Abstract
Background
Adjuvant aromatase inhibitors (AI) improve survival compared to tamoxifen in postmenopausal women with hormone receptor‐positive stage I to III breast cancer. In approximately half of these women, AI are associated with aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), often described as symmetrical pain and soreness in the joints, musculoskeletal pain and joint stiffness. AIMSS may have significant and prolonged impact on women's quality of life. AIMSS reduces adherence to AI therapy in up to a half of women, potentially compromising breast cancer outcomes. Differing systemic therapies have been investigated for the prevention and treatment of AIMSS, but the effectiveness of these therapies remains unclear.
Objectives
To assess the effects of systemic therapies on the prevention or management of AIMSS in women with stage I to III hormone receptor‐positive breast cancer.
Search methods
We searched CENTRAL, MEDLINE, Embase, WHO International Clinical Trials Registry Platform (ICTRP) and Clinicaltrials.gov registries to September 2020 and the Cochrane Breast Cancer Group (CBCG) Specialised Register to March 2021.
Selection criteria
We included all randomised controlled trials that compared systemic therapies to a comparator arm. Systemic therapy interventions included all pharmacological therapies, dietary supplements, and complementary and alternative medicines (CAM). All comparator arms were allowed including placebo or standard of care (or both) with analgesia alone. Published and non‐peer‐reviewed studies were eligible.
Data collection and analysis
Two review authors independently screened studies, extracted data, and assessed risk of bias and certainty of the evidence using the GRADE approach. Outcomes assessed were pain, stiffness, grip strength, safety data, discontinuation of AI, health‐related quality of life (HRQoL), breast cancer‐specific quality of life (BCS‐QoL), incidence of AIMSS, breast cancer‐specific survival (BCSS) and overall survival (OS). For continuous outcomes, we used vote‐counting by reporting how many studies reported a clinically significant benefit within the confidence intervals (CI) of the mean difference (MD) between treatment arms, as determined by the minimal clinically importance difference (MCID) for that outcome scale. For dichotomous outcomes, we reported outcomes as a risk ratio (RR) with 95% CI.
Main results
We included 17 studies with 2034 randomised participants. Four studies assessed systemic therapies for the prevention of AIMSS and 13 studies investigated treatment of AIMSS. Due to the variation in systemic therapy studies, including pharmacological, and CAM, or unavailable data, meta‐analysis was limited, and only two trials were combined for meta‐analysis. The certainty of evidence for all outcomes was either low or very low certainty.
Prevention studies
The evidence is very uncertain about the effect of systemic therapies on pain (from baseline to the end of the intervention; 2 studies, 183 women). The two studies, investigating vitamin D and omega‐3 fatty acids, showed a treatment effect with 95% CIs that did not include an MCID for pain. Systemic therapies may have little to no effect on grip strength (RR 1.08, 95% CI 0.37 to 3.17; 1 study, 137 women) or on women continuing to take their AI (RR 0.16, 95% 0.01 to 2.99; 1 study, 147 women). The evidence suggests little to no effect on HRQoL and BCS‐QoL from baseline to the end of intervention (the same single study; 44 women, both quality of life outcomes showed a treatment effect with 95% CIs that did include an MCID).
The evidence is very uncertain for outcomes assessing incidence of AIMSS (RR 0.82, 95% CI 0.63 to 1.06; 2 studies, 240 women) and the safety of systemic therapies (4 studies, 344 women; very low‐certainty evidence). One study had a US Food and Drug Administration alert issued for the intervention (cyclo‐oxygenase‐2 inhibitor) during the study, but there were no serious adverse events in this or any study.
There were no data on stiffness, BCSS or OS.
Treatment studies
The evidence is very uncertain about the effect of systemic therapies on pain from baseline to the end of intervention in the treatment of AIMSS (10 studies, 1099 women). Four studies showed an MCID in pain scores which fell within the 95% CI of the measured effect (vitamin D, bionic tiger bone, Yi Shen Jian Gu granules, calcitonin). Six studies showed a treatment effect with 95% CI that did not include an MCID (vitamin D, testosterone, omega‐3 fatty acids, duloxetine, emu oil, cat's claw).
The evidence was very uncertain for the outcomes of change in stiffness (4 studies, 295 women), HRQoL (3 studies, 208 women) and BCS‐QoL (2 studies, 147 women) from baseline to the end of intervention. The evidence suggests systemic therapies may have little to no effect on grip strength (1 study, 107 women). The evidence is very uncertain about the safety of systemic therapies (10 studies, 1250 women). There were no grade four/five adverse events reported in any of the studies. The study of duloxetine reported more all‐grade adverse events in this treatment group than comparator group.
There were no data on the incidence of AIMSS, the number of women continuing to take AI, BCCS or OS from the treatment studies.
Authors' conclusions
AIMSS are chronic and complex symptoms with a significant impact on women with early breast cancer taking AI. To date, evidence for safe and effective systemic therapies for prevention or treatment of AIMSS has been minimal. Although this review identified 17 studies with 2034 randomised participants, the review was challenging due to the heterogeneous systemic therapy interventions and study methodologies, and the unavailability of certain trial data. Meta‐analysis was thus limited and findings of the review were inconclusive. Further research is recommended into systemic therapy for AIMSS, including high‐quality adequately powered RCT, comprehensive descriptions of the intervention/placebo, and robust definitions of the condition and the outcomes being studied.
Keywords: Female, Humans, Aromatase Inhibitors, Aromatase Inhibitors/adverse effects, Breast Neoplasms, Breast Neoplasms/drug therapy, Musculoskeletal Pain, Musculoskeletal Pain/chemically induced, Musculoskeletal Pain/drug therapy, Musculoskeletal Pain/prevention & control, Quality of Life, Tamoxifen, Tamoxifen/adverse effects
Plain language summary
Systemic therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer
What was the aim of this review?
Hormonal therapy with aromatase inhibitors is used to treat a type of early breast cancer (hormone‐receptor positive) in women after the menopause. AI cause side effects including joint and muscle pains and stiffness (aromatase inhibitors musculoskeletal symptoms, or so‐called AIMSS), which may cause some women to stop taking their aromatase inhibitors, and potentially worsen survival. The aim of this Cochrane Review was to examine whether systemic therapies (treatments that reach cells throughout the body by travelling through the bloodstream) can prevent or treat AIMSS. The authors collected and analysed all relevant studies to answer this question.
Key messages
It is very unclear if systemic therapies improve, worsen or make no difference to pain or quality of life for women taking aromatase inhibitors. Most of the evidence was of very low quality. It was very unclear if systemic therapies for AIMSS were safe.
What did the review study?
We looked at research studies of systemic therapies, which included medicines, vitamins, and complementary and alternative medicines, to see if these could prevent or treat the joint and muscle pains and stiffness of women taking aromatase inhibitors. We included trials of systemic therapies compared to placebo (dummy treatment), or to standard treatments. Women treated with aromatase inhibitors for early‐stage hormone receptor‐positive breast cancer were included. Most studies were for treatment of AIMSS.
Outcomes that were studied included changes in pain, stiffness, hand strength (grip strength), safety and side effects of the study treatments, number of women continuing to take their aromatase inhibitors, quality of life for women, how many women developed muscle and joint aches from their aromatase inhibitors, and survival.
What were the main results of this review?
After collecting and analysing all the relevant studies, we found 17 studies with 2034 women included. Different numbers of women were involved in these studies, ranging from 37 to 299. Four studies looked at systemic therapies to prevent the joint and muscle pains from aromatase inhibitors; 13 studies investigated systemic therapies to treat these symptoms. Ten studies were carried out in the USA, three in China, two in Australia, one in Italy and one in Brazil. Many of the studies had low numbers of women and this may have made it difficult to find small differences. There were problems with some studies being at risk of bias. Other problems were because several studies had not fully published information about their treatment ingredients or their results, so that some data were not available for review or analysis. In addition, studies used many different types of treatment, and it was not appropriate to combine their results in analysis.
AIMSS prevention studies
It is unclear whether any of these studies found a positive or negative effect on pain, and on the number of women who developed AIMSS because of the very low quality evidence. Systemic therapies may have little to no effect on grip strength, quality of life or on women continuing to take their aromatase inhibitors (low‐quality evidence). None of the studies looked at stiffness.
AIMSS treatment studies
It is unclear whether any of these studies found a positive or negative effect on pain, stiffness and quality of life of women because of the very low‐quality evidence. Systemic therapies likely result in little to no change on grip strength in women with AIMSS (low‐quality evidence). None of the studies looked at the number of women continuing to take aromatase inhibitors or who developed AIMSS, or their survival.
Safety
We do not know if systemic therapies for AIMSS are safe as the evidence is very uncertain. There were no serious side effects. One treatment, duloxetine, resulted in an increase in side effects for women, and one treatment, etoricoxib, had a safety alert during the trial. Length of monitoring of women for many studies was short. Safety data should be interpreted with caution.
How up‐to‐date is this review?
The last search for studies (published and ongoing) in this review was in September 2020 within the specified databases and in March 2021 in the Cochrane Breast Cancer's Specialised Register.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Systemic therapy compared to control for treating aromatase inhibitor‐induced musculoskeletal symptoms in women with early breast cancer.
| Systemic therapy compared to control for treating aromatase inhibitor‐induced musculoskeletal symptoms in women with early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: Intervention: systemic therapy Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with systemic therapy | |||||
| Change in pain from baseline to end of intervention assessed with: Brief Pain Inventory (BPI) worst pain; BPI severity follow‐up: 24 weeks | There were 2 studies (omega‐3 fatty acids, vitamin D) that showed a treatment effect with 95% CI that did not include a minimal clinically important difference (MCID) for BPI pain scale. | 183 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The evidence is very uncertain about the effect of systemic therapies on change in pain from baseline to end of intervention. | ||
| Change in grip strength from baseline to end of intervention follow‐up: 24 weeks | 85 per 1000 | 91 per 1000 (31 to 268) | RR 1.08 (0.37 to 3.17) | 137 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | The evidence suggests that systemic therapies results in little to no difference in grip strength from baseline to end of intervention. |
| Safety of systemic therapies in AIMSS | 1 study had a US Food and Drug Administration alert issued for the class of drug which the study drug belonged to (cyclo‐oxygenase‐2 inhibitors), but the study reported no serious adverse effects. No serious adverse events noted in any study. | 344 (4 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | The evidence is very uncertain about the effect of systemic therapies on safety of systemic therapies in AIMSS. | ||
| Effect on discontinuation of aromatase inhibitors (AI) follow‐up: 24 weeks | 39 per 1000 | 6 per 1000 (0 to 116) | RR 0.16 (0.01 to 2.99) | 147 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | The evidence suggests that systemic therapies results in little to no difference in effect on discontinuation of AIs. |
| Effect on breast cancer‐specific quality of life (BCS‐QoL) assessed with: Functional Assessment of Cancer Therapy – Breast (FACT‐B) follow‐up: 24 weeks | 1 study (omega‐3 fatty acids) showed a treatment effect with 95% CI that did include an MCID for this outcome measure. | 44 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | The evidence suggests that systemic therapies results in little to no difference in effect on BCS‐QoL. | ||
| Health‐related quality of life (HRQoL) ‐ HRQoL: Total Functional Assessment of Cancer Therapy – General(FACT‐G) score assessed with: Functional Assessment of Cancer Therapy – General (FACT‐G) follow‐up: 24 weeks | A single study (omega 3 fatty acids) showed a treatment effect with 95% CI which did include a MCID for this outcome measure. | (1 RCT) | ⊕⊕⊝⊝ Lowa,c | The evidence suggests that systemic therapies results in little to no difference in effect on HRQoL. | ||
| Incidence of AIMSS follow‐up: range 24 weeks to 52 weeks | 537 per 1000 | 440 per 1000 (338 to 569) | RR 0.82 (0.63 to 1.06) | 240 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | The evidence is very uncertain about the effect of systemic therapies on change in Incidence of AIMSS. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424110183232321857. | ||||||
a High risk of reporting bias and attrition bias in single study. Downgraded one level. b Downgraded one level for indirectness (restricted population dependent on vitamin D level). c Downgraded one level for imprecision (small sample sizes). d Downgraded one level for risk of bias (high risk of bias across multiple domains). e High suspicion of publication bias (too few studies for funnel plot; one study in abstract form only).
Summary of findings 2. Summary of findings table ‐ Systemic therapy compared to control for preventing aromatase inhibitor‐induced musculoskeletal symptoms in women with early breast cancer.
| Systemic therapy compared to control for preventing aromatase inhibitor‐induced musculoskeletal symptoms in women with early breast cancer | ||||||
| Patient or population: health problem or population Setting: Intervention: Systemic therapy Comparison: Control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Control | Risk with Systemic therapy | |||||
| Change in pain from baseline to end of intervention follow‐up: range 30 days to 6 months | 4 studies showed a minimal clinically important difference (MCID) in pain scores that fell within the 95% CIs of the measured effect (calcitonin, bionic tiger bone, Yi Shen Jian Gu granules and vitamin D). 6 studies showed a treatment effect with 95% CIs that did not include an MCID (testosterone, vitamin D, duloxetine, omega‐3 fatty acids, emu oil, Cat's claw). Due to the variation in systemic therapies, including pharmacological, complementary and alternative medicines, the studies could not be combined for meta‐analysis. | 1099 (10 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | The evidence is very uncertain about the effect of systemic therapies on change in pain from baseline to the end of intervention in the treatment of AIMSS. | ||
| Change in stiffness from baseline to end of intervention | 2 studies showed a minimal clinically important difference (MCID) in stiffness scores that fell within the 95% CIs of the measured effect (bionic tiger bone, Yi Shen Jian Gu granules). 2 studies that showed a treatment effect with 95% CIs that did not include an MCID (vitamin D, emu oil). | 295 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,c,e,f | The evidence is very uncertain about the effect of systemic therapies on change in stiffness from baseline to the end of the intervention in the treatment of AIMSS. | ||
| Grip strength ‐ Vitamin D | There was a single study which did not show a MCID for grip strength which falls within the 95% CI for the measured effect (vitamin D). | (1 RCT) | ⊕⊕⊝⊝ Lowc,f | The evidence is uncertain about the effect of systemic therapies on change in grip strength from baseline to the end of the intervention in the treatment of AIMSS 'little to no effect' | ||
| Effect on breast cancer‐specific quality of life (BCS‐QoL) | 2 studies investigated the effect on BCS‐QoL (bionic tiger bone, Yi Shen Jian Gu granules). All subscales of the same quality of life tool utilised in both of these studies (Functional Assessment of Cancer Therapy – Breast (FACT‐B)) showed an MCID for this tool that falls within the 95% CIs of the measured effect. | 147 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,g,h | The evidence is very uncertain about the effect of systemic therapies on effect on BCS‐QoL from baseline to the end of the intervention in the treatment of AIMSS. | ||
| Effect on health‐related quality of life (HRQoL) | 2 studies investigated the effect of BCS‐QoL (bionic tiger bone, Yi Shen Jian Gu granules) using the Functional Assessment of Cancer Therapy – General (FACT‐G) tool. All subscales of this quality of life tool showed an MCID for this tool that fell within the 95% CIs of the measured effect. 1 study used the 36‐item Short Form (SF‐36) and showed most individual subscales in this outcome showing effect size that did not include MCID for this tool within the 95% CIs of the measured effect. | 208 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,g,h | The evidence is very uncertain about the effect of systemic therapies on HRQoL from baseline to the end of the intervention in the treatment of AIMSS. | ||
| Safety of systemic therapies for the treatment of AIMSS | There were no grade 4/5 adverse events reported in any studies. 1 study investigating duloxetine reported significantly more all‐grade adverse events in the systemic therapy arm (78%) than the control arm (50%) (P < 0.001). | 1250 (10 RCTs) | ⊕⊝⊝⊝ Very lowa,c,i | The evidence is very uncertain about the safety data in the use of systemic therapies for the treatment of AIMSS. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424113354545582340. | ||||||
a Downgraded one level for risk of bias (high risk of bias across multiple domains). b Downgraded one level for inconsistency (heterogeneity in interventions). c Downgraded one level for imprecision (small sample sizes and unable to be combined for meta‐analysis). d Downgraded one level for publication bias (funnel plot displayed asymmetry; multiple studies only written in abstract form). e Downgraded one level for risk of bias (one study had high risk of both performance and detection bias). f Downgraded one level for indirectness (one study with poorly defined AIMSS). g Downgraded one level for indirectness (no criteria for an AIMSS in one study and exclusion of bisphosphonates, which are frequently utilised in the target population). h Downgraded for imprecision (small sample size). i Downgraded one level for publication bias (multiple studies published in abstract form only).
Background
Description of the condition
Despite advances in screening and treatment, breast cancer continues to significantly impact the global community. There was an estimated 1.67 million new cases diagnosed in 2012 making breast cancer the most common non‐skin cancer in women, and it is the fifth most common cause of cancer death globally (Ferlay 2012). In women in high‐income regions of the world, breast cancer is second to lung cancer as the leading cause of death, and in lower income regions, breast cancer remains the leading cause of death (Ferlay 2012). Eighty percent of breast cancer is hormone receptor (i.e. oestrogen receptor or progesterone receptor, or both)‐positive, which is often described as 'endocrine‐sensitive' breast cancer (Nadji 2005). Treatment of postmenopausal women with hormone receptor‐positive breast cancer with aromatase inhibitor (AI) medications is effective. When compared to treatment with another hormonal therapy, tamoxifen, five years of AI therapy in early breast cancer improves disease‐free‐survival (DFS) and breast cancer‐specific survival (BCSS) (EBCTCG 2015). An AI medication called exemestane, used in combination with ovarian suppression in women with higher risk premenopausal breast cancer has also shown an improvement in DFS compared with tamoxifen (Francis 2015; Francis 2018). These results may see the adoption of AI therapy in a greater proportion of women with early breast cancer.
However, AIs are commonly associated with joint and muscular symptoms, commonly referred to as aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS) (Lintermans 2013). Approximately half of all women treated with AIs experience these musculoskeletal effects (Beckwee 2017), which can significantly impact on their quality of life. AIMSS usually presents as symmetrical pain or soreness in multiple joints within the first two to three months of initiating the AI (Burstein 2007). Women may also experience early‐morning stiffness and difficulty sleeping (Burstein 2007). Despite the survival advantage of AIs, between a quarter and a half of all women on this treatment choose to discontinue therapy (Chim 2013; Henry 2012; Kadakia 2016). AIMSS is the most common reason for women to discontinue AI therapy (Kwan 2017).
Description of the intervention
We aimed to investigate the impact of systemic therapies on the prevention or management of AIMSS. Systemic therapies range from prescription medications to dietary supplements such as vitamins. We also included complementary and alternative medicines (CAM) in our review. The National Centre for Complementary and Integrative Health defines CAM as "a group of diverse medical and health care systems, practices and products that are not presently considered to be part of conventional medicine" (NCCIH 2016).
How the intervention might work
There is a body of research investigating the mechanisms of action and the effectiveness of systemic therapies on other musculoskeletal conditions such as osteoarthritis, but quality evidence is still lacking for the optimal systemic therapy management of AIMSS. Examples of research into the management of other musculoskeletal conditions include reviews on glucosamine therapy for osteoarthritis (Towheed 2005), opioids for osteoarthritis of the knee or hip (Da Costa 2014), and muscle relaxants for pain management in rheumatoid arthritis (Richards 2012). Reviews such as these have enabled evidence‐based guidelines to be developed for the management of conditions such as osteoarthritis (NICE 2014). Systemic therapy approaches for AIMSS investigated by researchers have included interventions previously studied in osteoarthritis, rheumatological arthritis and chronic pain conditions (Hershman 2015b).
The exact cause of AIMSS remains unknown, but has been hypothesised to be related to multiple factors, including oestrogen deprivation, vitamin D insufficiency and the activation of molecules within the body that promote inflammation (Borrie 2017; Hershman 2015b). Of these, oestrogen deprivation appears to be the key mediator. Oestrogen has an effect on both inflammation and the neural processing of a painful stimulus, which could result in heightened pain in the setting of oestrogen depletion (Felson 2005). Researchers have also identified an association between AIMSS and various genetic variations in women, which may result in women being more susceptible to experiencing musculoskeletal toxicity from AIs (Borrie 2017; Lintermans 2016).
Risk factors that have been attributed to developing AIMSS include previous hormone replacement therapy (Sestak 2008), previous taxane chemotherapy (Crew 2007; Lintermans 2014), younger age (Kanematsu 2011; Lintermans 2014), fewer years since last menstrual period (Kanematsu 2011; Mao 2011), body mass index less than 25 or greater than 30 kg/m2 (Beckwee 2017; Crew 2007), severity of menopausal symptoms (Castel 2013), presence of joint‐related comorbidity at baseline (Castel 2013; Lintermans 2014), stage two breast cancer (Beckwee 2017), and fear of recurrence (Lopez 2015). However, clinicopathological associations have been inconsistent across studies (Beckwee 2017), except for time since last menstrual period (Kanematsu 2011; Mao 2009).
With only postulated causes of AIMSS, the interventions that have been investigated to date vary widely. Research interventions with systemic therapies have attempted to address various postulated causes of AIMSS, sometimes potentially via multiple complex and postulated pharmacological targets or pathways (or both).
Omega‐3 fatty acids (O3‐FA), as found in fish oil (particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have anti‐inflammatory properties (Hershman 2015a; Hershman 2015b), and have shown effect on pain and stiffness in inflammatory joint pain (Goldberg 2007). EPA and DHA inhibit conversion of arachidonic acid to prostaglandin and leukotrienes, reducing inflammation (Cleland 1988; Sundrarjun 2004). O3‐FA supplements have thus been investigated in AIMSS (Hershman 2015a; Lustberg 2015).
Other anti‐inflammatory systemic therapies have been investigated, including a non‐steroidal anti‐inflammatory drug (NSAID), etoricoxib (Rosati 2011) and prednisolone (Kubo 2012). NSAIDS are utilised as part of the management strategy for osteoarthritis, with traditional NSAIDS inhibiting the cyclo‐oxygenase‐1 (COX‐1) enzyme (Song 2016). Etoricoxib is selective for the cyclo‐oxygenase‐2 (COX‐2) inducible form of cyclo‐oxygenase, potentially improving the central hyperalgesic action (Song 2016). Rosati 2011 examined the effect of AI on musculoskeletal events as a secondary endpoint in a trial where the primary outcome was to investigate whether etoricoxib or placebo improved DFS in addition to adjuvant anastrozole in early breast cancer. Similarly, glucosamine with chondroitin has been investigated in AIMSS (Greenlee 2013). Chondroitin with glucosamine slightly improves pain in osteoarthritis (Singh 2015), with multiple postulated mechanisms of action in preclinical research, including anti‐inflammatory effects in joints and glucosamine increasing the synthesis of proteoglycans in articular cartilage (Greenlee 2013; Reginster 2012).
Oestrogen is thought to play a role in the modulation of nociceptive pain pathways (Felson 2005). With a higher incidence of autoimmune disease in women than men, hormonally active androgens are believed to be anti‐inflammatory and oestrogens are pro‐inflammatory (Schmidt 2006). The balance between oestrogen and androgen mediated by the AI enzyme is thought important in joint health (Schmidt 2006). Hence, testosterone has been investigated as it was hypothesised that this could improve AIMSS (Birrell 2009; Cathcart‐Rake 2020).
Duloxetine, as investigated by Henry 2018, is a serotonin‐noradrenaline reuptake inhibitor (SNRI) with antidepressant and analgesic properties (Bellingham 2010). The analgesic effect of duloxetine is postulated to be achieved by augmentation of the tone of the descending pain inhibitory pathways from the central nervous system (Bellingham 2010). Duloxetine is indicated for treatment of fibromyalgia, diabetic peripheral neuropathic pain and chronic musculoskeletal pain (Lilly 2020), and has been investigated for management of AIMSS (Henry 2018).
Low vitamin D levels have been associated with higher levels of joint pain in postmenopausal women (Chlebowski 2011). Vitamin D deficiency has been found to be prevalent in women with breast cancer undergoing adjuvant chemotherapy (Crew 2009). Oestrogen activates both vitamin D and it's receptor (Gallagher 1980), with AIs causing a subsequent functional deficiency of vitamin D. As vitamin D deficiency and insufficiency can contribute to musculoskeletal symptoms (Hershman 2015b; Holick 2007), vitamin D supplementation for prevention and management of AIMSS has thus been investigated in randomised controlled trials (RCTs) (Khan 2017; Niravath 2019; Rastelli 2011; Shapiro 2016).
Calcitonin acts with parathyroid hormone and 1,25 dihydroxycholecalciferol to regulate short‐term calcium homeostasis, mediated in part by inhibiting bone resorption by an effect on osteoclasts (Chesnut 2008). Salmon calcitonin has thus been used as an anti‐resorptive therapy in osteoporosis and other bone‐associated pain conditions (Chesnut 2008), and has been studied by Liu 2014.
Use of CAM is anecdotally widely noted by care providers and, therefore, reviewing the evidence of effect and toxicities is important (Boon 2000; Kremser 2008). Women with AIMSS may be unwilling to take medications with side effects to treat side effects, and hence may seek CAM as an alternative potential management strategy (Hershman 2015b).
Traditional Chinese medicine (TCM) has been widely used in care of people with cancer in China (Li 2013), and Chinese patients have sought out TCM practitioners due to lack of effective therapies for AIMSS (Peng 2018). Investigators have trialled various TCM for AIMSS (bionic tiger bone, Li 2017; Yi ShenJian Gu (YSJG) granules, Peng 2018; Blue Citrus capsules, Massimino 2011).
Tiger bone, whose "main ingredients are calcium and collagen", has been utilised in TCM for proposed strengthening of muscles and bones (Li 2017). However, as tigers are protected animals, "scientists adopted bionic research method to develop bionic tiger bone powder, which has similar ingredients to natural tiger bone"(Li 2017). "Bionic TB [tiger bone] powder has more than 20 kinds of amino‐acid and microelements essential to human. Besides, calcium to phosphorus ratio of TB makes it suitable for body to absorb, whereas it also contains various organic components, such as collagen, analgesic peptide, bone morphogenetic protein, bone growth factors, and polyose" (Li 2017). Li 2017 investigated bionic tiger bone powder for management of AIMSS because of proposed anti‐inflammatory, analgesic and anti‐osteoporotic effects.
YSJG granules, patented by the Beijing Hospital of Traditional Chinese Medicine, are composed of an empiric formula of 12 herbs, including Radix rehmanniae Preparata (ShuDiHuang), Fructus Corni (ShanZhuYu), Semen cuscutae (TuSiZi), Radix Achyranthis Bidentatae (NiuXi), Rhizoma cyperi (XiangFu), Radix Angelicae Sinensis (Dang‐Gui), Poria (FuLing), Radix Paeoniae Alba (BaiShao), Rhizoma chuanxiong (ChuanXiong), Rhizoma corydalis (YanHuSuo), Phryma leptostachya (TouGuCao) and Caulis trachelospermi (LuoShiTeng) (Peng 2014). YSGJ granules have been used in TCM treatment of musculoskeletal symptoms of postmenopausal women with osteoporosis and arthrosis based on TCM principles, and have been investigated for management of AIMSS (Peng 2018). "Because the formula for YSJG is currently being patented, the ingredients in YSJG cannot be published at this time" (Peng 2014), and therefore it is not possible to postulate on the proposed action of this intervention in AIMSS due to the large number and complexity of the ingredients, and the uncertainty arising from the lack of information on the ingredients with the pending patent.
Similarly, the TCM Blue Citrus has been investigated for AIMSS, due to anecdotal reports of improvements of AIMSS (Massimino 2011). According to the National Cancer Institute drug dictionary (NCI 2021), Blue Citrus is "An oral capsule formulation of a traditional Chinese herbal medicine with potential analgesic activity. In addition to other herbs, seeds and fruits, blue citrus‐based herbal capsule contains the Chinese herb blue citrus (qing pi), which is produced from the dried immature green peel of the tangerine Citrus reticulata Blanco. Blue citrus contains large amounts of limonene, citral and synephrine, which may attribute to its analgesic activity. However, due to the complexity of its chemical components, the exact mechanism of action of this agent remains to be determined".
Emu oil, obtained from the fat of emus, a large bird indigenous to the Australian continent, has been used by Australian Aboriginal people as a traditional medicine (Turner 2015; Whitehouse 1998). The liquid fat was applied topically by Aboriginal people for musculoskeletal disorders and to assist wound healing (Turner 2015; Whitehouse 1998) with transdermal absorption considered to be anti‐inflammatory in rat models of arthritis (Whitehouse 1998). There were anecdotal evidence of effectiveness in osteoarthritis (Power 2004), and hence topical application for treatment of AIMSS has been investigated (Chan 2017). Emu oil "contains several fatty acids (myristic, palmitic, stearic, palmitoleic, oleic, linoleic, and linolenic), and it is not known which specific component provides symptomatic relief" (Chan 2017).
Uncaria tomentosa (Cat's claw) is a plant species found in the Amazon (Sordi 2019), and has been used for centuries by the Incas as a traditional medicine to treat arthritis, arthrosis and other inflammatory conditions. The active metabolites (including pentacyclic and indole oxindole alkaloids and quinovic acid glycosides) have reported antioxidant, immunomodulatory, antineoplastic, anti‐inflammatory and antiviral activities (Aguilar 2002; Aquino 1989; Sordi 2019). Sordi 2019 investigated the use of the dry extract of Cat's claw for women with AIMSS.
Cherries contain many bioactive compounds with reported health benefits (Kelley 2018). Flavonoids and anthocyanins in tart cherry extract reportedly reduce inflammation, and some clinical trials suggest improvement in joint pain in osteoarthritis and gout (Kelley 2018; Shenouda 2019). Shenouda 2019 investigated tart cherry extract in AIMSS.
As the cause of AIMSS remains unknown, it is important to further review the evidence for systemic prevention and management of musculoskeletal symptoms specific to AIMSS, rather than extrapolating evidence from other non‐AIMSS musculoskeletal conditions.
Why it is important to do this review
The high prevalence of musculoskeletal symptoms secondary to AIs can result in detrimental outcomes for patients. Several studies have shown poor participant adherence to AI therapy (Brier 2017; Hadji 2014; Henry 2012; Hershman 2011; Partridge 2008; Presant 2007). This is particularly concerning in the setting of early breast cancer, where non‐compliance with hormonal therapies such as AIs, used in the curative setting, have been shown to be detrimental to patient survival (Hershman 2011). Given the prevalence of breast cancer in the community, the implications of the potential impact of non‐adherence on breast cancer outcomes are important.
While there has been a few reviews to assist clinicians with the management of AIMSS (Roberts 2017; Yang 2017), there have been no reviews dedicated solely to the systemic management of AIMSS. Several of the authors from this review have conducted a separate Cochrane Review entitled "Exercise therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer" (Roberts 2020). There has been a meta‐analysis on acupuncture for AIMSS (Chen 2017). Identifying current evidence and potential areas for further research in this field is required for the optimisation of management of women with endocrine‐sensitive breast cancer.
Objectives
To assess the effects of systemic therapies on the prevention or management of AIMSS in women with stage I to III hormone receptor‐positive breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
RCTs examining the prevention or management of AIMSS in women with stage I to III hormone receptor‐positive breast cancer. AIMSS was defined by the study authors of each trial. We excluded animal and in vitro studies. We considered studies in all languages for inclusion.
Types of participants
Women with stage I to III oestrogen‐receptor (ER) or progesterone‐receptor (PR) (or both)‐positive breast cancer, being treated adjuvantly with AIs.
Types of interventions
Intervention: all systemic therapy interventions, including pharmacological therapies, dietary supplements, and CAM. We excluded acupuncture administered as a sole intervention as it was not considered a systemic therapy.
Comparator: all comparator groups were allowed, including placebo or standard of care (or both) with analgesia alone.
Types of outcome measures
Primary outcomes
Symptoms of AIMSS (specifically pain, stiffness and grip strength) from baseline. These were preferably assessed by validated questionnaires, such as the Visual Analogue Scale (VAS), Brief Pain Inventory (BPI), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Functional Assessment of Cancer Therapy – General (FACT‐G), Medical Outcome Study Short Form 36 (SF‐36) and the Modified Score for the Assessment of Chronic Rheumatoid Affections of the Hands (M‐SACRAH). These questionnaires identified participant symptoms including, but not limited to, pain (e.g. severity of pain, change in pain scores), physical function (e.g. using stairs, sitting up, performing domestic duties) and stiffness. These outcomes were assessed for both the impact on AIMSS immediately after the intervention, and in the long‐term, where available.
Safety of the intervention, including adverse effects. All grade of adverse events were considered from each intervention.
Secondary outcomes
Persistence and adherence of participants continuing to take their AI medication due to the intervention.
Patient health‐related quality of life (HRQoL), also preferably assessed by validated patient‐reported outcome (PRO) questionnaires. Where available, this was analysed for the impact on participants during the intervention, immediately after the intervention and in the long term.
Incidence of AIMSS.
Breast cancer‐specific survival (BCSS).
Overall survival (OS).
Search methods for identification of studies
Electronic searches
The Information Specialist (KR) designed and conducted systematic searches in the selected databases and trial registries without language, publication year or publication status restrictions on 10 September 2020. Cochrane Breast Cancer's Information Specialist conducted the search of the Specialised Register on 27 March 2021. Where appropriate, the search strategies also included adaptations of the Highly Sensitive Search Strategy designed by the Cochrane Collaboration (Lefebvre 2011), and the search filter for CINAHL (EBSCO) created by Mark Clowes at SIGN for identifying RCTs and controlled clinical trials.
We searched the following databases and trial registries up to 10 September 2020.
CENTRAL (the Cochrane Library, 2020, Issue 8). See Appendix 1.
MEDLINE (via PubMed) from 1946. See Appendix 2.
Embase (via EMBASE.com) from 1947. See Appendix 3.
CINAHL (via EBSCO) from 1981. See Appendix 4.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials. See Appendix 5.
ClinicalTrials.gov (clinicaltrials.gov/). See Appendix 6.
The following was searched up to 27 March 2021.
The Cochrane Breast Cancer Group's (CBCG's) Specialised Register. Trials with the keywords “breast cancer” and related terms, “aromatase inhibitors”, “exemestane”, “anastrozole”, “letrozole”, were extracted and considered for inclusion in the review.
Searching other resources
Adverse effects
We did not perform a separate search for adverse effects of interventions used for the treatment of AIMSS. We considered adverse effects in included studies only.
Searching within other reviews
We scanned the reference lists of existing systematic reviews relevant to this systematic review that the search identified, or reference/citation lists, for additional trials and to check robustness of search strategy.
Bibliographic searching
We identified further studies from reference and citation lists of identified relevant trials or reviews. We obtained a copy of the full article for each reference reporting a potentially eligible trial for those studies selected for full‐text review. Where this was not possible, such as with the inclusion of conference abstracts, we contacted the authors for additional information and reviewed additional information from clinical trial databases.
Grey searching
The San Antonio Breast Cancer Symposium (SABCS) and American Society of Clinical Oncology (ASCO) conference proceedings are included in the Embase database and, therefore, were not searched separately. See Electronic searches for searching of clinical trials databases.
Data collection and analysis
Selection of studies
Two review authors (KER and NW) screened and retrieved abstracts from the literature search to assess whether they met the specified selection criteria. Subsequently, two review authors (KER and NW) reviewed full‐texts of all remaining studies, ensuring they still met the selection criteria. Any disagreements on study selection were resolved by a separate review author (IA or SC). The selection process was recorded in a PRISMA flow diagram (Figure 1). We documented the reason for excluding studies in the Characteristics of excluded studies table. Clinical trials that were terminated are detailed as Excluded studies, and those that are ongoing and awaiting publication, are included in the Studies awaiting classification table.
1.
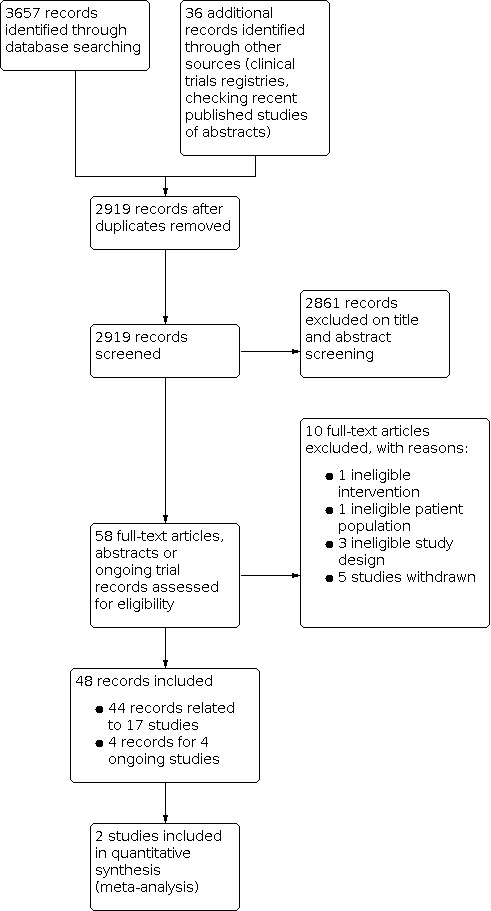
Study flow diagram.
No studies required translation from other languages.
Data extraction and management
Three review authors (IA, SC, NW) used a standardised data extraction form and collected the following information. A fourth review author (KER) resolved disagreements.
Characteristics of the study
Study sponsors and author affiliations.
Study funding.
Methods, including study design, method of sequence generation, allocation concealment, blinding of outcome, participant attrition and exclusions, and selective outcome reporting.
Full‐text available versus abstract only.
Characteristics of the study population
Country of enrolment.
Inclusion/exclusion criteria.
Study definition of AIMSS.
Number of participants in each treatment arm.
Mean and range of participant ages.
Number of participants aged less than 40 years; aged 40 to 60 years; and aged greater than 60 years.
Menopausal status (i.e. requirement for biochemical ovarian suppression versus no requirement).
Previous use of taxane (yes/no).
Type of AI prescribed, and time since commencement of AI.
Characteristics of the intervention
Description of the intervention.
Details of control group.
Ingredients of placebo, if applicable.
Compliance with intervention.
Safety.
Characteristics of the outcomes
Scoring systems used (and documentation of PRO versus investigator‐reported outcomes).
Outcomes of pain, stiffness, functioning and HRQoL.
Timing of outcome data collection, including length of time between intervention and last collected outcome measurement.
Follow‐up period.
Two review authors (IA and NW) entered the data into Review Manager Web (Review Manager 2014). Where there was more than one publication for a study, the data was extracted from all publications as required, but the most recent version of the study was considered the primary reference. Where possible, records relating to the same study were combined under an overall trial name or study.
Assessment of risk of bias in included studies
Two review authors (of NW, SC or IA) independently assessed risk of bias for all RCTs using the Cochrane's risk of bias assessment tool (Higgins 2011a). A third review author (KR) resolved any disagreements. This tool included seven specific domains: random sequence generation; allocation concealment; blinding of outcome assessment; blinding of participant and personnel; incomplete outcome data; selective reporting and other sources of bias. Each risk of bias domain was assessed as high risk, low risk or unclear risk. For risk of bias for cross‐over RCTs, we referred to guidance in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Where there was incomplete reporting of a study's conduct, we attempted to contact the study authors to clarify such uncertainties. Risk of bias tables for each study were presented in the Characteristics of included studies table. Risk of bias domain‐level judgements were presented in forest plots, and a summary table listing the risk of bias judgement for all studies is presented in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias domain, for each included trial.
3.

Risk of bias summary: review authors' judgements about each risk of bias item, presented as percentages across all included trials.
Measures of treatment effect
It was expected that studies would use a variety of different tools to measure the outcomes of interest (pain, stiffness, grip strength and HRQoL) and mostly report continuous outcomes. Because of the clinical heterogeneity of the included studies, a random‐effects model was considered.
The measurement of the treatment effect was performed using a mean difference (MD) analysis. This is different from our protocol, which we had intended to measure the treatment effect by performing a standardised mean difference (SMD) analysis and the random‐effects model to combine data from different scoring systems measuring the same outcome of interest. But, due to inconsistent reporting of standard deviations (SD), with some studies reporting only 'end‐of‐treatment' SD and others reporting only 'change score' SD, we were unable to combine these results for the calculation of SMD. Multiple studies did not report SD of change scores and only provided SD from baseline or end‐of‐treatment SD. If change score means with SD were not available, we reported end‐of‐treatment means and SD for both groups. As discussed in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions, "in a randomized study, MD based on changes from baseline can usually be assumed to be addressing exactly the same underlying intervention effects as analyses based on post‐intervention measurements" (Deeks 2021). If end‐of‐treatment means and SD were used, this was highlighted in the analysis.
If SDs could not be obtained for studies, imputing the SD was attempted as per Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). In studies where only CIs were available, we used the following formula to determine the SD: SD = √n × (upper limit 95% CI − lower limit 95% CI)/2 × T value calculated by the T distribution), where n was the sample size and CI was the confidence interval. We estimated appropriate T values for smaller sample sizes using the TINV function (TINV(1‐0.95,n‐1)) in Excel. If the standard error (SE) was reported instead of SD, then SD was calculated with the following formula, SE = SD × √n, as per guidance from Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). One study reported the use of mean and SD, but the data were more consistent with the reporting of median values and interquartile ranges (IQR) (Liu 2014). Reporting the median is often an indicator that the data are skewed, so it should be incorporated into a meta‐analysis with caution (Higgins 2021). To calculate SD from IQR, we used the following formula: SD = (q3‐q1)/1.35, where q3 is quartile 3 and q1 is quartile 1 (Higgins 2021).
If studies reported dichotomous outcomes (e.g. incidence rates), the treatment outcome was measured by the risk ratio (RR), in combination with a 95% CI. We reported the ratio of treatment effect so that RR less than 1.0 favoured the intervention group for relief of AIMSS symptoms and RR greater than 1.0 favoured the control group.
The most appropriate time point across all studies for each outcome depended on the availability of suitable data. As recommended in Section 16.7.1 of the Cochrane Handbook for Systematic Reviews of Interventions, we avoided multiple testing of the treatment effect at each time point (Higgins 2011c). We collected data on the treatment effect at baseline, immediately following the intervention and long‐term data, if available. The only study that we did not use end‐of‐treatment outcomes was Rastelli 2011. Instead, we utilised outcome data at two months to eliminate any differences in the stratums in the intervention arm, which received differing doses of intervention between two and four months of the study.
Unit of analysis issues
There were two studies that would have created unit of analysis issues, including one study with a cross‐over trial design (Massimino 2011), and one study with multiple treatment arms (Birrell 2009). However, both studies were only published in abstract form without pursuing full publication. We were unable to obtain information or data from either of the study authors by correspondence, and, therefore, did not have adequate data to analyse the results. Our planned approach for unit of analysis issues that were not utilised in this review can be found in our protocol (Roberts 2018).
Dealing with missing data
In the case of missing data, we attempted to contact the study authors to source additional information through clinical trial registries or data repositories. If the required data were still not available, we contacted original investigators via email and gave three weeks to reply to the request. If the corresponding authors did not reply, we attempted further contact with the original investigators and either the first or last author of each paper (if not the primary corresponding author).
Assessment of heterogeneity
We assessed clinical heterogeneity using the I2 statistic, Chi2 test and visual inspection of forest plots, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). An I2 statistic of 30% to 60% may represent moderate heterogeneity, a result of 50% to 90% may represent substantial heterogeneity and a result of 75% to 100% may represent considerable heterogeneity (Higgins 2011a). The importance of the I2 statistic depended on the magnitude and direction of effects and the strength of evidence for heterogeneity. For the Chi2 test, we used P < 0.1 to indicate significant heterogeneity. We planned to use both a random‐effects model and a fixed‐effect model for analysis of results of meta‐analysis, but there were only two studies combined for meta‐analysis of one outcome, and there was no evidence of statistical heterogeneity; therefore, fixed‐effect and random‐effects models did not need to be compared.
Assessment of reporting biases
We included one funnel plot in the assessment of reporting biases for the outcome with the largest number of studies. We could not undertake any further assessments due to the small number of studies contributing data to each outcome (fewer than 10 studies).
Data synthesis
We used Review Manager Web to perform statistical analysis (Review Manager Web 2021). We assessed analyses for clinical heterogeneity (see Assessment of heterogeneity). We performed a fixed‐effect meta‐analysis using the inverse variance method to combine data results for one outcome where at least two studies were appropriate to be combined for meta‐analysis. We reported the meta‐analysis using a forest plot and in the summary of findings table.
There needed to be sufficient data available for meta‐analysis between studies. If there were insufficient outcome data, we attempted to contact authors for additional data. If there were insufficient studies, or where the data could not be combined due to insufficient data for comparison, we presented the findings in a narrative manner.
To enable synthesis of our outcomes where meta‐analysis could not be done for the majority of outcomes, we applied the vote‐counting method as detailed by McKenzie 2021. We used vote‐counting by reporting continuous outcomes as the number of studies that showed a treatment effect that included a clinically significant benefit within the CIs of the MD between studies as determined by reported minimal clinically importance difference (MCID) for that outcome scale, compared to the number of studies that did not include an MCID within the CIs of the treatment effect. The MCID is a change in score that has been determined to represent a meaningful change in quality of life.
For binary outcomes, we reported RR as per our initial protocol. There were no trials with binary outcomes that reported zero events in both arms.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses, as there were insufficient studies and participants to undertake any meaningful subgroup analysis within this review. Our planned subgroup analyses can be found in our protocol (Roberts 2018).
Sensitivity analysis
There were not enough studies of each intervention in our review to undertake meaningful sensitivity analyses. Our planned sensitivity analyses can be found in our protocol (Roberts 2018).
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro GDT software to present summary of findings tables to illustrate the main outcomes and grade the certainty of the evidence. We assessed the level of evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) as per the Cochrane Handbook of Systematic Reviews of Interventions (Schünemann 2021). At least two review authors (KR, IA) independently assessed the evidence using the GRADE technique, and a third review author (NW) resolved any disputes.
Main outcomes in the summary of findings tables: prevention studies
Change in pain from baseline to end of intervention (symptoms of AIMSS)
Adverse effects secondary to the intervention (safety data)
Change in grip strength from baseline to end of intervention (symptoms of AIMSS)
Effect on discontinuation of AIs
Effect on BCS‐QoL
Effect on HRQoL
Change in incidence of AIMSS.
Main outcomes in the summary of findings table: treatment studies
Change in pain from baseline to end of intervention (symptoms of AIMSS)
Change in stiffness from baseline to end of intervention (symptoms of AIMSS)
Overall change in grip strength (symptoms of AIMSS)
Adverse effects secondary to the intervention (safety data)
Effect on BCS‐QoL
Effect on HRQoL
Results
Description of studies
Results of the search
The search retrieved 3693 records from four databases (3350 records; see Appendix 1; Appendix 2; Appendix 3; Appendix 4 in Electronic searches), the Specialised Register (307 records), clinical trial registries and reference lists of included studies (36 records). Once duplicates were removed, there were 2919 records. We excluded 2861 records during title and abstract screening, and obtained the full text (where possible) of the remaining 58 records. At full‐text review, we excluded 10 studies (see Characteristics of excluded studies table). We identified four ongoing studies (see Characteristics of ongoing studies table).
We included 17 studies reported in 44 references (see Characteristics of included studies table) and four ongoing studies (see Characteristics of ongoing studies table). The full details of our screening process is detailed in the study flow diagram (Figure 1).
Included studies
The review included 17 studies (see Characteristics of included studies table).
Thirteen studies investigated the treatment of AIMSS (Birrell 2009; Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Li 2017; Liu 2014; Massimino 2011; Peng 2018; Rastelli 2011; Shapiro 2016; Shenouda 2019; Sordi 2019), and all of these studies investigated at least one symptom of AIMSS as a primary outcome.
Four studies investigated the prevention of AIMSS (Khan 2017; Lustberg 2018; Niravath 2019; Rosati 2011). One of these studies investigated the five‐year event‐free survival (EFS) as a result of adding adjuvant etoricoxib versus placebo to adjuvant anastrozole, and examined the musculoskeletal events as a secondary outcome (Rosati 2011); another trial investigated adherence and tolerability as a primary outcome, and investigated pain as a secondary outcome (Lustberg 2018); the other two studies investigated prevention of AIMSS as a primary outcome (Khan 2017; Niravath 2019).
Ten studies enrolled participants in the USA (Cathcart‐Rake 2020; Henry 2018; Hershman 2015a; Khan 2017; Lustberg 2018; Massimino 2011; Niravath 2019; Rastelli 2011; Shapiro 2016; Shenouda 2019), three studies in China (Li 2017; Liu 2014; Peng 2018), two in Australia (Birrell 2009; Chan 2017), and one each in Italy (Rosati 2011) and Brazil (Sordi 2019). One trial used a multi‐arm study design (Birrell 2009), and another trial employed a cross‐over design (Massimino 2011).
Thirteen studies were published as full texts (Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Khan 2017; Li 2017; Liu 2014; Lustberg 2018; Niravath 2019; Peng 2018; Rastelli 2011; Shapiro 2016; Sordi 2019), whereas four studies were published as an abstract or in poster form only (Birrell 2009; Massimino 2011; Rosati 2011; Shenouda 2019). Author correspondence resulted in additional data information from four studies (Chan 2017; Khan 2017; Niravath 2019; Sordi 2019).
Population
This review included 2034 randomised participants in 17 studies. The sample sizes ranged from 37 to 299 participants.
Participant age ranges were from 27 to 83 years. Eight studies reported the mean age of participants, which ranged from 59 to 61.5 years (Cathcart‐Rake 2020; Khan 2017; Liu 2014; Lustberg 2018; Peng 2018; Rastelli 2011; Shapiro 2016; Sordi 2019). Six studies reported the median age of the participants, which ranged from 56.9 to 64 years (Chan 2017; Henry 2018; Hershman 2015a; Li 2017; Niravath 2019; Rosati 2011). Nine studies reported age ranges (Chan 2017; Henry 2018; Khan 2017; Li 2017; Lustberg 2018; Niravath 2019; Peng 2018; Rosati 2011; Sordi 2019). Three studies reported only in abstract/poster format provided no participant baseline characteristics data (Birrell 2009; Massimino 2011; Shenouda 2019).
Most participants were already receiving AI treatment at enrolment into the studies, except for the three prevention studies, which were investigating women commencing AI (Khan 2017; Niravath 2019; Rosati 2011). The prevention study by Lustberg 2018 included women with short duration (less than 21 days) of AI exposure. The treatment study by Li 2017 included women with a duration of AI treatment of less than one month, despite being designed to investigate the treatment of AIMSS. Six treatment studies reported the duration of AI therapy at baseline, with mean durations ranging from 47.9 weeks to 20.6 months (Henry 2018; Rastelli 2011; Shapiro 2016; Sordi 2019), and median durations reported as 1.2 years (Hershman 2015a) and 13.3 months (emu oil group) to 16.9 months (placebo group; Chan 2017). Inclusion criteria of note related to two of the vitamin D studies that required participants to have specific serum 25‐hydroxyvitamin D (25‐OHD) levels at baseline (Khan 2017; Rastelli 2011), particularly 25‐OHD levels of 40 ng/mL or less for Khan 2017 and between 10 ng/mL and 29 ng/mL for Rastelli 2011. Mean baseline 25‐OHD levels in the studies investigating vitamin D supplementation ranged from 22.5 ng/mL to 36.6 ng/mL (Khan 2017; Niravath 2019; Rastelli 2011; Shapiro 2016).
Several studies excluded women with potentially confounding comorbid musculoskeletal conditions such as rheumatoid arthritis, fibromyalgia and connective tissue disorders (Chan 2017; Henry 2018; Li 2017; Lustberg 2018; Massimino 2011; Peng 2018; Rastelli 2011; Shapiro 2016; Sordi 2019), or specifically the use of COX‐2 inhibitors for arthritis (Rosati 2011). All studies excluded women with metastatic disease; however, one woman with metastatic breast cancer was randomised and included in the study by Liu 2014. Inclusion criteria for Lustberg 2018 was stage I to III breast cancer; however, one women with ductal carcinoma in situ (DCIS) only was randomised; however, DCIS was allowed in the inclusion criteria in the study by Sordi 2019 but only one participant with DCIS was enrolled.
Definition of aromatase inhibitor‐induced musculoskeletal symptoms
Studies that included participants with AIMSS at baseline varied in their definitions of AIMSS. Most studies specified the requirement of arthralgia/myalgias or musculoskeletal symptoms to be related to the AI as an inclusion criterion, although the specific definition of AIMSS and the determination of the relationship to AI therapy varied markedly (Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Li 2017; Liu 2014; Peng 2018; Rastelli 2011; Shapiro 2016; Shenouda 2019; Sordi 2019). Several of these studies specified a minimum pain score to qualify for inclusion (Cathcart‐Rake 2020; Henry 2018; Hershman 2015a; Peng 2018; Shapiro 2016; Sordi 2019). Two other studies included women experiencing any joint symptoms while taking an AI (Birrell 2009; Massimino 2011), and one of these stipulated a minimum pain score to qualify for inclusion (Birrell 2009); however, both studies were reported in abstract form with minimal details.
Interventions
The interventions investigated for systemic therapy for AIMSS varied widely. These were:
one study on oral duloxetine for the treatment of AIMSS (Henry 2018);
one study on etoricoxib as part of an adjuvant trial with anastrozole, with musculoskeletal adverse effects as a secondary outcome (Rosati 2011);
four studies investigating vitamin D on AIMSS, either for prevention (Khan 2017; Niravath 2019) or treatment (Rastelli 2011; Shapiro 2016). The dose, scheduling and duration of vitamin D intervention varied substantially between these studies (Khan 2017; Niravath 2019; Rastelli 2011; Shapiro 2016);
one study on calcitonin for the treatment of AIMSS (Liu 2014);
two studies investigated testosterone for treatment of AIMSS, either administered as a subcutaneous implant or as gel (Birrell 2009; Cathcart‐Rake 2020);
two studies investigated O3‐FA supplementation for AIMSS, one for prevention (Lustberg 2018) and one for treatment (Hershman 2015a). For Hershman 2015a, the O3‐FA intervention dose was 3.3 grams (g) per day; for Lustberg 2018 4.3 g per day;
-
six studies investigated a range of complementary medicines, all for the treatment of AIMSS (Chan 2017; Li 2017; Massimino 2011; Peng 2018; Shenouda 2019; Sordi 2019) covering:
one study on topical application of emu oil, a traditional medicine of Australian Aboriginal and Torres Strait Islander people, massaged into joints, with possible anti‐inflammatory properties via local or systemic (or both) absorption (Chan 2017);
three studies on TCM (Li 2017; Massimino 2011; Peng 2018), which included bionic tiger bone powder (Li 2017), YSJG (Peng 2018) and Blue Citrus capsules (Massimino 2011);
one study on traditional Incan medicine Uncaria tomentosa (Cat's claw) administered as dry extract tablets (Sordi 2019); and
one study on tart cherry concentrate, added to water (Shenouda 2019).
Thirteen of the 17 studies were placebo controlled (Birrell 2009; Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Khan 2017; Lustberg 2018; Massimino 2011; Peng 2018; Rastelli 2011; Rosati 2011; Shenouda 2019; Sordi 2019). Seven studies described the placebo, in varying detail (Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Lustberg 2018; Peng 2018; Sordi 2019). The control arm of the other studies included calcium carbonate daily orally (Li 2017), oral caltrate D 600 mg/day (Liu 2014), oral vitamin D3 800 IU daily (Niravath 2019), and oral vitamin D3 600 IU daily (Shapiro 2016). The duration of the intervention varied, from four weeks to two years.
The duration of follow‐up varied between four weeks and five years.
Excluded studies
The reasons for excluding studies are listed in the Characteristics of excluded studies table. Ten studies were excluded from the analysis. Most studies were not RCTs (four studies) or the studies were withdrawn/terminated early (three studies). One study had an incorrect participant population (women with chemotherapy‐induced arthralgia) and one had an incorrect intervention (local rather than systemic treatment).
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
There are four ongoing studies (NCT02831582; NCT03865992; NCT04205786; UMIN000027481). See Characteristics of ongoing studies table.
Risk of bias in included studies
Details of the risk of bias are available in the risk of bias tables in the Characteristics of included studies table.
We requested further information to clarify unclear risk of bias from 11 studies, which authors of four studies provided (Chan 2017; Khan 2017; Niravath 2019; Sordi 2019). We also requested further information from another five studies to clarify potential bias including those at high risk of bias from selective reporting; however, we received no responses. The risk of bias summary can be viewed in Figure 2 and risk of bias graph in Figure 3.
Allocation
Random sequence generation and allocation concealment
We judged six studies at unclear risk of selection bias because there was insufficient information to permit judgement about the adequacy of methods of random sequence generation or allocation concealment (Birrell 2009; Liu 2014; Massimino 2011; Rosati 2011; Shenouda 2019; Sordi 2019). Four of these studies were published as abstract alone or in poster format and there was no further information (Birrell 2009; Massimino 2011; Rosati 2011; Shenouda 2019). One study had insufficient information available in the publication to permit judgement and no further information could be obtained by author correspondence (Liu 2014). One study had insufficient information available to permit judgement on methods of random sequence generation or allocation concealment, although author correspondence did provide additional information for this study (Sordi 2019).
Eleven studies were at low risk of selection bias for both methods of random sequence generation and allocation concealment (Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Khan 2017; Li 2017; Lustberg 2018; Niravath 2019; Peng 2018; Rastelli 2011; Shapiro 2016). These 11 studies all described in sufficient detail methods to generate randomisation sequences that produced comparable groups, and adequate methods to conceal allocation.
Blinding
Blinding of participants and personnel
We judged three studies at high risk of performance bias (Henry 2018; Li 2017; Niravath 2019). One study was not blinded, with participants randomised to receive either oral vitamin D3 at 50,000 International Units (IU) per week for 12 weeks followed by 2000 IU daily for 40 weeks, or vitamin D3 at 800 IU daily for 52 weeks (Niravath 2019). A second study had differences in the dosing between the intervention (bionic tiger bone) and the control which could foreseeably have led to unblinding of participants and personnel (Li 2017). The third study examining duloxetine versus placebo found that more participants in the duloxetine group experienced adverse effects (78% with duloxetine versus 50% with placebo; Henry 2018). More participants in the duloxetine group compared with the placebo arm believed they were receiving duloxetine (79% with duloxetine versus 50% with placebo; P < 0.001). Likely due to adverse events experienced, it was foreseeable that participants and personnel were at risk of unblinding.
Five studies were at unclear risk of performance bias due to insufficient information available to permit judgement on measures used to blind participants and personnel (Birrell 2009; Liu 2014; Massimino 2011; Rosati 2011; Shenouda 2019). We judged nine studies at low risk of performance bias due to effective blinding of participants and personnel (Cathcart‐Rake 2020; Chan 2017; Hershman 2015a; Khan 2017; Lustberg 2018; Peng 2018; Rastelli 2011; Shapiro 2016; Sordi 2019).
Blinding of outcome assessment
Most outcomes were PROs. We judged three studies at high risk of detection bias (Henry 2018; Li 2017; Niravath 2019). The primary outcomes for all three of these studies were PROs where participants were the outcome assessors. One of these studies had lack of blinding with outcome assessors, that is, participants, having knowledge of the assigned intervention (Niravath 2019). In two other studies, it was highly likely that participants, and therefore the outcome assessors, had potential knowledge of the assigned intervention (Henry 2018; Li 2017). Five studies were at unclear risk of detection bias (Birrell 2009; Liu 2014; Massimino 2011; Rosati 2011; Shenouda 2019). There was insufficient information to permit judgement on measures used to blind the participants who were the outcome assessors in four of these studies (Birrell 2009; Liu 2014; Massimino 2011; Shenouda 2019), and insufficient information on blinding procedures related to personnel who were the outcome assessors in the remaining study (Rosati 2011). We judged nine studies at low risk of detection bias due to effective blinding of outcome assessors (Cathcart‐Rake 2020; Chan 2017; Hershman 2015a; Khan 2017; Lustberg 2018; Peng 2018; Rastelli 2011; Shapiro 2016; Sordi 2019).
Incomplete outcome data
We assessed seven studies at high risk of attrition bias (Cathcart‐Rake 2020; Liu 2014; Lustberg 2018; Niravath 2019; Rastelli 2011; Rosati 2011; Shenouda 2019). We based these judgements on high drop‐out rates of 20% or greater (Cathcart‐Rake 2020; Lustberg 2018; Rosati 2011; Shenouda 2019; Niravath 2019), high proportional rates of exclusion from analysis with disparities between groups (Liu 2014), and a high drop‐out rate of 20% of greater and disparity in dropout rates between the intervention and control groups (Rastelli 2011). Two studies published in abstract/poster only were at unclear risk of attrition bias due to insufficient information to permit judgement (Birrell 2009; Massimino 2011).
We judged eight studies at low risk of attrition bias as handling of incomplete outcome data was adequately described and unlikely to have produced bias (Chan 2017; Henry 2018; Hershman 2015a; Khan 2017; Li 2017; Peng 2018; Shapiro 2016; Sordi 2019).
Selective reporting
We judged two studies at high risk of selective reporting (Lustberg 2018; Rastelli 2011). One study of high‐ versus standard‐dose vitamin D reported an improvement in two‐month pain scores for the high‐dose group; however, the two‐month PRO pain outcome data scores did not appear to be a prespecified outcome on the trial registry (Rastelli 2011). The six‐month PRO outcomes were the registered primary outcomes. The positive effect of high‐dose vitamin D supplementation was not maintained at six months. The second study of O3‐FA compared to placebo reported safety and tolerability as the primary outcomes (Lustberg 2018); however, the trial registration listed pain score change after six months based on the Functional Assessment of Cancer Therapy – Breast (FACT‐B) and Functional Assessment of Cancer Treatment – Endocrine Symptoms (FACT‐ES) instrument as the primary outcome, and did not specify specifically safety and tolerability as secondary outcomes on the trial registry. No further information could be obtained by author correspondence.
Nine studies were at unclear risk of selective reporting (Birrell 2009; Khan 2017; Li 2017; Liu 2014; Massimino 2011; Peng 2018; Rosati 2011; Shenouda 2019; Sordi 2019). The reasons for judgement were as follows: insufficient information to permit judgement as study published only in abstract/poster form and no further information available (Birrell 2009; Massimino 2011; Rosati 2011; Shenouda 2019), or insufficient information available in the publication to permit judgement and no further information able to be obtained (Liu 2014); at least one relevant missing unreported outcome among a very high number of planned outcomes in the protocol (Khan 2017); insufficient information whether certain outcomes had been analysed in accordance with a prespecified statistical plan (Li 2017); uncertainties about the time points for the primary outcomes with insufficient information available to permit judgement (Peng 2018); and inability to access the trial registry or statistical analysis plan to permit judgement (Sordi 2019).
We assessed six studies at low risk of selective reporting because these studies reported all of their proposed outcomes (Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Niravath 2019), or only secondary/exploratory outcomes included in the initial trial registration were not reported in the study and these outcomes were not of relevance to our review (Shapiro 2016).
Other potential sources of bias
We judged two studies at high risk of additional bias due to authors holding patents for either the intervention itself (Birrell 2009), or for patents relevant to the intervention (Cathcart‐Rake 2020). Birrell 2009 was published only in abstract/poster form and no further information could be obtained by author correspondence.
Eight studies were at unclear risk of additional sources of bias (Hershman 2015a; Liu 2014; Lustberg 2018; Massimino 2011; Peng 2018; Rosati 2011; Shapiro 2016; Shenouda 2019). Four of these studies were deemed to be unclear risk due to insufficient information to permit judgement (Liu 2014; Massimino 2011; Rosati 2011; Shenouda 2019). Two of these studies were considered at unclear risk of contamination, due to the unclear exposure to O3‐FA in the placebo arm (Hershman 2015a; Lustberg 2018). Two studies had run‐in designs, whereby participants were provided with vitamin D (Shapiro 2016) or calcium and vitamin D supplementation (Peng 2018) prior to randomisation. The authors in one study stated the study was designed to mimic the high prevalence of the use of supplemental vitamin D among women with AIMSS (Shapiro 2016). As it was possible that vitamin D had a role in altering AIMSS in women with low 25(OH)D (less than 30 ng/mL), and this may have enhanced or diminished the effect of subsequent randomised intervention, these studies were judged at unclear risk of other bias (Peng 2018; Shapiro 2016).
We considered the remaining seven studies at low risk of additional sources of bias, as the introduction of possible bias was thought to be adequately assessed through the primary domains of bias consideration (Chan 2017; Henry 2018; Khan 2017; Li 2017; Niravath 2019; Rastelli 2011; Sordi 2019).
Effects of interventions
Prevention of aromatase inhibitor‐induced musculoskeletal symptoms
See Table 1.
Pain (from baseline to the end of the intervention)
See Table 3; Analysis 1.1; Figure 4.
1. Prevention studies: pain.
| Study | Intervention vs control | Treatment duration | Intervention | Control | ||||
| Baseline pain, mean (SD) | Change from baseline, mean (SD) | n | Change from baseline, mean (SD) | n | Outcome measure (scale) | |||
| Khan 2017 | Vitamin D3 30,000 IU weekly vs placebo | 24 weeks | 2.39 (2.34) | 0.8 | 67 | 0.86 | 72 | BPI worst pain (0–10) |
| Lustberg 2018 | Omega‐3 fatty acids vs placebo containing mixture of fats and oils typical of USA diet | 24 weeks | 1.1 (1.55) | 0.11 (1.88*) | 22 | −0.70 (1.88*) | 22 | BPI severity (0–10) |
*Calculated from standard error (SE). SE of change scores in intervention arm 0.40; SE of change scores in control arm 0.41. BPI: Brief Pain Inventory; IU: international units; n: number of participants; SD: standard deviation.
1.1. Analysis.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 1: Pain
4.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 1: Pain
Two studies reported sufficient data (vitamin D3, Khan 2017; O3‐FA, Lustberg 2018) and showed no clinically meaningful difference in the change in mean pain scores between treatment arms (Analysis 1.1). A clinically meaningful change in BPI pain scales is considered to be two points (Mease 2011; Williams 2011).
PROs for pain included:
Categorical Pain Intensity Scale (CPIS; Khan 2017);
BPI (Khan 2017);
Brief Pain Inventory – Short Form (BPI‐SF; Lustberg 2018);
FACT‐ES pain subscale (Lustberg 2018).
Omega‐3 fatty acids
In Lustberg 2018, there was no clinically meaningful improvement in pain scores over the course of the study in either the O3‐FA arm or the control arm (Table 3). The change in pain scores between arms was MD 0.81 (95% CI −0.30 to 1.92; Analysis 1.1).
Vitamin D3
Author correspondence from Khan 2017 provided us with unpublished mean pain scores for BPI subscales. The difference in the change in mean pain scores between arms was MD −0.31 (95% CI −1.23 to 0.61; Analysis 1.1).
We rated the certainty of evidence for pain as very low due to concerns with risk of bias, including high risk of attrition and reporting bias in one study. One study only included women with 25‐OHD levels of 40 ng/mL or less, which resulted in concerns with indirectness, and the small sample sizes in the study raised concerns for imprecision. See Table 1.
Two other studies only reported the incidence of pain, rather than the mean pain scores for patients overall (Niravath 2019; Rosati 2011), despite Niravath 2019 utilising a VAS to record pain scores. We were unable to obtain the mean pain scores from the authors for this study.
Stiffness (from baseline to the end of the intervention)
None of the prevention studies investigated stiffness.
Grip strength
See Analysis 1.2.
1.2. Analysis.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 2: Grip strength
Vitamin D
Two studies investigated grip strength with the initiation of vitamin D plus an AI (Khan 2017; Niravath 2019); however, there were limited results for both studies, which prohibited these studies from being combined for meta‐analysis. Khan 2017 reported the incidence of worsening grip strength (−6.2 kg) of about 9% in the intervention and treatment arms (RR 1.08, 95% CI 0.37 to 3.17; 1 study, 147 participants; Analysis 1.2; low‐certainty evidence). The evidence was downgraded due to concerns with indirectness, as one study only included women with 25‐OHD levels of 40 ng/mL or less. The small sample size also raised concerns for imprecision. See Table 1.
Niravath 2019 tested participants' grip strengths at baseline, week 12 and week 52, but only reported the mean change in grip strength in the group who had shown symptoms of AIMSS (mean change −1.3 mmHg) compared to the group without symptoms of AIMSS (mean change −3.5 mmHg) (P = 0.37). It was not possible to incorporated this study in the summary of findings table for this outcome due to lack of information.
Safety
See Table 4.
2. Prevention: safety.
| Studies | Intervention vs control | Treatment duration | Intervention | Control | ||
| Safety reporting | n | Safety reporting | n | |||
| Khan 2017 | Vitamin D3 30,000 IU weekly vs placebo | 24 weeks | No AEs attributed to Vitamin D | 70 | 1 case of hypercalcaemia. No discussion of other AEs | 77 |
| Lustberg 2018 | Omega‐3 fatty acids vs placebo containing mixture of fats and oils typical of the American diet | 24 weeks | 1 grade 3 AE (diarrhoea) No grade 4/5 AEs |
22 | No grade 3/4/5 AEs |
22 |
| Niravath 2019 | Vitamin D3 50,000 IU weekly for 12 weeks then 2000 daily for 40 weeks vs 800 IU daily | 52 weeks | No grade 4/5 events 8 grade 3 events, deemed unrelated to study drugs |
44 | No grade 4/5 events 4 grade 3 events, deemed unrelated to study drugs |
43 |
| Rosati 2011 | Etoricoxib (COX‐2 inhibitor) 60 mg/day vs placebo | 2 years | FDA alert on COX‐2 inhibitors Study reported 0 serious AE |
16 | No report of safety data in control arm | 50 |
AE: adverse event; COX: cyclo‐oxygenase; FDA: Food and Drug Administration; IU: international unit; n: number of participants.
Four studies reported safety data (Khan 2017; Niravath 2019; Lustberg 2018; Rosati 2011). No study reported serious adverse events. Niravath 2019 included 87 participants and reported no grade four or five events. There were 12 grade three adverse events, with eight of these occurring in the high‐dose vitamin D arm, and the remaining four occurring in the standard‐dose vitamin D arm. All of these events were deemed unrelated to the study drugs. Grade three events included arthralgia, peripheral neuropathy, hypertension, hyperglycaemia and skin infection. Rosati 2011 reported "none of the patients in the treatment arm developed serious adverse event", but did not give any further details. During the course of the study, there was an FDA alert for COX‐2 inhibitors (FDA 2018), resulting in 38% of study participants withdrawing from the trial. Lustberg 2018 investigating O3‐FA reported a detailed list of adverse events. There was only one grade three event, which was in the intervention arm, due to diarrhoea. There were no grade four events.
The evidence for this outcome was very low certainty, due to risk of bias concerns, with high risk of bias in multiple studies, including one study with high risk of attrition bias following a significant number of participants dropping out following an FDA warning for the intervention (FDA 2018). Two other studies had high risk of attrition bias, and one study had high risk of both performance and detection bias. We downgraded for imprecision, due to small study samples, and also for indirectness due to one study only including women with 25‐OHD levels of 40 ng/mL or less. See Table 1.
Discontinuation of aromatase inhibitors
See Analysis 1.3.
1.3. Analysis.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 3: Discontinuation of aromatase inhibitors
Vitamin D
Two studies investigated discontinuation of AIs with vitamin D for AIMSS (Khan 2017; Niravath 2019). Khan 2017 reported 3/77 (4%) participants in the placebo arm versus 0/70 (0%) participants in the intervention arm (high‐dose vitamin D3 weekly) discontinued AI due to adverse events (RR 0.16, 95% CI 0.01 to 2.99; 1 study, 147 participants; Analysis 1.3; low‐certainty evidence). We downgraded the evidence due to concerns with indirectness, as one study only included women with 25‐OHD levels of 40 ng/mL or less. The small sample size raised concerns for imprecision. See Table 1.
Niravath 2019 attempted to investigate patient compliance with AI therapy using tablet counting, but only had data available for 14/93 (13%) randomised participants. Of the available data, the participants in the standard‐dose vitamin D arm had taken 96.5% of their AI therapy, and the participants in the high‐dose vitamin D arm had taken 98.1% of their AI therapy. As this study did not provide data on the number of participants who discontinued AI, it was not included in the summary of findings table for this outcome.
The other two prevention studies did not investigate adherence (Lustberg 2018; Rosati 2011).
Breast cancer‐specific quality of life
See Analysis 1.5; Table 5.
1.5. Analysis.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 5: Breast cancer‐specific quality of life
3. Prevention: breast cancer‐specific quality of life (BCS‐QoL).
| Study | Intervention vs control | Treatment duration | Intervention | Control | MD (95% CI) |
Outcome measure (scale) |
|||
| Baseline pain, mean (SD) | Change from baseline to end of intervention, mean (SD) | n | Change from baseline to end of intervention, mean (SE) | n | |||||
| Lustberg 2018 | Omega‐3 fatty acids vs placebo containing mixture of fats and oils typical of the USA diet | 24 weeks | 119 (12.66) |
2.5 (10.79) | 22 | 0.95 (10.79) | 22 | 1.55 (−4.83 to 7.93) | FACT‐B (0–148) |
SD calculated from SE. Baseline intervention arm SE = 2.7; change score SE = 2.3. FACT‐B: Functional Assessment of Cancer Therapy – Breast; MD: mean difference; n: number of participants; SD: standard deviation; SE: standard error.
Two studies investigated BCS‐QoL (vitamin D3, Khan 2017; O3‐FA, Lustberg 2018), but one study did not report values for this outcome (Khan 2017). In Lustberg 2018, the MD total FACT‐B change scores from baseline until the end of intervention between arms was 1.55 (95% CI −4.83 to 7.93; 1 study, 44 participants; Analysis 1.5; low‐certainty evidence). For the FACT tools, a high score is equivalent to a higher quality of life. There was also no clinically meaningful improvement in mean scores in either group from baseline until the end of the intervention (Table 5). The MCID for FACT‐B total score is 7 to 8 points (Eton 2004). We downgraded the evidence due to concern with risk of bias, high risk of attrition and reporting bias in one study, and concern with imprecision due to small study sample. See Table 1.
The other studies did not collect BCS‐QoL data (Niravath 2019; Rosati 2011).
Health‐related quality of life
See Analysis 1.6; Table 6.
1.6. Analysis.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 6: Health‐related quality of life (HRQoL)
4. Prevention: health‐related quality of life (HRQoL).
| Study | Intervention vs control | Treatment duration | FACT‐G subscale | Intervention | Control | Difference in change scores between arms, MD (95% CI) |
Outcome measure (scale) |
|||
| Baseline pain, mean (SD) | Change from baseline to end of intervention, mean (SD) | n | Change from baseline to end of intervention, mean (SD) | n | ||||||
| Lustberg 2018 | Omega‐3 fatty acids vs placebo containing mixture of fats and oils typical of the American diet | 24 weeks | FACT‐G overall score | 92 (10.32) | 1.9 (7.97) | 22 | 0.047 (8.44) | 22 | 1.85 (−3.00 to 6.70) | FACT‐G overall score (0–108) |
| Physical Well‐being | 25 (2.77) | −0.027 (3.24) | 22 | 0.015 (3.33) | 22 | −0.04 (−1.98 to 1.90) | FACT‐G physical well‐being subscale (0–28) |
|||
| Social Well‐being | 24 (4.5) | 0.39 (5.16) | 22 | −0.96 (5.16) | 22 | 1.35 (−1.70 to 4.40) | FACT‐G social well‐being subscale (0–28) |
|||
| Emotional Well‐being | 20 (2.53) | 0.76 (2.81) | 22 | 0.36 (2.86) | 22 | 0.40 (−1.28 to 2.08) | FACT‐G emotional well‐being subscale (0–24) |
|||
| Functional Well‐being | 23 (5.16) | 1.1 (3.19) | 22 | 0.72 (3.28) | 22 | 0.38 (−1.53 to 2.29) | FACT‐G functional well‐being subscale (0–28) |
|||
SD calculated from standard error (SE). CI: confidence interval; FACT‐B: Functional Assessment of Cancer Therapy – Breast; FACT‐G: Functional Assessment of Cancer Therapy – General (higher scores equate to better quality of life); SD: standard deviation.
Two studies investigated HRQoL (vitamin D, Khan 2017; O3‐FA, Lustberg 2018), but one study did not report values for this outcome (Khan 2017). In Lustberg 2018, the MD in FACT‐G overall scores from baseline until the end of intervention between arms was 1.85 (95% CI −3.00 to 6.70; 1 study, 44 participants; Analysis 1.6; low‐certainty evidence). The MCID for a FACT‐G overall score is 5 to 6 points (Eton 2004). We downgraded the evidence due to concern with risk of bias, high risk of attrition and reporting bias in one study, and concern with imprecision due to small study sample. See Table 1.
The other studies did not collect data on overall HR‐QoL (Niravath 2019; Rosati 2011).
Incidence of aromatase inhibitor‐induced musculoskeletal symptoms
See Analysis 1.4; Table 7.
1.4. Analysis.

Comparison 1: Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 4: Incidence of AIMSS
5. Prevention: incidence of aromatase inhibitor‐induced musculoskeletal symptoms.
| Study | Intervention vs control | Treatment duration | Intervention | Control | ||
| Incidence of AIMSS (%) | n | Incidence of AIMSS (%) | n | |||
| Khan 2017 | Vitamin D3 30,000 IU weekly vs placebo | 24 weeks | (37%) | 70 | (51%) | 77 |
| Niravath 2019 | Vitamin D3 50,000 IU weekly for 12 weeks then 2000 IU daily for 40 weeks vs 800 IU daily | 52 weeks | 25 (54%) | 47 | 27 (57%) | 46 |
| Rosati 2011 | Etoricoxib 60 mg/day vs placebo | 2 years | (31%) | 16 | (76%) | 50 |
AIMSS: aromatase inhibitor‐induced musculoskeletal symptoms; IU: international units; n: number of participants.
Three studies reported the incidence of AIMSS (Khan 2017; Niravath 2019; Rosati 2011; Table 7). Two of these studies, which both investigated the use of high‐dose vitamin D in the prevention of AIMSS, were combined for meta‐analysis (Khan 2017; Niravath 2019). The third study was not included in the meta‐analysis as it investigated a different systemic therapy, etoricoxib (Rosati 2011).
Vitamin D
The RR for the incidence of AIMSS between the vitamin D and control arms was 0.82 (95% CI 0.63 to 1.06; I2 = 0%; 2 studies, 240 participants; Analysis 1.4; very low‐certainty evidence). There was no statistical heterogeneity among the studies involved in the meta‐analysis. We downgraded the certainty of the evidence due to high risk of performance bias, detection bias and attrition bias. We downgraded for indirectness, due to one study only including women with 25‐OHD levels of 40 ng/mL or less, and downgraded for imprecision due to small study samples. See Table 1.
Etoricoxib
Rosati 2011 used the investigator‐reported outcome of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v3.0 to record musculoskeletal pain incidence (Table 7). Limited information is available for this trial as it has not progressed to the publication of a manuscript. There were no details regarding the breakdown of severity of pain experienced by participants in each arm. The study had a high risk of attrition bias due to a high number of participants withdrawing from the study due to a US Food and Drug Administration (FDA) alert on COX‐2 inhibitors (FDA 2018). There was insufficient data on the time point of the data collection for musculoskeletal symptoms.
Breast cancer‐specific survival and overall survival
None of the prevention studies reported BCSS or OS. One study investigating etoricoxib collected data on survival in the form of EFS (Rosati 2011), but the definition of EFS was unclear as the study was only presented at a conference without pursuing full publication. Rosati 2011 reported five‐year EFS of 83% in the etoricoxib arm versus 71% in the placebo arm (hazard ratio 1.9, 95% CI 1.03 to 3.59; P = 0.03). This was after nearly 40% of participants (37/93 in the intervention arm versus 33/89 in the control arm) had dropped out of the study due to an FDA alert regarding COX‐2 inhibitor safety concerns (FDA 2018). Due to the lack of information from this study, this outcome was not presented in the summary of findings table.
Treatment of aromatase inhibitor‐induced musculoskeletal symptoms
See Table 2.
Pain (from baseline to end of intervention due to aromatase inhibitor‐induced musculoskeletal symptoms)
See Analysis 2.1; Table 8.
2.1. Analysis.

Comparison 2: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 1: Pain
6. Treatment: pain.
| Studies | Intervention vs control | Treatment duration | Intervention | Control | Difference in change scores between arm, mean |
Outcome measure (scale) |
|||
| Baseline pain, mean (SD) | Change from baseline, Mean (SD) | n | Change from baseline, mean (SD) | n | |||||
| Cathcart‐Rake 2020 | Testosterone: 120 mg subcutaneous pellets, changed to topical daily gel due to slow accrual vs placebo | 6 months | 5.4 (1.7) | −1.9 (2.2) | 80 | −2.2 (2.7) | 77 | 0.3 | BPI‐AIA mean pain (0–10) |
| Shapiro 2016 | Vitamin D3 4000 IU daily vs 600 IU daily | 6 months | 10.8 (3) | −1.2 (3.8) | 39 | −0.6 (3.8) | 36 | −0.6 | WOMAC pain (0–20) |
| Hershman 2015a | Omega‐3 fatty acids 3.3 g daily vs soybean/cornoil placebo | 24 weeks | 7.06 (1.53a) | −2.23 | 94 | −1.81 | 98 | −0.42 | BPI worst pain (0–10) |
| Peng 2018 | Yi Shen Jian Gu granules twice daily vs placebo | 12 weeks | 6.18 (1.58) | −3.10 | 40 | −1.65 | 37 | −1.47 | BPI‐SF worst pain (0–10) |
| Chan 2017 | Emu oil topically 3 times daily vs placebo | 8 weeks | N/A | −0.82 (1.9a) | 36 | −0.93 (1.7a) | 37 | 0.11 | BPI severity (0–10) |
| Sordi 2019 | Cat's claw (Uncaria tomentosa) 3 times daily vs placebo |
30 days | 8 (2.1) | 0 | 32 | −3 | 29 | 3 | VAS (0–10) |
| Li 2017 | Bionic tiger bone capsules 3 times daily vs calcium carbonate 600 mg daily | 12 weeks | 6.59 (2.11) | −2.72 | 35 | 0.87 | 35 | −3.59 | BPI worst pain (0–10) |
| Henry 2018 | Duloxetine 30 mg daily for 1 week then 60 mg daily for 11 weeks vs placebo | 12 weeks | 5.53 (N/A) | −2.6 | 127 | −1.97 | 128 | −0.63 | BPI mean pain (0–10) |
| Rastelli 2011 | Vitamin D2: either 50,000 IU weekly for 8 weeks if vitamin D insufficiency then monthly; or 50,000 IU weekly for 16 weeks if vitamin D deficiency then monthly; or control, 400 IU daily | 6 months | 5.2 (2.4) | −1.5 | 28 | −0.9 | 29 | −0.4 | BPI worst pain (0–10) |
| Liu 2014 | Calcitonin 200 IU + 600 mg daily caltrate D vs 600 mg daily caltrate D | 3 months | 5.0 (0.74) | −3.00 (1.48) | 42 | −1.00 (1.11) | 40 | −2 | VAS scale (0–10) |
Where SD is not available, this was due to lack of change score SD reported in the study. For these studies, end of treatment MD were used in the analysis. Rastelli 2011: 2 months of BPI data used to eliminate differences between dosing in Stratum A and B. Liu 2014: although study reported mean (SD) being utilised, data more consistent with median and interquartile ranges. Data were treated as such. aSD calculated from provided confidence intervals. BPI: Brief Pain Inventory; BPI‐AIA: Brief Pain Inventory for Aromatase Inhibitor Arthralgia; BPI‐SF: Brief Pain Inventory – Short Form; IU: international unit; MD: mean difference; n: number of participants; N/A: not available; SD: standard deviation; VAS: Visual Analogue Scale.
Thirteen studies investigated the treatment of AIMSS (Birrell 2009; Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Li 2017; Liu 2014; Massimino 2011; Peng 2018; Rastelli 2011; Shapiro 2016; Shenouda 2019; Sordi 2019). A summary of the pain outcomes and tools used to measure pain is shown in Table 8. Three studies were not included in this table or in the assessment of this outcome due to lack of information, as each study was only published as an abstract (Birrell 2009; Massimino 2011; Shenouda 2019). The authors were either unable to be contacted or unable to provide further information. None of the studies investigating pain could be combined for meta‐analysis due either to the wide‐ranging interventions among studies, or because for the testosterone studies (Birrell 2009; Cathcart‐Rake 2020), there was insufficient information from the Birrell 2009 to allow meta‐analysis.
PROs for pain included:
Modified Brief Pain Inventory for Aromatase Inhibitor Arthralgia (BPI‐AIA; Cathcart‐Rake 2020);
BPI (Chan 2017; Henry 2018; Sordi 2019);
BPI‐SF (Hershman 2015a; Li 2017; Peng 2018; Rastelli 2011);
Fibromyalgia Impact Questionnaire (FIQ; Rastelli 2011);
Health Assessment Questionnaire – Disability Index (HAQ‐DI; Rastelli 2011);
WOMAC (Henry 2018; Hershman 2015a; Peng 2018);
Global Rating of Change Scale (GRCS; Henry 2018; Hershman 2015a);
Breast Cancer Prevention Trial Symptom Scales – Musculoskeletal subscale (BCPT‐MS; Shapiro 2016);
Australian/Canadian osteoarthritis hand index version 3.1 (AUSCAN; Shapiro 2016);
WOMAC version 3.1 (WOMAC; Henry 2018; Hershman 2015a; Shapiro 2016);
M‐SACRAH (Henry 2018; Hershman 2015a; Peng 2018); and
VAS (Birrell 2009; Chan 2017; Li 2017; Liu 2014; Massimino 2011; Shenouda 2019; Sordi 2019).
Testosterone
Two studies investigated testosterone (Birrell 2009; Cathcart‐Rake 2020). In Cathcart‐Rake 2020, the MD between arms for the change in mean joint pain from baseline to the end of intervention was 0.30 (95% CI −0.47 to 1.07; Analysis 2.1; very low‐certainty evidence). There were limited data available from Birrell 2009, which was published as an abstract only; the study reported decreased VAS scores of 35% in the placebo arm compared with a decrease of 43% (P = 0.06) with testosterone undecanoate 40 mg daily and 70% (P = 0.04) with testosterone undecanoate 80 mg daily. Due to the lack of data, testosterone study results were not combined for meta‐analysis, and the study by Birrell 2009 was not included in the Table 2 for this outcome.
Vitamin D
Two studies investigated vitamin D (Rastelli 2011; Shapiro 2016). Rastelli 2011 used high‐dose vitamin D2 supplementation, and the difference in the change in pain scores from baseline to two months between arms was MD −1.50 (95% CI −2.59 to −0.41; P = 0.041; Analysis 2.1; very low‐certainty evidence; Table 8). Shapiro 2016 investigated the use of high‐dose (4000 IU) vitamin D3 supplementation versus a usual‐dose (600 IU) vitamin D3. Using the WOMAC v3.1 tool, which is commonly used to investigate symptoms of arthritis in the lower limbs, the MD in pain scores from baseline to the end of the intervention between arms was −0.60 (95% CI −2.32 to 1.12; Analysis 2.1; very low‐certainty evidence; Table 8). There were similar results using the AUSCAN tool, which was developed to investigate osteoarthritis of the hand with the change in mean pain scores between baseline and the end‐of‐intervention in the control group of −0.2 (SD 3.3) compared to −0.9 (SD 3.3) in the intervention group (P = 0.21). These two vitamin D studies were not combined for meta‐analysis, due to the differing interventions and patient populations, with one study only including women with vitamin D deficiency and then stratifying women into different treatment doses based on their baseline vitamin D result (Rastelli 2011), and the other comparing a high‐ and low‐dose vitamin D supplementation in a patient population that was not selected based on baseline vitamin D levels (Shapiro 2016).
Duloxetine
One study investigated duloxetine to treat AIMSS pain (Henry 2018). Using the data we had available, including baseline SDs only, we calculated the MD between groups at the end of treatment (12 weeks) using BPI mean pain as −0.63 (95% CI −0.97 to −0.29; Analysis 2.1; very low‐certainty evidence; Table 8). This was similar to the effect reported by the study in a supplemental table for outcomes at 12 weeks of therapy (MD −0.56, 95% CI −1.11 to −0.01) using a multivariate linear regression including covariates for baseline mean pain and prior taxane use. The study's statistical plan was powered to test a difference by arm at 12 weeks only. A clinically significant change in mean pain in this study was defined as a decrease from baseline of at least two points, which is consistent with the literature (Mease 2011; Williams 2011). A decrease in pain scores of at least two points was seen in a higher proportion of participants in the duloxetine arm at six weeks (68% with duloxetine versus 49% with placebo; P = 0.003); however, this benefit was not sustained by the end of study intervention period at 12 weeks (68% with duloxetine versus 59% with placebo; P = 0.18). Change in pain score subscales were not provided for the WOMAC tool.
Omega‐3 fatty acids
One study on O3‐FA which gave the control group a placebo capsule made of soybean and corn oil, measured pain with the BPI‐SF and GRCS (Hershman 2015a). The difference in pain change scores between the two groups from baseline to the end of intervention was MD −0.24 (95% CI −1.0 to −0.52; Analysis 2.1; very low‐certainty evidence; Table 8). The study authors reported similar change in MD between arms at the end of intervention using a linear regression adjusting for baseline BPI score and stratification factors (MD −0.36, 95% CI −1.08 to 0.38).
Bionic tiger bone
One study investigated the use of bionic tiger bone capsules versus placebo (calcium carbonate tablets 600 mg) for 12 weeks (Li 2017). There was a reduction in mean pain scores between arms measured by BPI worst pain (MD −4.09, 95% CI −5.25 to −2.93; 70 participants; Analysis 2.1; very low‐certainty evidence; Table 8). This is consistent with an MCID for BPI pain in this study (Mease 2011; Williams 2011). There were similar results for the change in mean VAS scores at 12 weeks (−3.31 with bionic tiger bone vs 0.21 with placebo; P < 0.001) and BPI mean pain scores at 12 weeks (−2.62 with bionic tiger bone vs 0.75 with placebo; P < 0.001). This was listed as a blinded study, yet the intervention group was given bionic tiger bone capsules three times a day and the control group was given calcium carbonate tablets once daily.
Yi Shen Jian Gu
One study examined the use of YSJG granules (Peng 2018). Worst pain mean scores decreased between arms, with the CIs including the MCID for a BPI scale (MD −1.34, 95% CI −2.10 to −0.58; Analysis 2.1; very low‐certainty evidence; Table 8). Pain severity and pain‐related interference scores also decreased at 12 weeks (−2.41 with YSJG versus −1.22 with placebo; P = 0.003) and 24 weeks (−2.6 with YSJG versus −1.34 with placebo; P = 0.001). The decrease in the mean pain score in the intervention arm remained clinically meaningful at 24‐week follow‐up, even though the duration of intervention was only 12 weeks. Pain scores from both the WOMAC and M‐SACRAH pain subscales reflected similar findings.
Emu oil
One study investigated the use of emu oil drops applied topically to affected joints versus a placebo oil (Chan 2017). Between baseline and eight weeks of treatment, the MD in BPI severity scores between arms was 0.11 (95% CI −0.72 to 0.94; P = 0.78; Analysis 2.1; very low‐certainty evidence; Table 8). There were similar results with the use of the VAS tool. VAS scores improved by −1.46 in the control arm (95% CI −2.12 to −0.81) compared to −−1.09 in the emu oil arm (95% CI −1.83 to −0.35) (P = 0.45).
Calcitonin
One study investigated the use of calcitonin (Liu 2014). Although the study authors documented the reporting of mean and SD for results, the results were more consistent with median and IQR. The study reported a change in VAS scores of −1.00 (IQR (probably) −1.50 to 0.00) in the control group compared to a change in VAS score of −3.00 (IQR (probably) −4.00 to −2.00) in the calcitonin arm (P < 0.001). Assuming that the data were indeed reported using median/IQR, our analysis showed a difference between groups at the end of the intervention of MD −2.0 (95% CI −2.56 to −1.44; Analysis 2.1; very low‐certainty evidence). The Cochrane Handbook for Systematic Reviews of Interventions warns that the reporting of median values and IQR is often associated with skewed data (Higgins 2021).
Cat's claw
One study investigated the use of cat's claw (Uncaria tomentosa) for four weeks versus placebo (Sordi 2019). The change in mean VAS score in the control arm was −3 compared to no change in the intervention arm (MD 3.00, 95% CI 1.66 to 4.34; P = 0.02), which means that the placebo arm experienced an improvement in pain scores measured with the VAS tool and the intervention arm experienced no change in pain scores. This study also reported BPI severity scores; however, the baseline mean pain score in the control arm had an SD of 8.3 and the intervention arm end‐of‐treatment pain score had an SD of 7.3, using a pain scale of 0 to 10 with the BPI severity tool, as confirmed in the BPI user guide (Cleeland 2009). These SDs were not plausible for this pain scale, therefore the BPI data were not used. Further clarification could not be obtained from the study author.
Three studies had not been published as a manuscript and there was only limited information available (Birrell 2009; Massimino 2011; Shenouda 2019). We were unable to obtain further information from study authors and, therefore, these studies were not included in Table 2 for pain.
Overall, we were unable to determine the effect of systemic therapies on the treatment of pain from AIMSS, because we rated the evidence as very uncertain due to a number of concerns. There were concerns with risk of bias in multiple studies, the sample sizes of studies were small with serious concerns of imprecision, and significant heterogeneity in interventions used in these studies, therefore, concerns regarding inconsistency. A funnel plot showed an asymmetrical scatter of small studies with more studies showing a positive result than those showing a negative result (Figure 5), which raises suspicion of publication bias. In an extensive literature search, there were multiple studies in this outcome which have only been published in abstract form, without pursuing full/peer‐reviewed publication. See Table 2.
5.

Funnel plot of comparison: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 2.1 Pain.
Stiffness (from baseline to end of intervention)
See Analysis 2.2; Table 9.
2.2. Analysis.

Comparison 2: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 2: Stiffness
7. Treatment: stiffness.
| Study | Intervention vs control | Treatment duration | Intervention | Control | Difference in change scores between arms, MD (95% CI) |
Outcome measure (scale) |
|||
| Baseline pain, mean (SD) | Change from baseline to end of intervention, mean (SD) | n | Change from baseline to end of intervention, mean (SD) | n | |||||
| Shapiro 2016 | Vitamin D3 4000 IU daily vs 600 IU daily | 6 months | 5.5 (1.5) | −0.5 (1.7) | 39 | −0.5 (2.0) | 36 | 0 (−0.82 to 0.82) | WOMAC (0–8) |
| Hershman 2015a | Omega‐3 fatty acids 3.3 g daily vs soybean/cornoil placebo | 24 weeks | N/Aa | 0.59 (1.25)b | 109 | 0.46 (1.25)b | 114 | 0.13 (−0.20 to 0.46) | GRCS at 6 weeks |
| Peng 2018 | Yi Shen Jian Gu granules twice daily vs placebo | 12 weeks | 70.43 (43.49) | −37.53 | 40 | −20.82 | 37 | −16.71 | WOMAC (0–200) |
| Li 2017 | Bionic tiger bone capsules 3 times daily vs calcium carbonate 600 mg daily | 12 weeks | 5.49 (1.58) | −3.08 | 35 | 1.87 | 35 | −4.95 | Modified BPI with stiffness (0–10) |
| Henry 2018 | Duloxetine 30 mg daily for 1 week then 60 mg daily for 11 weeks | 12 weeks | N/Aa | — | — | — | — | OR 3.38 (1.85 to 6.18; P = 0.0001)c | — |
| Chan 2017 | Emu oil topically 3 times daily vs placebo | 8 weeks | 1.9 (0.67) | −0.31 (0.82) | 36 | −0.41 (0.82) | 37 | 0.10 (−0.28 to 0.48) | VAS scale (0–3) |
a Evaluates changes in symptoms since last visit, so no baseline scores were collected.
bSome standard deviations (SD) not available, due to lack of reported change score SD. For these studies, end of treatment means (SD) were used in the analysis of effect size. See Analysis 2.2. cReported as a binary outcome, OR. Henry 2018; Hershman 2015a could not be assessed for the effect of stiffness on the treatment of AIMSS, including confidence intervals and, therefore, were not included in the final assessment of this outcome. BPI: Brief Pain Inventory; CI: confidence interval; GRCS: Global Rating of Change Scale; IU: international unit; MD: mean difference; n: number of participants; N/A: not available/applicable; OR: odds ratio; SD: standard deviation; VAS: Visual Analogue Scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis scale.
Seven studies investigating the treatment of AIMSS reported stiffness (Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Li 2017; Peng 2018; Shapiro 2016), but none could be combined for meta‐analysis due to vastly differing interventions (Table 9). Cathcart‐Rake 2020 included BPI‐AIA which has a question on stiffness, but did not publish these data. They did report that "testosterone supplementation did not improve the average pain or joint stiffness, compared with placebo". As the CIs could not be assessed, this study was not included in the assessment for this outcome in Table 2.
PROs for stiffness in the treatment studies included:
WOMAC (Henry 2018; Hershman 2015a; Peng 2018; Shapiro 2016);
AUSCAN (Shapiro 2016);
GRCS for Stiffness (Henry 2018; Hershman 2015a);
BPI Stiffness subscale (Li 2017);
BPI‐AIA (Cathcart‐Rake 2020);
M‐SACRAH (Henry 2018; Hershman 2015a; Peng 2018);
VAS (Chan 2017).
Vitamin D
In Shapiro 2016, using the WOMAC tool, there was no difference in change scores between arms from baseline until the end of the six months' intervention (MD 0.00, 95% CI −0.84 to 0.84; Analysis 2.2; very low‐certainty evidence; Table 9). Results were similar using the AUSCAN tool, which investigates arthritic symptoms of the hand, with a change in stiffness scores from baseline to the end of intervention in the control group of −0.1 (SD 1.0) compared to −0.1 (SD 0.9) in the vitamin D group (P = 0.91).
Duloxetine
Henry 2018 used the GRCS for Stiffness and due to the nature of the tool, the effect of stiffness from baseline until the end of the intervention could not be measured. The GRCS for Stiffness requires a patient to recall their symptoms at a previous time point and compare this with their current symptoms, therefore no baseline recording is required (Kamper 2009). The study reported an improvement in stiffness scores with an odds ratio of 3.38 (95% CI 1.85 to 6.18; P = 0.0001) at two weeks. It is not clear what the criteria for 'improvement in GRCS for stiffness' were for the calculation of this as a binary outcome, and if this was consistent with the literature (Kamper 2009). Because this study could not be assessed for its effect on stiffness from baseline until the end of intervention, it was not included in Table 2.
Omega‐3 fatty acids
Hershman 2015a used the GRCS for Stiffness and recorded data at week six, week 12 and week 24. The study reported between‐group MDs at six weeks of 0.13 (95% CI −0.20 to 0.46; P = 0.44); at 12 weeks of 0.28 (95% CI −0.07 to 0.62; P = 0.12); and at 24 weeks of −0.003 (95% CI −0.41 to 0.41; P = 0.99). Stiffness was also measured using WOMAC and M‐SACRAH tools, but subscales were not provided. As this study could not be assessed for its effect on stiffness from baseline until the end of intervention, it was not included in Table 2.
Bionic tiger bone
Using the modified BPI scale, Li 2017 reported an MD in stiffness scores of −4.53 (95% CI −5.69 to −3.37; Analysis 2.2; very low‐certainty evidence; Table 9). It is unclear what the MCID would be for this modified BPI with stiffness tool, but it is likely that the 95% CIs for this treatment effect includes a clinically important benefit.
Yi Shen Jian Gu
Using the WOMAC stiffness subscale, Peng 2018 reported an MD in stiffness scores between arms of −28.82 (95% CI −47.13 to −10.51; Analysis 2.2; very low‐certainty evidence; Table 9). This was consistent with findings from the M‐SACRAH subscale, showing a change in mean scores of −47.28 in the treatment arm at 12 weeks, compared to −31.75 in the control arm (P = 0.011). The change in mean stiffness scores between treatment arms persisted at the 24‐week follow‐up. There has been wide‐ranging estimates of the MCID for stiffness using the WOMAC tool, from 12.9 to 25 (Maredupaka 2020), with the results of this study showing a clinically meaningful benefit in stiffness.
Emu oil
Chan 2017 used a VAS scale measuring 0 to 3 points. The results were not published, but we obtained data via author correspondence. The MD in stiffness scores between arms was 0.10 (95% CI −0.28 to 0.48; Analysis 2.2; very low‐certainty evidence; Table 9).
Overall, we rated the evidence for this outcome of stiffness as very low certainty due to concern with risk of bias; indirectness as one study had poorly defined criteria for AIMSS which means that participants may have experienced musculoskeletal symptoms from causes other than AIMSS; inconsistency due to heterogeneity in interventions used between studies; and imprecision due to the small sample sizes and wide CIs. See Table 2.
Grip strength
See Analysis 2.3.
2.3. Analysis.

Comparison 2: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 3: Grip strength
One study investigated the effect of vitamin D on grip strength (Shapiro 2016). The study reported no difference in the mean change in grip strength between the control and intervention arms over the course of the study using a Jamar hydraulic hand dynamometer (MD 0.80, 95% CI −2.69 to 4.29; P = 0.3; Analysis 2.3; low‐certainty evidence). An MCID for grip strength is estimated to be 5 kg to 6.5 kg (Bohannon 2019). We downgraded the certainty of the evidence due to imprecision and indirectness as the single study included women experiencing musculoskeletal symptoms regardless of temporal association with the start of the AI, which may have resulted in women without AIMSS being enrolled. See Table 2.
Safety
Eleven studies reported safety data (Birrell 2009; Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Li 2017; Liu 2014; Peng 2018; Rastelli 2011; Shapiro 2016; Sordi 2019). Shenouda 2019 and Massimino 2011 did not report safety data. Overall, there were no grade four or five adverse events in any of the treatment studies.
Testosterone
Cathcart‐Rake 2020 reported "no significant differences" between treatment arms in specific symptoms associated with testosterone toxicity as assessed on the Symptom Experience Diary, but incidence and P values were not provided. The testosterone study by Birrell 2009 is published in abstract only; however, they stated "no significant androgenic side effects (hirsutism, alopecia and acne) were observed and serum lipids remained unaltered". No values were given.
Vitamin D
In Shapiro 2016, the most frequent adverse events were musculoskeletal symptoms (18%) and gastrointestinal symptoms (17%) and the authors reported that these symptoms "did not differ in incidence between arms". No P values were given. In Rastelli 2011, they reported no toxicities in the vitamin D group. However, at two months, four participants in the high‐dose vitamin D group and one participant in the placebo group were removed from the study due to asymptomatic hypercalciuria.
Duloxetine
Henry 2018 reported no grade four or five adverse events. Grade three adverse events occurred in 12 (9%) participants in the duloxetine arm versus five (4%) participants in the placebo arm (P = 0.08). Adverse events of any grade occurred in 78% of the duloxetine group versus 50% of the placebo group (P < 0.001). The most common adverse events in the duloxetine arm were fatigue, nausea, dry mouth and headache. The toxicity profile of duloxetine may have unmasked the blinding of participants in this study, as more participants in the duloxetine arm compared with the placebo arm thought they were receiving duloxetine (79% with duloxetine versus 50% with placebo; P < 0.001).
Omega‐3 fatty acids
Hershman 2015a reported no grade four or grade five adverse events. With regard to grade three adverse events, there was one case each of diarrhoea, dyspepsia and pain in the extremity in the O3‐FA arm and one case each of arthralgia, pain, peripheral motor neuropathy and rash in the placebo arm.
Bionic tiger bone
In Li 2017, the only adverse event reported was 'stomach discomfort' in six participants overall (two in the treatment arm, four in the control arm). The grade of this adverse event was not listed. The study stated there were no other adverse events.
Yi Shen Jian Gu
Peng 2018 reported that 33% of participants in the intervention arm experienced all‐grade toxicities compared with 39% of participants in the placebo arm (P = 0.589). One participant in the intervention arm experienced an increase in CA‐125 levels during the study (27.4 U/mL at baseline versus 40.78 U/mL). Follicle‐stimulating hormone and oestradiol levels remained in the postmenopausal range throughout the study.
Emu oil
Chan 2017 reported no treatment‐emergent adverse events associated with emu oil and no serious adverse events in the total patient population.
Calcitonin
In Liu 2014, there was limited safety data. The study authors reported no difference in biochemistry parameters between the treatment and control arms over the course of the study (including serum calcium, P = 0.8758; serum phosphorus, P = 0.5128; serum osteocalcin, P = 0.9224; and alkaline phosphatase, P = 0.6528), but did not discuss other adverse events.
Cat's claw
In Sordi 2019, there were no grade three or grade four adverse events reported. Two participants in the experimental arm and three participants in the placebo arm discontinued treatment because of adverse events, but the adverse events were not detailed further.
We downgraded the certainty of the evidence for this outcome to very low, due to concerns with risk of bias in multiple studies, imprecision due to small sample sizes, and publication bias as multiple studies were published in abstract form only without pursuing full publication. See Table 2.
Persistence and adherence to aromatase inhibitor medication
None of the treatment studies reported persistence or adherence to AI medication.
Breast cancer‐specific quality of life
See Analysis 2.4; Table 10.
2.4. Analysis.

Comparison 2: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 4: Breast cancer‐specific quality of life
8. Treatment: breast cancer‐specific quality of life (BCS‐QoL).
| Studies | Intervention vs control | Treatment duration |
FACT‐B subscale (scale) |
Intervention | Control | Difference in change scores between arms, MD | |||
| Baseline BCS‐QoL, mean (SD) | Change from baseline, mean (SD) | n | Change from baseline, mean (SD) | n | |||||
| Peng 2018 | Yi Shen Jian Gu granules twice daily vs placebo | 12 weeks | Physical Well‐being (0–28) | 18.85 (4.53) | 4.77 | 40 | 3.41 | 37 | 2.46 |
| Social/Family Well‐being (0–24) | 16.95 (4.62) | 3.1 | — | 2.54 | — | 2.27 | |||
| Emotional Well‐being (0–24) | 21.78 (4.31) | 21.78 | — | 1.40 | — | 1.54 | |||
| Functional Well‐being (0–28) | 17.15 (4.42) | 4.26 | — | 3 | — | 4.27 | |||
| Additional Concerns (0–36) | 23.58 (4.93) | 2.93 | — | 1.84 | — | 2.51 | |||
| Li 2017 | Bionic tiger bone capsules 3 times daily vs calcium carbonate 600 mg daily | 12 weeks | Physical Well‐being (0–28) | 19.23 (5.02) | 3 | 35 | −0.1 | 35 | 2.30 |
| Social/Family Well‐being (0–24) | 19.81 (3.98) | 0.93 | — | −0.97 | — | 0.90 | |||
| Emotional Well‐being (0–24) | 15.34 (4.41) | 0.49 | — | 0.12 | — | 0.81 | |||
| Functional Well‐being (0–28) | 15.26 (4.67) | 0.71 | — | −0.09 | — | 0.10 | |||
| Additional Concerns (0–36) | 26.73 (4.61) | 0.73 | — | 0.75 | — | −0.40 | |||
Where SD are not available for change scores, end of treatment means (SD) were used in the analysis, see Analysis 2.4. In FACT scales, higher scores equate to better quality of life. BCS‐QoL: breast cancer‐specific quality of life; FACT‐B: Functional Assessment of Cancer Therapy – Breast; n: number of participants; SD: standard deviation.
Two studies investigated quality of life using FACT‐B (Li 2017; Peng 2018). These studies could not be combined for meta‐analysis due to using different interventions (Table 10).
Bionic tiger bone
In Li 2017, the Physical Well‐being subscale showed an MD between arms of 2.30 (95% CI 0.05 to 4.55; Analysis 2.4; very low‐certainty evidence; Table 10). For all the other FACT‐B subscales, the effect sizes between groups reported CIs that crossed zero, but all had CIs that included a potentially clinically meaningful benefit according to the MCID for FACT‐B (Social/Family Well‐being MD 0.90, 95% CI −1.37 to 3.17; Emotional Well‐being MD 0.81, 95% CI −1.66 to 3.28; Functional Well‐being MD 0.10, 95% CI −2.36 to 2.56; Additional Concerns MD −0.40, 95% CI −2.88 to 2.08; Analysis 2.4; very low‐certainty evidence). The MCID estimate for the overall FACT‐B tool is 7 to 8 points, and FACT‐B subscales is 2 to 3 points (Eton 2004; Yost 2014). For the FACT‐B tool, increased scores equate to better quality of life.
Yi Shen Jian Gu
In Peng 2018, all the FACT‐B subscales showed effect size between arms at the end of the intervention with CIs that included the MCID for FACT‐B subscales (Physical Well‐being MD 2.46, 95% CI 0.73 to 4.19; Social/Family Well‐being MD 2.27, 95% CI 0.07 to 4.47; Emotional Well‐being MD 1.54, 95% CI 0.06 to 3.02; Functional Well‐being MD 4.27, 95% CI 2.54 to 6.00; Additional Concerns MD 2.51, 95% CI −0.14 to 5.16; Analysis 2.4; very low‐certainty evidence; Table 10).
We downgraded the certainty of the evidence for BCS‐QoL due to high risk of both performance and detection bias in one study and unclear reporting bias. We also downgraded the certainty of the evidence for indirectness, as one study did not include any criteria for an AIMSS diagnosis and excluded use of agents influencing bone metabolism, including bisphosphonate, which are frequently utilised in the target population. We also downgraded for imprecision, due to small sample sizes. See Table 2.
Breast cancer‐specific quality of life
None of the other studies assessed BCS‐QoL.
Health‐related quality of life
See Analysis 2.5; Table 10; Figure 6.
2.5. Analysis.

Comparison 2: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 5: Health‐related quality of life
6.

Comparison 2: Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS), Outcome 2.5: Health‐related quality of life.
HR‐QoL results are represented in Figure 6 though none of the studies could be combined for meta‐analysis. Overall, HR‐QoL was not investigated in the studies by Birrell 2009, Chan 2017, Cathcart‐Rake 2020, Liu 2014, Massimino 2011, or Shenouda 2019.
Bionic tiger bone
Li 2017 utilised FACT‐B as a quality of life measure, which incorporates the overall HR‐QoL tool FACT‐G plus a BCS‐QoL specific subscale (Additional Concerns). The FACT subscales have been reported above in BCS‐QoL.
Yi Shen Jian Gu
Peng 2018 also used the FACT‐B tool. The FACT subscales have been reported above in BCS‐QoL.
Cat's claw
Sordi 2019 used the 36‐Item Short Form Survey (SF‐36) to assess HRQoL. In our review of the data, it appears that either incorrect data points were published for the SF‐36 (Physical Limitations) or the recorded data was not mean (SD), despite confirmation by study authors, as baseline placebo mean scores were published as 0 (SD 35.8). See Analysis 2.5 for the results for the eight SF‐36 domains (very low‐certainty evidence).
Two other studies did not report FACT‐G total score of subscales.
We downgraded the certainty of the evidence due to concerns with risk of bias, imprecision due to small sample sizes, inconsistency due to heterogeneity in intervention between studies and indirectness because one study did not include any criteria for an AIMSS diagnosis and excluded use of agents influencing bone metabolism, including bisphosphonates which are frequently utilised in the target population. See Table 2.
Incidence of aromatase inhibitor‐induced musculoskeletal symptoms
None of the treatment studies reported change in incidence of AIMSS.
Breast cancer‐specific survival and overall survival
None of the treatment studies reported BCSS or OS.
Discussion
Summary of main results
This Cochrane Review investigated systemic therapies for the prevention and treatment of AIMSS in early breast cancer, and included 17 RCTs with 2034 randomised participants. Systemic therapies investigated were diverse and included 'conventional' pharmacological therapies and CAM, and also included studies of vitamin D in the setting of insufficiency. There was limited information about certain study interventions or placebo ingredients (or both). In most studies, the comparator arm was a placebo, however, in some studies, the comparator was a vitamin supplement. Meta‐analysis was limited in this review. Due to the clinical and methodological heterogeneity of the studies and the insufficient data available from several studies, only one outcome had data that could be combined for meta‐analysis. Overall, the certainty of the evidence was very low for multiple outcomes for systemic therapies assessing prevention and treatment of AIMSS.
For preventing AIMSS, we found that the evidence for the effect of systemic therapies on change in pain from baseline to the end of the intervention was very uncertain. The evidence suggests systemic therapies for preventing AIMSS may have little to no effect on grip strength, little to no effect on change in HRQoL and BCS‐QoL from baseline to the end of the intervention, and little to no effect on women continuing to take their AI, although the certainty of this evidence was low. The evidence was very uncertain on the outcome of change in incidence of AIMSS. The evidence is very uncertain for the safety of systemic therapies for the prevention of AIMSS. One study of a COX‐2 inhibitor had an FDA safety alert issued for this class of medications during the study leading to significant participant withdrawal/discontinuation; however, there were no serious adverse events in this study, or any other prevention study. There were no data to assess the impact of systemic therapies for prevention of AIMSS on stiffness, BCSS or OS from the prevention studies.
For treating AIMSS, the evidence was very uncertain about the effect of systemic therapies on the effect of change in pain, stiffness, HRQoL and BCS‐QoL from baseline to the end of the intervention. The evidence suggests systemic therapies may have little to no effect on grip strength, although the certainty of this evidence was low. The evidence is very uncertain about the safety data for the use of systemic therapies for the treatment of AIMSS. There were no grade four or five adverse events in any of the treatment studies. One study reported significantly more all‐grade adverse events with duloxetine than with control (78% with duloxetine versus 50% with control; P < 0.001). There were no data on the incidence of AIMSS, number of women continuing to take AI, or BCS‐QoL or OS from the treatment studies.
The follow‐up interval was relatively short for most studies, and hence long‐term safety data are not available. Safety results should thus be interpreted with caution for both prevention and treatment studies.
Overall completeness and applicability of evidence
This review included 17 trials on 2034 participants. Insufficient data was a difficulty in this review, although additional data/information was obtained by author correspondence for four studies (Chan 2017; Khan 2017; Niravath 2019; Sordi 2019). The studies in this review were diverse in terms of clinical and methodological heterogeneity. There is considerable heterogeneity in the type of systemic therapy studied. These included 'conventional' pharmacological therapies and CAM, with the vitamin D studies not all fitting the Cochrane definition of CAM (Wieland 2011). There was also heterogeneity in the route of administration of the studied systemic therapy with most being oral therapy (16 trials) and only one trial of topical therapy with possible or postulated systemic absorption and effects (Chan 2017); the proposed mechanism of action of the systemic therapy; and the duration of intervention and the follow‐up. Several studies had limited details about the intervention, including the active compounds or the comparator arms (or both) (YSJG granules (Peng 2018), bionic tiger bone (Li 2017), Blue Citrus (Massimino 2011), tart cherry (Shenouda 2019), Cat's claw (Sordi 2019)). There were no known systemic interventions investigating the treatment of AIMSS missed in this review, but given the limited information from some studies and the incomplete understanding of the aetiology of AIMSS (Hershman 2015b; Niravath 2013), it is, therefore, not possible to make definitive conclusions about whether all relevant systemic or potentially effective therapy studies have been completed. We identified no studies that required translation into English, which raises the possibility that this review may have missed potential studies investigating a complementary or alternative medicine reported in a language other than English. Although non‐English papers were not excluded in this review, we searched only Western databases. There are ongoing studies of other systemic therapies with differing proposed mechanisms of action (see Characteristics of ongoing studies table). This review only included the investigation of systemic therapies for the treatment of AIMSS and, therefore, excluded other interventions that have been trialled for AIMSS that were not considered to be a systemic therapy, such as acupuncture and exercise. Most studies included in this analysis were conducted in hospital/oncology outpatient clinic settings. The limited information provided by some studies may limit the applicability to certain clinical settings.
The studies of vitamin D were methodologically and clinically heterogeneous (Khan 2017; Niravath 2019; Rastelli 2011; Shapiro 2016), and were performed either to prevent or treat AIMSS. Inclusion criteria in two of the vitamin D studies required participants to have specific 25‐OHD levels at baseline (Khan 2017; Rastelli 2011), particularly 25‐OHD levels of 40 ng/mL or less for the prevention trial of Khan 2017 and between 10 ng/mL and 29 ng/mL for the treatment trial of Rastelli 2011. Vitamin D deficiency is defined by most experts as a serum 25‐OHD level less than 20 ng/mL, and relative insufficiency of vitamin D as a level of 25‐OHD of 21 ng/mL to 29 ng/mL (Holick 2007). All women included in Rastelli 2011 had vitamin D deficiency or insufficiency, and many women in Khan 2017 also met these criteria. "Vitamin D deficiency in adults can precipitate or exacerbate osteopenia and osteoporosis, cause osteomalacia and muscle weakness, and increase the risk of fracture" (Holick 2007) and pain associated with these conditions could potentially be confused with AIMSS. Both studies were performed in the USA in predominantly white women (Khan 2017; Rastelli 2011). A nested case‐control correlative study has suggested vitamin D levels were not significantly associated with development of AI arthralgia (Niravath 2018). The studies that selected women with inclusion criteria related to serum vitamin D levels may therefore have uncertain generalisability and applicability to the entire population of women with AIMSS, and limit the overall completeness of the evidence related to vitamin D.
Patient populations included in this review had ethnic and racial diversity; however, diversity was sometimes related to particular characteristics of certain studied systemic therapy interventions, notably CAM, and to the ethnic, racial and cultural characteristics of the study populations (e.g. studies of TCM conducted in China) (Peng 2018; Li 2017); study of traditional Incan medicine, Cat's claw, conducted in Brazil (Sordi 2019). However, this was not consistent across all studies (e.g. study of TCM Blue Citrus conducted in the USA) (Massimino 2011). For studies examining TCM, choice of this intervention for AIMSS research in Chinese populations may relate to beliefs that TCMs for cancer care based on "deep cultural grounding", and beliefs that TCM is safe and effective (Xu 2006). Chinese herbal medicine was used by 76.8% (Chen 2008) and 86.7 % (Cui 2004) of Chinese women with breast cancer surveyed in Shanghai. In contrast, in a Canadian survey, although more than 80% of women with breast cancer reported using CAM (41% specifically to manage their breast cancer), only 2.2% of women with breast cancer consulted a TCM practitioner (Boon 2007). CAM use in ethnically and racially diverse populations has been found to be complex and nuanced; however, ethnicity has an independent role in the type of CAM used (Kronenberg 2006). One systematic review has highlighted the importance of traditional and CAM to indigenous people with cancer of Australia, Canada, New Zealand and the USA (Gall 2018). Ethnic, racial, cultural and linguistic diversity may therefore have relevance in limiting applicability, acceptability and generalisability of certain studied systemic therapy interventions (particularly traditional medicines and CAM) to other diverse populations with diverse cultural belief systems and corresponding traditional and CAM preferences. In addition, certain ethnic and racial groups were not adequately represented (or represented at all) in other studies of certain systemic therapies. As examples, in the treatment studies by Henry 2018 of duloxetine, Hershman 2015a of O3FA, Khan 2017 and Rastelli 2011 of vitamin D, greater than 85% of participants were white. However, in the prevention study of vitamin D by Niravath 2019 conducted in the USA, most participants enrolled were from minorities with 44% Latina people and 18% African American people enrolled.
The incidence of AIMSS has generally been reported to be approximately 50% (Beckwee 2017; Crew 2007; Henry 2008); however, one Chinese cohort reported an incidence of 72% (Xu 2014). A degree of current uncertainty about racial and ethnic variability in the incidence of AIMSS may affect data applicability and generalisability from this review. One placebo‐controlled trial of letrozole after five years of tamoxifen demonstrated in an unplanned subgroup analysis that women from minority ethnic and racial groups receiving letrozole had less arthritis however compliance was poorer than in Caucasian women receiving letrozole, and highlighted the low number of minority participants enrolled in this trial (Moy 2006). Pharmacogenetic predictors of AI toxicity have been reported but have not been adequately validated (Hertz 2017). Therefore, there is insufficient evidence from this review about differences in adverse events and other outcomes with the clinically heterogeneous systemic therapy interventions studied between different ethnic and racial participant groups and this may also limit generalisability and applicability.
The median age of women diagnosed with breast cancer in the USA is 62 years (Howlader 2019), similar to this review, and also similar to postmenopausal women diagnosed with breast cancer in other high‐income countries. However, similar to the Cochrane Review "Exercise therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer"(Roberts 2020), very few postmenopausal women older (greater than 75 years) or younger (less than 35 years) were included. Younger women at higher risk of recurrence are increasingly being treated with AI in combination with ovarian function suppression rather than with tamoxifen alone, due to improvements in DFS reported by landmark trials (Francis 2015; Francis 2018). There were high rates of musculoskeletal symptoms reported in these trials (88.7% for ovarian suppression with exemestane treatment versus 69% with tamoxifen treatment alone). The studies in this review included very few young women rendered postmenopausal and treated with AI, likely as a result of when the landmark trials were published (Francis 2015; Francis 2018). Young women treated with AI and ovarian function suppression or ablation represent an area of unmet clinical need, and an increasing population with AIMSS whose baseline incidence may be different to women included in this review. AIMSS may be inversely correlated with time since menopause (Mao 2009), and younger women with abrupt oestrogen withdrawal may be at higher risk of symptoms. Systemic therapy interventions for AIMSS may therefore have different applicability or even effectiveness in younger women.
Data were available for the primary outcome of pain for many of the studies. However, unfortunately data for certain important missing outcomes was unable to be obtained (e.g. Birrell 2009) to allow meta‐analysis (e.g. for testosterone in treatment – Birrell 2009; Cathcart‐Rake 2020). Many studies did not assess BCS‐QoL or HRQoL outcomes. Women experiencing pain from taking AI experience changes in QoL, with the degree of change in QoL depending on the type of pain experienced (Laroche 2017). The minimal QoL evidence available for this review raises concerns with completeness of the evidence. Many studies did not report important AIMSS PROs such as joint stiffness. There was no evidence available on the effect of systemic therapies for treatment of AIMSS on the incidence of AIMSS. There was no evidence available on the effect of systemic therapies for AIMSS on OS.
Studies that included participants with AIMSS at baseline varied in their definitions of AIMSS, and the comorbid conditions included. As noted in the Cochrane Review "Exercise therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer" (Roberts 2020), the lack of consensus on standardised definitions for AIMSS, the absence of objective outcome measures, and the multiple PROs reported in different studies meant it was not possible to make conclusions on completeness of the evidence of outcome measures in this review. These research barriers have been noted previously by Hershman 2015b and Niravath 2013. As the trials assessed multiple varied outcomes, and interventions were heterogeneous, it was neither possible nor appropriate to combine the majority of the outcomes for meta‐analysis. Long‐term safety data were lacking for many of the studies. Due to small sample sizes and heterogeneity of the trials, planned subgroup analysis was not possible, which further limits applicability of the findings.
There was considerable heterogeneity in the timing of the outcome measures of the included trials, which also limited comparability. The duration of the intervention in the included trials ranged between four weeks and two years. AIMSS are chronic symptoms with an unpredictable course. There was also considerable heterogeneity in follow‐up in the included studies. Evidence for long‐term benefits and harms is of increasing importance given the guideline recommendations for extended duration of AI therapy in certain women with breast cancer (Burstein 2019); however, limited evidence was available from this review in this setting. Two of the prevention studies reported adherence rates to AI therapy (Khan 2017; Niravath 2019), and in none of the treatment studies. Adherence to AI therapy is an important outcome of an intervention for AIMSS, as AI adherence impacts breast cancer survival (Chirgwin 2016). Therefore, evidence is incomplete given the very low‐certainty evidence from the small number of studies reporting AI adherence as an outcome.
There were notable limitations in the data obtained from this review. Many studies were of small size and either had some concerns or were at high risk of bias, and evidence was of very low certainty across multiple outcomes. As a result, caution needs to be taken in the interpretation of the outcome data from this review, and in assessment of the long‐term risks and harms of systemic therapies for AIMSS. These considerations also limit the generalisability and applicability of the studied systemic therapy interventions.
The outcomes analysed in this review were chosen based on which outcome domains the studies researching AIMSS used at the time of our protocol development, in addition to guidance from working groups such as Outcome Measures in Rheumatology (OMERACT 2021). In updates of this review, we would consider utilising Cochrane Musculoskeletal Group's core outcome list (CMSG 2021), although outcomes would have to extrapolated from other predefined musculoskeletal conditions, such as osteoarthritis or rheumatoid arthritis.
Quality of the evidence
Very low‐certainty evidence does not support the use of systemic therapy to prevent or treat AIMSS. Studies were generally of small size with the number of participants ranging from 37 to 299. Clinically heterogeneous systemic therapies or the clinically heterogenous settings in which they were studied (or both) meant that it was not appropriate to combine most outcomes for meta‐analysis. Hence, outcome assessments had low numbers of participants included, which led to downgrading of the evidence due to concerns with imprecision.
Many studies were either at moderate risk of bias, or had some concerns. A few of the studies were not blinded. One study had higher rates of adverse events with the intervention (duloxetine) than with the placebo, leading to higher numbers of participants successfully guessing which intervention they were receiving, and potentially unblinding these participants (Henry 2018). Similarly, another study used an intervention dosed three times daily versus a comparator with single daily dosing, which posed a high risk of unblinding the participants and assessors in this 'double‐blinded' trial (Li 2017). For these studies, there was a high risk of performance bias. There was also a high risk of detection bias as most outcomes were PROs, and the remaining outcomes were not blinded to outcome assessors. Several of the studies had inadequate outcome data and selective reporting of outcomes. There was high risk of attrition bias in several studies, which had a high risk of affecting outcome data, particularly in such small studies. We downgraded the evidence by at least one level for all our outcomes related to these studies due to serious concerns with the risk of bias.
Several studies had restrictive entry criteria which meant that the evidence obtained may not directly answer our review question. Particularly several of the vitamin D studies had criteria that specified low vitamin D levels for enrolment, specifically 25‐OHD levels at baseline (Khan 2017; Rastelli 2011), particularly 25‐OHD levels of 40 ng/mL or less for Khan 2017 and between 10 ng/mL and 29 ng/mL for Rastelli 2011. We downgraded the evidence by at least one level for all of our outcomes related to these studies due to indirectness, as these studies investigated a patient population that is not representative of all people with AIMSS. A number of outcomes were also downgraded for indirectness due to studies including patient populations that were not well‐defined for AIMSS. For example, one study included people experiencing musculoskeletal symptoms regardless of temporal association with the start of the AI, which resulted in a patient population that may have had pre‐existing musculoskeletal conditions rather than AIMSS.
There was no statistical heterogeneity in the single meta‐analysis performed in this review. However, there was significant clinical heterogeneity between interventions investigated in the review, which resulted in downgrading of the evidence for inconsistency for outcomes that incorporated multiple clinically heterogeneous interventions.
Four of the 17 studies have been published only in abstract form (Birrell 2009; Massimino 2011; Rosati 2011; Shenouda 2019). A considerable period of time has elapsed since the publication of three of these studies (Birrell 2009; Massimino 2011; Rosati 2011), and we were unable to obtain data. Some of these outcomes were of significant interest to this review; however, these were unable to be obtained. One study of etoricoxib had an FDA safety alert issued for the class of drug (COX‐2 inhibitors) during the study leading to high rates of participant withdrawal (Rosati 2011). Missing outcomes raise concerns about the overall quality of the evidence, and the potential for influence of publication bias on our outcomes. The only outcome with enough studies to warrant investigation with a funnel plot for evidence of publication bias confirmed our suspicions of the presence of publication bias. Concerns around publication bias led to downgrading of the evidence by one level.
Potential biases in the review process
This review undertook a comprehensive search strategy led by an Information Specialist (KR), with no language limitation. This is a strength of this review. Several authors (KER, SC, IA and NW) independently screened reference lists of all included studies and any systematic reviews (two authors for all references). We handsearched conference and meeting abstracts for the relevant organisations. Two review authors (IA, SC, KER, NW) independently performed data extraction and risk of bias assessments on each study. The search strategy should have identified most studies, however all studies identified were published in English language journals. Although several studies were conducted in non‐English speaking countries (e.g. China (Li 2017; Liu 2014; Peng 2018) and Brazil (Sordi 2019)), and were published in English, this observation does raise the possibility of a potential bias of missed publications in languages other than English.
The inconsistent definition of AIMSS (Hershman 2015b; Niravath 2013), and the potentially wide definition of systemic therapies may have been potential sources of bias in the search strategy. A wide search strategy was employed to attempt to account for these potential biases. All potential generally accepted definitions of systemic therapies were included in the search strategy, as were all AI in current clinical practice.
We contacted 15 study authors for further information. We received replies from four authors. A limitation of this review is that certain data were unavailable, which introduced selection bias. Certain outcomes were not available for meta‐analysis, due to our inability to obtain further data on request.
We did not systematically evaluate the measurement instruments for AIMSS in this review due to resource constraints. This is a potential limitation of our review; however, there is significant variability in the outcome assessments for AIMSS and no consensus (Hershman 2015b; Niravath 2013). Assessment of outcome measurements would therefore need to be interpreted within this clinical and research heterogeneity.
Agreements and disagreements with other studies or reviews
This Cochrane Review is the first of systemic therapies for prevention or treatment of AIMSS in postmenopausal women with early breast cancer. We searched other studies and reviews (systematic reviews and meta‐analyses) for comparison with this review. A broad search strategy identified the references, and we handsearched the identified studies for systematic reviews and meta‐analyses on the research question. The same reviews for treatment of AIMSS (which included systemic therapies and exercise) (Kim 2018; Nahm 2018; Roberts 2017; Yang 2017) were also identified by several of the current authorship team involved in a previous Cochrane Review of exercise for the prevention and management of AIMSS in early breast cancer (Roberts 2020), albeit the search date was earlier. One systematic review of systematic reviews (Kim 2018) identified the same three systematic reviews that included systemic therapies (Nahm 2018; Roberts 2017; Yang 2017). We excluded systematic reviews of acupuncture alone for AIMSS.
Roberts 2017 published a review entitled "Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: a systematic review and meta‐analysis". Several of the authors from the current Cochrane Review were also involved in this review. The author's search strategy was designed to include both prospective and retrospective clinical trials, including RCTs, cohort and case‐control studies and preventive trials of interventions for AIMSS including pharmacological, non‐pharmacological (including exercise), and CAM interventions. Meta‐analysis was conducted when there were sufficient data. Publications in English language only were considered. Studies of systemic therapy were grouped narratively into pharmacological therapies and CAM, and AIMSS outcomes reported. In contrast to our review, no meta‐analysis of any AIMSS outcomes was undertaken for systemic therapies. There was significant between‐study heterogeneity. Non‐randomised data was included in the overall systematic review of Roberts 2017 and the scope of studies included was broader, and hence this review is not directly comparable to our current Cochrane Review. In agreement with our review, evidence quality was determined to be poor overall, and there was limited evidence found to recommend systemic therapies for management of AIMSS. In contrast to our current Cochrane Review, Roberts 2017 undertook no reporting or analysis of stiffness, QoL outcomes or adverse event reporting.
The review by Yang 2017 entitled "Interventions for the treatment of aromatase inhibitor associated arthralgia in breast cancer survivors. A systematic review and meta‐analysis" included all studies that were RCTs and "quasi‐experimental design". The primary outcome was pain mean score as assessed by BPI at the end time point of the intervention. Yang 2017 separated the studies into "pharmacological approaches" and "nutritional supplementation". Four non‐randomised studies were said to be included in pharmacological approaches in the meta‐analysis (Briot 2010; Henry 2011; Kubo 2012; Zhang 2010), with pharmacological approaches reported to show "a very large effect in improving pain" (SMD −1.186, 95% CI −2.312 to −0.060). This subgroup meta‐analysis, with the inclusion of non‐randomised data, is different to our Cochrane Review. Three studies of diverse nutritional supplements were included together in meta‐analysis including a single‐arm non‐randomised open‐label phase two trial of glucosamine with chondroitin (Greenlee 2013), and RCTs of O3‐FA (Hershman 2015a) and vitamin D (Rastelli 2011). Nutritional supplementation showed no significant effect on joint pain, although there was "a tendency to decrease joint pain" (SMD −0.124, 95% CI −0.341 to 0.092). The review by Yang 2017 differs from our Cochrane Review as we have not analysed such diverse systemic therapies together due their postulated differing mechanisms of action, and we have not included non‐randomised data. In addition, in contrast to our current Cochrane Review, Yang 2017 did not undertake adverse event reporting.
In contrast to our current review, the systematic review by Nahm 2018 performed no meta‐analyses. This systematic review is not directly comparable to our current Cochrane Review as Nahm 2018 included all management interventions for AIMSS, and included non‐ randomised studies. In agreement with our review, the authors concluded, "While several trials had positive findings, the major methodological limitations of the studies meant that no definitive evidence could be found supporting any of the interventions".
Kim 2018 performed the most recent review with a search date of June 2018. Kim 2018 systematically reviewed all eligible systematic reviews and then subjected these to evidence mapping. The RCTs included in the reviews were handsearched for network meta‐analysis. The search strategy, statistical methodology and included studies therefore are different to our current Cochrane Review. Mean and SD of BPI was the outcome measure. Kim 2018 identified six trials that provided information on BPI for inclusion in their network meta‐analysis. Two of the six RCT included in the network meta‐analysis were systemic therapy studies, which were an RCT of O3‐FA (Lustberg 2018), and vitamin D (Rastelli 2011). These RCTs were not combined but compared separately to wait list control. Kim 2018 concluded "omega‐3 fatty acids (MD −2.10, 95% CI −3.23 to −0.97) showed statistically significant improvement in pain severity scores". However, a larger RCT of O3‐FA by Hershman 2015a of 262 participants which had BPI‐SF as a primary endpoint was not included in this network meta‐analysis, which is in contrast to our current Cochrane Review which included Hershman 2015a. For network meta‐analysis, the authors concluded "all interventions had a better effect than vitamin D" with only the trial by Rastelli 2011 included in their network meta‐analysis. The review by Kim 2018 is not comparable to our Cochrane Review as in contrast to our current review, it did not include vitamin D studies by Khan 2017, Shapiro 2016 (did not use BPI as an endpoint) and Niravath 2019 (published subsequently). The conclusions of Kim 2018 are not in agreement with our Cochrane Review findings. Adverse event reporting was concluded to be poor, and as a result of this, Kim 2018 did not perform network meta‐analysis of adverse events. We found that adverse event reporting was overall of very low‐certainty evidence, and data were not available from certain studies.
Authors' conclusions
Implications for practice.
Aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS) is a chronic symptom complex with a significant impact on women with early breast cancer taking aromatase inhibitors (AI). Poor adherence to AI therapy due to AIMSS impacts breast cancer survival. To date, evidence for safe and effective systemic therapies for prevention or treatment of AIMSS has been minimal.
This review has demonstrated that there is overall very low‐certainty evidence for systemic therapies for prevention or management of AIMSS in postmenopausal women with early breast cancer for most outcomes. Studies were clinically and methodologically heterogeneous and investigated diverse pharmacological and complementary and alternative medicines (CAM) therapies, which were often not appropriate to combine for meta‐analysis. Certain data were not available, which also limited meta‐analysis. Consequent to this, we were very uncertain of the effect of systemic therapies for either prevention or treatment of AIMSS on many important AIMSS outcomes including change in pain, health‐related quality of life and cancer‐specific quality of life. There was also limited information on long term benefits and harms of studied interventions. Certain studies had limited information available about the ingredients of the intervention and/or the placebo. Based on the very low certainty of evidence across multiple outcomes, no definitive recommendations for systemic therapies for either prevention or treatment of AIMSS on the basis of this review can be made.
An evidence‐based summary on management of AIMSS suggested that duloxetine (as per the study by Henry 2018) is one of the treatment options for women with AIMSS among other options, particularly if women have comorbid anxiety, depression or chronic osteoarthritis (Gupta 2020). The trial by Henry 2018 demonstrated the mean joint pain score was 0.82 points lower for women who received duloxetine compared with those who received placebo by 12 weeks (95% CI −1.24 to −0.40; P = 0.0002). However, this result did not indicate a clinically significant benefit, with the clinically significant change in mean pain defined as a decrease from baseline of at least two points. Within 12 weeks of discontinuing either duloxetine or placebo, pain scores were equivalent between the two arms. There were higher adverse event rates with duloxetine over placebo, which potentially led to unblinding. A subsequent retrospective exploratory analysis suggested women with obese range body mass index (BMI) of 30 kg/m2 of greater received more benefit from duloxetine (Henry 2019), however the original study was not designed to examine differences in pain scores by BMI. Given the short‐term duration of the study (Henry 2018), no evidence is available for the effectiveness of duloxetine for long‐term treatment of AIMSS. Similarly, no evidence is available that indicates whether duloxetine will improve AI adherence. The implications for practice of the evidence for duloxetine for AIMSS treatment are potentially limited based on our Cochrane Review. Comorbid conditions that may be treated by duloxetine or alter its efficacy in the setting of AIMSS have not been addressed in this Cochrane Review.
Vitamin D was investigated in both the prevention (Khan 2017; Niravath 2019) and treatment (Rastelli 2011; Shapiro 2016) of AIMSS. Clinical and methodological heterogeneity was present in these studies, and overall evidence was very uncertain on the effect of vitamin D across multiple outcomes. Hence, measurement of vitamin D concentration and routine vitamin D oral supplementation cannot be recommended for prevention or management of AIMSS on the basis of our review; however, it may be indicated for bone health management in early breast cancer and for documented vitamin D insufficiency/deficiency. Similar recommendations with respect to vitamin D have been made by Gupta 2020.
Further research into optimal systemic therapies to prevent and treat AIMSS are recommended to improve outcomes and quality of life for women with early breast cancer.
Implications for research.
This Cochrane Review has highlighted many of the research priorities for postmenopausal women with early breast cancer experiencing AIMSS. Further adequately powered, high‐quality, randomised phase three trials are required to definitively answer research questions on whether systemic therapies can prevent or treat AIMSS in postmenopausal women with breast cancer. AIMSS remains a poorly understood symptom complex with an unclear aetiology (Hershman 2015b, Niravath 2013). Without a clear understanding of the aetiology, researchers are yet to design targeted interventions or drug modifications with systemic therapies that comprehensively address the various aspects of AIMSS.
Ongoing clinical trials of systemic therapy would likely be enhanced by further research into the aetiology of AIMSS. However, as noted by Hershman 2015b, it may be the lowering of oestradiol caused by AI, the desirable effect for breast cancer efficacy, is also the inciting factor for AIMSS. Careful attention would then be needed with any future trials directed against this potential mechanism of action of AIMSS as these could affect the efficacy of AI (Hershman 2015b).
Similar to the Cochrane Review of exercise for AIMSS (Roberts 2020) and as noted by Hershman 2015b, research has been limited by heterogeneous populations. Prior chemotherapy, prior hormone replacement therapy and increased bodyweight are associated with an increased risk of AIMSS (Hershman 2015b; Niravath 2013). One large observational study demonstrated that CAM use prior to the initiation of letrozole did not prevent or improve the development of AIMSS; however, the CAM group had higher pain levels throughout the observation period (Hack 2020). Exploratory subgroup analyses of trials of omega‐3 fatty acid (Hershman 2015a; Shen 2018) and duloxetine (Henry 2018; Henry 2019) suggested obese participants gained more analgesic benefit than non‐obese participants. Understanding the impact of host factors on personalised risk of AIMSS and targeting research interventions to those most at risk are research priorities for future clinical trials. Stratification for risk factors, particularly obesity, should similarly be considered for future trials (Henry 2019).
Future research into AIMSS would also be improved by enhancing methodological rigor and reducing bias. All trials should ideally be prospectively registered and published in peer‐reviewed journals on completion, regardless of size. Of particular focus, research would be improved by standardisation of measurement of outcomes in AIMSS. Importantly, there remains no consensus on the constellation of symptoms and signs that define AIMSS (Hershman 2015b; Niravath 2013). Studies have used multiple different outcome measures, predominantly patient‐reported outcomes (PROs) (Hershman 2015b). PROs measure subjective experiences such as symptoms from the patient's perspective, and considered the gold standard for quantifying symptomatic effects of treatments (Basch 2010; Patrick 2007) as physicians under‐report treatment‐emergent toxicities in people with cancer (Basch 2016; Basch 2017). As PROs are the most reliable tools for quality of life research (Deshpande 2011), consensus on the definition of the syndrome and the most appropriate outcome measures utilising PROs remains a research priority. Development of more reliable instruments is underway (Castel 2015); however, further research is required. The systematic incorporation of PROs, including electronic platforms, with better standardisation, will improve research quality, and improve patient and carers' research experience (Brandt 2019; Rocque 2018). Standardisation of outcome measures would be required to reduce the heterogeneity that has thus far hampered meta‐analysis in research efforts into AIMSS. In addition, the interpretation of the clinical importance or meaningfulness of results for many of the PROs utilised in AIMSS research has not been specifically established in AIMSS. Interpretation of meaningfulness of trial data has been extrapolated from other chronic pain, rheumatological or cancer pain syndromes (Arnold 2005; Dworkin 2009; Farrar 2010; Mease 2011; Olsen 2018). Ongoing research is hence required to continue to develop and evaluate the appropriate outcome instruments in AIMSS.
Research questions should also address the time points most appropriate for measurement of outcomes. AIMSS have variable and fluctuating clinical progress (Hershman 2015b), and studies thus far have included variable assessment timing, making comparison and meta‐analysis challenging. Future research studies of systemic therapies should also include outcome measurements beyond the completion of the intervention to quantify if the effects of the intervention on AIMSS are sustained. Trials of supportive systemic therapies for AIMSS thus far have been predominantly of 6 months' duration or less and do not address pertinent research questions of establishing safe and effective long‐term therapies. Research challenges will be significant to design systemic therapy trials that continue for the entire duration of AI adjuvant therapy; in reality these may not be feasible. However, it is a research priority to design interventions of longer duration, as only then is it likely that knowledge gaps can be addressed – for instance, whether a systemic therapy for AIMSS can affect AI compliance.
Many of the trials in this review, 10/17 (Birrell 2009; Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Khan 2017; Liu 2014; Niravath 2019; Rastelli 2011; Shenouda 2019), were funded by industry sources, or had researchers with disclosed conflicts of interests with industry of varying relevance. In three trials, information was insufficient to make a full determination of relationships (Massimino 2011; Peng 2018; Rosati 2011). Several pharmaceutical company‐ or industry‐sponsored trials had design biases – the main example being four trials with inclusion criteria restricting entry to participants being treated with the sponsor's AI (Birrell 2009; Khan 2017; Liu 2014; Rastelli 2011). Rosati 2011 was similarly restricted to the sponsor's AI as an entry criterion but AIMSS was not the primary outcome of the study. For‐profit funded trials have a higher chance of a favourable outcome than not‐for‐profit or mixed funding sponsored trials (Bhandari 2004; Delgado 2017), although we acknowledge that research interventions in this review were designed to address a treatment‐emergent toxicity. Future research should preferentially access not‐for‐profit or mixed funding sources. This will require recognition and prioritisation of trials to address cancer treatment‐emergent toxicities by institutional and governmental bodies (as per the American Society of Clinical Oncology (ASCO) statement (Markham 2020)).
Investigators designing trials of systemic therapy for AIMSS need to adequately acknowledge and account for the 'placebo effect'. Improvement due to the placebo effect has been noted in multiple trials in this review (Birrell 2009; Cathcart‐Rake 2020; Chan 2017; Henry 2018; Hershman 2015a; Peng 2018; Shenouda 2019), and has been previously documented in trials of other systemic therapies for cancer treatment emergent toxicities to be up to 30% to 50% (Cruciani 2012; Loprinzi 2009; Smith 2013). Better understanding of the aetiology of the placebo effect may potentially improve outcomes for women with AIMSS, particularly if the effect could be harnessed. Failure to account for the placebo effect will likely result in statistically underpowered trials that do not detect small differences between interventions, and thus may not answer the pertinent research questions (Henry 2015). The ethical implications of the placebo effect as prevention or treatment for AIMSS also requires further research.
Ideally, further research into systemic therapy interventions for AIMSS would involve translational research aiming to understand the pharmacology of, and adequately document and study all potential active ingredients of, the intervention. This is of particular importance with trials of CAM with many potentially active compounds (e.g. Chan 2017; Massimino 2011; Peng 2018; Shenouda 2019; Sordi 2019). Such research would allow any potentially effective traditional or CAM for AIMSS to then align with World Health Organization traditional and complementary medicine policies which highlight the role and importance of product regulation (WHO 2013). To avoid contamination, the choice of the placebo itself remains an important issue for research into systemic therapy for AIMSS. The placebo arm(s) of future trials should ideally contain no ingredients that potentially have activity (Wan 2013) in prevention or treatment of AIMSS in order to reduce research heterogeneity and uncertainty. As examples, in the trial by Hershman 2015a, possible concerns were raised about contamination from ingredients in the placebo which contained soybean oil and therefore potentially plant phyto‐oestrogens (Henry 2018; Hershman 2015a). A trial of TCM had a placebo containing compounds that may have had uncertain activity, with the placebo granules were made from dextrin (95%) and Herba pogostemonis (5%) (Peng 2018). Herba pogostemonis may have anti‐inflammatory effects in in vitro models (Xian 2011). Careful reporting of all placebo and excipient details of any placebo will be important for any ongoing or further research into systemic therapy which includes a placebo arm. The challenges of matching the placebo to achieve blinding need to continue to be addressed in future research of systemic therapies in AIMSS, especially as most primary outcomes are PROS. Attention needs to be paid to matching all sensory aspects of the placebo to the investigational product (Wan 2013).
Future research should necessarily include rigorous monitoring for evidence of harm. Trials thus far have often included minimal long‐term adverse event monitoring. There has been conflicting evidence on the effect of supplement/antioxidant use on breast cancer recurrence and mortality in women with early breast cancer (Ambrosone 2020; Greenlee 2012; Poole 2013). Hence, monitoring safety and tolerability is thus of utmost importance for future trials of systemic therapy for AIMSS. However, the authors acknowledge the potential research challenges due to studies being potentially underpowered to detect possible small survival differences in studies of systemic therapies for prevention or treatment of AIMSS in women who are being treated with AI for early breast cancer.
History
Protocol first published: Issue 11, 2018
Acknowledgements
We would like to acknowledge and thank Ms Ava Tan‐Koay, Information Specialist/Assistant Managing Editor with the Cochrane Breast Cancer Group (CBCG), for peer reviewing the search strategies and for running the searches in the CBCG Specialist Register for us.
We would also like to acknowledge and thank our peer reviewers: Dr Rachel Dear, Medical Oncologist, St Vincent's Hospital, Darlinghurst (senior editor); Rebecca Seago‐Coyle (consumer editor) and Jacquie Chirgwin (content specialist).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Aromatase Inhibitors] explode all trees
#2 aromatase inhibit*
#3 anastrozole or exemestane or letrozole or aminoglutethimide* or atamestane or fadrozole or formestane or vorozole or arimidex or aromasin or femara or fadrozole or lentaron or rivizor or cytadren
#4 #1 or #2 or #3
#5 MeSH descriptor: [Breast Neoplasms] explode all trees
#6 breast near neoplasm*
#7 breast near (carcinoma* or adenocarcinoma*)
#8 breast near cancer*
#9 breast near tumour*
#10 breast near tumor*
#11 breast near malignan*
#12 #5 or #6 or #7 or #8 or #9 or#10 or#11
#13 MeSH descriptor: [Musculoskeletal Diseases] 1 tree(s) exploded
#14 myalg*
#15 fibromyalg*
#16 arthropath*
#17 joint or muscl* or arm* or leg* or shoulder* or elbow* or knee* or hip* or hand* or feet or foot
#18 (pain* or stiff* or sore* or discomfort* or symptom)
#19 15 near #16
#20musculoskeletal or musculo‐skeletal
#21 ("trigger finger" or "carpal tunnel” or “tendin*”)
#22 tenosynov*
#23 MeSH descriptor: [Range of Motion, Articular] this term only
#24 #13 or #14 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #4 and #12 and #24
Appendix 2. MEDLINE search strategy
((((((((("Feasibility Studies"[Mesh] OR "Pilot Projects"[Mesh] OR randomized controlled trial[Publication Type] OR controlled clinical trial[Publication Type] OR randomized[Title/Abstract] OR randomised[Title/Abstract] OR Clinical Trials as Topic[Mesh] OR randomly[Title/Abstract] OR placebo[Title/Abstract] OR trial[Title/Abstract] OR Pragmatic Clinical Trials as Topic[Mesh] OR pragmatic clinical trial [Publication Type] OR crossover[Title/Abstract] OR cross‐over[Title/Abstract] OR groups[Title/Abstract]) NOT ("Animals"[Mesh] NOT "Humans"[Mesh]))))))) AND (((((("Postmenopause"[Mesh] OR Breast neoplasms[mh] OR ((breast[mh] OR breast diseases[mh]) AND neoplasms[mh])) AND humans[mh]) OR DCIS[tiab] OR LCIS[tiab] OR ductal carcinoma in situ[tiab] OR lobular carcinoma in situ[tiab] OR (breast[tiab] AND (ductal carcinoma*[ti] OR lobular carcinoma*[ti])) OR ((Breast[ti] OR mammary[ti]) AND (cancer*[ti] OR neoplas*[ti] OR tumor*[ti] OR tumour*[ti] OR carcinoma*[ti] OR malignan*[ti] OR sarcoma[ti] OR lymphoma[ti]))))))) AND ((((((((((((((((((("Musculoskeletal Pain"[Mesh]) OR "Musculoskeletal Diseases"[Mesh] OR "Carpal Tunnel Syndrome"[Mesh]) OR ARTHRALGIA[Mesh] OR Carpal Tunnel Syndrome[Mesh] OR Range of Motion, Articular[Mesh] OR "Tendinopathy"[Mesh]) OR ((joint* OR hand OR hands OR elbow* OR knee* OR wrist* OR finger* OR ankle*OR hip OR hips) AND (pain* OR discomfort* OR tender* OR stiff*)) OR tendinitis) OR tendinopath*) OR tenosynovitis) OR arthralg*) OR rheumat*) OR "trigger finger") OR "carpal tunnel") OR fibromyalg*) OR mylag*) OR polyarthral*) OR arthriti*))))))) AND ((("Aromatase Inhibitors"[Mesh]) OR "Fadrozole"[Mesh] OR "Aminoglutethimide"[Mesh] OR exemestane OR anastrozole OR arimidex OR letrozole OR femara OR fadrozole OR formestane OR rivizor OR cytadren OR vorozole OR atamestane OR lentaron OR aminoglutethimide))
Appendix 3. Embase search strategy
#1 random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign* OR allocat* OR volunteer* OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'feasibility study'/exp OR 'pilot study'/exp
AND
#2 ('breast cancer'/exp OR (breast OR mammary) NEAR/3 (cancer* OR carcinoma* OR malignan* OR tumo*r* OR adenocarcinoma*) OR ('neoplasm'/exp AND ('breast'/exp OR 'breast disease'/exp))
AND
#3 (aromatase NEAR/2 inhibit* OR 'aromatase inhibitor'/exp OR anastrozole OR exemestane OR 'letrozole' OR aminoglutethimide* OR atamestane OR fadrozole OR formestane OR vorozole OR arimidex OR aromasin OR femara OR fadrozole OR lentaron OR rivizor OR cytadren)
AND
(musculo*skeletal:ab,ti OR arthralg*:ab,ti OR 'carpal tunnel syndrome':ab,ti OR 'trigger finger':ab,ti OR tendin*:ab,ti OR myalg*:ab,ti OR fibromyalg*:ab,ti OR tenosynov*:ab,ti OR 'arthropathy'/exp OR 'morning stiffness'/exp OR 'myalgia'/exp OR 'musculoskeletal stiffness'/exp OR 'tendon disease'/exp OR 'arm pain'/de OR 'hand pain'/de OR 'limb pain'/de OR 'wrist pain'/de OR ((joint* OR muscl* OR hand* OR knee* OR hip* OR shoulder* OR feet OR foot OR elbow* OR wrist* OR hip* OR ankle* OR finger*) NEAR/3 (pain* OR stiff* OR sore* OR discomfort* OR symptom* OR tender*)):ab,ti OR 'musculoskeletal disease'/de OR 'musculoskeletal system inflammation'/exp OR 'myalgia'/exp OR 'neuralgia'/exp OR 'shoulder pain'/de OR arthralgia OR rheumat*:ti,ab OR arthrit*:ti,ab OR polymyalg*:ti,ab OR polyarthralg*:ti,ab OR polyarthrit*:ti,ab OR (('muscle'/exp OR 'joint'/exp) OR ('quality of life'/exp OR 'pain'/exp))) OR ‘joint characteristics and functions‘/
Appendix 4. CINAHL search strategy
S36 S12 AND S25 AND S35 S35 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 S34 (MH "Range of Motion") S33 (tendinitis or tendinopath* or tenosynovitis or arthralg* or rheumat* or trigger finger or carpal tunnel or fibromyalg* or mylag* or polyarthral* or arthriti*) S32 ((joint* or hand or hands or elbow* or knee* or wrist* or finger* or ankle* OR hip or hips) N3 (pain* or discomfort* or tender* or stiff*)) S31 (MH "Tendinopathy+") S30 (MH "Carpal Tunnel Syndrome") S29 (MH "Arthralgia+") S28 "musculoskeletal pain" S27 (MH "Treatment Related Pain") S26 (MH "Muscle Pain") S25 S19 AND S24 S24 S20 OR S21 OR S22 OR S23 S23 TX (hormon* W1 therap*) S22 S20 OR S21 OR S22 OR S23 S21 TX (exemestane) or TX (anastrozole) or TX (arimidex) or TX (letrozole) or TX (aromasin) or TX (femara) or TX (fadrozole) or TX (formestane) or TX (rivizor) or TX (cytadren) or TX (vorozole) or TX (atamestane) or TX (lentaron) or TX (aminoglutethimide*) S20 (MH "Aromatase Inhibitors+") S19 S13 OR S14 OR S15 OR S16 OR S17 OR S18 S18 TX "breast tumor" S17 TX "breast tumour" S16 TX "breast carcinoma" S15 TX "breast neoplasm" S14 TX "breast cancer" S13 (MH "Breast Neoplasms+") S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 TX allocat* random* S10 (MH "Quantitative Studies") S9 (MH "Placebos") S8 TX placebo* S7 TX random* allocat* S6 (MH "Random Assignment") S5 TX randomi* control* trial* S4 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S3 TX clinic* n1 trial* S2 PT Clinical trial S1 (MH "Clinical Trials+")
Appendix 5. WHO ICTRP search strategy
breast cancer AND aromatase AND muscl*
breast cancer AND aromatase AND arthralg*
breast cancer AND aromatase AND joint*
breast cancer AND aromatase AND pain*
breast cancer AND aromatase AND stiff*
Appendix 6. ClinicalTrials.gov search strategy
1. breast cancer [Condition or disease] AND (aromatase OR AIMSS) AND (muscul* OR arthralgia OR pain OR joint* or muscl*) [Other terms]
Data and analyses
Comparison 1. Prevention of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Grip strength | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Discontinuation of aromatase inhibitors | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Incidence of AIMSS | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 1.5 Breast cancer‐specific quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Health‐related quality of life (HRQoL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 HRQoL: Total Functional Assessment of Cancer Therapy – General (FACT‐G) score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 FACT‐G Physical Well‐being subscale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.3 FACT‐G Social Well‐being subscale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.4 FACT‐G Emotional Well‐being subscale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.5 FACT‐G Functional Well‐being subscale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Treatment of aromatase inhibitor‐induced musculoskeletal symptoms (AIMSS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Testosterone | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.47, 1.07] |
| 2.1.2 Vitamin D | 2 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐2.16, ‐0.32] |
| 2.1.3 Duloxetine | 1 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.97, ‐0.29] |
| 2.1.4 Calcitonin | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.56, ‐1.44] |
| 2.1.5 Omega‐3 fatty acids | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.00, 0.52] |
| 2.1.6 Bionic tiger bone | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐4.09 [‐5.25, ‐2.93] |
| 2.1.7 Emu oil | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.72, 0.94] |
| 2.1.8 Yi Shen Jian Gu granules | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐2.10, ‐0.58] |
| 2.1.9 Cat's claw (Uncaria tomentosa) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [1.53, 4.47] |
| 2.2 Stiffness | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 Vitamin D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.2 Omega‐3 fatty acids | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.3 Yi Shen Jian Gu granules | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.4 Bionic tiger bone | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.5 Emu oil | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Grip strength | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 Vitamin D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 Breast cancer‐specific quality of life | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 Physical Well‐being | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.2 Social/ Family Well‐being | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.3 Emotional Well‐being | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.4 Functional Well‐being | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.5 Additional Concerns | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Health‐related quality of life | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.1 FACT Physical Well‐being | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.2 FACT Social/Family Well‐being | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.3 FACT Emotional Well‐being | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.4 FACT Functional Well‐being | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.5 SF36 Functional Capacity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.6 SF36 Physical Limitations | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.7 SF36 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.8 SF36 Overall Health Status | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.9 SF36 Vitality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.10 SF36 Social Aspects | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.11 SF‐36 Emotional Aspects | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.12 SF‐36 Mental Health | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Birrell 2009.
| Study characteristics | ||
| Methods | Phase II, placebo‐controlled, parallel RCT 3 arms Duration of intervention: 3 months TREATMENT TRIAL Recruited at Burnside Breast Centre Adelaide, South Australia, Australia |
|
| Participants |
Inclusion criteria
Exclusion criteria
90 participants; 30 in placebo group, 30 in testosterone 40 mg group, 30 in testosterone 80 mg group. |
|
| Interventions |
Intervention Arm 1: testosterone undecanoate 40 mg daily orally + anastrozole 1 mg daily orally Arm 2: testosterone undecanoate 80 mg daily orally + anastrozole 1 mg daily orally Comparator Placebo: 1 tablet daily orally + anastrozole 1 mg daily orally |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Abstract only. Only women receiving anastrozole were enrolled. Sponsors/collaborators: Havah Therapeutics Pty Ltd; AstraZeneca. NCT01573442. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract stated participants "were randomised", no information on process of randomisation. Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on methods to conceal allocation to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial register; quote: "Masking: Triple (Participant, care‐provider, investigator)". Abstract; quote: "No significant androgenic side effects (hirsutism, alopecia and acne) were observed". Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | VAS – PRO. Insufficient information on blinding and administration of scoring systems to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | High risk | Quote: "Dr. Birrell reports personal fees from Havah Therapeutics, that is developing androgen‐based therapies for women, and has a patent AU2005905768A0" (Cathcart‐Rake 2020). |
Cathcart‐Rake 2020.
| Study characteristics | ||
| Methods | Placebo controlled, parallel RCT Duration of intervention: 6 months After completion of the 6‐month active trial period, participants could choose to continue being followed for an additional 6‐month observation period. TREATMENT TRIAL Recruitment in USA centres with collaboration of NCI and Alliance for Clinical Trials in Oncology between 10 September 2013 and 29 November 2017 |
|
| Participants |
Inclusion criteria
Exclusion criteria
227 participants randomised. 114 in testosterone group, 113 in placebo group. 19 participants "canceled". 208 participants were eligible; 104 in testosterone group, 104 in placebo group. Recruited 55 participants prior to 15 January 2016, amendment; 172 participants thereafter Mean age: 59.9 (SD 8.7) years in testosterone group; 60.1 (SD 9.7) years in placebo group Baseline patient characteristics (Table 1 of publication) relatively well‐balanced between arms Most participants were white (> 90%) and 79% had been on an AI for ≥ 6 months at entry > 12 months of AI at entry: testosterone group 63 (61%) participants; placebo group 52 (50%) participants Quote: "Baseline symptomatology and quality of life measures at study entry were balanced between the two arms, with the exception of patients assigned to placebo being more significantly bothered by breast tenderness, dissatisfaction with their personal life, feeling depressed or anxious, and by changes in skin appearance, texture, or tone (Supplementary Table S2)" (Cathcart‐Rake 2020). No information about prior chemotherapy exposure of participants |
|
| Interventions |
Intervention Initial protocol: 2 subcutaneous pellets containing total of testosterone 120 mg and anastrozole 8 mg (provided to prevent aromatisation of the testosterone to oestrogen) inserted by surgical procedure at 2 time points – at end of first week on study (following completion of the hot flush baseline week ascertainment) and 3 months later. Each pellet contained testosterone 60 mg and anastrozole 4 mg, with stearic acid as an inactive ingredient. Total of 2 doses. Amendment to protocol on 15 January 2016 due to slow accrual attributed to the need for minor surgical procedure for implantation of the pellet: subcutaneous pellet as per the original design, or topical testosterone gel (without anastrozole) 10.4 mg/0.264 mL applied topically daily to the skin for 6 months. The testosterone gel was delivered by an Accupen dispensing device to allow accuracy of dosing. Comparator Placebo Initial protocol: 2 pellets as subcutaneous implant every 3 months for a total of 2 doses. Placebo pellets were similar in appearance to the testosterone–anastrozole pellets. Placebo pellets contained approximately 98% cholesterol and 2% stearic acid according to the protocol. Protocol amendment: subcutaneous placebo pellet or placebo gel applied daily for 6 months. |
|
| Outcomes |
Primary endpoints
Secondary endpoints
|
|
| Notes | Quote: "The research reported in this publication was financially supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA232760, U24CA196171, UG1CA189858, UG1CA189997, U10CA180820, and UG1CA189861 (ECOG‐ACRIN), U10CA180868 (NRG), and U24CA196171, as well as the Alliance biorepository resource" (Cathcart‐Rake 2020). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This study is supported by the NCI Cancer Trials Support Unit". "The patient then will be assigned to one of the following treatment groups using the Pocock and Simon65 dynamic allocation procedure which balances the marginal distributions of the stratification factors between the treatment groups". p.23 and 24 of protocol explains randomisation process via CTSU. "Further instructional information is provided on the OPEN tab of the CTSU members’ side of the CTSU website at https://www.ctsu.org or at https://open.ctsu.org" (p.23 protocol). |
| Allocation concealment (selection bias) | Low risk | Randomisation via external computerised site. Procedures for ensuring double blinding outlined on p.24 of protocol. Quote: "Participants were randomly allocated to receive two surgically implanted pellets containing either a combination of testosterone 120 mg and anastrozole 8 mg or a placebo. Treatment assignments were blinded to both the patient and medical professional. As such, after patient randomization, registration personnel assigned the patient a pellet from the Alliance Research Base Pharmacy, which was marked with a deidentified kit number and the label of “testosterone OR placebo". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To ensure that both the patient and the medical professionals who care for the patient are blinded to the identity of the treatment assignment, the randomization specialist will follow the double‐blinding procedures outlined below". "After the treatment assignment has been determined by the registration/randomization application, Alliance registration office personnel will be notified of the treatment assignment, and they will then communicate the treatment assignment to the Alliance Research Base Pharmacy. The registration personnel will also notify the participating institution of the patient specific kit number to be used on the order form. Institutions will then order testosterone/placebo using the A221102 Clinical Drug Order Form" (p.24 protocol). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | PROs as measurements. Blinding procedures as per above quotes, placebo controlled. Adverse events, e.g. hirsutism that may have led investigators/participants to suspect the assigned arm of testosterone were not significantly different between the arms. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Figure 1: Consort diagram. 227 enrolled, 208 evaluated. However placebo arm: 113 participants, 9 "cancels" with insufficient information on "cancels". 104 eligible participants with 77 completing 6 months on protocol (quote: "Patients not completing per protocol 19 refusals 6 had AE [adverse events] 2 went off study treatment for other reasons)". Quote: "104 evaluable for primary endpoint, as last values were carried forward". Testosterone arm: 114 participants with 10 "cancels". 104 eligible participants with 80 completing 6 months on protocol. (quote: "Patients not completing per protocol 17 refusals 3 had AE 4 went off study treatment for other reasons"). Quote: "104 evaluable for primary endpoint, as last values were carried forward". Insufficient information on reasons for refusals. Although balanced between groups, the Consort diagram indicates > 20% in each group not completing per protocol at 6 months. Figure 2. BPI mean pain scores – 75/99 participants' data available at 3 months in placebo group vs 80/98 in intervention arm (primary endpoint). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary endpoints reported in article match those endpoints outlined in protocol/trial registry – attached appendix provides further detail. |
| Other bias | High risk | Quote: "The authors have no financial relationship with any private company regarding this research with the exception that Dr. Birrell reports personal fees from Havah Therapeutics, that is developing androgen‐based therapies for women, and has a patent AU2005905768A0; additionally, Dr. Glaser has a patent (US 10,071,104 B2) related to this topic". |
Chan 2017.
| Study characteristics | ||
| Methods | Phase II, placebo‐controlled, parallel RCT Duration of intervention: 8 weeks, option to continue open‐label emu oil for further 8 weeks TREATMENT TRIAL Recruited from 3 breast cancer centres in Australia from December 2010 to February 2015 |
|
| Participants |
Inclusion criteria
Exclusion criteria
87 participants randomised; 43 in emu oil group, 44 in placebo oil group. Evaluable for primary endpoint: 36 in emu oil group, 37 in placebo oil group Median age: emu oil group 60.3 (range 46–74) years; placebo oil group 56.9 (range 43–68) years Baseline characteristics only reported for those that were evaluable for primary endpoint (36 in emu oil group, 37 in placebo oil group) Most participants received anastrozole or letrozole Median time on AI at the commencement of the study: 16.9 months in the placebo group, 13.3 monthsin the emu oil group; no difference between groups More participants received non‐taxane chemotherapy in placebo group (10.8% in emu oil group, 29.7% in placebo oil group), while more participants in emu oil group received endocrine therapy only (24.3% in emu oil group, 10.8% in placebo oil group); differences were of borderline significance More participants receiving placebo reported symptoms in the hand (35.9% in emu oil group, 46.9% in placebo il group; statistically significant) |
|
| Interventions |
Intervention
Comparator
|
|
| Outcomes |
Primary outcome
Secondary outcome
Change in VAS and BPI Severity and Interference for whole group also assessed Exploratory assessment by treatment groups stratified for receipt of prior chemotherapy or not, taxane vs non‐taxane chemotherapy, age and type of AI also planned |
|
| Notes | Trial registry indicates funding from Novartis Pharmaceuticals Australia Pty Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Direct communication with trialist: quote: "Our oncology pharmacist performed the randomisation sequence list taking into account the stratification factors with patients consecutively enrolled. Unfortunately the documentation of the randomisation process that the pharmacist used has been archived and it is not easy to extract". Trial protocol indicates "Patients will be randomized to receive either emu oil or placebo oil within 14 days of consent. Stratification will be based on 2 factors: 1) centre, 2) received chemotherapy: yes vs no" (p.5). Quote: "There were no significant imbalances between the placebo and treatment group … Slightly more patients in the placebo group received non‐taxane chemotherapy, while more emu oil patients received endocrine therapy only; these differences being of borderline significance (p=0.07)" (p.3787). Participant and tumour characteristics appeared balanced between groups (Table 1 of publication). |
| Allocation concealment (selection bias) | Low risk | Quote from trial protocol: "Treatment kits will be prepared in advance, and sent to each site pharmacy … Half the kits sent to the site will have an Emu oil bottle for phase A and the other half of the kits will have the placebo oil for phase A. Each kit will be assigned a unique identification code, and all bottles within the kit will have this same number … Once patients are enrolled, they will be randomized (1:1 randomization) by the Mount Hospital Pharmacy to a drug kit number. Notification of this number will occur by return of the enrolment/randomization fax. This number will also be recorded on pharmacy accountability logs and the patient’s CRF" (p.6). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial was double blinded for the first 8 weeks. Quote: "At the 8‐week clinic visit, treatment allocation was unblinded and all participants were offered open‐label emu oil for a further 8 weeks" (p.3786). The primary outcome (difference between baseline and week 8 joint pain using VAS) was based upon the results obtained during the double‐blind period of the trial. Authors provided evidence that they used methods to blind participants to treatment received; quote: "Placebo oil contained: shea butter 43g, medium chain triglycerides 30g and almond oil (sweet) 190g, and carrot oil blend 0.35g (to match the colour of active emu oil)" (p.3786). Quote: "there were no observed toxicities" (p.3788) that could have potentially unblinded the participants to the treatment they had received. Direct communication with trialist: quote: "All staff (other than the pharmacist) and investigators were blinded to randomisation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial utilised PRO measures – VAS for joint pain and BPI for the severity of joint pain and its interference. Given the use of subjective PROs, there was a risk of bias in the measurement of the outcome. However, there were no treatment‐emergent adverse effects that may have led to additional follow‐up prior to the 8‐week assessment point, neither was there evidence of unblinding of the participants to the treatment they had received. Thus, it is unlikely that the measurement of the outcome has differed between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "87 eligible patients were recruited. 14 patients ceased allocated treatment within 3 weeks of registration or randomization and did not complete an 8‐week assessment. The reasons for cessation of study treatment were unrelated to toxicity (withdrew after randomization 7, inconvenience of topical application 2, arthralgia resolved 1, reason not specified 4)" (p.3787). Quote: "The intent‐to‐treat population was thus the 73 patients who completed the 8‐week treatment period" (p.3787). All data for the 73 participants who reached the first assessment point at 8 weeks were included in the primary outcome analysis. 73/87 participants represented a 16% attrition of the total participant population, with near equal losses in each treatment group. |
| Selective reporting (reporting bias) | Low risk | The VAS and BPI both assess joint pain, and thus are multiple measurements of the same outcome domain, however, all of these data are presented in Table 2 of the publication, so did not appear to be selectively reported. The results obtained after 8 weeks of treatment were not statistically significantly different between treatment groups, therefore less likely that result was subject to reporting bias. The article indicates that "the primary objective was the difference between baseline and week 8 joint pain for each treatment group as assessed by the VAS, with pain difference between these two time points and difference in function as assessed by the BPI as secondary endpoints" (p.3786). The trial registry indicated that the change from baseline to week 8 joint pain according to the BPI was also included as a primary outcome. The trial protocol indicated that the change in joint pain and stiffness at 8 weeks according to the BPI were secondary endpoints (p.5). Thus, the data presented in the article were analysed in a manner that was in accordance with a prespecified statistical analysis plan. |
| Other bias | Low risk | No other bias. |
Henry 2018.
| Study characteristics | ||
| Methods | Phase III, placebo‐controlled RCT Duration of intervention: 14 weeks Follow‐up: 24 weeks TREATMENT TRIAL Participants enrolled from May 2013 to October 2015 from 43 institutions in the USA |
|
| Participants |
Inclusion criteria Disease‐related criteria
Clinical/laboratory criteria
Exclusion criteria
299 participants randomised, 150 in duloxetine group, 149 in placebo group. 5 ineligible in each group (CONSORT diagram Figure 1). 289 eligible; 145 in duloxetine group, 144 in placebo group Median age: 60 years, range 27–83 years. 71% were aged < 65 years. 86% white Mean duration of current AI therapy: 47.9 (SD 36.3) weeks 55% in duloxetine group and 53% in placebo group had received prior taxane. The majority (86% in duloxetine group vs 88% in placebo group) had not received prior tamoxifen 64% (65% in duloxetine group vs 63% in placebo group) had been on AI therapy for < 1 year, while 28% (26% in duloxetine group vs 29% in placebo group) had been on AI therapy for 1–2 years. Majority (82%) received their first AI Most participants (76%) had a baseline pain score (BPI‐SF) of 4–6 |
|
| Interventions |
Intervention
Comparator
Commercially available, open‐label duloxetine was allowed after study treatment ended. |
|
| Outcomes |
Primary outcome (Questionnaires administered at baseline, 2, 6, 12 and 24 weeks)
Secondary outcomes
NCI‐CTCAE v4.0 utilised for adverse event reporting |
|
| Notes | Funding from: "National Cancer Institute of the National Institutes of Health (Award Numbers: CA189974, CA180820, CA189821, CA189854, CA189858, CA189808, CA189830, CA189971, CA189952, CA189953, CA189957, CA189872, CA180835, CA189804, CA189873, CA190002, CA189853, CA189848, CA180801, CA189822, CA189954, CA189972, CA189829, CA189856, CA189960, CA189816, CA189823, CA180868, CA189867, CA180818); Damon Runyon‐Lilly Clinical Investigator Award No. CI‐53‐10 to N. Lynn Henry and in part by Lilly USA, manufacturer of Cymbalta (duloxetine)". Conflict of interest: "N. Lynn Henry Research Funding: BioMarin (Inst), Celldex (Inst), Innocrin. Pharmaceutical (Inst), Pfizer (Inst). Joseph M. Unger No relationship to disclose. Anne F. Schott Research Funding: Dompe Farmaceutici (Inst), Novartis (Inst), Pfizer (Inst), Genentech (Inst). Patents, Royalties, Other Intellectual Property: Systems and Methods for Tissue Imaging, 8,185,186, Inventor, submitted on 04/2008. This is a patent for use of diffusion magnetic resonance imaging technology to quantitate response to neoadjuvant breast cancer therapy. Louis Fehrenbacher Research Funding: Roche (Inst) Patrick J. Flynn. Employment: ARIAD (I), Sanofi (I)Stock or Other Ownership: ARIAD (I), Sanofi (I) Consulting or Advisory Role: Shire Speakers' Bureau: Eli Lilly, Novartis. Debra M. Prow. Employment: McFarland Clinic. Carl W. Sharer Employment: Community Health Systems Stock or Other Ownership: Aetna. Gary V. Burton No relationship to disclose. Charles S. Kuzma No relationship to disclose. Anna Moseley No relationship to disclose. Danika L. Lew No relationship to disclose. Michael J. Fisch Employment: AIM Specialty Health Stock or Other Ownership: Anthem. Carol M. Moinpour Stock or Other Ownership: Amgen (I), Pfizer (I), Merck (I). Dawn L. Hershman No relationship to disclose. James L. Wade Employment: Johnson & Johnson (I) Stock or Other Ownership: Seattle Genetics, Celgene". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned 1:1 to receive duloxetine or placebo" (p.327). Quote: "Random assignment was dynamically balanced according to the two stratification factors: baseline BPI average pain score (4 to 6 v 7 to 10) and prior taxane use (yes vs no)" (p.327). Quote: "All site staff will use OPEN to enroll patients to this study. OPEN is a web‐based application and can be accessed at https://open.ctsu.org, or from the OPEN tab on the CTSU members’ side of the website at https://www.ctsu.org, or from the OPEN Patient Registration link on the SWOG CRA Workbench The OPEN system will provide the site with a printable confirmation of registration and treatment information" (p.73). Quote: "There was no evidence that patterns of baseline stratification factors differed by arm for those with versus without 2, 6 and 12 week scores, or by one or more of the three scores (interaction P>0.05 in all instances" (p.328). Table 1 (p.329 of the publication) presents baseline patient characteristics by arm. Quote: "There were no sizeable imbalances in the baseline characteristics between study arms" (p.328). |
| Allocation concealment (selection bias) | Low risk | Quote from trial protocol details process of preparing concealed drug bottles (p.15): "Upon receipt of patient registration and randomization notification, Biologics will: Place a call to the study site confirming the order received … prepare shipment of patient‐specific drug. Shipments will include 3 bottles (Bottles 1 and 3 will have 7 tablets of 30mg of duloxetine or placebo; Bottle 2 will have 154 tablets of 30mg of duloxetine or placebo) of study drug to cover the 12 week treatment period accompanied by a patient‐specific labeled resealable bag. Each shipment includes a patient label on the resealable bags". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and providers were blinded to treatment allocation" (p.327). Quotes: "During treatment, AEs occurred in 78% of the duloxetine group and 50% of the placebo group (Fisher’s exact test, p <0.001)" (p.329). "More patients in the duloxetine arm compared with the placebo arm thought they were receiving duloxetine (79% v 50%; p <0.001)" (data supplement p.329). Comment: this statistically significant difference in occurrence of adverse events and participants successfully 'guessing' which intervention they had received would tend to suggest a difference in adverse effects that had the potential to unblind individuals to treatment received. Trial staff administering questionnaires possibly could have been unblinded to the treatment allocation, as participants reporting adverse events may have been considered to have received the drug. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study used PROs, primary outcome was "reduction in average joint pain according to the Brief Pain Inventory – Short Form (BPI‐SF) average pain score" (protocol p.30). Quote: "Patients completed post registration questionnaires at weeks 2 (+/‐ 7 days), 6 (+/−14 days), 12 (+/− 14 days), and 24 (12 weeks after discontinuing the full‐dose intervention)" (p.327). Quote: "More patients in the duloxetine arm compared with the placebo arm thought they were receiving duloxetine (79% v 50%; p < .001; Data Supplement. In addition, more patients in the duloxetine arm compared with the placebo arm reported that the study treatment was beneficial and had limited or no AEs (52% v 36%) or that it was beneficial despite the AEs (19% v 13%; Data Supplement)" (p.329). Comment: given the use of PROs, assessment of the outcome could have been influenced by knowledge of intervention received, e.g. those that thought they were receiving drug may be more likely to report a beneficial effect on their pain. Given that (quote) "more patients in the duloxetine arm compared with the placebo arm thought they were receiving duloxetine" (p.329), it seems possible that participants could have assessed their pain with knowledge of receiving duloxetine. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 299 registered patients, 10 were ineligible. Therefore, 145 patients in the duloxetine arm and 144 patients in the placebo arm were eligible" (p.327). From CONSORT diagram, Figure 1: 127/145 in the duloxetine arm were evaluable for primary endpoint. Represents 12.4% attrition. 128/144 in placebo arm were evaluable for primary endpoint. Represents 11.1% attrition There was a comparable degree of attrition in each of the intervention groups, but this represents a modest attrition of 11.8% overall. Quote: "Given the primary analysis is based on longitudinal measures, a sensitivity analysis will consider all observations within 14 weeks of registration" (protocol p.31). Quote with regards to BPI mean pain score reduction in the duloxetine group: "Results were similar if all follow‐up scores within 14 weeks were included (−0.76, 95% CI −1.17 to −0.34)" (p.328). Sensitivity analyses were also conducted on the secondary endpoints and were consistent with the results attained from the in‐window measurements. |
| Selective reporting (reporting bias) | Low risk |
NCT01598298/protocol (p.6) indicated endpoints. All major endpoints reported other than exploratory in references. Multiple domains examined joint pain (BPI‐SF, GRCS‐Pain, WOMAC and M‐SACRAH), but all of these measures at individual time points are presented in data supplement. Quote: "Section 11.0 Statistical Considerations" (trial protocol p.30–33) details how data will be analysed (version from 2015). All reported data were analysed according to this protocol. |
| Other bias | Low risk | No other bias. |
Hershman 2015a.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel assignment, phase III RCT Duration of intervention: 24 weeks TREATMENT TRIAL Participants recruited from February 2012 to February 2013 at 52 sites in the USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
262 participants randomised, 131 in O3‐FA group, 131 in placebo group. 4 participants ineligible, 7 participants withdrew consent for all follow‐up, leaving 249 evaluable participants; 122 in O3‐FA group, 127 in placebo group Median age: 59.2 years, range not stated AI therapy (in evaluable participants): 146 (59%) anastrozole, 29 (12%) exemestane, 74 (30%) letrozole Median 1.2 years since commencement of AI No notable imbalances in baseline characteristics by arm were observed by age, ethnicity, prior osteoarthritis or prior taxane use Most participants were white (87%); fewer black women were randomly assigned to the O3‐FA arm (4%in O3‐FA arm, 12% in placebo arm). 58% of participants had received prior taxane |
|
| Interventions |
Intervention O3‐FAs 3.3 g per day (6 capsules; Ocean Nutrition, Dartmouth, Nova Scotia, Canada) orally daily for 24 weeks. Each active capsule contained 560 mg of EPA + DHA in a 40:20 ratio. Comparator 6 matching placebo capsules daily for 24 weeks. Placebo capsule contained a blend of soybean and corn oil Active and placebo capsules both coloured with carob and flavoured with natural lemon–lime |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Supported by the Breast Cancer Research Foundation, by the NCI Division of Cancer Prevention, and by NCI Community Oncology Research Program Research Base Grant No.1UG1CA189974‐01. Conflict of interest statements: "Dawn L. Hershman No relationship to disclose. Joseph M. Unger No relationship to disclose. Katherine D. Crew No relationship to disclose. Danielle Awad No relationship to disclose. Shaker R. Dakhil No relationship to disclose. Julie Gralow Consulting or Advisory Role: Roche/Genentech, Novartis Research Funding: Roche/Genentech (Inst), Novartis (Inst), Amgen (Inst). Heather Greenlee Consulting or Advisory Role: EHE International. Danika L. Lew No relationship to disclose. Lori M. Minasian No relationship to disclose. Cathee Till No relationship to disclose. James L. Wade III Employment: Johnson & Johnson (I) Stock or Other Ownership: Seattle Genetics, Celgene. Frank L. Meyskens No relationship to disclose. Carol M. Moinpour No relationship to disclose". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All site staff will use OPEN to enroll patients to this study. OPEN is a web based application and can be accessed at https://open.ctsu.org, or from the OPEN tab on the CTSU members’ side of the website at https://www.ctsu.org, or from the OPEN Patient Registration link on the SWOG CRA Workbench" (p.26 protocol). Quote: "SWOG Statistical Center will notify UVI electronically early each morning of the randomizations from the previous day. This notification will result in an order for a patient specific kit" (p.12 protocol). |
| Allocation concealment (selection bias) | Low risk | Quote: "All site staff will use OPEN to enroll patients to this study. OPEN is a web based application and can be accessed at https://open.ctsu.org, or from the OPEN tab on the CTSU members’ side of the website at https://www.ctsu.org, or from the OPEN Patient Registration link on the SWOG CRA Workbench" (p.26 protocol). Quote: "SWOG Statistical Center will notify UVI electronically early each morning of the randomizations from the previous day. This notification will result in an order for a patient specific kit" (p.12 protocol). Comment: indicates allocation concealment likely done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "double‐blind multicenter trial". "Both the active and placebo capsules were colored with carob and flavored with natural lemon‐lime" (p.1911 primary publication). Quote protocol (p.11): "Formulation of omega‐3‐fatty acid: Each 1,000 mg capsule contains a combination of 560 mg ethyl esters of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) in a 40:20 ratio. Omega‐3 fatty acid capsules also contain mixed natural tocopherols. Capsules are colored with carob and flavored with natural lemon‐lime. Formulation of placebo: The matching placebo capsules are a blend of soybean: corn oil, in a 1:1 ratio. All capsules are colored with carob and flavored with lemon‐lime". Protocol (p.18): Same number of capsules for intervention or placebo, at same time intervals. Quote: "Patients will be randomized to receive 6 capsules (at 1,000 mg each) of omega3‐fatty acid or placebo". Quote: "There were 27 cases of grade ≥ 1 of these adverse events among patients receiving O3‐FAs (23%) and 33 cases among those receiving placebo (27%; P =.68); there were 13 cases of grade≥ 2 of these adverse events among those receiving O3‐FAs (11%) and 25 cases among those receiving placebo (20%; P =.09)" (p.1914). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded as per above comments, placebo identical. Outcomes were PROs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "BPI worst pain scores were not available for 20 (16%) of the 122 evaluable patients in the O3 ‐FA arm (dropout, n = 18; nonadherence, n = 2); in the placebo arm, scores were not available for 20 (16%) of 127 eligible patients (dropout, n = 19; nonadherence, n = 1). Therefore, dropout and nonadherence were lower than anticipated and did not differ by arm" (p.1913. Comment: however, note that there appears to be a potential discrepancy/ error in the Consort diagram (Fig 1) in O3FA arm for "BPI not available" group number. No response to requests for further information from authors. This is judged to be a simple error. Quote: "Dropout and nonadherence were lower than anticipated and did not differ by arm" (p.1913). |
| Selective reporting (reporting bias) | Low risk | Trial registry NCT01385137 Primary outcome: BPI worst pain/ stiffness 12 weeks Secondary outcomes: adverse events, WOMAC 12 weeks, M‐SACRAH 12 weeks, FACT‐ES 12 weeks All outcomes reported Table A1 of the publication |
| Other bias | Unclear risk | Unclear risk of contamination in the placebo arm re O3‐FA exposure. Quote: "It is also possible that the placebo contained ingredients (soy/corn oil) that were active in reducing arthralgia. It is also possible there was some contamination in the placebo arm, although the lower triglyceride levels in the O3‐FA arm suggests that any contamination was limited" (p.1914). |
Khan 2017.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel assignment RCT Duration of intervention: 24 weeks PREVENTION TRIAL Recruited from the University of Kansas Cancer Center and the Cancer Center of Kansas in Wichita, Kansas, USA between April 2009 and July 2010 |
|
| Participants |
Inclusion criteria
Exclusion criteria
160 participants randomised; 80 in vitamin D3 group, 80 in placebo group Mean age: 60.5 years (range 55–71) years in vitamin D3 group, 62 years (range 54–69) years in placebo group Majority of participants were white (95%) and more than half had received adjuvant chemotherapy (Table 1 of the publication; 41 (51%) participants in vitamin D3 group, 45 (56%) participants in placebo group More participants in the placebo arm were taking vitamin D at baseline (29) than the high‐dose vitamin D group (20). Mean baseline 25‐OHD levels: vitamin D3 group 21.9 (SD 9.1) ng/mL, placebo group 23.9 (SD 9.1) ng/mL |
|
| Interventions |
Intervention Calcium 1200 mg daily + vitamin D3 600 IU daily + vitamin D3 30,000 IU (3 capsules of 10,000 IU vitamin D3) once per week for 24 weeks Comparator Calcium 1200 mg daily + vitamin D3 600 IU daily + placebo (3 capsules matching placebo) once per week for 24 weeks All participants received with letrozole 2.5 mg daily orally |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Only participants on letrozole included Quote: "Letrozole and funding for this study were provided by Novartis Pharmaceutical Corporation (East Hanover, NJ). Vitamin D3 (10,000 IU capsules) and matched, blinded placebo were provided by BTR Group, Inc. (Pittsfield, IL)" (p.499) (Khan 2017). Conflict of interest statement: "The following authors are without financial interests or conflicts of interest related to this trial: Kimler, Reddy, Klemp, Nydegger, and Yeh. Within the past three years, Dr. Khan has served as a consultant to Novartis Pharmaceutical Company, Inc. and Pfizer. Drs. Khan and Sharma have received during the past three years, via their institution, funding for support of research and clinical trials from the following companies: AstraZeneca; Bristol‐Myers Squibb; Celgene, Inc.; Novartis Pharmaceutical Company, Inc.; Genentech‐Roche, GlaxoSmithKline, and Pfizer. Study agent but no funding has been provided by DSM and Pfizer for trials conducted by Dr. Fabian" (p.499) (Khan 2017). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were stratified by site and use of adjuvant chemotherapy and randomized 1:1 to either 30,000 IU VitD3 (VitD3) or placebo weekly for 24 weeks (three capsules of 10,000 IU VitD3 or three capsules of matched placebo weekly). Only the study biostatistician and investigational pharmacists were aware of drug assignments" (p.493). Author correspondence: "Randomization was via a block design after stratification for accrual site (two sites) and use of chemotherapy (yes/no). Performed by a biostatistician and sent to investigational pharmacies only. Blind broken after initial formal analyses of primary endpoints by biostatistician". |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were stratified by site and use of adjuvant chemotherapy and randomized 1:1 to either 30,000 IU VitD3 (VitD3) or placebo weekly for 24 weeks (three capsules of 10,000 IU VitD3 or three capsules of matched placebo weekly). Only the study biostatistician and investigational pharmacists were aware of drug assignments" (p.493). Author correspondence: "Randomization was via a block design after stratification for accrual site (two sites) and use of chemotherapy (yes/no). Performed by a biostatistician and sent to investigational pharmacies only. Blind broken after initial formal analyses of primary endpoints by biostatistician". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were stratified by site and use of adjuvant chemotherapy and randomized 1:1 to either 30,000 IU VitD3 (VitD3) or placebo weekly for 24 weeks (three capsules of 10,000 IU VitD3 or three capsules of matched placebo weekly). Only the study biostatistician and investigational pharmacists were aware of drug assignments" (p.493). Confirmed by author correspondence. Quote: "Excluding the adverse events related to letrozole, there were no adverse events attributed to vitamin D3. One subject in placebo group had mild hypercalcemia at 12 week assessment. She was taking a thiazide diuretic and had serum 25(OH)D level of 20 ng/ml at the time" (p.494). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | PROs and investigator reported (hand‐grip). Quote: "Participants were stratified by site and use of adjuvant chemotherapy and randomized 1:1 to either 30,000 IU VitD3 (VitD3) or placebo weekly for 24 weeks (three capsules of 10,000 IU VitD3 or three capsules of matched placebo weekly). Only the study biostatistician and investigational pharmacists were aware of drug assignments" (p.493). Confirmed by author correspondence. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Consort diagram Figure 1. 160 participants randomised. Quote: "Of the 160 subjects enrolled and randomized (Fig. 1), one subject was determined to be not eligible because she consumed high dose VitD between screening assay and baseline visit. An additional 12 subjects did not complete the study for reasons unrelated to study agents and/or AIMSS (withdrawal of consent, never started study agent, personal decision, etc.). Additionally, all quality of life assessments were not available for all participants at all visits. Specifically, 72 and 70 complete sets of assessments were available for the vitD and placebo arms at week 12, and 73 and 65 at week 24. The number of missing values was not significantly different between arms at week 12 or 24 (2‐sided p = 0.80 and 0.11 by Fisher’s exact test). Overall, sufficient information was available to allow assessment of protocol defined worsening of AIMSS for 147 evaluable subjects, with 77 in the placebo group and 70 in the VitD3 group" (p.494). |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome as per trial registry updated in 2018: "Number of Participants With Worsening of Musculoskeletal Symptoms (MS) [ Time Frame: Change from Baseline to 24 Weeks ] Worsening of Musculoskeletal Symptoms (MS) is defined as any one of the following three events: (a) an increase by at least 0.25 in the Health Assessment Questionnaire II (HAQ II, a measure of disability from joint pain) score, (b) an increase in patient reported severity of joint and/or muscle pain, or (c) discontinuation from trial prior to 24 weeks specifically because of problems with musculoskeletal symptoms. Secondary outcome as per NCT: Visual analog pain scale; hand grip strength; menopausal Quality of Life (MEN ‐QOL); serum 25OHD levels; serum letrozole levels; single nucleotide polymorphisms of vitamin D receptor genes [ Time Frame: 6 months ]" In addition to those listed on Clinical Trials Registry, the protocol also listed 3 additional validated tools: CPIS, BPI and VAS as well as additional QoL assessment with FACT‐B. The article stated that the questionnaires included: HAQ ‐II, CPIS, BPI, BFI, FACT‐B and MENQOL Quote: "Four other measures including hand grip strength showed a similar lack of difference between groups (Table 4), as did quality of life assessments using FACT‐B and MENQOL (data not shown)" (p.495 primary publication). Raw scores were provided by author correspondence for BPI, BFI and MENQOL; however, author correspondence stated that the FACT‐B results were not available. Therefore, not all prespecified outcomes are available for assessment/have been reported, therefore, risk in this category is considered unclear due to missing information. |
| Other bias | Low risk | No other bias. |
Li 2017.
| Study characteristics | ||
| Methods | Parallel RCT Duration of intervention: 12 weeks TREATMENT TRIAL Enrolled from Guang'anmen Hospital, Beijing, China from May 2015 to May 2016 |
|
| Participants |
Inclusion criteria
Exclusion criteria
72 participants; 36 in each group. 70 participants evaluable Median age: 57 years (range 27–73 years); > 50% had undergone natural menopause Most stage I–II disease Most had received adjuvant chemotherapy, with slightly more participants in the bionic tiger bone group receiving adjuvant taxane (37.1% in bionic tiger bone group vs 22.9% in placebo group) More than half received anastrozole (60% in bionic tiger bone group vs 51.4% in placebo group) |
|
| Interventions |
Intervention
Comparator
|
|
| Outcomes |
Primary outcomes (Questionnaires at baseline, 6 and 12 weeks)
Secondary outcome
E2 and FSH assays were performed on serum samples at baseline, 6 and 12 weeks to see if the intervention influenced patient's hormone levels |
|
| Notes | Dr Wenping Lu received financial support from National Natural Science Foundation of China (NSFC), H2902 and H2704. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization list was prepared using random permuted blocks" (p.2). Quote: "Baseline demographic and clinical characteristics were comparable between the two groups" (p.2). Baseline characteristics Table 1 (p.3 of the publication). The baseline characteristics of age, BMI, type of menopause, AI type and pathological states seemed well balanced. Slightly numerically more participants in the tiger bone group received adjuvant chemotherapy (25 (71.4%) in tiger bone group vs 21 (60%) in placebo group, and adjuvant taxane (13 (37.1%) in tiger bone group vs 8 (22.9%) in placebo group. |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutive assignments were placed into separate numbered, sealed envelopes. And group assignments will not be revealed until the entire study is completed" (p.2). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants, investigators, statisticians and all study staff are blinded, under the supervision of Ethics Committee of Guang’anmen Hospital" (p.2) Quotes: "TB group were given tiger bone powder made into capsule…the recommended dose is 1.2g, 3 times daily" (p.2). "Participants in placebo group were given calcium carbonate tablets, whose main ingredients are calcium and vitamin D, with recommended dose of 600mg daily" (p.2). Compared to taking the standard calcium carbonate 600 mg tablet once a day, there was a difference in dosing of the tiger bone group with participants taking 1.2 g capsules 3 times a daily. This had the potential to unblind the participants, and the personnel administering the medication to the treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "M‐BPI and VAS were used as the primary outcomes. FACT‐B was used as the secondary outcome" (p.1). Outcomes were all PROs. Participants who had received tiger bone potentially knew they had received this intervention, and may have reported more positive effects on pain. In contrast, participants who knew they had received placebo may have anticipated worse or no improvement on pain, and could have biased the direction of effect in favour of the intervention. Given the exclusive use of PROs, it is possible that knowledge of the intervention received may have biased the reporting of the pain outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "72 participants were enrolled and completed baseline questionnaires; 70 of them were evaluable. One patient immigrated to Japan, and another patient had scheduling difficulties to continue. All evaluable patients were randomly assigned" (p.2). CONSORT diagram (Figure 1; p.6) shows that 1 participant receiving tiger bone dropped out at week 3, while 1 participant receiving placebo dropped out at week 8. Quote: "2 participants failed to continue the intervention. One in TB [tiger bone] group lost her mother at 3 weeks, so that she had difficulties in scheduling. Another participant in placebo group, her husband had a job change, and the whole family had moved to Japan on week 8. Other 70 participants had finished the intervention, and there were no lost cases" (p.3). |
| Selective reporting (reporting bias) | Unclear risk | Mean M‐BPI and VAS data, the primary outcomes, were presented at all the relevant time points when questionnaires were administered (baseline, week 6 and week 12). In the methods section, the primary study publication stated that HRQoL as assessed by the FACT‐B was used as a secondary outcome measure. However, the trial registry stated that the FACT‐B was to be a primary outcome measure. Therefore, it was unclear whether these FACT‐B data have been analysed in accordance with a prespecified analysis plan. |
| Other bias | Low risk | No other bias. |
Liu 2014.
| Study characteristics | ||
| Methods | Parallel RCT Duration of intervention: 3 months TREATMENT TRIAL Recruitment at Peking University People's Hospital, China |
|
| Participants |
Inclusion criteria
Exclusion criteria None listed 91 participants enrolled; 45 in calcitonin group, 46 in placebo group. 9 excluded from study (3 in calcitonin group, 6 in placebo group). 82 participants included in the analysis (42 in calcitonin group, 40 in placebo group). 1 participant included in calcitonin group had metastatic disease (Table 1 of publication). Mean age: 59.6 (SD 9.2) years in calcitonin group, 61.3 (SD 6.9) years in placebo group Baseline characteristics only on participants included in the analysis Prior chemotherapy/taxane: see Table 1 of the publication 26/42 (62%) participants in calcitonin arm had prior chemotherapy, 19 of these (45%) had taxane 24/40 (60%) participants in placebo arm had prior chemotherapy, 12 of these (30%) had taxane Only included participants receiving anastrozole, duration of AI therapy not stated |
|
| Interventions |
Intervention Calcitonin 200 IU/day salmon calcitonin + caltrate D 600 mg/day for 3 months Comparator Caltrate D 600 mg/day for 3 months |
|
| Outcomes |
|
|
| Notes | Unable to contact study authors as email on publication no longer valid. Research supported by AstraZeneca. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated" (p.5287). Comment: insufficient information to permit judgement. Author email invalid. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information in publication to permit judgement. Quotes: "Patients were randomly allocated to one of two groups according to the drug received for three months: the calcitonin group received 200 IU/day salmon calcitonin and 600 mg/day Caltrate D, and the control group received 600 mg/day Caltrate D" (p.5287 in Methods section). "200 IU calcitonin per day or placebo" (p.5289 in Discussion section). Insufficient information regarding placebo. No information on blinding procedures. No adverse event/toxicity data provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | PRO of pain. No information on blinding procedures. Insufficient information regarding placebo details, insufficient information to permit judgement regarding blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 91 participants enrolled. Quote: "Nine patients were excluded from the study, three in the calcitonin group and six in the control group. Only 82 cases were analysed" (p.5287). No consort diagram; or reasons for exclusion documented. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. VAS was an outcome; however, no information documented about whether this was a prespecified outcome or a primary or secondary outcome. Author email invalid. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Lustberg 2018.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel feasibility RCT Duration of intervention: 24 weeks PREVENTION TRIAL Recruited between 9 November 2011 and 29 October 2013 at Ohio State University Wexner Medical Center, Columbus, Ohio, US |
|
| Participants |
Inclusion criteria
Exclusion criteria
44 participants randomised; 22 in O3‐FA group, 22 in placebo group Baseline clinical and demographic characteristics similar between the 2 groups (Table 1 of the publication) Mean age: 59.5 (SD 8.1) years, range 43–76 years Most participants had stage I breast cancer (27 (61%)). 98% of participants were white. History of medical comorbidities, osteoarthritis, was similar between groups, as was chemotherapy exposure (34% overall group taxanes) |
|
| Interventions |
Intervention 6 capsules per day in divided doses for 24 weeks to provide either EPA + DHA 4.3 g/day. Each active capsule contained approximately EPA 430 mg and DHA 230 mg in triglyceride form Comparator 6 capsules per day in divided doses for 24 weeks of placebo containing a mixture of fats and oils formulated to mirror the ratio of fatty acids typical of the American diet (Ervin 2004) Both types of capsules were produced by Marine Ingredients, Mt Bethel, PA, and were matched in appearance and lemon flavouring. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Supported by the NCI of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant). Conflict of interest statement: all authors declared that they had no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomization was utilised" (p.710). |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the pharmacist who dispensed the study agent was aware of randomisation assignment" (p.710). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "only the pharmacist who dispensed the study agent was aware of randomization assignment. Women were advised to take six capsules per day in divided doses for 24 weeks to provide either 4.3 g/day of EPA + DHA or a placebo containing a mixture of fats and oils formulated to mirror the ratio of fatty acids typical of the American diet". "Both types of capsules were produced by Marine Ingredients, Mt. Bethel, PA, and were matched in appearance and lemon flavoring" (p.710). Toxicities Table 2 of the publication – most similar between groups; numerical difference in the number of participants who experienced fishy aftertaste – experienced by 6 participants in O3‐FA group vs 1 in placebo group (P = 0.09). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Majority of primary and secondary outcomes were PROs, and we assumed participants and assessors were blinded because it stated that only the pharmacist was aware of the randomization assignment. Blood/serum markers objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "44 women were enrolled, 44 randomized, and 35 (80%) were evaluable at 24 weeks (Fig. 1)" (p.712). Fig 1 Consort‐ follow‐up incomplete in 5/22 participants in O3‐FA group, 7/22 in placebo. Quote: "There was no differential dropout between the treatment groups (p=0.88). The main reasons for not continuing treatment were difficulty remembering to take supplements (n=2), significant arthralgias resulting in therapy switch (n=1), intolerance of supplements (n=3) and scheduling difficulty (n=1)" (p.712). |
| Selective reporting (reporting bias) | High risk | Trial registry NCT01478477 Primary outcome stated as pain scores after 6 months based on FACT‐B/FACT‐ES baseline and 6 months. Final journal report states that primary outcomes are safety and tolerability. Additional information available in appendix. No response from authors to email seeking clarification. |
| Other bias | Unclear risk | Unclear risk of contamination in the placebo arm regarding O3‐FA exposure. Uncertain effect of placebo ingredients on AI arthralgia. Quote: "placebo containing a mixture of fats and oils formulated to mirror the ratio of fatty acids typical of the American diet" (p.710). |
Massimino 2011.
| Study characteristics | ||
| Methods | Single centre, pilot cross‐over RCT Duration of intervention: 6 months, cross‐over at 3 months TREATMENT TRIAL Participants recruited from 'Legacy Health System' hospital network across Oregon and Washington, USA between April 2008 and June 2011 |
|
| Participants |
Inclusion criteria
Exclusion criteria
37 participants (no indication of number in each treatment arm). 6 early withdrawals, which left 31 evaluable participants No baseline characteristic data provided |
|
| Interventions |
Intervention
Comparator
|
|
| Outcomes |
Primary outcome
|
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract and clinical trial registry states this was randomised trial but no information provided on randomisation sequence generation. No information provided on baseline patient characteristics to suggest whether there was a problem with the randomisation process. Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on methods employed to conceal allocation. Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind crossover placebo controlled trial". No information on process of blinding, and no information about occurrence of adverse events and whether there were differences between groups Abstract quote: "At crossover the BC+P [Blue Citrus then placebo] group experienced a spike in symptomology when changed to placebo that didn’t appear for the P+BC [placebo + Blue Citrus] group". Comment: potential that participants in the blue citrus group may have been unblinded to treatment received. Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | VAS for arthralgia is a PRO. Given uncertainty surrounding blinding and insufficient information on performance bias, there is insufficient information to permit judgement on whether the assessment of pain was influenced by knowledge of intervention received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only – insufficient information to permit judgement. Quote: "A total of 37 patients enrolled on the study. There were 6 early withdrawals, which left 31 evaluable patients". It is not clear when these women withdrew and their reasons for withdrawal. 6/37 (16.2%) total attrition. This is a moderate degree of attrition; however, without reasons stated for withdrawal difficult to determine whether the absence of such data could have made a substantial impact. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only – insufficient information to permit judgement. No protocol or statistical analysis plan available. Trial registry stated that primary outcome is to "determine if Blue Citrus decreases musculoskeletal symptoms while on AIT as compared to placebo". Main outcome measure reported in abstract was VAS for joint pain (arthralgia), reported at 90 days (3 months of initial treatment) and 180 days (3 months of additional cross‐over treatment), so have included data post‐cross‐over. |
| Other bias | Unclear risk | Cross‐over trial; however, insufficient information to permit judgement of the impact of cross‐over design. |
Niravath 2019.
| Study characteristics | ||
| Methods | Phase II, parallel RCT Duration of intervention: 52 weeks PREVENTION TRIAL Participants recruited at 3 centres: Breast Oncology Clinics at Baylor College of Medicine, Harris Health Systems' Smith Clinic and Siteman Cancer Center at Washington University, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
93 participants randomised from February 2014 through July 2017 at time of futility analysis, enrolment terminated after futility analysis (trial designed to enrol 184 participants). 46 in high‐dose vitamin D group, 47 in standard‐dose vitamin D group Median age: 64 years, range (44–82) years Baseline characteristics similar between groups (Table 1 of publication) Most participants were ethnic minorities (44% Latina participants and 18% African American participants). Most participants were overweight or obese (86% of participants with BMI > 25) 43% of participants received chemotherapy, which included a taxane in 95% Mean baseline serum 25‐OHD level: 21.7 ng/mL in high‐dose vitamin D group, 24.2 ng/mL in standard‐dose group |
|
| Interventions |
Intervention Vitamin D3 50,000 IU per week orally for 12 weeks followed by 2000 IU daily for 40 weeks. Participants instructed to take vitamin D 50,000 IU (10 capsules of 5000 IU) orally on a weekly basis for 12 weeks, on the same day each week, and at approximately the same time for each dose. Vitamin D should have been taken shortly after a meal. Participants then completed 40 additional weeks of 2000 IU daily (1 tablet daily of 2000 IU dose), with food Comparator Vitamin D3 800 IU daily orally for 52 weeks All women on both arms of the trial were also given calcium carbonate 600 mg daily. |
|
| Outcomes |
Primary outcomes Number of participants with AIA after 12 weeks of therapy as defined by: increase in HAQ‐II score from baseline by ≥ 0.2 or increase in VAS pain score by ≥ 0.3 Secondary outcomes Compliance with AI therapy at 52 weeks (tablet counting) Serum 25‐OHD level at baseline and week 12 Exploratory endpoint Grip strength correlated with AIA at baseline, week 12 and week 52 |
|
| Notes | Funding source of study (personal communication with Dr Niravath): career development grant from Baylor College of Medicine, for USD 3000 Conflict of interest statements: "Dr. Nangia has had a consultant/advisory role with Puma, and she has received funding from Paxman Coolers. Dr. Ademuyiwa has had a consultant/advisory role with Immunomedics, AstraZeneca, Jounce, Eisai, and Best Doctors; she has received funding from Pfizer, Abbvie, Seattle Genetics, Immunomedics, and Polyphor. Dr. Ellis has had a consultant/advisory role with NanoString, Novartis, AstraZeneca, Pfizer, Abbvie, Sermonix, and Puma; he has stock ownership in Bioclassifier with Royalty income from Prosigna/NanoString. Dr. Osborne has had a consultant/advisory role with Puma, AstraZeneca, and Genentech; stock ownership in GENETEX; and funding fromPuma. Dr. Rimawi has had a consultant/advisory role in MacroGenics, Daiichi, and Novartis; he has received funding from Novartis and Pfizer. Dr. Ma has had a consultant/advisory role with Pfizer, Novartis, and Lilly; she has received funding from Eisai, Puma, and Pfizer". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Prior to enrollment in the electronic database, the eligibility worksheet must be reviewed and signed by the study coordinator and a Breast Center physician investigator, in accordance with Lester & Sue Smith Breast Center clinical research standard operating procedures. Eligibility data will then be entered in the electronic database and the subject will be randomized" (p.16 protocol). Further correspondence with the author: "We had an automated randomisation process through the clinical trial software, OnCore". |
| Allocation concealment (selection bias) | Low risk | Author correspondence: use of "clinical trial software, OnCore". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded. No placebo. Dosing schedules differed between groups. Quote: "Patients were randomised 1:1 to receive either 50,000 International Units (IU) oral vitamin D3 per week for 12 weeks followed by 2000 IU daily for 40 weeks, or 800 IU Vitamin D3 daily for 52 weeks. All women on both arms of the trial were also given calcium carbonate 600mg daily, per Institute of Medicine Guidelines. See Fig. 1 for study design" (journal article p.428). Quote: "Patients will be instructed to take Vitamin D 50,000 IU (10 capsules) PO on a weekly basis, on the same day each week, and at approximately the same time each dose". "After completing 12 weeks of the high dose vitamin D, the patients randomised to this arm will then complete 40 additional weeks of 2000 IU strength, and the patients will take 1 pill daily, with food". "Patients on the high dose Vitamin D arm will be asked to take over‐the counter Calcium supplement, as is recommended as standard of care" (study protocol p.21–22: high‐dose group). Quote: "The patients who are randomised to the standard dose Vitamin D arm will take 1 Caltrate‐D pill daily" (study protocol p.21–22: standard‐dose group). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary outcomes were PROs and participants were not blinded. Quote: "The HAQ‐II is the primary measure used to determine development of AIA in this study"p.429. Furthermore, the protocol (p.20) stated "The questionnaire will be completed in the office during the clinic visit and it will be administered by the study coordinator" who was also unblinded. Quote: "Because the patients and the physicians knew which study treatment each subject was receiving, there could have been some bias in assessing arthralgia, as per the questionnaire" (p.434). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All patients who were randomized were considered evaluable for response, regardless of how much treatment they received, consistent with an intention to treat analysis. Any patient who was randomized but did not complete the HAQ ‐II at 12 weeks was designated as a treatment failure, meaning that they were assumed to have developed AIA" (p.428). Quote: "Most randomized patients completed at least 12 weeks of vitamin D treatment (83 of 93 patients, or 89%). The patients who did not complete treatment were equally split between the two arms, with five patients in each group completing less than 12 weeks of vitamin D" (p.430). See Figure 2 CONSORT diagram. Quote: "At 12 weeks, 67 of the 93 randomized patients completed the HAQ‐II questionnaire. Thirty‐two patients in the standard‐dose arm and 35 patients in the high‐dose arm completed their 12‐week questionnaires. As per protocol, the remaining patients were counted as failures. At 52 weeks, only 47 of the 93 trial patients completed the questionnaire. This was composed of 26 patients in the standard‐dose arm, and 21 patients in the high‐dose arm" (p.431). Attrition at 12 weeks: 35/46 (76%) in high‐dose group, 32/47 (68%) in standard‐dose group. Quote: "There were several limitations to our study. Firstly, per the protocol, many patients had to be counted as failures because they did not complete the 12‐week or 52‐week questionnaires. Also, there were ten patients on the trial who did not complete 12 weeks of study treatment for various reasons. These patients also were counted as failures" (p.434). |
| Selective reporting (reporting bias) | Low risk | Proposed outcomes reported. Data obtained for primary and secondary endpoints available; author communication provided additional data. |
| Other bias | Low risk | No other bias. |
Peng 2018.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel RCT Duration of intervention: 2‐week run‐in with 2 tablets of calcium 600 mg and vitamin D3 125 IU supplement. 12‐week randomised intervention. 12‐week follow‐up postintervention TREATMENT TRIAL Recruited from 3 hospitals in Beijing, China between August 2013 and October 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
84 participants randomised; 42 in each group (see Figure 2 of publication) Mean age: 59 years (range 43–76 years) Baseline characteristics only reported for analysed participants (40 in YSJG group, 37 in placebo group) All participants Asian, duration of AI therapy not stated There were no statistically significant differences in baseline characteristics between groups Almost all had stage I or II disease. Most had received previous chemotherapy (80% in YSJG group vs 78.4% in placebo group) Anastrozole was the most commonly used AI (42.5% in YSJG group vs 56.8% in placebo group), followed by letrozole (35.0% in YSJG group vs 27.0% in placebo group) All participants reported arthralgia, and the mean time to onset of AIMSS was 6.5 for YSJG group and 7.9 months for placebo group |
|
| Interventions |
Intervention
Comparator
|
|
| Outcomes |
Primary outcome (Questionnaires at baseline, 4, 8, 12 and 24 weeks)
Secondary outcomes
Blood count, urinalysis, faecal occult blood and microscopy, renal function, liver function tests, E2, FSH, tumour markers (CEA, CA125 and CA153) also performed at baseline and end of treatment (week 12) Adverse effects recorded at each visit during study period; including severity, duration and causality with experimental agent |
|
| Notes | Quote: "The study was funded by Beijing Municipal Science and Technology Commission, China (D131100002213001). The sponsor had a role in estimating rationality and practicability of the study design, financial support, and promoting achievements". Authors' disclosures of potential conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomization was carried out in a 1:1 ratio according to the sequence generated with SAS 7.0" (p.22). |
| Allocation concealment (selection bias) | Low risk | Quote: "Drug codes, numbered from 1 to 84, and contained group assignments were generated according to randomization numbers. Eligible participants were randomized into the YSJG group or the Placebo group by obtaining medicines associated with the given drug codes in accordance with the order of visits" (p.22). Quote: "Group assignments will not be revealed until the entire study is completed" (p.5) (Peng 2018). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, investigators, statisticians, and all study staff were blinded" (p.22). Placebo granules were manufactured "in order to achieve the maximum similarity in color, smell, taste and texture with that of YSJG granules" (p.20). Quote: "The blinding was evaluated at 12 weeks by comparing the percentage of participants in each group who thought they had received YSJG granules. 79% participants in the YSJG group thought that they had received YSJG granules as compared with 74% in the Placebo group (P=0.638)" (p.23). Quote: "There was a total of 14 participants reporting adverse events among those receiving YSJG granules (33%) and 16 participants among those receiving placebo granules (39%, P=0.589)" (p.23). Comments: given there were no differences in guessing which intervention was received, and no differences in treatment‐related adverse events between groups, likely that blinding was effective. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we selected the Brief Pain Inventory‐Short Form (BPI‐SF) as the primary outcome measure to evaluate general pain" (p.20–21). WOMAC, M‐SACRAH, FACT‐B were all secondary outcomes. Quote: "Bone mineral density (BMD) of the L2‐L4 region of the spine and hip was assessed before and after treatment (at weeks 0 and 12)" (p.21) was also secondary outcome. Most outcomes (BPI‐SF, WOMAC, M‐SACRAH, FACT‐B) were PROs – given effective blinding of participants, likely that these outcome assessments were also effectively blinded. BMD, as assessed by investigators who were blinded – it is likely this outcome was also appropriately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy and safety analyses were conducted according to the intention‐to‐treat (ITT) principle. Participants who took granules and completed at least one outcome assessment were considered analyzable. Missing values were imputed by the last‐observation carried‐forward method. All statistical analyses were performed using Statistical Packages of Social Sciences (SPSS 18.0)" (p.22). Quote: "84 eligible participants were randomly assigned. Nine of these participants discontinued the trial. Seven of the nine were excluded from efficacy analysis because they failed to complete one outcome assessment. Eventually, a total of 77 were evaluable at 12 weeks" (p.22). Figure 2 of the publication shows that: of the 42 randomly assigned to YSJG, 39 completed the study but 40 were analysable (meaning they had ≥ 1 primary outcome measure available), the other 2 participants were either lost to follow‐up (1) or had an adverse event (1). Of the 42 randomly assigned to placebo, 36 completed the study but 37 were analysable, the other 5 had been lost to follow‐up (1), had an adverse event (1), withdrew consent (1), had tumour recurrence (1) and had been removed due to protocol violation (1). The attrition rates of those randomised were: 4.8% in YSJG and 11.9% in placebo. Although there was a greater proportion of attrition in the placebo group, the reasons for loss were well documented. Additionally participants who were not analysable for a primary outcome measure, but experienced adverse events (1 in YSJG and 1 in placebo), were included in the safety assessment. |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome in the primary publication was BPI; this is the same as the 2014 publication; however, there is discrepancy with registry ISRCTN06129599 (doi.org/10.1186/ISRCTN06129599). Quote from trial registry: "Primary outcome measures 1. The Brief Pain Inventory‐Short Form (BPI‐SF) to evaluate general pain 2. Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index to assess joint pain, stiffness, and functional status in the knees. 3. The Modified Score for the Assessment and quantification of Chronic Rheumatoid Affections for the hands (M‐SACRAH) to assess joint pain, stiffness, and functional status in the hands. The outcome measures above will be assessed before the treatment, at 1 month, 2 and 3 months during the treatment, the assessments will be repeated at the third month after the treatment. Secondary outcome measures 1. The Functional Assessment of Cancer Therapy breast cancer‐specific quality of life tool (FACT‐B) to evaluate quality of life of patients with breast cancer. 2. TCM symptoms scale to evaluate TCM syndrome (deficiency of liver and kidney, qi and collaterals stagnation). 3. Bone Mineral Density (BMD) to evaluate bone density and bone metabolism objectively 4. Bone Metabolic Markers (calcium, phosphorus, alkaline phosphatase, osteocalcin, calcitonin) to evaluate bone density and bone metabolism objectively FACT‐B and TCM syndrome will be assessed before the treatment, at 1 month, 2 and 3 months during the treatment, the assessments will be repeated at the third month after the treatment. BMD and bone metabolic markers will be assessed before and after the treatment". Peng 2014 publication (although not trial protocol) (p.3–4): primary outcome stated as BPI – no time point stated. WOMAC and M‐SACRAH listed as secondary. Quote: statistical plan (p.4–5) "The primary analysis will be to compare the mean pain scores from BPI‐SF, the WOMAC, and the M‐SACRAH and the mean joint stiffness and function scores from the WOMAC and the M‐SACRAH between the TCM group and the placebo group. The secondary analysis will be to compare the mean scores from FACT‐B and changes in BMD before and after treatment". Insufficient information within the same publication. Peng 2018 Quote: "The primary analysis was to compare the mean worst pain scores from BPI‐SF at 12 weeks between the YSJG group and the Placebo group. All other analyses were secondary objectives, including the mean joint pain, stiffness and function scores from the WOMAC and the M‐SACRAH, the mean scores from FACT‐B and changes in BMD before and after treatment at 12 weeks between two groups" (p.22). Time points for the primary outcome not clearly stated. Insufficient information to permit judgement. |
| Other bias | Unclear risk | Quote: "The authors indicated no potential conflicts of interest" (p.26). Quote: "YSJG granules, a patent belongs to Beijing Hospital of Traditional Chinese Medicine" (p.20). Quote: "We thank Beijing Hospital of Traditional Chinese Medicine, Beijing Cancer Hospital, and Guang'Anmen Hospital, all clinicians who consented or referred their patients to our study …" (p.26). Insufficient information to permit judgement regarding above. Run‐in period design of study with calcium and vitamin D for 2 weeks – prerandomisation administration of an intervention that could enhance or diminish the effect of the subsequent, randomised intervention. Although this is probably unlikely to have an effect, vitamin D postulated to have a role in altering AIMSS in women with low 25‐OHD (< 30 ng/mL) and so judged as unclear risk of bias. |
Rastelli 2011.
| Study characteristics | ||
| Methods | Phase II, placebo‐controlled, parallel RCT Duration of intervention: 6 months for RCT. Participants were permitted to continue on an open‐label extension of the trial for 6 months. TREATMENT TRIAL Recruited between January 2006 and February 2009 at Washington University School of Medicine, St Louis, Missouri, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
60 participants randomised; 30 in vitamin D group, 30 in control group (Figure 1) Baseline subject characteristics similar between groups (Table 1 of the publication) Mean age: 60 (SD 8.8) years in vitamin D group, 63 (SD 7.8) years in control group > 85% participants were white 16 (53%) participants in the vitamin D group and 19 (63%) participants in the control group had previous chemotherapy. Chemotherapy type not stated Duration of anastrozole at entry to study: vitamin D group 15.2 (SD 13.1) months, control group 21 (SD 15.4) months Mean 25‐OHD level: vitamin D group 23 (SD 4.6) ng/mL; control group 22 (SD 4.7) ng/mL |
|
| Interventions | All participants received calcium carbonate 1000 mg and vitamin D3 400 IU tablets daily Participants stratified according to the baseline 25‐OHD level Stratum A, vitamin D insufficiency – baseline 25‐OHD levels 20–29 ng/mL Intervention Vitamin D2, 1 × 50,000 IU capsule orally once a week for 8 consecutive weeks, and then once a month for the rest of the study Comparator 1 matching placebo orally once a week for 8 consecutive weeks, and then once a month for the rest of the study Stratum B, vitamin D deficiency – 25‐OHD levels 10–19 ng/mL Intervention Vitamin D2, 1 × 50,000 IU capsule orally once a week for 16 consecutive weeks and then once a month for the rest of the study Comparator 1 matching placebo orally once a week for 16 consecutive weeks and then once a month for the rest of the study |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Included only women being treated with anastrozole. The study was supported by Astra‐Zeneca. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was also performed by Investigational Drug Services (IDS) in balanced blocks of 4 by means of a random number generator" (p.109). |
| Allocation concealment (selection bias) | Low risk | Quote: "Vitamin D2 capsules, matching placebo capsules and calcium with vitamin D supplements were dispensed by the Investigational Drug Services (IDS) at the Siteman Cancer Pharmacy. The randomization was also performed by IDS in balanced blocks of 4 by means of a random number generator. Both patients and investigators were blinded to the treatment group assignment" (p.109). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Vitamin D2 capsules, matching placebo capsules" (p.109). Quote: "Both patients and investigators were blinded to the treatment group assignment" (p.109). Quote: "There were no toxicities or significant adverse events in the vitamin D group" (p.111). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded PRO. Quote: "There were no toxicities or significant adverse events in the vitamin D group" (p.111). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The retention rates at 2, 4, and 6 months of the study were, respectively, 95, 83, and 78% of the subjects" (p.110). CONSORT diagram – see Figure 1. Data available for analysis in 21/30 participants in the high‐dose vitamin D group at 6 months and 26/30 in the placebo group at 6 months. 5 participants in the high‐dose group excluded due to high serum/urinary calcium vs 1 participant in placebo group. |
| Selective reporting (reporting bias) | High risk | Quote from primary publication: "The primary outcome measures included the FIQ pain score, BPI pain severity and interference scores, and the HAQ‐DI total score at 2 months after correction for baseline scores. Scores were also analyzed at and 6 months" (p.110). Quote from trial registry: "Primary Outcome Measures:
Quote: "At 2 months, FIQ pain (P = 0.0045), BPI worst‐pain (P = 0.04), BPI average‐pain (P = 0.0067), BPI pain severity (P = 0.04), and BPI interference (P = 0.034) scores were better in the HDD than placebo group" (p.107). Quote: "The positive effect of vitamin D supplementation was not maintained at 4 and 6 months once subjects were switched to 50,000 IU monthly" (p.112). Protocol unavailable. The 2‐month data do not appear to be a prespecified outcome. |
| Other bias | Low risk | No other bias. |
Rosati 2011.
| Study characteristics | ||
| Methods | Phase III, placebo‐controlled, parallel RCT Duration of intervention: 5 years. Duration of anastrozole with etoricoxib or placebo 24 months, then continued anastrozole for further 3 years ADJUVANT TRIAL (secondary endpoint of specific interest to this review) Enrolled from Policlinico Umberto I di Roma Hospital, Italy from 2003 to 2006 |
|
| Participants |
Inclusion criteria
Exclusion criteria
182 participants randomised; 93 in etoricoxib and anastrozole "ETAN" arm, 89 in placebo and anastrozole "PAN" arms. FDA alert on COX‐2 inhibitors in 2004 – 37 participants in the treatment arm and 33 participants in the control arm subsequently discontinued etoricoxib Median age: 58 years in ETAN group, 61 years in PAN group; range 51–70 years Most had ductal carcinoma (78 in ETAN group vs 72 in PAN group), and most were HER2 negative (60 in ETAN group vs 64 in PAN group). Most had stage IIA–IIIA disease (79 in ETAN group vs 72 in PAN group) Most had received previous chemotherapy (73 in ETAN group vs 62 in PAN group), of which 60 (35 in ETAN group vs 25 in PAN group) had received CMF (cyclophosphamide, methotrexate and 5‐fluorouracil), 42 (20 in ETAN group vs 22 in PAN group) had received anthracycline alone, 33 (18 in ETAN group vs 15 in PAN group) had received anthracycline and taxane, and 21 (12 in ETAN group vs 9 in PAN group) had received trastuzumab Some participants had known osteoarthritis prior to commencing AI (23 in ETAN group vs 19 in PAN group). Other comorbidities included diabetes, heart failure and hyperlipidaemia; these were balanced across treatment groups |
|
| Interventions |
Intervention
Comparator
|
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Abstract and ASCO presentation only. No indication of funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only – insufficient information to permit judgement. Quote: "women were randomized 1:1 to receive anastrozole (1mg/day) upfront in combination with placebo or etoricoxib (60mg/day)". No information provided on how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on methods employed to conceal allocation. Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement. Quote: "placebo". Quote: "None of the patients in the treatment arm developed serious adverse event. No thrombotic events were recorded" (slide 17 ASCO presentation); however, NSAIDS do have common mild adverse events. No information about blinding procedures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: [slide 9] "Primary end‐point was the 5‐years event free survival (EFS). The secondary end‐point was to compare the risk of fracture and the musculoskeletal events in the two groups". Quote: [slide 10] "Musculoskeletal events … were recorded according to NCI CTC 3.0". Insufficient information about blinding procedures to permit judgement. Pain – subjective assessment by investigators by NCI CTCAE V3.0, adverse events profile of NSAIDs makes it plausible that investigators were aware which treatment participants were receiving. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "data from clinical trial are poor, mainly due to the severe restrictions that the FDA imposed to the COX‐2 inhibitors in 2004 because of their serious adverse events". "Patients were allowed to discontinue the etoricoxib or placebo for toxicity (censored). Anastrozole was continued for 5 years". "A number of 93 patients were enrolled in the treatment arm and N=89 in control". "A number of 37 patients in the treatment arm and 33 in the control discontinued etoricoxib after the FDA alert". However, outcome data on musculoskeletal pain appears to be available for some of the individuals who discontinued treatment. "Musculoskeletal pain was significantly lower in the treatment arm (50/73) than the control (16/67)". 20 participants not evaluated for musculoskeletal pain in etoricoxib + anastrozole (ETAN) arm (73/93 participants evaluated). 22 participants not evaluated for musculoskeletal pain in placebo + anastrozole (PAN) arm (67/89 participants evaluated). 20/93 = 21.5% attrition ETAN arm. 22/89 = 24.7% attrition PAN arm. |
| Selective reporting (reporting bias) | Unclear risk | No protocol, trial registry or statistical analysis plan was available to compare whether data was analysed in accordance with a prespecified plan. Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Shapiro 2016.
| Study characteristics | ||
| Methods | Phase III, placebo‐controlled, parallel RCT Duration of intervention: 7 months. 4‐week 'run‐in' of control treatment after enrolment then 6 months of randomised intervention TREATMENT TRIAL Recruited between March 2012 and March 2014 at a single site – Frauenshuh Cancer Center, a community‐based oncology practice of Park Nicollet Health Services in Minneapolis, MN, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
116 participants randomised; 57 in high‐dose vitamin D group, 59 in usual‐dose comparator arm 55 (48%) participants taking letrozole, 47 (41%) participants taking anastrozole, and 11 (9%) participants taking exemestane at baseline Mean age: 60.9 (SD 8.8) years Baseline characteristics similar between groups (Table 1 of the publication) Mean duration of AI treatment at entry: 19.9 (SD 17.0) months 61 (54%) participants had received prior chemotherapy. 32% of participants had prior taxane, did not differ between groups. 49 (43%) participants had musculoskeletal comorbidities Mean baseline serum total 25‐OHD level for all participants was 36.6 (SD 13.0) ng/mL after the run‐in period 5 participants in the high‐dose group and 4 participants in the control group had insufficient 25‐OHD levels of ≤ 20 ng/mL |
|
| Interventions |
Intervention 4‐week run‐in period of vitamin D3 600 IU orally daily then high dose of vitamin D3 4000 IU orally daily for 6 months 4‐week run‐in of vitamin D3 600 IU was the recommended dietary allowance to "allow serum levels to begin to normalize" (p.502) Comparator 4‐week run‐in period of vitamin D3 600 IU orally daily then vitamin D3 600 IU orally daily for 6 months Both groups received calcium carbonate 500 mg orally daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Quote: "Funding Research relating to this analysis was co‐funded by Grants from the National Cancer Institute and the National Institutes of Health Office of Dietary Supplements (R21 CA149934) and the Park Nicollet Institute and Park Nicollet Foundation. This work was supported in part by NIHP30CA77598, using the following University of Minnesota Masonic Center Resource: Clinical Pharmacology and the National Center for Advancing Translational Sciences(NCATS) of the National Institutes of Health (NIH UL1TR000114)" (p.509). Conflicts of interest: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned (1:1)" (p.502). Quote: "cholecalciferol (D3) supplements were encapsulated and coded bottles prepared at Daily Manufacturing, Inc. (Rockwell, NC) – randomly assigned (1:1) to the usual care dose of 600 IU D3 or high dose of 4000 IU D3, plus 1000 mg calcium carbonate" (p.502). Quote: "The unblinded biostatistician assigned coded bottles according to a blocked‐randomization design in SAS v9.3 (2011, Cary, North Carolina)" (p.502). |
| Allocation concealment (selection bias) | Low risk | Quote: "The unblinded biostatistician assigned coded bottles according to a blocked‐randomization design in SAS v9.3 (2011, Cary, North Carolina)" (p.502). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study participants, their providers, and research staff remained blinded throughout the study" (p.502). Quote: "The most frequent adverse events according to CTCAEv. 4.0 were MS [musculoskeletal] (18 %) and gastrointestinal (17 %) in nature and these did not differ between groups" (p.507). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | PROs – participants blinded, placebo. Grip strength – research staff blinded. AI adherence/laboratory measures – personnel/participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 1: CONSORT diagram. Data available for analysis in high‐dose vitamin D group: 57/57 participants at 6 months. Data available for usual‐dose vitamin D group: 56/59 participants at 6 months (3 dropped out during run‐in period therefore excluded, 1 lost to follow‐up but included in analysis). |
| Selective reporting (reporting bias) | Low risk |
NCT01509079; clinicaltrials.gov trial registry available with primary and secondary outcomes listed as follows.
In the 2016 publication, the endpoints were listed as follows.
Quote: "The AUSCAN [Australian / Canadian osteoarthritis hand index version 3.1] and WOMAC [Western Ontario and McMaster osteoarthritis index version 3.1] were later added to the study schedule" (p.502). Primary and secondary relevant outcomes as registered reported in publication. |
| Other bias | Unclear risk | Run‐in period design of study – prerandomisation administration of an intervention that could enhance or diminish the effect of the subsequent, randomised, intervention. Authors acknowledged that this may have had a role in altering AIMSS. Quote: "This study has limitations. The run‐in period was designed to accommodate the prevailing use of vitamin D supplementation in women receiving AIs. It is possible that vitamin D has a role in altering AIMSS in women with low 25‐(OH)D (<30 ng/mL). Although our data cannot address this due to the run‐in period, D insufficiency does not currently appear as prevalent in this population" (p.509). |
Shenouda 2019.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel RCT Duration of intervention: 6 weeks TREATMENT TRIAL Study conducted in association with Edwards Comprehensive Cancer Center, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
60 participants; number per group not reported No data presented on baseline participant characteristics Numbers of evaluable participants in each group indicated but not number randomised |
|
| Interventions |
Intervention Tart cherry concentrate – equivalent to 50 tart cherries – flavonoids and anthocyanins in tart cherry reportedly exert an anti‐inflammatory effect Participants received 1 ounce (25 g) of tart cherry concentrate in 8 ounces (200 mL) of water daily for 6 weeks Comparator Participants received 1 ounce of placebo syrup in 8 ounces of water daily for 6 weeks No information provided on ingredients of placebo syrup |
|
| Outcomes |
Primary outcome
|
|
| Notes | Abstract and ASCO poster only. Funded by Cherry Marketing Institute, Dewitt, Michigan, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only; insufficient information to permit judgement. Quote: "eligible patients … were randomized to TC concentrate (equivalent to 50 tart cherries) versus placebo (p) [syrup] in a 1:1 model". No information provided on process of randomisation, and no baseline characteristics presented. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on methods employed to conceal allocation. Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind trial". Quote: "Pts [patients] were instructed to take 1 ounce of TC [tart cherry] or placebo in 8 ounces of water daily for 6 weeks". Volumes consistent among placebo and intervention, but no mention of methods employed to match taste/colour of placebo to control. Quote: "2 pts did not complete the study due to diarrhoea", not clear which treatment group these participants belonged to and whether diarrhoea occurred at an increased rate in either treatment group. No other information presented on blinding or whether blinding was maintained effectively. Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients documented their pain intensity at baseline, weekly and at study completion in a diary using a Visual Analog Scale (VAS)". VAS is a PRO measure. Without information on whether participants were effectively blinded to intervention received, insufficient to permit judgement on whether the outcome assessment was also effectively blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "60 pts were enrolled from May 2016 to August 2018"; "48 pts were included in the final analysis. Of the 12 pts excluded from the analysis, 2 pts did not complete the study due to diarrhea and 10 pts were non‐compliant"; 12/60 = 20% excluded. Given small cohort, the number of participants who dropped out would suggest a high degree of attrition. It is also difficult to comment on what the study defined as "non‐compliance". It is also not clear the proportion who were non‐compliant according to the treatment received (e.g. was this due to adverse events), and whether there were any differences between the treatment groups. Abstract suggested "pts documented their pain intensity at baseline, weekly and at study completion", not clear whether those who were non‐compliant had any outcome data available and whether they should have been included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unable to access on trial registry or access statistical analysis plan so cannot make judgement on whether data were analysed in accordance with a prespecified plan. VAS was only outcome measure used for assessment of arthralgia. Mann‐Whitney U test is only analysis mentioned to assess statistically significant differences between groups. Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Sordi 2019.
| Study characteristics | ||
| Methods | Phase II, placebo‐controlled, parallel RCT Duration of intervention: 4 weeks TREATMENT TRIAL Recruited from Instituto Brasileiro de Controle do Cancer outpatient clinics in Brazil, randomised from October 2016 to March 2017 |
|
| Participants |
Inclusion criteria
Exclusion criteria
70 participants randomised; 36 in Cat's claw group, 34 in placebo group Mean age: 59.2 years; 59.2 years (range 33–79 years) in Cat's claw group; 60.79 years (range 43–80 years) in placebo group 58% were Caucasian (believed to be white people) More participants in the placebo group had stage I disease (25% in Cat's claw group vs 41% in placebo group), luminal B was the most common subtype (49.3% of population) Most participants had received previous taxane chemotherapy (58% in Cat's claw group vs 56% in placebo group) Mean time on AI: 20.6 months (range 2–96 months) and all but 1 of the participants received anastrozole |
|
| Interventions |
Intervention
Comparator
|
|
| Outcomes |
Primary outcomes (Questionnaires at baseline and 4 weeks)
Secondary outcomes
|
|
| Notes | Study was self‐funded by Centro de Estudos e Pesquisa de Hematologia (CEPHO) – clinical study unit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "70 patients were randomized" (p.27, Figure 1); 36 randomised to U tomentosa, 34 to placebo. No information on methods used to generate randomisation sequence. Baseline characteristics presented in Table 1 of the publication (p.28). Age, BMI, cancer staging, previous chemotherapy, previous radiotherapy and predominance of anastrozole as the AI use similar across treatment groups. There was a greater range of time on AIs, and more white participants in the placebo group than the intervention group. 1 participant with carcinoma in situ was included in the placebo group, and 1 participant receiving exemestane was included in the intervention group. Given only slight differences between treatment groups, likely that there was not an inherent problem with the process of randomisation. Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | No information on methods to conceal allocation. Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study was double blinded" – personal communication with trialist. Quote: "The placebo was manipulated in 100mg capsules identical to U. tomentosa capsules manufactured using cornstarch and food coloring" (p.26) – evidence of efforts to match placebo to treatment in appearance to intervention. Quote: "Use of U. tomentosa in this patient group was safe and no cases of grade 3 or 4 toxicity occurred. Adverse events were analysed in 32 patients who underwent AI therapy and completed the study, two patients who discontinued AI therapy because of adverse events, 29 patients who used a placebo and completed the study and three patients who discontinued placebo treatment because of adverse events" (p.27). Table 5 of the publication (p.30) shows that more participants reported adverse events with placebo than with drug (8 in Cat's claw group vs 17 in placebo group), and all the grade 2 adverse events (6) were reported by participants using placebo. Quote: "U. tomentosa dry extract was not more effective than the placebo when evaluated using the questionnaires" (p.27) – likely that participants were effectively blinded (not biased towards intervention) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The primary objective of this study is to evaluate arthralgia reduction by U. tomentosa dry extract …" "The secondary objectives were to (detection bias) evaluate the drug’s safety and to determine the relationship between arthralgia and inflammatory markers" (p.25). Quote: "The efficacy parameters in this study were classified using five tools: Brief Pain Inventory (BPI), Visual Analog Scale (VAS) for pain, Lequesne (osteoarthritis), Disabilities of the Arm, Shoulder and Hand (DASH), SF36 questionnaire for assessing the quality of life" (p.25–26). All these efficacy parameters are PROs. Given participants were likely effectively blinded to intervention received, likely that outcome assessment was also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The primary analysis was based on outcome data from 4 weeks (at end of treatment), the first appointment where participants completed questionnaires on the effects of intervention. Follow‐up at weeks 2 and 3; Quote: to "ensure treatment adherence and side effects were evaluated by phone" (p.25), these safety assessments were secondary outcomes, no primary outcome data were collected at these time points. Consort diagram (Figure 1 in publication, p.27) shows of the 36 participants randomised to U tomentosa, "32 patients completed the study, 2 patients discontinued because of adverse events, 1 patient discontinued because of the lack of clinical improvement, 1 patient discontinued treatment for unknown reasons". This represented attrition of 11.1% for intervention. Consort diagram (Figure 1 of publication, p.27): of the 34 participants randomised to placebo "29 patients completed the study, 3 patients discontinued treatment because of adverse events, 2 patients discontinued treatment for unknown reasons". This represented an attrition of 14.7% for placebo. These were modest attrition rates, and given that no primary outcome data were assessed prior to treatment completion it is unlikely that there was a significant proportion of missing primary outcome data. Furthermore, those who discontinued due to adverse events were still included in the safety analysis (secondary outcome). |
| Selective reporting (reporting bias) | Unclear risk | No statistical analysis plan was accessed to determine whether data were analysed in accordance with a prespecified plan. Multiple efficacy parameters within the same pain outcome domain; however, all results from each were presented in Table 2 of the publication (p.29). Quote: "We evaluated associated between continuous and discrete variable through the ANOVA test. Associations between discrete variables were evaluated by the Chi‐square test or the Fisher Exact test" (p.26). Means and SDs for each of the efficacy parameters were presented in Table 2 of the publication, but unclear which statistical test had been used for each of the specific parameters Personal communication from trialist indicated that the study was registered on Platforma Brasil; however, unable to access this information in Portuguese. Insufficient information to permit judgement. |
| Other bias | Low risk | No other bias. |
25‐OHD: 25‐hydroxyvitamin D; AI: aromatase inhibitor; AIA: aromatase inhibitor arthralgia; AIMSS: aromatase inhibitor‐induced musculoskeletal symptoms; ALT: alanine transaminase; AST: aspartate aminotransferase; AUSCAN: Australian/Canadian osteoarthritis hand index version 3.1; BCPT‐MS: Breast Cancer Prevention Trial Symptom Scales – Musculoskeletal; BFI: Brief Fatigue Inventory; BMD: bone mineral density; BMI: body mass index; BPI: Brief Pain Inventory; BPI‐AIA: Brief Pain Inventory for Aromatase Inhibitor Arthralgia; BPI‐SF: Brief Pain Inventory – Short Form; COX: cyclo‐oxygenase; CPIS: Categorical Pain Intensity Score; CTCAE: Common Terminology Criteria for Adverse Events; CTSU: Cancer Trials Support Unit; DCIS: ductal carcinoma in situ; DHA: docosahexaenoic acid; E2: oestradiol; ECOG: Eastern Cooperative Oncology Group; EPA: eicosapentaenoic acid; ER: oestrogen receptor; FACT‐B: Functional Assessment of Cancer Therapy – Breast; FACT‐ES: Functional Assessment of Cancer Therapy – Endocrine Scale; FDA: Food and Drug Administration; FIQ: Fibromyalgia Impact Questionnaire; FSH: follicle‐stimulating hormone; GRCS: Global Rating of Change Scale; HAQ‐II: Health Assessment Questionnaire‐II; HAQ‐DI: Health Assessment Questionnaire – Disability Index; Hb: haemoglobin; HDL: high‐density lipoprotein; HRQoL: health‐related quality of life; LCIS: lobular carcinoma in situ; LDL: low‐density lipoprotein; LH: luteinising hormone; M‐BPI: Modified Brief Pain Inventory; M‐SACRAH: Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands; MENQOL: Menopause‐specific Quality of Life Questionnaire; NCI: National Cancer Institute; NCI‐CTC: National Cancer Institute Common Toxicity Criteria; NSAID: non‐steroidal anti‐inflammatory drug; O3‐FA: omega‐3 fatty acid; PHQ: Patient Health Questionnaire; POMS: Profile of Mood States; PR: progesterone receptor; PRO: patient‐reported outcome; PROMIS: Patient‐Reported Outcomes Measurement Information System; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation; SF‐12: 12‐item Short Form; SF‐36: 36‐item Short Form; SHBG: sex hormone binding globulin; TCM: traditional Chinese medicine; ULN: upper limit of normal; VAS: Visual Analogue Scale; WCC: white cell count; WOMAC: Western Ontario and McMaster Universities Osteoarthritis scale; YSJG: Yi Shen Jian Gu.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12611000891921 | Study withdrawn due to lack of funding/staff/facilities. |
| Altundag 2019 | Wrong study design. Not an RCT (letter to editor). |
| Barbor 2018 | Wrong study design. Not an RCT (commentary). |
| Becze 2017 | Wrong study design. Not an RCT (commentary). |
| KCT0003698 | Wrong intervention (not a systemic therapy). |
| Kim 2012 | Wrong patient population. |
| NCT01809171 | Recruitment terminated early and study discontinued. 15 participants only. Completed August 2015. |
| NCT03384095 | Study withdrawn prior to opening to accrual or study start. |
| UMIN000004416 | Wrong study design. Not an RCT. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT02831582.
| Study name | Omega‐3 supplementation in prevention of aromatase inhibitor‐induced toxicity in patients with stage I–III breast cancer |
| Methods | RCT |
| Participants | 120 participants, early breast cancer, initiating adjuvant AI |
| Interventions | Omega‐3 fatty acid vs placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 12 October 2016 |
| Contact information | Ohio State University Comprehensive Cancer Center; 800‐293‐5066; OSUCCCClinicaltrials@osumc.edu Evelyn Nguyen; 614‐685‐4852; Evelyn.Nguyen@osumc.edu |
| Notes | USA |
NCT03865992.
| Study name | Curcumin for breast cancer survivors with aromatase inhibitor‐induced joint arthropathy – a randomized, double‐blinded, controlled pilot study |
| Methods | RCT (pilot study) |
| Participants | 40 participants, early breast cancer, on AI for ≥ 3 months with AIMSS |
| Interventions | 3 months of nanoemulsion curcumin orally vs placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 4 March 2019 |
| Contact information | Lisa D Yee MD: 626‐218‐7461; lyee@coh.org |
| Notes | USA |
NCT04205786.
| Study name | A randomized study of oral vitamin B12 for the treatment of aromatase inhibitors (AI)‐associated musculoskeletal symptoms (AIMSS) in women with early stage breast cancer |
| Methods | RCT |
| Participants | 150 participants, early breast cancer, on AI ≥ 14 days with AIMSS |
| Interventions | Vitamin B12 2500 μg orally daily for 90 days vs standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | May 2021 |
| Contact information | Zeina Nahleh, Cleveland Clinic Taussig Cancer Center; 1 954‐659‐5840; NAHLEHZ@ccf.org |
| Notes | USA |
UMIN000027481.
| Study name | Randomized study of Sokei‐Kakketsu‐To for the reduction of aromatase inhibitor – associated joint symptoms in women with breast cancer |
| Methods | RCT |
| Participants | 68 participants with breast cancer, on AI who develop AIMSS |
| Interventions | Sokei‐Kakketsu‐To (TJ‐53) vs joint exercises |
| Outcomes | HAQ‐DI at 3 months |
| Starting date | 19 May 2017 |
| Contact information | Shinji Ozaki, National Hospital Organization Kure Medical Center/Chugoku Cancer Center; 0823‐22‐3111; ozakis@kure‐nh.go.jp |
| Notes | Japan |
AI: aromatase inhibitor; AIIA: aromatase inhibitor‐induced arthralgias; AIMSS: aromatase inhibitor‐induced musculoskeletal symptoms; BPI: Brief Pain Inventory; BPI‐SF: Brief Pain Inventory – Short Form; CRP: C‐reactive protein; DASH: Disabilities of the Arm, Shoulder and Hand; FACT‐ES: Functional Assessment of Cancer Therapy – Endocrine Symptoms; HAQ‐DI: Health Assessment Questionnaire – Disability Index; RCT: randomised controlled trial; SNP: single nucleotide polymorphism.
Differences between protocol and review
We changed the name of some of our main outcomes from our initial protocol, from 'overall change' to 'change from baseline until the end of intervention', with the intent of making the outcome clearer for the reader. We also changed the first proposed outcome from 'worst pain scores' to 'pain', as we felt that using the word 'worst' could obscure our intended outcome, as multiple different pain scores were used to assess this outcome across multiple studies. Another outcome, "persistence and adherence to aromatase inhibitors" was changed to "effect on discontinuation of AI" to provide more clarity for the reader.
The measurement of the treatment effect was performed using a mean difference (MD) analysis. This is different from our protocol, which we had intended to measure the treatment effect by performing a standardised mean difference (SMD) analysis and the random‐effects model to combine data from different scoring systems measuring the same outcome of interest. But due to inconsistent reporting of standard deviations (SD), with some studies reporting only 'end‐of‐treatment' SD and others reporting only 'change score' SD, we were unable to combine these results for the calculation of SMD. Multiple studies did not report SD of change scores and only provided SD from baseline or end‐of‐treatment SD. If change score means with SD were not available, we reported end‐of‐treatment means and SD for both groups. As discussed in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 10 "in a randomized study, MD based on changes from baseline can usually be assumed to be addressing exactly the same underlying intervention effects as analyses based on post‐intervention measurements" (Deeks 2021). If end‐of‐treatment means and SD were used, this was highlighted in the analysis.
In the protocol, we aimed to combine our studies in meta‐analysis if there were sufficient studies and sufficient data available. In this review, we found that the interventions were heterogeneous and most outcomes were unable to be combined for meta‐analysis. To appropriately synthesise and present the data, we undertook vote‐counting, by assessing the number of studies showing a minimal clinically important difference (MCID) within a treatment outcome which fell within the 95% confidence intervals of the measured effect.
Due to the limited meta‐analysis, we were unable to perform subgroup analyses. In our protocol, we had planned to undertake further subgroup analysis on different doses of a particular systemic intervention; menopausal status; participants who had received previous chemotherapy including a taxane versus those who had not; times since commencement of AI; and age group of participants.
Contributions of authors
Draft the protocol: KER, KR, MC, NW.
Study selection: KER, NW.
Extract data from studies: IA, SC, NW.
Enter data into Review Manager 2014: IA, NW.
Carry out the analysis: KER, MC.
Interpret the analysis: KER, MC, NW.
Draft the final review: KER, NW, KR, MC, IA, SC.
Disagreement resolution: KER, NW.
Update the review: KER, KR, MC, NW.
Sources of support
Internal sources
-
Mater Adults Hospital, Brisbane, Australia
Salary for NW
-
Princess Alexandra Hospital, Australia
Salary for KER
-
University of Queensland, Australia
Salary for KR
-
Queensland Institute of Medical Research, Australia
Salary for MC
External sources
No sources of support provided
Declarations of interest
KER: travel/accommodation/meeting expenses by Roche, Amgen, Pfizer; advisory board Amgen; conference registration by Novartis.
IA: none.
KR: none.
SC: none.
MC: none.
NW: consultancy fees by Roche and Novartis; grant for department trials from Medivation; expert panel review for Roche; stock in CSL, travel/accommodation/meeting expenses by Roche and Novartis.
New
References
References to studies included in this review
Birrell 2009 {published data only (unpublished sought but not used)}
- Birrell S, Tilley W. Testosterone undecanoate treatment reduces joint morbidities induced by anastrozole therapy in postmenopausal women with breast cancer: results of a double-blind, randomized phase II trial. Cancer Research 2009;69(24 Suppl):Abstract no. 804. [Google Scholar]
- Birrell SN. Reduction of side effects from aromatase inhibitors used for treating breast cancer. United States Patent Application Publication (date published 17 May 2012):US 2012/0122824 A1.
- NCT00497458. Androgen therapy for breast cancer patients with aromatase inhibitor induced side effects. clinicaltrials.gov/show/NCT00497458 (first received 6 July 2007).
Cathcart‐Rake 2020 {published data only}
- Cathcart-Rake E, Novotny P, Leon-Ferre R, Le-Rademacher J, Storrick EM, Adjei AA, et al. A randomized, double-blind, placebo controlled trial of testosterone for treatment of postmenopausal women with aromatase inhibitor-induced arthralgias: Alliance study A221102. Supportive Care in Cancer 2020;29(1):387-96. [DOI: 10.1007/s00520-020-05473-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Ferre RA, Le-Rademacher J, Terstriep S, Glaser R, Novotni P, Giuliano A, et al. A randomized, double-blind, placebo-controlled trial of testosterone (T) for aromatase inhibitor-induced arthralgias (AIA) in postmenopausal women: Alliance A221102. Cancer Research 2019;79(4 Suppl):P4-16-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01573442. Testosterone in treating postmenopausal patients with arthralgia caused by adjuvant aromatase inhibitor treatment. clinicaltrials.gov/show/NCT01573442 (first received 9 April 2012).
Chan 2017 {published and unpublished data}
- ACTRN12611000019909. Emu oil for joints pain in postmenopausal women with early breast cancer. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336281 (first received 1 December 2010).
- Chan A, De Boer R, Gan A, Willsher P, Martin R, Zissiadis Y, et al. Randomized phase II placebo-controlled study to evaluate the efficacy of topical pure emu oil for joint pain related to adjuvant aromatase inhibitor use in postmenopausal women with early breast cancer: JUST (Joints Under Study). Supportive Care in Cancer 2017;25(12):3785-91. [DOI] [PubMed] [Google Scholar]
Henry 2018 {published data only (unpublished sought but not used)}
- Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. Journal of Clinical Oncology 2018;36(4):326‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry NL, Unger JM, Till C, Schott AF, Crew KD, Lew DL, et al. Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202. Cancer 2019;125(12):2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01598298. S1202: duloxetine hydrochloride for muscle/joint pain in early-stage breast cancer receiving hormone therapy. clinicaltrials.gov/show/NCT01598298 (first received 15 May 2012).
Hershman 2015a {published data only (unpublished sought but not used)}
- Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, et al. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. Journal of Clinical Oncology 2015;33(17):1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman DL, Unger JM, Crew KD, Dakhil SR, Awad D, Greenlee H, et al. Omega-3 fatty acids for aromatase inhibitor-induced musculoskeletal symptoms in women with early-stage breast cancer (SWOG S0927). Journal of Clinical Oncology 2014;32(15 Suppl):Abstract no. 9532. [Google Scholar]
- Hershman DL, Unger JM, Crew KD, Moinpour CM, Minasian LM, Hansen L, et al. SWOG S0927: a randomized double blind placebo-controlled trial of omega-3-fatty acid for the control of aromatase inhibitor (AI)-induced musculoskeletal pain in women with early stage breast cancer. Cancer Research 2011;71(24 Suppl):Abstract no. OT2-07-02. [Google Scholar]
- NCT01385137. S0927: a randomized placebo-controlled trial of omega-3-fatty acid for the control of aromatase inhibitor-induced musculoskeletal pain and stiffness in women with early stage breast cancer, phase III. clinicaltrials.gov/ct2/show/NCT01385137 (first received 29 June 2011).
- Shen S, Unger JM, Crew KD, Till C, Greenlee H, Gralow J, et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Research and Treatment 2018;172(3):603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2017 {published and unpublished data}
- Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Fabian CJ. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms and fatigue in women with breast cancer starting adjuvant letrozole: the VITAL trial. Journal of Clinical Oncology 2012;30(15 Suppl):Abstract no. 9000. [DOI] [PubMed] [Google Scholar]
- Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Nydegger JL, et al. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Research and Treatment 2017;166(2):491-500. [DOI] [PubMed] [Google Scholar]
- NCT00867217. Vitamin D3 for aromatase inhibitor induced arthralgias. clinicaltrials.gov/show/NCT00867217 (first received 23 March 2009).
Li 2017 {published data only (unpublished sought but not used)}
- Li Y, Zhang Z, Cui F, Liu J, Wang Y, Jiang J, et al. Traditional Chinese medicine bionic tiger bone powder for the treatment of AI-associated musculoskeletal symptoms. Evidence-Based Complementary and Alternative Medicine 2017;2017:Article ID 2478565. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014 {published data only (unpublished sought but not used)}
- Liu P, Yang DQ, Xie F, Zhou B, Liu M. Effect of calcitonin on anastrozole-induced bone pain during aromatase inhibitor therapy for breast cancer. Genetics and Molecular Research 2014;13(3):5285-91. [DOI] [PubMed] [Google Scholar]
Lustberg 2018 {published data only (unpublished sought but not used)}
- Lustberg MB, Orchard T, Pan X, Reinbolt R, Logan A, Lester J, et al. Prevention of aromatase Inhibitor (AI)-induced joint symptoms with omega-3 fatty acid supplementation: a randomized placebo-controlled pilot study. Cancer Research 2015;75(9 Suppl):Abstract no. P1-09-03. [Google Scholar]
- Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, et al. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Research and Treatment 2018;167(3):709-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01478477. Omega-3 fatty acids in preventing joint symptoms in patients with stage I-III breast cancer receiving anastrozole, exemestane, or letrozole. clinicaltrials.gov/show/NCT01478477 (first received 23 November 2011).
- Orchard TS, Pan X, Lester J, Logan A, Shapiro CL, Jackson RD, et al. Relationship of dietary and red blood cell polyunsaturated fatty acids to inflammatory markers in breast cancer survivors taking aromatase inhibitors. Cancer Research 2015;75(9 Suppl):Abstract no. 14-P1-09-30. [Google Scholar]
Massimino 2011 {published data only (unpublished sought but not used)}
- Massimino K, Glissmeyer M, Wagie T, Karamlou K, Look RM, Sorenson L, et al. Use of Blue Citrus, a Chinese herbal remedy, to reduce side effects of aromatase inhibitors. Journal of Clinical Oncology 2011;29(27 Suppl):Abstract no. 170. [Google Scholar]
- NCT00702858. Trial of Blue Citrus compared to placebo in patients receiving aromatase inhibitor therapy for estrogen receptor positive post-menopausal breast cancer. clinicaltrials.gov/show/NCT00702858 (first received 20 June 2008).
Niravath 2019 {published and unpublished data}
- NCT01988090. High dose vitamin D vs standard dose vitamin D study. clinicaltrials.gov/ct2/show/NCT01988090 (first received 20 November 2013).
- Niravath P, Hilsenbeck SG, Wang T, Jiralerspong S, Nangia J, Pavlick A, et al. Randomized controlled trial of high-dose versus standard-dose vitamin D3 for prevention of aromatase inhibitor-induced arthralgia. Breast Cancer Research and Treatment 2019;177(2):427‐35. [DOI] [PubMed] [Google Scholar]
- Niravath PA, Hilsenbeck S, Wang T, Rimawi M. A randomized, controlled trial of high dose vs. standard dose vitamin D for aromatase inhibitor-induced arthralgia in breast cancer survivors. Cancer Research 2014;74(19 Suppl):Abstract no. CT319. [DOI] [PubMed] [Google Scholar]
Peng 2018 {published data only (unpublished sought but not used)}
- ISRCTN06129599. Traditional Chinese medicine for the management of aromatase inhibitor-associated musculoskeletal symptoms. isrctn.com/ISRCTN06129599 (first received 24 July 2013).
- Peng N, Yu M, Yang G, Fu Q, Xu Y, Yu J, et al. Effects of the Chinese medicine Yi Shen Jian Gu granules on aromatase inhibitor-associated musculoskeletal symptoms: a randomized, controlled clinical trial. Breast 2018;37:18-27. [DOI] [PubMed] [Google Scholar]
- Peng N, Zhang Y, Ma C, Yu MW, Yang GW, Fu Q, et al. Effects of the traditional Chinese medicine Yi Shen Jian Gu granules on aromatase inhibitor-associated musculoskeletal symptoms: a study protocol for a multicenter, randomized, controlled clinical trial. Trials 2014;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rastelli 2011 {published data only (unpublished sought but not used)}
- NCT00263185. High dose vit D musculoskeletal symptoms & bone density in anastrozole-treated breast cancer with marginal vit D status. clinicaltrials.gov/show/NCT00263185 (first received 7 December 2005).
- Rastelli A, Taylor M, Villareal R, Jamalabadi-Majidi S, Gao F, Ellis M. A double-blind, randomized, placebo-controlled trial of high dose vitamin D therapy on musculoskeletal pain and bone mineral density in anastrozole-treated breast cancer patients with marginal vitamin D status. Cancer Research 2010;69(24 Suppl):Abstract no. 803. [Google Scholar]
- Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Research and Treatment 2011;129(1):107-16. [DOI] [PubMed] [Google Scholar]
Rosati 2011 {published data only (unpublished sought but not used)}
- Rosati MS, Di Seri M, Baciarello G, Lo Russo V, Grassi P, Marchetti L, et al. Etoricoxib and anastrozole in adjuvant early breast cancer: ETAN trial (phase III). Journal of Clinical Oncology 2011;29(15 Suppl):Abstract no. 533. [Google Scholar]
Shapiro 2016 {published data only}
- NCT01509079. Vitamin D3 effects on musculoskeletal symptoms with use of aromatase inhibitors. clinicaltrials.gov/ct2/show/NCT01509079 (first received 12 January 2012).
- Shapiro A, Adlis S, Robien K, Kirstein M, Liang S, Richter S, et al. Erratum: randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Research and Treatment 2016;157(2):403. [DOI] [PubMed] [Google Scholar]
- Shapiro AC, Adlis SA, Liang S, Robien K, Kirstein MN, Anderson E, et al. A randomized trial of vitamin D3 in aromatase inhibitor-associated musculoskeletal symptoms. Journal of Clinical Oncology 2015;33(15 Supp):Abstract no. 9608. [Google Scholar]
- Shapiro AC, Adlis SA, Robien K, Kirstein MN, Liang S, Richter SA, et al. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Research and Treatment 2016;155(3):501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AC, Kirstein MN, Robien K, Swenson KK, Nissen MJ, Menk JS, et al. Vitamin D3 supplementation, musculoskeletal (MS) symptoms and aromatase inhibitor (AI) pharmacokinetics from the vitamin D3 AI study. Cancer Research 2013;73(24 Suppl):Abstract no. P5-09-18. [Google Scholar]
Shenouda 2019 {published data only (unpublished sought but not used)}
- Shenouda M, Tria Tirona MR. Effect of tart cherry on aromatase inhibitor-induced arthralgia (AIA) in nonmetastatic hormone-positive breast cancer patients: a randomized double-blind placebo-controlled trial. Journal of Clinical Oncology 2019;37(15 Suppl):Abstract no. 525. [DOI] [PubMed] [Google Scholar]
Sordi 2019 {published and unpublished data}
- Sordi R, Castro SN, Lera AT, Irene MN, Farinazzo MM, Sette C, et al. Randomized, double-blind, placebo-controlled phase ii clinical trial on the use of Uncaria tomentosa (Cat's claw) for aromatase inhibitor-induced arthralgia: a pilot study. Journal of Natural Remedies 2019;19(1):24-31. [Google Scholar]
References to studies excluded from this review
ACTRN12611000891921 {published data only}
- ACTRN12611000891921. A trial to determine the effect of glucosamine versus placebo on aromatase inhibitor induced arthralgia (joint pain) in postmenopausal women with early breast cancer who are on letrozole. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611000891921 (first received 9 August 2011).
Altundag 2019 {published data only}
- Altundag K. Combined use of vitamin D and omega-3 fatty acid in breast cancer patients might be more beneficial for reducing aromatase inhibitors-associated arthralgia. Journal of BUON 2019;24(2):862. [PubMed] [Google Scholar]
Barbor 2018 {published data only}
- Barbor M. Omega-3 fatty acids significantly reduced pain in obese patients with breast cancer. Oncology Nurse-APN/PA 2018;11(5):1-6. [Google Scholar]
Becze 2017 {published data only}
- Becze E. Antidepressant may relieve joint pain from aromatase inhibitors. ONS Voice 2017;32(3):11-11. [Google Scholar]
KCT0003698 {published data only}
- KCT0003698. Effect of moxibustion therapy for aromatase inhibitor-induced arthralgia of postmenopausal breast cancer patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=KCT0003698 (date of registration 1 April 2019).
Kim 2012 {published data only}
- Kim HA, Choi HJ, Lim MJ, Park W, Lee J, Choi SJ, et al. Nonsteroidal antiinflammatory drugs (NSAID) versus NSAID with hydroxychloroquine in treatment of chemotherapy-related arthropathy: open-label multicenter pilot study. Journal of Rheumatology 2012;39(9):1902-3. [DOI] [PubMed] [Google Scholar]
NCT01809171 {published data only}
- NCT01809171. Placebo-controlled trial with vitamin D to prevent worsening/relieve aromatase inhibitor-induced musculoskeletal symptoms in breast cancer patients. clinicaltrials.gov/ct2/show/NCT01809171 (first received 12 March 2013).
NCT03384095 {published data only}
- NCT03384095. Trial of oral hyaluronic acid for the prevention of aromatase inhibitor-associated arthralgias. clinicaltrials.gov/ct2/show/NCT03384095 (first received 27 December 2017).
UMIN000004416 {published data only}
- UMIN000004416. Japanese study for usefulness of vitamin E in reducing aromatase inhibitor-associated joint symptoms. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_his_list.cgi?recptno=R000005255 (date uploaded 19 October 2010). [TRIAL REGISTRY: UMIN000004416]
References to ongoing studies
NCT02831582 {published data only}
- NCT02831582. Omega-3 supplementation in prevention of aromatase inhibitor-induced toxicity in patients with stage I–III breast cancer. clinicaltrials.gov/show/NCT02831582 (first received 13 July 2016).
NCT03865992 {published data only}
- NCT03865992. Curcumin in reducing joint pain in breast cancer survivors with aromatase inhibitor-induced joint disease. clinicaltrials.gov/ct2/show/NCT03865992 (first received 7 March 2019).
NCT04205786 {published data only}
- NCT04205786. Vitamin B12 for aromatase inhibitors associated musculoskeletal symptoms in breast cancer. clinicaltrials.gov/ct2/show/NCT04205786 (first received 19 December 2019).
UMIN000027481 {published data only}
- UMIN000027481. Randomized study of Sokei-Kakketsu-To for the reduction of aromatase inhibitor – associated joint symptoms in women with breast cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031481 (first received 25 May 2017).
Additional references
Aguilar 2002
- Aguilar JL, Rojas P, Marcelo A, Plaza A, Bauer R, Reininger E, et al. Anti-inflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae). Journal of Ethnopharmacology 2002;81(2):271-6. [DOI] [PubMed] [Google Scholar]
Ambrosone 2020
- Ambrosone CB, Zirpoli GR, Hutson AD, McCann WE, McCann SE, Barlow WE, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a Cooperative Group Clinical Trial (SWOG S0221). Journal of Clinical Oncology 2020;38(8):804-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aquino 1989
- Aquino R, De Simone F, Pizza C, Conti C, Stein ML. Plant metabolites. Structure and in vitro antiviral activity of quinovic acid glycosides from Uncaria tomentosa and Guettarda platypoda. Journal of Natural Products 1989;52(4):679-85. [DOI] [PubMed] [Google Scholar]
Arnold 2005
- Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119(1-3):5-15. [DOI] [PubMed] [Google Scholar]
Basch 2010
- Basch E. The missing voice of patients in drug-safety reporting. New England Journal of Medicine 2010;362(10):865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basch 2016
- Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. Journal of Clinical Oncology 2016;34(6):557-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basch 2017
- Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318(2):197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beckwee 2017
- Beckwee D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Supportive Care in Cancer 2017;25(5):1673-86. [DOI] [PubMed] [Google Scholar]
Bellingham 2010
- Bellingham GA, Peng PW. Duloxetine: a review of its pharmacology and use in chronic pain management. Regional Anaesthesia and Pain Medication 2010;35(3):294-303. [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ 2004;170(4):477-80. [PMC free article] [PubMed] [Google Scholar]
Bohannon 2019
- Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. Journal of Physical Therapy Science 2019;31(1):75-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boon 2000
- Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. Journal of Clinical Oncology 2000;18(13):2515-21. [DOI] [PubMed] [Google Scholar]
Boon 2007
- Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borrie 2017
- Borrie AE, Kim RB. Molecular basis of aromatase inhibitor associated arthralgia: known and potential candidate genes and associated biomarkers. Journal of Expert Opinion on Drug Metabolism & Toxicology 2017;13(2):149-56. [DOI] [PubMed] [Google Scholar]
Brandt 2019
- Brandt J, Scotté F, Jordan K. Patient-reported outcomes (PROs) as a routine measure for cancer inpatients: the final missing piece of the puzzle? Annals of Oncology 2019;30(2):167-9. [DOI] [PubMed] [Google Scholar]
Brier 2017
- Brier MJ, Chambless DL, Gross R, Chen J, Mao JJ. Perceived barriers to treatment predict adherence to aromatase inhibitors among breast cancer survivors. Cancer 2017;123(1):169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briot 2010
- Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Research and Treatment 2010;120(1):127-34. [DOI] [PubMed] [Google Scholar]
Burstein 2007
- Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast 2007;16(3):223-34. [DOI] [PubMed] [Google Scholar]
Burstein 2019
- Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. Journal of Oncology Practice 2019;15(2):106-7. [DOI] [PubMed] [Google Scholar]
Castel 2013
- Castel LD, Hartmann KE, Mayer IA, Saville BR, Alvarez J, Boomershine CS, et al. Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer 2013;119(13):2375-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castel 2015
- Castel LD, Wallston KA, Saville BR, Alvarez JR, Shields BD, Feurer ID, et al. Validity and reliability of the Patient-Reported Arthralgia Inventory: validation of a newly-developed survey instrument to measure arthralgia. Patient Related Outcome Measures 2015;6:205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008
- Chen Z, Gu K, Zheng Y, Zheng W, Lu W, Shu XO. The use of complementary and alternative medicine among Chinese women with breast cancer. Journal of Alternative and Complementary Medicine 2008;14(8):1049-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2017
- Chen L, Lin CC, Huang TW, Kuan YC, Huang YH, Chen HC, et al. Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast 2017;33:132-8. [DOI] [PubMed] [Google Scholar]
Chesnut 2008
- Chesnut CH, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L. Salmon calcitonin: a review of current and future therapeutic indications. Osteoporosis International 2008;19(4):479-91. [DOI] [PubMed] [Google Scholar]
Chim 2013
- Chim K, Xie SX, Stricker CT, Li QS, Gross R, Farrar JT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer 2013;13:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chirgwin 2016
- Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, et al. Treatment adherence and Its Impact on disease-free survival in the Breast International Group 1-98 Trial of tamoxifen and letrozole, alone and in sequence. Journal of Clinical Oncology 2016;34(21):2452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2011
- Chlebowski RT, Johnson KC, Lane D, Pettinger M, Kooperberg CL, Wactawski-Wende J, et al. 25-Hydroxyvitamin D concentration, vitamin D intake and joint symptoms in postmenopausal women. Maturitas 2011;68(1):73-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleeland 2009
- Cleeland CS. The Brief Pain Inventory: user guide, 2009. www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf (accessed 18 December 2021).
Cleland 1988
- Cleland LG, French JK, Betts WH, Murphy GA, Elliott MJ. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. Journal of Rheumatology 1988;15(10):1471-5. [PubMed] [Google Scholar]
CMSG 2021
- Cochrane Musculoskeletal Group. Proposed outcomes. musculoskeletal.cochrane.org/proposed-outcomes (accessed 16 October 2021).
Crew 2007
- Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. Journal of Clinical Oncology 2007;25(25):3877-83. [DOI] [PubMed] [Google Scholar]
Crew 2009
- Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. Journal of Clinical Oncology 2009;27(13):2151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruciani 2012
- Cruciani RA, Zhang JJ, Manola J, Cella D, Ansari B, Fisch MJ. L-carnitine supplementation for the management of fatigue in patients with cancer: an Eastern Cooperative Oncology Group phase III, randomized, double-blind, placebo-controlled trial. Journal of Clinical Oncology 2012;30(31):3864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cui 2004
- Cui Y, Shu XO, Gao Y, Wen W, Ruan ZX, Jin F, et al. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Research and Treatment 2004;85(3):263-70. [DOI] [PubMed] [Google Scholar]
Da Costa 2014
- Da Costa BR, Nuesch E, Kasteler R, Husni E, Welch V, Rutjes AW, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD003115. [DOI: 10.1002/14651858.CD003115.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021 Available from www.training.cochrane.org/handbook.
Delgado 2017
- Delgado AF, Delgado AF. The association of funding source on effect size in randomized controlled trials: 2013–2015 – a cross-sectional survey and meta-analysis. Trials 2017;18(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deshpande 2011
- Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspectives in Clinical Research 2011;2(4):137-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2009
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238-44. [DOI] [PubMed] [Google Scholar]
EBCTCG 2015
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386(10001):1341-52. [DOI] [PubMed] [Google Scholar]
Ervin 2004
- Ervin RB, Wright JD, Wang C, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Advance Data 2004;348:1-6. [PubMed] [Google Scholar]
Eton 2004
- Eton D, Cella D, Jost K, Yount S, Peterman A, Neuberg D, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. Journal of Clinical Epidemiology 2004;57(9):898-910. [DOI: ] [DOI] [PubMed] [Google Scholar]
Excel [Computer program]
- Microsoft Excel. Version 16.17. Redmond (WA): Microsoft Corporation, 2018.
Farrar 2010
- Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. Journal of Pain 2010;11(2):109-18. [DOI] [PubMed] [Google Scholar]
FDA 2018
- Food and Drug Administration. COX-2 selective (includes Bextra, Celebrex, and Vioxx) and non-selective non-steroidal anti-inflammatory drugs (NSAIDs). www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/cox-2-selective-includes-bextra-celebrex-and-vioxx-and-non-selective-non-steroidal-anti-inflammatory (accessed 18 December 2021).
Felson 2005
- Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis and Rheumatism 2005;52(9):2594-8. [DOI] [PubMed] [Google Scholar]
Ferlay 2012
- Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN, Cancer Incidence and Mortality Worldwide. 1.0 edition. Lyon, France: International Agency for Research on Cancer, 2012. [Google Scholar]
Francis 2015
- Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. New England Journal of Medicine 2015;372(5):436-46. [DOI] [PMC free article] [PubMed]
Francis 2018
- Francis P, Pagani O, Fleming G, Walley B, Colleoni M, Láng I, et al for the SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. New England Journal of Medicine 2018;379(2):122-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gall 2018
- Gall A, Leske S, Adams J, Matthews V, Anderson K, Lawler S, et al. Traditional and complementary medicine use among Indigenous cancer patients in Australia, Canada, New Zealand, and the United States: a systematic review. Integrative Cancer Therapies 2018;17(3):568-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 1980
- Gallagher JC, Lawrence Riggs B, Deluca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. Journal of Clinical Endocrinology and Metabolism 1980;51(6):1359-64. [DOI] [PubMed] [Google Scholar]
Goldberg 2007
- Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007;129(1-2):210-23. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 10 September 2018. Hamilton (ON): GRADE Working Group, McMaster University, 2014. Available at gradepro.org.
Greenlee 2012
- Greenlee H, Kwan ML, Kushi LH, Song J, Castillo A, Weltzien E, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort. Cancer 2012;118(8):2048-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenlee 2013
- Greenlee H, Crew KD, Shao T, Kranwinkel G, Kalinsky K, Maurer M, et al. Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Supportive Care in Cancer 2013;21(4):1077-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2020
- Gupta A, Henry NL, Loprinzi CL. Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncology Practice 2020;16(11):733-9. [DOI] [PubMed] [Google Scholar]
Hack 2020
- Hack CC, Häberle L, Brucker SY, Janni W, Volz B, Loehberg CR, et al. Complementary and alternative medicine and musculoskeletal pain in the first year of adjuvant aromatase inhibitor treatment in early breast cancer patients. Breast 2020;50:11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadji 2014
- Hadji P, Jackisch C, Bolten W, Blettner M, Hindenburg HJ, Klein P, et al. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Annals of Oncology 2014;25(2):372-7. [DOI] [PubMed] [Google Scholar]
Henry 2008
- Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Research and Treatment 2008;111(2):365-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 2011
- Henry NL, Banerjee M, Wicha M, Poznak C, Smerage JB, Schott AF, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer 2011;117(24):5469-75. [DOI] [PubMed] [Google Scholar]
Henry 2012
- Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. Journal of Clinical Oncology 2012;30(9):936-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 2015
- Henry NL, Griggs JJ. The power of the placebo in symptom management. Journal of Clinical Oncology 2015;33(17):1870-2. [DOI] [PubMed] [Google Scholar]
Henry 2019
- Henry NN, Unger JM, Till C, Schott AF, Crew KD, Lew DL, et al. Association between body mass index and response to duloxetine for aromatase inhibitor‐associated musculoskeletal symptoms in SWOG S1202. Cancer 2019;125(12):2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hershman 2011
- Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Research and Treatment 2011;126(2):529-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hershman 2015a
- Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, et al. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. Journal of Clinical Oncology 2015;33(17):1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hershman 2015b
- Hershman DL, Loprinzi C, Schneider BP. Symptoms: aromatase inhibitor induced arthralgias. Advances in Experimental Medicine and Biology 2015;862:89-100. [DOI] [PubMed] [Google Scholar]
Hertz 2017
- Hertz DL, Henry NL, Rae JM. Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients. Pharmacogenomics 2017;18(5):481-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021), Cochrane, 2021. Available from training.cochrane.org/handbook.
Holick 2007
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266-81. [DOI] [PubMed] [Google Scholar]
Howlader 2019
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M. Median age of cancer patients at diagnosis (2012–2016). SEER Cancer Statistics Review (CSR) 1975–2016 2019:seer.cancer.gov/archive/csr/1975_2016/. Table 1.11.
Kadakia 2016
- Kadakia KC, Snyder CF, Kidwell KM, Seewald NJ, Flockhart DA, Skaar TC, et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist 2016;21(5):539-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamper 2009
- Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. Journal of Manual & Manipulative Therapy 2009;17(3):163-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanematsu 2011
- Kanematsu M, Morimoto M, Honda J, Nagao T, Nakagawa M, Takahashi M, et al. The time since last menstrual period is important as a clinical predictor for non-steroidal aromatase inhibitor-related arthralgia. BMC Cancer 2011;11:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelley 2018
- Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients 2018;10(3):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2018
- Kim TH, Kang JW, Lee TH. Therapeutic options for aromatase inhibitor-associated arthralgia in breast cancer survivors: a systematic review of systematic reviews, evidence mapping, and network meta-analysis. Maturitas 2018;118:29-37. [DOI] [PubMed] [Google Scholar]
Kremser 2008
- Kremser T, Evans A, Moore A, Luxford K, Begbie S, Bensoussan A, et al. Use of complementary therapies by Australian women with breast cancer. Breast 2008;17(4):387-94. [DOI] [PubMed] [Google Scholar]
Kronenberg 2006
- Kronenberg F, Cushman LF, Wade CM, Kalmuss D, Chao MT. Race/ethnicity and women's use of complementary and alternative medicine in the United States: results of a national survey. American Journal of Public Health 2006;96(7):1236-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubo 2012
- Kubo M, Onishi H, Kuroki S, Okido M, Shimada K, Yokohata K, et al. Short-term and low-dose prednisolone administration reduces aromatase inhibitor-induced arthralgia in patients with breast cancer. Anticancer Research 2012;32(6):2331-6. [PubMed] [Google Scholar]
Kwan 2017
- Kwan ML, Roh JM, Laurent CA, Lee J, Tang L, Hershman D, et al. Patterns and reasons for switching classes of hormonal therapy among women with early-stage breast cancer. Cancer Causes & Control 2017;28(6):557-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laroche 2017
- Laroche F, Perrot S, Medkour T, Cottu PH, Pierga JY, Lotz JP, et al. Quality of life and impact of pain in women treated with aromatase inhibitors for breast cancer. A multicenter cohort study. PLoS One 2017;12(11):e0187165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS One 2013;8(4):e60338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lilly 2020
- Lilly Pharmaceuticals USA. Highlights of prescribing information: CYMBALTA (duloxetine delayed-release capsules), for oral use. pi.lilly.com/us/cymbalta-pi.pdf (accessed 18 December 2021).
Lintermans 2013
- Lintermans A, Laenen A, Calster B, Hoydonck M, Pans S, Verhaeghe J, et al. Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Annals of Oncology 2013;24(2):350-5. [DOI] [PubMed] [Google Scholar]
Lintermans 2014
- Lintermans A, Asten K, Wildiers H, Laenen A, Paridaens R, Weltens C, et al. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Research and Treatment 2014;146(1):109-16. [DOI] [PubMed] [Google Scholar]
Lintermans 2016
- Lintermans A, Asten K, Jongen L, Brussel T, Laenen A, Verhaeghe J, et al. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. European Journal of Cancer 2016;56:31-6. [DOI] [PubMed] [Google Scholar]
Lopez 2015
- Lopez C, Charles C, Rouby P, Boinon D, Laurent S, Rey A, et al. Relations between arthralgia and fear of recurrence: results of a cross-sectional study of breast cancer patients treated with adjuvant aromatase inhibitors therapy. Supportive Care in Cancer 2015;23(12):3581-8. [DOI] [PubMed] [Google Scholar]
Loprinzi 2009
- Loprinzi CL, Sloan J, Stearns V, Slack R, Iyengar M, Diekmann B, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. Journal of Clinical Oncology 2009;27(17):2831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lustberg 2015
- Lustberg MB, Orchard T, Pan X, Reinbolt R, Logan A, Lester J, et al. Prevention of aromatase Inhibitor (AI)-induced joint symptoms with omega-3 fatty acid supplementation: a randomized placebo-controlled pilot study. Cancer Research 2015;75(9 Suppl):Abstract P1-09-03. [Google Scholar]
Lustberg 2018
- Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, et al. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Research and Treatment 2018;167(3):709-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2009
- Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 2009;115(16):3631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2011
- Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, et al. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Research 2011;13(1):R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maredupaka 2020
- Maredupaka S, Meshram P, Chatte M, Kim WH, Kim TK. Minimal clinically important difference of commonly used patient-reported outcome measures in total knee arthroplasty: review of terminologies, methods and proposed values. Knee Surgery & Related Research 2020;32(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Markham 2020
- Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology 2020;38(10):1081. [DOI] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Mease 2011
- Mease PJ, Spaeth M, Clauw DJ, Arnold LM, Bradley LA, Russell IJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care & Research 2011;63(6):821-6. [DOI] [PubMed] [Google Scholar]
Moy 2006
- Moy B, Tu D, Pater JL, Ingle JN, Shepherd LE, Whelan TJ, et al. Clinical outcomes of ethnic minority women in MA.17: a trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer. Annals of Oncology 2006;17(11):1637-43. [DOI] [PubMed] [Google Scholar]
Nadji 2005
- Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. American Journal of Clinical Pathology 2005;123(1):21-7. [DOI] [PubMed] [Google Scholar]
Nahm 2018
- Nahm N, Mee S, Marx G. Efficacy of management strategies for aromatase inhibitor-induced arthralgia in breast cancer patients: a systematic review. Asia-Pacific Journal of Clinical Oncology 2018;14(6):374-82. [DOI] [PubMed] [Google Scholar]
NCCIH 2016
- National Centre for Complementary and Integrative Health (NCCIH). Complementary, alternative, or integrative health: what’s In a name? nccih.nih.gov/health/integrative-health (accessed prior to 10 September 2018).
NCI 2021
- National Cancer Institute. Drug dictionary: Blue Citrus-based herbal capsule. www.cancer.gov/publications/dictionaries/cancer-drug/def/blue-citrus-based-herbal-capsule (accessed 27 March 2021).
NICE 2014
- National Institute for Health and Care Excellence. Osteoarthritis: care and management. www.nice.org.uk/guidance/cg177 (accessed prior to 10 September 2018).
Niravath 2013
- Niravath P. Aromatase inhibitor-induced arthralgia: a review. Annals of Oncology 2013;24(6):1443-9. [DOI] [PubMed] [Google Scholar]
Niravath 2018
- Niravath P, Chen B, Chapman JA, Agarwal SK, Welschhans RL, Bongartz T, et al. Vitamin D levels, vitamin D receptor polymorphisms, and inflammatory cytokines in aromatase inhibitor-induced arthralgias. Clinical Breast Cancer 2018;18(1):78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsen 2018
- Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. Journal of Clinical Epidemiology 2018;101:87-106. [DOI] [PubMed] [Google Scholar]
OMERACT 2021
- Outcome Measures in Rheumatology. OMERACT Outcome Measurement. omeract.org/ (accessed 16 October 2021).
Partridge 2008
- Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. Journal of Clinical Oncology 2008;26(4):556-62. [DOI] [PubMed] [Google Scholar]
Patrick 2007
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value in Health 2007;10(Suppl 2):S125-37. [DOI] [PubMed] [Google Scholar]
Peng 2014
- Peng N, Zhang Y, Ma C, Yu M, Yang G, Fu Q, et al. Effects of the traditional Chinese medicine Yi Shen Jian Gu granules on aromatase inhibitor-associated musculoskeletal symptoms: a study protocol for a multicenter, randomized, controlled clinical trial. Trials 2014;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poole 2013
- Poole EM, Shu X, Caan BJ, Flatt SW, Holmes MD, Lu W, et al. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Research and Treatment 2013;139(2):529-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Power 2004
- Power R. Emu oil for osteoarthritic hand pain [Masters thesis]. Melbourne (Australia): Victoria University, 2004. [URL: vuir.vu.edu.au/id/eprint/869] [Google Scholar]
Presant 2007
- Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, et al. Aromatase inhibitor-associated arthralgia and/ or bone pain: frequency and characterization in non-clinical trial patients. Clinical Breast Cancer 2007;7(10):775-8. [DOI] [PubMed] [Google Scholar]
Rastelli 2011
- Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Research and Treatment 2011;129(1):107-16. [DOI] [PubMed] [Google Scholar]
Reginster 2012
- Reginster JY, Neuprez A, Lecart M, Sarlet N, Bruyere O. Role of glucosamine in the treatment for osteoarthritis. Rheumatology International 2012;32(10):2959-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Version 2.5.1. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Richards 2012
- Richards BL, Whittle SL, Buchbinder R. Muscle relaxants for pain management in rheumatoid arthritis. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD008922. [DOI: 10.1002/14651858.CD008922.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2017
- Roberts K, Rickett K, Greer R, Woodward N. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: a systematic review and meta-analysis. Critical Reviews in Oncology/Hematology 2017;111:66-80. [DOI] [PubMed] [Google Scholar]
Roberts 2018
- Roberts KE, Rickett K, Chatfield MD, Woodward NE. Systemic therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013167. [DOI: 10.1002/14651858.CD013167] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2020
- Roberts KE, Rickett K, Feng S, Vagenas D, Woodward NE. Exercise therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD012988. [DOI: 10.1002/14651858.CD012988.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rocque 2018
- Rocque G. What is the role of symptom management and patient-reported outcomes in adherence to aromatase inhibitors? Journal of Clinical Oncology 2018;36(4):308-9. [DOI] [PubMed] [Google Scholar]
Rosati 2011
- Rosati MS, Di Sri M, Baciarello G, Russo VL, Grassi P, Marchetti L. Etoricoxib and anastrozole in adjuvant early breast cancer: ETAN trial (phase III). Journal of Clinical Oncology 2011;29(15 Suppl):533. [Google Scholar]
Schmidt 2006
- Schmidt M, Naumann H, Weidler C, Schellenberg M, Anders S, Straub RH. Inflammation and sex hormone metabolism. Annals of the New York Academy of Sciences 2006;1069:236-46. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook 2019:375-402. [DOI: 10.1002/9781119536604.ch14] [DOI]
Sestak 2008
- Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncology 2008;9(9):866-72. [DOI] [PubMed] [Google Scholar]
Shen 2018
- Shen S, Unger JM, Crew KD, Till C, Greenlee H, Gralow J, et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Research and Treatment 2018;172(3):603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2015
- Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD005614. [DOI: 10.1002/14651858.CD005614.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013;309(13):1359-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2016
- Song GG, Seo YH, Kim J, Choi SJ, Ji JD, Lee YH. Relative efficacy and tolerability of etoricoxib, celecoxib, and naproxen in the treatment of osteoarthritis: a Bayesian network meta-analysis of randomized controlled trials based on patient withdrawal. Zeitschrift fur Rheumatologie 2016;75(5):508-16. [DOI] [PubMed] [Google Scholar]
Sundrarjun 2004
- Sundrarjun T, Komindr S, Archararit N, Dahlan W, Puchaiwatananon O, Angthararak S, et al. Effects of n-3 fatty acids on serum interleukin-6, tumour necrosis factor-alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. Journal of International Medical Research 2004;32(5):443-54. [DOI] [PubMed] [Google Scholar]
Towheed 2005
- Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD002946. [DOI: 10.1002/14651858.CD002946.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2015
- Turner A, Hancock G, Wells J, Whitehouse M. Traditional medicinal oils sourced from birds: anti-inflammatories and potential immunoregulants. Progress in Drug Research 2015;70:155-78. [DOI] [PubMed] [Google Scholar]
Wan 2013
- Wan M, Orlu-Gul M, Legay H, Tuleu C. Blinding in pharmacological trials: the devil is in the details. Archives of Disease in Childhood 2013;98(9):656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitehouse 1998
- Whitehouse MW, Turner AG, Davis CK, Roberts MS. Emu oil(s): a source of non-toxic transdermal anti-inflammatory agents in Aboriginal medicine. Inflammopharmacology 1998;6(1):1-8. [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. WHO traditional medicine strategy: 2014–2023. apps.who.int/iris/bitstream/handle/10665/92455/9789241506090_eng.pdf?sequence=1 (accessed prior to 18 December 2021).
Wieland 2011
- Wieland LS, Manheimer E, Berman BM. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane collaboration. Alternative Therapies in Health and Medicine 2011;17(2):50-9. [PMC free article] [PubMed] [Google Scholar]
Williams 2011
- Williams DA, Arnold LM. Measures applied to the assessment of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), the Multidimensional Fatigue Inventory (MFI-20), the MOS Sleep Scale, and the Multiple Ability Self-Report Questionnaire (MASQ; cognitive dysfunction). Arthritis Care & Research 2011;63(0 11):S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xian 2011
- Xian YF, Li YC, Ip SP, Lin ZX, Lai XP, Su ZR. Anti-inflammatory effect of patchouli alcohol isolated from Pogostemonis Herba in LPS-stimulated RAW264.7 macrophages. Experimental and Therapeutic Medicine 2011;2(3):545-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2006
- Xu W, Towers AD, Li P, Collet JP. Traditional Chinese medicine in cancer care: perspectives and experiences of patients and professionals in China. European Journal of Cancer Care 2006;15(4):397-403. [DOI] [PubMed] [Google Scholar]
Xu 2014
- Xu L, Wang J, Xue DD, He W. Aromatase inhibitors associated musculoskeletal disorders and bone fractures in postmenopausal breast cancer patients: a result from Chinese population. Medical Oncology 2014;31(9):128. [DOI] [PubMed] [Google Scholar]
Yang 2017
- Yang GS, Kim HJ, Griffith KA, Zhu S, Dorsey SG, Renn CL. Interventions for the treatment of aromatase inhibitor-associated arthralgia in breast cancer survivors: a systematic review and meta-analysis. Cancer Nursing 2017;40(4):E26-41. [DOI] [PubMed] [Google Scholar]
Yost 2014
- Yost J, Ciliska D, Dobbins M. Evaluating the impact of an intensive education workshop on evidence-informed decision making knowledge, skills, and behaviours: a mixed methods study. BMC Medical Education 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010
- Zhang Q, Tang D, Zhao H. Immunological therapies can relieve aromatase inhibitor-related joint symptoms in breast cancer survivors. American Journal of Clinical Oncology 2010;33(6):557-60. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Roberts
- Roberts KE, Rickett K, Chatfield MD, Woodward NE. Systemic therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013167. [DOI: 10.1002/14651858.CD013167] [DOI] [PMC free article] [PubMed] [Google Scholar]


