6110 with both secondary typing methods and compared the results with the IS6110 fingerprinting results and epidemiologic data from the corresponding patients. Thus, we determined the discriminating power of the PGRS and DR spoligotyping methods when combined with IS6110 fingerprinting for low-copy-number strains.
MATERIALS AND METHODS
Study sample.
Eighty-eight isolates of M. tuberculosis were included in this study. The sample represents 100 percent of the isolates having fewer than six copies of IS6110 encountered during 1996 and 1997. During this time frame IS6110 fingerprints were obtained for 322 isolates (98.5% of the total culture-positive cases). All the patients included in the study were residents of Arkansas at the time of their diagnosis. The records of the Arkansas Health Department were used to ascertain the study population.
DNA fingerprinting and spoligotyping.
Isolates of M. tuberculosis were cultured on Lowenstein-Jensen medium. Chromosomal DNA was extracted from the isolates with chloroform-isoamyl alcohol as described previously (19). IS6110 fingerprinting and pTBN12 fingerprinting were performed according to standard procedures (10, 26). Restriction endonucleases PvuII and AluI were used to cleave DNA for IS6110 DNA fingerprinting and pTBN12 secondary typing, respectively. The IS6110 probe used is a PCR product complementary to the sequence on the right side of the PvuII site within IS6110, extending from bp 568 to 1089. The PGRS probe, designated pTBN12 probe, is a 3.4-kb insert of a PGRS contained in the recombinant plasmid pTBN12. The preparation of the two probes was as described previously (30). Both probes were labeled with the enhanced chemiluminescence direct nucleic acid labeling system (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England). Spoligotyping to detect 43 known spacer sequences in the DR locus of M. tuberculosis was performed as described previously (17). Approximately 20 ng of DNA from each isolate was used as a target for spoligotyping.
Analysis of genotyping results.
IS6110 fingerprints were analyzed by computer-assisted analyses using Bioimage (Ann Arbor, Mich.) Whole Band Analyzer software (version 3.4). Fingerprints were matched by the average linkage clustering method. Any two fingerprints that were 100% matched by computer analysis at a band deviation of 2.5% were considered to be identical. Fingerprints generated by pTBN12 secondary typing were compared by visual inspection. Bands that were larger than 1.6 kbp were taken into consideration in the comparison. Spoligotyping results were read manually. Results expressed as the presence (by using the letter “O”) or absence (by a dot) of each of the 43 spacer sequences were entered into Microsoft Excel software and sorted by the software according to the similarity of the patterns. Spoligotypes that matched exactly were considered to be identical.
To compare the diversity of DNA polymorphism associated with each of the three genetic markers, genotype clustering of 88 isolates was done respectively for each of the three methods. To evaluate the usefulness of pTBN12 secondary typing for subtyping isolates having fewer than six copies of IS6110, isolates were grouped based on combined results of IS6110 and pTBN12 fingerprinting and combined results of IS6110 fingerprinting and spoligotyping. Isolates having identical IS6110 fingerprints and pTBN12 patterns were defined as IS6110-pTBN12 clusters, and isolates having identical patterns and identical spoligotypes were considered to be in IS6110-spoligo clusters.
Pattern designation.
Each isolate was assigned an IS6110, a pTBN12, and a spoligotype pattern designation. Each different IS6110 pattern was designated with an arabic numeral, each new pTBN12 pattern was indicated with an uppercase letter, and a lowercase letter was used to designate each distinct spoligotype.
Epidemiological investigation.
Demographic information, details of tuberculosis infection and disease, contact information, and social history of the patients were obtained by review of medical records and interview of the patients using an intensive standardized questionnaire (4). Patients who lived in the same household or had a close work or social contact were considered to have definite epidemiological links. Patients who had neither a close contact (personal contact) nor a casual contact (attending the same church or the same public places of any other type) were classified as having no epidemiologic links.
RESULTS
Diversity of DNA fingerprints.
Based on IS6110 fingerprinting alone, a total of 34 IS6110 fingerprint patterns were observed among 88 low-copy-number isolates. Of the 34 patterns, 10 patterns are shared by more than 1 isolate, including a total of 64 (73%) of the isolates studied. Of the 88 isolates, 40 different spoligotypes and 68 different pTBN12 patterns were seen. Correlation among IS6110, pTBN12, and spoligotyping results among the 64 isolates clustered by IS6110 is shown in Table 1.
TABLE 1.
Comparison of IS6110 typing, pTBN12 typing, and spoligotypinga
| IS6110 copy no. | IS6110 type (no. of isolates) | Spoligotype (no. of isolates) | pTBN12 type (no. of isolates) | Epidemiology (no. linked/no. available) |
|---|---|---|---|---|
| 2 | 1 (28) | A (17) | a (8) | 2/7 |
| b (2) | 0/1 | |||
| c (1) | 0/1 | |||
| d (3) | 3/3 | |||
| e (1) | 0/1 | |||
| f (1) | NAb | |||
| g (1) | 0/1 | |||
| B (6) | h (1) | 0/1 | ||
| i (1) | 0/1 | |||
| j (1) | 0/1 | |||
| k (1) | 0/1 | |||
| l (2) | 0/2 | |||
| C (1) | m (1) | 0/1 | ||
| D (1) | n (1) | 0/1 | ||
| E (1) | o (1) | 0/1 | ||
| F (1) | p (1) | 0/1 | ||
| G (1) | q (1) | 0/1 | ||
| 3 | 2 (5) | H (5) | r (5) | 2/4 |
| 3 (2) | A (2) | s (1) | 0/1 | |
| t (1) | NA | |||
| 4 (2) | I (1) | u (1) | NA | |
| J (1) | v (1) | NA | ||
| 4 | 5 (10) | B (2) | w (1) | 0/1 |
| x (1) | NA | |||
| K (4) | y (2) | NA | ||
| z (2) | 0/1 | |||
| L (2) | aa (2) | 2/2 | ||
| M (1) | bb (1) | 0/1 | ||
| N (1) | w (1) | NA | ||
| 6 (7) | B (6) | cc (1) | 0/1 | |
| dd (1) | 1c/1 | |||
| ee (3) | 1c/2 | |||
| ff (1) | 0/1 | |||
| O (1) | gg (1) | 0/1 | ||
| 7 (3) | P (1) | hh (1) | 0/1 | |
| Q (2) | ii (2) | NA | ||
| 5 | 8 (2) | A (2) | jj (2) | 2/2 |
| 9 (2) | B (1) | kk (1) | NA | |
| R (1) | ll (1) | 0/1 | ||
| 10 (3) | M (3) | mm (2) | 0/2 | |
| nn (1) | 0/1 |
Data for the 64 isolates in IS6110 clusters (unique isolates are not shown).
NA, not available.
Intercluster epidemiological links.
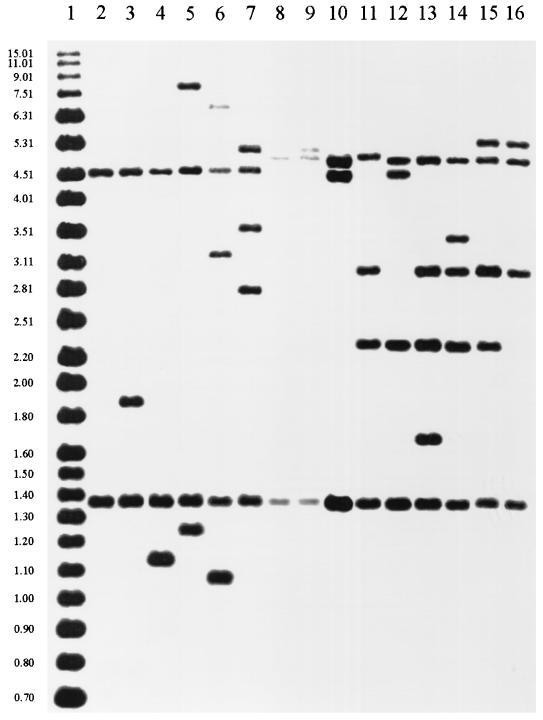
Among the 28 isolates that share the same IS6110 pattern consisting of two hybridizing fragments, seven different spoligotypes and 17 different pTBN12 patterns were observed. For the nine isolates having three different IS6110 patterns with three hybridizing fragments, four spoligotypes and five pTBN12 patterns were seen. Twenty isolates were grouped in three four-copy IS6110 clusters. These isolates showed six spoligotypes and 10 pTBN12 patterns. For the seven isolates in three five-copy clusters, three spoligotypes and five pTBN12 patterns were found. Twenty-four of the 88 isolates were found to have unique IS6110 patterns, and pTBN12 patterns of these isolates were also unique. Of the 24 isolates with unique IS6110 patterns, 22 were identified as unique by spoligotyping and two shared spoligotypes with isolates having different IS6110 patterns and pTBN12 patterns. Of the 64 IS6110 clustered isolates, 13 were identified as unique by both pTBN12 fingerprinting and spoligotyping. Identical spoligotypes were found among isolates having different IS6110 patterns, as well as among isolates showing different pTBN12 patterns. The two most prevalent spoligotypes were seen among 44 (50%) of the 88 isolates, among which 14 different IS6110 patterns consisting of two to five bands and 31 unique pTBN12 patterns were observed. IS6110-hybridizing fragments of common size were seen among isolates having different IS6110 copy numbers but identical spoligotypes (Fig. 1). Two IS6110-hybridizing fragments of common size shared by 25 isolates demonstrated spoligotype pattern A. Isolates with spoligotype pattern B shared two to four common size IS6110 hybridizing fragments. Computer analysis of the images grouped all of the isolates with two IS6110-hybridizing bands into a single cluster. When these isolates are electrophoresed on the same gel, two separate two-band clusters are resolved that differ in the size of one of the hybridizing fragments (Fig. 1 lanes 2 and 8).
FIG. 1.
IS6110 fingerprints of M. tuberculosis isolates that shared identical spoligotypes. Lane 1, molecular size standard DNA; lanes 2 to 7, isolates sharing spoligotype A; lanes 8 to 14, isolates sharing spoligotype B; lanes 15 and 16, isolates sharing spoligotype M.
Clustering analysis of isolates.
Primary clustering analysis was based on data from IS6110 fingerprinting. Subclustering of isolates was based on a combination of (i) IS6110 fingerprinting and spoligotyping, (ii) IS6110 fingerprinting and pTBN12, and (iii) all three methods. Differences in clustering rate were observed among the different combinations of typing methods (Table 2). A combination of IS6110 fingerprinting and spoligotyping did not decrease the clustering rate significantly (P > 0.1) compared to the rate observed with IS6110 alone. In contrast, a combination of IS6110 and pTBN12 typing significantly increased the discriminating power of genotyping, reducing the clustering rate determined by IS6110 fingerprinting by 42% (P < 0.01). Using all three typing methods provided no significant change in clustering rate compared with data obtained with a combination of IS6110 and pTBN12 fingerprinting. The size of the largest cluster identified by IS6110-pTBN12 combined typing is the same as that identified using a combination of all three methods, equal to approximately half of the size of the largest cluster determined by IS6110-spoligotyping and a third of the size of the largest cluster determined based on IS6110 typing alone.
TABLE 2.
Comparison of discriminating power among four different typing systems
| System(s) | No. of clustered isolates (%) | Size of clustera |
|---|---|---|
| IS6110 typing | 64 (73) | 2–28 |
| IS6110 typing-spoligotyping | 51 (58) | 2–17 |
| IS6110 typing-pTBN12 typing | 37 (42) | 2–8 |
| IS6110 typing-pTBN12 typing-spoligotyping | 35 (40) | 2–8 |
Number of isolates.
Correlation between epidemiological data and fingerprinting results.
Epidemiological data were obtained for 67 (76%) of the 88 patients included in this study. The correlation between epidemiological data and fingerprinting results was studied among the isolates for which epidemiological data were available. There were no known epidemiological links among patients whose isolates were unique by IS6110 fingerprinting-spoligotyping (31 isolates). Of the 43 patients who had unique isolates according to IS6110-pTBN12 typing, two were epidemiologically linked. Epidemiological links were found in 11 (46%) of 24 isolates clustered by IS6110-pTBN12 typing and in 13 of the 35 (37%) isolates that were clustered by IS6110 fingerprinting-spoligotyping (Table 1).
DISCUSSION
In a previous study, a combination of IS6110 fingerprinting and spoligotyping reduced the clustering rate among 249 M. tuberculosis isolates having low IS6110 copy number from 84% (determined solely based on IS6110 fingerprints) to 55% (2). In the present investigation, all low-copy-number isolates found in a population-based study in Arkansas during a 2-year period were examined. A systematic comparison among the three typing methods showed that pTBN12 typing provides the best discrimination for low-copy-number isolates. Combined typing with IS6110 and pTBN12 decreases significantly the clustering rate, while combining IS6110 and spoligotyping does not change the clustering of isolates significantly; combining all three of the methods only slightly decreases the clustering rate over that achieved with IS6110 and pTBN12. These data indicate pTBN12 fingerprinting is better than spoligotyping with regard to identifying different strains of M. tuberculosis.
Multiple tandem repeats of the PGRS are present in about 100 genes in the M. tuberculosis genome (11). This is reflected in the diversity of PGRS fingerprints among clinical isolates of M. tuberculosis with low IS6110 copy numbers. Further improvement of the pTBN12 typing method could be directed towards increasing the resolution of complex PGRS-containing restriction fragments by electrophoresis and in developing more-specific PGRS probes. Use of additional DR spacer sequences in the spoligotyping technique may increase the differentiating power of spoligotyping.
In this study, all isolates that had unique IS6110 fingerprints had unique pTBN12 patterns. Since spoligotyping is more rapid and easier to perform than is pTBN12 fingerprinting, screening of low-copy-number isolates by spoligotyping prior to pTBN12 typing would reduce the tedious work associated with pTBN12 secondary typing and analyzing pTBN12 fingerprints. Thirteen (20%) isolates that were in IS6110 clusters were identified as unique by both spoligotyping and pTBN12 fingerprinting. Thus, the number of isolates requiring pTBN12 subtyping could have been reduced by 20% if spoligotyping had been used as a screening technique prior to pTBN12 typing.
Among the 17 isolates with two copies of IS6110, pTBN12 subtyping subdivided the spoligotype cluster into three subclusters and four unique isolates. Epidemiologic links were found in two of the pTBN12 subclusters but in none of the pTBN12 unique patterns in that spoligotype cluster. Epidemiologic links were found in three other IS6110 clusters that were not subdivided by pTBN12, so in these cases, spoligotyping was as useful as pTBN12. Epidemiologic links were absent among the isolates that were unique by spoligotyping and pTBN12 fingerprinting except for a link between two isolates in one spoligotype cluster that was subdivided by pTBN12.
Even though the spoligotyping is less discriminatory than pTBN12 fingerprinting it allows one to differentiate unique isolates and leads to the fast detection and direct typing of M. tuberculosis in clinical specimens. One can envision that this would be a practical approach to genotyping in laboratories outside the research setting where multiple typing methods are not available. As described in a previous report (14a), spoligotyping could be used for initial screening, and then isolates with identical spoligotypes could be sent to a specialized genotyping laboratory for further differentiation with IS6110 and pTBN12 fingerprinting. In laboratories where all three typing methods are available, spoligotyping could be used as the first-line secondary typing method to reduce the number of isolates requiring pTBN12 fingerprinting. A combination of two or three methods would be necessary for the differentiation of low-copy-number isolates in a detailed epidemiologic study.
ACKNOWLEDGMENTS
This study was supported in part by the Centers for Disease Control and Prevention, National Tuberculosis Genotyping and Surveillance Network cooperative agreement.
We thank Bill Starrett and Michael Freeland for their excellent technical assistance in laboratory work during the study. We are also indebted to Don Cunningham and Stewart Matthews for their help in obtaining isolates and interviewing patients.
REFERENCES
- 1.Barnes P F, Yang Z, Preston-Martin S, Pogoda J M, Jones B E, Otaya M, Eisenach K D, Knowles L, Harvey S, Cave M D. Patterns of tuberculosis transmission in Central Los Angeles. JAMA. 1997;278:1159–1163. [PubMed] [Google Scholar]
- 2.Bauer J, Andersen A B, Kremer K, Miorner H. Usefulness of spoligotyping to discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. J Clin Microbiol. 1999;37:2602–2606. doi: 10.1128/jcm.37.8.2602-2606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer J, Yang Z, Poulsen S, Andersen A B. Results from 5 years of nationwide DNA fingerprinting of Mycobacterium tuberculosis complex isolates in a country with a low incidence of M. tuberculosis infection. J Clin Microbiol. 1998;36:305–308. doi: 10.1128/jcm.36.1.305-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braden C R, Templeton G L, Cave M D, Valway S, Onorato I M, Castro K G, Moers D, Yang Z H, Stead W W, Bates J H. Interpretation of restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates from a state with a large rural population. J Infect Dis. 1997;175:1446–1452. doi: 10.1086/516478. [DOI] [PubMed] [Google Scholar]
- 5.Braden C R, Templeton G L, Stead W W, Bates J H, Cave M D, Valway S E. Retrospective identification of laboratory cross-contamination of M. tuberculosis cultures with use of DNA fingerprint analysis. Clin Infect Dis. 1997;24:35–40. doi: 10.1093/clinids/24.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Burman W J, Stone B L, Reves R R, Wilson M L, Yang Z H, El-Hajj H, Bates J H, Cave M D. The incidence of false-positive cultures for Mycobacterium tuberculosis. Am J Respir Crit Care Med. 1997;155:321–326. doi: 10.1164/ajrccm.155.1.9001331. [DOI] [PubMed] [Google Scholar]
- 7.Burman W J, Reves R R, Hawkes A P, Rietmeijer C A, Yang Z H, El-Hajj H, Bates J H, Cave M D. DNA fingerprinting with two probes decreases clustering of Mycobacterium tuberculosis. Am J Respir Crit Care Med. 1997;155:1140–1146. doi: 10.1164/ajrccm.155.3.9117000. [DOI] [PubMed] [Google Scholar]
- 8.Cave M D, Eisenach K D, McDermott P F, Bates J H, Crawford J T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991;5:73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- 9.Cave M D, Eisenach K D, Templeton G, Salfinger M, Mazurek G, Bates J H, Crawford J T. Stability of DNA fingerprint patterns produced with IS6110 in strains of Mycobacterium tuberculosis. J Clin Microbiol. 1994;32:262–266. doi: 10.1128/jcm.32.1.262-266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaves F, Yang Z H, El Hajj H, Alonso M, Burman W J, Eisenach K D, Dronda F, Bates J H, Cave M D. Usefulness of the secondary probe pTBN12 in DNA fingerprinting of Mycobacterium tuberculosis. J Clin Microbiol. 1996;34:1118–1123. doi: 10.1128/jcm.34.5.1118-1123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krog A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Dooley S W, Villarino M E, Lawrence M, Salinas L, Amil S, Rullan J V, Jarvis W R, Block A B, Cauthen G M. Nosocomial transmission of tuberculosis in a hospital unit for HIV-infected patients. JAMA. 1992;267:2632–2635. [PubMed] [Google Scholar]
- 13.Edlin B R, Tokars J I, Grieco M H, Crawford J T, Williams J, Sordillo E M, Ong K R, Kilburn J O, Dooley S W, Castro K G, Jarvis W R, Holmberg S D. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 14.Frothingham C R, Meeker-O'Connell W A. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- 14a.Goguet de la Salmonière Y-O, Li H M, Torrea G, Bunschoten A, van Embden J, Gicquel B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J Clin Microbiol. 1997;35:2210–2214. doi: 10.1128/jcm.35.9.2210-2214.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groenen P M A, Bunschoten A E, van Soolingen D, van Embden J D A. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 16.Hermans P W M, van Soolingen D, Bik E M, de Hass P E W, Dale J W, van Embden J D A. The insertion element IS987 from M. bovis BCG is located in a hot spot integration region for insertion elements in M. tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamerbeek J, Schouls L, Kolk A, van Agtervveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazurek G H, Cave M D, Eisenach K D, Wallace R J, Jr, Bates J H, Crawford J T. Chromosomal DNA fingerprint patterns produced with IS6110 as strain-specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991;29:2030–2033. doi: 10.1128/jcm.29.9.2030-2033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray M G, Thompson W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otal I, Martin C, Vincent-Levy-Frebault V, Thierry D, Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991;29:1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson M L, Jereb J A, Freiden T R, Crawford J T, Davis B J, Dooley S W, Jarvis W R. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis. Ann Intern Med. 1992;117:191–196. doi: 10.7326/0003-4819-117-3-191. [DOI] [PubMed] [Google Scholar]
- 22.Ross B C, Raios K, Jackson K, Dwyer B. Molecular cloning of a highly repeated element from Mycobacterium tuberculosis and its use as an epidemiological tool. J Clin Microbiol. 1992;31:329–334. doi: 10.1128/jcm.30.4.942-946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Small P M, McClenny N B, Singh S P, Schoolnik G K, Tomkins L S, Mickelsen P A. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol. 1993;31:1677–1682. doi: 10.1128/jcm.31.7.1677-1682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Small P M, Hopewell P C, Singh S P, Paz A, Parsonnet J, Ruston D C, Schechter G F, Daley C L, Schoolnik G K. The epidemiology of tuberculosis in San Francisco, a population-based study using conventional and molecular methods. N Engl J Med. 1994;30:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 25.Small P M, Shafer R W, Hopewell P C, Singh S P, Murphy M J, Desmond E, Sierra M F, Schoolnik G K. Exogenous reinfection with multidrug-resistant M. tuberculosis in patients with advanced HIV infection. N Engl J Med. 1993;328:1137–1144. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
- 26.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P W M, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendation for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rie A, Warren R, Richardson M, Victor T C, Gie R P, Enarson D A, Beyers N, van Helden P D. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–1179. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 28.van Soolingen D, Borgdorff M W, de Haas P E W, Sebek M M G G, Veen J, Dessens M, Kremer K, van Embden J D A. Molecular epidemiology of tuberculosis in The Netherlands: a nation-wide study from 1993 through 1997. J Infect Dis. 1999;180:726–736. doi: 10.1086/314930. [DOI] [PubMed] [Google Scholar]
- 29.van Soolingen D, de Haas P E W, Hermans P W M, Groenen P M A, van Embden J D A. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993;29:2578–2586. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D A. The occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of IS-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z H, Chaves F, Barnes P F, Burman W, Koehler J, Eisenach K D, Bates J H, Cave M D. Evaluation of method for secondary DNA typing of Mycobacterium tuberculosis with pTBN12 in epidemiologic study of tuberculosis. J Clin Microbiol. 1996;34:3044–3048. doi: 10.1128/jcm.34.12.3044-3048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z H, de Haas P E W, Wachmann C H, van Soolingen D, van Embden J D A, Andersen A B. Molecular epidemiology of tuberculosis in Denmark in 1992. J Clin Microbiol. 1995;33:2077–2081. doi: 10.1128/jcm.33.8.2077-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]