To the Editor:
Although vaccines against COVID-19 have led to dramatic decreases in COVID cases and mortality in the United States, most patients in the vaccine trials did not have chronic underlying diseases—patients at the highest risk of morbidity and mortality due to COVID-19.1 Host factors in these high-risk patients may result in reduced responsiveness to vaccines and “breakthrough” COVID cases. As precautionary masking and distancing measures are lifted, individuals who fail to mount an immune response to the vaccines may change their protective behavior without being aware of their potential vulnerability. As of October 12, 2021, there have been at least 31,895 individuals with SARS-CoV-2 breakthrough infections who were hospitalized or died in the United States.2 , 3 A recent report showed that 39 fully vaccinated health care workers had breakthrough infections, and neutralizing antibody titers were lower during the peri-infection period than those in matched uninfected control subjects.4 This aligns with data indicating a strong correlation between antibody titers and vaccine efficacy.5 Although a recent study found that 46% of transplant patients had no antibody response after two doses of messenger RNA (mRNA) vaccines,6 no studies have investigated the effects of underlying chronic medical conditions on antibody response. This study used real-world data to evaluate risk factors of impaired antibody response to SARS-CoV-2 mRNA vaccines in individuals with chronic medical conditions evaluated in a respiratory specialty clinic.
Methods
We used National Jewish Health electronic medical record database (Allscripts and dataSCOUT) to identify patients who received two doses of SARS-CoV-2 mRNA vaccines between December 16, 2020 and July 24, 2021, and had a spike in IgG antibody results at least 14 days after the second dose. These tests were ordered by individual physicians to evaluate vaccine immunity based on patient request or significant chronic disease. Two different enzyme-linked immunosorbent assay (ELISA) tests (EUROIMMUN, New Jersey) detecting IgG to spike protein recombinant S1 domain, a surrogate for neutralizing antibodies to COVID vaccine, were used in our clinical laboratory: (1) Anti-SARS-CoV-2 ELISA (qualitative) with the ratio of sample optical density to calibration optical density provided with the kit was interpreted as positive (≥ 0.8) or negative (< 0.8) before July 2021, and (2) Anti-SARS-CoV-2 QuantiVac ELISA (semiquantitative) with the relative unit/mL (RU/mL) provided with the kit was interpreted as positive (≥ 0.8) or negative (< 0.8) after July 1, 2021. A negative IgG spike protein from ELISA has been correlated with a lack of neutralizing antibody; this was validated in a recent comparative study7 and is widely used.6 Medical conditions were based on physician diagnosis; medications were defined as in a previous study.8 A multivariate logistic regression model was used to identify clinical characteristics associated with a negative spike IgG protein adjusted for all variables listed in Figure 1 . To minimize confounding by indication, given that patients prescribed medications are more likely to have underlying comorbidities associated with impaired antibody response, overlap propensity score weighting9 was used. The propensity score was interpreted as the likelihood of receiving the medication of interest. The propensity score was estimated from a multivariate logistic regression model using age, sex, comorbidities, and other medication classes mentioned (except for the medication of interest). Each patient’s weight was the likelihood of being assigned to the opposite medication group. The propensity score weighting method was then applied to test the association between the medication of interest and impaired antibody response. We did a post hoc power analysis for interstitial lung disease (ILD) as a risk factor for lack of antibody response using G∗Power 3.1.10 The outcome is a positive antibody response, and the testing variable is ILD. The parameter (calculated) for the post hoc power calculation are: (1) OR of ILD = 0.37; (2) Probability of positive antibody response when someone is a non-ILD = 194/220 = 0.88; (3) X-distribution = binominal for ILD (yes/no); (4) X param ∏ = the proportion of patients with a positive antibody response who have ILD = 89/283 = 0.31; (5) α = 0.05; (6) sample size = 360. We assumed the R 2 other X = R 2 between the main categorical predictor (ILD) and all other covariates. We calculated the power based on low (R 2 = 0.01) and moderate (R 2 = 0.25) associations with the above-mentioned parameters; the power was 0.95 and 0.90, respectively.
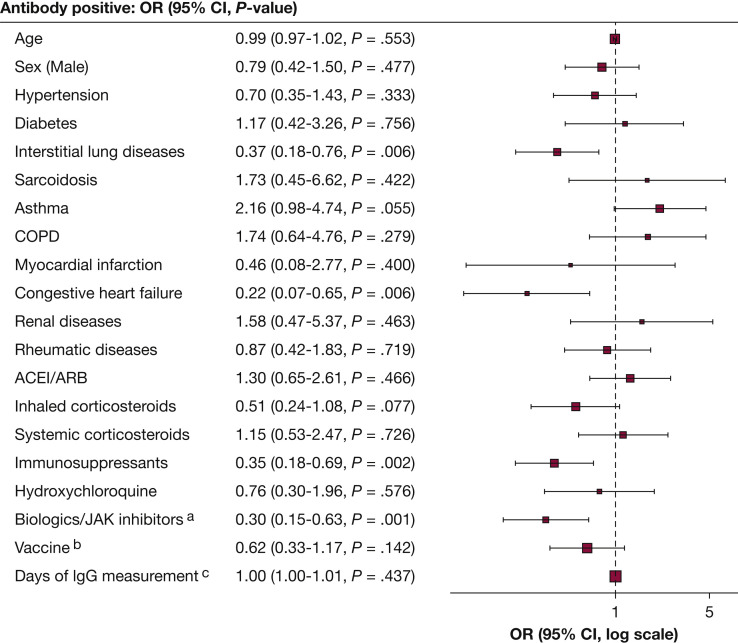
Figure 1.
Multivariate logistic regression of the association between antibody response and clinical characteristics. ACE/ARB = angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers. aBiologics: Anti- IL-5, -IL-6, -IL-12/23, -IL-17, -IgE, -CD20, and -TNF-α inhibitors. bBNT162b2 (Pfizer-BioNTech) compared with mRNA-1273 (Moderna). cDays after second vaccine dose.
Results
We identified 360 patients (mean age, 62 years; 63% female) who received two doses of mRNA vaccines and had antibody testing (Table 1 ). BNT162b2 (Pfizer-BioNTech) was administered to 64% of the patients, and 36% received mRNA-1273 (Moderna). At a mean (SD) of 96 (58) days, range 14-237 days, after dose 2, 21% had no antibody detected. Negative antibody responses were found in 24% of patients receiving the BNT162b2 (Pfizer-BioNTech) vaccination and 16% of patients receiving the mRNA-1273 (Moderna) vaccination. Eight patients had documented COVID-19 infection before the vaccination. The percentage of patients without antibodies detected ranged from 14% in asthma to 48% in congestive heart failure (CHF) (Table 1). Multivariate logistic regression (Fig 1) showed that ILD (OR 0.37; 95% CI, 0.18-0.76), CHF (OR 0.22; 95% CI, 0.07-0.65), use of biologics (anti-IL-5, -IL-6, -IL-12/23, -IL-17, -IgE, -CD20, and -tumor necrosis factor alpha [TNF-α] inhibitors)/Janus kinase (JAK) inhibitors (OR 0.30; 95% CI, 0.15-0.63), and use of other immunosuppressants (except corticosteroids) (OR 0.35; 95% CI, 0.18-0.69) are significant risk factors for negative antibody response adjusted for age, sex, vaccine types, days after vaccination, other medication use, and comorbidities. Using overlap propensity score weighting, rituximab (anti-CD20) use is a significant risk for negative antibody response (OR 0.59; 95% CI, 0.50-0.70), but other biologics/JAK inhibitors use (excluding rituximab) are not associated with negative antibody response. No significant effects on antibody response were observed with other medications, including systemic/inhaled corticosteroids. Immunosuppressants, including methotrexate, mycophenolate mofetil, azathioprine, leflunomide, cyclosporine, tacrolimus, mycophenolic acid, sirolimus, and everolimus showed a trend toward significance (OR, 0.88; 95% CI, 0.77-1.00). Given the finding that ILD is an independent risk factor for impaired antibody response, we further investigated the subpopulation with ILD. Among the 140 patients with ILD (mean age, 66 years; 62% female), 51 (36%) had negative antibodies. Clinical features such as age, sex, lung function, comorbidity, and medication use were similar in patients with ILD with and without antibody responses.
Table 1.
Characteristics of the Study Population (N = 360) and Antibody Responsea
| Characteristics | Negative (N = 77) | Positive (N = 283) |
|---|---|---|
| Age, years; mean (SD) | 64 (13) | 62 (15) |
| Sex | ||
| Female | 46 (20%)b | 181 (80%) |
| Male | 31 (23%) | 102 (77%) |
| Hypertension | 26 (23%) | 85 (77%) |
| Diabetes | 7 (22%) | 25 (78%) |
| Asthma | 18 (14%) | 113 (86%) |
| COPD | 7 (15%) | 40 (85%) |
| Interstitial lung diseases | 51 (36%) | 89 (63%) |
| Sarcoidosis | 5 (19%) | 21 (81%) |
| Congestive heart failure | 10 (48%) | 11 (52%) |
| Renal diseases | 6 (27%) | 16 (73%) |
| Rheumatic diseases | 31 (35%) | 57 (65%) |
| ACEI/ARB | 27 (23%) | 92 (77%) |
| Inhaled corticosteroids | 26 (18%) | 115 (82%) |
| Systemic corticosteroids (exclude tapering dose) | 18 (27%) | 49 (73%) |
| Immunosuppressants | 49 (36%) | 89 (64%) |
| Hydroxychloroquine | 11 (31%) | 24 (69%) |
| Biologicsc /JAK inhibitors | 27 (39%) | 42 (61%) |
| Vaccine type | ||
| mRNA-1273 (Moderna); | 21 (16%) | 110 (84%) |
| BNT162b2 (Pfizer-BioNTech) | 56 (24%) | 173 (76%) |
| Days after 2nd vaccine dose, mean (SD) | 93 (46) | 97 (61) |
ACEI/ARB = angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers; JAK = Janus kinase.
A negative IgG spike protein from enzyme-linked immunosorbent assay has been correlated with a lack of neutralizing antibody; this was validated in a recent comparative study7 and is widely used.6
No. and (%) of patients by row.
Biologics: Anti- IL-5, -IL-6, -IL-12/23, -IL-17, -IgE, -CD20, and -TNF-α inhibitors
Discussion
Recognizing that vaccination may not necessarily confer immunity, at least as assessed by commercially available spike protein antibody tests, in patients with certain chronic medical conditions and certain medication use, is critical. In these patients (81% with chronic pulmonary diseases), 21% had no antibody detected 14 or more days after the second dose of mRNA vaccines. Although these data show that many vaccinated individuals may be at risk for impaired antibody production, we found that ILD (independent of medication use), CHF, and rituximab use are independent risk factors for impaired antibody response. Our study is the first one to identify ILD as an independent risk factor. More studies are needed to identify other potential factors. One possibility is that fibrosis, a common complication seen in ILD, is regulated by interactions between T cells and cytokines released by relevant immunocompetent cells; ILD patients may have dysregulation or imbalance of this immunoprocess.11 Reduced influenza vaccine antibody titers have been observed in CHF,12 which may be due to sympathetic activation in heart failure leading to inhibition of antibody production. For the medications, the effect of rituximab use is consistent with a previous study.13 In our study, systemic corticosteroid use was not associated with impaired antibody response after controlling for concomitant immunosuppressant use and comorbidities. This finding is different from other recent studies, which showed reduced antibody responses in corticosteroid users; however, the failure to fully control for contributions of potential confounders such as disease-specific factors or concomitant immunosuppressant use was an acknowledged limitation of these studies.14 , 15 Although the exact antibody level conferring protection against SARS-CoV-2 is unknown, and other B cell and T cell-mediated factors are involved in protection,16 a recent study showed that among fully vaccinated health care workers, the occurrence of breakthrough COVID-19 infection was correlated with the titer of antibodies.4 Limitations of our study include the potential for bias because only those patients specifically chosen by physicians had antibody levels drawn, and only one part of vaccine immune response (ie, antibodies) was analyzed, as opposed to other components such as T cell immunity. We have observed four breakthrough COVID-19 infections in our study cohort, with a higher rate of 2/77 (2.6%) in negative vs 2/283 (0.7%) in the positive antibody responses group. However, drawing any meaningful statistical inference is difficult because of low breakthrough rate and relatively small sample size. Our study raises concerns that SARS-CoV-2 vaccination may not result in protective immunity in patients with certain chronic medical conditions. Further studies of immunologic response (including neutralizing antibodies and other measures of B cell and T cell response) and protection in vulnerable populations are needed to define ongoing COVID-19 risk and inform recommendations regarding additional vaccination (ie, boosters) and behaviors to mitigate risk. Such studies are particularly important with the predicted seasonal and variant-driven spikes in SARS-CoV-2 infection rates. We are analyzing B and T cell responses to SARS-CoV-2 in the patients and hope this report will stimulate others to further investigate immune responses in patients with chronic medical conditions.
Acknowledgments
Author contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Concept and design: All authors. Acquisition and interpretation of data: All authors. Drafting of the manuscript: S. Y. L. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: S. Y. L. Supervision: A. N. G., B. M., M. E. W.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The study was approved by the National Jewish Health Institutional Review Board. The authors would like to thank Joy Zimmer for her help with the electronic health record data query.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: None declared.
FUNDING/SUPPORT: This work was supported by funding from the National Jewish Health Department of Medicine and Division of Environmental and Occupational Health Sciences, and Jin Hua Foundation.
References
- 1.Gerayeli F.V., Milne S., Cheung C., et al. COPD and the risk of poor outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;33:100789. doi: 10.1016/j.eclinm.2021.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Hospitalized or fatal COVID-19 vaccine breakthrough cases reported to CDC as of October 12, 2021. https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html Accessed October 30, 2021.
- 3.Strickler L. NBC News; 2021. Breakthrough Covid cases: data shows how many vaccinated Americans have tested positive. [Google Scholar]
- 4.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 6.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel E.U., Bloch E.M., Clarke W., et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59(2) doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao S.Y., Petrache I., Fingerlin T.E., Maier L.A. Association of inhaled and systemic corticosteroid use with Coronavirus Disease 2019 (COVID-19) test positivity in patients with chronic pulmonary diseases. Respir Med. 2021;176:106275. doi: 10.1016/j.rmed.2020.106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas L.E., Li F., Pencina M.J. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi: 10.1001/jama.2020.7819. [DOI] [PubMed] [Google Scholar]
- 10.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 11.Semenzato G., Adami F., Maschio N., Agostini C. Immune mechanisms in interstitial lung diseases. Allergy. 2000;55(12):1103–1120. doi: 10.1034/j.1398-9995.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht C.M., Sweitzer N.K., Johnson M.R., Vardeny O. Lack of persistence of influenza vaccine antibody titers in patients with heart failure. J Card Fail. 2014;20(2):105–109. doi: 10.1016/j.cardfail.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021;80(10):1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 14.Deepak P., Kim W., Paley M.A., et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174(11):1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruddy J.A., Connolly C.M., Boyarsky B.J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(10):1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cromer D., Juno J.A., Khoury D., et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]