Abstract
Animal groups are often organized hierarchically, with dominant individuals gaining priority access to resources and reproduction over subordinate individuals. Initial dominance hierarchy formation may be influenced by multiple interacting factors, including an animal's individual attributes, conventions and self-organizing social dynamics. After establishment, hierarchies are typically maintained over the long-term because individuals save time, energy and reduce the risk of injury by recognizing and abiding by established dominance relationships. A separate set of behaviours are used to maintain dominance relationships within groups, including behaviours that stabilize ranks (punishment, threats, behavioural asymmetry), as well as signals that provide information about dominance rank (individual identity signals, signals of dominance). In this review, we describe the behaviours used to establish and maintain dominance hierarchies across different taxa and types of societies. We also review opportunities for future research including: testing how self-organizing behavioural dynamics interact with other factors to mediate dominance hierarchy formation, measuring the long-term stability of social hierarchies and the factors that disrupt hierarchy stability, incorporating phenotypic plasticity into our understanding of the behavioural dynamics of hierarchies and considering how cognition coevolves with the behaviours used to establish and maintain hierarchies.
This article is part of the theme issue ‘The centennial of the pecking order: current state and future prospects for the study of dominance hierarchies’.
Keywords: aggression, social structure, contests, resource holding potential, social instability, dominance relationships
1. Introduction
Many animal social interactions are organized hierarchically based on dominance rank. Dominance is typically defined as asymmetry in aggression by one animal towards another animal [1,2]. However, the term dominance is used in different ways across taxa and contexts. For example, in social insects, dominant individuals monopolize reproduction within their group, but may not be involved in aggressive competition [3,4]. More typically, dominance within groups is defined as a long-lasting position associated with asymmetric aggression and priority access to physical or social commodities that increase fitness, including food, water, shelter, space, receptive mates, alloparental care, etc [5]. In other cases, dominance refers to individuals that win short-term dyadic contests [1]. The winner of dyadic contests gains priority access to resources or reproduction, but there may be no long-term hierarchical relationship between the two competitive individuals. In groups, there is variation in the structure of dominance hierarchies [6]. Many social groups have linear or near-linear dominance hierarchies. However, hierarchies need not always be linear [2,7]. Nonlinear hierarchies with dominance reversals and intransitivities can also occur [8].
Much research on dominance relationships focuses on the initial establishment of hierarchies. Initial rank is based, in large part, on intrinsic individual attributes like resource holding potential (RHP) or motivation [9–11]. Individual attributes are assessed via direct agonistic interactions, signals of fighting ability and social information acquired by watching the interactions of others [12,13]. Other factors such as conventions and social dynamics also play a role in hierarchy formation [14,15]. The relative importance of individual attributes, conventions and social dynamics in the establishment of hierarchies vary across taxa and social contexts.
In large or unstable groups with repeated, low-stakes competitive interactions, individuals repeatedly establish dominance relationships without necessarily forming persistent hierarchies. However, in taxa that live in smaller groups with consistent membership, hierarchies are typically maintained over the long term. Continued conflict over rank is costly (e.g. time, energy, risk of injury) [16,17]. As a result, stable hierarchies are favoured. Hierarchies are maintained via social dynamics like punishment and threats [18,19] as well as signals that provide information about rank, including signals of individual identity and dominance [20,21].
In this review, we describe the behaviours used to establish and maintain dominance hierarchies (figure 1). Some behaviours are involved in both establishing and maintaining hierarchies. For example, differences in fighting ability influence initial rank formation and longer term rank maintenance [9]. Other behaviours are only involved in a single context. For example, signals of fighting ability influence dominance rank establishment, but are not involved in maintaining hierarchies within groups of known individuals [28–30]. We also describe how factors like group size, consistency of group membership, the costs and benefits of hierarchy position and individual cognitive capacity influence the behaviours involved in hierarchy formation and maintenance. Finally, we highlight several approaches and opportunities for future research on dominance hierarchies.
Figure 1.
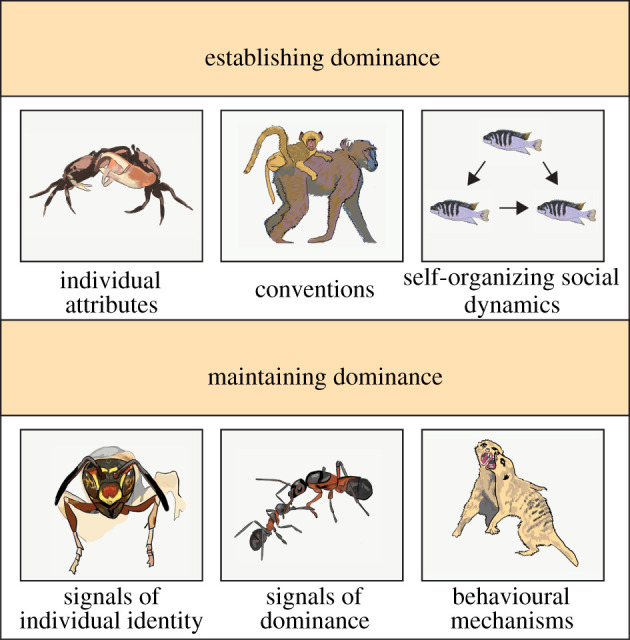
Mechanisms involved in establishing and maintaining dominance hierarchies. Establishing dominance. Individual attributes are traits such as RHP and/or motivation, assessed via aggressive interactions, signals of fighting ability and social information (e.g. claw waving displays provide information about fighting ability during contests between rival crabs [22]). Conventions are unique attributes that single out an individual as the next dominant without reflecting intrinsic characteristics that allow individuals to win contests (e.g. maternal rank inheritance in baboons [23]). Self-organizing social dynamics are social processes at the group level that increase hierarchy linearity (e.g. fishes that interact in groups are more likely to form transitive dominance relationships than fishes that only interact in dyadic contests [14]). Maintaining dominance. Signals of dominance provide information about dominance rank (e.g. dominant ant queens have cuticular hydrocarbons that provide information about rank and influence queen/worker interactions [24]). Signals of individual identity are unique phenotypes that receivers learn and associate with individual-specific information about the sender like dominance rank. (e.g. Polistes fuscatus wasps learn the dominance rank of individuals via aggressive contests and social eavesdropping and associate rank information with the unique facial patterns of conspecifics [25,26]). Behavioural mechanisms help dominants maintain their rank like threats, punishment and self-reinforcing behavioural mechanisms (e.g. in meerkats, dominants aggressively evict subordinates that try to reproduce [27]. (Online version in colour.)
2. Establishing dominance relationships
(a) . Individual attributes
The individual attributes of interacting individuals have a strong and consistent effect on dominance rank (figure 2). The most straightforward way to establish dominance is through competitive dyadic interactions with rivals. Individuals with greater RHP and/or motivation are more likely to win fights and become dominant than individuals with lower RHP and/or motivation [9,33]. RHP, or fighting capacity, is based on a composite of many morphological, physiological and behavioural traits (e.g. weapons, body size, skill, hormones, fat reserves, etc.) [34]. As a result, many RHP-linked attributes are associated with dominance rank, including body size [35,36], age [35], personality [37], hormone titres [38] and physical condition [39]. Motivation in contests is influenced by individual state, context and the pay-off individuals receive for winning a contest. Highly motivated individuals will invest more in attaining high dominance rank than less motivated individuals. For example, a hungry individual will be more motivated and fight harder than a satiated individual during competition over food. An individual defending a nest with offspring will be more motivated than an individual defending a potential nesting site. Establishing dominance ranks through direct contests is costly in terms of time, energy and potential physical damage, or death [40,41]. Nevertheless, dyadic contests ultimately guide the establishment of social dominance hierarchies in many species.
Figure 2.
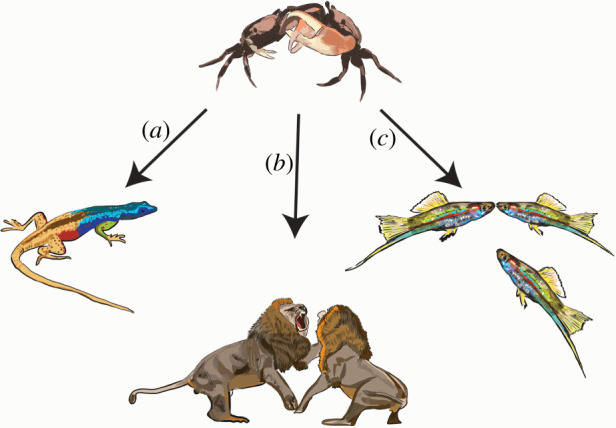
Three common ways animals assess the individual attributes of conspecifics. (a) Signals of fighting ability. Platysaurus broadleyi signal fighting ability with UV throat coloration [31]. (b) Competitive interactions. Panthera leo fight with rivals. (c) Social information. Xiphophorus helleri gain information about the fighting ability of potential rivals by observing and remembering how conspecifics behave during contests with other individuals [32]. (Online version in colour.)
The outcome of contests over dominance rank may be influenced by an individual's own RHP or the difference in RHP between rivals [42,43]. Some animals persist in contests based on their own abilities such that individuals with low RHP give up before individuals with high RHP (self-assessment). Other animals compare their own abilities with the abilities of their rival (mutual assessment). Self-assessment is less accurate than mutual-assessment but is also simpler [42,43]. Mutual assessment may require substantial time, energy and cognitive resources to process the cues and signals that provide information about an opponent's fighting ability [43]. A large body of literature has investigated the mechanisms used to determine contest outcomes, finding that self-assessment is more common than mutual assessment [43–45], but even animals thought to have limited cognitive capacity are capable of mutual assessment [45,46].
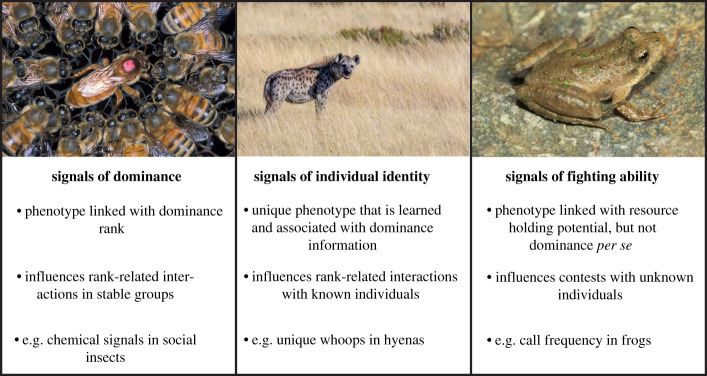
Some animals minimize the cost of conflict by using signals of fighting ability to directly assess rivals. Signals of fighting ability provide information about RHP and/or motivation during a contest and the signals alter receiver behaviour (figure 3) [28,47]. Receivers are more likely to avoid or submit to rivals that signal high fighting ability than individuals that signal low fighting ability. Signals of fighting ability are widespread across taxa and sensory modalities. For example, fighting ability is signalled by call frequency in frogs, black facial spots in paper wasps and claw size in crabs [46,48,49]. Some signals are inherently linked with fighting ability. For example, weapons like antlers or claws signal high fighting ability and weapons also directly influence an individual's ability to win a fight. As a result large weapons always signal high fighting ability. Other signals of fighting ability are termed ‘conventional signals' because they provide information about fighting ability but there is no logical, a priori, link between the signal phenotype and the information the signal conveys [50]. For example, soft song is a signal of aggressive intent in some songbirds, though there is no required link between aggression and singing quietly [51]. Some taxa even have multiple different traits that are used as conventional status signals in different contexts [52]. Although there has been some debate about the accuracy of conventional signals, there is growing evidence that conventional signals provide reliable information about fighting ability and/or aggressive motivation and often mediate conflict over rank [28].
Figure 3.
Signals involved in dominance hierarchy establishment and maintenance. (Online version in colour.)
The individual attributes of rivals can also be assessed indirectly using social information. For example, during social eavesdropping, bystanders gain information about the agonistic ability of particular rivals by observing and remembering how conspecifics behave during contests with other individuals [53,54]. For example, contest behaviour of Polistes fuscatus wasps is influenced by observing fights between conspecifics. Wasps are less aggressive towards individuals they observed initiate more aggression and receive less aggression. Control trials illustrate that the link between bystander behaviour and observed aggression is caused by social eavesdropping rather than alternative behaviours like winner/loser effects or cueing on physical traits (e.g. size) [25]. Social eavesdropping occurs in many birds, fishes and primates. Individuals often change their contest behaviour based on information about fighting ability obtained by watching or listening to interactions between others [32,55,56].
Social eavesdropping may be facilitated by ‘victory displays’, stereotyped behaviours performed by winners following fights that advertise the outcome of a contest to observers [57,58]. For example, little blue penguins give victory calls after they win a fight. Experimental playback of victory calls suggests that advertising victories may help males develop a reputation for winning fights within the social group, potentially reducing the likelihood of being challenged by eavesdroppers in future contests [59]. Victory displays are widespread, though less research has tested the functional consequences of these displays. Additional research will be useful to assess whether victory displays commonly play a role in social eavesdropping.
Some taxa combine social information and transitive inference to make inferences about the likely fighting ability of individuals without directly observing all potential fights. For example, if A dominates B, and B dominates C, then you can make the inference that A will probably dominate C, even if A and C have never been observed interacting. Transitive inference was originally thought to be based on logical deduction and confined to taxa with ‘advanced’ cognitive abilities like primates [60]. More recent work has shown that transitive inference occurs in many social species, including primates, birds, fishes and paper wasps [60–62]. Transitive inference may be favoured in social species with linear dominance hierarchies because it allows animals to keep track of dominance relationships while minimizing direct conflict. Further, hierarchies may form much more quickly when animals use transitive inference and social observation to assess rival ranks than when ranks are determined via direct aggressive competition [63]. Consistent with transitive inference providing a benefit during hierarchy formation, social complexity is linked with the capacity for transitive inference [64,65]. For example, highly social pinyon jays have more accurate transitive inference abilities than less social western scrub-jays [64].
Although many taxa use social eavesdropping and transitive inference to minimize conflict over dominance, there are cognitive costs associated with social information use. Social eavesdropping involves learning and remembering unique individuals, observing interactions, making appropriate deductions and remembering those deductions [53,54]. Social eavesdropping also requires animals to adopt a non-egocentric perspective and assess interactions they do not directly participate in. Keeping track of a broad network of social interactions has potential to dramatically increase the cognitive challenges of social life compared with only keeping track of personal interactions [66]. As a result, social information use influences cognitive evolution. Species that keep track of many individually differentiated social relationships often have larger brains and/or enhanced cognitive capacity compared with other species [67–70]. Within-species variation in cognitive performance may also be linked with social knowledge. For example, Australian magpies that live in larger social groups performed better on multiple intelligence tests than birds from smaller groups, suggesting that keeping track of others' social relationships may influence general intelligence [71].
Thus far, it is unclear how cognitive costs limit which taxa use social information. Social information use is common in taxa with ‘advanced’ cognitive capacity like primates, but taxa with relatively small brains like paper wasps and fishes are also capable of social eavesdropping and transitive inference [25,54,60–62]. Therefore, brain size does not strictly limit social information use. There are exciting opportunities for future research exploring the relationships between dominance, social information use and cognitive evolution across taxa and social contexts [72].
(b) . Conventions
Dominance rank can also be acquired through conventions. In societies with convention-based dominance, individuals have unique attributes that single them out as the next dominant, (e.g. age, tenure in a group or maternal rank) without reflecting intrinsic characteristics that allow individuals to win contests [73,74]. For example, some social insects determine dominance based on the seniority convention. The oldest worker is the most dominant and will take over if the queen disappears [15,75]. ‘Nepotistic hierarchies' are common dominance conventions where dominance rank is inherited from the mother. Juveniles acquire status immediately below their mother, with younger offspring outranking older siblings [76]. Although nepotistic hierarchies are considered convention-based, rank inheritance depends on support from mother, kin and coalition members to ensure offspring acquire the appropriate rank [77,78].
There has been a long-standing interest in the evolutionary stability of dominance conventions. If high dominance rank is beneficial and rank is not based on intrinsic attributes, what prevents individuals with high fighting ability from ignoring the convention and asserting their dominance? One explanation is that conventions are more likely to occur when the costs of competition over dominance rank outweigh the benefits of high dominance rank [79]. For example, conventions are particularly common in taxa that live in long-term social groups with many relatives because there are both direct and indirect fitness costs to group conflict (e.g. primates, social insects) [75,78]. Conventions may also be more likely to mediate dominance when there are weaker benefits of high dominance rank. For example, meerkats aggressively compete over rank rather than relying on conventions. Reproductive success is strongly influenced by rank because dominant meerkats kill the offspring of subordinates [80]. By contrast, savannah baboons use convention-based hierarchies and individuals of all ranks reproduce [81,82]. In chimpanzees, females largely use a seniority convention for social status in the female hierarchy, while males aggressively compete for status in the male hierarchy. The sex difference in behaviours chimpanzees use for hierarchy formation may occur because male reproductive success is strongly influenced by rank, while female social status is more strongly influenced by longevity than rank [83].
In some societies with convention-based hierarchies, aggressive competition also influences hierarchy position [78]. For example, rank in hyenas is inherited from the mother, but maintaining a particular rank requires support from kin and other coalition members. Without sufficient coalitionary support, dominance reversals can occur [77]. Therefore, rank may often be influenced by a combination of conventions and competition rather than purely conventions.
(c) . Self-organizing social dynamics
Recent research suggests that self-organizing social dynamics play a role in establishing linear dominance hierarchies. In a dominance hierarchy, individuals are arrayed in a line from most to least dominant; individuals are dominant to those below them in the hierarchy and subordinate to those above them in the hierarchy. In most social groups, dominance hierarchies are more linear than expected by chance [84]. Some of this linearity is owing to differences in individual attributes, as described above. However, a growing body of literature suggests that at least some of the linearity in dominance hierarchies occurs because of social processes that go beyond dyadic contests [8]. For example Chase et al. [14], experimentally demonstrated that social interactions between group members facilitate the formation of highly linear hierarchies in Metriaclima zebra fish. When fish interact in groups, nearly all groups formed linear dominance hierarchies. When fish interact in dyadic, round-robin competition alone, without any group interactions, only half the hierarchies were linear. Field studies in Pukeko birds also suggest that social interactions enhance the formation of orderly dominance hierarchies. Birds are more likely to form transitive dominance relationships (A dominates B, B dominates C, A dominates C) than cyclical relationships (A dominates B, B dominates C, but C dominates A). Their results suggest that dominance ranks are at least partially based on structural dependence between relationships rather than individual attributes alone [85].
Winner–loser effects are one key mechanism by which social interactions facilitate the formation of linear dominance hierarchies. Winner–loser effects mean that winners are more likely to win future encounters with any individual and losers are more likely to lose [86]. Winner–loser effects can occur when contest experience influences fighting behaviour or physiological traits like androgen titers [86]. Theory suggests winner–loser effects will generate linear dominance hierarchies via self-organizing dynamics, even without individual differences in intrinsic abilities [87]. Empirical work has shown that winner–loser effects occur in many species [86,88] and may contribute to stable dominance hierarchies [89,90]. Initial differences between individuals based on intrinsic attributes or random conditions are amplified with each social interaction, leading to stable, linear rankings. Intriguingly, the occurrence of winner and loser effects varies within and between species based on factors like social context [91,92] and genotype [89], suggesting that winner–loser effects may not be an inevitable consequence of competition. Instead, winner–loser effects may be an adaptive mechanism that minimizes conflict and stabilizes social relationships in situations where they are beneficial.
Winner–loser effects are unlikely to account for all patterns of self-organization in dominance hierarchies, so additional behaviours probably play a role. Bystander effects and localized social network properties have been proposed as potential mechanisms [8]. In taxa with bystander effects, an animal that observes a dominance contest between two others behaves differently than a non-observer when it meets the interactants [93]. Bystander effects are common across diverse taxa and encompass multiple specific behaviours. Priming occurs when observing any contest alters future contest behaviour. For example, bystander fish that observe contests between conspecifics are ‘primed’ to be more aggressive and more dominant than naive fish that do not observe contests [94]. Social eavesdropping occurs when individuals observe and remember the behaviour of specific individuals [13,53,94]. Some bystander effects, like social eavesdropping, may primarily influence dominance relationships by providing information about individual attributes, while other bystander effects, like priming, may mediate dominance relationships without involving assessment of individual attributes. Highly localized social network properties also mediate dominance hierarchies. Factors like aggressive state, fighting success, social status, motivation and random conditions influence rank via social feedback loops rather than having static effects on rank [95,96]. As a result, initial small differences between individuals are amplified over time via feedback loops, leading to differentiated ranks.
Given the accumulating evidence that dominance is influenced, in part, by self-organizing social dynamics, additional research will be important to understand both the behaviours that underlie these patterns [95] as well as the circumstances where self-organizing social dynamics are more versus less important. For example, social dynamics may be more likely to influence dominance hierarchies when orderly dominance hierarchies are beneficial [97]. In group-living taxa, orderly, stable dominance relationships minimize aggression and improve efficiency [16,17,98], so self-organizing dominance hierarchies may be common. Large groups that lack orderly hierarchies or groups where rank instability is less costly may be less likely to have self-organizing dynamics.
3. Maintaining dominance relationships
After dominance hierarchies are established, other mechanisms are used to maintain hierarchy stability. Maintaining stable dominance hierarchies is particularly valuable in taxa that live in small groups with consistent membership. Social instability has negative effects on traits like reproductive success, offspring survival, stress responses and longevity [16,17]. For example, zebra finches in flocks with an established dominance hierarchy are more efficient foragers because stable social relationships improve the coordination and synchronization of foraging groups [99]. Subordinates sometimes aggressively test dominants because subordinates would benefit if they rose in rank [100]. Accordingly, individuals may preferentially direct aggression towards individuals immediately below themselves in the hierarchy [101] to avoid such rank reversals. However, individuals in stable groups typically recognize and abide by established dominance relationships because of the costs of repeated conflict.
Empirical work indicates that dominance hierarchies are largely stable. Challenges by subordinates are rare and dominants often maintain their status for much of their lifetime [81,102–104]. Often, long periods of hierarchy stability are interspersed with short periods of competition following the death of dominant or other changes in group membership [105]. Strauss & Holekamp [77] used 27 years of field data to show that rank reversals are relatively uncommon in hyena groups, but some individuals can improve their position in the hierarchy by working together with top allies. The stability of dominance hierarchies has also been studied by experimentally inducing conflict over rank. Cant et al. [106] experimentally induced conflict in groups of queen wasps by removing the dominant queen, allowing a subordinate to take over as the new dominant, then releasing the original dominant. In 34 of 35 trials, the original dominant retook her dominance position. Future research using long-term field observation and experiments inducing conflict over rank will provide additional insight into the long-term stability of dominance ranks and the factors that influence hierarchy stability [79].
There are two types of mechanisms that maintain hierarchy stability. First, multiple behaviours maintain the supremacy of the dominant, including punishment, threats and self-reinforcing behavioural differences. Second, social taxa have signals that provide information about rank and maintain stable hierarchies, especially individual identity signals and signals of dominance.
(a) . Behaviours that maintain hierarchies
Dominants maintain their rank through threats, punishment and self-reinforcing behavioural mechanisms. A range of threat behaviours including dominance displays and minor aggression may maintain dominance relationships by providing information about an impending threat of eviction or attack by the dominants toward subordinates [18,107]. Threats are easily overlooked by researchers because many threat behaviours are subtle and effective threats rarely need to be carried out. Therefore, although threats may play key a role in maintaining dominance hierarchies [18,107], we have less direct experimental evidence of threats than other dominance maintaining behaviours.
While subtle threats can be difficult to quantify, there is strong evidence that punishment is involved in maintaining dominance hierarchies. We use Clutton-Brock's definition of punishment [19], retaliatory infliction of fitness reduction. Dominants punish subordinates via numerous behaviours, including eviction, aggression, social stress and infanticide. For example, some fishes have size-based dominance hierarchies where subordinates restrain their growth to maintain a minimum size difference between dominants and subordinates. When researchers created groups where the difference between dominant and subordinate size was smaller than the minimum difference observed in natural groups, dominants responded by forcibly evicting subordinates [108]. In taxa where dominants monopolize reproduction, dominants may punish subordinates attempting to maintain higher reproductive potential with aggression or by killing their eggs or offspring [19,39]. For example, subordinate wasps experimentally forced to maintain high reproductive potential are aggressively attacked by dominants [109]. Finally, subordinates that fail to contribute to the group by avoiding alloparental care or foraging may be aggressively attacked by dominants [110].
Dominance hierarchies can also be maintained without direct punishment or threats by dominants. In fact, dominance hierarchy position is often self-reinforcing because there are rank-linked disparities in feeding, work, and physical condition [111]. Dominants typically have priority access to food resources [102,103,112,113]. Access to more, higher quality food will accentuate initial differences in RHP or fertility between dominants and subordinates. In many taxa, subordinates also perform energetically expensive tasks like foraging more frequently than dominants [102,103,114]. The disparities between dominants and subordinates maintain reproductive and behavioural dominance hierarchies, so it is more difficult for individuals to increase their rank after a prolonged period as a subordinate.
(b) . Signals that provide information about dominance rank
(i) . Individual identity signals
The most common way to identify dominance rank in stable groups is individual recognition (figure 3) [20]. During individual recognition, receivers learn the unique phenotype of conspecifics (termed individual identity signals), associate the phenotype with individual-specific information and recall the phenotype-information link during subsequent interactions [20]. Learning the unique phenotype and dominance rank of conspecifics provides a precise, non-cheatable method of assessing dominance relationships [115]. As a result, individual recognition plays an important role in dominance interactions in diverse taxa [20].
Individual recognition is a nearly universal mechanism involved in dominance relationships in vertebrate taxa where there are repeated interactions between a limited number of individuals. For example, individual recognition via plumage and calls is thought to maintain hierarchies in many stable bird flocks [116]. Primates individually identify group members using visual, olfactory and acoustic information [66]. Many taxa have surprisingly sophisticated knowledge about other individuals and their dominance relationships. For example, ravens know the unique calls and relative dominance ranks of individuals in their own group and neighbouring groups [117]. Baboons learn the individual identity of all group members and classify individuals according to both rank and kinship [118]. Individual recognition also occurs in invertebrates, though it is less widespread than vertebrates. Lobsters and crayfish use unique odours to maintain stable relationships with territorial neighbours [119]. Polistes fuscatus wasps learn the unique facial patterns and of many rivals and remember individual rivals over a week of separation [26,120].
Using individual recognition to keep track of dominance relationships is thought to have substantial cognitive costs. Individual recognition may be more costly than other types of recognition because it requires precise perception, learning and memory. Developing and maintaining the sensory and nervous tissues required to assess and remember individual identity signals involves constitutive costs [121,122]. For example, P. fuscatus wasps have visual and cognitive adaptations to facilitate individual recognition [123–125]. There are also operating costs associated with learning the unique features of many individuals, including the time, energy and resources required to collect, store and recall information. Long-term memory formation also reduces immunity, survival and fecundity [126,127].
The cognitive challenge of keeping track of many individuals has long been thought to play a key role in the evolution of brain size and cognitive abilities (social intelligence hypothesis). The social intelligence hypothesis proposes that enhanced cognition is favoured in species that live in complex societies because individuals with superior cognitive capacity can keep track of more individual relationships and respond appropriately during interactions [66,128,129]. Consistent with the social intelligence hypothesis, species that keep track of more individually differentiated social relationships often have larger brains, brain regions or enhanced cognition compared with species that have less complex social behaviour [68–70]. Although there is strong evidence that social behaviour influences cognition, social behaviour is only one of multiple selection pressures that shapes cognitive evolution [130,131].
Individual recognition often maintains dominance relationships in vertebrate groups when a limited number of individuals interact repeatedly, but the use of individual recognition is highly context-dependent. For example, Gelada baboons individually recognize close group members, but do not individually identify all the individuals they regularly encounter [118]. Many birds use individual recognition to mediate dominance relationships in small, stable groups, but the same species use status signals to assess rivals in large, unstable groups [29,30]. Polistes fuscatus nest-founding queens use individual recognition in multiple social contexts, but workers are much less adept at individual recognition and rely primarily on signals of dominance [132]. These studies highlight the importance of testing recognition in multiple social contexts instead of assuming that recognition measured in a single situation is universally applicable. As a result, there is much room for future work exploring what animals know about themselves and other individuals as well as how this social knowledge varies across contexts.
(ii) . Signals of dominance
Signals of dominance provide information about the signaller's dominance status and also influence receiver behaviour (figure 2). Chemical signals of dominance are ubiquitous in social insects. Dominance in social insect colonies is commonly defined as the individual that monopolizes reproduction [3], so dominance signals provide information about queen fertility and dominance rank [133–135]. Dominance signals also influence the behaviour of nest-mates, as workers and subordinate queens limit their own fertility in response to the queen's dominance signal [21]. Dominance is inherently relational, so the phenotype of reliable signals of dominance change as an individual's rank changes. For example, if a new individual takes over as the dominant queen, her chemical profile becomes queen-like [136].
Signals of dominance are a straightforward mechanism to rapidly and accurately assess dominance rank with relatively little cognitive investment. Responding appropriately to signals of dominance requires little learning and memory. Instead, individuals may treat any individual with a particular signal phenotype as the dominant without needing to learn the specific phenotype of their dominant. Consistent with a conserved receiver response, dominance signals in social insects are similar across at least three independent origins of eusociality and may have originated as fertility cues in the common solitary ancestor of all ants, bees and wasps, which lived approximately 145 Ma [135]. Although a few insects are capable of individual recognition [26,137], current work suggests that social insects primarily rely on dominance signals to maintain stable hierarchies on nests. Dominance signals provide a straightforward way to identify rank with lower cognitive cost than individual recognition [132].
‘Dominance signals’, ‘status signals’ and ‘signals of fighting ability’ are terms that are sometimes used interchangeably, but they are quite distinct (figure 3). Signals of dominance are flexible traits that provide information about current dominance status, but not an individual's intrinsic fighting ability or RHP. Status signals or signals of fighting ability provide information about aspects of intrinsic ability like RHP [28,47]. Status signals are used to minimize conflict during dominance establishment and are often correlated with dominance rank. However, status signals do not provide information about rank per se. Polistes dominula paper wasps have both chemical signals of dominance and visual signals of status. On stable nests, Polistes use chemical signals to assess which individual is the dominant queen and chemical signals of dominance change as an individual's rank changes [138,139]. Polistes dominula also have visual signals that are used to assess the fighting ability of unknown rivals. The visual signals of fighting ability probably minimize the cost of conflict by allowing individuals to avoid escalated conflict with strong rivals [46,140]. Signals of fighting ability are one of multiple factors that mediate dominance hierarchy establishment, but they are not involved in maintaining hierarchies on nests. In fact, wasps ignore visual status signals on stable nests [141].
4. Opportunities for future research
Dominance hierarchies have been the focus of much research in the 100 years since Schjelderup-Ebbe's [2,142] pioneering work in domestic fowl. It has become clear that dominance relationships are common in social taxa, as they provide a way for interacting animals to manage the trade-offs inherent in social interactions. New methods and analyses have facilitated recent advances in our understanding of the diversity and complexity of dominance interactions [77,143,144]. Here we review several approaches and opportunities for future research on dominance hierarchies.
(a) . Self-organizing social dynamics
While it is clear that individual attributes have a huge effect on dominance hierarchy position, less is known about how other factors, especially self-organizing social dynamics, interact with individual attributes to influence rank [8]. This topic was tackled in wild baboons, finding that baboon hierarchies are based on both individual differences in fighting ability and winner–loser effects [89]. Notably, individual susceptibility to both mechanisms may have a genetic basis, suggesting that self-organizing dynamics co-evolve with individual attributes. Additional theory and experiments will be useful to test whether there are certain types of societies where self-organizing dynamics are more versus less important. For example, self-organization may be more important in societies where orderly hierarchies with transitive properties are most beneficial. By contrast, large or unstable social groups may be less likely to have self-organizing social dynamics. Newer network analysis methods will allow us to consider the role of self-organization in a way that was not previously possible [145].
(b) . Hierarchy stability
Thus far, there has been more research on the factors that influence dominance hierarchy establishment than the factors involved in maintaining hierarchy stability [146]. Both theoretical and empirical work suggests that maintaining stable social hierarchies is fundamental to successful, long-lasting social groups. Hierarchy stability reduces conflict, saves energy, promotes survival and increases reproductive success [16,17,99,147]. At the same time, it is clear that there is extensive variation in both the stability of hierarchies and the factors that maintain stability. We have much to learn about the types of hierarchies that are the most stable as well as the factors that disrupt stability. Experiments can provide insight into both the behaviours that maintain social stability and the potential costs of instability. For example, when the queen Streblognathus peetersi ant is treated with a hormone that alters the queen's signal of dominance and fertility, queens are aggressively removed from their position as dominant and a new individual takes over as queen [148]. Network analyses also offer new opportunities to analyse the nuances of hierarchy structure as well as how and why stability varies [84,144,149].
(c) . Plasticity
Animal behaviour is highly plastic, with individuals expressing different behavioural phenotypes in response to differences in the social environment, ecology or an individual's internal state [150] but see [151]. The mechanisms involved in dominance hierarchies are unlikely to be static. Instead, the way animals establish and maintain hierarchies may vary with traits like ecology (e.g. habitat saturation, food availability), social behaviour (e.g. group size, group consistency, costs and benefits of dominance rank) or individual characteristics (e.g. age, RHP, cognition, experience, genotype). Although relatively little is known about intra-specific plasticity in hierarchy formation and maintenance, there is some intriguing evidence of plasticity [152]. For example, the strength of winner/loser effects varies with the social environment [89,91,92], suggesting that the role of self-organizing social dynamics in hierarchy formation may also vary. In addition, animals communicate about dominance in different ways across different social contexts [29,30,116]. For example, P. fuscatus nest-founding queens use individual recognition to identify the rank of conspecifics prior to nest foundation [25,26], while workers largely ignore individual identity signals [132]. Instead, workers probably use chemical signals of dominance to assess dominance rank on stable nests [139]. Plasticity can be an experimental challenge because it is difficult to draw broad conclusions based on research conducted in a single situation. However, plasticity also presents exciting experimental opportunities to examine how and why dominance behaviour varies.
(d) . Cognition
Cognition has an important role in the behaviours used to establish and maintain hierarchies. Although there is debate about what constitutes ‘advanced’ versus ‘simple’ cognitive tasks, it is clear that there is variation in how much learning, memory, sensory perception, abstraction and deduction are required for different dominance behaviours. For example, self-assessment during fights and signals of dominance are traditionally considered to be cognitively simple behaviours, while individual recognition, transitive inference, social eavesdropping and mutual assessment during fights are typically considered more cognitively challenging tasks. The capacity for cognitively sophisticated behaviour was originally thought to be limited by taxonomic group or brain size. However, there is growing evidence that behaviours traditionally considered to be complex are used by diverse taxa [141,153]. For example, Mantis shrimp use mutual assessment [154]. Astatotilapia burtoni fish are capable of social eavesdropping and transitive inference [62]. Polistes paper wasps use individual recognition, social eavesdropping, transitive inference and mutual assessment to manage dominance relationships [25,46,61]. Therefore, we need to think about the cognitive challenge of apparently complex behaviours in more nuanced ways.
There is much room for future work at the intersection of dominance and cognition [66,72,143]. First, we have much to learn about animals' capacity for complex social knowledge and how this knowledge influences social relationships. For example, what do animals know about themselves and other individuals? How does this social knowledge vary across contexts? How do differences in social knowledge influence the way animals form and maintain hierarchies? Second, we need a more nuanced perspective on the cognitive costs or limitations associated with the behaviours involved in hierarchy formation and maintenance. It is clear that learning, memory and sensory systems involve substantial costs [122,126] and the challenge of keeping track of dominance relationships can influence cognitive evolution [67–70]. However, animals could also minimize the costs by using simple mechanisms to mediate apparently complex behaviours [153,155].
Data accessibility
This article has no additional data.
Authors' contributions
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This material is based in part upon work supported by the National Science Foundation under grant no. IOS-1557564.
References
- 1.Drews C. 1993. The concept and definition of dominance in animal behaviour. Behaviour 125, 283-313. ( 10.1163/156853993X00290) [DOI] [Google Scholar]
- 2.Schjelderup-Ebbe T. 1922. Beiträge zur sozialpsychologie des haushuhns. Z. Psychol. Physiol. Sinnesorgane, Abt, 1. Z. Psychol. 88, 225-252. [Google Scholar]
- 3.Wilson EO. 1971. The insect societies. Cambridge, MA: Belknap Press.
- 4.Shimoji H, Dobata S. 2021. The build-up of dominance hierarchies in eusocial insects. Phil. Trans. R. Soc. B 377, 20200437. ( 10.1098/rstb.2020.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann JH. 1983. On the definitions and functions of dominance and territoriality. Biol. Rev. 58, 1-20. ( 10.1111/j.1469-185X.1983.tb00379.x) [DOI] [Google Scholar]
- 6.de Vries H, Stevens J, Vervaecke H. 2006. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585-592. ( 10.1016/j.anbehav.2005.05.015) [DOI] [Google Scholar]
- 7.Appleby M. 1983. The protability of linearity in hierarchies. Anim. Behav. 31, 600-608. ( 10.1016/S0003-3472(83)80084-0) [DOI] [Google Scholar]
- 8.Chase ID, Seitz K. 2011. Self-structuring properties of dominance hierarchies: a new perspective. Adv. Genet. 75, 51-81. ( 10.1016/B978-0-12-380858-5.00001-0) [DOI] [PubMed] [Google Scholar]
- 9.Parker GA. 1974. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223-243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 10.Enquist M. 1985. Communication during aggressive interactions with particular reference to variation in choice of behaviour. Anim. Behav. 33, 1152-1161. ( 10.1016/S0003-3472(85)80175-5) [DOI] [Google Scholar]
- 11.Hurd PL. 2006. Resource holding potential, subjective resource value, and game theoretical models of aggressiveness signalling. J. Theor. Biol. 241, 639-648. ( 10.1016/j.jtbi.2006.01.001) [DOI] [PubMed] [Google Scholar]
- 12.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication. Sunderland, MA: Sinauer. [Google Scholar]
- 13.Johnstone RA. 2001. Eavesdropping and animal conflict. Proc. Natl Acad. Sci. USA 98, 9177-9180. ( 10.1073/pnas.161058798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. 2002. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl Acad. Sci. USA 99, 5744-5749. ( 10.1073/pnas.082104199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strassmann JE, Meyer DC. 1983. Gerontocracy in the social wasp, Polistes exclamans. Anim. Behav. 31, 431-438. ( 10.1016/S0003-3472(83)80063-3) [DOI] [Google Scholar]
- 16.Forkman B, Haskell M. 2004. The maintenance of stable dominance hierarchies and the pattern of aggression: support for the suppression hypothesis. Ethology 110, 737-744. ( 10.1111/j.1439-0310.2004.01009.x) [DOI] [Google Scholar]
- 17.Beaulieu M, Mboumba S, Willaume E, Kappeler PM, Charpentier MJ. 2014. The oxidative cost of unstable social dominance. J. Exp. Biol. 217, 2629-2632. [DOI] [PubMed] [Google Scholar]
- 18.Cant MA. 2011. The role of threats in animal cooperation. Proc. R. Soc. B 278, 170-178. ( 10.1098/rspb.2010.1241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clutton-Brock TH, Parker GA. 1995. Punishment in animal societies. Nature 373, 209-216. ( 10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- 20.Tibbetts EA, Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529-537. ( 10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 21.Keller L, Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787-794. ( 10.1006/anbe.1993.1092) [DOI] [Google Scholar]
- 22.Pratt AE, McLain DK, Lathrop GR. 2003. The assessment game in sand fiddler crab contests for breeding burrows. Anim. Behav. 65, 945-955. ( 10.1006/anbe.2003.2152) [DOI] [Google Scholar]
- 23.Cheney DL. 1977. The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behav. Ecol. Sociobiol. 2, 303-318. ( 10.1007/BF00299742) [DOI] [Google Scholar]
- 24.Peeters C, Monnin T, Malosse C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266, 1323-1327. ( 10.1098/rspb.1999.0782) [DOI] [Google Scholar]
- 25.Tibbetts EA, Wong E, Bonello S. 2020. Wasps use social eavesdropping to learn about individual rivals. Curr. Biol. 30, 3007-3010.e3002. ( 10.1016/j.cub.2020.05.053) [DOI] [PubMed] [Google Scholar]
- 26.Tibbetts EA. 2002. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. Lond. B 269, 1423-1428. ( 10.1098/rspb.2002.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005-12 010. ( 10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tibbetts EA. 2013. The function, development, and evolutionary stability of conventional signals of fighting ability. Adv. Study Behav. 45, 49-80. ( 10.1016/B978-0-12-407186-5.00002-1) [DOI] [Google Scholar]
- 29.Vedder O, Schut E, Magrath MJ, Komdeur J. 2010. Ultraviolet crown colouration affects contest outcomes among male blue tits, but only in the absence of prior encounters. Funct. Ecol. 24, 417-425. ( 10.1111/j.1365-2435.2009.01660.x) [DOI] [Google Scholar]
- 30.Chaine AS, Shizuka D, Block TA, Zhang L, Lyon BE. 2018. Manipulating badges of status only fools strangers. Ecol. Lett. 21, 1477-1485. ( 10.1111/ele.13128) [DOI] [PubMed] [Google Scholar]
- 31.Stapley J, Whiting MJ. 2006. Ultraviolet signals fighting ability in a lizard. Biol. Lett. 2, 169-172. ( 10.1098/rsbl.2005.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earley RL, Dugatkin LA. 2002. Eavesdropping on visual cues in green swordtail (Xiphophorus helleri) fights: a case for networking. Proc. R. Soc. Lond. B 269, 943-952. ( 10.1098/rspb.2002.1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu C, Ligneul R, Van der Henst J-B, Dreher J-C. 2017. An integrative interdisciplinary perspective on social dominance hierarchies. Trends Cogn. Sci. 21, 893-908. ( 10.1016/j.tics.2017.08.004) [DOI] [PubMed] [Google Scholar]
- 34.Vieira MC, Peixoto PE. 2013. Winners and losers: a meta-analysis of functional determinants of fighting ability in arthropod contests. Funct. Ecol. 27, 305-313. ( 10.1111/1365-2435.12051) [DOI] [Google Scholar]
- 35.Favre M, Martin JG, Festa-Bianchet M. 2008. Determinants and life-history consequences of social dominance in bighorn ewes. Anim. Behav. 76, 1373-1380. ( 10.1016/j.anbehav.2008.07.003) [DOI] [Google Scholar]
- 36.Schein MW, Fohrman MH. 1955. Social dominance relationships in a herd of dairy cattle. Br. J. Anim. Behav. 3, 45-55. ( 10.1016/S0950-5601(55)80012-3) [DOI] [Google Scholar]
- 37.David M, Auclair Y, Cézilly F. 2011. Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Anim. Behav. 81, 219-224. ( 10.1016/j.anbehav.2010.10.008) [DOI] [Google Scholar]
- 38.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 39.Young A. 2009. The causes of physiological suppression in vertebrate. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB), pp. 397-436. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 40.Archer J. 1988. The behavioural biology of aggression. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 41.Hardy IC, Briffa M. 2013. Animal contests. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Arnott G, Elwood RW. 2009. Assessment of fighting ability in animal contests. Anim. Behav. 77, 991-1004. ( 10.1016/j.anbehav.2009.02.010) [DOI] [Google Scholar]
- 43.Taylor P, Elwood RW. 2003. The mismeasure of animal contests. Anim. Behav. 65, 1195-1202. ( 10.1006/anbe.2003.2169) [DOI] [Google Scholar]
- 44.Pinto NS, Palaoro AV, Peixoto PE. 2019. All by myself? Meta-analysis of animal contests shows stronger support for self than for mutual assessment models. Biol. Rev. 94, 1430-1442. [DOI] [PubMed] [Google Scholar]
- 45.Elwood RW, Arnott G. 2012. Understanding how animals fight with Lloyd Morgan's canon. Anim. Behav. 84, 1095-1102. ( 10.1016/j.anbehav.2012.08.035) [DOI] [Google Scholar]
- 46.Tibbetts EA, Mettler A, Levy S. 2010. Mutual assessment via visual status signals in Polistes dominulus wasps. Biol. Lett. 6, 10-13. ( 10.1098/rsbl.2009.0420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 48.Backwell PR, Christy JH, Telford SR, Jennions MD, Passmore J. 2000. Dishonest signalling in a fiddler crab. Proc. R. Soc. Lond. B 267, 719-724. ( 10.1098/rspb.2000.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner WE. 1989. Fighting, assessment, and frequency alteration in Blanchard's cricket frog. Behav. Ecol. Sociobiol. 25, 429-436. ( 10.1007/BF00300189) [DOI] [Google Scholar]
- 50.Guilford T, Dawkins MS. 1995. What are conventional signals? Anim. Behav. 49, 1689-1695. ( 10.1016/0003-3472(95)90090-X) [DOI] [Google Scholar]
- 51.Anderson RC, Searcy WA, Hughes M, Nowicki S. 2012. The receiver-dependent cost of soft song: a signal of aggressive intent in songbirds. Anim. Behav. 83, 1443-1448. ( 10.1016/j.anbehav.2012.03.016) [DOI] [Google Scholar]
- 52.Chaine AS, Roth AM, Shizuka D, Lyon BE. 2013. Experimental confirmation that avian plumage traits function as multiple status signals in winter contests. Anim. Behav. 86, 409-415. ( 10.1016/j.anbehav.2013.05.034) [DOI] [Google Scholar]
- 53.Peake T. 2005. Eavesdropping in communication. In Animal communication networks (ed. McGregor PK), pp. 13-37. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 54.Bonnie KE, Earley RL. 2007. Expanding the scope for social information use. Anim. Behav. 74, 171-181. ( 10.1016/j.anbehav.2006.12.009) [DOI] [Google Scholar]
- 55.Oliveira RF, McGregor PK, Latruffe C. 1998. Know thine enemy: fighting fish gather information from observing conspecific interactions. Proc. R. Soc. Lond. B 265, 1045-1049. ( 10.1098/rspb.1998.0397) [DOI] [Google Scholar]
- 56.Valone TJ. 2007. From eavesdropping on performance to copying the behavior of others: a review of public information use. Behav. Ecol. Sociobiol. 62, 1-14. ( 10.1007/s00265-007-0439-6) [DOI] [Google Scholar]
- 57.Bower J. 2005. The occurrence and function of victory displays within communication networks. In Animal communication networks (ed. McGregor PK), pp. 114-126. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.Mesterton-Gibbons M, Sherratt TN. 2013. Signalling victory to ensure dominance: a continuous model. In Advances in dynamic games (eds Cardaliaguet P, Cressman R), pp. 25-38. Berlin, Germany: Springer. [Google Scholar]
- 59.Mouterde SC, Duganzich DM, Molles LE, Helps S, Helps F, Waas JR. 2012. Triumph displays inform eavesdropping little blue penguins of new dominance asymmetries. Anim. Behav. 83, 605-611. ( 10.1016/j.anbehav.2011.11.032) [DOI] [Google Scholar]
- 60.Vasconcelos M. 2008. Transitive inference in non-human animals: an empirical and theoretical analysis. Behav. Process. 78, 313-334. ( 10.1016/j.beproc.2008.02.017) [DOI] [PubMed] [Google Scholar]
- 61.Tibbetts EA, Agudelo J, Pandit S, Riojas J. 2019. Transitive inference in Polistes paper wasps. Biol. Lett. 15, 20190015. ( 10.1098/rsbl.2019.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosenick L, Clement TS, Fernald RD. 2007. Fish can infer social rank by observation alone. Nature 445, 429-432. ( 10.1038/nature05511) [DOI] [PubMed] [Google Scholar]
- 63.Nakamaru M, Sasaki A. 2003. Can transitive inference evolve in animals playing the hawk–dove game? J. Theor. Biol. 222, 461-470. ( 10.1016/S0022-5193(03)00059-6) [DOI] [PubMed] [Google Scholar]
- 64.Bond AB, Kamil AC, Balda RP. 2003. Social complexity and transitive inference in corvids. Anim. Behav. 65, 479-487. ( 10.1006/anbe.2003.2101) [DOI] [Google Scholar]
- 65.MacLean EL, Merritt DJ, Brannon EM. 2008. Social complexity predicts transitive reasoning in prosimian primates. Anim. Behav. 76, 479-486. ( 10.1016/j.anbehav.2008.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seyfarth RM, Cheney DL. 2015. Social cognition. Anim. Behav. 103, 191-202. ( 10.1016/j.anbehav.2015.01.030) [DOI] [Google Scholar]
- 67.Dunbar RI. 1998. The social brain hypothesis. Evol. Anthropol.: Issues News Rev. 6, 178-190. () [DOI] [Google Scholar]
- 68.MacLean EL, Barrickman NL, Johnson EM, Wall CE. 2009. Sociality, ecology, and relative brain size in lemurs. J. Hum. Evol. 56, 471-478. ( 10.1016/j.jhevol.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 69.Perez-Barberia FJ, Shultz S, Dunbar RIM. 2007. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution 61, 2811-2821. ( 10.1111/j.1558-5646.2007.00229.x) [DOI] [PubMed] [Google Scholar]
- 70.Sakai ST, Arsznov BM, Lundrigan BL, Holekamp KE. 2011. Brain size and social complexity: a computed tomography study in Hyaenidae. Brain Behav. Evol. 77, 91-104. ( 10.1159/000323849) [DOI] [PubMed] [Google Scholar]
- 71.Ashton BJ, Ridley AR, Edwards EK, Thornton A. 2018. Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature 554, 364-367. ( 10.1038/nature25503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hobson EA. 2020. Differences in social information are critical to understanding aggressive behavior in animal dominance hierarchies. Curr. Opin. Psychol. 33, 209-215. ( 10.1016/j.copsyc.2019.09.010) [DOI] [PubMed] [Google Scholar]
- 73.Lewis D. 2008. Convention: a philosophical study. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 74.Horrocks J, Hunte W. 1983. Maternal rank and offspring rank in vervet monkeys: an appraisal of the mechanisms of rank acquisition. Anim. Behav. 31, 772-782. ( 10.1016/S0003-3472(83)80234-6) [DOI] [Google Scholar]
- 75.Taylor BA, Cini A, Cervo R, Reuter M, Sumner S. 2020. Queen succession conflict in the paper wasp Polistes dominula is mitigated by age-based convention. Behav. Ecol. 31, 992-1002. ( 10.1093/beheco/araa045) [DOI] [Google Scholar]
- 76.Holekamp KE, Smale L. 1991. Dominance acquisition during mammalian social development: the ‘inheritance’ of maternal rank. Am. Zool. 31, 306-317. ( 10.1093/icb/31.2.306) [DOI] [Google Scholar]
- 77.Strauss ED, Holekamp KE. 2019. Social alliances improve rank and fitness in convention-based societies. Proc. Natl Acad. Sci. USA 116, 8919-8924. ( 10.1073/pnas.1810384116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapais, B. 1992. The role of alliances in social inheritance of rank among female primates. In Coalitions and alliances in humans and other mammals (eds Harcourt A, de Waal FBM), pp. 29-60. Oxford, UK: Oxford University Press. [Google Scholar]
- 79.Broom M, Koenig A, Borries C. 2009. Variation in dominance hierarchies among group-living animals: modeling stability and the likelihood of coalitions. Behav. Ecol. 20, 844-855. ( 10.1093/beheco/arp069) [DOI] [Google Scholar]
- 80.Thavarajah NK, Fenkes M, Clutton-Brock TH. 2014. The determinants of dominance relationships among subordinate females in the cooperatively breeding meerkat. Behaviour 151, 89-102. ( 10.1163/1568539X-00003124) [DOI] [Google Scholar]
- 81.Hausfater G, Altmann J, Altmann S. 1982. Long-term consistency of dominance relations among female baboons (Papio cynocephalus). Science 217, 752-755. ( 10.1126/science.217.4561.752) [DOI] [PubMed] [Google Scholar]
- 82.Silk JB. 2009. Nepotistic cooperation in non-human primate groups. Phil. Trans. R. Soc. B 364, 3243-3254. ( 10.1098/rstb.2009.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE. 2016. Chimpanzee females queue but males compete for social status. Sci. Rep. 6, 1-11. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shizuka D, McDonald DB. 2012. A social network perspective on measurements of dominance hierarchies. Anim. Behav. 83, 925-934. ( 10.1016/j.anbehav.2012.01.011) [DOI] [Google Scholar]
- 85.Dey CJ, Quinn JS. 2014. Individual attributes and self-organizational processes affect dominance network structure in pukeko. Behav. Ecol. 25, 1402-1408. ( 10.1093/beheco/aru138) [DOI] [Google Scholar]
- 86.Hsu Y, Earley RL, Wolf LL. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33-74. ( 10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 87.Dugatkin LA. 1997. Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 8, 583-587. ( 10.1093/beheco/8.6.583) [DOI] [Google Scholar]
- 88.Rutte C, Taborsky M, Brinkhof MW. 2006. What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16-21. ( 10.1016/j.tree.2005.10.014) [DOI] [PubMed] [Google Scholar]
- 89.Franz M, McLean E, Tung J, Altmann J, Alberts SC. 2015. Self-organizing dominance hierarchies in a wild primate population. Proc. R. Soc. B 282, 20151512. ( 10.1098/rspb.2015.1512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dugatkin LA, Earley RL. 2003. Group fusion: the impact of winner, loser, and bystander effects on hierarchy formation in large groups. Behav. Ecol. 14, 367-373. ( 10.1093/beheco/14.3.367) [DOI] [Google Scholar]
- 91.Fuxjager MJ, Mast G, Becker EA, Marler CA. 2009. The ‘home advantage'is necessary for a full winner effect and changes in post-encounter testosterone. Horm. Behav. 56, 214-219. ( 10.1016/j.yhbeh.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 92.Oliveira RF. 2009. Social behavior in context: hormonal modulation of behavioral plasticity and social competence. Integr. Comp. Biol. 49, 423-440. ( 10.1093/icb/icp055) [DOI] [PubMed] [Google Scholar]
- 93.Dugatkin LA. 2001. Bystander effects and the structure of dominance hierarchies. Behav. Ecol. 12, 348-352. ( 10.1093/beheco/12.3.348) [DOI] [Google Scholar]
- 94.Clotfelter ED, Paolino AD. 2003. Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Anim. Behav. 66, 343-347. ( 10.1006/anbe.2003.2227) [DOI] [Google Scholar]
- 95.Chase ID. 1982. Dynamics of hierarchy formation: the sequential development of dominance relationships. Behaviour 80, 218-239. ( 10.1163/156853982X00364) [DOI] [Google Scholar]
- 96.Hobson EA, DeDeo S. 2015. Social feedback and the emergence of rank in animal society. PLoS Comput. Biol. 11, e1004411. ( 10.1371/journal.pcbi.1004411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borrelli JJ, et al. 2015. Selection on stability across ecological scales. Trends Ecol. Evol. 30, 417-425. ( 10.1016/j.tree.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 98.Meese G, Ewbank R. 1973. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav. 21, 326-334. ( 10.1016/S0003-3472(73)80074-0) [DOI] [Google Scholar]
- 99.Maldonado-Chaparro AA, Alarcón-Nieto G, Klarevas-Irby JA, Farine DR. 2018. Experimental disturbances reveal group-level costs of social instability. Proc. R. Soc. B 285, 20181577. ( 10.1098/rspb.2018.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cant MA, Johnstone RA. 2000. Power struggles, dominance testing, and reproductive skew. Am. Nat. 155, 406-417. ( 10.1086/303328) [DOI] [PubMed] [Google Scholar]
- 101.Dehnen T; Papageorgiou D, Nyaguthii B, Cherono W, Penndorf J, Boogert NJ, Farine DR. 2021. Costs dictate strategic investment in dominance interactions. Phil. Trans. R. Soc. B 377, 20200447. ( 10.1098/rstb.2020.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Solomon N, French J. 1997. Cooperative breeding in mammals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 103.Stacey PB, Koenig WD. 1990. Cooperative breeding in birds: long term studies of ecology and behaviour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 104.Chiarati E, Canestrari D, Vera R, Marcos JM, Baglione V. 2010. Linear and stable dominance hierarchies in cooperative carrion crows. Ethology 116, 346-356. ( 10.1111/j.1439-0310.2010.01741.x) [DOI] [Google Scholar]
- 105.Samuels A, Silk JB, Altmann J. 1987. Continuity and change in dominance relations among female baboons. Anim. Behav. 35, 785-793. ( 10.1016/S0003-3472(87)80115-X) [DOI] [Google Scholar]
- 106.Cant M, English S, Reeve H, Field J. 2006. Escalated conflict in a social hierarchy. Proc. R. Soc. B 273, 2977-2984. ( 10.1098/rspb.2006.3669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cant M, Johnstone RA. 2009. How threats influence the evolutionary resolution of within-group conflict. Am. Nat. 173, 759-771. ( 10.1086/598489) [DOI] [PubMed] [Google Scholar]
- 108.Wong MY, Buston PM, Munday PL, Jones GP. 2007. The threat of punishment enforces peaceful cooperation and stabilizes queues in a coral-reef fish. Proc. R. Soc. B 274, 1093-1099. ( 10.1098/rspb.2006.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tibbetts EA, Fearon ML, Wong E, Huang ZY, Tinghitella RM. 2018. Rapid juvenile hormone downregulation in subordinate wasp queens facilitates stable cooperation. Proc. R. Soc. B 285, 20172645. ( 10.1098/rspb.2017.2645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reeve HK. 1992. Queen activation of lazy workers in colonies of the eusocial naked mole-rat. Nature 358, 147-149. ( 10.1038/358147a0) [DOI] [PubMed] [Google Scholar]
- 111.Young AJ. 2009. The causes of physiological suppression in vertebrate societies: a synthesis. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB), pp. 397-436. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 112.Ekman J, Liliendahl K. 1993. Using priority to food access: fattening strategies in dominance-structured willow tit (Parus montanus) flocks. Behav. Ecol. 4, 232-238. ( 10.1093/beheco/4.3.232) [DOI] [Google Scholar]
- 113.Barrette C, Vandal D. 1986. Social Rank, dominance, antler size, and access to food in snow-bound wild woodland caribou. Behaviour 97, 118-145. ( 10.1163/156853986X00342) [DOI] [Google Scholar]
- 114.West-Eberhard M. 1969. The social biology of polistine wasps. Miscellaneous Publications of the Museum of Zoology University Michigan 140, pp. 1-101. [Google Scholar]
- 115.Barnard C, Burk T. 1979. Dominance hierarchies and the evolution of ‘individual recognition’. J. Theor. Biol. 81, 65-73. ( 10.1016/0022-5193(79)90081-X) [DOI] [PubMed] [Google Scholar]
- 116.Whitfield DP. 1987. Plumage variability, status signalling and individual recognition in avian flocks. Trends Ecol. Evol. 2, 13-18. ( 10.1016/0169-5347(87)90194-7) [DOI] [PubMed] [Google Scholar]
- 117.Massen JJ, Pašukonis A, Schmidt J, Bugnyar T. 2014. Ravens notice dominance reversals among conspecifics within and outside their social group. Nat. Commun. 5, 1-7. ( 10.1038/ncomms4679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bergman TJ. 2010. Experimental evidence for limited vocal recognition in a wild primate: implications for the social complexity hypothesis. Proc. R. Soc. B 277, 3045-3053. ( 10.1098/rspb.2010.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karavanich C, Atema J. 1998. Individual recognition and memory in lobster dominance. Anim. Behav. 56, 1553-1560. ( 10.1006/anbe.1998.0914) [DOI] [PubMed] [Google Scholar]
- 120.Sheehan MJ, Tibbetts EA. 2008. Robust long-term social memories in a paper wasp. Curr. Biol. 18, R851-R852. ( 10.1016/j.cub.2008.07.032) [DOI] [PubMed] [Google Scholar]
- 121.Snell-Rood EC, Papaj DR, Gronenberg W. 2009. Brain size: a global or induced cost of learning? Brain Behav. Evol. 73, 111-128. ( 10.1159/000213647) [DOI] [PubMed] [Google Scholar]
- 122.Dukas R. 2004. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 35, 347-374. ( 10.1146/annurev.ecolsys.35.112202.130152) [DOI] [Google Scholar]
- 123.Sheehan MJ, Tibbetts EA. 2011. Specialized face learning is associated with individual recognition in paper wasps. Science 334, 1272-1275. ( 10.1126/science.1211334) [DOI] [PubMed] [Google Scholar]
- 124.Gronenberg W, Ash LE, Tibbetts EA. 2008. Correlation between facial pattern recognition and brain composition in paper wasps. Brain Behav. Evol. 71, 1-14. ( 10.1159/000108607) [DOI] [PubMed] [Google Scholar]
- 125.Sheehan MJ, Jinn J, Tibbetts EA. 2014. Coevolution of visual signals and eye morphology in Polistes paper wasps. Biol. Lett. 10, 20140254. ( 10.1098/rsbl.2014.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mery F, Kawecki TJ. 2005. A cost of long-term memory in Drosophila. Science 308, 1148. ( 10.1126/science.111131) [DOI] [PubMed] [Google Scholar]
- 127.Snell-Rood EC, Davidowitz G, Papaj DR. 2011. Reproductive tradeoffs of learning in a butterfly. Behav. Ecol. 22, 291-302. ( 10.1093/beheco/arq169) [DOI] [Google Scholar]
- 128.Jolly A. 1966. Lemur social behavior and primate intelligence. Science 153, 501. ( 10.1126/science.153.3735.501) [DOI] [PubMed] [Google Scholar]
- 129.Dunbar RIM. 2003. The social brain: mind, language, and society in evolutionary perspective. Annu. Rev. Anthropol. 32, 163-181. ( 10.1146/annurev.anthro.32.061002.093158) [DOI] [Google Scholar]
- 130.DeCasien AR, Williams SA, Higham JP. 2017. Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 1, 1-7. ( 10.1038/s41559-017-0112) [DOI] [PubMed] [Google Scholar]
- 131.Rosati AG. 2017. Foraging cognition: reviving the ecological intelligence hypothesis. Trends Cogn. Sci. 21, 691-702. ( 10.1016/j.tics.2017.05.011) [DOI] [PubMed] [Google Scholar]
- 132.Tibbetts EA, Injaian A, Sheehan MJ, Desjardins N. 2018. Intraspecific variation in learning: worker wasps are less able to learn and remember individual conspecific faces than queen wasps. Am. Nat. 191, 595-603. ( 10.1086/696848) [DOI] [PubMed] [Google Scholar]
- 133.Oi CA, da Silva RC, Stevens I, Ferreira HM, Nascimento FS, Wenseleers T. 2021. Hormonal modulation of reproduction and fertility signaling in polistine wasps. Curr. Zool. 67, 519-530. ( 10.1093/cz/zoab026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Korb J. 2018. Chemical fertility signaling in termites: idiosyncrasies and commonalities in comparison with ants. J. Chem. Ecol. 44, 818-826. ( 10.1007/s10886-018-0952-2) [DOI] [PubMed] [Google Scholar]
- 135.Van Oystaeyen A, et al. 2014. Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287-290. ( 10.1126/science.1244899) [DOI] [PubMed] [Google Scholar]
- 136.Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B. 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl Acad. Sci. USA 97, 4124-4131. ( 10.1073/pnas.97.8.4124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.D'Ettorre P, Heinze J. 2005. Individual recognition in ant queens. Curr. Biol. 23, 2170-2174. ( 10.1016/j.cub.2005.10.067) [DOI] [PubMed] [Google Scholar]
- 138.Izzo A, Wells M, Huang Z, Tibbetts E. 2010. Cuticular hydrocarbons correlate with fertility, not dominance, in a paper wasp, Polistes dominulus. Behav. Ecol. Sociobiol. 64, 857-864. ( 10.1007/s00265-010-0902-7) [DOI] [Google Scholar]
- 139.Smith A, Liebig J. 2017. The evolution of cuticular fertility signals in eusocial insects. Curr. Opin. Insect Sci. 22, 79-84. ( 10.1016/j.cois.2017.05.017) [DOI] [PubMed] [Google Scholar]
- 140.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature 432, 218-222. ( 10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 141.Simons M, Tibbetts EA. 2021. Signal response is context-dependent in Polistes dominula. J. Ethol. 39, 417-422. [Google Scholar]
- 142.Hobson EA. 2021. Quantifying the dynamics of nearly 100 years of dominance hierarchy research. Phil. Trans. R. Soc. B 377, 20200433. ( 10.1098/rstb.2020.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hobson EA, Monster D, DeDeo S. 2021. Aggression heuristics underlie animal dominance hierarchies and provide evidence of group-level social information. Proc. Natl Acad. Sci. USA 118, e2022912118. ( 10.1073/pnas.2022912118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pinter-Wollman N, et al. 2014. The dynamics of animal social networks: analytical, conceptual, and theoretical advances. Behav. Ecol. 25, 242-255. ( 10.1093/beheco/art047) [DOI] [Google Scholar]
- 145.McDonald DB, Shizuka D. 2013. Comparative transitive and temporal orderliness in dominance networks. Behav. Ecol. 24, 511-520. ( 10.1093/beheco/ars192) [DOI] [Google Scholar]
- 146.Strauss ED, Shizuka D. 2021. The dynamics of dominance: open questions, challenges and solutions. Phil. Trans. R. Soc. B 377, 20200445. ( 10.1098/rstb.2020.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sapolsky RM. 1983. Endocrine aspects of social instability in the olive baboon (Papio anubis). Am. J. Primatol. 5, 365-379. ( 10.1002/ajp.1350050406) [DOI] [PubMed] [Google Scholar]
- 148.Cuvillier-Hot V, Lenoir A, Peeters C. 2004. Reproductive monopoly enforced by sterile police workers in a queenless ant. Behav. Ecol. 15, 970-975. ( 10.1093/beheco/arh072) [DOI] [Google Scholar]
- 149.Farine DR, Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144-1163. ( 10.1111/1365-2656.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004-1011. ( 10.1016/j.anbehav.2012.12.031) [DOI] [Google Scholar]
- 151.Sih A, Bell J, Johnson J. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372-378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 152.Milewski TM, Lee W, Champagne FA, Curley JP. 2021. Behavioural and physiological plasticity in social hierarchies. Phil. Trans. R. Soc. B 377, 20200443. ( 10.1098/rstb.2020.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995-R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 154.Green P, Patek S. 2018. Mutual assessment during ritualized fighting in mantis shrimp (Stomatopoda). Proc. R. Soc. B 285, 20172542. ( 10.1098/rspb.2017.2542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fawcett T, Mowles S. 2013. Assessments of fighting ability need not be cognitively complex. Anim. Behav. 5, e1-e7. ( 10.1016/j.anbehav.2013.05.033) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.