Abstract
We present a patient with acute heart failure and new onset atrial fibrillation secondary to giant cell myocarditis with lone atrial involvement. The diagnosis was managed with cardiac magnetic resonance and confirmed by interventionally guided biopsy. In the future, diagnosis could be managed noninvasively for this rare entity as the gold standard. (Level of Difficulty: Advanced.)
Key Words: atrial giant cell myocarditis, cardiac magnetic resonance, heart failure, magnetic resonance imaging
Abbreviations and Acronyms: AF, atrial fibrillation; ANCA, antineutrophil cytoplasmic antibody; CMR, cardiac magnetic resonance; GCM, giant cell myocarditis; HF, heart failure; Ig, immunoglobulin; LA, left atrium; RA, right atrium; TEE, transesophageal echocardiography
Graphical abstract
History of Presentation
A 79-year-old woman presented to the emergency department with exertional dyspnea and palpitations. Additionally, she reported a weight loss of 4 kg (weight, 50 kg; body mass index, 17.5 kg/m2), night sweats, and low appetite for 4 weeks. She was afebrile, with a blood pressure of 120/70 mm Hg and a heart rate of 126 beats/min. Electrocardiography showed newly diagnosed persistent atrial fibrillation (AF) (CHA2DS2-VASc-score of 3).
Learning Objectives
-
•
To diagnose precisely in future this rare entity of atrial GCM and to rule out ventricular inflammation or storage diseases noninvasively by imaging evaluation with CMR as the gold standard.
-
•
To pay special attention to atrial and septal wall thickening as signs of atrial inflammation for clinicians conducting the imaging.
Past Medical History
There was no cardiac or other relevant medical history, no recent infections or COVID-19. The patient had undergone regular cancer screening in the past.
Differential Diagnosis
Possible differential diagnoses included acute heart decompensation resulting from new onset AF or myocarditis or a malignant disease.
Investigations
Natriuretic peptide levels were elevated (N-terminal pro–B-type natriuretic peptide, 3,063 pg/mL). There were no laboratory signs of infection (no leukocytosis and no value elevation of C-reactive protein, procalcitonin, interleukin 6, or blood sedimentation rate) or acute kidney injury. Results of an extensive infective work-up, including immunoglobulin (Ig) levels (IgM and IgG) for myocarditis-relevant viral agents (ie, coxsackievirus, parvovirus B19, human herpesvirus-6, human cytomegalovirus, adenovirus) and the rheumatologic panel (including cytoplasmic antineutrophil cytoplasmic antibody [c-ANCA], perinuclear ANCA [p-ANCA], antinuclear antibody, rheumatic factor) were negative. Thoracic and abdominal computed tomography, as well as gastroscopy and colonoscopy, ruled out concomitant malignant disease.
Transthoracic echocardiography (Figures 1A to 1C, Videos 1 and 2) revealed a 7-mm pericardial effusion and dilatation of both the left atrium (LA) (surface area, 22 cm2) and the right atrium (RA) (surface area, 18 cm2), with thickening of the free atrial wall (RA, 6 mm; LA, 6 mm). Transesophageal echocardiography (TEE) confirmed the markedly thickened atrial wall, including thickening of the atrial septum (Figure 1F, Videos 3 and 4). Additionally, no valve vegetations were detected. Cardiac magnetic resonance (CMR) showed distinct inflammation isolated to the atrium without ventricular involvement (Figure 2, Videos 5, 6, and 7). The pathognomonic sign was biatrial wall thickening, with isolated bright T2 edema and enhanced late gadolinium enhancement in both atria. Diffuse myocardial or pericardial inflammation was ruled out by noninvasive tissue characterization. Additionally, CMR showed normal ventricular function.
Figure 1.
Echocardiographic Images and Guided Biopsy
(A) Transthoracic echocardiography showing biatrial dilatation, wall thickening, and pericardial effusion. Transesophageal short-axis images depict (B) left atrial wall thickening and (C) the left atrial appendage (LAA). Biopsies of the atrial septum and left ventricle (LV) were guided by (DandE) fluoroscopy and (F) transesophageal echocardiography. LA = left atrium; LAO = left anterior oblique; RA = right atrium; RAO = right anterior oblique.
Figure 2.
“Virtual Histology”: Noninvasive Tissue Characterization With Cardiac Magnetic Resonance
(A to I) Ventricle. (AandB) 3-chamber and short-axis views of T2-weighted black blood sequence with no ventricular or pericardial focal edema. (CandD) Color-coded magnetic resonance strain (tagging, fast strain encoded imaging by MyoHealth) with normal longitudinal and circumferential contractility. (AandF) T2- and T1-weighted color-coded quantitative map with no signs of diffuse edema or storage disease. (GandH) 4-chamber and short-axis views without ventricular, or myocardial, or pericardial late gadolinium enhancement (LGE). (I) 3-chamber-view T2-weighted black blood edema image with a bright left atrium and a normal left ventricle (LV). (J to L) Atrium. (J) Short-axis-view with thickening of the left atrium and interatrial septum. (K) Short-axis late gadolinium enhanced left atrium and interatrial septum. (L) 4-chamber view T2-weighted black blood sequence with a bright left atrium, interatrial septum, and right atrium.
Management
Heart failure (HF) medication with an oral angiotensin receptor antagonist (candesartan, 8 mg/d) and a beta-blocker (metoprolol, 95 mg/d), as well as oral anticoagulation with a direct oral anticoagulant agent (apixaban, 10 mg/d) was initiated. AF heart rate control was achieved by digitoxin (0.07 mg/d).
The case was discussed within the institutional heart team, and we proposed performing an interventional endomyocardial biopsy of the atrial septum and ventricle. For this purpose, the patient was sedated, and vascular access was obtained through the right femoral vein and artery (Cook 16-F, 45-cm Check Flo Performer). Guided by TEE and fluoroscopy, multiple biopsy specimens were acquired throughout the atrial and ventricular septum with the biopsy catheter system (Maslanka flexible biopsy forceps) (Figures 1D and 1E). Histopathologic examination of the atrial biopsy specimens revealed necrotic areas, lymphocytic infiltration, and multinuclear giant cells leading to the final diagnosis of giant cell myocarditis (GCM) (Figures 3A to 3F). Ventricular biopsy results were negative, proving lone involvement of the atrium.
Figure 3.
Histology of Atrial Giant Cell Myocarditis
Atrial biopsy revealed necrotic areas, with lymphocytic infiltrations (CD8− and CD4+ cells) and multinuclear giant cells, with positive expression of CD68. (A) Hematoxylin and eosin stain of myocardial biopsy. (B) Period acid–Schiff stain. (C and D) Immunohistochemical staining with CD68 antibody, highlighting macrophages and multinuclear giant cells. (E) CD4+ infiltrating lymphocytes. (F) CD8+ infiltrating lymphocytes.
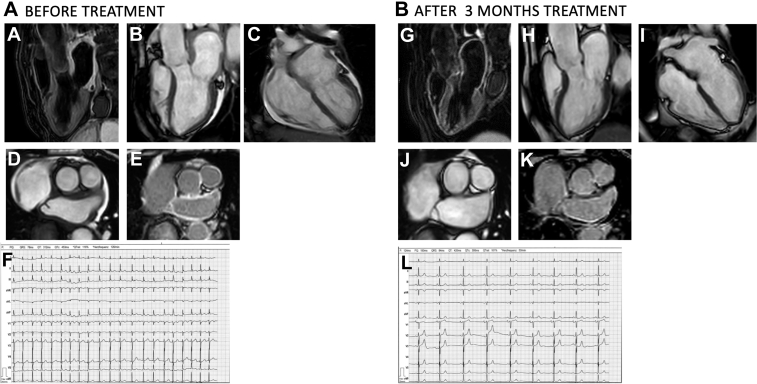
Symptomatic HF and the ongoing atrial inflammation, as assessed by CMR and confirmed by biopsy, were the key factors for specific treatment initiation. The patient was treated with prednisolone, starting with 50 mg/d and reduced to 10 mg/d over the course of 3 months. HF symptoms resolved, and spontaneous conversion to sinus rhythm was observed. Follow-up CMR at 3 months revealed normal thickness of both left and right atrial walls, with no signs of inflammatory T2 edema, compared with pretreatment findings (Figures 4A to 4L, Videos 8, 9, and 10).
Figure 4.
Images and Electrocardiography Before and After Treatment
Cardiac magnetic resonance (A to E) before and (G to K) after treatment. (AandG) 3-chamber view T2-weighted image (A) with and (G) without a bright left atrium. (B and H) 3-chamber view cine (B) with and (H) without thickening of the left atrial walls. (AandI) 4-chamber view cine with (C) and (I) without thickening of the atrial walls. (DandJ) Short-axis view of the left atrium (D) with and (J) without thickening of the left atrial walls. (EandK) Short-axis view late gadolinium enhancement (E) with and (K) without enhanced left atrial walls. Electrocardiography showing (F) atrial fibrillation before treatment and (L) sinus rhythm at 3-month follow-up.
Discussion
Novelty
GCM is known as a rapidly progressive and frequently fatal myocardial disease in young and middle-aged adults.1 However, atrial GCM represents a rare, distinct clinicopathologic entity with a more favorable prognosis than the classic ventricular GCM.2 Only a few cases have been described so far, and the definitive histopathologic diagnosis was achieved mostly after surgical procedures.2,3 Here we present a patient with atrial GCM and an accurate diagnostic pathway, managed primarily with CMR and confirmed by interventional biopsy, followed by selective treatment with prednisolone that led to a full recovery.
Atrial fibrillation differential diagnosis
Atrial cardiomyopathy may be a cause of recurrent AF. Atrial GCM was first described in 1964 in a case report.4 The pathophysiology of GCM is not well understood beyond its recognition as an autoimmune disorder attributable to T-lymphocyte–mediated myocardial inflammation.1 In published reports, some atrial GCM cases have been associated with autoimmune disorders4,5; however, another series of cases indicated that atrial GCM was a distinct entity.2
A new specific diagnostic pathway
When GCM involves only the atria, clinical and imaging findings are different from those of ventricular GCM. AF seems to be a nearly universal finding in all cases of atrial GCM.2,6 Atrial GCM should be included in the differential diagnosis of AF and dilatation, and CMR should be performed, particularly in association with atrial wall thickening.2 Ventricular function was preserved in our patient and in all other cases described.2 Diagnosis in our patient was achieved by CMR, which showed atrial wall thickening, dilatation, and inflammation limited to the atria and sparing the ventricles. Findings were in line with a previous described CMR case of atrial GCM,2 and are in our opinion they were pathognomonic for this rare distinct entity. Although our belief is that in future the diagnosis of atrial GCM can be managed noninvasively by clinical and imaging evaluation, with CMR tissue characterization as the gold standard for this rare entity, following our diagnostic pathway, endomyocardial biopsy may be considered in patients without early clinical improvement or resolution after treatment initiation.
Follow-Up
Treatment with prednisolone monotherapy in our patient led to full recovery and conversion to sinus rhythm at 3-month follow-up. However, the role of immunosuppressive treatment remains unknown for this rare entity, and additional studies will therefore be required before conclusions can be made regarding the long-term efficacy of this approach.
Conclusions
GCM affecting only the atria differs from the ventricular GCM entity. We show a case of atrial GCM leading to AF and HF in a patient whose accurate diagnosis was managed primarily noninvasively by CMR, followed by atrial septal and ventricular biopsies and selected treatment with prednisolone monotherapy, leading to full recovery.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic Echocardiography, 4-Chamber View
Transthoracic Echocardiography, Parasternal Long-Axis View
Transesophageal Echocardiography, Short-Axis View Showing the Aortic Valve
Transesophageal Echocardiography, Short-Axis View Showing the Left Atrial Appendage
Cardiac Magnetic Resonance, Baseline Short-Axis View
Cardiac Magnetic Resonance, Baseline 4-Chamber View
Cardiac Magnetic Resonance, Baseline 3-Chamber View
Cardiac Magnetic Resonance, Follow-up Short-Axis View
Cardiac Magnetic Resonance, Follow-up Short 4-Chamber View
Cardiac Magnetic Resonance, Follow-up 3Chamber View
References
- 1.Kandolin R., Lehtonen J., Salmenkivi K., Raisanen-Sokolowski A., Lommi J., Kupari M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 2.Larsen B.T., Maleszewski J.J., Edwards W.D., et al. Atrial giant cell myocarditis: a distinctive clinicopathologic entity. Circulation. 2013;127:39–47. doi: 10.1161/CIRCULATIONAHA.112.128900. [DOI] [PubMed] [Google Scholar]
- 3.Tanyeli O., Dereli Y., Gormus N., Avunduk M.C. Left atrial giant cell myocarditis presenting as a tumor: First-in-man case report. Braz J Cardiovasc Surg. 2018;33:306–308. doi: 10.21470/1678-9741-2017-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrea P.C., Childers R.W. Two unusual cases of giant cell myocarditis associated with mitral stenosis and with Wegener's syndrome. Br Heart J. 1964;26:490–498. doi: 10.1136/hrt.26.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillie I., Fox H. Mitral stenosis together with a giant cell myocarditis limited to the left atrium. J Clin Pathol. 1968;21:750–752. doi: 10.1136/jcp.21.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumagai K., Shirakura T., Minami K., Oshima S. Atrial giant cell myocarditis after atrial fibrillation ablation. Eur Heart J Case Rep. 2018;2:yty065. doi: 10.1093/ehjcr/yty065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic Echocardiography, 4-Chamber View
Transthoracic Echocardiography, Parasternal Long-Axis View
Transesophageal Echocardiography, Short-Axis View Showing the Aortic Valve
Transesophageal Echocardiography, Short-Axis View Showing the Left Atrial Appendage
Cardiac Magnetic Resonance, Baseline Short-Axis View
Cardiac Magnetic Resonance, Baseline 4-Chamber View
Cardiac Magnetic Resonance, Baseline 3-Chamber View
Cardiac Magnetic Resonance, Follow-up Short-Axis View
Cardiac Magnetic Resonance, Follow-up Short 4-Chamber View
Cardiac Magnetic Resonance, Follow-up 3Chamber View