Abstract
Reproductive division of labour is a hallmark of eusocial insects. However, its stability can often be hampered by the potential for reproduction by otherwise sterile nest-mates. Dominance hierarchy has a crucial role in some species in regulating which individuals reproduce. Compared with those in vertebrates, the dominance hierarchies in eusocial insects tend to involve many more individuals, and should require additional selective forces unique to them. Here, we provide an overview of a series of studies on dominance hierarchies in eusocial insects. Although reported from diverse eusocial taxa, dominance hierarchies have been extensively studied in paper wasps and ponerine ants. Starting from molecular physiological attributes of individuals, we describe how the emergence of dominance hierarchies can be understood as a kind of self-organizing process through individual memory and local behavioural interactions. The resulting global structures can be captured by using network analyses. Lastly, we argue the adaptive significance of dominance hierarchies from the standpoint of sterile subordinates. Kin selection, underpinned by relatedness between nest-mates, is key to the subordinates' acceptance of their positions in the hierarchies.
This article is part of the theme issue ‘The centennial of the pecking order: current state and future prospects for the study of dominance hierarchies’.
Keywords: dominance hierarchy, dominance network, kin selection, self-organization
1. Introduction
Reproductive division of labour is a hallmark of eusocial insects such as ants, bees, wasps and termites. Typically, the reproductive castes (‘reproductives’, hereafter referred to as ‘Rs’) devote themselves to reproduction, whereas the non-reproductive castes (‘non-reproductives’ or ‘NRs’) take charge of colony maintenance, such as raising broods, guarding the nest and foraging for food [1]. This system has been regarded as key to the ecological and evolutionary success of eusocial insects [1–3]. The peaceful maintenance of the reproductive division of labour is, however, often hampered by the presence of adults who are expected to be NRs but retain the physiological potential for reproduction. For example, in eusocial Hymenoptera the haplodiploid system requires mating for the production of diploid daughters, which is allowed only for Rs in a colony. Nevertheless, in many species, female NRs retain functional ovaries and can produce haploid sons in the same colony without mating [4]. Under this tension, dominance behaviours find their role in maintaining the reproductive division of labour.
Since the pioneering work of Pardi [5] on Polistes wasps, dominance behaviours of dominants towards subordinates have been reported in a wide range of eusocial Hymenoptera. The resulting dominance hierarchies are remarkable for their size: the number of individuals in the hierarchy often reaches 70 ([6], see also [7]). The formation and maintenance of such large hierarchies would require unique selective forces that are not present in other animal societies.
In this review, we first list the occurrences of dominance behaviours in eusocial insects along the timeline of colony development, and give an overview of their taxonomic diversity. We then describe the build-up of dominance hierarchy in a bottom-up manner, from individual attributes through interaction dynamics to the global network structures. Finally, we discuss the adaptive significance of the dominance behaviours and hierarchy from the standpoint of sterile subordinates.
2. Dominance behaviours in the colony life cycle
Generally, dominance behaviours manifest in both the formation and the maintenance of a dominance hierarchy. In eusocial insects, dominance behaviours are typically observed at three phases in the colony's life cycle (figure 1). The first phase occurs at the colony founding stage. In many species, an initial colony contains a single female (called a foundress). In some species, however, multiple seminated females, related or unrelated, come together and create a new colony (pleometrosis). Dominance behaviours are observed among these foundresses (formation phase: figure 1a). All founding nest-mates are reproductively equal at the onset, and dominance behaviours occur once the colony is established. In the resulting linear dominance hierarchy, a differentiation arises between top-ranked Rs and other NRs that is associated with physiological and behavioural modifications. In some species, aggressive interactions between foundresses sometimes result in the death of some individuals or even a single survivor [8].
Figure 1.
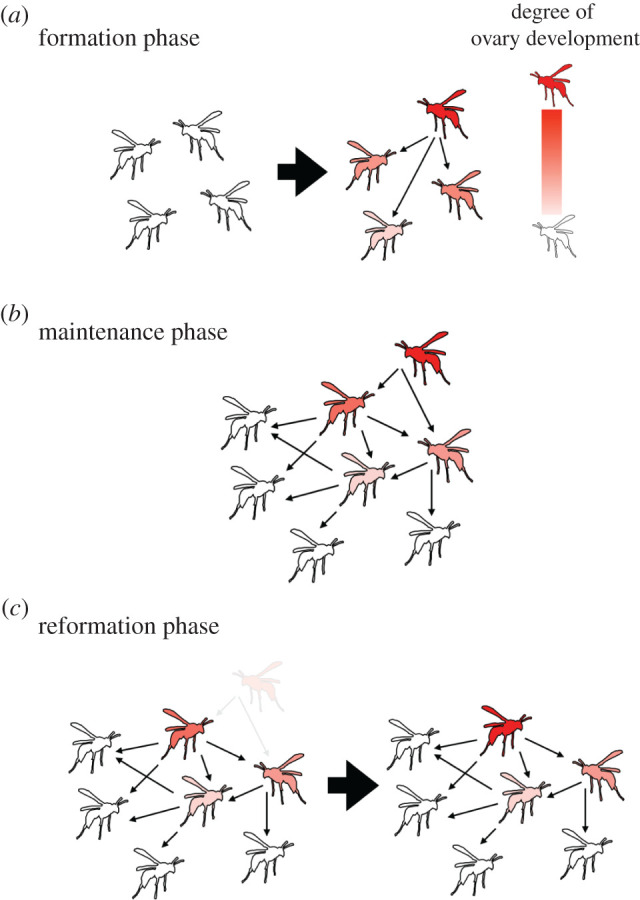
Typical formation of dominance hierarchies at three phases in the life cycle of a colony of eusocial insects. (a) Formation phase: foundresses compete for top ranking in the hierarchy. The resulting one dominant individual can take over reproduction as a ‘reproductive’ (R) and the others engage in non-reproductive roles as ‘NRs’ in the colony. (b) Maintenance phase: in addition to the R, dominant NRs perform dominance behaviours, leading to a stable hierarchical structure that allows the highest individual to maintain the R position. (c) Reformation phase: after the R is lost from the colony, the remaining dominant NRs compete and one NR takes over the top-ranked position in the hierarchy. The reconstructed hierarchy will move into maintenance phase again. The red colour scale indicates reproductive ability, with red indicating full development of ovaries and white indicating non-development. Arrows point from actors to recipients of dominance behaviours. (Online version in colour.)
The second phase occurs after the reproductive division of labour has been established in a colony (maintenance phase: figure 1b). In this phase, each individual R or NR performs dominance behaviours towards lower-ranked NRs in the hierarchy, and thereby represses their reproductive ability. A unique feature of this phase is that birth–death of individuals in the colony gives a certain local instability to the dominance hierarchy.
The third phase arises after the top-ranked R is lost from the colony (reformation phase: figure 1c). This event destabilizes the dominance hierarchy among the remaining NRs, but dominance behaviours quickly reconstruct a new hierarchy and determine a new R among the previous NRs. This agility is in part attributed to the physiological heterogeneity that is already established among NRs during the second phase.
3. Taxonomic distributions
Dominance behaviours have been found in a wide range of taxa in eusocial Hymenoptera (electronic supplementary material, table S1). They are particularly enriched in phylogenetically basal (so-called ‘primitive’) eusocial species that are characterized by small colony size (up to the order of 102 individuals) and low degrees of morphological differentiation between Rs (usually queens) and NRs (workers). In this section, we focus on several species of wasps and ants to illustrate the role of dominance behaviours in their colonies.
(a) . Wasps
Dominance behaviours in wasps are arguably the best studied among eusocial insects [5,9,10]. In the genus Polistes, colony foundation occurs in two ways: by a single foundress or by multiple foundresses. As with other eusocial species, dominance behaviours at the formation phase are expected in the latter case, which results in a linear dominance hierarchy under which the top- or higher-ranked female(s) become(s) the R(s). The dominance hierarchy is then maintained by dominance behaviours among Rs and NRs (maintenance phase). Dominance behaviours are also observed after the Rs are lost, i.e. at the reformation phase. Jandt et al. [10] provides a good review of the dominance behaviours in Polistes.
In the same subfamily Polistinae, Ropalidia marginata is another species whose dominance behaviours have been well studied. Multiple females initiate a new colony, and a mature colony consists of a single R (queen) and fewer than 100 NRs (workers) [11]. Dominance behaviours manifest in all of the above three phases [12,13]. Foundresses frequently perform dominance behaviours, and then a top-ranked individual becomes the R in a colony (formation phase). However, unlike in Polistes, once the dominance hierarchy is established, the R rarely acts aggressively towards NRs. Instead of physical interactions, hydrocarbon profiles secreted from Dufour's gland of the R are known to suppress the reproductive ability of NRs [14].
(b) . Ants
Ants, a large family of eusocial Hymenoptera, exhibit diverse social systems [2]. Cole [15] found a dominance hierarchy among NRs (workers) in a myrmicine ant, Temnothorax (formerly Leptothorax) allardycei (maintenance and reformation phases). Since this discovery, dominance behaviours have been studied in a variety of ants covering all of the above three phases (electronic supplementary material, table S1). The studied species share common features such as small colony size and low degrees of morphological differentiation between Rs and NRs. By contrast to eusocial wasps, reports of dominance behaviours at the formation phase, i.e. when the colony is initiated by multiple foundresses, are relatively rare in ants (electronic supplementary material, table S1). This is chiefly because the losers are typically killed by the winner and also workers [8]. However, Hölldobler & Carlin [16] reported a case of dominance behaviours at the formation phase in the formicine ant Iridomyrmex purpureus.
There are many examples of dominance behaviours in ants at both the maintenance and reformation phases. Among them are those from queenless ants of the subfamily Ponerinae, which are considered to represent ancestral societies [17,18]. Here we focus on two species, Pachycondyla sublaevis and Diacamma cf. indicum, whose dominance behaviours in these two phases are well characterized.
Colonies of P. sublaevis contain 2–18 adult females morphologically classified as workers; only one mated worker (called a gamergate) becomes an R that dominates reproduction in the colony [19,20]. The gamergate continues to perform dominance behaviours after mating (maintenance phase), forming an almost linear dominance hierarchy with her at the top [20]. Newly emerged females often take over positions of higher-ranked elder females during this maintenance phase. The gamergate can be lost (i.e. the colony is orphaned) when she dies or when a colony splits into two through colony fission. A dominance hierarchy is then reconstructed among the remaining unmated NRs in the colony, but usually the second-ranked NR takes over the reproductive position (reformation phase) [19]. Although all females in a colony are at least physiologically capable of mating, it is only the new top-ranking female that is expected to encounter her mating partner and become a new R [20].
Another example comes from D. cf. indicum from Japan [21,22], which has a well-documented colony life cycle [23]. This species has long been reported as ‘Diacamma sp. from Japan’ owing to taxonomic issues, but the elusive taxonomic status does not hinder the unique identification of this species (or at least population), because the study sites are located in a limited area on Okinawa Island, in southern Japan. Extensive population genetic studies on the island [24,25] have not yet identified a cryptic species. Colonies of this species contain one gamergate (R) and 50–300 unmated workers (NRs) in the field [26]. All females on emergence possess a pair of tiny rudimentary or vestigial wings (called gemmae) on the thorax, which are surgically bitten off by the gamergate. Consequently, the ‘mutilated’ adults lose their mating ability and differentiate into NRs (maintenance phase). When the gamergate is lost, the first adult that emerges in the colony successfully retains her gemmae without being targeted and becomes a gamergate-to-be. This female performs dominance behaviours towards the other elder sisters (reformation phase). Interestingly, aggressiveness of the new gamergate declines with her age [27] (a similar pattern is observed in the congeneric species Diacamma ceylonense [28]), and the reproductive division of labour seems to be mediated by mechanisms other than behavioural interactions [29]. NRs in the maintenance phase engage in dominance behaviours, leading to an almost perfectly linear dominance hierarchy among the NRs [6]. In the hierarchy, a few higher-ranked females attain the right to male production [30]. The dominance hierarchy among NRs in the presence of the non-aggressive R is reminiscent of those observed in R. marginata and T. allardycei. Dominance behaviours among NRs continue at the reformation phase.
4. Individual-level attributes affecting ranking in the hierarchy
Recent advances in the study of the molecular underpinnings of social behaviours have opened up the possibility of understanding the formation of dominance hierarchies from the bottom up. Here, we focus on individual age and physiological state as the individual-level attributes of the dominance hierarchies.
In eusocial insect colonies, unlike in vertebrate societies, ranking in a dominance hierarchy is usually associated with individual age rather than body size ([10,31,32], but see also [33], regarding the effect of body size on dominance status). The effect of individual age on ranking in the dominance hierarchy becomes clear when the birth of individuals during the maintenance phase is taken into account: in a colony with Rs, new adults are continuously supplied and challenge the pre-existing dominance hierarchy.
Interestingly, the relationship between age and hierarchical ranking is taxon-specific. In Polistes wasps that live in temperate regions and show an annual colony life cycle, older individuals tend to be higher ranked and become Rs when the current R is lost from the colony [33,34]. By contrast, in ants that typically show a perennial life cycle, younger workers rank higher in the dominance hierarchy [20,35,36]. This taxonomic difference in the age effect can be understood as adaptation to relative life expectancy of individuals and colonies [37]. However, those rules may have some flexibility rather than being fixed by age. For example, Monnin & Peeters [35] showed that in colonies of the ponerine ant Dinoponera quadriceps consisting of 50–112 workers, although the callows (very young workers) took over higher positions from older workers (greater than three months old), relatively younger workers defended their positions against the callows.
Physiological correlates of individual ranking in the dominance hierarchy would give us an insight into the mechanisms of formation and maintenance of the hierarchy (figure 2a). Surprisingly, however, information on the molecular physiological mechanisms triggering dominance behaviours is still scarce. One possible reason would be that the expression of dominance behaviours depends on the rank of the partner, making it difficult to identify causal relationships.
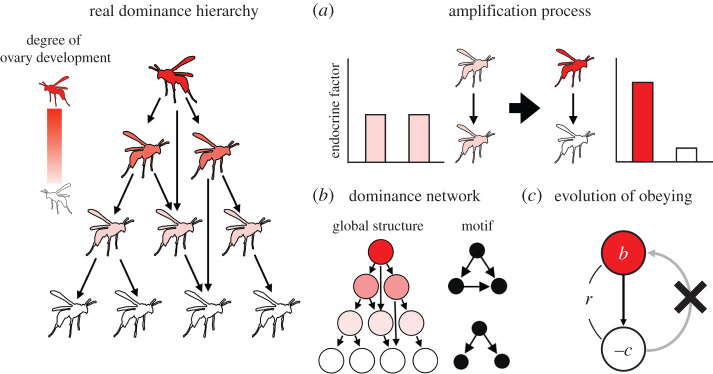
Figure 2.
Build-up of a dominance hierarchy (as represented by the leftmost diagram). The colour scale indicates reproductive ability, with white indicating non-development of ovaries. (a) Amplification of physiological states by dominance behaviour is an example of how changes in individual-level attributes affect ranking in the hierarchy. (b) A dominance hierarchy can be abstracted into a directed network, whose global structure is characterized by several quantitative measures such as motif frequencies. (c) Evolution of costly (–c) obeying, i.e. acceptance of a subordinate position, in dominance interactions requires special explanation. Inclusive fitness benefit (r × b) to the subordinate provides a general solution. Arrows point from actors to recipients of dominance behaviours. (Online version in colour.)
Generally, the ovarian development of eusocial insects is regulated by several factors, such as biogenic amines, juvenile hormones and insulin-like peptides [38–40]. These factors serve as activators of reproduction (i.e. have a gonadotropic effect), and their production is affected not only by the nutritional state of individuals but also by social interactions with other individuals [27,39,41,42] (figure 2a). Some studies have shown an association between individual ranking in the dominance hierarchy and titres of the gonadotropic factors. For example, the correlation between dopamine and dominance rank has been shown in studies of D. cf. indicum [43]. Moreover, in the paper wasp Pistacia chinensis, Tsuchida et al. [44] showed that in addition to dopamine, juvenile hormone titres positively associated with rank. The above physiological factors have also been reported to regulate individual behaviours in eusocial insects (biogenic amines: [45]; juvenile hormones: [46]). For example, serotonin regulates the aggressiveness of workers in the trap-jaw ant Odontomachus kuroiwae [47]. This aggressiveness might well be a component of dominance behaviours. Moreover, in the paper wasp Polistes annularis, Barth et al. [48] showed that application of juvenile hormone and ecdysone to foundresses increased both ovary development and frequency of dominance behaviours, and that the oocyte length was positively correlated with the behavioural score. Nevertheless, the causal relationship between physiological factors and dominance behaviours deserves further study.
5. Patterns of interactions shaping hierarchy structure
As is the case for other social behaviours, understanding patterns of interactions among individuals is essential to the study of dominance behaviours. We begin this section by considering recognition systems of ranking in the hierarchy. Based on the signal-receiver systems, repeated interactions alter the individuals' physiological states, which serves as an amplification mechanism of difference between individuals (figure 2a). The reinforcement of individual ranking contributes to the formation of stable dominance hierarchies. The global structure of the resulting dominance hierarchies can be summarized by directed networks (figure 2b). We argue how recent network analyses applied to dominance hierarchies in eusocial insects can improve our understanding of their social systems.
(a) . Recognition system
Generally, dominance behaviours are directed selectively towards the lower-ranked individuals in a stable dominance hierarchy. Therefore, each individual in the hierarchy is expected to have some cues that enable others to recognize rank relationships and prevent subordinates from challenging higher-ranking individuals. When a cue has been evolutionarily modified in accordance with the interest of the bearer, it can be regarded as a signal that alters its receiver's response in favour of the signaller [49]. This evolutionary process entails that the signal can be either honest or deceptive as a result of coevolution between senders and receivers. In eusocial insects, interactions between relatives and selection at the level of the colony make this evolutionary process even more complex.
Nest-mate communications in eusocial insects are known to be mediated by their cuticular hydrocarbons (CHCs) [50]. With regard to the reproductive division of labour, queen-specific CHC profiles are highly conserved across species in eusocial Hymenoptera [51,52]. Workers, even with functional ovaries, refrain from laying their own eggs in the presence of the chemicals. From an evolutionary perspective, the queen-specific profiles can be regarded as an honest signal, not a manipulative agent, to inform the presence of active queens ([53], see also [54]). This means that workers can flexibly change their behaviours depending on the quality or the quantity of the signal, leading to worker reproduction in certain situations. Likewise, in a dominance hierarchy, CHCs serve as an honest signal to inform individual ranking [50,55–57]. The signal pattern among individuals changes gradually with dominance rank rather than showing a distinct difference between Rs and NRs, thus providing a good indicator for deciding targets by dominants. In other words, the higher-ranked individuals can effectively suppress the physiological states of their rivals on the basis of the signal patterns.
In addition to chemical profiles, individuals can rely on visual features to identify individuals' ranking in the dominance hierarchy. The most spectacular case of visual discrimination is reported from Polistes fuscatus and Polistes dominulus wasps, where facial colour patterns are used by nest-mates as a good visual indicator of individual ranking ([58,59]; see also [60] for the relationships between recognition systems and dominance hierarchies [60]).
(b) . Reinforcement of dominance hierarchy
Adding the time dimension to dominance hierarchies highlights their dynamical aspects, which involve temporal changes in both individual-level attributes and interactions among individuals. An initially subtle difference in power relationships between individuals can be amplified over time in a positive feedback manner (figure 2a), which will contribute to the global stability of the dominance hierarchy, especially in its maintenance phase (figure 1b). Self-organizing mechanisms of amplification behind the dominance hierarchy have been a main topic in the study of biological self-organization [61].
The primary factor amplifying initial differences among individuals is the winner and loser effect, in which an individual who experienced winning (losing) in a previous interaction retains the information and increases (decreases) its probability of winning in the next interaction. Being solely based on individual memory, this effect amplifies between-individual differences and thus stabilizes the ranking of individuals in a dominance hierarchy [62,63]. Bonabeau et al. [64] provide a basic model for understanding this process. They modelled the formation of dominance hierarchies in Polistes colonies. In the model, each individual i is given an initial value of the intrinsic parameter F, which changes depending on the outcome of a contest with another individual j during dominance interactions. When i wins, Fi is increased and Fj is decreased, both by a constant δ. The larger the difference between opponents in the values of F, the higher the probability of winning for the individual with the larger F. Simulations clearly demonstrated that initially subtle differences between individuals became amplified towards the formation of a linear dominance hierarchy in a self-organizing manner.
The classic model of Bonabeau et al. [64] can be extended in various ways by incorporating features observed in real colonies. First, the assumption of the constant shift of Fi per interaction can be modified to reflect the empirical observations that the memory of losing is better retained than that of winning [63,65–67]. Incorporating this fact might contribute to better stability of the dominance hierarchy. Second, Fi and δ can change intrinsically depending on an individual's age. This modification could explain how an individual's age is often associated with her ranking in the dominance hierarchy in eusocial Hymenoptera [20,31,34,35]. Third, the birth and death of individuals during the maintenance phase, which were not incorporated in the model of Bonabeau et al. [64], can act as a destabilizing force to the hierarchy. How these factors affect the process of reinforcement deserves further theoretical consideration.
(c) . Network structure of dominance hierarchies
In the past two decades, network sciences have revolutionized our view of social interactions in group-living animals [68,69]. In the study of eusocial insects, network analysis is increasingly used to detect patterns of social interactions [70–74], including dominance interactions [6,75,76]. Individuals and interactions in a dominance hierarchy are, respectively, represented by nodes and links of a network, and the structure of a dominance hierarchy, or more generally a dominance network, is characterized by several statistics known as network properties, such as degree, clustering coefficient, centrality and type/frequency of motifs [69,77,78] (figure 2b). Here, the network can be regarded as a colony-level ‘phenotype’ that can be compared among colonies and among species.
A few studies have focused on global network structures of dominance hierarchies to characterize the role of individuals and the functioning of the dominance networks. Shimoji et al. [6] examined dominance networks in the ponerine ant D. cf. indicum through behavioural observation for 4 days. The networks that they studied had direct links from actors to recipients of unilateral dominance behaviours (typically bite and jerk; [79]). They found that higher-ranked NRs, but not top-ranked Rs, had a greater number of out-degrees than expected from corresponding positions in a randomized network (i.e. they served as ‘hub dominants’), and that there were no specific NRs targeted by many dominant NRs, making the network structure tree-like. These results suggest that their dominance hierarchies are maintained by the higher-ranked NRs rather than by the top-ranked R in the colony. Nandi et al. [75] showed another property of dominance networks using the polistine wasp R. marginata. By applying Boolean network modelling, they found that the dominance network of this wasp is highly efficient in terms of information flow in the colony. Interestingly, network structures in both species shared the same characteristics in motif distribution, i.e. over-representation of the ‘feed-forward loop’. This characteristic is also revealed from motif analysis of directed contact networks in the seed harvester ant Pogonomyrmex californicus [71]. These results support the idea that these interaction networks in eusocial insect colonies, and even their dominance networks, serve as regulatory networks selected to maximize information processing at the colony level [71].
One challenge for future studies will be longer-term observations of dominance networks, which have been enabled by the recent development of automated tracking systems (e.g. [73]). Generally, the network structure of a group is affected by the group size, which changes through individual birth–death and immigration–emigration processes [80]. The individual rank would also vary over time as described above. Long-term observations, ideally throughout the three phases of the colony (figure 1), will help to capture such temporal dynamics of dominance hierarchies. In addition, experimental removal of nodes with crucial roles in a dominance network will allow examination of the resilience and stability of the network (e.g. [81]), which is exactly what we observe in the reformation phase (figure 1c). These analyses could be combined with the assessment of colony-level productivity and efficiency as functional measures. Moreover, future studies should clarify how changes in individual attributes (see above) affect global network structures in the modelling framework of biological self-organization. Finally, it would be of interest to subject dominance networks to analysis by phylogenetic comparative methods, in which network statistics are treated as multivariate phenotypes [82]. To facilitate such comparative analyses, observation methods should be standardized (e.g. [83]). These approaches would help to gain a more complete understanding of the developmental and evolutionary build-up of dominance hierarchies.
6. Evolution of subordination
The initial driving force for the evolution of hostile interactions among individuals within a group would have most likely been interference competition over resources for reproduction, which is simply understood by adaptation at the level of individuals. In most dominance hierarchies, however, one characteristic distinguishing dominance behaviours from interference competition, in general, is that the former do not escalate into immediate lethality; that is, the loser does not resist and instead seems to ‘obey’ in its subordinate position [84]. In the general context of interference competition, the evolution of ‘obeying’ behaviours is understood under the hawk–dove game in evolutionary game theory [85], in which a severe cost of combat compared with the individual's resource-holding potential should favour obeying behaviours as a dove strategy. However, the resulting evolutionary stable state requires that all individuals in the population realize the same fitness, which is not the case under the reproductive division of labour in most dominance hierarchies. Therefore, additional factors should be considered. Historically, this consideration has been elaborated in the reproductive skew theory [86–88]. Here, we have attempted to simplify the discussion by focusing on subordinates.
Recent analyses of dominance hierarchies in animal societies have been increasingly focusing on the efficiency of information transfer at the colony level [71,75], where the colony is typically viewed as a ‘superorganism’. However, it is not obvious whether or how such group-level traits have been achieved through adaptive evolution that is chiefly driven by the evolutionary interests of individuals. A unique feature characterizing eusocial insect colonies is that the evolutionary interest of a colony member is shared by the other members to varying degrees. In the following sections, we compare the outcome of docile behaviour of subordinates with those of two possible alternative strategies. The first is to leave the natal group and reproduce independently, and the second is to ‘rebel’ against the hierarchy while staying in the group. We clarify how docile behaviours can be adaptive by considering the shared evolutionary interest among individuals in terms of inclusive fitness theory.
(a) . Stay versus leave
We start our consideration with a group composed of unrelated individuals. If the lifetime reproductive success of staying exceeds that of leaving, individual-level selection should favour staying. The ‘staying’ subordinates, even currently with NR status, can eventually reproduce in their group when the original R is lost, and the benefits of takeover (known as nest inheritance) can exceed those obtained by leaving the group. This explanation, based on direct fitness benefit, has been applied to the evolution of helpers in some vertebrate societies [89], and in eusocial insects seems to best fit the dominance hierarchies observed among unrelated Polistes wasp foundresses. Subordinates are considered to ‘make the best of a bad job’ by waiting (known as social queuing) [90] to take over the top rank after the current dominant dies [91–93]. If the focal subordinate can contribute to the inherited benefit simply by staying, the degree of staying can be adaptively raised on the basis of her eventual direct fitness benefits. It is possible that this process leads to more efficient dominance hierarchies.
However, there is a more substantial factor by which the evolution of staying can be promoted even further, namely the genetic relatedness between the focal subordinate and her nest-mates. If the staying behaviour (and associated behaviours such as helping) of the focal subordinate improves the fitness of genetically related dominants, the frequency of alleles responsible for staying can increase. According to the inclusive fitness argument, even lifelong sterility (with cost c, which would have been obtained by leaving and producing offspring in an independent colony) should pay for a subordinate when Hamilton's rule rb > c is fulfilled, where the subordinate gives fitness benefit b (measured by reproductive offspring of the dominant) to the dominants whose genetic relatedness is r (figure 2c). Dominance hierarchies in Polistes wasps are often formed among sisters [4]. The evolution of staying behaviour and hence the formation of their dominance hierarchies should be promoted even without the direct fitness benefit for the staying subordinates when independent colony foundation is risky. The above inclusive fitness argument is a special case of the general explanation of the evolution of sterile workers in eusocial insects [94], where a daughter of the queen can enjoy inclusive fitness benefit, even if she gives up independent colony foundation as a next-generation R to become a sterile worker, by helping her younger siblings develop into new Rs in the next generation. Likewise, the subordinate status of daughter workers during dominance interactions with their mother queen can be understood as the improvement of their inclusive fitness through their younger siblings (but see [95]).
(b) . Obey versus rebel
Even if subordinates stay in their natal nest, they can still enjoy their direct fitness by self-reproducing there. In many species of eusocial Hymenoptera, workers cannot mate but retain the ability to produce males, which is indeed predicted by inclusive fitness theory. This situation is known as the evolutionary conflict over who reproduces in the colony [96]. In this case, even the inclusive fitness benefit for subordinates obtained by staying might be insufficient to result in the formation of stable reproductive division of labour. Nevertheless, in real dominance hierarchies, the ability of self-reproduction seems to be well suppressed during their maintenance phase (figure 1b), and the ‘obeying’ behaviour is even correlated with the helping behaviours. At the extreme, the subordinate status would be achieved without dominance behaviours. This is realized through mechanisms such as differential nutrition during pre-adult development (e.g. royal-jelly feeding in honeybee larvae) and completely non-aggressive communications among individuals mediated only by ‘honest’ chemical signals (e.g. queen mandibular pheromone in honeybees) [97–99]. In all of these cases, the question is why subordinates finally ‘obey’ the rule, i.e. refrain from reproducing, despite their potential for self-reproduction that is realized in the reformation phase (figure 1c).
If the evolutionary conflict were realized, the resulting state of the colony would be the persistent absence of reproductive division of labour. As discussed above, the individual's reproductive activity correlates with the dominance behaviours. Consequently, all colony members would be involved in ‘the war of all against all’. Artificial removal of dominants from a dominance hierarchy (i.e. artificial induction of the reformation phase; figure 1c) enables us to get a glimpse of how the resulting instability of the hierarchy could impair reproductive outputs of the Rs in a ‘tragedy of the commons' [100] manner. Cole [101] found that among individuals of the ant T. allardycei in a queenless colony, the time spent on dominance behaviours was negatively correlated with the time spent on brood care. In orphaned colonies of the ponerine ant D. cf. indicum, Tsuji et al. [102] found that the instability of the colonies resulted in excessive brood-care workload on the subordinate NRs and shortened their lifespans. These results, often referred to as a colony-level cost of worker reproduction [103], illustrate the necessity of an efficient stabilizing mechanism in the evolutionary conflict. The obeying behaviours of subordinates in dominance hierarchies provide an implementation of such a mechanism. Again, it should be stressed that the resulting stability of the colonies would be impossible without the inclusive fitness benefit for the subordinates, which is underpinned by the relatedness of their siblings (the inclusive fitness is equivalent to the group selection argument that stresses the emergence of selection and heritability components for the group-level phenotype such as the proportion of subordinate individuals [104]). This view is corroborated by observations of lethal combat between unrelated foundresses when peaceful behaviour no longer ensures either direct or inclusive fitness benefits for a foundress [8].
The adaptive significance of dominance behaviours has already been relatively well argued in previous studies [7,98,105,106]. Our view of dominance hierarchies as a mechanism for ‘conflict resolution’ is close to the concept of policing in eusocial insects [98,105]. On the basis of these earlier studies, it is necessary to further clarify the relationship between dominance and policing behaviours, especially the concepts of ‘selfish worker policing’ (eating of worker-laid eggs by reproductively active workers) [107], ‘second-order altruism’ (policing behaviour with fitness cost) [108] and ‘social enforcement’ in more general contexts [109]. In addition, the very idea that real dominance hierarchies in eusocial insects have been adaptively fine-tuned in their efficiency of information transfer at the colony level remains to be directly tested. The self-organization models for the formation of dominance hierarchies should be considered in the light of evolution, and the inclusive fitness concept outlined above will play a crucial role.
7. Conclusion
Ever since the discovery of pecking order in the domestic chicken by Schjelderup-Ebbe [110], dominance hierarchies have been reported in various animal taxa, which has led us to a deeper understanding of animal societies. In eusocial insects, the dominance hierarchy plays a crucial role in regulating which individuals become Rs in the colony. Figure 2 summarizes our bottom-up approach to understanding the developmental and evolutionary build-up of these dominance hierarchies. The dominance behaviours suppress ovary development of NRs by modulating their physiological states (bar graphs in figure 2a), and self-organizing processes connect the local behavioural interactions and the global heterogeneity in reproduction among colony members (thick arrow in figure 2a). Our understanding of the dominance hierarchy has been revolutionized by network analysis, which has provided quantitative measures of this colony-level phenotype (figure 2b). With the notable exception of some colonies in Polistes paper wasps where foundresses are unrelated, dominance hierarchies of eusocial insects are typically composed of genetic relatives, and inclusive fitness benefit of subordinates explains why they accept their positions in the hierarchy (figure 2c). The resulting reproductive harmony enables their dominance hierarchies to achieve larger sizes than those in other animal societies.
Acknowledgements
We thank two anonymous reviewers, and Daizaburo Shizuka for kind and insightful comments.
Contributor Information
Hiroyuki Shimoji, Email: shimojih@kwansei.ac.jp.
Shigeto Dobata, Email: dobata@g.ecc.u-tokyo.ac.jp.
Data accessibility
This article has no additional data.
Authors' contributions
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Wilson EO. 1971. The insect societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Roisin Y, Korb J. 2011. Social organisation and the status of workers in termites. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N), pp. 133-164, 2nd edn. Berlin, Germany: Springer. [Google Scholar]
- 4.Crozier RH, Pamilo P. 1996. Evolution of social insect colonies: sex allocation and kin selection. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Pardi L. 1948. Dominance order in Polistes wasps. Physiol. Zool. 21, 1-13. ( 10.1086/physzool.21.1.30151976) [DOI] [PubMed] [Google Scholar]
- 6.Shimoji H, Abe MS, Tsuji K, Masuda N. 2014. Global network structure of dominance hierarchy of ant workers. J. R. Soc. Interface 11, 20140599. ( 10.1098/rsif.2014.0599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnin T, Ratnieks FLW, Brandoã CRF. 2003. Reproductive conflict in animal societies: hierarchy length increases with colony size in queenless ponerine ants. Behav. Ecol. Sociobiol. 54, 71-79. ( 10.1007/s00265-003-0600-9) [DOI] [Google Scholar]
- 8.Bernasconi G, Strassmann JE. 1999. Cooperation among unrelated individuals: the ant foundress case. Trends Ecol. Evol. 14, 477-482. ( 10.1016/S0169-5347(99)01722-X) [DOI] [PubMed] [Google Scholar]
- 9.West EMJ. 1969. The social biology of polistine wasps. Misc. Publ. Mus. Zool. Univ. Mich. 140, 1-101. [Google Scholar]
- 10.Jandt, JM, Tibbetts EA, Toth AL. 2014. Polistes paper wasps: a model genus for the study of social dominance hierarchies. Insect. Soc. 61, 11-27. ( 10.1007/s00040-013-0328-0) [DOI] [Google Scholar]
- 11.Gadagkar R, Gadgil M, Joshi, NV, Mahabal, AS. 1982. Observations on the natural history and population ecology of the social wasp Ropalidia marginata (Lep.) from peninsular India. (Hymenoptera: Vespidae). Proc. Anim. Sci. 91, 539-552. ( 10.1007/BF03186154) [DOI] [Google Scholar]
- 12.Chandrashekara K, Gadagkar R. 1991. Behavioural castes, dominance and division of labour in a primitively eusocial wasp. Ethology 87, 269-283. ( 10.1111/j.1439-0310.1991.tb00252.x) [DOI] [Google Scholar]
- 13.Premnath S, Sinha A, Gadagkar R. 1996. Dominance relationship in the establishment of reproductive division of labour in a primitively eusocial wasp (Ropalidia marginata). Behav. Ecol. Sociobiol. 39, 125-132. ( 10.1007/s002650050274) [DOI] [Google Scholar]
- 14.Bhadra A, et al. 2010. Regulation of reproduction in the primitively eusocial wasp Ropalidia marginata: on the trail of the queen pheromone. J. Chem. Ecol. 36, 424-431. ( 10.1007/s10886-010-9770-x) [DOI] [PubMed] [Google Scholar]
- 15.Cole BJ. 1981. Dominance hierarchies in Leptothorax ants. Science 212, 83-84. ( 10.1126/science.212.4490.83) [DOI] [PubMed] [Google Scholar]
- 16.Hölldobler B, Carlin NF. 1985. Colony founding, queen dominance and oligogyny in the Australian meat ant Iridomyrmex purpureus. Behav. Ecol. Sociobiol. 18, 45-58. ( 10.1007/BF00299237) [DOI] [Google Scholar]
- 17.Peeters C. 1997. Morphologically ‘primitive’ ants: comparative review of social characters, and the importance of queen-worker dimorphism. In The evolution of social behavior in insects and arachnids (eds Choe J, Crespi B), pp. 372-391. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Moreau C, Bell C, Vila R, Archibald S, Pierce N. 2006. Phylogeny of the ants: diversification in the age of angiosperms. Science 312, 101-104. ( 10.1126/science.1124891) [DOI] [PubMed] [Google Scholar]
- 19.Ito F, Higashi SA. 1991. linear dominance hierarchy regulating reproduction and polyethism of the queenless ant Pachycondyla sublaevis. Naturwissenschaften 78, 80-82. ( 10.1007/BF01206263) [DOI] [Google Scholar]
- 20.Higashi S, Ito F, Sugiura N, Ohkawara K. 1994. Worker's age regulates the linear dominance hierarchy in the queenless ponerine ant, Pachycondyla sublaevis (Hymenoptera: Formicidae). Anim. Behav. 47, 179-184. ( 10.1006/anbe.1994.1020) [DOI] [Google Scholar]
- 21.Fujioka H, Abe MS, Okada Y. 2021. Individual ants do not show activity-rest rhythms in nest conditions. J. Biol. Rhythms 36, 297-310. ( 10.1177/07487304211002934) [DOI] [PubMed] [Google Scholar]
- 22.Shimoji H, et al. 2021. Worker-dependent gut symbiosis in an ant. Isme Commun. 1, 60. ( 10.1038/s43705-021-00061-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumoto Y, Abe T, Taki A. 1989. A novel form of colony organization in the ‘queenless’ ant Diacamma rugosum. Physiol. Ecol. Jpn 26, 55-61. [Google Scholar]
- 24.Viginier B, Peeters C, Brazier L, Doums C. 2004. Very low genetic variability in the Indian queenless ant Diacamma indicum. Mol. Ecol. 13, 2095-2100. ( 10.1111/j.1365-294X.2004.02201.x) [DOI] [PubMed] [Google Scholar]
- 25.Dobata S, Shimoji H, Ohnishi H, Hasegawa E, Tsuji K. 2012. Paternally inherited alleles in male body parts of an ant (Diacamma sp.) sex mosaic: implication for androgenetic male production in the Hymenoptera. Insect. Soc. 59, 55-59. ( 10.1007/s00040-011-0187-5) [DOI] [Google Scholar]
- 26.Kikuchi T, Nakagawa T, Tsuji K. 2008. Changes in relative importance of multiple social regulatory forces with colony size in the ant Diacamma sp. from Japan. Anim. Behav. 76, 2069-2077. ( 10.1016/j.anbehav.2008.08.029) [DOI] [Google Scholar]
- 27.Okada Y, et al. 2015. Social dominance and reproductive differentiation mediated by dopaminergic signaling in a queenless ant. J. Exp. Biol. 218, 1091-1098. ( 10.1242/jeb.118414) [DOI] [PubMed] [Google Scholar]
- 28.Cuvillier-Hot V, Gadagkar R, Peeters C, Cobb M. 2002. Regulation of reproduction in a queenless ant: aggression, pheromones and reduction in conflict. Proc. R. Soc. B 269, 1295-1300. ( 10.1098/rspb.2002.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji K, Egashira K, Hölldobler B. 1999. Regulation of worker reproduction by direct physical contact in the ant Diacamma sp. from Japan. Anim. Behav. 58, 337-343. ( 10.1006/anbe.1999.1161) [DOI] [PubMed] [Google Scholar]
- 30.Shimoji H, Kikuchi T, Ohnishi H, Kikuta N, Tsuji K. 2018. Social enforcement depending on the stage of colony growth in an ant. Proc. R. Soc. B 285, 20172548. ( 10.1098/rspb.2017.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes CR, Strassmann JE. 1988. Age is more important than size in determining dominance among workers in the primitively eusocial wasp, Polistes instabilis. Behaviour 107, 1-14. ( 10.1163/156853988X00151) [DOI] [Google Scholar]
- 32.Taylor BA, Cini A, Cervo R, Reuter M, Sumner S. 2020. Queen succession conflict in the paper wasp Polistes dominula is mitigated by age-based convention. Behav. Ecol. 31, 992-1002. ( 10.1093/beheco/araa045) [DOI] [Google Scholar]
- 33.Reeve HK. 1991. Polistes. In The social biology of wasps (eds Ross KG, Matthews RW), pp. 99-148. Comstock, Ithaca, NJ: Cornell University Press. [Google Scholar]
- 34.Strassmann JE, Meyer DC. 1983. Gerontocracy in the social wasp, Polistes exclamans. Anim. Behav. 31, 431-438. ( 10.1016/S0003-3472(83)80063-3) [DOI] [Google Scholar]
- 35.Monnin T, Peeters C. 1999. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 10, 323-332. ( 10.1093/beheco/10.3.323) [DOI] [Google Scholar]
- 36.Cournault L, Peeters C. 2012. Aggression regulates monogyny in non-mutilating Diacamma ants. Insect. Soc. 59, 533-539. ( 10.1007/s00040-012-0251-9) [DOI] [Google Scholar]
- 37.Tsuji K, Tsuji N. 2005. Why is dominance hierarchy age-related in social insects? The relative longevity hypothesis. Behav. Ecol. Sociobiol. 58, 517-526. ( 10.1007/s00265-005-0929-3) [DOI] [Google Scholar]
- 38.Corona M, Libbrecht R, Wheeler DE. 2016. Molecular mechanisms of phenotypic plasticity in social insects. Curr. Opin. Insect Sci. 13, 55-60. ( 10.1016/j.cois.2015.12.003) [DOI] [PubMed] [Google Scholar]
- 39.Chandra V, et al. 2018. Social regulation of insulin signaling and the evolution of eusociality in ants. Science 361, 398-402. ( 10.1126/science.aar5723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki K, et al. 2021. Social evolution with decoupling of multiple roles of biogenic amines into different phenotypes in Hymenoptera. Front. Ecol. Evol. 9, 659160. ( 10.3389/fevo.2021.659160) [DOI] [Google Scholar]
- 41.Dombroski TCD, Simões ZLP, Bitondi MMG. 2003. Dietary dopamine causes ovary activation in queenless Apis mellifera workers. Apidologie 34, 281-289. ( 10.1051/apido:2003024) [DOI] [Google Scholar]
- 42.Sasaki K, Harano K. 2007. Potential effects of tyramine on the transition to reproductive workers in honeybees (Apis mellifera L.). Physiol. Entomol. 32, 194-198. ( 10.1111/j.1365-3032.2007.00566.x) [DOI] [Google Scholar]
- 43.Shimoji H, et al. 2017. Queen contact and among-worker interactions dually suppress worker brain dopamine as a potential regulator of reproduction in an ant. Behav. Ecol. Sociobiol. 71, 35. ( 10.1007/s00265-016-2263-3) [DOI] [Google Scholar]
- 44.Tsuchida K, et al. 2020. Reproductive workers insufficiently signal their reproductive ability in a paper wasp. Behav. Ecol. 31, 577-590. ( 10.1093/beheco/arz212) [DOI] [Google Scholar]
- 45.Kamhi JF, Arganda S, Moreau CS, Traniello JFA. 2017. Origins of aminergic regulation of behavior in complex insect social systems. Front. Syst. Neurosci. 11, 74. ( 10.3389/fnsys.2017.00074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson GE, Vargo EL. 1997. Juvenile hormone in adult eusocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 35, 559-583. () [DOI] [PubMed] [Google Scholar]
- 47.Aonuma H. 2020. Serotonergic control in initiating defensive responses to unexpected tactile stimuli in the trap-jaw ant Odontomachus kuroiwae. J. Exp. Biol. 223, jeb228874. ( 10.1242/jeb.228874) [DOI] [PubMed] [Google Scholar]
- 48.Barth RH, Lester LJ, Sroka P, Kessler T, Hearn R. 1975. Juvenile hormone promotes dominance behavior and ovarian development in social wasps (Polistes annularis). Exprientia 31, 691-692. ( 10.1007/BF01944632) [DOI] [PubMed] [Google Scholar]
- 49.Maynard-Smith J, Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Monnin T. 2006. Chemical recognition of reproductive status in social insects. Ann. Zool. Fennici 43, 515-530. [Google Scholar]
- 51.Holman L, Jørgensen CG, Nielsen J, d'Ettorre P. 2010. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. B 277, 3793-3800. ( 10.1098/rspb.2010.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Oystaeyen A, et al. 2014. Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287-290. ( 10.1126/science.1244899) [DOI] [PubMed] [Google Scholar]
- 53.Oi CA, et al. 2015. The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. Bioessays 37, 808-821. ( 10.1002/bies.201400180) [DOI] [PubMed] [Google Scholar]
- 54.Keller L, Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787-794. ( 10.1006/anbe.1993.1092) [DOI] [Google Scholar]
- 55.Peeters C, Monnin T, Malosse C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266, 1323-1327. ( 10.1098/rspb.1999.0782) [DOI] [Google Scholar]
- 56.Cuvillier-Hot V, Lenoir A, Crewe R, Malosse C, Peeters C. 2004. Fertility signalling and reproductive skew in queenless ants. Anim. Behav. 68, 1209-1219. ( 10.1016/j.anbehav.2003.11.026) [DOI] [Google Scholar]
- 57.D'Ettorre P, Heinze J. 2005. Individual recognition in ant queens. Curr. Biol. 15, 2170-2174. ( 10.1016/j.cub.2005.10.067) [DOI] [PubMed] [Google Scholar]
- 58.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature 432, 218-222. ( 10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 59.Sheehan MJ, Tibbetts EA. 2010. Selection for individual recognition and the evolution of polymorphic identity signals in Polistes paper wasps. J. Evol. Biol. 23, 570-577. ( 10.1111/j.1420-9101.2009.01923.x) [DOI] [PubMed] [Google Scholar]
- 60.Tibbetts EA, Pardo-Sanchez J, Weise C. 2022. The establishment and maintenance of dominance hierarchies. Phil. Trans. R. Soc. B 377, 20200450. ( 10.1098/rstb.2020.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G, Bonabeau E. 2001. Self-organization in biological systems. Princeton studies in complexity. Princeton, NJ: Princeton University Press. [Google Scholar]
- 62.Chase ID. 1982. Dynamics of hierarchy formation: the sequential development of dominance relationships. Behaviour 80, 218-240. ( 10.1163/156853982X00364) [DOI] [Google Scholar]
- 63.Dugatkin LA. 1997. Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 8, 583-587. ( 10.1093/beheco/8.6.583) [DOI] [Google Scholar]
- 64.Bonabeau E, Theraulaz G, Deneubourg L. 1999. Dominance orders in animal societies: the self-organization hypothesis revisited. Bull. Math. Biol. 61, 727-757. ( 10.1006/bulm.1999.0108) [DOI] [PubMed] [Google Scholar]
- 65.Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. 2002. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl Acad. Soc. USA 99, 744-5749. ( 10.1073/pnas.082104199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasumovic MM, Elias DO, Sivalinghem S, Mason AC, Andrade MCB. 2010. Examination of prior contest experience and the retention of winner and loser effects. Behav. Ecol. 21, 404-409. ( 10.1093/beheco/arp204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trannoy S, Penn J, Lucey K, Popovic D, Kravitz EA. 2016. Short and long-lasting behavioral consequences of agonistic encounters between male Drosophila melanogaster. Proc. Natl Acad. Sci. USA 113, 4818-4823. ( 10.1073/pnas.1520953113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shizuka D, McDonald DB. 2013. A social network perspective on measurements of dominance hierarchies. Anim. Behav. 83, 925-934. ( 10.1016/j.anbehav.2012.01.011) [DOI] [Google Scholar]
- 69.Pinter-Wollman N, et al. 2014. The dynamics of animal social networks: analytical, conceptual, and theoretical advances. Behav. Ecol. 25, 242-255. ( 10.1093/beheco/art047) [DOI] [Google Scholar]
- 70.Pinter-Wollman N, Wollman R, Guetz A, Holmes S, Gordon DM. 2011. The effect of individual variation on the structure and function of interaction networks in harvester ants. J. R. Soc. Interface 8, 1562-1573. ( 10.1098/rsif.2011.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waters JS, Fewell JH. 2012. Information processing in social insect networks. PLoS ONE 7, e40337. ( 10.1371/journal.pone.0040337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blonder B, Dornhaus A. 2011. Time-ordered networks reveal limitations to information flow in ant colonies. PLoS ONE 6, e20298. ( 10.1371/journal.pone.0020298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mersch DP, Crespi A, Keller L. 2013. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340, 1090-1093. ( 10.1126/science.1234316) [DOI] [PubMed] [Google Scholar]
- 74.Stroeymeyt N, et al. 2018. Social network plasticity decreases disease transmission in a eusocial insect. Science 362, 941-945. ( 10.1126/science.aat4793) [DOI] [PubMed] [Google Scholar]
- 75.Nandi AK, Sumana A, Bhattacharya K. 2014. Social insect colony as a biological regulatory system: modelling information flow in dominance networks. J. R. Soc. Interface 11, 20140951. ( 10.1098/rsif.2014.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiocca K, et al. 2020. Reproductive physiology corresponds to adult nutrition and task performance in a Neotropical paper wasp: a test of dominance–nutrition hypothesis predictions. Behav. Ecol. Sociobiol. 74, 114. ( 10.1007/s00265-020-02898-x) [DOI] [Google Scholar]
- 77.McDonald DB, Shizuka D. 2013. Comparative transitive and temporal orderliness in dominance networks. Behav. Ecol. 24, 511-520. ( 10.1093/beheco/ars192) [DOI] [Google Scholar]
- 78.Holekamp KE, Strauss ED. 2016. Aggression and dominance: an interdisciplinary overview. Curr. Opin. Behav. Sci. 12, 44-51. ( 10.1016/j.cobeha.2016.08.005) [DOI] [Google Scholar]
- 79.Peeters C, Tsuji K. 1993. Reproductive conflict among ant workers in Diacamma sp. from Japan: dominance and oviposition in the absence of the gamergate. Insectes Soc. 40, 119-136. ( 10.1007/BF01240701) [DOI] [Google Scholar]
- 80.Shizuka D, Johnson AE. 2020. How demographic processes shape animal social networks. Behav. Ecol. 31, 1-11. ( 10.1093/beheco/arz083) [DOI] [Google Scholar]
- 81.Naug D. 2009. Structure and resilience of the social network in an insect colony as a function of colony size. Behav. Ecol. Sociobiol. 63, 1023-1028. ( 10.1007/s00265-009-0721-x) [DOI] [Google Scholar]
- 82.Adams DC, Collyer ML. 2019. Phylogenetic comparative methods and the evolution of multivariate phenotypes. Annu. Rev. Ecol. Evol. Syst. 50, 405-425. ( 10.1146/annurev-ecolsys-110218-024555) [DOI] [Google Scholar]
- 83.Strauss ED, Holekamp KE. 2019. Inferring longitudinal hierarchies: framework and methods for studying the dynamics of dominance. J. Anim. Ecol. 88, 521-536. ( 10.1111/1365-2656.12951) [DOI] [PubMed] [Google Scholar]
- 84.Clutton-Brock TH, Parker GA. 1995. Punishment in animal societies. Nature 373, 209-216. ( 10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- 85.Maynard SJ. 1982. Evolution and the theory of games. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 86.Vehrencamp SL. 1983. A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667-682. ( 10.1016/S0003-3472(83)80222-X) [DOI] [Google Scholar]
- 87.Keller L, Reeve HK. 1994. Partitioning of reproduction in animal societies. Trends Ecol. Evol. 9, 98-102. ( 10.1016/0169-5347(94)90204-6) [DOI] [PubMed] [Google Scholar]
- 88.Nonacs P, Harger R. 2011. The past, present and future of reproductive skew theory and experiments. Biol. Rev. 86, 271-298. ( 10.1111/j.1469-185X.2010.00144.x) [DOI] [PubMed] [Google Scholar]
- 89.Cant MA. 2012. Cooperative breeding systems. In The evolution of parental care (eds Royle N, Smiseth PT, Kölliker M), pp. 206-225. Oxford, UK: Oxford University Press. [Google Scholar]
- 90.Kokko H, Johnstone RA. 1999. Social queuing in animal societies: a dynamic model of reproductive skew. Proc. R. Soc. Lond. B 266, 571-578. ( 10.1098/rspb.1999.0674) [DOI] [Google Scholar]
- 91.Queller DC, et al. 2000. Unrelated helpers in a social insect. Nature 405, 784-787. ( 10.1038/35015552) [DOI] [PubMed] [Google Scholar]
- 92.Field J, Cronin A, Bridge C. 2006. Future fitness and helping in social queues. Nature 441, 214-217. ( 10.1038/nature04560) [DOI] [PubMed] [Google Scholar]
- 93.Leadbeater E, Carruthers JM, Green JP, Rosser NS, Field J. 2011. Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874-876. ( 10.1126/science.1205140) [DOI] [PubMed] [Google Scholar]
- 94.Hamilton WD. 1971. The geometry of the selfish herd. J. Theor. Biol. 31, 295-311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 95.Alexander RD. 1974. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325-383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 96.Pamilo P. 1991. Evolution of colony characteristics in social insects. II. Number of reproductive individuals. Am. Nat. 137, 83-107. ( 10.1086/285224) [DOI] [Google Scholar]
- 97.Wheeler DE. 1986. Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. Am. Nat. 128, 13-34. ( 10.1086/284536) [DOI] [Google Scholar]
- 98.Ratnieks FLW, Foster KR, Wenseleers T. 2006. Conflict resolution in insect societies. Annu. Rev. Entomol. 51, 581-608. ( 10.1146/annurev.ento.51.110104.151003) [DOI] [PubMed] [Google Scholar]
- 99.Le Conte Y, Hefetz A.. 2008. Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 53, 523-542. ( 10.1146/annurev.ento.52.110405.091434) [DOI] [PubMed] [Google Scholar]
- 100.Wenseleers T, Ratnieks FLW. 2004. Tragedy of the commons in Melipona bees. Proc. R. Soc. B 271, S310-S312. ( 10.1098/rsbl.2003.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cole BJ. 1986. The social behavior of Leptothorax allardycei (Hymenoptera, Formicidae): time budgets and the evolution of worker reproduction. Behav. Ecol. Sociobiol. 18, 165-173. ( 10.1007/BF00290820) [DOI] [Google Scholar]
- 102.Tsuji K, Kikuta N, Kikuchi T. 2012. Determination of the cost of worker reproduction via diminished life span in the ant Diacamma sp. Evolution 66, 1322-1331. ( 10.1111/j.1558-5646.2011.01522.x) [DOI] [PubMed] [Google Scholar]
- 103.Ratnieks FLW. 1988. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 132, 217-236. ( 10.1086/284846) [DOI] [Google Scholar]
- 104.Queller DC. 1992. Quantitative genetics, inclusive fitness, and group selection. Am. Nat. 139, 540-558. ( 10.1086/285343) [DOI] [Google Scholar]
- 105.Monnin T, Ratnieks FL. 2001. Policing in queenless ponerine ants. Behav. Ecol. Sociobiol. 50, 97-108. ( 10.1007/s002650100351) [DOI] [Google Scholar]
- 106.Cant MA, Field J. 2005. Helping effort in a dominance hierarchy. Behav. Ecol. 16, 708-715. ( 10.1093/beheco/ari051) [DOI] [Google Scholar]
- 107.Stroeymeyt N, Brunner E, Heinze J. 2007. ‘Selfish worker policing’ controls reproduction in a Temnothorax ant. Behav. Ecol. Sociobiol. 61, 1449-1457. ( 10.1007/s00265-007-0377-3) [DOI] [Google Scholar]
- 108.Eldakar OT, Wilson DS. 2009. Selfishness as second-order altruism. Proc. Natl Acad. Soc. USA 105, 6982-6986. ( 10.1073/pnas.0712173105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ågren JA, Davies NG, Foster KR. 2019. Enforcement is central to the evolution of cooperation. Nat. Ecol. Evol. 3, 1018-1029. ( 10.1038/s41559-019-0907-1) [DOI] [PubMed] [Google Scholar]
- 110.Schjelderup-Ebbe T. 1922. Beitrage zur Sozialpsychologie des Haushuhns. Z. Psychol. 88, 225-252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.