Abstract
We present the case of a 25-year-old woman with desmoplakin cardiomyopathy–related myocarditis. Her high-sensitivity troponin and symptoms improved with pulse steroid therapy and mycophenolate mofetil. The literature lacks data to effectively guide the management of recurrent myocarditis in desmoplakin cardiomyopathy. (Level of Difficulty: Advanced.)
Key Words: desmoplakin cardiomyopathy, genetic cardiomyopathy, late gadolinium enhancement, myocarditis
Abbreviations and Acronyms: DSP, desmoplakin; LGE, late gadolinium enhancement; LV, left ventricular
Graphical abstract
History of Presentation
A 25-year-old Caucasian woman presented with palpitations and intermittent retrosternal chest tightness of 3 weeks duration. No presyncope, orthopnea, or paroxysmal nocturnal dyspnea was reported. Her high-sensitivity troponin on presentation was 65,616 ng/mL.
Learning Objectives
-
•
To recognize the importance of arrhythmogenic and genetic cardiomyopathy and consider testing for these conditions in the right clinical setting
-
•
To understand the role of immunosuppressant therapy in DSP-related myocarditis
Her initial vital signs were as follows: temperature 36.9 °C, heart rate 66 beats/min, respiratory rate 18 breaths/min, blood pressure 104/71 mmHg, and oxygen saturation 95%. The patient was a physically fit young woman. Her hair was thick, wavy, and mildly coarse. The result of skin examination was unremarkable. Her lungs were clear to auscultation bilaterally, with no crackles, wheezes, or rales. On electrocardiogram, S1 and S2 were normal, with regular rate and rhythm, and no murmurs, rubs or gallops. The patient was clinically euvolemic without peripheral edema or jugular venous distension. The results of a 12-point comprehensive physical examination were unremarkable on admission.
Medical History
The patient’s medical history was notable for a pathogenic heterozygous variant in the form of truncating mutation c.4518del (p.Arg1506Serfs∗20) for desmoplakin (DSP) gene and a missense mutation c.826C>A (p.Leu276Ile) for gene fukutin-related protein, resulting in recurrent episodes of myocarditis (3 episodes over 5 years before the current presentation). The patient also had a history of primary hypothyroidism.
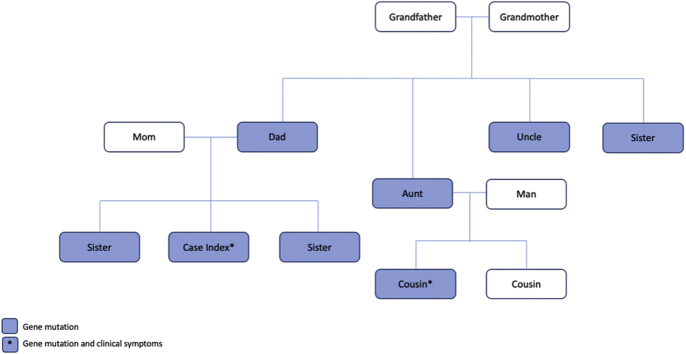
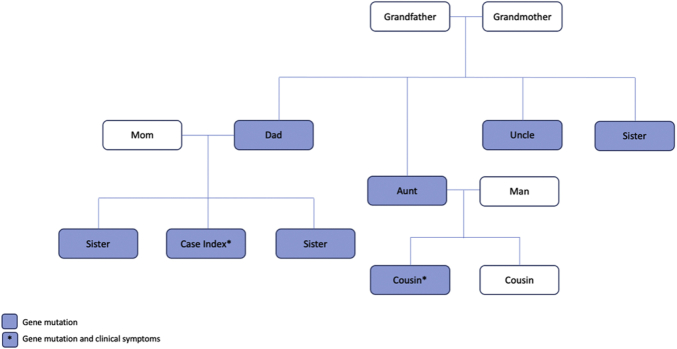
The family history was positive for DSP and fukutin-related protein gene mutations, which were present exclusively in the patient’s paternal lineage. After a positive pathogenic DSP mutation was identified in our patient, a genetic counselor recommended screening other family members. The patient’s father, sister, paternal uncle, both paternal aunts, and a paternal cousin all had the mutation genotype (as shown in Figure 1). Only the paternal cousin was symptomatic, with an episode of myocarditis and preserved ejection fraction of 52%.
Figure 1.
Family-Pedigree of Desmoplakin Mutation
Differential Diagnosis
Myocarditis, sarcoidosis, acute coronary syndrome, and pericarditis.
Investigations
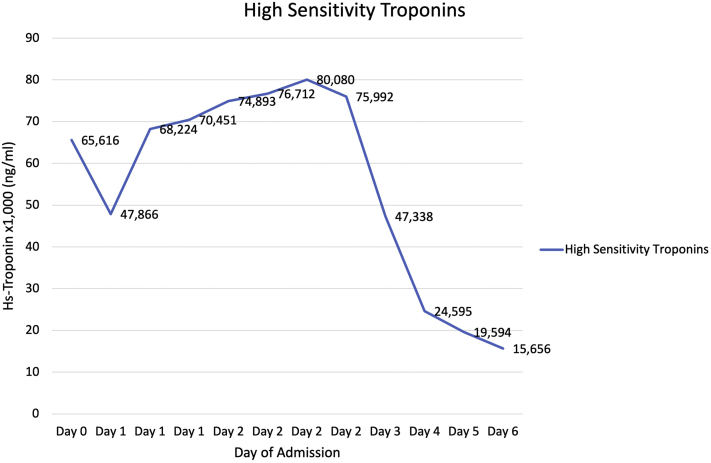
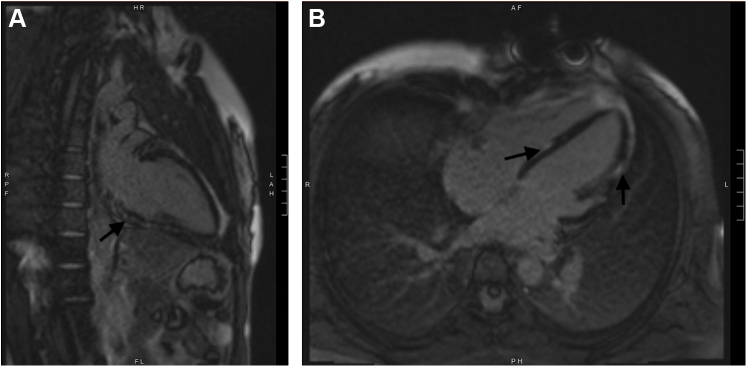
A transthoracic echocardiogram showed an ejection fraction of 55% to 60%, focal hypokinesis of the basal inferoseptal wall, and apical septal hypokinesis of the basal inferior wall. The high-sensitivity troponin curve throughout the patient’s hospitalization is shown in Figure 2. The erythrocyte sedimentation rate, C-reactive protein, brain natriuretic peptide, and creatine kinase were within normal limits. Prior cardiac MRI showed patchy epicardial to midwall late gadolinium enhancement (LGE) within the basal anteroseptal and inferoseptal walls, in addition to LGE throughout the lateral and inferior walls, with enhancement ranging from subepicardial to transmural. Cardiac MRI during current flare showed extensive patchy LGE in both lateral and inferior walls of the LV ranges from subepicardial to transmural as shown in Figure 3.
Figure 2.
High-Sensitivity Troponins Curve During Current Hospitalization
Figure 3.
Cardiac Magnetic Resonance Images During Current Myocarditis Flare
(A) 2 -chamber cardiac magnetic resonance image showing patchy late gadolinium enhancement involving the inferior left ventricular wall during current myocarditis episode. (B) 4-chamber cardiac MRI showing patchy late gadolinium enhancement involving the interventricular septum and lateral wall of the left ventricle during the current myocarditis episode.
Management
A multidisciplinary team conference involving general cardiology and advanced heart failure teams started a successful regimen that included an initial loading period for 3 days of 1,000 mg intravenous methylprednisolone daily with a taper to oral steroids over a 2- to 3-week period. Overlying this regimen was a maintenance dosage of mycophenolate mofetil 500 mg twice daily. After these interventions, the patient’s high-sensitivity troponin curve peaked and then trended down, as shown in Figure 2.
Discussion
Desmoplakin (DSP) is an intracellular protein essential in anchoring intermediate filaments to desmosome in cardiomyocytes (1). A pathogenic mutation in the DSP gene can result in recurrent left ventricular (LV) inflammation and myocardial injury followed by myocardial fibrosis and eventually LV systolic dysfunction. A DSP mutation has been reported to be associated with dilated cardiomyopathy, left-dominant arrhythmogenic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy (2,3). The most sensitive signs of DSP cardiomyopathy are LV systolic dysfunction, LV fibrosis on MRI, and frequent premature ventricular contractions on rhythm monitoring (2). The RV is usually unaffected. The most sensitive test for DSP cardiomyopathy is the cardiac MRI. Up to 90% of patients show LGE involving the LV subepicardium rather than the midmyocardium as is seen in nonischemic cardiomyopathy (2). The findings on transthoracic echocardiogram vary depending on the disease stage. Up to 61% of cases may show global or inferoposterior hypokinesia, and systolic function can be normal or moderately to severely reduced (4). The presence of curly hair and thick calloused skin on the hands and soles of the feet increases the likelihood of DSP cardiomyopathy (4). Molecular diagnosis is essential in guiding the treatment of these patients. The pancardiomyopathy genetic testing panel includes evaluation for DSP gene mutation. Variations in mutations are classified into truncating (a more aggressive phenotype) or missense (2).
Acute myocarditis can be the initial manifestation of DSP cardiomyopathy (5). The management of myocarditis secondary to DSP cardiomyopathy is not fully agreed upon. The most successful regimen to treat DSP cardiomyopathy–related myocarditis is still unknown. It remains unclear whether or not tailored immunosuppressive therapy or watchful waiting has equivalent results. Previous reports and studies propose conflicting data. For example, a previous study shows no improvement on PET scan after tailored immunosuppressive therapy (2,6). In our case, the patient responded clinically to high-dose steroids and mycophenolate mofetil. It is difficult to assess the efficacy of this approach in comparison with watchful waiting. Multicentric retrospective studies and randomized clinical trials are needed to shed some light on the management of this condition.
Follow-Up
The patient was discharged home and received outpatient follow-up care in the advanced heart failure clinic. She described significant improvement in her symptoms after her discharge. Her repeated troponin T fifth-generation level was 9 ng/L.
Conclusions
DSP cardiomyopathy is a rare arrhythmogenic entity associated with episodic myocarditis. Myocarditis can be the initial presentation of this disease. Managing these episodes may be challenging because of the lack of data in the literature and uncertainty of the effect of treatment on long-term outcomes. Arrhythmogenic cardiomyopathy should be considered in the differential of first/acute and recurrent episodes of myocarditis, DSP cardiomyopathy being one such example.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Favre B., Begré N., Marsili L., van Tintelen J.P., Borradori L. Desmoplakin gene variants and risk for arrhythmogenic cardiomyopathy. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.118.002241. [DOI] [PubMed] [Google Scholar]
- 2.Smith E.D., Lakdawala N.K., Papoutsidakis N., et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norgett E.E., Hatsell S.J., Carvajal-Huerta L., et al. Recessive mutation in desmoplakin disrupts desmoplakin–intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Gene. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 4.López-Ayala J.M., Gómez-Milanés I., Sánchez Muñoz J.J., et al. Desmoplakin truncations and arrhythmogenic left ventricular cardiomyopathy: characterizing a phenotype. Europace. 2014;16:1838–1846. doi: 10.1093/europace/euu128. [DOI] [PubMed] [Google Scholar]
- 5.Reichl K., Kreykes S.E., Martin C.M., Shenoy C. Desmoplakin variant-associated arrhythmogenic cardiomyopathy presenting as acute myocarditis. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.118.002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschöpe C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]